Abstract
We explored determinants of depressive mood in adults with coronary artery disease and obstructive sleep apnea and response to positive airway pressure treatment in sleepy and non‐sleepy phenotypes. In this secondary analysis of the RICCADSA trial conducted in Sweden, 493 cardiac patients with obstructive sleep apnea (n = 386) or no obstructive sleep apnea (n = 107) with complete Epworth Sleepiness Scale and Zung Self‐rating Depression Scale questionnaires were included. Sleepy (Epworth Sleepiness Scale ≥10) versus non‐sleepy (Epworth Sleepiness Scale <10) patients with depressive mood (Zung Self‐rating Depression Scale score ≥50) were evaluated after 3 and 12 months of positive airway pressure treatment. In all, 133 patients (27.0%) had depressive mood (29.3% of obstructive sleep apnea versus 18.7% of no obstructive sleep apnea; p = 0.029), with a higher percentage among the sleepy phenotype (36.9% versus 24.5%; p = 0.009). In multivariate analysis, depressive mood was significantly associated with female sex, body mass index and Epworth Sleepiness Scale. Among 97 obstructive sleep apnea patients with depressive mood at baseline, there was a significant reduction in the scores at follow‐up both in the sleepy and non‐sleepy patients allocated to positive airway pressure treatment, whereas no significant changes were observed in the untreated group (p = 0.033). The device use (hr/night) predicted improvement in mood (odds ratio, 1.33; 95% confidence interval, 1.10–1.61; p = 0.003) adjusted for age, female sex, body mass index, left ventricular ejection fraction, apnea–hypopnea index and delta Epworth Sleepiness Scale score. We conclude that obstructive sleep apnea was associated with depressive mood in adults with coronary artery disease. Treatment with positive airway pressure improved mood in both phenotypes, independent of the confounding factors.
Keywords: coronary artery disease, daytime sleepiness, depression, obstructive sleep apnea, positive airway pressure
1. INTRODUCTION
Obstructive sleep apnea (OSA) is a common condition characterized by repeated cessation of breathing during sleep as a result of complete or partial pharyngeal obstruction and is associated with increased cardiovascular and neurocognitive morbidity (Banno & Kryger, 2007). OSA may cause excessive daytime sleepiness (EDS) and fatigue (Stoohs, Guilleminault, Itoi, & Dement, 1994) and it has been associated with anxiety and depression (Ohayon, 2003). Similarly, OSA has been reported to be more common in patients with depression (Gupta, Simpson, & Lyons, 2016).
It has also been suggested that depression may be an independent risk factor for incident coronary artery disease (CAD), as well as for increased mortality in CAD (Barth, Schumacher, & Herrmann‐Lingen, 2004). Increased plasma levels of inflammatory markers have been demonstrated in conditions with increased stress activity exacerbated by depression (Frisbee et al., 2015). Moreover, coexisting depression has been associated with increased risk of mortality in patients with CAD adjusted for the other independent predictors (Bush et al., 2001).
Continuous positive airway pressure (CPAP) is the first‐line treatment for patients with OSA with EDS (Loube et al., 1999). Randomized controlled trials (RCTs) have demonstrated favourable effects of CPAP on quality of life in addition to improvement in EDS in sleep‐clinic cohorts (Celik et al., 2016; Jenkinson, Davies, Mullins, & Stradling, 1999). Less is known regarding the contribution of OSA to depression in CAD patients and impact of CPAP treatment in the sleepy and non‐sleepy phenotypes. In the SAVE trial, comprising a large number of patients with CAD and cerebrovascular disease, CPAP treatment improved mood in the intention‐to‐treat (ITT) population (McEvoy et al., 2016).
The Randomized Intervention with CPAP in CAD and OSA (RICCADSA) trial primarily aimed to explore the impact of CPAP on the composite of repeat revascularization, myocardial infarction, stroke and cardiovascular mortality in patients with CAD and OSA (Peker et al., 2016). The current subprotocol evaluated the determinants of depressive mood at baseline and additionally addressed response to 3 and 12 months of CPAP treatment in sleepy versus non‐sleepy OSA phenotypes.
2. METHODS
As previously described (Peker et al., 2009), CAD patients with a history of percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) within 6 months prior to recruitment in the Skaraborg County of West Götaland, Sweden, were invited to participate. The patient enrollment was conducted between 2005 and 2010 and the follow‐up for the primary outcomes was ended in May 2013 (Peker et al., 2016). As shown in Figure 1a, the CAD patients were classified as OSA (apnea–hypopnea index [AHI] ≥15/h) and no‐OSA (AHI <15/h) based on the baseline cardiorespiratory polygraphy. The non‐sleepy OSA patients (Epworth Sleepiness Scale [ESS] <10) were randomized to CPAP or no‐CPAP and the sleepy OSA patients (ESS ≥10) were offered CPAP (Figure 1b). For the first part of the current protocol, all patients who completed Zung Self‐rating Depression Scale (SDS) questionnaires at baseline (n = 493) were included for evaluation of variables associated with depressive mood at baseline and 107 no‐OSA patients were chosen as controls (Figure 1a). Additional baseline comparisons were performed within the OSA group (n = 386) between the sleepy (n = 123) and non‐sleepy (n = 203) phenotypes. For the second part, the OSA patients with sleepy versus non‐sleepy phenotype, who started CPAP treatment and who had completed Zung SDS questionnaires at baseline and after 3 and 12 months (n = 123 sleepy OSA versus 99 non‐sleepy OSA), were further evaluated with regard to response to CPAP treatment (Figure 1b). The non‐sleepy OSA arm randomized to no‐CPAP (n = 104) served as an additional control group. The Ethics Committee of the Medical Faculty of the University of Gothenburg approved the study protocol (approval nr 207‐05; 09.13.2005; amendment T744‐10; 11.26.2010; amendment T512‐11; 06.16.2011) and written informed consent was provided by all patients. The trial was registered (ClinicalTrials.gov; NCT 00519597).
Figure 1.
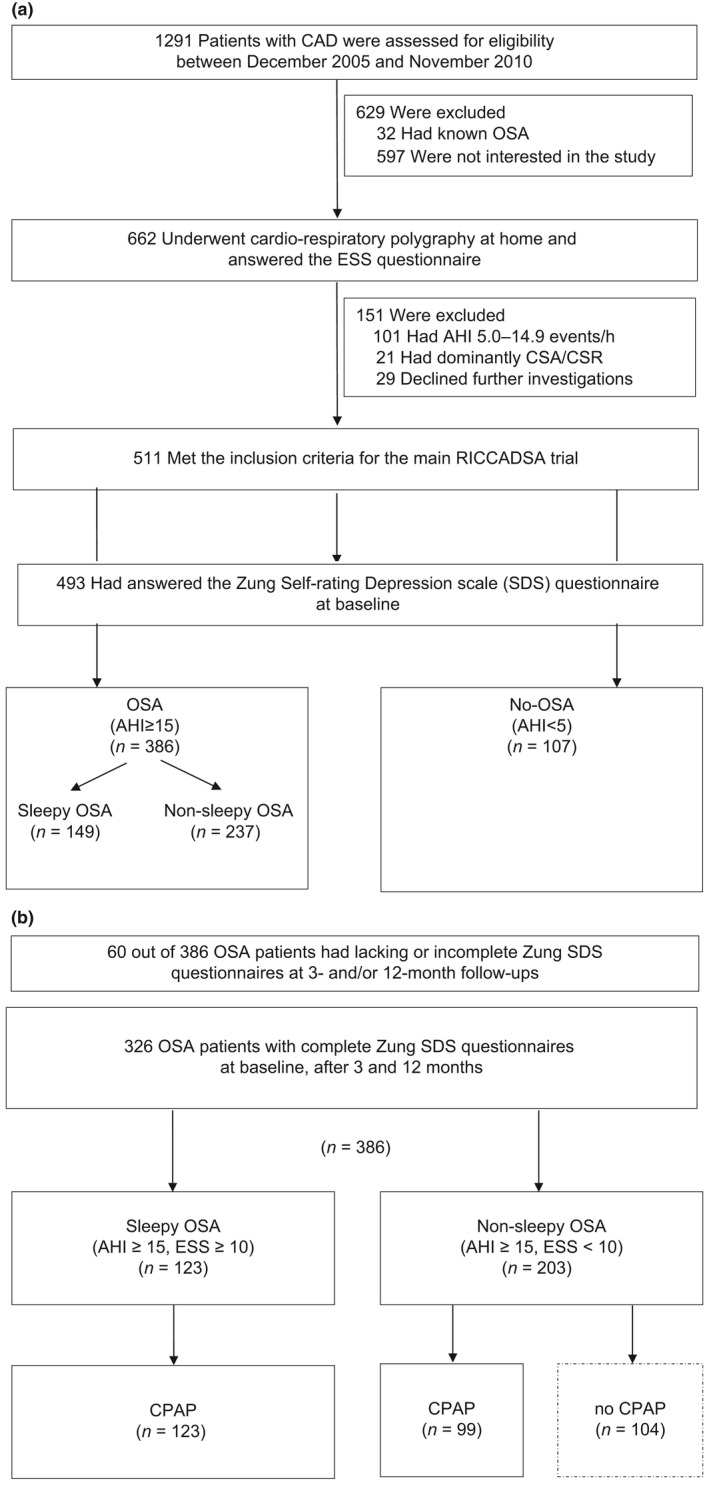
Flow of patients through the study. (a) Baseline study cohort. (b) Follow‐up population. AHI, apnea–hypopnea index; CAD; coronary artery disease; CPAP, continuous positive airway pressure; CSA‐CSR, central sleep apnea–Cheyne Stokes respiration; ESS, Epworth Sleepiness Scale; OSA, obstructive sleep apnea; RICCADSA, Randomized Intervention with CPAP in Coronary Artery Disease and Sleep Apnea; SDS, Self‐rating Depression Scale
2.1. Cardiorespiratory polygraphy
Overnight sleep studies at baseline were conducted by using the Embletta® Portable Digital System device (Embla, Broomfield, CO, USA). As previously described in detail (Peker et al., 2009), the Chicago criteria were used for apnea and hypopnea definitions (American Academy of Sleep Medicine Task Force, 1999).
2.2. Epworth Sleepiness Scale
Daytime sleepiness was assessed by the ESS questionnaire (Johns, 1991) containing eight questions regarding the chance of dozing off under eight different scenarios in the past month. The total score ranges between 0 and 24 and a cut‐off value of 10 for the ESS score was used for categorizing the patients with excessive daytime sleepiness in this protocol.
2.3. Zung Self‐rating Depression Scale
All participants were requested to complete Zung SDS questionnaire at baseline and at follow‐ups. The Zung SDS is a widely accepted questionnaire, that provides both a total score and a categorical rate of depression (Zung et al., 1965). In summary, 20 items are included and the rating scale is scored from 1 to 4 points, resulting in a real total score from 20 to 80. The real score is multiplied by 1.25, resulting in a total range of 25–100 (Zung et al., 1965). The individuals with SDS score <50 were categorized as normal, the ones with scores between 50 and 59 as having mild depressive mood, those with scores between 60 and 69 as moderate depressive mood and those with scores ≥70 as severe depressive mood (the details of the questionnaire as well as the scoring manual are provided in Supporting Information Table S1). As answers to question number 2 (“Morning is when I feel the best”) and question number 4 (“I have trouble sleeping at night”) may overlap, a separate calculation was performed excluding these items from the total scores. All answers were entered into the database by a research nurse blinded to the group allocation and clinical data.
2.4. Comorbidities
Baseline anthropometrics, smoking habits and medical histories of the entire study population were obtained from the medical records (Peker et al., 2009). Obesity was defined as a body mass index (BMI) ≥30 kg/m2 (World Health Organization, 2000).
2.5. Group assignment, randomization, interventions and follow‐up
The group assignment was based on the baseline polygraphy results and the ESS scores (Figure 1). Non‐sleepy OSA patients randomized to CPAP were provided with a self‐titrating CPAP device (S8® or S9®; San Diego, CA, USA) with a nasal or full‐face mask and humidifier. All patients receiving CPAP were contacted by telephone after 1 week and all patients were followed after 1 month, 3 months, 6 months and 1 year, and then yearly to the end of the main study (Peker et al., 2009).
2.6. Adherence to CPAP
All patients receiving CPAP brought their devices to the clinic at each scheduled follow‐up visit and CPAP hr/night and CPAP days/period were downloaded from the device and accumulated CPAP hr/night/all nights was calculated. All technical adjustments were carried out according to the clinical routines by the Sleep Medicine Unit staff. Patients who were not using the devices were followed as part of the treatment arm as defined in the intention‐to‐treat (ITT) analysis (Peker et al., 2009).
2.7. Outcomes and the sample size
The main outcomes of the current protocol were the occurrence and determinants of depressive mood at baseline in the entire RICCADSA cohort in the first part of the study and the absolute change in the Zung SDS scores in response to CPAP treatment in sleepy versus non‐sleepy OSA phenotypes in the second part of the study. In addition, a categorical variable, “improvement in mood”, was defined for the depressive mood group in terms of change into a better SDS category (no or milder depressive mood) versus worsening/no change (mild or moderate‐to‐severe depressive mood) at 1‐year follow‐up. No specific power estimate was made for the current study, as the sample size estimation for the RICCADSA trial was based on the calculations for the primary endpoints.
2.8. Statistical analysis
Data are given as mean ± standard deviation or standard error of mean for continuous variables and categorical variables are represented as numbers and percentages. An independent sample t test was used for between‐group differences in means and the chi‐squared test (or when appropriate, Fisher's exact test) was used to compare categorical variables. Only patients who had answered all 20 SDS items were included in the study. Repeated measures of SDS scores were analysed using a two‐way analysis of covariance (ANCOVA). A logistic regression analysis was applied for the patients with depressive mood at baseline to determine the variables associated with improvement in mood and odds ratios (ORs) with 95% confidence intervals (CIs) were reported. All statistical tests were two‐sided and p < 0.05 was considered statistically significant. Statistical analysis was performed using the Statistical Package for Social Sciences, version 22.0 for Windows® system (SPSS® Inc., Chicago, Illinois, USA).
3. RESULTS
3.1. Patient characteristics and variables associated with depressive mood at baseline
As illustrated in Figure 1, a total of 511 patients were included in the main study. After exclusion of 18 patients with missing SDS questionnaires at baseline, 493 (mean age 63.9 ± 8.6 years; female, 16.8%) remained for the current protocol. As shown in Table 1, OSA patients were slightly older and had higher BMI, AHI and ESS score values compared to those in the no‐OSA group. Hypertension, diabetes mellitus, CABG at baseline and history of atrial fibrillation were significantly more common in the OSA group, whereas the percentage of current smokers was higher in the no‐OSA group. In the entire study cohort, 133 (27.0%) had depressive mood (29.3% of OSA versus 18.7% of no‐OSA; p = 0.029). As shown in Table 2, depressive mood was associated with OSA and AHI, as well as with female sex, obesity, BMI, ESS and EDS, in the univariate analysis. In the multivariate model, female sex (OR, 2.50), BMI (OR, 1.01) and ESS (OR, 1.10) remained significant. When excluding the two items overlapping regarding sleep, raw total scores decreased from 34.8 ± 7.7 to 30.5 ± 7.2 in the OSA group and from 32.6 ± 8.6 to 28.6 ± 7.8 in the no‐OSA group without any meaningful between‐group difference. In total, 17.6% in the OSA group and 13.1% in the no‐OSA group had answered “little of the time” to the item “morning is when I feel the best” with no between‐group difference regarding the sleepy and non‐sleepy phenotypes (16.1% versus 18.6%). Regarding the item “I have trouble sleeping at night”, 4.9% in the OSA group and 0.9% in the no‐OSA group had replied “most of the time” with no significant between‐group difference in the sleepy and non‐sleepy OSA phenotypes (5.4% versus 4.6%, respectively). A detailed distribution of the items within the groups as well as within the OSA phenotypes is provided in Supporting Information Tables S2 and S3, respectively.
Table 1.
Baseline clinical characteristics of the study population with coronary artery disease (n = 493)
| OSA (n = 386) | No‐OSA (n = 107) | p value | |
|---|---|---|---|
| Age, years | 64.6 ± 8.2 | 61.4 ± 9.6 | 0.001 |
| Age ≥ 65 years, % | 48.7 | 38.3 | 0.057 |
| Female, % | 14.2 | 26.2 | 0.004 |
| AHI, events/hr | 30.0 ± 14.5 | 3.0 ± 1.3 | <0.001 |
| ESS score | 8.1 ± 4.1 | 5.5 ± 2.9 | <0.001 |
| EDS (ESS ≥ 10), % | 38.6 | 6.5 | <0.001 |
| BMI, kg/m2 | 28.9 ± 4.0 | 25.5 ± 3.0 | <0.001 |
| Obesity, % | 33.2 | 6.5 | <0.001 |
| Current smoker, % | 16.3 | 26.2 | 0.020 |
| Pulmonary disease, % | 8.0 | 14.0 | 0.060 |
| Hypertension, % | 61.4 | 45.8 | 0.004 |
| Diabetes mellitus, % | 24.9 | 13.1 | 0.010 |
| Acute MI at baseline, % | 50.3 | 59.8 | 0.080 |
| History of atrial fibrillation, % | 18.9 | 8.4 | 0.010 |
| Stroke, % | 7.3 | 3.8 | 0.267 |
| CABG at baseline, % | 26.7 | 15.9 | 0.021 |
| Former revascularization, % | 19.7 | 16.8 | 0.504 |
| Antidepressive medication, % | 5.3 | 3.9 | 0.798 |
| Zung SDS score | 42.8 ± 9.8 | 40.7 ± 10.7 | 0.058 |
| Depression (Zung SDS score ≥ 50), % | 29.3 | 18.7 | 0.029 |
Bold indicate significance values.
AHI, apnea–hypopnea index; BMI, body mass index; CABG, coronary artery bypass grafting; EDS, excessive daytime sleepiness; ESS, Epworth Sleepiness Scale; MI, myocardial infarction; OSA, obstructive sleep apnea; SDS, Self‐rating Depression Scale.
Table 2.
Logistic regression analysis of covariables associated with depression at baseline (n = 493)
| Odds ratio | 95% CI | p value | |
|---|---|---|---|
| Univariate | |||
| Age | 0.99 | 0.96–1.01 | 0.295 |
| Age ≥ 65 years | 0.93 | 0.62–1.39 | 0.717 |
| Female sex | 2.05 | 1.25–3.35 | 0.005 |
| OSA | 1.80 | 1.06–3.07 | 0.031 |
| BMI | 1.12 | 1.06–1.18 | <0.001 |
| Obesity | 2.14 | 1.40–3.28 | <0.001 |
| Current smoking | 1.03 | 0.62–1.72 | 0.906 |
| Hypertension | 0.72 | 0.43–1.22 | 0.231 |
| Diabetes mellitus | 1.28 | 0.81–2.04 | 0.293 |
| AMI at baseline | 1.15 | 0.77–1.72 | 0.490 |
| CABG at baseline, % | 0.82 | 0.51–1.33 | 0.426 |
| Former revascularization | 1.19 | 0.72–1.95 | 0.495 |
| Pulmonary disease | 1.21 | 0.62–2.34 | 0.579 |
| Stroke | 0.61 | 0.24–1.51 | 0.285 |
| AHI | 1.01 | 1.00–1.03 | 0.026 |
| ESS | 1.10 | 1.04–1.15 | <0.001 |
| EDS (ESS score ≥10) | 1.98 | 1.31–2.99 | 0.001 |
| Multivariate (Model 1) | |||
| AHI | 1.00 | 0.99–1.02 | 0.654 |
| Age | 1.01 | 0.98–1.03 | 0.629 |
| Female sex | 2.45 | 1.44–4.17 | 0.001 |
| BMI | 1.10 | 1.04–1.16 | 0.001 |
| ESS | 1.09 | 1.04–1.15 | 0.001 |
| Multivariate (Model 2) | |||
| OSA | 1.16 | 0.63–2.16 | 0.601 |
| Age | 1.01 | 0.98–1.03 | 0.674 |
| Female sex | 2.48 | 1.45–4.24 | 0.001 |
| BMI | 1.10 | 1.04–1.16 | 0.001 |
| ESS | 1.09 | 1.03–1.15 | 0.002 |
Bold indicate significance values.
AHI, apnea–hypopnea index; AMI, acute myocardial infarction; BMI, body mass index; CABG, coronary artery bypass grafting; EDS, excessive daytime sleepiness; ESS, Epworth Sleepiness Scale; OSA, obstructive sleep apnea; SDS, Self‐rating Depression Scale.
As shown in Table 3, the non‐sleepy patients were older and had slightly lower BMI and AHI. Zung SDS scores, as well as the percentage of patients with depressive mood, were significantly higher in the sleepy group. Other comorbidities and use of antidepressive medication did not differ significantly between the groups at baseline (Table 3). Demographic and clinical characteristics at baseline did not differ significantly between the excluded patients with incomplete data (n = 60) and the ones remaining in the study (Zung SDS scores 44.2 ± 12.2 versus 43.2 ± 9.3).
Table 3.
Clinical characteristics of the coronary artery disease patients with OSA phenotypes (n = 386)
| Non‐sleepy OSA (n = 237) | Sleepy OSA (n = 149) | p value | |
|---|---|---|---|
| Age, years | 65.9 ± 8.4 | 62.5 ± 7.3 | <0.001 |
| Age ≥ 65 years, % | 54.9 | 38.9 | 0.002 |
| Female, % | 16.5 | 10.7 | 0.118 |
| AHI, events/hr | 28.8 ± 13.5 | 31.9 ± 15.9 | 0.040 |
| ESS score at baseline | 5.5 ± 2.3 | 12.2 ± 2.6 | <0.001 |
| BMI, kg/m2 | 28.4 ± 3.7 | 29.8 ± 4.3 | 0.001 |
| Obesity, % | 27.8 | 41.6 | 0.005 |
| Current smoker, % | 15.6 | 17.4 | 0.634 |
| Pulmonary disease, % | 6.8 | 10.1 | 0.243 |
| Hypertension, % | 64.1 | 57.0 | 0.164 |
| Atrial fibrillation, % | 19.0 | 18.8 | 0.962 |
| AMI at baseline, % | 50.6 | 49.7 | 0.853 |
| Former revascularization, % | 20.3 | 18.8 | 0.725 |
| Stroke, % | 9.3 | 4.1 | 0.053 |
| CABG at baseline, % | 26.6 | 26.8 | 0.955 |
| Diabetes mellitus, % | 24.5 | 25.5 | 0.820 |
| Antidepressive medication, % | 3.9 | 7.4 | 0.133 |
| Zung SDS score | 41.7 ± 9.7 | 44.7 ± 9.8 | 0.003 |
| Depression (Zung SDS score ≥50), % | 24.5 | 36.9 | 0.009 |
Bold indicate significance values.
AHI, apnea–hypopnea index; AMI, acute myocardial infarction; BMI, body mass index; CABG, coronary artery bypass grafting; EDS, excessive daytime sleepiness; ESS, Epworth Sleepiness Scale; OSA, obstructive sleep apnea; SDS, Self‐rating Depression Scale.
3.2. Adherence to CPAP treatment
Among patients with depressive mood at baseline, 19 out of the 29 non‐sleepy patients (65.5%) and 43 out of the 46 with a sleepy phenotype (93.5%) were remaining on CPAP at 1‐year follow‐up (p = 0.002). CPAP usage in hr/night was lower in the non‐sleepy phenotype but the difference was not statistically significant (3.5 ± 3.1 in non‐sleepy OSA versus 4.4 ± 2.4 hr/night in sleepy OSA, p = 0.173).
3.3. Response to CPAP treatment
3.3.1. Intention to treat population
Among 97 OSA patients with depressive mood at baseline, there was a significant decline in the scores in both phenotypes allocated to CPAP (Figure 2a). SDS scores decreased from 54.6 to 46.9 after 3 months and to 45.0 after 1 year in the sleepy group and from 55.1 to 46.9 after 3 months and to 44.2 after 1 year in the non‐sleepy group. The corresponding values were 53.9 at baseline, 53.7 after 3 months and 53.9 at 1‐year follow‐up in the non‐sleepy OSA group allocated to no‐CPAP (p = 0.033 for the between‐group difference in ANCOVA, adjusted for age, sex, BMI, AHI and ESS at baseline). CPAP prescription, regardless of usage, was significantly associated with improvement in mood at 1‐year (OR, 6.86; 95% CI, 2.36–19.09; p < 0.001) (Table 4). No changes were observed regarding the antidepressive medications during the follow‐up period and none of the patients was hospitalized because of depression.
Figure 2.
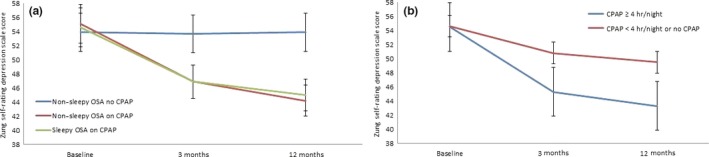
Mean values with standard error of means at baseline and after 3 and 12 months. (a) Among the sleepy and non‐sleepy OSA patients with depressive mood at baseline allocated to continuous positive airway pressure (CPAP) and no‐CPAP (control) groups in intention‐to‐treat population. (b) Among the patients using CPAP ≥4 hr/night/all nights versus CPAP <4 hr/night or no‐CPAP in the on‐treatment population who had depressive mood at baseline (p‐values were calculated using ANCOVA analysis)
Table 4.
Significant predictors of improvement in mood after 12 months in the sleepy and non‐sleepy OSA patients with Zung SDS score ≥50 at baseline (n = 97)
| Odds ratio | 95% CI | p value | |
|---|---|---|---|
| Univariate | |||
| Age | 1.01 | 0.96–1.06 | 0.612 |
| Age ≥ 65 years | 0.86 | 0.38–1.96 | 0.726 |
| Female sex | 1.38 | 0.49–3.89 | 0.543 |
| BMI | 0.97 | 0.88–1.07 | 0.533 |
| Obesity | 0.63 | 0.28–1.44 | 0.276 |
| LVEF, % | 1.00 | 0.96–1.05 | 0.866 |
| Pulmonary disease | 0.09 | 0.01–0.76 | 0.027 |
| AHI | 0.99 | 0.97–1.01 | 0.439 |
| ESS | 1.07 | 0.98–1.18 | 0.148 |
| ESS score change from baseline | 1.15 | 1.02–1.30 | 0.026 |
| CPAP prescription | 6.86 | 2.36–19.09 | <0.001 |
| CPAP hr/night/all nights | 1.34 | 1.14–1.59 | <0.001 |
| CPAP 3 hr/night | 3.43 | 1.45–8.09 | 0.005 |
| CPAP 4 hr/night | 4.14 | 1.63–10.53 | 0.003 |
| CPAP 5 hr/night | 4.52 | 1.65–12.42 | 0.003 |
| Multivariate model 1a | |||
| CPAP hr/night/all nights | 1.33 | 1.10–1.61 | 0.003 |
| CPAP 3 hr/night/all nights | 2.96 | 1.13–7.72 | 0.027 |
| CPAP 4 hr/night/all nights | 3.77 | 1.36–10.49 | 0.011 |
| CPAP 5 hr/night/all nights | 3.72 | 1.25–11.13 | 0.019 |
| Multivariate model 2b | |||
| CPAP hr/night/all nights | 1.36 | 1.10–1.68 | 0.005 |
| ESS score change from baseline | 1.12 | 0.96–1.02 | 0.139 |
| Pulmonary disease | 0.03 | 0.00–0.37 | 0.006 |
| Multivariate model 3b | |||
| CPAP 4 hr/night/all nights | 3.59 | 1.22–10.56 | 0.020 |
| ESS score change from baseline | 1.17 | 1.02–1.35 | 0.025 |
| Pulmonary disease | 0.04 | 0.00–0.45 | 0.009 |
Bold indicate significance values.
AHI, apnea–hypopnea index; BMI, body mass index; EDS, excessive daytime sleepiness; ESS, Epworth Sleepiness Scale; LVEF, left ventricular ejection fraction; OSA, obstructive sleep apnea; SDS, Self‐rating Depression Scale.
Adjusted for age, female sex, BMI, AHI at baseline, LVEF and ESS score change from baseline.
Adjusted for age, female sex, BMI and AHI at baseline.
3.3.2. On treatment population
In post hoc analysis of the patients with depressive mood at baseline, there was a decline from 54.5 to 45.3 after 3 months and to 43.3 after 1 year among 40 patients using the CPAP device ≥4 hr/night, whereas the corresponding values were 54.6, 50.8 and 49.5, respectively, among 57 patients with CPAP use <4 hr/night or no‐CPAP allocation (p = 0.002) (Figure 2b). As shown in Table 4, pulmonary disease (asthma or chronic obstructive pulmonary disease) at baseline was negatively correlated with improvement in mood, whereas CPAP hr/night and ESS score change from baseline were significant predictors of improvement at 1‐year follow‐up. Other comorbidities as well as drugs were not related to change in depressive mood (data not shown). In multivariate analysis, CPAP hr/night (OR, 1.33) as well as CPAP usage cut‐off categories (3, 4, 5 hr/night, respectively) remained as significant predictors of improvement in mood (ORs, 2.96, 3.77 and 3.72, respectively), adjusted for age, female sex, BMI, left ventricular ejection fraction, AHI and ESS score change from baseline (Table 4). Pulmonary disease remained inversely correlated with improvement in all models tested and delta ESS score predicted improvement in mood first in the model with CPAP use at least 4 hr/night (Table 4).
4. DISCUSSION
In the current study, 27% of the revascularized CAD patients demonstrated depressive mood at baseline with a higher percentage among the OSA patients with a sleepy phenotype. Female sex, BMI and ESS score were significant determinants of depressive mood at baseline. CPAP treatment improved mood in both phenotypes independent of the confounding factors and ESS change from baseline.
To our best knowledge, this is the first study addressing the occurrence of depressive mood in revascularized CAD patients with OSA and response to CPAP treatment in sleepy and non‐sleepy phenotypes. We observed depressive mood among approximately one‐fourth of the entire population and the vast majority of those patients had minor depressive scores. In a systemic meta‐analysis of CAD patients, including 22 studies, major depression was found among 20% and the prevalence was even higher (30%) among the hospitalized patients with CAD (Thombs et al., 2006). Persisting depression after acute myocardial infarction was also defined (Lesperance, Frasure‐Smith, & Talajic, 1996) and increased risk of cardiac mortality in such cases has been reported (Frasure‐Smith et al., 2000).
4.1. Determinants of depressive mood at baseline in a revascularized CAD cohort
In the current study, the percentage of CAD patients with depressive mood was significantly higher among the OSA patients compared with no‐OSA. Previous studies suggesting an association between OSA and depression were conducted mainly in sleep clinics or community‐based cohorts. Edwards et al. used the patient health questionnaire (PHQ)‐9 in a sleep‐clinic cohort and reported a significant relationship between severity of depression (PHQ‐9 scores) and AHI (Edwards et al., 2015). In the APPLES study, impact of CPAP on neurocognitive function as well as on EDS was evaluated (Kushida et al., 2012) and the baseline report from a subgroup of the same cohort showed no relationship between mild OSA and worse mood (Quan et al., 2014). Chen et al. compared 2,818 OSA and 14,090 non‐OSA patients using a longitudinal health‐insurance database in Thailand and observed an increased incident depression rate in the OSA group, which was more common in women (Chen, Keller, Kang, Hsieh, & Lin, 2013). In our cohort, the association between OSA and depressive mood was dependent on sex, BMI and EDS. The presented relationship between female sex and depressive mood is in line with a few previous studies in this context. Strik et al. reported that incident depression was more common in women than in men in patients with myocardial infarction, but there was no impact of sex on cardiac events (Strik, Lousberg, Cheriex, & Honig, 2004). In a multicentre study, the relationship between anxiety/depression and all‐cause mortality in 1,125 CABG patients was addressed and although female sex was associated with preoperative depression (OR, 1.43; 95% CI, 1.06–1.92), this was not predictive for all‐cause mortality (Geulayov, Novikov, Dankner, & Dankner, 2018). The relationship between BMI and depression has also been shown previously (Dragan & Akhtar‐Danesh, 2007). The other significant determinant of depressive mood in our cohort was ESS score, which is also in line with the previous reports. In one study, 50 patients who were referred to the sleep clinic for sleep disturbances and EDS were evaluated based on the Hospital Anxiety and Depression Scale questionnaires and a significant association between EDS and depression was demonstrated (Smith et al., 2018). In a large cross‐sectional survey of 8,937 adults, sleepiness was associated with an increased risk of major depression (Ohayon, 2012).
4.2. Impact of CPAP on depressive mood in sleepy versus non‐sleepy OSA patients
In the RCT arm of the RICCADSA trial, we have recently demonstrated significant reductions in the SDS scores in non‐sleepy OSA patients randomized to CPAP compared with those in the no‐CPAP group in the ITT population (Balcan, Thunström, Strollo, & Peker, 2019). In the SAVE trial comprising 2,410 patients with CAD and cerebrovascular disease, CPAP treatment was related to improvement in mood in the ITT population after 2 years (McEvoy et al., 2016). Of note, approximately 20% of the entire population had mild sleepiness (ESS 10‐15) and no distinction was made between the participants with regard to ESS levels when evaluating the impact of CPAP on mood in that trial (McEvoy et al., 2016).
As previously summarized, CPAP was effective in some studies (Campos‐Rodriguez et al., 2016; Diamanti et al., 2013) and not in some others (Barnes et al., 2004; Gagnadoux et al., 2014). CPAP treatment did not resolve depressive symptoms in many OSA patients and persistent depressive symptoms were strongly associated with EDS (Gagnadoux et al., 2014). On the other hand, beneficial effects of CPAP on mood based on the Zung SDS scores were reported in a 2‐year follow‐up study of 47 patients with OSA (Yamamoto, Akashiba, Kosaka, Ito, & Horie, 2000), as well as in another sleep‐clinic cohort of 132 patients after 8 weeks of CPAP treatment (Kawahara, Akashiba, Akahoshi, & Horie, 2005). In a recent RCT, with 307 female patients with moderate to severe OSA, Campos‐Rodriguez et al. showed significant improvement in depression after 3 months of CPAP therapy compared to conservative treatment (Campos‐Rodriguez et al., 2016). In a meta‐analysis, including 19 trials, CPAP treatment was shown to be effective in improving depression and a greater benefit of treatment was observed especially in patients with higher depression scores at baseline (Povitz et al., 2014).
As described above, EDS is one of the most critical symptoms of OSA and it is very likely that there exists an interaction between EDS and depression in OSA patients. The benefits of CPAP treatment for EDS are already recognized (Pecotic et al., 2018). We observed improvement in both SDS and ESS scores following 1 year of CPAP treatment in both phenotypes. The between‐group differences regarding the improvement in depressive mood in response to CPAP treatment in both sleepy and non‐sleepy patients versus untreated OSA are indeed important. In on‐treatment analysis, there was a significant association between CPAP use in hr/night and improvement in mood in multivariate analysis adjusted for the confounding factors. The cut‐off value of CPAP usage for improvement in mood was 4 hr/night when ESS score change from baseline was entered into the model, suggesting that improvement in depression is strongly dependent on the improvement in EDS in such cases. In other words, the required “dose effect” of CPAP seems to be higher in sleepy apneic patients with a history of heart disease.
Our study has a number of limitations. First, the power estimate for the entire RICCADSA cohort was conducted for the primary outcome (i.e. reduction in composite of repeat revascularization, myocardial infarction, stroke and cardiovascular mortality) for the RCT arm and not for the secondary outcomes assessed in this study for comparison of the sleepy and non‐sleepy phenotypes. Second, the diagnostic procedure at baseline was based on the cardiorespiratory sleep studies in comparison to polysomnography and consequently, the total sleep time could not be given exactly. However, the cut‐off value for AHI (15/h) chosen for OSA diagnosis was previously shown to be reliable (Dingli et al., 2003). Third, the distinction between “non‐sleepy” and “sleepy” OSA in this cohort was based on an ESS threshold, which may not reflect an objective sleepiness. However, the ESS questionnaire is a generally accepted tool for subjective daytime sleepiness and other methods, such as the Multiple Sleep Latency Test (Wise, 2006), which is suggested as an objective tool, are time consuming and not reasonable to run for large‐scale intervention studies in cardiac populations. Likewise, it may also be argued that the Zung SDS questionnaire is a subjective tool and may not necessarily reflect a real depression state. Nevertheless, the survey is a largely accepted tool to assess depressive mood in clinical cohorts.
5. CONCLUSIONS
In this revascularized CAD cohort, OSA was associated with depressive mood but this was dependent on sex, BMI and ESS. CPAP treatment improved mood in both sleepy and non‐sleepy OSA phenotypes after 3 months in patients who had depressive scores at baseline. The improvement in mood remained significant at the 12‐month follow‐up and was predicted by CPAP hr/night independent of sex, BMI and improvement in daytime sleepiness.
AUTHORS’ CONTRIBUTIONS
YP, conception and design; BB, ET, PS and YP, analysis and interpretation; BB, ET, PS and YP, drafting the manuscript for important intellectual content. All authors approved this manuscript in its final form.
CONFLICT OF INTEREST
BB reports no conflicts of interest. ET received consultant fees from ResMed and Pfizer. PS received institutional grants from Philips‐Respironics, ResMed, Inspire Medical Systems and National Football League PinMed, and advisory fees from ResMed, Emmi Solutions, Jazz Pharmaceuticals, Itamar Medical, Inspire Medical Systems and Seperation Design Group (all outside the current work). YP received institutional grants from ResMed for the current work and consultant fees from BresoTec and lecture fees from ResMed and Philips‐Respironics outside the current work.
FUNDING INFORMATION
None of the funders had any direct influence on the design of the study, the analysis of the data, the data collection, drafting of the manuscript or the decision to publish.
Supporting information
ACKNOWLEDGEMENTS
The authors would like to gratefully acknowledge the research nurse Alen Salkic for data entry of the Zung SDS questionnaires and the research secretary Eija Magnusson for her professional assistance in data entry and coordinating quality control of patient records and data follow‐up during the whole trial period.
Balcan B, Thunström E, Strollo PJ Jr, Peker Y. Determinants of depressive mood in coronary artery disease patients with obstructive sleep apnea and response to continuous positive airway pressure treatment in non‐sleepy and sleepy phenotypes in the RICCADSA cohort. J Sleep Res. 2019;28:e12818 10.1111/jsr.12818
REFERENCES
- American Academy of Sleep Medicine Task Force . (1999). Sleep‐related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep, 22, 667–89. [PubMed] [Google Scholar]
- Balcan, B. , Thunström, E. , Strollo, P. J. Jr , & Peker, Y. (2019). Continuous positive airway pressure treatment and depression in adults with coronary artery disease and nonsleepy obstructive sleep apnea. A secondary analysis of the RICCADSA trial. Annals of the American Thoracic Society, 16, 62–70. 10.1513/AnnalsATS.201803-174OC [DOI] [PubMed] [Google Scholar]
- Banno, K. , & Kryger, M. H. (2007). Sleep apnea: Clinical investigations in humans. Sleep Medicine, 8, 400–26. 10.1016/j.sleep.2007.03.003 [DOI] [PubMed] [Google Scholar]
- Barnes, M. , McEvoy, R. D. , Banks, S. , Tarquinio, N. , Murray, C. G. , Vowles, N. , & Pierce, R. J. (2004). Efficacy of positive airway pressure and oral appliance in mild to moderate obstructive sleep apnea. American journal of respiratory and critical care medicine, 170, 656–64. 10.1164/rccm.200311-1571oc [DOI] [PubMed] [Google Scholar]
- Barth, J. , Schumacher, M. , & Herrmann‐Lingen, C. (2004). Depression as a risk factor for mortality in patients with coronary heart disease: A meta‐analysis. Psychosomatic Medicine, 66, 802–13. 10.1097/01.psy.0000146332.53619.b2 [DOI] [PubMed] [Google Scholar]
- Bush, D. E. , Ziegelstein, R. C. , Tayback, M. , Richter, D. , Stevens, S. , Zahalsky, H. , & Fauerbach, J. A. (2001). Even minimal symptoms of depression increase mortality risk after acute myocardial infarction. The American Journal of Cardiology, 88, 337–41. 10.1016/s0002-9149(01)01675-7 [DOI] [PubMed] [Google Scholar]
- Campos‐Rodriguez, F. , Queipo‐Corona, C. , Carmona‐Bernal, C. , Jurado‐Gamez, B. , Cordero‐Guevara, J. , Reyes‐Nuñez, N. , & Martin‐Romero, M. (2016). Continuous positive airway pressure improves quality of life in women with obstructive sleep apnea. A randomized controlled trial. American Journal of Respiratory and Critical Care Medicine, 194, 1286–94. 10.1164/rccm.201602-0265oc [DOI] [PubMed] [Google Scholar]
- Celik, M. , Sarikaya, Y. , Acar, M. , Kalenderoğlu, A. , Doğan, S. , Kaskalan, E. , & Karataş, M. (2016). Effect of continuous positive airway pressure treatment on depression, anxiety and perceived stress levels in patients with obstructive sleep apnea syndrome. Turkish Journal of Psychiatry, 27, 244–50. [PubMed] [Google Scholar]
- Chen, Y. H. , Keller, J. K. , Kang, J. H. , Hsieh, H. J. , & Lin, H. C. (2013). Obstructive sleep apnea and the subsequent risk of depressive disorder: A population‐based follow‐up study. Journal of clinical sleep medicine, 9, 417–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamanti, C. , Manali, E. , Ginieri‐Coccossis, M. , Vougas, K. , Cholidou, K. , Markozannes, E. , & Alchanatis, M. (2013). Depression, physical activity, energy consumption, and quality of life in OSA patients before and after CPAP treatment. Sleep and breathing, 2013(17), 1159–68. 10.1007/s11325-013-0815-6 [DOI] [PubMed] [Google Scholar]
- Dingli, K. , Coleman, E. L. , Vennelle, M. , Finch, S. P. , Wraith, P. K. , Mackay, T. W. , & Douglas, N. J. (2003). Evaluation of a portable device for diagnosing the sleep apnoea/hypopnoea syndrome. European Respiratory Journal, 21, 253–9. 10.1183/09031936.03.00298103 [DOI] [PubMed] [Google Scholar]
- Dragan, A. , & Akhtar‐Danesh, N. (2007). Relation between body mass index and depression: A structural equation modeling approach. BMC Medical Research Methodology, 7, 17 10.1186/1471-2288-7-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards, C. , Mukherjee, S. , Simpson, L. , Palmer, L. J. , Almeida, O. P. , & Hillman, D. R. (2015). Depressive Symptoms before and after Treatment of Obstructive Sleep Apnea in Men and Women. Journal of Clinical Sleep Medicine, 11, 1029–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasure‐Smith, N. , Lespérance, F. , Gravel, G. , Masson, A. , Juneau, M. , Talajic, M. , & Bourassa, M. G. (2000). Social support, depression, and mortality during the first year after myocardial infarction. Circulation, 101, 1919–24. 10.1161/01.cir.101.16.1919 [DOI] [PubMed] [Google Scholar]
- Frisbee, J. C. , Brooks, S. D. , Stanley, S. C. , & D'audiffret, A. C. (2015). An unpredictable chronic mild stress protocol for instigating depressive symptoms, behavioral changes and negative health outcomes in rodents. Journal of Visualized Experiments, 106 10.3791/53109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnadoux, F. , Le Vaillant, M. , Goupil, F. , Pigeanne, T. , Chollet, S. , Masson, P. , & Meslier, N. (2014). Depressive symptoms before and after long‐term CPAP therapy in patients with sleep apnea. Chest, 145, 1025–31. 10.1378/chest.13-2373 [DOI] [PubMed] [Google Scholar]
- Geulayov, G. , Novikov, I. , Dankner, D. , & Dankner, R. (2018). Symptoms of depression and anxiety and 11‐year all‐cause mortality in men and women undergoing coronary artery bypass graft (CABG) surgery. Journal of Psychosomatic Research, 105, 106–14. 10.1016/j.jpsychores.2017.11.017 [DOI] [PubMed] [Google Scholar]
- Gupta, M. A. , Simpson, F. C. , & Lyons, D. C. (2016). The effect of treating obstructive sleep apnea with positive airway pressure on depression and other subjective symptoms: A systematic review and meta‐analysis. Sleep Medicine Reviews, 28, 55–68. 10.1016/j.smrv.2015.07.002 [DOI] [PubMed] [Google Scholar]
- Jenkinson, C. , Davies, R. J. , Mullins, R. , & Stradling, J. R. (1999). Comparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: A randomised prospective parallel trial. Lancet (London, England), 353, 2100–5. 10.1016/s0140-6736(98)10532-9 [DOI] [PubMed] [Google Scholar]
- Johns, M. W. (1991). A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep, 14, 540–5. 10.1093/sleep/14.6.540 [DOI] [PubMed] [Google Scholar]
- Kawahara, S. , Akashiba, T. , Akahoshi, T. , & Horie, T. (2005). Nasal CPAP improves the quality of life and lessens the depressive symptoms in patients with obstructive sleep apnea syndrome. Internal Medicine (Tokyo, Japan), 44, 422–7. 10.2169/internalmedicine.44.422 [DOI] [PubMed] [Google Scholar]
- Kushida, C. A. , Nichols, D. A. , Holmes, T. H. , Quan, S. F. , Walsh, J. K. , Gottlieb, D. J. , & Schweitzer, P. K. (2012). Effects of continuous positive airway pressure on neurocognitive function in obstructive sleep apnea patients: The Apnea Positive Pressure Long‐term Efficacy Study (APPLES). Sleep, 35, 1593–602. 10.5665/sleep.2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesperance, F. , Frasure‐Smith, N. , & Talajic, M. (1996). Major depression before and after myocardial infarction: Its nature and consequences. Psychosomatic Medicine, 58, 99–110. 10.1097/00006842-199603000-00001 [DOI] [PubMed] [Google Scholar]
- Loube, D. I. , Gay, P. C. , Strohl, K. P. , Pack, A. I. , White, D. P. , & Collop, N. A. (1999). Indications for positive airway pressure treatment of adult obstructive sleep apnea patients: A consensus statement. Chest, 115, 863–6. 10.1378/chest.115.3.863 [DOI] [PubMed] [Google Scholar]
- McEvoy, R. D. , Antic, N. A. , Heeley, E. , Luo, Y. , Ou, Q. , Zhang, X. , & Chen, G. (2016). CPAP for prevention of cardiovascular events in obstructive sleep apnea. New England Journal of Medicine, 375, 919–31. 10.1056/nejmoa1606599 [DOI] [PubMed] [Google Scholar]
- Ohayon, M. M. (2003). The effects of breathing‐related sleep disorders on mood disturbances in the general population. The Journal of Clinical Psychiatry, 64, 1195–200; quiz, 274‐6. [DOI] [PubMed] [Google Scholar]
- Ohayon, M. M. (2012). Determining the level of sleepiness in the American population and its correlates. Journal of Psychiatric Research, 46, 422–7. 10.1016/j.jpsychires.2011.06.008 [DOI] [PubMed] [Google Scholar]
- Pecotic, R. , Dodig, I. P. , Valic, M. , Galic, T. , Kalcina, L. L. , Ivkovic, N. , & Dogas, Z. (2018). Effects of CPAP therapy on cognitive and psychomotor performances in patients with severe obstructive sleep apnea: A prospective 1‐year study. Sleep and Breathing. [DOI] [PubMed] [Google Scholar]
- Peker, Y. , Glantz, H. , Eulenburg, C. , Wegscheider, K. , Herlitz, J. , & Thunstrom, E. (2016). Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive sleep apnea. The RICCADSA Randomized Controlled Trial. American Journal of Respiratory and Critical Care Medicine, 194, 613–20. [DOI] [PubMed] [Google Scholar]
- Peker, Y. , Glantz, H. , Thunstrom, E. , Kallryd, A. , Herlitz, J. , & Ejdeback, J. (2009). Rationale and design of the Randomized Intervention with CPAP in Coronary Artery Disease and Sleep Apnoea–RICCADSA trial. Scandinavian Cardiovascular Journal, 43, 24–31. 10.1080/14017430802276106 [DOI] [PubMed] [Google Scholar]
- Povitz, M. , Bolo, C. E. , Heitman, S. J. , Tsai, W. H. , Wang, J. , & James, M. T. (2014). Effect of treatment of obstructive sleep apnea on depressive symptoms: Systematic review and meta‐analysis. PLoS Medicine, 11, e1001762 10.1371/journal.pmed.1001762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan, S. F. , Budhiraja, R. , Batool‐Anwar, S. , Gottlieb, D. J. , Eichling, P. , Patel, S. , & Kushida, C. A. (2014). Lack of impact of mild obstructive sleep apnea on sleepiness, mood and quality of life. Southwest Journal of Pulmonary & Critical Care, 9, 44–56. 10.13175/swjpcc082-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S. , Rossdale, J. , Serry, Y. , Sekaran, A. , Drakatos, P. , & Steier, J. (2018). Multiple dimensions of excessive daytime sleepiness. Journal of Thoracic Disease, 10, S170–S76. 10.21037/jtd [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoohs, R. A. , Guilleminault, C. , Itoi, A. , & Dement, W. C. (1994). Traffic accidents in commercial long‐haul truck drivers: The influence of sleep‐disordered breathing and obesity. Sleep, 17, 619–23. [PubMed] [Google Scholar]
- Strik, J. J. , Lousberg, R. , Cheriex, E. C. , & Honig, A. (2004). One year cumulative incidence of depression following myocardial infarction and impact on cardiac outcome. Journal of Psychosomatic Research, 56, 59–66. 10.1016/s0022-3999(03)00380-5 [DOI] [PubMed] [Google Scholar]
- Thombs, B. D. , Bass, E. B. , Ford, D. E. , Stewart, K. J. , Tsilidis, K. K. , Patel, U. , & Ziegelstein, R. C. (2006). Prevalence of depression in survivors of acute myocardial infarction. Journal of General Internal Medicine, 21, 30–8. 10.1111/j.1525-1497.2005.00269.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise, M. S. (2006). Objective measures of sleepiness and wakefulness: Application to the real world? Journal of Clinical Neurophysiology, 23, 39–49. 10.1097/01.wnp.0000190416.62482.42 [DOI] [PubMed] [Google Scholar]
- World Health Organization . (2000). Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. World Health Organization Technical Report Series, 894, i‐xii–1‐253. [PubMed] [Google Scholar]
- Yamamoto, H. , Akashiba, T. , Kosaka, N. , Ito, D. , & Horie, T. (2000). Long‐term effects nasal continuous positive airway pressure on daytime sleepiness, mood and traffic accidents in patients with obstructive sleep apnoea. Respiratory medicine, 94, 87–90. 10.1053/rmed.1999.0698 [DOI] [PubMed] [Google Scholar]
- Zung, W. W. , Richards, C. B. , & Short, M. J. (1965). Self‐rating depression scale in an outpatient clinic. Further validation of the SDS. Archives of General Psychiatry, 13, 508–15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


