Summary
Background
Palmoplantar pustulosis (PPP) is a chronic inflammatory skin disease‐related to psoriasis. Its treatment is challenging, and little is known about the sustainability of different medications. The aim of this study was to analyze drug survival rates and drug discontinuation in the treatment of PPP under real‐world conditions.
Patients and Methods
Patients with PPP treated in the dermatology departments of five German university medical centers between 01/2005 and 08/2017 were included in our retrospective study. Drug survival of systemic therapies was assessed with Kaplan‐Meier analysis and multivariate regression.
Results
Overall, 347 patients with 935 treatment courses were identified. Within the group of non‐biologic systemic agents, apremilast showed the highest median drug survival (15 months), followed by cyclosporine (12 months), the combination of acitretin and topical PUVA (9 months), MTX (8 months), acitretin monotherapy (6 months), alitretinoin (5 months), and fumaric acid esters (3 months). Among biologicals, the highest maintenance rate was detected for certolizumab pegol (restricted mean: 47.4 months), followed by infliximab (median: 26 months), golimumab (22 months), ustekinumab (21 months), adalimumab (18 months), secukinumab (9 months), and etanercept (8 months).
Conclusions
Biologicals and apremilast may serve as second‐line options for treatment of PPP and should be further evaluated.
Introduction
Palmoplantar pustulosis (PPP), also termed palmoplantar pustular psoriasis, is a chronic inflammatory skin disease of unknown etiology with a prevalence of 0.01–0.05 % 1. The clinical morphology is characterized by sterile pustules, erythema, and hyperkeratosis of palms and/or soles, causing physical discomfort, functional disability, and impaired quality of life. Palmoplantar pustulosis co‐occurs with psoriasis vulgaris (PsO), psoriatic arthritis (PsA) and other comorbidities including depression, diabetes and cardiovascular disease 2. Whether PPP is a subtype of psoriasis is controversial, but most authors believe it is a distinct entity 3, 4.
Treatment of PPP is challenging. The therapeutic repertoire typically comprises highly potent topical corticosteroids, phototherapy, and oral retinoids, as well as other systemic therapies approved for PsO and PsA such as methotrexate (MTX), fumaric acid esters (FAE), cyclosporine (CyA), TNF antagonists (adalimumab (ADA), etanercept (ETA), infliximab (INX)), ustekinumab (UST), and interleukin‐17 antagonists such as secukinumab (SEC) 5, 6, 7. Due to the chronic course, long‐term control with continuous treatment is necessary.
Drug survival, defined as the interval between initiation and discontinuation of a drug, can be used as a broad indicator of therapeutic success, since it reflects the drug's effectiveness over time, safety, and tolerability, as well as quality of life and other patient‐oriented factors 8, 9. Drug survival rates for systemic medications were recently investigated for PsO 10, 11, 12, 13, 14. With PsO, biologicals were shown to yield lower discontinuation rates than traditional systemic medications 10; among biologicals, the longest maintenance rates were reported for ustekinumab 11, 12, 13, 14. Although there is evidence on the efficacy and safety of different treatments for PPP 5, 6, 7, drug survival of therapies prescribed for this indication has not yet been investigated in a real‐world setting.
The aim of this study was to analyze drug survival rates and drug discontinuation related to treatment of PPP in a real‐life setting.
Patients and methods
Study cohort
Patients with PPP who were treated in the dermatology departments of five German university medical centers (Berlin, Bonn, Göttingen, Kiel, and Mannheim) between 01/2005 and 08/2017 were identified retrospectively by their ICD‐10 code (L40.3). Their medical records were reviewed, and patients with non‐pustular palmoplantar psoriasis (plaque type) or onset of pustulosis under biological treatment as a paradoxical reaction were excluded. Treatment with at least one systemic medication administered in‐label or off‐label to treat PPP (e.g. FAE, MTX, acitretin [ACI], CyA, apremilast [APR], ALI, ADA, ETA, INX, UST, golimumab [GOL], SEC, or certolizumab pegol [CER]) was required for inclusion. Drugs received by less than eight patients were excluded from analysis. The study was performed according to the principles of the Declaration of Helsinki 15 and approved by the ethics committees of all participating medical centers.
Data extraction
Patient characteristics extracted from medical records comprised gender, age at onset of disease and age at first and last visit, family history, involvement of hands, feet, or both, nail involvement, BMI (body mass index), physician‐diagnosed comorbidities (other types of psoriasis, PsA, diabetes, cardiovascular disease, depression, liver disease, hypertension, dyslipidemia), number of comedications as well as alcohol and tobacco consumption. Treatment characteristics were assessed with regard to type of systemic treatment, dosage, concomitant MTX, topical PUVA (psoralen plus ultraviolet light A), or prednisolone, duration of treatment, and adverse events. If more than one antipsoriatic therapy was documented for an individual patient, all treatments and their sequences were extracted. Due to infrequent documentation of PASI (Psoriasis Area and Severity Index) or ppPASI (palmoplantar pustulosis Psoriasis Area and Severity Index), treatment success at the end of a treatment course (if discontinued) or at last visit (if not discontinued) was categorized as improvement of (a) > 75 % (excellent response), (b) 25–75 % (partial response), or (c) < 25 % or exacerbation (non‐response) according to the physicians’ descriptions or photographic evidence. The duration of treatment (i.e. drug survival) was calculated as the time from first to last dose of medication. Treatment courses that were continued past the time of last observation were censored. The reason for drug discontinuation (if applicable) was categorized as “lack of effectiveness in PPP”, “lack of effectiveness in concomitant PsA”, “adverse events”, or “other reasons”. Multiple answers were permitted.
Comparison with psoriasis vulgaris
Data on patient and treatment characteristics, drug survival, and reasons for drug discontinuation of the PPP cohort were compared with those of patients with PsO. For the cohort with PsO we included all patients from a previously published cohort 10 recruited at the Department of Dermatology of the University Medical Center Mannheim, who suffered from PsO but not from PPP or psoriasis palmoplantaris (PsO cohort: n = 351 from the previously published cohort n = 373) (Table 1).
Table 1.
Cohort characteristics
| Characteristic | PPP cohort | PsO cohort |
|---|---|---|
| Cohort size, n | 347 | 351 |
| Female, n (%) | 281 (81.0) | 139 (39.6) |
| Age at disease onset (yrs), mean (SD) | 44.3 (15.1) | 32.7 (18.2) |
| Age at last visit (yrs), mean (SD) | 54.9 (14.0) | NR |
| Disease duration (yrs), median (range) | 7 (0–66) | NR |
| Connection to center (months), median (range) | 27 (0–295) | NR |
| Only palmar involvement, n (%) | 21 (6.1) | NA |
| Only plantar involvement, n (%) | 66 (19.0) | NA |
| Palmoplantar involvement, n (%) | 260 (74.9) | NA |
| Involvement of nails, n (%) | 176 (50.7) | NR |
| No other type of psoriasisa, n (%) | 195 (56.2) | 258 (73.5) |
| Psoriasis vulgaris, n (%) | 129 (37.2) | 351 (100) |
| Interval (yrs) between diagnosis of PPP and PsOb, median (range) | 0 (–47–36) | NA |
| Family history, n (%) | 96 (27.7) | 115 (32.8) |
| BMI, mean (SD) | 27.6 (5.2) | NR |
| BMI ≥ 30c, n / n (%) | 69/248 (27.8) | |
| Alcohol/Tobacco | ||
| Alcohol use disorder, n (%) | 12 (3.5) | 11 (3.1) |
| No alcohol use disorder, n (%) | 263 (75.8) | 32 (9.1) |
| Alcohol consumption not documented, n (%) | 72 (20.8) | 308 (87.8) |
| Current smoker, n (%) | 222 (64.0) | 78 (22.2) |
| Ex‐smoker, n (%) | 39 (11.2) | NR |
| Never smoked, n (%) | 33 (9.5) | 26 (7.4) |
| Tobacco use not documented, n (%) | 53 (15.3) | 247 (70.4) |
| Comorbidity | ||
| Psoriatic arthritis, n (%) | 108 (31.1) | 107 (30.5) |
| Interval (yrs) between diagnosis of PPP and PsAb, median (range) | 2 (–12–47) | NR |
| Diabetes, n (%) | 52 (15.0) | 46 (13.1) |
| Cardiovascular disease, n (%) | 37 (10.7) | 48 (13.7) |
| Depression, n (%) | 61 (17.6) | 34 (9.7) |
| Liver disease, n (%) | 41 (11.8) | 36 (10.3) |
| Hypertension, n (%) | 144 (41.5) | 131 (37.3) |
| Dyslipidemia, n (%) | 75 (21.6) | 63 (18.0) |
| Number of comedications, mean (SD) | 2.1 (2.4) | 1.8 (2.7) |
No other type of psoriasis than PPP or PsO in the respective cohort.
A positive value implies diagnosis of PsA/PsO before PPP.
Number of patients with BMI ≥ 30 as percentage of all patients with documented BMI.
Abbr.: SD, standard deviation; yrs, years; NA, not applicable; NR, not reported.
Statistical analysis
Statistical analyses were performed with commercially available software (R®, version 3.4.2). Drug survival was calculated as restricted mean with standard deviation and median with 95 % confidence intervals (CI), and displayed in actual survival curves using Kaplan‐Meier analysis. Cox regression was carried out to estimate hazard ratios (HRs) for treatment discontinuation with ACI, the only medication approved for systemic therapy of PPP in Germany except for corticosteroids, as reference. The regression model was fitted with the independent variables age, gender, PsA, diabetes, cardiovascular disease, depression and number of comedications. Subgroup analysis was performed with respect to the treatment sequence. For this purpose, non‐biological agents were stratified into “first”, “second”, “third”, and “≥ fourth systemic medication” while biologicals were grouped into “first”, “second”, “third”, and “≥ fourth biological”. Differences compared to the first systemic treatment or the first biological were calculated using log‐rank tests. Moreover, drug survival of monotherapy with TNF‐α antagonists was compared to the combination of TNF‐α antagonists with MTX. The significance level was set to 5 %. Due to multiple testing, significance levels were adjusted consecutively with the Bonferroni approach. Comparison of twelve drug survivals resulted in a significance level of 0.05/12 = 0.004. For other analyses, corresponding significance levels, depending on the actual number of tests performed, are presented in figures and tables. Results that are significant at the corresponding level are highlighted in the text with an asterisk after the p‐value* and marked bold in the tables.
Results
Cohort characteristics
Overall, 347 patients with 935 treatment courses were identified (Table 1). 81 % of patients were female; the mean age at onset of disease was 44.3 years, and median disease duration was 7 years. 74.9 % had involvement of both hands and feet. Nail involvement was present in every second patient. Concomitant PsO was documented in 37.2 % and a positive family history of PsO in 27.7 % of patients. Current or previous tobacco use was common (75.6 % and 13.3 % of documented cases, respectively). PsA was diagnosed in 31.1 %. Metabolic and psychological comorbidities were frequent (hypertension: 41.5 %, depression: 17.6 %, diabetes: 15.0 %, cardiovascular disease: 10.7 %). Cohort characteristics with respect to specific treatments are displayed in Table S1 (online only).
Treatment characteristics
Patients were treated most frequently with MTX (n = 220 courses, Table 2) and ACI (n = 205), followed by FAE (n = 96), CyA (n = 70), ALI (n = 53), and APR n = 35). Among biologicals, ADA was prescribed most frequently (n = 69 courses), followed by ETA (n = 62), UST (n = 42), INX (n = 32), SEC (n = 31), GOL (n = 12), and CER (n = 8). 36.6 % of the patients on ACI concomitantly received topical PUVA. TNF‐α inhibitors were commonly combined with MTX (INX: 62.5 %, ETA: 35.5 %, GOL: 33.3 % and ADA: 30.4 %). Most patients treated with biologicals or APR received the standard maintenance dosage for PsO. ACI was most frequently given as first systemic treatment (57.6 % of all courses with ACI), followed by MTX (53.6 %), FAE (40.6 %), ALI (39.6 %), and CyA (25.7 %). Biologicals and APR were usually administered as third (GOL) or ≥ fourth systemic therapy (APR, ADA, CER, ETA, INX, UST, SEC). Among biologicals, ETA, ADA, SEC, and UST were prescribed as the first biological in 68.3 %, 55.1 %, 54.8 %, and 47.6 % of all treatment courses with the respective drug.
Table 2.
Treatment characteristics
| Characteristic, n (%) | ACI | MTX | FAE | CyA | ALI | APR | ADA | ETA | INX | GOL | CER | UST | SEC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of treatment courses | 205 | 220 | 96 | 70 | 53 | 35 | 69 | 62 | 32 | 12 | 8 | 42 | 31 |
| + PUVAa | 75 (36.6) | 31 (14.1) | 18 (18.8) | 0 (0.0) | 9 (17.0) | 6 (17.1) | 0 (0.0) | 6 (9.7) | 3 (9.4) | 0 (0.0) | 0 (0.0) | 1 (2.4) | 5 (16.1) |
| + MTXb | 0 (0.0) | NA | 0 (0.0) | 1 (1.4) | 0 (0.0) | 2 (5.7) | 21 (30.4) | 22 (35.5) | 20 (62.5) | 4 (33.3) | 1 (12.5) | 3 (7.1) | 2 (6.5) |
| + prednisolonea | 3 (1.5) | 16 (7.3) | 0 (0.0) | 2 (2.9) | 1 (1.9) | 5 (14.3) | 7 (10.1) | 8 (12.9) | 5 (15.6) | 2 (16.7) | 3 (37.5) | 0 (0.0) | 2 (6.5) |
| Dosage 1b | 60 (29.3) | 15 (6.8) | 10 (10.4) | 14 (20.0) | 3 (5.7) | 34 (97.1) | 64 (92.8) | 56 (90.3) | 25 (78.1) | 11 (91.7) | 8 (50.0) | 36 (85.7) | 30 (96.8) |
| Dosage 2b | 115 (56.1) | 140 (63.6) | 20 (20.8) | 40 (57.1) | 5 (9.4) | 1 (2.9) | 5 (7.3) | 6 (9.7) | 7 (21.9) | 1 (8.3) | 8 (50.0) | 6 (14.3) | 1 (3.2) |
| Dosage 3b | 2 (1.0) | 38 (17.3) | 37 (38.5) | 7 (10.0) | 37 (69.8) | NA | NA | NA | NA | NA | NA | NA | NA |
| 1st syst. treatment | 118 (57.6) | 118 (53.6) | 39 (40.6) | 18 (25.7) | 21 (39.6) | 1 (2.9) | 4 (5.8) | 2 (4.1) | 2 (6.3) | 1 (8.3) | 1 (12.5) | 0 (0.0) | 5 (16.1) |
| 2nd syst. treatment | 51 (24.9) | 62 (28.2) | 28 (29.2) | 21 (30.0) | 10 (18.9) | 11 (31.4) | 14 (20.3) | 13 (26.5) | 5 (15.6) | 3 (25.0) | 1 (12.5) | 4 (9.5) | 7 (22.6) |
| 3rd syst. treatment | 21 (10.2) | 24 (10.9) | 20 (20.8) | 15 (21.4) | 7 (13.2) | 5 (14.3) | 18 (26.1) | 8 (16.3) | 2 (6.3) | 7 (58.3) | 3 (37.5) | 4 (9.5) | 6 (19.4) |
| ≥ 4th syst. treatment | 15 (7.3) | 16 (7.3) | 9 (9.4) | 16 (22.9) | 15 (28.3) | 18 (51.4) | 33 (47.8) | 26 (53.1) | 23 (71.9) | 1 (8.3) | 3 (37.5) | 34 (81.0) | 13 (41.9) |
| 1st biological | NA | NA | NA | NA | NA | NA | 38 (55.1) | 41 (68.3) | 12 (37.5) | 2 (16.7) | 1 (12.5) | 20 (47.6) | 17 (54.8) |
| 2nd biological | NA | NA | NA | NA | NA | NA | 24 (34.8) | 11 (18.3) | 14 (43.8) | 3 (25.0) | 3 (37.5) | 9 (21.4) | 5 (16.1) |
| 3rd biological | NA | NA | NA | NA | NA | NA | 5 (7.3) | 5 (8.3) | 3 (9.4) | 5 (41.7) | 2 (25.0) | 10 (23.8) | 5 (16.1) |
| ≥ 4th biological | NA | NA | NA | NA | NA | NA | 2 (2.9) | 3 (5.0) | 3 (9.4) | 2 (16.7) | 2 (25.0) | 3 (7.1) | 4 (12.9) |
Number of treatment courses combined with topical PUVA, MTX, or prednisolone, respectively.
Non‐biologicals were categorized into 3 groups according to the dosage: acitretin <0.25/0.25‐0.5/>0.5 mg/kg body weight, MTX < 10/10–15/> 15 mg weekly, fumaric acid esters initial tablets/1–2 tablets/> 2 tablets, cyclosporine < 2.5/2.5–3.5/> 3.5 mg/kg body weight, alitretinoin ≤ 10/> 10–< 30/≥ 30 mg; biologicals and apremilast were categorized into standard maintenance dosage for psoriasis (Dosage 1) and any other regime (Dosage 2).
Abbr.: ACI, acitretin; MTX, methotrexate; FAE, fumaric acid esters; CyA, cyclosporine A; ALI, alitretinoin; APR, apremilast; ADA, adalimumab; ETA, etanercept; INX, infliximab; GOL, golimumab; CER, certolizumab pegol; UST, ustekinumab; SEC, secukinumab; syst. treatment, systemic treatment; NA, not applicable.
Treatment outcomes and discontinuation
Overall, the effectiveness of non‐biological systemic treatments was rather low with the exception of CyA (Table 3). An excellent response was documented most frequently for CyA (51.4 % of all courses; 36/70), followed by APR (31.4 %; 11/35), ALI (22.6 %; 12/53), ACI (19.5 %; 40/205), FAE (17.7 %; 17/96) and MTX (16.8 %; 37/220). Among biologicals, CER achieved excellent responses in 62.5 % of courses (5/8), followed by GOL (41.7 %; 5/12), INX (40.6 %; 13/32), ADA (33.3 %; 23/69), UST (31 %; 13/42), SEC (29 %; 9/31), and ETA (19.4 %; 12/62). Systemic treatment was frequently discontinued. Discontinuation rates were higher for non‐biologicals (CyA: 85.7 %; FAE: 83.3 %; MTX: 79.6 %; ACI: 76.1 %; ALI: 73.6 %) than for most biologicals (ETA: 83.9 %; ADA: 68.1 %; INX: 62.5 %; SEC: 58.1 %; GOL and UST: 50 % each; CER: 12.5 %).
Table 3.
Treatment outcomes and reasons for discontinuation
| ACI | MTX | FAE | CyA | ALI | APR | ADA | ETA | INX | GOL | CER | UST | SEC | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total number of treatment courses, n | 205 | 220 | 96 | 70 | 53 | 35 | 69 | 62 | 32 | 12 | 8 | 42 | 31 |
| Non‐responsea, n (%) | 66 (32.2) | 82 (37.3) | 36 (37.5) | 8 (11.4) | 20 (37.7) | 9 (25.7) | 19 (27.5) | 16 (25.8) | 7 (21.9) | 3 (25.0) | 0 (0.0) | 7 (16.7) | 11 (35.5) |
| Partial responsea, n (%) | 87 (42.4) | 78 (35.5) | 34 (35.4) | 23 (32.9) | 19 (35.9) | 10 (28.6) | 23 (33.3) | 33 (53.2) | 12 (37.5) | 4 (33.3) | 3 (37.5) | 16 (38.1) | 8 (25.8) |
| Excellent responsea, n (%) | 40 (19.5) | 37 (16.8) | 17 (17.7) | 36 (51.4) | 12 (22.6) | 11 (31.4) | 23 (33.3) | 12 (19.4) | 13 (40.6) | 5 (41.7) | 5 (62.5) | 13 (31.0) | 9 (29.0) |
| Treatment discontinued, n (%) | 156 (76.1) | 175 (79.6) | 80 (83.3) | 60 (85.7) | 39 (73.6) | 14 (40.0) | 47 (68.1) | 52 (83.9) | 20 (62.5) | 6 (50.0) | 1 (12.5) | 21 (50.0) | 18 (58.1) |
| ▸Adverse eventsb, n (%) | 93 (59.6) | 72 (41.1) | 55 (68.8) | 28 (46.7) | 11 (28.2) | 4 (28.6) | 18 (38.3) | 11 (21.2) | 9 (45.0) | 1 (16.7) | 1 (100) | 3 (14.3) | 2 (11.1) |
| ▸Ineffectiveness skinb, n (%) | 83 (53.2) | 96 (54.9) | 28 (35.0) | 14 (23.3) | 29 (74.4) | 8 (57.1) | 30 (63.8) | 36 (69.2) | 11 (55.0) | 3 (50.0) | 1 (100) | 14 (66.7) | 12 (66.7) |
| ▸Ineffectiveness jointsb, n (%) | 9 (5.8) | 30 (17.1) | 2 (2.5) | 1 (1.7) | 0 (0.0) | 2 (14.3) | 16 (34.0) | 22 (42.3) | 3 (15.0) | 5 (83.3) | 1 (100) | 9 (42.9) | 2 (11.1) |
| ▸Remissionb, n (%) | 5 (3.2) | 7 (4.0) | 7 (8.8) | 9 (15.0) | 6 (15.4) | 0 (0.0) | 0 (0.0) | 1 (1.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (4.8) | 0 (0.0) |
Response was evaluated at the end of treatment or time of last observation according to physician's description and/or photographic evidence.
Percentage of all patients who discontinued the respective treatment. Multiple reasvons could be stated.
Abbr.: ACI, acitretin; MTX, methotrexate; FAE, fumaric acid esters; CyA, cyclosporine A; ALI, alitretinoin; APR, apremilast; ADA, adalimumab; ETA, etanercept; INX, infliximab; GOL, golimumab; CER, certolizumab pegol; UST, ustekinumab; SEC, secukinumab.
The most common reason for treatment discontinuation was ineffectiveness for cutaneous lesions (Table 3). Adverse events were frequently mentioned as a reason for discontinuing FAE (68.8 %), ACI (59.6 %), and CyA (46.7 %). Among biological therapies, adverse events were reported most frequently for INX (45 % of all discontinued treatments). Details of adverse events are provided in Table S2 (online only). Remission was indicated as the reason for discontinuation in 5.2 % (36 of 689) of all discontinued treatment courses (Table 3).
Drug survival
The median length of all treatment courses was eight months (95 % CI 7–9 months). Among non‐biologic systemic agents (median drug survival six months), APR showed the highest median drug survival (15 months; p = 0.025 vs. ACI) (Table 4; Figure 1a), followed by CyA (12 months); combination of ACI and topical PUVA (9 months; p = 0.047 vs. ACI monotherapy); MTX (8 months; p = 0.045 vs. ACI); ACI monotherapy (6 months); ALI (5 months), and FAE (3 months; p = 0.019 vs. ACI). Drug survival of biologicals was longer than with non‐biological therapy (median: 12 months; p < 0.0001* vs. non‐biological therapy) (Figure 1b). The median for CER was not reached (restricted mean: 47.4 months; p = 0.014 vs. ACI). INX had a median survival of 26 months (p = 0.001* vs. ACI), followed by GOL (22 months, p = 0.012 vs. ACI); UST (21 months; p = 0.006 vs. ACI); ADA (18 months; p < 0.001* vs. ACI); SEC (9 months), and ETA (8 months). Cumulative 3‐, 6‐, 12‐, 24‐, 36‐, and 60‐month survival rates of all medications are shown in Table S3 (online only).
Table 4.
Drug survival
| ACIa (months) | MTX (months) | FAE (months) | CyA (months) | ALI (months) | APR (months) | ADA (months) | ETA (months) | INX (months) | GOL (months) | CER (months) | UST (months) | SEC (months) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | + PUVA | – PUVA | |||||||||||||
| PPP cohort: RMST (SD) | 29.0 (5.5) | 41.2 (10.1) | 16.3 (2.4) | 31.6 (4.9) | 8.9 (1.4) | 19.3 (3.5) | 16.9 (4.1) | 14.3 (2.0) | 37.7 (6.2) | 25.0 (5.9) | 42.5 (8.5) | 41.3 (10.8) | 47.38 (6.2) | 25.6 (4.6) | 11.7 (1.8) |
| PPP cohort: median (95% CI) | 6 (5–9) | 9 (6–14) | 6 (4–7) | 8 (6–11) | 3 (3–6) | 12 (7–19) | 5 (3–10) | 15 (6–NA) | 18 (11–35) | 8 (6–12) | 26 (8–NA) | 22 (8–NA) | NA | 21 (9–NA) | 9 (5–NA) |
| p‐value compared to ACI without PUVAb | NA | 0.047 | NA | 0.045 | 0.019 | 0.170 | 0.904 | 0.025 | < 0.001 | 0.292 | 0.001 | 0.012 | 0.014 | 0.006 | 0.214 |
| PsO cohort: RMST (SD) | 21.2 (3.7) | NR | NR | 27.0 (4.1) | 40.1 (4.8) | 8.4 (1.3) | NR | NR | 53.6 (4.2) | 37.8 (7.2) | 28.7 (6.3) | NR | NR | 51.8 (3.3) | NR |
| PsO cohort: median (95 % CI) | 6 (5–11) | NR | NR | 10 (8–14) | 10 (8–24) | 7 (4–12) | NR | NR | 26 (17–NA) | 22 (11–42) | 14 (9–40) | NR | NR | NA (NA–NA) | NR |
| p‐value comparing drug survival of the PPP and PsO cohort | 0.6872 | NR | NR | 0.3786 | < 0.001 | 0.009 | NA | NA | 0.014 | 0.017 | 0.424 | NA | NA | < 0.001 | NA |
Drug survival of acitretin was presented for all treatment courses and stratified according to concomitant topical photochemotherapy (PUVA).
The p‐value refers to the difference of the median of each treatment compared to acitretin without PUVA. Significant results according to an adjusted p‐value < 0.004 using the Bonferroni approach for multiple testing are highlighted in bold.
Abbr.: ACI, acitretin; MTX, methotrexate; FAE, fumaric acid esters; CyA, cyclosporine A; ALI, alitretinoin; APR, apremilast; ADA, adalimumab; ETA, etanercept; INX, infliximab; GOL, golimumab; CER, certolizumab pegol; UST, ustekinumab; SEC, secukinumab; NR, not reported; NA, not applicable (e.g. median not reached); RMST, restricted mean; SD, standard deviation; CI, confidence interval.
Figure 1.
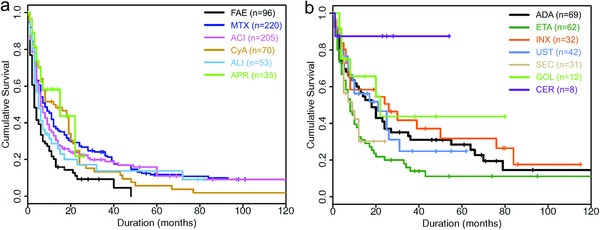
Cumulative probability of drug survival of non‐biological (a) and biological therapy (b) in the PPP cohort based on the number of treatment courses (Kaplan‐Meier analysis).
Abbr.: ACI, acitretin; MTX, methotrexate; FAE, fumaric acid esters; CyA, cyclosporine A; ALI, alitretinoin; APR, apremilast; ADA, adalimumab; ETA, etanercept; INX, infliximab; GOL, golimumab; CER, certolizumab pegol; UST, ustekinumab; SEC, secukinumab.
Drug survival was much lower in the PPP cohort than in the PsO cohort (median: 8 vs. 14 months, p < 0.0001*). In particular, FAE (3 vs. 10 months, p < 0.001*) (Table 4), ADA (18 vs. 26 months, p = 0.014), ETA (8 vs. 22 months, p = 0.017), and UST (median not calculable, restricted means: 25.6 vs. 51.8 months, p < 0.001*) were administered for a shorter period of time in patients with PPP than those with PsO, while CyA showed a longer drug survival in the PPP cohort (12 vs. 7 months, p = 0.009, see Figure S1 [online only] for corresponding drug survival curves in the PsO cohort).
Hazard ratios for treatment discontinuation were calculated with Cox regression and ACI as reference category (Tables 5, 6). Compared to ACI, FAE had a higher risk of treatment discontinuation (HR: 1.68; p < 0.001*), whereas the hazard of discontinuation was lower for ADA (HR: 0.55, p = 0.002*); APR (HR: 0.54, p = 0.034); INX (HR: 0.46, p = 0.002*); UST (HR: 0.46, p = 0.003*); GOL (HR: 0.38, p = 0.035), and CER (HR: 0.12, p = 0.035). Patients with PsA were more likely to switch therapy than those without this comorbidity when treated with ACI (HR: 1.41, p = 0.021); ALI (HR: 1.52, p = 0.027); INX (HR: 1.60, p = 0.013) and UST (HR: 1.60, p = 0.014). Patients with diabetes were less likely to discontinue therapy with ACI (HR: 0.67, p = 0.032) and ETA (HR: 0.65, p = 0.042) than those without diabetes. Depression was associated with a higher risk of discontinuing ETA (HR: 1.51, p = 0.032); UST (HR: 1.55, p = 0.035); ALI (HR: 1.58, p = 0.021); FAE (HR: 1.59, p = 0.016); ACI (HR: 1.60, p = 0.004); GOL (HR: 1.60, p = 0.037); CyA (HR: 1.62, p = 0.009) and APR (HR: 1.64, p = 0.021). However, independent variables other than treatment did not reach the level of significance.
Table 5.
Multivariate regression model predicting treatment discontinuation in non‐biologicals
| ACI | MTX | FAE | CyA | ALI | APR | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95 % CI) | p‐value | HR (95 % CI) | p‐value | HR (95 % CI) | p‐value | HR (95 % CI) | p‐value | HR (95 % CI) | p‐value | HR (95 % CI) | p‐value | |
| Comparison with ACI | 1.00 (0.80, 1.26) | 1.000 | 0.94 (0.73, 1.19) | 0.598 | 1.68 (1.26, 2.23) | < 0.001 | 0.87 (0.64, 1.19) | 0.379 | 1.13 (0.79, 1.63) | 0.494 | 0.54 (0.30, 0.95) | 0.034 |
| Age | 1.01 (1.00, 1.01) | 0.272 | 1.00 (1.00, 1.01) | 0.251 | 1.01 (1.00, 1.02) | 0.198 | 1.00 (0.99, 1.01) | 0.899 | 1.00 (0.99, 1.01) | 0.929 | 1.01 (0.99, 1.02) | 0.253 |
| Gender (female)a | 1.18 (0.90, 1.55) | 0.230 | 1.01 (0.76, 1.33) | 0.972 | 1.18 (0.85, 1.62) | 0.325 | 1.15 (0.83, 1.59) | 0.405 | 1.15 (0.82, 1.60) | 0.418 | 1.19 (0.82, 1.72) | 0.349 |
| PsA | 1.41 (1.05, 1.89) | 0.021 | 0.96 (0.75, 1.24) | 0.775 | 1.26 (0.88, 1.81) | 0.208 | 1.40 (0.98, 1.99) | 0.063 | 1.52 (1.05, 2.20) | 0.027 | 1.41 (0.96, 2.08) | 0.082 |
| Diabetes | 0.67 (0.46, 0.97) | 0.032 | 1.13 (0.79, 1.61) | 0.499 | 0.93 (0.61, 1.42) | 0.741 | 0.77 (0.49, 1.21) | 0.255 | 0.63 (0.39, 1.01) | 0.053 | 0.66 (0.40, 1.08) | 0.097 |
| Cardiovascular disease | 0.96 (0.64, 1.42) | 0.821 | 0.86 (0.59, 1.25) | 0.418 | 0.96 (0.59, 1.58) | 0.882 | 0.82 (0.51, 1.34) | 0.430 | 1.01 (0.59, 1.72) | 0.986 | 0.97 (0.56, 1.66) | 0.909 |
| Depression | 1.60 (1.17, 2.20) | 0.004 | 1.27 (0.95, 1.7) | 0.108 | 1.59 (1.09, 2.32) | 0.016 | 1.62 (1.13, 2.32) | 0.009 | 1.58 (1.07, 2.32) | 0.021 | 1.64 (1.08, 2.50) | 0.021 |
| Total number of comedications | 0.99 (0.93, 1.05) | 0.686 | 0.97 (0.92, 1.03) | 0.305 | 0.94 (0.87, 1.02) | 0.122 | 1.00 (0.92, 1.09) | 0.962 | 1.01 (0.93, 1.09) | 0.842 | 0.98 (0.90, 1.07) | 0.684 |
Age, gender, psoriatic arthritis, diabetes, cardiovascular disease, depression, and the total number of comedications served as independent variables. The hazard ratio of discontinuing treatment in comparison to acitretin was used as dependent variable. Values > 1 indicate a higher risk of treatment discontinuation compared to acitretin. Significant results according to an adjusted p‐value < 0.004 using the Bonferroni approach for multiple testing are highlighted in bold.
The reference category for female was male.
Abbr.: ACI, acitretin; MTX, methotrexate; FAE, fumaric acid esters; CyA, cyclosporine A; ALI, alitretinoin; APR, apremilast; HR, hazard ratio; CI, confidence interval; PsA, psoriatic arthritis.
Table 6.
Multivariate regression model predicting treatment discontinuation in biologicals
| ADA | ETA | INX | GOL | CER | UST | SEC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95 % CI) | p‐value | HR (95 % CI) | p‐value | HR (95 % CI) | p‐value | HR (95 % CI) | p‐value | HR (95 % CI) | p‐value | HR (95 % CI) | p‐value | HR (95 % CI) | p‐value | |
| Comparison to ACI | 0.55 (0.37, 0.81) | 0.002 | 0.92 (0.61, 1.39) | 0.695 | 0.46 (0.28, 0.74) | 0.002 | 0.38 (0.16, 0.94) | 0.035 | 0.12 (0.02, 0.86) | 0.035 | 0.46 (0.28, 0.77) | 0.003 | 0.71 (0.40, 1.25) | 0.240 |
| Age | 1.01 (0.99, 1.02) | 0.377 | 1.00 (0.99, 1.01) | 0.614 | 1.00 (0.99, 1.02) | 0.663 | 1.00 (0.99, 1.02) | 0.601 | 1.00 (0.99, 1.02) | 0.517 | 1.00 (0.99, 1.01) | 0.684 | 1.00 (0.99, 1.01) | 0.998 |
| Gender (female)a | 1.16 (0.82, 1.64) | 0.403 | 1.22 (0.88, 1.70) | 0.234 | 1.22 (0.85, 1.74) | 0.281 | 1.18 (0.81, 1.72) | 0.380 | 1.18 (0.81, 1.73) | 0.389 | 1.17 (0.80, 1.70) | 0.419 | 1.19 (0.82, 1.73) | 0.349 |
| PsA | 1.34 (0.94, 1.91) | 0.105 | 1.17 (0.81, 1.70) | 0.405 | 1.60 (1.10, 2.31) | 0.013 | 1.40 (0.92, 2.11) | 0.114 | 1.41 (0.93, 2.14) | 0.101 | 1.60 (1.10, 2.32) | 0.014 | 1.26 (0.85, 1.88) | 0.249 |
| Diabetes | 0.80 (0.51, 1.25) | 0.321 | 0.65 (0.43, 0.98) | 0.042 | 0.73 (0.45, 1.19) | 0.207 | 0.68 (0.41, 1.13) | 0.139 | 0.67 (0.40, 1.13) | 0.135 | 0.66 (0.41, 1.07) | 0.090 | 0.81 (0.50, 1.30) | 0.387 |
| Cardiovascular disease | 0.91 (0.57, 1.47) | 0.710 | 0.87 (0.55, 1.38) | 0.562 | 0.97 (0.57, 1.64) | 0.910 | 1.05 (0.61, 1.79) | 0.863 | 0.93 (0.53, 1.63) | 0.806 | 1.00 (0.60, 1.69) | 0.991 | 1.00 (0.57, 1.75) | 0.997 |
| Depression | 1.35 (0.92, 1.98) | 0.121 | 1.51 (1.04, 2.21) | 0.032 | 1.47 (0.97, 2.23) | 0.070 | 1.60 (1.03, 2.49) | 0.037 | 1.55 (0.99, 2.43) | 0.054 | 1.55 (1.03, 2.34) | 0.035 | 1.43 (0.93, 2.21) | 0.107 |
| Total number of comedications | 0.99 (0.91, 1.07) | 0.752 | 1.01 (0.94, 1.09) | 0.738 | 0.99 (0.90, 1.08) | 0.752 | 0.99 (0.91, 1.09) | 0.887 | 0.99 (0.9, 1.08) | 0.799 | 0.98 (0.90, 1.07) | 0.681 | 0.97 (0.89, 1.05) | 0.409 |
Age, gender, psoriatic arthritis, diabetes, cardiovascular disease, depression, and the total number of comedications served as independent variables. The hazard ratio of discontinuing treatment in comparison to acitretin was used as dependent variable. Values >1 indicate a higher risk of treatment discontinuation compared to acitretin. Significant results according to an adjusted p‐value < 0.004 using the Bonferroni approach for multiple testing are highlighted in bold.
The reference category for female was male.
Abbr.: ACI, acitretin; ADA, adalimumab; ETA, etanercept; INX, infliximab; GOL, golimumab; CER, certolizumab pegol; UST, ustekinumab; SEC, secukinumab; HR, hazard ratio; CI, confidence interval; PsA, psoriatic arthritis.
When non‐biological therapies were stratified according to first, second, third, and ≥ fourth systemic treatment, drug survival of MTX was found to be higher when administered as first‐line treatment (median: 12 months) than as second‐ or third‐line treatment (6 months, p = 0.017 and 4 months, p = 0.023; Figure S2, online only). Among biologicals, ADA showed a shorter drug survival when given as first biological than as second biological (median: 6 vs. 55 months; p = 0.0006). The same applied for GOL (mean: 4 vs. 29 months; p = 0.046; Figure S3, online only).
We also investigated the impact of concomitant MTX on drug survival of TNF‐α inhibitors (Figure 2). Combining MTX with ADA was associated with a longer drug survival (median: 55 vs. 12 months, p = 0.033, Figure 2a). Similarly, patients receiving INX plus MTX had better drug survival than patients who received INX alone (median: 50 vs. 7 months, p = 0.012, Figure 2b).
Figure 2.
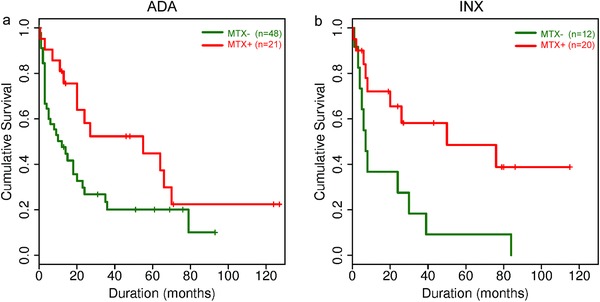
Cumulative probability of drug survival based on the number of treatment courses (Kaplan‐Meier analysis) depending on administration of MTX concomitant with adalimumab (a) and infliximab (b). Adjusted significance level according to Bonferroni correction of multiple testing, p < 0.01.
Abbr.: MTX, methotrexate; ADA, adalimumab; INX, infliximab; MTX‐, monotherapy with biological; MTX+, concomitant MTX administration.
Discussion
To our knowledge, this is the first study to investigate drug survival of systemic treatments for PPP in a real‐world setting.
The sociodemographic and disease‐related characteristics of our cohort were well in line with published epidemiological data 2, 5, 16, 17. Effectiveness of systemic treatments was low, with approximately one of five patients who received non‐biological therapy and one of three patients who received biological treatment having an excellent response. Among non‐biologicals, CyA, APR, ACI, and ALI were more effective than FAE and MTX. Except for corticosteroids, ACI is the only drug licensed for PPP in Germany, and is therefore considered to be standard first‐line treatment. Marsland and colleagues conducted a systematic review of interventions in PPP, and reported superior efficacy for the combination treatment with retinoids and topical PUVA than for retinoids alone 6. Similarly, we found better drug survival with acitretin and topical PUVA than with acitretin alone. The efficacy of CyA was good, which was also demonstrated in double‐blind, placebo‐controlled studies 18, 19. However, treatment was frequently discontinued due to adverse events. Accordingly, concerns about the safety profile of CyA, including hypertension and nephrotoxicity, are mentioned in the current literature on treatment recommendations for PPP 5, 17.
Drug survival of biologicals was longer than that of non‐biological treatments except for ETA. Among biologicals, CER was most effective in our cohort, followed by GOL, INX, ADA, UST, SEC, and ETA. However, only a few patients were treated with CER (n = 8) and GOL (n = 12), and the vast majority of these (n = 18) had concomitant PsA, so that efficacy for PsA may have influenced treatment duration. Moreover, CER and GOL were often administered as third or further biologicals. Thus, the lack of further promising therapeutic choices may also have contributed to high drug survival. A recent review of biological treatment of PPP reported the greatest efficacy for INX, followed by UST, consistent with our data 7. A recent review emphasized interleukin‐17 and phosphodiesterase‐4 inhibition as promising new options 17; however, evidence for the efficacy of novel biologicals and APR in PPP is weak 17, 20.
Patients with PPP had significantly shorter drug survival than the cohort with PsO. Drug survival in PsO has been extensively studied 10, 11, 12, 13, 14, 21, 22, 23, 24. For example, according to the Danish DERMBIO registry, median drug survival was 30 months for ETA, 44 months for INX, and 59 months for ADA. UST showed the best retention time (median not reached) 12. Our study suggests higher drug survival rates for INX, ADA, and UST than for ETA in patients with PPP, consistent with data for PsO 11, 12, 13, 21, 23, 24.
In regression analysis, PsA was a predictor for switching therapy with ACI, ALI, INX, and UST. A study from Israel consistently found a higher risk of drug discontinuation in patients with PsO and concomitant PsA than in patients with PsO alone when treated with ACI 25. In contrast, biological therapy was reported to be maintained longer if patients with PsO had concomitant PsA 24, 26, 27.
Interestingly, patients with diabetes were less likely to discontinue therapy with ACI and ETA than those without diabetes; this is surprising, considering the possible metabolic adverse events of retinoids 28. In patients with depression, discontinuation of ETA, UST, ALI, FAE, ACI, GOL, CyA, and APR was more likely than in patients without this comorbidity. Depression has been reported to predict low treatment adherence in immune‐mediated diseases 29.
Stratification according to the treatment sequence of biological therapies revealed longer drug survival of ADA and GOL when prescribed as the second biological. Literature on drug survival comparing patients with psoriasis who are naïve to biologicals with those who are not is conflicting. According to some studies, survival rates of ADA and other biologicals are independent of the treatment sequence 23, 30, whereas others report decreased maintenance of biologicals prescribed ≥ second‐line 11, 12. In particular, previous therapy with a TNF‐α antagonist predicted lower drug survival of other TNF‐α antagonists prescribed subsequently 11, 12, 31.
Drug survival of INX and ADA was higher when MTX was administered concomitantly, which is in line with data for PsO and other immune‐mediated diseases 10, 22, 32, 33. MTX is thought to prolong survival of TNF‐α antagonists, particularly INX, presumably at least in part by preventing formation of anti‐drug antibodies 32, and should therefore be considered as a comedication 34.
The results of this study should be interpreted with caution. Firstly, data were collected retrospectively in an observational setting without control groups, which may introduce selection bias 35. Identification of patients by their ICD‐10 code was the most feasible though possibly incomplete strategy. Due to the retrospective nature of this study and thus the possibility of missing or imprecise outcome data collected from medical records, the results, particularly on clinical efficacy, should be considered as rough estimates rather than precise predictions. Secondly, patients were recruited at tertiary care centers specialized in psoriasis; these patients might suffer from severe and recalcitrant PPP, which could have contributed to low drug survival rates. Thirdly, some subgroups contained small numbers of patients. Fourthly, several new drugs, particularly biologicals, became available during the retrospective time frame. The opportunity to switch to these new treatments might result in decreased survival rates of traditional systemic therapies 9, 36.
Another critical aspect of the concept of drug survival is the intermittent administration of therapies, e.g. due to concerns of cumulative toxicity with cyclosporine 36, which limits the significance of this measure for certain treatments which are intermittently planned a priori. Finally, drug maintenance is not only influenced by efficacy, tolerability and safety, but also by a plethora of physician‐, patient‐ and disease‐related characteristics such as the physician's attitude towards individual systemic therapies, patient preferences, treatment experience, and comorbidities. Although our regression models were adjusted for several covariates, other unmeasured confounders cannot be excluded 9, 36.
A major strength is the multicenter design of the study. A wide range of systemic treatments were investigated in a real‐world setting over a sufficiently long period of time to adequately capture the chronic course of PPP. The cohort was characterized in detail and drug survival data were controlled for patient‐ and treatment‐inherent confounding variables.
In summary, drug survival of systemic antipsoriatic therapies in PPP is low. ACI combined with topical PUVA represents a reasonable first‐line treatment. Biologicals, particularly anti‐ TNF monoclonal antibodies and UST, as well as APR show higher drug survival rates than traditional systemic therapies and should be considered as second‐line treatment. More research with a prospective design is needed to further evaluate traditional and newer antipsoriatic drugs for the treatment of PPP 37.
Funding sources
Celgene. The funding source had no role in the design of this study, execution, analysis or interpretation of data or decision to submit results.
Conflict of interest
C. Kromer has received honoraria for presentations from Janssen‐Cilag. D. Wilsmann‐Theis has been advisor and/or received speakers’ honoraria or travel expense reimbursements and/or received grants and/or participated in clinical trials by the companies AbbVie, Almirall, Amgen, Beiersdorf, Biogen, Boehringer‐Ingelheim Pharma, Celgene, Forward Pharma, GlaxoSmithKline, Janssen‐Cilag, Leo, Lilly, Medac, Merck Sharp & Dohme Corp., Novartis, Pfizer, UCB Pharma, and VBL. S. Gerdes has been an advisor and/or received speakers’ honoraria and/or received grants and/or participated in clinical trials by the following companies: Abbott/AbbVie, Almirall‐Hermal, Amgen, Baxalta, Bayer Health Care, Biogen Idec, Bioskin, Boehringer Ingelheim, Celgene, Centocor, Dermira, Eli Lilly, Foamix, Forward Pharma, Galderma, Hexal AG, Isotechnika, Janssen‐Cilag, Leo Pharma, Medac, Merck Serono, Mitsubishi Tanabe, MSD, Novartis, Pfizer, Polichem SA, Regeneron Pharmaceutical, Sandoz Biopharmaceuticals, Schering‐Plough, Takeda, Teva, UCB Pharma, VBL therapeutics, Wyeth Pharma. S. Philipp has been an advisor and/or received speakers’ honoraria and/or received grants and/or participated in clinical trials by the following companies: AbbVie Germany, Almirall, Amgen, Biogen Idec, Boehringer‐Ingelheim, BMS, Celgene, Dermira, Eli Lilly, Forward Pharma, GSK, Hexal, Janssen‐Cilag, Leo Pharma, Maruho, Medac, Merck, MSD, mundipharma, Novartis, Pfizer, UCB Biopharma, VBL Therapeutics. ML. Schaarschmidt conducted clinical trials for Abbvie, Boehringer‐Ingelheim, Celgene, Eli Lilly, Janssen‐Cilag, Merck, Novartis and UCB Pharma; obtained honoraria from Janssen‐Cilag and Novartis; and received financial support for participation in conferences from Abbvie, ALK‐Abello, Biogen Inc., Janssen‐Cilag and MSD. A. Schmieder served as an investigator for Abbvie, Eli Lilly, Janssen‐Cilag, Merck, Novartis and Pfizer; obtained honoraria from Abbvie, Janssen‐Cilag, and Novartis, and received support for conferences from Abbvie, ALK‐Abello, Alma Lasers, ARC Lasers, Asclepion, BMS, GSK, Janssen‐Cilag, L’Oreal, LEO Pharma, Medac, Merck, MSD, Novartis, P&M Cosmetics, and Pfizer. W. K. Peitsch served as an investigator for Abbvie, Boehringer Ingelheim, Eli Lilly, Janssen‐Cilag, Merck, Novartis, Pfizer and UCB Pharma, and was member of an advisory board of Abbvie, Eli Lilly, LEO Pharma, MSD and Novartis. She received speaker's honoraria from ALK‐Abello, Abbvie, Janssen‐Cilag, MSD, Novartis and Roche; and received support for conferences from Abbvie, Actelion, ALK‐Abello, Allergika, Alma Lasers, ARC Lasers, Asclepion, Beiersdorf, BMS, Celgene, Dermapharm, Dermasence, Eli Lilly, Galderma, GSK, IGEA, Interlac, Janssen‐Cilag, L’Oreal, La Roche Posay, LEO Pharma, Medac, Merck, MSD, Novartis, Pierre Fabre, P&M Cosmetics, Pfizer, and Roche. R. Mössner has been an advisor and/or received speakers’ honoraria and/or received grants and/or participated in clinical trials by the following companies: Abbott/Abbvie, Allmirall, Biogen Idec GmbH, Böhringer‐Ingelheim, Celgene, Essex Pharma GmbH, Janssen‐Cilag GmbH, Leo Pharma GmbH, Lilly, Merck Serono GmbH, MSD SHARP & DOHME GmbH, Novartis Pharma GmbH, Pfizer GmbH and UCB. M. Dakna and T. Arnold declared no conflicts of interest.
Supporting information
Figure S1
Figure S2
Figure S3
Supporting Information
References
- 1. Hellgren L, Mobacken H. Pustulosis palmaris et plantaris. Prevalence, clinical observations and prognosis. Acta Derm Venereol 1971; 51: 284–8. [PubMed] [Google Scholar]
- 2. Wilsmann‐Theis D, Jacobi A, Frambach Y et al. Palmoplantar pustulosis – a cross‐sectional analysis in Germany. Dermatol Online J 2017; 23(4). pii: 13030/qt0h15613d. [PubMed] [Google Scholar]
- 3. de Waal AC, van de Kerkhof PC. Pustulosis palmoplantaris is a disease distinct from psoriasis. J Dermatolog Treat 2011; 22: 102–5. [DOI] [PubMed] [Google Scholar]
- 4. Mrowietz U, van de Kerkhof PC. Management of palmoplantar pustulosis: do we need to change? Br J Dermatol 2011; ; 164: 942–6. [DOI] [PubMed] [Google Scholar]
- 5. Weisenseel P, Wilsmann‐Theis D, Kahl C et al. [Pustular psoriasis]. Hautarzt 2016; 67: 445–53. [DOI] [PubMed] [Google Scholar]
- 6. Marsland AM, Chalmers RJ, Hollis S et al. Interventions for chronic palmoplantar pustulosis. Cochrane Database Syst Rev. 2006: CD001433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sanchez IM, Sorenson E, Levin E et al. The efficacy of biologic therapy for the management of palmoplantar psoriasis and palmoplantar pustulosis: a systematic review. Dermatol Ther (Heidelb) 2017; 7: 425–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yeung H, Wan J, Van Voorhees AS et al. Patient‐reported reasons for the discontinuation of commonly used treatments for moderate to severe psoriasis. J Am Acad Dermatol 2013; 68: 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van den Reek J, Kievit W, Gniadecki R et al. Drug survival studies in dermatology: principles, purposes, and pitfalls. J Invest Dermatol 2015; 135: 1–5. [DOI] [PubMed] [Google Scholar]
- 10. Arnold T, Schaarschmidt ML, Herr R et al. Drug survival rates and reasons for drug discontinuation in psoriasis. J Dtsch Dermatol Ges 2016; 14: 1089–99. [DOI] [PubMed] [Google Scholar]
- 11. Egeberg A, Ottosen MB, Gniadecki R et al. Safety, efficacy, and drug survival of biologics and biosimilars for moderate‐to‐severe plaque psoriasis. Br J Dermatol 2018; 178(2): 509–19. [DOI] [PubMed] [Google Scholar]
- 12. Gniadecki R, Bang B, Bryld LE et al. Comparison of long‐term drug survival and safety of biologic agents in patients with psoriasis vulgaris. Br J Dermatol 2015; 172: 244–52. [DOI] [PubMed] [Google Scholar]
- 13. Menter A, Papp KA, Gooderham M et al. Drug survival of biologic therapy in a large, disease‐based registry of patients with psoriasis: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Eur Acad Dermatol Venereol 2016; 30: 1148–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. No DJ, Inkeles MS, Amin M et al. Drug survival of biologic treatments in psoriasis: a systematic review. J Dermatolog Treat 2018; 29(5): 460–6. [DOI] [PubMed] [Google Scholar]
- 15. World Medical A. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013; 310: 2191–4. [DOI] [PubMed] [Google Scholar]
- 16. Kubota K, Kamijima Y, Sato T et al. Epidemiology of psoriasis and palmoplantar pustulosis: a nationwide study using the Japanese national claims database. BMJ Open 2015; 5: e006450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Raposo I, Torres T. Palmoplantar psoriasis and palmoplantar pustulosis: current treatment and future prospects. Am J Clin Dermatol 2016; 17: 349–58. [DOI] [PubMed] [Google Scholar]
- 18. Erkko P, Granlund H, Remitz A et al. Double‐blind placebo‐controlled study of long‐term low‐dose cyclosporin in the treatment of palmoplantar pustulosis. Br J Dermatol 1998; 139: 997–1004. [DOI] [PubMed] [Google Scholar]
- 19. Reitamo S, Erkko P, Remitz A et al. Cyclosporine in the treatment of palmoplantar pustulosis. A randomized, double‐blind, placebo‐controlled study. Arch Dermatol 1993; 129: 1273–9. [PubMed] [Google Scholar]
- 20. Gottlieb A, Sullivan J, van Doorn M et al. Secukinumab shows significant efficacy in palmoplantar psoriasis: Results from GESTURE, a randomized controlled trial. J Am Acad Dermatol 2017; 76: 70–80. [DOI] [PubMed] [Google Scholar]
- 21. Menting SP, Sitaram AS, Bonnerjee‐van der Stok HM et al. Drug survival is not significantly different between biologics in patients with psoriasis vulgaris: a single‐centre database analysis. Br J Dermatol 2014; 171: 875–83. [DOI] [PubMed] [Google Scholar]
- 22. Spertino J, Lopez‐Ferrer A, Vilarrasa E et al. Long‐term study of infliximab for psoriasis in daily practice: drug survival depends on combined treatment, obesity and infusion reactions. J Eur Acad Dermatol Venereol 2014; 28: 1514–21. [DOI] [PubMed] [Google Scholar]
- 23. van den Reek JM, Zweegers J, Kievit W et al. ’Happy’ drug survival of adalimumab, etanercept and ustekinumab in psoriasis in daily practice care: results from the BioCAPTURE network. Br J Dermatol 2014; 171: 1189–96. [DOI] [PubMed] [Google Scholar]
- 24. Warren RB, Smith CH, Yiu ZZN et al. Differential drug survival of biologic therapies for the treatment of psoriasis: a prospective observational cohort study from the British Association of Dermatologists Biologic Interventions Register (BADBIR). J Invest Dermatol 2015; 135: 2632–40. [DOI] [PubMed] [Google Scholar]
- 25. Shalom G, Zisman D, Harman‐Boehm I et al. Factors associated with drug survival of methotrexate and acitretin in patients with psoriasis. Acta Derm Venereol 2015; 95: 973–7. [DOI] [PubMed] [Google Scholar]
- 26. Brunasso AM, Puntoni M, Massone C. Drug survival rates of biologic treatments in patients with psoriasis vulgaris. Br J Dermatol 2012; 166: 447–9. [DOI] [PubMed] [Google Scholar]
- 27. Jacobi A, Rustenbach SJ, Augustin M. Comorbidity as a predictor for drug survival of biologic therapy in patients with psoriasis. Int J Dermatol 2016; 55: 296–302. [DOI] [PubMed] [Google Scholar]
- 28. Dunn LK, Gaar LR, Yentzer BA et al. Acitretin in dermatology: a review. J Drugs Dermatol 2011; 10: 772–82. [PubMed] [Google Scholar]
- 29. Vangeli E, Bakhshi S, Baker A et al. A systematic review of factors associated with non‐adherence to treatment for immune‐mediated inflammatory diseases. Adv Ther 2015; 32: 983–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van Lumig PP, van de Kerkhof PC, Boezeman JB et al. Adalimumab therapy for psoriasis in real‐world practice: efficacy, safety and results in biologic‐naive vs. non‐naive patients. J Eur Acad Dermatol Venereol 2013; 27: 593–600. [DOI] [PubMed] [Google Scholar]
- 31. Gniadecki R, Kragballe K, Dam TN et al. Comparison of drug survival rates for adalimumab, etanercept and infliximab in patients with psoriasis vulgaris. Br J Dermatol 2011; 164: 1091–6. [DOI] [PubMed] [Google Scholar]
- 32. Garces S, Demengeot J, Benito‐Garcia E. The immunogenicity of anti‐TNF therapy in immune‐mediated inflammatory diseases: a systematic review of the literature with a meta‐analysis. Ann Rheum Dis 2013; 72: 1947–55. [DOI] [PubMed] [Google Scholar]
- 33. Philipp S, Wilsmann‐Theis D, Weyergraf A et al. Combination of adalimumab with traditional systemic antipsoriatic drugs – a report of 39 cases. J Dtsch Dermatol Ges 2012; 10: 821–37. [DOI] [PubMed] [Google Scholar]
- 34. Armstrong AW, Bagel J, Van Voorhees AS et al. Combining biologic therapies with other systemic treatments in psoriasis: evidence‐based, best‐practice recommendations from the Medical Board of the National Psoriasis Foundation. JAMA Dermatol 2015; 151: 432–8. [DOI] [PubMed] [Google Scholar]
- 35. van den Reek J, Kievit W, de Jong E. Comment on “Drug survival analysis is not a good method for assessing the safety or effectiveness of systemic therapies in psoriasis”. Actas Dermosifiliogr 2017; 108: 695–6. [DOI] [PubMed] [Google Scholar]
- 36. Davila‐Seijo P, Garcia‐Doval I. Drug survival analysis is not a good method for assessing the safety or effectiveness of systemic therapies in psoriasis. Actas Dermosifiliogr 2017; 108: 3–5. [DOI] [PubMed] [Google Scholar]
- 37. Davila‐Seijo P, Dauden E, Carretero G et al. Survival of classic and biological systemic drugs in psoriasis: results of the BIOBADADERM registry and critical analysis. J Eur Acad Dermatol Venereol 2016; 30: 1942–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Figure S2
Figure S3
Supporting Information


