Abstract
Objective
Since the 1980s, dengue incidence has increased 30‐fold. However, in 2017, there was a noticeable reduction in reported dengue incidence cases within the Americas, including severe and fatal cases. Understanding the mechanism underlying dengue's incidence and decline in the Americas is vital for public health planning. We aimed to provide plausible explanations for the decline in 2017.
Methods
An expert panel of representatives from scientific and academic institutions, Ministry of Health officials from Latin America and PAHO/WHO staff met in October 2017 to propose hypotheses. The meeting employed six moderated plenary discussions in which participants reviewed epidemiological evidence, suggested explanatory hypotheses, offered their expert opinions on each and developed a consensus.
Results
The expert group established that in 2017, there was a generalised decreased incidence, severity and number of deaths due to dengue in the Americas, accompanied by a reduction in reported cases of both Zika and chikungunya virus infections, with no change in distribution among age groups affected. This decline was determined to be unlikely due to changes in epidemiological surveillance systems, as similar designs of surveillance systems exist across the region. Although sudden surveillance disruption is possible at a country or regional level, it is unlikely to occur in all countries simultaneously. Retrospective modelling with epidemiological, immunological and entomological information is needed. Host or immunological factors may have influenced the decline in dengue cases at the population level through immunity; however, herd protection requires additional evidence. Uncertainty remains regarding the effect on the outcome of sequential infections of different dengue virus (DENV) types and Zika virus (ZIKV), and vice versa. Future studies were recommended that examine the epidemiological effect of prior DENV infection on Zika incidence and severity, the epidemiological effect of prior Zika virus infection on dengue incidence and severity, immune correlates based on new‐generation ELISA assays, and impact of prior DENV/other arbovirus infection on ZIKV immune response in relation to number of infections and the duration of antibodies in relation to interval of protection. Follow‐up studies should also investigate whether increased vector control intensification activities contributed to the decline in transmission of one or more of these arboviruses. Additionally, proposed studies should focus on the potential role of vector competence when simultaneously exposed to various arboviruses, and on entomological surveillance and its impact on circulating vector species, with a goal of applying specific measures that mitigate seasonal occurrence or outbreaks.
Conclusions
Multifactorial events may have accounted for the decline in dengue seen in 2017. Differing elements might explain the reduction in dengue including elements of immunity, increased vector control, and even vector and\or viruses changes or adaptations. Most of the results of this expert consensus group meeting are hypothetical and based on limited evidence. Further studies are needed.
Keywords: Dengue, decline, Americas, hypotheses
Abstract
Objectif
Depuis les années 1980, l'incidence de la dengue a été multipliée par 30. Cependant, en 2017, il y a eu une réduction notable du nombre de cas d'incidence de dengue rapportés dans les Amériques. Nous voulions fournir des explications plausibles à la baisse en 2017.
Méthodes
Un groupe d'experts constitué de représentants d'institutions scientifiques et académiques, d'officiels des Ministères de la Santé d'Amérique Latine et de membres du personnel de l’OPS/OMS s'est réuni en octobre 2017 pour proposer et évaluer des hypothèses.
Résultats
En 2017, il y a eu une baisse généralisée de l'incidence, de la sévérité et du nombre de décès dus à la dengue dans les Amériques, accompagnée d'une réduction des cas rapportés d'infections par le virus Zika et par le virus du chikungunya, sans modification dans la répartition entre les groupes d’âge affectés. Il a été déterminé que ce déclin était peu probablement dû aux changements dans les systèmes de surveillance épidémiologique, étant donné que des systèmes de surveillance similaires existaient dans toute la région. Bien que des perturbations soudaines dans la surveillance soient possibles au niveau national ou régional, il est peu probable que cela se produise simultanément dans tous les pays. Une modélisation rétrospective avec des informations épidémiologiques, immunologiques et entomologiques est nécessaire. Des facteurs liés à l'hôte ou immunologiques peuvent avoir influencé le déclin des cas de dengue au niveau de la population par le biais de l'immunité; cependant, l’évidence d'une protection conférée par l'effet du troupeau nécessite des données supplémentaires. Une incertitude subsiste quant à l'effet sur le résultat des infections séquentielles de différents types du virus de la dengue (DENV) et du virus Zika (ZIKV), et vice‐versa. Les études à venir devraient examiner (1) l'effet épidémiologique d'une infection antérieure par le DENV sur l'incidence et la sévérité du virus Zika, (2) l'effet épidémiologique d'une infection antérieure par le virus Zika sur l'incidence et la sévérité de la dengue, (3) les corrélats immunitaires basés sur des tests ELISA de nouvelle génération, (4) l’ impact d'une infection antérieure à DENV/autres arbovirus sur la réponse immunitaire au ZIKV en fonction du nombre d'infections et de la durée des anticorps en fonction de l'intervalle de protection, (5) si des activités d'intensification de la lutte antivectorielle ont contribué à la diminution de la transmission d'un ou plusieurs de ces arbovirus, (6) le rôle potentiel de la compétence vectorielle lorsqu'ils sont exposés simultanément à différents arbovirus, (7) la surveillance entomologique et son impact sur la circulation d'espèces de vecteurs, dans le but d'appliquer des mesures spécifiques qui réduisent l'occurrence saisonnière d’épidémies.
Conclusions
Des événements multifactoriels pourraient expliquer le déclin observé de la dengue en 2017. La plupart des résultats de cette réunion du groupe de consensus d'experts sont hypothétiques, reposent sur des données limitées et requièrent des investigations supplémentaires.
Keywords: dengue, déclin, Amériques, hypothèses
Introduction
Dengue virus (DENV) infections are a major international public health concern. During the past 40 years, the global incidence of dengue has increased 30‐fold 1. Up to 100 million new infections are estimated to occur annually in tropical and subtropical areas of the world 2, with documented further spread to previously unaffected areas 3. Dengue can be caused by any of the four DENV serotypes (DENV −1 to −4), an RNA virus of the Flaviviridae family. Aedes mosquitoes, primarily Aedes aegypti and Aedes albopictus, transmit the disease. Dengue infection can result in a broad spectrum of symptoms, ranging from asymptomatic infections to a self‐limited illness to severe dengue and potentially lethal outcomes. Without rapid clinical diagnosis and appropriate therapy, severe dengue case fatality rates can exceed 20% 2. The economic impact of dengue in the Americas is estimated at $2.1 billion (USD) per year on average 4.
Between the 1950s and 1960s, the Americas were a virtually dengue‐free zone due to a continental Aedes aegypti eradication campaign supported and led by the Pan American Health Organization (PAHO), that began in 1947 5, 6. Unfortunately, after the campaign's end in the 1960s, vector control efforts were not maintained. This, along with the rapid population growth and acceleration of uncontrolled urbanisation in Latin America, contributed to the re‐infestation with Aedes aegypti and the return of DENV circulation 7. During the early and mid‐1970s, outbreaks associated with DENV‐2 were reported in Colombia and in the Caribbean, and until 1977, DENV‐2 and DENV‐3 serotypes continued to circulate in the region. By 1978, DENV‐1 had spread to South America, Central America, and Mexico. Overall, the countries in the Americas reported approximately 702 000 cases during 1977–1980, with DENV‐1 being the predominant serotype 8, 9.
During the 1980s and early 1990s, reported dengue incidence was relatively stable, apart from 1981 when Cuba reported over 340 000 cases caused by DENV‐2. However, since the 1990s, the Americas have experienced sharp increases in dengue case numbers. The region reported over 4.7 million dengue cases from 2000 to 2007 vs. 3.76 million cases during 1980–1999 10. The increasing trend within the Region has continued in recent years, with reported dengue cases rising nearly three‐fold from 857 534 cases in 2008 to 2.3 million during 2016 11 (in 2016, cases of Zika could have been misclassified as dengue). The year 2016 was characterised by large dengue outbreaks with Brazil alone contributing slightly less than 1.5 million cases, approximately three times more than in 2014. The region also reported 1032 dengue‐related deaths 11.
The introduction of chikungunya virus (CHIKV) and Zika virus (ZIKV) in 2013 and 2015, respectively, has created new public health challenges. The similarity of symptoms between the three viral diseases hindered accurate clinical diagnosis and appropriate patient treatment by healthcare providers (at least initially). Serological diagnosis is difficult due to cross‐reaction between IgM and IgG antibodies against ZIKV and DENV, which complicates laboratory confirmation and case reporting. More than 534 000 Zika and 351 000 chikungunya cases were reported during 2016 12, 13. Yet, despite the recent emergence of these two viruses, dengue remains the most common mosquito‐borne disease in the Americas.
In 2017, however, there was a noticeable reduction in dengue incidence in the Americas (Figure 1). In comparison to the 2 177 118 cases reported during 2016, total dengue virus cases reported in the region during 2017 fell by 73% 11; by a total of 584 263 reported cases. This was the lowest case number since 2006, when 537 412 cases were reported. Moreover, the number of severe and fatal dengue cases during 2017 was the lowest since the Region's Integrated Management Strategy for dengue prevention and control in the Americas (IMS‐Dengue) was implemented in 2003 (Table 1 and Figure 2). Aruba, Panama and Peru are the only countries in the Americas that observed increases in dengue cases in 2017 vs. 2016 11 (Tables 2, 3).
Figure 1.
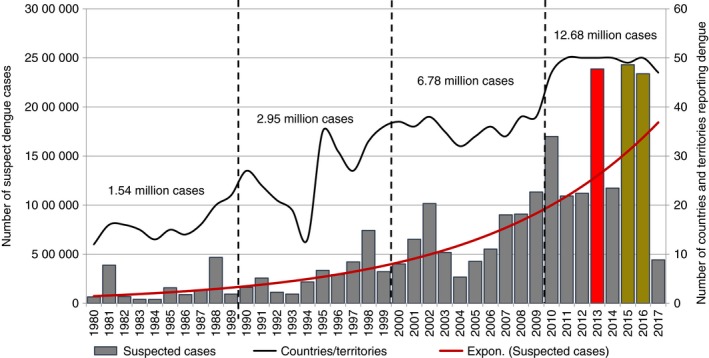
Dengue epidemiological situation in the Americas, 1980–2017. Source: Regional PAHO/WHO Dengue Program. 2017 updated to SE # 36. [Colour figure can be viewed at http://wileyonlinelibrary.com]
Table 1.
Percentage change of total number of deaths due to dengue reported in 2017 compared to 2016, by country and subregion
| Country/Subregion | Epidemiological week | 2016 | 2017 | Percentage change |
|---|---|---|---|---|
| Central America Isthmus and Mexico | 52 | 64 | 62 | −3% |
| Belize | 52 | 0 | 0 | 0% |
| Costa Rica | 52 | 0 | 0 | 0% |
| El Salvador | 52 | 1 | 0 | −100% |
| Guatemala | 52 | 1 | 24 | 2300% |
| Honduras | 52 | 3 | 0 | −100% |
| Mexico | 52 | 34 | 34 | 0% |
| Nicaragua | 52 | 16 | 2 | −88% |
| Panama | 51 | 9 | 2 | −78% |
| Andean subregion | 52 | 142 | 113 | −20% |
| Bolivia | 52 | 2 | 2 | 0% |
| Colombia | 52 | 60 | 15 | −75% |
| Ecuador | 52 | 4 | 4 | 0% |
| Peru | 52 | 37 | 76 | 105% |
| Venezuela | 52 | 39 | 16 | −59% |
| Southern Cone subregion | 52 | 668 | 133 | −80% |
| Argentina | 52 | 10 | 0 | −100% |
| Brazil | 52 | 642 | 133 | −79% |
| Paraguay | 52 | 16 | 0 | −100% |
| Latin Caribbean subregion | 52 | 39 | 3 | −92% |
| Cuba | 52 | 0 | 0 | 0% |
| Dominican Republic | 52 | 39 | 3 | −92% |
| Puerto Rico | 52 | 0 | 0 | 0% |
| Non‐Latin Caribbean subregion | 52 | 0 | 0 | 0% |
| The AMERICAS | 52 | 913 | 311 | −66% |
Figure 2.
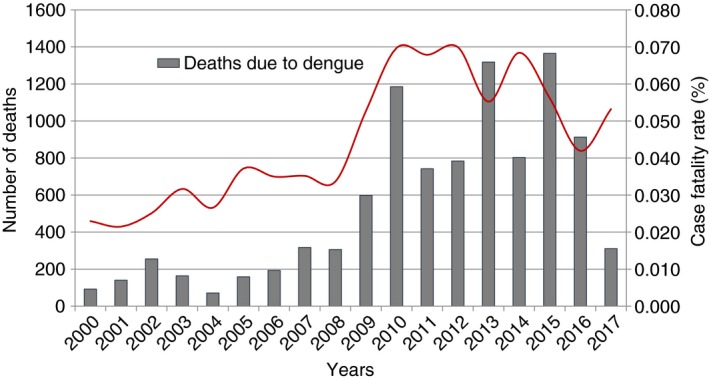
Number and case fatality rate (%) due to dengue in the Americas, 2000–2017. [Colour figure can be viewed at http://wileyonlinelibrary.com]
Table 2.
Number of cases of dengue and Zika, and percentage change of total number of dengue cases reported in 2017 compared to 2016, by country and subregion
| Country/Subregion | Epidemiological week | Zika cases 2016 | Dengue cases 2016 | Dengue cases 2017 | Percentage change (%) |
|---|---|---|---|---|---|
| North America subregion | 52 | 217 | 990 | 348 | −65 |
| United States of America | 52 | 217 | 990 | 348 | −65 |
| Central America Isthmus and Mexico | 52 | 68 376 | 295 042 | 186 088 | −37 |
| Belize | 52 | 824 | 4713 | 2966 | −37 |
| Costa Rica | 52 | 6835 | 23 319 | 5561 | −76 |
| El Salvador | 52 | 11 485 | 8789 | 4300 | −51 |
| Guatemala | 52 | 4131 | 8844 | 4214 | −52 |
| Honduras | 52 | 32 234 | 22 961 | 5217 | −77 |
| Mexico | 52 | 7475 | 130 069 | 89 893 | −31 |
| Nicaragua | 52 | 2053 | 88 463 | 64 712 | −27 |
| Panama | 51 | 3339 | 7278 | 9225 | 27 |
| Andean subregion | 52 | 174 577 | 210 864 | 132 312 | −37 |
| Bolivia | 52 | 897 | 31 756 | 9938 | −69 |
| Colombia | 52 | 106 552 | 103 822 | 26 279 | −75 |
| Ecuador | 52 | 3555 | 14 150 | 11 387 | −20 |
| Peru | 52 | 1958 | 31 868 | 76 093 | 139 |
| Venezuela | 52 | 61 615 | 29 268 | 8615 | −71 |
| Southern Cone subregion | 52 | 323 782 | 1 651 575 | 258 064 | −84 |
| Argentina | 52 | 1847 | 79 455 | 557 | −99 |
| Brazil | 52 | 321 366 | 1 500 535 | 255 665 | −83 |
| Paraguay | 52 | 569 | 70 215 | 1832 | −97 |
| Latin Caribbean subregion | 52 | 41 570 | 8685 | 2617 | −70 |
| Cuba | 52 | 3 | 1836 | 1248 | −32 |
| Dominican Republic | 52 | 5241 | 6645 | 1359 | −80 |
| Puerto Rico | 52 | 36 326 | 204 | 10 | −95 |
| Non‐Latin Caribbean subregion | 52 | 106 114 | 10 025 | 4834 | −52 |
| Anguilla | 52 | 42 | 50 | 22 | −56 |
| Antigua and Barbuda | 52 | 479 | 103 | 1 | −99 |
| Aruba | 52 | 704 | 1319 | 1734 | 31 |
| Bahamas | 52 | 22 | 82 | 14 | −83 |
| Barbados | 52 | 745 | 1433 | 538 | −62 |
| Bermuda | 52 | 0 | 2 | 0 | −100 |
| Jamaica | 52 | 7238 | 2269 | 70 | −97 |
| The Americas | 52 | 714 636 | 2 177 181 | 584 263 | −73 |
Table 3.
Percentage change of total number of severe dengue cases reported in 2017 compared to 2016, by countries and subregions
| Country or Subregion | Epidemiological week | 2016 | 2017 | Percentage change (%) |
|---|---|---|---|---|
| Central America Ithsmus and México | 1405 | 595 | −58 | |
| Costa Rica | 52 | 21 | 0 | −100 |
| El Salvador | 52 | 206 | 7 | −97 |
| Guatemala | 52 | 47 | 64 | 36 |
| Honduras | 52 | 313 | 126 | −60 |
| Mexico | 52 | 806 | 375 | −53 |
| Panama | 51 | 12 | 21 | 75 |
| Andean Subregion | 1381 | 960 | −30 | |
| Bolivia | 52 | 56 | 46 | −18 |
| Colombia | 52 | 1047 | 286 | −73 |
| Ecuador | 52 | 39 | 18 | −54 |
| Peru | 52 | 124 | 251 | 102 |
| Venezuela | 52 | 115 | 359 | 212 |
| Southern Cone Subregion | 909 | 378 | −58 | |
| Argentina | 52 | 0 | 0 | 0 |
| Brazil | 52 | 861 | 378 | −56 |
| Paraguay | 52 | 48 | 0 | −100 |
| Latin Caribbean Subregion | 670 | 107 | −84 | |
| Cuba | 52 | 29 | 19 | −34 |
| Dominican Republic | 52 | 641 | 88 | −86 |
| Puerto Rico | 52 | 0 | 0 | 0 |
| The Americas | 4368 | 2040 | −53 |
Understanding the mechanism(s) underlying the decline in reported dengue incidence in the Americas is key for public health planning. Questions arising from this observation need to be answered to better guide future dengue programmatic actions and outbreak management and control. It is currently unknown whether vector control measures reduced the magnitude of the epidemic, and if so, when such measures should be initiated in future to reduce the risk of large dengue outbreaks developing. The aim of the present discussion and analysis was to hypothesise and present some plausible explanations for this decline.
Methods
A group of dengue experts convened at the PAHO headquarters in Washington D.C., USA from October 3–5, 2017. This meeting brought together representatives from scientific and academic institutions, Ministry of Health officials from Latin America, and PAHO staff to build a common understanding of the issue, as well as to identify, discuss and address proposed hypotheses. A draft hypothesis was generated by a core group of experts from PAHO based on evidence from the literature search. We used the nominal group technique (known as the expert panel) to gather information from relevant experts and determine the extent to which they agreed on a given topic 14. A purposive sampling method was used to recruit the experts in the field of virology, epidemiology, clinical science, entomology and laboratory for this study. They were identified based on diversity of expertise and affiliations. Participation was voluntary. All experts were asked for informed consent to use the information provided and be included as co‐authors of the study. After each panel discussion, feedback was processed and summarised within the expert group. The meeting employed moderated plenary discussions. Deliberations took place in a total of six sessions of panel discussions in which the discussants offered their expert opinions. The meeting's objective was to analyse the virological, epidemiological, and entomological factors that could be linked with the decline in dengue cases throughout the Americas in 2017. The data were thematically analysed to identify all important themes mentioned by the experts. No non‐main stream opinions were identified in the discussions. Several hypotheses were proposed to explain the reduction in incidence, and with each, specific questions were thoroughly addressed and discussed:
Hypothesis 1: Changes in epidemiological surveillance systems.
-
1
What were the trends in the notification of dengue, chikungunya and Zika cases?
-
a
Were there any changes in time, geographic location and age‐group distribution?
-
a
-
2
Were there any changes (e.g. case definition, surveillance sites) in epidemiological surveillance systems?
Hypothesis 2: Temporary or lasting cross‐immunity generated by the simultaneous circulation of several arboviruses.
-
Do host or immunological factors exist that may have an influence at the population level; that is, does immunity or herd protection play a role in the decline in cases?
-
a
If these effects exist, what is the expected duration?
-
a
Hypothesis 3: Changes in the density and competencies of vectors.
Do climatic factors or changes in vector control practices exist that could have influenced or diminished the vector population density in recent years?
How does the simultaneous circulation of various arboviruses and vectors impact vector competence and human transmission?
PAHO staff taped all meetings. Recordings were transcribed verbatim, translated into English, and analysed and edited for common themes.
Results
Hypothesis 1: Changes in epidemiological surveillance systems
Ministry of Health representatives from Brazil, Colombia, Nicaragua, Panama and Venezuela delivered presentations addressing Hypothesis 1. A summary of these presentations follows.
Countries generally reported large declines in dengue incidence during 2017 vs. previous years. Brazil, Colombia and Venezuela have all experienced considerable declines in dengue incidence. Between 2016 and 2017, the number of dengue cases in Brazil dropped from 1 500 535 to 252 054 Between 2016 and 2017, total number of dengue cases in Colombia declined from 103 822 to 26 279 (75% reduction). In Venezuela, although dengue is the most common mosquito‐borne viral illness, disease frequency was well below the minimum expected levels with approximately 8600 dengue cases reported during 2017, which is a 70% decline from the previous year, when 29 268 dengue cases were reported. Similarly, dengue incidence also fell in Nicaragua over the same period, albeit by a smaller magnitude (27% decrease in cases). Dengue cases in Panama also steadily declined since 2014, yet approximately 1300 more cases were reported in 2017 than in the previous year. These declines have occurred despite high vector presence measured in countries such as Colombia and Panama. The expert group agreed that correspondingly, reductions in severe or fatal dengue have also been observed in areas in the region.
In 2017, various DENV serotypes were circulating throughout the region. DENV‐1 circulated in Brazil and Panama; however, Panama also reported concurrent circulation of DENV‐2. In 2013 and 2014, DENV 1, 2 and 3 were all co‐circulating in Nicaragua, with a shift from DENV‐1 to DENV‐2 as the primary serotype. DENV‐2 has since been responsible for most cases. DENV‐2 and DENV‐3 have been the primary DENV serotypes circulating in Colombia since 2015, with DENV‐3 more predominant during 2017.
The expert group agreed that the region also experienced large declines in chikungunya and Zika disease incidence in 2017. Brazil observed declines between 81% and 97% in Zika virus incidence for all five regions (North, Northeast, Southeast, South and Midwest). In contrast, chikungunya incidence rose sharply in 2017 in both the Brazilian North (129% increase) and Midwest (89% increase). In Colombia, approximately 2000 Zika cases were reported in 2017, vs. 82 059 one year earlier, representing a 98% decline. Chikungunya and Zika epidemics peaked in Nicaragua during 2015 and 2016, respectively, with a subsequent diminishment of cases 15, 16. Chikungunya emerged in Panama during April 2014, of which most cases were travel‐acquired. As of epidemiological week 43 (2017), Panama continued to experience autochthonous transmission of chikungunya, with 58 cases reported. Between 2015 and 2017, Panama reported 1266 Zika cases. Although more cases occurred in 2016, cases were more frequent between epidemiological weeks 24–32 of 2017 than in the previous year. Zika was still occurring in 2017 in some regions, including Panama City and Herrera. Incidence of chikungunya and Zika in Venezuela were also well below 2016 levels. Frequency of cases for both diseases declined 98% since their epidemic peaks.
Based on the epidemiological surveillance data analysed, the expert group in consensus decided that the decline in dengue incidence was unlikely due to surveillance system changes for several reasons including:
Similar design of surveillance systems within the region operating at a country level.
Sudden disruption of the system is possible at a country or state/regional level but unlikely to occur simultaneously in all countries.
There is no evidence of significant changes that have occurred in any of the surveillance systems in countries of the Americas, that would explain a decrease in reporting similar to what has been observed.
Epidemiological studies have also shown that dengue can produce endemic and epidemic cycles. A reduction in the number of cases is typically expected after large outbreaks due to low transmission. New outbreaks generally occur every 3–5 years due to changes in herd immunity. The expert group established that in 2017, a generalised decrease in the incidence, severity and number of deaths of dengue occurred in the Americas, accompanied by a reduction in both Zika and chikungunya. Despite this decline, affected age groups generally remain unchanged.
Although high incidences of dengue were reported in 2015 and 2016, the numbers should be interpreted with some caution. The expert group concluded that levels of over‐reporting might have transpired during this period because of initial unnoticed chikungunya and Zika virus's arrival to the Region, resulting in numerous cases possibly misclassified as dengue due to antibody test cross‐reactivity. Surveillance systems in place through each epidemic's introduction were prepared to notify primarily dengue. Thus, countries in the region had no established report form and health care workers were not properly trained to correctly report these new diseases, particularly during their emergence. A major challenge for an epidemiological surveillance system when multiple viruses are circulating simultaneously is the initial case detection during an epidemic.
When an arboviral epidemic begins, there is a cover‐up of the emerging disease in favour of dengue. However, the proportion of cases misclassified is unknown. Assuming that 20–40% of Zika or chikungunya cases were misclassified as dengue in 2015 and 2016, the decline in dengue would not have been as abrupt in 2017. Therefore, the group agreed that part of the decline in dengue incidence during 2017 resulted from over‐reporting between 2015 and 2016. It was recommended that interventions modifying surveillance systems be carried out to account for this adjustment. Therefore, hypothesis 1 was discarded by the expert group as being the reason for the decline in dengue cases at this time, that is, a postulation that a change in the surveillance systems caused a reduction in dengue incidence was neither sufficient nor demonstrated.
Hypothesis 2: Temporary or lasting cross‐immunity generated by the simultaneous circulation of several arboviruses
Addressing Hypothesis 2 began with a review of scientific evidence by the expert group pertaining to the existence and duration of cross‐immunity between dengue viruses and other flaviviruses. Prior DENV infection reduced risk of symptomatic ZIKV infection in a cohort study in Nicaragua 16. Several studies suggest greater antibody responses to DENV and other arboviruses after ZIKV infections. For example, in one study recent ZIKV infections in DENV‐immune individuals elicited stronger neutralising DENV antibody responses for up to 6 months than in DENV‐naïve persons 17, but no data are available on the durability of these responses beyond 6 months. A recent study postulates that the 2015 outbreak of Zika in Brazil might have inhibited transmission of DENV or of symptomatic infection and that ZIKV infections could induce cross‐protective immunity against dengue virus 18. Moreover, strong neutralising antibody responses to ZIKV infection were observed regardless of prior DENV exposure. ZIKV monoclonal antibody (mAbs) studies found that in DENV‐immune persons, ZIKV neutralising antibodies are ZIKV‐specific and come from naive B cells. In contrast, DENV‐cross reactive mAbs were derived from memory B cells 19.
Other studies suggest that neutralising antibodies (NAbs) against DENV can provide protection against symptomatic infection. Katzelnick et al. 20 demonstrated that pre‐infection cross‐reactive dengue NAbs titres correlated with a lower risk of symptomatic secondary infection in a longitudinal pediatric dengue cohort in Nicaragua. The protective effect remained significant after controlling for age, and number of years between infections. It was observed that levels of cross‐reactive neutralising antibodies are maintained over time, possibly due to re‐exposure 20. Furthermore, individuals with higher dengue NAb titres immediately after primary infection had delayed symptomatic DENV infections compared with children with lower titres. It has previously been suggested that the window of cross‐protection induced by a first infection with DENV against a second symptomatic infection is approximately 2 years 21, 22, 23.
Although NAbs are correlated with protection against symptomatic dengue, heterotypic secondary infection (i.e., infection with DENV serotype different than the primary serotype) are more likely to result in severe disease 24. This is known as Antibody‐Dependent Enhancement (ADE). For instance, Katzelnick et al. showed that a specific range of low‐titre anti‐DENV binding antibodies were predictive of increased risk of severe dengue disease in a Nicaraguan cohort study 25. Such antibodies are now been analysed following ZIKV infection in DENV‐immune and DENV‐naïve individuals (L. Katzelnick, A. Balmaseda, and E. Harris, unpublished data) to explore whether preexisting anti DENV antibodies might also enhance ZIKV. However, recent studies in non‐human primates and in humans do not support this hypothesis 26, 27. To better understand the existence and duration of cross immunity for dengue and other flaviviruses, the expert group concluded that future studies should examine:
Epidemiological effect of prior DENV infection on Zika incidence and severity;
Epidemiological effect of prior ZIKV infection on dengue incidence and severity;
Immune correlates based on new‐generation ELISA assays: quality of antibodies in relation to clinical outcome (cohort studies);
Duration of antibodies in relation to interval of protection;
Role of T‐cells.
A distinction exists between cross‐reaction and cross‐protection. It is well‐established that cross‐reaction does not necessarily indicate cross‐protection. There are a few studies that postulate that cross‐protection from Zika occurs after a DENV infection 16, 18. However, evidently immunity resulting from DENV infection was not sufficient to prevent the introduction and rapid spread of Zika in the Americas. Nonetheless, prior DENV infection and anti‐DENV immunity is associated with protection from symptomatic DENV infection, but not with protection against infection. Similarly, ZIKV immunity may reduce symptomatic DENV infection 25. However, antibody depletion studies have shown that in DENV‐immune individuals, DENV cross‐reactive antibodies do not contribute significantly to ZIKV neutralisation 28. Thus, the mechanisms of immunological cross‐protection are still not fully understood and the contribution of CD8+ T cells responses has also been postulated 29, 30. Based on the elements analysed, and based on a limited number of studies, the group concluded that the hypothesis of cross‐immunity has the greatest weight in the reduction of the incidence of dengue. To this are added other elements such as an increase in vector interventions during the period in question.
Hypothesis 3: Changes in the density and competencies of vectors
During the past half century, there has been a rise in both atmospheric concentration of carbon dioxide and average world temperature. Climate change can produce extreme meteorological conditions, heat waves, drought, food and water contamination, and changes in vector distribution (increase or decrease). During this time, emerging arbovirus disease patterns have changed significantly 31. Due to increasing temperatures and precipitation through warming oceans and extreme climatic variability, problems with arthropod vectors, along with arboviruses, will continue to emerge in new regions. This is illustrated by the influence of climatic factors such as temperature and humidity on dengue transmission and distribution. Chandler found that only freezing temperatures kill eggs and larvae of Aedes aegypti 32. In contrast, rising temperatures increases the length of the dengue transmission season in temperate regions 33.
Temperature and humidity may also affect the behaviour, maturation and duration of infectivity in vectors. Feeding behaviour is more frequent at higher temperatures, which further affects transmission risk 34. It may also affect the initial time of mosquito infectivity. The time between feeding and virus detection in the salivary glands of Aedes aegypti decreased from 9 days at 26°C and 28°C to 5 days at 30°C for DENV‐1 and DENV‐4 35. The expert group concluded that there is incomplete knowledge to relate climate change to a decrease in dengue and unfortunately, few long‐term studies regarding predictions of climate change on vector‐borne diseases.
Vector competence is the intrinsic ability of the vector for infection, replication and transmission of a specific virus. It begins with establishment of an infection followed by replication in the epithelial cells. Afterwards, the infecting agent traverses the midgut escape barrier then replicates in other tissues. Finally, invasion and infection of mosquito salivary glands followed by the release in saliva transpires, resulting in the vector being able to transmit the virus. According to Mitchell 36, a species is considered a biological vector of arboviruses when all the following facts occur:
Virus is isolated from mosquitoes collected in nature;
A mosquito is infected after artificial feeding using the blood of a host in the viremic phase or a virus suspension;
The virus can be transmitted to a host through a bite, or detection of the virus in the a mosquito's salivary glands;
Evidence in the field confirming an association between a mosquito species and the population of vertebrates in which the viral infection is occurring.
Several studies examining DENV in Aedes aegypti have found high minimum infection rates (i.e., MIR = Number of positive samples/total number of mosquitoes tested × 1000) for adult females 37, 38. However, high MIR rates in collected mosquito larvae indicate that vertical transmission of DENV from an adult female to her eggs may also occur 39.
Both Aedes aegypti and Aedes albopictus are able to transmit dengue, Zika, chikungunya and even Yellow Fever viruses with similar efficiency. Colder temperatures may however, decrease the local transmission of chikungunya by European Aedes albopictus, possibly explaining the lack of autochthonous transmission of the disease in Europe despite hundreds of imported cases returning from the Caribbean. Vega‐Rúa et al. found that exposing European Aedes albopictus to lower temperatures (20°C) significantly reduced CHIKV transmission potential in the Asian genotype isolated from Saint‐Martin Island 40, 41.
The recent emergence of both chikungunya and Zika viruses has increased the possibility that individuals may become infected by more than one Aedes aegypti‐borne virus at a time. Recent clinical data support that Aedes mosquitoes can be infected with and concurrently transmit all three viruses 39.
Although the Aedes aegypti mosquito is susceptible to climatic variables, elevated mosquito populations in the Region continue, and within many areas, mosquito density remained at levels that maintain and support epidemics, suggesting that climatic factors did not result in diminishing cases. Moreover, based on discussions after literature review, the simultaneous circulation of three arboviruses likely does not interfere in viral transmission by mosquitoes 41, 42. Nevertheless, evidence suggested a recent intensification of vector control measures along with new strategies, implemented (probably simultaneously) within several countries. Due to the arrival of chikungunya followed by Zika, vector control units in countries throughout the Americas intensified activities. Among these, the Networking to Combat Zika and other mosquito borne disease in the Caribbean, the Action Plan for Prevention and control of the Zika virus in Central America and the Dominican Republic, the National Plan for Dengue, Chikungunya and Zika in Brazil and the National Plan for Preparedness and Response to vector‐borne diseases in Colombia and Peru 43, 44, 45. All these countries had in common the following strategies: incorporating additional training for vector control technicians and staff, reinforcement of the entomological surveillance activities through larval surveys, vector control interventions with the suppression of breeding sites using chemical and/or mechanical methods, use of insecticides to reduce the adult mosquito population, community mobilisation and health education by strengthening communication activities related to personal measures of prevention and protection and implementing integrated plans or contingency plans for vector control and other activities to improve entomological surveillance systems. Several countries also implemented the use of pesticides on a large scale, although the precise numbers are not available. Overall, these interventions have previously been demonstrated to reduce dengue incidence rates 46. Furthermore, also apparent is greater and improved communication both between and within countries. What is unknown, however, is the impact of these measures on the potential reduction in dengue burden and incidence. Several suggestions to estimate this impact were provided by the expert group, including:
Intensify entomological surveillance and obtain information regarding the presence of viruses in mosquitoes in a more systematic way.
Investigate the vector control intensification/improvement practices within countries that resulted in mosquito density reductions. Despite many countries reporting intensification of mosquito control activities, specific details are not readily available. Obtaining this information would assist other countries in following similar protocols.
Examine impact of the intensification/improvement of vector control activities on dengue and other arboviral disease incidence.
The expert group concluded that prior to implementing vector control interventions, it is necessary to identify: (i) where they are required and (ii) the surveillance data needed to estimate their impact. Since vector control programs are evaluated using several variables (e.g., biological, chemical, physical and personnel), improved data collection, verification of variables producing an impact, and identification of levels of intervention are essential.
Conclusions and next steps
A consensus was reached by the expert group that multifactorial explanations may account for the decline in dengue observed during 2017. Different elements were described that might explain the reduction, including elements of immunity and increased vector control activities. However, the degree of strength of these elements is unknown. Mathematical modelling using retrospective epidemiological information, along with existing immunological and entomological data, could address and quantify these uncertainties. In an epidemiological model, one can try to estimate future forecasts of disease incidence (by country or even by specific geographic region). When new information is added, the model then can be readjusted and subsequently becomes more accurate. Increased availability of epidemiological models can allow public health workers to identify and target high‐risk areas with appropriate and timely control measures. Nevertheless, proper training in the use of these models is necessary for cautious interpretation. Consequently, a recommendation was set forth to create a working group for mathematical space‐time model development to estimate these effects and dynamics. Countries in attendance were asked to provide weekly epidemiological data during the past 10 years by state/province (i.e., first geopolitical level of data). Specific data requested included circulating serotypes, incidence of dengue, severe dengue, and deaths stratified by gender and age group.
The expert group agreed that much uncertainty remains regarding the sequence of infections between different types and ZIKV. To better understand the cellular and functional properties of an acute DENV and ZIKV infection and its role in cross‐protection and immunopathology, several proposals were recommended. In DENV‐1 followed by ZIKV infections, a subset of B cells generated cross‐neutralising antibodies 47; a similar relation has been observed for DENV‐3 and ZIKV (P. Andrade and E. Harris, unpublished data). The basis for this observation is not known; therefore, further studies are needed. Additionally, several cohort studies exist pertaining to the behaviour of antibodies after an arboviral infection. To better analyse this behaviour, systematic reviews and meta‐analyses of existing cohort studies are recommended.
Case–control studies stratified by previous immune status were also suggested. Two sets of case–controls were proposed: individuals with a laboratory confirmation of dengue (cases) vs. healthy persons (controls) and severe (cases) vs. non‐severe (controls) dengue. Through laboratory tests, there is a need to demonstrate: cross‐immunity between dengue and Zika viruses that this cross‐immunity between the two viruses is protective, that this possible protection could result in a large reduction in dengue incidence and undertake follow‐up studies to document the duration of this protective effect once demonstrated.
Changes in mosquito density and competence may have played a role in the reduction in dengue throughout the Americas due to intensification of vector control activities. Whether specific vector control elements were performed with quality, time, place, and simultaneously with other coinciding control actions in a manner that impacted and reduced infection and stopped local epidemics or reduced transmission is unknown. Follow‐up research studies should investigate the question of whether increased vector control intensification and/or a combination of activities contributed to the decline in transmission of arboviruses. Proposed studies should focus on whether enhanced entomological surveillance has an impact on incidence of arboviruses with a goal of applying specific measures that mitigate seasonal occurrence or an outbreak. Examples of studies proposed by the expert group include:
Determine whether the population of mosquitoes at a local level was infected and infectious by conducting a prospective study examining the infectivity of mosquitoes by various types of arboviruses.
Conduct cross‐sectional studies to examine which mosquitoes are currently transmitting DENV, via data obtained from the country entomological surveillance system.
Conduct research to assess whether Cx. quinquefasciatus plays a role in ZIKV transmission, as some studies suggest this possibility 41.
Develop cross‐sectional evaluations comparing vector control interventions to define which entomological indicators are best assessed and measured over time.
We used the expert consensus methodology in this study considering that it is a valuable approach when other evidence is unavailable and is considered adequate to validate hierarchies of evidence. The number of panel members in our study was higher than the recommended 8–12 members for a consensus panel 48. We increased this number to constitute a multidisciplinary group and obtain a wider range of opinions as has been suggested in previous reports 49.
However, the method was subject to limitations such as potential for bias in the selection of participants, the possibility of having been a random variation in panel behaviour and not ranking the feedback of the experts to weight their agreement using a scale. We consider that we have included experts with considerable experience and a thorough knowledge of the study topic from academia, and government institutions who contributed equally to the product of the study. As has been cited in other reports, this method has acceptable construct validity 50 and reliability 51. It is important to mention that unless the findings can be tested against observed data, we can never be sure that the methods have produced the correct answer.
In conclusion, using the expert group, it was agreed that the decrease in dengue cases in the Americas has been evidenced by the epidemiological surveillance systems of the countries of the countries of the Americas. This decrease initiated after two major epidemics; Chikungunya (2013) and Zika (2015). Among the hypotheses reviewed that may explain this unprecedented phenomenon, the working group concluded that the greatest weight is the existence of cross‐immunity between Zika and Dengue. This hypothesis establishes that patients with a history of dengue infection who are subsequently infected with Zika have protective immunity against a new dengue infection. Another important hypothesis that cannot be ruled out is the increase and strengthening of vector control interventions which included an important community response in combating the vector through the elimination of breeding sites. These vector control actions were characterised by a significant dissemination of information campaigns for the general public through various means. Nevertheless, a recent publication reviewed possible causes of the decline of dengue in Brazil considering herd immunity, cross‐reactivity between Zika and Dengue, vector control measures and environmental factors and concludes that these are not sufficient to explain the dengue scenario in Brazil in 2017 52. There is a need to provide additional evidence concerning the immune response and protection to new infections in the community as well as information on the duration of the acquired immunity. Finally, it is necessary to better understand the impact of vector control actions on the transmission of arboviruses. For this, the development of new scientific research is decisive now and before the current transmission pattern changes.
Acknowledgement
The authors thank Dr. Marcos Espinal, director of the Department of Communicable Diseases and Environmental Determinants of Health of the Pan American Health Organization for his comments on draft versions of this manuscript and his continuous support of this initiative.
References
- 1. World Health Organization (WHO) . Global strategy for dengue prevention and control, 2012. (Available from: http://www.who.int/denguecontrol/9789241504034/en/) [12 April 2018].
- 2. World Health Organization (WHO) . Dengue and severe dengue [factsheet no. 117, revised January 2012]. World Health Organization: Geneva: (Available from: http://www.who.int/mediacentre/factsheets/fs117/en/) [12 April 2018] [Google Scholar]
- 3. Bhatt S, Gething PW, Brady OJ et al The global distribution and burden of dengue. Nature 2013: 496: 504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shepard DS, Coudeville L, Halasa YA, Zambrano B, Dayan GH. Economic impact of dengue illness in the Americas. Am J Trop Med Hyg 2011: 84: 200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schneider J, Droll D. (2001). A Time Line for Dengue in the Americas to December 31, 2000 and Noted First Occurrences, 2001. Pan American Health Organization: Washington, DC: (Available from: http://www.paho.org/English/HCP/HCT/VBD/dengue_finaltime.doc) [6 April 2018]. [Google Scholar]
- 6. Pan American Health Organization (PAHO) . Direct Council of the Pan American Health Organization. Resolution CD1.R1. Continental Aedes aegypti eradication. Sept–Oct 1947 Pub. 247, 1947. (Available from: http://www.paho.org/English/GOV/CD/ftcd_1.htm#R1) [12 April 2018].
- 7. Gubler DJ. Dengue, urbanization and globalization: the unholy trinity of the 21st century. Trop Med Health 2011: 39(Suppl. 4): 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brathwaite‐Dick O, San Martin JL, Montoya RH, Del Diego J, Zambrano B, Dayan GH. Review: the history of dengue outbreaks in the Americas. Am J Trop Med Hyg 2012: 87: 584–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pan American Health Organization (PAHO) . Dengue and dengue hemorrhagic fever in the Americas – Guidelines for prevention and control, 1995. (Available from: http://www.paho.org/hq/index.php?option=com_docman&task=doc_download&Itemid=270&gid=27350&lang=en) [6 May 2018].
- 10. San Martin JL, Brathwaite O, Zambrano B et al The epidemiology of dengue in the Americas over the last three decades: a worrisome reality. Am J Trop Med Hyg 2010: 82: 128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pan American Health Organization (PAHO) . PLISA – Plataforma de Información en Salud de las Américas, 2017. (Available from: http://www.paho.org/data/index.php/es/) [6 May 2018]
- 12. PAHO Pan American Health Organization (PAHO) . Zika Cumulative Cases, 2017. (Available from: http://www.paho.org/hq/index.php?option=com_content&view=article&id=12390&Itemid=42090&lang=en) [6 May 2018].
- 13. PAHO Pan American Health Organization (PAHO) . Chikungunya: Data, Maps and Statistics, 2017. (Available from: http://www.paho.org/hq/index.php?option=com_topics&view=readall&cid=5927&Itemid=40931&lang=en) [6 May 2018].
- 14. Jones J, Hunter D. Consensus methods for medical and health services research. BMJ 1995: 311: 376–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gordon A, Gresh L, Ojeda S et al Differences in transmission and disease severity between 2 successive waves of Chikungunya. Clin Infect Dis 2018: 67: 1760–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gordon A, Gresh L, Ojeda S et al Prior dengue virus infection and risk of Zika: a pediatric cohort in Nicaragua. Submitted for Publication. [DOI] [PMC free article] [PubMed]
- 17. Montoya M, Collins M, Dejnirattisai W et al Longitudinal analysis of antibody cross‐neutralization following Zika and dengue virus infection in Asia and the Americas. J Infect Dis 2018: 4: 536–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ribeiro GS, Kikuti M, Tauro LB et al Does immunity after Zika virus infection cross‐protect against dengue? Lancet Glob Health 2018: 6: e140–e141. [DOI] [PubMed] [Google Scholar]
- 19. Rogers TF, Goodwin EC, Briney B et al Zika virus activates de novo and cross‐reactive memory B cell responses in dengue‐experienced donors. Sci Immunol 2017: 2: eaan6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Katzelnick LC, Montoya M, Gresh L, Balmaseda A, Harris E. Neutralizing antibody titers against dengue virus correlate with protection from symptomatic infection in a longitudinal cohort. Proc Natl Acad Sci 2016: 113: 728–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sabin AB. Research on dengue during World War II. Am J Trop Med Hyg 1952: 1: 30–50. [DOI] [PubMed] [Google Scholar]
- 22. Montoya M, Gresh L, Mercado JC , et al Symptomatic versus inapparent outcome in repeat dengue virus infections is influenced by the time interval between infections and study year. PLoS Negl Trop Dis 2013: 7: e2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Anderson KB, Gibbons RV, Cummings DA et al A shorter time interval between first and second dengue infections is associated with protection from clinical illness in a school‐based cohort in Thailand. J Infect Dis 2014: 209: 360–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guzman MG, Harris E. Dengue. Lancet 2015: 385: 453–465. [DOI] [PubMed] [Google Scholar]
- 25. Katzelnick LC, Gresh L, Halloran ME et al Antibody‐dependent enhancement of severe dengue disease in humans. Science 2017: 358: 929–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pantoja P, Pérez‐Guzmán EX, Rodríguez IV et al Zika virus pathogenesis in rhesus macaques is unaffected by pre‐existing immunity to dengue virus. Nat Commun 2017: 8: 15674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Terzian ACB, Schanoski AS, Mota MTO et al Viral load and cytokine response profile does not support antibody‐dependent enhancement in dengue‐primed Zika virus‐infected patients. Clin Infect Dis 2017: 65: 1260–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Collins MH, McGowan E, Jadi R et al Lack of durable cross‐neutralizing antibodies against Zika virus from dengue virus infection. Emerg Infect Dis 2017: 23: 773–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Grifoni A, Pham J, Sidney J et al Prior Dengue virus exposure shapes T cell immunity to Zika virus in humans. J Virol 2017: 91: e01469‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wen J, Tang WW, Sheets N et al Identification of Zika virus epitopes reveals immunodominant and protective roles for dengue virus cross‐reactive CD8+ T cells. Nat Microbiol 2017: 2: 17036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gould EA, Higgs S. Impact of climate change and other factors on emerging arbovirus diseases. Trans R Soc Trop Med Hyg 2009: 2009: 109–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chandler AC. Factors influencing the uneven distribution of Aedes aegypti in Texas cities. Am J Trop Med Hyg 1945: 25: 145–149. [Google Scholar]
- 33. Jetten TH, Focks DA. Potential changes in the distribution of dengue transmission under climate warming. Am J Trop Med Hyg 1997: 57: 295–297. [DOI] [PubMed] [Google Scholar]
- 34. Christophers SR (1960). Aedes aegypti (L.), the Yellow Fever Mosquito. Its Life History, Bionomics, and Structure. Cambridge University Press: Cambridge. [Google Scholar]
- 35. Sutherst RW. Global change and human vulnerability to vector‐borne diseases. Clin Microbiol Rev 2004: 17: 136–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mitchell CJ. Geographic spread of Aedes albopictus and potential for involvement in arbovirus cycles in the Mediterranean basin. J Vector Ecol 1995: 1995: 44–58. [Google Scholar]
- 37. Chung YK, Pang FY. Dengue virus infection rate in field populations of female Aedes aegypti and Aedes albopictus in Singapore. Trop Med Int Health 2002: 2002: 322–330. [DOI] [PubMed] [Google Scholar]
- 38. Lourenço‐de‐Oliveira R, Vazeille M, de Filippis AMB, Failloux AB. Oral susceptibility to yellow fever virus of Aedes aegypti from Brazil. Mem Inst 2002: 97: 437–439. [DOI] [PubMed] [Google Scholar]
- 39. Cecílio AB, Campanelli ES, Souza KP, Figueiredo LB, Resende MC. Natural vertical transmission by Stegomyia albopicta as dengue vector in Brazil. Braz J Biol 2009: 69: 123–127. [DOI] [PubMed] [Google Scholar]
- 40. Vega‐Rúa A, Lourenço‐de‐Oliveira R, Mousson L, Vazeille M et al Chikungunya virus transmission potential by local Aedes mosquitoes in the Americas and Europe. PLoS Negl Trop Dis 2015: 9: e0003780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rückert C, Weger‐Lucarelli J, Garcia‐Luna S et al Impact of simultaneous exposure to arboviruses on infection and transmission by Aedes aegypti mosquitoes. Nat Commun 2017: 8: 15412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Göertz GP, Vogels CBF, Geertsema C, Koenraadt CJM, Pijlman GP. Mosquito co‐infection with Zika and Chikungunya virus allows simultaneous transmission without affecting vector competence of Aedes aegypti . PLoS Negl Trop Dis 2017: 11: e0005654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pan American Health Organization . Response to the Epidemic of Zika Virus in the Americas December 2015 – 2016. Pan American Health Organization: Washington DC, 2016. (Available from: https://www.paho.org/hq/index.php?option=com_docman&view=download&category_slug=informes-tecnicos-8345&alias=43927-reporte-ops-respuesta-a-epidemia-virus-zika-americas-diciembre-2015-2016-disponible-solo-ingles-927&Itemid=270&lang=es) [15 Dec 2018] [Google Scholar]
- 44. Ministerio de Salud de Perú . Plan Nacional de Preparación y Respuesta frente a la enfermedad por el virus Zika ‐ Perú, 2016. (Available from: http://bvs.minsa.gob.pe/local/MINSA/3468.pdf) [15 December 2018]
- 45. Ministry of Health of Brazil . Sala Nacional de Coordenacao e controle para o enfrentamento da dengue, do virus Chikungunya e do Zika virus. Informe No. 19 ‐10 de Janeiro de 2016, monitoramento das ativiaddes do 7° ciclo de vistas a imoveis no Brasil (Available from: http://www.combateaedes.saude.gov.br/images/informes/informe-sncc-19-avaliacao-do-7-ciclo.pdf) [15 December 2018]
- 46. Roiz D, Wilson AL, Scott TW et al Integrated Aedes management for the control of Aedes‐borne diseases. PLoS Negl Trop Dis 2018: 12: e0006845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Robbiani DF, Bozzacco L, Keeffe JR et al Recurrent potent human neutralizing antibodies to Zika virus in Brazil and Mexico. Cell 2017: 169: 597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Murphy MK, Black NA, Lamping DL et al Consensus development methods, and their use in clinical guideline development. Health Technol Assess 1998:2:i1–iv88. [PubMed] [Google Scholar]
- 49. Hutchings A, Raine R, Sanderson C, Black N. A comparison of formal consensus methods used for developing clinical guidelines. J Health Serv Res Policy 2006: 4: 218–224. [DOI] [PubMed] [Google Scholar]
- 50. Cross H. Consensus methods: a bridge between clinical reasoning and clinical research? Int J Lepr Other Mycobact Dis 2005: 73: 28–32. [DOI] [PubMed] [Google Scholar]
- 51. Hutchings A, Raine R. A systematic review of factors affecting the judgments produced by formal consensus development methods in health care. J Health Serv Res Policy 2006: 11: 172–179. [DOI] [PubMed] [Google Scholar]
- 52. Lopes TRR, Silva CS, Pastor AF, Silva Júnior JVJ. Dengue in Brazil in 2017: what happened? Rev Inst Med Trop Sao Paulo 2018: 60: e43. [DOI] [PMC free article] [PubMed] [Google Scholar]


