Abstract
Background
Knowledge of the level of healthcare utilization (HCU) and the predictors of high HCU use in patients with an implantable cardioverter defibrillator (ICD) is lacking. We examined the level of HCU and predictors associated with increased HCU in first‐time ICD patients, using a prospective study design.
Methods
ICD patients (N = 201) completed a set of questionnaires at baseline and 3, 6, and 12 months after inclusion. A hierarchical multiple linear regression with three models was performed to examine predictors of HCU.
Results
HCU was highest between baseline and 3 months postimplantation and gradually decreased during 12 months follow‐up. During the first year postimplantation, only depression (β = 0.342, P = 0.002) was a significant predictor. Between baseline and 3 months follow‐up, younger age (β = −0.220, P < 0.01), New York Heart Association class III/IV (β = 0.705, P = 0.01), and secondary indication (β = 0.148, P = 0.05) were independent predictors for increased HCU. Between 3 and 6 months follow‐up, younger age (β = −0.151, P = 0.05) and depression (β = 0.370, P < 0.001) predicted increased HCU. Between 6 and 12 months only depression (β = 0.355, P = 0.001) remained a significant predictor.
Conclusions
Depression was an important predictor of increased HCU in ICD patients in the first year postimplantation, particularly after 3 months postimplantation. Identifying patients who need additional care and provide this on time might better meet patients’ needs and lower future HCU.
Keywords: depression, healthcare utilization, implantable cardioverter defibrillator, mental health
1. INTRODUCTION
The implantable cardioverter defibrillator (ICD) is the treatment of choice for the prevention of potentially life‐threatening cardiac tachyarrhythmias in high‐risk patients (primary prevention) and in patients who have experienced cardiac tachyarrhythmias in the past (secondary prevention).1 The ICD constantly monitors the heart rate and in case of a ventricular tachyarrhythmia delivers a shock in order to restore a normal rhythm.2 Because of the proven benefits of the ICD as compared to antiarrhythmic medication terminating tachyarrhythmias,3 the number of ICD implants has increased over the past years, although a plateau now has been reached in at least many European countries.4
Besides the unequivocal medical benefits, ICD therapy is associated with a risk of complications and increased healthcare costs have been reported.5 Receiving an ICD is associated with high healthcare costs as the implantation device itself can be as expensive as 30 000 euros.6 In addition, after implantation, patients are generally followed‐up at 1‐to‐4‐month intervals (depending on patients’ clinical status and device model) at the outpatient clinic.2 These follow‐ups are generally performed by the ICD technicians but may also involve a consult by the cardiologist in case of complications (eg, wound infections, lead failure), which may result in even higher healthcare expenses.5 Besides patients’ medical needs with respect to living with an ICD, a significant subgroup (1 in 5) reports symptoms of anxiety and depression at a level that warrants treatment7 and that affects patients’ physical and mental functioning.8 In turn, this may lead to a further increase in healthcare utilization (HCU) and associated costs.
Previous studies have shown that an increase of HCU depends on patients’ demographic, medical, and psychological characteristics (eg, multimorbidity, mental health disorders).9 As some of these factors are modifiable, it is important to timely identify this subset of patients at the time of implantation and to provide patients with relevant care in order to reduce HCU and associated costs. To the best of our knowledge, no studies to date have examined the level of HCU and its predictors in the ICD population. Hence, in the current study we examined the level of HCU and the predictors of increased HCU and costs in patients with an ICD.
2. METHODS
2.1. Participants and study procedure
Data for the current study have been collected as part of the WEB‐based distress management program for implantable CARdioverter dEfibrillator patients (WEBCARE), which was a multicenter randomized controlled trial.10 The study sample consisted of patients who were admitted for a first‐time implantation of an ICD. Patients were recruited between April 2010 and February 2013 from six Dutch referral hospitals (Amphia Hospital, Breda; Canisius‐Wilhelmina Hospital, Nijmegen; Catharina Hospital, Eindhoven; Erasmus Medical Centre, Rotterdam; Onze Lieve Vrouwe Gasthuis, Amsterdam; Vlietland Hospital, Schiedam).
The ICD technician or ICD nurse of the participating hospitals approached all patients between 18 and 75 years who received a first‐time ICD implant for participation. Exclusion criteria were a history of psychiatric illness other than depressive or anxiety disorders, significant cognitive impairments (eg, dementia), being on the waiting list for heart transplantation, life‐threatening comorbidities (eg, malignancies), life expectancy less than 1 year, lack of internet/computer skills, and insufficient knowledge of the Dutch language. In the 1‐year follow‐up period after the ICD implantation, patients were requested to complete a set of validated and standardized questionnaires at four time‐points (baseline, 3, 6, and 12 months after inclusion). The study procedure has been described elsewhere in more detail.10 The study protocol was approved by the Medical Ethical Committees of all participating centers and the study was conducted in accordance to the Helsinki declaration.
2.2. Demographic and clinical variables
Information on demographic (age, gender, educational level) and clinical variables (Charlson Comorbidity Index [CCI], New York Heart Association [NYHA] functional class [NYHA‐class I/II vs III/IV], ICD indication [primary vs secondary indication], and total shocks [appropriate and inappropriate]) was obtained from purpose‐designed questions in the questionnaires and patients’ medical records.
2.3. Healthcare utilization
An adjusted version of the Trimbos/iMTA questionnaire for Costs associated with Psychiatric Illness (TiC‐P) was used to assess HCU in the WEBCARE population.11 This generic patient self‐report survey includes 14 structured yes/no questions on relevant medical resources (eg, “Did you consult with a General Practitioner at any time during the past three months”).11 Each item is followed by a question on the frequency of utilization of that specific medical resource over the past 3 months. For example, the baseline score represents the HCU within the 3 months prior to ICD implantation, while the 3‐month score reflects HCU between baseline and 3 months postimplantation. For the current study, only the items referring to medical resource use (eg, General Practitioner, company doctor, physiotherapist, and outpatient hospital visits) were used, whereas current sample is not a psychiatric population and psychological HCU was reported sporadically. The reliability and validity of the medical resource items are considered satisfactory (Cohen's kappa ranges from 0.597 to 0.795) within the population of patients with mild to moderate mental health problems,11 and is previously used within the cardiac population.12
2.4. Anxiety
Symptoms of anxiety were assessed with the 7‐item General Anxiety Disorder scale (GAD‐7). The GAD‐7 items (eg, “Feeling afraid, as if something awful might happen”) are rated on a 4‐point Likert scale ranging from 0 (not at all) to 3 (almost every day).13 The total score implies severity of anxiety and ranges from 0 to 21, with higher scores indicating higher anxiety levels. With a Cronbach's alpha of 0.92, the internal consistency of the GAD‐7 is considered excellent.13
2.5. Depression
Depressive symptoms were assessed with the Patient Health Questionnaire (PHQ‐9), a patient self‐report survey which is comprised of 9 items (eg, “Feeling down, depressed, or hopeless”) that are answered on a 4‐point Likert scale ranging from 0 (not at all) to 3 (nearly every day).14 The score range is between 0 and 27, with higher scores representing a higher level of depression symptom severity. The internal consistency is considered excellent, with Cronbach's alpha of 0.90.14
2.6. Type D personality
Type D (distressed) personality was assessed with the DS14 (Type D scale).15 This questionnaire consists of 14 items with two 7‐item subscales measuring Social Inhibition (eg, “I find it hard to start a conversation”) and Negative Affectivity (eg, “I am often in a bad mood”).15 The items are rated on a 5‐point Likert scale from 0 (false) to 4 (true). The total score of both subscales range from 0 to 28. Patients are classified as Type D when scoring ≥10 on both subscales. The internal consistency of SI and NA are considered satisfactory, with reported Cronbach's alphas of 0.86 and 0.88, respectively.15
2.7. Statistical analysis
Descriptive statistics of the baseline variables were evaluated using frequencies (categorical variables) and mean scores with standard deviations (SD) (continuous variables). They are presented as percentages and means ± SDs, respectively. Differences between nonresponders and participants on baseline characteristics were calculated by using the χ2 test for independence (categorical variables) and independent sample t‐tests (continuous variables). Missing data were handled by using pairwise deletion (available‐case analysis). In order to assess whether a priori determined demographic characteristics (age, gender, and education), clinical variables (NYHA class, ICD indication, total shocks [appropriate and inappropriate], CCI), and psychological variables (anxiety, depression, and Type D personality) were associated with total HCU in ICD patients the first year after implantation, a sum score was calculated for the number of healthcare use during the 12‐month period. Baseline measurement for the sum score was excluded, as this score reflects HCU before the ICD implantation. Generalized linear modelling was used to investigate the change in HCU over time. As HCU is considered count data, a Poisson distribution with log linear link function was used to model HCU scores over time. Time was modelled as a continuous variable because the intervals between the measurements were unequally spaced. A hierarchical multivariable regression with three models was performed in order to examine the predictors of HCU. Demographic characteristics were entered in the first model. In the second model, clinical variables were entered, followed by the psychological variables in the third model. Subsequently, more detailed information about the course of HCU over the year was provided by performing the same analyses for time‐point specific reported HCU at 3 (reflecting HCU between baseline and 3 months follow‐up), 6 (reflecting HCU between 3 and 6 months follow‐up), and 12 months (reflecting HCU between 9 and 12 months follow‐up) after ICD implantation, respectively. All analyses were conducted using IBM SPSS Statistics 22.0 (IBM Corp., Armonk, NY, USA). A P value < 0.05 was considered statistically significant.
3. RESULTS
3.1. Patient characteristics
A total of 1024 patients were approached for participation, with 562 patients being eligible and 340 patients signing the informed consent. Of these 340 patients, 15% (51/340) did not return the baseline questionnaire, an additional 11% (31/289) were lost to follow‐up, and 3% (9/280) passed away in the period between baseline and 12 months follow‐up (see Figure 1 for a detailed description of the sample selection). A total of 201 patients was included in the current analyses. The majority of the patients were men (274/340; 80.6%), with a mean age of 58 ± 10 years. Nonresponders, compared to participants, were more likely to have a NYHA functional class III/IV (58.1% vs 18.9%; χ2 (1, 221) = 30.88, P < 0.001, phi = −0.39), more likely to be diagnosed with diabetes mellitus (35.5% vs 14.0%; χ2 (1, 262) = 12.79, P < 0.001, phi = −0.23), and more likely to have peripheral vascular disease (12.9% vs 4.5%; χ2 (1, 262) = 4.21, P = 0.040, phi = −0.15). A detailed overview of the baseline sample characteristics is presented in Table 1.
Figure 1.
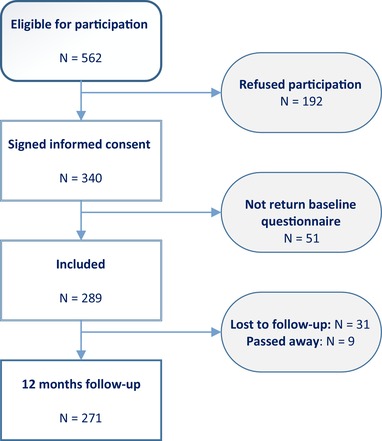
Flowchart of patient recruitment [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 1.
Baseline patient characteristics of the total sample
| Variable | Mean ± SD; N (%) |
|---|---|
| Demographic | |
| Age | 58.16 ± 10.30 |
| Gender (men) | 274 (80.6) |
| Partner (yes)N = 288 | 244 (71.8) |
| Education level (High)N = 288 | 208 (61.2) |
| Work (yes)N = 288 | 141 (41.5) |
| Clinical | |
| Heart failure (yes)N = 339 | 181 (53.2) |
| NYHA class III/IVN = 267 | 57 (21.3) |
| Ischemic heart disease (yes)N = 339 | 204 (60.0) |
| Atrial fibrillation (yes) N = 339 | 80 (23.5) |
| Secondary prevention indication N = 339 | 47 (13.8) |
| Any shocksa | 28 (8.2) |
| Anemia (yes)N = 339 | 17 (5.0) |
| CVA in past N = 339 | 15 (4.4) |
| TIA in past N = 339 | 26 (7.6) |
| PAD N = 339 | 18 (5.3) |
| COPD (yes) N = 339 | 26 (7.6) |
| Diabetes mellitus (yes) N = 339 | 51 (15.0) |
| Dyslipidemia (yes) N = 339 | 71 (20.9) |
| Hypertension (yes) N = 339 | 76 (22.4) |
| Malignancy, excluding metastatic cancer N = 339 | 14 (4.1) |
| Psychological | |
| Anxiety (GAD‐7)N = 288 | 4.30 ± 4.54 |
| Depression (PHQ‐9) N = 289 | 5.45 ± 4.83 |
| Type D personality (yes)N = 288 | 45 (13.2) |
| Cardiac medication | |
| Psychotrophics N = 339 | 33 (9.7) |
| ACE‐inhibitors N = 339 | 206 (60.6) |
| Beta‐blockers N = 339 | 279 (82.1) |
| Statins N = 339 | 209 (61.5) |
| Diuretics N = 339 | 172 (50.6) |
| Amiodarone N = 339 | 31 (9.1) |
ACE = angiotensin‐converting enzyme; COPD = chronic obstructive pulmonary disease; CVA = cerebrovascular accident; GAD–7 = 7–item General Anxiety Disorder scale; NHYA = New York Heart Association; PAD = peripheral arterial disease; PHQ–9 = Patient Health Questionnaire; TIA = transient ischemic attack.
Shocks received between implantation and 12 months.
3.2. Healthcare utilization
The mean frequency of HCU (number of visits to General Practitioner, company doctor, physiotherapist, and outpatient hospital visits), within the first year following the ICD implantation, was 6.20 three months after implantation (0 to 3 months), 5.36 at 6 months follow‐up (3 to 6 months), and 4.76 12 months postimplantation (9 to 12 months), respectively (Table 2). These HCU frequencies did not significantly differ across time (F (2, 680) = 2.738, P = 0.065).
Table 2.
Overview of healthcare utilization over 12 months post‐ICD implantation
| Measurement point | Mean ± SD |
|---|---|
| HCU Baseline | 5.39 ± 5.92 |
| HCU 3 months after implantation | 6.20 ±7.89 |
| HCU 6 months after implantation | 5.36 ± 7.34 |
| HCU 12 months after implantation | 4.76 ± 7.38 |
HCU = healthcare utilization; ICD = implantable cardioverter defibrillator; SD = standard deviation.
3.3. Predictors of total HCU within 12 months postimplantation
Assessing the predictors of total HCU within 12 months post‐ICD implantation, analyses showed that sociodemographic variables (Model 1) did not explain a significant part of the variance in HCU (F (3, 155) = 0.91, P = 0.437, ∆R 2 = 0.017). However, after adding clinical characteristics (Model 2), the regression model improved significantly (F (4, 151) = 2.02, P = 0.027, ∆R 2 = 0.068) and explained 9% of the total variance in HCU. Age (β = ‐0.187, P = 0.029), NYHA class (β = 0.179, P = 0.026), and CCI (β = 0.176, P = 0.034) emerged as independent predictors of postimplantation HCU. With the addition of psychological variables (Model 3), 15% of the variance in HCU was explained (F (3, 148) = 2.67, P = 0.010, ∆R 2 = 0.067), adding to the level of prediction of the model. Of all the predictors in the final model, only depression appeared to be significantly associated with total HCU (β = 0.342, P = 0.002). This association indicates that higher depression scores at baseline are associated with higher HCU the first year postimplantation, independent of demographic, clinical, and psychological variables (see Table 3 for a detailed overview of the regression model).
Table 3.
Predictors of HCU over the entire 12 months, at 3, 6, and 12 months after ICD implantation
| Total | 3 months | 6 months | 12 months | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE B | Β | B | SE B | β | B | SE B | β | B | SE B | β | |
| Model 1: | ||||||||||||
| Age | −0.241 | 0.156 | −0.129 | −0.135 | 0.059 | −0.177* | −0.091 | 0.054 | −0.128 | 0.023 | 0.057 | 0.032 |
| Gendera | 0.596 | 4.001 | 0.012 | −0.702 | 1.525 | −0.035 | −0.930 | 1.398 | −0.050 | 1.858 | 1.469 | 0.100 |
| Educationb | −1.380 | 3.494 | −0.032 | 0.428 | 1.332 | 0.024 | −1.641 | 1.221 | −0.099 | 1.037 | 1.283 | 0.063 |
| Model 2: | ||||||||||||
| Age | −0.348 | 0.158 | −0.187* | −0.169 | 0.060 | −0.220** | −0.126 | 0.056 | −0.177* | −0.007 | 0.059 | −0.009 |
| Gender | 0.405 | 4.002 | 0.008 | −0.779 | 1.524 | −0.039 | −0.989 | 1.410 | −0.053 | 2.054 | 1.502 | 0.110 |
| Education | −0.540 | 3.432 | −0.012 | 0.689 | 1.307 | 0.039 | −1.363 | 1.209 | −0.083 | 1.300 | 1.288 | 0.078 |
| CCI | 3.611 | 1.683 | 0.176* | 1.055 | 0.641 | 0.125 | 1.235 | 0.593 | 0.157* | 1.302 | 0.632 | 0.165 |
| NYHA class | 8.385 | 3.741 | 0.179* | 3.607 | 1.424 | 0.188* | 2.512 | 1.318 | 0.140 | 0.128 | 1.404 | 0.007 |
| ICD indicationc | 2.389 | 1.851 | 0.102 | 1.421 | 0.705 | 0.148* | 0.803 | 0.652 | 0.090 | −0.115 | 0.695 | −0.013 |
| Total shocksd | −0.180 | 2.712 | −0.005 | −0.030 | 1.033 | −0.002 | −0.340 | 0.955 | −0.026 | 0.076 | 1.018 | 0.006 |
| Model 3: | ||||||||||||
| Age | −0.306 | 0.156 | −0.164 | −0.173 | 0.061 | −0.226** | −0.107 | 0.054 | −0.151* | 0.019 | 0.058 | 0.026 |
| Gender | −1.650 | 3.960 | −0.034 | −.882 | 1.551 | −0.044 | −1.851 | 1.387 | −0.100 | 1.088 | 1.479 | 0.058 |
| Education | −0.760 | 3.358 | −0.018 | 0.499 | 1.316 | 0.028 | −1.430 | 1.176 | −0.087 | 1.339 | 1.254 | 0.081 |
| CCI | 3.277 | 1.683 | 0.159 | 1.160 | 0.659 | 0.137 | 1.056 | 0.589 | 0.135 | 1.068 | 0.629 | 0.135 |
| NYHA class | 5.652 | 3.760 | 0.121 | 3.127 | 1.473 | 0.163* | 1.391 | 1.317 | 0.078 | −0.866 | 1.404 | −0.048 |
| ICD indication | 2.379 | 1.801 | 0.101 | 1.399 | 0.706 | 0.145* | 0.799 | 0.631 | 0.089 | −0.100 | 0.673 | −0.011 |
| Total shocks | −.446 | 2.642 | −0.013 | −0.071 | 1.035 | −0.005 | −0.459 | 0.925 | −0.035 | −0.018 | 0.987 | −0.100 |
| Anxietye | −0.621 | 0.441 | −0.146 | −0.196 | 0.173 | −0.112 | −0.254 | 0.155 | −0.157 | −0.148 | 0.165 | −0.091 |
| Depressionf | 1.362 | 0.422 | 0.342* | 0.186 | 0.166 | 0.114 | 0.561 | 0.148 | 0.370** | 0.542 | 0.158 | 0.355** |
| Type D personalityg | −6.795 | 4.347 | −0.128 | −1.833 | 1.703 | −0.084 | −2.301 | 1.522 | −0.114 | −2.317 | 1.623 | −0.114 |
Male vs female.
High vs low level.
Secondary vs primary indication.
total shocks (both appropriate and inappropriate) received between implantation and 12 months
Continuous.
Continuous.
Type D personality vs non‐Type D personality.
Total: R 2 = 0.02 for model 1, ΔR 2 = 0.07 (P = 0.03) for model 2, ΔR 2 = 0.07 (P = 0.01) for model 3.
3 months: R 2 = 0.03 for model 1, ΔR 2 = 0.07 (P = 0.02) for model 2, ΔR 2 = 0.02 (P = 0.41) for model 3.
6 months: R 2 = 0.02 for model 1, ΔR 2 = 0.07 (P = 0.05) for model 2, ΔR 2 = 0.08 (P < 0.01) for model 3.
12 months: R 2 = 0.01 for model 1. ΔR 2 = 0.03 (P = 0.36) for model 2, ΔR 2 = 0.08 (P < 0.01) for model 3.
*
P ≤ 0.05.
**
P ≤ 0.01.
CCI = Charlson Comorbidity Index (continuous); HCU = healthcare utilization; ICD = implantable cardioverter defibrillator; NYHA class = New York Heart Association functional class (III–IV vs I–II).
3.4. HCU at 3, 6, and 12 months
3.4.1. Baseline–3 months follow‐up
Assessing the predictors of HCU within the first 3 months post‐ICD implantation revealed that sociodemographic characteristics were not associated with HCU (Model 1: F(3, 177) = 1.91, P = 0.130, ∆R 2 = 0.031). In Model 2, clinical characteristics were added to the sociodemographic characteristics and this model explained 10% of the total variance in HCU (F (4, 173) = 2.65, P = 0.016, ∆R 2 = 0.066). Younger age (β = −0.220, P = 0.006), NYHA class III/IV (β = 0.188, P = 0.012), and secondary indication for ICD (β = 0.148, P = 0.045) were independent predictors in this model. Adding anxiety, depression, and Type D personality in Model 3 did not significantly increase the explained variance in HCU (F (3, 170) = 2.15, P = 0.406, ∆R 2 = 0.015). Younger age (β = ‐0.226, P = 0.005), NYHA class III/IV (β = 0.163, P = 0.035), and secondary indication for an ICD (β = 0.145, P = 0.049) remained significant predictors of HCU. See Table 3 for a detailed overview.
3.4.2. Three to 6 months follow‐up
For HCU between 3 and 6 months postimplantation, demographic variables (Model 1) did not explain a significant proportion of the variance (F (3, 184) = 1.34, P = 0.263, ∆R 2 = 0.021). When adding clinical characteristics in Model 2, the model did not explain a significant proportion of the variance (F (4, 180) = 1.96, P = 0.052, ∆R 2 = 0.049). Younger age (β = −0.177, P = 0.024) and CCI (β = 0.157, P = 0.039) were significantly associated with HCU. Sociodemographic, clinical, and psychological characteristics combined in Model 3 accounted for a significant 15% of the variance in HCU (F (3, 177) = 3.04, P = 0.002, ∆R 2 = 0.076). In this model, younger age (β = −0.151, P = 0.050) and depression (β = 0.370, P < 0.001) were associated with increased HCU, independent of other demographic, clinical, and psychological variables (see Table 3).
3.4.3. Six to 12 months follow‐up
Between 6 and 12 months, sociodemographic characteristics in Model 1 were not associated with HCU (F (3, 170) = 0.72, P = 0.544, ∆R 2 = 0.012). Addition of clinical characteristics in Model 2 did not significantly improve the level of prediction of the model (F (4, 166) = 0.94, P = 0.358, ∆R 2 = 0.026). When combining sociodemographic, clinical, and psychological characteristics in Model 3, a significant 12% of the variance in HCU was explained (F (3, 163) = 2.13, P = 0.003, ∆R 2 = 0.078). Depression (β = 0.355, P = 0.001) was the only variable associated with increased HCU independent of demographic, clinical, and psychological variables (see Table 3).
Although the influence of depression on HCU was larger at 6 and 12 month than at 3 months, these differences failed to reach significance.
4. DISCUSSION
To our knowledge, this is the first study to tap into HCU and its predictors in patients with an ICD. The results of the current study showed that HCU was the highest between baseline and 3 months postimplantation and that it gradually decreased over the 12‐months follow‐up. Focusing on the predictors of HCU, the results showed that for total HCU in the first year postimplantation only depression was a significant predictor after controlling for demographic and clinical variables. When focusing on HCU within the first 12 month postimplantation, analyses showed that between baseline and 3 months postimplantation, younger age, NYHA class, and secondary indication were associated with increased HCU, adjusting for baseline demographic and clinical and psychological characteristics. However, between 3 and 6 months postimplantation only younger age and depression were significant predictors of HCU after controlling for other relevant variables. In addition, between 6 and 12 months only depression was significantly associated with increased HCU independent of other relevant variables.
The current findings are in line with some previous studies in the general cardiac population that focused on HCU. For example, in patients with chronic stable angina, depression was an independent predictor of an increase in mean cumulative 1‐year healthcare costs, with a 33% increase in patients with depression as compared to non‐depressed patients.16 This increase was observed in several healthcare sectors after controlling for other healthcare expenditure costs (eg, physician costs, outpatient care, chronic care, inpatient care, and medications).16 A possible mechanism underlying the association between depression and HCU is the association between depression and unhealthy lifestyle behaviors (eg, smoking, reduced exercise, unhealthy diet, alcohol consumption, and sedentary lifestyle), as depression is a known barrier for lifestyle changes, which may result in poor physical health and eventually in increased HCU.17 In addition, patients with depression may experience associated somatic symptoms (eg, headaches, sleep disturbances) for which additional care is sought. Another explanation could be that depressed cardiac patients obtain lower quality of care as compared to cardiac patients who are not depressed.18 This may lead to undertreatment of these patients, resulting in more physical complaints as a consequence. Remarkably, in contrast with existing literature on the relation between anxiety and high HCU in both cardiac and noncardiac populations,19, 20 the current study did not find this association. The relatively healthy recruited population could possibly explain this. Furthermore, the majority of patients in the current sample received beta‐blockers as part of their cardiac medication regimen.21 As beta‐blockers are known for their anxiolytic properties,22 this may have further reduced anxiety levels. Speculatively, one might also reason that increased anxiety levels might lead to less medical resource seeking as part of an avoidant coping strategy.
Within the ICD population specifically, appropriate and inappropriate shocks are well known predictors of HCU.23, 24 Yet, in our study, only 8% of the total sample received a shock of any kind, which may explain why no association between shocks and HCU within the predictor models was found. Nevertheless, it would be valuable for future research to conduct a sub analysis in a larger sample with only ICD recipients who have received shocks, exploring the specific contribution of appropriate and inappropriate shocks to HCU. Previous studies found older age and comorbidities9 to be predictors of increased HCU. By contrast, our results showed that younger age was associated with increased HCU, while no association was found with comorbidities. Again, a possible explanation for the latter could be that the WEBCARE sample was relatively healthy. With respect to age, within the ICD population younger age has been associated with poor adjustment postimplantation and increased distress.25 As discussed previously, this may lead to increase of somatic symptoms and associated HCU.
The outcomes of this study stress the necessity for clinicians to be alert to signs of depression (eg, sadness, loss of interest, withdrawing from relatives, nonadherence to treatment, etc.), particularly as of 3 months post‐ICD implantation. Since studies show that psychological interventions like cognitive behavioral therapy are beneficial in reducing depressive symptoms in ICD patients,26 referral for psychological help in case of a suspected mood disorder is warranted.
The results of the current study must be interpreted with the following limitations in mind. HCU was assessed using the TiC‐P self‐report questionnaire. While this is easy and low‐cost to use, it may be prone to recall bias and therefore not an entirely accurate representation of HCU. Furthermore, the TiC‐P questionnaire has been designed for psychiatric populations and has not been validated for cardiac patients. Therefore, future research should use more objective sources of information to quantify HCU. In addition, HCU over 12 months may be underestimated. As the TiC‐P questionnaire assesses HCU 3 months in retrospect, at month 12 the scores reflect HCU between month 9 and 12. As in the WEBCARE study no assessment took place at 9 months; we were not able to assess the level of HCU between 6 and 9 months postimplant. Furthermore, as mentioned previously the WEBCARE sample was relatively higher educated and healthy as compared to other ICD samples. This might have influenced the results and their generalizability to the general ICD population. Final, given improvements in technology of the ICD during the last decade, the representativeness of the WEBCARE cohort for the current ICD population might be questionable. These innovations have led to a reduction in shocks, and could therefore have an influence on the generalizability of current findings. However, similar levels of depression have been reported by a study in ICD recipients that did use new programming strategies.27 This study also have some advantages. It is the first study to examine HCU in the ICD population, using a prospective study design in a well‐described population. In addition, research shows that only a small number of high‐cost utilizers in general keeps using medical resources after a year.9
Future studies are warranted that focus on the mechanisms underlying the association between depression and HCU. In addition, it would be valuable to examine which patient profiles are associated with increased HCU in order to develop programs that could provide more personalized care at the right time. Finally, research is warranted to replicate the current findings, using more objective indicators of HCU and associated costs and compare whether there is a discrepancy between objective and subjective indicators of HCU.
In conclusion, this study showed that depression is an important predictor of HCU in ICD patients within the first 12 months postimplantation. The impact of depression on HCU is particularly prominent from 3 months and up to 12 months postimplantation. Within the first 3 months, younger age, NYHA classification (III‐IV), and secondary indication showed to be associated with HCU. Future research should focus identifying patients who need additional care and provide this on time in order to better meet patients’ needs and lower future HCU.
AUTHOR CONTRIBUTIONS
EB: data analysis/interpretation, statistics, drafting article, critical revision, approval of article, data collection. PL: data analysis/interpretation, statistics, critical revision, approval of article. VS: data interpretation, critical revision, approval of article. JW: data interpretation, critical revision of article, approval of article. SP: concept/design, data interpretation, critical revision of article, approval of article, funding secured by. MH: concept/design, data interpretation, drafting article, critical revision of article, approval of article, data collection.
ACKNOWLEDGMENTS
We would like to thank all the patients for their participation and the (ICD) nurses in the participating hospitals for helping with recruitment.
CONFLICTS OF INTEREST
None declared.
Broers ER, Lodder P, Spek VRM, Widdershoven JWMG, Pedersen SS, Habibović M. Healthcare utilization in patients with first‐time implantable cardioverter defibrillators (data from the WEBCARE study). Pacing Clin Electrophysiol. 2019;42:439–446. 10.1111/pace.13636
REFERENCES
- 1. Goldberger Z, Lampert R. Implantable cardioverter‐defibrillators. JAMA. 2006;295:809 Available at http://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.295.7.809. Accessed October 22, 2018. [DOI] [PubMed] [Google Scholar]
- 2. Priori SG, Blomström‐Lundqvist C, Mazzanti A, Bloom N, Borggrefe M, Camm J, et al. 2015. ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J 2015;36:2793‐867. Available at https://academic.oup.com/eurheartj/article-lookup/doi/10.1093/eurheartj/ehv316. Accessed October 17, 2018. [Google Scholar]
- 3. Pedersen CT, Kay GN, Kalman J, et al. EHRA/HRS/APHRS expert consensus on ventricular arrhythmias. Europace. 2014;16:1257‐1283. Available at https://academic.oup.com/europace/article-lookup/doi/10.1093/europace/euu194. Accessed June 27, 2018. [DOI] [PubMed] [Google Scholar]
- 4. Mond HG, Proclemer A. The 11th World Survey of cardiac pacing and implantable cardioverter‐defibrillators: Calendar year 2009–A World Society of Arrhythmia's Project. Pacing Clin Electrophysiol. 2011;34:1013‐1027. Available at http://www.ncbi.nlm.nih.gov/pubmed/21707667. Accessed October 22, 2018. [DOI] [PubMed] [Google Scholar]
- 5. Koneru JN, Jones PW, Hammill EF, Wold N, Ellenbogen KA. Risk factors and temporal trends of complications associated with transvenous implantable cardiac defibrillator leads. J Am Heart Assoc. 2018;7 Available at:e007691 http://www.ncbi.nlm.nih.gov/pubmed/29748177. Accessed October 22, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gold MR, Padhiar A, Mealing S, Sidhu MK, Tsintzos SI, Abraham WT. Economic value and cost‐effectiveness of cardiac resynchronization therapy among patients with mild heart failure: Projections from the reverse long‐term follow‐up. JACC Hear Fail. 2017;5:204‐212. Available at https://www.sciencedirect.com/science/article/pii/S2213177916305728. Accessed October 10, 2018. [DOI] [PubMed] [Google Scholar]
- 7. Magyar‐Russell G, Thombs BD, Cai JX, et al. The prevalence of anxiety and depression in adults with implantable cardioverter defibrillators: A systematic review. J Psychosom Res. 2011;71:223‐231. Available at http://www.ncbi.nlm.nih.gov/pubmed/21911099. Accessed June 22, 2018. [DOI] [PubMed] [Google Scholar]
- 8. Habibović M, Pedersen SS, van den Broek KC, et al. Anxiety and risk of ventricular arrhythmias or mortality in patients with an implantable cardioverter defibrillator. Psychosom Med. 2013;75:36‐41. Available at https://insights.ovid.com/crossref?an=00006842-201301000-00007. Accessed June 29, 2018. [DOI] [PubMed] [Google Scholar]
- 9. Johnson TL, Rinehart DJ, Durfee J, et al. For many patients who use large amounts of health care services, the need is intense yet temporary. Health Aff. 2015;34:1312‐1319. Available at http://content.healthaffairs.org/cgi/doi/10.1377/hlthaff.2014.1186. Accessed June 20, 2017. [DOI] [PubMed] [Google Scholar]
- 10. Habibović M, Denollet J, Cuijpers P, et al. Web‐based distress management for implantable cardioverter defibrillator patients: A randomized controlled trial. Heal Psychol. 2017;36:392‐401. Available at http://psycnet.apa.org/doiLanding?doi=10.1037%2Fhea0000451. Accessed June 27, 2018. [DOI] [PubMed] [Google Scholar]
- 11. Bouwmans C, De Jong K, Timman R, et al. Feasibility, reliability and validity of a questionnaire on healthcare consumption and productivity loss in patients with a psychiatric disorder (TiC‐P). BMC Health Serv Res. 2013;13:217 Available at http://bmchealthservres.biomedcentral.com/articles/10.1186/1472-6963-13-217. Accessed June 27, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kraal JJ, Peek N, Van Den Akker‐Van Marle E, Kemps HM, Effects and costs of home‐based training with telemonitoring guidance in low to moderate risk patients entering cardiac rehabilitation: The FIT@Home study. 2013;13:82 Available at http://www.biomedcentral.com/1471-2261/13/82. Accessed October 17, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder. Arch Intern Med. 2006;166:1092 Available at http://archinte.jamanetwork.com/article.aspx?doi=10.1001/archinte.166.10.1092. Accessed April 20, 2018. [DOI] [PubMed] [Google Scholar]
- 14. Stafford L, Berk M, Jackson HJ. Validity of the hospital anxiety and depression scale and patient health questionnaire‐9 to screen for depression in patients with coronary artery disease. Gen Hosp Psychiatry. 2007;29:417‐424. Available at https://www.sciencedirect.com/science/article/pii/S0163834307001296. Accessed June 27, 2018. [DOI] [PubMed] [Google Scholar]
- 15. Denollet J, DS14: Standard assessment of negative affectivity, social inhibition, and Type D personality. Psychosom Med 2005;67:89‐97. Available at https://insights.ovid.com/crossref?an=00006842-200501000-00013. Accessed June 30, 2018. [DOI] [PubMed] [Google Scholar]
- 16. Szpakowski N, Qiu F, Masih S, Kurdyak P, Wijeysundera HC. Economic impact of subsequent depression in patients with a new diagnosis of stable angina: A population‐based study. J Am Heart Assoc. 2017;6:e006911. Available at http://www.ncbi.nlm.nih.gov/pubmed/29021276. Accessed June 15, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cabello M, Miret M, Caballero FF, et al. The role of unhealthy lifestyles in the incidence and persistence of depression: A longitudinal general population study in four emerging countries. Global Health. 2017;13:18 Available at http://www.ncbi.nlm.nih.gov/pubmed/28320427. Accessed June 29, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Druss BG, Bradford WD, Rosenheck RA, Radford MJ, Krumholz HM. Quality of medical care and excess mortality in older patients with mental disorders. Arch Gen Psychiatry. 2001;58:565 Available at http://archpsyc.jamanetwork.com/article.aspx?doi=10.1001/archpsyc.58.6.565. Accessed June 15, 2018. [DOI] [PubMed] [Google Scholar]
- 19. Tremblay M‐A, Denis I, Turcotte S, et al. Heart‐focused anxiety and health care seeking in patients with non‐cardiac chest pain: A prospective study. Gen Hosp Psychiatry. 2018; 50:83‐89. Available at https://linkinghub.elsevier.com/retrieve/pii/S0163834317302761. Accessed January 18, 2018. [DOI] [PubMed] [Google Scholar]
- 20. Chamberlain AM, Vickers KS, Colligan RC, Weston SA, Rummans TA, Roger VL. Associations of preexisting depression and anxiety with hospitalization in patients with cardiovascular disease. Mayo Clin Proc. 2011;86:1056‐1062. Available at https://www.sciencedirect.com/science/article/abs/pii/S002561961165194X. Accessed January 18, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Habibović M, Denollet J, Cuijpers P, Spek VRM, van den, Broek KC, Warmerdam L, et al. E‐health to manage distress in patients with an implantable cardioverter‐defibrillator:. Psychosom Med 2014;75:593‐602. Available at https://insights.ovid.com/crossref?an=00006842-201410000-00004. Accessed June 27, 2018. [DOI] [PubMed] [Google Scholar]
- 22. Steenen SA, van Wijk AJ, van der Heijden GJMG, van Westrhenen R, de Lange J, de Jongh A. Propranolol for the treatment of anxiety disorders: Systematic review and meta‐analysis. J Psychopharmacol. 2016;30:128‐139. Available at http://www.ncbi.nlm.nih.gov/pubmed/26487439. Accessed January 18, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rickard J, Whellen D, Sherfesee L, et al. Characterization of health care utilization in patients receiving implantable cardioverter‐defibrillator therapies: An analysis of the managed ventricular pacing trial. Heart Rhythm. 2017;14:1382‐1387. Available at https://www.sciencedirect.com/science/article/pii/S1547527117304174. Accessed January 18, 2019. [DOI] [PubMed] [Google Scholar]
- 24. Turakhia MP, Zweibel S, Swain AL, Mollenkopf SA, Reynolds MR. Healthcare utilization and expenditures associated with appropriate and inappropriate implantable defibrillator shocks. Circ Cardiovasc Qual Outcomes. 2017;10:e002210. Available at https://www.ahajournals.org/doi/10.1161/CIRCOUTCOMES.115.002210. Accessed January 21, 2019. [DOI] [PubMed] [Google Scholar]
- 25. Sears SF, Burns JL, Handberg E, Sotile WM, Conti JB. Young at heart: understanding the unique psychosocial adjustment of young implantable cardioverter defibrillator recipients. Pacing Clin Electrophysiol. 2001;24:1113‐1117. Available at http://www.ncbi.nlm.nih.gov/pubmed/11475828. Accessed June 29, 2018. [DOI] [PubMed] [Google Scholar]
- 26. Sears S, Sowell L, Kuhl E, et al. The ICD shock and stress management program: A randomized trial of psychosocial treatment to optimize quality of life in ICD patients. Pacing Clin Electrophysiol. 2007;30:858‐864. Available at http://doi.wiley.com/10.1111/j.1540-8159.2007.00773.x. Accessed June 22, 2018. [DOI] [PubMed] [Google Scholar]
- 27. Mastenbroek MH, Pedersen SS, van der Tweel I, Doevendans PA, Meine M. Results of ENHANCED implantable cardioverter defibrillator programming to reduce therapies and improve quality of life (from the ENHANCED‐ICD study). Am J Cardiol. 2016;117:596‐604. Available at https://www.sciencedirect.com/science/article/pii/S000291491502336X#tbl1. Accessed January 21, 2019. [DOI] [PubMed] [Google Scholar]


