Abstract
Essentials.
Predictive ability of pro‐hemostatic Gas6 for recurrent venous thromboembolism (VTE) is unknown.
We measured Gas6 levels in 864 patients with VTE over 3 years.
High Gas6 (> 157%) at diagnosis is associated with VTE recurrence, major bleeding and mortality.
Gas6 plasma levels measured 12 months after the index VTE are discriminatory for VTE recurrence.
Summary
Background
Growth arrest‐specific gene 6 (Gas6) is a prohemostatic protein with an unknown predictive ability for recurrent venous thromboembolism (VTE). In the elderly, VTE results in higher mortality but does not have a higher rate of recurrence than in younger patients. Consequently, anticoagulation management in the elderly is challenging.
Objective
To prospectively investigate the performance of Gas6 in predicting VTE recurrence, major bleeding and mortality in the elderly.
Methods
Consecutive patients aged ≥ 65 years with acute VTE were followed for a period of 3 years. Primary outcomes were symptomatic VTE recurrence, major bleeding, and mortality. Plasma Gas6 was measured with ELISA.
Results
Gas6 levels were measured in 864 patients at the time of the index VTE (T1) and, in 70% of them, also 12 months later (T2). The Gas6 level at T1 was discriminatory for VTE recurrence (C‐statistic, 0.56; 95% confidence interval [CI] 0.51–0.62), major bleeding (0.60, 95% CI 0.55–0.65) and mortality (0.69, 95% CI 0.65–0.73) up to 36 months. VTE recurrence up to 24 months after T2 was discriminated by the Gas6 level at T2 (0.62, 95% CI 0.54–0.71). High Gas6 levels (> 157%) and continuous Gas6 levels at T1 were associated with VTE recurrence up to 6 months and 12 months, respectively.
Conclusions
In elderly patients, a high Gas6 level is associated with higher risks of VTE recurrence, major bleeding, and death. These findings support further studies to assess the performance of Gas6 in adjusting the length of anticoagulation.
Keywords: aged, cohort studies, growth arrest‐specific gene 6, mortality, venous thromboembolism
Introduction
Venous thromboembolism (VTE), comprising deep vein thrombosis (DVT) and pulmonary embolism (PE), represents a worldwide major health issue, and is a leading cause of cardiovascular death 1. VTE incidence rises with age 2, 3, 4, 5. In the elderly population, VTE results in higher mortality but does not have a higher rate of recurrence than in younger patients 4. Elderly patients more often present with comorbidities, and therefore a higher risk of bleeding 4. Consequently, management of the anticoagulation in the elderly constitutes a challenge.
Because the risk of VTE recurrence is greatest in the first 6–12 months following the initial event and progressively decreases afterwards 6, the benefit of extended anticoagulation may be exceeded by the risk of clinically important bleeding 7, 8, 9, 10, 11, 12.
Growth arrest‐specific gene 6 (Gas6), the product of the gene GAS6 13, is a secreted vitamin K‐dependent protein. The Gas6 plasma level is known to be elevated in a variety of clinical conditions, including inflammation or sepsis 14, 15, 16, 17, 18, obesity 19, chronic renal failure, and cancer 20. Importantly, no change in the Gas6 plasma level with increasing age was previously observed 21. Nevertheless, the Gas6 level progressively decreased with increasing International Normalized Ratio under warfarin therapy 21.
Gas6 has multiple functions, including regulation of cell growth 13 and inflammation 22. It also has effects on platelet function and coagulation, enhancing platelet aggregation and tissue factor expression in endothelial cells, as well as promoting the recruitment of platelets and leukocytes to the endothelial cell membrane 23, 24, 25, 26, 27, 28. In mice, the absence of Gas6 is protective against thrombosis without causing excessive bleeding, pointing to Gas6 as an attractive target for antithrombotic therapy 23, 25.
In a cross‐sectional study including 279 patients and 79 controls, Blostein et al. 29 measured a higher Gas6 plasma level in patients 4 months after VTE than in healthy controls. In addition, they observed that subjects with elevated Gas6 levels in plasma had an increased risk of VTE as compared with those with lower Gas6 levels after adjustment for age, sex, medications, and comorbidities. However, elevated Gas6 plasma levels were not predictive of VTE recurrence 29. Finally, most of the patients included in this study were aged < 65 years.
Here, in a cohort of 864 patients aged ≥ 65 years with VTE, we prospectively investigated the performance of Gas6 plasma levels at admission and 1 year after the index VTE in predicting the risk of VTE recurrence, major bleeding, and mortality.
Methods
Cohort sample
The study was conducted between September 2009 and December 2013 as part of the Swiss Cohort of Elderly Patients with VTE (SWITCO65+), which was a prospective multicenter cohort study to assess medical outcomes and quality of life in elderly patients with acute VTE from all five university hospitals and from four high‐volume non‐university hospitals in Switzerland 30.
Consecutive patients aged ≥ 65 years with acute VTE were identified in the inpatient and outpatient services of all participating study sites, and followed for a period of 3 years. We defined DVT as acute onset of leg pain or swelling plus incomplete compressibility of a venous segment on ultrasonography or an intraluminal filling defect on contrast venography 31.
Because iliac veins and the inferior vena cava may be technically difficult to compress, additional diagnostic criteria for iliac/caval DVT comprised abnormal duplex flow patterns compatible with thrombosis or an intraluminal filling defect on spiral computed tomography (CT) or magnetic resonance imaging venography 32, 33, 34.
Given that ultrasonography has reduced sensitivity and specificity for distal DVT 35, patients with isolated distal DVT were included only if the incompressible distal deep vein transverse diameter was at least 5 mm 36, 37.
Symptomatic PE was defined as: acute onset of dyspnea, chest pain, or syncope, coupled with a new high‐probability ventilation/perfusion lung scan; a new contrast filling defect on spiral CT or pulmonary angiography; or the new documentation of a proximal DVT either by venous ultrasound or by contrast venography 37, 38. Radiographic studies used to diagnose VTE were interpreted by on‐site vascular specialists or radiologists.
Exclusion criteria were inability to provide informed consent (i.e. severe dementia), conditions incompatible with follow‐up (i.e. terminal illness or place of residence too far from the study center), insufficient German‐speaking or French‐speaking ability, thrombosis at a site other than a lower limb, and catheter‐related thrombosis.
Treatment of VTE, e.g. the type of anticoagulant used (i.e. parenteral anticoagulant followed by vitamin K antagonists, parenteral anticoagulant alone, or direct oral anticoagulant), the duration of the anticoagulation, and the prescription of compression stocking, was entirely left to the discretion of the managing physicians.
Eligible patients were approached for informed consent to participate in the study. The ethics committees at each study site approved the study, and written informed consent was obtained from all participants. A detailed description of the study methods has previously been published 30.
Data collection
For all enrolled patients, trained study nurses prospectively collected baseline demographic information (age and sex), type, history and complication of VTE (distal DVT, proximal DVT, overt PE, presence of post‐thrombotic syndrome, prior VTE, provoked index VTE, or cancer‐related VTE), concomitant use of estrogen therapy during the past 3 months, immobilization during the last 3 months, major surgery during the last 3 months, comorbid conditions (history of major bleeding, chronic liver disease, renal disease, chronic or acute heart failure, cerebrovascular disease, diabetes mellitus, body mass index of > 30, acute rheumatic disease during the last 3 months, inflammatory bowel disease, or severe infection or sepsis during the last 3 months), a high risk of falling, laboratory findings (anemia or low platelet count), concomitant use of antiplatelet drugs, arterial hypertension, a heart rate of ≥ 110 beats min−1, systolic blood pressure of < 100 mmHg, a respiratory rate of ≥ 30 min−1, a temperature of < 36 °C, arterial oxygen saturation of < 90%, and VTE‐related treatment, by the use of standardized data collection forms. Follow‐up included one telephone interview and two face‐to‐face evaluations during the first year of study participation, and then semi‐annual contacts, alternating between face‐to‐face evaluations (clinic visits or home visits in house‐bound patients) and telephone calls, as well as periodic reviews of the patient's hospital chart. During each visit/contact, study nurses interviewed patients to obtain information about the date and type of clinical events (recurrent VTE, bleeding, or death). If a clinical event had occurred, this information was complemented by reviewing medical charts and interviewing patients’ primary‐care physicians and family members. Collected data were recorded on standardized forms.
Blood samples
Blood was collected after minimal venostasis into 1/9 of its volume of 0.0160 m trisodium citrate (Sarstedt; Thermo Fischer Scientific, Waltham, Massachusetts, USA) at the time of the index VTE diagnosis and 12 months later 39. Citrated platelet‐poor plasma (PPP) was prepared by centrifugation for 10 min at 2700 × g and room temperature, and recentrifugation of the supernatant plasma for 10 min at 2700 × g to remove remaining platelets 39. The resulting citrated PPP was stored in aliquots of 2 mL at − 80 °C within 1 h of blood collection 39. Citrated PPP was used for Gas6 ELISA.
Gas6 ELISA
To measure Gas6, we used the ELISA method developed by Clauser et al. 40, with some modifications 17. Wells from 96‐well plates (Maxisorp; Nunc, Nümbrecht, Germany) were coated with 100 μL per well of polyclonal goat anti‐human Gas6 antibody (AB885; R&D Systems, Abington, UK) diluted in 0.1 m NaHCO3 (pH 8.2), and incubated overnight at 4 °C. After two washes with phosphate‐buffered saline (PBS)–Tween 0.05%, 100 μL of PBS–bovine serum albumin (BSA) 1%/5% was added to the wells, and plates were incubated for 2 h at room temperature. After three washes, samples diluted 50‐fold and 100‐fold and a normal plasma serial dilution with PBS–BSA 1% were added to the wells, and this was followed by overnight incubation at 4 °C. After three washes, 100 μL of biotinylated polyclonal goat antibody (BAF885; R&D Systems) was added to each well, and plates were left for 2 h at room temperature. Signals were amplified with avidin–horseradish peroxidase (BD Pharmingen, Oxford, UK), and plates were incubated for 20 min at 37 °C. Finally, o‐phenylenediamine dihydrochloride (Sigma‐Aldrich St. Louise, Missouri, USA) was added. Reactions were stopped by the addition of 50 μL of 3 m HCl. Absorbance was measured at 492 nm, and the results were expressed as percentages relative to normal plasma, with its serial dilution as standard curve 17, 40. This ELISA was specific for human Gas6, with no cross‐reactivity with human protein S.
D‐dimer
D‐dimer was measured by ELISA (Vidas D‐dimer exclusion test; bioMérieux, Marcy‐l'Etoile, France).
Outcome variables
We defined objectively confirmed, symptomatic VTE recurrence, major bleeding and overall mortality up to 3 years as primary study outcomes.
VTE recurrence was defined as a fatal or new non‐fatal PE or new DVT 41. The diagnosis of recurrent VTE during follow‐up was established according to the following criteria: for DVT, on the basis of abnormal results on ultrasonography; and for PE, on the basis of CT or angiography showing new intraluminal defects, or on the basis of a ventilation–perfusion lung scan showing a high‐probability pattern with new perfusion defects. A new proximal DVT, based on abnormal results on ultrasonography, associated with new PE symptom(s) (shortness of breath, chest pain, and syncope) was also considered as recurrent PE.
Major bleeding was defined as fatal bleeding, symptomatic bleeding at critical sites (intracranial, intraspinal, intraocular, retroperitoneal, intra‐articular, pericardial, or intramuscular with compartment syndrome), or clinically overt bleeding with a reduction in hemoglobin level of at least 20 g L−1, or leading to transfusion of two or more units of packed red blood cells 42.
We assessed the outcomes by using patient or proxy interviews, interview of the patient's primary‐care physician, and/or hospital chart review 30. A committee of three blinded clinical experts confirmed all outcomes and classified all deaths as being definitely attributable to PE, possibly attributable to PE (e.g. sudden death without an obvious cause), attributable to major bleeding, or attributable to another cause 30. The final classification was made on the basis of the full consensus of this committee 30.
Statistical analyses
We compared the baseline characteristics of patients in relation to elevated plasma Gas6 (above versus below the median) by using the chi‐squared test and the non‐parametric Wilcoxon rank‐sum test as appropriate. We calculated incidence rates of a first VTE recurrence, a first major bleed or death up to 3 years after the index event in relation to the level of Gas6. Gas6 was categorized into low, medium and high levels on the basis of lower and upper quartiles. We estimated the cumulative incidence of these outcomes by using the Kaplan–Meier method, and compared survivor functions across groups by using the log‐rank test.
The discriminative power of Gas6 for predicting VTE recurrence, major bleeding and mortality was assessed by the use of Harrell's C concordance statistic.
Associations between Gas6 and the time to a first VTE recurrence and major bleeding were assessed by the use of competing risk regression accounting for non‐PE‐related and non‐bleeding‐related death, respectively, as a competing event, according to the method of Fine and Gray 43. The method yields subhazard ratios with corresponding 95% confidence intervals (CIs) and P‐values for the failure event of primary interest. For mortality, an ordinary Cox regression with robust standard errors was calculated. We adjusted the model for previously published predictors of VTE recurrence or major bleeding [6,41,42,44–52]. For overall mortality, analyses were adjusted for age, gender, cancer, provoked VTE, prior VTE, overt PE, renal disease, history of major bleeding, heart failure, chronic lung disease, elevated heart rate, low blood pressure, low oxygen and periods of anticoagulation as a time‐varying covariate 49, 53.
All analyses were performed with stata 14 (Stata Corporation, College Station, Texas, USA).
Results
Study sample
Of 1003 enrolled patients aged ≥ 65 years with acute VTE, we excluded 139 patients at the time of index VTE diagnosis (eight patients did not allow the use of data, four withdrew their consent within 1 day, and 127 had no Gas6 measurement), leaving a study sample of 864 patients (Fig. 1). Of these patients, 601 (69.6%) had Gas6 measurement 12 months after the index VTE.
Figure 1.
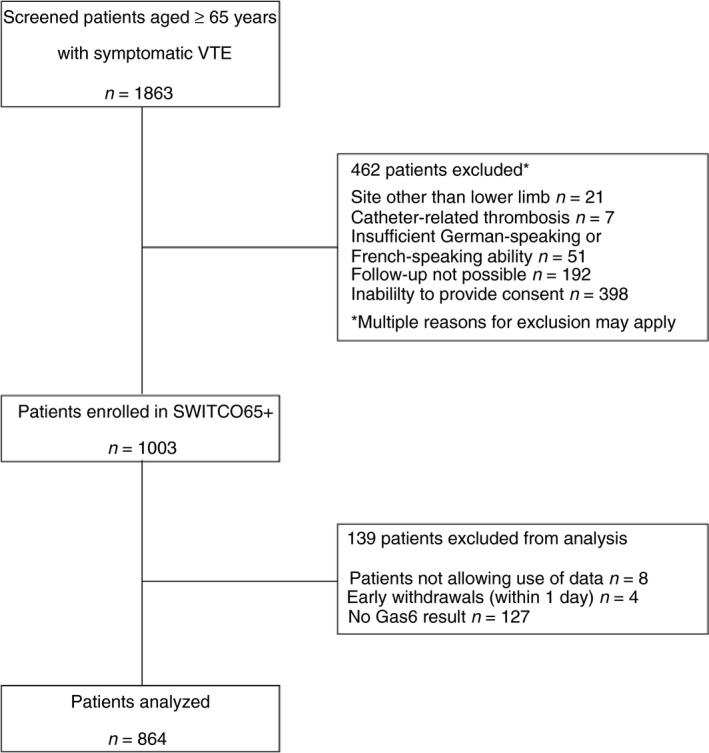
Flow diagram of patients included in the study. Gas6, growth arrest‐specific gene 6; VTE, venous thromboembolism.
Characteristics at the time of the index VTE diagnosis are shown in Table 1. Overall, 476 patients (44.9%) were women, and the median age was 75.0 years (interquartile range [IQR] 69.0–81.0 years). Five hundred and ninety‐nine patients (69.3%) presented with an index PE. Two hundred and fifty‐one patients (29.1%) had experienced prior VTE. Five hundred and twenty‐two patients (60.4%) had an unprovoked index VTE, 185 (21.4%) had provoked VTE, and 157 (18.2%) had cancer‐related VTE. Patients with an unprovoked index VTE or with prior VTE were more likely to present with PE (70%) than with proximal (24%) or distal DVT (6%) only. PE was more frequent in patients with unprovoked VTE (72%) than in patients with provoked (66%) or cancer‐related (64%) VTE (P = 0.01). Twelve months after the index VTE, 432 patients (50%) were still receiving anticoagulation, most of them with vitamin K antagonists.
Table 1.
Patient characteristics at the time of the index venous thromboembolism (VTE) by growth arrest‐specific gene 6 (Gas6) plasma level (above versus below or at the median)
| Characteristic* | All | Gas6 level above median (> 129%) | Gas6 level below or at median (≤ 129%) | P‐value |
|---|---|---|---|---|
| n (%) or median (IQR) | n (%) or median (IQR) | |||
| Total number of patients | 864 | 435 | 429 | |
| Patient age (years) | 75.0 (69.0–81.0) | 76.0 (70.0–82.0) | 74.0 (69.0–80.0) | 0.001 |
| Female sex | 388 (44.9) | 207 (47.6) | 181 (42.2) | 0.111 |
| VTE location | ||||
| Distal DVT only | 70 (8.1) | 29 (6.7) | 41 (9.6) | 0.053 |
| Proximal DVT | 195 (22.6) | 111 (25.5) | 84 (19.6) | |
| Pulmonary embolism | 599 (69.3) | 295 (67.8) | 304 (70.9) | |
| Type of VTE† | ||||
| Unprovoked | 522 (60.4) | 242 (55.6) | 280 (65.3) | < 0.001 |
| Provoked | 185 (21.4) | 92 (21.1) | 93 (21.7) | |
| Cancer‐related* | 157 (18.2) | 101 (23.2) | 56 (13.1) | |
| Estrogen therapy during the last 3 months* | 27 (3.1) | 9 (2.1) | 18 (4.2) | 0.073 |
| Immobilization during the last 3 months | 190 (22.0) | 115 (26.4) | 75 (17.5) | 0.001 |
| Major surgery during the last 3 months | 131 (15.2) | 72 (16.6) | 59 (13.8) | 0.251 |
| Prior VTE | 251 (29.1) | 125 (28.7) | 126 (29.4) | 0.837 |
| Presence of PTS* , ‡ | 453 (52.4) | 251 (57.7) | 202 (47.1) | 0.003 |
| History of major bleeding* | 89 (10.3) | 54 (12.4) | 35 (8.2) | 0.039 |
| Chronic liver disease | 13 (1.5) | 10 (2.3) | 3 (0.7) | 0.053 |
| Renal disease§ | 170 (19.7) | 97 (22.3) | 73 (17.0) | 0.051 |
| Chronic or acute heart failure | 103 (11.9) | 57 (13.1) | 46 (10.7) | 0.280 |
| Cerebrovascular disease (stroke, TIA) | 84 (9.7) | 44 (10.1) | 40 (9.3) | 0.695 |
| Diabetes mellitus | 137 (15.9) | 79 (18.2) | 58 (13.5) | 0.062 |
| BMI > 30* | 201 (23.3) | 107 (24.6) | 94 (21.9) | 0.360 |
| High risk of falling* , ¶ | 406 (47.0) | 233 (53.6) | 173 (40.3) | < 0.001 |
| Acute rheumatic disease during the last 3 months | 29 (3.4) | 17 (3.9) | 12 (2.8) | 0.365 |
| Inflammatory bowel disease | 31 (3.6) | 9 (2.1) | 22 (5.1) | 0.016 |
| Severe infection or sepsis during the last 3 months | 71 (8.2) | 42 (9.7) | 29 (6.8) | 0.121 |
| Anemia* , ** | 335 (38.8) | 206 (47.4) | 129 (30.1) | < 0.001 |
| Platelet count of < 150 G L−1 * | 132 (15.3) | 78 (17.9) | 54 (12.6) | 0.039 |
| Antiplatelet therapy†† | 275 (31.8) | 147 (33.8) | 128 (29.8) | 0.212 |
| Arterial hypertension | 552 (63.9) | 289 (66.4) | 263 (61.3) | 0.116 |
| Heart rate of ≥ 110 beats min−1 * | 79 (9.1) | 49 (11.3) | 30 (7.0) | 0.031 |
| Systolic BP of < 100 mmHg* | 28 (3.2) | 13 (3.0) | 15 (3.5) | 0.664 |
| Respiratory rate of ≥ 30 min−1 * | 28 (3.2) | 16 (3.7) | 12 (2.8) | 0.469 |
| Temperature of < 36 °C* | 65 (7.5) | 27 (6.2) | 38 (8.9) | 0.119 |
| Arterial oxygen saturation of < 90%* | 93 (10.8) | 62 (14.3) | 31 (7.2) | 0.001 |
BP, blood pressure; BMI, body mass index; DVT, deep vein thrombosis; IQR, interquartile range, PTS, post‐thrombotic syndrome; TIA, transient ischemic attack. *Values were missing for estrogen therapy during the last 3 months (0.1%), presence of PTS (1.9%), history of major bleeding (0.1%), BMI > 30 (0.6%), high risk of falling (0.1%), anemia (5.8%), platelet count (5.8%), heart rate of ≥ 110 beats min−1 (2.1%), systolic BP of < 100 mmHg (1.6%), respiratory rate of ≥ 30 min−1 (21.1%), temperature of < 36°C (7.8%), and arterial oxygen saturation of < 90% (21.3%). †Provoked VTE is defined as immobilization, surgery or estrogen therapy during the last 3 months. Cancer is defined as any solid or hematological cancer that required chemotherapy, radiation therapy, surgical treatment or palliative treatment during the last 3 months. ‡Defined as a Villalta score of > 5 or the presence of an ulcer on the left or right side. §Chronic renal disease or creatinine clearance of < 30 mL min−1. ¶Defined as answering yes to at least one screening question: (i) Did you fall during the last year? (ii) Did you notice any problem with gait, balance, or mobility? **Anemia: a hemoglobin level of < 12 g dL−1 for females or of < 13 g dL−1 for males. ††Defined as antiplatelet therapy such as aspirin 100–300 mg daily, clopidogrel, prasugrel or aspirin/dipyridamole at the time of the index VTE.
Gas6 plasma levels in study samples
At the time of the index VTE diagnosis, the median Gas6 level was 129.3% (IQR 108.9–156.6) (T1 in Fig. 2). Patients with elevated Gas6 levels (> 129%) at the time of the index VTE were slightly older (median age of 76 years versus 74 years, P = 0.001). However, the correlation between Gas6 level and age was weak both at the time of the index VTE (Spearman correlation, r s = 0.12) and 12 months later (r s = 0.09). Patients with elevated Gas6 levels at the time of the index VTE diagnosis were more likely to have cancer‐related VTE. They were also more immobilized during the last 3 months and showed higher prevalences of post‐thrombotic syndrome, history of major bleeding, anemia, thrombocytopenia, heart rate of ≥ 110 beats min−1, and oxygen saturation of < 90% (Table 1). In contrast, these patients were less likely to be still receiving oral anticoagulation 12 months after the index VTE (180 [41.4%] patients with Gas6 levels above the median versus 230 [53.6%] patients with Gas6 levels below or at the median, P < 0.001). Interestingly, patients with inflammatory bowel disease were more likely to have a lower Gas6 level (P = 0.016). Twelve months after the index VTE, the median Gas6 level was 93% (IQR 77.1–111.7) (T2 on Fig. 2). However, at this time point, the median Gas6 level was lower in patients receiving anticoagulation than in patients not receiving anticoagulation (86.1% [IQR 70.4–107.3] versus 100.2% [IQR 85.3–116.8], P < 0.001).
Figure 2.
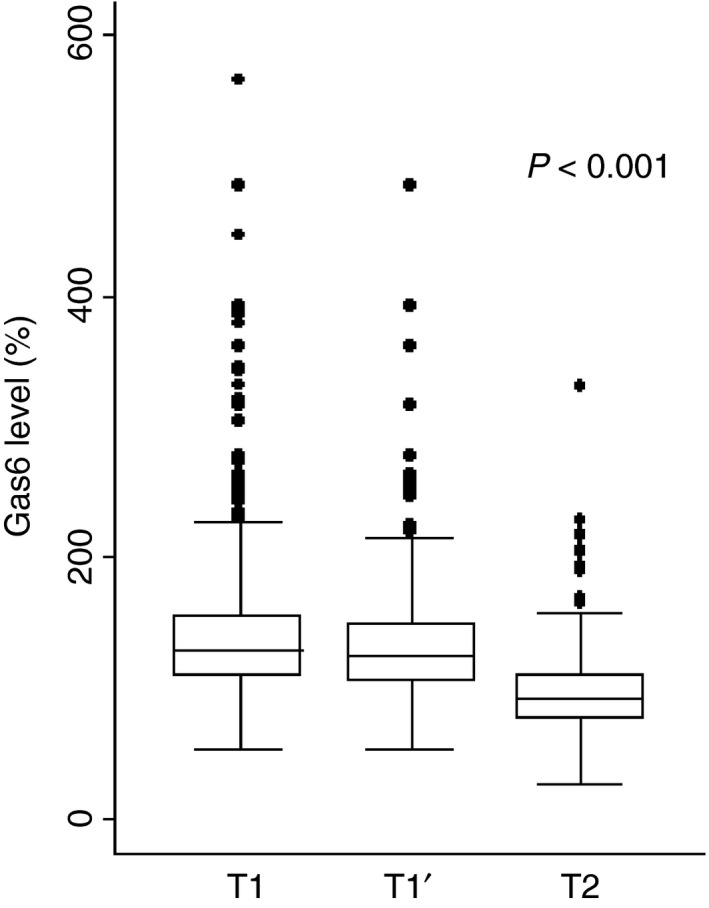
Growth arrest‐specific gene 6 (Gas6) plasma levels at the time of venous thromboembolism (VTE) diagnosis and 12 months later. Box‐plot of Gas6 levels presented as median with interquartile range (IQR) and whiskers with a maximum length of 1.5 IQR. T1, Gas6 level at the time of the index VTE of all patients (Gas6: n = 864). T1′, Gas6 level at the time of the index VTE of patients who also had the Gas6 level measured at T2 (Gas6: n = 601). T1′ and T were compared using the Wilcoxon matched pairs signed‐ranks test. The P‐values indicate that the differences were significant. The Spearman correlation between T1′ and T2 was r s = 0.33 for Gas6.
Gas6 plasma levels were generally lower 12 months after the index VTE (T2) than at the time of the index VTE (T1′ versus T2, P < 0.001; Fig. 2). In a minority of patients (n = 97, 11%), the Gas6 level increased from the time of VTE diagnosis to 12 months later.
The correlation between Gas6 and D‐dimer was weak, both at the time of the index VTE (Spearman correlation, r s = 0.06) and 12 months later (r s = 0.24).
Incidence rates of VTE recurrence, major bleeding, and mortality
After a follow‐up of 3 years, 100 patients had developed recurrent VTE, resulting in an incidence rate of 5.6 per 100 person‐years (95% CI 4.6–6.8). During the same period, 170 of 864 patients had died (mortality rate of 9.0 per 100 person‐years; 95% CI 7.8–10.5). The mortality rate was higher during the initial 6 months, whereas the VTE recurrence rate remained stable over the observation period (Table S1). During the whole follow‐up, the incidence rates of VTE recurrence and major bleeding were higher in patients with high Gas6 levels than in patients with medium or low Gas6 levels measured at the time of the index VTE (Table S1). Likewise, the 2‐year cumulative incidence of VTE recurrence was higher for patients with high (> 157%) than for patients with medium (109–157%) and low (< 109%) Gas6 levels measured at the time of the index VTE, although not significantly (P = 0.087) (Fig. 3A). The 2‐year cumulative incidence of major bleeding was higher for patients with high (> 157%) than for patients with medium (109–157%) and low (< 109%) Gas6 levels measured at the time of the index VTE (P = 0.0004) (Fig. 3B).
Figure 3.
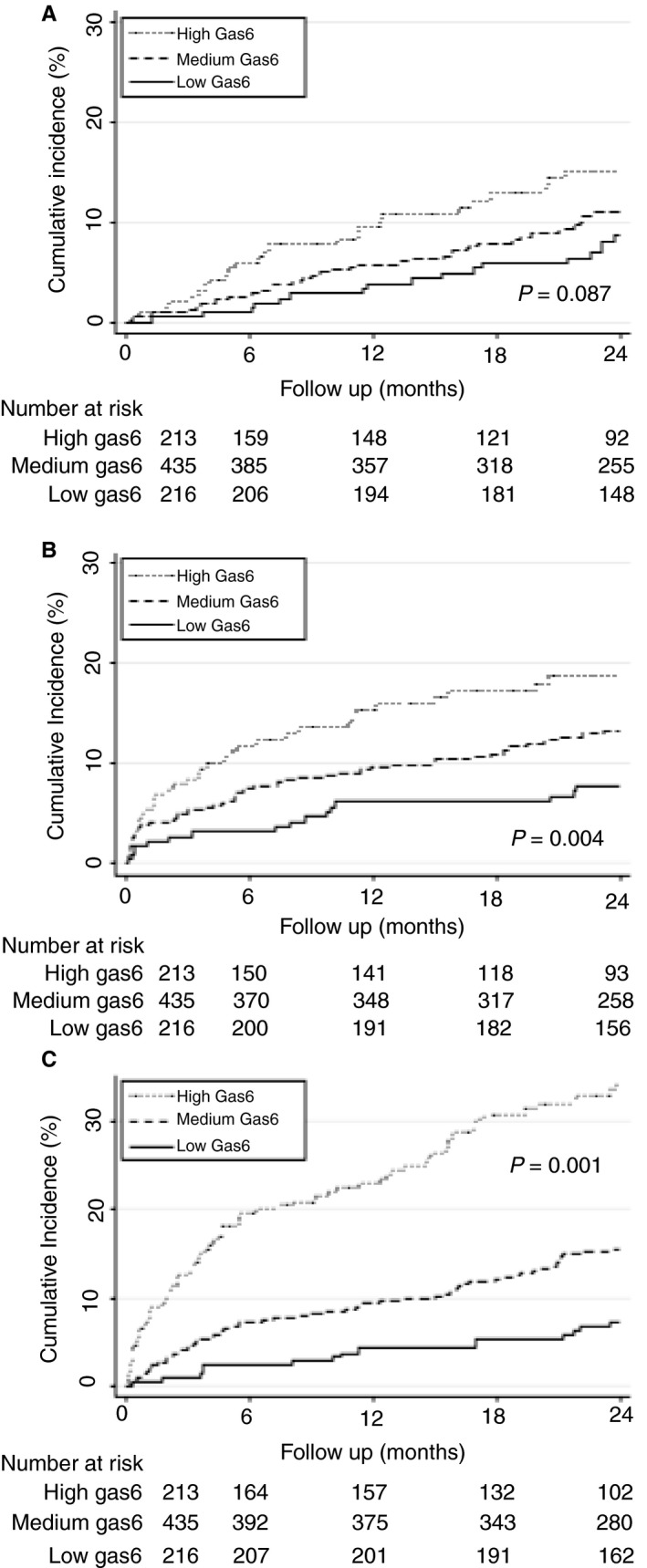
Cumulative incidence rates of venous thromboembolism (VTE), major bleeding and mortality for strata of growth arrest‐specific gene 6 (Gas6). The cumulative incidence rates of VTE (A), major bleeding (B) and mortality (C) for strata of Gas6 levels were estimated with the Kaplan–Meier method, and survivor functions across groups were compared by use of the log‐rank test. Gas6 levels were categorized on the basis of the lower and upper quartiles as low (< 109%), medium (109–157%), and high (> 157%).
The 2‐year cumulative incidence rates of overall mortality were 7%, 15% and 35% (P < 0.001) for patients with low, medium and high Gas6 levels, respectively (Fig. 3C).
Discriminative power of Gas6 levels for outcomes
In order to evaluate the discriminative power of Gas6 levels, C‐statistic (95% CI) values were calculated (Table 2). Gas6 levels measured at the time of the index VTE were discriminatory for VTE recurrence, major bleeding and mortality up to 36 months.
Table 2.
Discriminative power of growth arrest‐specific gene 6 (Gas6) plasma level for outcomes
| No. of events/no. of patients | C‐statistics (95% confidence interval) | P‐value* | |
|---|---|---|---|
| From the time of the index VTE (T1) onwards using measurements performed at the time of VTE diagnosis (T1) | |||
| Gas6 at the time of VTE diagnosis | |||
| VTE recurrence | |||
| Up to 6 months | 24/864 | 0.67 (0.57–0.78) | 0.001 |
| Up to 12 months | 48/864 | 0.61 (0.52–0.69) | 0.010 |
| Up to 24 months | 83/864 | 0.58 (0.52–0.64) | 0.010 |
| Up to 36 months | 100/864 | 0.56 (0.51–0.62) | 0.031 |
| Major bleeding | |||
| Up to 6 months | 62/864 | 0.62 (0.55–0.69) | < 0.001 |
| Up to 12 months | 82/864 | 0.60 (0.54–0.66) | 0.001 |
| Up to 24 months | 103/864 | 0.60 (0.55–0.65) | < 0.001 |
| Up to 36 months | 118/864 | 0.60 (0.55–0.65) | < 0.001 |
| Overall mortality | |||
| Up to 6 months | 77/864 | 0.73 (0.67–0.78) | < 0.001 |
| Up to 12 months | 97/864 | 0.71 (0.65–0.76) | < 0.001 |
| Up to 24 months | 149/864 | 0.70 (0.66–0.74) | < 0.001 |
| Up to 36 months | 170/864 | 0.69 (0.65–0.73) | < 0.001 |
| From 12 months after the index VTE (T2) onwards using measurements performed 12 months after the index VTE (T2) | |||
| Gas6 12 months after the index VTE | |||
| VTE recurrence | |||
| Up to 12 months | 32/601 | 0.66 (0.56–0.75) | 0.002 |
| Up to 24 months | 49/601 | 0.62 (0.54–0.71) | 0.003 |
| Major bleeding | |||
| Up to 12 months | 18/601 | 0.58 (0.43–0.72) | 0.294 |
| Up to 24 months | 32/601 | 0.57 (0.47–0.68) | 0.173 |
| Overall mortality | |||
| Up to 12 months | 33/601 | 0.57 (0.47–0.68) | 0.181 |
| Up to 24 months | 48/601 | 0.56 (0.48–0.65) | 0.159 |
VTE, venous thromboembolism. *The P‐value is from a test of the null hypothesis of no discrimination (i.e. a C‐statistics of 0.5).
The Gas6 level measured 12 months after the index VTE was discriminatory for VTE recurrence up 24 months. In contrast, when measured 12 months later, the Gas6 level was not discriminatory for major bleeding and mortality up to 24 months (Table 2).
Association between Gas6 plasma levels and outcomes
High Gas6 levels (> 157%) measured at the time of the index VTE were associated with an increased risk of VTE recurrence up to 6 months (Table 3), and an increased risk of major bleeding up to 36 months (crude analysis) (Table 4). In continuous analysis (log‐transformed Gas6 levels), the risk of VTE recurrence was increased up to 12 months (Table 3), and the risk of major bleeding was increased up to 36 months (crude analysis) (Table 4).
Table 3.
Association between growth arrest‐specific gene 6 (Gas6) plasma level and venous thromboembolism (VTE) recurrence – from the time of the index VTE (T1) onwards using Gas6 measured at the time of VTE diagnosis (T1)
| n/N (%) | Crude subhazard ratio (95% confidence interval) | P‐value | Adjusted subhazard ratio (95% confidence interval) | P‐value | |
|---|---|---|---|---|---|
| Up to 6 months | |||||
| Gas6 at the time of the index VTE (categorized) | |||||
| Low (< 109%) | 2/216 (0.9) | Reference | Reference | ||
| Medium (109–157%) | 11/435 (2.5) | 2.77 (0.61–12.51) | 0.185 | 2.95 (0.62–13.95) | 0.172 |
| High (> 157%) | 11/213 (5.2) | 5.74 (1.27–25.95) | 0.023 | 6.65 (1.44–30.80) | 0.015 |
| Log‐transformed Gas6 at the time of the index VTE | |||||
| Continuous (per log unit) | 24/864 (2.8) | 4.71 (1.98–11.19) | < 0.001 | 5.04 (2.14–11.88) | < 0.001 |
| Up to 12 months | |||||
| Gas6 at the time of the index VTE (categorized) | |||||
| Low (< 109%) | 8/216 (3.7) | Reference | Reference | ||
| Medium (109–157%) | 23/435 (5.3) | 1.46 (0.65–3.25) | 0.355 | 1.50 (0.66–3.40) | 0.335 |
| High (> 157%) | 17/213 (8.0) | 2.26 (0.98–5.23) | 0.056 | 2.42 (1.00–5.89) | 0.051 |
| Log‐transformed Gas6 at the time of the index VTE | |||||
| Continuous (per log unit) | 48/864 (5.6) | 2.42 (1.12–5.24) | 0.025 | 2.47 (1.08–5.64) | 0.032 |
Adjustments: VTE recurrence was adjusted for age, cancer, provoked VTE, prior VTE, overt pulmonary embolism, renal disease and periods of anticoagulation (oral or parenteral anticoagulation) as a time‐varying covariate [6,41,44–52].
Table 4.
Association between growth arrest‐specific gene 6 (Gas6) plasma level and major bleeding up to 6 months
| n/N (%) | Crude SHR (95% CI) | P‐value | Adjusted SHR (95% CI) | P‐value | |
|---|---|---|---|---|---|
| From the time of the index VTE (T1) onwards using Gas6 measured at the time of VTE diagnosis (T1) | |||||
| Gas6 at the time of the index VTE (categorized) | |||||
| Low (< 109%) | 7/216 (3.2) | Reference | Reference | ||
| Medium (109–157%) | 32/435 (7.4) | 2.33 (1.03–5.28) | 0.043 | 2.07 (0.89–4.82) | 0.093 |
| High (> 157%) | 23/213 (10.8) | 3.47 (1.49–8.10) | 0.004 | 2.58 (1.04–6.37) | 0.040 |
| Log‐transformed Gas6 at the time of the index VTE | |||||
| Continuous (per log unit) | 62/864 (7.2) | 2.79 (1.42–5.46) | 0.003 | 2.05 (0.95–4.41) | 0.067 |
CI, confidence interval; SHR, subhazard ratio; VTE, venous thromboembolism. Adjustments: major bleeding was adjusted for age, cancer, provoked VTE, prior VTE, overt pulmonary embolism, renal disease, history of major bleeding, anemia, antiplatelet therapy and periods of anticoagulation as a time‐varying covariate [51,59–73].
In addition, medium (109–157%) and high Gas6 levels were associated with increased overall mortality up to 36 months (Table 5).
Table 5.
Association between growth arrest‐specific gene 6 (Gas6) plasma level and overall mortality up to 36 months
| n/N (%) | Crude hazard ratio (95% confidence interval) | P‐value | Adjusted hazard ratio (95% confidence interval) | P‐value | |
|---|---|---|---|---|---|
| From the time of the index VTE (T1) onwards using Gas6 measured at the time of VTE diagnosis (T1) | |||||
| Gas6 at the time of the index VTE (categorized) | |||||
| Low (< 109%) | 20/216 (9.3) | Reference | Reference | ||
| Medium (109–157%) | 73/435 (16.8) | 1.96 (1.20–3.19) | 0.007 | 1.69 (1.00–2.84) | 0.048 |
| High (> 157%) | 77/213 (36.2) | 4.95 (3.04–8.05) | < 0.001 | 3.44 (2.03–5.82) | < 0.001 |
| Log‐transformed Gas6 at the time of the index VTE | |||||
| Continuous (per log unit) | 170/864 (19.7) | 7.21 (4.48–11.60) | < 0.001 | 5.00 (3.16–7.92) | < 0.001 |
| From the time of the index VTE onwards using Gas6 as a time‐varying covariate (at the time of the index VTE and 12 months later) | |||||
| Gas6 time‐varying covariate (categorized) | |||||
| Low (< 109%) | Reference | Reference | |||
| Medium (109–157%) | 1.88 (1.26–2.80) | 0.002 | 1.68 (1.09–2.57) | 0.017 | |
| High (> 157%) | 5.55 (3.63–8.47) | < 0.001 | 3.55 (2.21–5.71) | < 0.001 | |
| Log‐transformed Gas6 time‐varying covariate | |||||
| Continuous (per log unit) | 8.50 (5.51–13.11) | < 0.001 | 5.18 (3.17–8.46) | < 0.001 | |
VTE, venous thromboembolism. Adjustments: mortality was adjusted for age, gender, cancer, provoked VTE, prior VTE, overt pulmonary embolism, renal disease, history of major bleeding, heart failure, chronic lung disease, high pulse, low blood pressure, low oxygen, and periods of anticoagulation as a time‐varying covariate 49, 53.
These associations also remained after adjustment for potential confounding factors for the risk of VTE recurrence and overall mortality (Tables 3 and 5).
Regarding the risk of major bleeding, only the association with high Gas6 levels measured at the time of the index VTE remained up to 6 months after adjustment for potential confounding factors (Table 4).
We assessed the relationship between continuous log‐transformed Gas6 values and risks of VTE recurrence and overall mortality by using fractional polynomial competing risk and Cox proportional hazards models, which showed that (sub)‐hazards and Gas6 levels increased linearly (Fig. S1).
The findings of the sensitivity analyses revealed that these associations also remained after the exclusion of patients with cancer (Table S2) or with cancer and provoked VTE (Table S3). Moreover, in the subgroup of patients not receiving oral anticoagulation 12 months after the index VTE, continuous (log‐transformed) Gas6 levels were associated with VTE recurrence up to 12 months (Table S4). This association also remained after adjustment for potential confounding factors (Table S5). Finally, medium, high and continuous (log‐transformed) Gas6 levels were associated with increased mortality up to 36 months (Table 5).
Discussion
We prospectively followed 864 elderly patients with VTE for a period of 3 years, and observed that patients with higher Gas6 levels were more likely to have cancer‐related VTE and comorbidities. Our findings are consistent with previous publications reporting high Gas6 levels in a number of clinical conditions, most of them associated with inflammation and organ damage 14, 17, 54, 55.
Our data showed that an elevated Gas6 level was independently associated with recurrent VTE up to 12 months, with major bleeding up to 6 months and with mortality up to 36 months after the index VTE. Considering that patients with more comorbidities were more likely to have higher Gas6 levels, neither the association with VTE recurrence, the association with major bleeding nor the association with overall mortality was surprising. However, the observed association remained significant after adjustment for a large number of comorbidities (Table 3, 4, 5). The Gas6 level was also still associated with VTE recurrence and mortality after the exclusion of patients with cancer (Table S2) or with cancer and provoked VTE (Table S3). Because Gas6 is a prohemostatic protein 23, 24, 25, we may assume that the association between high Gas6 levels and VTE recurrence might be at least partly causal.
Another important finding of this study is that Gas6 levels measured at the time of diagnosis were discriminatory for VTE recurrence and mortality. In addition, Gas6 levels measured 12 months after the index VTE were discriminatory only for VTE recurrence. A previous study comprising a lower number of patients than this study did not demonstrate the predictive ability of Gas6 levels for VTE recurrence 29.Thus, the data of the present study point to an elevated Gas6 level as an independent predictor for VTE recurrence, major bleeding and mortality up to 36 months in the elderly. The Gas6 level might therefore be useful in adjusting the intensity of surveillance in this group of high‐risk patients. However, before considering the Gas6 level as an additional marker with which to predict recurrence and guide therapy, the Gas6 level would need to be compared with or integrated into established risk scores such as the DASH 56, HERDOO‐2 57 and Vienna 58 scores.
Our study has some limitations. First, the scope of the study was limited to elderly patients, and 18.2% of them had cancer; the mortality resulting from comorbid diseases is naturally higher than the VTE recurrence rate, as persons with limited life‐expectancy often do not have the time to develop recurrent VTE. Thus, it is indeed unclear whether the results can be extrapolated to younger persons with VTE. In addition, although the Gas6 plasma level was previously reported not to be influenced by age 21, both its predictive ability for VTE recurrence and its association with VTE recurrence would need to be studied in younger patients. Second, the Gas6 level was previously reported to be elevated in several other medical conditions. Nevertheless, in this study, we were able to demonstrate that the association between the Gas6 level and VTE recurrence and mortality remained after adjustment for these conditions. However, this needs to be verified in younger patients. Third, VTE treatment has changed since this cohort was constituted; that is, direct oral anticoagulants have replaced vitamin K antagonists for most patients. Therefore, it is unclear whether the results can be extrapolated to patients treated with direct oral anticoagulants. Fourth, as we enrolled patients with VTE in inpatient and outpatient hospital services, the proportion of patients with PE was relatively high, and represented 69% of our study sample. Fifth, Gas6 testing was performed only at the time of the index VTE and 12 months later, when 50% of the patients were still receiving oral anticoagulation. Because we and others 21 have demonstrated that Gas6 levels are affected by oral anticoagulation with vitamin K antagonists, we can assume that the significantly lower Gas6 level 12 months after the index VTE was at least partly attributable to the anti‐vitamin K effect. Interestingly, Gas6 levels in the subgroup of patients not receiving oral anticoagulation at this time point were significantly lower than those in patients receiving anticoagulation. Thus, the correct interpretation of Gas6 levels would require patients to interrupt anticoagulation, exposing those with increased risk to the possibility of a VTE recurrence. Finally, even though we adjusted our analyses for many covariates, we might have missed important predictor variables.
In conclusion, in the elderly, a high Gas6 level is associated with higher risks of VTE recurrence and major bleeding, but only up to 6 months, a period of time during which most patients were still anticoagulated, and death. Our data suggest that a clinical decision to avoid prolonged anticoagulation could be attempted on the basis of Gas6 plasma levels in the elderly. Further studies are required to confirm whether the use of Gas6 levels for adjusting the length of anticoagulation leads to better outcomes, especially in younger patients.
Addendum
A. Schnegg‐Kaufmann, S. Calzavarini, and A. Angelillo‐Scherrer designed the protocol and the analysis plan, conducted the analyses, and drafted the manuscript. S. Calzavarini performed Gas6 measurements. A. Limacher performed the statistical analysis. A. Schnegg‐Kaufmann, S. Calzavarini, and A. Angelillo‐Scherrer interpreted the data. M. Méan, M. Righini, B. Frauchiger, J. Osterwalder, N. Kucher, and N. Rodondi organized data collection, intellectually reviewed the manuscript, and participated in funding procedures. A. Schnegg‐Kaufmann, S. Calzavarini, A. Limacher, D. Staub, J. H. Beer, C. M. Matter, M. Husmann, M. Banyai, M. Aschwanden, L. Mazzolai, O. Hugli, M. Nagler, and M. Daskalakis organized data collection and intellectually reviewed the manuscript. D. Aujesky was principal investigator of the SWITCO65+ cohort, and was responsible for planning of the study, data collection, drafting of the manuscript, and obtaining funding. A. Angelillo‐Scherrer was in charge of the Gas6 nested study, and was responsible for planning of the study, data collection, drafting of the manuscript, and obtaining funding. All authors approved the final version of the manuscript.
Disclosure of Conflict of Interests
C. M. Matter reports receiving: grants from the Swiss National Science Foundation, during the conduct of the study; and grants from MSD, Bayer, AstraZeneca, EliLilly, and Sanofi, and personal fees from MSD, AstraZeneca, Roche, Sanofi, Amgen, and Novartis, outside the submitted work. A. Limacher reports receiving grants from the Swiss National Science Foundation, during the conduct of the study. The other authors state that they have no conflict of interest.
Supporting information
Table S1. Incidence rates of VTE recurrence, major bleeding and mortality rates by level of Gas6 measured at the time of the index VTE.
Table S2. Sensitivity analyses: from baseline onwards using baseline Gas6, excluding patients with cancer.
Table S3. Sensitivity analyses: from baseline onwards using baseline Gas6, excluding patients with cancer and provoked VTE.
Table S4. Association between Gas6 measured 12 months after the index VTE in patients not receiving oral anticoagulation and VTE recurrence from 12 months after the index VTE onwards.
Fig. S1. Relative subhazards for VTE recurrence and relative hazards for overall mortality.
Acknowledgements
This work was supported by grants from the Swiss National Science Foundation (33CSCO‐122659/139 470 and 310030_153436, 314730_173127).
Schnegg‐Kaufmann A, Calzavarini S, Limacher A, Mean M, Righini M, Staub D, Beer JH, Frauchiger B, Osterwalder J, Kucher N, Matter CM, Husmann M, Banyai M, Aschwanden M, Mazzolai L, Hugli O, Nagler M, Daskalakis M, Rodondi N, Aujesky D, Angelillo‐Scherrer A. A high Gas6 level in plasma predicts venous thromboembolism recurrence, major bleeding and mortality in the elderly: a prospective multicenter cohort study. J Thromb Haemost 2019; 17: 306–18. 10.1111/jth.14365
Manuscript handled by: M. Carrier
Final decision: F. R. Rosendaal, 29 November 2018
References
- 1. Roger VL, Go AS, Lloyd‐Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, et al Heart disease and stroke statistics – 2012 update: a report from the American Heart Association. Circulation 2012; 125: e2–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Deitelzweig SB, Johnson BH, Lin J, Schulman KL. Prevalence of clinical venous thromboembolism in the USA: current trends and future projections. Am J Hematol 2011; 86: 217–20. [DOI] [PubMed] [Google Scholar]
- 3. Spencer FA, Gore JM, Lessard D, Emery C, Pacifico L, Reed G, Gurwitz JH, Goldberg RJ. Venous thromboembolism in the elderly. A community‐based perspective. Thromb Haemost 2008; 100: 780–8. [PMC free article] [PubMed] [Google Scholar]
- 4. Spencer FA, Gurwitz JH, Schulman S, Linkins LA, Crowther MA, Ginsberg JS, Lee AY, Saczynski JS, Anand S, Lessard D, Emery C, Huang W, Goldberg RJ. Venous thromboembolism in older adults: a community‐based study. Am J Med 2014; 127: 530–7. [DOI] [PubMed] [Google Scholar]
- 5. White RH. The epidemiology of venous thromboembolism. Circulation 2003; 107: I4–8. [DOI] [PubMed] [Google Scholar]
- 6. Heit JA, Mohr DN, Silverstein MD, Petterson TM, O'Fallon WM, Melton LJ 3rd. Predictors of recurrence after deep vein thrombosis and pulmonary embolism: a population‐based cohort study. Arch Intern Med 2000; 160: 761–8. [DOI] [PubMed] [Google Scholar]
- 7. Schulman S, Rhedin AS, Lindmarker P, Carlsson A, Larfars G, Nicol P, Loogna E, Svensson E, Ljungberg B, Walter H. A comparison of six weeks with six months of oral anticoagulant therapy after a first episode of venous thromboembolism. Duration of Anticoagulation Trial Study Group. N Engl J Med 1995; 332: 1661–5. [DOI] [PubMed] [Google Scholar]
- 8. Kearon C, Gent M, Hirsh J, Weitz J, Kovacs MJ, Anderson DR, Turpie AG, Green D, Ginsberg JS, Wells P, MacKinnon B, Julian JA. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med 1999; 340: 901–7. [DOI] [PubMed] [Google Scholar]
- 9. Agnelli G, Prandoni P, Santamaria MG, Bagatella P, Iorio A, Bazzan M, Moia M, Guazzaloca G, Bertoldi A, Tomasi C, Scannapieco G, Ageno W; Warfarin Optimal Duration Italian Trial Investigators . Three months versus one year of oral anticoagulant therapy for idiopathic deep venous thrombosis. Warfarin Optimal Duration Italian Trial Investigators. N Engl J Med 2001; 345: 165–9. [DOI] [PubMed] [Google Scholar]
- 10. Pinede L, Ninet J, Duhaut P, Chabaud S, Demolombe‐Rague S, Durieu I, Nony P, Sanson C, Boissel JP; Investigators of the ‘Durée Optimale du Traitement AntiVitamines K’ (DOTAVK) Study . Comparison of 3 and 6 months of oral anticoagulant therapy after a first episode of proximal deep vein thrombosis or pulmonary embolism and comparison of 6 and 12 weeks of therapy after isolated calf deep vein thrombosis. Circulation 2001; 103: 2453–60. [DOI] [PubMed] [Google Scholar]
- 11. Palareti G, Leali N, Coccheri S, Poggi M, Manotti C, D'Angelo A, Pengo V, Erba N, Moia M, Ciavarella N, Devoto G, Berrettini M, Musolesi S. Bleeding complications of oral anticoagulant treatment: an inception‐cohort, prospective collaborative study (ISCOAT). Italian Study on Complications of Oral Anticoagulant Therapy. Lancet 1996; 348: 423–8. [DOI] [PubMed] [Google Scholar]
- 12. Kearon C, Ginsberg JS, Kovacs MJ, Anderson DR, Wells P, Julian JA, MacKinnon B, Weitz JI, Crowther MA, Dolan S, Turpie A, Geerts W, Solymoss S, van Nguyen P, Demers C, Kahn SR, Kassis J, Rodger M, Hambleton J, Gent M; Extended Low‐Intensity Anticoagulation for Thrombo‐Embolism Investigators . Comparison of low‐intensity warfarin therapy with conventional‐intensity warfarin therapy for long‐term prevention of recurrent venous thromboembolism. N Engl J Med 2003; 349: 631–9. [DOI] [PubMed] [Google Scholar]
- 13. Schneider C, King RM, Philipson L. Genes specifically expressed at growth arrest of mammalian cells. Cell 1988; 54: 787–93. [DOI] [PubMed] [Google Scholar]
- 14. Borgel D, Clauser S, Bornstain C, Bieche I, Bissery A, Remones V, Fagon JY, Aiach M, Diehl JL. Elevated growth‐arrest‐specific protein 6 plasma levels in patients with severe sepsis. Crit Care Med 2006; 34: 219–22. [DOI] [PubMed] [Google Scholar]
- 15. Ekman C, Linder A, Akesson P, Dahlback B. Plasma concentrations of Gas6 (growth arrest specific protein 6) and its soluble tyrosine kinase receptor sAxl in sepsis and systemic inflammatory response syndromes. Crit Care 2010; 14: R158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gibot S, Massin F, Cravoisy A, Dupays R, Barraud D, Nace L, Bollaert PE. Growth arrest‐specific protein 6 plasma concentrations during septic shock. Crit Care 2007; 11: R8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stalder G, Que YA, Calzavarini S, Burnier L, Kosinski C, Ballabeni P, Roger T, Calandra T, Duchosal MA, Liaudet L, Eggimann P, Angelillo‐Scherrer A. Study of early elevated Gas6 plasma level as a predictor of mortality in a prospective cohort of patients with sepsis. PLoS ONE 2016; 11: e0163542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Uehara S, Handa H, Gotoh K, Tomita H, Sennshuu M. Plasma concentrations of growth arrest‐specific protein 6 and protein S in patients with acute pancreatitis. J Gastroenterol Hepatol 2009; 24: 1567–73. [DOI] [PubMed] [Google Scholar]
- 19. Wu KS, Hung YJ, Lee CH, Hsiao FC, Hsieh PS. The involvement of GAS6 signaling in the development of obesity and associated inflammation. Int J Endocrinol 2015; 2015: 202513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jiang T, Liu G, Wang L, Liu H. Elevated serum Gas6 is a novel prognostic biomarker in patients with oral squamous cell carcinoma. PLoS ONE 2015; 10: e0133940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Balogh I, Hafizi S, Stenhoff J, Hansson K, Dahlback B. Analysis of Gas6 in human platelets and plasma. Arterioscler Thromb Vasc Biol 2005; 25: 1280–6. [DOI] [PubMed] [Google Scholar]
- 22. Lemke G, Rothlin CV. Immunobiology of the TAM receptors. Nat Rev Immunol 2008; 8: 327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Angelillo‐Scherrer A, de Frutos P, Aparicio C, Melis E, Savi P, Lupu F, Arnout J, Dewerchin M, Hoylaerts M, Herbert J, Collen D, Dahlback B, Carmeliet P. Deficiency or inhibition of Gas6 causes platelet dysfunction and protects mice against thrombosis. Nat Med 2001; 7: 215–21. [DOI] [PubMed] [Google Scholar]
- 24. Angelillo‐Scherrer A, Burnier L, Flores N, Savi P, DeMol M, Schaeffer P, Herbert JM, Lemke G, Goff SP, Matsushima GK, Earp HS, Vesin C, Hoylaerts MF, Plaisance S, Collen D, Conway EM, Wehrle‐Haller B, Carmeliet P. Role of Gas6 receptors in platelet signaling during thrombus stabilization and implications for antithrombotic therapy. J Clin Invest 2005; 115: 237–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Robins RS, Lemarie CA, Laurance S, Aghourian MN, Wu J, Blostein MD. Vascular Gas6 contributes to thrombogenesis and promotes tissue factor up‐regulation after vessel injury in mice. Blood 2013; 121: 692–9. [DOI] [PubMed] [Google Scholar]
- 26. Laurance S, Aghourian MN, Jiva Lila Z, Lemarie CA, Blostein MD. Gas6‐induced tissue factor expression in endothelial cells is mediated through caveolin‐1‐enriched microdomains. J Thromb Haemost 2014; 12: 395–408. [DOI] [PubMed] [Google Scholar]
- 27. Cosemans JM, Van Kruchten R, Olieslagers S, Schurgers LJ, Verheyen FK, Munnix IC, Waltenberger J, Angelillo‐Scherrer A, Hoylaerts MF, Carmeliet P, Heemskerk JW. Potentiating role of Gas6 and Tyro3, Axl and Mer (TAM) receptors in human and murine platelet activation and thrombus stabilization. J Thromb Haemost 2010; 8: 1797–808. [DOI] [PubMed] [Google Scholar]
- 28. Tjwa M, Bellido‐Martin L, Lin Y, Lutgens E, Plaisance S, Bono F, Delesque‐Touchard N, Herve C, Moura R, Billiau AD, Aparicio C, Levi M, Daemen M, Dewerchin M, Lupu F, Arnout J, Herbert JM, Waer M, Garcia de Frutos P, Dahlback B, et al Gas6 promotes inflammation by enhancing interactions between endothelial cells, platelets, and leukocytes. Blood 2008; 111: 4096–105. [DOI] [PubMed] [Google Scholar]
- 29. Blostein MD, Rajotte I, Rao DP, Holcroft CA, Kahn SR. Elevated plasma gas6 levels are associated with venous thromboembolic disease. J Thromb Thrombolysis 2011; 32: 272–8. [DOI] [PubMed] [Google Scholar]
- 30. Mean M, Righini M, Jaeger K, Beer HJ, Frauchiger B, Osterwalder J, Kucher N, Lammle B, Cornuz J, Angelillo‐Scherrer A, Rodondi N, Limacher A, Trelle S, Matter CM, Husmann M, Banyai M, Aschwanden M, Egloff M, Mazzolai L, Hugli O, et al The Swiss cohort of elderly patients with venous thromboembolism (SWITCO65+): rationale and methodology. J Thromb Thrombolysis 2013; 36: 475–83. [DOI] [PubMed] [Google Scholar]
- 31. Dauzat M, Laroche JP, Deklunder G, Ayoub J, Quere I, Lopez FM, Janbon C. Diagnosis of acute lower limb deep venous thrombosis with ultrasound: trends and controversies. J Clin Ultrasound 1997; 25: 343–58. [DOI] [PubMed] [Google Scholar]
- 32. Enden T, Sandvik L, Klow NE, Hafsahl G, Holme PA, Holmen LO, Ghanima W, Njaastad AM, Sandbaek G, Slagsvold CE, Sandset PM. Catheter‐directed venous thrombolysis in acute iliofemoral vein thrombosis – the CaVenT study: rationale and design of a multicenter, randomized, controlled, clinical trial (NCT00251771). Am Heart J 2007; 154: 808–14. [DOI] [PubMed] [Google Scholar]
- 33. Fraser DG, Moody AR, Davidson IR, Martel AL, Morgan PS. Deep venous thrombosis: diagnosis by using venous enhanced subtracted peak arterial MR venography versus conventional venography. Radiology 2003; 226: 812–20. [DOI] [PubMed] [Google Scholar]
- 34. Fraser DG, Moody AR, Morgan PS, Martel AL, Davidson I. Diagnosis of lower‐limb deep venous thrombosis: a prospective blinded study of magnetic resonance direct thrombus imaging. Ann Intern Med 2002; 136: 89–98. [DOI] [PubMed] [Google Scholar]
- 35. Kearon C, Ginsberg JS, Hirsh J. The role of venous ultrasonography in the diagnosis of suspected deep venous thrombosis and pulmonary embolism. Ann Intern Med 1998; 129: 1044–9. [DOI] [PubMed] [Google Scholar]
- 36. Righini M, Paris S, Le Gal G, Laroche JP, Perrier A, Bounameaux H. Clinical relevance of distal deep vein thrombosis. Review of literature data. Thromb Haemost 2006; 95: 56–64. [PubMed] [Google Scholar]
- 37. Buller HR, Davidson BL, Decousus H, Gallus A, Gent M, Piovella F, Prins MH, Raskob G, van den Berg‐Segers AE, Cariou R, Leeuwenkamp O, Lensing AW; Matisse Investigators . Subcutaneous fondaparinux versus intravenous unfractionated heparin in the initial treatment of pulmonary embolism. N Engl J Med 2003; 349: 1695–702. [DOI] [PubMed] [Google Scholar]
- 38. Le Gal G, Righini M, Sanchez O, Roy PM, Baba‐Ahmed M, Perrier A, Bounameaux H. A positive compression ultrasonography of the lower limb veins is highly predictive of pulmonary embolism on computed tomography in suspected patients. Thromb Haemost 2006; 95: 963–6. [DOI] [PubMed] [Google Scholar]
- 39. Mean M, Aujesky D, Lammle B, Gerschheimer C, Trelle S, Angelillo‐Scherrer A. Design and establishment of a biobank in a multicenter prospective cohort study of elderly patients with venous thromboembolism (SWITCO65+). J Thromb Thrombolysis 2013; 36: 484–91. [DOI] [PubMed] [Google Scholar]
- 40. Clauser S, Peyrard S, Gaussem P, Crespin M, Emmerich J, Aiach M, Borgel D. Development of a novel immunoassay for the assessment of plasma Gas6 concentrations and their variation with hormonal status. Clin Chem 2007; 53: 1808–13. [DOI] [PubMed] [Google Scholar]
- 41. Prandoni P, Noventa F, Ghirarduzzi A, Pengo V, Bernardi E, Pesavento R, Iotti M, Tormene D, Simioni P, Pagnan A. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica 2007; 92: 199–205. [DOI] [PubMed] [Google Scholar]
- 42. Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis . Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. J Thromb Haemost 2005; 3: 692–4. [DOI] [PubMed] [Google Scholar]
- 43. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94: 496–509. [Google Scholar]
- 44. Agnelli G, Prandoni P, Becattini C, Silingardi M, Taliani MR, Miccio M, Imberti D, Poggio R, Ageno W, Pogliani E, Porro F, Zonzin P; Warfarin Optimal Duration Italian Trial Investigators . Extended oral anticoagulant therapy after a first episode of pulmonary embolism. Ann Intern Med 2003; 139: 19–25. [DOI] [PubMed] [Google Scholar]
- 45. Boutitie F, Pinede L, Schulman S, Agnelli G, Raskob G, Julian J, Hirsh J, Kearon C. Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants’ data from seven trials. BMJ 2011; 342: d3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Christiansen SC, Lijfering WM, Helmerhorst FM, Rosendaal FR, Cannegieter SC. Sex difference in risk of recurrent venous thrombosis and the risk profile for a second event. J Thromb Haemost 2010; 8: 2159–68. [DOI] [PubMed] [Google Scholar]
- 47. Hansson PO, Sorbo J, Eriksson H. Recurrent venous thromboembolism after deep vein thrombosis: incidence and risk factors. Arch Intern Med 2000; 160: 769–74. [DOI] [PubMed] [Google Scholar]
- 48. Huang W, Goldberg RJ, Anderson FA, Cohen AT, Spencer FA. Occurrence and predictors of recurrence after a first episode of acute venous thromboembolism: population‐based Worcester Venous Thromboembolism Study. J Thromb Thrombolysis 2016; 41: 525–38. [DOI] [PubMed] [Google Scholar]
- 49. Insam C, Mean M, Limacher A, Angelillo‐Scherrer A, Aschwanden M, Banyai M, Beer JH, Bounameaux H, Egloff M, Frauchiger B, Husmann M, Kucher N, Lammle B, Matter C, Osterwalder J, Righini M, Staub D, Rodondi N, Aujesky D. Anticoagulation management practices and outcomes in elderly patients with acute venous thromboembolism: a clinical research study. PLoS ONE 2016; 11: e0148348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lopez‐Jimenez L, Montero M, Gonzalez‐Fajardo JA, Arcelus JI, Suarez C, Lobo JL, Monreal M; Riete Investigators . Venous thromboembolism in very elderly patients: findings from a prospective registry (RIETE). Haematologica 2006; 91: 1046–51. [PubMed] [Google Scholar]
- 51. Prandoni P, Lensing AW, Piccioli A, Bernardi E, Simioni P, Girolami B, Marchiori A, Sabbion P, Prins MH, Noventa F, Girolami A. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood 2002; 100: 3484–8. [DOI] [PubMed] [Google Scholar]
- 52. Wattanakit K, Cushman M, Stehman‐Breen C, Heckbert SR, Folsom AR. Chronic kidney disease increases risk for venous thromboembolism. J Am Soc Nephrol 2008; 19: 135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gussoni G, Frasson S, La Regina M, Di Micco P, Monreal M; RIETE Investigators . Three‐month mortality rate and clinical predictors in patients with venous thromboembolism and cancer. Findings from the RIETE registry. Thromb Res 2013; 131: 24–30. [DOI] [PubMed] [Google Scholar]
- 54. Palmiere C, Augsburger M. Postmortem serum protein growth arrest‐specific 6 levels in sepsis‐related deaths. Int J Legal Med 2015; 129: 1079–84. [DOI] [PubMed] [Google Scholar]
- 55. Lee IJ, Hilliard B, Swami A, Madara JC, Rao S, Patel T, Gaughan JP, Lee J, Gadegbeku CA, Choi ET, Cohen PL. Growth arrest‐specific gene 6 (Gas6) levels are elevated in patients with chronic renal failure. Nephrol Dial Transplant 2012; 27: 4166–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tosetto A, Iorio A, Marcucci M, Baglin T, Cushman M, Eichinger S, Palareti G, Poli D, Tait RC, Douketis J. Predicting disease recurrence in patients with previous unprovoked venous thromboembolism: a proposed prediction score (DASH). J Thromb Haemost 2012; 10: 1019–25. [DOI] [PubMed] [Google Scholar]
- 57. Rodger MA, Kahn SR, Wells PS, Anderson DA, Chagnon I, Le Gal G, Solymoss S, Crowther M, Perrier A, White R, Vickars L, Ramsay T, Betancourt MT, Kovacs MJ. Identifying unprovoked thromboembolism patients at low risk for recurrence who can discontinue anticoagulant therapy. CMAJ 2008; 179: 417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Eichinger S, Heinze G, Jandeck LM, Kyrle PA. Risk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: the Vienna prediction model. Circulation 2010; 121: 1630–6. [DOI] [PubMed] [Google Scholar]
- 59. Beyth RJ, Quinn LM, Landefeld CS. Prospective evaluation of an index for predicting the risk of major bleeding in outpatients treated with warfarin. Am J Med 1998; 105: 91–9. [DOI] [PubMed] [Google Scholar]
- 60. Fang MC, Go AS, Chang Y, Borowsky LH, Pomernacki NK, Udaltsova N, Singer DE. A new risk scheme to predict warfarin‐associated hemorrhage: the ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) study. J Am Coll Cardiol 2011; 58: 395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gage BF, Yan Y, Milligan PE, Waterman AD, Culverhouse R, Rich MW, Radford MJ. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J 2006; 151: 713–19. [DOI] [PubMed] [Google Scholar]
- 62. Hutten BA, Prins MH, Gent M, Ginsberg J, Tijssen JG, Buller HR. Incidence of recurrent thromboembolic and bleeding complications among patients with venous thromboembolism in relation to both malignancy and achieved international normalized ratio: a retrospective analysis. J Clin Oncol 2000; 18: 3078–83. [DOI] [PubMed] [Google Scholar]
- 63. Kuijer PM, Hutten BA, Prins MH, Buller HR. Prediction of the risk of bleeding during anticoagulant treatment for venous thromboembolism. Arch Intern Med 1999; 159: 457–60. [DOI] [PubMed] [Google Scholar]
- 64. Landefeld CS, Goldman L. Major bleeding in outpatients treated with warfarin: incidence and prediction by factors known at the start of outpatient therapy. Am J Med 1989; 87: 144–52. [DOI] [PubMed] [Google Scholar]
- 65. Lip GY, Frison L, Halperin JL, Lane DA. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS‐BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score. J Am Coll Cardiol 2011; 57: 173–80. [DOI] [PubMed] [Google Scholar]
- 66. Nieto JA, Bruscas MJ, Ruiz‐Ribo D, Trujillo‐Santos J, Valle R, Ruiz‐Gimenez N, Monreal M; RIETE Investigators . Acute venous thromboembolism in patients with recent major bleeding. The influence of the site of bleeding and the time elapsed on outcome. J Thromb Haemost 2006; 4: 2367–72. [DOI] [PubMed] [Google Scholar]
- 67. Olesen JB, Lip GY, Hansen PR, Lindhardsen J, Ahlehoff O, Andersson C, Weeke P, Hansen ML, Gislason GH, Torp‐Pedersen C. Bleeding risk in ‘real world’ patients with atrial fibrillation: comparison of two established bleeding prediction schemes in a nationwide cohort. J Thromb Haemost 2011; 9: 1460–7. [DOI] [PubMed] [Google Scholar]
- 68. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user‐friendly score (HAS‐BLED) to assess 1‐year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010; 138: 1093–100. [DOI] [PubMed] [Google Scholar]
- 69. Ruiz‐Gimenez N, Suarez C, Gonzalez R, Nieto JA, Todoli JA, Samperiz AL, Monreal M; RIETE Investigators . Predictive variables for major bleeding events in patients presenting with documented acute venous thromboembolism. Findings from the RIETE Registry. Thromb Haemost 2008; 100: 26–31. [DOI] [PubMed] [Google Scholar]
- 70. Shireman TI, Mahnken JD, Howard PA, Kresowik TF, Hou Q, Ellerbeck EF. Development of a contemporary bleeding risk model for elderly warfarin recipients. Chest 2006; 130: 1390–6. [DOI] [PubMed] [Google Scholar]
- 71. Torn M, Bollen WL, van der Meer FJ, van der Wall EE, Rosendaal FR. Risks of oral anticoagulant therapy with increasing age. Arch Intern Med 2005; 165: 1527–32. [DOI] [PubMed] [Google Scholar]
- 72. White RH, Beyth RJ, Zhou H, Romano PS. Major bleeding after hospitalization for deep‐venous thrombosis. Am J Med 1999; 107: 414–24. [DOI] [PubMed] [Google Scholar]
- 73. van der Meer FJ, Rosendaal FR, Vandenbroucke JP, Briet E. Bleeding complications in oral anticoagulant therapy. An analysis of risk factors. Arch Intern Med 1993; 153: 1557–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Incidence rates of VTE recurrence, major bleeding and mortality rates by level of Gas6 measured at the time of the index VTE.
Table S2. Sensitivity analyses: from baseline onwards using baseline Gas6, excluding patients with cancer.
Table S3. Sensitivity analyses: from baseline onwards using baseline Gas6, excluding patients with cancer and provoked VTE.
Table S4. Association between Gas6 measured 12 months after the index VTE in patients not receiving oral anticoagulation and VTE recurrence from 12 months after the index VTE onwards.
Fig. S1. Relative subhazards for VTE recurrence and relative hazards for overall mortality.


