Abstract
Aim
These international clinical practice recommendations (CPR) for developmental coordination disorder (DCD), initiated by the European Academy of Childhood Disability (EACD), aim to address key questions on the definition, diagnosis, assessment, intervention, and psychosocial aspects of DCD relevant for clinical practice.
Method
Key questions in five areas were considered through literature reviews and formal expert consensus. For recommendations based on evidence, literature searches on ‘mechanisms’, ‘assessment’, and ‘intervention’ were updated since the last recommendations in 2012. New searches were conducted for ‘psychosocial issues’ and ‘adolescents/adults’. Evidence was rated according to the Oxford Centre for Evidence‐Based Medicine (level of evidence [LOE] 1–4) and transferred into recommendations. For recommendations based on formal consensus, two meetings of an international, multidisciplinary expert panel were conducted with a further five Delphi rounds to develop good clinical practice (GCP) recommendations.
Results
Thirty‐five recommendations were made. Eight were based on the evidence from literature reviews (three on ‘assessment’, five on ‘intervention’). Twenty‐two were updated from the 2012 recommendations. New recommendations relate to diagnosis and assessment (two GCPs) and psychosocial issues (three GCPs). Additionally, one new recommendation (LOE) reflects active video games as adjuncts to more traditional activity‐oriented and participation‐oriented interventions, and two new recommendations (one GCP, one LOE) were made for adolescents and adults with DCD.
Interpretation
The CPR–DCD is a comprehensive overview of DCD and current understanding based on research evidence and expert consensus. It reflects the state of the art for clinicians and scientists of varied disciplines. The international CPR–DCD may serve as a basis for national guidelines.
What this paper adds
Updated international clinical practice guidelines on developmental coordination disorder (DCD).
Refined and extended recommendations on clinical assessment and intervention for DCD.
A critical synopsis of current research on mechanisms of DCD.
A critical synopsis of psychosocial issues in DCD, with implications for clinical practice.
The first international recommendations to consider adolescents and adults with DCD.
What this paper adds
Updated international clinical practice guidelines on developmental coordination disorder (DCD).
Refined and extended recommendations on clinical assessment and intervention for DCD.
A critical synopsis of current research on mechanisms of DCD.
A critical synopsis of psychosocial issues in DCD, with implications for clinical practice.
The first international recommendations to consider adolescents and adults with DCD.
This article's abstract has been translated into Spanish and Portuguese.
Follow the links from the http://onlinelibrary.wiley.com/doi/10.1111/dmcn.14132/abstract to view the translations.
A pocket version of these guidelines is available as Appendix S1 (https://onlinelibrary.wiley.com/doi/full/10.1111/dmcn.14132#support-information-section)
Resumen
Recomendaciones internacionales para la práctica clínica sobre la definición, diagnóstico, evaluación, intervención y aspectos psicosociales del trastorno del desarrollo de la coordinación
Objetivo
Estas recomendaciones internacionales para la práctica clínica (RPC) sobre el trastorno del desarrollo de la coordinación (TDC), iniciadas por la Academia Europea de Discapacidad Infantil (EACD), tienen como objetivo abordar preguntas clave sobre la definición, diagnóstico, evaluación, intervención y aspectos psicosociales de TDC relevantes para la práctica clínica.
Método
Las preguntas clave en cinco áreas fueron tratadas a través de revisiones bibliográficas y consenso formal de expertos. Para las recomendaciones basadas en la evidencia, las búsquedas en la literatura sobre “mecanismos”, “evaluación” e “intervención” se actualizaron desde las últimas recomendaciones en 2012. Se realizaron nuevas búsquedas para “problemas psicosociales” y “adolescentes / adultos”. La evidencia se calificó de acuerdo con la gradación del Centro de Oxford para Medicina Basada en la Evidencia (nivel de evidencia [LOE] 1–4) y en ello se basaron las recomendaciones. Para recomendaciones basadas en el consenso formal, se llevaron a cabo dos reuniones de un panel multidisciplinario internacional de expertos con cinco rondas Delphi adicionales para desarrollar recomendaciones de buena práctica clínica (BPC).
Resultados
Se realizaron 35 recomendaciones. Ocho de ellas se basaron en la evidencia de las revisiones de la literatura (tres en “evaluación”, cinco en “intervención”). Veintidós fueron actualizadas a partir de las recomendaciones de 2012. Las nuevas recomendaciones se relacionan con el diagnóstico y la evaluación (dos BPC) y las cuestiones psicosociales (tres BPC). Además, una nueva recomendación (LOE) trata acerca de los videojuegos activos como complemento de las intervenciones más tradicionales orientadas a la actividad y la participación, y se hicieron dos nuevas recomendaciones (una BCP, una LOE) para adolescentes y adultos con TDC.
Interpretación
Estas recomendaciones internacionales para la práctica clínica sobre TDC aportan una visión general completa sobre TDC y el conocimiento actual basado en evidencia de investigación y consenso de expertos. Brinda actualización para clínicos y científicos de diversas disciplinas. Las recomendaciones internacionales para la práctica clínica TDC pueden servir como base para recomendaciones nacionales.
Recomendações internacionais para a prática clínica na definição, diagnóstico, avaliação, intervenção e aspectos psicossociais do transtorno do desenvolvimento da coordenação
Objetivo
Essas recomendações internacionais para a prática clínica (RPC) no transtorno do desenvolvimento da coordenação (TDC), iniciadas pela Academia Européia de Deficiência Infantil (EACD), objetiva direcionar questões chave na definição, diagnóstico, avaliação, intervenção e aspectos psicossociais do TDC relevantes para a prática clínica.
Métodos
Questões chave em cinco áreas foram consideradas através de revisões da literatura e consensos formais de especialistas. Para recomendações baseadas em evidências, buscas na literatura em “mecanismos”, “avaliação” e “intervenção” foram atualizadas desde as últimas recomendações de 2012. Novas buscas foram conduzidas para “problemas psicossociais” e “adolescentes/adultos”. Evidências foram classificadas de acordo com o Centro Oxford para Medicina Baseada em Evidência (nível de evidência [NE] 1‐4) e transferidas em recomendações. Para recomendações baseadas em consensos formais, dois encontros de um painel de especialistas internacional e multidisciplinar foram conduzidos com posteriormente cinco sessões Delphi para desenvolver recomendações de boa prática clínica (BPC).
Resultados
Trinta e cinco recomendações foram feitas. Oito foram baseadas em evidências de revisões da literatura (três em “avaliação”, cinco em “intervenção). Vinte e duas foram atualizadas das recomendações de 2012. Novas recomendações são relacionadas com diagnóstico e avaliação (duas BPC) e problemas psicossociais (três BPCs). Adicionalmente, uma nova recomendação (NE) se refere a jogos de videogame ativos como adjuntos à mais tradicional terapia orientada à tarefa e intervenção orientada à participação, e duas novas recomendações (uma BPC, um NE) foram feitas para adolescentes e adultos com TDC.
Interpretação
A RPC‐TDC apresenta uma visão geral do TDC e o conhecimento atual baseado em evidências de pesquisas e consenso de especialistas. Reflete o estado de arte dos clínicos e cientistas de disciplinas variadas. A RPC‐TDC internacional deverá servir como uma base para as diretrizes nacionais.
Abbreviations
- ADC
Adult Developmental Coordination Disorder/Dyspraxia Checklist
- ADL
Activities of daily living
- ASD
Autism spectrum disorder
- AWMF
Associaiton of the Scientific Medical Societies in Germany
- BOT‐2
Bruininks‐Oseretsky Test of Motor Proficiency, Second Edition
- CPR
Clinical practice recommendations
- DCD
Developmental coordination disorder
- DCDQ(‐R)
Developmental Coordination Disorder Questionnaire, Revised Version
- EACD
European Academy of Childhood Disability
- GCP
Good clinical practice
- GRADE
Grading of Recommendations Assessment, Development and Evaluation
- ICF
International Classification of Functioning, Disability and Health
- KTK
Körperkoordinationstest für Kinder
- LOE
Level of evidence
- MABC‐1
Movement Assessment Battery for Children
- MABC‐2
Movement Assessment Battery for Children, Second Edition
- MABC‐2‐C
Movement Assessment Battery for Children, Second Edition, Checklist
- MNS
Mirror neuron system
- NTT
Neuromotor Task Training
- SOS
Systematische Opsporing Schrijfproblemen
- ZNA
Zurich Neuromotor Assessment Battery
Authors
Coordinators
Rainer Blank (Germany), Sabine Vinçon (Germany)
International representatives
Mechanisms
Peter Wilson (Australia), David Sugden (UK), Jane Clark (USA), Bert Steenbergen (the Netherlands), Bouwien Smits‐Engelsman (South Africa, the Netherlands), Karen Caeyenberghs (Australia)
Assessments
Rainer Blank (Germany), Sabine Vinçon (Germany), Sara Rosenblum (Israel), Reint Geuze (the Netherlands), Sheila Henderson (UK), Oskar Jenni (Switzerland), Lívia C Magalhães (Brazil), Stefania Zoia (Italy)
Interventions
Bouwien Smits‐Engelsman (South Africa, the Netherlands), Helene Polatajko (Canada), Sabine Vinçon (Germany), Motohide Miyahara (New Zealand; served in the intervention group until May 2016), Peter Wilson (Australia)
Psychosocial issues
Dido Green (UK), John Cairney (Canada), Paulene Kamps (Canada), Sabine Vinçon (Germany)
Adolescents and adults
Anna L Barnett (UK), Amanda Kirby (UK), Hilde van Waelvelde (Belgium), Naomi Weintraub (Israel)
International Society
European Academy of Childhood Disability (EACD)
External Supervision
Ina Kopp, Association of the Scientific Medical Societies in Germany (Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften), past chair Guidelines International Network (G‐I‐N).
Duration of the Validity
These international clinical practice recommendations were written and received consent in 2017. They are valid until the next revision, at the latest until 31st December 2022. A revision is planned about every 5 years by the international representative group. If new knowledge or experience has considerable influence on the current recommendations, the representative group will quickly disseminate the latest information.
Contents
| I INTRODUCTION |
| II AIM, TARGET GROUP, SCOPE, AND PATIENT EXPECTATIONS |
| III KEY QUESTIONS |
| IV UPDATE PROCEDURE, EVIDENCE, AND METHODOLOGICAL BASIS |
| A CHILDREN |
| 1 Epidemiology, consequences, outcome, and burden for society |
| 1.1 Epidemiology |
| 1.2 Outcomes |
| 1.3 Burden for society |
| 2 Definition and terminology |
| 3 Underlying mechanisms in DCD |
| 3.1 Overview |
| 3.2 Towards a unified account of DCD |
| 3.3 Clinical implications of mechanisms research |
| 4 Diagnosis and assessment |
| 4.1 Definition and criteria |
| 4.2 The process of assessment |
| 4.3 Comorbidities/co‐occurring conditions |
| 4.4 Psychosocial issues |
| 4.5 Flow chart assessment, treatment indication and planning |
| 5 Intervention |
| 5.1 Intervention: general principles |
| 5.2 Therapeutic approaches |
| 5.3 Interventions delivery mode: (group) settings |
| 5.4 Interventions: intensity and scheduling |
| 5.5 The role of environmental factors |
| 5.6 Somatic interventions: drugs, additives |
| 5.7 Monitoring |
| 5.8 Cost‐effectiveness |
| 5.9 Flow chart treatment planning, intervention, and evaluation |
| B TRANSITION FROM CHILD TO ADULT |
| C ADOLESCENTS AND ADULTS |
| 1 Terminology, diagnosis, and assessment |
| 1.1 Motor assessment in the literature on adolescents and adults with DCD |
| 2 Intervention |
| References |
1. Introduction
The present document is the long version of the international clinical practice recommendations (CPR) for developmental coordination disorder (DCD). A pocket version (algorithm) of these recommendations is available (Appendix S1, online supporting information).
The terminology in this document is consistent with that of the International Classification of Functioning, Disability and Health (ICF).1 The current classification systems, the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM‐5),2 and the International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD‐10),3 use different terminology to describe the population of concern.
The term ‘developmental coordination disorder’ is used throughout this document. The ICD‐10 term ‘specific developmental disorder of motor function’ is used only once at the beginning as this term is almost never used in the research literature. Regardless, the ICD‐10 term may be more precise and adequate to describe the nature of the disorder as DCD is not only a disorder of ‘coordination’.
Although this document is concerned with individuals with DCD across the lifespan, it is sometimes necessary to refer solely to children or adolescents and adults. Since children and adults tend to be treated by different specialists in different contexts, these recommendations are presented in two sections: (1) children and (2) adolescents and adults. Within these two sections, the recommendations are specific for these target groups.
Goals of the International CPR–DCD
The general goals of this document are as follows: (1) to determine and prioritize key questions on aetiology, diagnosis, and intervention; (2) to raise high‐priority practice questions; (3) to provide knowledge on the best evidence‐based practice; (4) to point out research gaps; (5) to define individual diagnostic and intervention strategies based on clinical decision rules and evidence‐based knowledge; (6) to make recommendations for a variety of different disciplines and to define their roles within clinical practice; (7) to recognize the value of an interdisciplinary approach with physicians and therapists of different disciplines; (8) to provide an effective implementation strategy of these recommendations by involving all medical and paramedical organizations relevant in assessment and treatment; (9) to identify possible barriers for implementation; (10) to provide a basis for clinical training and for implementation in quality management systems.
In addition, specific goals of the international CPR–DCD are: (1) to improve the identification of individuals with DCD; (2) to increase the use of effective treatments and reduce the use of ineffective treatments; (3) to decrease the burden of the disorder and increase quality of life; (4) to improve performance of everyday activities and participation at home, school, education, employment, and at leisure; (5) to improve personal and environmental resources; (6) to improve access to services, in particular health care provisions; (7) to help clarify responsibilities and propose models of cooperation among the various relevant professionals (e.g. by defining clinical pathways); (8) to help prevent long‐term consequences of DCD (e.g. by timely and effective intervention); (9) to raise community awareness for DCD.
As a clinical practice guideline, the international CPR–DCD are not designed as a rule explaining what to do or how to act in a legal situation. These recommendations cannot be a basis for legal sanctions.
These international recommendations are based on expert consensus and evidence drawn from systematic literature search and evaluation (see ‘Update procedure, evidence, and methodological basis’). On the basis of these international recommendations, national guidelines can be adapted according to culture‐specific needs, country‐specific legal issues, etc., and established through a systematic group discussion process of all relevant national stakeholders and interest groups. This procedure is desirable to ensure best possible national implementation.4
The international CPR–DCD follow the methodological recommendations of the Association of the Scientific Medical Societies in Germany (Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften [AWMF]) and the German Instrument for Methodological Guideline Appraisal (Deutsches Instrument zur methodischen Leitlinien‐Bewertung).5 The AWMF represents Germany in the Council for International Organizations of Medical Sciences. The AWMF follows similar standards as other national associations (e.g. the National Institute for Health and Care Excellence in the UK) to ensure best evidence health and social care.
Target Audience
The international CPR–DCD may be used by health care professionals involved in the care of individuals diagnosed with or suspected as having DCD. The pocket version of these guidelines is designed for a general audience in which the most important content of the recommendations are summarized and may be more appropriate for specific target groups.
Implementation
These CPR are based on the consensus of international experts in the field of DCD and current evidence. This long version does not include proposals for implementation strategies and quality indicators/quality management. Given country‐specific and culture‐specific service provision for individuals with DCD, these international standards need to be adapted for national conditions (Fig. 1).
Figure 1.
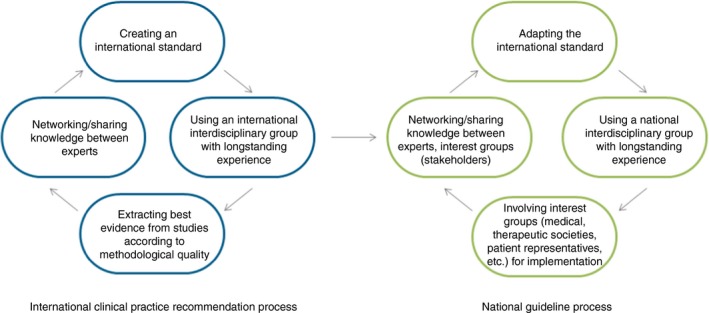
From international recommendations to national guidelines. [Colour figure can be viewed at http://www.wileyonlinelibrary.com]
2. Aim, Target Group, Scope, AND Patient Expectations
Target group
The international CPR–DCD should apply to individuals with long‐standing non‐progressive problems of specific motor skill performance, not attributable to any other known medical or psychosocial condition. Individuals may demonstrate motor problems for which these recommendations do not apply such as cerebral palsy, neurodegenerative disorders, traumatic brain injuries, inflammatory brain diseases, toxic and teratogenic disorders, malignancies, or any motor problem due to other diagnosed medical conditions that may explain poor motor performance. Individuals with severe intellectual developmental disorder are generally not identified as having DCD because of assessment difficulties (pragmatic reasons). These individuals, however, may also have symptoms of poor motor coordination. Therefore, general recommendations for treatment indications and specific intervention methods may also be applied to the group of individuals with intellectual developmental disorder, although the research so far has excluded these individuals from evaluation.
Clinical relevance
DCD is a common and chronic disorder resulting in considerable consequences in daily life; prevalence estimates of 5% to 6% of children are most frequently quoted in the literature.2, 6 At least 2% of all individuals with typical intelligence experience severe consequences in everyday living including academic productivity, and a further 3% have a degree of functional impairment in activities of daily living (ADL) or school work.7 Nonetheless, DCD is largely underrecognized by health care and educational professionals.8, 9, 10
There are, however, considerable costs for long‐term treatment with questionable effectiveness. For example, the German Therapy Report 2016 (also known as Heilmittelbericht 2016) reports on the largest health insurance in Germany (Allgemeine Ortskrankenkasse). According to Waltersbacher,11 this document states sensorimotor disorders are treated in about 50% of all occupational therapy sessions and are therefore ranked as the primary concern overall. Waltersbacher11 also reported that for those being treated for sensorimotor disorders, 90% include therapeutic sessions for children and adolescents under 15 years; within this 90% allotment, hemiparesis accounts for 10.3% and DCD accounts for 8.6% of all occupational therapy intervention sessions. When the number of occupational therapy assessments was analysed, DCD was found to be the leading diagnosis, accounting for 7.4% of all of the children and young people tested.
Scope
There are several questions and issues about DCD and particularly important issues to be answered and addressed for adolescents and adults: (1) diagnosis and assessment (how to assess for diagnosis for there is still no criterion standard; how to monitor during development and treatment); (2) outcome and prognosis (what consequences; in which areas of everyday living and participation); (3) underlying mechanisms (which brain networks are responsible; genetic and environmental influences; etc.); (4) treatment indication (when and what to treat; especially in adolescents and adults); (5) intervention methods (which; how long; how intensive); (6) psychosocial issues in DCD; and implications for outcomes, interventions, and participation.
These questions were the primary reasons for the development of the international CPR–DCD in 2012.6 and are especially addressed in the current revision. The authors hope to achieve improvements in national and international definitions of DCD, the assessment and diagnostic practices for DCD, and the treatment indications and specific interventions that show benefit for individuals with DCD.
Further, the international CPR–DCD should help to increase professional attention to this topic and suggest future directions for research.
Expectations of the patient representative
To ensure that these recommendations are responsive to the expectations of the individuals and their parents, a parent organization for individuals with learning disorders participated during the entire process. Up to now, patient stakeholder groups for DCD are not common or known. However, it would be desirable for future action also to include adolescents and adults organized in stakeholder groups.
The following expectations were identified: (1) more awareness and recognition of the problems experienced by people with DCD by medical professionals, nursery and young people‐care staff, parents, educators, coaches, sports instructors, relevant others, and members within the general community; (2) improved access to services, particularly health care provisions; (3) establishing a clear diagnostic pathway (e.g. transparency about and explanation of diagnostic criteria, and initiating the necessary examinations); (4) better information about therapeutic options for parents and relevant others; (5) information about the effectiveness of interventions.
3. Key Questions
The international expert group focused on five key questions. These are described below.
(1) How is DCD defined? Which functions are impaired in individuals with DCD?
The definition of DCD was the subject of an expert consensus. To enhance communication between experts, health care professionals, patients, and relatives, it was deemed important to develop a generally recognized definition of DCD based on the DSM‐5 and the ICD‐10.2, 3
The underlying mechanisms of DCD and the findings of impaired functions related to DCD were extracted from a systematic literature search. Impairments should reflect the levels of the ICF such as body function and structure (e.g. brain structure and function; motor, sensory, and cognitive function; emotional/affective function), ADL (e.g. basic and instrumental skills), participation (e.g. at home, school/employment, and community), and acknowledge personal and environmental factors. The question of impairment does not aim at specific CPRs, but aims to increase understanding of the disorder, its severity, and its natural course.
(2) How is DCD assessed and monitored? How should individuals with DCD with and without treatment (natural course) be monitored (qualitative/quantitative aspects)?
Applicability and test criteria of assessment instruments were subject to a systematic literature search and, where not possible, then addressed by expert opinion and consensus.
The question of how DCD can be identified should be answered by interviews, examining the individual's developmental history, medical history, questionnaires, clinical examination, and motor tests. Assessment instruments should investigate ADL, involvement in play/leisure activities, functioning in school, and the role of laboratory versus natural settings. Decisions for how and when to measure progress should reflect the levels of the ICF such as body function and structure (e.g. brain structure and function; motor, sensory, cognitive function; emotional/affective function), ADL (e.g. basic and instrumental skills), participation (e.g. at home, school/employment, and community), and acknowledge personal and environmental factors.
(3) How effective are the treatment methods for DCD?
The treatment effectiveness should be answered by systematic evaluation of the literature and, where not possible, answered by a nominative group process during expert consensus.
The levels of the ICF should be considered such as body function and structure (e.g. brain structure and function; motor, sensory, cognitive function; emotional/affective function), ADL (e.g. basic and instrumental skills), participation (e.g. at home, school/employment, and community), and acknowledge personal and environmental factors. Effectiveness should also be discussed with respect to efficiency (cost–benefit).
(4) What are the psychosocial issues of DCD and their impacts?
The consideration of psychosocial issues present in DCD was done through a scoping review and systematic evaluation of the literature and addressed by experts’ opinions and consensus conference.
As in the key question on assessment, determination of methods for identifying psychosocial and mental health issues in DCD should be answered by interviews, examining the individual's medical and psychiatric history, questionnaires, observational assessments, and reports offered by self and/or significant others.
The levels of the ICF should be considered such as body function and structure (e.g. global and specific psychosocial functions including sleep, emotional/affective function), ADL (e.g. basic and instrumental skills), participation (e.g. at home, school/employment, and community), and acknowledge personal and environmental factors (including attitudinal).
(5) How does DCD present in adolescence and adulthood? What motor assessments have been used and what intervention programmes have been developed for adolescents and adults?
The study of DCD in adolesence and adulthood is relatively new. To gather information on the presentation of the condition beyond childhood, a scoping review was conducted. This was designed to map: (1) areas of research related to adolescents and adults with DCD (and areas that are lacking); (2) the assessments used for measuring motor skills in this body of literature; and (3) intervention programmes developed for this population.
Further questions of interest
Many other questions were of great interest but could only be addressed to a limited extent in this document. For example: how and which interactions are affected when treating comorbid conditions (e.g. pharmacological treatment with stimulants of individuals with attention‐deficit/hyperactivity disorder [ADHD])? What barriers do individuals with DCD face when trying to access health care or other treatment services (e.g. parental education, language, cultural, geographic, socio‐economic status, health services policies)? What specific views and opinions do parents, relatives, patients, and teachers have about DCD?
Areas of interest and relevance of outcomes
On the basis of the key questions, the main areas of interest for clinical recommendations are identification/diagnosis, treatment indication, and treatment outcome for all individuals with DCD: children, adolescents, and adults.
In recent years, the expanded studies of neuroimaging (mechanism group), psychosocial consequences (new working group), and of adolescents and adults with DCD (new working group) have become more important.
The study results within these areas of interest were classified according to the ICF. The relevance of outcome within the different ICF areas have been rated. For example, for assessment studies, outcomes on body function and activity levels were seen as most important for desicion making. The ratings of 20126 were adopted (Tables 1 and 2).
Table 1.
Target variables for outcome
| Body function and structure | Motor performance, basic motor functions, perceptual functions, executive functions |
| Personal factors | Quality of life (well‐being, satisfaction), coping, motivation for treatment |
| Activities | Activities of daily living, school performance, activity limitation, prevocational and vocational activities, leisure activities |
| Participation | Social integration, social burden of disorder, sports participation, participation restrictions |
| Environmental factors | Socio‐economic resources (nursery/school facilities, financial resources, therapeutic resources, availability of sports club, etc.), coping/compensation (by family, teachers, adaptive materials, sports equipment, etc.) |
Table 2.
Relevance of outcomes: areas of interest according to the ICF and target variables as rated by the expert group in 20126
| Diagnosis | Treatment indication | Treatment outcome | |
|---|---|---|---|
| Body function and structure | × | ||
| Deficit in motor performance and psychomotor functions | |||
| Poor basic motor skills and perceptual/motor functions | |||
| Activities | × | × | × |
| ADL (basic ADL,a school performance, leisure instrumental ADL)b | |||
| Participation | × | × | |
| Social integration (e.g. sport participation)c | |||
| Personal factors | × | × | |
| Coping (individual resources, intelligence, etc.) | |||
| Quality of life, well‐being, satisfaction | |||
| Environmental factors | × | ||
| Socio‐economic resources (nursery/school facilities, financial resources, therapeutic resources, availability of sports club, etc.) | |||
| Coping/compensation (by family, teachers, adaptive materials, sports equipment, etc.) |
×, very important: critical for making a decision. aBasic activites of daily living (ADL) (self‐care, toileting, eating – drinking, etc.). bInstrumental ADL (using a pen, scissors, playing with toys, cooking, driving, etc.). cPossible participation restriction as a consequence of activity limitations.
4. Update Procedure, Evidence, and Methodological Basis
Under the umbrella of the European Academy of Childhood Disability (EACD), and based on the work of worldwide experts in the field of DCD, recommendations on the definition, diagnosis, and intervention of DCD were previously published in 2012.6 The present document is a major revision and further advancement of that work.
In July 2015, an international expert panel was founded at the International DCD Conference in Toulouse, France. The international experts were selected according to scientific background, representation of countries, and, if possible, of all continents. Finally, all invited experts, scientists, as well as clinicians from North America and South America, Asia, Europe, Africa, and Australia agreed to take part and were involved in this work (Fig. 2).
Figure 2.
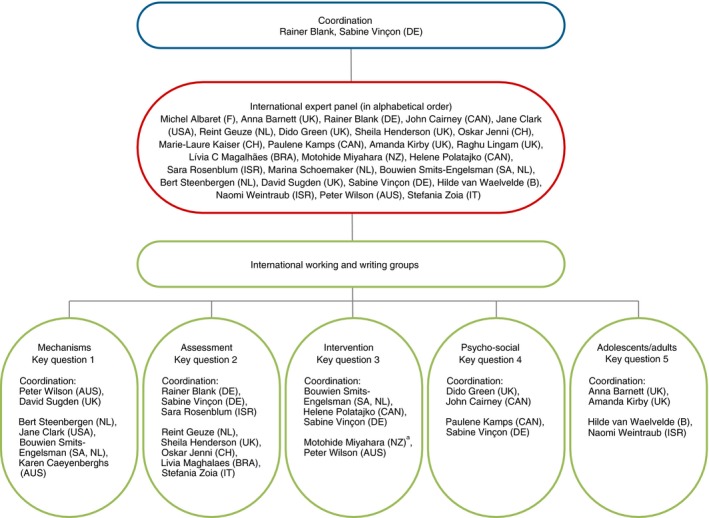
International expert panel. aServed in the intervention group until May 2016. [Colour figure can be viewed at http://www.wileyonlinelibrary.com]
During the following revision process, two consensus meetings were held: (1) in Toulouse (International DCD Conference in Toulouse, France, 2nd–4th July 2015); and (2) in Stockholm (combined meeting of the EACD and the International Academy of Childhood Disability, 1st–4th June 2016).
Within the initial consensus meeting in Toulouse, France, the recommendations of 2012 served as the basis for discussion and the starting point of the revision of the good clinical practice (GCP) recommendations.
Recommendations based on formal consensus
The vast majority of the resulting CPRs were based on group consensus along with other processes. Specifically, following basic decisions made at the first consensus meeting in Toulouse, five Delphi rounds followed; these rounds were performed by electronic mail and focused on the development of the GCP recommendations. Following the methodological guideline of the AWMF,12 experts were asked to vote for or against the proposed GCP recommendation and provide alternatives if possible with references, in case of refusal. Recommendations with 90% consensus (⇑⇑) were accepted and this content was then not included in further Delphi rounds.
At the second consensus meeting in Stockholm, all GCP final recommendations were revised and received consent (>90% consensus), both in terms of content and language.
Recommendations based on evidence
During the entire process of developing the GCP recommendations, the five established working groups (mechanisms, assessments, interventions, psychosocial issues, adolescents and adults; Fig. 2) reviewed the literature and new studies (Fig. 3) published since the previous CPR–DCD6 to prepare recommendations on the basis of evidence.
Figure 3.
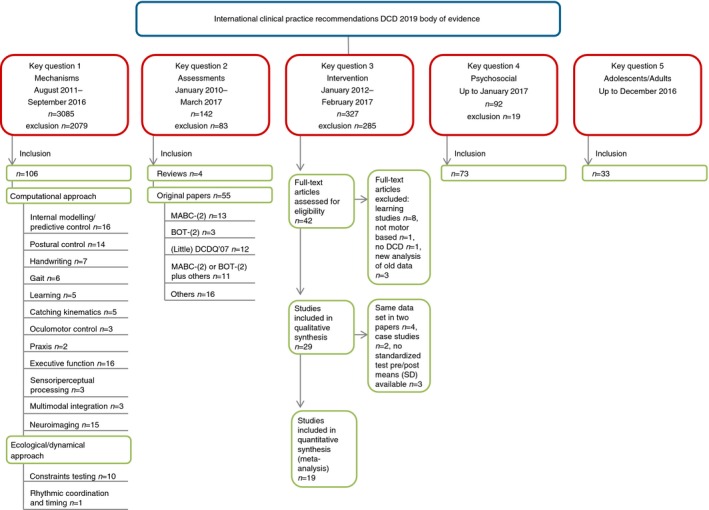
Literature review. DCD, developmental coordination disorder; MABC, Movement Assessment Battery for Children; BOT, Bruininks‐Oseretsky Test of Motor Proficiency; DCDQ, Developmental Coordination Disorder Questionnaire; SD, standard deviation. [Colour figure can be viewed at http://www.wileyonlinelibrary.com]
As in the previous version in 2012, original papers addressing key question 2 (assessment) were categorized according to the Oxford Levels of Evidence system13 (Table 3). Intervention studies were classified using an adapted version of the Scottish Intercollegiate Guidelines Network14 (Table 3). Therefore only original studies related to the specific key questions of the recommendations were included in the systematic analysis of the literature. For more information about the literature search, methodological background, and the evidence tables on assessments and interventions, see Figure 3, Appendices S2 and S3 (online supporting information), and Tables SI to SIII (online supporting information).
Table 3.
Classification of the body of evidence
| Level of evidence (GRADE) | Body of evidence | Oxford level | Oxford definition (diagnostic studies)13 | Adapteda SIGN criteria14 |
|---|---|---|---|---|
| 1 (high) |
Evidence from a meta‐analysis or systematic review of randomized controlled or other well‐controlled studies with homogenous findings; homogeneity of the results. Very good quality of the results (e.g. validity and reliability measures >0.8) |
I a | Systematic review (with homogeneity) of Level 1 diagnostic studies; CDR with 1b studies from different clinical centres | 1++ High quality meta‐analyses, systematic reviews of RCTs, or RCTs with a very low risk of bias |
|
Evidence from at least two RCTs (intervention studies) or well‐controlled trials with well‐described sample selection (diagnostic study);b confirmatory data analysis, good standards. Very good quality of the results (e.g. validity and reliability measures >0.8) |
I b | Validating cohort study with good reference standards or CDR tested within one clinical centre | 1+ Well conducted meta‐analyses, systematic reviews of RCTs, or RCTs with a low risk of bias | |
| I c | ‘Absolute SpPins’ and ‘Absolute SnNouts’c | 1− Meta‐analyses, systemtic reviews or RCTs, or RCTs with a high risk of bias | ||
| 2 (moderate) |
Evidence from at least two well‐designed, controlled studies without randomization from different working groups. Sufficient standards; homogeneity of the results. Good quality of the results (e.g. validity and reliability measures >0.6) |
II a | Systematic review (with homogeneity) of Level >2 diagnostic studies |
2++ High quality systematic reviews of case–control or cohort studies or High quality case–control or cohort studies with a very low risk of confounding bias, or chance and a high probability that the relationship is causal |
| Evidence from at least one well‐designed other type of quasi‐experimental study (non‐randomized, non‐controlled). Good quality of the results (e.g. validity and reliability measures >0.6) | II b | Exploratory cohort study with good reference standards. CDR after derivation, or validated only on split‐sample or databases | 2+ Well‐conducted case–control or cohort studies with a low risk of confounding, bias, or chance and a moderate probability that the relationship is causal | |
| 3 (low) |
Evidence from well‐designed non‐experimental descriptive or observational studies (e.g. correlational studies, case‐control‐studies). Moderate homogeneity of the results. Moderate quality of the results (e.g. validity and reliability measures >0.4) |
III a | Systematic review (with homogeneity) of 3b and better studies | 2− Case–control or cohort studies with a high risk of confounding, bias, or chance and a significant risk that the relationship is not causal |
| III b | Non‐consecutive study or without consistently applied reference standards | 3 Non‐analytic studies, e.g. case reports, case series | ||
| 4 (very low) | Evidence from expert committee reports or experts | IV/V | Case–control study, poor or non‐independent reference standard/expert opinion without explicit critical appraisal, or based on physiology, bench research or ‘first principles’ | 4 Expert opinion |
aOnly original studies related to the specific key questions of the recommendations were included in the systematic analysis of the literature. bThe expert panel agreed to require at least two well‐controlled studies from different study groups in order to reduce bias. cAn ‘Absolute SpPin’ is a diagnostic finding whose specificity is so high that a positive result rules‐in the diagnosis. An ‘Absolute SnNout’ is a diagnostic finding whose sensitivity is so high that a negative result rules out the diagnosis. CDR, Clinical Decision Rule; RCT, randomized controlled trial.
Each recommendation is based on the highest level of available evidence; a group of original papers or systematic reviews (if applicable) were summarized giving an overall level of evidence (LOE) using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system (Table 3) where possible. These GRADE levels (LOE) were transferred into CPRs (Tables 4 and 5). The levels and strength of recommendations correspond directly to the GRADE LOE.
Table 4.
Levels of recommendations
| Level of evidence (GRADE) | Recommendation for/against | Recommendation level |
|---|---|---|
| 1 | ‘should’, ‘should not’, or ‘is not indicated’ | A |
| 2 | ‘may’/‘suggest’ or ‘may not’/‘not suggest’ | B |
| 3 or 4 | ‘may be considered’ or ‘do not know’ | 0 |
Levels of evidence for recommendations are based on the analysis of the literature and transferred into detailed wording in the clinical recommendations with defined levels of recommendations.
Table 5.
Description of the levels of recommendations in relation to the strength of the evidence
| Strength of recommendation | Description | Criteria |
|---|---|---|
| A (A−) | Strongly recommended that clinicians (do not) routinely provide the intervention/the assessment to eligible individuals | Good quality of evidence and substantial net benefits or costs |
| B (B−) | Recommended that clinicians (do not) routinely provide the intervention/the assessment to eligible individuals |
Fair quality of evidence and substantial net benefit or costs or Good quality of evidence and moderate net benefit or costs or Fair quality of evidence and moderate net benefit or costs |
| 0 | No recommendation for or against routine provision of the intervention/the assessment |
Good quality of evidence and small net benefit or costs or Fair quality of evidence and small net benefit or costs |
| Insufficient evidence for recommendation of the intervention/the assessment | Poor quality of evidence (conflicting results; balance between benefits and risks difficult to determine; and poor study design) |
Adaptation from the Canadian Guide to Clinical Preventive Health Care and from US Preventive Services Resources.6
The CPR–DCD includes eight recommendations based on evidence from systematic literature searches in the sections on assessments and interventions. The GRADE levels of these recommendations could directly be transformed to the corresponding recommendation level (e.g. GRADE level 1 has led to strong recommendation [A], GRADE level 2 to a [moderate] recommendation [B], and lower GRADE levels to an inconclusive recommendation [0]).15
Usually an LOE of 1 is related to recommendation level A. However, in specified cases there can be a downgrade or upgrade of the clinical recommendation level. For example, if a highly effective medication (LOE 1) has unacceptable side effects or if cost:benefit ratios are extremely high, the recommendation level can be downgraded from A to B. In the present CPR‐DCD adaptations, for example upgrading or downgrading of the recommendation level, were not necessary.
For information about systematic literature searches and evidence tables of the other three working groups, with no LOE recommendations, underlying mechanisms, psychosocial issues, and adolescents and adults, see Figure 3, Appendices [Link], [Link], [Link] (online supporting information), and Tables SIV to SXVIII (online supporting information). For a comparison of the current CPRs with the 2012 CPRs see Table SXIX (online supporting information).
The whole process was supervised by the AWMF (regional association; members: 177 specialist societies). The AWMF represents Germany in the Council for International Organizations of Medical Sciences (see http://www.awmf.de for further information).
A Children
1. Epidemiology, consequences, outcome, and burden for society
1.1. Epidemiology
Current prevalence estimates for DCD range from 2% to 20% of children, with 5% to 6% being the most frequently quoted percentage in the literature.2, 6 It is generally recognized that these children have problems with motor skills that are significant enough to interfere with both social and academic functioning.16 Kadesjö and Gillberg17 found a prevalence rate of 4.9% for severe DCD and of 8.6% for moderate DCD in a population‐based study of 7‐year‐old children in Sweden. The Avon Longitudinal Study of Parents and Children found 1.8% of children aged 7 years had severe DCD, with another 3% defined as having probable DCD with consequences for everyday life.7 A recent study in South India came to very different estimates using DSM‐5 criteria (0.8%).18
We note that epidemiological information is largely dependent on how strictly selection criteria are applied.
DCD is more common in males than in females, with male:female ratios varying from 2:1 to 7:1.7, 16 Only Girish et al.18 found more females than males had DCD (male:female ratio 1:2).
1.2. Outcomes
There are only a few studies that have examined the natural course of DCD. There is evidence that in many cases DCD persists well into adolescence,19, 20, 21, 22, 23 with 50% to 70% of children continuing to have motor difficulties.22 Studies of adults with DCD reveal continuing difficulties with a range of motor skills and when learning new skills, such as driving. DCD is often associated with other learning or behavioural disorders. At kindergarten/preschool age, motor problems seem to be associated with language and communication difficulties.24, 25 These can persist into school age. Kadesjö and Gillberg17 found restricted reading comprehension in children diagnosed with DCD at the age of 7 years. There are further indications that some school‐aged children with DCD show poorer outcome in scholastic achievements26 than their typically developing peers, especially in reading27 and mathematics (i.e. symbolic and non‐symbolic number processing).28, 29
In adults with DCD, a range of non‐motor problems are commonly reported. These include problems with executive functioning, attention, and anxiety, as well as symptoms of depression and low global self‐esteem.
The systematic search conducted for the international CPR–DCD in 20126 found numerous studies presenting data on the limitations shown by individuals with DCD in different areas listed by the ICF. There is no doubt that DCD leads to an impaired functional performance in ADL.30, 31 These children require a higher level of structure and assistance in these activities than their typically developing peers.32
The impact of motor incoordination on physical activity engagements throughout life is influenced by a multitude of factors (social, cultural, physical environments, individual characteristics, etc.).33 There is evidence that children with DCD show less physical activity, especially participation in team sports.34, 35 Reduced physical activity has been associated with poor self‐efficacy in children with DCD36, 37 and lower life satisfaction.38 Behavioural problems, as well as problems in social interactions, persisted in a long‐term follow‐up.39 This affected the whole family system, especially the parents, over a long period,31, 39 but also resulted in parental concerns about their children's participation in society.40
Some studies highlight the possible negative effect of DCD on body fitness41, 42 which is mostly ascribed to less physical activity than in typically developing peers.
Cairney et al.43 report a correlation between DCD and subsequent development of obesity in males, although there was no such correlation observed in females. One explanation may be that the participation in team play activities and sport teams is diminished in children with DCD.26, 44, 45, 46 Studies on adults with DCD also report higher rates of obesity, and lower endurance, flexibility, and strength compared with typically developing adults, as well as poorer general health (both mental and physical).
An over‐representation of obesity in children with DCD and adults with a history of coordination difficulties47 requires further investigation as a bidirectional effect may occur in which propensity for obesity may lead to inactivity which exacerbates coordination difficulties by reducing participation in skilled activities.48, 49 However, there is no evidence that a lack of physical activity causes DCD.
According to the agreed diagnostic criteria, it would not be possible to make the diagnosis of DCD if the motor deficit is probably due to a lack of practice (Recommendation 3, criterion I).
1.3. Burden for society
Motor performance difficulties of individuals with DCD are often viewed as ‘mild’ and, thus, not warranting attention compared with the needs of individuals with more severe movement impairments such as cerebral palsy. Therefore, it may be argued that the net benefits for assessment and intervention in DCD may not be justified as an investment for society.
However, the numerous data on epidemiology (DCD is by far the most frequent motor disorder relevant for daily activities) and the findings on the outcome of DCD clearly suggest that DCD is a considerable burden and therefore it is also important to intervene from the viewpoint of the society.
The marked influence of DCD on everyday activities and school performance, and, secondarily, on social participation, physical health, and mental health concerns, combined with the high prevalence rate indicate that the social and economic burden is considerable.
2. Definition and terminology
DCD occurs across cultures, races, and socio‐economic conditions. The disorder is idiopathic in nature, although several hypotheses for the cause of DCD have been proposed (see ‘Underlying mechanisms in DCD’). Evidence suggests that DCD is a unique and separate neurodevelopmental disorder which can, and often does, co‐occur1 with one or more other neurodevelopmental and neurobehavioural disorders. Commonly, these disorders include ADHD, specific language impairment, learning disorders, autism spectrum disorder (ASD), and developmental dyslexia or reading disorder. Some comorbidities are so strongly associated with incoordination that DCD has even been regarded as a part of certain disorders.
The the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM‐IV)50 did not permit a dual diagnosis of DCD with ASD; however, the DSM‐52 now permits this co‐occurrence.51 Additionally, the concept of deficits in attention, motor control, and perception (DAMP) included aspects of ADHD and DCD together; this term is seldom used anymore, except in a few Scandinavian regions.52, 53
2.1. Definition according to DSM‐5
DCD is listed within the classification section Neurodevelopmental Disorders and the first condition identified under a subsection called Motor Disorders (‘315.4 Developmental coordination disorder’). The term DCD was endorsed in the International Consensus Meeting in London, ON, Canada, in 1994. DCD, according to the DSM‐52 is defined by the following four criteria: (1) acquiring and execution of coordinated motor skills is far below expected level for age, given opportunity for skill learning; (2) motor skill difficulties significantly interfere with ADL and impact academic/school productivity, prevocational and vocational activities, leisure and play; (3) onset is in the early developmental period; (4) motor skill difficulties are not better explained by intellectual delay, visual impairment, or other neurological conditions that affect movement.
2.2. Definition according to ICD‐10
In the ICD‐103 DCD is referred to as a specific developmental disorder of motor function. According to the ICD‐10, specific developmental disorder of motor function is defined as a ‘disorder in which the main feature is a serious impairment in the development of motor coordination that is not solely explicable in terms of general intellectual retardation or of any specific congenital or acquired neurological disorder. Nevertheless, in most cases a careful clinical examination shows marked neurodevelopmental immaturities such as choreiform movements of unsupported limbs or mirror movements and other associated motor features, as well as signs of impaired fine and gross motor coordination’.3
The definition excludes abnormalities of gait and mobility (R26.‐), isolated lack of coordination (R27.‐), and lack of coordination secondary to intellectual disabilities (F70–F79) or to other medical and psychosocial disorders.
The definition of DCD according to ICD‐10 requires that the diagnosis is not solely explicable by moderate to severe intellectual disability or any specific congenital or acquired neurological disorder.
2.3. Other terms and definitions
It should be recognized that in some places there is debate and sometimes confusion around the different terms and definitions used in this field. The same term is sometimes used with various definitions and it is not always clear when a term is seen as equivalent to DCD. For example, the Dyspraxia Foundation (UK) uses the term ‘dyspraxia’,54 stipulating that this incorporates DCD. However, the definition provided is broader than that for DCD, including various non‐motor difficulties.
Some use the term ‘dyspraxia’ in a more specific way, postulating a distinction between developmental ‘dyspraxia’ and DCD.55 However, the term ‘dyspraxia’ has not become recognized as a separate entity or subgroup of DCD. The international consensus does not recommend use of the term ‘dyspraxia’.
Other terms that have been used in the literature include ‘motor learning difficulty’, ‘physical awkwardness’, and ‘movement difficulty’. These refer to a significant motor difficulty, which is the main feature of DCD. However, they are ambiguous about whether the formal diagnostic criteria for DCD have been met.
| Recommendation 1 | GCP |
|---|---|
|
We recommend the use of the term Developmental Coordination Disorder (DCD) for individuals fulfilling the DCD criteria (Recommendation 3) in all research publications For clinical and educational purposes we recommend the term DCD in countries which adhere to the DSM‐5 classification (315.4) In countries where ICD‐10 has legal status, we also recommend the term Specific Developmental Disorder of Motor Function (SDDMF) (F82, ICD‐10) |
⇑⇑ |
The term DCD is used because this wording is well recognized in the English literature (Table 6). The term DCD is taken from the DSM‐5 classification. However, in several European countries, the ICD‐10 has legal status. Thus, the terminology of the ICD‐10 must be used in those countries. Accordingly, the term ‘specific developmental disorder of motor functions’ has to be used (for countries using ICD‐10 terminology). Moreover, the following recommendations throughout this document are also related to the ICD‐10. Where concepts differ between DSM‐5 and ICD‐10, specific comments are provided.
Table 6.
Terminology for DCD according to language
| Language | Disorder | Abbreviation |
|---|---|---|
| English | Developmental coordination disorder | DCD |
| German | Umschriebene Entwicklungsstörung motorischer Funktionen (specific developmental disorder of motor function) | UEMF (SDDMF) |
| French | Trouble du développement de la coordination | TDC |
3. Underlying mechanisms in DCD
3.1. Overview
To better understand the neural and performance factors that may underlie DCD, and their implications for theory and practice, a large systematic review of the recent experimental literature was conducted.56 The review included a total of 106 studies published between June 2011 and September 2016 (Fig. 3 and Appendix S4). The following summary of evidence on mechanisms of DCD is persuasive but, it must be stressed, by no means conclusive. There has been an almost exponential growth in mechanisms research over the past 30 years, with the promise that tentative working models will become more refined as additional behavioural and neuroimaging data are integrated, and as experimental approaches address the very process of motor learning in children over multiple timescales and levels of analysis (behavioural, cognitive, and neural). Nonetheless, the body of recent work is impressive and affords several tentative conclusions.
Behavioural data from 91 studies showed a broad cluster of deficits across different aspects of motor control (including planning and anticipatory control of movement), basic processes of motor learning (including procedural learning), and cognitive control (or executive function). Importantly, however, performance issues were often shown to be moderated by task type and difficulty. As well, new evidence has emerged to show that children with DCD can adopt compensatory strategies/actions under certain task conditons, enabling response solutions that are adequate to the goal of the task in question, while perhaps being less efficient overall. The review showed a significant growth in neuroimaging studies over the past 5 to 6 years (15 studies). These studies have been of mixed quality, hindered by small sample sizes, lack of concurrent behavioural measures, and missing data. This suggests some caution with interpretation. However, there has been some converging evidence across studies with data showing reduced cortical thickness57 and hypoactivation across functional networks involving prefrontal, parietal, and cerebellar regions.58, 59, 60, 61 However, evidence for hypoactivation has not been uniform across all studies and tasks; for example, Licari et al.59 also showed increased activation in DCD in the right postcentral gyrus. Structural diffusion magnetic resonance imaging studies have demonstrated alterations of white matter microstructural organization, particularly in sensorimotor tracts that include the corticospinal tract, posterior thalamic radiation, and parietal subregion of the corpus callosum.62, 63, 64 Other structural magnetic resonance imaging data have also suggested a poorly integrated neural network involving sensorimotor structures.65 Taken together, these results provide some support for the hypothesis that children with DCD show differences in neural structure and function compared with typically developing children. Larger and longitudinal studies are needed to confirm these trends. Behaviourally, these emerging differences may affect anticipatory planning and observational learning, and reduce automatization of movement skill, prompting greater reliance on slower feedback‐based control and compensatory strategies. Findings of the review can be embedded in a multi‐component account of DCD that considers the interaction of individual, task, and environmental constraints. This account blends both cognitive neuroscience and classical dynamic systems (or ecological) theory.66, 67 At the individual level, neuromaturational factors are beginning to emerge as candidates in the aetiology of DCD.
3.2. Towards a unified account of DCD
The improved quality of experimental work on DCD over recent years has enhanced our ability to compare results across studies and to consider how findings might be integrated under a common explanatory framework (Fig. 4), one that considers more precisely the mutual interaction of individual, task, and environmental constraints. Whereas mechanisms research has had a traditional focus on individual‐level factors in performance, recent research suggests that we think about the notion of action constraints (and their interaction) more seriously when assessing and treating children with DCD.68 The upshot of an integrated framework (or hybrid model) is that individual‐level constraints (such as internal modelling or even executive function deficits) can affect performance (or not) in variable ways, especially as a function of task difficulty/type. The causal mechanisms that determine motor performance are not linear but dynamic and interactive. Several important conclusions can be made about the body of evidence, mainly related to the themes of predictive motor control, action representation, perceptual–motor coupling, task complexity, co‐occurring cognitive issues, compensation, and persistence into adulthood.
Figure 4.
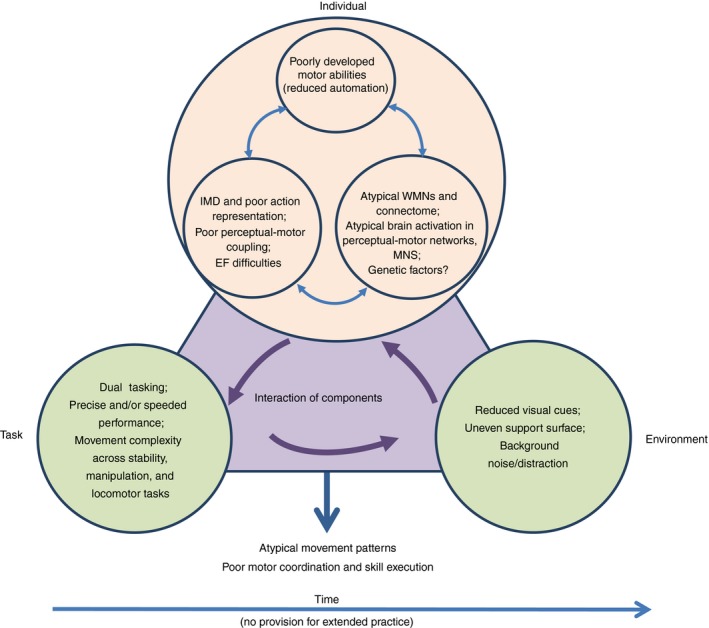
Multi‐component account of motor skill development showing correlates of performance in developmental coordination disorder (DCD). IMD, internal modeling deficit; EF, executive function; WMN, white matter network; MNS, mirror neuron system. [Colour figure can be viewed at http://www.wileyonlinelibrary.com]
Research has continued to address the internal modelling deficit hypothesis and the function of the mirror neuron system (MNS). The internal modelling deficit hypothesis holds reasonably well across different types of movement, but the deficit in forward planning (or predictive control) is more evident in tasks of higher complexity or those that require higher endpoint precision. Related to this, issues with action representation (in gesture imitation and motor imagery) reflect problems associated with the egocentric representation of space and possibly function of the MNS (Fig. 4); neuroimaging data show differences in microstructure associated with the MNS but, on the other hand, there is also very recent evidence from functional neuroimaging that shows no differences in MNS activation between DCD and non‐DCD groups.69 On balance, additional data are needed to make firmer conclusions about the role of the MNS in DCD.
In general, performance deficits tend to map to functional and structural issues in a distributed neural network that supports motor control and learning, a view that has garnered some support by structural and functional neuroimaging and electroencephalography data. This distributed network involves the MNS, visual–motor mapping, and predictive control (e.g. frontoparietal and parieto‐cerebellar structures) and corticospinal tract. However, it should be stressed again that the fidelity of neuroimaging studies is not yet sufficient to make firm conclusions. A delay in the maturation of these network structures is possible; however, we do not yet have sufficient longitudinal data to better clarify the causal connections.
Cognitive dysfunction, namely executive function, in DCD is a common finding across measures (experimental, questionnaire, and real‐world behaviour) that persists into early adulthood and, very importantly, is strongly linked to impaired planning and disorganization in daily life. With age, cognitive–motor coupling becomes increasingly important to goal‐directed action. For example, frontal executive systems are known to support the integration of working memory with attentional resource allocation – a process called branching – which is particularly important in dual‐task performance.70 A (whole‐brain) network approach will no doubt prove important when developing models that describe such processes in DCD.65 However, more cross‐cultural studies are needed to verify the impact of executive function on adaptive behaviours in young people and young adults. Moreover, it remains unclear whether dysfunction in executive function is a core symptom of DCD or a co‐occurring condition.
In general, we are seeing more evidence that motor control deficits in DCD depend on the nature of the task at hand. Deficits are especially apparent for dual tasks, and tasks that demand more precision (both spatial and temporal), more advanced planning, or that stress the system in a way that requires some adaptation/adjustment at a perceptual–motor level to maintain stability. As well, associated executive function issues (e.g. response inhibition) may also constrain the ability to implement motor control and to automate skill without the need for extended periods of practice. In general, with poor predictive control and reduced automatization, we see more reliance on slower feedback‐based control, and the need to enlist compensatory strategies to maintain ‘safety’ margins when performing complex or difficult skills. One hypothesis is that delays in the development of sensorimotor networks that underpin internal modelling and observational learning (via the MNS) may necessitate that the child relies more on external feedback. The weight of evidence would tend to support this broad hypothesis, but other converging data are needed. Indeed, we still know little of the specific mechanisms that explain these issues in motor control, especially in the context of development with age. Also, the issue of delay versus deviance is still unresolved. However, for the more than half of children with DCD whose motor difficulties persist into adulthood, it is tempting to say that the underlying mechanism is likely to be more than a mere developmental delay. However, until we take full account of the learning/activity history of individuals with DCD over childhood, adolescence, and young adulthood, a concrete conclusion is not possible. Clearly, well‐controlled longitudinal studies are needed to clarify the issue of deviance versus delay, particularly those that allow multilevel, growth curve analysis.
3.3. Clinical implications of mechanisms research
The review of research on basic processes and mechanisms has several very important clinical implications. The first relates to the co‐occurrence of cognitive issues in DCD. The imperative is to assess broadly across motor and cognitive functions, taking aspects of task organization and self‐regulation also into account, not only in childhood but through adolescence and into early adulthood.
Also tempering assessment and treatment is the issue of heterogeneity in the presentation of DCD and in severity, which is evident across studies. For instance, a child may be functionally impaired and yet perform within the typical range for motor control and cognition, or the reverse may apply (typical function but impaired control). Similarly, current data do not allow us to say whether a child with mild, moderate, or severe DCD will present with a particular cluster of motor and cognitive issues. In the absence of further evidence, it remains doubly prudent for clinicians to assess comprehensively across motor and cognitive functions.
Since motor control and executive function deficits are expressed as a function of task type and difficulty, a measured approach to assessment and intervention is recommended. Clinicians are encouraged to assess movement skill in different domains by varying systematically task and environmental constraints. Identifying those specific aspects of the task that present difficulty will directly inform approaches to training, especially the scaling of difficulty which is so important in many task‐oriented approaches to training. Finally, the suggestion of neurocognitive issues in DCD (as in ADHD and other neurodevelopmental disorders) suggests that clumsiness in children should not be ignored clinically, and that it be given due consideration on its own.
4. Diagnosis and assessment
4.1. Definition and criteria
These CPR–DCD aim to minimize differences in interpretation and classification between the ICD‐10 and DSM‐5, because the disorders are considered to represent similar conditions.
| Recommendation 2 | GCP |
|---|---|
| We recommend that the diagnosis of DCD is made by a medical professional or a multi‐professional team2 suitably qualified to assess the individual according to the specified criteria. | ⇑⇑ |
| Recommendation 3 | GCP |
|---|---|
|
We recommend the following criteria for the diagnosis of DCD. These criteria follow closely those proposed in DSM‐5 with some minor changes, including the order of criteria III and IV: IThe acquisition and execution of coordinated motor skills is substantially below that expected given the individual's chronological age and sufficient opportunities to acquire age‐appropriate motor skills. IIThe motor skills deficit described in criterion I significantly and persistently interferes with the activities of everyday living appropriate to chronological age (e.g. self‐care and self‐maintenance and mobility) and impacts upon academic/school productivity, prevocational and vocational activities, leisure, and play. IIIThe motor skills deficits are not better accounted for by any other medical, neurodevelopmental, psychological, social condition, or cultural background. IVOnset of symptoms in childhood (although not always identified until adolescence or adulthood). Comment: Criterion I: The symptoms of DCD may include slowness and/or inaccuracy of motor skills performed in isolation or in combination. Criterion III: This addresses issues of aetiology with regard to DCD and is designed to facilitate differential diagnosis. Examples of conditions which may rule out or influence the diagnosis of DCD are: (1) Medical conditions: movement disorders with known aetiologies (e.g. cerebral palsy, muscular dystrophy, childhood arthritis), side effects of drugs (e.g. neuroleptics, chemotherapy, sedatives), sensory problems (e.g. substantial visual impairments or impairments of the vestibular organ) (2) Other neurodevelopmental disorders (e.g. severe intellectual disabilities) or other psychological disorders (e.g. anxiety, depression), or other psychological conditions (e.g. attentional problems) as primary causes of motor problems (3) Social conditions (e.g. deprivation, cultural diversity) Note: It may be difficult to differentiate between conditions that may be causal and those that may co‐occur.3 For example, a child from a culture, which limits physical activity or which provides little opportunity for motor learning may present like a child with DCD (at least initially). A child with ADHD might appear to have movement problems, which are in fact caused by impulsivity and/or inattention. Especially in unclear cases, multiprofessional or repeated assessments can be helpful to differentiate. Criterion IV: The onset of symptoms is usually evidenced in infancy and childhood. The following recommendations are designed to offer guidance as to how to arrive at an accurate diagnosis of DCD. Instead of being listed according to the criteria I to IV they are given in the opposite order which is in line with how a medical professional would usually proceed with his/her examination. Thus, the process starts with: (1) considering the age and context of the child (criterion IV), (2) ruling out other medical conditions causing motor problems (criterion III), (3) taking into account the impact on activities and participation (criterion II), (4) quantifying the motor impairment (criterion I). It should be noted, however, that there are other pathways to diagnosis. For example, a child might be identified as having difficulties within a school system and be first assessed by a therapist or educational psychologist. Their assessments may show the child meets criteria I, II, and IV and only then might the child be referred to a medical doctor to exclude other conditions. |
⇑⇑ |
4.1.1. Clarification of criterion III
When DCD should not be diagnosed?
DCD should not be diagnosed if: (1) motor performance cannot be assessed by a motor test (e.g. because of intellectual disability or a medical disorder); or (2) after a comprehensive assessment including clinical history, examination, and consideration of teachers’ and parents’ reports, the motor dysfunction can be explained by another condition including a neurological or psychosocial disorder or moderate to severe intellectual disability.
In the definition of the disorder within the ICD‐10 (F82), it is mentioned that some children may show ‘marked neurodevelopmental immaturities such as choreiform movements of unsupported limbs or mirror movements and other associated motor features’.3 According to the current literature and clinical experience, the roles of these motor features are still largely unclear and need further evaluation.
DCD and intellectual developmental disorder
The problem of diagnosing DCD in children with severe cognitive delays or intellectual developmental disorder was discussed intensively. It was recognized that defining a specific IQ score below which the diagnosis of DCD is precluded seems artificial. Given the complexities of arbitrating between cut‐offs and determining discrepancy scores, it is recognized that categorical decision (above or below a specific IQ score) may be extremely difficult. Looking at the meta‐analysis on underlying mechanisms of DCD within the previous international CPR,71 a specific IQ score does not seem to be helpful in distinguishing between children with DCD and children with coordination problems due to intellectual developmental disorder as the construct of DCD is now increasingly supported by neurobiological and neurophysiological findings and not restricted to global intellectual abilities.
It was agreed that the motor dysfunction should be defined as DCD if the other criteria are fulfilled and if clinical history and examination cannot explain the motor problems and their impact on daily activities by cognitive status.
DCD and coexisting diagnoses
It is widely recognized that children with DCD often have coexisting diagnoses (Recommendation 14). It should be considered that ADHD, ASD, or conduct disorders may interfere with motor performance and testing, as well as with ADL, making interpretation of motor assessment findings difficult.
4.1.1.1.
| Recommendation 4 | GCP |
|---|---|
|
The symptoms of DCD are usually apparent in the early years. However, due to the large variability in normal motor development, we recommend that a formal diagnosis of DCD under the age of 5 years is only made in cases of severe impairment. In such instances, the decision to make a diagnosis should be based on the findings from at least two motor assessments carried out at least 3 months apart. Comment: Based on the persistence and the extent to which the motor problems interfere with daily life (criterion II), and after excluding other conditions that may explain the motor problems (criterion III), it should be decided whether any form of intervention should be recommended at this stage. Options will include formal intervention, the provision of opportunities for motor learning in a less formal setting, or advice plus clinical supervision. |
⇑⇑ |
DCD and age
As in the previous version of the recommendations, there are considerable problems for the diagnosis of DCD in children below 5 years of age for the following reasons.
First, young children may show delayed motor development with a spontaneous catch up (late bloomers).
Second, the cooperation and motivation of young children for motor assessments may be variable. Thus, test performance may be unreliable and then result in poor predictive validity (criterion I).72, 73 Nevertheless, a study by Ellinoudis et al.74 confirmed earlier results from Smits‐Engelsman et al.75 indicating that motor assessment by the Movement Assessment Battery for Children, Second Edition (MABC‐2) has a good test–retest reliability and a reasonable construct validity in 3‐year‐old to 5‐year‐old children.
Third, the rate of acquisition of ADL skills is variable in children at kindergarten age. Thus, the evaluation of criterion II of the diagnostic criteria in children under 5 years is unreliable.
Finally, there are no reliable data on the value of early intervention in preventing DCD. The lack of stability of DCD diagnosed at early ages has been shown, with the exception of DCD in cases with coexisting ASD.72, 73, 76 While the assessment itself may be reliable (e.g. using the Movement Assessment Battery for Children [MABC‐1], Chow and Henderson77 repeated the assessment within 3‐week intervals, but this is not recommended because of practice effects),78 a previous follow‐up study emphasized that only in definite (severe) cases of DCD being detected before school age is the disorder stable 2 to 3 years later.79
On the other hand, criterion IV emphasizes that symptoms of DCD should be present in early childhood. This means that the onset of DCD is usually during childhood but may not become fully manifest until movement challenges exceed limited capacities with respect to context and opportunities. This aspect is particularly important when assessing adolescents and adults.
| Recommendation 5 | GCP |
|---|---|
| For countries using ICD‐10: for motor problems in individuals who do not meet criteria for DCD but where criterion III is fullfilled, we recommend the ICD‐10 categories of R26, R27, or R29 be applied if appropriate | ⇑⇑ |
4.2. The process of assessment
4.2.1. Explanatory frameworks for different assessment approaches
According to the evaluative review by Wilson,80 the following five assessment approaches can be distinguished:
Normative functional skill approach. Assumptions about movement difficulties are largely process neutral. Approaches to assessment are descriptive, product oriented (focus on functional skills), and norm‐referenced. For example, the MABC‐2 is based on this approach.
General abilities approach. The guiding assumption here is that impaired sensory–motor integration underpins both perceptual–motor problems and learning difficulties. These impairments reflect neural damage. According to this approach, basic general abilities (such as sensory–motor integration) can be measured (e.g. by the Sensory Integration and Praxis Test) and then become the focus for treatment to improve motor functions.
Neurodevelopmental theory (biomedical model). Early neurological markers (e.g. clumsiness) predict disease states (e.g. minimal brain dysfunction). This may be assessed by neurodevelopmental examination. An eclectic blend of neurological and learning tasks (e.g. soft signs or minor neurological dysfunction) will be tested. Normative data on soft signs exist.81, 82, 83 A new version of the examination of the Child with Minor Neurological Dysfunction is available.84 The manual contains criteria, cut‐offs, and description of psychometric properties. Evidence is emerging that children with DCD often exhibit minor neurological dysfunction, in particular quite often the ‘complex form of minor neurological dysfunction’.85, 86, 87 This issue may deserve further attention. Advances in neuroimaging and functional imaging will provide insights into hard and soft signs of neural dysfunction. On the other hand, the role of minimal brain dysfunction and minor neurological dysfunction for the development of a theory of DCD has been questioned.80
Dynamical systems approach.88 This approach suggests that the child with DCD has had reduced opportunities to form movement synergies through interaction with learning tasks and environment. Assessments used within this framework include biomechanical, kinematic, and observational analyses.
Cognitive neuroscience approach. It is suggested that atypical brain development creates cognitive susceptibility. Reduced learning experiences exacerbate the risk for developing DCD. Approaches to assessment tend to be oriented towards brain systems that are of known importance to the development of movement skill (e.g. internal modelling or motor imagery, and timing control linked to parieto‐cerebellar loops).
The recommendations largely reflect assessment strategies based on the normative functional skill approach as the criteria of DCD are descriptive and rely on this approach.
4.2.2. General aspects on screening
Early identification of children with motor impairments has been recommended.89, 90 On the other hand, the diagnosis of DCD before the age of 5 years is not generally recommended. This has already been discussed (Recommendation 4).
Screening instruments for assessment of DCD are not sufficiently refined to enable highly valid and reliable results. Therefore, at present, it is not useful to screen the population for DCD. The diagnosis of DCD can only be made for a clinical population within a specified setting as described below.
Motor coordination test batteries are generally not feasible as screening protocols owing to both time and costs.
4.2.3. History taking: covering criteria IV, III, II, and I (previous tests)
| Recommendation 6 | GCP |
|---|---|
|
To begin any assessment process, we recommend careful history taking to support the application of all four criteria. Comment: Children: history should include the following aspects: (1) Parental report Reasons for referral and presenting problems. Family history – to include information about the presence of developmental disorders or other genetic conditions (e.g. muscular disorders in family members). Medical history – to include information about major accidents, diseases, neurological disorders, relevant or associated psychological problems, sensory problems (e.g. documented in previous assessments, new symptoms arising), medication regime where relevant. Developmental history – to include information about pregnancy, birth, milestones – motor and non‐motor; history of motor engagement (e.g. family habits, home environment, access to motor activities), social competences, and ability to interact with others. Educational history – educational progress through nursery, pre‐school, kindergarten, and grade school, and information about any measures of academic achievement. Impact of the condition – including impact on ADL and participation. Contextual factors – including amount and type of previous and current intervention/support; description of current family structure, social network and relationship status (e.g. social support, living with family, extended relatives, guardians, friends or others), social‐economic status, personal resources. (2) Sources other than parents Formal documents and reports from relevant professionals and significant others (e.g. nursery, pre‐school, kindergarten and school teachers, educational psychologists, therapists). These might include: (a) Written information or interview data from other family members or significant adults if appropriate and consent is given. (b) Reports about motor functions from physical education teachers or therapists as well as other areas of interest (as per the ICF): participation and levels of physical activity, environmental factors, support systems, individual/personal factors, etc. (c) Reports concerning cognitive functions (e.g. IQ data, scores on working memory, attention, other tests). (d) Reports concerning academic achievement. (e) Reports concerning behaviour that might bear on differential diagnosis and/or possible dual diagnosis (e.g. ADHD, ASD, learning disorders). (3) Child Self‐reports Adapted questionnaires |
⇑⇑ |
4.2.4. Clinical examination: covering criteria III and II
In addition to the exploration of the history, clinical examination is mainly necessary to exclude the presence of other medical conditions that may explain motor impairment. The aim of the neurological status is to rule out other movement disorders and to support criterion III. A comprehensive clinical examination should be performed to verify that the disturbance is not due to a psychosocial condition (e.g. deprivation, child abuse) and/or general medical condition (e.g. cerebral palsy, hemiplegia, or muscular dystrophy).
Exclusion of neurological disorders such as corticospinal, cerebellar, extrapyramidal, or neuromuscular origin. Signs of neurometabolic disorders or of acquired neurological disorders (prenatal, perinatal, postnatal), peripheral neurological disorders. Since the previous GCP, no further studies on the diagnostic value of minor neurological dysfunction or the soft signs exhibited by individuals with DCD have been found.
A behavioural and cognitive evaluation is recommended for all children with DCD because attentional disorders, learning disorders, and ASD are frequent co‐occurances. If features of maladaptive behavioural or emotional issues exist, further examination according to the respective guidelines is necessary.
If there is a normal history of school and academic achievements, cognitive function does not need to be evaluated by objective measures (e.g. IQ testing). However, a test for intellectual ability is recommended, if there is any doubt.
| Recommendation 7 | GCP |
|---|---|
|
We recommend problem‐oriented clinical observation and examination. Comment: The clinical observation/examination should include an evaluation of the following. Neurological status (e.g. exclusion of other movement disorders or neurological dysfunctions, a rapid change or deterioration in motor functioning). Medical status (e.g. obesity, hypothyroidism, genetic syndromes, malnutrition, joint problems). Sensory status (e.g. vision, audition, tactile and proprioceptive functioning, vestibular functioning). Other neurodevelopmental disorders and psychological status (e.g. ASD‐type behaviours, self‐esteem, depression, anxiety). Cognitive status (e.g. attention, memory, verbal and non‐verbal reasoning, executive functioning), especially if there is a history of learning difficulties at school. Observation of motor activities (e.g. playing, drawing, dressing, undressing). |
⇑⇑ |
4.2.5. Specific history and questionnaires: covering criterion II
| Recommendation 8 | GCP |
|---|---|
|
We recommend that the complete assessment considers ADL (e.g., self‐care and self‐maintenance), academic/school productivity, prevocational or vocational activities, leisure, sports, and play. We recommend that this information be gathered from multiple sources such as: self‐reports, reports of parents, health care/educational professionals, and relevant others. Comment: Because language is involved, handwriting and keyboarding are areas of motor competence that should be assessed separately. |
⇑⇑ |
By definition, ADL implies cultural differences. When applying this criterion, it is therefore crucial to consider the context in which the child is living and whether the child has had appropriate opportunities to learn and practise different skills that would be considered typical within their respective home and community settings (Recommendation 3, criterion I).
Establishing a direct link between poor motor incoordination and academic achievement is complex. However, the specific skill of handwriting is usually affected, and is known to adversely influence academic achievement owing to slow, inaccurate, and/or illegible penmanship and output. Therefore, academic achievement should be assessed.
The complete assessment should reflect culturally relevant developmental norms.
4.2.6. Evidence‐based analysis of DCD questionnaires
The results of the systematic review on DCD questionnaires are shown in Appendix S2 and Table SII. The sensitivity and specificity are highly variable and depend on the person who completed the questionnaire and the sample (clinical or population‐based). Researchers have previously argued for motor‐based questionnaires that are completed by the child,89, 91 teachers,92, 93, 94 and/or parents.90
While the Developmental Coordination Disorder Parent Questionnaire (DCDQ),95 its revised version (DCDQ‐R),4 96 and the teacher questionnaire MABC‐2 checklist (MABC‐2‐C)97, 98 all focus on the individual's activity level (e.g. self‐care, ball skills), they do contain items that refer and relate to underlying body functions.
The DCDQ/DCDQ‐R has been validated most frequently in the literature. In addition to the CPR–DCD in 2012, nine additional studies have been found (Appendix S2 and Table SII). Further, the DCDQ‐R has been extended towards children of 3 years and 4 years of age: the Little DCD Questionnaire or Little DCDQ.99 Three studies have been found so far.99, 100, 101 In contrast, the MABC‐2‐C has been much less examined. There is only one additional study on the MABC‐2‐C.102
Further questionnaires and assessments
Although several other questionnaires and observation forms exist, these instruments have only been researched in single studies (Appendix S2). Therefore, no recommendations can be made at present.
Other scales and questionnaires also exist; but these ‘unspecific’ instruments do not verify the diagnosis of DCD. However, the information gathered may be useful. Some examples are as follows: (1) Early Years Motor Skills Checklist;103 (2) Children Activity Scales for Parents and Children Activity Scales for Teachers;104 (3) The Handwriting Proficiency Screening Questionnaire105 for teachers/parents report and the Handwriting Proficiency Screening Questionnaire‐Children106 for the child's self‐report about handwriting difficulties; (4) My Child's Play, a parent questionnaire designed to detect the play characteristics of young children aged 3 to 6 years suspected for DCD.107, 108
Furthermore, there are self‐reports for children, most of which measure aspects of self‐efficacy for movement and self‐esteem: (1) The All About Me Scale;109, 110 (2) The Perceived Efficacy and Goal Setting System;109, 111 (3) The Childrens Self‐Perceptions of Adequacy in and Predilection for Physical Activity.89, 91 This last scale has been examined mainly by one research group. Several terms in it are specific to North America (e.g. the different settings for participation).
Although these instruments may provide an idea of how the child perceives their disorder, such self‐reports are not confirmed to be specific and sensitive assessment tools for the diagnosis of DCD.
There is a clear need for research that evaluates the validity of these assessment instruments, especially their associations with the relevant aspects of DCD.
The DCDDaily112, 113 is a parent questionnaire, which exclusively examines ADL. The DCDDaily Questionnaire seems to be a valid and reliable (only internal consistency) questionnaire about children's acquisition of performance and regular participation in ADL. It is the first questionnaire to provide insight into the broad range of ADL that children with DCD seem to struggle with every day.
Observation forms may become a useful tool to standardize clinical examination. An example completed by teachers is the Motor Observation Questionnaire for Teachers.114, 115, 116
The Performance Quality Rating Scale117 allows observers to rate a client's performance on a client‐selected activity according to a set scoring system. This tool may be very useful for treatment planning, monitoring, and evaluation.
The Do‐Eat is a real‐life performance‐based assessment with a parent questionnaire.118 It evaluates both the child's actual performance and the sensory–motor and executive control as underlying mechanisms. The Do‐Eat was validated among children with DCD.118
Kirby et al.119 created and studied an Adult Developmental Coordination Disorder/Dyspraxia Checklist (ADC).
In conclusion, the only questionnaire with a good LOE is the DCDQ‐R. Other questionnaires and assessments – especially those using direct observation to focus on ADL, play, and other childhood activities – may be very helpful and therefore should be examined further. Multiple questionnaires and assessments will help clinicians gain a more complete picture of the child's everyday activities and self‐perception. A broad range of assessments will also be useful in multidisciplinary centres and other settings.
| Recommendation 9 | GCP |
|---|---|
| We recommend that, if possible, the measure(s) used to collect information on the DCD related characteristics of an individual, has appropriate standardization. These measures (e.g., questionnaires, observational assessment tools) may be completed by parents, teachers, the child himself/herself, or significant others in the child's life. | ⇑⇑ |
| Recommendation 10 | LOE |
|---|---|
|
We suggest that that the DCDQ‐R is used in a clinical setting as supplementary information in the diagnosis of children with DCD. Comment: Athough many questionnaires (e.g. MABC‐2‐C, Motor Observation Questionnaire for Teachers, DCDDaily Questionnaire) are available, the DCDQ's psychometric properties have been studied most extensively and therefore can be suggested as offering supplementary information on motor‐related problems. The DCDQ‐R has been shown to be a useful adjunct in studies using clinical samples. However, the DCDQ‐R should not be used in population‐based screening as it has been shown that the sensitivity is too low to identify children with DCD in the general population. |
LOE 2, level B |
4.2.7. Criterion I: objective assessment of motor proficiency
Assessment with standardized tests
To substantiate the motor difficulties associated with the definition of DCD, an appropriate, valid, reliable, and standardized motor test (norm‐referenced) should be used. Numerous tests measure motor functioning, but only a few have been designed and tested for the assessment of the diagnosis DCD.
Assessments on motor functions according to criterion I
In addition to the clinical examination, which is focused primarily on the level of body structure and functions, assessment using one of the following standardized tests is more focused on the level of activities.
Within the literature search interval from 1995 to January 2010, 19 studies examining the MABC‐197 were found. Five studies examined the Bruininks‐Oseretsky Test of Motor Proficiency,120 three studies (including one from 2010) used the Körperkoordinationstest für Kinder (KTK),121 and three studies were conducted on the Züricher Neuromotorik (Zurich Neuromotor Assessment Battery [ZNA]).122 The last two tests have not been validated for the specific diagnosis of DCD. The McCarron Assessment of Neuromuscular Dysfunction123 has also been used in several studies of DCD and has shown good convergent validity (see, for example, Brantner et al.).124
Within the literature search interval from 2011 to March 2017, 13 studies were conducted on the MABC‐2, three examined the Bruininks‐Oseretsky Test of Motor Proficiency, Second Edition (BOT‐2), and 11 examined one of these tests along with others. Other tests such as the KTK (two studies), ZNA (two studies), or the McCarron Assessment of Neuromuscular Dysfunction (one study) have received little study or have not been used for validation in children with DCD.
The MABC‐2
The MABC‐197 and its revised version the MABC‐298 are by far the most commonly used and best‐examined test (Appendix S2 and Table SI). Apart from the English version, the MABC‐2 is published in 10 countries and languages (Slovenian, Italian, French, German, Dutch, Spanish, Danish, Norwegian, Swedish, and Czech).
The MABC‐2 is a norm‐referenced test for children aged 3 to 16 years, with three age group splits. The former version, MABC‐1, was designed for children aged 4 to 12 years and split into four age groups. Numerous studies conducted on the MABC‐2 were not designed to examine test criteria, but factors that influence the test criteria. Thus, only studies with representative samples and sound methodological background were included in the evaluation.
Psychometric properties of the MABC‐2
The studies on the MABC‐2 show good to excellent interrater reliability, good to excellent test–retest reliability, and fair to good validity (construct validity and concurrent validity with BOT‐2). The specificity seems to be good (0.8–0.9) and the sensitivity (0.7–0.8) is lower in general.75, 125, 126, 127, 128, 129, 130
Limitations of the MABC‐2
There is a lack of research on the discriminant validity of the MABC‐2. As for any motor test, attentional problems may interfere with performance on the MABC‐2 and there may be variability when repeating the measurement. However, in one study, the MABC‐2 produced reliable results in repeated assessments over a 20‐day interval and seemed responsive to change in children with DCD who were enrolled in a rehabilitation programme for a 6‐month period.131
A further problem may be the scaling of the reference values (e.g. with ‘floor effects’ in age band 1, which is for children aged 3–6y). The ‘discontinuation’ of the scales moving from one age band to another may be a problem in longitudinal comparisons, especially as children move from kindergarten to school age or when first‐grade children are compared (6–7y). These age ranges are often critical for DCD diagnosis and treatment monitoring. No effects of sex have been found. This finding is in contrast to the findings of the BOT‐2.132, 133
Comments on the MABC‐2
The MABC‐2 and its previous version the MABC‐1 are by far the most used and best studied standardized motor test for individuals with DCD. The MABC‐2 test includes eight tasks to assess manual dexterity (three tasks), aiming and catching (two tasks), and balance (three tasks) across three age bands (3–6y, 7–10y, 11–16y). The second edition was published with UK norms. However, the norms need to be adapted for different countries because, for example, Dutch norms have been found to be different from UK norms.134 The specificity seems to be very good; however, the sensitivity seems to be fair.
As it pertains to the diagnosis of DCD, the MABC‐2 is regarded as moderate to good overall.
BOT‐2
Apart from the MABC‐2, the Bruininks‐Oseretsky Test of Motor Proficiency120 and its revised version the BOT‐2 are often used in clinical practice and studies for individuals with DCD.
Through eight subtests, which measure 53 items and result in four motor composites and a total motor composite, this test assesses a wide array of motor skills in individuals. Skills measured include precision, coordination, speed and dexterity of upper limbs, the speed of response, and visual–motor control. It is also used to assess coordination of bilateral movements, the maintenance of an individual's balance, running and general agility, and strength of movement. The BOT‐2 provides broad norms (aged 4–21y). These age norms have 4‐month intervals in preschool children, half‐year intervals in school children, and full‐year intervals in adolescents older than 14 years. The instrument has separate norms for each sex. The BOT‐2 is available in English with American norms and in German with norms from Germany, Austria, and Switzerland.133 The German norms were provided for children from 4 to 14 years 11 months.
Psychometric properties of the BOT‐2
As in the previous version of the CPR, the BOT‐2 shows good to excellent reliability, fairly good validity (construct and concurrent validity with MABC‐2), and good specificity. However, the American version of the BOT‐2 has lower sensitivity than the MABC‐2. The German version has been shown to have very good sensitivity.135 Primary strengths of the BOT‐2 include: (1) the manual contains photographs which help to minimize language demands and provide extra cues for examiners that further support standard and efficient test administration; (2) the face validity of the items reflects typical childhood motor activities (e.g. ball skills, movement, paper/pencil activities, card sorting); (3) the construct validation of the test is good; (4) moderate to strong interrater and test–retest reliabilities for both the total motor composite and the short form; and (5) the norms are relatively up to date and reflect the demographics of the USA. In 2012 and 2013, the BOT‐2 was completely restandardized for children aged 4 to 14 years in German‐speaking countries.133 Reliability and validity measures being examined so far seem to be even better than in the original USA standardization sample.
Limitations of the BOT‐2
Limitations include: (1) weak test–retest reliabilities for some subtests as well as for certain motor composites for some age groups, which constrain confidence in the use of these scores; (2) the scoring process is time intensive and tedious (e.g. errors are likely to occur owing to the multiple step process and the characteristics of the record form and norm tables); (3) in contrast to the MABC‐2, single items are short and therefore less vulnerable to attentional fluctuations. However, the duration of the long version may be difficult in children with attentional problems; and (4) the items for 4‐year‐old children may be too difficult (floor effect).136
In sum, the LOE for the quality and suitability of the Bruininks‐Oseretsky Test of Motor Proficiency/BOT‐2 is rated as moderate (LOE 2); however, in general, the evidence is weaker than for the MABC‐1/MABC‐2. Regardless, the original American standardization population and recently conducted standardization sample for German‐speaking countries is large and seems convincing. This also applies to the reference values in young children, including those within the 4‐month interval groups.
Other tests
Several other tests that assess motor functions are found in the literature, but they have not been evaluated with respect to the diagnosis of DCD (level 0, LOE 3). In most tests, there are only one to three published papers on test criteria (LOE 2–3). However, they may be suitable for testing motor abilities. Examples of these tests include the following.
The ZNA122 examines motor abilities (e.g. finger tapping), motor skills (e.g. static balance, pegboard, rope jumping), and associated movements (e.g. movement quality, soft signs) in 5‐year‐old to 18‐year‐old Swiss children and adolescents. Several studies have been published assessing the test–retest, interobserver and intraobserver reliability,137 construct validity,138 and the validity of the ZNA in children born preterm.139, 140 Studies also presented age‐related normative values (centiles)81, 82, 141 and examined the influence of age, sex, and left‐handedness on the motor tasks.83, 141 There is now a study on concurrent validity of the ZNA with the MABC‐2 showing moderate correlations and suggesting that both tests possibly measure different contructs (e.g. the ZNA focuses more on body functions than the MABC‐2).142
The KTK121 assesses the general coordination of children, with four subtests. It was recently published as the third revised edition.143 The most important requirement for test procedures is the need for actual norms,144 because the KTK was criticized for having outdated norms from 1973 and 1974. In 2014, Schilling presented ‘norm values’ that were calculated as secondary data from other studies using similar test items in different studies.145 The KTK has not been specifically used to assess children with DCD.
The Peabody Developmental Motor Scales, Second Edition146 is a quantitative and qualitative assessment of gross motor and fine motor development in young children (birth–5y). It is based on an age‐stratified sample of 2000 children. It may be useful for descriptive and evaluative use in children younger than 4 years. Recently, it has been correlated with the MABC‐2, showing moderate concurrent validity. Furthermore, reliability was fairly good for the Peabody Developmental Motor Scales, Second Edition.134, 147
The Bayley Scales of Infant Development, Third Edition148 is a comprehensive developmental test, designed to evaluate motor, language, and cognitive functions in infants and toddlers, age up to 3 years. The motor subscale may be useful for descriptive and evaluative purposes, especially when identifying early motor dysfunctions within a general developmental assessment. Frijters et al.125 have shown it had a good correlation (p=0.7) with MABC‐2 results in children aged 36–48 months.
The Zuk Assessment149 tool is reliable when assessing typically developing children and it seems on par with the MABC‐2, for comparable reliability and validity measures were found when it was studied against the MABC‐2.149
The Test of Gross Motor Development, Second Edition150 has been examined in one study.151 It shows weak to moderate correlations with MABC‐2.
Handwriting
Handwriting is an important everyday activity. Therefore, testing may help to support criterion II. Handwriting has been shown as highly discriminative in children who have developmental disorders.152 The same researchers found a significant group difference in the kinematics, relative size, and other handwriting measures. There was an accuracy rate of 94.9% for the diagnosis of developmental disorders. Further studies indicate a predictive validity of handwriting with respect to the diagnosis of DCD.153, 154, 155 However, more studies are needed as it is not yet clear whether handwriting is an important ‘general’ marker for identifying children with DCD, or, if in certain cases, some children with DCD also present with co‐occurring handwriting problems.
The Handwriting Proficiency Screening Questionnaire may be used in school‐aged children with DCD and poor handwriting function.105 It is a practical, non‐language‐dependent observational questionnaire developed to detect handwriting difficulties and their impact. The 10 items in the Handwriting Proficiency Screening Questionnaire cover the most important indicators of handwriting deficiencies in three domains: (1) legibility (three items); (2) performance time (three items); and (3) physical and emotional well‐being (four items). These 10 items are scored on a five‐point Likert scale, ranging from 1 (‘never’) to 5 (‘always’), with higher scores indicating poorer performance. The Handwriting Proficiency Screening Questionnaire's content validity, internal reliability, and interrater and test–retest reliability have been established among school‐aged children,105 and discriminant validity was exhibited among children with DCD.154, 155 The reliability and validity of a child's self‐report version was recently confirmed.106
The Systematische Opsporing van Schrijfproblemen (SOS)/Beknopte Beoordelingsmethode voor KinderHandschriften156, 157, 158, 159 (Dutch norms, French norms) (Concise Assessment Methods for Children's Handwriting)156 is a tool designed to screen the handwriting quality of elementary‐school students on the basis of a completed piece of cursive writing. The writing task consists of copying a standard text in 5 minutes, or at least five lines if the child is a very slow writer. The text is copied on unruled paper. The test offers 13 criteria to evaluate the quality of the handwriting product. The test also evaluates speed of writing. The interrater agreement between pairs of raters has been reported to vary between r=0.71 and 0.89, with a median of r=0.82. Furthermore, the correlation between the Beknopte Beoordelingsmethode voor KinderHandschriften and the Dysgraphia Scale is reported to be 0.78.159
For the SOS, the most discriminating items were selected from the Beknopte Beoordelingsmethode voor KinderHandschriften, reformulated, and concretized to develop the SOS test.160 Writing speed is measured by counting the number of letters.161 Criterion validity with the Beknopte Beoordelingsmethode voor KinderHandschriften is good (r=0.80–0.88, p=0.01).160, 162 The SOS is now available as s revised version in Dutch (SOS‐2‐NL),163 Flemish (SOS‐2‐FL), and English.164 A German adaptation (Systematische Erfassung motorischer Schreibstörungen) is in preparation.165, 166
The Detailed Assessment of Speed of Handwriting167, 168 assesses speed of handwriting in 9‐year‐old to 16‐year‐olds, with an extension for older students aged 17 to 25 years.169 The Detailed Assessment of Speed of Handwriting includes a sentence‐copying task (with ‘best’ and ‘fast’ conditions), alphabet writing, and a 10‐minute ‘free’ writing task. The test yields a total standard score plus a profile across the different tasks. Interrater reliability is reported as above 0.99 for each of the four main tasks and test–retest reliability as 0.85 for the total score.167 The Detailed Assessment of Speed of Handwriting is sensitive to differences in age and distinguishes clinical and non‐clinical groups.167 It has been used to identify and describe handwriting difficulties in children with DCD.170, 171
Other useful instruments for diagnosing a handwriting disorder include the Minnesota Handwriting Assessment,172 the Diagnosis and Remediation of Handwriting Problems,173 Children's Handwriting Evaluation Scale‐Manuscript,174 Evaluation Tool of Children's Handwriting,175 and Test of Legible Handwriting.176
On the basis of the literature search, the following recommendations can be made.
| Recommendation 11 | GCP |
|---|---|
|
We recommend the use of an appropriate motor test that measures different areas of motor competence, has good reliability and validity, and has population‐based standardization (appropriately norm‐referenced). The test should measure different types of motor skills to describe one's motor competence or difficulties. Comment: Because language is involved, handwriting and keyboarding are areas of motor competence that should be assessed separately with standardized and psychometrically sound measures. |
⇑⇑ |
| Recommendation 12 | LOE |
|---|---|
|
We suggest criterion I be satisfied by using the MABC‐2 or the BOT‐2. Comment: At present there are no biological markers that provide definitive cut‐off points for diagnosing DCD (or any other developmental disorder). Consequently, statistically defined criteria must suffice. In the absence of generally accepted cut‐offs for identifying DCD, and in addition to the other criteria being satisfied, it is recommended that when using the MABC‐2 or other equivalent objective measures, the 16th centile (1SD) for the total score (standard score of ≤7) should be used as a cut‐off. Scores at or below the fifth centile should be considered as unequivocal evidence of DCD, provided the child meets all other criteria. |
LOE 2, level B |
In a comprehensive review, a distinction between clinical diagnostic criteria and research criteria was postulated.177 The international expert group also emphasizes that the purpose for clinicians and researchers may be different. For clinicians, it is important not to miss children in need of adequate support. Limited sensitivity of the present motor test battery and specific deficits relevant for daily activities in certain areas (e.g. balance or dexterity) would mean that a large number of children with moderate DCD would be missed if using the fifth centile. Several studies comparing the sensitivity and specificity of the MABC‐1/MABC‐2 with other measures also used 1SD (16th centile), finding reasonably good agreement between the measures.178, 179, 180, 181, 182 This view is also supported when population‐based data are analysed.7, 17 It is therefore plausible to use a cut‐off level of the 16th centile (1SD) in addition to criteria II and III.
| Recommendation 13 | GCP |
|---|---|
|
If there are clear indications of increased risk for DCD from the history and clinical examination (criteria IV, III, and II), and the results of one standardized motor test are above specified cut‐off criteria, we recommend the use of a second standardized motor test or a second examination by another expert. Comment: All studies confirm that the currently available motor tests have a sensitivity below 90%. That means at least 10% of children with relevant motor problems are missed by one test (e.g. the MABC‐2). If there are clear clinical signs, a second assessment should take place with a different test (e.g. BOT‐2) along with examination. |
⇑⇑ |
|
Research note 1 Further studies of reliability and validity on the clinical reference standard are required. |
4.3. Comorbidities/co‐occurring conditions
There is strong evidence that DCD is combined with several emotional, social, and learning disorders.183 In several children, it cannot always be determined to what extent behavioural problems are coexisting disorders or the consequences of long‐standing negative experiences with clumsiness in everyday life. Kaplan et al.184 questioned the term ‘comorbidity’ as there is large overlap between DCD, learning disorders, and ADHD. They prefer the term ‘atypical brain development’. However, the group of international experts working on these recommendations decided to adopt the concept of co‐ocurring conditions because it seems more appropriate to look for distinct disorders when conducting assessments and when setting and choosing priorities for intervention.
4.3.1. Co‐occurring disorders
ADHD has been reported to be the most frequent co‐occurring disorder with DCD. Several studies – mostly examining clinical samples – suggest a rate of 50% or higher.185 Data from population‐based studies also suggest that about half of those diagnosed with DCD and half of those diagnosed with ADHD have combined problems.16 Kadesjö and Gillberg16 describe the overlap of children with ADHD and those with motor difficulties (Fig. 5). These data suggest that children with motor problems alone (‘DCD’) have a similar prevalence as children with ADHD alone. The overlap seems to be about 50%. This means that ADHD and DCD have to be regarded as similarly frequent.
Figure 5.
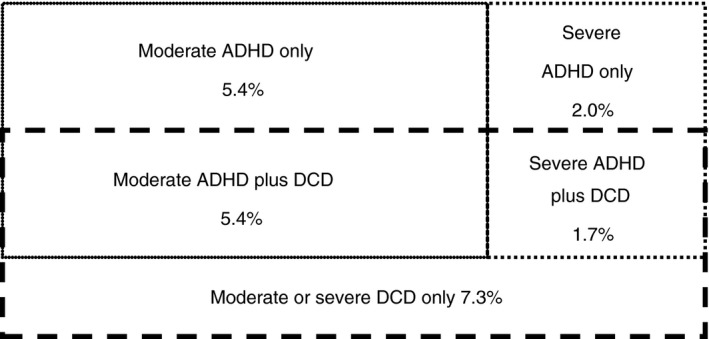
Prevalences in a population of 7‐year‐old children with attention‐deficit/hyperactivity disorder (ADHD) only, developmental coordination disorder (DCD) only, and ADHD/DCD combined.16
In a further paper, Kadesjö and Gillberg17 described and used the features of 7‐year‐old Swedish children diagnosed with DCD to predict their reading comprehension at age 10 years. They found that the features of DCD itself remained stable within an additional 1‐year follow‐up. In a further population‐based study, Kadesjö and Gillberg186 found that 87% of children with ADHD had comorbidities. In fact, Miyahara et al.187 also found that the ADHD and DCD combination seems to be more common in clinical and support groups (55% and 54% respectively) than in school groups (35%).
A further study emphasizes the important clinical role of DCD in context with ADHD. In a 22‐year longitudinal, community‐based follow‐up study, Rasmussen and Gillberg188 found that individuals with co‐occurring ADHD and DCD had a much worse outcome than those with ADHD without DCD. Antisocial personality disorder, alcohol abuse, criminal offending, reading disorders, and low educational level were over‐represented in the ADHD/DCD group (58% vs 13% in the ADHD group without DCD).
The co‐occurence of DCD and specific language impairment has been shown in up to 70% of the children with language problems.25, 189, 190, 191, 192 A recent study showed that DCD is present in about one‐third of children with specific language impairment.193 In this study, children with co‐occurring specific language impairment and DCD differed from children with specific language impairment without DCD; specifically, the specific language impairment and DCD group obtained significantly lower mean overall‐domain, motor‐domain, autonomy‐domain, and cognitive‐domain quality of life scores. Additionally, atypical speech lateralization in adults with DCD has recently been demonstrated.194
An important finding of a large British epidemiological study was that children with severe DCD were more likely to have co‐occurring ophthalmological abnormalities. Increased odds ratios were found for abnormal sensory fusion at near (odds ratio [95% confidence interval]) (1.98 [1.13–3.48]) and distance (2.59 [1.16–5.79]); motor fusion (1.74 [1.07–2.84]); reduced stereoacuity (2.75 [1.78–4.23]); hypermetropia (2.29 [1.1–4.57]) and anisometropia (2.27 [1.13–4.60]).195 Because of the co‐occurrence, common pathways of insufficient ocular accommodation and DCD are suggested.196
Increased ophthalmological problems have to be recognized when children with DCD have reading and writing problems. The presence of DCD and reading and/or writing disorders have been known for a long time.27, 184, 197, 198
A Taiwanese study, however, showed that there were no significant differences between children with DCD and typically developing children in their scores on the Chinese Reading Achievement Test and in their reading composite scores on the Basic Reading and Writing Test. These results contrasted interestingly with those obtained from English‐speaking children: English‐speaking children with DCD showed poorer reading and poorer writing than typically developing English‐speaking children.199 This suggests that there are fairly specific perceptual involvements in individuals with DCD.
More recently, specific coexisting mathematical problems have also been shown in children who struggle with incoordination. Specifically, children with DCD had lower performance in non‐symbolic and symbolic number comparison tasks than typically developing children. When compared with age‐matched individuals, children with DCD performed significantly worse for number fact retrieval and procedural calculation.28, 200
Coexisting learning disorders have been interpreted as an indicator for severity and for perceptual–motor dysfunction.201 This has been confirmed in a recent study,202 in which children with severe motor difficulties demonstrated a higher risk of difficulties in ADL, handwriting, attention, reading, and social cognition than those with moderate motor difficulties, who in turn had a higher risk of difficulties than comparison children in these five out of seven domains.
Kastner and Petermann203 looked for cognitive deficits in children with DCD. Children with DCD scored below average in the Hamburg‐Wechsler‐Intelligenztest für Kinder/Wechsler Intelligence Scale for Children, Fourth Edition (verbal comprehension, perception reasoning, working memory, and processing speed). Their general IQ scores were found to be 1SD below the same scores of the comparison group. Other studies report fewer differences in total IQ.204 Alloway et al.205 also found selective deficits in visuospatial short‐term and working memory in children with DCD. In the same study they found deficits in verbal short‐term and working memory in children with language impairments.
ASD is also reported to be associated with DCD.191, 206, 207 In a population‐based study, a comorbidity of ASD was found in 10 out of 122 children with severe DCD and in nine out of 222 children with moderate DCD.7 The inverse has also been found, with 79% of children diagnosed with an ASD having definite movement impairments consistent with DCD.208 On the other hand, these results show that more than 90% of children with DCD have no ASD. Taking into account these findings, the idea of a common aetiology of DCD, ADHD, learning disorders, and autism should be discussed with great care.
An over‐representation of DCD in preterm and low‐birthweight children (about 2:1) is known and has been emphasized in more recent studies.7, 209, 210, 211
4.3.2. Co‐occurring disorders and genetic aetiology of DCD
A genetic study212 of a large group of twins confirmed consistent co‐occurring disorders of DCD with other conditions in severe cases only (latent classes 5–7; Table 7). One cluster of children exhibited severe reading disorders with fine motor functions and handwriting problems, and one other cluster displayed problems with movement control and gross motor planning. Of interest is that this twin study was able to show that the motor symptoms of DCD were, in most cases, distinct from behavioural features such as conduct disorder and ADHD.
Table 7.
Co‐occurring disorders of DCD with learning and behavioural disorders, cluster analysis in a large twin study212
| Latent classa | Clinical feature | Frequencya | %a |
|---|---|---|---|
| 1 | Unaffected | 1957 | 62 |
| 2 | Moderate inattentive‐impulsive with ODD | 440 | 14 |
| 3 | Severe reading problems with moderate fine motor/handwriting | 267 | 9 |
| 4 | Control during movement with moderate gross motor planning | 201 | 6 |
| 5 | Inattentive‐impulsive with reading problems, ODD, fine motor and general control | 140 | 4 |
| 6 | Inattentive‐impulsive with ODD | 114 | 4 |
| 7 | Moderate to severe for combined ADHD, reading disorder, ODD, and DCD scales with some conduct disorder | 29 | 1 |
| Total | 3148 | 100 |
aFrequencies and percentages for a 7 latent class solution concerning different patterns in symptomatology analysing 1304 families of twins (3148 individuals) from the Australian Twin ADHD Project for developmental coordination disorder (DCD), oppositional defiant disorder (ODD), attention‐deficit–hyperactivity disorder (ADHD), and conduct disorder.212
Meanwhile, the genetic aetiology of DCD seems to be further supported. Findings suggest that there may be shared susceptibility genes for DCD and other neurodevelopmental disorders; this highlights the need for thorough phenotyping when investigating the genetics of neurodevelopmental disorders. Furthermore, these data provide growing evidence supporting a genetic basis for DCD.213
In conclusion, despite numerous co‐occurring disorders in children with DCD, there is some evidence that DCD exists as a distinct disorder. The presence of DCD seems to be a critical feature for outcomes when co‐occurring with neurodevelopmental disorders.
| Recommendation 14 | GCP |
|---|---|
|
Owing to the high degree of co‐occurrence among developmental disorders, we recommend that dual or multiple diagnoses including DCD and any other disorder be given when appropriate. To ensure that this is done properly, appropriate assessments should be undertaken and interpreted according to established clinical guidelines. Comment: To ensure that co‐occurrence is not missed when assessing a person referred for problems in the motor domain, difficulties in other areas of development and educational attainment should be recorded and any necessary further assessment and intervention planned. |
⇑⇑ |
4.4. Psychosocial issues
Accumulating research and evidence from clinical practice shows children with DCD (and those with poor motor coordination in general) seem to be at increased risk of psychosocial problems that impact negatively on participation and longer‐term outcomes.188, 214, 215, 216, 217 Studies emerging in the 1980s highlighted the increased incidence of social ‘immaturity’218 and hypoactivity or hyperactivity and emotional disturbance219 in children defined as ‘clumsy’ or those with minimal brain damage. Longitudinal studies arising from this early work and an increasing number of studies comparing children with DCD with their peers confirm the added risk for psychosocial problems concomitant with DCD and motor coordination difficulties.23, 185, 220
Co‐occurring mental health difficulties have been identified across internalizing problems, especially mood disorders (e.g. depression, anxiety) and externalizing behaviours (ADHD).221, 222 Figure 6 highlights the extensive overlap between emotional and behavioural symptoms and DCD. Research in this area has tended towards use of symptom scales but there is some evidence that children with DCD may be at greater risk for various mental disorders, defined as meeting a clinical threshold for caseness based upon DSM criteria.221, 223 In addition to the often‐reported high level of DCD co‐occurring with ADHD (50%–60%), Pratt and Hill223 also showed that among children with DCD, 30% met clinical criteria for social anxiety. Recruitment from clinical samples may confound estimates of prevalence in view of the higher risk of co‐occurrence in this group. Nevertheless, it would seem that additional psychosocial deficits are common, ranging from 25% to 85% depending on population or clinical sampling respectively.185, 224
Figure 6.
Overlap between emotional and behavioural disorders in children with developmental coordination disorder (DCD).185, 220
As well as symptoms of psychological problems and disorder, numerous studies have identified lower self‐concept and self‐efficacy in children with motor difficulties.23, 37, 225, 226, 227, 228, 229, 230, 231, 232, 233, 234, 235, 236 Lower levels of perceived competence have been associated with reduced participation, particularly in physical activity and social participation.37, 232, 237, 238, 239, 240 More recently, studies have gone on to look at bullying and victimization.241, 242, 243 Of note, Campbell et al.242 showed higher levels of depression and victimization among children suspected of having coordination difficulties. In contrast, a smaller but well‐controlled study did not find an association between victimization and DCD or between DCD and self‐worth, although some group and sex differences were evident.243 Females with movement difficulties may be particularly vulnerable to verbal peer victimization, influencing self‐worth.243 Psychosocial factors, such as self‐concept, efficacy, social support, and negative peer interactions, are important in their own right as they are related to participation and quality of life, but are also important in the context of psychological distress and disorder. Negative self‐esteem, for example, has been shown to predict depression and anxiety in adults and in children.244, 245 Table SXVII and Appendix S5 show several core studies in the field that have influenced our understanding of the associations between DCD, psychosocial factors, and related mental health problems.
The aetiology connecting motor coordination difficulties and DCD to mental health problems is wide ranging and complex. Undoubtedly, there will be transactional influences between multiple factors including the developing brain and positive and negative social and/or interpersonal experiences. Moreover, in view of the multiple areas of the brain involved in the coordination and execution of skilled movement, it is not implausible that areas of the brain involved in emotional and behavioural regulation may be affected by primary factors fundamental to the emergence of DCD. Evidence for this is reflected in the high level of persistent mental health problems in children with brain injury, particularly in other motor disorders such as cerebral palsy.246, 247 At the same time, the connections between DCD/poor motor coordination and the psychosocial situations previously identified suggest an integral role for social, environmental risk, and protective factors in the causal chain. Cairney et al.,221 Mancini et al.,248 and Cairney et al.249 developed ‘the environmental stress hypothesis’ over a series of papers to offer a theoretical framework for mapping the interrelationships among DCD, social‐level and individual‐level factors, and mental health. Drawing on the stress process framework,250 DCD or poor motor coordination is positioned as a primary stressor, which in turn exposes children and young people to an array of secondary stressors arising from interpersonal and intrapersonal conflicts (e.g. bullying; everyday hassles connected to functional limitations; poor concept of self). These stressors have both a direct and indirect effect on symptoms of internalizing problems such as depression and anxiety; indirectly, stress arising from problems with motor coordination can be buffered by factors such as supportive/caring parents and or by perceptions of competence. A particularly novel feature of the model is the inclusion of overweight/obesity and physical (in)activity as pathways connecting DCD to mental health problems through their influence on stress and psychosocial resources. Both insufficient physical activity and unhealthy weight have been shown to be common in children with DCD,251 and related to mental health problems in children and young people.252 Several studies have tested parts of the model,222, 228, 253 with the strongest and most consistent evidence showing self‐concept (e.g. self‐esteem; self‐worth) to mediate the relationship between DCD and mental health problems in children and young people.248 A recent study, in particular, found that low self‐esteem and poor social communication are associated with problems of mental health and well‐being.224 A comprehensive test of the model has yet to be conducted.
In conclusion, these findings provide evidence highlighting a potential multiplicative effect of DCD for children and young people in which impaired motor skills are associated not only with limited academic and physical skill achievements. Notably, significant psychosocial issues have also been linked to reduced participation across a range of physical and social activities that can have a lifelong impact on physical as well as mental health. Further research is required to understand the contributors and pathways of psychosocial problems in DCD, with interventions designed to offset the substantial negative consequences of these issues. At present, only one theoretical model has been offered to try to understand these connections, and testing of the model has been limited. Clearly, there is need to expand both theory and empirical research in this area.
|
Statement 1 (GCP) Research evidence shows that, for many children with DCD, substantial psychosocial difficulties often have an impact on engagement, participation, psychosocial well‐being, and quality of life. Individual and environmental factors will work together, influencing both the expression and management of these associated issues. |
4.5. Flow chart assessment, treatment indication and planning
See Figure 7.
Figure 7.
Flow chart assessment, treatment indication, and planning. DCD, developmental coordination disorder; ADL, activities of daily living; R, recommendation. [Colour figure can be viewed at http://www.wileyonlinelibrary.com]
5. Intervention
5.1. Intervention: general principles
Children with DCD meeting the diagnostic criteria for DCD usually need treatment. Indications for intervention are essentially dependent on criterion II: the influence of the diagnosis on activities of everyday living (self‐care, academic/school productivity, leisure, play, and other daily physical activity). However, in some cases diagnosis does not indicate treatment.
On the other hand, if not all the criteria for the diagnosis of DCD are met but motor problems exist in the performance of ADL tasks, and in educational and social support, then strategies for participation across environmental contexts should be implemented. This may be particularly useful for children below the age of 5 years who show significant motor problems without meeting all the diagnostic criteria of DCD.
The severity of motor impairment affects not only the presentation of DCD but also participation, which has important implications for treatment. In school‐aged children, specific fine motor problems may be more relevant for school achievement than gross motor problems. Gross motor problems, on the other hand, seem to be important for participation in play, sport and leisure, and development of social contact with peers.
| Recommendation 15 | LOE |
|---|---|
| Children with the diagnosis DCD should receive intervention if current indications are present. | LOE 1, level A |
Results of the literature review performed for this recommendations update254 reveal consistent findings that activity‐oriented interventions can have a positive effect on skill performance. Furthermore, studies showed that both activity and some body‐function‐oriented interventions may have positive effects on motor function. Importantly, relatively short interventions also demonstrated positive treatment effects. Twenty‐nine new intervention studies have been published since the 2012 EACD recommendations for intervention for DCD.6, 255 The overall effect size (Cohen's d) across intervention studies was large (1.06), but the range was wide as not all interventions were equally effective across populations and studies.
The review of the literature reaffirms that children with a diagnosis of DCD should receive intervention. If measured with valid and reliable norm‐referenced tests, many interventions have large effect sizes and some moderate. To evaluate treatment effects, measures that capture the levels both of activities and of participation should be used, because they provide a different source of information.
| Recommendation 16 | GCP |
|---|---|
|
When planning a programme of intervention, we recommend that both the strengths and weaknesses of the individual in their environmental context should be taken into account in order to improve motor function, activity, and participation. Comment: The environment in which the individual functions (e.g. family, school, community) should be considered so that the specific programme of intervention is consistent with the individual's goals and opportunities for learning. In some children with DCD, compensatory and environmental support with follow‐up may be sufficient. Information sources for planning a programme of intervention include history, clinical examination, motor test results, and, if possible, parents’ reports, self‐report, teachers’ reports, report of relevant others, and, if available, validated questionnaires. |
⇑⇑ |
| Recommendation 17 | GCP |
|---|---|
|
When planning intervention, evidence of effectiveness including regime and dose should be considered. In case of co‐occurring disorders, we recommend that priorities for intervention are set according to the type and severity of each disorder, and in consultation with the child and the family. The question of which problem has the most severe impact on the individual's functioning, activity, and participation must be addressed. However, if motor difficulties are not considered at one point in time, they may have an impact at a later date. Thus, priority setting should be reviewed over time as they may change. |
⇑⇑ |
| Recommendation 18 | GCP |
|---|---|
|
For intervention planning, individualized goal setting is essential. We recommend setting goals that address the levels both of activities and participation. We recommend taking into account the child's and family's viewpoint and the viewpoint of relevant others for goal setting. |
⇑⇑ |
When setting goals, a clear description of the tasks in the required context is needed. To assess performance issues of everyday living (self‐care, academic/school productivity, leisure, and play), assessment instruments such as the Canadian Occupational Performance Measure256 may be helpful. Although goals at the level of body functions may be defined, the main goals should be set at the level of activities and participation.
There is still not enough evidence for ‘goal setting’ with respect to treatment regime and/or dose, as it is unknown how goal setting or dosage amounts may influence the outcome of DCD intervention.
So far, there have been no reported studies that used a training programme until the children with DCD reached a plateau or no longer improved; as a result, it is unclear whether children with DCD will be able to reach the level of typically developing children. We do know that relatively short interventions (approximately 10 sessions) did demonstrate positive effects.
| Recommendation 19 | GCP |
|---|---|
|
When planning intervention, psychosocial factors that may accompany a child's motor difficulties should be considered. Where appropriate, standardized and validated assessments of these factors should be used, with referral to relevant and additional services made if needed. Comment: Monitoring and surveillance of psychosocial factors should be integral throughout intervention. |
⇑⇑ |
| Recommendation 20 | GCP |
|---|---|
|
We recommend that self‐concept be assessed and accommodated in treatment (planning). Self‐concept may be assessed through child‐reported rating scales. |
⇑⇑ |
There are very few assessment tools adapted for different cultures/languages. An example would be the Strengths and Difficulties Questionnaire.257 There are several self‐report tools, for example the Perceived Efficacy and Goal Setting System.109, 111 For the assessment of more specific self‐concepts of motor‐based tasks and physical activity, the Children's Self‐Perceptions of Adequacy in and Predilection for Physical Activity89, 91 questionnaire can be used. A further option would be an adequate exploration of self‐concept by interview.
| Recommendation 21 | GCP |
|---|---|
|
We recommend intervention priorities be established by considering both motor and non‐motor aspects of the individual's functioning. Comment: Individual dispositions and psychosocial factors (e.g. motivation, presence of psychiatric disorders) may limit the effectiveness of treatment. Compensatory techniques (e.g. equipment, environmental adjustments) and social support may enhance the treatment effects. |
⇑⇑ |
For tools to be used for intervention planning, we refer to ‘Diagnosis and assessment’, where the tools and their psychometric properties, if known, are described.
5.2. Therapeutic approaches
For this update the literature was searched for all interventions published since January 2012 addressing children with DCD. What follows is an update on previous recommendations; it is based on additonal findings from the newer studies.
The new literature affirmed the recommendations made in 2012:6 that is, the more recent literature either added no new information that suggested major changes or provided further support for the recommendations made in 2012. However, the new literature also supported some additional recommendations with respect to training in basic motor skills and the use of virtual reality. The recommendations reported below reflect the newer evidence in conjunction with the previous findings.
5.2.1. Labels for classification of interventions
Regarding the different interventions studied for effectiveness, various labels were found in the literature. Interventions to improve motor performance in DCD have many components and vary in type, intensity, duration, and frequency. Moreover, owing to length restrictions in most journals, descriptions of any intervention are often very limited. The Consolidated Standards of Reporting Trials258 states that precise information should be given about the intervention, but several studies in our review failed to do so and thus the interventions discussed were hard to classify. To mitigate against this problem, we strongly encourage researchers to use a reporting framework such as the Template for Intervention Description and Replication checklist.259 This would support future replication studies and interstudy comparison, enabling reviewers to unpack often heterogeneous results. Moreover clinicians would then know the important ingredients of the intervention, which would make translation into practice more feasible.
Traditionally, approaches to intervention for DCD71, 255 have been grouped into two broad areas: those that use activity to target the underlying performance problems, often referred to as process‐oriented approaches, and those that address the performance issue itself, often referred to as task‐oriented approaches.260 In the earlier stages of DCD research, intervention approaches were almost exclusively process‐oriented, or, in ICF terms, focused on reducing impairment and improving body function and structure.255 Studies examining these approaches tended to report on changes at the level of body function and structure only. Since the last review, several such studies have also reported on changes at the level of activity, and occasionally on participation. A similar trend was noted in the studies of task‐oriented approaches. While in the previous review such studies reported almost exclusively on changes at the ICF level of activity and or participation, several such studies in this review also started to report on effects at the level of body function and structure, namely impairment reduction.
To reflect this shift in the literature, we use a new taxonomy for interventions based on ICF terminology1 in this recommendations update. Specifically, we describe interventions based on the level of the ICF that is the primary target of the intervention. Interventions are therefore grouped into three categories: (1) body function and structure‐oriented, where the activity is designed to improve targeted body functions considered to underlie the reported functional motor problem; (2) activity‐oriented, where the activity is designed to improve performance of that activity; and (3) participation‐oriented, where the activity is designed to improve participation in that activity in an everyday life situation. In interventions identified as activity‐oriented or participation‐oriented, the primary interest is to improve performance of particular activities or participation and the content of the intervention involves direct training of the performance skill concerned.
5.2.2. Activity‐oriented or participation‐oriented interventions
In the 2013 review by Smits‐Engelsman et al., it was observed that task‐oriented approaches (now known as activity‐oriented or participation‐oriented approaches) were more efficient than process‐oriented approaches (now known as body‐function‐oriented approaches), yielding better functional performance outcomes in less time for children with DCD.255 In our new nomenclature, task‐oriented interventions are activity‐oriented but also clearly facilitate participation.
Mandatory characteristics of up‐to‐date task‐oriented or activity‐oriented interventions are as follows: (1) client‐oriented (meaningful for the client); (2) goal‐oriented (aiming at activities and participation as described in the Children and Youth Version of the ICF); (3) task and context specific (what is to be learned, and for which circumstances); (4) those that consider the client to take an active role; (5) aiming at functionality, not at normality; (6) active involvement of parent(s)/caretakers to enable transfer.
Since we published the first version of the CPR–DCD, new reviews and meta‐analyses have been published. The review by Lucas et al.261 addressed nine randomized controlled trials evaluating interventions that aim to improve gross motor performance in children with neurodevelopmental disorders (cerebral palsy and DCD). They found that some interventions with a task‐oriented framework improved gross motor outcomes; however, high quality intervention trials are urgently needed.
On the basis of a systematic review and meta‐analysis of 29 articles on physical therapy, Offor et al.262 concluded that task‐oriented approaches (Neuromotor Task Training [NTT]) and motor training programmes from traditional (e.g. strength or balance exercises) and contemporary or novel physical therapy frameworks (e.g. active virtual gaming, hippotherapy, and rebound therapy) are beneficial for children with DCD. Interventions based on physical therapy motor skills training and NTT are effective for gross motor problems. NTT is also effective for fine motor problems.262
Finally, a meta‐analysis of nine randomized controlled trials in DCD263 showed large effect sizes for NTT, task‐oriented motor training, and motor imagery plus task practice training.
Together with the new data analysis, this gives enough evidence for a level A recommendation for activity‐oriented or participation‐oriented interventions, such as task‐specific training, NTT, and, on the basis of our earlier review,255 for the Cognitive Orientation to daily Occupational Performance approach (CO‐OP).
| Recommendation 22 | LOE |
|---|---|
|
If an intervention is to be provided then we recommend that activity‐oriented and participation‐oriented approaches be used as a means to improve general, fundamental, and specific motor skills in individuals with DCD. Comment: Activity‐oriented or participation‐oriented approaches are interventions that focus on ADL (including personal care, play, leisure/sports, arts and crafts, and academic, prevocational, and vocational tasks) within the intervention process. Intervention must also aim to generalize to daily function, activity, and participation across environmental contexts in which the child needs to perform. Activity‐oriented or participation‐oriented approaches should involve family, teacher, significant others, and/or environmental support to cascade and promote essential opportunities for practice and generalization. This is necessary to give enough opportunity for motor learning and consolidation of skills. Formally investigated activity‐oriented or participation‐oriented approaches, based on this and the previous review, include but are not limited to task‐specific training, NTT, and cognitive orientation to daily occupational performance approach (CO‐OP). |
LOE 1, level A |
5.2.3. Handwriting
For individuals, especially children, with DCD, handwriting is a particular problem; indeed many children are referred to the therapies specifically for handwriting issues.
Since the first version of the CPR–DCD, no papers on children with DCD receiving intervention focused on handwriting have been published that would change the recommendation. Only one case study264 reported new data in training of handwriting skills, but only in three children. After very intensive training (2×45min per week plus daily home work for 13wk), two of the three children improved.
Although the use of modern technology with smartphones, tablets, and laptops has increased, and to some extent (depending on the cultural background and social environment) children are less dependent on their penmanship, participation problems caused by handwriting issues are still an issue for children with DCD. Keyboarding and swiping are also fine‐motor skills that may prove to pose problems in this group of children and who may need to be trained. To prevent children with DCD suffering the consequences of bad handwriting (and being labelled as underachievers or poor academic performers), parallel training of keyboarding and tablet skills is suggested.
| Recommendation 23 | LOE |
|---|---|
| If handwriting problems are present in children with DCD, we suggest activity‐oriented and participation‐oriented intervention (including ways to self‐evaluate performance) to improve the quality of the handwriting. | LOE 2, level B |
| Recommendation 24 | GCP |
|---|---|
| If handwriting problems are present in children with DCD, in addition to activity‐oriented and participation‐oriented handwriting intervention, we suggest teaching keyboarding from early on, to improve the legibility and neatness of schoolwork. | ⇑⇑ |
5.2.4. Interventions: body‐function‐oriented approaches
Children with DCD have a great number of symptoms connected with impaired body functions. Earlier developed treatment approaches focused on improving these body functions on the basis of the hypothesis that better body functions would lead to improvement of activities. Our latest review showed that some of these approaches may be effective. Given the unclear mechanisms about transfer, we recommend using activity‐oriented approaches that stay close to the selected activities to be improved and/or body‐function‐oriented approaches that apply/train the gained function also within the activities to be improved.
| Statement 2 (GCP) |
| Some interventions that aim to improve body functions and structures may be effective, but there is limited evidence whether body‐function‐oriented interventions are effective in improving activity and participation in children with DCD. |
Since our first version of the CPR–DCD, no new intervention studies comparing sensory integration therapy or kinesthetic sensitivity training with another intervention have been published, so the negative recommendation has not been altered.
| Statement 3 (GCP) |
| The following interventions cannot be recommended as empirical support, because their effectiveness is inconclusive, absent, or negative: (1) the evidence is inconclusive for the effectiveness of sensory integration therapy as an intervention for children with DCD; (2) the evidence is inconclusive for the effectiveness of kinesthetic sensitivity training for children with DCD. |
| Other approaches used in children with DCD (e.g. brain‐gym, complementary and alternative therapies) have not been systematically evaluated so cannot be recommended. |
5.2.5. Interventions: adjuncts to activity‐oriented and participation‐oriented approaches
The use of active video games has steadily grown as a popular form of entertainment and play, and is finding a place in movement rehabilitation across a range of conditions.265 As a result, studies on active video games‐based training has also increased as intervention for children with DCD. Although studies show promising effects, the issue of transfer to everyday performance and participation is an unanswered question. It has recently been shown that use of active video games leads to moderate to large improvement on balance tasks266, 267, 268, 269, 270, 271 in DCD, and that children also improved on more functional tasks such as standing up from a chair and going up and down stairs.267, 269 However, no studies are available on transfer effects to more complex tasks in everyday contexts.
| Recommendation 25 | LOE |
|---|---|
| Active video games may be recommended as a useful adjunct to more traditional activity‐oriented and participation‐oriented interventions in children with DCD, in supervised settings or group intervention. | LOE 2, level B |
Fitness outcomes are particularly relevant to DCD given that childhood overweight and obesity are over‐represented in this population of children. Overall, interventions led to moderate to strong improvements on fitness metrics, and associated changes in movement skill.268, 269, 272, 273, 274, 275, 276 Given the low levels of strength and condition in children with DCD, interventions to improve general fitness (cardiorespiratory fitness and functional strength) and empowerment and engagement in sports activities in daily life should be considered. This is because such interventions have been reported as being effective in children with DCD.269 Long‐term follow‐up is still missing.
| Recommendation 26 | GCP |
|---|---|
| We recommend that physical fitness (e.g. strength, endurance, flexibility) is considered as part of intervention planning. | ⇑⇑ |
5.2.6. Interventions: new perspectives
Motor imagery training.
Motor imagery training is a new cognitive approach developed by Wilson.80 It uses internal modelling of movements, which facilitates the child to predict consequences for actions in the absence of overt movement. In time and with practice, children use the knowledge of the relation between vision and internal feeling of the movement to make appropriate predictions about the consequences of self‐produced movements; this reduces the errors in feedforward planning. As a strategy for learning feedforward planning, it seems to work for some children. Three studies were found that investigated motor imagery training (two randomized controlled trials277, 278 and one multiple case study).279 These studies have shown positive effects of motor imagery training on measures of movement skill in children with DCD.
5.3. Interventions delivery mode: (group) settings
5.3.1. Personal factors
There have been some prominent changes in the type of training since 2011, with group‐based intervention adding to the treatment landscape. Overall, group‐based intervention produced large effects on motor performance. Recommendations on the ideal group size cannot be determined on the basis of the data, but we know that groups of between four and six children have been used, are manageable, and effective with one therapist and, if needed, an assistant.268, 273, 280, 281 Indeed, small groups enable instructors to move easily between participants and to monitor both group dynamics and individual progress. It is possible that children with very poor motor skill may feel more anxious in a larger group, as was found in one study.280 These same children did, however, improve their ability to deal with peer problems, which is a very useful life skill. As such, group settings should be considered but used carefully depending on age, severity of the disorder, the members of the group, and the goals of the intervention.
| Recommendation 27 | GCP |
|---|---|
| We recommend considering small group intervention because it can be effective. | ⇑⇑ |
| Recommendation 28 | GCP |
|---|---|
|
We suggest considering carefully if and when a group setting is appropriate for a child. Comment: We suggest considering the level of anxiety and movement skills of the children when composing groups (and their size) for group‐based intervention. The optimum staff to child ratio has yet to be ascertained. |
⇑⇑ |
5.4. Interventions: intensity and scheduling
At present, there remains a large gap in our knowledge about choice of, and/or how to optimize, therapies for individuals with DCD: in terms of dosage, timing, scheduling, and content. Training protocols used in clinical practice and research papers show much variability in scheduling and dose. While the average duration in our review was relatively short, at around 9 weeks, the length of training varied between 4 weeks and 18 weeks. The studies that were in the longer range (10–16wk) and occurred more frequently (two to three per week) were group‐based programmes targeting specific sports skills or general fitness. In particular, training fine motor skills tends to be more time intensive than gross motor training. Studies comparing different treatment approaches and different modes of delivery are needed in this area.
| Statement 4 (GCP) |
| Current information on the effectiveness of intervention does not allow clear recommendations on intensity, duration, and timing because comparison studies are lacking. Mean duration of new effective studies was 10 weeks (range 2–18wks). |
| Overall, long training protocols (20–30h) do not seem to be more effective than shorter ones (10–15h) when measured using standardized tests assessing body function/activity. However, these tests may not capture the transfer of skills to complex situations and the level of automaticity needed in everyday life. |
|
Research note 2 Additional comparison studies on types of intervention, intensity, and duration are required. Group versus individual approaches must also be evaluated. |
5.5. The role of environmental factors
Different treatment approaches can be seen as different strategies to support learning.282 Each treatment approach focuses on a special aspect in the learning process and requires special competencies from the child (e.g. verbal and cognitive skills in the cognitive orientation to daily occupational performance approach, self‐reflection used in NTT, or the concept of pretence in motor imagery). These prerequisites are dependent on age, experience, developmental stage, and personality of the child. Each child with DCD has individual difficulties and abilities, and preferred individual learning strategies and solutions.283 Therapists should try to find the right strategies and to adapt the circumstances to optimize the learning processes.
Regular exercise is essential for motor learning and skill acquisition, and exercise in various environments for transfer to the context of daily living. Support from parents, teachers, and other significant people in the child's environment is important for treatment success. Parents and teachers need to understand the child's problems and difficulties in motor learning and skill acquisition. They have to know how to support the child's learning process and exercise efforts, adapt the learning process and the environment, and advise in structuring the daily life activities. Whether this support can be given depends on the family structure and situation. There might be families who are not able to give the needed support.
| Recommendation 29 | GCP |
|---|---|
|
We recommend that individuals with DCD are given ample opportunity to practise movement skills in order to learn them and to participate in daily activities (e.g. at home, school, in community and leisure settings, and in sports). Comment: Once they have learned the basic skills through targeted intervention (which provided them with appropriate feedback and strategies), individuals with DCD should also be given additional opportunities and time to practise these required skills in context, to develop an adequate level of competency. This is particularly true of skills that are complex in nature or that require high levels of planning. We recommend professionals support parents, teachers, significant others, and other stakeholders to encourage the children to participate in relevant activities at home and school, and in the community (e.g. games that require diverse movement activities, extracurricular sports, cultural events, etc.) to promote their practice and newly acquired motor skills. |
⇑⇑ |
| Recommendation 30 | GCP |
|---|---|
| We suggest that involved professionals give parents and relevant others (teachers, etc.) advice on the specific abilities and the problems of the child with DCD and how to help them improve their motor functions and participation in daily activities (at home, school, leisure, sport, and cultural activities). | ⇑⇑ |
Only one study reported a health promotion intervention at school level with some positive results.275 Camden et al.284 investigated the impact of an evidence‐based online module on perceived knowledge and skills of parents of children with DCD, and its behavioural/health outcomes. Children start to compare their abilities with peers at the age of 5 years. This happens especially in sports, and in group and playground games. The experience of failing in these activities has an effect on the children's self‐esteem and self‐efficacy. The support of school and parents is important for motivation and to prevent avoidance of the activities.
Because children spend most of their time awake in school during weekdays, adapting the school environment to focus on motor skill literacy might be beneficial for individuals with DCD. There is no doubt that physical inactivity and the lack of opportunities to develop fundamental motor skills are modifiable risk factors in children. School‐based multi‐component programmes and community‐wide physical activity programmes are likely to be more effective than single‐component intervention.285 Accordingly, efforts aimed at enabling and encouraging young children to be more physically active should include multiple activities targeting fundamental movement skills because this may have lifelong impacts on motor development. However, evidence for the efficiency of school adaptation in children with DCD on motor skill development is not available so far. Lastly, because of the large differences in school systems around the world, generalizability of the evidence will be low. As for the preceding version of these recommendations, adaptations are recommended to apply these specific aspects to differing cultural contexts.286
|
Research note 3 There is a lack of studies reporting outcomes on motor skills after systematic intervention conducted at the school or parent level. There is only extrapolated evidence to show that it may work at the school level. |
5.6. Somatic interventions: drugs, additives
In the second review for these recommendations, no new evidence was found that supplements of fatty acids plus vitamin E have an effect on motor functions.
| Recommendation 31 | LOE |
|---|---|
| We do not suggest fatty acids + vitamin E to improve motor functions as there is no evidence for an effect on motor functions. | LOE 2, level B negative |
5.6.1. Methylphenidate
Medication is often used in children with co‐occurring disorders (e.g. ADHD). This is based on the knowledge that methylphenidate reduces difficulties with attention. There are indications that methylphenidate has a positive effect on behavioural ADHD symptoms, quality of life, and motor symptoms (handwriting). Additional motor therapy will still be needed in about 50% of the children with ADHD/DCD receiving methylphenidate, within interdisciplinary treatment settings and involving educational and psychosocial assistance.116 There are indications that the use of methylphenidate may be favourable for children with combined ADHD and DCD with specific problems in fine motor skills and in handwriting. Accuracy may improve, but writing could become less fluent.287 Methylphenidate should not be considered as the only therapy for children with both DCD and ADHD. These children need additional treatment and support to overcome specific functional problems for handwriting and drawing. Recent findings288 suggest that methylphenidate gives immediate effect on both attention and motor coordination in children with coexisting DCD and ADHD. Further studies should measure the medium‐term and long‐term effect of methylphenidate on a larger group of children with DCD and ADHD, perhaps comparing with groups having DCD without ADHD.
|
Statement 5 (GCP) Where there is co‐occurring DCD and ADHD, it is known that methylphenidate in combination with further intervention is helpful in overcoming functional problems. Methylphenidate has been shown to improve some aspects of apparent motor function. The effectiveness of other medications and/or supplements has not been systematically evaluated. |
5.7. Monitoring
DCD presents a risk factor for concomitant problems in psychosocial and behavioural function. Deficits in executive function and planning have been reported in children with DCD. Recent data suggest that these deficits remain evident in early adulthood, and are strongly linked to impaired planning and disorganization in daily life.289, 290
| Recommendation 32 | GCP |
|---|---|
| We recommend that ongoing behavioural observation be performed during the period of intervention to provide information about the necessity of adjustments to a treatment plan and/or to facilitate the adaptation of an individual's intervention goals. | ⇑⇑ |
For tools to be used for intervention and/or the evaluation of treatment, we refer to ‘Diagnosis and assessment’, where the tools and their psychometric properties, if known, are described.
| Recommendation 33 | GCP |
|---|---|
|
We recommend that formal standardized outcome measures are used for assessment, and are repeated at the end of intervention or at least every 3 months if intervention is longer, to evaluate the effects of an intervention programme and goal attainment and to determine whether further intervention is required. We recommend to evaluate intervention effects using psychometrically sound outcome assessment tools that capture the levels of both activities and participation. We also recommend other evaluation sources including clinical examination, the child's self‐report, family report, teacher/kindergarten reports, questionnaire information, activity monitoring, etc. |
⇑⇑ |
5.8. Cost‐effectiveness
No studies were found comparing different treatment approaches in relation to cost‐effectiveness. Studies about the long‐term effect of the treatment approaches in relation to cost‐effectiveness are needed. Also, no studies were found about the cost‐effectiveness of medication in children with DCD and ADHD. The strong effect for group‐based training suggests that intervention in small groups may be a good option where the cost of treatment is an issue. Studies confirming cost‐effectiveness of individual and group treatments are needed.
5.9. Flow chart treatment planning, intervention, and evaluation
See Figure 8.
Figure 8.
Flow chart treatment planning, intervention, and evaluation. DCD, developmental coordination disorder; R, recommendation; S, statement.
B Transition From Child to Adult
It must be recognized that the nature and impact of DCD will change over the life course of an individual. Significant changes in the environment and the personal task demands on an individual may also alter the experience and impact of the condition. For example, transitions from school to higher education and from education to employment will bring new challenges such as increased demands on the person alongside reduced scaffolding and levels of support.
These current CPRs include a separate section for adolescents and adults with DCD. Much of the material is very similar to the CPRs for children, with minor adaptations. Some new material has also been added.
It must be recognized that the age an individual transitions from childhood to adolescence and from adolescence to adulthood may vary from country to country. Services for these different groups may also vary considerably. For instance, in some areas, services for children extend to those up to the age of 18 years, and in other areas it may just be to 14 years or 16 years. Some areas will have specialist services for adolescents: for example, in the age range 10 to 19 years (or in a more restricted age range, covering just the teenage years). Some services might be targeted specifically at young adults (19–25y), although many adult services are available to those from 18 years and onwards.
It is intended that the child and adult CPRs presented here should be used in a flexible manner to suit the available services and resources and the circumstances of the individual with DCD. For example, if a 15‐year‐old is involved in employment rather than education, then it will be appropriate to consider aspects of the adult CPRs.
Further research on these transition periods and on the experience and impact of DCD beyond early childhood is needed to help gain a better understanding of how best to support individuals to achieve their full potential.
C Adolescents and Adults
This section refers specifically to terminology, diagnostic criteria, assessment, and intervention for adolescents and adults with DCD.
There is enough evidence that many children do not grow out of their problems when they reach adolescence and adulthood.23, 291, 292, 293 Nevertheless, the kind of problems they experience in daily life, education, work, and leisure will change according to their circumstances. For example, there will be different challenges for those studying in higher education compared with individuals in a range of employment settings.
The impact of the condition will also vary depending on an individual's personal resources (resilience, self‐confidence) and the nature and extent of social support networks.
1. Terminology, diagnosis, and assessment
Currently there are no explicit diagnostic criteria for adults. However, in DSM‐5, adults are now mentioned. This implies that we can use the same criteria as for children, with small adaptations of the daily activities and examples.
| Recommendation 34 | GCP |
|---|---|
|
We recommend the following criteria for the diagnosis of DCD. These criteria follow closely those proposed in DSM‐5 with some minor changes, including the order of criteria III and IV. IThe acquisition and execution of coordinated motor skills is substantially below that expected given the individual's chronological age and sufficient opportunities to acquire age‐appropriate motor skills. IIThe motor skills deficit described in criterion I significantly and persistently interferes with the activities of everyday living appropriate to chronological age (e.g. self‐care, self‐maintenance, and mobility) and affects academic productivity, prevocational and vocational activities, leisure, and work. IIIThe motor skills deficits are not better accounted for by any other medical, neurodevelopmental, psychological, social condition, or cultural background. IVOnset of symptoms in childhood (although not always identified until adolescence or adulthood). Comment: Criterion I: the symptoms of DCD may include slowness and/or inaccuracy of performance of motor skills in isolation or in combination. Criterion III: this criterion addresses issues of aetiology with regard to DCD and is designed to facilitate differential diagnosis. Examples of conditions that may rule out or influence the diagnosis of DCD are: (1) Medical conditions: movement disorders with known aetiologies (e.g. cerebral palsy, muscular dystrophy, childhood arthritis), side effects of drugs (e.g. neuroleptics, chemotherapy, sedatives), sensory problems (e.g. substantial visual impairments or impairments of the vestibular organ); (2) Other neurodevelopmental disorders (e.g. severe intellectual disabilities) or other psychological disorders (e.g. anxiety, depression), or other psychological conditions (e.g. attentional problems) as primary causes of motor problems; (3) Social conditions (e.g. deprivation, cultural constraints); (4) Acquired motor difficulties (e.g. trauma or Parkinson disease, Huntingdon chorea, multiple sclerosis, stroke, brain tumours, arthropathies). Note: it may be difficult to differentiate between conditions that may be causal and those that may co‐occur. For example, an adult with ADHD might appear to have movement problems, which are in fact caused by impulsivity and/or inattention. Especially in unclear cases, multi‐professional assessments can be helpful in differentiating motor from attentional problems. Criterion IV: the onset of symptoms is usually evidenced in infancy and childhood. The following recommendations are designed to offer guidance for arriving at an accurate diagnosis of DCD. Instead of being listed according to the criteria I to IV, they are given in the opposite order, which is in line with how a medical professional would usually proceed with the diagnostic process. Thus, the process starts with: (1) considering the age and context of the individual (criterion IV); (2) ruling out other medical conditions causing motor problems (criterion III); (3) taking into account the impact on activities and participation (criterion II); (4) quantifying the motor impairment (criterion I). It should be noted, however, that there are other pathways to diagnosis. For example, an adult may be identified as having difficulties in employment or education and be first assessed by an occupational therapist, psychologist, or an educational psychologist. |
⇑⇑ |
Although there is less research evidence available beyond childhood, much of the information and the recommendations for children with DCD outlined in the previous sections are likely also to apply to adolescents and adults with DCD and are therefore relevant here. For example, the sections on history taking, clinical examination, and the use of motor tests are all relevant. The previous section on co‐occurring conditions is also relevant to adolescents and adults.
It should be noted that currently there are few appropriate standardized tests available for adults that provide objective information on the mentioned criteria.
| Recommendation 35 | LOE |
|---|---|
|
It is noted that some motor performance tests are currently being used to help identify and describe DCD in adolescence and adulthood. These include the BOT‐2 and the MABC‐2. These have been recommended for use with children with DCD but further work is needed to establish their use with adults. The ADC has been most widely used in research with adults with DCD, and other, shorter screening tools are available (e.g. Adolescents and Adults oordination Questionnaire, Functional Difficulties Questionnaire). Further work is needed with these instruments to establish their psychometric properties. Research note 4 There is a lack of standardized assessments for adults with DCD at present. Comment: History, examination, and criterion‐referenced assessments are required to gain a complete picture. Differentiating motor difficulties that are acquired in adulthood need to be checked where possible before a diagnosis of DCD can be made. |
LOE 2, level B |
A small number of studies have tracked development from childhood to adolescence and suggest persistence of motor difficulties in 50–70% of cases (quoted in DSM‐5). However, compared with the large body of literature available on DCD in childhood, the study of DCD in adolesence and adulthood is relatively sparce. Given the paucity of evidence to provide direction for practice, a scoping review was conducted294 to help bridge the knowledge‐to‐practice gap.295 This review had three objectives: (1) to map areas of research related to adolescents and adults with DCD (and areas that are lacking); (2) to map the assessments used for measuring motor skills in this body of literature; and (3) to map intervention programmes developed for this population. Relevant studies were identified by using selected keywords to conduct a scientific literature search of studies published in English, in three databases: PubMed, PsycNET, and CINAHL. Google Scholar was also searched and the ancestry method used. All peer‐reviewed publications up to the end of December 2016 were included.
As this is a relatively new area of study, which has lacked formal guidelines and appropriate assessment tools, a broad approach was taken to ensure that all studies that might be relevant to the description of DCD in adolescence and adulthood were captured. All studies researching individuals who were described as having a motor difficulty that seemed to meet the DSM‐5 criteria for DCD were included. Exclusion of studies was based on the age of participants (<16y and >65y), an indication of other known motor disorders (e.g. stroke, Huntington disease, Parkinson disease), or specific studies of populations with other factors known to be linked with motor problems (such as low birthweight, and physical, cognitive, or visual deficits). A total of 33 manuscripts were identified. The studies ranged from single case examinations to group studies using qualitative interviews, questionnaires, psychometric testing, and experimental manipulations. Findings from the scoping review were organized using the ICF framework, under the headings of ‘Body functions and structures’, ‘Activity’, and ‘Participation’.
Reported impairments in ‘Body functions and structures’ included perceptual–motor difficulties in balance, agility, and hand skills. Experimental studies have provided detailed descriptions of various tasks and highlight differences compared with typically developing individuals in aspects of walking, obstacle avoidance, and reach and grasp. In terms of control mechanisms there have been suggestions of poor motor imagery, and poorer use of visual information in fast responses and visual tracking which is slow and variable. These findings suggest a persistence of the motor difficulties and underlying constraints identified in studies of children with DCD (see ‘Underlying mechanisms in DCD’). A range of non‐motor impairments were also evident from several studies. Problems with executive function, attention, and anxiety were reported, as well as symptoms of depression and low global self‐esteem. These reflect the co‐occurring difficulties previously reported in the childhood literature and those resulting as a secondary impact of the condition. Clinicians should be aware of their common occurrence in DCD, and the need for comprehensive assessment and intervention that take into account the range of motor and non‐motor difficulties experienced by an individual. Another reflection of the childhood literature is reports of poorer physical health in adults with DCD compared with typically developing adults. This includes higher rates of obesity, and lower endurance, flexibility, and strength, as well as poorer general health (both mental and physical). Consideration of ways to maintain a physically active lifestyle through adulthood is thus an important element of intervention in adults with DCD. Finally, studies on the mechanisms possibly underlying impairments are beginning to emerge, with a focus on genetic contributions and brain imaging.
Limitations in specific types of ‘Activity’ such as handwriting, ball skills, driving, and playing sports were reported; some of these seem to have continued from childhood whereas others (such as driving) arise as new challenges presented in adulthood. More generally, there have been consistent reports of difficulties with organization in everyday activities such as managing money, planning ahead, and finding and arranging belongings.296 With increasing demands on such activities in emerging adulthood, difficulties in this area can have a severe impact on the ability to cope with independent living and seem to be a particular area of concern for the individuals themselves.
A major focus of much of the work in this field has been on restrictions in levels of ‘Participation’ and the impact this has on the everyday lives of adolescents and adults with DCD.296, 297 There are clear examples in the literature of negative impacts on independent living, employment, work performance, leisure and physical activities, and quality of life satisfaction.
It may be concluded that there is an emerging but limited literature on DCD in adolescents and adults. The findings suggest that a similar range of motor difficulties seen in childhood extend into adolescence and adulthood. As reported in children, there are also associated physical, cognitive, and psychosocial difficulties which continue to affect everyday life activity. All but one of the identified studies described a single ‘snapshot’ of the condition (one study included a 3–4y follow‐up). We lack, therefore, a good understanding of how the condition continues to develop through adolescence and adulthood. There is an urgent need for further research with adults with DCD of all ages. This will help guide the practice of educational and health professionals, enable employers and families to understand the needs of adults with DCD, and provide guidance to the individuals themselves.
1.1. Motor assessment in the literature on adolescents and adults with DCD
One of the major limitations of the body of literature reviewed is the lack of consistency across studies and the sometimes limited assessment of motor difficulties. In some studies, evidence that there are significant motor difficulties is rather weak. However, as the investigation of adults with DCD is a relatively new area, we have taken a broad approach to the inclusion of studies in this review. Some studies rely only on self‐report of current motor difficulties or a childhood diagnosis of DCD (or ‘dyspraxia’). Other studies reviewed have used self‐report screening questionnaires: the ADC, the Adolescents and Adults Coordination Questionnaire, or adolescent/adult versions of the DCDQ (this last questionnaire is widely used with children) (see ‘Evidence‐based analysis of DCD questionnaires’). The ADC is designed to consider the range of motor, organization, attention, and social difficulties commonly associated with DCD in adulthood, while the Adolescents and Adults Coordination Questionnaire is a shorter instrument with items relating to motor difficulties, physical activity, and general organization. Some good psychometric properties have been reported on both the ADC and Adolescents and Adults Coordination Questionnaire. The ADC is longer and in particular shows good discriminant validity and provides a useful description of the range of difficulties experienced. However, further study of aspects of validity is needed for both instruments.
Other studies on adults report the use of motor performance tests that have been extensively used in clinical and research contexts with children with DCD (e.g. the BOT‐2, MABC‐2, and McCarron Assessment of Neuromuscular Dysfunction). However these do have limitations when applied to an older age group. While the BOT‐2 has norms up to 21 years, the original norms are from the USA and the only other translation and normative data set is available for Germany. The MABC‐2 has norms and translations for several different countries but these extend only to 16 years of age. Norms for the McCarron Assessment of Neuromuscular Dysfunction include young adults (18–35y) but are now over 20 years old. While both the BOT‐2 and MABC‐2 test have been used with adults over the intended ages, the lack of appropriate norms means that interpretation of results is difficult. Until more appropriate assessment tools become available for adolescents and adults, UK guidelines suggest cautious use of the BOT‐2, MABC‐2, and other tests of specific aspects of performance such as handwriting,286 although results must be interpreted with caution when used beyond the age of norms provided.
2. Intervention
If adults with a diagnosis of DCD experience problems in daily life, they should receive intervention.
|
Research note 5 There is a lack of research on interventions in adolescents and adults with DCD. |
It is therefore not possible to make formal recommendations about the most effective approaches. However, there is evidence from children that task‐oriented (activity‐oriented or participation‐oriented) approaches are most effective in improving specific daily life skills.
|
Research note 6 Longitudinal studies are needed to obtain more information about the developmental course of DCD in adolescence and adulthood. Age‐appropriate standardized assessments are needed for diagnosis and evaluation of intervention of DCD in adolescence and adulthood. |
|
Statement 6 (GCP) It is acknowledged that access to services for adolescents and adults varies both within and between countries and is often very limited. However, it is recognized that most young people and adults with DCD benefit from individualized support to: (1) learn specific motor skills for ADL, education, or vocational activities (e.g. using tools, keyboarding, driving); (2) deal with associated problems (e.g. psychological/psychiatric disorders); (3) address the impact of DCD on psychosocial skills and participation in various activities; (4) minimize the risk of longer‐ term problems (e.g. weight gain, physical inactivity). |
No specific work on intervention was identified in the scoping review, although some individual coping strategies were identified from some studies. These included keyboarding instead of handwriting, using assistive applications (e.g. mobile phones and alarms), and focusing on individual strengths. This reflects the environmental and personal ‘contextual factors’ highlighted in the ICF framework, which can have a huge influence on the impact of the condition. Manipulation of environmental factors (both physical and social) could be a focus for intervention strategies, as well as building on personal factors, particularly those related to strengths and resilience. It is clear that further work is needed in this area to gain an understanding of the effectiveness of intervention approaches. In the meantime, it is recommended that, as for children, intervention is based on the needs and goals of the individual, and that there should be consideration of both motor and non‐motor issues and support for long‐term physical and psychological well‐being. Several of the papers reviewed demonstrate that in adolescence and adulthood (just as in childhood) DCD continues to often co‐occur with other disorders (including ASD, ADHD, dyslexia, anxiety, and depression). This emphasizes the importance of a broad assessment of the individual's needs and consideration of how to provide support beyond just the motor domain.
Supporting information
Appendix S1: Pocket version of the developmental coordination disorder recommendations.
Appendix S4: Mechanisms: strategy used to search for, select, and appraise the evidence.
Appendix S2: Assessments: strategy used to search for, select, and appraise the evidence.
Appendix S3: Interventions: strategy used to search for, select, and appraise the evidence.
Appendix S5: Psychosocial issues: strategy used to search for, select, and appraise the evidence.
Appendix S6: Adolescents/adults: strategy used to search for, select, and appraise the evidence.
Appendix S7: Disclosures of interest form.
Table SI: Assessments: evidence table on standardized tests/assessments
Table SII: Assessments: evidence table on questionnaires
Table SIII: Interventions: evidence table
Table SIV: Mechanisms: evidence table—study results for the internal modelling task category
Table SV: Mechanisms: evidence table—study results for the ecological–dynamic category
Table SVI: Mechanisms: evidence table—study results for the postural control task category
Table SVII: Mechanisms: evidence table—study results for the handwriting task category
Table SVIII: Mechanisms: evidence table—study results for the gait task category
Table SIX: Mechanisms: evidence table—study results for the motor learning task category
Table SX: Mechanisms: evidence table—study results for the catching dynamics task category
Table SXI: Mechanisms: evidence table—study results for the oculomotor task category
Table SXII: Mechanisms: evidence table—study results for the praxis task category
Table SXIII: Mechanisms: evidence table—study results for the executive function task category
Table SXIV: Mechanisms: evidence table—study results for the sensory–perceptual factors task category
Table SXV: Mechanisms: evidence table—study results for the multimodal integration task category
Table SXVI: Mechanisms: evidence table—study results for the neuroimaging category
Table SXVII: Psychosocial issues: evidence table
Table SXVIII: Adolescents/adults: evidence table
Table SXIX: Clinical practice recommendations 2019 and 2012 in comparison
Acknowledgements
We thank Paulene Kamps for proofreading and linguistic revisions. A full disclosure of interests document is provided online (Appendix S7, online supporting information). The authors have stated that they had no interests that might be perceived as posing a conflict or a bias.
Notes
The fact that developmental disorders frequently overlap and co‐occur is referred to throughout this document. Where overlap is mentioned, we do not provide an exhaustive list of possible childhood disorders. Instead, the list is varied from time to time.
In some countries the diagnosis can only be made by a medical doctor. This means that the multi‐professional team must include a medical doctor.
The term ‘co‐occurring’ has been used throughout these recommendations in preference to ‘comorbid’ to reflect that two or more conditions are present but a common aetiology is not known and that this term is consistent with concurrent or overlapping.
The term ‘DCDQ‐R’ is used throughout this document and is consistent with the term DCDQ'07, which is also used to refer to the revised version of the DCDQ.
References
- 1. World Health Organization . International Classification of Functioning, Disability and Health. Geneva: World Health Organization, 2001. [Google Scholar]
- 2. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, 5th edn Arlington, VA: American Psychiatric Association, 2013. [Google Scholar]
- 3. World Health Organization . International Statistical Classification of Diseases and Related Health Problems, 10th Revision. Geneva: World Health Organization, 2016. Available from: http://apps.who.int/classifications/icd10/browse/2016/en#/F82 (accessed 5 October 2017). [Google Scholar]
- 4. The ADAPTE Collaboration . The ADAPTE Process: Resource Toolkit for Guideline Adaptation. Version 2.0 2009. Available from: http://www.g-i-n.net/document-store/working-groups-documents/adaptation/adapte-resource-toolkit-guideline-adaptation-2-0.pdf (accessed 20 December 2017).
- 5. AWMF, AQuMed . German Instrument for Methodological Guideline Appraisal 2008 25.10.2017. Available from: http://www.leitlinien.de/mdb/edocs/pdf/literatur/german-guideline-appraisal-instrument-delbi.pdf (accessed 25 October 2017).
- 6. Blank R, Smits‐Engelsman BC, Polatajko H, Wilson PH. European Academy for Childhood Disability. European Academy for Childhood Disability (EACD): recommendations on the definition, diagnosis and intervention of developmental coordination disorder (long version). Dev Med Child Neurol 2012; 54: 54–93. [DOI] [PubMed] [Google Scholar]
- 7. Lingam R, Hunt L, Golding J, Jongmans M, Emond A. Prevalence of developmental coordination disorder using the DSM‐IV at 7 years of age: a UK population‐based study. Pediatrics 2009; 123: 693–700. [DOI] [PubMed] [Google Scholar]
- 8. Missiuna C, Moll S, Law M, King S, King G. Mysteries and mazes: parents’ experiences of children with developmental coordination disorder. Can J Occup Ther 2006; 73: 7–17. [DOI] [PubMed] [Google Scholar]
- 9. Missiuna C, Moll S, King S, King G, Law M. A trajectory of troubles: parents’ impressions of the impact of developmental coordination disorder. Phys Occup Ther Pediat 2007; 27: 81–101. [PubMed] [Google Scholar]
- 10. Wilson BN, Neil K, Kamps PH, Babcock S. Awareness and knowledge of developmental co‐ordination disorder among physicians, teachers and parents. Child Care Health Dev 2013; 39: 296–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Waltersbacher A. Heilmittelbericht 2016, Wissenschaftliches Institut der AOK (WIdO) 2015. Available from: https://www.wido.de/fileadmin/wido/downloads/pdf_heil_hilfsmittel/wido_hei_hmb16_1216.pdf (accessed 20 October 2017).
- 12. AWMF . AWMF‐Regelwerk ‘Leitlinien’. 1. Auflage 2012 26.10.2017. Available from: http://www.awmf.org/leitlinien/awmf-regelwerk.html (accessed 26 October 2017).
- 13. Centre for Evidence‐based Medicine . Oxford Centre for Evidence‐based Medicine – Levels of Evidence (March 2009): University of Oxford, 2017. Available from: http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/ (accessed 30 October 2017).
- 14. Harbour R, Miller J. A new system for grading recommendations in evidence based guidelines. BMJ 2001; 323: 334–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. AWMF‐Regelwerk Leitlinien: Graduierung der Empfehlungen. Available from: https://www.awmf.org/leitlinien/awmf-regelwerk/ll-entwicklung/awmf-regelwerk-03-%09leitlinienentwicklung/ll-entwicklung-graduierung-der-empfehlungen.html (accessed 6 August 2018).
- 16. Kadesjö B, Gillberg C. Attention deficits and clumsiness in Swedish 7‐year‐old children. Dev Med Child Neurol 1998; 40: 796–804. [DOI] [PubMed] [Google Scholar]
- 17. Kadesjö B, Gillberg C. Developmental coordination disorder in Swedish 7‐year‐old children. J Am Acad Child Adolesc Psychiatry 1999; 38: 820–8. [DOI] [PubMed] [Google Scholar]
- 18. Girish S, Raja K, Kamath A. Prevalence of developmental coordination disorder among mainstream school children in India. J Pediatr Rehabil Med 2016; 9: 107–16. [DOI] [PubMed] [Google Scholar]
- 19. Cantell M, Smyth MM, Ahonen T. Clumsiness in adolescence: educational, motor and social outcomes of motor delay detected at 5 years. Adapt Phys Activ Q 1994; 11: 115–29. [Google Scholar]
- 20. Geuze RH. Static balance and developmental coordination disorder. Hum Mov Sci 2003; 22: 527–48. [DOI] [PubMed] [Google Scholar]
- 21. Hellgren L, Gillberg C, Gillberg IC, Enerskog I. Children with deficits in attention, motor control and perception (DAMP) almost grown up: general health at 16 years. Dev Med Child Neurol 1993; 35: 881–92. [DOI] [PubMed] [Google Scholar]
- 22. Visser J, Geuze RH, Kalverboer AF. The relationship between physical growth, the level of activity and the development of motor skills in adolescence: differences between children with DCD and controls. Hum Mov Sci 1998; 17: 573–608. [Google Scholar]
- 23. Losse A, Henderson SE, Elliman D, Hall D, Knight E, Jongmans M. Clumsiness in children‐do they grow out of it? A 10‐year follow‐up study. Dev Med Child Neurol 1991; 33: 55–68. [DOI] [PubMed] [Google Scholar]
- 24. Gaines R, Missiuna C. Early identification: are speech/language‐impaired toddlers at increased risk for Developmental Coordination Disorder? Child Care Health Dev 2007; 33: 325–32. [DOI] [PubMed] [Google Scholar]
- 25. Scabar A, Devescovi R, Blason L, Bravar L, Carrozzi M. Comorbidity of DCD and SLI: significance of epileptiform activity during sleep. Child Care Health Dev 2006; 32: 733–9. [DOI] [PubMed] [Google Scholar]
- 26. Cantell M, Smyth MM, Ahonen TP. Two distinct pathways for developmental coordination disorder: persistence and resolution. Hum Mov Sci 2003; 22: 413–31. [DOI] [PubMed] [Google Scholar]
- 27. Tseng MH, Howe TH, Chuang IC, Hsieh CL. Cooccurrence of problems in activity level, attention, psychosocial adjustment, reading and writing in children with developmental coordination disorder. Int J Rehabil Res 2007; 30: 327–32. [DOI] [PubMed] [Google Scholar]
- 28. Gomez A, Piazza M, Jobert A, Dehaene‐Lambertz G, Dehaene S, Huron C. Mathematical difficulties in developmental coordination disorder: symbolic and nonsymbolic number processing. Res Dev Disabil 2015; 43–44: 167–78. [DOI] [PubMed] [Google Scholar]
- 29. Gomez A, Piazza M, Jobert A, Dehaene‐Lambertz G, Huron C. Numerical abilities of school‐age children with developmental coordination disorder (DCD): a behavioral and eye‐tracking study. Hum Mov Sci 2017; 55: 315–26. [DOI] [PubMed] [Google Scholar]
- 30. Wang TN, Tseng MH, Wilson BN, Hu FC. Functional performance of children with developmental coordination disorder at home and at school. Dev Med Child Neurol 2009; 51: 817–25. [DOI] [PubMed] [Google Scholar]
- 31. Summers J, Larkin D, Dewey D. Activities of daily living in children with developmental coordination disorder: dressing, personal hygiene, and eating skills. Hum Mov Sci 2008; 27: 215–29. [DOI] [PubMed] [Google Scholar]
- 32. Summers J, Larkin D, Dewey D. What impact does developmental coordination disorder have on daily routines? Intl J Disabil Dev Educ 2008; 55: 131–41. [Google Scholar]
- 33. Poulsen AA, Ziviani JM. Can I play too? Physical activity engagement of children with developmental coordination disorders. Can J Occup Ther 2004; 71: 100–7. [DOI] [PubMed] [Google Scholar]
- 34. Smyth MM, Anderson HI. Coping with clumsiness in the school playground: social and physical play in children with coordination impairments. Br J Dev Psychol 2000; 18: 389–413. [Google Scholar]
- 35. Cairney J, Hay J, Faught BE, Mandigo J, Flouris AD. Developmental coordination disorder, self‐efficacy toward physical activity, and play: does gender matter? Adapt Phys Activ Q 2005; 22: 67–82. [Google Scholar]
- 36. Hay J, Missiuna C. Motor proficiency in children reporting low levels of participation in physical activity. Can J Occup Ther 1998; 65: 64–71. [Google Scholar]
- 37. Cairney J, Hay J, Faught BE, Wade TJ, Corna L, Flouris A. Developmental coordination disorder, generalized self‐efficacy toward physical activity, and participation in organized and free play activities. J Pediatr 2005; 147: 515–20. [DOI] [PubMed] [Google Scholar]
- 38. Poulsen AA, Ziviani JM, Johnson H, Cuskelly M. Loneliness and life satisfaction of boys with developmental coordination disorder: the impact of leisure participation and perceived freedom in leisure. Hum Mov Sci 2008; 27: 325–43. [DOI] [PubMed] [Google Scholar]
- 39. Stephenson EA, Chesson RA. ‘Always the guiding hand’: parents’ accounts of the long‐term implications of developmental co‐ordination disorder for their children and families. Child Care Health Dev 2008; 34: 335–43. [DOI] [PubMed] [Google Scholar]
- 40. Segal R, Mandich A, Polatajko H, Valiant Cook J. Stigma and its management: a pilot study of parental perceptions of the experiences of children with developmental coordination disorder. Am J Occup Ther 2002; 56: 422–8. [DOI] [PubMed] [Google Scholar]
- 41. Schott N, Alof V, Hultsch D, Meermann D. Physical fitness in children with developmental coordination disorder. Res Q Exerc Sport 2007; 78: 438–50. [DOI] [PubMed] [Google Scholar]
- 42. Cairney J, Hay J, Faught BE, Flouris A, Klentrou P. Developmental coordination disorder and cardiorespiratory fitness in children. Pediatr Exerc Sci 2007; 19: 20–8. [DOI] [PubMed] [Google Scholar]
- 43. Cairney J, Hay J, Faught BE, Hawes R. Developmental coordination disorder and overweight and obesity in children aged 9‐14. Int J Obes Relat Metab Disord 2005; 29: 369–72. [DOI] [PubMed] [Google Scholar]
- 44. Imms C, Case‐Smith J, Poulsen A. Parents of children with developmental coordination disorder (i) experienced uncertainty as they came to understand their children and (ii) described a trajectory of changing difficulties as their children got older. Aust Occup Ther J 2007; 54: 242–4. [Google Scholar]
- 45. Cairney J, Hay J, Veldhuizen S, Missiuna C, Faught BE. Developmental coordination disorder, sex, and activity deficit over time: a longitudinal analysis of participation trajectories in children with and without coordination difficulties. Dev Med Child Neurol 2010; 52: e67–72. [DOI] [PubMed] [Google Scholar]
- 46. Chen H, Cohn ES. Social participation for children with developmental coordination disorder: conceptual, evaluation and intervention considerations. Phys Occup Ther Pediat 2003; 23: 61–78. [PubMed] [Google Scholar]
- 47. Osika W, Montgomery SM. Longitudinal Birth Cohort Study. Physical control and coordination in childhood and adult obesity: longitudinal birth cohort study. BMJ 2008; 337: a699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Metcalf BS, Hosking J, Jeffery AN, Voss LD, Henley W, Wilkin TJ. Fatness leads to inactivity, but inactivity does not lead to fatness: a longitudinal study in children (EarlyBird 45). Arch Dis Child 2011; 96: 942–7. [DOI] [PubMed] [Google Scholar]
- 49. Timmons BW, Leblanc AG, Carson V, et al. Systematic review of physical activity and health in the early years (aged 0‐4 years). Appl Physiol Nutr Metab 2012; 37: 773–92. [DOI] [PubMed] [Google Scholar]
- 50. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorder, 4th ed text revision. Washington, DC: American Psychiatric Association, 2000. [Google Scholar]
- 51. Kamps PH, Hart SR. DSM‐5 and school psychology: Developmental coordination disorder. NASP Communiqué 2015; 44: 30–1. [Google Scholar]
- 52. Gillberg C. ADHD and DAMP: a general health perspective. Child Adolesc Ment Health 2003; 8: 106–13. [DOI] [PubMed] [Google Scholar]
- 53. Gillberg C. Deficits in attention, motor control, and perception: a brief review. Arch Dis Child 2003; 88: 904–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dyspraxia Foundation . What is dyspraxia? Dyspraxia Foundation, 2017. Available from: https://dyspraxiafoundation.org.uk/about-dyspraxia/ (accessed 30 October 2017).
- 55. Miyahara M, Mobs I. Developmental dyspraxia and developmental coordination disorder. Neuropsychol Rev 1995; 5: 245–68. [DOI] [PubMed] [Google Scholar]
- 56. Wilson PH, Smits‐Engelsman BC, Caeyenberghs K, et al. Cognitive and neuroimaging findings in developmental coordination disorder: new insights from a systematic review of recent research. Dev Med Child Neurol 2017; 59: 1117–29. [DOI] [PubMed] [Google Scholar]
- 57. Langevin LM, MacMaster FP, Dewey D. Distinct patterns of cortical thinning in concurrent motor and attention disorders. Dev Med Child Neurol 2015; 57: 257–64. [DOI] [PubMed] [Google Scholar]
- 58. Debrabant J, Gheysen F, Caeyenberghs K, Van Waelvelde H, Vingerhoets G. Neural underpinnings of impaired predictive motor timing in children with developmental coordination disorder. Res Dev Disabil 2013; 34: 1478–87. [DOI] [PubMed] [Google Scholar]
- 59. Licari MK, Billington J, Reid SL, et al. Cortical functioning in children with developmental coordination disorder: a motor overflow study. Exp Brain Res 2015; 233: 1703–10. [DOI] [PubMed] [Google Scholar]
- 60. Pangelinan MM, Hatfield BD, Clark JE. Differences in movement‐related cortical activation patterns underlying motor performance in children with and without developmental coordination disorder. J Neurophysiol 2013; 109: 3041–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zwicker JG, Missiuna C, Harris SR, Boyd LA. Brain activation associated with motor skill practice in children with developmental coordination disorder: an fMRI study. Int J Dev Neurosci 2011; 29: 145–52. [DOI] [PubMed] [Google Scholar]
- 62. Debrabant J, Vingerhoets G, Van Waelvelde H, Leemans A, Taymans T, Caeyenberghs K. Brain connectomics of visual‐motor deficits in children with developmental coordination disorder. J Pediatr 2016; 169: 21–7.e2. [DOI] [PubMed] [Google Scholar]
- 63. Langevin LM, Macmaster FP, Crawford S, Lebel C, Dewey D. Common white matter microstructure alterations in pediatric motor and attention disorders. J Pediatr 2014; 164: 1157–64.e1. [DOI] [PubMed] [Google Scholar]
- 64. Zwicker JG, Missiuna C, Harris SR, Boyd LA. Developmental coordination disorder: a pilot diffusion tensor imaging study. Pediatr Neurol 2012; 46: 162–7. [DOI] [PubMed] [Google Scholar]
- 65. Caeyenberghs K, Taymans T, Wilson PH, Vanderstraeten G, Hosseini H, van Waelvelde H. Neural signature of developmental coordination disorder in the structural connectome independent of comorbid autism. Dev Sci 2016; 19: 599–612. [DOI] [PubMed] [Google Scholar]
- 66. Newell K. Motor skill acquisition. Ann Rev Psychol 1991; 42: 213–37. [DOI] [PubMed] [Google Scholar]
- 67. Davids KW. The constraints‐based approach to motor learning In Renshaw I, Davids KW, Savelsbergh GJP, editors. Motor Learning in Practice: A Constraints‐Led Approach. London, UK: Routledge (Taylor & Francis), 2010: 3–16. [Google Scholar]
- 68. Wilson PH, Smits‐Engelsman BC, Caeyenberghs K, Steenbergen B. Toward a hybrid model of developmental coordination disorder. Curr Dev Disord Rep 2017; 4: 64–71. [Google Scholar]
- 69. Reynolds JE, Billington J, Kerrigan S, et al. Mirror neuron system activation in children with developmental coordination disorder: a replication functional MRI study. Res Dev Disabil 2017. 10.1016/j.ridd.2017.11.012 [DOI] [PubMed] [Google Scholar]
- 70. Braver TS, Bongiolatti SR. The role of frontopolar cortex in subgoal processing during working memory. NeuroImage 2002; 15: 523–36. [DOI] [PubMed] [Google Scholar]
- 71. Wilson PH, Ruddock S, Smits‐Engelsman BC, Polatajko H, Blank R. Understanding performance deficits in developmental coordination disorder: a meta‐analysis of recent research. Dev Med Child Neurol 2013; 55: 217–28. [DOI] [PubMed] [Google Scholar]
- 72. Darrah J, Redfern L, Maguire TO, Beaulne AP, Watt J. Intra‐individual stability of rate of gross motor development in full‐term infants. Early Hum Dev 1998; 52: 169–79. [DOI] [PubMed] [Google Scholar]
- 73. Darrah J, Hodge M, Magill‐Evans J, Kembhavi G. Stability of serial assessments of motor and communication abilities in typically developing infants‐implications for screening. Early Hum Dev 2003; 72: 97–110. [DOI] [PubMed] [Google Scholar]
- 74. Ellinoudis T, Evaggelinou C, Kourtessis T, Konstantinidou Z, Venetsanou F, Kambas A. Reliability and validity of age band 1 of the Movement Assessment Battery for Children–second edition. Res Dev Disabil 2011; 32: 1046–51. [DOI] [PubMed] [Google Scholar]
- 75. Smits‐Engelsman BC, Niemeijer AS, Van Waelvelde H. Is the movement assessment battery for children‐2nd edition a reliable instrument to measure motor performance in 3 year old children? Res Dev Disabil 2011; 32: 1370–7. [DOI] [PubMed] [Google Scholar]
- 76. Van Waelvelde H, Oostra A, Dewitte G, Van Den Broeck C, Jongmans MJ. Stability of motor problems in young children with or at risk of autism spectrum disorders, ADHD, and or developmental coordination disorder. Dev Med Child Neurol 2010; 52: e174–8. [DOI] [PubMed] [Google Scholar]
- 77. Chow SM, Henderson SE. Interrater and test‐retest reliability of the movement assessment battery for Chinese preschool children. Am J Occup Ther 2003; 57: 574–7. [DOI] [PubMed] [Google Scholar]
- 78. Van Waelvelde H, Peersman W, Lenoir M, Smits Engelsman BC. The reliability of the movement assessment battery for children for preschool children with mild to moderate motor impairment. ClinRehabil 2007; 21: 465–70. [DOI] [PubMed] [Google Scholar]
- 79. Pless M, Carlsson M, Sundelin C, Persson K. Preschool children with developmental coordination disorder: a short‐term follow‐up of motor status at seven to eight years of age. Acta Paediatr 2002; 91: 521–8. [DOI] [PubMed] [Google Scholar]
- 80. Wilson PH. Practitioner review: approaches to assessment and treatment of children with DCD: an evaluative review. J Child Psychol Psychiatry 2005; 46: 806–23. [DOI] [PubMed] [Google Scholar]
- 81. Largo RH, Caflisch JA, Hug F, Muggli K, Molnar AA, Molinari L. Neuromotor development from 5 to 18 years. Part 2: associated movements. Dev Med Child Neurol 2001; 43: 444–53. [DOI] [PubMed] [Google Scholar]
- 82. Largo RH, Caflisch JA, Hug F, et al. Neuromotor development from 5 to 18 years. Part 1: timed performance. Dev Med Child Neurol 2001; 43: 436–43. [DOI] [PubMed] [Google Scholar]
- 83. Gasser T, Rousson V, Caflisch J, Jenni OG. Development of motor speed and associated movements from 5 to 18 years. Dev Med Child Neurol 2010; 52: 256–63. [DOI] [PubMed] [Google Scholar]
- 84. Hadders‐Algra M, Heineman KR, Bos AF, Middelburg KJ. The assessment of minor neurological dysfunction in infancy using the Touwen Infant Neurological Examination: strengths and limitations. Dev Med Child Neurol 2010; 52: 87–92. [DOI] [PubMed] [Google Scholar]
- 85. Van Hoorn J, Maathuis CG, Peters LH, Hadders‐Algra M. Handwriting, visuomotor integration, and neurological condition at school age. Dev Med Child Neurol 2010; 52: 941–7. [DOI] [PubMed] [Google Scholar]
- 86. Peters LH, Maathuis CG, Hadders‐Algra M. Limited motor performance and minor neurological dysfunction at school age. Acta Paediatr 2010; 100: 271–8. [DOI] [PubMed] [Google Scholar]
- 87. Uslu R, Kapci EG, Oztop D. Neurological soft signs in comorbid learning and attention deficit hyperactivity disorders. Turk J Pediatr 2007; 49: 263–9. [PubMed] [Google Scholar]
- 88. Thelen E, Smith LB. A Dynamic Systems Approach to the Development of Cognition and Action. London, UK: MIT Press, 1994. [Google Scholar]
- 89. Cairney J, Veldhuizen S, Kurdyak P, Missiuna C, Faught BE, Hay J. Evaluating the CSAPPA subscales as potential screening instruments for developmental coordination disorder. Arch Dis Child 2007; 92: 987–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Schoemaker MM, Flapper B, Verheij NP, Wilson BN, Reinders‐Messelink HA, de Kloet A. Evaluation of the developmental coordination disorder questionnaire as a screening instrument. Dev Med Child Neurol 2006; 48: 668–73. [DOI] [PubMed] [Google Scholar]
- 91. Hay J, Hawes R, Faught BE. Evaluation of a screening instrument for developmental coordination disorder. J Adolesc Health 2004; 34: 308–13. [DOI] [PubMed] [Google Scholar]
- 92. Junaid K, Harris SR, Fulmer KA, Carswell A. Teachers’ use of the MABC checklist to identify children with motor coordination difficulties. Pediatr Phys Ther 2000; 12: 158–63. [PubMed] [Google Scholar]
- 93. Wright HC, Sugden DA. The nature of developmental coordination disorder: inter‐ and intragroup differences. Adapt Phys Activ Q 1996; 13: 357–71. [Google Scholar]
- 94. Wright HC, Sugden DA. A two‐step procedure for the identification of children with developmental co‐ordination disorder in Singapore. Dev Med Child Neurol 1996; 38: 1099–105. [DOI] [PubMed] [Google Scholar]
- 95. Wilson BN, Kaplan BJ, Crawford SG, Campbell A, Dewey D. Reliability and validity of a parent questionnaire on childhood motor skills. Am J Occup Ther 2000; 54: 484–93. [DOI] [PubMed] [Google Scholar]
- 96. Wilson BN, Crawford SG, Green D, Roberts G, Aylott A, Kaplan BJ. Psychometric properties of the revised developmental coordination disorder questionnaire. Phys Occup Ther Pediat 2009; 29: 182–202. [DOI] [PubMed] [Google Scholar]
- 97. Henderson SE, Sugden DA. Movement Assessment Battery for Children: Manual. London, UK: Psychological Corporation, 1992. [Google Scholar]
- 98. Henderson SE, Sugden DA, Barnett AL. Movement Assessment Battery for Children. 2nd edn London, UK: Harcourt Assessment, 2007. [Google Scholar]
- 99. Rihtman T, Wilson BN, Parush S. Development of the little developmental coordination disorder questionnaire for preschoolers and preliminary evidence of its psychometric properties in Israel. Res Dev Disabil 2011; 32: 1378–87. [DOI] [PubMed] [Google Scholar]
- 100. Wilson BN, Creighton D, Crawford SG, et al. Psychometric properties of the Canadian little developmental coordination disorder questionnaire for preschool children. Phys Occup Ther Pediat 2015; 35: 116–31. [DOI] [PubMed] [Google Scholar]
- 101. Venter A, Pienaar AE, Coetzee D. Suitability of the ‘Little DCDQ'for the identification of DCD in a selected group of 3‐5‐year‐old South African children. Early Child Dev Care 2015; 185: 1359–71. [Google Scholar]
- 102. Schoemaker MM, Niemeijer AS, Flapper BC, Smits‐Engelsman BC. Validity and reliability of the movement assessment battery for children‐2 checklist for children with and without motor impairments. Dev Med Child Neurol 2012; 54: 368–75. [DOI] [PubMed] [Google Scholar]
- 103. Chambers M, Sugden DA. The identification and assessment of young children with movement difficulties. Int J Early Years Educ 2002; 10: 157–76. [Google Scholar]
- 104. Rosenblum S. The development and standardization of the children activity scales (ChAS‐P/T) for the early identification of children with developmental coordination disorders. Child Care Health Dev 2006; 32: 619–32. [DOI] [PubMed] [Google Scholar]
- 105. Rosenblum S. Development, reliability, and validity of the handwriting proficiency screening questionnaire (HPSQ). Am J Occup Ther 2008; 62: 298–307. [DOI] [PubMed] [Google Scholar]
- 106. Rosenblum S, Gafni‐Lachter L. Handwriting proficiency screening questionnaire for children (HPSQ–C): development, reliability, and validity. Am J Occup Ther 2015; 69: 6903220030. [DOI] [PubMed] [Google Scholar]
- 107. Schneider E, Rosenblum S. Development, reliability, and validity of the My Child's Play (MCP) questionnaire. Am J Occup Ther 2014; 68: 277–85. [DOI] [PubMed] [Google Scholar]
- 108. Rosenblum S, Waissman P, Diamond GW. Identifying play characteristics of pre‐school children with developmental coordination disorder via parental questionnaires. Hum Mov Sci 2017; 53: 5–15. [DOI] [PubMed] [Google Scholar]
- 109. Missiuna C. Development of ‘All About Me’, a scale that measures children's perceived motor competence. Occup Ther J Res 1998; 18: 85–108. [Google Scholar]
- 110. Pless M, Carlsson M, Sundelin C, Persson K. Pre‐school children with developmental co‐ordination disorder: self‐perceived competence and group motor skill intervention. Acta Paediatr 2001; 90: 532–8. [PubMed] [Google Scholar]
- 111. Missiuna C, Rivard L, Pollock N. They're bright but can't write: developmental coordination disorder in school aged children. Teach Except Child 2004; 1: 3. [Google Scholar]
- 112. van der Linde BW, van Netten JJ, Otten BE, Postema K, Geuze RH, Schoemaker MM. Development and psychometric properties of the DCDDaily: a new test for clinical assessment of capacity in activities of daily living in children with developmental coordination disorder. Clin Rehabil 2013; 27: 834–44. [DOI] [PubMed] [Google Scholar]
- 113. Van der Linde BW, Van Netten JJ, Otten BE, Postema K, Geuze RH, Schoemaker MM. Psychometric properties of the DCDDaily‐Q: a new parental questionnaire on children's performance in activities of daily living. Res Dev Disabil 2014; 35: 1711–19. [DOI] [PubMed] [Google Scholar]
- 114. GiofrèD Cornoldi C, Schoemaker MM. Identifying developmental coordination disorder: MOQ‐T validity as a fast screening instrument based on teachers’ ratings and its relationship with praxic and visuospatial working memory deficits. Res Dev Disabil 2014; 35: 3518–25. [DOI] [PubMed] [Google Scholar]
- 115. Asunta P, Viholainen H, Ahonen T, et al. Reliability and validity of the Finnish version of the motor observation questionnaire for teachers. Hum Mov Sci 2017; 53: 63–71. [DOI] [PubMed] [Google Scholar]
- 116. Schoemaker MM, Flapper BC, Reinders‐Messelink HA, Kloet A. Validity of the motor observation questionnaire for teachers as a screening instrument for children at risk for developmental coordination disorder. Hum Mov Sci 2008; 27: 190–9. [DOI] [PubMed] [Google Scholar]
- 117. Martini R, Rios J, Polatajko H, Wolf T, McEwen S. The performance quality rating scale (PQRS): reliability, convergent validity, and internal responsiveness for two scoring systems. Disabil Rehabil 2014; 37: 231–8. [DOI] [PubMed] [Google Scholar]
- 118. Josman N, Goffer A, Rosenblum S. Development and standardization of a ‘Do–Eat’ activity of daily living performance test for children. Am J Occup Ther 2010; 64: 47–58. [DOI] [PubMed] [Google Scholar]
- 119. Kirby A, Edwards L, Sugden DA, Rosenblum S. The development and standardization of the adult developmental co‐ordination disorders/dyspraxia checklist (ADC). Res Dev Disabil 2010; 31: 131–9. [DOI] [PubMed] [Google Scholar]
- 120. Bruininks RH. Bruininks‐Oseretsky Test of Motor Proficiency. Circle Pines, MN: American Guidance Service, 1978. [Google Scholar]
- 121. Kiphard EJ, Schilling F. KTK, Körperkoordinationstest für Kinder Hogrefe, 2007. [PubMed]
- 122. Largo RH, Rousson V, Caflisch J, Jenni O. Zurich Neuromotor Assessment. Zürich: AWE Verlag, 2007. [Google Scholar]
- 123. McCarron LT. MAND McCarron Assessment of Neuro Muscular Development: Fine and Gross Motor Abilities (rev. ed.) Dallas, TX: Common Market Press, 1997. [Google Scholar]
- 124. Brantner S, Piek JP, Smith LM. Evaluation of the validity of the MAND in assessing motor impairment in young children. Rehabil Psychol 2009; 54: 413–21. [DOI] [PubMed] [Google Scholar]
- 125. Frijters M, Westenberg Y, Smits‐Engelsman BC. Vergelijking van de movement‐ABC 2 test en de bayley scales of infant development motorische schaal (BSID‐II‐NL‐M) bij kinderen van 36 tot 43 maanden. Ned Tijdschr Kinderfysiother 2010; 3: 14. [Google Scholar]
- 126. Jelsma LD, Van Bergen‐Verhoef LLJ, Niemeijer AS, Smits‐Engelsman BC. Overeenstemming tussen de movement assessment battery for children second edition en de bruininks‐oseretsky test of motor proficiency second edition bij kinderen van 7–11 jaar. Ned Tijdschr Kinderfysiother 2010; 3: 16. [Google Scholar]
- 127. van Beek I, Booij JC, Niemeijer AS, Smits‐Engelsman BC. De movement ABC‐2 test en de Körperkoordinations Test für Kinder vergeleken bij 11–16 jarigen. Ned Tijdschr Kinderfysiother 2010; 3: 18. [Google Scholar]
- 128. Van Waelvelde H, Peersman W, Smits‐Engelsman BC. Factoranalytische validatie van de movement ABC‐ 2 test. Ned Tijdschr Kinderfysiother 2010; 3: 19. [Google Scholar]
- 129. Schulz J, Henderson SE, Sugden DA, Barnett AL. Structural validity of the movement ABC‐2 test: factor structure comparisons across three age groups. Res Dev Disabil 2011; 32: 1361–9. [DOI] [PubMed] [Google Scholar]
- 130. Wagner MO, Kastner J, Petermann F, Bös K. Factorial validity of the movement assessment battery for children‐2 (age band 2). Res Dev Disabil 2011; 32: 674–80. [DOI] [PubMed] [Google Scholar]
- 131. Wuang YP, Su JH, Su CY. Reliability and responsiveness of the movement assessment battery for children‐second edition test in children with developmental coordination disorder. Dev Med Child Neurol 2012; 54: 160–5. [DOI] [PubMed] [Google Scholar]
- 132. Bruininks RH, Bruininks BD. Bruininks‐Oseretsky Test of Motor Proficiency. 2nd edn Manual. Minneapolis, MN: Pearson, 2005. [Google Scholar]
- 133. Blank R, Jenetzky E, Vinçon S. Bruininks‐Oseretzky Test der motorischen Fähigkeiten, Zweite Ausgabe, Handbuch. Frankfurt am Main: Pearson, 2014. [Google Scholar]
- 134. Smits‐Engelsman BC. Movement Assessment Battery for Children‐2 (2nd edition) (Movement ABC‐2). Examiner's Manual. Dutch Translation and Standardisation. Boston: Pearson, 2010. [Google Scholar]
- 135. Vincon S, Jenetzky E, Link J, Blank R. Vergleich des M‐ABC‐2 und BOT‐2 bei Diagnose UEMF. Ergotherapie Kongress; Erfurt 2014.
- 136. Deitz JC, Kartin D, Kopp K. Review of the Bruininks‐Oseretsky test of motor proficiency, second edition (BOT‐2). Phys Occup Ther Pediat 2007; 27: 87–102. [PubMed] [Google Scholar]
- 137. Rousson V, Gasser T, Caflisch J, Largo R. Reliability of the Zurich neuromotor assessment. Clin Neuropsychol 2008; 22: 60–72. [DOI] [PubMed] [Google Scholar]
- 138. Rousson V, Gasser T. Simple component analysis. Appl Stat 2004; 53: 539–55. [Google Scholar]
- 139. Schmidhauser J, Caflisch J, Rousson V, Bucher HU, Largo RH, Latal B. Impaired motor performance and movement quality in very‐low‐birthweight children at 6 years of age. Dev Med Child Neurol 2006; 48: 718–22. [DOI] [PubMed] [Google Scholar]
- 140. Seitz J, Jenni OG, Molinari L, Caflisch J, Largo RH, Latal Hajnal B. Correlations between motor performance and cognitive functions in children born < 1250 g at school age. Neuropediatrics 2006; 37: 6–12. [DOI] [PubMed] [Google Scholar]
- 141. Rousson V, Gasser T, Caflisch J, Jenni OG. Neuromotor performance of normally developing left‐handed children and adolescents. Hum Mov Sci 2009; 28: 809–17. [DOI] [PubMed] [Google Scholar]
- 142. Kakebeeke TH, Egloff K, Caflisch J, et al. Similarities and dissimilarities between the Movement ABC‐2 and the Zurich Neuromotor Assessment in children with suspected developmental coordination disorder. Res Dev Disabil 2014; 35: 3148–55. [DOI] [PubMed] [Google Scholar]
- 143. Kiphard EJ, Schilling F. KTK, Körperkoordinationstest für Kinder, 3. Überarbeitete und ergänzte Auflage: Hogrefe, 2017. [Google Scholar]
- 144. Petermann F, Macha T. Entwicklungsdiagnstorik. Kindheit und Entwicklung 2005; 14: 131–9. [Google Scholar]
- 145. Schilling F. Körperkontrolle und kindliche Entwicklung. KTK‐Normentabellen erweitert. Motorik 2014; 4: 167–77. [Google Scholar]
- 146. Folio MR, Fewell RR. Peabody Developmental Motor Scales. 2nd edn (PDMS‐2). Austin, TX: Pro‐Ed, 2000. [Google Scholar]
- 147. Hua J, Gu G, Meng W, Wu Z. Age band 1 of the movement assessment battery for children‐second edition: exploring its usefulness in mainland China. Res Dev Disabil 2013; 34: 801–8. [DOI] [PubMed] [Google Scholar]
- 148. Bayley N. Bayley Scales of Infant and Toddler Development, Third Edition. Administration Manual. San Antonio, TX: Psychological Corporation, 2006. [Google Scholar]
- 149. Zuk L, Tlumek H, Katz‐Leurer M, Peretz C, Carmeli E. A new tool for identifying children with motor problems reliability and validity study. J Child Neurol 2014; 29: 592–8. [DOI] [PubMed] [Google Scholar]
- 150. Ulrich DA. Test of Gross Motor Development‐2 (TGMD‐2). Austin, TX: ProEd, 2000. [Google Scholar]
- 151. Logan SW, Robinson LE, Rudisill ME, Wadsworth DD, Morera M. The comparison of school‐age children's performance on two motor assessments: the test of gross motor development and the movement assessment battery for children. Phys Educ Sport Pedagogy 2014; 19: 48–59. [Google Scholar]
- 152. Dhall JK. Handwriting‐based model for identification of developmental disorders among North Indian children. School Psychol Int 2015; 37: 51–63. [Google Scholar]
- 153. Rosenblum S. Handwriting measures as reflectors of executive functions among adults with developmental coordination disorders (DCD). Front Psychol 2013; 4: 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Rosenblum S, Livneh‐Zirinski M. Handwriting process and product characteristics of children diagnosed with developmental coordination disorder. Hum Mov Sci 2008; 27: 200–14. [DOI] [PubMed] [Google Scholar]
- 155. Rosenblum S, Margieh JA, Engel‐Yeger B. Handwriting features of children with developmental coordination disorder–results of triangular evaluation. Res Dev Disabil 2013; 34: 4134–41. [DOI] [PubMed] [Google Scholar]
- 156. Hamstra‐Bletz L, De Bie J, Den Brinker B. Beknopte beoordelingsmethode voor kinderhandschriften: Experimentele versie [Concise Evaluation Scale for Children's Handwriting: Experimental Version]. Lisse, Netherlands: Swets & Zeitlinger, 1987. [Google Scholar]
- 157. Blöte AW, Hamstra‐Bletz L. A longitudinal study on the structure of handwriting. Percept Mot Skills 1991; 72: 983–94. [DOI] [PubMed] [Google Scholar]
- 158. Hamstra‐Bletz L, Blöte A. Development of handwriting in primary school: a longitudinal study. Percept Mot Skills 1990; 70: 759–70. [DOI] [PubMed] [Google Scholar]
- 159. Hamstra‐Bletz L, Blöte A. A longitudinal study on dysgraphic handwriting in primary school. J Learn Disabil 1993; 26: 689–99. [DOI] [PubMed] [Google Scholar]
- 160. Smits‐Engelsman BC, Stevens M, Vrenken I, van Hagen A. Systematische Opsporing Schrijfproblemen (SOS): een hulpmiddel voor leerkrachten bij het signaleren van motorische schrijfproblemen van leerlingen in het Basis en Speciaal Onderwijs. [Systematic screening of handwriting problems (SOS): an instrument for teachers for screening of handwriting problem of children in primary school and special education]. Kinderfysiotherapie 2005; December: 16–20.
- 161. Van Waelvelde H, De Mey A, Smits‐Engelsman BC. Handleiding SOS. Available from: http://wwwrevakiugentbe/files/research/SOS-handleidingpdf 2008 (accessed 17 August 2017).
- 162. Bommel‐Rutgers I, Smits‐Engelsman BC. Is de SOS (Systematische Opsporing Schrijfproblemen) een valide meetinstrument om motorische schrijfproblemen op te sporen bij kinderen uit groep 4 en 5? [Is the SOS a valid and reliable instrument to find children with motor based writing problems.] Stimulus 2005; 24: 222–32. [Google Scholar]
- 163. Smits‐Engelsman BC, van Bommel‐Rutgers I, Van Waelvelde H. Systematische Opsporing Schrijfproblemen, SOS‐2–NL; 2014.
- 164. Schrijven NL. Information: SOS‐2‐EN (English version). 2017. Available from: http://www.schrijvennl.nl/sos-2-nl/sos-2-en-english-version/ (accessed 31 October 2017).
- 165. Vincon S, Blank R, Jenetzky E. Systematische Erfassung motorischer Schreibstörungen [Systematic assessment of graphomotor dysfunctions]. In preparation.
- 166. Vincon S, Smits‐Engelsman BC, Blank R, Jenetzky E. Norm values for writing speed in German pupils. DCD Conference Toulouse, France 2015.
- 167. Barnett AL, Henderson SE, Scheib B, Schulz J. Detailed Assessment of Speed of Handwriting (DASH). Boston, MA: Pearson, 2007. [Google Scholar]
- 168. Barnett AL. Motor assessment in developmental coordination disorder: from identification to intervention. Intl J Disabil Dev Educ 2008; 55: 113–29. [Google Scholar]
- 169. Barnett AL, Henderson SE, Scheib B, Schultz J. The Detailed Assessment of Speed of Handwriting 17+ (DASH 17+). London, UK: Pearson Assessment, 2010. [Google Scholar]
- 170. Prunty MM, Barnett AL, Wilmut K, Plumb MS. An examination of writing pauses in the handwriting of children with developmental coordination disorder. Res Dev Disabil 2014; 35: 2894–905. [DOI] [PubMed] [Google Scholar]
- 171. Prunty MM, Barnett AL, Wilmut K, Plumb MS. The impact of handwriting difficulties on compositional quality in children with developmental coordination disorder. Br J Occup Ther 2016; 79: 591–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Reisman J. Minnesota Handwriting Assessment Manual. San Antonio, TX: Harcourt Assessment, 1999. [Google Scholar]
- 173. Stott D, Moyes FA, Henderson SE. Diagnosis and Remediation of Handwriting Problems. Cardiff, UK: DRAKE Educational Associates, 1985. [Google Scholar]
- 174. Phelps J, Stempel L. Children's Handwriting Evaluation Scale for Manuscript Writing. Dallas, TX: Texas Scottish Rite Hospital for Cripples Children, 1987. [Google Scholar]
- 175. Amundson SJ. Evaluation Tool of Children's Handwriting: A. Homer, OT Kids, 1995.
- 176. Larsen S, Hammill D. Test of Legible Handwriting. Austin, TX: PRO‐ED, 1989. [Google Scholar]
- 177. Geuze RH, Jongmans MJ, Schoemaker MM, Smits‐Engelsman BC. Clinical and research diagnostic criteria for developmental coordination disorder: a review and discussion. Hum Mov Sci 2001; 20: 7–47. [DOI] [PubMed] [Google Scholar]
- 178. Smits‐Engelsman BC, Henderson SE, Michels CGJ. The assessment of children with developmental coordination disorders in the Netherlands: the relationship between the movement assessment battery for children and the Körperkoordinations test für Kinder. Hum Mov Sci 1998; 17: 699–709. [Google Scholar]
- 179. Tan SK, Parker HE, Larkin D. Concurrent validity of motor tests used to identify children with motor impairment. Adapt Phys Activ Q 2001; 18: 168–82. [Google Scholar]
- 180. Van Waelvelde H, De Weerdt W, De Cock P, Smits‐Engelsman BC. Aspects of the validity of the movement assessment battery for children. Hum Mov Sci 2004; 23: 49–60. [DOI] [PubMed] [Google Scholar]
- 181. Chen YW, Tseng MH, Hu FC, Cermak SA. Psychosocial adjustment and attention in children with developmental coordination disorder using different motor tests. Res Dev Disabil 2009; 30: 1367–77. [DOI] [PubMed] [Google Scholar]
- 182. Cairney J, Hay J, Veldhuizen S, Missiuna C, Faught BE. Comparing probable case identification of developmental coordination disorder using the short form of the Bruininks‐Oseretsky test of motor proficiency and the movement ABC. Child Care Health Dev 2009; 35: 402–8. [DOI] [PubMed] [Google Scholar]
- 183. Lingam R, Golding J, Jongmans MJ, Hunt LP, Ellis M, Emond A. The association between developmental coordination disorder and other developmental traits. Pediatrics 2010; 126: e1109–18. [DOI] [PubMed] [Google Scholar]
- 184. Kaplan BJ, Dewey DM, Crawford SG, Wilson BN. The term comorbidity is of questionable value in reference to developmental disorders: data and theory. J Learn Disabil 2001; 34: 555–65. [DOI] [PubMed] [Google Scholar]
- 185. Green D, Baird G, Sugden DA. A pilot study of psychopathology in developmental coordination disorder. Child Care Health Dev 2006; 32: 741–50. [DOI] [PubMed] [Google Scholar]
- 186. Kadesjö B, Gillberg C. The comorbidity of ADHD in the general population of Swedish school‐age children. J Child Psychol Psychiatry 2001; 42: 487–92. [PubMed] [Google Scholar]
- 187. Miyahara M, Mobs I, Doll‐Tepper G. Severity of hyperactivity and the comorbidity of hyperactivity with clumsiness in three sample sources: school, support group and hospital. Child Care Health Dev 2001; 27: 413–24. [DOI] [PubMed] [Google Scholar]
- 188. Rasmussen P, Gillberg C. Natural outcome of ADHD with developmental coordination disorder at age 22 years: a controlled, longitudinal, community‐based study. J Am Acad Child Adolesc Psychiatry 2000; 39: 1424–31. [DOI] [PubMed] [Google Scholar]
- 189. Hill EL, Bishop DVM, Nimmo‐Smith I. Representational gestures in developmental coordination disorder and specific language impairment: error‐types and the reliability of ratings. Hum Mov Sci 1998; 17: 655–78. [Google Scholar]
- 190. Rintala P, Pienimäki K, Ahonen T, Cantell M, Kooistra L. The effects of a psychomotor training programme on motor skill development in children with develomental language disorders. Hum Mov Sci 1998; 17: 721–37. [Google Scholar]
- 191. Wisdom SN, Dyck MJ, Piek J, Hay D, Hallmayer J. Can autism, language and coordination disorders be differentiated based on ability profiles? Eur Child Adolesc Psychiatry 2007; 16: 178–86. [DOI] [PubMed] [Google Scholar]
- 192. Visscher C, Houwen S, Scherder EJ, Moolenaar B, Hartman E. Motor profile of children with developmental speech and language disorders. Pediatrics 2007; 120: e158–63. [DOI] [PubMed] [Google Scholar]
- 193. Flapper BC, Schoemaker MM. Developmental coordination disorder in children with specific language impairment: co‐morbidity and impact on quality of life. Res Dev Disabil 2013; 34: 756–63. [DOI] [PubMed] [Google Scholar]
- 194. Hodgson JC, Hudson JM. Atypical speech lateralization in adults with developmental coordination disorder demonstrated using functional transcranial Doppler ultrasound. J Neuropsychol 2017; 11: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Creavin AL, Lingam R, Northstone K, Williams C. Ophthalmic abnormalities in children with developmental coordination disorder. Dev Med Child Neurol 2014; 56: 164–70. [DOI] [PubMed] [Google Scholar]
- 196. Rafique SA, Northway N. Relationship of ocular accommodation and motor skills performance in developmental coordination disorder. Hum Mov Sci 2015; 42: 1–14. [DOI] [PubMed] [Google Scholar]
- 197. Montgomery D. Cohort analysis of writing in year 7 following two, four and seven years of the National Literacy Strategy. Support Learn 2008; 23: 3–11. [Google Scholar]
- 198. Iversen S, Berg K, Ellertsen B, Tønnessen FE. Motor coordination difficulties in a municipality group and in a clinical sample of poor readers. Dyslexia 2005; 11: 217–31. [DOI] [PubMed] [Google Scholar]
- 199. Cheng HC, Chen JY, Tsai CL, Shen ML, Cherng RJ. Reading and writing performances of children 7‐8 years of age with developmental coordination disorder in Taiwan. Res Dev Disabil 2011; 32: 2589–94. [DOI] [PubMed] [Google Scholar]
- 200. Pieters S, Desoete A, Van Waelvelde H, Vanderswalmen R, Roeyers H. Mathematical problems in children with developmental coordination disorder. Res Dev Disabil 2012; 33: 1128–35. [DOI] [PubMed] [Google Scholar]
- 201. Jongmans MJ, Smits‐Engelsman BC, Schoemaker MM. Consequences of comorbidity of developmental coordination disorders and learning disabilities for severity and pattern of perceptual‐motor dysfunction. J Learn Disabil 2003; 36: 528–37. [DOI] [PubMed] [Google Scholar]
- 202. Schoemaker MM, Lingam R, Jongmans MJ, van Heuvelen MJ, Emond A. Is severity of motor coordination difficulties related to co‐morbidity in children at risk for developmental coordination disorder? Res Dev Disabil 2013; 34: 3084–91. [DOI] [PubMed] [Google Scholar]
- 203. Kastner J, Petermann F. Development coordination disorder: relations between deficits in movement and cognition. Klin Pädiatr 2010; 222: 26–34. [DOI] [PubMed] [Google Scholar]
- 204. van Dellen T, Geuze RH. Motor response processing in clumsy children. J Child Psychol Psychiatry 1988; 29: 489–500. [DOI] [PubMed] [Google Scholar]
- 205. Alloway TP, Rajendran G, Archibald LM. Working memory in children with developmental disorders. J Learn Disabil 2009; 42: 372–82. [DOI] [PubMed] [Google Scholar]
- 206. Green D, Baird G, Barnett AL, Henderson L, Huber J, Henderson SE. The severity and nature of motor impairment in asperger's syndrome: a comparison with specific developmental disorder of motor function. J Child Psychol Psychiatry 2002; 43: 655–68. [DOI] [PubMed] [Google Scholar]
- 207. Kopp S, Beckung E, Gillberg C. Developmental coordination disorder and other motor control problems in girls with autism spectrum disorder and/or attention‐deficit/hyperactivity disorder. Res Dev Disabil 2009; 31: 350–61. [DOI] [PubMed] [Google Scholar]
- 208. Green D, Charman T, Pickles A, et al. Impairment in movement skills of children with autistic spectrum disorders. Dev Med Child Neurol 2009; 51: 311–16. [DOI] [PubMed] [Google Scholar]
- 209. Holsti L, Grunau RVE, Whitfield MF. Developmental coordination disorder in extremely low birth weight children at nine years. J Dev Behav Pediatr 2002; 23: 9–15. [DOI] [PubMed] [Google Scholar]
- 210. Edwards J, Berube M, Erlandson K, et al. Developmental coordination disorder in school‐aged children born very preterm and/or at very low birth weight: a systematic review. J Dev Behav Pediatr 2011; 32: 678–87. [DOI] [PubMed] [Google Scholar]
- 211. Zwicker JG, Yoon SW, Mackay M, Petrie‐Thomas J, Rogers M, Synnes AR. Perinatal and neonatal predictors of developmental coordination disorder in very low birthweight children. Arch Dis Child 2013; 98: 118–22. [DOI] [PubMed] [Google Scholar]
- 212. Martin NC, Piek J, Baynam G, Levy F, Hay D. An examination of the relationship between movement problems and four common developmental disorders. Hum Mov Sci 2010; 29: 799–808. [DOI] [PubMed] [Google Scholar]
- 213. Mosca SJ, Langevin LM, Dewey D, et al. Copy‐number variations are enriched for neurodevelopmental genes in children with developmental coordination disorder. J Med Genet 2016; 53: 812–19. [DOI] [PubMed] [Google Scholar]
- 214. Lingam R, Jongmans MJ, Ellis M, Hunt LP, Golding J, Emond A. Mental health difficulties in children with developmental coordination disorder. Pediatrics 2012; 129: e882–91. [DOI] [PubMed] [Google Scholar]
- 215. Hill EL, Brown D. Mood impairments in adults previously diagnosed with developmental coordination disorder. J Ment Health 2013; 22: 334–40. [DOI] [PubMed] [Google Scholar]
- 216. Sigurdsson E, Van Os J, Fombonne E. Are impaired childhood motor skills a risk factor for adolescent anxiety? Results from the 1958 U.K. birth cohort and the National Child Development Study. Am J Psychiatry 2002; 159: 1044–6. [DOI] [PubMed] [Google Scholar]
- 217. Zwicker JG, Harris SR, Klassen AF. Quality of life domains affected in children with developmental coordination disorder: a systematic review. Child Care Health Dev 2013; 39: 562–80. [DOI] [PubMed] [Google Scholar]
- 218. Henderson SE, Hall D. Concomitants of clumsiness in young school children. Dev Med Child Neurol 1982; 24: 448–60. [DOI] [PubMed] [Google Scholar]
- 219. Nichols PL, Chen TC. Minimal Brain Dysfunction: A Prospective Study. Mahwah, NJ: Lawrence Erlbaum Associates, 1981. [Google Scholar]
- 220. Green D, Baird G. DCD and overlapping conditions In Sugden D, Chambers M, editors. Children with Developmental Coordination Disorder. London, UK: Whurr Publications, 2005: 93–118. [Google Scholar]
- 221. Cairney J, Veldhuizen S, Szatmari P. Motor coordination and emotional‐behavioral problems in children. Curr Opin Psychiatry 2010; 23: 324–9. [DOI] [PubMed] [Google Scholar]
- 222. Piek J, Rigoli D. Psychosocial and Behavioral Problems in Children with DCD In Cairney J, editor. Developmental Coordination Disorder and Its Consequences. Toronto, ON: University of Toronto Press, 2015: 108–27. [Google Scholar]
- 223. Pratt ML, Hill EL. Anxiety profiles in children with and without developmental coordination disorder. Res Dev Disabil 2011; 32: 1253–9. [DOI] [PubMed] [Google Scholar]
- 224. Harrowell I, Hollén L, Lingam R, Emond A. The impact of developmental coordination disorder on mental health in late adolescence. Dev Med Child Neurol 2017; 59: 973–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Yu J, Sit CH, Capio CM, Burnett A, Ha AS, Huang WY. Fundamental movement skills proficiency in children with developmental coordination disorder: does physical self‐concept matter? Disabil Rehabil 2016; 38: 45–51. [DOI] [PubMed] [Google Scholar]
- 226. Green D, Arscott C, Barnett AL, et al. Impairment of movement and socialdifficulties in children with Autism Spectrum Disorder (Asperger Syndrome) and Developmental Coordination Disorder‐how are they perceived by the children and their teachers? In: Barnett ALS, Sugden DA, editors. Moving, Developing and Learning. A Festschrift in Celebration of the Career of Sheila E. Henderson. Oxford: Oxford Brookes University, 2015: ISBN: 978‐1‐873640‐87‐6. [Google Scholar]
- 227. Noordstar JJ, Stuive I, Herweijer H, et al. Perceived athletic competence and physical activity in children with developmental coordination disorder who are clinically referred, and control children. Res Dev Disabil 2014; 35: 3591–7. [DOI] [PubMed] [Google Scholar]
- 228. Viholainen H, Aro T, Purtsi J, Tolvanen A, Cantell M. Adolescents’ school‐related self‐concept mediates motor skills and psychosocial well‐being. Br J Educ Psychol 2014; 84: 268–80. [DOI] [PubMed] [Google Scholar]
- 229. Engel‐Yeger B, Hanna Kasis A. The relationship between developmental co‐ordination disorders, child's perceived self‐efficacy and preference to participate in daily activities. Child Care Health Dev 2010; 36: 670–7. [DOI] [PubMed] [Google Scholar]
- 230. Cocks N, Barton B, Donelly M. Self‐concept of boys with developmental coordination disorder. Phys Occup Ther Pediat 2009; 29: 6–22. [DOI] [PubMed] [Google Scholar]
- 231. Piek J, Baynam GB, Barrett NC. The relationship between fine and gross motor ability, self‐perceptions and self‐worth in children and adolescents. Hum Mov Sci 2006; 25: 65–75. [DOI] [PubMed] [Google Scholar]
- 232. Poulsen AA, Ziviani JM, Cuskelly M. General self‐concept and life satisfaction for boys with differing levels of physical coordination: the role of goal orientations and leisure participation. Hum Mov Sci 2006; 25: 839–60. [DOI] [PubMed] [Google Scholar]
- 233. Heath NL, Toste JR, Missiuna C. An exploration of the relationship between motor impairment and emotional/behavioural difficulties amongst children suspected of having DCD. Israel J Occup Ther 2005; 14: 153–70. [Google Scholar]
- 234. Skinner RA, Piek J. Psychosocial implications of poor motor coordination in children and adolescents. Hum Mov Sci 2001; 20: 73–94. [DOI] [PubMed] [Google Scholar]
- 235. Schoemaker MM, Kalverboer AF. Social and affective problems of children who are clumsy: how early do they begin? Adapt Phys Activ Q 1994; 11: 130–40. [Google Scholar]
- 236. Shaw L, Levine MD, Belfer M. Developmental double jeopardy: a study of clumsiness and self esteem in children with learning problems. J Dev Behav Pediatr 1982; 3: 191–6. [PubMed] [Google Scholar]
- 237. Barnett AL, Dawes H, Wilmut K. Constraints and facilitators to participation in physical activity in teenagers with developmental co‐ordination disorder: an exploratory interview study. Child Care Health Dev 2013; 39: 393–403. [DOI] [PubMed] [Google Scholar]
- 238. Soref B, Ratzon NZ, Rosenberg L, Leitner Y, Jarus T, Bart O. Personal and environmental pathways to participation in young children with and without mild motor disabilities. Child Care Health Dev 2012; 38: 561–71. [DOI] [PubMed] [Google Scholar]
- 239. Poulsen AA, Ziviani JM, Cuskelly M. Perceived freedom in leisure and physical co‐ordination ability: impact on out‐of‐school activity participation and life satisfaction. Child Care Health Dev 2007; 33: 432–40. [DOI] [PubMed] [Google Scholar]
- 240. Poulsen AA, Johnson H, Ziviani JM. Participation, self‐concept and motor performance of boys with developmental coordination disorder: a classification and regression tree analysis approach. Aust Occup Ther J 2011; 58: 95–102. [DOI] [PubMed] [Google Scholar]
- 241. Bejerot S, Humble MB. Childhood clumsiness and peer victimization: a case‐control study of psychiatric patients. BMC Psychiatry 2013; 13: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Campbell WN, Missiuna C, Vaillancourt T. Peer victimization and depression in children with and without motor coordination difficulties. Psychol Sch 2012; 49: 328–41. [Google Scholar]
- 243. Piek J, Barrett NC, Allen LS, Jones A, Louise M. The relationship between bullying and self‐worth in children with movement coordination problems. Br J Educ Psychol 2005; 75: 453–63. [DOI] [PubMed] [Google Scholar]
- 244. Petty KH, Davis CL, Tkacz J, Young‐Hyman D, Waller JL. Exercise effects on depressive symptoms and self‐worth in overweight children: a randomized controlled trial. J Pediatr Psychol 2009; 34: 929–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Biddle SJ, Asare M. Physical activity and mental health in children and adolescents: a review of reviews. Br. J Sports Med 2011; 45: 886–95. [DOI] [PubMed] [Google Scholar]
- 246. Goodman R, Graham P. Psychiatric problems in children with hemiplegia: cross sectional epidemiological survey. BMJ 1996; 312: 1065–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Parkes J, White‐Koning M, Dickinson H, et al. Psychological problems in children with cerebral palsy: a cross‐sectional European study. J Child Psychol Psychiatry 2008; 49: 405–13. [DOI] [PubMed] [Google Scholar]
- 248. Mancini VO, Rigoli D, Cairney J, Roberts LD, Piek JP. The elaborated environmental stress hypothesis as a framework for understanding the association between motor skills and internalizing problems: a mini‐review. Front Psychol 2016; 7: 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Cairney J, Rigoli D, Piek J. Developmental coordination disorder and internalizing problems in children: the environmental stress hypothesis elaborated. Dev Rev 2013; 33: 224–38. [Google Scholar]
- 250. Pearlin LI. The sociological study of stress. J Health Soc Behav 1989; 30: 241–56. [PubMed] [Google Scholar]
- 251. Cairney J, Veldhuizen S. Is developmental coordination disorder a fundamental cause of inactivity and poor health‐related fitness in children? Dev Med Child Neurol 2013; 55(Suppl. 4): 55–8. [DOI] [PubMed] [Google Scholar]
- 252. Ussher MH, Owen CG, Cook DG, Whincup PH. The relationship between physical activity, sedentary behaviour and psychological wellbeing among adolescents. Soc Psychiatry Psychiatr Epidemiol 2007; 42: 851–6. [DOI] [PubMed] [Google Scholar]
- 253. Wagner MO, Jekauc D, Worth A, Woll A. Elboration of the Environmental Stress Hypothesis – Results from a population‐based 6‐year follow‐up. 11th International Conference on DCD; Toulouse, France 2015. [DOI] [PMC free article] [PubMed]
- 254. Smits‐Engelsman BC, Vinçon S, Blank R, Quadrado VH, Polatajko H, Wilson PH. Evaluating the evidence for motor‐based interventions in developmental coordination disorder: a systematic review and meta‐analysis. Res Dev Disabil 2018; 3: 72–102. [DOI] [PubMed] [Google Scholar]
- 255. Smits‐Engelsman BC, Blank R, van der Kaay AC, et al. Efficacy of interventions to improve motor performance in children with developmental coordination disorder: a combined systematic review and meta‐analysis. Dev Med Child Neurol 2013; 55: 229–37. [DOI] [PubMed] [Google Scholar]
- 256. Law M, Baptiste S, Carswell A, McColl MA, Polatajko H, Pollock N. Canadian Occupational Performance Measure. 5th edn (COPM). Ottawa, ON: CAOT Publications ACE, 2014. [Google Scholar]
- 257. Goodman R. The strengths and difficulties questionnaire: a research note. J Child Psychol Psychiatry 1997; 38: 581–6. [DOI] [PubMed] [Google Scholar]
- 258. Consort . Consolidated Standards of Reporting Trials 2010. Available from: http://www.consort-statement.org/consort-2010 (accessed 18 May 2017).
- 259. Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014; 348: g1687. [DOI] [PubMed] [Google Scholar]
- 260. Polatajko H, Cantin N. Developmental coordination disorder (dyspraxia): an overview of the state of the art. Semin Pediatr Neurol 2005; 12: 250–8. [DOI] [PubMed] [Google Scholar]
- 261. Lucas BR, Elliott EJ, Coggan S, et al. Interventions to improve gross motor performance in children with neurodevelopmental disorders: a meta‐analysis. BMC Pediatr 2016; 16: 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Offor N, Williamson PO, Cacola P. Effectiveness of interventions for children with developmental coordination disorder in physical therapy contexts: a systematic literature review and meta‐analysis. J Mot Learn Dev 2016; 4: 169–96. [Google Scholar]
- 263. Preston N, Magallon S, Hill LJ, Andrews E, Ahern SM, Mon‐Williams M. A systematic review of high quality randomized controlled trials investigating motor skill programmes for children with developmental coordination disorder. Clin Rehabil 2016; 31: 857–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Baldi S, Nunzi M, Brina CD. Efficacy of a task‐based training approach in the rehabilitation of three children with poor handwriting quality: a pilot study. Percept Mot Skills 2015; 120: 323–35. [DOI] [PubMed] [Google Scholar]
- 265. Bonnechere B, Jansen B, Omelina L, Van Sint Jan S. The use of commercial video games in rehabilitation: a systematic review. Int J Rehabil Res 2016; 39: 277–90. [DOI] [PubMed] [Google Scholar]
- 266. Ashkenazi T, Weiss PL, Orian D, Laufer Y. Low‐cost virtual reality intervention program for children with developmental coordination disorder: a pilot feasibility study. Pediatr Phys Ther 2013; 25: 467–73. [DOI] [PubMed] [Google Scholar]
- 267. Bonney E, Jelsma LD, Ferguson GD, Smits‐Engelsman BC. Learning better by repetition or variation? Is transfer at odds with task specific training? PLoS ONE 2017; 12: e0174214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Ferguson GD, Jelsma LD, Jelsma J, Smits‐Engelsman BC. The efficacy of two task‐orientated interventions for children with developmental coordination disorder: neuromotor task training and Nintendo Wii Fit training. Res Dev Disabil 2013; 34: 2449–61. [DOI] [PubMed] [Google Scholar]
- 269. Smits‐Engelsman BC, Jelsma LD, Ferguson GD. The effect of exergames on functional strength, anaerobic fitness, balance and agility in children with and without motor coordination difficulties living in low‐income communities. Hum Mov Sci 2016; 55: 327–37. [DOI] [PubMed] [Google Scholar]
- 270. Smits‐Engelsman BC, Jelsma LD, Ferguson GD, Geuze RH. Motor learning: an analysis of 100 trials of a ski slalom game in children with and without developmental coordination disorder. PLoS ONE 2015; 10: e0140470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Jelsma LD, Geuze RH, Mombarg R, Smits‐Engelsman BC. The impact of Wii Fit intervention on dynamic balance control in children with probable developmental coordination disorder and balance problems. Hum Mov Sci 2014; 33: 404–18. [DOI] [PubMed] [Google Scholar]
- 272. Au MK, Chan WM, Lee L, Chen TM, Chau RM, Pang MY. Core stability exercise is as effective as task‐oriented motor training in improving motor proficiency in children with developmental coordination disorder: a randomized controlled pilot study. Clin Rehabil 2014; 28: 992–1003. [DOI] [PubMed] [Google Scholar]
- 273. Farhat F, Masmoudi K, Hsairi I, et al. The effects of 8 weeks of motor skill training on cardiorespiratory fitness and endurance performance in children with developmental coordination disorder. Appl Physiol Nutr Metab 2015; 40: 1269–78. [DOI] [PubMed] [Google Scholar]
- 274. Farhat F, Hsairi I, Baati H, et al. The effect of a motor skills training program in the improvement of practiced and non‐practiced tasks performance in children with developmental coordination disorder (DCD). Hum Mov Sci 2016; 46: 10–22. [DOI] [PubMed] [Google Scholar]
- 275. Ferguson GD, Naidoo N, Smits‐Engelsman BC. Health promotion in a low‐income primary school: children with and without DCD benefit, but differently. Phys Occup Ther Pediatr 2015; 35: 147–62. [DOI] [PubMed] [Google Scholar]
- 276. Fong SS, Chung JW, Chow LP, Ma AW, Tsang WW. Differential effect of taekwondo training on knee muscle strength and reactive and static balance control in children with developmental coordination disorder: a randomized controlled trial. Res Dev Disabil 2013; 34: 1446–55. [DOI] [PubMed] [Google Scholar]
- 277. Wilson PH, Thomas PR, Maruff P. Motor imagery training ameliorates motor clumsiness in children. J Child Neurol 2002; 17: 491–8. [DOI] [PubMed] [Google Scholar]
- 278. Wilson PH, Adams IL, Caeyenberghs K, Thomas P, Smits‐Engelsman B, Steenbergen B. Motor imagery training enhances motor skill in children with DCD: a replication study. Res Dev Disabil 2016; 57: 54–62. [DOI] [PubMed] [Google Scholar]
- 279. Adams ILJ, Smits‐Engelsman B, Lust JM, Wilson PH, Steenbergen B. Feasibility of motor imagery training for children with developmental coordination disorder ‐ a pilot study. Front Psychol 2017; 8: 1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Cacola P, Romero M, Ibana M, Chuang J. Effects of two distinct group motor skill interventions in psychological and motor skills of children with developmental coordination disorder: a pilot study. Disabil Health J 2016; 9: 172–8. [DOI] [PubMed] [Google Scholar]
- 281. Zwicker JG, Rehal H, Sodhi S, et al. Effectiveness of a summer camp intervention for children with developmental coordination disorder. Phys Occup Ther Pediat 2015; 35: 163–77. [DOI] [PubMed] [Google Scholar]
- 282. Becker H. Entwurf einer Theorie des körper‐ und leibbezogenen Lernens am Beispiel von Therapieansätzen aus der Ergotherapie und Physiotherapie. Berlin: Humboldt Universität, 2010. [Google Scholar]
- 283. Sangster CA, Beninger C, Polatajko H, Mandich A. Cognitive strategy generation in children with developmental coordination disorder. Can J Occup Ther 2005; 72: 67–77. [DOI] [PubMed] [Google Scholar]
- 284. Camden C, Foley V, Anaby D, et al. Using an evidence‐based online module to improve parents’ ability to support their child with developmental coordination disorder. Disabil Health J 2016; 9: 406–15. [DOI] [PubMed] [Google Scholar]
- 285. 2018 Physical Activity Guidelines Advisory Committee 2018 Physical Activity Guidelines Advisory Committee Scientific Report. Washington, DC: 2018.
- 286. Barnett AL, Hill EL, Kirby A, Sugden DA. Adaptation and extension of the European Recommendations (EACD) on developmental coordination disorder (DCD) for the UK context. Phys Occup Ther Pediat 2015; 35: 103–15. [DOI] [PubMed] [Google Scholar]
- 287. Flapper BC, Houwen S, Schoemaker MM. Fine motor skills and effects of methylphenidate in children with attention‐deficit–hyperactivity disorder and developmental coordination disorder. Dev Med Child Neurol 2006; 48: 165–9. [DOI] [PubMed] [Google Scholar]
- 288. Bart O, Daniel L, Dan O, Bar‐Haim Y. Influence of methylphenidate on motor performance and attention in children with developmental coordination disorder and attention deficit hyperactive disorder. Res Dev Disabil 2013; 34: 1922–7. [DOI] [PubMed] [Google Scholar]
- 289. Tal‐Saban M, Ornoy A, Parush S. Young adults with developmental coordination disorder: a longitudinal study. Am J Occup Ther 2014; 68: 307–16. [DOI] [PubMed] [Google Scholar]
- 290. Tal‐Saban M, Zarka S, Grotto I, Ornoy A, Parush S. The functional profile of young adults with suspected developmental coordination disorder (DCD). Res Dev Disabil 2012; 33: 2193–202. [DOI] [PubMed] [Google Scholar]
- 291. Cantell M, Kooistra L. Long‐term outcomes of developmental coordination disorder In: Cermak SA, Larkin D, editors. Developmental Coordination Disorder. Albany, NY: Delmar, 2002: 23–38. [Google Scholar]
- 292. Hill EL, Brown D, Sorgardt KS. A preliminary investigation of quality of life satisfaction reports in emerging adults with and without developmental coordination disorder. J Adult Dev 2011; 18: 130–4. [Google Scholar]
- 293. Kirby A, Williams N, Thomas M, Hill EL. Self‐reported mood, general health, wellbeing and employment status in adults with suspected DCD. Res Dev Disabil 2013; 34: 1357–64. [DOI] [PubMed] [Google Scholar]
- 294. Barnett AL, Kirby A, van Waelvelde H, Weintraub N. Features of DCD in adolescents and adults: a scoping review. 12th International Conference on DCD; Fremantle, Australia 2017.
- 295. Levac D, Colquhoun H, O'Brien KK. Scoping studies: advancing the methodology. Implement Sci 2010; 5: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Kirby A, Edwards L, Sugden DA. Emerging adulthood in developmental co‐ordination disorder: parent and young adult perspectives. Res Dev Disabil 2011; 32: 1351–60. [DOI] [PubMed] [Google Scholar]
- 297. Gagnon‐Roy M, Jasmin E, Camden C. Social participation of teenagers and young adults with developmental co‐ordination disorder and strategies that could help them: results from a scoping review. Child Care Health Dev 2016; 42: 840–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Pocket version of the developmental coordination disorder recommendations.
Appendix S4: Mechanisms: strategy used to search for, select, and appraise the evidence.
Appendix S2: Assessments: strategy used to search for, select, and appraise the evidence.
Appendix S3: Interventions: strategy used to search for, select, and appraise the evidence.
Appendix S5: Psychosocial issues: strategy used to search for, select, and appraise the evidence.
Appendix S6: Adolescents/adults: strategy used to search for, select, and appraise the evidence.
Appendix S7: Disclosures of interest form.
Table SI: Assessments: evidence table on standardized tests/assessments
Table SII: Assessments: evidence table on questionnaires
Table SIII: Interventions: evidence table
Table SIV: Mechanisms: evidence table—study results for the internal modelling task category
Table SV: Mechanisms: evidence table—study results for the ecological–dynamic category
Table SVI: Mechanisms: evidence table—study results for the postural control task category
Table SVII: Mechanisms: evidence table—study results for the handwriting task category
Table SVIII: Mechanisms: evidence table—study results for the gait task category
Table SIX: Mechanisms: evidence table—study results for the motor learning task category
Table SX: Mechanisms: evidence table—study results for the catching dynamics task category
Table SXI: Mechanisms: evidence table—study results for the oculomotor task category
Table SXII: Mechanisms: evidence table—study results for the praxis task category
Table SXIII: Mechanisms: evidence table—study results for the executive function task category
Table SXIV: Mechanisms: evidence table—study results for the sensory–perceptual factors task category
Table SXV: Mechanisms: evidence table—study results for the multimodal integration task category
Table SXVI: Mechanisms: evidence table—study results for the neuroimaging category
Table SXVII: Psychosocial issues: evidence table
Table SXVIII: Adolescents/adults: evidence table
Table SXIX: Clinical practice recommendations 2019 and 2012 in comparison


