Abstract
Globally, increasing acquired antimicrobial resistance among pathogenic bacteria presents an urgent challenge to human and animal health. As a result, significant efforts, such as the One Health Initiative, are underway to curtail and optimize the use of critically important antimicrobials for human medicine in all applications, including food animal production. This review discusses the rationale behind multiple and competing “critically important antimicrobial” lists and their contexts as created by international, regional, and national organizations; identifies discrepancies among these lists; and describes issues surrounding risk management recommendations that have been made by regulatory organizations on the use of antibiotics in food animal production. A more harmonized approach to defining criticality in its various contexts (e.g., for human versus animal health, enteric diseases versus other systemic infections, and direct versus indirect selection of resistance) is needed in order to identify shared contextual features, aid in their translation into risk management, and identify the best ways to maintain the health of food animals, all while keeping in mind the wider risks of antimicrobial resistance, environmental impacts, and animal welfare considerations.
Keywords: animal agriculture, antimicrobials, antimicrobial resistance, critically important antimicrobials
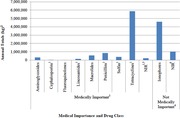
Most of the antibiotics sold for animal use in the US are either not considered medically important by the FDA (e.g., ionophores) or else are not included in the list of critically important antimicrobials for human medicine by the WHO (e.g., tetracyclines). Most of the highest priority critically important antimicrobials (HPCIA) have relatively low sales volumes (e.g., fluoroquinolones and cephalosporins), the exception to this being the macrolide class.
Introduction
The term antimicrobial refers to anything that inhibits bacteria, viruses, fungi, or microparasites. The term often is used synonymously with antibiotic, although the latter specifically refers to a microbial‐derived compound that is active against bacteria. Both terms are used interchangeably throughout the text here; in general—though not exclusively—“antimicrobial” is used in conjunction with resistance, whereas “antibiotic” is used when discussing a pharmacologically active agent. In recent years, there have been increasing concerns that the widespread use of antibiotics in food animal agriculture could lead to the emergence, spread, and propagation of bacteria that are antibiotic resistant. If these bacteria spread to human populations through food products, direct contact with animals, or via the environment, the resulting infections could be more difficult to treat if antibiotics of the same class or different classes of antimicrobials are used in both animals and in human medicine, leading to increased burdens of morbidity and mortality, and increased costs for care.1 To help navigate the complex set of medications involved and to ensure that the most essential drugs are used judiciously in both human and veterinary medicine, public and animal health organizations have created competing lists of “critically important antimicrobials” (CIAs) that rank these compounds according to their importance in human and veterinary medicine, respectively.2
This article will discuss how CIAs are defined and how international and national guidelines concerning their use are developed. The following is based on the presentations and discussions of the integrated discussion group meeting “Minimizing the Risk of Antimicrobial Resistance from Food Animal Production,” hosted by the New York Academy of Sciences on May 8 and 9, 2018. The authors—including veterinarians, microbiologists, epidemiologists, physicians, economists, and food safety and public health specialists—are from international academic and industry organizations.
Defining critically important antimicrobials
Antimicrobial classification from a global human and animal health perspective
Globally, despite arising from a public health agency, the World Health Organization (WHO) CIA list serves as the benchmark for food animal producers around the world and provides important guidance to global retail companies.2, 3 The WHO CIA list is the only one of its kind that considers global antimicrobial use and designates compounds as medically important if they have indications in human medicine anywhere in the world, and regardless of whether or not these are for infections caused by enteric bacteria common to animals and humans.3
The WHO published its first CIA list in 2005, and the fifth and most recent revision was released in 20162 and the 6th revision (2018) was scheduled for release in 2019. The 2016 list ranks antimicrobial compounds into four categories:
“Critically Important,” which includes two subcategories deemed “Highest Priority” and “High Priority.” An antimicrobial designated “Critically Important” must meet two criteria. The first is defined as “the sole, or one of limited available therapies, to treat serious bacterial infections in people” (Criterion 1). In addition, those infections must either “be transmitted to humans from nonhuman sources” or have the potential to “acquire resistance genes from nonhuman sources” (Criterion 2). Finer distinctions are made among the “Critically Important” antimicrobials, such as those with a “high frequency of use,” a “high proportion of use in patients with serious infections in health care settings,” or else those used “to treat infections in people for which there is evidence of transmission of resistant bacteria or resistance genes from nonhuman sources.” In turn, antimicrobials meeting any of these distinctions earn the designation “Highest Priority.”
“Highly Important” antimicrobials meet either Criterion 1 or 2 listed above but not both.
“Important” antimicrobials are any other products used in human medicine, meeting neither Criterion 1 nor 2.
“Currently not used in humans” is a category that reappeared on the 2016 list for the first time since 2005 and is listed in Annex 2 of the 2016 CIA.
The majority of antimicrobial classes on the WHO CIA list fall within the “Critically Important” category, with fewer classes in the “Highly Important” category, fewer still in the “Important,” and even fewer in the “Currently not used in humans” categories (Fig. 1).2 The Critically Important, Highly Important, and Important categories are also referred to as “Medically Important Antimicrobials.” The “Currently not used in humans” category is also referred to as “Non‐medically Important Antimicrobials.”
Figure 1.
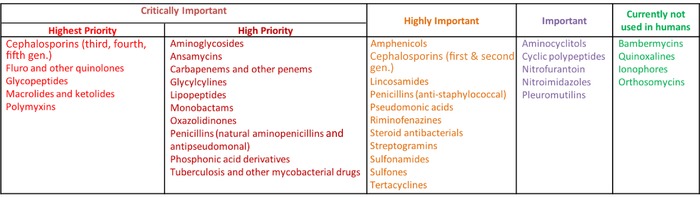
WHO list (5th revision, 2017) of critically important antimicrobials for human medicine. Adapted from Ref. 36.
Separate from the WHO CIA list, the World Organization for Animal Health (OIE) also created a CIA list of antimicrobials that are important in veterinary medicine. Although the hope from the scientific community was that the most important antimicrobials would not be on both lists, there is considerable overlap between the WHO and OIE lists.4 For example, macrolides, which have always been considered “Critically Important” on the WHO CIA list, were likewise placed in the “Veterinary Critically Important Antimicrobial Agents” (VCIA) category on the OIE list. However, bacitracin, a cyclic peptide, is low on the WHO CIA list (where it is classified as an “Important” antimicrobial), while it is ranked on the OIE list among the “Veterinary Highly Important Antimicrobial Agents” (VHIA).4 It should be noted that the two organizations used different criteria and goals to create their CIA lists, and in some instances there may be a need for further clarification and refinement in the interest of preventing antimicrobial resistance.
National and regional CIA lists
The WHO encourages countries and regions to create their own CIA lists based on the antibiotics used for human health in their area.2 Specifically, the WHO notes “that the implementation of the concept at national levels required that national considerations would be taken into account, and consequently lists may vary from country to country…”2 To ensure that the “national” or “regional” context is taken into account, these CIA lists should have precedence above other lists, including the WHO list.
To date, countries, such as the United States, Europe, Australia, and Japan, have country‐ or region‐specific CIA lists;5, 6, 7, 8 meanwhile, other countries are currently developing CIA lists, including China, Thailand, Malaysia, India, Philippines, and New Zealand. It is important to note that the national and regional CIA lists rarely are completely aligned with the WHO CIA list. On the other hand, such country‐specific lists are essential to address regional health priorities, resistance profiles, and approved drug uses that cannot be captured on a global scale. While this can create uncertainty among retailers and companies that source animal products locally, as well as import and export on a global scale, multinational companies deal with a highly complex compliance environment and are generally well equipped to deal with such variability.
U.S. FDA lists
An equivalent to the WHO CIA list for the United States is contained as Appendix A in FDA's 2003 Guidance for Industry #152.8 A much‐awaited revision was anticipated to begin before the end of 2018, but the process may still take several years. Currently, the FDA list, which is almost exclusively focused on foodborne pathogens (rather than all bacterial diseases of humans as per the WHO), includes three categories:
“Critically Important” antimicrobials are “used to treat enteric pathogens that cause foodborne disease” (Criterion 1), and are the “[s]ole therapy or one of few alternatives to treat serious human disease or drug is essential component among many antimicrobials in treatment of human disease” (Criterion 2).
“Highly Important” compounds meet either Criterion 1 or 2.
“Important” compounds are used to “treat enteric pathogens in nonfoodborne disease” (Criterion 3), and/or are not associated with “cross‐resistance within drug class” or “linked resistance with other drug classes” (Criterion 4), and/or there is “[d]ifficulty in transmitting resistance elements within or across genera and species of organisms” (Criterion 5).
The WHO (2016) and FDA (2003) lists agree in that they both rank third‐generation cephalosporins, fluoroquinolones, and macrolides in the “Critically Important” category. However, the WHO later designated fourth‐generation cephalosporins, glycopeptides (added in 2011), and polymyxins (added in 2016) as “Critically Important,” whereas the FDA categorized them in 2003 as only “Highly Important.”2 A decision taken by the Veterinary Medicines Advisory Committee (VMAC) of the FDA in 2006 to recommend against approval of a fourth‐generation cephalosporin product (cefquinome) for use in veterinary medicine suggests that its risk management was not affixed to 2003 data, which categorized fourth‐generation cephalosporins as highly important rather than critically important.9, 10 Several other contrasts exist between the WHO list, which designates pleuromutilins and cyclic polypeptides (e.g., bacitracin) as “medically important,” whereas the FDA considers the plueromutilins and bacitracin to be “non‐medically” important. In general, the rationale for differences among lists can be discerned through careful analysis of the regulatory process, stated purposes of each list, and the specified criteria. The effects on actual use and practice are much more difficult to measure or predict.
Other regional and national CIA lists
In Europe, the European Medicines Agency (EMA) released an antimicrobial ranking system in 2014, which classified antimicrobials according to three categories, whereby Category 1 poses the lowest risk to human health and Category 3 poses the highest risk and is “not approved for use in veterinary medicine.”7 As the EMA noted, Category 1 includes certain antimicrobials that are considered “Critically Important” by the WHO, such as macrolides and polymyxins, although some macrolides are seen as not critically important, based on their ring structure.7
Similarly, the CIA list in Japan classifies macrolides according to their ring structure.5 Fourteen‐ and fifteen‐member macrolides are considered “Critically Important,” with the exception of erythromycin, which is “Highly Important,” whereas 16‐member macrolides, such as tylosin, are deemed “Important.”
The Australian CIA list classifies macrolides as being “low” for human and veterinary medicine, which is a significant departure from other lists.6 Although Australia does not use fluoroquinolones in animal agriculture, the country still has low levels of fluoroquinolone‐resistant Campylobacter (especially when compared to Europe, South America, and most of the world except Canada and the United States); as a result, it is currently trying to understand the source of these resistant bacteria.11 Besides the macrolides, other notable differences between the Australian CIA and the WHO CIA include the ketolides and penicillins, which are “Critically Important” under the WHO criteria, but of “Low” importance under the Australian Importance Ratings. The difference in importance rating reflects the relatively low reliance on these antibacterials in Australia because resistance is widespread in many human pathogens causing infection in Australia. Although the rating for streptogramins has been reduced from “Critically Important” to “Highly Important” in the latest iteration of the WHO List, they remain in the highest category (high) in the Australian Importance Ratings. This is because pristinamycin is a reserve agent used for methicillin‐resistant Staphylococcus aureus infection in Australia.6
Given the different antibiotic treatment practices used in human medicine around the world, the disconnect between the WHO CIA list and the various national and regional lists seems inevitable. While each local CIA list is helpful from the perspective of human medicine, taken together they could potentially create difficulties when it comes to the trade of meat and livestock between countries with discrepant CIA lists. Indeed, there is anxiety in Latin American and Asian countries that they will be required to abide by the recommendations of countries they export to. To this end, the European parliament has recently proposed a resolution to ban the import of meat from countries that use antibiotic growth promoters, although the resolution was not approved.
Going forward, it is expected that the WHO, as well as national CIA lists, will be revised every 2–3 years. It will be unusual for a list to not be updated for 15‐plus years, as is the case with the FDA GFI 152 Appendix A.8 This fluidity will help keep these lists up to date, but could present challenges for food animal producers. For example, an antibiotic that played a key part in an animal health management system could be upgraded in importance because it was found to select for bacterial resistance after a resistance gene arose, which can occur without warning.12 Generally speaking, the uses of those antibiotics that are revised upward as to their criticality are restricted in turn from prevention (which is disallowed in many countries already) through disease control and finally to treatment. It is rare that antibiotic use for the treatment of infected and clinically ill animals is completely restricted; however, it remains an option especially for the class of highest priority CIAs.13, 14 Meanwhile, veterinarians in large food animal production companies are urging that antimicrobials that are used in human medicine but do not pose a threat for resistance, such as bacitracin, be granted protected status as safe for animal use.8
Factors affecting antibiotic use in animal agriculture
Several factors affect which antibiotics and how much of them are used in animal agriculture. Global and national regulations play an important role; but self‐imposed limitations within the private sector are also meaningful factors.
WHO recommendations for antimicrobial use in agriculture
In 2017, the WHO published four broad recommendations on the use of medically important antimicrobials in food animal production in order to maintain effectiveness of these antimicrobials in human medicine.3 To develop the basis for its recommendations, the WHO commissioned two independent systematic reviews, the first of which was published in Lancet Planetary Health in 2017.1, 15 The review concluded that interventions aimed at reducing antibiotic use in food‐producing animals were associated with reduced antibiotic resistance in these animals, but there was less compelling evidence that these interventions also reduced antibiotic resistance in human populations.
The first WHO recommendation calls for an “overall reduction in use of all classes of medically important antimicrobials in food‐producing animals.” Similarly, the second recommendation calls for the “complete restriction of use of all classes of medically important antimicrobials in food‐producing animals for growth promotion.” Those two recommendations were well received by experts in the food‐animal industry, particularly in the United States, where the FDA had already achieved a de facto ban on the use of medically important antibiotics for growth promotion in 2017 through voluntary withdrawal of labels by drug sponsors.
By contrast, Recommendation 3—which calls for the “complete restriction of use of all classes of medically important antimicrobials in food‐producing animals for prevention of infectious diseases that have not yet been clinically diagnosed”—has been rejected outright by many.16, 17 The main issue with this recommendation seems to be that there is no universally accepted definition of what is meant by prevention, as organizations each defines prevention in different ways and adds to the confusion. Nevertheless, on November 30, 2018, a major U.S. poultry producer committed to achieving the equivalent of the third WHO Guideline recommendation by March 2019 by removing its use of gentamicin and virginiamycin from its prevention protocols.18
Recommendation 4 from the WHO, which is a two‐part recommendation, is widely viewed as highly controversial.16, 19, 20 Recommendation 4a states that antimicrobials classified by WHO as “critically important for human medicine (…) should not be used for control of the dissemination of a clinically diagnosed infectious disease identified within a group of food‐producing animals.” Recommendation 4b states that antimicrobials that are the “highest priority critically important for human medicine should not be used for treatment of food‐producing animals with a clinically diagnosed infectious disease.” These latter two recommendations (4a and 4b) are supported by the lowest quality of evidence (per GRADE criteria) and are not strongly made by WHO (in contrast to Recommendation 3).21
The Chief Scientist of the U.S. Department of Agriculture (USDA) denounced these recommendations, stating they would “impose unnecessary and unrealistic constraints” on veterinarians’ professional judgment to treat, control, and prevent disease.16 The USDA also condemned the recommendations for being unsupported by sound data and out of alignment with U.S. policy.16 Similarly, the American Veterinary Medical Association (AVMA) criticized the recommendations as putting “unwarranted restrictions” on antibiotic use for protecting animal health and the food supply.17
Both the USDA and AVMA pointed out that the WHO developed the recommendations based on evidence that was by the WHO's own assessment of “low‐quality” (Recommendations 1–3) and “very low‐quality” (Recommendations 4a and 4b). However, the systematic review on which the recommendations were primarily based could only analyze available observational studies (cross‐sectional and longitudinal) that are by definition low quality according to the WHO grading system (GRADE). Some critics have suggested that the WHO change its grading system so that the types of studies needed here, which are not conducive to randomized trials, can be graded as being of higher quality. However, the philosophy at the WHO tends toward preemptive action, which leads to more cautious recommendations. Within this context, researchers are currently evaluating the grading system and the studies on which Recommendations 3 and 4 were based.
Clearly, there needs to be a more universally recognized definition of prevention. Equally important is a more universally accepted set of guidelines concerning prevention uses of medically important antibiotics, especially where alternative means of reducing the risk of disease are known to exist. McDonald's, in its 2015 Global Antibiotic Policy on stewardship, attempts to differentiate between “routine prevention” and “targeted prevention.”22 It would be appropriate that producers shift from using antimicrobials for routine prevention and instead identify critical life stages, or a set of risk factors, for when preventive use is justified. For instance, restricting antibiotic use to defined periods when animals are more vulnerable to infection because they are stressed or their immune systems are depressed, such as when chicks are moved into houses or when piglets are weaned. Alternatives to antibiotics, especially for prevention indications, are currently a topic of great interest to advocates, industry, and researchers.23 Notably, the WHO and many organizations do not currently distinguish between disease prevention, which is treating animals that do not have detectable pathogens but are at risk of being infected, and disease control, which involves treating groups of animals with varying numbers of detectable but often subclinical infections.3
U.S. regulatory framework
FDA regulations have also led to a reduction in domestic sales of antibiotics in animal agriculture,24 most notably through the FDA Guidance for Industry (GFI) 213 which called for the voluntary withdrawal of labels of medically important antibiotics used for growth promotion (AGP) in food animals.25 As of January 1, 2017, the FDA prohibited the use of medically important antibiotics for AGP, though a staged withdrawal of labels by sponsors was initiated over the previous 3‐year period and resulted in drastic reductions in the options available to producers. There remains a need for improved communication by the FDA about antibiotic practices that are permitted, including dosing and administration. While “over‐the‐counter” formulations of in‐feed and in‐water antibiotics for food‐producing animals have completely disappeared, veterinarians have been afforded a much greater role in decision making through Veterinary Feed Directives. However, the agency has approved new uses of medically important antibiotics in animal agriculture that appear counterintuitive to the goal of reducing antibiotic use. For example, a long‐duration formulation of the third‐generation cephalosporin ceftiofur is now labeled as requiring 2 doses 3 days apart, effectively doubling the mass of active ingredient used to treat metritis in dairy cows.26, 27 This approval was announced soon after the extra‐label use of cephalosporins in food‐producing animals was prohibited, albeit with several exceptions.28 Further, the agency has recently approved “control” labels for the fluoroquinolone enrofloxacin, opening the door to mass treatment (control) of groups of cattle and pigs to control respiratory disease.29
It should be noted that a number of countries have adopted the FDA model on the use of AGPs (e.g., Australia and Malaysia), and following a risk assessment approach have chosen to only ban the use of medically important antibiotics as AGPs. Other countries (e.g., New Zealand and Thailand) have taken a more precautionary approach, similar to the EU, and banned the use of all antibiotics for AGP use.
Assessing antibiotic use in animal agriculture
Historically, assessing the quantities and types of antibiotics that are used in animal agriculture has been challenging, which has made it very difficult to understand the effects of policies on antibiotic use. Antimicrobial drug sponsors are now required by the FDA to estimate the sales volume/mass of antimicrobials to each of the major food animal commodities (cattle, swine, chickens, and turkeys) to which they sell in the United States.30 This policy is aimed at tracking how drugs are used in veterinary medicine. These estimates are not likely to be precise, since the companies have little control over how drugs are actually used and for which indications. Animal health pharmaceutical companies have relations with their clients that currently permit reasonable estimates of sales to the target food animal species, if not for specific disease indications. As the data from veterinary feed directives (i.e., prescriptions) accumulate in the private sector, it is hoped that more refined estimates will be forthcoming regarding species‐specific sales data.31, 32 In addition, this means that sales data rather than usage data are used to infer antibiotic use; sales data are less exact, lack geographical specificity, and do not include information on companion animals. However, this is currently the best quality information on how antimicrobials are being used in food animal agriculture in the United States. While not ideal this is progress, since prior to this amendment data were aggregated across all species.
In the United States, domestic sales of all antimicrobial drug classes licensed for use in food‐producing animals in the United States decreased by 10% from 2015 to 2016, after steadily increasing from 2009 to 2015, according to an FDA 2016 Summary Report.24 Over this period, two antimicrobial classes have dominated sales in the United States for food‐producing animals in terms of total weight (kilograms) of drug for primarily domestic sales of: (1) ionophores (not medically important), which are predominantly sold for use in cattle and chickens; and (2) tetracyclines, which are primarily sold for use in cattle and swine and are considered medically important.24 Figure 2 shows the sales data for each of the antimicrobial classes from 2016. Notably, the antibiotics most commonly used in human medicine, such as β‐lactam antibiotics including cephalosporins and penicillin, make up less than 10% of sales by mass in food‐producing animals.33
Figure 2.
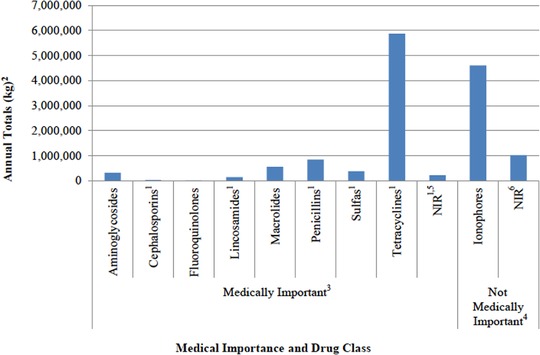
Domestic sales and distribution of antimicrobial drugs approved for use in food‐producing animals in 2016. Reproduced from U.S. FDA 2016 Summary Report.
1Includes antimicrobial drug applications, which are approved and labeled for use in both food‐producing
animals (e.g., cattle and swine) and nonfood‐producing animals (e.g., dogs and horses).
2kg = kilogram of active ingredient. Antimicrobials, which were reported in International Units (IU) (e.g., penicillins), were converted to kg. Antimicrobial class includes drugs of different molecular weights, with some drugs reported in different salt forms.
3Guidance for Industry #213 states that all antimicrobial drugs and their associated classes listed in Appendix A of FDA's Guidance for Industry #152 are considered “medically important” in human medical therapy.
4Not Medically Important refers to any antimicrobial class not listed in Appendix A of FDA's Guidance for Industry #152.
5NIR = Not Independently Reported. Antimicrobial classes for which there were fewer than three distinct sponsors actively marketing products domestically are not independently reported. These classes include the following: amphenicols, diaminopyrimidines, polymyxins, and streptogramins.
6NIR = Not Independently Reported. Antimicrobial classes for which there were fewer than three distinct sponsors are not independently reported. These classes include the following: aminocoumarins, glycolipids, orthosomycins, pleuromutilins, polypeptides, and quinoxalines.
Other FDA requirements pertaining to how antibiotics must be administered may actually drive up drug use in practice. For example, a recent study found that intermittent feeding of tylosin achieves reduction of liver abscesses in cattle comparable to continuous feeding, but this strategic antibiotic use may violate laws governing specific use practices.34 Given the regulations on antibiotic use, veterinarians may be required to administer higher doses than they feel is necessary, or for longer than needed, to prevent disease.35
Conclusions
In order to prioritize and protect the most essential antibiotics used in human medicine, the WHO and national governments have created CIA lists. Competing lists for animal health also exist at similar scales and serve to prioritize antibiotics among the range of acceptable uses in food animal agriculture. Additional recommendations and regulations have been put in place to curtail the use of antibiotics, which have helped to reduce the use of antimicrobial use in food animal production. However, more data are needed to better understand how antimicrobials are used in animal agriculture to find opportunities for further reduction. Additional research into alternatives to antibiotics in order to prevent disease, coupled with more judicious use of antibiotics needed to treat and control infectious diseases in food animals, is necessary.
Competing interests
J.G. is currently a member of the scientific advisory council of the National Pork Board, which is interested in antibiotic use and resistance in swine production. P.M. received support from JBS Five Rivers Cattle Feeding and Feedlot Health Management Services. M.J.S. is a former employee of Elanco Animal Health. S.S. is an employee of Elanco Animal Health. R.S.S. received funding from the Animal Agriculture Alliance, Elanco Animal Health, Zoetis, and Bayer Animal Health. T.C.S. received funding to assist in a clinical trial from GOJO related to general hand hygiene.
Acknowledgment
This work was funded by the New York Academy of Sciences through a grant from Elanco Animal Health.
References
- 1. Tang, K.L. , Caffrey N.P., Nóbrega D.B., et al 2017. Restricting the use of antibiotics in food‐producing animals and its associations with antibiotic resistance in food‐producing animals and human beings: a systematic review and meta‐analysis. Lancet Planet. Health 1: e316–e327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . 2017. WHO list of critically important antimicrobials for human medicine (WHO CIA list). 5th revision. World Health Organization.
- 3. World Health Organization . 2017. WHO guidelines on use of medically important antimicrobials in food‐producing animals. WHO. [PubMed]
- 4. World Organisation for Animal Health . 2015. OIE list of antimicrobial agents of veterinary importance. OIE International Committee. Vol. 33, pp. 1–9. [Google Scholar]
- 5. Food Safety Commission of Japan . 2014. Ranking of the importance of antimicrobials against bacteria which affect human health through food commodities.
- 6. Australian Strategic and Technical Advisory Group on AMR . 2018. Importance ratings and summary of antibacterial uses in human and animal health in Australia.
- 7. European Medicines Agency . 2014. Answers to the request for scientific advice on the impact on public health and animal health of the use of antibiotics in animals. Vol. 381884, pp. 1–84. [Google Scholar]
- 8. Food and Drug Administration Center for Veterinary Medicine . 2003. Evaluating the safety of antimicrobial new drugs with regard to their microbiological effects on bacteria of human health concern. Guidance for Industry #152. pp. 1–36. [Google Scholar]
- 9. U.S. Food and Drug Administration . 2006. Cefquinome formulations for parenteral injection for the treatment of bovine respiratory disease.
- 10. Snelson, H. 2006. American Association of Swine Veterinarians. Accessed December 11, 2018. https://www.aasv.org/news/story.php?id=2084.
- 11. Cheng, A.C. , Turnidge J., Collignon P., et al 2012. Control of fluoroquinolone resistance through successful regulation, Australia. Emerg. Infect. Dis. 18: 1453–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. European Medicines Agency . 2016. Accessed December 11, 2018. https://www.ema.europa.eu/en/news/countries-should-reduce-use-colistin-animals-decrease-risk-antimicrobial-resistance.
- 13. Agencia de Regulacion y Control Fito y ZoosAnit Ario Ecuador . 2019. Resolution 0003. Ecuador. [Google Scholar]
- 14. Government of Argentina . 2019. Accessed February 13, 2019. https://www.boletinoficial.gob.ar/#!DetalleNorma/200151/20190115.
- 15. Scott, A.M. , Beller E., Glasziou P., et al 2018. Is antimicrobial administration to food animals a direct threat to human health? A rapid systematic review. Int. J. Antimicrob. Agents 52: 316–323. [DOI] [PubMed] [Google Scholar]
- 16. U.S. Department of Agriculture . 2017. Accessed October 17, 2018. https://www.usda.gov/media/press-releases/2017/11/07/usda-chief-scientist-statement-who-guidelines-antibiotics.
- 17. American Veterinary Medical Association . 2017. AVMA@Work 2017. Accessed October 10, 2018. https://atwork.avma.org/2017/11/10/who-antimicrobial-guidelines-fall-short-of-protecting-human-and-animal-health/.
- 18. Sanderson Farms . 2018. Accessed December 11, 2018. http://ir.sandersonfarms.com/news-releases/news-release-details/sanderson-farms-inc-announces-change-antibiotic-use-program.
- 19. Wagstrom, L. 2017. Statement of NPPC Chief Veterinarian Dr. Liz Wagstrom on WHO call for ban on prevention uses of antibiotics.
- 20. International Poultry Council . 2017. IPC statement on WHO antimicrobial guidelines. Accessed March 1, 2019. http://www.internationalpoultrycouncil.com/ipc-on-the-press/ipc-press-releases/ipc-responds-to-who-guidelines-on-antimicrobials.
- 21. Schünemann, H. , Brożek J., Guyatt G., et al, Eds. 2013. GRADE Handbook . GRADE Working Group.
- 22. McDonald's . 2015. Accessed November 2, 2018. https://news.mcdonalds.com/media-statements/food-details/statement-antibiotic-use.
- 23. The Pew Charitable Trusts . 2017. Alternatives to antibiotics in animal agriculture.
- 24. U.S. Food and Drug Administration . 2016. 2016 Summary report on antimicrobials sold or distributed for use in food‐producing animals.
- 25. U.S. Food and Drug Administration . 2013. Guidance for industry #213 new animal drugs and new animal drug combination products administered in or on medicated feed or drinking water of food‐producing animals: recommendations for drug sponsors for voluntarily aligning product use conditions with. Guidance for Industry #213, pp. 1–18. [Google Scholar]
- 26. U.S. Food and Drug Administration . 2012. New animal drugs; Ceftiofur crystalline free acid; Gamithromycin; Tylosin. U.S. Food and Drug Administration. pp. 26161–26162. [Google Scholar]
- 27. Zoetisus . Accessed December 11, 2018. https://www.zoetisus.com/products/pages/excede_dairy/treat_metritis.aspx.
- 28. U.S. Food and Drug Administration . 2012. Accessed December 11, 2018. https://www.fda.gov/AnimalVeterinary/SafetyHealth/AntimicrobialResistance/ucm421538.htm.
- 29. U.S. Food and Drug Administration . 2012. New animal drugs; Enrofloxacin; Tylvalosin.
- 30. U.S. Food and Drug Administration . 2017. Accessed December 11, 2018. https://www.fda.gov/ForIndustry/UserFees/AnimalDrugUserFeeActADUFA/ucm446786.htm.
- 31. U.S. Food and Drug Administration . 2017. https://www.fda.gov/AnimalVeterinary/NewsEvents/CVMUpdates/ucm588086.htm.
- 32. U.S. Food and Drug Administration . 2012. Guidance for industry ‐ #209 the judicious use of medically important antimicrobial drugs in food‐producing animals. Federal Register: 1–19. [Google Scholar]
- 33. U.S. Food and Drug Administration . 2014. 2011 Summary report on antimicrobials sold or distributed for use in food‐producing animals.
- 34. Müller, H.C. , Van Bibber‐Krueger C.L., Ogunrinu O.J., et al 2018. Effects of intermittent feeding of tylosin phosphate during the finishing period on feedlot performance, carcass characteristics, antimicrobial resistance, and incidence and severity of liver abscesses in steers. J. Anim. Sci. 96: 2877–2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. U.S. Food and Drug Administration . 2017. Accessed October 10, 2018. https://www.fda.gov/animalveterinary/resourcesforyou/ucm380135.htm#Conditions_for_Extra-Label_Drug_Use_in_Food-Producing_Animals.
- 36. Doyle M.P., Loneragan G.H., Scott H.M., et al 2013. Antimicrobial resistance: challenges and perspectives. Compr. Rev. Food Sci. Food Saf. 12: 234–248. [Google Scholar]


