Abstract
Objectives
This longitudinal comparative study investigated the effect of preventive chemotherapy (PC) on covert tissue changes associated with lymphatic filariasis (LF) among young people living in an LF‐endemic area in Myanmar.
Methods
Tissue compressibility and extracellular free fluid in the lower limbs of people aged 10–21 years were measured using indurometry and bioimpedance spectroscopy (BIS). Baseline measures were taken in October 2014, annual mass drug administration (MDA) of PC was delivered in December, and in March 2015 further PC was offered to LF‐positive cases who had missed MDA. Follow‐up measures were taken in February and June 2015.
Results
A total of 50 antigen‐positive cases and 46 antigen‐negative controls were included. Self‐reported PC consumption was 60.1% during 2014 MDA and 66.2% overall. At second follow‐up, 24 of 34 cases and 27 of 43 controls had consumed PC. Significant and clinically relevant between‐group differences at baseline were not found post‐PC. Bayesian linear mixed models showed a significant change in indurometer scores at both calves for antigen‐positive cases who consumed any PC (dominant calf: −0.30 [95% CI −0.52, −0.07], P < 0.05 and non‐dominant calf: −0.35 [95% CI −0.58, −0.12], P < 0.01). Changes in antigen‐negative participants or those not consuming PC were not significant.
Conclusion
This study is the first attempt to use simple field‐friendly tools to track fluid and tissue changes after treatment of asymptomatic people infected with LF. Results suggested that PC alone is sufficient to reverse covert lymphatic disturbance. Longer follow‐up of larger cohorts is required to confirm these improvements and whether they persist over time. These findings should prompt increased efforts to overcome low PC coverage, which misses many infected young people, particularly males, who are unaware of their infection status, unmotivated to take PC and at risk of developing lymphoedema. Indurometry and BIS should be considered in assessment of lymphatic filariasis‐related lymphedema.
Keywords: lymphedema, lymphatic filariasis, preventive chemotherapy, mass drug administration, global programme to eliminate lymphatic filariasis, lower extremity, indurometry, bioimpedance spectroscopy
Abstract
Objectifs
Cette étude comparative longitudinale a investigué l'effet de la chimiothérapie préventive (CP) sur les modifications tissulaires cachées associées à la filariose lymphatique (FL) chez les jeunes vivant dans une zone d'endémie pour la FL au Myanmar.
Méthodes
La compressibilité des tissus et le liquide libre extracellulaire dans les membres inférieurs des personnes âgées de 10 à 21 ans ont été mesurés par indurométrie et spectroscopie de bioimpédance (BIS). Les mesures de base ont été prises en octobre 2014, la distribution en masse de médicament (DMM) annuelle a été administrée en décembre et en mars 2015, et une CP additionnelle a été offerte aux cas positifs pour la FL qui avaient manqué la DMM. Des mesures de suivi ont été prises en février et juin 2015.
Résultats
50 cas positifs pour l'antigène et 46 témoins négatifs ont été inclus. L'administration de CP auto‐déclarée était de 60,1% durant la DMM de 2014 et de 66,2% au total. Au deuxième suivi, 24 des 34 cas et 27 des 43 témoins avaient pris la CP. Des différences significatives et cliniquement pertinentes entre les groupes au départ n'ont pas été trouvées après la CP. Les modèles mixtes linéaires bayésiens ont montré un changement significatif des scores d'indurometrie aux deux mollets pour les cas positifs pour l'antigène qui prenaient une CP (mollet dominant: ‐0,30 [IC95%: ‐0,52, ‐0,07], p <0,05, mollet non dominant: ‐ 0,35 [IC95%: ‐0,58, ‐0,12], p <0,01). Les changements chez les participants négatifs pour l'antigène ou ceux qui ne prenaient pas de CP n’étaient pas significatifs.
Conclusion
Cette étude est la première tentative d'utilisation d'outils simples, conviviaux sur le terrain, pour suivre les modifications du tissu conjonctif après le traitement de personnes asymptomatiques infectées par la FL. Les résultats suggèrent que la CP seule est suffisante pour inverser les modifications lymphatiques cachées. Un suivi plus long de plus grandes cohortes est nécessaire pour confirmer ces améliorations et déterminer si elles persistent ou non. Ces résultats devraient inciter à redoubler d'efforts pour surmonter la faible couverture en CP, qui rate beaucoup de jeunes infectés, en particulier les hommes, qui ne sont pas au courant de leur statut d'infection, qui ne sont pas motivés pour prendre une CP et risquent de développer un lymphœdème. L'indurométrie et la BIS devraient être considérées dans l’évaluation du lymphoedème associé à la filariose lymphatique.
Keywords: lymphoedème, filariose lymphatique, chimiothérapie préventive, administration en masse de médicaments, programme mondial d’élimination de la filariose lymphatique, extrémités inférieurs, indurométrie, spectroscopie de bio‐impédance
Introduction
Lymphatic filariasis (LF) is a major cause of disability and the most widespread of the neglected tropical diseases 1. Several mosquito species are capable of transmitting microfilariae, and there are three main species of worm, the most common being Wuchereria bancrofti which is endemic in many countries throughout the western Pacific, Asia and Africa 2. Home for the thread‐like worms is the human lymphatic system where they typically reside in worm nests established close to lymph nodes in the groin. Of the two main sequelae, lymphedema and hydrocele, only hydrocele can be cured with surgical intervention. Manifest lymphedema is irreversible and current estimates put the number of people living with chronic filariasis‐related lymphedema (FRL) at 16.7 million worldwide 3. The global programme to eliminate LF (GPELF) as a public health problem operates at two levels. To interrupt transmission of microfilariae, annual mass drug administration (MDA) of preventive chemotherapy (PC) is distributed to all residents in LF‐endemic areas; WHO recommends PC coverage of 65% of the total population for 4–6 years. For people who already have chronic disease, morbidity management and disability prevention (MMDP) services should be provided 4.
Lymphedema is an accumulation of protein‐rich fluid in the skin and subcutaneous tissue that would normally be removed by continuous pumping of the lymphatic vessels 5. In LF, host inflammatory responses to worm antigen and the endosymbiotic Wolbachia bacteria can lead to lymphatic insufficiency 6, but lymphedema can also be caused by any damage or congenital malformation of the lymphatic system 7. In breast cancer‐related lymphedema (BCRL) of the arm, the most researched form of lymphedema, it is excision of lymph nodes in the arm pit, radiation treatments and subsequent tissue scarring that can disrupt lymph flow 8. Whatever the cause, the pathogenesis of lymphedema follows a largely predictable course. Early protein‐rich fluid accumulation in the tissue spaces is gradually replaced by hyperplasia of fibrous and fatty tissue and enlargement of the affected limb or body parts. In advanced stages, hyperkeratosis gives the skin a thick and scaly appearance and ulcerations and papillomatosis are common. As lymphedema progresses from transient swelling to irreversible limb enlargement, treatment becomes increasingly difficult and resource‐intensive 9. Skin pathologies increase the risk of secondary bacterial and fungal infections, and later stages are associated with significant disability 10.
From studies on BCRL, it is known that onset of lymphedema can be delayed years or even decades after the original exposure to the risk 11, 12 and a growing body of evidence indicates that early detection and intervention have the potential to reverse covert changes and prevent lymphedema from forming 13, 14, 15. This has led to development of monitoring protocols in those at risk of BCRL and employment of preventive interventions before any overt symptoms appear 16. In LF, parasitic infection usually begins in childhood 17, and children with LF typically remain asymptomatic until young adulthood with a long interval between infection and the onset of overt lymphedema. This presents an extended period in which to identify those at risk of progression and initiate preventive interventions, but in contrast to guidelines for BCRL, GPELF recommendations do not attempt to identify or intervene in covert FRL. The potential effectiveness of PC in reversing covert and early‐stage disease has been reported in two studies which both used a similar methodology 18, 19. Both studies were on cohorts of 100 children with filarial disease aged 5–18 years and both found that PC medication reversed covert tissue changes (assessed by lymphoscintigraphy) and some mild, overt lymphedema. However, neither included a control group to assess the effect over time of either no PC or PC on uninfected individuals. Furthermore, one was conducted on children with Brugia malayi infection 19 and both studies involved 100% PC coverage, so neither would reflect actual MDA coverage in countries endemic for W. bancrofti 18, 19.
All mainland South‐East Asian countries are endemic for LF 20. In Myanmar, this includes 45 of the 64 districts and the central western ‘dry zone’ is highly endemic, with pre‐MDA prevalence estimates of 20–30% 21. The mean national prevalence has fallen from 7.1% in 2001 to 2.7% in 2011, but MDA coverage has been inconsistent in some areas 20. A recent survey of a representative sample of individuals in Mandalay Region indicated that PC was consumed, on average, fewer than half of the six times MDA had been delivered between 2001 and 2015 22. A cross‐sectional study in Myanmar on asymptomatic young people infected with W. bancrofti found covert increases in tissue compressibility and extracellular free fluid in the lower extremities of antigen‐positive young people compared to their antigen‐negative peers 23. This confirmed similar findings of a pilot study in an endemic region in Papua New Guinea (PNG) where increased tissue compressibility was also identified in lower limbs of young asymptomatic people with LF 24.
The transient nature of these covert tissue changes in relation to progression to FRL, or the effect of PC medications on covert disease in actual MDA conditions, has not been investigated. Therefore, we conducted a comparative cohort study to investigate changes in lower limb tissue compressibility and extracellular free fluid in asymptomatic individuals with different LF antigen status before and after annual MDA in Mandalay Region, Myanmar.
Methods
During October 2014 (Oct 14), a convenience sample of young people aged 10–21 years residing in Amarapura Township, Central Myanmar, was invited to be screened for LF infection using a rapid field immunochromatographic test (ICT) for the presence of W. bancrofti antigen (Binax NOW, Alere, USA). Antigen‐positive cases and a similar cohort (by age and gender) of antigen‐negative controls were invited to continue in the longitudinal study. A timeline of study activities can be seen in Figure 1. The study was approved by the James Cook University Human Research Ethics Committee approval number H5261 and the Myanmar Ministry of Health (now known as the Ministry of Health and Sports (MOHS)).
Figure 1.
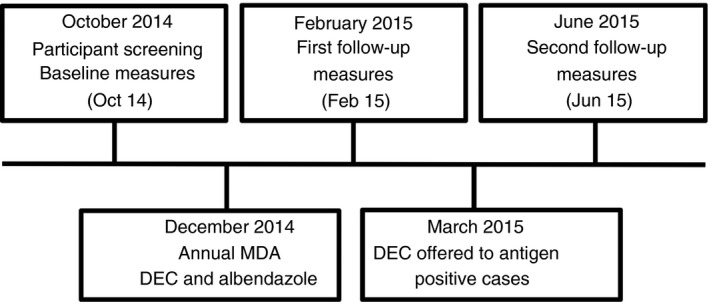
Timeline of data collection activities.
Detailed accounts of sample size calculation, recruitment, consent and screening methods have been reported previously 23, 25. The annual MDA, a single dose of albendazole and diethylcarbamazine citrate (DEC) 26, 27, was distributed in Mandalay Region during December 2014 (Dec 14), and follow‐up measures were obtained during February 2015 (Feb 15). At this follow‐up, fewer than the recommended 65% of participants reported PC consumption during the Dec 14 MDA. The Myanmar MOHS was advised of this, and a course of DEC (12 daily doses as per Myanmar MOHS policy) was offered to positive cases (as detected by ICT) during March 2015. Further follow‐up data were then collected in June 2015 (Jun 15).
Data collection
Procedures for physical measures and blood collection used at baseline (Oct 14) 23 were repeated at each follow‐up. Local research assistants interviewed each participant to ascertain any changes in their medical status and recorded height and weight measures. Leg dominance was determined by asking which leg was used to kick a ball. A 10 ml venous blood sample was collected into a cooled ethylenediaminetetraacetic acid (EDTA) anticoagulant vacutainer (BD Biosciences, North Ryde, Australia) and used to confirm the LF antigen status of all participants using Og4C3 ELISA (Cellabs, Australia). Detailed blood collection and plasma handling procedures are reported in the cross‐sectional analysis of baseline data 23 and in a report on the results of the serological analyses 25.
The procedure for collecting device measures has also been reported in detail 23, 28, 29. A tape measure was used to locate the mid‐point of the anterior thigh, posterior thigh and calf segments of each leg. An indurometer (SA Biomedical Engineering, Australia) was used to assess tissue compressibility at each of the marked mid‐points. This electromechanical device uses an indenter pressed into the skin with a defined force to measure the resistance (stiffness) or compliance (compressibility) in the underlying skin and tissue. The hand‐held unit has a 7‐cm‐diameter reference plate with a 1‐cm‐diameter indenter. With the reference plate rested flat to the skin over the marked point, the indenter is pushed into the skin by the operator and a force sensor detects when the equivalent to a 200 g force has been applied. The displacement between the indenter and the reference plate (to what extent the indenter is able to compress the underlying tissue) is detected in 0.01 increments and displayed on a digital light‐emitting diode (LED) screen. This device has been previously shown to have excellent reliability in uninfected young people in Australia and Myanmar 28.
Whole leg extracellular free fluid was assessed by BIS using the SBF7 (Impedimed, Australia), a portable battery‐operated device. Self‐adhesive electrodes were placed on the skin according to the manufacturers’ instructions for whole‐limb analysis. When the device is activated, a multifrequency low‐level current passes between the electrodes and through the epifascial compartment. The resistances (impedance) in the extracellular and intracellular fluid compartments are recorded separately and expressed as a ratio of ICF:ECF (Ri:Re). Images of the indurometer and SBF7 and their use in Myanmar can be seen in additional Figures S1 and S2.
At baseline (Oct 14), participants were asked whether they had consumed PC during the previous MDA (September 2013). After device measures and blood samples had been collected, advice was given to each participant and the attending guardian of minors on their LF status and the importance of everyone – regardless of infection status – consuming the PC which would be offered during the 2014 MDA (the following December). At the Feb 15 follow‐up, they were asked about their participation in the 2014 MDA, and at the Jun 15 follow‐up, they were asked whether they had consumed the additional PC medication offered in March.
Data on average monthly temperature and relative humidity for Amarapura Township were collected retrospectively using an online source 30. The average monthly temperature and humidity data were used to estimate the heat index for each time point using an online calculator 31. A heat index can be used to determine the risk of heat stress in varying conditions 32.
Analysis
A sample size of 32 in each group was calculated to detect a 10% difference between groups at baseline with 80% power, based on a mean mid‐calf indurometer value of 2.5 with SD of 0.7 23, 29. BMI was calculated using the formula kg/m2, and participants were classified as underweight if they were more than two standard deviations below the mean BMI‐for‐age using WHO definitions and growth tables 33. Systemic hydration was approximated by the length of time since the last drink was taken with less than or more than 60 min defining the more‐ or less‐hydrated groups, respectively. Consumption of PC was determined by self‐report.
At each time point, participant characteristics and device scores were analysed using independent t‐tests to identify any between‐infection group differences, and one‐way ANOVA was used to compare within‐group characteristics over three time points. t‐Tests and one‐way ANOVA were performed using SPSS (v24, IBM Corp). A clinically relevant difference in device score was determined as ≥ 5% for indurometer measures 7 and ≥3% for BIS measures 13. Device scores were further analysed using Bayesian linear mixed models to account for time, age, gender, BMI, infection status and PC consumption. Study participants were specified as participant‐level random intercepts. Leg dominance is known to influence device measures 29, 34, and therefore, before and after comparisons were considered by individual limb. The R statistical software v.3.4.4 was used to perform the analyses 35. Bayesian linear mixed models were fit with the Stan program v.2.17.0 36 through the Rstan v.2.17.3 37 and brms v.2.2.0 38 interfaces.
Participants
Volunteers (n = 104) at baseline (Oct 14) were resident in 11 villages in Amarapura Township located within the village tracts shown on the map in Figure 2 39. All data were collected out at the Nge Toe Village administration centre.
Figure 2.
Map of Amarapura Township area (coloured) showing the village tracts (shaded) where study participants were resident at the time of recruitment into the cross‐sectional study. Mandalay city centre is also identified (star).
A flow chart of participants through the study is provided in Figure 3. Participants were classified as LF antigen‐positive if they were positive by either ICT or Og4C3 (confirmed by repeat tests) 23. Data from two controls that tested negative by ICT were excluded from analysis due to discordant Og4C3 results on repeat testing as this discrepancy could not be resolved. At the Feb 15 follow‐up, eight participants could not be found or declined to return. At the Jun 15 follow‐up, four people who had missed the Feb 15 follow‐up presented for measures, but 14 others could not be found or did not return for unknown reasons. At the Jun 15 follow‐up, one participant revealed that she was five months pregnant which meant that she had been pregnant during the Feb 15 follow‐up measures but not at baseline and her data set was excluded from the follow‐up analysis. This provided 96 participants at baseline, 87 participants at the Feb 15 follow‐up and 77 participants at the Jun 15 follow‐up for the longitudinal analysis.
Figure 3.
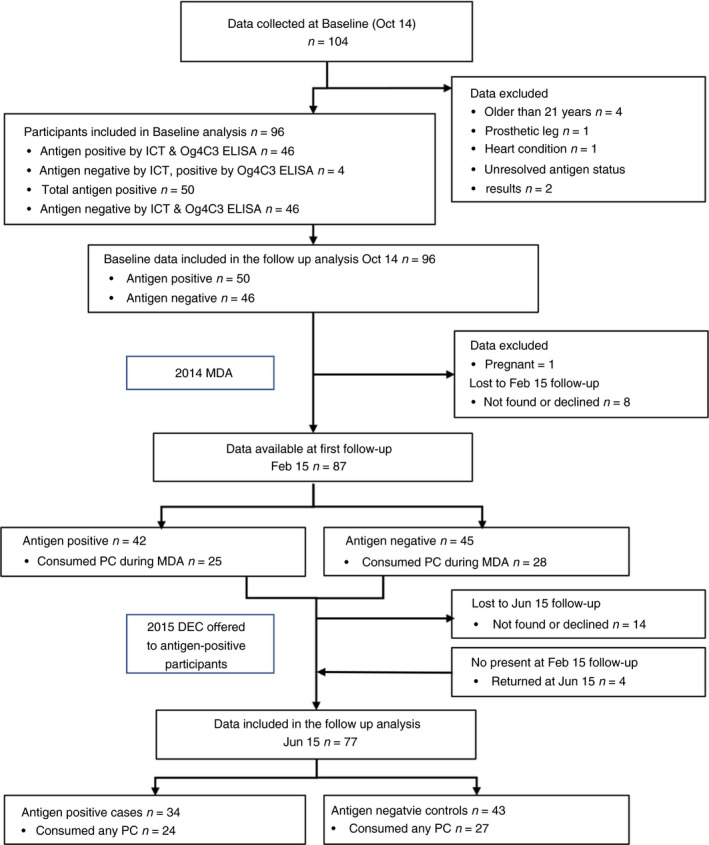
Flow chart of participants through the study. [Colour figure can be viewed at http://wileyonlinelibrary.com]
Participant characteristics are given in Table 1. At baseline, the mean cohort age was 15.3 (SD 3.41) years and 55.2% were female. There were no significant between‐infection group differences for age, gender, height, weight, BMI, BMI‐for‐age, occupation (student or working), time since the last menstrual period (females ≥14 days since last menses) or hydration (drank ≤60 min before the measures were taken) and this remained true at the Feb 15 and Jun 15 follow‐ups. Between‐infection group characteristics at each time point are given in additional Table S1. Within‐infection group characteristics did not change significantly over time other than the level of recent hydration (see Table 1). At the Jun 15 follow‐up, significantly more people had consumed a drink within the hour prior to measurement in both groups than at baseline (whole cohort; Oct 14 26.0%, Jun 15 59.7%, P < 0.000).
Table 1.
Group characteristics and moderating factors at three time points
| Participant characteristic | Ag status | Oct 14 n = 96 Ag− n = 46 Ag+ n = 50 | Feb 15 n = 87 Ag− n = 45 Ag+ n = 42 | Jun 15 n = 77 Ag− n = 43 Ag+ n = 34 | P |
|---|---|---|---|---|---|
| Age in years, mean (SD) | Negative | 15.41 (3.47) | 15.33 (3.30) | 16.28 (3.35) | 0.350a |
| Positive | 15.20 (3.38) | 14.98 (3.50) | 15.38 (3.68) | 0.879a | |
| Female, n = (%) | Negative | 26 (56.5%) | 27 (60.0%) | 28 (65.1%) | 0.707b |
| Positive | 27 (54.0%) | 23 (54.8%) | 22 (64.7%) | 0.579b | |
| Height in cm, mean (SD) | Negative | 152.55 (11.65) | 153.28 (11.34) | 154.10 (9.58) | 0.798a |
| Positive | 151.80 (12.56) | 151.56 (12.57) | 149.65 (12.07) | 0.712a | |
| Weight in kg, mean (SD) | Negative | 42.62 (10.16) | 44.05 (9.81) | 44.61 (9.30) | 0.612a |
| Positive | 42.27 (12.81) | 43.29 (12.98) | 40.36 (11.83) | 0.598a | |
| BMI, mean (SD) | Negative | 18.04 (2.79) | 18.50 (2.68) | 18.67 (2.78) | 0.531a |
| Positive | 18.04 (3.33) | 18.40 (3.36) | 17.57 (3.23) | 0.562a | |
| Underweight for age n = (%) | Negative | 6 (13.0%) | 6 (13.3%) | 3 (7.0%) | 0.567b |
| Positive | 7 (14.0%) | 5 (11.9%) | 7 (20.6%) | 0.554b | |
| Occupation, student n = (%) | Negative | 13 (28.3%) | 12 (26.7%) | 9 (20.9%) | 0.609b |
| Positive | 14 (28.0%) | 14 (33.3%) | 9 (26.4%) | 0.152b | |
| Menstruation ≥ 2 weeks n = (%) | Negative | 7 (26.9%) | 5 (18.5%) | 7 (25.0%) | 0.597b |
| Positive | 8 (16.0%) | 9 (21.4%) | 2 (5.9$) | 0.425b | |
| Hydration ≤ 60 min n = (%) | Negative | 12 (26.1%) | 19 (42.2%) | 26 (60.5%) | 0.016b |
| Positive | 13 (26.0%) | 15 (35.7%) | 20 (58.8%) | 0.009b |
Ag, antigen status; BMI, body mass index; SD, standard deviation.
One‐way ANOVA.
Pearson chi‐square.
Consumption of PC
At the first follow‐up (Feb 15), 60.1% of all returning participants reported they had taken PC during the 2014 MDA. Table 2 shows the PC consumption for antigen‐positive and antigen‐negative groups and by gender. There was no significant between‐infection group difference in the proportion of PC consumed during the 2014 MDA (antigen‐positive 59.5% and antigen‐negative 62.2%, P = 0.800); however, there were large differences between consumption by sex (male = 29.7%, female = 64% P = 0.392.). At the Jun 15 follow‐up after the ICT‐positive cases were offered further PC, a total of 66.2% of participants had taken PC either during the 2014 MDA or in March 2015.
Table 2.
Self‐reported consumption of PC by infection group and sex
| Participants | 2013 MDA | 2014 MDA | 2014 MDA or DEC | |
|---|---|---|---|---|
| Antigen‐negative | All n = (%) | 22 (47.8%) | 28 (62.2%) | 27 (62.8%) |
| Males n = (%) | 13 (65.0%) | 2 (11.1%) | 11 (73.3%) | |
| Females n = (%) | 9 (34.6%) | 16 (59.3%) | 16 (57.1%) | |
| Antigen‐positive | All n = (%) | 17 (34.0%) | 25 (59.5%) | 24 (70.6%) |
| Males n = (%) | 15 (65.2%) | 9 (47.4%) | 8 (66.7%) | |
| Females n = (%) | 2 (7.4%) | 16 (69.6%) | 16 (72.7%) | |
DEC, diethylcarbamazine citrate; MDA, mass drug administration of albendazole and DEC.
Device measures
There was a prevailing pattern of tissue compressibility at all time points. Highest indurometer values (most compressible tissue) were always found over the anterior thighs, and the lowest values (stiffest tissue) were always at the mid‐calf where there is little underlying fat. Whole leg free fluid was higher in the dominant leg. This pattern of tissue compressibility and free fluid in the lower limbs is consistent with previous reports on young people without infection in Australia and Myanmar 29. Whole cohort mean values for indurometer and BIS scores at all time points are provided in additional Table S2.
There was significant variation in unadjusted device scores for the whole cohort associated with time, but the direction of the association was not consistent with natural linear growth (see Figure 4a,b). Rather, a nonlinear effect was detected where tissue compressibility was lower and free fluid was higher at the Feb 15 follow‐up than at baseline, but this had reversed again at the Jun 15 follow‐up. Compared to baseline, at the Jun 15 follow‐up there was a significant increase in tissue compressibility at the dominant anterior thigh (9.1%, P < 0.000) (Figure 4a), and a statistically and clinically significant reduction in free fluid in both legs (dominant leg −13.6%, P < 0.000) (non‐dominant leg −7.7%, P = 0.019) (Figure 4b).
Figure 4.
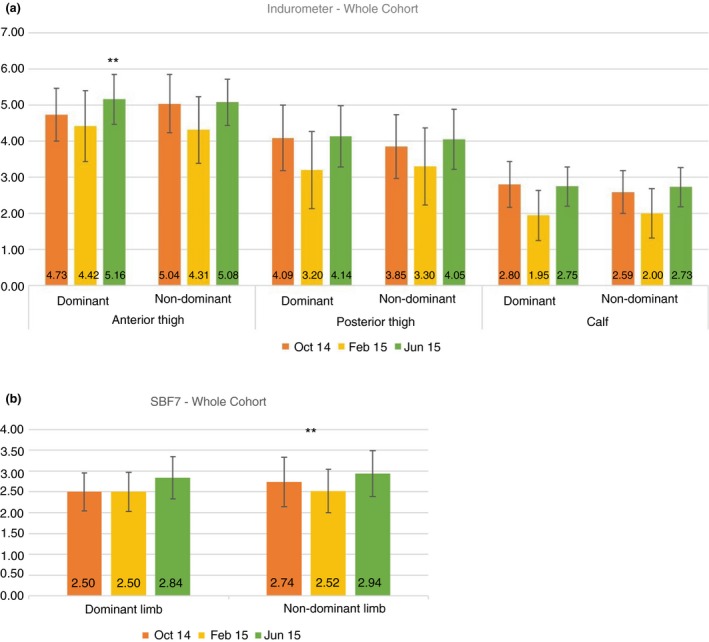
(a) Whole cohort mean indurometer scores for each measurement site at all three time points. Higher values = higher tissue compressibility. **P < 0.000, *P < 0.05 Oct 14 vs. Jun 15. (b) Whole cohort mean BIS scores for each leg at all three time points. Higher values = less extracellular free fluid. **P < 0.000, *P < 0.05 Oct 14 vs. Jun 15.
Myanmar has a range of climate regions from tropical monsoons in the south to subtropical highlands in the north. Mandalay Region, in Central Myanmar, is known as the dry zone and classified as hot and semi‐arid by the Köppen–Geiger scale 40. Baseline data were collected in October at the end of the rainy season, the Feb 15 follow‐up was in the middle of the cool dry season, and Jun 15 follow‐up measures were taken during the hot, humid build‐up to the wet season. Average monthly temperature, relative humidity and the heat index score for each data collection time are shown in Table 3.
Table 3.
Average monthly temperature, humidity and heat index for Amarapura during each month of data collection
| Average monthly | |||||
|---|---|---|---|---|---|
| Humidity (%) | Temperature (°C) | Temp range (°C) | Heat index | Heat warning | |
| Oct 14 | 72 | 31 | 35–24 | 39 | Caution |
| Feb 15 | 36 | 29 | 34–19 | 28 | No warning |
| Jun 15 | 60 | 34 | 38–29 | 42 | Extreme caution |
Comparison of device scores between‐infection groups at baseline (Oct 14) was previously reported 23 and showed that antigen‐positive cases had higher tissue compressibility at all measuring points, and more free fluid in both legs, than their antigen‐negative peers. This was both clinically relevant and statistically significant for indurometer scores at the non‐dominant calf. Unadjusted between‐infection group comparisons at the Jun 15 follow‐up showed that tissue compressibility was still higher among the positive cases at both calves, and free fluid was higher in the dominant leg but none were statistically significant. Figure 5a summarises the unadjusted between‐group differences at baseline (Oct 14) and second follow‐up (Jun 15) for indurometer scores and Figure 5b for BIS scores.
Figure 5.
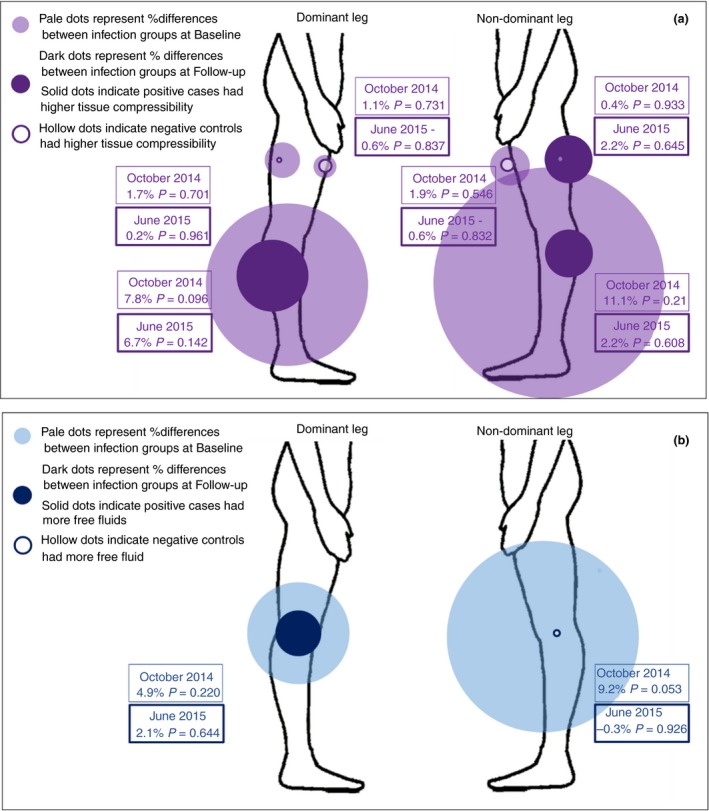
(a) Unadjusted comparison of indurometer scores between‐infection groups at baseline (Oct 14) and second follow‐up (Jun 15). (b) Unadjusted comparison of BIS scores between‐infection groups at baseline (Oct 14) and second follow‐up (Jun 15).
Previous reports on indurometry and BIS measures in the lower limbs of young people have identified moderating factors associated with variation in device scores. Linear regression was used to determine factors associated with variation in device scores in this cohort, and results were consisted with previous reports 23, 29. Results of the linear regression for age, gender, body composition and recent hydration are given in additional Table S3. In summary, over the nine months of the study, there was an association with increased age and small increases in tissue compressibility and increased free fluid. Being female was significantly associated with increased tissue compressibility and less free fluid in both legs, and all associations were large enough to be considered clinically relevant and statistically significant. There were notable associations with being underweight and increased free fluid in both legs and between less recent hydration and increased tissue stiffness.
Effects of preventive chemotherapy
A Bayesian linear mixed model was used to determine the marginal effect of PC consumption on device scores over time. There was a significant change in indurometer scores at both calves for antigen‐positive cases who consumed any PC (dominant calf: −0.30 [95% CI −0.52, −0.07], P < 0.05 and non‐dominant calf: −0.35 [95% CI −0.58, −0.12], P < 0.01). Changes in all scores for antigen‐positive cases who consumed any PC are given in additional Table S4. Figures 6a,b shows box and whisker plots of the marginal effects for all device scores in antigen‐positive and antigen‐negative groups who did or did not consume PC (either during the 2014 MDA or March 2015). Considering the non‐dominant calf where the between‐group differences had been significant at baseline, the box plots suggest that there was little change in tissue compressibility among antigen‐negative participants who did or did not take any PC. However, antigen‐positive cases who did not take PC had small increases in tissue compressibility while those who had taken PC (either at the 2014 MDA or in March 2015) had a significant reduction in tissue compressibility (Figure 6a).
Figure 6.
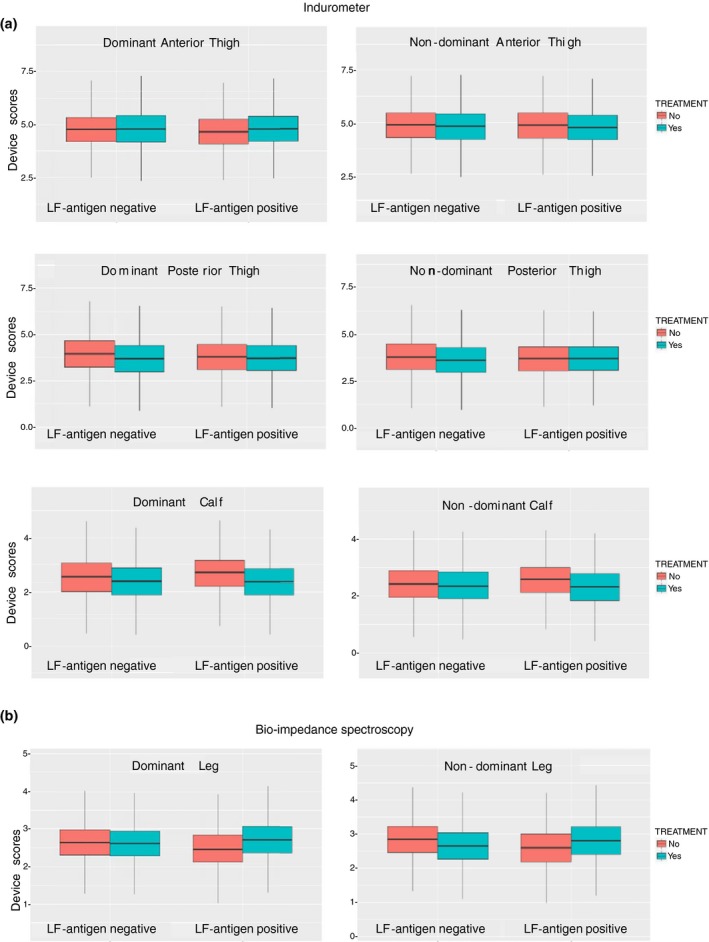
(a) Box and whisper plots showing the marginal change in indurometer scores. Higher scores indicate increased tissue compressibility. (b) Box and whisper plots showing the marginal change in BIS scores. Higher scores indicate reduced free fluid.
Likewise, among antigen‐positive cases who took any PC, free fluid, which had been higher in both legs than in their antigen‐negative peers at baseline, was reduced in Jun 15. For antigen‐positive cases who had not taken PC, there was little change, and for antigen‐negative controls, the non‐dominant leg had an increase in free fluid while the dominate leg had remained the same, although none of these changes were statistically significant (Figure 6b).
The direction of these changes in tissue compressibility and free fluid among antigen‐positive participants who had consumed any PC suggests that some of the covert tissue changes associated with LF antigenaemia which had been found at baseline had resolved by the Jun 15 follow‐up.
Discussion
It was previously shown that young Myanmar people who were LF antigen‐positive but asymptomatic had covert increases in both tissue compressibility and free fluid in the lower limbs compared to their antigen‐negative peers 23. These changes were detected using an indurometer and with bioimpedance spectroscopy (BIS) and are consistent with an imperceptible accumulation of fluid in the tissues such as occurs when lymphatic transport is reduced. Similar covert changes have been identified in the at‐risk arm of women after breast cancer treatment using BIS. In the breast cancer population, it has been shown that responding with a prophylactic intervention (light compression sleeve) to a small increase in free fluid (3%) prevents the onset of overt lymphatic disease 13. This study followed the Myanmar cohort longitudinally to assess the effect of PC, at coverage levels recommend under GPELF, on indurometer and BIS scores. There were significant absolute changes in device scores in the antigen‐positive group who took any PC (either during the 2014 MDA or in March 2015). The significant between‐infection group differences, which were detected before the 2014 MDA, were not found at the Jun 15 follow‐up, which suggests that PC may have a beneficial effect on early fluid and tissue changes in the lower limbs among young people who are LF antigen‐positive. PC consumption had little or no effect on device scores among antigen‐negative cases while antigen‐positive cases who did not consume PC had further increases in tissue compressibility at both calves.
Self‐reported MDA participation in this cohort was lower than the WHO‐recommended coverage levels. Only 40.1% of participants reported taking PC during the 2013 MDA (see Table 2). Despite gaining knowledge of their infection status in October 2014, and receiving information on the importance of the MDA, the proportion of young males who participated in the MDA in December 2014 went down (antigen‐positive males from 65.2% in 2013 to 47.4% in 2014 and antigen‐negative males from 65.0% in 2013 to 11.1% in 2014). Conversely, consumption went up among all females from 13.8% in 2013 to 64% in 2014, which could imply that young women are more responsive to health‐related information. Preliminary reports on 2014 MDA participation reported in this cohort and a morbidity survey conducted in Myanmar at the same time 22 were delivered to the Myanmar MOHS and greater emphasis has been given to increasing community awareness of LF and the MDA programme in the Mandalay Region since 2015 41.
Variation associated with time constituted the greatest proportion of the observed variation when all three time points were included in the models. However, a causal effect of season cannot be confirmed with current data, and this variation could be due to another unmeasured factor. Nonetheless, tissue compressibility was significantly reduced in the cooler months (first follow‐up Feb 15) and increased during the hot humid weather at the Jun 15 follow‐up. This is consistent with the effect of seasonal changes noted among women with BCRL in Australia 42 where increased swelling was associated with warmer temperatures on the day before measurement (r = 0.27, P < 0.001). However, BIS scores indicated that free fluid was higher during the cooler Feb 15 measures but then reduced during the hot humid Jun 15 measures which is in the opposite direction to what might be expected, especially considering that significantly more participants in both groups had reported having a drink within the hour prior to measures. It may be that during June in Central Myanmar, when the heat index indicated extreme caution, it is difficult to remaining well hydrated. This unanticipated limitation in study design (ambient temperature and relative humidity were not recorded within the data collection centre) highlights the importance of accounting for seasonal variation when assessing tissue compressibility and extracellular free fluid. This important variable may have been missed entirely if data collection had stopped in February after the 2014 MDA as originally planned. There are few published data around the effect of weather on LF other than a 1998 report by Shenoy and colleagues 43 on an increase in episodes of adeno‐dermato‐lymphangitis (acute attacks) in Brugian filariasis during the rainy season in southern India. How ambient temperature affects free fluid and tissue compressibility in people with filariasis‐related lymphoedema is not known.
Other limitations to this study were the poor MDA coverage during the 2014 MDA, which required additional DEC (administered by the Myanmar MOHS) to achieve more than the recommended 65% PC coverage among the study population. This means the results are not based on the before‐and‐after MDA data alone. The study was also limited by the 26% dropout rate, which was higher than expected and reduced the data available for analysis at follow‐up. Group sizes at follow‐up were large enough for analysis of between‐infection groups, but once subgroups such as sex and PC consumption were considered, these became too small, and studies on larger cohorts are required. The short time frame of nine months was insufficient to definitively answer questions regarding the reversal of early fluid and tissue changes using MDA alone, and long‐term follow‐up on this and future research cohorts would be ideal.
At the covert stage of lymphatic disease from LF, it is unlikely that the lymphatic vessels themselves have undergone irreversible damage. Young people in particular may recover fully if the cause, in this case the presence of adult worms, can be removed as has been shown using lymphoscintigraphy 18. Our study used changes in tissue compressibility and extracellular fluid load as early indicators of lymphatic pathology. These subcutaneous tissue changes are less specific to the lymph vessels themselves than lymphoscintigraphy. However, during analysis of such changes when known moderating factors were taken into account 23, there was adequate signal within the data to support further exploration in larger studies of longer duration.
Our study thus suggests that PC medications, delivered at coverage levels consistent with WHO recommendations, may be sufficient to achieve reversal of covert tissue changes. However, given the low rate of MDA participation in this cohort, the risk of progression to overt lymphedema may still persist for some of the participants in this study. A more holistic approach to morbidity prevention may be needed, including community‐wide education in hygiene protocols, and early self‐care should symptoms manifest. Country programmes may need to make greater efforts to target young males with gender appropriate education regarding the necessity to participate in MDA activities, even if they do not see themselves as being at risk. Adolescents and young adults should be a focus of increased testing in highly endemic areas as well as for increased promotion of behavioural change interventions such as preventing mosquito bites to avoid infection altogether.
Conclusion
Baseline comparison of LF antigen‐positive and antigen‐negative young people showed significant and clinically relevant covert differences in the superficial tissues among the antigen‐positive cases compared to negative controls. These differences were no longer detectable six months after consumption of PC. Thus, treatment was associated with reversal over time of the previously observed effects of infection. Longer follow‐up of such cohorts is required to confirm whether these improvements persist over time. Low PC coverage as observed during the 2014 MDA may miss many infected young people, as well as being unlikely to interrupt disease transmission to those currently uninfected. Young people should be a focus of increased education and awareness campaigns in endemic areas. Indurometry and BIS have shown good reliability to detect tissue changes in young people living in an LF‐endemic region in Central Myanmar. These methods should therefore be considered in operational research on, and implementation of, clinical services to fulfil the second pillar of the GPELF.
Supporting information
Figure S1. Tissue compressibility was assessed using the indurometer (SA Biomedical Engineering Australia).
Figure S2. Whole leg extracellular free fluid was assessed by BIS using the SBF7 (Impedimed, Australia).
Table S1. Between‐infection group participant characteristics at each time point.
Table S2. Whole cohort mean values for tissue compressibility and free fluid at three time points.
Table S3. Linear regression for whole group effects of age, gender, being underweight and hydration on device scores.
Table S4. Bayesian analysis of change in indurometer and BIS scores from October 14 to June 15 in participants who were antigen positive and consumed any PC.
Acknowledgements
We thank Peter Wood for development of the map in Figure 2; Luke Becker for training of Myanmar research assistants in blood collection protocols; staff at the Public Health Laboratory in Mandalay for plasma separation; Maureen Roineau, Dr Khin Saw Aye and staff at Department of Medical Research; Yangon for laboratory testing in Myanmar; and Jesse Masson for the laboratory testing in Cairns. The majority of funds for the study were donated by private individuals through crowdfunding campaigns. James Cook University provided the ICT cards and contributed to fieldwork and laboratory expenses. Impedimed Australia donated a portion of the BIS electrodes and provided a backup BIS unit. We thank GlaxoSmithKline for support to the WHO Collaborating Centre at JCU. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. WHO . Global programme to eliminate lymphatic filariasis. Relevé épidémiologique hebdomadaire/Section d'hygiène du Secrétariat de la Société des Nations = Weekly epidemiological record/Health Section of the Secretariat of the League of Nations. 2009;84(42):437‐444. [PubMed]
- 2. WHO . Global programme to eliminate lymphatic filariasis: progress report, 2014. Wkly Epidemiol Rec. Geneva: World Health Organization 2015. p. 489‐504.
- 3. Ramaiah KD, Ottesen EA. Progress and impact of 13 years of the global programme to eliminate lymphatic filariasis on reducing the burden of filarial disease. PLoS Negl Trop Dis 2014: 8: e3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brady M, Global Alliance to Eliminate Lymphatic F . Seventh meeting of the Global Alliance to Eliminate Lymphatic Filariasis: reaching the vision by scaling up, scaling down, and reaching out. Parasit Vectors 2014: 7: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ridner SH. Pathophysiology of lymphedema. Semin Oncol Nurs 2013: 29: 4–11. [DOI] [PubMed] [Google Scholar]
- 6. Taylor MJ, Cross HF, Ford L, Makunde WH, Prasad GB, Bilo K. Wolbachia bacteria in filarial immunity and disease. Parasite Immunol 2001: 23: 401–409. [DOI] [PubMed] [Google Scholar]
- 7. ISL . The diagnosis and treatment of peripheral lymphedema: 2016 consensus document of the international society of lymphology. Lymphology 2016: 49: 170–184. [PubMed] [Google Scholar]
- 8. DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta‐analysis. Lancet Oncol 2013: 14: 500–515. [DOI] [PubMed] [Google Scholar]
- 9. Shih YCT, Xu Y, Cormier JN et al Incidence, treatment costs and complications of lymphedema after breast cancer among women of working age: a 2‐year follow‐up study. J Clin Oncol 2010: 22: 303–304. [DOI] [PubMed] [Google Scholar]
- 10. Budge PJ, Little KM, Mues KE et al Impact of community‐based lymphedema management on perceived disability among patients with lymphatic filariasis in Orissa State, India. PLoS Negl Trop Dis 2013: 7: e2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brennan MJ, Weitz J. Lymphedema 30 years after radical mastectomy. Am J Phys Med Rehabil 1992: 71: 12–14. [DOI] [PubMed] [Google Scholar]
- 12. Lawenda BD, Mondry TE, Johnstone PAS. Lymphedema: a primer on the identification and management of a chronic condition in oncologic treatment. CA Cancer J Clin 2009: 59: 8–24. [DOI] [PubMed] [Google Scholar]
- 13. Stout‐Gergich NL, Pfalzer LA, McGarvey C, Springer B, Gerber LH, Soballe P. Preoperative assessment enables the early diagnosis and successful treatment of lymphedema. Cancer 2008: 112: 2809–2819. [DOI] [PubMed] [Google Scholar]
- 14. Castro‐Sanchez AM, Moreno‐Lorenzo C, Mataran‐Penarrocha GA, Aguilar‐Ferrandiz ME, Almagro‐Cespedes I, Anaya‐Ojeda J. Preventing lymphoedema after breast cancer surgery by elastic restraint orthotic and manual lymphatic drainage: a randomized clinical trial. Med Clin (Barc) 2011: 137: 204–207. [DOI] [PubMed] [Google Scholar]
- 15. Zhang L, Fan A, Yan J et al Combining manual lymph drainage with physical exercise after modified radical mastectomy effectively prevents upper limb lymphedema. Lymphat Res Biol 2016: 14: 104–108. [DOI] [PubMed] [Google Scholar]
- 16. Stout NL, Binkley JM, Schmitz KH et al A prospective surveillance model for rehabilitation for women with breast cancer. Cancer 2012: 118(8 Suppl): 2191–2200. [DOI] [PubMed] [Google Scholar]
- 17. Witt C, Ottesen EA. Lymphatic filariasis: an infection of childhood. Trop Med Int Health 2001: 6: 582–606. [DOI] [PubMed] [Google Scholar]
- 18. Kar SK, Dwibedi B, Das BK, Agrawala BK, Ramachandran CP, Horton J. Lymphatic pathology in asymptomatic and symptomatic children with Wuchereria bancrofti infection in children from Odisha, India and its reversal with DEC and albendazole treatment. PLoS Negl Trop Dis 2017: 11: e0005631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shenoy RK, Suma TK, Kumaraswami V, Rahmah N, Dhananjayan G, Padma S. Antifilarial drugs, in the doses employed in mass drug administrations by the Global Programme to Eliminate Lymphatic Filariasis, reverse lymphatic pathology in children with Brugia malayi infection. Ann Trop Med Parasitol 2009: 103: 235–247. [DOI] [PubMed] [Google Scholar]
- 20. Dickson B, Graves P, McBride W. Lymphatic filariasis in mainland Southeast Asia: a systematic review and meta‐analysis of prevalence and disease burden. Trop Med Infect Dis 2017: 2: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aye NN, Lin Z, Lon KN et al Mapping and modelling the impact of mass drug administration on filariasis prevalence in Myanmar. Infect Dis Poverty 2018: 7: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dickson B, Graves P, Aye N et al The Prevalence of Lymphatic Filariasis Related Hydrocele, Lymphedema and Infection in Mandalay Region, Myanmar. In: BFR D, editor. 64th Annual Meeting of the American Tropical Society of Medicine and Hygiene; October 25–29, 2015; Philadelphia2015.
- 23. Douglass J, Graves P, Lindsay D et al Lymphatic filariasis increases tissue compressibility and extracellular fluid in lower limbs of asymptomatic young people in Central Myanmar. Trop Med Infect Dis 2017a: 2: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gordon S, Melrose W, Warner J, Buttner P, Ward L. Lymphatic filariasis: a method to identify subclinical lower limb change in PNG adolescents. PLoS Negl Trop Dis 2011: 5: e1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Masson J, Douglass J, Roineau M et al Relative performance and predictive values of plasma and dried blood spots with filter paper sampling techniques and dilutions of the lymphatic filariasis Og4C3 antigen ELISA for samples from Myanmar. Trop Med Infect Dis 2017: 2: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bhumiratana A, Intarapuk A, Koyadun S, Maneekan P, Sorosjinda‐Nunthawarasilp P. Current bancroftian filariasis elimination on Thailand–Myanmar border: public health challenges toward postgenomic MDA evaluation. ISRN Trop Med 2013: 2013: 13. [Google Scholar]
- 27. Ottesen EA, Duke BO, Karam M, Behbehani K. Strategies and tools for the control/elimination of lymphatic filariasis. Bull World Health Organ 1997: 75: 491–503. [PMC free article] [PubMed] [Google Scholar]
- 28. Douglass J, Graves P, Gordon S. Intrarater reliability of tonometry and bioimpedance spectroscopy to measure tissue compressibility and extracellular fluid in the legs of healthy young people in Australia and Myanmar. Lymphat Res Biol 2017b: 15: 57–63. [DOI] [PubMed] [Google Scholar]
- 29. Douglass J, Graves P, Gordon S. Moderating factors in tissue tonometry and bioimpedance spectroscopy measures in the lower extremity of healthy young people in Australia and Myanmar. Lymphat Res Biol 2018: 16: 309–316. [DOI] [PubMed] [Google Scholar]
- 30. World Weather Online . Mandalay Region, Amarapura, (Available from: https://www.worldweatheronline.com/amarapura-weather-averages/mandalay/mm.aspx.) [22 Sep 2018]
- 31. Calculator.net. (Available from: https://www.calculator.net/heat-index-calculator.html.) [22 Sep 2018]
- 32. US Dept of Commerce . Heat Index 1325 East‐West Hwy, 18th floor, Communications Office, Silver Spring, MD 20910: National Oceanic and Atmospheric Administration National Weather Service (Available from: https://www.wrh.noaa.gov/psr/general/safety/heat/heatindex.png.)
- 33. Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school‐aged children and adolescents. Bull World Health Organ 2007: 85: 660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ward L, Winall A, Isenring E et al Assessment of bilateral limb lymphedema by bioelectrical impedance spectroscopy. Int J Gynecol Cancer 2011: 21: 409–418. [DOI] [PubMed] [Google Scholar]
- 35. Team RC . R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2013. 2014.
- 36. Carpenter B, Gelman A, Hoffman MD et al Stan: a probabilistic programming language. J Stat Softw 2017: 76: 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Luo Y, Jiao H. Using the Stan program for Bayesian item response theory. Educ Psychol Measur 2018: 78: 384–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bürkner P‐C. brms: an R package for Bayesian multilevel models using Stan. J Stat Softw 2017: 80: 1–28. [Google Scholar]
- 39. Sandvik B. Thematic Mapping. Simplified Country Borders 2018 (Available from: http://www.thematicmapping.org/downloads/world_borders.php.)
- 40. Kottek M, Grieser J, Beck C, Rudolf B, Rubel F. World map of the Köppen‐Geiger climate classification updated. Meteorol Z 2006: 15: 259–263. [Google Scholar]
- 41. WHO . Global programme to eliminate lymphatic filariasis: progress report, 2016. Wkly Epidemiol Rec. Geneva: World Health Organization; 2017. p. 594‐608.
- 42. Czerniec SA, Ward LC, Kilbreath SL. Breast cancer‐related arm lymphedema: fluctuation over six months and the effect of the weather. Lymphat Res Biol 2016: 14: 148–155. [DOI] [PubMed] [Google Scholar]
- 43. Shenoy RK, Suma TK, Rajan K, Kumaraswami V. Prevention of acute adenolymphangitis in brugian filariasis: comparison of the efficacy of ivermectin and diethylcarbamazine, each combined with local treatment of the affected limb. Ann Trop Med Parasitol 1998: 92: 587–594. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Tissue compressibility was assessed using the indurometer (SA Biomedical Engineering Australia).
Figure S2. Whole leg extracellular free fluid was assessed by BIS using the SBF7 (Impedimed, Australia).
Table S1. Between‐infection group participant characteristics at each time point.
Table S2. Whole cohort mean values for tissue compressibility and free fluid at three time points.
Table S3. Linear regression for whole group effects of age, gender, being underweight and hydration on device scores.
Table S4. Bayesian analysis of change in indurometer and BIS scores from October 14 to June 15 in participants who were antigen positive and consumed any PC.


