Abstract
Essentials.
The role of statins in hemostasis and venous thromboembolism (VTE) prophylaxis is not clear.
This trial assessed whether rosuvastatin use affects thrombin generation in patients with VTE.
Endogenous thrombin potential and peak were decreased by 10% and 5% with rosuvastatin therapy.
These results provide basis for trials on the efficacy of statins in reducing recurrent VTE risk.
Summary
Background
Statin therapy could form an alternative prophylactic treatment for venous thromboembolism (VTE) if statins are proven to downregulate hemostasis and prevent recurrent VTE, without increasing bleeding risk.
Objectives
The STAtins Reduce Thrombophilia (START) trial investigated whether statin affects coagulation in patients with prior VTE.
Patients/methods
After anticoagulation withdrawal, patients were randomized to rosuvastatin 20 mg day−1 for 4 weeks or no intervention. Plasma samples taken at baseline and at the end of the study were analyzed employing thrombin generation assay.
Results and conclusions
The study comprised 126 rosuvastatin users and 119 non‐users. Mean age was 58 years, 61% were men, 49% had unprovoked VTE and 75% had cardiovascular (CV) risk factors. Endogenous thrombin potential (ETP) increased from baseline to end of study in non‐statin users (mean 97.22 nm*min; 95% CI, 40.92–153.53) and decreased in rosuvastatin users (mean −24.94 nm*min; 95% CI, −71.81 to 21.93). The mean difference in ETP change between treatments was −120.24 nm*min (95% CI, −192.97 to −47.51), yielding a 10.4% ETP reduction by rosuvastatin. The thrombin peak increased in both non‐statin (mean 20.69 nm; 95% CI, 9.80–31.58) and rosuvastatin users (mean 8.41 nm; 95% CI −0.86 to 17.69). The mean difference in peak change between treatments was −11.88 nm (95% CI, −26.11 to 2.35), yielding a 5% peak reduction by rosuvastatin. Other thrombin generation parameters did not change substantially. The reduction in ETP and peak by rosuvastatin was more pronounced in the subgroups of participants with CV risk factors and with unprovoked VTE. We conclude that rosuvastatin reduces thrombin generation potential in patients who had VTE.
Keywords: hydroxymethylglutaryl‐CoA reductase inhibitors, randomized clinical trial, thrombin generation, thrombophilia, venous thrombosis
Introduction
Venous thromboembolism (VTE) contributes significantly to the global disease burden and, therefore, preventive measures and adequate treatment are warranted 1. Anticoagulation is the treatment of choice for preventing VTE episodes 2. Bleeding complications are a major concern and may lead to treatment avoidance in many cases 3. The latter underscores the need for alternative treatment options for VTE prophylaxis. Statins may provide a promising alternative treatment for thromboprophylaxis because these drugs are alleged to have pleiotropic effects on hemostasis and may reduce VTE risk, although strong clinical evidence supporting these effects is still scarce 4.
Previous studies have reported that statins reduce the risk of first VTE by 14–54% 5, 6, 7, 8, 9 and the risk of recurrent VTE by 27% 10. However, healthy user effects, survivor bias and adherence bias could have influenced these results 11. Moreover, the strongest evidence on the effect of rosuvastatin on first VTE still comes from one randomized clinical trial 8, whereas no randomized trials have investigated the impact of statin therapy on the risk of recurrent VTE. Despite the need for additional randomized trials, the lack of knowledge on the mechanisms that are the basis of the supposed causal association between statin use and a reduced risk of VTE may discourage the conduction of such interventional studies.
Recently, we have shown in the STAtins Reduce Thrombophilia (START) trial that 1 month of treatment with rosuvastatin at 20 mg day−1 led to an improved coagulation profile as compared with non‐statin users in patients with prior VTE, most notably by reducing factor VIII plasma levels 12. These observations from the START trial were the first randomized evidence indicating that rosuvastatin reduces coagulation factor levels in patients with prior VTE and confirmed similar findings previously observed for other statins 13, 14, 15. To better understand the effect of rosuvastatin on individual prothrombotic profiles, we evaluated here whether rosuvastatin could interfere with thrombin generation, a global coagulation test that reflects not only the coagulation potential 16, 17, 18 of an individual but also predicts the risk of a first and recurrent VTE 19, 20, 21.
Methods
Trial design
The START trial is a randomized, open label, controlled, clinical trial conducted in the Netherlands that investigated whether the coagulation profile in persons with a history of VTE and not taking anticoagulants is improved when using rosuvastatin. Details of the study design are described elsewhere 12. The study was undertaken in accordance with the Declaration of Helsinki and International Conference on Harmonization guidelines for Good Clinical Practice. All participants gave written informed consent prior to participation. START was approved by the Medical Ethics Committee of the Leiden University Medical Center, Leiden, the Netherlands, and is registered at http://www.clinicaltrials.gov as NCT01613794.
Participants
Participants were recruited at three anticoagulation clinics in the Netherlands (Leiden, Hoofddorp and Rotterdam) between June 2012 and January 2017. Individuals aged 18 years or older with confirmed symptomatic proximal deep vein thrombosis or pulmonary embolism, were eligible for inclusion in the study if their physicians approved the cessation of oral anticoagulant treatment. Exclusion criteria were: current use of statins or lipid‐lowering drugs, or any contraindications for rosuvastatin at 20 mg day−1 as provided in the instruction leaflet from the manufacturer.
Intervention
Informed consent was obtained at the study baseline visit. The study baseline visit was defined at the time of the last regular visit of the patient to the anticoagulation clinic. After informed consent, participants were screened for acquired risk factors for thrombosis through a questionnaire and tested for liver and kidney functions. At randomization, participants were allocated to receive either rosuvastatin at 20 mg day−1 or no study medication. The random allocation sequence was implemented by a central telephone and the sequence was concealed until interventions were assigned.
The duration of the study was 28 days, based on the consideration that some small non‐randomized studies showed beneficial effects of statins on the coagulation system as early as after 3 days of statin administration 12.
Measurements
Patients stopped using their vitamin K antagonist for 1 month (to allow the anticoagulant drugs to wear off), after which a blood sample was drawn at the randomization visit and at the end of the study period (i.e. 28 days later). All blood draws were performed between 08:00 and 15:00. Blood was collected in tubes containing sodium citrate (3.2%) and centrifuged within 3 h of venepuncture at 2500 g for 15 min at 18 °C, after which plasma was immediately stored at −80 °C. Laboratory technicians, who were unaware of which participants were rosuvastatin users, performed the assays after all participants had completed the study.
The thrombin generation potential was assessed by means of the thrombin generation assay (TGA), which is a global coagulation test that reproduces the kinetics of thrombin formation 22, 23, using the Calibrated Automated Thrombogram® (Diagnostica Stago, Asinères, France) according to the manufacturer's specifications 24. Briefly, plasma samples were mixed with the assay reagents (tissue factor and phospholipids) and tested in duplicate. As internal control, normal pooled plasma, derived from citrated plasma from 64 healthy men and women not taking oral contraceptives, was tested in each assay and a thrombin calibrator was used for each plasma duplicate. The fluorescent signal representing generated thrombin was monitored in a Fluoroskan Ascent fluorometer (Thermo Scientific, Waltham, MA, USA) and the parameters were calculated with the Thrombinoscope software (Thrombinoscope BV, Maastricht, the Netherlands). The TGA parameters determined were: endogenous thrombin potential (ETP), thrombin peak, time to peak, lag time and velocity index. ETP, or area under curve, represents the total amount of thrombin generated over time. The thrombin peak represents the maximum amount of thrombin that can be generated. Time to peak indicates the time required to reach the maximum amount of thrombin formed. The lag time measures the length of time between the start of the assay (addition of triggers) and the initiation of thrombin generation. The velocity index is defined as [peak height/(time to peak − lag time)] and represents the rate of thrombin generation 20.
Outcomes
Because the ETP and thrombin peak have been consistently associated with VTE risk 25, 26, 27, 28, 29, 30, 31, the primary endpoints were defined as the difference in change in ETP and thrombin peak from baseline to the end of the study between rosuvastain users and non‐users. The differences in the change in lag time, time to peak or velocity index were considered secondary endpoints. The study was originally powered on factor VIII 12. Nevertheless, we observed in the non‐statin users that the mean ETP was 1245 mm*min (SD 322) at randomization. Therefore, we a priori expected to find a powered mean difference of at least 76 nm*min or 6% decrease between participants at the end of the study with a two‐sided alpha of 0.05 and 80% power.
Statistical analysis
Final analyses were carried out by modified intention‐to‐treat because there were post‐randomization exclusions. The mean levels and 95% confidence intervals (95% CIs) of all prespecified thrombin generation assay parameters were calculated at the time of randomization (baseline), at the end of the study period and for the change between these two time periods within each treatment group. We also calculated the percentage of change within groups by subtracting the baseline value from the end of the study value, dividing it by the baseline value and multiplying the result by 100%.
To determine the between‐groups difference in thrombin generation parameters, the mean difference in change and 95% CI between treatment groups (rosuvastatin users vs. non‐users) was calculated by means of linear regression methods. We performed both unadjusted and age and sex‐adjusted analyses, because more men were randomized to non‐rosuvastatin use and non‐rosuvastatin users were slightly older than those who were randomized to rosuvastatin. In a predefined sensitivity analysis, we excluded all participants who reported signs or symptoms of an infection during the study, as infections may affect thrombin generation 32, 33.
Next, we plotted the end‐of‐study‐expected and the end‐of‐study‐observed thrombin generation among rosuvastatin users. To do so, we assumed that if patients assigned to rosuvastatin had not received the drug, they would have had the same change in thrombin generation as those assigned to non‐statin treatment. Thus, the expected end‐of‐study thrombin generation among rosuvastatin users was estimated by adding the mean change in thrombin values (at each time‐point of the thrombin generation curve) within non‐statin users to the corresponding baseline thrombin value in rosuvastatin users.
Additionally, we performed a subgroup analysis according to the following potential or established prognostic determinants of recurrent venous thrombosis: male/female sex, unprovoked/provoked first event, deep vein thrombosis or pulmonary embolism, and presence or absence of self‐reported cardiovascular risk factors.
A post hoc analysis was performed to investigate whether the coagulation factors VIII, VII, XI and D‐dimer were associated with the effect of rosuvastatin on ETP. For this purpose, we performed linear regression with those coagulation factors entered as independent variables, along with the randomization groups and sex and age, and ETP entered as dependent variable. All analyses were performed with SPSS version 23.0 (SPSS Inc, Chicago, IL, USA).
Results
Study population
A total of 255 patients were randomized between December 2012 and December 2016, 131 were assigned to receive rosuvastatin and 124 were allocated to non‐statin treatment. Figure 1 shows the trial profile. Two participants allocated to rosuvastatin treatment did not start treatment and another six randomized, three in each study arm, did not complete the study. The thrombin generation assay could not be performed in two patients because of technical issues; they both had been assigned to non‐statin treatment. Table 1 presents baseline characteristics in the 245 participants who completed the study: 126 assigned to rosuvastatin and 119 assigned to non‐rosuvastatin treatment.
Figure 1.
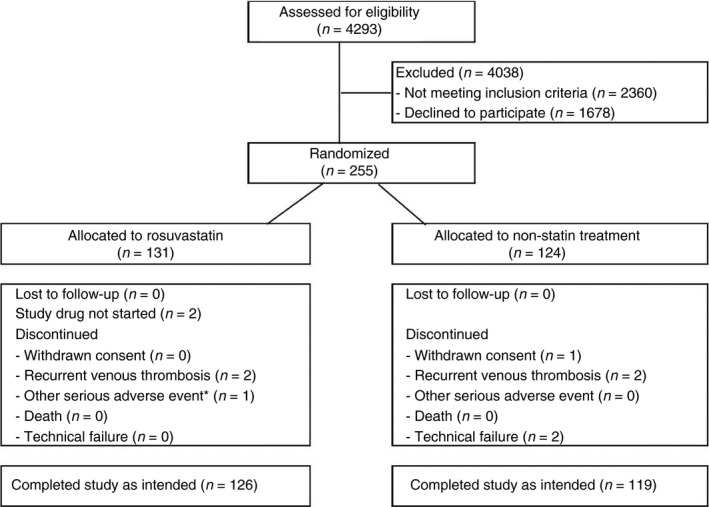
Trial profile. Study enrolment, randomization, follow‐up and reasons for withdrawal (*one participant admitted to hospital with a diagnosis of acute asthma exacerbation).
Table 1.
Baseline characteristics of participants
| Rosuvastatin users (n = 126) | Non‐users (n = 119*) | |
|---|---|---|
| General | ||
| Age (years) | 56.8 (19–82) | 58.4 (21–80) |
| Male | 68 (54) | 82 (69) |
| Body mass index (kg m−2) | 27.4 (19.2–43.5) | 27.7 (17.2–43.3) |
| Aspirin use | 5 (4) | 5 (4) |
| Venous thrombosis characteristics | ||
| Type of venous thromboembolism | ||
| Deep vein thrombosis | 72 (57) | 64 (54) |
| Pulmonary embolism | 54 (43) | 55 (46) |
| Unprovoked | 57 (45) | 62 (52) |
| Provoked, by | 69 (55) | 57 (48) |
| Surgery/trauma/immobilization | 32 (25) | 30 (25) |
| Travel >4 h | 22 (18) | 14 (12) |
| Estrogen use (% in women) | 24 (41) | 14 (38) |
| Pregnancy/puerperium (% in women) | 0 (0) | 2 (5) |
| Malignancy | 2 (2) | 8 (7) |
| Recurrent venous thrombosis | 10 (8) | 8 (7) |
| Cardiovascular risk factors | ||
| Cardiovascular risk | 89 (71) | 94 (78) |
| Current smoking | 18 (14) | 16 (13) |
| Hypertension | 24 (19) | 20 (17) |
| Diabetes | 3 (2) | 0 (0) |
| Overweight† | 54 (43) | 51 (43) |
| Obesity‡ | 29 (23) | 34 (28) |
Continuous variables denoted as mean (range), categorical variables as n (%). *Technical issues in two non‐users. †Overweight was defined as body mass index (BMI) between 25 and 30 kg m−2. ‡Obesity was defined as BMI above 30 kg m−2.
Non‐rosuvastatin users were slightly older than rosuvastatin users; the mean ages were 58.4 years (range 21–80) and 56.8 years (range 19–82), respectively. More men were assigned to non‐statin treatment; the proportion of men was 54% among rosuvastatin users and 69% among non‐users. Other reported exposures, such as body mass index (BMI), type and classification of venous thromboembolism, and presence of cardiovascular risk factors, were balanced at baseline (Table 1).
Outcomes
Results of all measured thrombin generation parameters are shown in Table 2. ETP increased 7.8% from baseline to end of study in non‐statin users (mean change, or intraindividual variability, within non‐users, 97.22 nm*min; 95% CI, 40.92–153.53) and decreased 1.9% from baseline to end of study in rosuvastatin users (mean change in rosuvastatin users, −24.94 nm*min; 95% CI, −71.81 to 21.93). The mean difference between treatments, after adjustment for age and sex, was −120.24 nm*min (95% CI, −192.97 to −47.51). After the exclusion of patients who reported an infection at the end of the study, as prespecified by the study protocol, the age and sex‐adjusted mean difference in ETP between treatments was −129.39 nm*min (95% CI, −202.29 to −56.49). The mean difference between treatments yielded a treatment effect of 10.4% (95% CI, 4.5–16.2%) reduction in ETP by rosuvastatin, when compared with non‐statin treatment (Fig. 2).
Table 2.
Effects of rosuvastatin on measures of thrombin generation parameters
| Mean levels (SD) | Mean* | Mean difference† | Mean‡ | Mean difference§ | ||
|---|---|---|---|---|---|---|
| Baseline | End of study | change (95% CI) | in change (95% CI) | change (95% CI) | in change (95% CI) | |
| Thrombin generation | ||||||
| ETP (nm*min) | ||||||
| Non‐users | 1245.01 (321.47) | 1343.85 (290.17) | 97.22 (40.92, 153.53) | Reference | 94.62 (37.78, 151.46) | Reference |
| Rosuvastatin users | 1284.04 (263.97) | 1259.10 (205.37) | −24.94 (−71.81, 21.93) | −120.24 (−192.97, −47.51) | −38.49 (−85.19, 8.21) | −129.39 (−202.29, −56.49) |
| Thrombin peak (nm) | ||||||
| Non‐users | 273.33 (62.09) | 294.47 (52.32) | 20.69 (9.80, 31.58) | Reference | 20.39 (9.42, 31.37) | Reference |
| Rosuvastatin users | 288.86 (62.68) | 297.27 (52.25) | 8.41 (−0.86, 17.69) | −11.88 (−26.11, 2.35) | 5.99 (−3.31, 15.29) | −13.69 (−27.98, 0.60) |
| Lag time (min) | ||||||
| Non‐users | 2.23 (0.49) | 2.19 (0.72) | −0.04 (−0.16, 0.08) | Reference | −0.04 (−0.17, 0.08) | Reference |
| Rosuvastatin users | 2.16 (0.43) | 2.05 (0.38) | −0.11 (−0.15, −0.07) | −0.07 (−0.20, 0.05) | −0.12 (−0.16, −0.08) | −0.08 (−0.21, 0.05) |
| Time to peak (min) | ||||||
| Non‐users | 4.55 (0.89) | 4.48 (1.06) | −0.07 (−0.23, 0.09) | Reference | −0.06 (−0.22, 0.10) | Reference |
| Rosuvastatin users | 4.37 (0.77) | 4.09 (0.71) | −0.28 (−0.35, −0.21) | −0.21 (−0.38, −0.03) | −0.28 (−0.35, −0.21) | −0.22 (−0.39, −0.04) |
| Velocity index | ||||||
| Non‐users | 126.68 (47.07) | 137.39 (43.90) | 10.37 (3.64, 17.09) | Reference | 9.96 (3.15, 16.77) | Reference |
| Rosuvastatin users | 140.33 (57.35) | 154.69 (50.87) | 14.36 (7.38, 21.34) | 4.41 (−5.35, 14.17) | 12.52 (5.60, 19.44) | 3.07 (−6.66, 12.80) |
SD, standard deviation; ETP, endogenous thrombin potential. *Paired analysis. †Between comparison analysis, adjusted for age and sex. ‡Paired analysis to eight participants who reported an infection at time of end of study excluded. §Between comparison analysis, adjusted for age and sex to eight participants who reported an infection at time of end of study excluded.
Figure 2.
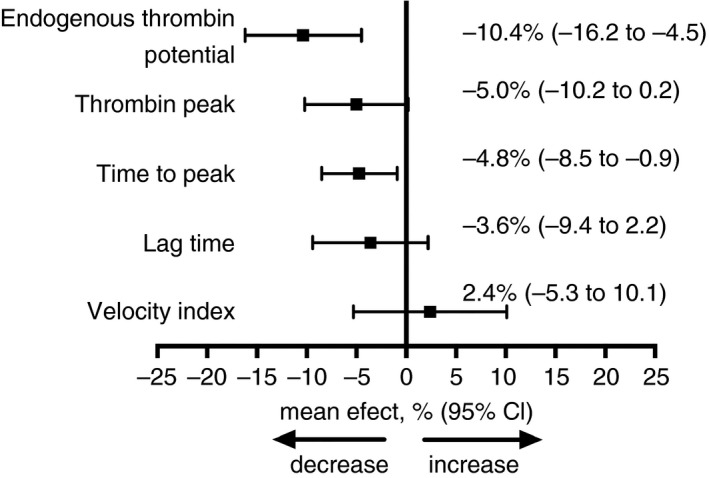
Relative effect of rosuvastatin treatment on thrombin generation. This figure illustrates the changes in endogenous thrombin potential, from baseline to the end of treatment, compared between rosuvastatin users and non‐statin users.
Although the thrombin peak increased in both rosuvastatin and non‐statin users from baseline to the end of the study, the percentage change was higher for non‐users (7.6%) relative to the rosuvastatin users (2.9%). The mean change in thrombin peak was 20.69 nm (95% CI, −9.80 to 31.58) for the non‐users and 8.41 nm (95% CI, −0.86 to 17.69) for the rosuvastatin users, which resulted in a mean difference in change between both treatments, adjusted for age and sex, of −11.88 nm (95% CI, −26.11 to 2.35). The mean difference between the treatments yielded a treatment effect of 5.0% (95% CI, −0.2 to 10.2%) reduction in thrombin peak by rosuvastatin, when compared with non‐statin treatment (Fig. 2).
The time to peak decreased 6.4% from baseline to the end of the study in rosuvastatin users (mean change, −0.28 min; 95% CI, −0.35 to −0.21), and 1.5% in non‐statin users (mean change, −0.07 min; 95% CI, −0.23 to 0.09). The mean difference in these changes between treatments was −0.21 min (95% CI, −0.38 to −0.03), which was equivalent to a treatment effect of 4.8% (95% CI, 0.9–8.5) reduction in time to peak by rosuvastatin, when compared with non‐statin treatment (Fig. 2). The results were not materially affected by excluding the eight participants who reported an infection. Changes in lag time and velocity index were not different between treatments (Fig. 2).
Figure 3 illustrates the difference between expected and observed thrombin generation in rosuvastatin users by the end of the study.
Figure 3.
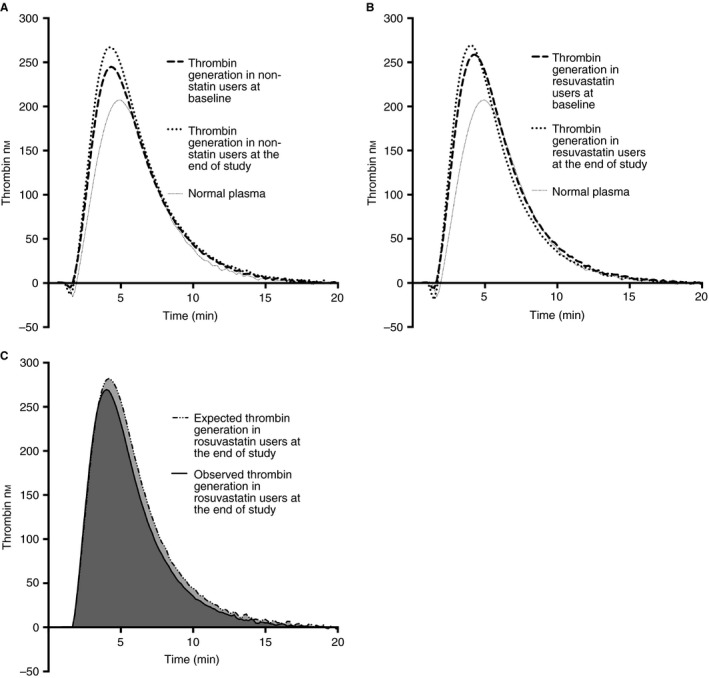
Thrombin generation curves: (A) mean values of thrombin generation over time in non‐statin users at baseline and at the end of the study, (B) mean values of thrombin generation over time in rosuvastatin users at baseline and at the end of the study, and (C) expected mean thrombin generation values (if rosuvastatin would not have a treatment effect on thrombin generation) and observed mean thrombin generation values by the end of the study in patients receiving rosuvastatin.
Tables S1–S5 show all measures of thrombin generation parameters in the subgroups of sex, unprovoked or provoked first VTE, deep vein thrombosis or pulmonary embolism, and presence or absence of self‐reported cardiovascular risk factors. These subgroup analyses revealed that the decrease in ETP and thrombin peak by rosuvastatin was more pronounced in patients with unprovoked venous thrombosis, pulmonary embolism or cardiovascular risk factors, than in those with provoked venous thrombosis, deep vein thrombosis or without cardiovascular risk factors (Fig. 4). A relative decrease in ETP following rosuvastatin use was also more pronounced in men than in women, whereas the effects of rosuvastatin on thrombin peak were similar between the sexes. Subgroup analysis of the effect of rosuvastatin on other thrombin generation parameters revealed similar results as in the main analysis.
Figure 4.
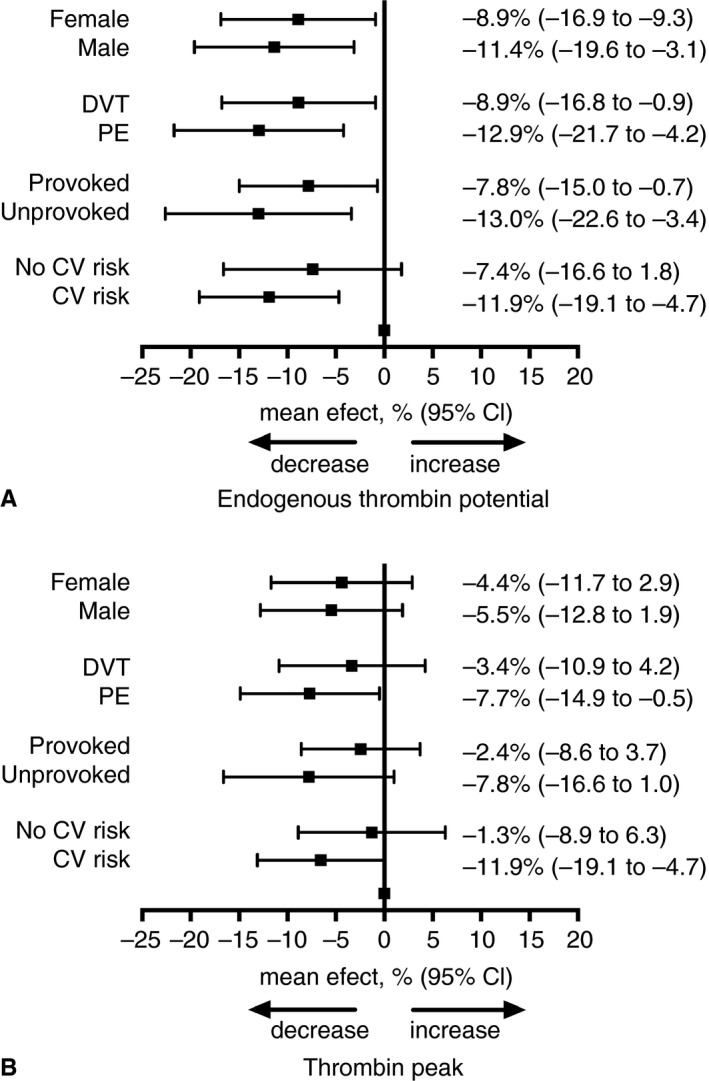
Relative effect of rosuvastatin treatment on thrombin generation potential by subgroups. The relative effect of rosuvastatin treatment on endogenous thrombin potential (A) and on thrombin peak (B) in prespecified subgroups: sex (female/male), type of VTE (DVT/PE), VTE classification (provoked/unprovoked) and presence of cardiovascular (CV) risk factors (no CV risk/CV risk) compared with non‐statin treatment.
As we have recently reported that treatment with rosuvastatin led to a decrease in the levels of D‐dimer and coagulation factors VIII, VII and XI as compared with non‐statin in START, we performed a post hoc analysis to evaluate whether the observed effect of rosuvastatin on thrombin generation could be explained by the levels of these factors at the end of the study. As shown in Table 3, the effect of rosuvastatin on thrombin generation was reduced by 33% with factor VII, but not by the other coagulation factors/D‐dimer.
Table 3.
Mean difference in endogenous thrombin potential between rosuvastatin users and non‐users (reference) at the end of the study, adjusted for coagulation factors
| Mean difference (95% CI)‡ | |
|---|---|
| ETP (nm*min) at the end of the study | |
| No coagulation factor | −89.46 (−153.18, −25.74) |
| +Factor VIII | −87.66 (−148.43, −26.89) |
| +Factor XI | −73.13 (−133.58, −12.68) |
| +Factor VII | −59.93 (−120.02, 0.17) |
| +DD | −87.20 (−151.41, −22.99) |
| +Factors VIII, XI, VII, DD | −54.98 (−111.99, 2.03) |
DD, D dimer; CI, confidence interval. ‡Comparison between rosuvastatin treatment and no treatment at the end of the study, adjusted for age and sex.
Discussion
In this randomized clinical trial (START), we have shown that treatment with rosuvastatin leads to a relative reduction in thrombin generation potential, decreasing the ETP by 10.4% (adjusted mean difference between treatments, −129.39 nm*min) and decreasing the thrombin peak by 5% (adjusted mean difference between treatments, −13.69 nm), in comparison with non‐statin treatment. Our results confirm previous clinical studies that also demonstrated that statin therapy, either with rosuvastatin 30, simvastatin 34, atorvastatin 35, 36 or cerivastatin 37, affects coagulation factors and thrombin generation.
Additionally, these findings are consistent with previous results from the START trial, in which rosuvastatin treatment was shown to decrease the plasma factor VIII levels by 6% (adjusted mean difference in change between treatments, −8.2 IU dL−1; 95% CI, −13.6 to −2.9) and those of FXI by 4% (adjusted mean difference in change between treatments, −4.9 IU dL−1; 95% CI, 9.9 to −0.1), coinciding with a decrease in D‐dimer by 3% and factor VII levels by 4% 12. The results from the START trial point to the same direction of an effect of rosuvastatin on the individual coagulation profile, but the observed decrease in thrombin generation potential was only partially mediated by factor VII and by D‐dimer, factor VIII or XI. Because thrombin generation is a product of a synergic combination of multiple coagulation factors 18, 38, it is possible that the mechanism behind the effect of rosuvastatin on decreasing thrombin generation potential relies on the reduction of several coagulation factors, some of them not measured in the START trial. Whether this effect of rosuvastatin on the coagulation profile has clinical significance in terms of reducing VTE risk deserves to be addressed in clinical trials that aim to evaluate this question. However, it is possible to speculate on a potential clinical impact of statins on VTE risk if the current findings are evaluated in the light of previous studies. Studies on thrombin generation and VTE risk have demonstrated that both the ETP and thrombin peak are associated with a first VTE 16, 28, 29, 31 and can predict the risk of recurrent VTE 25, 26, 27, 30.
A cohort study of 188 patients with VTE 28 reported that the risk of recurrent VTE increased by 25% per 100 nm*min increase in ETP (hazard ratio, 1.25 per 100 nm*min increase; 95% CI, 1.01–1.55). The Austrian Study on Recurrent Venous Thromboembolism (AUREC), which is a cohort study with patients with an unprovoked first episode of VTE, showed that the risk of recurrent VTE increased by 1.4% for each 1% increase in ETP (hazard ratio 1.014 per 1% increase in ETP; 95% CI, 1.0–1.03; P = 0.06) 25. Another study derived from the AUREC cohort showed that the relative risk of recurrent VTE increased by 4% (relative risk [RR], 1.04; 95% CI, 1.02–1.06) for each 10 nm increase in thrombin peak 27. The Vienna Cancer and Thrombosis Study (CATS), a prospective cohort study of patients with cancer, demonstrated that patients who developed VTE had 10% higher thrombin peak at baseline than those without VTE events (peak values 556 nm, 95% CI 432–677 and 499 nm, 95% CI 360–603, respectively) 39. Considering ETP and thrombin peak as surrogate markers of recurrent VTE risk, as described in the aforementioned trials, our results suggest that rosuvastatin has the potential to decrease the risk of recurrent VTE by 14–25%. Interestingly, a meta‐analysis of observational studies reported that statins reduced the overall risk of recurrent VTE by 27% (RR, 0.73; 95% CI, 0.68–0.79) 10. Therefore, our finding that statins are capable of modulating the prothrombotic profile in patients after a first VTE episode could be interpreted as statins having the potential to decrease the risk of recurrence.
We also observed that the relative treatment effect of rosuvastatin on ETP was mainly driven by the absence of an increase in this parameter among rosuvastatin users, in contrast to a significant increase in ETP in patients not using statins. This is consistent with a previous observation from this trial demonstrating that the difference in D‐dimer levels between the treatment groups was driven by the absence of an increase in D‐dimer following rosuvastatin use 12. As both thrombin generation and D‐dimer are markers of hypercoagulability 25, 26, the current results provide further evidence that rosuvastatin may prevent a rebound phenomenon; that is, a shift to a more procoagulant profile along with increased risk of a recurrence of VTE after the sudden withdrawal of anticoagulant treatment 40, 41. Preventing such a rebound hypercoagulability may be a further benefit to patients with previous VTE in whom anticoagulation is withdrawn.
It is worth noting that the decrease in ETP and thrombin peak appeared strongest in participants with unprovoked VTE and in those with cardiovascular risk factors. This potential benefit for patients who had unprovoked VTE is interesting because these patients are at high risk of recurrent VTE 2, and anticoagulants may not be prescribed if a patient is considered to be at high risk of anticoagulation‐related bleeding 42. Secondary prevention with statin therapy may be a convenient alternative treatment, as statins do not increase the risk of bleeding complications 43. In addition, a benefit among patients with cardiovascular risk factors is noteworthy because most of these patients are already likely to receive statins 44. Therefore, the possibility of using one single drug to prevent both cardiovascular diseases and VTE could diminish the medication burden associated with the use of several classes of drugs and decrease the risk of adverse effects, thus increasing the changes in treatment efficacy 45.
Although our results point to a decrease in thrombin generation potential by rosuvastatin, not all thrombin generation parameters were modified after the treatment. The lag time and velocity index did not change substantially, whereas the time to peak decreased in rosuvastatin users, in comparison with non‐statin users. Despite the fact that a reduced time to peak may indicate a hypercoagulable state 20, the real significance of this parameter is not known, because it is not associated with the risk of VTE. Conversely, as time to peak is calculated based on the thrombin values, a shortened time to peak may be explained by a relative reduction in ETP and thrombin peak 46; a similar phenomenon was reported in a previous study, wherein a protraction of the thrombin generation curve lengthened the time to peak 47.
There are some aspects of this study that need to be highlighted. First, the trial was not blind to the participants and physicians involved; however, it was considered unlikely that knowledge of the treatment could affect a surrogate laboratory outcome. Second, we previously noticed that the distribution of sex and age after randomization was different between the groups, and we a priori decided to adjust the analysis for these potential confounding factors 12. These adjustments did not influence our results. Third, we decided a priori to perform a sensitivity analysis excluding participants who developed an infection during the follow‐up because of the possibility of an acute‐phase reaction affecting the thrombin generation potential, which did not materially change the results. Fourth, although the results from our subgroup analyses suggest that statins may have the strongest potential to decrease thrombin potential in individuals with CV risk factors or unprovoked VTE, these subgroup analyses must be handled with caution as the study was not designed or powered to analyze differences in subgroups 48. Finally, the assessment of thrombin generation potential is dependent on the assay conditions, which vary according to different laboratory protocols and may affect the clinical interpretation of the results 49. Besides the potential limitations, the START trial evaluated the effect of rosuvastatin on six coagulation parameters related to the risk of VTE: VWF, factors VIII, VII and XI, D‐dimer, ETP and thrombin peak. The values of all parameters were consistently pointing towards a decreased level with rosuvastatin treatment, as compared with no statin. Altogether, these results confirm that rosuvastatin is capable of affecting several components of coagulation and modifying the coagulation profile of patients with a prior VTE.
We conclude that rosuvastatin 20 mg day−1 improves the coagulation profile in patients with VTE by reducing the thrombin generation potential after anticoagulation withdrawal. These results of the START trial suggest that statin therapy might be beneficial in patients at risk of recurrent VTE and provide a clinical rationale for the conduction of a randomized controlled trial to evaluate the effectiveness of rosuvastatin in decreasing the risk of recurrent VTE.
Addendum
F. A. Orsi performed the statistical analyses and drafted the manuscript. J. S. Biedermann performed the statistical analyses and revised the manuscript. M. J. H. A. Kruip, F. J. van der Meer, F. R. Rosendaal, A. van Hylckama Vlieg and F. W. G. Leebeek revised the manuscript. M. H. A. Bos was responsible for the laboratory analyses and revised the manuscript. S. C. Cannegieter designed the analyses and revised the manuscript, and W. M. Lijfering was responsible for the START study concept, design of the analyses and revision of the manuscript.
Disclosure of Conflict of Interests
The authors state that they have no conflict of interests.
Supporting information
Table S1. Effects of rosuvastatin on the endogenous thrombin potential in prespecified subgroups.
Table S2. Effects of rosuvastatin on the thrombin peak in prespecified subgroups.
Table S3. Effects of rosuvastatin on the lag time in prespecified subgroups.
Table S4. Effects of rosuvastatin on the time to peak in prespecified subgroups.
Table S5. Effects of rosuvastatin on the velocity index in prespecified subgroups.
Acknowledgements
This study was supported by a grant from the Dutch Heart Foundation (NHS 2011T012). F. A. Orsi received financial support from the São Paulo Research Foundation (FAPESP grant #2017/09506‐5). The sponsors had no role in study design, data collection, data analysis, data interpretation or the writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Orsi FA, Biedermann JS, Kruip MJHA, van der Meer FJ, Rosendaal FR, van Hylckama Vlieg A, Bos MHA, Leebeek FWG, Cannegieter SC, Lijfering WM. Rosuvastatin use reduces thrombin generation potential in patients with venous thromboembolism: a randomized controlled trial. J Thromb Haemost 2019; 17: 319–28.
Manuscript handled by: J. Douketis
Final decision: J. Douketis, 1 December 2018
References
- 1. ISTH Steering Committee for World Thrombosis Day . Thrombosis: a major contributor to the global disease burden. J Thromb Haemost 2014; 12: 1580–90. [DOI] [PubMed] [Google Scholar]
- 2. Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, Huisman M, King CS, Morris TA, Sood N, Stevens SM, Vintch JRE, Wells P, Woller SC, Moores L. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016; 149: 315–52. [DOI] [PubMed] [Google Scholar]
- 3. Kearon C, Akl EA. Duration of anticoagulant therapy for deep vein thrombosis and pulmonary embolism. Blood 2014; 123: 1794–801. [DOI] [PubMed] [Google Scholar]
- 4. Lijfering WM, Biedermann JS, Kruip MJ, Leebeek FW, Rosendaal FR, Cannegieter SC. Can we prevent venous thrombosis with statins: an epidemiologic review into mechanism and clinical utility. Expert Rev Hematol 2016; 9: 1023–30. [DOI] [PubMed] [Google Scholar]
- 5. Kunutsor SK, Seidu S, Khunti K. Statins and primary prevention of venous thromboembolism: a systematic review and meta‐analysis. Lancet Haematol 2017; 4: e83–93. [DOI] [PubMed] [Google Scholar]
- 6. Pai M, Evans NS, Shah SJ, Green D, Cook D, Crowther MA. Statins in the prevention of venous thromboembolism: a meta‐analysis of observational studies. Thromb Res 2011; 128: 422–30. [DOI] [PubMed] [Google Scholar]
- 7. Hippisley‐Cox J, Coupland C. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database. BMJ 2010; 340: c2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Glynn RJ, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Ridker PM. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med 2009; 360: 1851–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rahimi K, Bhala N, Kamphuisen P, Emberson J, Biere‐Rafi S, Krane V, Robertson M, Wikstrand J, McMurray J. Effect of statins on venous thromboembolic events: a meta‐analysis of published and unpublished evidence from randomised controlled trials. PLoS Med 2012; 9: e1001310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kunutsor SK, Seidu S, Khunti K. Statins and secondary prevention of venous thromboembolism: pooled analysis of published observational cohort studies. Eur Heart J 2017; 38: 1608–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rosendaal FR. Statins and venous thrombosis: a story too good to be true? PLoS Med 2012; 9: e1001311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Biedermann JS, Kruip M, van der Meer FJ, Rosendaal FR, Leebeek FWG, Cannegieter SC, Lijfering WM. Rosuvastatin use improves measures of coagulation in patients with venous thrombosis. Eur Heart J 2018; 39: 1740–7. [DOI] [PubMed] [Google Scholar]
- 13. Adams NB, Lutsey PL, Folsom AR, Herrington DH, Sibley CT, Zakai NA, Ades S, Burke GL, Cushman M. Statin therapy and levels of hemostatic factors in a healthy population: the Multi‐Ethnic Study of Atherosclerosis. J Thromb Haemost 2013; 11: 1078–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sahebkar A, Serban C, Mikhailidis DP, Undas A, Lip GY, Muntner P, Bittner V, Ray KK, Watts GF, Hovingh GK, Rysz J, Kastelein JJ, Banach M; Lipid and Blood Pressure Meta‐analysis Collaboration (LBPMC) Group . Association between statin use and plasma D‐dimer levels. A systematic review and meta‐analysis of randomised controlled trials. Thromb Haemost 2015; 114: 546–57. [DOI] [PubMed] [Google Scholar]
- 15. Sahebkar A, Serban C, Ursoniu S, Mikhailidis DP, Undas A, Lip GY, Bittner V, Ray K, Watts GF, Hovingh GK, Rysz J, Kastelein JJ, Banach M; Lipid and Blood Pressure Meta‐analysis Collaboration (LBPMC) Group . The impact of statin therapy on plasma levels of von Willebrand factor antigen. Systematic review and meta‐analysis of randomised placebo‐controlled trials. Thromb Haemost 2016; 115: 520–32. [DOI] [PubMed] [Google Scholar]
- 16. Segers O, van Oerle R, ten Cate H, Rosing J, Castoldi E. Thrombin generation as an intermediate phenotype for venous thrombosis. Thromb Haemost 2010; 103: 114–22. [DOI] [PubMed] [Google Scholar]
- 17. ten Cate‐Hoek AJ, Dielis AW, Spronk HM, van Oerle R, Hamulyak K, Prins MH, ten Cate H. Thrombin generation in patients after acute deep‐vein thrombosis. Thromb Haemost 2008; 100: 240–5. [PubMed] [Google Scholar]
- 18. Dielis AW, Castoldi E, Spronk HM, van Oerle R, Hamulyak K, Ten Cate H, Rosing J. Coagulation factors and the protein C system as determinants of thrombin generation in a normal population. J Thromb Haemost 2008; 6: 125–31. [DOI] [PubMed] [Google Scholar]
- 19. Ten Cate H. Thrombin generation in clinical conditions. Thromb Res 2012; 129: 367–70. [DOI] [PubMed] [Google Scholar]
- 20. Tripodi A. Thrombin generation assay and its application in the clinical laboratory. Clin Chem 2016; 62: 699–707. [DOI] [PubMed] [Google Scholar]
- 21. van Veen JJ, Gatt A, Makris M. Thrombin generation testing in routine clinical practice: are we there yet? Br J Haematol 2008; 142: 889–903. [DOI] [PubMed] [Google Scholar]
- 22. Brummel‐Ziedins K, Vossen CY, Rosendaal FR, Umezaki K, Mann KG. The plasma hemostatic proteome: thrombin generation in healthy individuals. J Thromb Haemost 2005; 3: 1472–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Morishima Y, Kamisato C. Laboratory measurements of the oral direct factor Xa inhibitor edoxaban: comparison of prothrombin time, activated partial thromboplastin time, and thrombin generation assay. Am J Clin Pathol 2015; 143: 241–7. [DOI] [PubMed] [Google Scholar]
- 24. Spronk HM, Dielis AW, De Smedt E, van Oerle R, Fens D, Prins MH, Hamulyak K, ten Cate H. Assessment of thrombin generation II: validation of the Calibrated Automated Thrombogram in platelet‐poor plasma in a clinical laboratory. Thromb Haemost 2008; 100: 362–4. [PubMed] [Google Scholar]
- 25. Eichinger S, Hron G, Kollars M, Kyrle PA. Prediction of recurrent venous thromboembolism by endogenous thrombin potential and D‐dimer. Clin Chem 2008; 54: 2042–8. [DOI] [PubMed] [Google Scholar]
- 26. van Hylckama Vlieg A, Baglin CA, Luddington R, MacDonald S, Rosendaal FR, Baglin TP. The risk of a first and a recurrent venous thrombosis associated with an elevated D‐dimer level and an elevated thrombin potential: results of the THE‐VTE study. J Thromb Haemost 2015; 13: 1642–52. [DOI] [PubMed] [Google Scholar]
- 27. Hron G, Kollars M, Binder BR, Eichinger S, Kyrle PA. Identification of patients at low risk for recurrent venous thromboembolism by measuring thrombin generation. JAMA 2006; 296: 397–402. [DOI] [PubMed] [Google Scholar]
- 28. Besser M, Baglin C, Luddington R, van Hylckama Vlieg A, Baglin T. High rate of unprovoked recurrent venous thrombosis is associated with high thrombin‐generating potential in a prospective cohort study. J Thromb Haemost 2008; 6: 1720–5. [DOI] [PubMed] [Google Scholar]
- 29. Lutsey PL, Folsom AR, Heckbert SR, Cushman M. Peak thrombin generation and subsequent venous thromboembolism: the Longitudinal Investigation of Thromboembolism Etiology (LITE) study. J Thromb Haemost 2009; 7: 1639–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tripodi A, Legnani C, Chantarangkul V, Cosmi B, Palareti G, Mannucci PM. High thrombin generation measured in the presence of thrombomodulin is associated with an increased risk of recurrent venous thromboembolism. J Thromb Haemost 2008; 6: 1327–33. [DOI] [PubMed] [Google Scholar]
- 31. van Hylckama Vlieg A, Christiansen SC, Luddington R, Cannegieter SC, Rosendaal FR, Baglin TP. Elevated endogenous thrombin potential is associated with an increased risk of a first deep venous thrombosis but not with the risk of recurrence. Br J Haematol 2007; 138: 769–74. [DOI] [PubMed] [Google Scholar]
- 32. Visser MR, Tracy PB, Vercellotti GM, Goodman JL, White JG, Jacob HS. Enhanced thrombin generation and platelet binding on herpes simplex virus‐infected endothelium. Proc Natl Acad Sci USA 1988; 85: 8227–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Regnault V, Boehlen F, Ozsahin H, Wahl D, de Groot PG, Lecompte T, de Moerloose P. Anti‐protein S antibodies following a varicella infection: detection, characterization and influence on thrombin generation. J Thromb Haemost 2005; 3: 1243–9. [DOI] [PubMed] [Google Scholar]
- 34. Szczeklik A, Musial J, Undas A, Gajewski P, Gora P, Swadzba J, Jankowski M. Inhibition of thrombin generation by simvastatin and lack of additive effects of aspirin in patients with marked hypercholesterolemia. J Am Coll Cardiol 1999; 33: 1286–93. [DOI] [PubMed] [Google Scholar]
- 35. Cortellaro M, Cofrancesco E, Arbustini E, Rossi F, Negri A, Tremoli E, Gabrielli L, Camera M. Atorvastatin and thrombogenicity of the carotid atherosclerotic plaque: the ATROCAP study. Thromb Haemost 2002; 88: 41–7. [PubMed] [Google Scholar]
- 36. Macchia A, Laffaye N, Comignani PD, Cornejo Pucci E, Igarzabal C, Scazziota AS, Herrera L, Mariani JA, Bragagnolo JC, Catalano H, Tognoni G, Nicolucci A. Statins but not aspirin reduce thrombotic risk assessed by thrombin generation in diabetic patients without cardiovascular events: the RATIONAL trial. PLoS ONE 2012; 7: e32894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ural AU, Yilmaz MI, Avcu F, Yalcin A. Treatment with cerivastatin in primary mixed hyperlipidemia induces changes in platelet aggregation and coagulation system components. Int J Hematol 2002; 76: 279–83. [DOI] [PubMed] [Google Scholar]
- 38. Mann KG, Butenas S, Brummel K. The dynamics of thrombin formation. Arterioscler Thromb Vasc Biol 2003; 23: 17–25. [DOI] [PubMed] [Google Scholar]
- 39. Ay C, Pabinger I. Predictive potential of haemostatic biomarkers for venous thromboembolism in cancer patients. Thromb Res 2012; 129(Suppl 1): S6–9. [DOI] [PubMed] [Google Scholar]
- 40. Martinez C, Katholing A, Folkerts K, Cohen AT. Risk of recurrent venous thromboembolism after discontinuation of vitamin K antagonist treatment: a nested case‐control study. J Thromb Haemost 2016; 14: 1374–83. [DOI] [PubMed] [Google Scholar]
- 41. Palareti G, Legnani C, Guazzaloca G, Frascaro M, Grauso F, De Rosa F, Fortunato G, Coccheri S. Activation of blood coagulation after abrupt or stepwise withdrawal of oral anticoagulants–a prospective study. Thromb Haemost 1994; 72: 222–6. [PubMed] [Google Scholar]
- 42. Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galie N, Gibbs JS, Huisman MV, Humbert M, Kucher N, Lang I, Lankeit M, Lekakis J, Maack C, Mayer E, Meneveau N, Perrier A, Pruszczyk P, Rasmussen LH, Schindler TH, et al 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014; 35: 3033–69, 69a–69k. [DOI] [PubMed] [Google Scholar]
- 43. Undas A, Brummel‐Ziedins KE, Mann KG. Anticoagulant effects of statins and their clinical implications. Thromb Haemost 2014; 111: 392–400. [DOI] [PubMed] [Google Scholar]
- 44. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd‐Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC Jr, Watson K, Wilson PW; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63: 2889–934. [DOI] [PubMed] [Google Scholar]
- 45. Chapman RH, Benner JS, Petrilla AA, Tierce JC, Collins SR, Battleman DS, Schwartz JS. Predictors of adherence with antihypertensive and lipid‐lowering therapy. Arch Intern Med 2005; 165: 1147–52. [DOI] [PubMed] [Google Scholar]
- 46. Hemker HC, Kremers R. Data management in thrombin generation. Thromb Res 2013; 131: 3–11. [DOI] [PubMed] [Google Scholar]
- 47. Cohen H, Hunt BJ, Efthymiou M, Arachchillage DRJ, Mackie IJ, Clawson S, Sylvestre Y, Machin SJ, Bertolaccini ML, Ruiz‐Castellano M, Muirhead N, Dore CJ, Khamashta M, Isenberg DA; RAPS Trial Investigators . Rivaroxaban versus warfarin to treat patients with thrombotic antiphospholipid syndrome, with or without systemic lupus erythematosus (RAPS): a randomised, controlled, open‐label, phase 2/3, non‐inferiority trial. Lancet Haematol 2016; 3: E426–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schulz KF, Altman DG, Moher D; CONSORT Group . CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Obstet Gynecol 2010; 115: 1063–70. [DOI] [PubMed] [Google Scholar]
- 49. Kintigh J, Monagle P, Ignjatovic V. A review of commercially available thrombin generation assays. Res Pract Thromb Haemost 2018; 2: 42–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Effects of rosuvastatin on the endogenous thrombin potential in prespecified subgroups.
Table S2. Effects of rosuvastatin on the thrombin peak in prespecified subgroups.
Table S3. Effects of rosuvastatin on the lag time in prespecified subgroups.
Table S4. Effects of rosuvastatin on the time to peak in prespecified subgroups.
Table S5. Effects of rosuvastatin on the velocity index in prespecified subgroups.


