Abstract
Background and objectives
Several sources of haematopoietic stem cells have been used for static culture of megakaryocytes to produce platelets in vitro. This study compares and characterizes platelets produced in shear flow using precursor cells from either umbilical (UCB) or adult peripheral blood (PB).
Materials and methods
The efficiency of platelet production of the cultured cells was studied after perfusion in custom‐built von Willebrand factor‐coated microfluidic flow chambers. Platelet receptor expression and morphology were investigated by flow cytometry and microscopy, respectively.
Results
Proliferation of stem cells isolated out of UCB was significantly higher (P < 0·0001) compared to PB. Differentiation of these cells towards megakaryocytes was significantly lower from PB compared to UCB where the fraction of CD42b/CD41 double positive events was 44 ± 9% versus 76 ± 11%, respectively (P < 0·0001). However, in vitro platelet production under hydrodynamic conditions was more efficient with 7·4 platelet‐like particles per input cell from PB compared to 4·2 from UCB (P = 0·02). The percentage of events positive for CD42b, CD41 and CD61 was comparable between both stem cell sources. The mean number of receptors per platelet from UCB and PB was similar to that on blood bank platelets with on average 28 000 CD42b, 57 000 CD61 and 5500 CD49b receptors. Microscopy revealed platelets appearing similar to blood bank platelets in morphology, size and actin cytoskeleton, alongside smaller fragments and source megakaryocytes.
Conclusion
This characterization study suggests that platelets produced in vitro under flow either from UCB or from PB share receptor expression and morphology with donor platelets stored in the blood bank.
Keywords: haematopoiesis, megakaryopoiesis, platelets, stem cells, tissue engineering
Introduction
Transfusion of platelet concentrates (PC) can be a life‐saving treatment for patients suffering from thrombocytopenia 1. Platelet inventory management is a major challenge because donor attendance is unpredictable and the demand for PC is increasing, in addition to the limited shelf life of PC 2, 3. This has stimulated researchers to investigate in vitro production of platelets as an alternative to donor‐derived PC. The platelet moreover offers an ideal model cell for tissue engineering with limited risks of carcinogenesis because platelets are anucleate and thus do not replicate and the final product could be irradiated using low intensity gamma irradiation before transfusion. 4, 5.
Platelets are produced by megakaryocytes (MK) that reside outside the blood vessel but protrude cellular extensions that release (pro)platelets in circulation 6, 7. When this physiological production process can be mimicked in vitro, an attractive alternative for the current platelet blood banking chain is offered. The ideal unlimited cell source for MK is a self‐renewable embryonic 8 or induced pluripotent stem cell 9, 10 or immortalized or chemically defined MK cell line 5, 11. However, research into the platelet production process currently still requires isolated primary haematopoietic stem cells (HSC) until limitations of the current self‐renewable cell sources are overcome. Umbilical cord blood (UCB) is often used as HSC source 12, 13, 14, 15, 16 but requires specialized access and is thus not always available to research teams. Leuko‐depletion filters as a waste product of whole blood donations therefore offer an attractive alternative source 17. But even simpler, buffy coats from routine whole peripheral blood donations (PB) are available daily in many blood banks in Europe and may be source material for stem cell isolation 18 and in vitro platelet production on a research scale 19.
In this study, the efficiency and quality of in vitro platelet production in a microfluidic flow chamber chip coated with von Willebrand factor (VWF) 20 was evaluated for cells derived from UCB compared to PB. The platelets were studied by differential interference contrast (DIC) and fluorescent microscopy and flow cytometry. Data were compared to standard blood bank platelets (bPLT).
Materials and methods
Isolation of CD34+ haematopoietic stem cells
Human HSC were obtained from UCB or from buffy coats isolated from PB. The research protocols were approved by ethical committees of Ghent University Hospital (2016/0448) and Antwerp University Hospital (B300201420404). Isolation of HSC from UCB was by selection of CD34+ cells using an immunomagnetic cell‐sorting system (QuadroMACS, Miltenyi Biotech, Bergisch Gladbach, Germany) according to the manufacturer's instructions. Briefly, red blood cell lysis was induced with 2% dextran (w/v) before performing density‐gradient centrifugation using ficoll (970 g – 20 min – room temperature (RT)). The mononucleated cell layer was aspirated and washed. Total nucleated cell number was determined in a Malassez haemocytometer. The nucleated cells were resuspended to 108 cells in 300 μl phosphate‐buffered saline (PBS) (1·5 mm KH2PO4, 137 mm NaCl, 2·7 mM KCl and 8 mm Na2HPO4 at pH 7·4) supplemented with 2 mm ethylenediaminetetraactic acid (EDTA) and 0·5% bovine serum albumin (BSA) (w/v) before addition of 100 μl anti‐CD34 labelled magnetic beads and 100 μl Fc‐receptor blocking agent per 108 cells. Cells were incubated for 90 min at 4°C during constant agitation before removal of unbound cells by centrifugation (300 g – 10 min – RT) and resuspension in PBS with EDTA and BSA. Bound CD34+ cells were collected and counted in the Malassez haemocytometer. After isolation, the cells were cryopreserved at 0·5–1·0 × 106 cells per mL in fetal bovine serum and 10% (v/v) dimethylsulphoxide at −80°C (≥24 h) before transfer to our liquid nitrogen (−196°C) long time storage facility. Three buffy coats prepared from voluntary whole blood donations by the Belgian Red Cross‐Flanders Blood Service were pooled prior to isolation of HSC according to the protocol described previously. Whole blood donations contain approximately 14% (v/v) citrate‐phosphate‐dextrose‐acid as anticoagulant. After storage overnight at RT, whole blood donations were centrifuged (4556 g – 13 min – RT). Automated separators were used for separation into red blood cell concentrate, plasma and a buffy coat. Excess buffy coats not used for platelet concentrate preparation were used in this work. The number of magnetic beads used per total number of nucleated cells was lower, and additional washing steps were used compared to isolation from UCB to compensate for the low ratio HSC versus total nucleated cells in PB 21.
Cell culture towards megakaryocytes
Cryopreserved HSC were thawed in a 37°C water bath before addition of 10 ml prewarmed HP01 medium (Macopharma, Tourcoing, France) and centrifugation (300 g – 10 min – RT). HSC were resuspended in HP01 supplemented with 10 nm thrombopoietin peptide (TPO, Sigma‐Aldrich, Saint Louis, MO), 25 ng/ml stem cell factor (SCF, Miltenyi Biotech) and 2 ng/ml interleukin 3 (IL3, Miltenyi Biotech) and were seeded at 300 000 cells/ml for 6 days of expansion. On day 7 (D7), cells were split at 500 000 cells/ml in HP01 containing 50 nm TPO and 2 ng/ml SCF for differentiation towards MK. All cells were harvested on D12 and subjected to BSA gradient centrifugation for removal of small particles 22.
Definition of platelets and cultured cells
In this manuscript, the term (input) cells is used for particles with a diameter ≥ 7 μm counted in a Malassez haemocytometer. Particles ≤ 7 μm are called platelet‐like particles (PLP). Size of particles was measured using the analytical software tools for microscopy (Zen 2, Blue edition, Carl Zeiss). The same term (PLP) is also used for flow cytometry events appearing inside a polygon gate set as a contour around bPLT as source material 23 (Figs S1a and b). This polygon gate is further referred to as the morphology gate. Particles within the morphology gate that stain positive for both surface receptor CD42b and CD41 (see below) were then defined as platelets (Fig S1c).
Standard blood bank platelets
Platelets from PC were used as reference material to compare with in vitro platelet production and are defined as standard blood bank platelets. The PC were prepared according to standard blood banking methods as described previously 24, 25, in this case by pooling of six buffy coats. A final 65% (v/v) of additive solution (SSP+, Macopharma, Tourcoing) was targeted.
Phenotyping by flow cytometry
Differentiation of cultured cells was determined by flow cytometry (Attune, Life Technologies, Carlsbad, CA) on D0, D4, D7, D10, D11 and D12. The haematopoietic precursor lineage was determined with an antibody against CD34 (phycoerythrin (PE)‐anti‐CD34, BD, Biosciences, Erembodegem, Belgium). Determination of MK differentiation was with fluorescein (FITC)‐labelled anti‐CD42b (Life Technologies) and PE‐Cy7‐labelled anti‐CD41 (BD Biosciences). Cells and antibodies were incubated for 10 min in 4‐(2‐hydroxyethyl)‐1‐piperazineethanesulfonic acid buffered saline (HBS) (10 mm HEPES, 155 mm NaCl and 1 mm MgSO4, pH 7·4) at RT 26. Expression of CD42b (GPIbα), CD41 (GPIIb) and CD61 (GPIIIa) on PLP was detected using FITC‐labelled anti‐CD42b, PE‐Cy7‐labelled anti‐CD41 and allophycocyanin (APC)‐labelled anti‐CD61 (Life Technologies).The median number of CD42b, CD41 and CD49b (GPIa) receptors per PLP was quantified using the GP screen kit (Biocytex, Marseille, France). Threshold gates were set including 0·5% of 10 000 events incubated with corresponding isotype antibody negative controls.
In vitro platelet production
Microfluidic flow chamber chips were manufactured from polydimethylsiloxane as described before 27. One chip (length = 17·3 cm) consisted of 16 parallel channels composed of a structured network of pillars. The radius of one pillar is 15 μm, and the distance between neighbouring pillars, from centre to centre, is 85 μm. The pillars are oriented so that the angle between the pillar array and the flow direction of the channel is 10°. Prior to cell perfusion, the chip was coated overnight with 40 μg/ml purified VWF (Wilfactin, LFB Biotechnologies, France). The perfusion experiment was performed according to Blin et al. 27. In brief, the harvested cell suspension on D12 of culture was placed on an orbital shaker and was connected to the perfusion chamber inlet and outlet by tubing. A peristaltic pump (Watson‐Marlow, Falmouth, UK) created continuous perfusion of the cell suspension at a wall shear rate of 1800 s−1 for two hours during which platelets were formed. Adhering cells were visualized by phase contrast microscopy at 100× magnification (Axio observer A1, Carl Zeiss). After perfusion, the suspension was centrifuged at 110 g for 20 min to remove MK. Next, the supernatant was centrifuged at 1240 g for 15 min to pellet the platelets.
Differential interference contrast microscopy
The morphology of PLP was determined by DIC microscopy (Axio observer A1, Carl Zeiss). Cell suspensions were resuspended in Tyrode's washing buffer (135 mm NaCl, 2·7 mm KCl, 12 mm NaHCO3, 430 μm NaH2PO4, 1 mm MgCl2, 5 mm HEPES with 0·1% (w/v) D‐glucose and 0·35% (w/v) BSA) at pH 7·4 before fixation with 2% (w/v) paraformaldehyde. Fixed cells were centrifuged at 2000 g for 20 min onto glass coverslips, coated with 0·1% (w/v) poly‐L‐lysine (Sigma Aldrich, Saint Louis). These coverslips were then mounted on glass carriers. A 100× oil immersion objective (numerical aperture = 1·4) was used to a final magnification of 1000×. To control for potential side‐effects downstream of platelet production caused by centrifugations, bPLT were subjected to the same centrifugation protocol prior to preparation for DIC imaging.
Cytoskeleton staining
Actin was visualized on PLP from UCB and on bPLT by Alexa Fluor (AF) 405 labelled‐phalloidin (Invitrogen, Carlsbad, CA). Coverslips were prepared as described under differential interference contrast microscopy. Cells were permeabilized with 0·5% (v/v) triton X‐100 (Sigma Aldrich, Saint Louis) followed by staining with 165 μm AF405~phalloidin and mounting onto glass carriers.
Statistical analysis
Results are reported as mean with standard deviation (SD). Comparison between stem cell sources was by two‐tailed t‐test (with Welch's correction for unequal variances), by one‐way anova for comparison of mean number of receptors per PLP (Tukey's correction for multiple comparison) or with two‐way anova (Sidak's correction for multiple comparison) for comparison of both HSC sources at different time points of culture. Correlation was by Spearman's algorithm. Statistical analyses was performed with Prism version 7.04 (GraphPad Software Inc., La Jolla, CA).
Results
Haematopoietic stem cell isolation, proliferation and differentiation towards megakaryocytes
Table 1 shows that the mean number of nucleated cells in PB was at least 4‐fold higher in comparison to UCB. However, the mean absolute number of cells isolated per PB is 3·93 × 105 compared to 16·97 × 105 per UCB. The percentage of isolated cells per total nucleated cells for UCB and PB source samples is presented in Fig. 1a. A significantly higher (P < 0·0001) yield was found for UCB (1·33 ± 0·74%) compared to PB (0·06 ± 0·02%). Proliferation in static culture was significantly faster for UCB‐derived HSC compared to PB (P < 0·0001 on D7, Fig. 1b). As expected, proliferation decreased when the TPO, SCF and IL3 concentration were changed on D7 (Fig. 1b). Consequently, CD34 expression decreased (Fig. 1c) while expression of CD42b/CD41 markers increased (Fig. 1d). The fraction of CD42b/CD41 double positive events was significantly lower in cultures from PB than in UCB on D10, 11 & 12 (Fig. 1d) with a difference of 44 ± 9% vs 76 ± 11% CD42b/CD41 positive events on D12 for PB and UCB, respectively (P < 0·0001).
Table 1.
Absolute number of total nucleated cells before isolation and of CD34+ cells after isolation from PB or UCB
| UCB | PB | |
|---|---|---|
| Absolute number of total nucleated cells per sample before isolation (×108) | 1·49 ± 0·65 | 6·93 ± 1·73 |
| Absolute number of CD34+ cells per sample after isolation (×105) | 16·97 ± 6·49 | 3·93 ± 1·35 |
Data are shown as mean ± SD (n ≥ 14).
Figure 1.
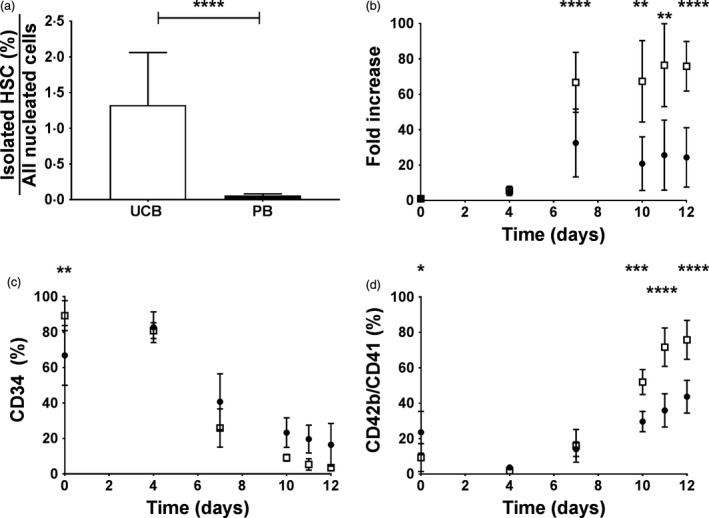
Static cell culture of HSC derived from PB or UCB. (a) The percentage HSC obtained by magnetic isolation per total nucleated cells in UCB (□) or PB (●) samples. (b) The proliferation (all cells), in function of time, expressed as fold increase relative to the number of cells seeded on D0. (c) Expression of CD34 and (d) CD42b/CD41 markers on cultured cells as measured by flow cytometry as a function of time. Data are shown as mean with SD (n ≥ 5). Statistical analysis results are shown as *P < 0·05, **P < 0·01, ***P < 0·001 and ****P < 0·0001.
Production of platelet‐like particles
On D12 of culture, cells were perfused for two hours resulting in cell anchorage, elongation and release of PLP, starting from both UCB and PB (Fig. 2a and b). The PLP yield defined as the number of PLP over the number of input cells was significantly higher for PB (7·4 ± 4·4) than for UCB (4·2 ± 2·2) (P = 0·02) (Fig. 2c). The PLP yield was independent of the proportion of mature MK when defined as events positive for CD41/CD42b present in the input sample. This held for cells isolated from UCB (r = −0·13, P = 0·62) (Fig. 2d) and from PB (r = 0·03, P = 0·85) (Fig. 2e) at D12.
Figure 2.
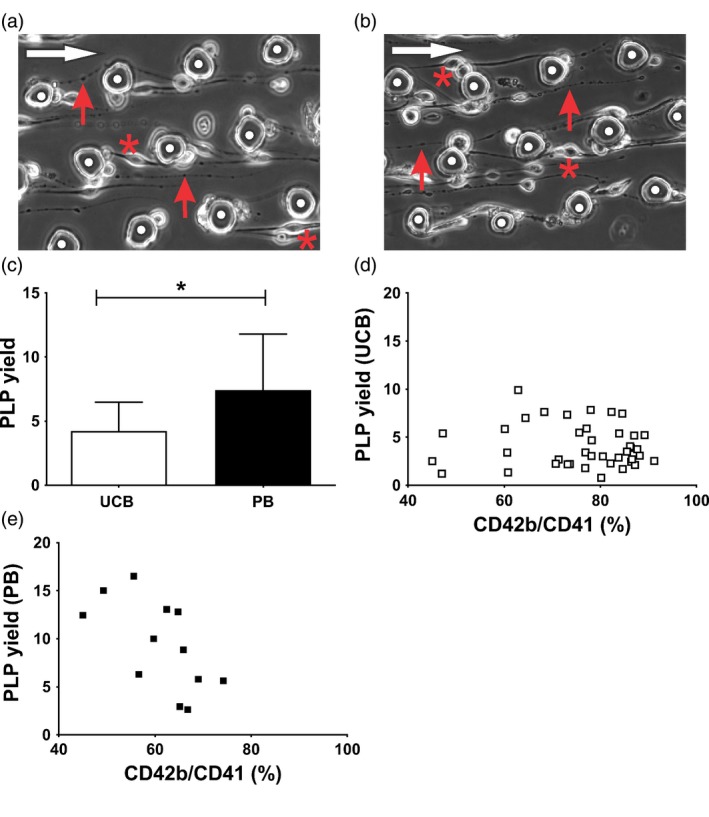
Platelet production in hydrodynamic flow. (a) Representative image of cell anchorage (*) to VWF‐coated pillars (white dots) of a cultured cell (D12) started from UCB and (b) PB. Cell elongation and (pro)platelet formation is indicated by red arrows. The flow direction is shown by a white arrow. (c) The number of PLP produced per input cell during the perfusion experiment. Data are given as mean with SD (n ≥ 12). Statistical analysis results are shown as *P < 0·05. (d and e) The PLP yield was plotted as a function of the number of CD42b/CD41 double positive input cells for (d) UCB (n = 36) and (e) PB (n = 13).
Platelet receptor expression
The fraction of events in the morphology gate (Fig. S1) staining positive for either CD41, CD42b or both was comparable between PLP from UCB or PB sources (Fig. 3a and b). Consequently, based on our definition (Fig. S1) approximately 60% of PLP could be defined as platelets (Fig. 3c). Expression of CD61 was also comparable between both HSC sources (Fig. 3d). The mean number of CD42b, CD61 and CD49b receptors per PLP did not significantly differ between both stem cell sources and is comparable to the number of receptors on bPLT (Fig. 4). The number of CD42b receptors per PLP is on average 28 000 per PLP (Fig. 4a) while this is approximately 57 000 and 5500 per PLP for CD61 and CD49b, respectively (Fig. 4b and c).
Figure 3.
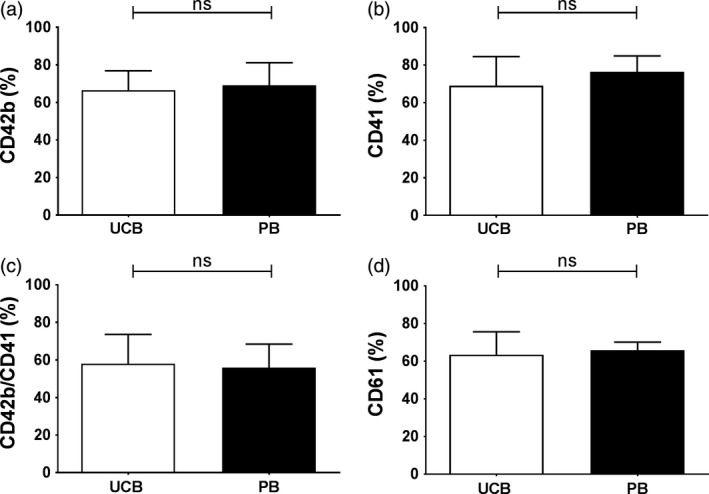
Expression of platelet markers. The fraction of PLP that was staining positive for (a) CD42b, (b) CD41, (c) CD42b/CD41 and (d) CD61 produced from UCB (open bars) and PB (closed bars). Data are shown as mean with SD (n ≥ 5).
Figure 4.
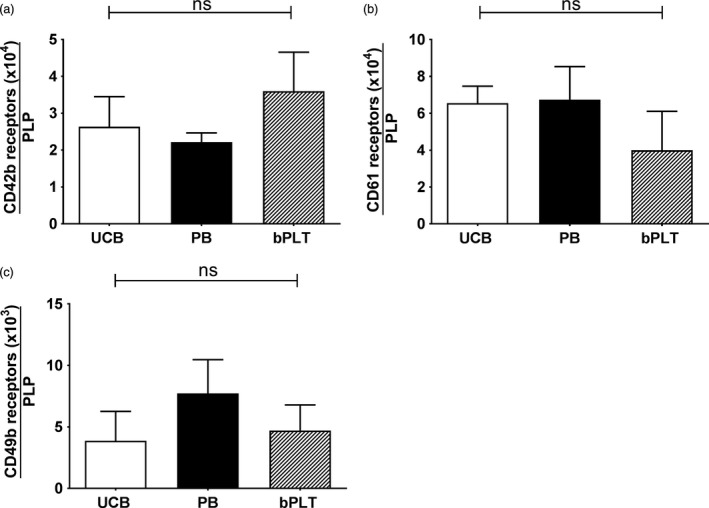
Number of platelet receptors. The number of (a) CD42b, (b) CD61 and (c) CD49b receptors was determined using GP screen kit on PLP produced from UCB (open bars) and PB (closed bars). Data were compared to the levels in bPLT (hatched bars). Data are shown as mean with SD (n = 4).
Morphology and cytoskeleton stain
Cell suspensions after perfusion were analysed by DIC microscopy at 1000× magnification. All suspensions contained a heterogeneous cell population by size. Particles structurally comparable to platelets were found both in UCB and PB derived samples (Fig. 5a and b). These contained what appeared to be granules as intense dark zones inside and near the cell's periphery. Alongside these, the suspension also contained large cells (*) characteristic of remainder MK. The ratio of remaining MK compared to PLP was approximately 7:3 and was indistinguishable between UCB or PB. Particles smaller than typical platelets could either be extracellular vesicles (also called microparticles) or cell debris. Reference bPLT were prepared as a control sample (Fig. 5c). Additionally, the cytoskeleton protein actin was visualized on the DIC images (Fig. S2). All cells from the bPLT suspension stained positive for actin while in the cell suspension from UCB only particles with comparable structure to platelets were found positive for actin.
Figure 5.
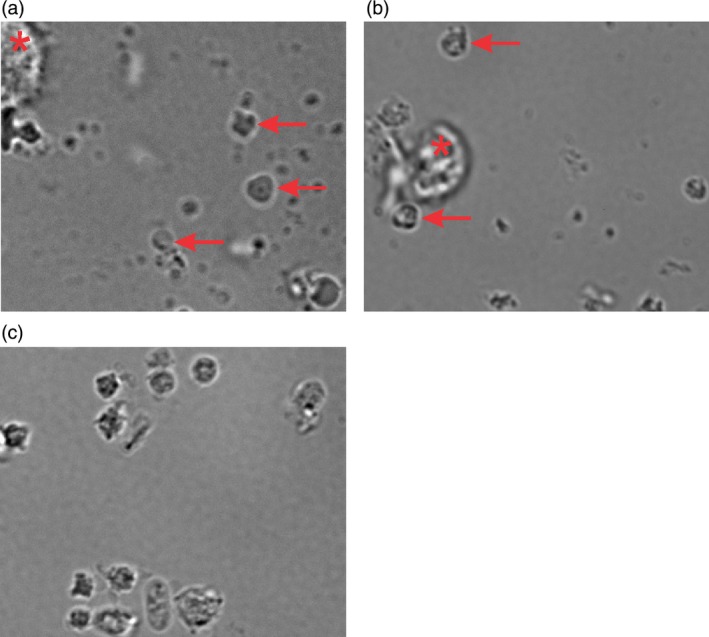
Differential interference microscopy. Representative images of the cell suspensions from (a) UCB or (b) PB after perfusion and double centrifugation. PLP are indicated by arrows. The larger cells were most likely MK (*). (c) Representative image of bPLT prepared in the same way as the PLP. Pictures were taken from DIC microscopy studies 1000× magnification.
Discussion
To manufacture platelets in vitro, three major steps are needed 28: first, appropriate stem cells are expanded in tissue culture. In a second step, these are differentiated to generate haematopoietic and MK precursor cells. As an alternative, immortalized MK can be used as demonstrated by Nakamura et al. 11. In any case, the final step is to produce platelets from these MK in an efficient and safe manner.
Ultimately, the technology will use self‐renewable stem cell sources like hESC or iPSC although some advocate the use of UCB and PB as well 29. Ethical concerns limit the use of hESC 30 and current platelet yields obtained from iPSC are not (yet) reaching platelet yields obtained in vivo 5, 10, 11, 31. Therefore, to enable research and development to the final step in the process (platelet production), primary HSC can still be used and offer an inexpensive and accessible source. Furthermore, for rare phenotypes, PB may still be needed as a source of autologous stem cells. Bearing this in mind, this study's objective was to compare the yield and quality of platelets produced in hydrodynamic flow conditions using precursor cells from either UCB or PB and to compare these to current standard of care bPLT.
As expected from research by van den Oudenrijn et al. 13, in vitro expansion of stem cells in static conditions was more efficient from UCB than from PB. Relative to D0, a 68‐fold and 32‐fold expansion was found on D7 for UCB and PB, respectively. From D7 on, the TPO concentration was raised to promote differentiation to MK. In those conditions, platelet production happens spontaneously, albeit slowly and asynchronously 16, 32, 33. In our flow chambers, the combination of shear and immobilized VWF results in synchronized and efficient platelet production 14, 27. BSA gradient centrifugation was performed on culture cell prior to perfusion in the flow chamber to remove PLP produced during static culture conditions and focus investigation on PLP produced in the flow chamber. When comparing precursor cells sourced from UCB and PB, a higher platelet yield for PB was found. These data are comparable to findings in static conditions 19. Hence, phenotypic differences between source cells are translated in the same way in static as in dynamic conditions. MK ploidy has been suggested to correlate with platelet production 19, albeit not in all conditions 34, 35. We hypothesized that the fraction of CD42b/CD41 positive MK in the input suspension could predict PLP yield but the data show this was not the case. Consequently, although these markers are specific for platelets, these do not predict platelet production efficiency in our system. But expression of CD42b/CD41 as a predictor for PLP production cannot be ruled out completely because the exact expression levels of CD42b/CD41 on the MK may still be correlating. Despite differences in HSC expansion and platelet production efficiency, platelet phenotypic characteristics are quite similar between UCB and PB. Foremost, these are also comparable to standard bPLT, although a trend was found for lower GPIbα (CD42b) and higher integrin β3 (CD61 or GPIIIa) expression. Decreased GPIbα expression levels have also been reported in murine platelets produced in vitro in static conditions from mouse ESC 36. This was caused by ectodomain shedding and could be prevented by co‐incubation with a metalloproteinase inhibitor. Importantly, GPIbα levels were not decreased to levels relevant to impede haemostasis 37. Contrary to GPIbα, an increase in integrin β3 expression was found. The β3 integrin dynamically forms heterodimers with αIIb and αV, the former being utmost important for platelet aggregation as the fibrinogen receptor. Platelet aggregation studies are required next to demonstrate whether this moderate increase translates into altered function compared with bPLT.
The input cell suspension contains MK in several stages of maturation 33, 38, 39 and senescence. After the 2 h of perfusion to produce platelets, a double centrifugation is performed to remove remainder MK and concentrate PLP. Our microscopic images indicate that large cells as well as small particles are nonetheless still found. Using flow cytometry, a fraction of 70% was consequently defined as platelets while the other 30% are remaining MK (with exclusion cellular debris and microparticles). The same ratio of PLP and MK was found in the microscopic images for both UCB and PB. Therefore, future clinical applications will require optimized filtration and concentration steps of the outflow 40. Such alternatives are spinning‐membrane filtration devices 41 or microfluidic‐based separations whether or not in combination with acoustopheresis 42.
However, the microscopic images indicate mainly platelet‐like cells. These are similar in size and aspect to bPLT with this imaging technique. These platelet‐like particles were present in both suspensions from UCB and PB. Furthermore, actin staining showed that these platelet‐like cells are positively stained while no actin was detected in smaller particles. Ultrastructural analyses using electron microscopy must be used to further identify the subcellular organelles as alpha and/or dense granules characteristic of healthy platelets.
We conclude that platelet production efficiency by perfusion of cultured cells in VWF‐coated microfluidic flow chambers is higher starting from PB compared to UCB, like in static conditions. Despite this, the absolute overall PLP number is higher starting from UCB because of higher number of isolated cells and higher proliferation rates in the preceding static culture. Preliminary platelet characterization indicates surface receptor expression and morphology are comparable to reference bPLT. Future experiments should focus on platelet ultrastructure and platelet function. In the long run, increasing the platelet yield and lowering production costs are essential prior to proceeding to clinical trials comparing these in vitro produced platelets to standard PC.
Author contributions
KRS & RD performed the experiments; KRS, GS, HBF & DB interpreted results; HBF, VC & DB designed the research; VC & DB provided essential materials and supported the study; KRS & HBF wrote the manuscript. All authors critically reviewed the manuscript and agreed upon submission.
Supporting information
Fig. S1 Representative scatter plots to define PLP and platelets.
Fig. S2 Cytoskeleton staining.
Acknowledgements
This research was supported by the Foundation for Scientific Research of the Belgian Red Cross‐Flanders, the Special Research Fund of Ghent University (BOF 30290744) and the ME TO YOU foundation, Tielt, Belgium. This work was partially supported by LFB‐Biotechnologies through a service agreement with PlatOD. Special thanks to all voluntary donors of the Belgian Red Cross‐Flanders for their invaluable contribution.
References
- 1. Tiberghien P, Follea G, Muller JY: Platelet transfusions in acute leukemia. N Engl J Med 2016; 375:96–97 [DOI] [PubMed] [Google Scholar]
- 2. Murphy S, Gardner FH: Effect of storage temperature on maintenance of platelet viability–deleterious effect of refrigerated storage. N Engl J Med 1969; 280:1094–1098 [DOI] [PubMed] [Google Scholar]
- 3. Abbot S: The three “R”s of blood transfusion in 2020; routine, reliable and robust. Clin Lab Med 2010; 30:405–417 [DOI] [PubMed] [Google Scholar]
- 4. Avanzi MP, Mitchell WB: Ex vivo production of platelets from stem cells. Br J Haematol 2014; 165:237–247 [DOI] [PubMed] [Google Scholar]
- 5. Moreau T, Evans AL, Vasquez L, et al: Large‐scale production of megakaryocytes from human pluripotent stem cells by chemically defined forward programming. Nat Commun 2016; 7:11208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pease DC: An electron microscopic study of red bone marrow. Blood 1956; 11:501–526 [PubMed] [Google Scholar]
- 7. Richardson JL, Shivdasani RA, Boers C, et al: Mechanisms of organelle transport and capture along proplatelets during platelet production. Blood 2005; 106:4066–4075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lu SJ, Li F, Yin H, et al: Platelets generated from human embryonic stem cells are functional in vitro and in the microcirculation of living mice. Cell Res 2011; 21:530–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Takayama N, Nishimura S, Nakamura S, et al: Transient activation of c‐MYC expression is critical for efficient platelet generation from human induced pluripotent stem cells. J Exp Med 2010; 207:2817–2830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Feng Q, Shabrani N, Thon JN, et al: Scalable generation of universal platelets from human induced pluripotent stem cells. Stem Cell Rep 2014; 3:817–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nakamura S, Takayama N, Hirata S, et al: Expandable megakaryocyte cell lines enable clinically applicable generation of platelets from human induced pluripotent stem cells. Cell Stem Cell 2014; 14:535–548 [DOI] [PubMed] [Google Scholar]
- 12. Balduini A, d'Apolito M, Arcelli D, et al: Cord blood in vitro expanded CD41 cells: identification of novel components of megakaryocytopoiesis. J Thromb Haemost 2006; 4:848–860 [DOI] [PubMed] [Google Scholar]
- 13. van den Oudenrijn S, von dem Borne AE, de Haas M: Differences in megakaryocyte expansion potential between CD34(+) stem cells derived from cord blood, peripheral blood, and bone marrow from adults and children. Exp Hematol 2000; 28:1054–1061 [DOI] [PubMed] [Google Scholar]
- 14. Dunois‐Larde C, Capron C, Fichelson S, et al: Exposure of human megakaryocytes to high shear rates accelerates platelet production. Blood 2009; 114:1875–1883 [DOI] [PubMed] [Google Scholar]
- 15. Nichol JL, Hornkohl AC, Choi ES, et al: Enrichment and characterization of peripheral blood‐derived megakaryocyte progenitors that mature in short‐term liquid culture. Stem Cells 1994; 12:494–505 [DOI] [PubMed] [Google Scholar]
- 16. Thon JN, Mazutis L, Wu S, et al: Platelet bioreactor‐on‐a‐chip. Blood 2014; 124:1857–1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ivanovic Z, Duchez P, Morgan DA, et al: Whole‐blood leuko‐depletion filters as a source of CD 34 + progenitors potentially usable in cell therapy. Transfusion 2006; 46:118–125 [DOI] [PubMed] [Google Scholar]
- 18. Barr RD, Whang‐Peng J, Perry S: Hemopoietic stem cells in human peripheral blood. Science 1975; 190:284–285 [DOI] [PubMed] [Google Scholar]
- 19. Mattia G, Vulcano F, Milazzo L, et al: Different ploidy levels of megakaryocytes generated from peripheral or cord blood CD34 + cells are correlated with different levels of platelet release. Blood 2002; 99:888–897 [DOI] [PubMed] [Google Scholar]
- 20. Pietrzyk‐Nivau A, Poirault‐Chassac S, Gandrille S, et al: Three‐dimensional environment sustains hematopoietic stem cell differentiation into platelet‐producing megakaryocytes. PLoS ONE 2015; 10:e0136652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Philpott NJ, Prue RL, Marsh JC, et al: G‐CSF‐mobilized CD34 peripheral blood stem cells are significantly less apoptotic than unstimulated peripheral blood CD34 cells: role of G‐CSF as survival factor. Br J Haematol 1997; 97:146–152 [DOI] [PubMed] [Google Scholar]
- 22. Robert A, Cortin V, Garnier A, et al: Megakaryocyte and platelet production from human cord blood stem cells. Methods Mol Biol 2012; 788:219–247 [DOI] [PubMed] [Google Scholar]
- 23. Feys HB, Coene J, Devloo R, et al: Persistent aggregates in apheresis platelet concentrates. Vox Sang 2015; 108:368–377 [DOI] [PubMed] [Google Scholar]
- 24. Van Aelst B, Feys HB, Devloo R, et al: Riboflavin and amotosalen photochemical treatments of platelet concentrates reduce thrombus formation kinetics in vitro. Vox Sang 2015; 108:328–339 [DOI] [PubMed] [Google Scholar]
- 25. Hardwick J: Blood processing. ISBT Sci Ser 2008; 3:148–176 [Google Scholar]
- 26. Goodall AH, Appleby J: Flow‐cytometric analysis of platelet‐membrane glycoprotein expression and platelet activation. Methods Mol Biol 2004; 272:225–253 [DOI] [PubMed] [Google Scholar]
- 27. Blin A, Le Goff A, Magniez A, et al: Microfluidic model of the platelet‐generating organ: beyond bone marrow biomimetics. Sci Rep 2016; 6:21700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sim X, Poncz M, Gadue P, et al: Understanding platelet generation from megakaryocytes: implications for in vitro‐derived platelets. Blood 2016; 127:1227–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Strassel C, Gachet C, Lanza F: On the way to in vitro platelet production. Front Med 2018; 5:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Karagiannis P, Eto K: Manipulating megakaryocytes to manufacture platelets ex vivo. J Thromb Haemost 2015; 13(Suppl 1):S47–S53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sugimoto N, Eto K: Platelet production from induced pluripotent stem cells. J Thromb Haemost 2017; 15:1717–1727 [DOI] [PubMed] [Google Scholar]
- 32. Choi ES, Nichol JL, Hokom MM, et al: Platelets generated in vitro from proplatelet‐displaying human megakaryocytes are functional. Blood 1995; 85:402–413 [PubMed] [Google Scholar]
- 33. Thon JN, Montalvo A, Patel‐Hett S, et al: Cytoskeletal mechanics of proplatelet maturation and platelet release. J Cell Biol 2010; 191:861–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zimmet JM, Ladd D, Jackson CW, et al: A role for cyclin D3 in the endomitotic cell cycle. Mol Cell Biol 1997; 17:7248–7259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Leysi‐Derilou Y, Duchesne C, Garnier A, et al: Single‐cell level analysis of megakaryocyte growth and development. Differentiation 2012; 83:200–209 [DOI] [PubMed] [Google Scholar]
- 36. Nishikii H, Eto K, Tamura N, et al: Metalloproteinase regulation improves in vitro generation of efficacious platelets from mouse embryonic stem cells. J Exp Med 2008; 205:1917–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. van Zanten GH, Heijnen HF, Wu Y, et al: A fifty percent reduction of platelet surface glycoprotein Ib does not affect platelet adhesion under flow conditions. Blood 1998; 91:2353–2359 [PubMed] [Google Scholar]
- 38. Kellner J, Li S, Zweidler‐McKay PA, et al: Phenotypic and functional comparison of mobilized peripheral blood versus umbilical cord blood megakaryocyte populations. Cytotherapy 2015; 17:418–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sim X, Jarocha D, Hayes V, et al: Identifying and enriching the platelet‐producing human stem cell‐derived megakaryocytes using factor V uptake. Blood 2017; 130:192–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Thon JN, Medvetz DA, Karlsson SM, et al: Road blocks in making platelets for transfusion. J Thromb Haemost 2015; 13(Suppl 1):S55–S62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schlinker AC, Radwanski K, Wegener C, et al: Separation of in‐vitro‐derived megakaryocytes and platelets using spinning‐membrane filtration. Biotechnol Bioeng 2015; 112:788–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dykes J, Lenshof A, Astrand‐Grundstrom IB, et al: Efficient removal of platelets from peripheral blood progenitor cell products using a novel micro‐chip based acoustophoretic platform. PLoS ONE 2011; 6:e23074 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Representative scatter plots to define PLP and platelets.
Fig. S2 Cytoskeleton staining.


