Abstract
This consensus paper provides an overview of the state of the art in research on the aetiology and treatment of nightmare disorder and outlines further perspectives on these issues. It presents a definition of nightmares and nightmare disorder followed by epidemiological findings, and then explains existing models of nightmare aetiology in traumatized and non‐traumatized individuals. Chronic nightmares develop through the interaction of elevated hyperarousal and impaired fear extinction. This interplay is assumed to be facilitated by trait affect distress elicited by traumatic experiences, early childhood adversity and trait susceptibility, as well as by elevated thought suppression and potentially sleep‐disordered breathing. Accordingly, different treatment options for nightmares focus on their meaning, on the chronic repetition of the nightmare or on maladaptive beliefs. Clinically, knowledge of healthcare providers about nightmare disorder and the delivery of evidence‐based interventions in the healthcare system is discussed. Based on these findings, we highlight some future perspectives and potential further developments of nightmare treatments and research into nightmare aetiology.
Keywords: anxiety, evidence‐based medicine, exposure, imagery (psychotherapy), nightmare scripts, parasomnias, posttraumatic stress disorders, PTSD, sleep‐disorders
1. INTRODUCTION
Nightmare disorder is defined by the repeated occurrence of nightmares that cause clinically significant distress or impairment in social, occupational or other important areas of functioning, which are not attributable to the physiological effects of a substance (e.g. drug abuse or medication) and which cannot be adequately explained by coexisting mental and medical disorders (fifth revision of the Diagnostic and Statistical Manual of Mental Disorders [DSM‐5], American Psychiatric Association [APA], 2013, third] revision of the International Classification of Sleep Disorders [ICSD‐3], American Academy of Sleep Medicine [AASM], 2014).
Both ICSD‐3 (AASM, 2014) and DSM‐5 (APA, 2013) define a nightmare as an “extended, extremely dysphoric” dream that “usually involves efforts to avoid threats to survival, security, or physical integrity”. Nightmares usually occur during REM sleep (Nielsen, 2000; APA, 2013, but see Solms, 2000) and are associated with symptoms of physical arousal such as sweating and shortness of breath and more elevated indices of periodic leg movements during rapid eye movement (REM) sleep (Germain & Nielsen, 2003). The prevalent emotion is fear, although other emotions such as anger, shame and sadness may also arise (Köthe & Pietrowsky, 2001; Robert & Zadra, 2014, but see Phelps et al., 2018). These physiological symptoms and emotions can occur during the dreaming episode, upon awakening from the disturbing dream, or upon later recollection of the dream experience. One can differentiate between posttraumatic nightmares and idiopathic nightmares. Posttraumatic nightmares are either direct replications of a traumatic event or they contain trauma‐related emotion or content that is symbolically related to the trauma. Idiopathic nightmares depict stories that are more imaginative in nature and that do not necessarily reflect a traumatic event. Posttraumatic nightmares lead to more severe arousal, more nocturnal awakenings, stronger aggression and more elevated helplessness than do idiopathic nightmares (Wittmann & De Dassel, 2015).
There is an ongoing debate about whether the dreamer needs to awaken from a dysphoric dream for the dream to be considered a nightmare (Robert & Zadra, 2014). Although the previous DSM diagnostic manual (DSM‐IV, American Psychiatric Association (APA), 1994) described “Repeated awakenings from the major sleep period or naps with detailed recall of extended and extremely frightening dreams […]”, the more recent DSM‐5 (APA, 2013) formulated this criterion A as follows: “Repeated occurrences of extended, extremely dysphoric, and well‐remembered dreams […]” (p. 404). Thus, although nightmares often lead to awakenings according to the DSM‐5, waking up is not a necessary diagnostic criterion for nightmare disorder. There is no frequency criterion, although the DSM‐5 suggests a mild nightmare disorder as less than one episode per week on average, a moderate disorder as one or more episodes per week, but less than nightly, and a severe disorder as nightly episodes. An acute episode has a duration of 1 month or less, a subacute episode a duration of at least 1 month but less than 6 months, and chronic nightmares endure for 6 months or longer (APA, 2013).
Contrary to night terrors that manifest with confusional arousals, incomplete awakenings and difficulties in being comforted, individuals diagnosed with nightmare disorder awaken completely, orient immediately and remember the dream vividly. Nightmares are also frequently misidentified as sleep paralysis and sleep‐onset or sleep‐offset hallucinations, which are commonly associated with narcolepsy, but a sleep‐paralysis type of nightmares is recognized (APA, 2013).
Notwithstanding the above nosologies, in practice it is rare to diagnose a chronic nightmare disorder because practitioners are often unaware of the fact that chronic nightmares often constitute an independent mental disorder or sleep disorder or co‐occurring disorder with another psychiatric condition. Instead, practitioners frequently subsume most types of disturbing dreams as a secondary symptom to a primary mental disorder such as anxiety disorder or posttraumatic stress disorder (PTSD, Krakow, 2006; Spoormaker, Schredl, & Van Den Bout, 2006; Schredl, 2013; Thünker, Norpoth, Von Aspern, Özcan, & Pietrowsky, 2014; Nadorff, Nadorff, & Germain, 2015; Schredl & Göritz, 2014; Krakow, Kellner, Pathak, & Lambert, 1995). Overall, 3.5%–8.3% of the general adult population (Li, Zhang, Li, & Wing, 2010; Munezawa et al., 2011; Sandman et al., 2013; Schredl, 2010), 6.7%–11.3% of children (Wiechers et al., 2011) and 15.6%–66.7% of adult psychiatric patients (anxiety disorders and PTSD, respectively, Swart, Van Schagen, Lancee, & Van Den Bout, 2013) report recurrent nightmares. Across all epidemiological findings, nightmares occur more frequently in women than in men (Li et al., 2010; Munezawa et al., 2011; Sandman et al., 2013; Schredl, 2010; Swart et al., 2013; Wiechers et al., 2011). Frequent nightmares are associated with a wide range of mental complaints, sleep disruptions and insomnia, that is, difficulties in sleep onset and maintenance together with compromised daytime functioning (Cattarius & Schlarb, 2016; Krakow, 2006; Lancee & Schrijnemaekers, 2013; Lancee, Spoormaker, & Van Den Bout, 2010b). Further consequences are tiredness upon getting up, daytime sleepiness, lack of energy, petulance, difficulties in concentrating, worries about having enough sleep (Lancee & Schrijnemaekers, 2013), increased mental distress, anxiety, depression (Blagrove, Farmer, & Williams, 2004; Levin & Fireman, 2002), poor academic performance (Wiechers et al., 2011) and maladaptive personality functioning (Köthe & Pietrowsky, 2001; Van Schagen, Lancee, Swart, Spoormaker, & Van Den Bout, 2017). Finally, recurrent nightmares increase the risk of attempting and re‐attempting suicide (Sjöström, Hetta, & Waerna, 2009), even after controlling for sex, age, relationship status, employment status, smoking, use of alcohol, amount of exercise, symptoms of insomnia, symptoms of depression, traumatic experiences, posttraumatic symptoms, other mental disorders and use of psychotropic medication (Nadorff, Nazem, & Fiske, 2011; Sandman et al., 2017).
Given their high prevalence and associated features of impaired mental and physical health, nightmares are of substantial clinical relevance. Several theoretically and empirically based attempts to treat nightmare disorder have been developed since the 1980s. This paper reflects the results of an international symposium on nightmare treatment attended by scientists and practitioners from Austria, Canada, Denmark, France, Germany, the Netherlands, Switzerland, the United Kingdom and the United States. It outlines current knowledge on the aetiology and treatment of nightmare disorder. It further provides an outlook on current developments and relevant perspectives in future nightmare research and treatment.
1.1. Assessment of nightmares and nightmare disorder
There are several instruments to assess nightmare frequency and nightmare distress. Nightmare frequency can be assessed by the Nightmare Frequency Questionnaire (NFQ, Krakow, Schrader et al., 2002). In addition, current and childhood nightmare frequency and the relative number of recurrent nightmares in adults are part of the Mannheim Dream Questionnaire (MADRE, Schredl, Berres, Klingauf, Schellhaas, & Göritz, 2010). To address the distress that is associated with nightmares, some authors use a single question asking whether participants have a problem with nightmares (Wood & Bootzin, 1990). Alternatively, psychometrically constructed instruments such as the Nightmare Distress Questionnaire (NDQ, Belicki, 1992) assess general concerns about nightmares, including their impact on sleep quality or on daytime beliefs and perceptions (Böckermann, Gieselmann, & Pietrowsky, 2014). A subscale of the SLEEP‐50 assesses nightmare distress with reference to DSM‐IV criteria (Lancee, Spoormaker, & Van Den Bout, 2010a). Other questionnaires focus on the impact nightmares have on social, sleep and health‐related domains and include the Nightmare Effects Survey (NES, Krakow et al., 2000) and the Trauma Related Nightmare Survey (TRNS, Davis & Wright, 2007). The Disturbing Dream and Nightmare Severity Index (DDNSI, Krakow, 2006) combines nightmare frequency and nightmare effects in adults and the Nightmare Effects Questionnaire (NEQ, Schlarb et al., 2016) combines nightmare frequency with nightmare effects in adolescents from 14 to 18 years of age. Other instruments focus on anxiety symptoms, such as the Van Dream Anxiety Scale (VDAS, Agargün et al., 1999), or on behavioural consequences, such as the Nightmare Behavior Questionnaire (NBQ, Pietrowsky & Köthe, 2003).
The most frequently used distress instrument, the NDQ (Belicki, 1992), demonstrates that nightmare distress is more strongly associated with other mental health complaints, including anxiety and depression, than is nightmare frequency. Furthermore, the relationship between nightmare frequency and well‐being is mediated by NDQ‐assessed nightmare distress (Blagrove et al., 2004) and nightmare‐specific treatment reduces both nightmare frequency and nightmare distress, whereas non‐specific treatment only reduces nightmare frequency (Gieselmann, Böckermann, Sorbi, & Pietrowsky, 2017; Van Schagen, Lancee, De Groot, Spoormaker, & Van Den Bout, 2015). Against this background, many authors conclude that the distress caused by the nightmares is more relevant to mental health than is the sheer number of nightmares (Belicki, 1992; Blagrove et al., 2004; Gieselmann et al., 2017; Levin & Fireman, 2002; Van Schagen et al., 2015).
2. NIGHTMARE AETIOLOGY
According to Solms’ (2000) neurocognitive theory of dream generation, dreams are not mainly generated by the brainstem that controls REM sleep, but rather by complex forebrain mechanisms independently of the REM‐sleep state. He underpins his rationale with the neuropsychological finding from his large clinical–anatomical study that “the mesocortical‐mesolimbic dopamine system plays a causal role in the generation of dreams” (Solms, 2000, p. 847). Lesions of these pathways inhibit dreaming but do not affect REM frequency, duration and density (Solms, 2000). Solms (1995, p. 61) understands his findings as providing “confirmation of the classical theory of dreams that was introduced by Freud almost one hundred years ago”, according to which, dreams provide a sleep‐protecting function that offer a gateway to the knowledge of the unconscious mind (Freud, 1900).
Other research groups have explained the aetiology of nightmare disorder by (a) increased hyperarousal and (b) impaired fear extinction.
2.1. Hyperarousal
The aetiology of nightmare disorder may be influenced by increased hyperarousal that accumulates during the day and is maintained at night. Elevated hyperarousal is discussed as a central pathophysiological factor in PTSD (Buckley & Kaloupek, 2001; Kendall‐Tackett, 2009; Pacella, Hruska, & Delahanty, 2013; Pole, 2007), but also in insomnia disorder (see Riemann et al., 2010, for an integrative review). Patients diagnosed with insomnia disorder were found to produce more frequent (micro‐) arousals during REM sleep than good sleepers and were found to produce more fragmented REM‐sleep periods than were good sleepers (Feige et al., 2008, 2013). This hyperarousal enhanced information processing and beta activity during sleep (Perlis, Giles, Mendelson, Bootzin, & Wyatt, 1997). So, just as hyperarousal is typical for both PTSD and insomnia, it may also help explain the aetiology of nightmare disorder.
2.2. Impaired fear extinction
Although normal sleep and dreaming may enable fear extinction through a process of recombining fearful memories with novel and dissociated contexts, individuals with nightmare disorder continue to activate arousing memory fragments during sleep, reinforcing fear memories (Germain, Buysse, & Nofzinger, 2008; Nielsen, 2017a; Nielsen & Levin, 2007). As stipulated by the affect network dysfunction model (AND; Nielsen & Levin, 2007), individuals high in affect load and affect distress are particularly prone to such impaired fear extinction. “Affect load” is defined as a state factor referring to the influence of stressful and negative‐emotion‐eliciting events on one's capacity to regulate emotions, whereas “affect distress” is defined as a trait‐like tendency to react to stressors with negative affect and distress. Neurologically, this process can be understood as a hyperactive amygdala together with compromised frontal regulatory pathways, particularly of the medial prefrontal cortex (mPFC). A detrimental consequence of this brain state is that memory fragments incompatible with negative arousal cannot be retrieved from the associative network and fear‐laden memory fragments cannot be integrated into the network. Thus, fear extinction is impaired (Germain et al., 2008; Nielsen, 2017a; Nielsen & Levin, 2007).
2.3. Factors facilitating hyperarousal and impaired fear extinction
Several factors have been proposed as facilitating hyperarousal and impaired fear extinction in patients diagnosed with nightmare disorder. Although we are not able to draw a complete picture of these facilitating factors, here we address trait affect distress as caused by (a) traumatic experiences and childhood adversity, (b) trait susceptibility, (c) maladaptive cognitive factors and (d) physiological factors.
2.3.1. Traumatic experiences and childhood adversity
According to the AND model, fear extinction capacity is impaired by the consequences of traumatic experiences, although developmental stress such as early childhood adversity may initiate some similar mechanisms. Childhood adversity is known to induce changes in mPFC–amygdala circuits, which are associated with heightened threat perception (Ochsner & Gross, 2005) and, presumably, dysphoric dreaming (Nielsen & Levin, 2007). Accordingly, childhood adversity may disrupt the normal development of emotion regulation, including emotional expression, and thus reinforce the development of a fear memory. This theory was recently articulated by the stress acceleration hypothesis of nightmares (SAH, Nielsen, 2017a). The SAH model suggests that memories of early events, which are normally suppressed after the infantile amnesia period (Madsen & Kim, 2016), may exert a persistent influence on dreaming and waking memory. Supporting evidence comes from clinical (Hartmann, 1991) and empirical work (Nielsen, 2017b) demonstrating that nightmare sufferers have an unusually good memory of early childhood events. Likewise, a history of severe childhood maltreatment is associated with more frequent disturbed dreams, higher nightmare distress and heightened psychopathology (Duval, Mcduff, & Zadra, 2013), and the severity and amount of trauma are both correlated with several dream and pathology measures (Yu, 2014). Such results are consistent with the assumption that both traumatic experiences and early childhood adversity facilitate affect distress and the development of a nightmare disorder (Nielsen, 2017a; Nielsen & Levin, 2007).
2.3.2. Trait susceptibility
The theory of differential susceptibility (Carr & Nielsen, 2017, c.f. Belsky & Pluess, 2009) is consistent with the developmental tenets of the AND and SAH models, which suppose that traumatic experiences and early childhood adversity augment trait sensitivity in susceptible individuals. However, it stipulates further that such trait sensitivity is responsive not only to negative, but also to positive or supportive contexts. Empirically, higher novelty seeking and reward‐related behaviours were found to characterize those suffering from nightmare disorder and anticipatory worry (Perogamvros et al., 2015). Furthermore, individuals with frequent nightmares display an increased depth of processing of both negative and positive semantic stimuli (Carr, Blanchette‐Carrière, Marquis, Ting, & Nielsen, 2016), and display more errors in response to both negative and positive distractors on an emotional Stroop task (Simor, Pajkossy, Horváth, & Bódizs, 2012). These studies suggest not only that trait susceptibility may facilitate affect distress and the development of a nightmare disorder but also that nightmare sufferers are sensitive to both negatively and positively toned emotional stimuli. Their sensitivity to positive stimuli may make them particularly susceptible to positive psychology, supportive social environments and other interventions that enhance emotion regulation, such as mindfulness training (Carr & Nielsen, 2017).
2.3.3. Maladaptive beliefs
Maladaptive beliefs may play a role in the aetiology of nightmare disorder. Current research addressing the volitional attempt to suppress unwanted thoughts and feelings (“thought suppression”) finds that such attempts increase the likelihood that the suppressed thoughts reoccur in a person's dreams, especially those in REM sleep (Wegner, Wenzlaff, & Kozak, 2004). Furthermore, the intentional avoidance of internal and external stimuli is associated with the presence of recurrent nightmares (Kramer & Kinney, 2003). In experimental trials, deliberate thought suppression and subsequent dream rebound were enhanced by cognitive load (Bryant, Wyzenbeek, & Weinstein, 2011) and deliberate thought suppression worsened sleep quality and mental health, when practised over a period of 1 week (Kröner‐Borowik et al., 2013). Thus, deliberate thought suppression may be another trigger that facilitates the development of nightmare disorder.
2.3.4. Physiological factors
One physiological factor that may explain some aspects of hyperarousal in nightmare patients and which may impede fear extinction is the severe sleep fragmentation caused by obstructive and central sleep apnea (OSA and CSA), by its subtler variant referred to as upper airway resistance syndrome (UARS) or by complex sleep‐related hypoventilation syndromes. It is noteworthy that OSA, CSA or UARS occur in unusually high rates in PTSD patients (Jaoude, Vermont, Porhomayon, & El‐Solh, 2015; Krakow, Melendrez et al., 2002) and lead to (micro‐) awakenings. Several studies have drawn an association between sleep‐disordered breathing and nightmares, and treatment with positive airway pressure therapy (PAP; Bahammam, Al‐Shimemeri, Salama, & Sharif, 2013; Krakow et al., 2000) and mood stabilizers (Gupta, 2017) improved both OSA/CSA/UARS and PTSD symptoms, including nightmares.
At the same time, drugs influencing the neurotransmitters norepinephrine, serotonin and dopamine are associated with the occurrence of nightmares in some patients, whereas compounds affecting the neurotransmitters gamma‐aminobutyric acid (GABA), acetylcholine and histamine are less likely to induce nightmares, although not all results are consistent on this point. Furthermore, withdrawal from REM‐sleep‐suppressing medications has been reported to cause nightmares (Morgenthaler et al., 2018; Pagel & Helfter, 2003). Together, these findings suggest that there are chemosensoric pathways that facilitate the occurrence of nightmares.
2.4. The formation of nightmare scripts
All the above factors may contribute to the condensing of recurrent nightmare elements into a nightmare script. For some individuals, nightmares are thought to persist as a learned behaviour, that is, they have developed a “life of their own” (e.g. Ehlers & Clark, 2000; Hartmann, 1996; Krakow & Neidhardt, 1992). More recently, according to the cognitive model of recurrent dreams (Spoormaker, 2008), the persistence of nightmares is caused by loops of replaying fixed expectation patterns (i.e. nightmare scripts), even when the original stressor that first initiated the nightmare has long faded. In this respect, nightmares occur when a nightmare script is activated in response to dream elements that are similar to the original stressor; the nightmare is thus perpetuated in its habitual form.
Taken together, the preceding nightmare‐inducing factors constitute a pathophysiologic patchwork, which is summarized in Figure 1.
Figure 1.
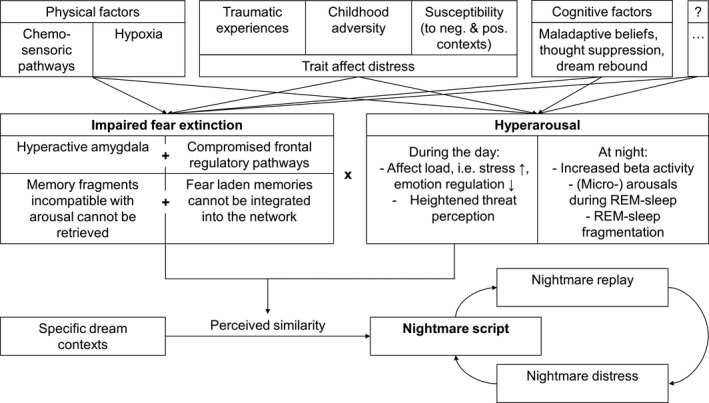
Integrative model of nightmare aetiology
3. THE TREATMENT OF NIGHTMARE DISORDER
Untreated, nightmare disorder often persists for decades (Schreuder, Kleijn, & Rooijmans, 2000) and, although specific treatment approaches exist, patients are often not treated or are inadequately treated (Krakow et al., 1995). The following considerations may help focus attention on the main impacts of different treatment approaches; these are treatments that emphasize: (a) the subjective meaning of nightmares, (b) the pathologic repetition of the nightmares and (c) maladaptive beliefs about nightmares.
3.1. Treatment approaches targeting the meaning of nightmares
The particular meaning of a nightmare is one focus in psychodynamic approaches. Given that posttraumatic nightmares are often described by patients as having stereotypic replicative content (Wittmann, Schredl, & Kramer, 2007), many treatment approaches neglect the potential subjective meanings of this replicative content. Yet, Lansky and Bley (1995) reported extensive clinical evidence that so‐called replicative nightmares may differ from the original episodic trauma memory and constitute an important source of information. From a psychodynamic perspective, the occurrence of nightmares could be considered an alarm signal for a disturbance of current psychic equilibrium and psychodynamic approaches could aim to identify and solve the underlying conflicts rather than addressing the alarm signal alone. This work includes processing of multiple levels, such as the developmental and trauma history of an individual (Wittmann & De Dassel, 2015), discovering similarities and differences to episodic memory traces (Lansky & Bley, 1995), relating the occurrence of nightmares to therapeutic processes, current life situation and interpersonal patterns (Wittmann & De Dassel, 2015), making sense of repetitions (Gardner & Ørner, 2009), and elaborating implicit meanings in order to gain insight into one's affect regulation processes (Moser & Von Zeppelin, 1996).
Changes in dream content were found to correspond to the adaptation versus maladaptation that patients experienced after traumatic events (Mellman, David, Bustamante, Torres, & Fins, 2001; Terr, 1983) and to indicate symptom changes and personal growth in the course of psychoanalytic treatment. However, evidence of the effectiveness of psychodynamic approaches in treating nightmares is restricted to uncontrolled case series (e.g. Gardner & Ørner, 2009; Lansky & Bley, 1990; Wittmann & De Dassel, 2015).
3.2. Treatment approaches targeting the chronic repetition of nightmares
Treatment approaches that address the chronic repetition of nightmare content often consider nightmares to be an overlearned behaviour and aim to unlearn these habits. These approaches are mainly based on the principles of (a) desensitization and exposure therapy, (b) imagery rehearsal therapy (IRT) or (c) lucid dreaming, as described below.
3.2.1. Desensitization and exposure therapy
Desensitization and exposure therapy were the first approaches used to treat nightmares directly (Kellner, Neidhardt, Krakow, & Pathak, 1992; Miller & Dipalato, 1983). For a desensitization exercise, the patient is first introduced to muscular relaxation. Then he/she is asked to imagine the nightmare, without modifying it, and to relax whenever it causes tension. During exposure therapy, the patient is also asked to imagine the nightmare vividly but without an accompanying relaxation exercise and also without actively changing the story line (Kunze, Arntz, Morina, Kindt, & Lancee, 2017). This method is adapted from traditional techniques for treating PTSD (e.g. Foa & Rothbaum, 1989). The common treatment rationales of desensitization and exposure therapy are based on the principles of extinction and/or inhibitory learning, by which the symptoms of fear and arousal accompanying the nightmare are overridden by more adaptive behavioural, cognitive and emotional processes. Several trials have demonstrated the effectiveness of desensitization and exposure therapies (Davis & Wright, 2007; Hansen, Höfling, Kröner‐Borowik, Stangier, & Steil, 2013). Kunze et al. (2017) showed a pure exposure protocol had similar effect sizes to a pure IRT protocol. However, one major disadvantage of desensitization and exposure therapies is that the arousal elicited during treatment may cause severe distress in the patient (Arntz, Tiesema, & Kindt, 2007, but see Kunze et al., 2017).
3.2.2. Imagery rehearsal therapy (IRT)
The IRT approach to treating nightmares is underpinned by the best available evidence and therefore recommended as level A treatment for nightmare disorder by the Oxford Centre for Evidence Based Medicine (Cranston, Davis, Rhudy, & Favorite, 2011), as well as by the AASM (Aurora et al., 2010; Morgenthaler et al., 2018). With IRT, patients are instructed to rescript the story of the nightmare during wakefulness. The original instruction was to: “Change the nightmare anyway you wish” (Kellner et al., 1992), which offered no specific targeting of the nightmare's ending or any other specified component of the disturbing dream, whereas others have proposed to change the nightmare to possess a “triumphant” (Marks, 1978), “satisfactory” or “more neutral or even pleasant” (Thünker & Pietrowsky, 2012) ending. This rescripted nightmare (which no longer appears to be a nightmare) is then rehearsed in the patients’ imagination a couple of times each day. In their meta‐analysis, Hansen et al. (2013) reported large pre–post within‐group effect sizes for IRT for nightmare frequency (Hedges’ g = 1.04) and nights per week with nightmares (g = 0.99, gs ≥ 0.80 are considered a large effect) (see Augedal, Hansen, Kronhaug, Harvey, and Pallesen (2013), Cranston et al. (2011), Casement and Swanson (2012), Nadorff, Lambdin, and Germain (2014), Seda, Sanchez‐Ortuno, Welsh, Halbower, and Edinger (2015), for comparable results of other meta‐analyses). IRT not only decreased nightmare frequency, but also decreased PTSD severity (g = 0.92, Hansen et al., 2013), increased sleep quality (Cohen's d = 0.68, Casement & Swanson, 2012) and decreased more general mental health complaints. Neither adverse effects nor symptom substitutions were reported in patients being treated with IRT.
There is an ongoing debate about active treatment mechanisms in IRT. Because IRT is most often offered in a multicomponent treatment package, specific treatment mechanisms are difficult to identify: nightmare modification rarely occurs without any exposure to the aversive nightmare content (Lancee, Van Den Bout, & Spoormaker, 2010) and most treatment manuals also include psycho‐education, the keeping of sleep diaries, relaxation exercises and imagination techniques taken from the treatment of PTSD. Attempts to identify such treatment components have demonstrated that the simple recording of nightmares in a diary decreases nightmare frequency and nightmare distress (Lancee et al., 2010a; Neidhardt, Krakow, Kellner, & Pathak, 1992); nonetheless, IRT as an adjunct therapy outperformed a comparison treatment that also included diaries (Gieselmann et al., 2017; Van Schagen et al., 2015). On the other hand, keeping detailed narrative sleep diaries was as effective as IRT (with the diaries) in decreasing nightmare frequency, whereas IRT outperformed detailed diaries in reducing nightmare distress. Interestingly, a pure IRT protocol stripped of any other treatment components such as diaries or homework assignments, and only including a minimum amount of psycho‐education, was also effective (Kunze et al., 2017). Thus, the keeping of diaries may not be a necessary treatment component of IRT. Furthermore, it has been argued that exposure to nightmare content is not a necessary treatment component, as IRT is effective with minimal exposure (Krakow, Hollifield et al., 2001). However, some studies suggest that an intervention of relaxation training and sleep habit modification does not perform worse than the full IRT protocol including exposure and rescripting (Miller & Dipalato, 1983; Pruiksma, Cranston, Rhudy, Micol, & Davis, 2018). The literature thus remains unclear and sometimes conflicting.
3.2.3. Lucid dreaming
Lucid dreaming therapy takes a notably different approach, focusing on enabling individuals to influence their dreams directly while dreaming (Holzinger, 2014). A lucid dream is characterized by an awareness of orientation, that is, of being in the process of dreaming; it is often also accompanied by an awareness of being able to make decisions and therefore to take charge of or responsibility for the dream plot. This may lead to the ability to act volitionally; for example, to alter or even control the dream's content or to simply wake oneself up. In practice, patients are invited to go to bed with the explicit intent to improve their dream recall by writing down their dreams directly upon awakening. Also, adopting the conviction that one can realize when one is dreaming was found to increase the frequency of lucid dreams. Checking whether or not one has become lucid can be encouraged during wakefulness with reality testing methods that are repeated frequently: ‘‘Is this really happening or am I dreaming?’’, ‘‘When I look at something twice, does it still look the same (e.g. Does the clock still indicate the same time or not? Is the book title still the same or is it not)?’’ Lucid dreaming as an add‐on to Gestalt therapy was found to improve nightmare and sleep‐related symptoms more quickly than was Gestalt therapy without explicit focus on the nightmares (Holzinger, Klösch, & Saletu, 2015). However, one major concern was that not every dreamer is able to reach the lucid dreaming state, as demonstrated by Spoormaker and Van Den Bout (2006), where only six of 16 patients were able to become lucid in a nightmare. Interestingly though, treatment effects occurred independent of whether or not patients were able to become lucid in their dreams. Meanwhile, a mere introduction to the concept of lucid dreaming was found to cause relief (Holzinger et al., 2015).
3.3. Treatment approaches targeting maladaptive beliefs
Another aspect seldom targeted in theory and practice is the holding of maladaptive beliefs. Does maladaptive ruminating and catastrophizing (e.g. “After a nightmare, I am drenched in sweat”, “After a night with nightmares, the next day will be a torture”) have an impact on patients’ distress? Do maladaptive idiosyncratic models about the meaning of nightmares (e.g. “I need the nightmares to prepare for future events in real life”) help or hurt treatment effectiveness? Do changes in these beliefs decrease distress? Harvey, Tang, and Browning (2005) introduced cognitive therapy for the treatment of insomnia. In this vein, they recommend changing maladaptive beliefs by means of behavioural experiments and cognitive restructuring. Negative self‐ascriptions are also addressed in the original IRT protocol (Krakow & Zadra, 2006), and both exposure and IRT protocols were able to ameliorate negative beliefs about nightmares (Kunze et al., 2017). Paradoxical intention, that is, encouraging nightmare sufferers to have nightmares, has been recommended as a strategy to tackle negative appraisals in recurrent nightmares (Spoormaker, 2008).
3.4. Active treatment mechanisms of direct nightmare treatment
In their attempt to explain reported effects of specific nightmare treatments, Rousseau and Belleville (2018) conducted a qualitative analysis and concluded that increased feelings of mastery, as proposed by Germain et al. (2004) and Krakow, Hollifield et al. (2001), was the most often cited mechanism. The authors state that the conviction that one is in control of one's nightmares does include cognitive elements but also emotional processing. Emotional processing leads to memory consolidation, that is, a reorganization of conscious and unconscious fear structures in the memory network. They discussed their approach with reference to the AND model (Nielsen & Levin, 2007). Beyond that, mechanisms similar to emotional processing are also discussed as active treatment mechanisms in the treatment of PTSD (see below, c.f. Kleim et al., 2013), as well as in relation to REM sleep as a facilitating factor in the restructuring of cognitive‐affective memory content in overcoming hyperarousal (Feige et al., 2008, 2013; Perlis et al., 1997; Riemann et al., 2010).
3.5. Pharmacological treatment
Regarding the specific pharmacological treatment of nightmares, most trials have made use of prazosin, a CNS active alpha‐1 adrenoreceptor antagonist. The AASM recommended prazosin as an adjunct therapy for PTSD, including nightmares (Aurora et al., 2010, on behalf of the AASM). However, there was a recent large‐scale multicentre trial that did not yield any improvements (Raskind et al., 2018) and another trial in which patients returned to their baseline levels after treatment was discontinued (Raskind et al., 2018). These conflicting data caused the AASM to downgrade their advice regarding prazosin from “recommended” to “may be used” and to name other pharmaceuticals that “may be used” as well (Morgenthaler et al., 2018, on behalf of the AASM).
When evaluating the effects of pharmacological treatment, one should take into account that pharmaceuticals are commonly tested against placebos in a double‐blinded manner. Given that most psychotherapy trials deliver narrative‐based interventions, they often cannot meet these high standards: Therapists are commonly aware of the group the patients are randomized to and most interventions are tested against waiting‐list control conditions which cannot be compared with a placebo pill. At the same time, there are currently no pharmaceuticals that can be recommended without reservation for the treatment of nightmare disorder.
4. NIGHTMARE TREATMENT IN DIFFERENT PATIENT POPULATIONS
4.1. Nightmare treatment in PTSD
Up to 67% of patients diagnosed with PTSD re‐experience intrusive trauma memories in the form of distressing dreams. Further, the sleep fragmentation associated with nightmares is likely to interfere with recovery from PTSD symptoms (Babson & Feldner, 2010; Germain et al., 2008; Harvey, Jones, & Schmidt, 2003).
Just as with idiopathic nightmares, IRT is a powerful strategy for the treatment of nightmares in PTSD patients. A single session including IRT in victims of violent crimes (Germain, Shear, Hall, & Buysse, 2007) as well as IRT without any add‐on procedures in sexual assault survivors improved both nightmare symptoms and other sleep parameters (Krakow, Hollifield et al., 2001). Furthermore, IRT was found to generalize to other PTSD symptoms without directly targeting them, that is, by causing improvements in intrusive, avoidance and arousal symptoms (Seda et al., 2015). Numerous studies show significant effects of IRT on nightmares and PTSD symptoms (e.g. Nappi, Drummond, Thorp, & Mcquaid, 2010).
When comparing the short‐term effects of prazosin versus IRT (Seda et al., 2015), both were found to show comparable and moderate effects on nightmares and PTSD symptoms, but studies that combined IRT with CBT for insomnia (CBT‐i) performed better at improving overall sleep quality compared to those using prazosin. IRT studies show considerably more heterogeneity and variation in treatment protocol delivery (e.g. combining IRT with CBT‐i or with other types of psychological interventions), which may influence results. Initial findings from controlled studies show that CBT‐i alone (Ho, Chan, & Tang, 2016), as well as CBT‐i combined with IRT, effectively reduces insomnia and PTSD symptoms (Margolies, Rybarczyk, Vrana, Leszczyszyn, & Lynch, 2013).
4.2. Nightmare treatment in patients with diverse other mental disorders
IRT had moderate effects on nightmare frequency and nightmare distress when used as an add‐on treatment in outpatients with diverse mental disorders. These patients improved more in nightmare frequency and nightmare distress than did patients who received treatment as usual (Van Schagen et al., 2015). Improvements were maintained in the long term, that is, at 6‐ and 9‐months follow‐up (Van Schagen, Lancee, Spoormaker, & Van Den Bout, 2016). Furthermore, treating nightmares as an add‐on to a standard inpatient psychiatric treatment also leads to greater reductions in suicidal ideation (Ellis, Rufino, & Nadorff, 2019). Thus, nightmare treatment may help improve treatment outcome in general psychiatric practice. On the other hand, treatment effects were found to vary across patients with and without comorbidities: higher comorbidities predicted worsened treatment outcome (Thünker & Pietrowsky, 2012).
4.3. Nightmare treatment in refugees
Refugees are another population that often suffers from diverse mental disorders, including recurrent nightmares. Their number reached a new peak by the end of 2017, with a total of 25.4 million refugees worldwide (United Nations High Commissioner for Refugees (UNHCR), 2018). It is estimated that roughly 30% of the world's refugees suffer from PTSD. In a sample of 752 trauma‐affected refugees undergoing psychiatric treatment at an outpatient ambulance service in Denmark in 2008 to 2012, 99% reported sleep disturbances and recurrent nightmares as their most prevalent symptoms (Sandahl, Vindbjerg, & Carlsson, 2017). However, common treatment manuals cannot account for the special circumstances experienced by refugees, such as the language, cultural and organizational barriers that complicate effective treatment. In the case of refugees’ nightmares, preliminary investigations have addressed the effects of prazosin (Boynton, Bentley, Strachan, Barbato, & Raskind, 2009) and clonidine (Kinzie, Sack, & Riley, 1994).
4.4. Nightmare treatment in children and adolescents
Nightmares and other parasomnias are frequently reported in children and adolescents. Often children and adolescents are not only afraid of nightmares, but also of sleeping alone, or express fears of the dark (Wiechers et al., 2011). Frequently, not only the child but at least one other member of the family will suffer from non‐restorative sleep because of the child's sleep (Cattarius & Schlarb, 2016). Symptoms usually decrease with advancing age. Yet, the presence of nightmares at the age of 8–11 years was predictive of having nightmares 2 years later, indicating that nightmares at an earlier age are likely to remain years later (Schredl, Fricke‐Oerkermann, Mitschke, Wiater, & Lehmkuhl, 2009). Diverse treatment techniques have been reported for use with children and adolescents, including systematic desensitization, IRT, relaxation techniques, extinction and eye movement desensitization; however, findings are mainly restricted to case studies and trials with small sample sizes (see Sadeh, 2005 for a review on sleep disorders in children and adolescents). Various studies applying combined treatments to address mainly insomnia showed significant decreases in nightmare symptoms for children aged up to 4 years (Schlarb, Brandhorst et al., 2011), in children aged 5 to 10 years (Schlarb, Velten‐Schurian et al., 2011) and in university students (Schlarb et al., 2017). Regarding treatment targeting nightmares specifically, IRT showed a significantly reduced nightmare frequency in children aged 6 to 11 years (Simard & Nielsen, 2009) and in children aged 13 to 18 years (Krakow, Sandoval et al., 2001).
5. LACK OF KNOWLEDGE ABOUT NIGHTMARE SUFFERING AND TREATMENT AVAILABILITY
Despite the fact that up to 70% of patients in psychiatric samples suffer from recurrent nightmares (Swart et al., 2013; Van Schagen et al., 2017), nightmare screenings and treatments remain rare, even in sleep centres. Nonetheless, nightmares can be treated with a minimum‐contact intervention. The treatment of nightmare disorder has been effective with a single session (Neidhardt et al., 1992), with posted self‐help books (Burgess, Marks, & Gill, 1998; Lancee, Spoormaker, & Van Den Bout, 2011; Lancee et al., 2010a) and with Internet‐based self‐help (Gieselmann et al., 2017). However, the majority of nightmare sufferers do not seek professional help. Many patients choose to live with their nightmares, often because they are not aware of any other option. Less than one‐third believed their nightmares are treatable and only 38% reported them to a healthcare provider (Nadorff et al., 2015; Schredl, 2013; Thünker et al., 2014). If help was sought, most patients consulted general practitioners (41%) or medical specialists (38%), who possessed scant expertise in the treatment of nightmare disorder. Less than one‐third to one‐fifth of nightmare sufferers who sought professional help reported having received helpful advice (Nadorff et al., 2015; Schredl & Göritz, 2014; Thünker et al., 2014).
6. FUTURE PERSPECTIVES
Nightmare aetiology and treatment are still relatively new fields and numerous research questions remain unanswered. Thus, the last section will present possible future perspectives regarding (a) the aetiology of nightmare disorder, (b) the continued development of nightmare treatments, (c) application of nightmare treatments to different subpopulations and (d) the integration of nightmare treatments into the healthcare system.
6.1. Future perspectives regarding the aetiology of nightmare disorder
From a basic science perspective, research on nightmares is often hampered by the fact that the occurrence of nightmares solely depends on subjective reports. However, many other phenomena have similar issues of subjectivity (e.g. fear, pain, mind‐wandering, episodic memory, consciousness) that do not prevent the development of valid experimental models and assessment tools to study various aspects of the topic. Thus, (a) we should more clearly define nightmare criteria that help to objectify common agreement. Given the fact that nightmare distress is a key feature of nightmare disorder, the diverse number of assessment tools limits the comparability of nightmare distress across different trials and well established instruments have been criticized for methodological reasons (Schredl, Landgraf, & Zeiler, 2003). Thus, a common consensus on how to define nightmare criteria and how to operationalize nightmare distress is warranted. Furthermore, (b) the fields of psychoneuroimmunology and psychoneuroendocrinology (Buckley & Kaloupek, 2001; Kendall‐Tackett, 2009; Pacella et al., 2013; Pole, 2007) may help provide further insight into the distress evoked by nightmares and probable causal relationships between psychoneuroimmunologic and psychoneuroendocrinologic mechanisms and nightmare occurrence. Here, the role of nightmare‐inducing medications may help clarify such mechanisms, although results are mixed as nightmares occur in some patients but not in others. (c) The interplay between the pathophysiology of sleep‐disordered breathing and nightmares may add another layer of understanding from the basic science framework (Gupta, 2017; Jaoude et al., 2015).
We propose that in developing experimental models for nightmares, much can be learned from the adjacent fields of hyperarousal and fear extinction. Regarding hyperarousal, EEG studies are warranted to clarify whether phenomena such as (micro‐) arousals during REM sleep and REM sleep fragmentation (Feige et al., 2008, 2013) are also observed in nightmares. Furthermore, there must be clarification of why patients with insomnia disorder more frequently perceive REM sleep as being awake, whereas patients with nightmare disorder more frequently perceive nightmares during REM sleep. The theory formulated above that impaired fear extinction and elevated hyperarousal are necessary to elicit nightmares remains hypothetical and should be investigated empirically. Regarding fear extinction, opportunities for knowledge translation from afflicted individuals to healthy individuals, and from animals to human beings, are encouraged by strong overlap in both experimental procedures and underlying neural circuitry. However, the unconditioned stimuli used in studies with humans are commonly mild electric shocks to the wrist and to our knowledge, this does not result in intrusive memory traces in human subjects (e.g. flashbacks and spontaneous thoughts about the aversive event). Thus, a standard fear‐learning paradigm may prove to be suboptimal for studying intrusive memories and nightmares in humans.
Interestingly, several research groups have started to address relations between fear conditioning and intrusive memory formation by using highly aversive film clips as unconditioned stimuli, for instance, with brief film fragments (Wegerer, Blechert, Kerschbaum, & Wilhelm, 2013) or reminder cues of previously watched long‐duration movies (Kunze, Arntz, & Kindt, 2015). Moreover, there is an increasing focus on the effects of emotional stimuli (film fragments) on sleep parameters (Talamini, Bringmann, De Boer, & Hofman, 2013; Werner, Schabus, Blechert, Kolodyazhniy, & Wilhelm, 2015). A central question remains the extent to which fear‐conditioned intrusive memory traces are subjectively reported during the night, because their spontaneous occurrence in the daytime does not necessarily imply spontaneous occurrence during sleep. This research on fear conditioning and fear extinction could be an interesting topic for examining individual differences; moreover, future research could increase experimental control in the sleep laboratory by means of external stimulation methods.
Additionally, a better understanding of the role of thought suppression is warranted. To date, the impact of thought suppression in idiopathic and posttraumatic nightmares can be considered as the most speculative among all discussed concepts. In posttraumatic nightmares, cognitive avoidance was not found to predict nightmare frequency (Babson et al., 2011), although it remains unclear whether cognitive avoidance is congruent with what is subsumed under thought suppression. Yet, the available theoretical frameworks could guide the development of testable research procedures to address the role of thought suppression and cognitive avoidance in both idiopathic and posttraumatic nightmares.
6.2. Future perspectives regarding the treatment of nightmare disorder
6.2.1. The case of different treatment approaches
Despite medium to large effect sizes, there is still room for improvement of treatment outcomes. In particular, the large variance across the different work groups (Hansen et al., 2013) is still not well understood.
Alternative treatments to IRT should be considered and studied further. More precise knowledge is needed regarding the effects of pharmaceutical treatments, which will enable the formulation of more precise recommendations. Furthermore, RCTs should be developed to explore how PAP therapy provides clinical improvement in nightmare disorder (Bahammam et al., 2013; Krakow et al., 2000), especially against the background that many patients do not tolerate PAP therapy (Jaoude et al., 2015); nonetheless, a recent study showed higher than usual adherence rates in PTSD patients using advanced PAP modes such as auto‐bilevel or adaptive servo‐ventilation (Krakow, Obando, Ulibarri, & Mciver, 2017). Furthermore, the possibilities of lucid dreaming as treatment deserve more attention. If possible, one could develop technologies that reliably allow a direct modification of dreaming while it occurs. Speculatively, these technologies may prove more effective than changing the nightmare plot during the day. Currently, the necessity of becoming lucid remains in doubt because many patients fail to become lucid (Spoormaker & Van Den Bout, 2006), and because the mere introduction to the concept of lucid dreaming may itself bring about some relief (Holzinger et al., 2015).
Studies that address the effects of psychodynamic approaches are lacking. Relatedly, there is little research on effects of nightmare treatment in relation to the content of nightmares. Results obtained from a large sample (N=1214) of most recent nightmares indicated that the topics of danger and loss of significant others are frequently named, although they are not well represented in the diagnostic criteria (Mathes & Schredl, 2014) and it is not yet clear whether nightmare treatment works as well for these topics as for other nightmare topics. In addition, several nightmares included suicidal themes and should be considered more specifically against the background of the cathartic functions the nightmares may have: do the occurence or treatment of such nightmares help to prevent suicide? Thus, nightmare content analyses should focus on possible "affect‐regulating functions" of nightmares (Moser & Von Zeppelin, 1996). Furthermore, an evaluation of the specific life situation in which the nightmare occurred may offer insights into the precise nature of related underlying conflicts (c.f. Lansky & Bley, 1995), and changes in nightmare content during therapy may offer prognostic information (Mellman et al., 2001; Terr, 1983). Analogies between dream content and therapeutic processes, current life situation and interpersonal patterns of a patient can help to enhance awareness of significant current experiences (Wittmann & De Dassel, 2015).
Finally, to the best of our knowledge, there is no research on treatment approaches that target maladaptive beliefs, idiosyncratic models of meaning and false self‐ascriptions. Thus, it remains to be tested whether association splitting helps to demolish gridlocked nightmare scripts beyond and above IRT. Association splitting, which was originally developed to treat obsessive thoughts in obsessive compulsive disorder (OCD, Moritz, Jelinek, Klinge, & Naber, 2007) but was also effective in alleviating other symptoms such as unwanted intrusive thoughts (Rodríguez‐Martín, Moritz, Molerio‐Pérez, & Gil‐Pérez, 2013), may be a particularly good candidate for future research. Similarly, Harvey et al.'s (2005) cognitive restructuring approach may help patients to alter assumptions such as nightmares foretell the future or that nights with nightmares make it impossible to concentrate on work and life issues the next day. It remains to be investigated whether such strategies are better applied as a stand‐alone treatment, as an add‐on method or as a combined approach.
6.3. Future perspectives regarding subpopulations of nightmare sufferers
6.3.1. The case of PTSD patients
To date, the majority of trauma survivors with PTSD receiving psychotherapy, such as trauma‐focused CBT, are treated with a focus on their daytime symptoms. It is assumed that improvements generalize to symptoms at night (i.e. sleep disturbances) and this is supported by initial evidence (Woodward et al., 2017). IRT treatment, on the other hand, mainly addresses night‐time symptoms and treatment gains, in turn, were found to generalize to daytime symptoms (Krakow, Hollifield et al., 2001). Further randomized controlled trials are needed to better identify to what extent the treatment of daytime PTSD symptoms generalizes to nightmare symptoms and vice versa. Moreover, it would be useful to establish predictors of treatment response and determine which patients are more likely to respond to one treatment compared to another. So far, there is limited knowledge about the effects of combining treatments.
With regard to a stepped‐care approach and given that IRT interventions can be delivered quickly and easily, future studies should establish the potential benefit of providing a brief course of IRT in combination with, or possibly prior to, trauma‐focused psychotherapy (Nadorff et al., 2014). By improving sleep quality, PTSD patients may also benefit from improved memory encoding and memory consolidation (Diekelmann, Wilhelm, & Born, 2009; Walker & Stickgold, 2006), as suggested by findings that sleep reshapes brain processes involved in learning and memory (Walker & Stickgold, 2006). Improvements in sleep quality were found to reduce PTSD symptoms (Ho et al., 2016) and this process may be moderated by cognitive appraisal, which was found to precede changes in PTSD symptoms (Kleim et al., 2013). It is conceivable that following effective IRT treatment, a number of key PTSD symptoms would already be normalized. Remaining symptoms could be treated using trauma‐focused CBT or other evidence‐based treatment approaches (see Figure 2), with a potentially reduced number of treatment sessions. Conducting IRT prior to trauma‐focused CBT may reward and motivate patients and limit unwanted adverse effects that are sometimes caused by longer, more demanding, more complex and more distressing trauma‐focused interventions. Indeed, there are ongoing clinical trials investigating the effects of treating nightmares as a first‐line intervention for PTSD, as well as the effects in reducing suicidal ideation (e.g. https://tango.uthscsa.edu/ssads/507, University of Texas; Nadorff et al., 2014).
Figure 2.
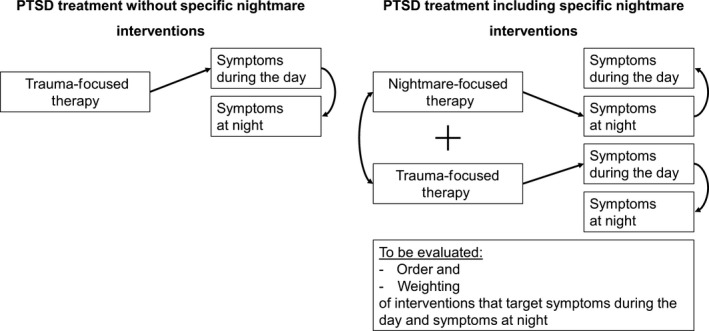
Frequently applied trauma‐focused interventions and proposed alternative approaches
To date, there are no treatment algorithms, no stepped‐care recommendations and insufficient empirical evidence to recommend whether IRT or CBT for PTSD may be best for starting treatment. Future studies should determine the optimal choice and timepoint for IRT within a PTSD treatment regimen. In addition, interventions for PTSD are emerging that offer non‐exposure options for patients and therapists who are unable to engage in exposure and who thus prefer, for example, imagery‐based or mindfulness‐based interventions. Future studies are needed to derive reliable treatment algorithms and treatment choices for specific patient characteristics, including information on potential synergistic effects of both nightmare interventions and trauma‐focused CBT or similar first‐line PTSD treatments.
6.3.2. The case of different patient populations
Although nightmares have been shown to be associated with many forms of psychopathology, such as schizophrenia, borderline personality disorder and dissociative disorders, little research has examined the impact of nightmare treatments on these disorders (Swart et al., 2013; Van Schagen et al., 2017). Future research may also help clarify why treated patients with diverse other mental complaints do not benefit as much as patients without multiple comorbidities (Thünker & Pietrowsky, 2012) and elucidate the effects of nightmare treatment in patients who suffer from other chronic diseases (e.g. epilepsy and pain disorders). More studies are needed to shed light on precisely how the treatment of nightmare disorder affects the other physical and mental complaints of patients (e.g. are depressive symptoms reduced by treating nightmares?).
Approaches to treating refugees often fail to address the specific needs of trauma‐affected refugees as the latter often suffer from prolonged, repeated, cross‐generational and, often, non‐verbalizable trauma, as well as from stress during and after migration, including uncertainty about asylum status and residence, concerns about family members still unsafe in their home country, cultural and language difficulties, and perceived discrimination and racism (Boynton et al., 2009; Crumlish & O'rourke, 2010; Palic & Elklit, 2011). With respect to different age groups, the special needs of unaccompanied young refugees should be highlighted. Further, studies on the treatment of sleep disturbances and nightmares in refugees suffering from PTSD and sleep disorders are lacking and require future attention. Challenges to overcome are the lack of availability of translated standardized instruments and the optimal employment of translators. Because of language and cultural barriers, trials comparing pharmacological and psychological treatment are of particular interest (Sandahl, Jennum, Baandrup, Poschmann, & Carlsson, 2017).
Most studies have addressed adults but have not compared age groups; for example, the effects of treating nightmare disorder in children and adolescents are rarely studied. Yet given the promising results for adult patients and early studies in adolescents, the use of IRT for treating nightmares in children and adolescents should receive high priority. Nightmare treatment should be adapted for various age groups, such as toddlers and infants, schoolchildren, adolescents and students of an older age, because early treatment onset may help to prevent young people from developing chronic nightmare disorder.
6.4. Future perspectives regarding integration into the healthcare system
The rarity of screening for nightmares within the healthcare system, together with the commonality of patients who do not complain about their nightmares to healthcare providers, has caused innumerable patients to go untreated. Thus, one major focus in future research should be on understanding these diagnostic shortcomings and how to overcome them. This will require two policy objectives: (a) make screening more efficient so as to fit within a primary care or paediatrician visit and (b) educate healthcare professionals so they are equipped to make appropriate referrals. Given that the presence of nightmares is either comorbid with severe forms of psychopathology or is an exacerbating influence on other mental disorders (Van Schagen et al., 2017), disturbing dreams may serve as an alarm signal to practitioners that an individual needs mental health treatment. On the other hand, if nightmares are a simple byproduct of normal childhood development, guidelines should be developed to help paediatricians determine whether nightmares should be addressed or not. In short, we suggest that referrals for mental health evaluations are often warranted for nightmares when suffering exceeds the normal bounds of developmental change.
In the attempt to improve access to treatment, low‐threshold interventions such as telephone counselling (Schredl et al., 2016), Internet‐based applications and self‐help approaches (Gieselmann et al., 2017; Lancee et al., 2010a) should be further developed and offered to the general public. There is an abundance of evidence that Internet‐based treatment is effective, with effect sizes comparable to those of face‐to‐face treatments; such novel approaches may help with disseminating, simplifying and, perhaps, increasing the effects of treatments (Andersson, Cuijpers, Carlbring, Riper, & Hedman, 2014; Cuijpers, Kleiboer, Karyotaki, & Riper, 2017). At the same time, such Internet‐based approaches bring about new challenges of data security, ethics, legal responsibility, the availability of professionals in emergencies, and the integration of such treatments into the mental healthcare system in accordance with existing rules of professional conduct and compensation.
In sum, there are numerous research questions that remain unanswered concerning the aetiology of nightmare disorder, the improvement of nightmare treatments, the application of nightmare treatment to various subpopulations and the integration of nightmare treatments into the healthcare system. Prompt attention to these outstanding questions will enhance our understanding of nightmare suffering and will facilitate the delivery of mental healthcare to many underserved populations. We hope that the current consensus paper helps to highlight the nature of the continuing obstacles and to stimulate much‐needed research in the field.
AUTHOR CONTRIBUTION
All authors contributed by producing text blocks regarding their specific topic of expertise; all authors were involved in the revision of the drafts and approved the final version of the manuscript.
CONFLICT OF INTEREST
Anne Germain owns equity in and serves as CEO for Rehat, LLC and received a consulting honorarium from Jazz Pharmaceuticals. Barry Krakow sells products and services for the treatment of nightmares via his website and owns and operates the commercial sleep center Maimonides Sleep Arts & Sciences, Ltd. Annika Gieselmann, Malik Ait Aoudia, Michelle Carr, Robert Gorzka, Brigitte Holzinger, Birgit Kleim, Anna E. Kunze, Jaap Lancee, Michael R. Nadorff, Tore Nielsen, Dieter Riemann, Hinuga Sandahl, Angelika A. Schlarb, Carolin Schmid, Michael Schredl, Victor I. Spoormaker, Regina Steil, Annette M. van Schagen, Lutz Wittmann, Maria Zschoche and Reinhard Pietrowsky declare that they do not have any conflict of interests.
ACKNOWLEDGEMENTS
This consensus paper is based on an international symposium on the treatment of nightmare disorder that was held in Hanover, Germany, July 14th—15th, 2016, organized by Annika Gieselmann and Reinhard Pietrowsky (http://www.nts.hhu.de). The symposium was supported by the Volkswagen Foundation. The authors thank Thomas Mäder and Gerhard Klösch, who substantially contributed to the conducted studies reported in the manuscript.
Gieselmann A, Ait Aoudia M, Carr M, et al. Aetiology and treatment of nightmare disorder: State of the art and future perspectives. J Sleep Res. 2019;28:e12820 10.1111/jsr.12820
REFERENCES
- Agargün, M. Y. , Kara, H. , Bilici, M. , Çilli, A. S. , Telci, M. , Semiz, Ü. B. , & Başoğlu, C. (1999). The Van Dream Anxiety Scale: A subjective measure of dream anxiety in nightmare sufferers. Sleep and Hypnosis, 1, 204–211. [Google Scholar]
- American Academy of Sleep Medicine (AASM) (2014). International classification of sleep disorders – third edition. ICSD‐3. Darien, IL: AASM. [Google Scholar]
- American Psychiatric Association (APA) (1994). Diagnostic and statistical manual of mental disorders. DSM‐IV. Washington, DC: American Psychiatric Press. [Google Scholar]
- American Psychiatric Association (APA) (2013). Diagnostic and statistical manual of mental disorders. DSM‐5. Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Andersson, G. , Cuijpers, P. , Carlbring, P. , Riper, H. , & Hedman, E. (2014). Guided internet‐based vs. face‐to‐face cognitive behavior therapy for psychiatric and somatic disorders: A systematic review and meta‐analysis. World Psychiatry, 13, 288–295. 10.1002/wps.20151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arntz, A. , Tiesema, M. , & Kindt, M. (2007). Treatment of PTSD: A comparison of imaginal exposure with and without imagery rescripting. Journal of Behavior Therapy and Experimental Psychiatry, 38, 345–370. 10.1016/j.jbtep.2007.10.006 [DOI] [PubMed] [Google Scholar]
- Augedal, A. W. , Hansen, K. S. , Kronhaug, C. R. , Harvey, A. G. , & Pallesen, S. (2013). Randomized controlled trials of psychological and pharmacological treatments for nightmares: A meta‐analysis. Sleep Medicine Reviews, 17, 143–152. 10.1016/j.smrv.2012.06.001 [DOI] [PubMed] [Google Scholar]
- Aurora, R. N. , Zak, R. S. , Auerbach, S. H. , Casey, K. R. , Chowdhuri, S. , Karippot, A. , … Standards of Practice Committee; American Academy of Sleep Medicine (2010). Best practice guide for the treatment of nightmare disorder in adults. Journal of Clinical Sleep Medicine, 38, 9–401. [PMC free article] [PubMed] [Google Scholar]
- Babson, K. A. , & Feldner, M. T. (2010). Temporal relations between sleep problems and both traumatic event exposure and PTSD: A critical review of the empirical literature. Journal of Anxiety Disorders, 24, 1–15. 10.1016/j.janxdis.2009.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babson, K. A. , Feldner, M. , Badour, C. , Trainor, C. , Blumenthal, H. , Sachs‐Ericsson, N. , & Schmidt, N. (2011). Posttraumatic stress and sleep: Differential relations across types of symptoms and sleep problems. Journal of Anxiety Disorders, 25, 706–713. 10.1016/j.janxdis.2011.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahammam, A. S. , Al‐Shimemeri, S. A. , Salama, R. I. , & Sharif, M. M. (2013). Clinical and polysomnographic characteristics and response to continuous positive airway pressure therapy in obstructive sleep apnea patients with nightmares. Sleep Medicine, 14, 149–154. 10.1016/j.sleep.2012.07.007 [DOI] [PubMed] [Google Scholar]
- Belicki, K. (1992). Nightmare frequency versus nightmare distress: Relations to psychopathology and cognitive style. Journal of Abnormal Psychology, 101, 592–597. 10.1037/0021-843x.101.3.592 [DOI] [PubMed] [Google Scholar]
- Belsky, J. , & Pluess, M. (2009). Beyond diathesis stress: Differential susceptibility to environmental influences. Psychological Bulletin, 135, 885–908. 10.1037/a0017376 [DOI] [PubMed] [Google Scholar]
- Blagrove, M. , Farmer, L. , & Williams, E. (2004). The relationship of nightmare frequency and nightmare distress to well‐being. Journal of Sleep Research, 13, 129–136. 10.1111/j.1365-2869.2004.00394.x [DOI] [PubMed] [Google Scholar]
- Böckermann, M. , Gieselmann, A. , & Pietrowsky, R. (2014). What does nightmare distress mean? Factorial structure and psychometric properties of the Nightmare Distress Questionnaire (NDQ). Dreaming, 24, 279–289. 10.1037/a0037749 [DOI] [Google Scholar]
- Boynton, L. , Bentley, J. , Strachan, E. , Barbato, A. , & Raskind, M. (2009). Preliminary findings concerning the use of prazosin for the treatment of posttraumatic nightmares in a refugee population. Journal of Psychiatric Practice, 15, 454–459. 10.1097/01.pra.0000364287.63210.92 [DOI] [PubMed] [Google Scholar]
- Bryant, R. A. , Wyzenbeek, M. , & Weinstein, J. (2011). Dream rebound of suppressed emotional thoughts: The influence of cognitive load. Consciousness and Cognition, 20, 515–522. 10.1016/j.concog.2010.11.004 [DOI] [PubMed] [Google Scholar]
- Buckley, T. C. , & Kaloupek, D. G. (2001). A meta‐analytic examination of basal cardiovascular activity in posttraumatic stress disorder. Psychosomatic Medicine, 63, 585–594. 10.1097/00006842-200107000-00011 [DOI] [PubMed] [Google Scholar]
- Burgess, M. , Marks, I. , & Gill, M. (1998). Postal self‐exposure treatment of recurrent nightmares: Randomised controlled trial. British Journal of Psychiatry, 172, 257–262. 10.1192/bjp.172.3.257 [DOI] [PubMed] [Google Scholar]
- Carr, M. , Blanchette‐Carrière, C. , Marquis, L.‐P. , Ting, C.‐T. , & Nielsen, T. (2016). Nightmare sufferers show atypical emotional semantic breadth and prolonged REM sleep‐dependent emotional priming. Sleep Medicine Reviews, 20, 80–87. 10.1016/j.sleep.2015.11.013 [DOI] [PubMed] [Google Scholar]
- Carr, M. , & Nielsen, T. (2017). A novel differential susceptibility framework for the study of nightmares: Evidence for trait sensory processing sensitivity. Clinical Psychology Review, 58, 86–96. 10.1016/j.cpr.2017.10.002 [DOI] [PubMed] [Google Scholar]
- Casement, M. D. , & Swanson, L. M. (2012). A meta‐analysis of imagery rehearsal for post‐trauma nightmares: Effects on nightmare frequency, sleep quality, and posttraumatic stress. Clinical Psychology Review, 32, 566–574. 10.1016/j.cpr.2012.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattarius, B. G. , & Schlarb, A. A. (2016). Gegenseitige Beeinflussung von Eltern und Babys in ihrem Schlafverhalten. Der heimliche Blick ins Schlafzimmer [Mutual influence of parents and babies on their sleep behaviour. A look behind closed doors]. Somnologie, 20, 189–198. 10.1007/s11818-016-0064-6 [DOI] [Google Scholar]
- Cranston, C. C. , Davis, J. L. , Rhudy, J. L. , & Favorite, T. K. (2011). Replication and expansion of “best practice guide for the treatment of nightmare disorder in adults”. Journal of Clinical Sleep Medicine, 7, 549–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumlish, N. , & O'rourke, K. (2010). A systematic review of treatments for post‐traumatic stress disorder among refugees and asylum‐seekers. The Journal of Nervous and Mental Disease, 198, 237–251. 10.1097/nmd.0b013e3181d61258 [DOI] [PubMed] [Google Scholar]
- Cuijpers, P. , Kleiboer, A. , Karyotaki, E. , & Riper, H. (2017). Internet and mobile interventions for depression: Opportunities and challenges. Depression and Anxiety, 34, 596–602. 10.1002/da.22641 [DOI] [PubMed] [Google Scholar]
- Davis, J. L. , & Wright, D. C. (2007). Randomized clinical trial of chronic nightmares in trauma‐exposed adults. Journal of Traumatic Stress, 20, 123–133. 10.1002/(issn)1573-6598 [DOI] [PubMed] [Google Scholar]
- Diekelmann, S. , Wilhelm, I. , & Born, J. (2009). The whats and whens of sleep‐dependent memory consolidation. Sleep Medicine Reviews, 13, 309–321. 10.1016/j.smrv.2008.08.002 [DOI] [PubMed] [Google Scholar]
- Duval, M. , Mcduff, P. , & Zadra, A. (2013). Nightmare frequency, nightmare distress, and psychopathology in female victims of childhood maltreatment. The Journal of Nervous and Mental Disease, 201, 767–772. 10.1097/nmd.0b013e3182a214a1 [DOI] [PubMed] [Google Scholar]
- Ehlers, A. , & Clark, D. M. (2000). A cognitive model of posttraumatic stress disorder. Behavior Research and Therapy, 38, 319–345. 10.1016/s0005-7967(99)00123-0 [DOI] [PubMed] [Google Scholar]
- Ellis, T. E. , Rufino, K. A. , & Nadorff, M. R. (2019). Treatment of nightmares in psychiatric inpatients with imagery rehearsal therapy: An open trial and case series, Behavioral Sleep Medicine, 17, 112–123. 10.1080/15402002.2017.1299738 [DOI] [PubMed] [Google Scholar]
- Feige, B. , Al‐Shajlawi, A. , Nissen, C. , Voderholzer, U. , Hornyak, M. , Spiegelhalder, K. , … Riemann, D. (2008). Does REM sleep contribute to subjective wake time in primary insomnia? A comparison of polysomnographic and subjective sleep in 100 patients. Journal of Sleep Research, 17, 180–190. 10.1111/j.1365-2869.2008.00651.x [DOI] [PubMed] [Google Scholar]
- Feige, B. , Baglioni, C. , Spiegelhalder, K. , Hirscher, V. , Nissen, C. , & Riemann, D. (2013). The microstructure of sleep in primary insomnia: An overview and extension. International Journal of Psychophysiology, 89, 171–180. 10.1016/j.ijpsycho.2013.04.002 [DOI] [PubMed] [Google Scholar]
- Foa, E. B. , & Rothbaum, B. O. (1989). Behavioural psychotherapy for post‐traumatic stress disorder. International Review of Psychiatry, 1, 219–226. 10.3109/09540268909110412 [DOI] [Google Scholar]
- Freud, S. Die Traumdeutung [The interpretation of dreams], 13th edn Frankfurt a. M.: Fischer; 1900/2005. [Google Scholar]
- Gardner, S. E. , & Ørner, R. J. (2009). Searching for a new evidence base about repetitions. An exploratory survey of patients’ experiences of traumatic dreams before, during and after therapy. Counselling and Psychotherapy Research, 9, 27–32. [Google Scholar]
- Germain, A. , Buysse, D. J. , & Nofzinger, E. A. (2008). Sleep‐specific mechanisms underlying posttraumatic stress disorder: Integrative review and neurobiological hypotheses. Sleep Medicine Reviews, 12, 185–195. 10.1016/j.smrv.2007.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain, A. , Krakow, B. , Faucher, B. , Zadra, A. , Nielsen, T. , Hollifield, M. , … Koss, M. (2004). Increased mastery elements associated with imagery rehearsal treatment for nightmares in sexual assault survivors with PTSD. Dreaming, 14, 195–206. 10.1037/1053-0797.14.4.195 [DOI] [Google Scholar]
- Germain, A. , & Nielsen, T. (2003). Sleep pathophysiology in posttraumatic stress disorder and idiopathic nightmare sufferers. Biological Psychiatry, 54, 1092–1098. 10.1016/s0006-3223(03)00071-4 [DOI] [PubMed] [Google Scholar]
- Germain, A. , Shear, M. K. , Hall, M. , & Buysse, D. J. (2007). Effects of a brief behavioral treatment for PTSD‐related sleep disturbances: A pilot study. Behavior Research and Therapy, 45, 627–632. 10.1016/j.brat.2006.04.009 [DOI] [PubMed] [Google Scholar]
- Gieselmann, A. , Böckermann, M. , Sorbi, M. , & Pietrowsky, R. (2017). The effects of an Internet‐based imagery rehearsal intervention: A randomized controlled trial. Psychotherapy and Psychosomatics, 86, 231–240. 10.1159/000470846 [DOI] [PubMed] [Google Scholar]
- Gupta, M. A. (2017). Treatment of PTSD‐related OSA with CPAP is associated with only a modest improvement in PTSD: Possible adjunctive treatment with mood stabilizers. Journal of Clinical Sleep Medicine, 13, 841 10.5664/jcsm.6636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen, K. , Höfling, V. , Kröner‐Borowik, T. , Stangier, U. , & Steil, R. (2013). Efficacy of psychological interventions aiming to reduce chronic nightmares: A meta‐analysis. Clinical Psychology Review, 33, 146–155. 10.1016/j.cpr.2012.10.012 [DOI] [PubMed] [Google Scholar]
- Hartmann, E. (1991). Boundaries in the mind. New York: Basic Books. [Google Scholar]
- Hartmann, E. (1996). Outline for a theory on the nature and functions of dreaming. Dreaming, 6, 147–160. 10.1037/h0094452 [DOI] [Google Scholar]
- Harvey, A. G. , Jones, C. , & Schmidt, D. A. (2003). Sleep and posttraumatic stress disorder: A review. Clinical Psychology Review, 23, 377–401. 10.1016/s0272-7358(03)00032-1 [DOI] [PubMed] [Google Scholar]
- Harvey, A. G. , Tang, N. K. Y. , & Browning, L. (2005). Cognitive approaches to insomnia. Clinical Psychology Review, 25, 593–611. 10.1016/j.cpr.2005.04.005 [DOI] [PubMed] [Google Scholar]
- Ho, F. Y. , Chan, C. S. , & Tang, K. N. (2016). Cognitive‐behavioral therapy for sleep disturbances in treating posttraumatic stress disorder symptoms: A meta‐analysis of randomized controlled trials. Clinical Psychology Review, 43, 90–102. 10.1016/j.cpr.2015.09.005 [DOI] [PubMed] [Google Scholar]
- Holzinger, B. (2014). Lucid dreaming in psychotherapy In Hurd R., & Bukeley K. (Eds.), Lucid dreaming: New perspectives on consciousness in sleep (pp. 37–62). Westport: Praeger. [Google Scholar]
- Holzinger, B. , Klösch, G. , & Saletu, B. (2015). Studies with lucid dreaming as add‐on therapy to Gestalt therapy. Acta Neurologica Scandinavica, 131, 355–363. 10.1111/ane.12362 [DOI] [PubMed] [Google Scholar]
- Jaoude, P. , Vermont, L. N. , Porhomayon, J. , & El‐Solh, A. A. (2015). Sleep‐disordered breathing in patients with post‐traumatic stress disorder. Annals of the American Thoracic Society, 2, 259–268. 10.1513/annalsats.201407-299fr [DOI] [PubMed] [Google Scholar]
- Kellner, R. , Neidhardt, J. , Krakow, B. , & Pathak, D. (1992). Changes in chronic nightmares after one session of desensitization or rehearsal instructions. American Journal of Psychiatry, 149, 659–663. [DOI] [PubMed] [Google Scholar]
- Kendall‐Tackett, K. (2009). Psychological trauma and physical health: A psychoneuroimmunology approach to etiology of negative health effects and possible interventions. Psychological Trauma: Theory, Research, Practice, and Policy, 1, 35–48. 10.1037/a0015128 [DOI] [Google Scholar]
- Kinzie, J. D. , Sack, R. L. , & Riley, C. M. (1994). The polysomnographic effects of clonidine on sleep disorders in posttraumatic stress disorder: A pilot study with Cambodian patients. The Journal of Nervous and Mental Disease, 182, 585–587. 10.1097/00005053-199410000-00010 [DOI] [PubMed] [Google Scholar]
- Kleim, B. , Grey, N. , Wild, J. , Nussbeck, F. W. , Stott, R. , Hackmann, A. , … Ehlers, A. (2013). Cognitive change predicts symptom reduction with cognitive therapy for posttraumatic stress disorder. Journal of Consulting and Clinical Psychology, 81, 383–393. 10.1037/a0031290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köthe, M. , & Pietrowsky, R. (2001). Behavioral effects of nightmares and their correlations to personality patterns. Dreaming, 11, 43–52. 10.1023/a:1009468517557 [DOI] [Google Scholar]
- Krakow, B. (2006). Nightmare complaints in treatment‐seeking patients in clinical sleep medicine settings: Diagnostic and treatment implications. Sleep, 29, 1313–1319. 10.1093/sleep/29.10.1313 [DOI] [PubMed] [Google Scholar]
- Krakow, B. , Hollifield, M. , Johnston, L. , Koss, M. , Schrader, R. , Warner, T. D. , … Cheng, D. (2001). Imagery rehearsal therapy for chronic nightmares in sexual assault survivors with posttraumatic stress disorder: A randomized controlled trial. JAMA, 286, 537–545. 10.1001/jama.286.5.537 [DOI] [PubMed] [Google Scholar]
- Krakow, B. , Kellner, R. , Pathak, D. , & Lambert, L. (1995). Imagery rehearsal treatment for chronic nightmares. Behavior Research and Therapy, 33, 837–843. 10.1016/0005-7967(95)00009-m [DOI] [PubMed] [Google Scholar]
- Krakow, B. , Lowry, C. , Germain, A. , Gaddy, L. , Hollifield, M. , Koss, M. , … Melendrez, D. (2000). A retrospective study on improvements in nightmares and post‐traumatic stress disorder following treatment for co‐morbid sleep‐disordered breathing. Journal of Psychosomatic Research, 49, 291–298. 10.1016/s0022-3999(00)00147-1 [DOI] [PubMed] [Google Scholar]
- Krakow, B. , Melendrez, D. , Johnston, L. , Warner, T. D. , Clark, J. O. , Pacheco, M. , … Schrader, R. (2002). Sleep‐disordered breathing, psychiatric distress, and quality of life impairment in sexual assault survivors. The Journal of Nervous and Mental Disease, 190, 442–452. 10.1097/00005053-200207000-00004 [DOI] [PubMed] [Google Scholar]
- Krakow, B. , Melendrez, D. , Pedersen, B. , Johnston, L. , Hollifield, M. , Germain, A. , … Schrader, R. (2001). Complex insomnia: Insomnia and sleep‐disordered breathing in a consecutive series of crime victims with nightmares and PTSD. Biological Psychiatry, 49, 948–953. 10.1016/s0006-3223(00)01087-8 [DOI] [PubMed] [Google Scholar]
- Krakow, B. , & Neidhardt, E. J. (1992). Conquering bad dreams & nightmares: A guide to understanding, interpretation, and cure. New York: Berkley Books. [Google Scholar]
- Krakow, B. , Obando, J. J. , Ulibarri, V. A. , & Mciver, N. D. (2017). Positive airway pressure adherence and subthreshold adherence in posttraumatic stress disorder patients with comorbid sleep apnea. Patient Preference and Adherence, 11, 1923–1932. 10.2147/ppa [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakow, B. , Sandoval, D. , Schrader, R. , Keuhne, B. , Mcbride, L. , Yau, C. L. , & Tandberg, D. (2001). Treatment of chronic nightmares in adjudicated adolescent girls in a residential facility. Journal of Adolescent Health, 29, 94–100. 10.1016/s1054-139x(00)00195-6 [DOI] [PubMed] [Google Scholar]
- Krakow, B. , Schrader, R. , Tandberg, D. , Hollifield, M. , Koss, M. P. , Yau, C. L. , & Cheng, D. T. (2002). Nightmare frequency in sexual assault survivors with PTSD. Journal of Anxiety Disorders, 16, 175–190. 10.1016/s0887-6185(02)00093-2 [DOI] [PubMed] [Google Scholar]
- Krakow, B. , & Zadra, A. (2006). Clinical management of chronic nightmares: Imagery rehearsal therapy. Behavioral Sleep Medicine, 4, 45–70. 10.1207/s15402010bsm0401_4 [DOI] [PubMed] [Google Scholar]
- Kramer, M. , & Kinney, L. (2003). Vigilance and avoidance during sleep in US Vietnam war veterans with posttraumatic stress disorder. The Journal of Nervous and Mental Disease, 191, 685–687. 10.1097/01.nmd.0000092179.74348.20 [DOI] [PubMed] [Google Scholar]
- Kröner‐Borowik, T. , Gosch, S. , Hansen, K. , Borowick, B. , Schredl, M. , & Steil, R. (2013). The effects of suppressing intrusive thoughts on dream content, dream distress and psychological parameters. Journal of Sleep Research, 22, 600–604. 10.1111/jsr.12058 [DOI] [PubMed] [Google Scholar]
- Kunze, A. E. , Arntz, A. , & Kindt, M. (2015). Fear conditioning with film clips: A complex associative learning paradigm. Journal of Behavior Therapy and Experimental Psychiatry, 47, 42–50. 10.1016/j.jbtep.2014.11.007 [DOI] [PubMed] [Google Scholar]
- Kunze, A. E. , Arntz, A. , Morina, N. , Kindt, M. , & Lancee, J. (2017). Efficacy of imagery rescripting and imaginal exposure for nightmares: A randomized wait‐list controlled trial. Behavior Research and Therapy, 97, 14–25. 10.1016/j.brat.2017.06.005 [DOI] [PubMed] [Google Scholar]
- Lancee, J. , & Schrijnemaekers, N. C. M. L. (2013). The association between nightmares and daily distress. Sleep and Biological Rhythms, 11, 14–19. 10.1111/j.1479-8425.2012.00586.x [DOI] [Google Scholar]
- Lancee, J. , Spoormaker, V. I. , & Van Den Bout, J. (2010a). Cognitive behavioral self‐help treatment for nightmares: A randomized controlled trial. Psychotherapy and Psychosomatics, 79, 371–377. 10.1159/000320894 [DOI] [PubMed] [Google Scholar]
- Lancee, J. , Spoormaker, V. I. , & Van Den Bout, J. (2010b). Nightmare frequency is associated with subjective sleep quality but not with psychopathology. Sleep and Biological Rhythms, 8, 187–193. 10.1111/j.1479-8425.2010.00447.x [DOI] [Google Scholar]
- Lancee, J. , Spoormaker, V. I. , & Van Den Bout, J. (2011). Long‐term effectiveness of cognitive‐behavioural self‐help intervention for nightmares. Journal of Sleep Research, 20, 454–459. 10.1111/j.1365-2869.2010.00894.x [DOI] [PubMed] [Google Scholar]
- Lancee, J. , Van Den Bout, J. , & Spoormaker, V. (2010). Expanding self‐help imagery rehearsal therapy for nightmares with sleep hygiene and lucid dreaming: A waiting‐list controlled trial. International Journal of Dream Research, 3, 111–120. [Google Scholar]
- Lansky, M. R. , & Bley, C. R. (1990). Exploration of nightmares in hospital treatment of borderline patients. Bulletin of the Menninger Clinic, 54, 466–478. [PubMed] [Google Scholar]
- Lansky, M. R. , & Bley, C. R. (1995). Posttraumatic nightmares: Psychodynamic explorations. Hillsdale, NJ: Analytic Press. [Google Scholar]
- Levin, R. , & Fireman, G. (2002). Nightmare prevalence, nightmare distress, and self‐reported psychological disturbance. Sleep, 25, 205–212. [PubMed] [Google Scholar]
- Li, S. X. , Zhang, B. , Li, A. M. , & Wing, Y. K. (2010). Prevalence and correlates of frequent nightmares: A community‐based 2‐phase study. Sleep, 33, 774–780. 10.1093/sleep/33.6.774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen, H. B. , & Kim, J. H. (2016). Ontogeny of memory: An update on 40 years of work on infantile amnesia. Behavioral Brain Research, 298, 4–14. 10.1016/j.bbr.2015.07.030 [DOI] [PubMed] [Google Scholar]
- Margolies, S. O. , Rybarczyk, B. , Vrana, S. R. , Leszczyszyn, D. J. , & Lynch, J. (2013). Efficacy of a cognitive‐behavioral treatment for insomnia and nightmares in Afghanistan and Iraq veterans with PTSD. Journal of Clinical Psychology, 69, 1026–1042. 10.1002/jclp.21970 [DOI] [PubMed] [Google Scholar]
- Marks, I. (1978). Rehearsal relief of a nightmare. British Journal of Psychiatry, 133, 461–465. 10.1192/bjp.133.5.461 [DOI] [PubMed] [Google Scholar]
- Mathes, J. , & Schredl, M. (2014). Analysis of a large sample of diary dreams – how typical are these typical dreams? Somnologie, 18, 107–112. 10.1007/s11818-013-0653-6 [DOI] [Google Scholar]
- Mellman, T. A. , David, D. , Bustamante, V. , Torres, J. , & Fins, A. (2001). Dreams in the acute aftermath of trauma and their relationship to PTSD. Journal of Traumatic Stress, 14, 241–247. 10.1023/a:1007812321136 [DOI] [Google Scholar]
- Miller, W. , & Dipalato, M. (1983). Treatment of nightmares via relaxation and desensitization: A controlled evaluation. Journal of Consulting and Clinical Psychology, 51, 870–877. 10.1037/0022-006x.51.6.870 [DOI] [PubMed] [Google Scholar]
- Morgenthaler, T. I. , Auerbach, S. , Casey, K. R. , Kristo, D. , Maganti, R. , Ramar, K. , … Kartje, R. (2018). Position paper for the treatment of nightmare disorder in adults: An American Academy of Sleep Medicine position paper. Journal of Clinical Sleep Medicine, 14, 1041–1055. 10.5664/jcsm.7178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz, S. , Jelinek, L. , Klinge, R. , & Naber, D. (2007). Fight fire with fireflies! Association splitting: A novel cognitive technique to reduce obsessive thoughts. Behavioural and Cognitive Psychotherapy, 35, 631–635. [Google Scholar]
- Moser, U. , & Von Zeppelin, I. (1996). Der geträumte Traum [The dreamt dream]. Stuttgart, Germany: Kohlhammer. [Google Scholar]
- Munezawa, T. , Kaneita, Y. , Osaki, Y. , Kanda, H. , Ohtsu, T. , Suzuki, H. , … Ohida, T. (2011). Nightmare and sleep paralysis among Japanese adolescents: A nationwide representative survey. Sleep Medicine, 12, 56–64. 10.1016/j.sleep.2010.04.015 [DOI] [PubMed] [Google Scholar]
- Nadorff, M. R. , Lambdin, K. K. , & Germain, A. (2014). Pharmacological and non‐pharmacological treatments for nightmare disorder. International Review of Psychiatry, 26, 225–236. 10.3109/09540261.2014.888989 [DOI] [PubMed] [Google Scholar]
- Nadorff, M. R. , Nadorff, D. K. , & Germain, A. (2015). Nightmares: Under‐reported, undetected, and therefore untreated. Journal of Clinical Sleep Medicine, 11, 747–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadorff, M. R. , Nazem, S. , & Fiske, A. (2011). Insomnia symptoms, nightmares, and suicidal ideation in a college student sample. Sleep, 34, 93–98. 10.1093/sleep/34.1.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nappi, C. M. , Drummond, S. P. , Thorp, S. R. , & Mcquaid, J. R. (2010). Effectiveness of imagery rehearsal therapy for the treatment of combat‐related nightmares in veterans. Behavior Therapy, 41, 237–244. 10.1016/j.beth.2009.03.003 [DOI] [PubMed] [Google Scholar]
- Neidhardt, E. J. , Krakow, B. , Kellner, R. , & Pathak, D. (1992). The beneficial effects of one treatment session and recording of nightmares on chronic nightmare sufferers. Sleep, 15, 470–473. [PubMed] [Google Scholar]
- Nielsen, T. (2000). A review of mentation in REM and NREM sleep: “covert” REM sleep as a possible reconciliation of two opposing models. Behavioral and Brain Sciences, 23, 851–866. 10.1017/s0140525x0000399x [DOI] [PubMed] [Google Scholar]
- Nielsen, T. (2017a). The stress acceleration hypothesis of nightmares. Frontiers in Neurology, 8, 201 10.3389/fneur.2017.00201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen, T. (2017b). When was your earliest dream? Association of very early dream recall with frequent current nightmares supports a stress‐acceleration explanation of nightmares. Dreaming, 27, 122–136. 10.1037/drm0000051 [DOI] [Google Scholar]
- Nielsen, T. , & Levin, R. (2007). Nightmares: A new neurocognitive model. Sleep Medicine Reviews, 11, 295–310. 10.1016/j.smrv.2007.03.004 [DOI] [PubMed] [Google Scholar]
- Ochsner, K. N. , & Gross, J. J. (2005). The cognitive control of emotion. Trends in Cognitive Sciences, 9, 242–249. 10.1016/j.tics.2005.03.010 [DOI] [PubMed] [Google Scholar]
- Pacella, M. L. , Hruska, B. , & Delahanty, D. L. (2013). The physical health consequences of PTSD and PTSD symptoms: A meta‐analytic review. Journal of Anxiety Disorders, 27, 33–46. 10.1016/j.janxdis.2012.08.004 [DOI] [PubMed] [Google Scholar]
- Pagel, J. F. , & Helfter, P. (2003). Drug induced nightmares – an etiology based review. Human Psychopharmacology, 18, 59–67. 10.1002/(issn)1099-1077 [DOI] [PubMed] [Google Scholar]
- Palic, S. , & Elklit, A. (2011). Psychosocial treatment of posttraumatic stress disorder in adult refugees: A systematic review of prospective treatment outcome studies and a critique. Journal of Affective Disorders, 131, 8–23. 10.1016/j.jad.2010.07.005 [DOI] [PubMed] [Google Scholar]
- Perlis, M. L. , Giles, D. E. , Mendelson, W. B. , Bootzin, R. R. , & Wyatt, J. K. (1997). Psychophysiological insomnia: The behavioural model and a neurocognitive perspective. Journal of Sleep Research, 6, 179–188. 10.1046/j.1365-2869.1997.00045.x [DOI] [PubMed] [Google Scholar]
- Perogamvros, L. , Aberg, K. , Gex‐Fabry, M. , Perrig, S. , Cloninger, C. R. , & Schwartz, S. (2015). Increased reward‐related behaviors during sleep and wakefulness in sleepwalking and idiopathic nightmares. PLoS ONE, 10, e0134504 10.1371/journal.pone.0134504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps, A. J. , Kanaan, R. A. A. , Worsnop, C. , Redston, S. , Ralph, N. , & Forbes, D. (2018). An ambulatory PSG study of the posttraumatic nightmares of posttraumatic stress disorder. Sleep, 41, zsx188 10.1093/sleep/zsx188 [DOI] [PubMed] [Google Scholar]
- Pietrowsky, R. , & Köthe, M. (2003). Personal boundaries and nightmare consequences in frequent nightmare sufferers. Dreaming, 13, 245–254. 10.1023/b:drem.0000003146.11946.4c [DOI] [Google Scholar]
- Pole, N. (2007). The psychophysiology of posttraumatic stress disorder: A meta‐analysis. Psychological Bulletin, 133, 725–746. 10.1037/0033-2909.133.5.725 [DOI] [PubMed] [Google Scholar]
- Pruiksma, K. E. , Cranston, C. C. , Rhudy, J. L. , Micol, R. L. , & Davis, J. L. (2018). Randomized controlled trial to dismantle exposure, relaxation, and rescripting therapy (ERRT) for trauma‐related nightmares. Psychological Trauma, 10, 67–75. 10.1037/tra0000238 [DOI] [PubMed] [Google Scholar]
- Raskind, M. A. , Peskind, E. R. , Chow, B. , Harris, C. , Davis‐Karim, A. , Holmes, H. A. , & Huang, G. D. (2018). Trial of prazosin for post‐traumatic stress disorder in military veterans. New England Journal of Medicine, 378, 507–517. 10.1056/nejmoa1507598 [DOI] [PubMed] [Google Scholar]
- Riemann, D. , Spiegelhalder, K. , Feige, B. , Voderholzer, U. , Perlis, M. , & Nissen, C. (2010). The hyperarousal model of insomnia: A review of the concept and its evidence. Sleep Medicine Reviews, 14, 19–31. 10.1016/j.smrv.2009.04.002 [DOI] [PubMed] [Google Scholar]
- Robert, G. , & Zadra, A. L. (2014). Thematic and content analysis of idiopathic nightmares and bad dreams. Sleep, 37, 409–417. 10.5665/sleep.3426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez‐Martín, B. C. , Moritz, S. , Molerio‐Pérez, O. , & Gil‐Pérez, P. (2013). Effectiveness of association splitting in reducing unwanted intrusive thoughts in a nonclinical sample. Behavioural and Cognitive Psychotherapy, 4, 433–440. 10.1017/s1352465812000513 [DOI] [PubMed] [Google Scholar]
- Rousseau, A. , & Belleville, G. (2018). The mechanisms of action underlying the efficacy of psychological nightmare treatments: A systematic review and thematic analysis of discussed hypotheses. Sleep Medicine Reviews, 39, 122–133. 10.1016/j.smrv.2017.08.004 [DOI] [PubMed] [Google Scholar]
- Sadeh, A. (2005). Cognitive‐behavioral treatment for childhood sleep disorders. Clinical Psychology Review, 25, 612–628. 10.1016/j.cpr.2005.04.006 [DOI] [PubMed] [Google Scholar]
- Sandahl, H. , Jennum, P. , Baandrup, L. , Poschmann, I. S. , & Carlsson, J. (2017). Treatment of sleep disturbances in trauma‐affected refugees: Study protocol for a randomised controlled trial. Trials, 18, 520 10.1186/s13063-017-2260-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandahl, H. , Vindbjerg, E. , & Carlsson, J. (2017). Treatment of sleep disturbances in refugees suffering from posttraumatic stress disorder. Transcultural Psychiatry, 54, 806–823. 10.1177/1363461517746314 [DOI] [PubMed] [Google Scholar]
- Sandman, N. , Valli, K. , Kronholm, E. , Ollila, H. M. , Revonsuo, A. , Laatikainen, T. , & Paunio, T. (2013). Nightmares: Prevalence among the Finnish general adult population and war veterans during 1972‐2007. Sleep, 36, 1041–1050. 10.5665/sleep.2806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman, N. , Valli, K. , Kronholm, E. , Vartiainen, E. , Laatikainen, T. , & Paunio, T. (2017). Nightmares as predictors of suicide: An extension study including war veterans. Scientific Reports, 7, 44756 10.1038/srep44756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlarb, A. A. , Brandhorst, I. , & Hautzinger, M. (2011). Mini‐KiSS – ein multimodales Gruppentherapieprogramm für Eltern von Kleinkindern mit Schlafstörungen: Eine Pilotstudie [Mini‐KiSS–a multimodal group therapy intervention for parents of young children with sleep disorders: A pilot study]. Zeitschrift fur Kinder‐ und Jugendpsychiatrie und Psychotherapie, 39, 197–206. 10.1024/1422-4917/a000106 [DOI] [PubMed] [Google Scholar]
- Schlarb, A. A. , Friederich, A. , & Claßen, M. (2017). Sleep problems in university students – an intervention. Neuropsychiatric Disease and Treatment, 13, 1989–2000. 10.2147/ndt [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlarb, A. A. , Velten‐Schurian, K. , Poets, C. F. , & Hautzinger, M. (2011). First effects of a multicomponent treatment for sleep disorders in children. Nature and Science of Sleep, 3, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlarb, A. A. , Zschoche, M. , & Schredl, M. 2016. Der Nightmare Effects Questionnaire (NEQ). Pilotstudie zu ersten psychometrischen Kennwerten bei Jugendlichen und jungen Erwachsenen [The Nightmare Effects Questionnaire (NEQ). A pilot study on the first psychometric characteristics among adolescents and young adults]. Somnologie, 20, 251–257. 10.1007/s11818-016-0086-0 [DOI] [Google Scholar]
- Schredl, M. (2010). Nightmare frequency and nightmare topics in a representative German sample. European Archives of Psychiatry and Clinical Neuroscience, 260, 565–570. 10.1007/s00406-010-0112-3 [DOI] [PubMed] [Google Scholar]
- Schredl, M. (2013). Seeking professional help for nightmares: A representative study. The European Journal of Psychiatry, 27, 259–264. 10.4321/s0213-61632013000400004 [DOI] [Google Scholar]
- Schredl, M. , Berres, S. , Klingauf, A. , Schellhaas, S. , & Göritz, A. S. (2010). The Mannheim Dream Questionnaire (MADRE): Retest reliability, age and gender effects. International Journal of Dream Research, 7, 141–147. [Google Scholar]
- Schredl, M. , Dehmlow, L. , & Schmitt, J. (2016). Interest in information about nightmares in patients with sleep disorders. Journal of Clinical Sleep Medicine, 12, 973–977. 10.5664/jcsm.5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schredl, M. , Fricke‐Oerkermann, L. , Mitschke, A. , Wiater, A. , & Lehmkuhl, G. (2009). Longitudinal study of nightmares in children: Stability and effect of emotional symptoms. Child Psychiatry and Human Development, 40, 439–449. 10.1007/s10578-009-0136-y [DOI] [PubMed] [Google Scholar]
- Schredl, M. , & Göritz, A. S. (2014). Umgang mit Alpträumen in der Allgemeinbevölkerung: Eine Online‐Studie. [Coping with nightmares in the general population: An online study]. Psychotherapie, Psychosomatik, Medizinische Psychologie, 64, 192–196. [DOI] [PubMed] [Google Scholar]
- Schredl, M. , Landgraf, C. , & Zeiler, O. (2003). Nightmare frequency, nightmare distress and neuroticism. North American Journal of Psychology, 5, 345–350. [Google Scholar]
- Schreuder, B. J. N. , Kleijn, W. C. , & Rooijmans, H. G. M. (2000). Nocturnal re‐experiencing more than forty years after war trauma. Journal of Traumatic Stress, 3, 453–463. 10.1023/a:1007733324351 [DOI] [PubMed] [Google Scholar]
- Seda, G. , Sanchez‐Ortuno, M. M. , Welsh, C. H. , Halbower, A. C. , & Edinger, J. D. (2015). Comparative meta‐analysis of prazosin and imagery rehearsal therapy for nightmare frequency, sleep quality, and posttraumatic stress. Journal of Clinical Sleep Medicine, 15, 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard, V. , & Nielsen, T. (2009). Adaptation of imagery rehearsal therapy for nightmares in children: A brief report. Psychotherapy: Theory, Research, Practice, Training, 46, 492–497. 10.1037/a0017945 [DOI] [PubMed] [Google Scholar]
- Simor, P. , Pajkossy, P. , Horváth, K. , & Bódizs, R. (2012). Impaired executive functions in subjects with frequent nightmares as reflected by performance in different neuropsychological tasks. Brain and Cognition, 78, 274–283. 10.1016/j.bandc.2012.01.006 [DOI] [PubMed] [Google Scholar]
- Sjöström, N. , Hetta, J. , & Waerna, M. (2009). Persistent nightmares are associated with repeat suicide attempt: A prospective study. Psychiatry Research, 170, 208–211. 10.1016/j.psychres.2008.09.006 [DOI] [PubMed] [Google Scholar]
- Solms, M. (1995). New findings on the neurological organization of dreaming: Implications for psychoanalysis. The Psychoanalytic Quarterly, 64, 43–67. 10.1080/21674086.1995.11927443 [DOI] [PubMed] [Google Scholar]
- Solms, M. (2000). Dreaming and REM sleep are controlled by different brain mechanisms. Behavioral and Brain Sciences, 23, 843–850. 10.1017/s0140525x00003988 [DOI] [PubMed] [Google Scholar]
- Spoormaker, V. I. (2008). A cognitive model of recurrent nightmares. International Journal of Dream Research, 1, 15–22. [Google Scholar]
- Spoormaker, V. I. , Schredl, M. , & Van Den Bout, J. (2006). Nightmares: From anxiety symptom to sleep disorder. Sleep Medicine Reviews, 10, 19–31. 10.1016/j.smrv.2005.06.001 [DOI] [PubMed] [Google Scholar]
- Spoormaker, V. I. , & Van Den Bout, J. (2006). Lucid dreaming treatment for nightmares: A pilot study. Psychotherapy and Psychosomatics, 75, 389–394. 10.1159/000095446 [DOI] [PubMed] [Google Scholar]
- Swart, M. L. , Van Schagen, A. M. , Lancee, J. , & Van Den Bout, J. (2013). Prevalence of nightmare disorder in psychiatric outpatients. Psychotherapy and Psychosomatics, 82, 267–268. 10.1159/000343590 [DOI] [PubMed] [Google Scholar]
- Talamini, L. M. , Bringmann, L. F. , De Boer, M. , & Hofman, W. F. (2013). Sleeping worries away or worrying away sleep? Physiological evidence on sleep‐emotion interactions. PLoS ONE, 8, e62480 10.1371/journal.pone.0062480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terr, L. C. (1983). Chowchilla revisited: The effects of psychic trauma four years after a school‐bus kidnapping. American Journal of Psychiatry, 140, 1543–1550. [DOI] [PubMed] [Google Scholar]
- Thünker, J. , Norpoth, M. , Von Aspern, M. , Özcan, T. , & Pietrowsky, R. (2014). Nightmares: Knowledge and attitudes in health care providers and nightmare sufferers. Journal of Public Health and Epidemiology, 6, 223–228. [Google Scholar]
- Thünker, J. , & Pietrowsky, R. (2012). Effectiveness of a manualized imagery rehearsal therapy for patients suffering from nightmare disorders with and without a comorbidity of depression or PTSD. Behavior Research and Therapy, 50, 558–564. 10.1016/j.brat.2012.05.006 [DOI] [PubMed] [Google Scholar]
- United Nations High Commissioner for Refugees (UNHCR) (2018). Global Trends forced displacement in 2017. Retrieved from: http://www.unhcr.org/figures-at-a-glance.html.
- Van Schagen, A. M. , Lancee, J. , De Groot, I. W. , Spoormaker, V. I. , & Van Den Bout, J. (2015). Imagery rehearsal therapy in addition to treatment as usual for patients with diverse psychiatric diagnoses suffering from nightmares: A randomized controlled trial. Journal of Clinical Psychiatry, 76, e1105–e1113. 10.4088/jcp.14m09216 [DOI] [PubMed] [Google Scholar]
- Van Schagen, A. M. , Lancee, J. , Spoormaker, V. I. , & Van Den Bout, J. (2016). Long‐term treatment effects of imagery rehearsal therapy for nightmares in a population with diverse psychiatric disorders. International Journal of Dream Research, 9, 67–70. [Google Scholar]
- Van Schagen, A. M. , Lancee, J. , Swart, M. , Spoormaker, V. I. , & Van Den Bout, J. (2017). Nightmare disorder, psychopathology levels, and coping in a diverse psychiatric sample. Journal of Clinical Psychology, 73, 65–75. 10.1002/jclp.22315 [DOI] [PubMed] [Google Scholar]
- Walker, M. P. , & Stickgold, R. (2006). Sleep, memory, and plasticity. Annual Review of Psychology, 57, 139–166. 10.1146/annurev.psych.56.091103.070307 [DOI] [PubMed] [Google Scholar]
- Wegerer, M. , Blechert, J. , Kerschbaum, H. , & Wilhelm, F. H. (2013). Relationship between fear conditionability and aversive memories: Evidence from a novel conditioned‐intrusion paradigm. PLoS ONE, 8, e79025 10.1371/journal.pone.0079025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner, D. M. , Wenzlaff, R. M. , & Kozak, M. (2004). Dream rebound: The return of suppressed thoughts in dreams. Psychological Science, 15, 232–236. 10.1111/j.0963-7214.2004.00657.x [DOI] [PubMed] [Google Scholar]
- Werner, G. G. , Schabus, M. , Blechert, J. , Kolodyazhniy, V. , & Wilhelm, F. H. (2015). Pre‐ to postsleep change in psychophysiological reactivity to emotional films: Late‐night REM sleep is associated with attenuated emotional processing. Psychophysiology, 52, 813–825. 10.1111/psyp.12404 [DOI] [PubMed] [Google Scholar]
- Wiechers, S. , Schlarb, A. A. , Urschitz, M. S. , Eggebrecht, E. , Schlaud, M. , & Poets, C. F. (2011). Sleep problems and poor academic performance in primary school children. Somnologie, 15, 243–248. 10.1007/s11818-011-0535-8 [DOI] [Google Scholar]
- Wittmann, L. , & De Dassel, T. (2015). Posttraumatic nightmares: From scientific evidence to clinical significance In Kramer M., & Glucksman M. L. (Eds.), Dream research. Contributions to clinical practice (pp. 135–148). New York: Routledge. [Google Scholar]
- Wittmann, L. , Schredl, M. , & Kramer, M. (2007). Dreaming in posttraumatic stress disorder: A critical review of phenomenology, psychophysiology and treatment. Psychotherapy and Psychosomatics, 76, 25–39. 10.1159/000096362 [DOI] [PubMed] [Google Scholar]
- Wood, J. M. , & Bootzin, R. R. (1990). The prevalence of nightmares and their independence from anxiety. Journal of Abnormal Psychology, 99, 64–68. 10.1037/0021-843x.99.1.64 [DOI] [PubMed] [Google Scholar]
- Woodward, E. , Hackmann, A. , Wild, J. , Grey, N. , Clark, D. M. , & Ehlers, A. (2017). Effects of psychotherapies for posttraumatic stress disorder on sleep disturbances: Results from a randomized clinical trial. Behavior Research and Therapy, 97, 75–85. 10.1016/j.brat.2017.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, C. K.‐C. (2014). Normality, pathology, and dreaming. Dreaming, 24, 203–216. 10.1037/a0037306 [DOI] [Google Scholar]


