Abstract
Background
The Beers Criteria is used as a reference to identify potentially inappropriate medications (PIMs) prescribed to older people, and anticholinergic risk measurement scales (ARMSs) have been continuously made for measuring the anticholinergic burden. This study aimed to evaluate the concordance between any anticholinergics among PIMs identified by the Beers Criteria and those assessed by 9 different ARMSs.
Methods
This study was retrospectively conducted with Korean older patients hospitalized in the long-term care facility between March 2014 and August 2015. The data were collected through the chart review of electronic medical records of the patients. The Beers Criteria 2003, 2012, and 2015 were used to detect PIMs, and the following ARMSs were also employed to assess their potential anticholinergic effects: Anticholinergic Cognitive Burden Scale (2008), Anticholinergic Risk Scale (2008), Chew’s Scale (2008), Anticholinergic Drug Scale (ADS; 2006), Anticholinergic Activity Scale (AAS; 2010), Anticholinergic Load Scale (2011), Clinician-Rated Anticholinergic Scale (2008), Duran’s Scale (2013), and Anticholinergic Burden Classification (2006).
Results
The eligible patients who met inclusion and exclusion criteria were 216 during the study period. Most patients were females (70.4%), and the mean age was 81.0 ± 6.7 years. Approximately 70%, 86%, and 87% of the patients included were identified as using at least one PIM according to the Beers Criteria 2003, 2012, and 2015, respectively. Compared with the Beers Criteria 2003, the versions of 2012 and 2015 showed more improved concordance associated with the ARMSs. When the Beers Criteria 2015 was compared with the ARMSs, the lowest concordance was found for AAS (κ = 0.153; 95% CI, 0.079–0.227), whereas the highest concordance was observed for ADS (κ = 0.530; 95% CI, 0.406–0.654).
Conclusion
The heterogeneity between the Beers Criteria and the ARMSs was observed. Compared with the Beers Criteria 2003, the versions of 2012 and 2015 showed more enhanced concordance associated with the ARMSs.
Keywords: Beers Criteria, potentially inappropriate medications, anticholinergic burden scale, anticholinergics, older patients, concordance
Introduction
The population of the elderly is increasing worldwide. Today, most people can live up to 60 years or longer. It is expected that the proportion of people over 60 years in the world’s population will almost double from 12% in 2015 to 22% in 2050.1 This change in the percentage constituting the elderly will occur in low- and middle-income countries (eg, Chile, China, Iran, and Russia). These countries will have an elderly-people proportion similar to that of Japan, where 30% of the population is already older than 60 years.1
It is well known that the incidence of preventable chronic diseases (eg, cardiovascular diseases, cancer, chronic respiratory diseases, and diabetes mellitus) increases markedly with age.2 Consequently, the majority of patients over 65 years have at least one chronic disease.2 This may lead elderly patients to take multiple medications in order to manage their chronic conditions. This polypharmacy can cause adverse drug reactions and drug–drug interactions as well as poor medication compliance. In particular, these drug-related problems are likely to occur more frequently among the elderly owing to altered pharmacokinetics and pharmacodynamics, changes in body composition, and weaker physiological functions.3
Of the drug-related problems in older adults, anticholinergic side effects (eg, cognitive impairment, mydriasis, flushing, dry mouth, constipation, and urinary retention) are more prevalent due to altered anticholinergic sensitivity by ageing.4–8 Therefore, anticholinergics are generally categorized as potentially inappropriate medications (PIMs).4 Nonetheless, their use remains prevalent among the elderly.8,9 Accordingly, one simple strategy to reduce the use of PIMs may be to avoid them prior to prescription through interventions on the basis of reliable criteria.
The American Geriatrics Society (AGS) Beers Criteria are some of the most popular reference sources for the prevention of PIM prescription to patients of 65 years of age and older.10 The Beers Criteria were initially published in 1991; after multiple revisions were implemented in 1997, 2003, and 2012, the latest version was released in 2015.11 The Beers Criteria categorize PIMs as avoided in older adults in general and in those with certain circumstances, prescribed at lower doses or cautiously, or monitored carefully.10 The Beers Criteria also include separate categories to assess medications with anticholinergic properties; however, these categories cannot comprehensively cover all anticholinergic medications prescribed in real-world settings.10,11 Besides the Beers Criteria, multiple attempts to produce reliable anticholinergic risk measurement scales (ARMSs) have been continuously made, such as Anticholinergic Cognitive Burden Scale (ACB; 2008),12 Anticholinergic Risk Scale (ARS; 2008),13 Chew’s Scale (2008),14 Anticholinergic Drug Scale (ADS; 2006),15 and Anticholinergic Activity Scale (AAS; 2010),16 for measuring the anticholinergic burden and predicting the potential adverse effects.17
Several studies have assessed the prevalence of PIM prescriptions by means of Beers Criteria 2003, 2012, and 2015 and compared the concordance of PIMs identified by the Beers Criteria.18–20 Some studies have evaluated the prevalence of anticholinergic medication use via the ARMSs and compared the concordance of anticholinergics identified by the scales.4,21,22 To our knowledge, no studies have compared the Beers Criteria with various ARMSs. Therefore, this study aimed to evaluate the concordance between any anticholinergics among PIMs identified by the Beers Criteria and those assessed by the anticholinergic scales in Korean older patients hospitalized in the long-term care facility.
Methods
Ethics
Ethical approval for the study was obtained from the Institutional Review Board (IRB) of Chosun University (2-1041055-AB-N-01-2015-0042). Informed consent was deemed unnecessary for the study patients because their records were anonymized and deidentified before analysis.
Study Population
Among patients who were hospitalized during the study period, those who met the following inclusion criteria were selected for this study: patients ≥65 years of age and patients who had prescription information in electronic medical records (EMRs). The following exclusion criteria were applied: patients <65 years of age, patients without prescription information in EMRs, the same patients who were readmitted to the hospital during the study period, or patients whose duration of hospitalization was shorter than 14 days.
In the facility, 7-day medications at a time had been prescribed, filled, and dispensed although there was an exception depending on patients’ conditions. After the patients finished all medications prescribed in previous hospitals, the pharmacy in the facility started to fill and dispense new medications in accordance with a doctor’s prescription order. From this point, the patient’s medication history was recorded in the EMR chart of the facility. Usually, it took about 2 weeks for a patient to be hospitalized, receive an inpatient prescription, and have a record about the medication prescription on the EMR chart. After considering these special conditions of the facility, we decided to select the patients who had stayed for at least 14 days.
Study Procedures
This study retrospectively analyzed the patients who had been hospitalized at the long-term care facility between March 2014 and August 2015. This facility is located in Gwangju, South Korea, and was equipped with approximately 290 beds. Through the chart review of EMRs of the patients, the following information was collected by a trained hospital pharmacist by means of paper case report forms: demographic characteristics (eg, age and gender), disease(s) present, and prescribed medications. In case of patients who were readmitted, the most recent prescription information was collected. Prescription information was not collected about injections that were administered under special clinical conditions.
The Beers Criteria were employed in this study to identify PIMs taken by elderly patients. The Beers Criteria are some of the most frequently used tools for the identification of PIMs that should be avoided by older adults in general and especially by people with certain diseases or syndromes, when the drugs are prescribed at a reduced dose or taken with caution or careful monitoring. These criteria were updated in 2003,23 2012,24 and 2015.10 The changes implemented in the 2015 update were not as extensive as those in two previous versions. The PIMs that should be avoided by elderly persons in general and by those with certain diseases or syndromes were selected to determine the differences among the Beers Criteria 2003, 2012, and 2015 versions. In particular, in case that the medications which should be avoided at certain durations or in elderly persons with certain medical conditions were present, these were still defined as PIMs irrespective of treatment durations or medical conditions.
The following ARMSs were administered to quantify potential anticholinergic effects of medications taken by the patients: ACB,12 ARS,13 Chew’s Scale,14 ADS,15 AAS,16 Anticholinergic Load Scale (ALS; 2011),25 Clinician-Rated Anticholinergic Scale (CrAS; 2008),26 Duran’s Scale (2013),8 and Anticholinergic Burden Classification (ABC; 2006).27 Each instrument has a different range for measuring anticholinergic activity (AA); however, a higher score indicates a stronger AA. The evaluation ratings of most scales except for AAS, Chew’s Scale, and Duran’s Scale were defined as follows: no AA (0), mild AA (1), moderate AA (2), and severe AA (3). The AAS is a 5-point scale (0–4), and Duran’s Scale is a 3-point instrument (0–2). Chew’s Scale defines medications as having no AA at therapeutic doses (0), no or minimal AA (0/+), low AA (+), moderate AA (++), and high AA (+++).
Lastly, the proportion of anticholinergic medications identified by the Beers Criteria was compared with that of anticholinergics evaluated by 9 ARMSs.
Statistical Analyses
Frequencies (n) and percentages (%) were used to present categorical variables, whereas means and standard deviations (mean ± SD) served to describe continuous variables. The χ2 test or Fisher’s exact test were performed to assess the differences in proportions, and the independent-sample t-test was carried out to evaluate the differences in means. The levels of concordance between the proportions of anticholinergic medications identified by the AGS Beers Criteria and the nine ARMSs were assessed by Cohen’s kappa statistic (k). The 95% confidence interval (CI) for Cohen’s kappa was computed via standard error. Cohen’s kappa was interpreted as follows: poor (<0.20), fair (0.20–0.40), moderate (0.41–0.60), good (0.61–0.80), and very good (0.81–1.00). Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated to evaluate the accuracy of anticholinergic identification by the Beers Criteria and by the ARMSs. Sensitivity is defined as the ability of a test to detect a true positive result, specificity as the ability of a test to detect a true negative result, PPV as the proportion of positive test results that is true positive and represents the presence of an event, and NPV is defined as the proportion of negative test results that is true negative and represents the absence of an event.28 All analyses were performed in the SAS software, version 9.3 (SAS Institute Inc., Cary, NC). Statistical significance was set at a p-value <0.05.
Results
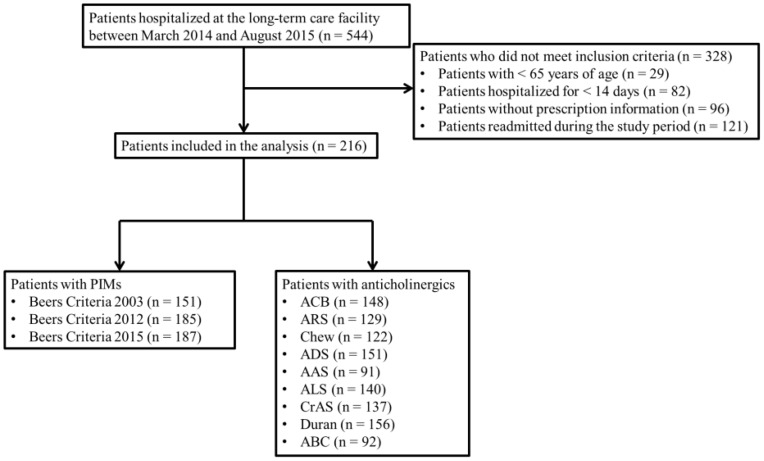
In this study, the number of eligible patients who met the inclusion criteria (and did not match the exclusion criteria) was 216 during the study period (Figure 1). The complete list of prescribed medications is presented in Table S1. The PIMs identified by Beers Criteria 2003, 2012, and 2015 are listed in Table S2, and the PIMs with anticholinergic properties classified according to the nine ARMSs are shown in Table S3.
Figure 1.
Flow chart of selecting the study subjects.
Abbreviations: PIMs, potentially inappropriate medications; ACB, Anticholinergic Cognitive Burden Scale; ARS, Anticholinergic Risk Scale; Chew, Chew’s Scale; ADS, Anticholinergic Drug Scale; AAS, Anticholinergic Activity Scale; ALS, Anticholinergic Load Scale; CrAS, Clinician-Rated Anticholinergic Scale; Duran, Duran’s Scale; ABC, Anticholinergic Burden Classification.
The patients’ characteristics are given in Table 1. Most patients were females (70.4%), and the mean age was 81.0 ± 6.7 years (mean ± SD) among all the study subjects. The number of comorbidities was 4.4 ± 2.7, and the number of prescribed drugs was 5.3 ± 2.7. The anticholinergic drugs identified by the nine ARMSs were classified into PIMs and non-PIMs on the basis of Beers Criteria 2003, 2012, and 2015 versions. As the Beers Criteria were updated, most anticholinergics identified by the ARMSs moved from non-PIMs to PIMs. In particular, when the anticholinergics identified by Chew’s Scale and AAS were categorized into PIMs and non-PIMs according to Beers Criteria 2003, there were no significant differences between the two groups; however, significant differences were found after classification by Beers Criteria 2012 and 2015.
Table 1.
Demographic Characteristics Of Patients
| Characteristic | Total | Beers Criteria 2003 | Beers Criteria 2012 | Beers Criteria 2015 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PIMs | Non-PIMs | p-Value | PIMs | Non-PIMs | P-Value | PIMs | Non-PIMs | p-Value | ||
| Gender | ||||||||||
| Male, n (%) | 64 (29.6) | 39 (25.8) | 25 (38.5) | 0.062 | 57 (30.8) | 7 (22.6) | 0.353 | 59 (31.6) | 5 (17.2) | 0.116 |
| Female, n (%) | 152 (70.4) | 112 (74.2) | 40 (61.5) | 128 (69.2) | 24 (77.4) | 128 (68.4) | 24 (82.8) | |||
| Age, mean ± SD | 81.0 ± 6.7 | 81.2 ± 6.3 | 80.6 ± 7.6 | 0.561 | 81.1 ± 6.8 | 80.5 ± 5.8 | 0.662 | 81.1 ± 6.8 | 80.4 ± 6 | 0.590 |
| 65–74, n (%) | 40 (18.5) | 24 (15.9) | 16 (24.6) | 0.308 | 34 (18.4) | 6 (19.4) | 0.477 | 34 (18.2) | 6 (20.7) | 0.591 |
| 75–84, n (%) | 114 (52.8) | 83 (55.0) | 31 (47.7) | 95 (51.4) | 19 (61.3) | 97 (51.9) | 17 (58.6) | |||
| ≥85, n (%) | 62 (28.7) | 44 (29.1) | 18 (27.7) | 56 (30.3) | 6 (19.4) | 56 (29.9) | 6 (20.7) | |||
| No. of comorbidity, mean ± SD | 4.4 ± 2.7 | 4.2 ± 2.6 | 5.0 ± 3.0 | 0.046 | 4.3 ± 2.7 | 4.8 ± 2.9 | 0.415 | 4.3 ± 2.7 | 4.9 ± 3 | 0.333 |
| 0–5, n (%) | 152 (70.4) | 116 (76.8) | 36 (55.4) | 0.006 | 134 (72.4) | 18 (58.1) | 0.209 | 136 (72.7) | 16 (55.2) | 0.120 |
| 6–9, n (%) | 54 (25.0) | 30 (19.9) | 24 (36.9) | 43 (23.2) | 11 (35.5) | 43 (23.0) | 11 (37.9) | |||
| ≥10, n (%) | 10 (4.6) | 5 (3.3) | 5 (7.7) | 8 (4.3) | 2 (6.5) | 8 (4.3) | 2 (6.9) | |||
| No. of prescribed drugs, mean ± SD | 5.3 ± 2.7 | 5.8 ± 2.7 | 4.1 ± 2.3 | <0.001 | 5.7 ± 2.7 | 3.2 ± 1.5 | <0.001 | 5.7 ± 2.7 | 3.1 ± 1.3 | <0.001 |
| 1–4, n (%) | 96 (44.4) | 53 (35.1) | 43 (66.2) | <0.001 | 71 (38.4) | 25 (80.6) | <0.001 | 72 (38.5) | 24 (82.8) | <0.001 |
| 5–9, n (%) | 101 (46.8) | 81 (53.6) | 20 (30.8) | 95 (51.4) | 6 (19.4) | 96 (51.3) | 5 (17.2) | |||
| ≥10, n (%) | 19 (8.8) | 17 (11.3) | 2 (3.1) | 19 (10.3) | 0 (0.0) | 19 (10.2) | 0 (0.0) | |||
| No. of anticholinergic drugs, mean ± SD | 129.6 ± 24.0 | 104.9 ± 19.5 | 25.8 ± 8.6 | <0.001 | 126.8 ± 24.1 | 2.8 ± 1.7 | <0.001 | 127.2 ± 24.4 | 2.3 ± 1.8 | <0.001 |
| ACB, n (%) | 148 (68.5) | 123 (81.5) | 25 (38.5) | <0.001 | 144 (77.8) | 4 (12.9) | <0.001 | 144 (77.0) | 4 (13.8) | <0.001 |
| ARS, n (%) | 129 (59.7) | 110 (72.8) | 19 (29.2) | <0.001 | 129 (69.7) | 0 (0.0) | <0.001 | 129 (69.0) | 0 (0.0) | <0.001 |
| Chew, n (%) | 122 (56.5) | 91 (60.3) | 31 (47.7) | 0.087 | 118 (63.8) | 4 (12.9) | <0.001 | 118 (63.1) | 4 (13.8) | <0.001 |
| ADS, n (%) | 151 (69.9) | 121 (80.1) | 30 (46.2) | <0.001 | 150 (81.1) | 1 (3.2) | <0.001 | 151 (80.7) | 0 (0.0) | <0.001 |
| AAS, n (%) | 91 (42.1) | 68 (45.0) | 23 (35.4) | 0.188 | 88 (47.6) | 3 (9.7) | <0.001 | 88 (47.1) | 3 (10.3) | <0.001 |
| ALS, n (%) | 140 (64.8) | 114 (75.5) | 26 (40.0) | <0.001 | 135 (73.0) | 5 (16.1) | <0.001 | 136 (72.7) | 4 (13.8) | <0.001 |
| CrAS, n (%) | 137 (63.4) | 110 (72.8) | 27 (41.5) | <0.001 | 136 (73.5) | 1 (3.2) | <0.001 | 137 (73.3) | 0 (0.0) | <0.001 |
| Duran, n (%) | 156 (72.2) | 123 (81.5) | 33 (50.8) | <0.001 | 152 (82.2) | 4 (12.9) | <0.001 | 153 (81.8) | 3 (10.3) | <0.001 |
| ABC, n (%) | 92 (42.8) | 84 (55.6) | 8 (12.5) | <0.001 | 89 (48.4) | 3 (9.7) | <0.001 | 89 (47.8) | 3 (10.3) | <0.001 |
Abbreviations: PIMs, potentially inappropriate medications; ACB, Anticholinergic Cognitive Burden Scale; ARS, Anticholinergic Risk Scale; Chew, Chew’s Scale; ADS, Anticholinergic Drug Scale; AAS, Anticholinergic Activity Scale; ALS, Anticholinergic Load Scale; CrAS, Clinician-Rated Anticholinergic Scale; Duran, Duran’s Scale; ABC, Anticholinergic Burden Classification.
The results from the evaluations of concordance between the proportions of anticholinergics identified by the Beers Criteria and the ARMSs are listed in Table 2. Overall, the concordance between the Beers Criteria and the ARMSs varied from poor to moderate. As the Beers Criteria were updated from 2003 to 2015, improved concordance levels were noted for most ARMSs except for ABC where the concordance decreased from kappa [κ] = 0.341 in 2003 to κ = 0.158 in 2015. When Beers Criteria 2003 were compared with the nine ARMSs, the lowest concordance was observed for AAS (κ = 0.076; 95% CI, 0.036–0.189), whereas the highest concordance was found for ACB (κ = 0.424; 95% CI, 0.294–0.555). When Beers Criteria 2012 were compared with the ARMSs, the lowest concordance was noted for AAS (κ = 0.168; 95% CI, 0.092–0.244), and the highest concordance was observed for ADS (κ = 0.535; 95% CI, 0.411–0.659). When the Beers Criteria 2015 version was compared with the nine ARMSs, the lowest concordance was found for AAS (κ = 0.153; 95% CI, 0.079–0.227), whereas the highest concordance was noted for ADS (κ = 0.530; 95% CI, 0.406–0.654).
Table 2.
Concordance Between The Proportions Of Anticholinergic Identified By The Beers Criteria And Various Anticholinergic Scales
| Anticholinergic Scale | Beers Criteria 2003 | Beers Criteria 2012 | Beers Criteria 2015 | |||
|---|---|---|---|---|---|---|
| PIMs | Non-PIMs | PIMs | Non-PIMs | PIMs | Non-PIMs | |
| ACB, n (%) | ||||||
| Point = 0 | 28 (13.0) | 40 (18.5) | 41 (19.0) | 27 (12.5) | 43 (19.9) | 25 (11.6) |
| Point > 0 | 123 (56.9) | 25 (11.6) | 144 (66.7) | 4 (1.9) | 144 (66.7) | 4 (1.9) |
| Agreement (%) | 75.463 | 79.167 | 78.241 | |||
| Kappa (95% CI) | 0.424 (0.294–0.555) | 0.434 (0.307–0.561) | 0.403 (0.275–0.531) | |||
| ARS, n (%) | ||||||
| Point = 0 | 41 (19.0) | 46 (21.3) | 56 (25.9) | 31 (14.4) | 58 (26.9) | 29 (13.4) |
| Point > 0 | 110 (50.9) | 19 (8.8) | 129 (59.7) | 0 (0.0) | 129 (59.7) | 0 (0.0) |
| Agreement (%) | 72.222 | 74.074 | 73.148 | |||
| Kappa (95% CI) | 0.398 (0.274–0.522) | 0.398 (0.290–0.506) | 0.374 (0.267–0.481) | |||
| Chew, n (%) | ||||||
| Point = 0 | 60 (27.8) | 34 (15.7) | 67 (31.0) | 27 (12.5) | 69 (31.9) | 25 (11.6) |
| Point > 0 | 91 (42.1) | 31 (14.4) | 118 (54.6) | 4 (1.9) | 118 (54.6) | 4 (1.9) |
| Agreement (%) | 57.870 | 67.130 | 66.204 | |||
| Kappa (95% CI) | 0.112 (−0.017–0.240) | 0.276 (0.171–0.380) | 0.253 (0.151–0.356) | |||
| ADS, n (%) | ||||||
| Point = 0 | 30 (13.9) | 35 (16.2) | 35 (16.2) | 30 (13.9) | 36 (16.7) | 29 (13.4) |
| Point > 0 | 121 (56.0) | 30 (13.9) | 150 (69.4) | 1 (0.5) | 151 (69.9) | 0 (0.0) |
| Agreement (%) | 72.222 | 83.333 | 83.333 | |||
| Kappa (95% CI) | 0.340 (0.205–0.475) | 0.535 (0.411–0.659) | 0.530 (0.406–0.654) | |||
| AAS, n (%) | ||||||
| Point = 0 | 83 (38.4) | 42 (19.4) | 97 (44.9) | 28 (13.0) | 99 (45.8) | 26 (12.0) |
| Point > 0 | 68 (31.5) | 23 (10.6) | 88 (40.7) | 3 (1.4) | 88 (40.7) | 3 (1.4) |
| Agreement (%) | 50.926 | 53.704 | 52.778 | |||
| Kappa (95% CI) | 0.076 (−0.036–0.189) | 0.168 (0.092–0.244) | 0.153 (0.079–0.227) | |||
| ALS, n (%) | ||||||
| Point = 0 | 37 (17.1) | 39 (18.1) | 50 (23.1) | 26 (12.0) | 51 (23.6) | 25 (11.6) |
| Point > 0 | 114 (52.8) | 26 (12.0) | 135 (62.5) | 5 (2.3) | 136 (63.0) | 4 (1.9) |
| Agreement (%) | 70.833 | 74.537 | 74.537 | |||
| Kappa (95% CI) | 0.339 (0.207–0.470) | 0.354 (0.233–0.476) | 0.350 (0.230–0.470) | |||
| CrAS, n (%) | ||||||
| Point = 0 | 41 (19.0) | 38 (17.6) | 49 (22.7) | 30 (13.9) | 50 (23.1) | 29 (13.4) |
| Point > 0 | 110 (50.9) | 27 (12.5) | 136 (63.0) | 1 (0.5) | 137 (63.4) | 0 (0.0) |
| Agreement (%) | 68.519 | 76.852 | 76.852 | |||
| Kappa (95% CI) | 0.295 (0.163–0.427) | 0.427 (0.312–0.543) | 0.424 (0.310–0.538) | |||
| Duran, n (%) | ||||||
| Point = 0 | 28 (13.0) | 32 (14.8) | 33 (15.3) | 27 (12.5) | 34 (15.7) | 26 (12.0) |
| Point > 0 | 123 (56.9) | 33 (15.3) | 152 (70.4) | 4 (1.9) | 153 (70.8) | 3 (1.4) |
| Agreement (%) | 71.759 | 82.870 | 82.870 | |||
| Kappa (95% CI) | 0.314 (0.177–0.451) | 0.499 (0.366−0.631) | 0.492 (0.360–0.625) | |||
| ABC, n (%) | ||||||
| Point = 0 | 67 (31.2) | 56 (26.0) | 95 (44.2) | 28 (13.0) | 97 (45.1) | 26 (12.1) |
| Point > 0 | 84 (39.1) | 8 (3.7) | 89 (41.4) | 3 (1.4) | 89 (41.4) | 3 (1.4) |
| Agreement (%) | 65.116 | 54.419 | 53.488 | |||
| Kappa (95% CI) | 0.341 (0.237–0.445) | 0.173 (0.096–0.251) | 0.158 (0.083–0.234) | |||
Abbreviations: PIMs, potentially inappropriate medications; ACB, Anticholinergic Cognitive Burden Scale; ARS, Anticholinergic Risk Scale; Chew, Chew’s Scale; ADS, Anticholinergic Drug Scale; AAS, Anticholinergic Activity Scale; ALS, Anticholinergic Load Scale; CrAS, Clinician-Rated Anticholinergic Scale; Duran, Duran’s Scale; ABC, Anticholinergic Burden Classification.
The accuracy characteristics of anticholinergic identification by the Beers Criteria and by the ARMSs are presented in Table 3. When Beers Criteria 2003 were compared with the ARMSs, ACB and Duran’s Scale manifested the highest sensitivity (81.46% and 81.46%, respectively), ABC showed the highest specificity (87.50%), ABC the highest PPV (91.30%), and ACB the highest NPV (58.82%). When the Beers Criteria 2012 version was compared with the nine ARMSs, the highest sensitivity was observed for Duran’s Scale (82.16%), the highest specificity for ARS (100.00%), the highest PPV for ARS (100.00%), and the highest NPV for ADS (46.15%). When the Beers Criteria 2015 version was compared with the ARMSs, the highest sensitivity was registered for Duran’s Scale (81.82%); the highest specificity for ARS (100.00%), ADS (100.00%), and CrAS (100.00%); the highest PPV for ARS (100.00%), ADS (100.00%), and CrAS (100.00%); and the highest NPV for ADS (44.62%).
Table 3.
Accuracy And Predictability Of Anticholinergic Identified By The Beers Criteria And Various Anticholinergic Scales
| Anticholinergic Scale | Beers Criteria 2003 | Beers Criteria 2012 | Beers Criteria 2015 | |||
|---|---|---|---|---|---|---|
| PIMs | Non-PIMs | PIMs | Non-PIMs | PIMs | Non-PIMs | |
| ACB, n (%) | ||||||
| Point = 0 | 28 (13.0) | 40 (18.5) | 41 (19.0) | 27 (12.5) | 43 (19.9) | 25 (11.6) |
| Point > 0 | 123 (56.9) | 25 (11.6) | 144 (66.7) | 4 (1.9) | 144 (66.7) | 4 (1.9) |
| Sensitivity (%) | 81.46 | 77.84 | 77.01 | |||
| Specificity (%) | 61.54 | 87.10 | 86.21 | |||
| PPV (%) | 83.11 | 97.30 | 97.30 | |||
| NPV (%) | 58.82 | 39.71 | 36.76 | |||
| ARS, n (%) | ||||||
| Point = 0 | 41 (19.0) | 46 (21.3) | 56 (25.9) | 31 (14.4) | 58 (26.9) | 29 (13.4) |
| Point > 0 | 110 (50.9) | 19 (8.8) | 129 (59.7) | 0 (0.0) | 129 (59.7) | 0 (0.0) |
| Sensitivity (%) | 72.85 | 69.73 | 68.98 | |||
| Specificity (%) | 70.77 | 100.00 | 100.00 | |||
| PPV (%) | 85.27 | 100.00 | 100.00 | |||
| NPV (%) | 52.87 | 35.63 | 33.33 | |||
| Chew, n (%) | ||||||
| Point = 0 | 60 (27.8) | 34 (15.7) | 67 (31.0) | 27 (12.5) | 69 (31.9) | 25 (11.6) |
| Point > 0 | 91 (42.1) | 31 (14.4) | 118 (54.6) | 4 (1.9) | 118 (54.6) | 4 (1.9) |
| Sensitivity (%) | 60.26 | 63.78 | 63.10 | |||
| Specificity (%) | 52.31 | 87.10 | 86.21 | |||
| PPV (%) | 74.59 | 96.72 | 96.72 | |||
| NPV (%) | 36.17 | 28.72 | 26.60 | |||
| ADS, n (%) | ||||||
| Point = 0 | 30 (13.9) | 35 (16.2) | 35 (16.2) | 30 (13.9) | 36 (16.7) | 29 (13.4) |
| Point > 0 | 121 (56.0) | 30 (13.9) | 150 (69.4) | 1 (0.5) | 151 (69.9) | 0 (0.0) |
| Sensitivity (%) | 80.13 | 81.08 | 80.75 | |||
| Specificity (%) | 53.85 | 96.77 | 100.00 | |||
| PPV (%) | 80.13 | 99.34 | 100.00 | |||
| NPV (%) | 53.85 | 46.15 | 44.62 | |||
| AAS, n (%) | ||||||
| Point = 0 | 83 (38.4) | 42 (19.4) | 97 (44.9) | 28 (13.0) | 99 (45.8) | 26 (12.0) |
| Point > 0 | 68 (31.5) | 23 (10.6) | 88 (40.7) | 3 (1.4) | 88 (40.7) | 3 (1.4) |
| Sensitivity (%) | 45.03 | 47.57 | 47.06 | |||
| Specificity (%) | 64.62 | 90.32 | 89.66 | |||
| PPV (%) | 74.73 | 96.70 | 96.70 | |||
| NPV (%) | 33.60 | 22.40 | 20.80 | |||
| ALS, n (%) | ||||||
| Point = 0 | 37 (17.1) | 39 (18.1) | 50 (23.1) | 26 (12.0) | 51 (23.6) | 25 (11.6) |
| Point > 0 | 114 (52.8) | 26 (12.0) | 135 (62.5) | 5 (2.3) | 136 (63.0) | 4 (1.9) |
| Sensitivity (%) | 75.50 | 72.97 | 72.73 | |||
| Specificity (%) | 60.00 | 83.87 | 86.21 | |||
| PPV (%) | 81.43 | 96.43 | 97.14 | |||
| NPV (%) | 51.32 | 34.21 | 32.89 | |||
| CrAS, n (%) | ||||||
| Point = 0 | 41 (19.0) | 38 (17.6) | 49 (22.7) | 30 (13.9) | 50 (23.1) | 29 (13.4) |
| Point > 0 | 110 (50.9) | 27 (12.5) | 136 (63.0) | 1 (0.5) | 137 (63.4) | 0 (0.0) |
| Sensitivity (%) | 72.85 | 73.51 | 73.26 | |||
| Specificity (%) | 58.46 | 96.77 | 100.00 | |||
| PPV (%) | 80.29 | 99.27 | 100.00 | |||
| NPV (%) | 48.10 | 37.97 | 36.71 | |||
| Duran, n (%) | ||||||
| Point = 0 | 28 (13.0) | 32 (14.8) | 33 (15.3) | 27 (12.5) | 34 (15.7) | 26 (12.0) |
| Point > 0 | 123 (56.9) | 33 (15.3) | 152 (70.4) | 4 (1.9) | 153 (70.8) | 3 (1.4) |
| Sensitivity (%) | 81.46 | 82.16 | 81.82 | |||
| Specificity (%) | 49.23 | 87.10 | 89.66 | |||
| PPV (%) | 78.85 | 97.44 | 98.08 | |||
| NPV (%) | 53.33 | 45.00 | 43.33 | |||
| ABC, n (%) | ||||||
| Point = 0 | 67 (31.2) | 56 (26.0) | 95 (44.2) | 28 (13.0) | 97 (45.1) | 26 (12.1) |
| Point > 0 | 84 (39.1) | 8 (3.7) | 89 (41.4) | 3 (1.4) | 89 (41.4) | 3 (1.4) |
| Sensitivity (%) | 55.63 | 48.37 | 47.85 | |||
| Specificity (%) | 87.50 | 90.32 | 89.66 | |||
| PPV (%) | 91.30 | 96.74 | 96.74 | |||
| NPV (%) | 45.53 | 22.76 | 21.14 | |||
Abbreviations: PIMs, potentially inappropriate medications; ACB, Anticholinergic Cognitive Burden Scale; ARS, Anticholinergic Risk Scale; Chew, Chew’s Scale; ADS, Anticholinergic Drug Scale; AAS, Anticholinergic Activity Scale; ALS, Anticholinergic Load Scale; CrAS, Clinician-Rated Anticholinergic Scale; Duran, Duran’s Scale; ABC, Anticholinergic Burden Classification; PPV, positive predictive value; NPV, negative predictive value.
Discussion
To the best of our knowledge, the present study is the first to compare any anticholinergic medications among PIMs identified by Beers Criteria 2003, 2012, and 2015 and anticholinergics identified by nine ARMSs among Korean elderly patients hospitalized in a long-term care facility. Some differences were uncovered in the prevalence of PIMs identified by the Beers Criteria 2003, 2012, and 2015. Of note, differences were also found in the total prevalence of anticholinergic use evaluated by the anticholinergic scales: the lowest (42.1%) was yielded by AAS and the highest (72.2%) by Duran’s Scale. Generally, the highest concordance between the proportions of anticholinergics identified by the Beers Criteria and the ARMSs was observed for ADS, whereas the lowest concordance was found for AAS.
The prevalence rates of PIMs in this study are higher than those reported in other studies. As many as 70%, 86%, and 87% of the patients included in this study were found to take at least one PIM as determined by Beers Criteria 2003, 2012, and 2015, respectively. A study conducted in China revealed that 45% and 54% of patients take at least one PIM as determined by Beers Criteria 2012 and 2015, respectively.18 Two separate studies conducted in China indicate that PIMs identified by the Beers Criteria 2012 version are taken by 53% and 72% of elderly patients.29,30 According to a study on 38,250 inpatients in the United States, the prevalence of PIM use as assessed by Beers Criteria 2012 was 37.6% in 2007 and 34.2% in 2012.31 In a study out of India, 29% and 40% of elderly patients were found to take at least one PIM as determined by Beers Criteria 2003 and 2012, respectively.19 The higher prevalence of PIM prescription in the present study can be explained as follows. Most of the elderly patients had been hospitalized for at least 14 days at the long-term care facility because of serious diseases such as cancer, thus possibly receiving palliative care. This setting might lead to more frequent prescription of PIMs. A lack of knowledge regarding PIMs among physicians at this facility was also likely to result in the greater number of PIMs prescribed to the patients. Moreover, a lack of appropriate intervention provided by pharmacists using validated screening tools might contribute to the higher prevalence of PIM prescription. As reported in another study, pharmacist interventions can decrease the number of PIMs prescribed to elderly people.32,33
The Beers Criteria are some of the most popular guidelines for the prevention of PIM prescription to older patients. Several ARMSs have been developed to evaluate anticholinergic drugs prescribed to older patients. As revealed by the results from the comparison of the Beers Criteria with the nine ARMSs in this study, some heterogeneity was observed when these analyses were administered to the elderly patients hospitalized at the long-term care facility. Overall, the concordance between the Beers Criteria and the ARMSs varied from poor to moderate. Specifically, when the Beers Criteria 2015 version was compared with the ARMSs, the lowest concordance was found for AAS (κ = 0.153; 95% CI, 0.079–0.227) and the highest concordance for ADS (κ = 0.530; 95% CI, 0.406–0.654). This tendency may be caused by the difference in the number of medications listed and the rating of anticholinergic activity assigned to them in each ARMS.17,22 The number of medications ranges from 27 to 117 across the ARMSs.17 In the ARMSs, the inclusion and rating of medications with anticholinergic properties were decided based on expert opinions from an interdisciplinary team consisting of various healthcare professionals such as geriatricians, pharmacists, primary care physicians, and nurses.22 Therefore, the subjective decision on their inclusion and rating was predominantly affected by the panel’s knowledge about the adverse effects of anticholinergic medications.
Overall, compared with Beers Criteria 2003, the 2012 and 2015 versions showed improved concordance with the ARMSs in our study. All medications with anticholinergic properties according to ARS, ADS, or CrAS were classified as PIMs by Beers Criteria 2015; however, the number of PIMs with anticholinergic properties (ARS, 129; ADS, 151; CrAS, 137) varied. This finding could be explained by the number of anticholinergics listed in each ARMS and the subjects analyzed to compile the anticholinergic medication lists. ARS, ADS, and CrAS include 49, 117, and 60 anticholinergics, respectively.13,15,26 The medication list of ARS has been derived from the patients over 65 years of age who have visited geriatric and primary care clinics.13 Rudolph and colleagues have retrospectively reviewed medical records of geriatric patients to include medications into ARS and prospectively evaluated this instrument on elderly patients in primary care clinics.13 Carnahan and colleagues have derived the medication list of ADS from older residents of a long-term care facility, and Han et al have established the medication list of CrAS on the basis of medical records of men older than 65 years who received a diagnosis of hypertension.15,26
The Beers Criteria 2015 version was compared with the ARMSs. The highest sensitivity was found for Duran’s Scale (81.82%) followed by ADS (80.75%). The highest specificity was observed for ARS (100.00%), ADS (100.00%), and CrAS (100.00%). These data indicate that 81.82% and 80.75% of the 187 patients taking PIMs as revealed by Beers Criteria 2015 might be identified as patients taking anticholinergics as determined by Duran’s Scale and ADS, respectively. Furthermore, all 29 patients taking non-PIMs as determined by Beers Criteria 2015 might be classified as people not taking anticholinergics as determined by ARS, ADS, or CrAS. Because both sensitivity and specificity in this study were defined only on the basis of patients taking or not taking PIMs, these parameters could not serve for estimating the probability of PIM use by individual patients.34 Therefore, PPV and NPV were also calculated. ARS (100.00%), ADS (100.00%), and CrAS (100.00%) yielded the highest PPV, and the highest NPV was observed for ADS (44.62%). These findings suggest that all the patients who take anticholinergics as determined by ARS, ADS, or CrAS probably do not take any PIMs, and 44.62% of the 65 patients who did not take anticholinergics as determined by ADS might be taking non-PIMs. Nonetheless, it is important to bear in mind that both PPV and NPV can vary depending on the prevalence of PIM use.34 According to another study (conducted by Sumukadas et al), the proportion of the elderly taking anticholinergic medications has increased over time.35 Thus, the use of anticholinergics may increase further, and the concordance between the Beers Criteria and the ARMSs may change, too. In particular, it is possible that PPV and NPV show pronounced disparities because they are affected by the prevalence of PIM prescription.
In terms of clinical applicability of the findings from this study, these nine ARMSs, especially ADS and Duran’s Scale, can serve as supplementary tools for the latest version of the Beers Criteria to more closely monitor anticholinergics prescribed to older patients. Nevertheless, there are big discrepancies between their lists and ratings. For example, atenolol, a β-blocker, was identified as anticholinergic medication only by ACB and CrAS. Quetiapine, an atypical antipsychotic, was identified as an anticholinergic drug by all ARMSs except for ALS and ABC, but its anticholinergic activity was ranked differently. In addition, because older populations are poly-medicated and have individual biological differences,3 the effects of dosing and of the administration route on the anticholinergic activity have to be considered when this activity is rated.22
This study has some limitations, which must be considered when our data are interpreted. First, the cross-sectional design of this study was likely to detect only medication use patterns in the 2 weeks after the patients were hospitalized instead of examination of their chronic medication patterns. Nevertheless, most of the analyzed medications (eg, amitriptyline, nortriptyline, oxybutynin, tolterodine, amiodarone, and haloperidol) are usually taken chronically. Therefore, this limitation may be mild. Second, the patients included in this study were elderly people with serious diseases who had stayed for at least 14 days at the long-term care facility in Korea. Consequently, it may be difficult to extrapolate the results of this study to better-functioning elderly people. Third, the selection procedure of the medications avoided at certain durations or in people with certain medical conditions might affect the prevalence rates of PIMs. Fourth, if implicit criteria such as medication appropriateness index36 had been applied instead of using only explicit criteria, more comprehensive results might be obtained. Lastly, the new version of the Beers Criteria 2019 had been already published, but this could not be applied in this study due to the date of the study cohort.
Conclusion
Among Korean elderly patients, this study evaluated the concordance in identified anticholinergic PIMs among various versions of the Beers Criteria and concordance in identified anticholinergics between the Beers Criteria and other anticholinergic scales. Heterogeneity was noted between the Beers Criteria and the ARMSs, and the concordance between them varied from poor to moderate. Compared with Beers Criteria 2003, the 2012 and 2015 versions manifested higher concordance with the ARMSs. Currently, there is no standardized rating scale for the measurement of the anticholinergic burden; therefore, further research is necessary to develop a useful and comprehensive tool identifying medications with anticholinergic properties.
Disclosure
The authors report that they have no conflicts of interest in this work.
References
- 1.Ageing and health. World Health Organization. Available from: https://www.who.int/en/news-room/fact-sheets/detail/ageing-and-health Accessed January13, 2019.
- 2.Prasad S, Sung B, Aggarwal BB. Age-associated chronic diseases require age-old medicine: role of chronic inflammation. Prev Med. 2012;54(Suppl):S29–S37. doi: 10.1016/j.ypmed.2011.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Page RL 2nd, Linnebur SA, Bryant LL, Ruscin JM. Inappropriate prescribing in the hospitalized elderly patient: defining the problem, evaluation tools, and possible solutions. Clin Interv Aging. 2010;5:75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naples JG, Marcum ZA, Perera S, et al. Concordance between anticholinergic burden scales. J Am Geriatr Soc. 2015;63(10):2012–2014. doi: 10.1111/jgs.2015.63.issue-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fox C, Richardson K, Maidment ID, et al. Anticholinergic medication use and cognitive impairment in the older population: the medical research council cognitive function and ageing study. J Am Geriatr Soc. 2011;59(8):1477–1483. doi: 10.1111/j.1532-5415.2011.03491.x [DOI] [PubMed] [Google Scholar]
- 6.Felton M, Hanlon JT, Perera S, Thorpe JM, Marcum ZA. Racial differences in anticholinergic use among community-dwelling elders. Consult Pharm. 2015;30(4):240–245. doi: 10.4140/TCP.n.2015.240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gray SL, Hanlon JT. Anticholinergic medication use and dementia: latest evidence and clinical implications. Ther Adv Drug Saf. 2016;7(5):217–224. doi: 10.1177/2042098616658399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durán CE, Azermai M, Vander Stichele RH. Systematic review of anticholinergic risk scales in older adults. Eur J Clin Pharmacol. 2013;69(7):1485–1496. doi: 10.1007/s00228-013-1499-3 [DOI] [PubMed] [Google Scholar]
- 9.Kumpula EK, Bell JS, Soini H, Pitkälä KH. Anticholinergic drug use and mortality among residents of long-term care facilities: a prospective cohort study. J Clin Pharmacol. 2011;51(2):256–263. doi: 10.1177/0091270010368410 [DOI] [PubMed] [Google Scholar]
- 10.The American Geriatrics Society 2015 Beers Criteria Updated Expert Panel. American geriatrics society 2015 updated beers critiera for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227–2246. doi: 10.1111/jgs.13702 [DOI] [PubMed] [Google Scholar]
- 11.Salbu RL, Feuer J. A closer look at the 2015 Beers critiera. J Pharm Pract. 2017;30(4):419–424. doi: 10.1177/0897190016663072 [DOI] [PubMed] [Google Scholar]
- 12.Boustani M, Campbell N, Munger S, Maidment I, Fox C. Impact of anticholinergics on the aging brain: a review and pratical application. Aging Health. 2008;4(3):311–320. doi: 10.2217/1745509X.4.3.311 [DOI] [Google Scholar]
- 13.Rudolph JL, Salow MJ, Angelini MC, McGlinchey RE. The anticholinergic risk scale and anticholinergic adverse effects in older persons. Arch Intern Med. 2008;168(5):508–513. doi: 10.1001/archinternmed.2007.106 [DOI] [PubMed] [Google Scholar]
- 14.Chew ML, Mulsant BH, Pollock BG, et al. Anticholinergic activity of 107 medications commonly used by older adults. J Am Geriatr Soc. 2008;56(7):1333–1341. doi: 10.1111/j.1532-5415.2008.01737.x [DOI] [PubMed] [Google Scholar]
- 15.Carnahan RM, Lund BC, Perry PJ, Pollock BG, Culp KR. The anticholinergic drug scale as a measure of drug-related anticholinergic burden: associations with serum anticholinergic activity. J Clin Pharmacol. 2006;46(12):1481–1486. doi: 10.1177/0091270006292126 [DOI] [PubMed] [Google Scholar]
- 16.Ehrt U, Broich K, Larsen JP, Ballard C, Aarsland D. Use of drugs with anticholinergic effect and impact on cognition in parkinson’s disease: a cohort study. J Neurol Neurosurg Psychiatry. 2010;81(2):160–165. doi: 10.1136/jnnp.2009.186239 [DOI] [PubMed] [Google Scholar]
- 17.Villalba-Moreno AM, Alfaro-Lara ER, Pérez-Guerrero MC, Nieto-Martín MD, Santos-Ramos B. Systematic review on the use of anticholinergic scales in poly pathological patients. Arch Gerontol Geriatr. 2016;62:1–8. doi: 10.1016/j.archger.2015.10.002 [DOI] [PubMed] [Google Scholar]
- 18.Zhang X, Zhou S, Pan K, et al. Potentially inappropriate medications in hospitalized older patients: a cross-sectional study using the Beers 2015 criteria versus the 2012 criteria. Clin Interv Aging. 2017;12:1697–1703. doi: 10.2147/CIA.S146009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Momin TG, Pandya RN, Rana DA, Patel VJ. Use of potentially inappropriate medications in hospitalized elderly at a teaching hospital: a comparison between Beers 2003 and 2012 criteria. Indian J Pharmacol. 2013;45(6):603–607. doi: 10.4103/0253-7613.121372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baldoni Ade O, Ayres LR, Martinez EZ, Dewulf Nde L, Dos Santos V, Pereira LR. Factors associated with potentially inappropriate medications use by the elderly according to Beers criteria 2003 and 2012. Int J Clin Pharm. 2014;36(2):316–324. doi: 10.1007/s11096-013-9880-y [DOI] [PubMed] [Google Scholar]
- 21.Pasina L, Djade CD, Lucca U, et al. Association of anticholinergic burden with cognitive and functional status in a cohort of hospitalized elderly: comparison of the anticholinergic cognitive burden scale and anticholinergic risk scale: results from the REPOSI study. Drugs Aging. 2013;30(2):103–112. doi: 10.1007/s40266-012-0044-x [DOI] [PubMed] [Google Scholar]
- 22.Salahudeen MS, Duffull SB, Nishtala PS. Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: a systematic review. BMC Geriatr. 2015;15:31. doi: 10.1186/s12877-015-0029-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fick DM, Cooper JW, Wade WE, Waller JL, Maclean JR, Beers MH. Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts. Arch Intern Med. 2003;163(22):2716–2724. doi: 10.1001/archinte.163.22.2716 [DOI] [PubMed] [Google Scholar]
- 24.Campanelli CM. American geriatrics society updated Beers criteria for potentially inappropriate medication use in older adults: the American Geriatrics Society 2012 Beers criteria update expert panel. J Am Geriatr Soc. 2012;60(4):616–631. doi: 10.1111/j.1532-5415.2012.03923.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sittironnarit G, Ames D, Bush AI, et al. Effects of anticholinergic drugs on cognitive function in older Australians: results from the AIBL study. Dement Geriatr Cogn Disord. 2011;31(3):173–178. doi: 10.1159/000325171 [DOI] [PubMed] [Google Scholar]
- 26.Han L, Agostini JV, Allore HG. Cumulative anticholinergic exposure is associated with poor memory and executive function in older men. J Am Geriatr Soc. 2008;56(12):2203–2210. doi: 10.1111/j.1532-5415.2008.02009.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ancelin ML, Artero S, Portet F, Dupuy AM, Touchon J, Ritchie K. Non-degenerative mild cognitive impairment in elderly people and use of anticholinergic drugs: longitudinal cohort study. Bmj. 2006;332(7539):455–459. doi: 10.1136/bmj.38740.439664.DE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trevethan R. Sensitivity, specificity, and predictive values: foundations, pliabilities, and pitfalls in research and practice. Front Public Health. 2017;5:307 eCollection 2017. doi: 10.3389/fpubh.2017.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mo L, Yang X, He J, Dong B. Evaluation of potentially inappropriate medications in older inpatients in China. J Am Geriatr Soc. 2014;62(11):2216–2218. doi: 10.1111/jgs.13053 [DOI] [PubMed] [Google Scholar]
- 30.Li H, Pu S, Liu Q, et al. Potentially inappropriate medications in Chinese older adults: the beers criteria compared with the screening tool of older persons’ prescriptions criteria. Geriatr Gerontol Int. 2017;17(11):1951–1958. doi: 10.1111/ggi.12999 [DOI] [PubMed] [Google Scholar]
- 31.Jirón M, Pate V, Hanson LC, Lund JL, Jonsson Funk M, Stürmer T. Trends in prevalence and determinants of potentially inappropriate prescribing in the United States: 2007 to 2012. J Am Geriatr Soc. 2016;64(4):788–797. doi: 10.1111/jgs.14077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gustafsson M, Sjölander M, Pfister B, Schneede J, Lövheim H. Effects of pharmacists’ interventions on inappropriate drug use and drug-related readmissions in People with Dementia – a secondary analysis of a randomized controlled trial. Pharm (Basel). 2018;6(1):E7. doi: 10.3390/pharmacy6010007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gillespie U, Alassaad A, Hammarlund-Udenaes M, et al. Effects of pharmacists’ interventions on appropriateness of prescribing and evaluation of the instruments’ (MAI, STOPP and STARTs’) ability to predict hospitalization – analyses from a randomized controlled trial. PLoS One. 2013;8(5):e62401. doi: 10.1371/journal.pone.0062401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akobeng AK. Understanding diagnostic tests 1: sensitivity, specificity and predictive values. Acta Paediatr. 2007;96(3):338–341. doi: 10.1111/j.1651-2227.2006.00180.x [DOI] [PubMed] [Google Scholar]
- 35.Sumukadas D, McMurdo ME, Mangoni AA, Guthrie B. Temporal trends in anticholinergic medication prescription in older people: repeated cross-sectional analysis of population prescribing data. Age Ageing. 2014;43(4):515–521. doi: 10.1093/ageing/aft199 [DOI] [PubMed] [Google Scholar]
- 36.Hanlon JT, Schmader KE. The medication appropriateness index at 20: where it started, where it has been, and where it may be going. Drugs Aging. 2013;30(11):893–900. doi: 10.1007/s40266-013-0118-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Ageing and health. World Health Organization. Available from: https://www.who.int/en/news-room/fact-sheets/detail/ageing-and-health Accessed January13, 2019.