Abstract
Background
Hyperleukocytic acute myeloid leukemia (AML) (initial white blood cell count≥100 × 109/L) is a clinical emergency often accompanied by leukostasis syndrome, tumor lysis syndrome (TLS), and disseminated intravascular coagulation (DIC), with a poor clinical prognosis. The aim of this study retrospectively analyzed the clinical features of hyperleukocytic AML, focusing on high-risk factors affecting prognosis, the selection of initial induction therapy, and the impact of hematopoietic stem cell transplantation (HSCT) on prognosis.
Patients and methods
A total of 558 AML patients at our center from January 2013 to December 2017 were diagnosed, and 52 (9.32%) patients presented with hyperleukocytosis were retrospectively reviewed.
Results
The 3-year overall survival (OS) rate in the 15–39 years old and 40–60 years old group was 58.8% and 25.4%, respectively; the longest survival time in patients aged >60 years was only 8 months, and the 8-month OS rate was 8.3% (p=0.002). The 3-year OS rate of the patients in the favorable risk group, intermediate risk group and high risk group, according to the 2017 ELN risk stratification, was 50%, 28.0%, and 29.5%, respectively (p=0.374). The 3-year OS rate of patients carrying CEBPA or NPM1 mutation and those with FLT3-ITD or MLL mutation was 37.5% and 30.0%, respectively (p=0.63). The 3-year OS rate of patients employing an induction regimen of a standard IA regimen was 58.4%, and of those employing a non-standard IA regimen was 22.2% (p=0.065). The 3-year OS rate of the transplantation patients reached 73.8%, while the 9-month OS rate of patients without transplantation was 11.4% (p<0.001).
Conclusion
This study suggest that hyperleukocytosis is an independent risk factor for AML patients, regardless of the risk stratification based on cytogenetic or molecular abnormalities. Age is the main factor influencing the prognosis of hyperleukocytic AML. The use of a standard IA regimen and HSCT can significantly improve the patient’s prognosis.
Keywords: acute myeloid leukemia, hyperleukocytosis, ELN risk stratification, induction chemotherapy, hematopoietic stem cell transplantation
Introduction
Hyperleukocytic acute myeloid leukemia (AML) with white blood cell (WBC) count more than 100 × 109/L is a clinical emergency, often accompanied by leukostasis syndrome, tumor lysis syndrome (TLS), and disseminated intravascular coagulation (DIC), with a poor clinical prognosis.1 Despite many clinical studies on hyperleukocytic AML, the following clinical issues remain unresolved: 1) the existing European LeukemiaNet (ELN) risk stratification does not include hyperleukocytosis as a prognostic factor, and it is unclear whether a favorable genetic abnormality can improve the poor prognosis of hyperleukocytic AML; 2) whether anthracycline plus cytarabine (commonly referred to as “7+3” regimens) with a standard dose is the optimal initial induction therapy for patients with hyperleukocytic AML in actual clinical practice; and 3) whether hematopoietic stem cell transplantation can improve the poor prognosis and prolong the survival time of patients with hyperleukocytic AML. To solve the aforementioned issues, we retrospectively analyzed the clinical features of 52 cases of hyperleukocytic AML at our center from January 2013 to December 2017, focusing on high-risk factors affecting prognosis, the selection of initial induction therapy, and the impact of hematopoietic stem cell transplantation on prognosis.
Patients And Methods
Patient Eligibility
Patients with newly diagnosed AML at our center from January 2013 to December 2017 were retrospectively studied. The screening criteria were 14 years or older, and patients with acute promyelocytic leukemia were excluded. Hyperleukocytosis was defined as a white blood cell (WBC) count of more than 100 × 109/L. The classification criteria for AML were based on standards developed by the French-American-British (FAB; 1976) and the World Health Organization (WHO; 2016),2 and the prognostic stratification was derived from the 2017 ELN recommendations on diagnosis and management of AML in adults.3 This study was reviewed and approved by the research ethics committee at Anhui Provincial Hospital according to the Declaration of Helsinki. Due to the retrospective nature of the study, informed consent was waived by the Ethics Committee.
Definitions Of TLS, DIC, And Leukostasis
The diagnosis of TLS refers to the Cairo-Bishop standard:4 laboratory TLS (LTLS) is defined as the presence of two of the following abnormal laboratory indicators appearing within 3 days to 1 week: uric acid >8.0 mg/L (475.8 mmol/L), serum potassium >6.0 mmol/L, blood phosphorus >4.6 mg/dL (1.75 mmol/L), or calcium ion <1.12 mg/dL (0.3 mmol/L). Clinical TLS (CTLS) is diagnosed as symptomatic hypocalcemia or as LTLS with one or more of the following abnormalities: (1) serum creatinine 1.5 times higher than the normal upper limit; (2) convulsion; (3) arrhythmia; and (4) sudden death.
The diagnosis of DIC is based on the International Society of Thrombosis and Hemostasis (ISTH) score.5,6
The diagnosis of leukostasis relies on the patient’s clinical symptoms,1,7 such as the respiratory system manifesting dyspnea, hypoxemia, diffuse alveolar hemorrhage, and respiratory failure; the central nervous system manifesting confusion, lethargy, dizziness, headache, delirium, coma, and localized nerve function impairment symptoms; manifestations involving the eyes, including impaired vision and retinal hemorrhage; and hearing system manifestations such as tinnitus; and circulatory system manifestations such as myocardial ischemia/infarction, limb ischemia, and renal vein thrombosis. When the patient demonstrated one or more of the above symptoms, which could not be explained by other diseases, leukostasis was diagnosed.
Transplantation Procedures
After reached complete remission (CR), the patient was administered a high-dose cytarabine (HDAC, 2.0~3.0 g/m2 every 12 h) for 2 courses as consolidation and then received hematopoietic stem cell transplantation. Patients with relapsed and refractory AML were also offered hematopoietic stem cell transplantation when a HLA-matched sibling donor (MSD) was available. If the patient did not have HLA-matched siblings, or if there was not sufficient time to wait an unrelated donor due to leukemia progression, unrelated umbilical cord blood transplantation (CBT) was scheduled. All patients in the allogeneic transplant cohort received an intensified myeloablative conditioning regimen, which included a BUCY2 (busulfan and cyclophosphamide) or TBICY (total body irradiation and cyclophosphamide)-based conditioning, and cyclosporine and mycophenolate mofetil were used for graft-versus-host disease (GVHD) prophylaxis as previously described.8
Statistical Analyses
Definitions of neutrophil and platelet engraftment, acute GVHD (aGVHD), chronic GVHD (cGVHD), TRM (Transplant-related mortality), relapse, OS (overall survival), and LFS (leukemia-free survival) were defined according to previously published criteria.9–11 Patient-, disease-, and transplant-related variables were measured using χ2 test (categorical variables) or Mann–Whitney U-test (continuous variables). The probabilities of neutrophil and platelet engraftment, aGVHD, cGVHD, TRM, and relapse were generated by the cumulative-incidence function method, taking into account competing risks. The probabilities of OS and LFS were generated by the Kaplan-Meier method. R statistical software was used for statistical analysis (R Foundation for Statistical Computing). Differences were considered statistically significant at p< 0.05.
Results
Patient Characteristics
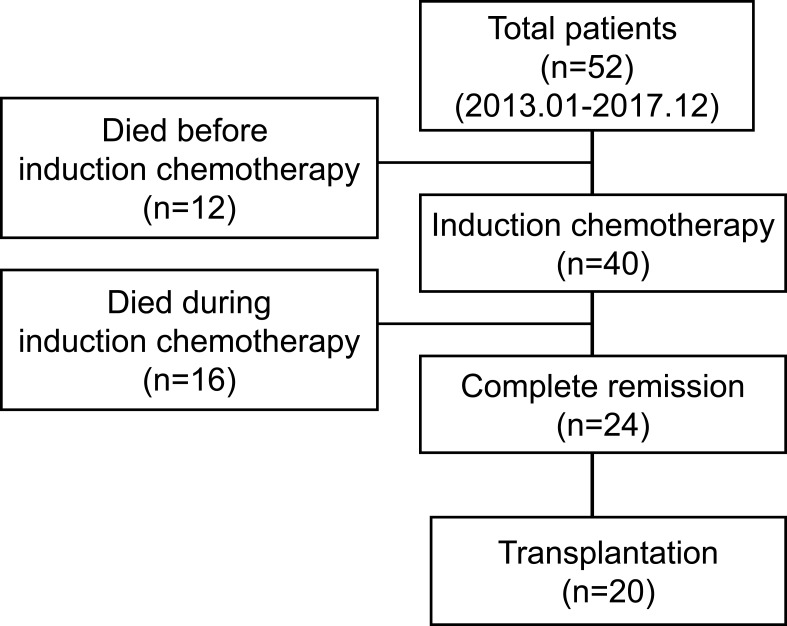
A total of 558 AML patients at our center from January 2013 to December 2017 were diagnosed, and 52 (9.32%) patients presented with hyperleukocytosis were retrospectively reviewed (Figure 1). The baseline patient related characteristics were showed in Table 1. The average age of the patients was 46 years (range: 16–77 years) and included 17 patients (32.7%) who were 15–39 years old, 23 patients (44.2%) who were 40–60 years old, and 12 patients (23.1%) who were older than 60 years. The median white blood cell (WBC) count at the first visit was 172.3×109 (range: 100–477.3×109), the platelet count was 44.7×109 (range: 5-125×109), and the initial LDH level was 934U/L (range: 285-1802U/L).
Figure 1.
Flow diagram of the study.
Table 1.
Clinical Characteristics
| Characteristics | n=52 |
|---|---|
| Age (years), median (range) | 46 (16–77) |
| Age group, n (%) | |
| 15–39 | 17 (32.7) |
| 40–60 | 23 (44.2) |
| >60 | 12 (23.1) |
| Sex: M/F, n (%) | 24/28 (46.2/53.8) |
| WBC at first diagnosis(×109/L), median (range) | 172.3 (100.0–477.3) |
| PLT at first diagnosis(×109/L), median (range) | 44.7 (5–125) |
| LDH level at first diagnosis, median (range) | 934 (285–1802) |
| CD56 positive expression, n (%) | 10/34 (29.4) |
| FAB-classification, n (%) | |
| M5 | 28 (53.8) |
| M4 | 8 (15.4) |
| M2 | 12 (23.1) |
| M1 | 4 (7.7) |
| Molecular biology, n (%) | |
| NPM1 mutation | 4 (7.7) |
| FLT3-ITD mutation | 6 (11.5) |
| FLT3 mutation MPM1 mutation | 4 (7.7) |
| MLL arrangements | 3 (5.8) |
| CEBPA mutation | 4 (7.7) |
| Others | 4 (7.7) |
| Negative detection | 27 (51.9) |
| ELN risk assessment, n (%) | |
| Favorable-risk | 8 (15.4) |
| Intermediate-risk | 25 (48.1) |
| High-risk | 19 (36.5) |
| Tumor lysis syndrome (TLS), n (%) | 7 (13.5) |
| Leukostasis, n (%) | 26 (50.0) |
| DIC, n (%) | 9 (17.3) |
| Severe infection at first diagnosis, n (%) | 31 (59.6) |
| Pre-induction treatment before confirmed diagnosis, n (%) | |
| Hydroxyurea | 26 (50.0) |
| Hydroxyurea+ cytarabine+ etoposide | 12 (23.1) |
| Hydroxyurea+ cytarabine | 8 (15.4) |
| Cytarabine+ etoposide | 3 (5.8) |
| Hydroxyurea+ daunorubicin | 3 (5.8) |
| Intensive induction chemotherapy (n) | 40 |
| IDA 10~12mg/m2+ cytarabine 100mg/m2, n (%) | 22 (55.0) |
| IDA 8mg/m2+ cytarabine 100mg/m2 or other regimens, n (%) | 18 (45.0) |
| CNS events (infarction or bleeding) before or during induction, n (%) | 11 (21.2) |
| Death before induction, n (%) | 12 (23.1) |
| Death during induction, n (%) | 16 (30.8) |
| First CR (CR1), n | 24 |
| One course induction to reach CR1, n (%) | 15 (62.5) |
| More than one course induction to reach CR1, n (%) | 9 (37.5) |
| Transplant, n | 20 (38.5) |
| Time from diagnosis to transplant (months), median (range) | 7 (5–12) |
Abbreviations: WBC, white blood cell; PLT, platelet; LDH, lactate dehydrogenase; DIC, disseminated intravascular coagulation; IDA, idarubicin; CNS, central nervous system; CR, complete remission.
Molecular Biological Characteristics
According to the FAB classification, 28 patients (53.80%) were M5 subtype, 8 (15.4%) were M4 subtype, 12 (23.1%) were M2 subtype, and 4 (7.7%) were M1 subtype. Four patients (7.7%) carried an NPM1 mutation, 6 (11.5%) carried a FLT3-ITD mutation, 4(7.7%) carried a FLT3 and NPM1 double mutation, and 3 (5.8%) had MLL rearrangement. Four patients (7.7%) carried a CEBPA mutation, 4 (7.7%) carried other mutation types. Twenty-seven (51.90%) had no identified gene mutations. According to the 2017 ELN risk stratification, 8 patients (15.4%) were in the favorable risk group, 25 (48.1%) were in the intermediate risk group, and 19 (36.5%) were in the adverse risk group (Table 1).
Treatment Regimens
Seven patients (13.5%) had TLS on admission, 26 (50%) had leukostasis, and 9 (17.3%) had DIC. Thirty-one patients (59.1%) had severe infections at the initial visit. All patients received cytoreduction treatment to lower the tumor burden and reduce the risk of TLS before proper induction chemotherapy, among whom 26 (50.0%) received single-agent hydroxyurea (1.0~2.0g/d×3~5days), 12 (23.1%) received combined regimens of hydroxyurea (1.0~2.0g/d×3~5days), cytarabine (100mg/d×1~3 days) and etoposide (100mg/d×1~3 days), 8 (15.4%) received hydroxyurea (1.0~2.0g/d×3~5days) and cytarabine (100mg/d×1~3 days), 3 (5.8%) received cytarabine (100mg/d×1~3 days) and etoposide (100mg/d ×1~3 days), and 3 (5.8%) received hydroxyurea (1.0~2.0g/d ×3~5days) and low-dose daunorubicin (20mg/d ×1~3 days) (Table 1). Leukapheresis was not the standard of care in our institution and was not performed for these patients, even in patients with hyper-viscosity syndrome.
A total of 40 patients received induction chemotherapy, of whom 22 (55%) received an induction regimen of Idarubicin (IDA) 10–12 mg/m2.d (3 days) in combination with cytarabine 100~150 mg/m2.d (7 days) (standard IA regimen), and the remaining 18 (45%) used IDA 8 mg/m2.d (3 days) combined with cytarabine 100~150 mg/m2.d (7 days) or other induction protocols (non-standard IA regimen). Eleven patients (21.2%) developed central nervous system events before or during induction chemotherapy, including cerebral infarction (n=5) and cerebral hemorrhage (n=6). Twenty-four patients achieved CR, of whom 15 (62.5%) achieved CR after one induction course and 9 (37.5%) achieved CR after 2 or more courses (Figure 1, Table 1).
Transplant Characteristics
Twenty patients underwent hematopoietic stem cell transplantation, with a median time from diagnosis to transplantation of 7 months (5–12 months) and a median age of 32 years (range: 16–53 years). Thirteen transplant patients (65.0%) belonged to the favorable-risk and intermediate-risk group at the initial diagnosis, and 7 (35.0%) were high-risk patients. Fifteen patients were in first CR (CR1) when received transplant, and 3 patients were in CR2 or more (≥CR2), and 2 patients underwent transplantation after relapse without remission. Fifteen patients (75.0%) who had no HLA-matched donor underwent unrelated cord blood transplantation (UCBT), 4 patients (20.0%) underwent allogeneic HLA-matched peripheral blood stem cell transplantation (allo-PBSCT), and 1 patient (5.0%) underwent autologous PBSCT. Twelve patients employed a BUCY2-based myeloablative conditioning regimen, and 8 received a TBICY-based myeloablative conditioning regimen. Seventeen patients (85.0%) used the CSA+MMF regimen to prevent GVHD, and 2 patients (10%) received the CSA+MMF+MTX regimen (Table 2).
Table 2.
Transplant Characteristics
| Characteristics | Transplantation |
|---|---|
| Total, n | 20 |
| Age at transplantation (years): median (range) | 32 (16–53) |
| Sex: M/F, n (%) | 11/9 |
| ELN risk at first diagnosis, n (%) | |
| High risk | 7 (35.0) |
| Standard or intermediate risk | 13 (65.0) |
| Disease stage in transplant, n (%) | |
| CR1 | 15 (75.0) |
| ≥CR2 | 3 (15.0) |
| No remission after relapse | 2 (10.0) |
| Graft sources, n (%) | |
| Cord blood | 15 (75.0) |
| PBSC from matched sibling donor | 4 (20.0) |
| Autologous PBSC | 1 (5.0) |
| Myeloablative conditioning regimen, n (%) | |
| BUCY2-based conditioning | 12 (60.0) |
| TBICY-based conditioning | 8 (40.0) |
| GVHD Prophylaxis, n (%) | |
| CSA + MMF | 17 (85.0) |
| CSA + MMF+ MTX | 2 (10.0) |
| Total nucleated-cell dose, median (range) (×107/kg) | |
| Cord blood | 2.9 (2.1–3.7) |
| PBSC from matched sibling donor | 68.2 (45.6–93.1) |
| Total CD34+ cell dose, median (range) (×105/kg) | |
| Cord blood | 2.2 (0.83–4.6) |
| PBSC from matched sibling donor | 52.8 (22.6–88.5) |
| Neutrophil engraftment(days), median (range) | |
| Cord blood | 19.7 (16–25) |
| PBSC from matched sibling donor | 11.8 (11–14) |
| Platelet engraftment(days), median (range) | |
| Cord blood | 40.7 (19–65) |
| PBSC from matched sibling donor | 13.3 (12–15) |
| Grade Ⅱ-Ⅳacute GVHD, n | |
| Cord blood | 2 |
| PBSC from matched sibling donor | 0 |
| Chronic GVHD, n | |
| Cord blood | 3 |
| PBSC from matched sibling donor | 1 |
| Follow-up among survivors, (months), median (range) | 38.7 (15–70) |
Abbreviations: CR, complete remission; PBSC, peripheral blood stem cell; BUCY, busulfan and cyclophosphamide; TBICY, total body irradiation and cyclophosphamide GVHD, graft-versus-host disease CSA, cyclosporine; MMF, mycophenolate mofetil; MTX, methotrexate.
Survival
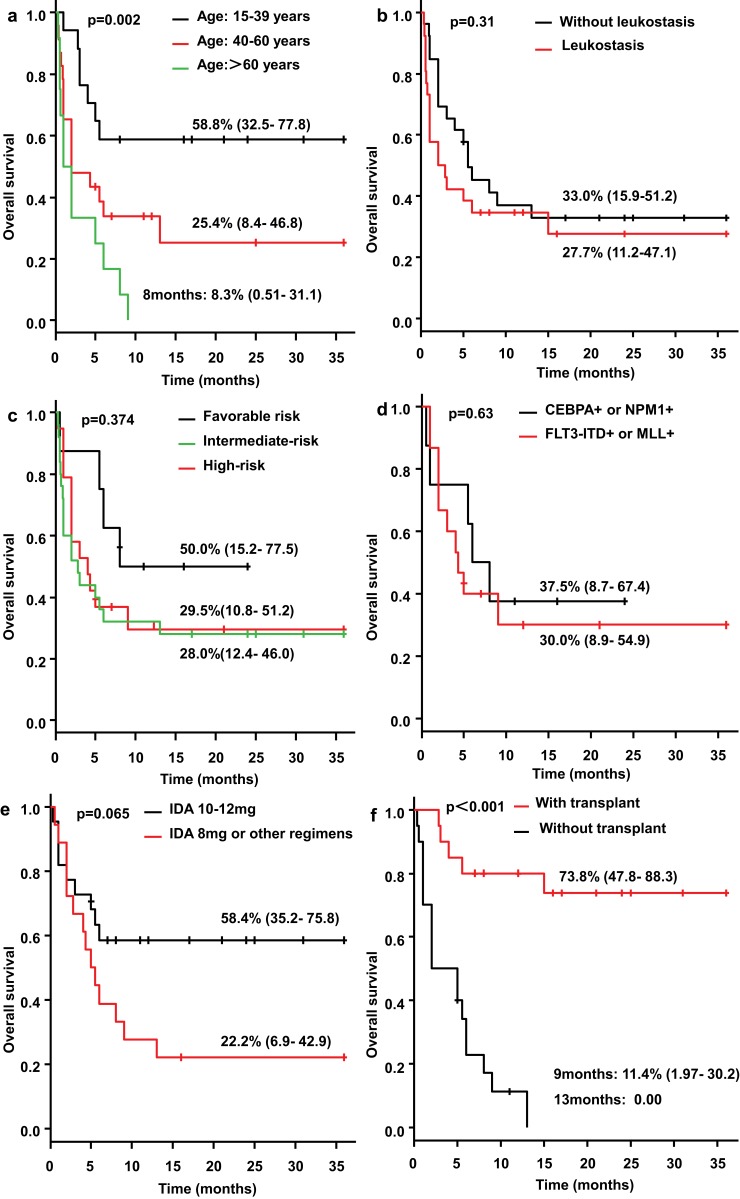
The 3-year overall survival (OS) rate in the 15–39 years old and 40–60 years old group was 58.8% (95% CI: 32.5–77.8%) and 25.4% (95% CI: 8.4–46.8%), respectively (Figure 2A). The longest survival time in patients aged >60 years was only 8 months, and the 8-month OS rate was 8.3%. The difference between these three groups was statistically significant (p=0.002) (Figure 2A).The 3-year OS rate of patients with and without leukostasis was 27.7% (95% CI: 11.2–47.1) and 33.0% (95% CI: 15.9–51.2%), respectively (p=0.31) (Figure 2B). The 3-year OS rate of the patients in the favorable risk group, intermediate risk group and high risk group, according to the 2017 ELN stratification, was 50% (95% CI: 15.2–77.5%), 28.0% (95% CI: 12.4–46.0%), and 29.5%(95% CI:10.8–51.2%), respectively (p=0.374) (Figure 2C). The 3-year OS rate of patients carrying CEBPA or NPM1 mutation and those with FLT3-ITD or MLL mutation was 37.5% (95% CI: 8.7–67.4) and 30.0% (95% CI: 8.9–54.9%), respectively (p=0.63) (Figure 2D). The 3-year OS rate of patients employing an induction regimen of a standard IA regimen was 58.4% (95% CI: 35.2–75.8%), and of those employing a non-standard IA regimen was 22.2% (95% CI: 6.9–42.9%) (Figure 2E); although the difference between the two groups was not statistically significant (p=0.065), patients employing induction chemotherapy with a standard IA regimen demonstrated a trend toward a longer survival time.
Figure 2.
Overall survival. The 3-year overall survival (OS) rate in the 15–39 years old and 40–60 years old group was 58.8% (95% CI: 32.5–77.8%) and 25.4% (95% CI: 8.4–46.8%), respectively; and the longest survival time in patients aged >60 years was only 8 months, and the 8-month OS rate was 8.3% (p=0.002) (A).The 3-year OS rate of patients with and without leukostasis was 27.7% (95% CI: 11.2–47.1) and 33.0% (95% CI: 15.9–51.2%), respectively (p=0.31) (B). The 3-year OS rate of the patients in the favorable risk group, intermediate risk group and high risk group was 50% (95% CI: 15.2–77.5%), 28.0% (95% CI: 12.4–46.0%), and 29.5%(95% CI:10.8–51.2%), respectively (p=0.374) (C). The 3-year OS rate of patients carrying CEBPA or NPM1 mutation and those with FLT3-ITD or MLL mutation was 37.5% (95% CI: 8.7–67.4) and 30.0% (95% CI: 8.9–54.9%), respectively (p=0.63) (D). The 3-year OS rate of patients employing an induction regimen of a standard IA regimen was 58.4% (95% CI: 35.2–75.8%), and of those employing a non-standard IA regimen was 22.2% (95% CI: 6.9–42.9%) (p=0.065) (E). The 3-year OS rate of the transplantation patients reached 73.8% (95% CI: 47.8–88.3%), while the 9-month OS rate of patients without transplantation was 11.4% (95% CI: 1.97–30.2%) (p<0.001) (F).
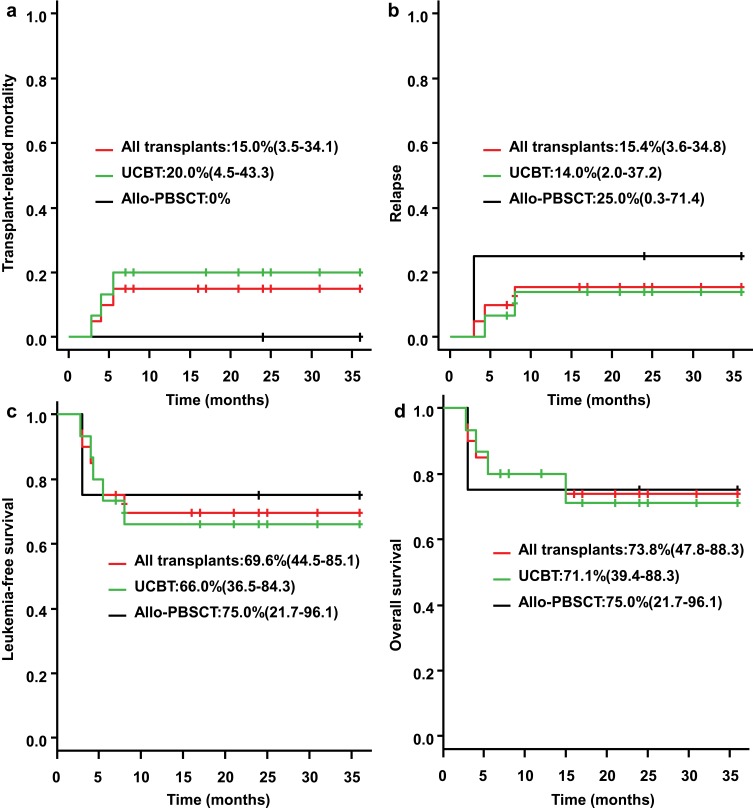
The 3-year OS rate of the transplantation patients reached 73.8% (95% CI: 47.8–88.3%), while the 9-month OS rate of patients without transplantation was 11.4% (95% CI: 1.97–30.2%), and all patients without transplantation died after 13 months (p<0.001) (Figure 2F), suggesting that hematopoietic stem cell transplantation can significantly improve the prognosis of patients. The median survival time after transplantation was 38.7 months (range: 15–70 months). The 3-year transplant-related mortality (TRM) rate was 15% (95% CI: 3.5–34.1%) in all transplant patients, and the 3-year TRM in UCBT patients was 20% (95% CI: 4.5–43.3%) (Figure 3A). Allo-PBSCT patients showed no transplant-related death. The 3-year relapse rate for transplant patients was 15.4% (95% CI: 3.6–34.8%), of whom the 3-year relapse rate for UCBT patients was 14.0% (95% CI: 2.0–37.2%), and the 3-year relapse rate for allo-PBSCT patients was 25% (95% CI: 0.3–71.4%) (Figure 3B). The 3-year leukemia-free survival (LFS) rate for all transplant patients was 69.6% (95% CI: 44.5–85.1%), including 66% for UCBT patients (95% CI: 36.5–84.3%) and 75% for allo-PBSCT patients (95% CI: 21.7–96.1%)(Figure 3C).
Figure 3.
TRM, relapse and survival of patients in transplant. The 3-year transplant-related mortality (TRM) rate was 15% (95% CI: 3.5–34.1%) in all transplant patients, and the 3-year TRM in UCBT patients was 20% (95% CI: 4.5–43.3%), and allo-PBSCT patients showed no transplant-related death (A). The 3-year relapse rate for transplant patients was 15.4% (95% CI: 3.6–34.8%), of whom the 3-year relapse rate for UCBT patients was 14.0% (95% CI: 2.0–37.2%), and the 3-year relapse rate for allo-PBSCT patients was 25% (95% CI: 0.3–71.4%) (B). The 3-year leukemia-free survival (LFS) rate for all transplant patients was 69.6% (95% CI: 44.5–85.1%), including 66% for UCBT patients (95% CI: 36.5–84.3%) and 75% for allo-PBSCT patients (95% CI: 21.7–96.1%) (C). The 3-year OS rate for UCBT patients was 71.1% (95% CI: 39.4–88.3%) and for allo-PBSCT patients was 75.0% (95% CI: 21.7–96.1%) (D).
The 3-year OS rate for UCBT patients was 71% (95% CI: 39.4–88.3%) and for allo-PBSCT patients was 75% (95% CI: 21.7–96.1%)(Figure 3D).
Discussion
Several reports have indicated that patients with hyperleukocytic AML are often associated with adverse cytogenetic or molecular abnormality which is an independent risk factor for disease recurrence and decreased long-term survival.12–16 However, it is unclear whether patients with hyperleukocytic AML who have favorable prognostic factors according to the 2017 ELN risk stratification would have a relatively good prognosis. In this study, we found that there were no significant differences of the 3-year OS rates among the favorable risk group, intermediate risk group and high risk group patients (p=0.374), and the OS time was similar between patients with CEBPA+/NPM1+ mutations and patients with FLT3-ITD+/MLL+ mutations (37.5% vs 30.0%) (p=0.63). These results suggest that patients with a high WBC count are associated with a worse prognosis, regardless of the risk stratification based on cytogenetic or molecular abnormalities. In clinical practice, hyperleukocytosis should be considered an independent risk factor for a poor prognosis in patients with AML.
This study showed that for patients with hyperleukocytic AML, age is an important prognostic factor affecting disease remission and survival. The 3-year OS rates for patients aged 15–39 years and 40–60 years were 58.8% and 25.4%, respectively, and the longest survival time for patients aged >60 years was only 8 months. Adverse molecular or cytogenetic abnormalities such as complex karyotypes, MLL gene rearrangements, and FLT3-ITD mutations are associated with poor prognosis and occur more frequently with increasing age.13,17 On the other hand, elderly AML patients may progress from myelodysplastic syndrome (MDS), and have a poor performance status or poor organ functions and cannot tolerate chemotherapy at a standard dosage.18–20 The above factors are probably the main causes of worse outcomes for elderly patients with AML. Moreover, in this study, we found that patients who received induction chemotherapy with the standard IA regimen (IDA 10–12 mg/m2.d with 3 days in combination with cytarabine 100~150 mg/m2.d with 7 days) had a higher CR rate and long-term survival rate than those with non-standard IA regimens (p=0.065). The lack of a statistically significant difference may be caused by the small sample size assessed in this study. Our previous clinical studies have also shown that AML patients who receive induction chemotherapy with a standard dose IA regimen achieve superior outcomes than those with non-standard dose IA regimens, with no significant difference between the two groups in red blood cell infusion, duration of neutropenia, and occurrence of severe infections.21
Our study findings suggest that hematopoietic stem cell transplantation can significantly improve the clinical outcomes of these patients. Tien et al12 studied patients with hyperleukocytic AML who achieved CR1, and they found that patients who underwent allo-HSCT demonstrated significantly better OS and DFS than those who did not undergo transplantation or who had a relapse after consolidation treatment. For AML patients complicated with hyperleukocytosis, regardless of their prognosis stratification according to ELN risk stratification, a more aggressive treatment regimen is necessary; for example, HSCT should be a readily available option after the patient achieves CR1. A retrospective study of 1275 AML patients who underwent HSCT reported by EBMT22 comparing the prognosis of hyperleukocytic AML patients based on donor types found that the incidence of relapse was both increased in hyperleukocytosis patients with matched sibling donors (MSDs) and matched unrelated donors (MUDs); and extensive chronic GVHD and GRFS were increased in patients with MSDs whereas it was not significantly different among patients transplanted from MUDs. In the present study, the TRM, relapse rate, LFS and OS time were not statistically significant between patients who underwent allo-PBSCT and UCBT in our center. Our previous clinical study8 has also shown that for AML patients, unrelated CBT is associated with a similar incidence of severe aGVHD and TRM but less cGVHD (especially extensive cGVHD) and a lower risk of relapse, which translates into better GRFS compared with HLA-matched sibling allo-PBSCT/BMT.
Conclusion
The results of this study suggest that hyperleukocytosis is an independent risk factor for AML patients, regardless of the risk stratification based on cytogenetic or molecular abnormalities. Age is the main factor influencing the prognosis of hyperleukocytic AML. The use of a standard IA regimen and HSCT can significantly improve the patient’s prognosis. However, this study has some limitations, such as a retrospective study and a relatively small number of patients, especially those receiving HSCT. In addition, the impact of mutant genes on prognosis was limited to a few common genes, leading to a lack of new cytogenetic or molecular abnormalities data. So the prospective randomized clinical trials are needed to confirm the results of this study.
Acknowledgments
This work was partly supported by the Fundamental Research Funds for the Central Universities of China (No. WK9110000003).
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Röllig C, Ehninger G. How I treat hyperleukocytosis in acute myeloid leukemia. Blood. 2015;125:3246–3252. doi: 10.1182/blood-2014-10-551507 [DOI] [PubMed] [Google Scholar]
- 2.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405. doi: 10.1182/blood-2016-03-643544 [DOI] [PubMed] [Google Scholar]
- 3.Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cairo MS, Bishop M. Tumour lysis syndrome: new therapeutic strategies and classification. Br J Haematol. 2004;127:3–11. doi: 10.1111/bjh.2004.127.issue-1 [DOI] [PubMed] [Google Scholar]
- 5.Taylor FB Jr, Toh CH, Hoots WK, Wada H, Levi M. Scientific subcommittee on Disseminated Intravascular Coagulation (DIC) of the International Society on Thrombosis and Haemostasis (ISTH). Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86:1327–1330. doi: 10.1055/s-0037-1616068 [DOI] [PubMed] [Google Scholar]
- 6.Toh CH, Hoots WK; SSC on Disseminated Intravascular Coagulation of the ISTH. The scoring system of the Scientific and Standardisation Committee on Disseminated Intravascular Coagulation of the International Society on Thrombosis and Haemostasis: a 5-year overview. J Thromb Haemost. 2007;5:604–606. doi: 10.1111/jth.2007.5.issue-3 [DOI] [PubMed] [Google Scholar]
- 7.Giammarco S, Chiusolo P, Piccirillo N, et al. Hyperleukocytosis and leukostasis: management of a medical emergency. Expert Rev Hematol. 2017;10:147–154. doi: 10.1080/17474086.2017.1270754 [DOI] [PubMed] [Google Scholar]
- 8.Zheng CC, Zhu XY, Tang BL, et al. Clinical separation of cGvHD and GvL and better GvHD-free/relapse-free survival (GRFS) after unrelated cord blood transplantation for AML. Bone Marrow Transplant. 2017;52:88–94. doi: 10.1038/bmt.2016.182 [DOI] [PubMed] [Google Scholar]
- 9.Iacobelli S; EBMT Statistical Committee. Suggestions on the use of statistical methodologies in studies of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2013;48:S1–37. doi: 10.1038/bmt.2012.282 [DOI] [PubMed] [Google Scholar]
- 10.Dignan FL, Clark A, Amrolia P, et al.; Haemato-oncology Task Force of British Committee for Standards in Haematology; British Society for Blood and Marrow Transplantation. Diagnosis and management of acute graft-versus-host disease. Br J Haematol. 2012;158:30–45. doi: 10.1111/bjh.2012.158.issue-1 [DOI] [PubMed] [Google Scholar]
- 11.Dignan FL, Amrolia P, Clark A, et al.; Haemato-oncology Task Force of British Committee for Standards in Haematology; British Society for Blood and Marrow Transplantation. Diagnosis and management of chronic graft-versus-host disease. Br J Haematol. 2012;158:46–61. doi: 10.1111/bjh.2012.158.issue-1 [DOI] [PubMed] [Google Scholar]
- 12.Tien FM, Hou HA, Tsai CH, et al. Hyperleukocytosis is associated with distinct genetic alterations and is an independent poor-risk factor in de novo acute myeloid leukemia patients. Eur J Haematol. 2018;101:86–94. doi: 10.1111/ejh.13073 [DOI] [PubMed] [Google Scholar]
- 13.Tsai CH, Hou HA, Tang JL, et al. Genetic alterations and their clinical implications in older patients with acute myeloid leukemia. Leukemia. 2016;30:1485–1492. doi: 10.1038/leu.2016.65 [DOI] [PubMed] [Google Scholar]
- 14.How J, Sykes J, Gupta V, et al. Influence of FLT3-internal tandem duplication allele burden and white blood cell count on the outcome in patients with intermediate-risk karyotype acute myeloid leukemia. Cancer. 2012;118:6110–6117. doi: 10.1002/cncr.27683 [DOI] [PubMed] [Google Scholar]
- 15.de Jonge HJ, Valk PJ, de Bont ES, et al. Prognostic impact of white blood cell count in intermediate risk acute myeloid leukemia: relevance of mutated NPM1 and FLT3-ITD. Haematologica. 2011;96:1310–1317. doi: 10.3324/haematol.2011.040592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fröhling S, Schlenk RF, Breitruck J, et al.; AML Study Group Ulm. Acute myeloid leukemia. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Ulm. Blood. 2002;100:4372–4380. doi: 10.1182/blood-2002-05-1440 [DOI] [PubMed] [Google Scholar]
- 17.Heiblig M, Labussière-Wallet H, Nicolini FE, et al. Prognostic value of genetic alterations in elderly patients with acute myeloid leukemia: a single institution experience. Cancers (Basel). 2019;11:570. doi: 10.3390/cancers11040570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mueller BU, Seipel K, Pabst T. Myelodysplastic syndromes and acute myeloid leukemias in the elderly. Eur J Intern Med. 2018;58:28–32. doi: 10.1016/j.ejim.2018.05.026 [DOI] [PubMed] [Google Scholar]
- 19.Rao AV. Fitness in the elderly: how to make decisions regarding acute myeloid leukemia induction. Hematology Am Soc Hematol Educ Program. 2016;2016:339–347. doi: 10.1182/asheducation-2016.1.339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Webster JA, Pratz KW. Acute myeloid leukemia in the elderly: therapeutic options and choice. Leuk Lymphoma. 2018;59:274–287. doi: 10.1080/10428194.2017.1330956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou L, Liu X, Liu H, et al. A comparative study of idarubicin 12 mg/m(2) and 8 mg/m(2) combined with cytarabine as the first induction regimen for adult acute myeloid leukemia patients. Onco Targets Ther. 2016;9:985–991. doi: 10.2147/OTT.S96176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Canaani J, Labopin M, Socié G, et al. Long term impact of hyperleukocytosis in newly diagnosed acute myeloid leukemia patients undergoing allogeneic stem cell transplantation: an analysis from the acute leukemia working party of the EBMT. Am J Hematol. 2017;92:653–659. doi: 10.1002/ajh.24737 [DOI] [PubMed] [Google Scholar]