Abstract
Background
Bronchopulmonary dysplasia (BPD) is the most frequent chronic lung disease in infancy and is associated with neonatal comorbidity and impairment in pulmonary and neurodevelopmental (ND) long‐term outcome.
Methods
This was a retrospective, single‐center, cohort study to compare a cohort of very preterm infants (gestational age [GA], 24+0–28+6 weeks) with BPD (n = 44), with a cohort of GA‐matched preterm infants without BPD (n = 44) with regard to neonatal morbidity, incidence of lower respiratory tract infection (LRTI), ND outcome and growth to 2 years' corrected age (CA) and preschool age.
Results
Bronchopulmonary dysplasia (incidence, 11.3%) was associated with a higher rate of neonatal pneumonia (26% vs 7%, P = 0.001), longer total duration of mechanical ventilation (mean days, 21 vs 13, P < 0.001), and a higher rate of pulmonary hypertension (20.5% vs 0%, P = 0.002) and of severe retinopathy of prematurity (13.6% vs 0%, P = 0.026). Incidence of LRTI was significantly higher in the BPD infants (50% vs 26%, P = 0.025). ND outcome did not differ between the two groups. Growth at neonatal intensive care unit discharge was similar. In the BPD cohort, rate of weight < 10th percentile was higher at 2 years' CA (52% vs 30%, P = 0.041) and rate of head circumference < 10th percentile was higher at preschool age (59% vs 27%, P = 0.028).
Conclusion
Neonatal respiratory morbidity was significantly higher in the BPD cohort, but long‐term ND outcome did not differ. Infants with BPD had poorer growth.
Keywords: bronchopulmonary dysplasia, growth, morbidity, neurodevelopment, preterm infant
Bronchopulmonary dysplasia (BPD) is the most frequent chronic lung disease (CLD) in infancy. Rate and severity are influenced by a number of variables such as grade of immaturity, underlying pathological conditions, genetic predisposition, perinatal management or BPD definition. Originally described and named by Northway et al.1 in 1967 in relatively mature preterm infants as severe chronic pulmonary disease (“old” or “classic” BPD), the clinical picture has changed over time. It is now a pulmonary disease occurring mainly in very or extremely low gestational age neonates (ELGAN) with severity‐based diagnostic criteria assessed at a postmenstrual age (PMA) of 36 weeks.2, 3 The most commonly used BPD definition in practice comes from Shennan et al.,4 who proposed that the requirement of supplemental oxygen (sO2) at 36 weeks' PMA was the best predictor of abnormal pulmonary outcomes at 2 years of age in very low‐birthweight infants (VLBWI). While the classic BPD was attributed to aggressive mechanical ventilation (MV) and high fraction of inspiratory oxygen, the new BPD in the era of surfactant and gentle ventilation is instead considered a consequence of disrupted lung development.5 Nevertheless, the pathophysiology of BPD is still complex, and its rate has not changed much in the last two decades, despite advances in perinatal management in countries with high‐quality neonatal intensive care units (NICU), with estimates persisting at approximately 40% in preterm infants between 22 and 29 weeks of gestation.6 In addition, BPD may still have a significant impact on mortality and short‐ and long‐term morbidity. The primary aim of the present study was to follow a cohort of preterm infants between 24+0 and 28+6 weeks' gestation with BPD from birth to preschool age, including neurodevelopmental (ND) outcome, in comparison with a gestational age (GA)‐matched cohort of preterm infants with no BPD. Parameters of secondary outcomes were incidence of hospitalization due to lower respiratory tract infection (LRTI), and the analysis of infant growth profile. We hypothesized that patients with BPD would have poorer outcomes in addition to a higher rate of neonatal comorbidities.
Methods
Inclusion and exclusion criteria
Patients between 24+0 and 28+6 weeks' GA, admitted to the present NICU between 1 January 2000 and 31 December 2011 with a diagnosis of BPD and surviving > 36 weeks' GA were recruited from the NICU electronic database. For post‐discharge follow up the medical hospital reports and data from the outpatient clinic on ND follow up were reviewed up to preschool age. For group comparisons we recruited GA‐matched (1:1) preterm infants, born in the same year, designated as having no BPD in the medical reports. According to our guidelines for the management of ELGAN at the limits of viability, provisional care was not provided for infants < 24 weeks' GA. The only exclusion criterion was multiple congenital malformations.
Definition of BPD
During the study period patients were generally coded as having BPD if they were still on sO2 at 36 weeks' PMA. For the purpose of this study we also implemented workshop‐based definition criteria published in 2001 for preterm infants < 32 weeks' GA,2 and differentiated moderate from severe BPD. Moderate BPD was defined as requirement for sO2 < 30%, and severe BPD as requirement for sO2 ≥ 30% and/or positive pressure support or nasal continuous positive airway pressure at 36 weeks' PMA or at discharge to home, whichever came first. The physiologic definition of BPD7 was not used.
Neonatal comorbidities and therapeutic interventions
Patients were treated according to the unit policy (NICU‐policy) with standardized protocols for invasive and non‐invasive respiratory support. Post‐natal steroids were given i.v. for ≥7 days throughout the study period as a prophylaxis for BPD in infants who could not be weaned after 1 week of continuous MV. Neonatal sepsis and pneumonia were diagnosed based on a combination of clinical symptoms, laboratory results and chest X‐ray.8 In the case of positive Ureaplasma urealyticum (UU) culture results from tracheal aspirates, and chest X‐ray showing streaky–patchy interstitial lung changes, patients received erythromycin 50 mg/kg bodyweight three times per day for 10 days. Cerebral ultrasound (US) was routinely performed on days 1,3,5, and thereafter once per week in cases of pathological findings such as intraventricular/periventricular hemorrhage (IVH/PVH) and periventricular leukomalacia (PVL). IVH was diagnosed according to the criteria of Papile et al.9 Severe IVH was graded as IVH ≥ III, severe PVL as being cystic (cPVL ≥ II).10 Necrotizing enterocolitis (NEC) was diagnosed according to clinical and radiographic gastrointestinal signs.11 For NEC prophylaxis a multimodal approach started in the first 24 h of life consisting of early trophic feeding with human breast milk, and enteral gentamycin, nystatin, and Lactobacillus casei rhamnosus was routinely given during the whole study period in all VLBWI.12 Retinopathy of prematurity (ROP) was diagnosed using indirect ophthalmoscopy by an experienced ophthalmologist and classified according to the International Classification of Retinopathy of Prematurity (ICROP).13 Severe ROP was defined as ROP ≥ stage III and treated by laser therapy.14 Growth parameters such as weight, length and head circumference (HCF) were documented on fetal–infant growth charts for preterm infants in the NICU and on standard growth charts in infants after discharge from the NICU. For the purpose of this study we analyzed the incidence of weight, length and HCF < 10th percentile. Small for gestational age (SGA) was defined as weight < 10th percentile.
Follow up to preschool age
From the medical hospital charts and outpatient controls the frequency of hospitalization due to LRTI was analyzed. After discharge from the NICU patients at risk were routinely seen in the outpatient clinic for ND follow up by experienced neuropediatricians at the corrected age (CA) of 4, 8, 12, 18 and 24 months. Thereafter they were seen once per year up to preschool or school age. For the purpose of this study, ND was analyzed at CA of 2 years (time point [TP]1) and at preschool age (TP2). At TP1, Griffith's Developmental Scales (Griffiths Scales of Infant Development‐rev. 197015) were used until 2005, and from 2005 to 2011 the Bayley Scales of Infant Development were used.16 At TP2 neurocognitive outcome (NCO) was measured using the Wechsler Preschool and Primary Scale of Intelligence–Version III, and on neurological and clinical examination according to Amiel‐Tison and Stewart, and to Touwen.17, 18, 19 NCO was categorized as normal, mild–moderate, and severe.20 Evaluation of neurological abnormality included cerebral palsy (CP), visual impairment (VI), and hearing impairment (HI). This study was approved by the local ethics committee (29‐486 ex18/17).
Ethics
The study has been approved by the local ethics committee and was performed in accordance with the ethics standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Statistical analysis
Continuous data are presented as mean ± SD or median (IQR), as appropriate. Categorical data are given in absolute and relative numbers. The Mann–Whitney U‐test or t‐test was used to compare continuous data between matched patient groups, and the chi‐squared test or Fisher's exact test to compare categorical data between matched groups. Test for independent samples was chosen because matching was done for only one variable (GA). P < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS version 24 (IBM, Armonk, NY, USA).
Results
During the study period 469 infants with GA 24+0–28+6 weeks were admitted to the NICU (Fig. 1). There were 89 deaths at <36 weeks' PMA. Of the remaining 380 patients, 43 (11.3%) were diagnosed with moderate BPD. Only one patient (0.3%) developed severe BPD, an SGA preterm infant (GA, 28 weeks; birthweight, 530 g). She died during the NICU stay at a PMA of 40.6 weeks due to severe sepsis associated with multiorgan failure. Nevertheless because of late death she was included in the BPD cohort. Only two patients (4.5%) with moderate BPD were discharged with home sO2. A total of 44 survivors with no BPD were matched for GA and year of birth (Table 1). Perinatal clinical characteristics are listed in Table 1. Table 2 lists additional respiratory diagnoses, medical treatment strategies during the NICU stay, anthropometrics and PMA at discharge from the NICU. There was a high but similar incidence of early and late onset neonatal sepsis in the cohorts 54.5% is the incidence of early and late‐onset sepsis in the BPD‐cohort and 42% the corresponding rate in the non‐BPD cohort, whereas pneumonia, mainly of late onset, was significant higher in the BPD cohort (36% vs 7%). Interestingly, >50% were associated with UU respiratory tract colonization (RTC). Table 3 lists the prevalence of major neonatal morbidities. There was a higher incidence of severe ROP in the BPD cohort (13.6% vs 0.0%, P = 0.026). Hospitalization rate due to LRTI including obstructive bronchitis, bronchiolitis and pneumonia was significantly higher in the BPD cohort (50% vs 26%, P = 0.025). Table 4 lists the ND outcome including neurocognitive impairment (NCI) and neurologic abnormality, and growth parameters, at TP1 at 2 years' CA (mean, 24 months; range, 21–30 months of age), and at TP2 at preschool age (mean, 6 years; range, 5–7 years of age). More patients in the BPD cohort had severe NCI at TP1 (17.5% vs 5.1%) and TP2 (17.1% vs 5.4%), but these differences did not reach statistical significance. Neurologic abnormality varied between 8% and 11%, but was not significantly different between the groups. The BPD cohort did show a higher incidence of growth failure (<10th percentile), and the difference was significant for the weight at 2 years' CA (52% vs 30%, P = 0.041), and for HCF at preschool age (59% vs 27%, P = 0.028; Fig. 2).
Figure 1.
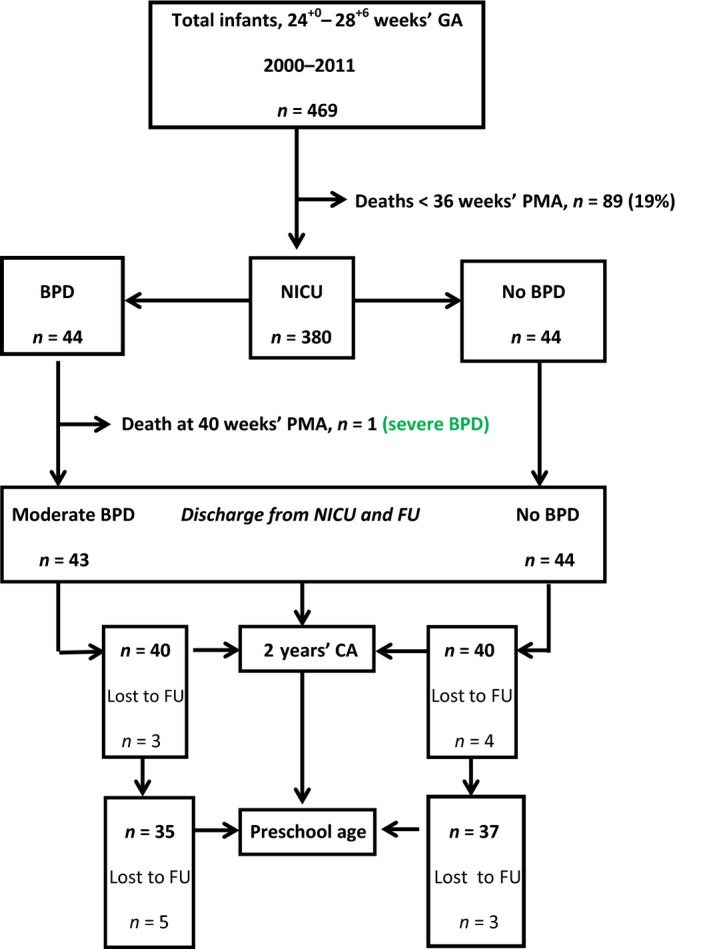
Flow chart of subject selection and follow up (FU). BPD, bronchopulmonary dysplasia; CA, corrected age; GA, gestational age; NICU, neonatal intensive care unit; PMA, post‐menstrual age.
Table 1.
Perinatal clinical characteristics
| Characteristics | BPD | No BPD | P‐value |
|---|---|---|---|
| (n = 44) | (n = 44) | ||
| n (%), mean ± SD or median (range) | n (%), mean ± SD or median (range) | ||
| Gestational age (weeks) | 25 (24–28) | 25 (24–28) | 0.754 |
| Birthweight (g) | 683 ± 213 | 836 ± 211 | 0.007 |
| Antenatal steroids | 39 (88.6) | 29 (72.5) | 0.060 |
| Cesarean section | 39 (88.6) | 37 (84.1) | 0.534 |
| SGA | 12 (27.3) | 5 (11.4) | 0.059 |
| Male | 26 (59.1) | 25 (56.8) | 0.829 |
| Apgar score at 5 min | 8 (4–10) | 8 (3–10) | 0.417 |
| Apgar score at 10 min | 9 (7–10) | 9 (5–10) | 0.140 |
| UA‐pH | 7.3 ± 0.1 | 7.3 ± 0.1 | 0.838 |
BPD, bronchopulmonary dysplasia; SGA, small for gestational age (birthweight < 10th percentile); UA‐ph, umbilical artery pH.
Table 2.
Neonatal characteristics at NICU discharge
| Outcome | BPD | No BPD | P‐value |
|---|---|---|---|
| n = 44 | n = 44 | ||
| n (%) or mean ± SD | n (%) or mean ± SD | ||
| RDS any stage | 37 (84.1) | 37 (84.1) | 1.000 |
| RDS stage III–IV | 24 (54.5) | 18 (40.9) | 0.200 |
| Surfactant therapy | 39 (88.6) | 31 (72.7) | 0.059 |
| PTX | 3 (6.9) | 2 (4.5) | >0.99 |
| Pneumonia | 16 (36) | 3 (7) | 0.001 |
| UU‐RTC | 9 | 2 | |
| Sepsis | 23 (54.5) | 18 (42) | 0.270 |
| Pulmonary hypertension | 9 (20.5) | 0 | 0.002 |
| iNO | 8 | – | |
| Sildenafil | 1 | – | |
| iMV (total days) | 21 ± 13.1 | 13.0 ± 9.8 | <0.01 |
| Rescue HFO | 12 (27.3) | 0 (0.0) | <0.01 |
| Postnatal steroids | 31 (70.5) | 10 (23.3) | <0.01 |
| Weight < 10th percentile† | 32 (76.2) | 32 (72.7) | 0.713 |
| Length < 10th percentile† | 31 (76) | 15 (69) | 0.544 |
| HCF < 10th percentile† | 25 (62.5) | 13 (48.6) | 0.402 |
| PMA (weeks) at discharge | 41.6 ± 7.0 | 36.8 ± 2.7 | 0.122 |
BPD, bronchopulmonary dysplasia; HCF, head circumference; HFO, high frequency oscillation; iMV, invasive Mechanical Ventilation; iNO, inhaled Nitric Oxide; NICU, neonatal intensive care unit; PMA, postmenstrual age; PTX, pneumothorax; RDS, respiratory distress syndrome; UU‐RTC, Ureaplasma urealyticum respiratory tract colonization.
Calculated from available data refers only to weigt, length and HCF.
Table 3.
Major neonatal comorbidities and medical treatment
| Major morbidity | BPD | No BPD | P‐value |
|---|---|---|---|
| Treatment | n = 44 | n = 44 | |
| n (%) | n (%) | ||
| IVH ≥ III/PVH | 5 (11.4) | 5 (11.4) | 1.000 |
| VP shunt | 2 (40) | 1 (20) | |
| cPVL ≥ grade II | 1 (2.3) | 2 (4.5) | 0.200 |
| ROP ≥ stage III | 6 (13.6) | 0 (0.0) | 0.026 |
| Laser therapy | 5 (83) | – | |
| Surgical NEC | 1 (2.3) | 1 (2.3) | 1.000 |
| PDA‐hs | 9 (20.5) | 6 (13.6) | 0.451 |
| PDA ligation | 1 (11.1) | 0 (0.0) |
BPD, bronchopulmonary dysplasia; cPVL, cystic periventricular leukomalacia; IVH, intraventricular hemorrhage; NEC, necrotizing enterocolitis; PDA‐hs, hemodynamically significant persistent ductus arteriosus; PVH, periventricular hemorrhage; ROP, retinopathy of prematurity; VP, ventriculoperitoneal.
Table 4.
Neurodevelopmental outcome at 2 years' CA (TP1) and preschool age (TP2)
| Outcome | Moderate BPD | No BPD | P‐value | ||
|---|---|---|---|---|---|
| TP1 | TP2 | TP1 | TP2 | TP1/TP2 | |
| n = 40 | n = 35 | n = 40 | n = 37 | ||
| n (%) | n (%) | n (%) | n (%) | ||
| Follow up (%) | 90.9 | 79.8 | 90.9 | 84.1 | |
| NCI | 0.241/0.350 | ||||
| None | 17 (42.5) | 15 (42.9) | 21 (53.8) | 19 (51.4) | |
| Mild–moderate | 16 (40.0) | 14 (40.0) | 16 (41.0) | 16 (43.2) | |
| Severe | 7 (17.5) | 6 (17.1) | 2 (5.1) | 2 (5.4) | |
| Neurologic abnormality | 4 (10.0) | 4 (11.4) | 4 (10.0) | 3 (8.1) | 1.000/0.707 |
| CP | 1 (2.5) | 1 (2.9) | 2 (5.0) | 2 (5.4) | |
| VI | 3 (7.5) | 3 (8.6) | 0 (0.0) | 0 (0.0) | |
| HI | 1 (2.5) | 1 (2.9) | 1 (2.5) | 1 (2.7) | |
BPD, bronchopulmonary dysplasia; CA, corrected age; CP, cerebral palsy; HI, hearing impairment; NCI, neurocognitive impairment; TP, time point; VI, visual impairment.
Figure 2.
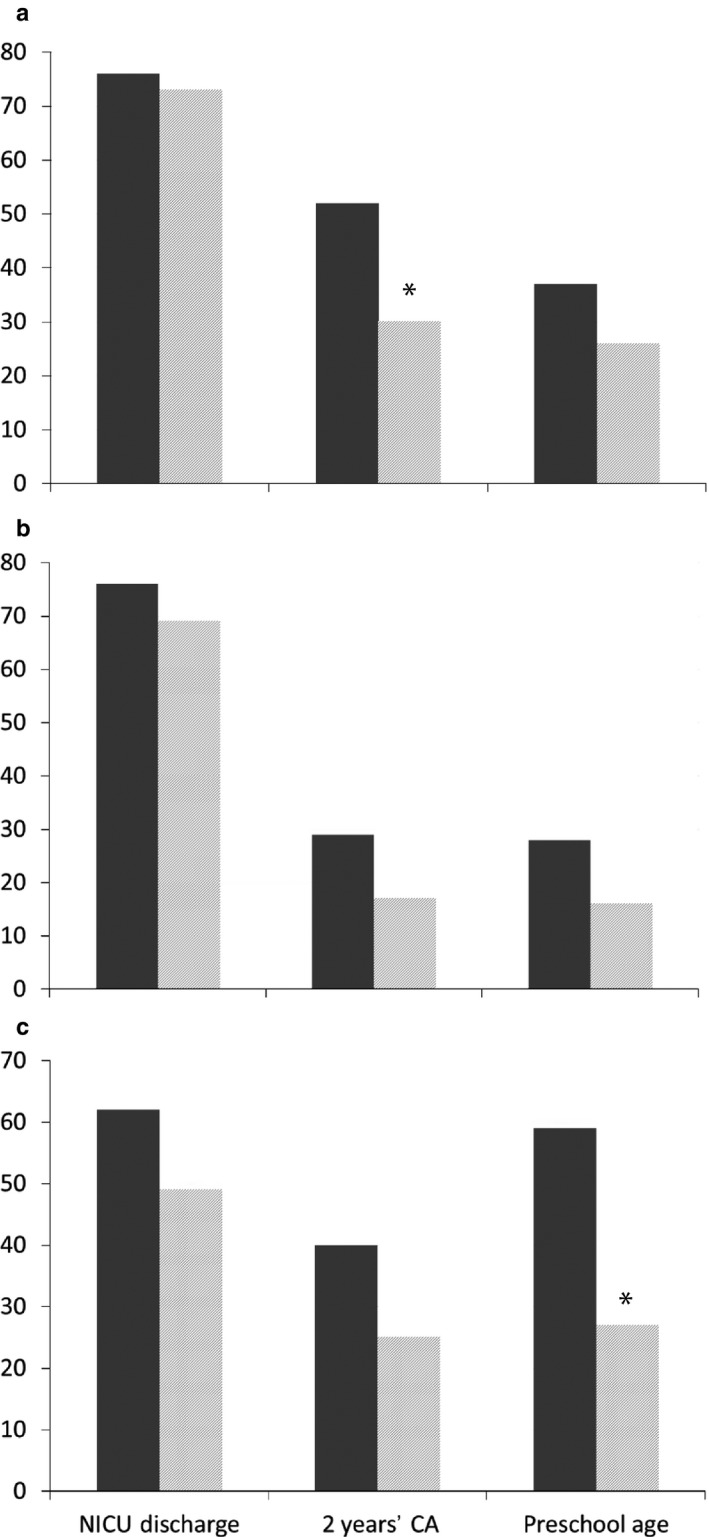
Percentage of patients with (a) weight < 10th percentile, (b) length < 10th percentile and (c) head circumference < 10th percentile in infants ( ) with and (
) with and ( ) without bronchopulmonary dysplasia at discharge from the neonatal intensive care unit (NICU), at 2 years' corrected age (CA) and at preschool age. *P < 0.05.
) without bronchopulmonary dysplasia at discharge from the neonatal intensive care unit (NICU), at 2 years' corrected age (CA) and at preschool age. *P < 0.05.
Discussion
The present clinical observational cohort study included preterm infants between 24+0 and 28+6 weeks' GA. Preterm infants with BPD had a significantly higher neonatal pulmonary morbidity. At discharge only severe ROP was significantly higher in the BPD group. Looking at long‐term clinical follow up, significantly more rehospitalizations due to LRTI were seen in that cohort. ND outcomes assessed at 2 years' CA and at preschool age did not differ significantly. Nevertheless the BPD cohort did show poorer growth during the observation period: weight < 10th percentile occurred significantly more often in the BPD group at 2 years' CA, and HCF < 10th percentile occurred significantly more often at preschool age.
BPD incidence and definition
The incidence of BPD varies widely in the literature, which may be explained by differences in local practices, prenatal and postnatal clinical risk factors, and the definition, but it mainly depends on the degree of immaturity.21, 22, 23 In the present study, the prevalence of moderate and severe BPD in surviving infants > 36 weeks' GA was low (we included only patients ≥ 24 weeks' GA). In a national prospective, observational study from Norway the prevalence of moderate BPD was 25.2%, but that study also included infants from 22 to 23 weeks' GA.24 It is well known that there is strong inverse relationship between BPD severity and GA. In a recent paper by Jobe and Steinhorn the authors articulate inadequacies in definition and classification for BPD, partly due to identifying infants only at a single point in time, and changing respiratory care practices.3 For a population‐based study like the present one, the use of a definition of BPD as requirement for sO2 at 36 weeks' PMA may be sufficient.
Neonatal respiratory morbidity, medical treatment
Bronchopulmonary dysplasia is mainly characterized by persistent respiratory symptoms requiring long‐term respiratory support and is also frequently associated with clinically relevant comorbidities. Despite similar GA, incidence, and severity of surfactant‐treated respiratory distress syndrome, infants with BPD had more persistent respiratory morbidity, and subsequently an increased need for respiratory support in the NICU. Although SGA is a known risk factor for BPD and other adverse clinical outcomes,25 we do not think that this was an important aspect in the present study, because there was no significant weight difference between the groups at discharge. To our knowledge there are few studies on the incidence of neonatal pneumonia in BPD. In a recent paper the incidence of pneumonia in a cohort of BPD patients with a mean GA of 25 weeks was 27%,26 which was similar to the present BPD cohort. The reported incidence of RTC with Ureaplasma species in VLBWI ranges from 20% to 45%.27 The role of Ureaplasma in the development of BPD remains controversial in the literature. Nevertheless, several meta‐analyses, reports, and a single‐center case–control study from the present NICU observed a significant association between Ureaplasma RTC and BPD, defined either as requirement for sO2 at 28 days or at 36 weeks' PMA.27, 28, 29
Major neonatal morbidities
We found a significant higher rate of severe ROP in the present BPD cohort (13.6% vs 0%). Other morbidities were similar. This was surprising, because one would expect a higher number of comorbidities in the BPD group. Nevertheless, the present ROP incidence was in agreement with the literature. In a study of infants < 27 weeks' gestation with BPD, severe ROP was significantly higher in the BPD cohort.30
Lower respiratory tract infection
We found a significantly higher incidence of LRTI in the BPD cohort (50% vs 26%). These findings parallel other reports in the literature, showing an increased short‐ and long‐term respiratory morbidity in BPD patients.31, 32
ND outcome assessments
We did not find significant differences between the two cohorts at TP1 and TP2. This may be partly explained by the fact that there were no patients with severe BPD in the long‐term follow up. Furthermore, no patients had a combined morbidity count of BPD, severe ROP and brain injury (severe IVH/cPVL). That combination normally is predictive for late neurosensory impairment in Extremely Low Birthweight (ELBW) infants.33 There are a number of long‐term studies in the literature in preterm infants with BPD at different ages reporting conflicting results in regard to the assumption that BPD may be an independent risk factor for poor ND outcome. In a retrospective cohort study of infants between 22 and 27 weeks' GA, 50% of patients had an Neurodevelopmental Impairment (NDI). But there was no significant difference between those patients with no–mild BPD versus moderate–severe BPD.23 In contrast, in a study by Anderson and Doyle, in survivors with BPD (defined as the need for sO2 at 28 days of life) the CP rate was 15% compared with 3%–4% in patients without BPD.34 In an updated publication by the same authors, children with BPD also higher rates of cognitive, educational and behavioral problems.35 Differences in reported ND outcomes may be due to a number of reasons such as the selected patient population, BPD definition and severity, incidence and severity of major comorbidities, treatment protocols, methods and time points of ND assessment, socioeconomic status, or the observation period.23, 25, 26 There are some ND follow‐up studies for BPD patients longer than 2 years for CA. In a long‐term outcome study up to 3 years' CA, ND disability, particularly cognitive impairment, was significantly more frequent in children with BPD compared with no BPD.37 In a longitudinal follow‐up study to 8 years in preterm infants with BPD, Short et al.38 reported poorer Neurocognitive outcome (NC) outcome in children with severe BPD compared with mild or moderate BPD, using a severity‐based classification system for BPD definition.
Growth assessment follow up
At discharge there were no differences in growth assessment. With increasing age, differences appeared. Whereas there were absolutely no significant differences in length growth (Fig. 2b), differences appeared in weight and HCF. Overall the incidence of growth failure diminished with increasing age. At TP1 the incidence of weight < 10th percentile was significantly higher in the BPD patients. Interestingly, this difference was not present at TP2 (Fig. 2a). Differences in HCF appeared later, only at TP2 (Fig. 2c). The prevalence of HCF < 10th percentile was significantly higher in the BPD group. In a study by Natarajan et al.,30 at the follow‐up visit at 18–22 months' CA, a significantly higher proportion of infants with BPD had weight and HCF < 10th percentile. In the study by Lodha et al.37 evaluating growth as a secondary outcome, children with BPD and with chronic oxygen dependence were significantly more likely to have weight and length below the 5th percentile, but the proportion of HCF below the 5th percentile was not significantly different. These studies and the present report highlight the importance of nutritional support and longitudinal follow up of growth in ELGAN, and particularly in BPD patients. They might have deficits in growth due to higher energy consumption or the more frequent use of postnatal steroids.
Limitations
This was a retrospective single‐center study with a small sample size due to a low BPD incidence. It may be representative only of a selected population of inborn preterm infants between 24+0 and 28+6 weeks' GA with moderate BPD. Matching on birthweight in addition to GA was not possible due to the limited sample size. Not all patients returned to follow up, but the follow‐up rate of 80–90% (Table 4) up to preschool age may be considered as very satisfactory and similar to other studies.23, 30, 38
Strengths
The present study had an appropriate control group without BPD comparable for several perinatal, medical variables. We used the same standardized NICU treatment protocols during the study period and report on longitudinal follow up of clinical outcomes to preschool age, with assessment of ND outcome and growth.
In conclusion, neonatal respiratory morbidity was significantly higher in the BPD cohort, but long‐term ND outcome did not differ significantly in infants with moderate BPD compared with a control group without BPD. Neonates with BPD had poorer growth charts with significantly smaller HCF at preschool age.
Disclosure
The authors declare no conflict of interest.
Author contributions
F.R. conceptualized the manuscript and wrote the original draft; A.S. performed the data collection; U.MF. analyzed the follow‐up data; B.R. assisted in writing and editing the manuscript; A.A. performed the statistical analysis; B.U. critically reviewed the manuscript. All authors read and approved the final manuscript.
References
- 1. Northway WH Jr, Rosan RC, Porter DY. Pulmonary disease following respiratory therapy of hyaline‐membrane disease: Bronchopulmonary dysplasia. N. Engl. J. Med. 1967; 267: 357–68. [DOI] [PubMed] [Google Scholar]
- 2. Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am. J. Resp. Crit. Care Med. 2001; 163: 1723–9. [DOI] [PubMed] [Google Scholar]
- 3. Jobe AH, Steinhorn R. Can we define bronchopulmonary dysplasia ? J. Pediatr. 2017; 188: 19–33. [DOI] [PubMed] [Google Scholar]
- 4. Shennan AT, Dunn MS, Ohlsson A et al Abnormal pulmonary outcomes in premature infants: Prediction from oxygen requirement in the neonatal period. Pediatrics 1988; 82: 527–32. [PubMed] [Google Scholar]
- 5. Jobe AH. The new bronchopulmonary dysplasia. Curr. Opin. Pediatr. 2011; 23: 167–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stoll BJ, Hansen NI, Bell EF et al Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993‐2012. JAMA 2015; 314: 1039–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Walsh MC, Wilson‐Costello D, Zadell A et al Safety, reliability, and validity of a physiologic definition of bronchopulmonary dysplasia. J. Perinatol. 2003; 23: 451–6. [DOI] [PubMed] [Google Scholar]
- 8. Reiterer F. Neonatal pneumonia In: Resch B. (ed). Neonatal Bacterial Infection. INTECH, Rijeka, Croatia, 2013; 19–32. [Google Scholar]
- 9. Papile L, Burstein J, Burstein R et al Incidence and evolution of subependymal and intraventricular hemorrhage in premature infants: A study of infants < 1500 gms. J. Pediatr. 1978; 92: 529–34. [DOI] [PubMed] [Google Scholar]
- 10. De Vries LS, Eken P, Dubowitz MS et al The spectrum of leukomalacia using cranial ultrasound. Behav. Brain Res. 1992; 49: 1–6. [DOI] [PubMed] [Google Scholar]
- 11. Walsh MC, Kiegman RM. Necrotizing enterocolitis: Treatment based on staging criteria. Pediatr. Clin. North Am. 1986; 33: 179–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schmoelzer G, Urlesberger B, Kuttnig‐Haim M et al Multimodal approach to prophylaxis of necrotizing enterocolitis: Clinical report and review of the literature. Pediatr. Surg. Int. 2006; 22: 573–80. [DOI] [PubMed] [Google Scholar]
- 13. Committee for the Classification of Retinopathy of Prematurity . An international classification of retinopathy of prematurity. Arch. Ophthalmol. 1982; 103: 1130–4. [DOI] [PubMed] [Google Scholar]
- 14. Early Treatment For Retinopathy of Prematurity Cooperative Group . Revised indications for the treatment of retinopathy of prematurity: Results of the early treatment for retinopathy of prematurity randomized trial. Arch. Ophthalmol. 2003; 121: 1684–94. [DOI] [PubMed] [Google Scholar]
- 15. Griffith A. The Abilities of Babies. University of London Press, London, UK, 1970. [Google Scholar]
- 16. Bayley N. Bayley Scales of Infant and Toddler Development®, 3rd edn Harcourt Assessment, San Antonio, TX, 2006. [Google Scholar]
- 17. Wechsler D. WPPSI‐III. Wechsler Preschool and Primary Scale of Intelligence – III. Psychological Corporation, San Antonio, TX, 2002. [Google Scholar]
- 18. Amiel‐Tison C, Stewart A. Follow up studies in the first five years of life: A pervasive assessment of neurological function. Arch. Dis. Child. 1989; 64: 496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Touwen BC. Neurological Development in Infancy. William Heineman Medical Books, London, UK, 1976. [Google Scholar]
- 20. Marlow N, Wolke D, Bracewell MA et al Neurologic and developmental disability at six years of age after extremely preterm birth. N. Engl. J. Med. 2005; 352: 9–19. [DOI] [PubMed] [Google Scholar]
- 21. Hines D, Modi N, Isayama T et al Scoping review shows wide variation in the definitions of bronchopulmonary dysplasia in preterm infants and calls for a consensus. Acta Paediatr. 2017; 106: 366–74. [DOI] [PubMed] [Google Scholar]
- 22. Gortner L, Misselwitz B, Milligan D et al Rates of bronchopulmonary dysplasia in very preterm infants in Europe: Results from the MOSAIC Cohort. Neonatology 2011; 99: 112–7. [DOI] [PubMed] [Google Scholar]
- 23. Trittman JK, Nelin LD, Klebanoff MA. Bronchopulmonary dysplasia and neurodevelopmental outcome in extremely preterm neonates. Eur. J. Pediatr. 2013; 172: 1173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Farstadt T, Bratlid D, Medbe S et al Bronchopulmonary dysplasia: Prevalence, severity and predictive factors in a national cohort of extremely preterm infants. Acta Paediatr. 2011; 100: 55–8. [DOI] [PubMed] [Google Scholar]
- 25. Nobile S, Marchionni P, Carnielli VP. Neonatal outcome of small for gestational age preterm infants. Eur. J. Pediatr. 2017; 176: 1083–8. [DOI] [PubMed] [Google Scholar]
- 26. Wagner B, Sontag M, Harris J et al Airway microbial community turnover differs by BPD severity in ventilated preterm infants. PLoS ONE 2017; 12: e0170120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang EL, Ohlsson A, Kellner JD. Association of Ureaplasma urealyticum colonization with chronic lung disease of prematurity: Results of a metaanalysis. J. Pediatr. 1995; 127: 640–4. [DOI] [PubMed] [Google Scholar]
- 28. Schelonka RL, Katz B, Waites KB et al Critical appraisal of the role of Ureaplasma in the development of bronchopulmonary dysplasia with metaanalytic techniques. Pediatr. Infect. Dis. J. 2005; 24: 1033–9. [DOI] [PubMed] [Google Scholar]
- 29. Resch B, Gutmann C, Reiterer F et al Neonatal Ureaplasma urealyticum colonization increases pulmonary and cerebral morbidity despite treatment with macrolide antibiotics. Infection 2015; 44: 323–7. [DOI] [PubMed] [Google Scholar]
- 30. Natarajan G, Pappas A, Shankaran S et al Outcomes of extremely low birthweight infants with bronchopulmonary dysplasia: Impact on the physiologic definition. Early Hum. Dev. 2012; 88: 509–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rodriguez‐Martinez CE, Acuna‐Coredeor R, Sossa‐Briceno MP. Predictors of prolonged length of hospital stay or readmissions for acute viral infections among infants with a history of bronchopulmonary dysplasia. J. Med. Virol. 2018; 90: 405–11. [DOI] [PubMed] [Google Scholar]
- 32. Durlak W, Klimek M, Kwinta P. Regional lung ventilation pattern in preschool children with bronchopulmonary dysplasia is modified by bronchial response. Pediatr. Pulmonol. 2017; 52: 353–9. [DOI] [PubMed] [Google Scholar]
- 33. Schmidt B, Asztalos EV, Roberts RS et al Trial of Indomethacin Prophylaxis in Preterms (TIPP) Investigators: Impact of bronchopulmonary dysplasia, brain injury and severe retinopathy on the outcome at 18 months: Results from the trial of indomethacin prophylaxis in preterms. JAMA 2003; 289: 1124–9. [DOI] [PubMed] [Google Scholar]
- 34. Anderson PJ, Doyle LW. Neurodevelopmental outcome of bronchopulmonary dysplasia. Semin. Perinatol. 2006; 30: 227–32. [DOI] [PubMed] [Google Scholar]
- 35. Doyle LW, Anderson PJ. Long‐term outcomes of bronchopulmonary dysplasia. Semin. Perinatol. 2009; 14: 391–5. [DOI] [PubMed] [Google Scholar]
- 36. Van Marter LJ, Kuban KC, Allred E et al Does bronchopulmonary dysplasia contribute to the occurrence of cerebral palsy among infants born before 28 weeks of gestation? Arch. Dis. Child. Fetal Neonatal Ed. 2011; 96: F20–9. [DOI] [PubMed] [Google Scholar]
- 37. Lodha A, Sauve R, Bhandari V et al Need for supplemental oxygen at discharge in infants with bronchopulmonary dysplasia is not associated with worse neurodevelopmental outcome at 3 years corrected age. PLoS ONE 2014; 9: e90843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Short EJ, Kirchner HI, Assad GR et al Developmental sequelae in preterm infants having a diagnosis of bronchopulmonary dysplasia: Analysis using a severity‐based classification system. Arch. Pediatr. Adolesc. Med. 2007; 161: 1082–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


