Abstract
Changes in the complexity of planktonic food webs may be expected in future aquatic systems due to increases in sea surface temperature and an enhanced stratification of the water column. Under these conditions, the growth of unpalatable, filamentous, N2‐fixing cyanobacterial blooms, and their effect on planktonic food webs will become increasingly important. The planktonic food web structure in aquatic ecosystems at times of filamentous cyanobacterial blooms is currently unresolved, with discordant lines of evidence suggesting that herbivores dominate the mesozooplankton or that mesozooplankton organisms are mainly carnivorous. Here, we use a set of proxies derived from amino acid nitrogen stable isotopes from two mesozooplankton size fractions to identify changes in the nitrogen source and the planktonic food web structure across different microplankton communities. A transition from herbivory to carnivory in mesozooplankton between more eutrophic, near‐coastal sites and more oligotrophic, offshore sites was accompanied by an increasing diversity of microplankton communities with aging filamentous cyanobacterial blooms. Our analyses of 124 biotic and abiotic variables using multivariate statistics confirmed salinity as a major driver for the biomass distribution of non‐N2‐fixing microplankton species such as dinoflagellates. However, we provide strong evidence that stratification, N2 fixation, and the stage of the cyanobacterial blooms regulated much of the microplankton diversity and the mean trophic position and size of the metabolic nitrogen pool in mesozooplankton. Our empirical, macroscale data set consistently unifies contrasting results of the dominant feeding mode in mesozooplankton during blooms of unpalatable, filamentous, N2‐fixing cyanobacteria by identifying the at times important role of heterotrophic microbial food webs. Thus, carnivory, rather than herbivory, dominates in mesozooplankton during aging and decaying cyanobacterial blooms with hitherto uncharacterized consequences for the biogeochemical functions of mesozooplankton.
Keywords: amino acids, Baltic Sea, bloom stage, cyanobacteria, food web structure, mesozooplankton, N2 fixation, stable nitrogen isotopes, stratification
R/V Meteor passing a decaying bloom of unpalatable, filamentous, N2‐fixing cyanobacteria in the Baltic Sea in August 2015 that are expected to increase in large areas of the global ocean in the future. While herbivory dominated the trophic structure of mesozooplankton in earlier bloom stages, direct estimates of carnivory in field mesozooplankton identified the at times important role of heterotrophic microbial food webs namely in such aging and decaying cyanobacterial blooms with hitherto uncharacterized consequences for the biogeochemical functions of mesozooplankton. Photo: Andreas Raeke, DWD.

1. INTRODUCTION
Approximately 93% of the excess heat energy trapped since the 1970s has been absorbed into the oceans, leading to a variety of changes in ocean conditions (Jewett & Romanou, 2017 and references therein). The most rapid warming of the mean sea surface temperature (>0.9°C) is observed in land‐locked or semienclosed seas such as the Baltic Sea, North Sea, Black Sea, Japan Sea/East Sea, and East China Sea and over the Newfoundland–Labrador Shelf (Belkin, 2009). The large heat absorption alters the fundamental physical properties of the ocean by increasing the stratification of the water column (Jewett & Romanou, 2017 and references therein). This process indirectly impacts chemical and biological properties such as changing nitrogen and carbon biogeochemical cycles that can result in increasing standing stocks of filamentous, N2‐fixing cyanobacteria such as Trichodesmium (Gattuso et al., 2015; Hutchins & Fu, 2017; Jewett & Romanou, 2017; Paerl & Huisman, 2008; Roy et al., 2011). So far, we lack a consistent understanding of how stratification and blooms of unpalatable, N2‐fixing, filamentous cyanobacteria affect the food web structure in aquatic ecosystems (Steinberg & Landry, 2017).
The number of trophic levels between autotrophs and mesozooplankton is a critical determinant to estimate the transfer of net primary production into the biological carbon pump and into higher trophic levels, such as fish, according to empirical data and ecosystem models (Jewett & Romanou, 2017; Peck et al., 2018; Steinberg & Landry, 2017). Direct grazing on filamentous, N2‐fixing cyanobacteria by mesozooplankton organisms only sporadically takes place by some specialists (Engstroem et al., 2000; Loick‐Wilde et al., 2012; O'Neil, 1999). The transfer of nutrients from unpalatable, filamentous, N2‐fixing cyanobacterial genera such as Trichodesmium, Nodularia, or Aphanizomenon into mesozooplankton is thought to be mainly indirect through grazing on a microbial food web (summarized by Motwani, Duberg, Svedén, & Gorokhova, 2018; Wannicke, Korth, Liskow, & Voss, 2013). In these microbial food webs, auto‐, mixo‐, and heterotrophic microorganisms incorporate and grow on ammonium, amino acids (AA), or other dissolved organic nitrogen (DON) forms (Stal, Staal, & Villbrandt, 1999) that can be exuded in large quantities from filamentous cyanobacteria during N2 fixation (Adam et al., 2016; Mulholland, Bernhardt, Heil, Bronk, & O'Neil, 2006; Ploug, 2008; Stal et al., 2003). The majority of studies suggest that mesozooplankton are herbivorous when filamentous, N2‐fixing cyanobacteria dominate a microplankton community because the animals rely on co‐occurring autotrophic phytoplankton species (Hannides, Popp, Landry, & Graham, 2009; McClelland, Holl, & Montoya, 2003; Mompeán, Bode, Gier, & McCarthy, 2016). All of these trophic studies used nitrogen stable isotope ratios in AAs to identify the mean trophic position (TP) of mixed field mesozooplankton samples without experimental manipulations (Chikaraishi et al., 2009; McClelland & Montoya, 2002; Steffan et al., 2015). Only recently, a study showed that mesozooplankton at times can also be carnivorous during a filamentous, N2‐fixing cyanobacterial bloom according to TP estimates (Eglite et al., 2018). Different from other studies this bloom was in a very late, decayed stage but with surprisingly fast amino acid turnover during N2 fixation by the remaining intact cells (Loick‐Wilde et al., 2018).
The TP approach is based on the stable nitrogen isotope composition (δ15N) of the AAs phenylalanine (Phe) and glutamic acid (Glu) but sometimes also on phenylalanine (Phe) and alanine (Ala, reviewed by Ohkouchi et al., 2017). The δ15N of Phe is determined during its synthesis by autotrophs from an inorganic nitrogen source, and its isotopy remains virtually unaltered across trophic levels because no carbon–nitrogen atomic bonds are cleaved during the metabolism of essential Phe in heterotrophs (Chikaraishi et al., 2009; McClelland & Montoya, 2002; Steffan et al., 2015). The δ15N‐Phe in heterotrophs such as mesozooplankton is therefore a proxy for the dominant inorganic nitrogen source (e.g., nitrate or N2) in an aquatic food web (Eglite et al., 2018; McClelland & Montoya, 2002; McMahon, McCarthy, Sherwood, Larsen, & Guilderson, 2015; Sherwood, Lehmann, Schubert, Scott, & McCarthy, 2011). Glu and Ala belong to a group of AAs called “trophic AAs.” Their isotopic signatures undergo large isotopic fractionation during the incorporation of a diet by a consumer (Montoya, Carpenter, & Capone, 2002; Steffan et al., 2015). In combination, Phe and Glu are the pair most often used for the stepless determination of the mean TP (TPGlu/Phe) of a mixed field plankton sample without experimental manipulations (Chikaraishi et al., 2009; McClelland & Montoya, 2002; Ohkouchi et al., 2017), while the mean TP based on the δ15N‐Ala and δ15N‐Phe (TPAla/Phe) was recently suggested to better resolve the protist level (Décima, Landry, Bradley, & Fogel, 2017).
The Baltic Sea is one of the largest brackish ecosystems in the world and overall not a nutrient‐poor sea; rather, it experiences over fertilization (Conley, 2012; Voss et al., 2011). However, in midsummer every year, its dissolved nitrate and phosphorus pools are largely depleted, and during this time, massive blooms of unpalatable, N2‐fixing, filamentous cyanobacteria, namely, Nodularia and Aphanizomenon, occur (Kahru, Elmgren, Di Lorenzo, & Savchuk, 2018; Karlson et al., 2015; Wasmund, 1997). In addition, the Baltic Sea faces warming already today (Belkin, 2009; Kahru, Elmgren, & Savchuk, 2016; Suikkanen et al., 2013). This scenario leads to an increased summer surface temperature and stratification, which are among the primary variables that regulate variations in the intensity and occurrence of Nodularia and Aphanizomenon blooms in the offshore waters of the central Baltic Sea (Kahru & Elmgren, 2014; Kononen et al., 1996; Vahtera et al., 2007; Wasmund, 1997). Despite large structural (e.g., average water depths of only 58 m) and functional (e.g., low rate of exchange with North Atlantic waters and predominantly brackish conditions) differences between the Baltic Sea compared to marine coastal and offshore ecosystems, these regular and extensive cyanobacterial blooms offer the opportunity to develop a more mechanistic understanding of the effect of stratification and unpalatable, N2‐fixing, filamentous cyanobacterial blooms on the number of trophic levels between autotrophs and mesozooplankton (Reusch et al., 2018).
In this study, we empirically identified how the trophic structure in planktonic food webs changed along with different abiotic and biotic factors across the Baltic Sea in summer. We determined the impact of N2 fixation and the trophic structure of planktonic food webs according to two AA nitrogen stable isotope‐based biogeochemical proxies (δ15N‐Phe and TPGlu/Phe) from two mesozooplankton size fraction samples from stations across the Baltic Sea. The proxies were connected to the results from Eglite et al. (2018) and Loick‐Wilde et al. (2018) and to a large set of environmental variables, for example, mixed layer depth, nutrient concentrations, N2 fixation rates (in parts from Loick‐Wilde et al., 2018), microplankton cell carbon concentrations and biodiversity, and the sizes of the bulk and metabolic nitrogen pools in mesozooplankton. This process allowed us to conceptualize how the mean TPGlu/Phe of mesozooplankton can change along with the stratification, N2 fixation, and filamentous cyanobacterial bloom stage in an aquatic ecosystem.
2. MATERIALS AND METHODS
Samples were collected on a transect including a total of 59 hydrographical stations (Stas.) across the Baltic Sea in July and August 2015 during cruise M117 on board the RV Meteor. Fourteen stations were sampled for biogeochemical variables (Figure 1). Hydrographic data and water samples for chlorophyll a (Chl. a) and nutrient determinations were obtained by deployment of a Seabird SBE‐911 plus CTD equipped with oxygen and fluorescence sensors and mounted on a rosette sampler with thirteen 5‐L GO‐FLO bottles (Hydro‐Bios, Kiel, Germany). Nutrient samples were analyzed directly on board according to Rhode and Nehring (1979) and Hansen and Koroleff (2007). Additional samples of particulate organic matter (POM) were taken from the Chl. a maximum by filtering 0.5–1.0 L of seawater through precombusted Whatman GF/F filters (0.7 μm pore size, 25 mm in diameter) for elemental (total carbon and total nitrogen) analyses. All filters were stored at −20°C after shock‐freezing in liquid nitrogen (−196°C). Additionally, 200–500 mL samples of surface water (from depths of 1 m, 5 m, 10 m, 15 m, and 20 m) were filtered for Chl. a concentrations on glass‐fiber filters (Whatman GF/F) that were shock‐frozen in liquid N2 and stored at −80°C. The 96% ethanol extracts were used for fluorometric analysis according to the guidelines of HELCOM (2017). Nano‐ and microplankton samples (summarized as microplankton) were collected from an integrated depth between 0 and 10 m at 12 stations across the Baltic Sea, including two upwelling sites (Table 1). At Stas. TF12, TF360, TF109, TF113, TF213, TF259, and TF271, additional microplankton samples from a 20 m depth were taken. All microplankton samples were fixed with acid Lugol's solution, and at least 500 counting units were counted in sedimentation chambers under the inverted microscope as described in the manual of HELCOM (2017). Microplankton organisms were assigned to genera (species, if possible) and size classes, for which the specific volume was estimated as shown by Olenina et al. (2006). From these biovolume estimates, the carbon content of the taxa (in the following cell carbon biomass) was calculated after Menden‐Deuer and Lessard (2000), as recommended by HELCOM (2017). Microplankton included autotrophic, mixotrophic, and heterotrophic species.
Figure 1.
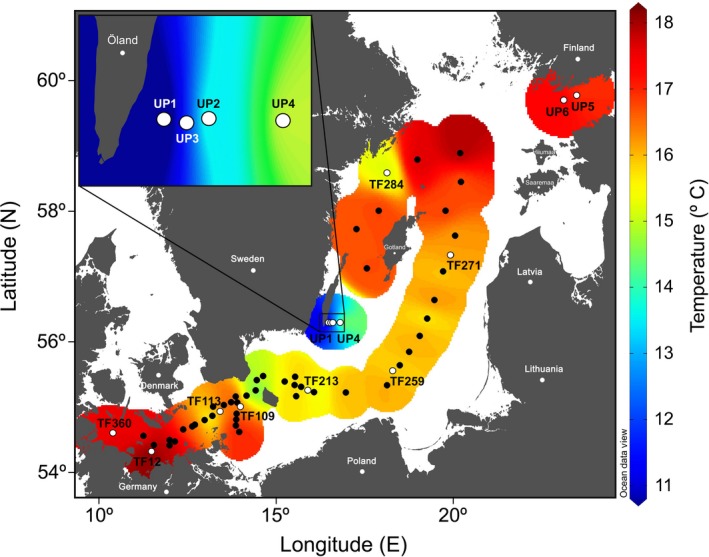
Station map superimposed on the sea surface temperature across the Baltic Sea during RV Meteor cruise M117 in July/August 2015. Biogeochemical variables were taken at 14 stations (marked in white), of which 11 contained all 124 variables for a detailed multivariate analysis including three upwelling stations off Öland (UP2‐UP4), two upwelling stations in the Gulf of Finland (UP5 and UP6), one western Baltic Sea station (TF12), and five central Baltic Sea stations (TF113, TF109, TF213, TF259, and TF271).
Table 1.
Hydrographic characteristics, nutrient and Chl. a concentrations, and microplankton biodiversity indices (H’: Shannon‐Wiener's diversity; J0: Pielou's evenness; and d: Margalef's species richness) found in the surface mixed layer (ML) in July/August 2015. Dashes stand for no data. Chl. a from fluorometrical data from discrete measurements are given in mg m−3 and from the water column sensor in relative units, and both variables were significantly correlated (Supporting Figure S1)
| Station | Sea area | *ML depth | *Temperature | *Salinity | *Oxygen | *PO4 3− | *SiO2 | *DIN | NH4 + | Chl. a | *Chl. a | *H’ | *Jo | *d |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (m) | (ºC) | (PSU) | (mL L−1) | (μM) | (mg m−3) | (rel. units) | ||||||||
| TF360 | wB | 13.3 | 17.6 | 15.1 | 3.8 | – | – | – | – | – | 1.9 | 3.6 | 0.67 | 9.0 |
| TF12* | wB | 11.0 | 17.9 | 10.8 | 6.2 | 0.13 | 10.5 | 0.11 | 0.36 | – | 1.4 | 3.8 | 0.67 | 10.4 |
| TF113* | AB | 16.5 | 16.3 | 8.3 | 6.5 | 0.19 | 11.2 | 0.06 | 0.35 | 2.1 | 1.6 | 3.6 | 0.69 | 7.9 |
| TF109* | AB | 17.0 | 16.1 | 7.9 | 6.6 | 0.27 | 11.5 | 0.07 | 0.28 | – | 1.6 | 3.5 | 0.68 | 8.1 |
| TF213* | BB | 27.8 | 15.6 | 7.6 | 6.4 | 0.28 | 13.2 | 0.07 | 0.29 | 2.3 | 1.4 | 4.0 | 0.76 | 9.1 |
| TF259* | sGB | 27.8 | 15.9 | 7.2 | 6.5 | 0.33 | 17.4 | 0.05 | 0.26 | 2.9 | 1.4 | 3.9 | 0.76 | 8.7 |
| TF271* | eGB | 22.3 | 16.3 | 7.8 | 6.5 | 0.04 | 9.9 | 0.10 | 0.34 | 3.2 | 2.4 | 3.8 | 0.75 | 7.6 |
| TF284 | wGB | 9.8 | 15.2 | 5.7 | 7.1 | 0.02 | 8.8 | 0.12 | 0.29 | 3.6 | 1.9 | – | – | – |
| UP1 | ÖL | 8.0 | 8.7 | 7.0 | 7.2 | 0.56 | 14.2 | 0.15 | – | 1.1 | 0.6 | – | – | – |
| UP2* | ÖL | 8.3 | 14.1 | 6.9 | 6.7 | 0.28 | 12.3 | 0.12 | – | 1.5 | 1.0 | 3.2 | 0.72 | 7.0 |
| UP3* | ÖL | 5.8 | 11.6 | 6.9 | 6.8 | 0.38 | 12.6 | 0.19 | – | 1.2 | 0.9 | 2.6 | 0.61 | 6.2 |
| UP4* | ÖL | 10.4 | 15.9 | 6.8 | 6.6 | 0.13 | 12.1 | 0.14 | – | 1.8 | 1.0 | 3.3 | 0.73 | 6.7 |
| UP5* | GoF | 8.5 | 16.9 | 6.0 | 6.5 | 0.16 | 10.3 | 0.19 | – | 3.2 | 1.8 | 2.7 | 0.56 | 6.0 |
| UP6* | GoF | 10.3 | 17.5 | 6.1 | 6.5 | 0.06 | 9.2 | 0.24 | – | 4.4 | 2.5 | 3.0 | 0.63 | 5.5 |
*Stations and variables (among others) included in the principal component analysis. Abbreviations: wB: western Baltic; AB: Arkona Basin; BB: Bornholm Basin; sGB: southern Gotland Basin, eGB: eastern Gotland Basin; wGB: western Gotland Basin; ÖL: off Öland; GoF: Gulf of Finland. DIN: dissolved inorganic nitrogen from nitrate and nitrite.
N2 fixation rates into particulate organic nitrogen were measured according to Montoya, Voss, Kaehler, and Capone (1996) at the upwelling Stas. UP2, UP3, and UP4 and were combined with published rates from Stas. TF109, TF213, TF259, and TF271 in the central Baltic Sea (Loick‐Wilde et al., 2018). Details of the experiments can be found in Loick‐Wilde et al. (2018).
Zooplankton were collected by vertical tows through surface waters using a UNESCO WP‐2 net (0.25 m2 mouth opening) fitted with a 100 μm mesh size at 14 stations (Table 2). Once on deck, the mesozooplankton were concentrated in a light trap for 3 h before further processing to avoid sample contamination with phytoplankton cells. Then, the samples were divided into 100–300 μm and >300 μm size groups by sieving the samples through a 300 μm nylon mesh and stored in the manner described for the aforementioned filters. Plankton samples were then analyzed in the laboratory for total organic nitrogen (TN) and carbon (TC), and AA composition, as well as for bulk and compound‐specific isotope ratios.
Table 2.
δ15N values (‰) of bulk nitrogen and of individual amino acids and the heterotrophic resynthesis index (ΣV) in the two mesozooplankton size fractions collected from the upper water column across the Baltic Sea in July/August 2015
| Station | Depth (m) | Bulk | Trophic AA | Source AA | Metabolic AA | ∑V | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ala | Asp | Glu | Ile | Leu | Pro | Val | Gly | Lys | Phe | Thr | ||||
| (δ 15N relative to atmospheric N2) | ||||||||||||||
| Zoo 100–300 μm | ||||||||||||||
| TF360 | 0–15 | 5.6 | 12.8 | 10.8 | 15.8 | 10.5 | 10.8 | 10.7 | 13.2 | 3.6 | 7.0 | 5.0 | −2.3 | 1.6 |
| TF12 | 0–25 | 6.1 | 11.2 | 10.8 | 15.7 | 12.9 | 8.9 | 10.3 | 10.3 | 3.0 | 6.0 | 5.0 | −0.9 | 1.6 |
| TF113 | 0–20 | 4.6 | 10.7 | 9.7 | 12.3 | 6.6 | 7.7 | 9.1 | 10.0 | −07 | 3.1 | 1.7 | −4.7 | 1.4 |
| TF109 | 0–43 | 3.5 | 10.7 | 7.8 | 12.7 | 7.7 | 8.5 | 8.8 | 11.3 | −0.3 | 1.9 | −0.8 | −1.7 | 1.5 |
| TF213 | 0–25 | 2.1 | 9.8 | 7.1 | 9.9 | 9.2 | 6.9 | 5.9 | 10.0 | −1.8 | 1.2 | −2.3 | −6.8 | 1.5 |
| TF259 | 0–20 | 4.8 | 14.4 | 11.5 | 14.8 | 12.9 | 10.4 | 9.9 | 13.5 | −0.9 | 2.4 | −2.6 | −10.2 | 1.6 |
| TF271a | 0–25 | 3.7 (±0.8) | 12.2(±1.9) | 9.1 (±1.7) | 12.9(±2.2) | 9.1 (±3.3) | 7.2 (±1.3) | 9.9 (±1.5) | 10.2(±2.2) | −2.5(±0.3) | 0.9 (±0.4) | −2.1(±0.7) | −9.3 (±0.8) | 1.8 (±0.1) |
| TF284 | 0–20 | 4.3 | 11.1 | 9.4 | 12.3 | 11.2 | 8.6 | 9.8 | 10.7 | −0.6 | 2.2 | −0.5 | −8.1 | 1.0 |
| UP1 | 0–15 | 4.6 | 12.6 | 11.1 | 13.4 | 9.4 | 7.9 | 9.4 | 11.1 | 1.1 | 1.9 | 0.0 | 4.4 | 1.5 |
| UP2 | 0–53 | 4.5 | 14.1 | 13.0 | 14.0 | 8.5 | 8.5 | 10.3 | 11.0 | −1.2 | 1.7 | 0.9 | −6.7 | 2.0 |
| UP3 | 0–35 | 4.6 | 12.1 | 9.8 | 13.1 | 8.4 | 7.4 | 9.1 | 9.6 | −0.4 | 1.0 | 0.0 | 3.8 | 1.5 |
| UP4 | 0–20 | 3.5 | 10.6 | 8.0 | 11.4 | 9.4 | 7.0 | 8.7 | 10.2 | −2.3 | 0.0 | −0.8 | −6.2 | 1.2 |
| UP5 | 0–30 | 6.4 | 12.0 | 11.7 | 13.2 | 12.1 | 9.7 | 12.0 | 13.0 | −1.9 | 4.2 | 2.8 | 0.0 | 0.7 |
| UP6 | 0–45 | 5.6 | 12.0 | 11.6 | 12.9 | 10.9 | 10.5 | 12.3 | 12.2 | −1.0 | 3.7 | 2.7 | −1.8 | 1.1 |
| Zoo 300 μm | ||||||||||||||
| TF360 | 0–15 | 7.3 | 14.6 | 11.9 | 15.1 | 11.8 | 12.2 | 12.1 | 14.1 | 2.0 | 4.6 | 5.8 | −0.8 | 1.3 |
| TF12 | 0–25 | 7.7 | 17.8 | 15.8 | 18.7 | 15.9 | 14.8 | 14.8 | 17.4 | 3.1 | 5.9 | 5.3 | −5.0 | 1.3 |
| TF113 | 0–20 | 5.8 | 16.8 | 13.5 | 17.0 | 14.5 | 12.0 | 12.5 | 15.3 | 1.0 | 4.1 | 2.5 | −5.9 | 1.6 |
| TF109 | 0–43 | 6.7 | 17.2 | 14.6 | 18.0 | 15.9 | 13.3 | 12.6 | 16.0 | 1.3 | 4.1 | 0.5 | −8.8 | 1.6 |
| TF213 | 0–25 | 4.7 | 16.4 | 12.7 | 15.9 | 16.0 | 12.5 | 10.9 | 15.8 | −0.0 | 3.2 | −0.5 | −9.0 | 1.9 |
| TF259 | 0–28 | 4.7 | 15.8 | 11.7 | 15.7 | 15.5 | 11.6 | 11.3 | 15.4 | −.5 | 2.0 | −1.5 | −10.6 | 2.0 |
| TF271[Link] | 0–25 | 5.4 (±0.1) | 15.5(±0.8) | 12.0(±0.8) | 16.9(±0.8) | 10.6(±0.8) | 13.0(±0.8) | 13.1(±0.8) | 13.0(±0.8) | −1.3(±0.8) | 1.5 (±0.8) | −1.4(±0.8) | −11.3 (±0.8) | 1.9 (±0.1) |
| TF284 | 0–20 | 5.9 | 15.7 | 12.9 | 15.8 | 15.6 | 12.5 | 12.9 | 15.6 | 0.8 | 3.4 | −0.5 | −7.7 | 1.4 |
| UP1 | 0–15 | 5.7 | 17.2 | 11.1 | 13.8 | 12.5 | 11.6 | 14.7 | 15.5 | 0.5 | 1.8 | 2.9 | −7.3 | 1.7 |
| UP2 | 0–53 | 5.3 | 15.0 | 13.0 | 15.6 | 13.1 | 10.7 | 13.1 | 13.1 | 0.6 | 3.2 | 2.4 | −6.0 | 1.2 |
| UP3 | 0–35 | 5.4 | 15.2 | 9.8 | 14.6 | 13.4 | 10.7 | 13.6 | 12.8 | −1.3 | 2.7 | 4.7 | −7.3 | 1.0 |
| UP4 | 0–20 | 5.1 | 14.3 | 8.0 | 14.6 | 13.8 | 10.4 | 12.0 | 13.6 | −1.3 | 2.5 | −0.6 | −10.6 | 1.6 |
| UP5 | 0–30 | 8.3 | 14.9 | 11.7 | 15.3 | 15.0 | 11.7 | 14.1 | 13.6 | 2.1 | 4.6 | 2.6 | −2.5 | 1.3 |
| UP6 | 0–45 | 8.1 | 15.8 | 11.6 | 16.8 | 15.3 | 12.4 | 14.3 | 14.7 | 1.6 | 5.3 | 3.2 | −2.6 | 1.1 |
Data taken from Eglite et al. (2018) and presented as the mean values with standard deviations from 3 to 4 samples.
2.1. Elemental and biochemical analyses of plankton samples
TN and TC and individual concentrations of 11 AAs as well as the respective stable nitrogen isotope values in POM and mesozooplankton samples were measured in samples from the upwelling stations UP1, UP2, UP3, and UP4; the western Baltic Sea Stas. TF360 and TF12; and the central Baltic Sea Stas. TF113, TF109, TF213, TF259, and TF284 and were combined with the published data from Station (Sta.) TF271 (Eglite et al., 2018). Individual concentrations and nitrogen stable isotopes of 11 AAs included the so‐called “source” AAs glycine ‐Gly‐, lysine ‐Lys‐, and phenylalanine ‐Phe‐; the “trophic” AAs alanine ‐Ala‐, aspartic acid ‐Asp‐, glutamic acid ‐Glu‐, isoleucine ‐Ile‐, leucine ‐Leu‐, proline ‐Pro‐, and valine ‐Val‐; and the “metabolic” AA threonine ‐Thr‐, categorized by Germain, Koch, Harvey, and McCarthy (2013), McClelland and Montoya (2002), and Chikaraishi et al. (2009) according to the sensitivity of each AA to trophic enrichment in 15N. It should be noted that Asp and Glu also include the amide forms asparagine and glutamine, respectively, with the N isotopic signature coming only from the α‐amino‐N from both compounds as the amide N is lost during hydrolysis. Details of the elemental analysis–isotope ratio mass spectrometry (EA‐IRMS), gas chromatography–mass spectrometry (GC‐MS), and gas chromatography–combustion–isotope ratio mass spectrometry (GC‐C‐IRMS) analyses can be found in Eglite et al. (2018) and in the Supporting Material S1.
2.2. Proxies derived from amino acid analyses
The δ15N values of Phe from the two mesozooplankton size fractions were used as time‐integrating proxies for the dominant inorganic nitrogen source sustaining the different planktonic food webs across the Baltic Sea (Eglite et al., 2018; McClelland & Montoya, 2002; McMahon et al., 2015; Sherwood et al., 2011). Different from the δ15N‐Phe in POM, the δ15N‐Phe in zooplankton integrates over transient events that can alter the isotopic baseline in autotrophs as described for bulk δ15N values in POM and thus provides a more robust proxy for the inorganic N source (summarized by Loick‐Wilde et al., 2016).
The proportion of Phe from N2 fixation in mesozooplankton can be estimated according to a modified two‐source mixing model after Montoya et al. (2002). For this model, it is assumed that the δ15N‐Phe value of the mesozooplankton is a mixture of the δ15N values of Phe that is synthesized by phytoplankton from the two new nitrogen sources nitrate and nitrogen from N2 fixation (diazotroph N). For this estimation, the δ15N‐Phe value based on its synthesis from nitrate and the δ15N‐Phe value based on its synthesis from N2 fixation must be known. Here, we assume that the Phe in Baltic Sea zooplankton based on a synthesis from nitrate (δ15N‐PheNitrate) is at least 2.6‰ as found in zooplankton in the subtropical North Pacific (McCarthy, Benner, Lee, & Fogel, 2007). The justification for this is that subsurface nitrate has the same δ15N value of 3.5 ‰ in both ecosystems (Casciotti, Trull, Glover, & Davies, 2008; Korth, Deutsch, Frey, Moros, & Voss, 2014). The δ15N‐Phe value in Baltic Sea zooplankton based on a synthesis from N2 fixation (δ15N‐PheDiazo) is assumed to be −3.6‰ as found for Trichodesmium (McClelland et al., 2003). The contribution of diazotroph Phe to mesozooplankton Phe (% Diazo Phe‐N) was calculated as follows:
| (1) |
where δ15N‐PheZoo is the δ15N‐Phe value of one of the two mesozooplankton size fractions, and the other variables are the two δ15N‐Phe endmember values as defined above.
The δ15N values of Glu and Phe together were used to estimate the mean trophic position of mesozooplankton samples based on equation (2) according to Chikaraishi et al. (2009):
| (2) |
where δ15N‐Glu and δ15N‐Phe are the measured values from a single sample, and β is the δ15N difference between Glu and Phe in the primary producers (−3.4 ± 0.9‰ for aquatic cyanobacteria and algae). TDF represents the trophic discrimination factor between Glu and Phe (7.6 ± 1.2‰) at each trophic shift between a consumer and its diet, and λ represents the first trophic level (=1) of the food web. Accordingly, a TP of 1.0 ± 0.3 represents the dominance of autotrophs in a mixed sample, a TP of 2.0 ± 0.3 represents the dominance of herbivores, and a TP of 3.0 ± 0.3 represents the dominance of carnivores (Chikaraishi et al., 2014; Eglite et al., 2018; McMahon & McCarthy, 2016).
TPs based on δ15N‐Ala and δ15N‐Phe (Décima et al., 2017) and a proxy for heterotrophic resynthesis (ΣV) that tracks to what degree the δ15N‐AA values in mesozooplankton samples were modified by heterotrophic microbial alterations (McCarthy et al., 2007; Mompeán et al., 2016) have also been calculated. However, no significant differences between either the TP estimation approach or no indication for heterotrophic resynthesis according to ΣV values of maximum 2 (Table 2) and coupled ΣV and TP values (Ohkouchi et al., 2017) were found (Supporting Figure S3). Therefore, we proceeded with the TPGlu/Phe estimates (in short TP) for both mesozooplankton size fractions.
The sum of nitrogen in AAs was used as a measure of the metabolic nitrogen pool size in mesozooplankton according to Mayzaud and Conover (1988). The metabolic nitrogen pool is a critical measure to estimate the availability of AAs as precursors for other macromolecules, such as nucleotides or fatty acids, or as an energy pool in mesozooplankton (Mayzaud & Conover, 1988). Normalization of the total hydrolyzable amino acid nitrogen (THAAN) concentration to total organic nitrogen (TN) as the wt% TN‐specific THAA nitrogen concentrations (μg THAAN (100 μg TN)−1 or THAAN wt%TN) was performed for comparability of the THAAN pools in the two mesozooplankton size fractions across the different stations (Cowie & Hedges, 1992).
2.3. Estimation of mixed layer depth
For the mixed layer depth (MLD) estimation, the density anomaly σT was calculated according to TEOS10 (International Thermodynamic Equation of Seawater‐2010). Standard MLD estimation methods applied in oceanic conditions (e.g., Kara, Rochford, & Hurlburt, 2000; Thomson & Fine, 2003) failed for some profiles of the data set due to the special conditions of the brackish Baltic Sea. Therefore, a slightly modified difference threshold method was applied. The mean σT of the upper layer was calculated stepwise down to depth zd, and the difference ΔσT(z d) to σT(z d) was derived:
| (3) |
Starting at the surface, the MLD was reached when ΔσT(z d) exceeded a threshold of 0.1 g kg−1 + the standard deviation of σT in the range 0 to z d (mixed layer). The results were verified by visual inspection of the density profiles.
2.4. Statistical analyses
A cluster analysis was used to identify cell carbon‐specific communities of microplankton taxa based on samples from the upper 10 m from 12 stations across the Baltic Sea, including two upwelling areas. A similarity (SIMPER) analysis additionally identified which taxa shaped the respective communities. Cluster analyses including the available microplankton data from 20 m depth have also been tested but did not show significant differences to the more consistent data set from the upper 10 m (Supporting Figure S2).
The diversity of the microplankton species/taxa in the upper 10 m at the different stations was estimated using Margalef's index for species richness (d), Shannon–Wiener's diversity index (H’), and Pielou's evenness index (J0). Notably, logarithms to base 2 were used in the calculation of H’. All biodiversity indices used were based on the microplankton cell carbon biomass (Clarke & Warwick, 2001).
A correlation‐based principal component analysis (PCA) was used to identify the biotic and abiotic factors that impact the mean trophic position of the two mesozooplankton size fractions across the Baltic Sea during blooms of unpalatable, filamentous, N2‐fixing cyanobacteria (Clarke & Warwick, 2001). The PCA included a total of 124 normalized environmental variables, of which the majority belonged to the 81 individually counted microplankton taxa. Additionally, microplankton taxa were summarized into 22 groups to resolve higher taxonomic levels (mainly orders) as well as the categories unicellular cyanobacteria, filamentous cyanobacteria, or flagellates (Supplemental Data SD1_1 and SD2_2). All 124 variables were available for 11 stations across the Baltic Sea, excluding Stas. TF360 (not sampled for nutrients), UP1, and TF284 (not sampled for microplankton). The number of factors (or principal components, PCs) included in the PCA was based on the a priori criterion to cover the abiotic variables from Table 1 that were included in the PCA and that typically explain much of the biotic variability in an aquatic ecosystem (e.g., Raes et al., 2018 and references therein). This result was crosschecked and confirmed with a scree test (Bryant & Yarnold, 1995). The leading abiotic and biotic variables of a PC were chosen according to eigenvectors above the cut‐point of ǀ0.100ǀ and were mainly attributed to the leading PC for which the eigenvector above ǀ0.100ǀ was highest. Post hoc linear correlations between the leading biotic variables and the leading abiotic variables of a PC further elucidated these relationships especially for biotic variables with eigenvectors above ǀ0.100ǀ in more than one of the leading factors. Post hoc linear correlation was further used to identify the leading variables that directly correlated with the mean TPs (TP100 and TP300) and the proxies for the dominant inorganic nitrogen source (δ15N‐Phe100 and δ15N‐Phe300) of the two mesozooplankton size fractions, which were of special interest for this study.
All biodiversity indices, community similarities, and the PCA were analyzed using PRIMER‐6 Software (Primer‐E Ltd., UK).
The data sets of N2 fixation rates from three upwelling stations (UP2, UP3, and UP4) and Sta. TF284 together with the published rates at Stas. TF109, TF213, TF259, and TF271 from Loick‐Wilde et al. (2018) were too small to be included in the PCA. Therefore, the correlation of the N2 fixation rates with the different abiotic and biotic variables was analyzed separately by regression analyses. Furthermore, three missing THAAN values for Stas. UP1 and UP5 (poor recovery of internal standards in the GC‐MS runs) precluded the inclusion of the THAAN data into the PCA, and therefore, the correlation of the THAAN with the different abiotic and biotic variables was also analyzed separately.
3. RESULTS
3.1. Environmental conditions
A typical thermal stratification was found in the Baltic Sea in July/August 2015, with a subsurface thermocline that separated the warm surface waters from the colder intermediate layer. The inflow of saline water from the Kattegat area into the central Baltic Sea resulted in the characteristic west to east salinity decrease (Table 1). The sea surface temperatures ranged from 15.6°C to 17.9°C, excluding the upwelling area off Öland (Figure 1). The stations referred to here as upwelling stations exhibited clearly lower temperatures and/or shallower thermoclines, especially the ones located off Öland. In the central Baltic Sea, notable temperature and mixed layer depth gradients occurred with lower temperatures and deeper mixed layer depths in the eastern and southern Gotland and Bornholm Basins (Stas. TF271, TF259, and TF213) compared to that in the Arkona Basin (Stas. TF109 and TF113, Table 1, Figure 1).
Dissolved, oxidized, inorganic nitrogen concentrations of nitrate and nitrite (DIN) were relatively low at all stations and ranged from 0.05 to 0.24 μM with lowest concentrations at the central Baltic Sea Stas. TF109, TF113, TF213, TF259, and TF271 (average of 0.07 ± 0.02 μM, Table 1). Upwelling stations had slightly higher DIN concentrations than most other stations, with DIN values between 0.12 μM and 0.24 μM (average of 0.17 ± 0.04 μM, Table 1) that were double the concentrations measured in the central Baltic basins. Phosphate concentrations had an average value of 0.22 ± 0.15 μM, and the lowest values coincided with the highest Chl. a concentrations at the eastern and western Gotland stations (Stas. TF271 and TF284) and at the upwelling Sta. UP6 in the Gulf of Finland (Table 1). The lowest Chl. a values were found at the upwelling stations off Öland.
Volumetric N2 fixation rates at the upwelling stations were very low, yet detectable, at 0.05–0.1 nmol L−1 h−1 at the upwelling Stas. UP2 and UP3 and were enhanced at 0.8 nmol L−1 h−1 further offshore at Sta. UP4 (Supporting Data SD2_2). In the central Baltic Sea, the rates ranged from 2.6 nmol L−1 h−1 to 9.3 nmol L−1 h−1 in the Chl. a maxima of the mixed layer (rates after 24 h of incubation from Loick‐Wilde et al., 2018). Linear regression analyses identified positive correlations of volumetric N2 fixation rates with Chl. a concentrations and the cell carbon biomasses of N2‐fixing Nostocales (namely, Nodularia and Aphanizomenon) (Supporting Data SD2_1).
THAAN wt% TN concentrations in mesozooplankton ranged from 54 to 77 wt% TN and from 65 to 81 wt% TN in the small and large mesozooplankton size fractions, respectively, and were uncorrelated with the corresponding TN and TC concentrations or atomic C/N ratios (Supporting Data SD2_1). The highest THAAN wt% TN concentrations were mainly found in animals from the central Baltic Sea (73.2 ± 4.1 THAAN wt% TN), and the lowest values were mainly found at the coast near stations (66.1 ± 6.0 THAAN wt% TN, Supporting Data SD2_1). Interesting outliers were the low concentrations in both mesozooplankton size fractions in the highly decayed Nodularia bloom at Sta. TF271 in the eastern Gotland Basin (65.2 ± 4.9 THAAN wt% TN). Excluding the outliers at Sta. TF271, significant positive correlations between the cell carbon concentrations of Nostocales from the upper 10 m and the THAAN concentrations from both size fractions were found. These correlations further improved by averaging the Nostocales cell carbon data from the upper 20 m depth where available (Figure 2 and Supporting Data SD2_3).
Figure 2.
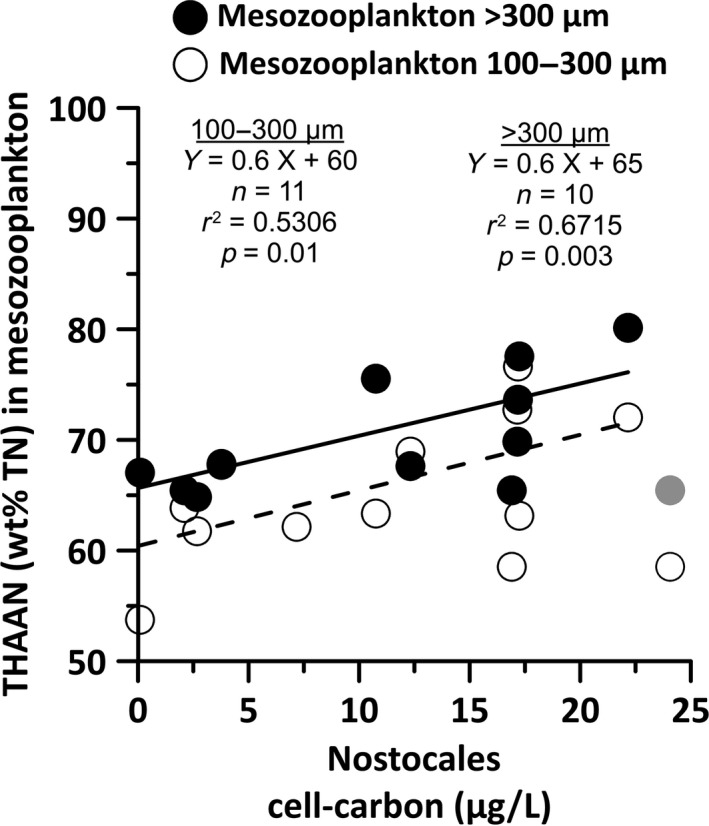
Total hydrolyzable amino acid nitrogen concentrations (THAAN in wt% TN) in two mesozooplankton size fractions relative to the cell carbon concentrations of Nostocales (μg cell‐C L−1) averaged from the upper 10 m (UP stations) or upper 20 m (other stations). The regression statistics for the respective regression lines for both size fractions are included in the panel. Data from the highly decayed cyanobacterial bloom at Station TF271 (gray symbols) were excluded from the regression analysis.
3.2. Microplankton communities across the Baltic Sea
The cluster analysis based on the microplankton cell carbon concentrations in the upper 10 m (Supporting Data SD1_1) identified four different microplankton clusters across the Baltic Sea in summer (Supporting Figure S2), with the dominant taxa in each cluster identified by SIMPER analysis (Supporting Data SD1_2). Only the cluster in the central Baltic Sea (Stas. TF109, TF113, TF213, TF259, TF271) was dominated by N2‐fixing, filamentous cyanobacteria (namely, Nodularia and/or Aphanizomenon). The other three more near‐coastal clusters in the western Baltic Sea (Stas. TF360 ad TF12), in the upwelling area off Öland (Stas. UP2‐UP4), and in the upwelling area off Finland (Stas. UP5 and UP6) were codominated by different combinations of non‐N2‐fixing species, such as dinoflagellates, unicellular cyanobacteria, Pseudanabaena, small diatoms, or Mesodinium rubrum (Supporting Data SD1_2). The Shannon diversity (H’) and the Margalef's species richness (d) indices (Table 1) were significantly lower at the upwelling stations (average H’ of 3.0 ± 0.3; average d of 6.3 ± 0.5) than at the western and central Baltic Sea communities (average H’ of 3.7 ± 0.2; average d of 8.7 ± 0.9; two‐tailed Student's t tests: p = 2.6E−3, df = 6 and p = 2.7E−4, respectively).
3.3. Nitrogen source and trophic position proxies in mesozooplankton
The lowest δ15N‐Phe values in both mesozooplankton size fractions were found at the central Baltic Sea Stas. TF213, TF259, TF271, and TF284 (range of −2.6‰ to −0.5‰), while the highest δ15N‐Phe values were found in the western Baltic Sea at Stas. TF360 and TF12 (range of 5.0‰ to 5.8‰, Table 2). Interestingly, decreasing δ15N‐Phe values in mesozooplankton were significantly correlated with increasing cell carbon biomasses of non‐N2‐fixing, unicellular cyanobacteria. These correlations were further improved by including the unicellular cyanobacterial cell carbon data from the upper 20 m depth where available (Figure 3A, Supporting Data SD2_4). According to equation (1), essential Phe that received its nitrogen from N2 fixation (diazotroph Phe) contributed up to 84% and up to 65% of the Phe pool in the small and large mesozooplankton size fraction samples, respectively, in the central Baltic Sea (Figure 3B).
Figure 3.
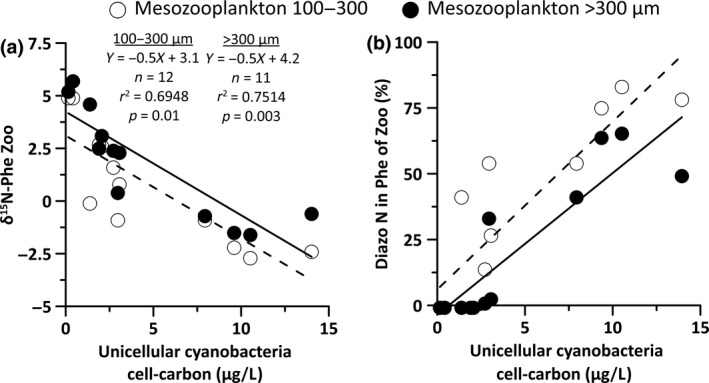
(a) The δ15N‐Phenylalanine (Phe) from two mesozooplankton size fractions relative to the cell carbon concentrations of unicellular cyanobacteria (μg L−1) averaged from the upper 10 m (UP stations) or upper 20 m (other stations). (b) Estimated share of Phe from diazotroph N in zooplankton based on a simple isotope mixing model.
The TPs in the zooplankton communities ranged from 1.8 to 2.9 and covered the range from predominantly herbivorous to predominantly carnivorous communities across the Baltic Sea with distinct regional patterns (Figure 4). Zooplankton from the near‐coastal microplankton communities of the western Baltic, the upwelling area off Öland, and the upwelling area in the Gulf of Finland were predominantly herbivorous in both size fractions (Figure 4). In contrast, animals from the central Baltic Sea microplankton community became increasingly carnivorous from the Arkona Basin (Stas. TF113 and TF109) toward the Bornholm (TF213) and southern (Sta. TF259) and eastern (Sta. TF271) Gotland Basin stations (Figure 4). Animals in the larger size fraction were dominated by carnivores more often (TP >2.5) compared to the smaller size fraction, in which animals more often were predominantly herbivores (TP <2.5).
Figure 4.
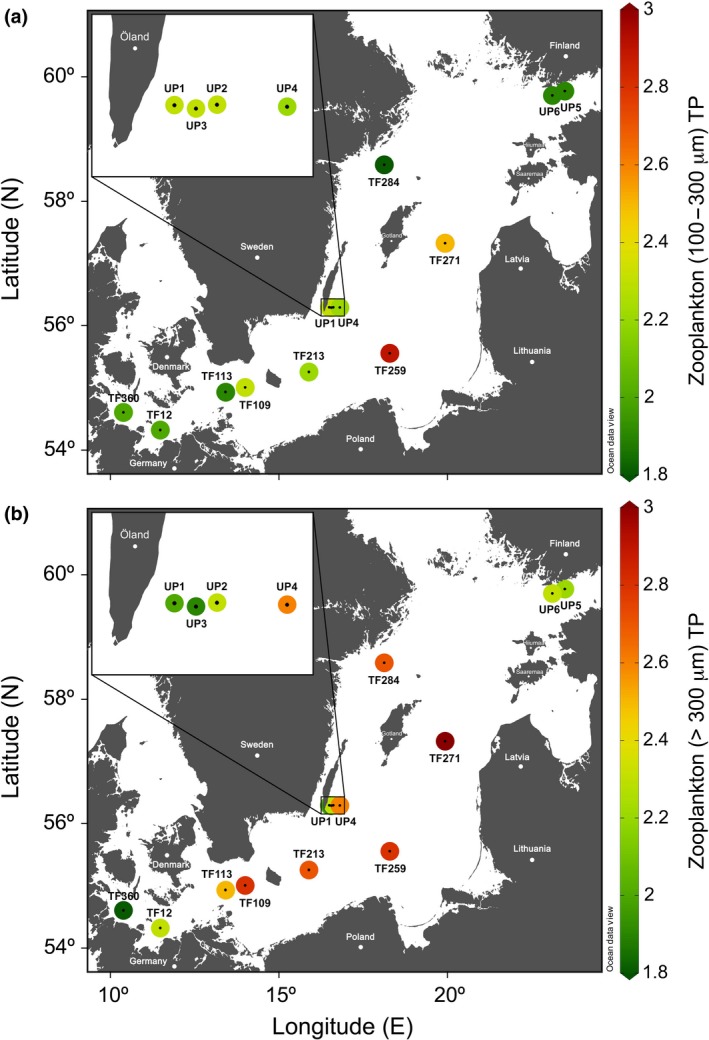
Distribution of the mean trophic positions (TPs) of the mesozooplankton size fractions 100–300 μm and >300 μm across the Baltic Sea in summer. Station names are shown below color‐coded TP estimates. TPs of 2.0 ± 0.3: herbivores; 3.0 ± 0.3: carnivores.
3.4. Statistical analyses to identify the environmental controls on the food web structure
A PCA was used to identify the environmental controls on the planktonic food web structure in the Baltic Sea during blooms of unpalatable, N2‐fixing, filamentous cyanobacteria. A total of four PCs were necessary to cover all abiotic variables from Table 1 that were included in the PCA (Supplemental Data SD2_5). Together, the four leading PCs explained 72.5% of the variability of the 124 variables from 11 stations that were included in the PCA (Supporting Data SD2_2). The eigenvalues of all PCs and the cumulative variance, as well as the loading components of the different variables making up all four PCs are shown in the Supporting Data SD2_5. Accordingly, PC 1 (26.3% of variability) was driven by decreasing salinity and to a lesser degree increasing oxygen concentrations, PC 2 (22.3% of variability) was driven by an increasing mixed layer depth and to a lesser degree increasing SiO2 concentrations at decreasing DIN concentrations, and PC 4 (PC 4: 10.6%) was driven by decreasing PO4 3− concentrations and to a lesser degree increasing temperature. Different from the other four PCs, PC 3 (13.3% of variability) was driven by the biotic variables for the (autocorrelated) biomasses of cyanobacteria (all) and filamentous cyanobacteria (Supporting Data SD2_5). While there is substantial variation explained by PC 3 and PC 4 (together explaining 23.9% of the variability), here we focused on PC 1 and PC 2 (together explaining 48.5% of the variability) to identify environmental controls specifically on the planktonic food web structure and its nitrogen supply across the Baltic Sea (Figure 5). The reason for this is that already PC 2 comprised both the mean trophic position and inorganic nitrogen source proxies for the two mesozooplankton size fractions as leading biotic variables, while the leading abiotic and biotic variables of PC 3 and PC 4 showed no significant relationship with the two proxies in either mesozooplankton size fraction (Supporting Data SD2_5).
Figure 5.
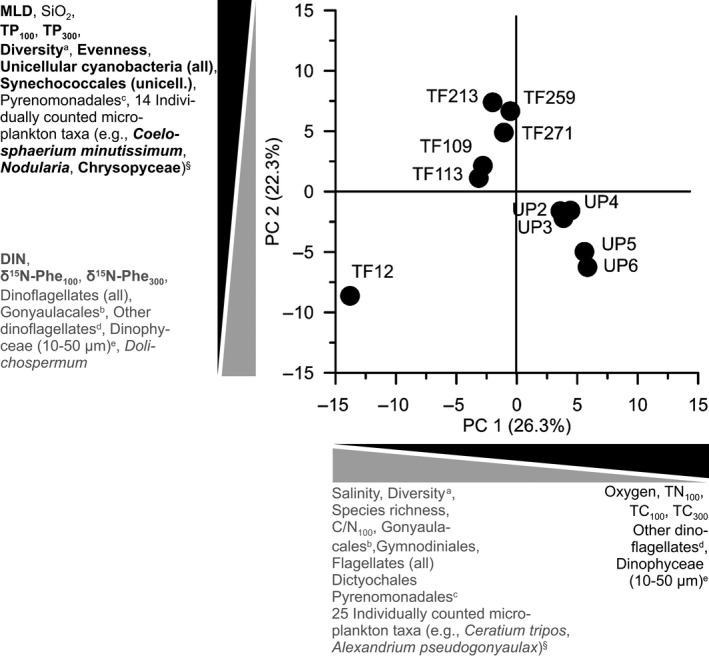
Factors regulating the planktonic food web structure and its nitrogen supply during blooms of unpalatable, filamentous, N2‐fixing cyanobacteria according to a two‐dimensional principal component analysis ordination of the first two leading principal components (PC 1: x‐axis; PC 2: y‐axis) of 124 environmental variables from the Baltic Sea in summer of 2015. All variables that dominated the eigenvectors of PC 1 and PC 2 are given along each axis. Variables directly involved in elucidating the environmental controls on the planktonic food web structure and the supply with nitrogen from N2 fixation are highlighted in bold. See text for more details. For biotic variables that were leading in both PCs, lowercase letters identify the leading abiotic variable(s) with most significant post hoc linear correlation(s): (a,c) mixed layer depth (MLD) and dissolved inorganic nitrogen (DIN), (b) salinity, or (d,e) DIN. §see text and Supporting Data SD2_5 for all individually counted microplankton taxa.
Besides decreasing salinity and increasing oxygen concentrations, PC 1 generally represented an axis of decreasing microplankton diversity and species richness, as well as of mainly decreasing biomasses for a number of summarized microplankton groups of dinoflagellates and flagellates and multiple individually counted taxa (Figure 5, Supporting Data SD2_5). Among the 81 individually counted microplankton taxa, 25 mainly non‐N2‐fixing species had eigenvalues <−0.100 and showed positive correlations between their biomasses with salinity (p < 0.05) primarily due to their high biomasses at the high saline Sta. TF12 in the western Baltic Sea (see Supporting Data SD2_5 for all 28 individual species). Among the leading variables for mesozooplankton, TN and TC of the small mesozooplankton size fraction robustly increased with decreasing salinity (e.g., correlations not driven by data from a single station), while C/N ratios decreased. Interestingly, larger animals had constant C/N ratios, although their TC also increased with decreasing salinity (Supporting Data SD2_2 and SD2_5). In contrast to salinity, oxygen only had very weak relationships with the biotic variables in general (e.g., all correlations driven by data from a single station, Supporting Data SD2_5).
Besides increasing mixed layer depth and SiO2 concentrations, PC 2 generally represented an axis of increasing microplankton diversity and species evenness, as well as of increasing biomasses for a number of summarized microplankton groups of unicellular cyanobacteria and flagellates (Figure 5, Supporting Data SD2_5). Furthermore, the mean TPs of both mesozooplankton size fractions (TP100 and TP300) increased along PC 2. In contrast, DIN concentrations as well as biomasses for a number of summarized microplankton groups of dinoflagellates together with the δ15N‐Phe values as N source proxies from both mesozooplankton size fractions (δ15N‐Phe100 and δ15N‐Phe300) decreased along PC 2 (Supporting Data SD2_5, Figure 5). Notably, none of the dinoflagellate taxa with significant positive or negative relationships with mixed layer depth or DIN showed any significant relationship with the TP or N source proxies in mesozooplankton (Figure 5, Supporting Data SD2_5). In contrast to the mixed layer depth and DIN, SiO2 only had weak relationships with very few biotic variables (e.g., all correlations driven by data from a single station, Supporting Data SD2_5). Among the 81 individually counted microplankton taxa, 12 out of 16 taxa with high loadings for PC 2 (>ǀ100ǀ) showed significant (p < 0.05) and robust (e.g., correlations not driven by data from a single station) increases in their biomasses with increasing mixed layer depth and fewer also with decreasing DIN concentrations (5 taxa). Only 10 of these 12 taxa correlated significantly and mainly robustly with one or more of the two biogeochemical proxies for the two mesozooplankton size fractions, the majority of which belonged to the group of unicellular cyanobacteria (6 taxa, Supporting Data SD2_5). The most prominent taxon (e.g., >10% contribution to the average biomass in a microplankton community, Supporting Data SD1_2) among the other four taxa was Nodularia, which showed strong positive and negative relationships with the TP and δ15N‐Phe proxies, respectively, of the large mesozooplankton size fraction (Figure 5, Supporting Data SD2_5). Finally, its noteworthy that despite its comparatively low density (e.g., max. 1.55 μg cell carbon at Sta. TF 259), the individually counted, mixotrophic flagellate group of Chrysophyceae also showed consistent and mainly robust positive and negative relationships with the TP and δ15N‐Phe proxies, respectively, of both mesozooplankton size fractions at increasing mixed layer depth (Figure 5, Supporting Data SD2_5). In summary, among the different microplankton taxa, the N source and TP proxies of both mesozooplankton size fractions consistently decreased and increased, respectively, namely with at times very dense biomasses of unicellular cyanobacteria (e.g., total unicellular biomass of max. 17.98 μg cell carbon at Sta. TF 213) at increasing mixed layer depth.
4. DISCUSSION
The mean trophic position of zooplankton is routinely used in biogeochemical models (Butenschön et al., 2016; Daewel & Schrum, 2013; Hjøllo, Huse, Skogen, & Melle, 2012; Neumann, Fennel, & Kremp, 2002), and yet, the degree of complexity of planktonic food webs under different environmental conditions that can lead to changes in phytoplankton biomass is still not well understood (Maar et al., 2018; Peck et al., 2018). Our examination of the mean trophic position of field mesozooplankton using amino acid δ15N revealed how the structure of planktonic food webs can alter with changing environmental conditions. Coastal and offshore habitats in the Baltic Sea in summer provided a large variety of microplankton communities and planktonic food web structures. Our results in combination with complementary studies from the same cruise (Eglite et al., 2018; Loick‐Wilde et al., 2018) indicate that changes from herbivory to carnivory are underlain by changes in stratification, N2 fixation, microplankton diversity and composition, and cyanobacterial bloom stage. Hence, amino acid nitrogen stable isotope‐based mean trophic position and nitrogen source estimates in mesozooplankton provide empirical data to study the environmental controls that can lead to changes in the structure of planktonic food webs.
4.1. Stratification, nitrogen sources, and microplankton biomass and diversity
Salinity determined the distribution of many non‐N2‐fixing microplankton species with the most profound impact on dinoflagellate biomass. However, stratification regulated much of the availability of new nitrogen sources, the biomass of phytoplankton key species such as N2‐fixing Nodularia and non‐N2‐fixing unicellular cyanobacteria, as well as of microplankton diversity with ultimate consequences for the planktonic food web structure.
More eutrophic, near‐coastal sites with shallow mixed layer depths, for example, in the upwelling areas off Öland and in the Gulf of Finland, were in contrast to more DIN‐depleted areas in the central Baltic Sea, including the Arkona, Bornholm, and southern and eastern Gotland Basins with deeper mixed layer depths. In addition to the slightly lower DIN concentrations in the central Baltic Sea compared to the more coastal near sites, three additional lines of evidence suggested spatially different new nitrogen sources. First, direct evidence was given by the very high N2 fixation rates in the central Baltic Sea including the highly degraded Nodularia bloom in the eastern Gotland Basin (Loick‐Wilde et al., 2018), in contrast to the comparatively low N2 fixation rates in the upwelling area off Öland. Second, the microplankton community structure was dominated by N2‐fixing, filamentous cyanobacteria in the central Baltic Sea only, while at the near‐coastal sites, non‐N2‐fixing microplankton species dominated. Finally, the high values in the δ15N‐Phe of zooplankton as a time‐integrating proxy for the dominant N source in a planktonic food web confirmed preferential growth on nitrate at the near‐coastal sites. In contrast, the negative or low δ15N‐Phe values in the central Baltic Sea confirmed a preferential growth of the food webs on N2 fixation.
Despite the well‐described positive effect of salinity on the diversity of plankton species in general (Herlemann et al., 2011 and references therein), the depth of the mixed layer had a more profound impact on microplankton diversity than salinity in the Baltic Sea in summer (Supporting data SD2_2 and SD2_5). Most likely, this result was related to the blooms of positively buoyant, N2‐fixing, filamentous cyanobacteria that can substantially increase the diversity in microbial food webs (Hewson et al., 2009; Loick‐Wilde et al., 2017; Sheridan, Steinberg, & Kling, 2002). Accordingly, higher microplankton diversities were found at the central Baltic Sea stations compared to that in most of the near‐coastal stations that largely lacked N2‐fixing, filamentous cyanobacteria, even when salinity was higher at the near‐coastal stations (Table 1). Microplankton biomass and diversity were especially low at the upwelling stations despite enhanced nutrients, which is typical for recent upwelling events in the Baltic Sea (Nausch et al., 2009; Vahtera, Laanemets, Pavelson, Huttunen, & Kononen, 2005) and elsewhere (Blasco, Estrada, & Jones, 2013; MacIsaac, Dugdale, Barber, Blasco, & Packard, 1985).
Interestingly, microplankton diversity further increased from the Arkona toward the Bornholm and southern and eastern Gotland Basins (Table 1). Concomitantly, an increase in the age of the cyanobacterial blooms from the Arkona toward the Bornholm and southern and eastern Gotland Basins has been documented based on organic matter degradation proxies, phosphate concentrations, and visual inspections of the cyanobacterial cells in a complementary study (Loick‐Wilde et al., 2018). The underlying physical mechanism for both patterns was likely a transition from late‐summer stratification toward a wind‐ and thermal‐induced deepening of the mixed layer with the onset of the fall mixing that typically starts at the end of July and beginning of August in the central Baltic Sea (Leppäranta & Myrberg, 2009). This deepening of the mixed layer probably proceeded more in the Bornholm and southern and eastern Gotland Basins than in the Arkona Basin and must have contributed to an earlier breakdown of the Nodularia bloom in the eastern Gotland Basin. The critical role of stratification and water temperatures <16°C for the breakdown of cyanobacterial blooms, namely of Nodularia, in the Baltic Sea has earlier been observed (Kanoshina, Lips, & Leppänen, 2003; Wasmund, 1997). However, in contrast to many diatoms, senescent unpalatable filamentous cyanobacteria are positively buoyant as long as their gas vacuole is intact (Sellner, 1997), which results in the degradation of their biomass components, such as AAs in surface waters, namely, in the highly decayed Nodularia bloom in the eastern Gotland Basin (Loick‐Wilde et al., 2018). The advancing decay of cyanobacterial cells, including a growing availability of their amino acids for the surrounding heterotrophs in surface waters, must have additionally fostered the complexity of the microbial food webs from the Arkona toward the Bornholm, and southern and eastern Gotland Basins with large consequences for the mean trophic positions of mesozooplankton.
4.2. Planktonic food web structure across the Baltic Sea in summer
Mesozooplankton across the Baltic Sea covered a large range of mean trophic positions from dominantly herbivorous animals at the near‐coastal sites to dominantly carnivorous animals in the central Baltic Sea, which points to fundamentally different factors regulating the food web structure during blooms of unpalatable, N2‐fixing, filamentous cyanobacteria compared to palatable, non‐N2‐fixing microplankton communities.
Herbivory in mesozooplankton at the near‐coastal sites probably indicates that mesozooplankton directly grazed on the nitrate‐grown primary producers. In contrast, filamentous, N2‐fixing cyanobacteria are hardly grazed directly (Loick‐Wilde et al., 2012; Mulholland, 2007; Wannicke et al., 2013). Their exuded ammonium or DON for growth is taken up by unicellular cyanobacteria that do not fix N2 in the Baltic Sea and from which the diazotroph nitrogen can be directly incorporated by mesozooplankton species (Motwani et al., 2018; Stal et al., 2003). Three lines of evidence support the idea that generally, this trophic relationship was also responsible for the incorporation of diazotroph nitrogen into mesozooplankton during our study. First, the group of Nostocales, namely, N2‐fixing, unpalatable, filamentous Nodularia and Aphanizomenon, were responsible for the high N2 fixation rates in the microplankton community in the central Baltic Sea according to significant positive correlations of their cell carbon biomasses with N2 fixation rates (Supporting Data SD2_1). Second, the negative δ15N‐Phe values in mesozooplankton in the nitrate‐depleted waters of the central Baltic Sea stations provide strong evidence for the intensive incorporation of essential Phe that must have been synthesized from diazotroph ammonium. Third, the δ15N‐Phe values in both mesozooplankton size fractions decreased with increasing cell carbon biomasses of unicellular cyanobacteria but not with Nodularia and Aphanizomenon cell carbon biomasses (Figure 2A). According to the simple isotope mixing model, diazotroph Phe accounted for a maximum of 83.9% and 66.1% of the essential Phe pool in the small and large mesozooplankton size fraction, respectively, when unicellular cyanobacteria were abundant. These numbers are at the high end of the contribution of diazotroph N to total nitrogen in mesozooplankton from the oligotrophic, tropical North Atlantic, where diazotroph N contributed a maximum of 65–67% to the organic nitrogen pool in mesozooplankton (Montoya et al., 2002). The high contribution of diazotroph Phe in animal tissues related to high unicellular cyanobacterial biomass further stresses the vector function of unicellular cyanobacteria for the supply of essential diazotroph amino acids for higher trophic levels in the Baltic Sea (Karlson et al., 2015; Loick‐Wilde et al., 2012; Motwani et al., 2018; Paul, Sommer, Garzke, Moustaka‐Gouni, Paul et al., 2016).
Interestingly, the mean trophic positions of mesozooplankton from the central Baltic Sea were not homogenously carnivorous; rather, spatial and zooplankton size‐specific differences occurred. In the Arkona Basin (Stas. TF113 and TF109) and Bornholm Basin (Sta. TF213), herbivorous TPs in animals from the smaller mesozooplankton‐sized fractions indicate that smaller animals must have intensively grazed directly on co‐occurring autotrophs such as unicellular cyanobacteria during the younger N2‐fixing cyanobacterial blooms. In contrast, TPs of 2.9 and 2.5 for small mesozooplankton in the southern (Sta. TF259) and eastern Gotland Basins (Sta. TF271), respectively, indicate that smaller animals must have predominantly preyed on mixo‐ and heterotrophic microorganisms in the more decayed cyanobacterial blooms (Figure 2). Additionally, larger animals showed a clear increase in their mean trophic position with aging cyanobacterial blooms according to a predominantly omnivorous feeding behavior in the Arkona Basin (Sta. TF113) and toward a predominantly carnivorous feeding behavior in the eastern Gotland Basin. In the southern and eastern Gotland Basins, carnivorous animals from both size fractions must have intensively preyed on diverse microbial biocenoses of bacteria, unicellular cyanobacteria, and mixo‐ and heterotrophic flagellates that had developed in association with the positively buoyant, aging or decaying Nodularia colonies (Figure 6).
Figure 6.
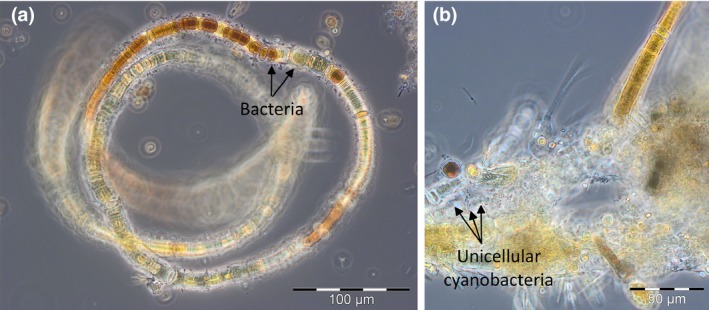
Biocenoses associated with the highly decayed Nodularia bloom in the mixed layer of the eastern Gotland Basin, including (a) unidentified bacteria and (b) unicellular cyanobacteria.
A common theme from bulk carbon and nitrogen pools in mesozooplankton was that larger animals rather than smaller animals in general were better able to keep body stoichiometry constant across the salinity gradient. The underlying causes for the variation in the C:N ratios with salinity in the smaller animals are potentially manifold (Steinberg & Saba, 2008) and may include changes in the mesozooplankton community structure (Walve & Larsson, 1999), changes in the lipid pools (Gismervik, 1997), or changes in ammonium excretion and growth efficiency (Checkley & Entzeroth, 1985; Koski, 1999; Walve & Larsson, 1999). In contrast, the metabolic nitrogen pool in mesozooplankton did not change with salinity but primarily with the biomass of N2‐fixing Nostocales outside the predominantly decayed bloom in the eastern Gotland Basin. An increase in the metabolic nitrogen pools of mesozooplankton due to the intensive incorporation of diazotroph amino acids from N. spumigena at constant C:N ratios has previously been observed in field mesozooplankton from the central Baltic Sea (Loick‐Wilde et al., 2012). This supports the idea that the amino acid nitrogen pool is a sensitive measure to quantify the impact of unpalatable, filamentous, N2‐fixing cyanobacterial blooms on mesozooplankton physiology. In the decayed bloom, the size of the metabolic nitrogen pools in carnivorous mesozooplankton was more similar to the small pools in the near‐coastal herbivorous animals. Thus, we can only speculate that differences in the turnover times of AAs associated with food quality were responsible for the relatively small metabolic nitrogen pools in mesozooplankton, which deserves further investigation.
Our data support the prevailing view that direct grazing of mesozooplankton on palatable phytoplankton species that co‐occur with the unpalatable, filamentous, N2‐fixing species dominates in a highly stratified, nitrate‐depleted water column. However, in association with the increasing decay of blooms of unpalatable N2‐fixing cyanobacteria, carnivory became the dominant feeding mode in mesozooplankton, which stresses the at times important role of heterotrophic microbial food webs for the planktonic food web structure. In the next step, empirical data of the mean trophic position of mesozooplankton and the dominant new nitrogen source for biological production will be used to calibrate and validate current biogeochemical models, including end‐to‐end models (e.g., physics to fish to human sectors, Peck et al., 2018).
Finally, an increase in sea surface stratification linked to sea surface warming, which tends to slow the nutrient supply to the surface, is projected for future oceans (Gattuso et al., 2015; Roy et al., 2011). Under these conditions, unpalatable N2‐fixing Trichodesmium are especially favored, and their large blooms are expected to further expand under future global warming scenarios because enhanced N2 fixation was found to persist under high CO2 irrespective of phosphorus limitation (Hutchins & Fu, 2017; Hutchins et al., 2017; Walworth et al., 2018). Analogously to Nodularia blooms in the Baltic Sea (Wasmund, Nausch, & Voss, 2012), blooms of Trichodesmium in other marine systems develop seasonally in association with mixing of DIN‐depleted but phosphorus‐rich upwelling waters into warm, stratified oceanic waters that contain seed populations of Trichodesmium (Deutsch, Sarmiento, Sigman, Gruber, & Dunne, 2007; Hegde et al., 2008; Hood, Coles, & Capone, 2004). Further, tropical and subtropical Trichodesmium blooms also decay in surface waters, which is namely triggered by the deepening of the mixed layer depth (Devassy, Bhattathiri, & Qasim, 1979; Hood et al., 2004) as found for Nodularia blooms (Kanoshina et al., 2003; Wasmund, 1997). It is a testable hypothesis that the observed changes in the planktonic food web structure in the Baltic Sea also take place in decaying Trichodesmium blooms in other marine systems, which would imply that a carnivorous feeding behavior in mesozooplankton can become more common under future global warming scenarios.
Supporting information
ACKNOWLEDGEMENTS
The authors wish to thank the captain and crew of the RV Meteor and the cruise leaders G. Nausch and O. Wurl for their invaluable assistance at sea. Susanne Busch is highly acknowledged for the microplankton counts and pictures. Fruitful discussions with C. Schrum and T. Neumann helped to consider the modeling perspective. This project was funded by the German Research Foundation (DFG) grant LO 1820/ 4‐1 to NLW.
Loick‐Wilde N, Fernández‐Urruzola I, Eglite E, Liskow I, Nausch M, Schulz‐Bull D, Wodarg D, Wasmund N, Mohrholz V. Stratification, nitrogen fixation, and cyanobacterial bloom stage regulate the planktonic food web structure. Glob Change Biol. 2019;25:794–810. 10.1111/gcb.14546
REFERENCES
- Adam, B. , Klawonn, I. , Sveden, J. B. , Bergkvist, J. , Nahar, N. , Walve, J. , Littmann, S. , Whitehouse, M. J. , Lavik, G. , Kuypers, M. M. M. , & Ploug, H. (2016). N2‐fixation, ammonium release and N‐transfer to the microbial and classical food web within a plankton community. ISME Journal, 10, 450–459. 10.1038/ismej.2015.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkin, I. M. (2009). Rapid warming of large marine ecosystems. Progress in Oceanography, 81, 207–213. 10.1016/j.pocean.2009.04.011 [DOI] [Google Scholar]
- Blasco, D. , Estrada, M. , & Jones, B. H. (2013). Short time variability of phytoplankton populations in upwelling regions—The example of Northwest Africa. Coastal Upwelling, 1, 339–347. [Google Scholar]
- Bryant, F. B. , & Yarnold, P. R. (1995). Principal‐components analysis and exploratory and confirmatory factor analysis In Grimm L. G. & Yarnold P. R. (Eds.), Reading and understanding multivariate statistics (pp. 99–136). Washington, DC: American Psychological Association. [Google Scholar]
- Butenschön, M. , Clark, J. , Aldridge, J. N. , Allen, J. I. , Artioli, Y. , Blackford, J. , Bruggeman, J. , Cazenave, P. , Ciavatta, S. , & Kay, S. (2016). ERSEM 15.06: A generic model for marine biogeochemistry and the ecosystem dynamics of the lower trophic levels. Geoscientific Model Development, 9, 1293–1339. 10.5194/gmd-9-1293-2016 [DOI] [Google Scholar]
- Casciotti, K. , Trull, T. , Glover, D. , & Davies, D. (2008). Constraints on nitrogen cycling at the subtropical North Pacific Station ALOHA from isotopic measurements of nitrate and particulate nitrogen. Deep Sea Research Part II: Topical Studies in Oceanography, 55, 1661–1672. 10.1016/j.dsr2.2008.04.017 [DOI] [Google Scholar]
- Checkley, D. M. , & Entzeroth, L. C. (1985). Elemental and isotopic fractionation of carbon and nitrogen by marine, planktonic copepods and implications to the marine nitrogen cycle. Journal of Plankton Research, 7, 553–568. 10.1093/plankt/7.4.553 [DOI] [Google Scholar]
- Chikaraishi, Y. , Ogawa, N. O. , Kashiyama, Y. , Takano, Y. , Suga, H. , Tomitani, A. , Miyashita, H. , Kitazato, H. , & Ohkouchi, N. (2009). Determination of aquatic food‐web structure based on compound‐specific nitrogen isotopic composition of amino acids. Limnology and Oceanography: Methods, 7, 740–750. [Google Scholar]
- Chikaraishi, Y. , Steffan, S. A. , Ogawa, N. O. , Ishikawa, N. F. , Sasaki, Y. , Tsuchiya, M. , & Ohkouchi, N. (2014). High‐resolution food webs based on nitrogen isotopic composition of amino acids. Ecology and Evolution, 4, 2423–2449. 10.1002/ece3.1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke, K. R. , & Warwick, R. M. (2001). Changes in marine communities: An approach to statistical analysis and interpretation. UK: Natural Environment Research Council. [Google Scholar]
- Conley, D. J. (2012). Ecology: Save the Baltic Sea. Nature, 486, 463–464. 10.1038/486463a [DOI] [PubMed] [Google Scholar]
- Cowie, G. L. , & Hedges, J. I. (1992). Sources and reactivities of amino acids in a coastal marine environment. Limnology and Oceanography, 37, 703–724. 10.4319/lo.1992.37.4.0703 [DOI] [Google Scholar]
- Daewel, U. , & Schrum, C. (2013). Simulating long‐term dynamics of the coupled North Sea and Baltic Sea ecosystem with ECOSMO II: Model description and validation. Journal of Marine Systems, 119, 30–49. 10.1016/j.jmarsys.2013.03.008 [DOI] [Google Scholar]
- Décima, M. , Landry, M. R. , Bradley, C. J. , & Fogel, M. L. (2017). Alanine δ15N trophic fractionation in heterotrophic protists. Limnology and Oceanography, 62, 2308–2322. 10.1002/lno.10567 [DOI] [Google Scholar]
- Deutsch, C. , Sarmiento, J. L. , Sigman, D. M. , Gruber, N. , & Dunne, J. P. (2007). Spatial coupling of nitrogen inputs and losses in the ocean. Nature, 445, 163–167. 10.1038/nature05392 [DOI] [PubMed] [Google Scholar]
- Devassy, V. P. , Bhattathiri, P. M. A. , & Qasim, S. Z. (1979). Succession of organisms following Trichodesmium phenomenon. Indian Journal of Marine Sciences, 8, 89–93. [Google Scholar]
- Eglite, E. , Wodarg, D. , Dutz, J. , Wasmund, N. , Nausch, G. , Liskow, I. , Schulz‐Bull, D. , & Loick‐Wilde, N. (2018). Strategies of amino acid supply in mesozooplankton during cyanobacteria blooms: A stable nitrogen isotope approach. Ecosphere, 9 10.1002/ecs2.2135 [DOI] [Google Scholar]
- Engstroem, J. , Koski, M. , Viitasalo, M. , Reinikainen, M. , Repka, S. , & Sivonen, K. (2000). Feeding interactions of the copepods Eurytemora affinis and Acartia bifilosa with the cyanobacteria Nodularia sp . Journal of Plankton Research, 22, 1403–1409. 10.1093/plankt/22.7.1403 [DOI] [Google Scholar]
- Gattuso, J.‐P. , Magnan, A. , Billé, R. , Cheung, W. W. , Howes, E. L. , Joos, F. , Allemand, D. , Bopp, L. , Cooley, S. R. , & Eakin, C. M. (2015). Contrasting futures for ocean and society from different anthropogenic CO2 emissions scenarios. Science, 349, aac4722 10.1126/science.aac4722 [DOI] [PubMed] [Google Scholar]
- Germain, L. R. , Koch, P. L. , Harvey, J. , & McCarthy, M. D. (2013). Nitrogen isotope fractionation in amino acids from harbor seals: Implications for compound‐specific trophic position calculations. Marine Ecology Progress Series, 482, 265–277. 10.3354/meps10257 [DOI] [Google Scholar]
- Gismervik, I. (1997). Stoichiometry of some marine planktonic crustaceans. Journal of Plankton Research, 19, 279–285. 10.1093/plankt/19.2.279 [DOI] [Google Scholar]
- Hannides, C. C. , Popp, B. N. , Landry, M. R. , & Graham, B. S. (2009). Quantification of zooplankton trophic position in the North Pacific Subtropical Gyre using stable nitrogen isotopes. Limnology and Oceanography, 54, 50–61. 10.4319/lo.2009.54.1.0050 [DOI] [Google Scholar]
- Hansen, H. P. , & Koroleff, F. (2007). Determination of nutrients In Grasshoff K., Kremling K. & Ehrhardt M. (Eds.), Methods of seawater analysis (pp. 159–228). Hoboken, NJ: John Wiley. [Google Scholar]
- Hegde, S. , Anil, A. C. , Patil, J. S. , Mitbavkar, S. , Krishnamurthy, V. , & Gopalakrishna, V. V. (2008). Influence of environmental settings on the prevalence of Trichodesmium spp. in the Bay of Bengal. Marine Ecology Progress Series, 356, 93–101. 10.3354/meps07259 [DOI] [Google Scholar]
- HELCOM (2017). Monitoring of phytoplankton species composition, abundance and biomass. http://www.helcom.fi/Documents/Action%20areas/Monitoring%20and%20assessment/Manuals%20and%20Guidelines/Guidelines%20for%20monitoring%20phytoplankton%20species%20composition,%20abundance%20and%20biomass.pdf
- Herlemann, D. P. R. , Labrenz, M. , Jürgens, K. , Bertilsson, S. , Waniek, J. J. , & Andersson, A. F. (2011). Transitions in bacterial communities along the 2000 km salinity gradient of the Baltic Sea. The ISME Journal, 5, 1571 10.1038/ismej.2011.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewson, I. , Poretsky, R. S. , Dyhrman, S. T. , Zielinski, B. , White, A. E. , Tripp, H. J. , Montoya, J. P. , & Zehr, J. P. (2009). Microbial community gene expression within colonies of the diazotroph, Trichodesmium, from the Southwest Pacific Ocean. The ISME Journal, 3, 1286–1300. 10.1038/ismej.2009.75 [DOI] [PubMed] [Google Scholar]
- Hjøllo, S. S. , Huse, G. , Skogen, M. D. , & Melle, W. (2012). Modelling secondary production in the Norwegian Sea with a fully coupled physical/primary production/individual‐based Calanus finmarchicus model system. Marine Biology Research, 8, 508–526. 10.1080/17451000.2011.642805 [DOI] [Google Scholar]
- Hood, R. R. , Coles, V. J. , & Capone, D. G. (2004). Modeling the distribution of Trichodesmium and nitrogen fixation in the Atlantic Ocean. Journal of Geophysical Research, 109 10.1029/2002jc001753 [DOI] [Google Scholar]
- Hutchins, D. A. , & Fu, F. (2017). Microorganisms and ocean global change. Nature Microbiology, 2, 17058 10.1038/nmicrobiol.2017.58 [DOI] [PubMed] [Google Scholar]
- Hutchins, D. A. , Fu, F. , Walworth, N. G. , Lee, M. D. , Saito, M. A. , & Webb, E. A. (2017). Comment on “The complex effects of ocean acidification on the prominent N2‐fixing cyanobacterium Trichodesmium”. Science, 357, eaao0067 10.1126/science.aao0067 [DOI] [PubMed] [Google Scholar]
- Jewett, L. , & Romanou, A. (2017). Ocean acidification and other ocean changes In Wuebbles D. J., Fahey D. W., Hibbard K. A., Dokken D. J., Stewart B. C., & Maycock T. K. (Eds.), Climate Science Special Report: Fourth National Climate Assessment (pp. 364–392). Washington, USA: U.S. Global Change Research Program. [Google Scholar]
- Kahru, M. , & Elmgren, R. (2014). Multidecadal time series of satellite‐detected accumulations of cyanobacteria in the Baltic Sea. Biogeosciences, 11, 3619–3633. 10.5194/bg-11-3619-2014 [DOI] [Google Scholar]
- Kahru, M. , Elmgren, R. , Di Lorenzo, E. , & Savchuk, O. (2018). Unexplained interannual oscillations of cyanobacterial blooms in the Baltic Sea. Scientific Reports, 8, 6365 10.1038/s41598-018-24829-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahru, M. , Elmgren, R. , & Savchuk, O. P. (2016). Changing seasonality of the Baltic Sea. Biogeosciences, 13, 1009–1018. 10.5194/bg-13-1009-2016 [DOI] [Google Scholar]
- Kanoshina, I. , Lips, U. , & Leppänen, J.‐M. (2003). The influence of weather conditions (temperature and wind) on cyanobacterial bloom development in the Gulf of Finland (Baltic Sea). Harmful Algae, 2, 29–41. 10.1016/S1568-9883(02)00085-9 [DOI] [Google Scholar]
- Kara, A. B. , Rochford, P. A. , & Hurlburt, H. E. (2000). An optimal definition for ocean mixed layer depth. Journal of Geophysical Research: Oceans, 105, 16803–16821. 10.1029/2000JC900072 [DOI] [Google Scholar]
- Karlson, A. M. L. , Duberg, J. , Motwani, N. H. , Hogfors, H. , Klawonn, I. , Ploug, H. , Barthel Svedén, J. , Garbaras, A. , Sundelin, B. , Hajdu, S. , Larsson, U. , Elmgren, R. , & Gorokhova, E. (2015). Nitrogen fixation by cyanobacteria stimulates production in Baltic food webs. Ambio, 44, 413–426. 10.1007/s13280-015-0660-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kononen, K. , Kuparinen, J. , Mäkelä, K. , Laanemets, J. , Pavelson, J. , & Nommann, S. (1996). Initiation of cyanobacterial blooms in a frontal region at the entrance of the Gulf of Finland, Baltic Sea. Limnology and Oceanography, 41, 98–112. 10.4319/lo.1996.41.1.0098 [DOI] [Google Scholar]
- Korth, F. , Deutsch, B. , Frey, C. , Moros, C. , & Voss, M. (2014). Nitrate source identification in the Baltic Sea using its isotopic ratios in combination with a Bayesian isotope mixing model. Biogeosciences, 11, 4913–4924. 10.5194/bg-11-4913-2014 [DOI] [Google Scholar]
- Koski, M. (1999). C: N ratios of Baltic Sea copepods—Indication of mineral limitation? Journal of Plankton Research, 8, 1565–1573. 10.1093/plankt/21.8.1565 [DOI] [Google Scholar]
- Leppäranta, M. , & Myrberg, K. (2009). Physical oceanography of the Baltic Sea. Berlin, Germany: Springer Science & Business Media; 10.1007/978-3-540-79703-6 [DOI] [Google Scholar]
- Loick‐Wilde, N. , Bombar, D. , Doan, H. N. , Nguyen, L. N. , Nguyen‐Thi, A. M. , Voss, M. , & Dippner, J. W. (2017). Microplankton biomass and diversity in the Vietnamese upwelling area during SW monsoon under normal conditions and after an ENSO event. Progress in Oceanography, 153, 1–15. 10.1016/j.pocean.2017.04.007 [DOI] [Google Scholar]
- Loick‐Wilde, N. , Dutz, J. , Miltner, A. , Gehre, M. , Montoya, J. P. , & Voss, M. (2012). Incorporation of nitrogen from N2 fixation into amino acids of zooplankton. Limnology and Oceanography, 57, 199–210. 10.4319/lo.2012.57.1.0199 [DOI] [Google Scholar]
- Loick‐Wilde, N. , Weber, S. C. , Conroy, B. J. , Capone, D. G. , Coles, V. J. , Medeiros, P. M. , Steinberg, D. K. , & Montoya, J. P. (2016). Nitrogen sources and net growth efficiency of zooplankton in three Amazon River plume food webs. Limnology and Oceanography, 61, 460–481. 10.1002/lno.10227 [DOI] [Google Scholar]
- Loick‐Wilde, N. , Weber, S. C. , Eglite, E. , Liskow, I. , Schulz‐Bull, D. , Wasmund, N. , Wodarg, D. , & Montoya, J. P. (2018). De novo amino acid synthesis and turnover during N2 fixation. Limnology and Oceanography, 63, 1076–1092. 10.1002/lno.10755 [DOI] [Google Scholar]
- Maar, M. , Butenschön, M. , Daewel, U. , Eggert, A. , Fan, W. , Hjøllo, S. S. , Hufnagl, M. , Huret, M. , Ji, R. , & Lacroix, G. (2018). Responses of summer phytoplankton biomass to changes in top‐down forcing: Insights from comparative modelling. Ecological Modelling, 376, 54–67. 10.1016/j.ecolmodel.2018.03.003 [DOI] [Google Scholar]
- MacIsaac, J. J. , Dugdale, R. C. , Barber, R. T. , Blasco, D. , & Packard, T. T. (1985). Primary production cycle in an upwelling center. Deep‐Sea Research, 5, 503–529. 10.1016/0198-0149(85)90042-1 [DOI] [Google Scholar]
- Mayzaud, P. , & Conover, R. J. (1988). O: N atomic ratio as a tool to describe zooplankton metabolism. Marine Ecology Progress Series, 45, 289–302. 10.3354/meps045289 [DOI] [Google Scholar]
- McCarthy, M. D. , Benner, R. , Lee, C. , & Fogel, M. L. (2007). Amino acid nitrogen isotopic fractionation patterns as indicators of heterotrophy in plankton, particulate, and dissolved organic matter. Geochimica et Cosmochimica Acta, 71, 4727–4744. 10.1016/j.gca.2007.06.061 [DOI] [Google Scholar]
- McClelland, J. W. , Holl, C. M. , & Montoya, J. P. (2003). Nitrogen sources to zooplankton in the Tropical North Atlantic: Stable isotope ratios of amino acids identify strong coupling to N2‐fixation. Deep‐Sea Res. I, 50, 849–861. 10.1016/S0967-0637(03)00073-6 [DOI] [Google Scholar]
- McClelland, J. W. , & Montoya, J. P. (2002). Trophic relationships and the nitrogen isotopic composition of amino acids in plankton. Ecology, 83, 2173–2180. 10.1890/0012-9658(2002)083[2173:TRATNI]2.0.CO;2 [DOI] [Google Scholar]
- McMahon, K. W. , & McCarthy, M. D. (2016). Embracing variability in amino acid δ15N fractionation: Mechanisms, implications, and applications for trophic ecology. Ecosphere, 7. [Google Scholar]
- McMahon, K. W. , McCarthy, M. D. , Sherwood, O. A. , Larsen, T. , & Guilderson, T. P. (2015). Millennial‐scale plankton regime shifts in the subtropical North Pacific Ocean. Science, 350, 1530–1533. 10.1126/science.aaa9942 [DOI] [PubMed] [Google Scholar]
- Menden-Deuer, S. , & Lessard, E. J. (2000). Carbon to volume relationships for dinoflagellates, diatoms, and other protist plankton. Limnology and Oceanography, 45, 569–579. [Google Scholar]
- Mompeán, C. , Bode, A. , Gier, E. , & McCarthy, M. D. (2016). Bulk vs. amino acid stable N isotope estimations of metabolic status and contributions of nitrogen fixation to size‐fractionated zooplankton biomass in the subtropical N Atlantic. Deep Sea Research Part I: Oceanographic Research Papers, 114, 137–148. 10.1016/j.dsr.2016.05.005 [DOI] [Google Scholar]
- Montoya, J. P. , Carpenter, E. J. , & Capone, D. G. (2002). Nitrogen‐fixation and nitrogen isotope abundances in zooplankton of the oligotrophic North Atlantic. Limnology and Oceanography, 47, 1617–1628. 10.4319/lo.2002.47.6.1617 [DOI] [Google Scholar]
- Montoya, J. P. , Voss, M. , Kaehler, P. , & Capone, D. G. (1996). A simple, high precision, high sensitivity tracer assay for dinitrogen fixation. Applied Environmental Microbiology, 62, 986–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motwani, N. H. , Duberg, J. , Svedén, J. B. , & Gorokhova, E. (2018). Grazing on cyanobacteria and transfer of diazotrophic nitrogen to zooplankton in the Baltic Sea. Limnology and Oceanography, 63, 672–686. 10.1002/lno.10659 [DOI] [Google Scholar]
- Mulholland, M. R. (2007). The fate of nitrogen fixed by diazotrophs in the ocean. Biogeosciences, 4, 37–51. 10.5194/bg-4-37-2007 [DOI] [Google Scholar]
- Mulholland, M. R. , Bernhardt, P. W. , Heil, C. A. , Bronk, D. A. , & O'Neil, J. M. (2006). Nitrogen fixation and release of fixed nitrogen by Trichodesmium spp. in the Gulf of Mexico. Limnology and Oceanography, 51, 1762–1776. 10.4319/lo.2006.51.4.1762 [DOI] [Google Scholar]
- Nausch, M. , Nausch, G. , Lass, H. U. , Mohrholz, V. , Nagel, K. , Siegel, H. , & Wasmund, N. (2009). Phosphorus input by upwelling in the eastern Gotland Basin (Baltic Sea) in summer and its effects on filamentous cyanobacteria. Estuarine, Coastal and Shelf Science, 83, 434–442. 10.1016/j.ecss.2009.04.031 [DOI] [Google Scholar]
- Neumann, T. , Fennel, W. , & Kremp, C. (2002). Experimental simulations with an ecosystem model of the Baltic Sea: A nutrient load reduction experiment. Global Biogeochemical Cycles, 16, 1033. [Google Scholar]
- Ohkouchi, N. , Chikaraishi, Y. , Close, H. G. , Fry, B. , Larsen, T. , Madigan, D. J. , McCarthy, M. D. , McMahon, K. W. , Nagata, T. , Naito, Y. I. , Ogawa, N. O. , Popp, B. N. , Steffan, S. , Takano, Y. , Tayasu, I. , Wyatt, A. S. J. , Yamaguchi, Y. T. , & Yokoyama, Y. (2017). Advances in the application of amino acid nitrogen isotopic analysis in ecological and biogeochemical studies. Organic Geochemistry, 113, 150–174. 10.1016/j.orggeochem.2017.07.009 [DOI] [Google Scholar]
- Olenina, I. , Hajdu, S. , Edler, L. , Andersson, A. , Wasmund, N. , Busch, S. , ... Niemkiewicz, E. (2006) Biovolumes and size-classes of phytoplankton in the Baltic Sea In HELCOM Balt. Sea Environ. Proc. No. 106, 144pp. [Google Scholar]
- O'Neil, J. M. (1999). Grazer interactions with nitrogen‐fixing marine cyanobacteria: Adaptation for N‐acquisition? Bulletin de l'Institut océanographique, 19, 293–317. [Google Scholar]
- Paerl, H. W. , & Huisman, J. (2008). Climate. Blooms like it hot. Science, 320, 57–58. 10.1126/science.1155398 [DOI] [PubMed] [Google Scholar]
- Paul, C. , Sommer, U. , Garzke, J. , Moustaka‐Gouni, M. , Paul, A. , & Matthiessen, B. (2016). Effects of increased CO2 concentration on nutrient limited coastal summer plankton depend on temperature. Limnology and Oceanography, 61, 853–868. 10.1002/lno.10256 [DOI] [Google Scholar]
- Peck, M. A. , Arvanitidis, C. , Butenschön, M. , Canu, D. M. , Chatzinikolaou, E. , Cucco, A. , Domenici, P. , Fernandes, J. A. , Gasche, L. , Huebert, K. B. , Hufnagl, M. , Jones, M. C. , Kempf, A. , Keyl, F. , Maar, M. , Mahévas, S. , Marchal, P. , Nicolas, D. , Pinnegar, J. K. , Rivot, E. , Rochette, S. , Sell, A. F. , Sinerchia, M. , Solidoro, C. , Somerfield, P. J. , Teal, L. R. , Travers‐Trolet, M. , & van de Wolfshaar, K. E. (2018). Projecting changes in the distribution and productivity of living marine resources: A critical review of the suite of modelling approaches used in the large European project VECTORS. Estuarine, Coastal and Shelf Science, 201, 40–55. 10.1016/j.ecss.2016.05.019 [DOI] [Google Scholar]
- Ploug, H. (2008). Cyanobacterial surface blooms formed by Aphanizomenon sp. and Nodularia spumigena in the Baltic Sea: Small‐scale fluxes, pH, and oxygen microenvironments. Limnology and Oceanography, 53, 914–921. 10.4319/lo.2008.53.3.0914 [DOI] [Google Scholar]
- Raes, E. J. , Bodrossy, L. , van de Kamp, J. , Bissett, A. , Ostrowski, M. , Brown, M. V. , Sow, S. L. S. , Sloyan, B. , & Waite, A. M. (2018). Oceanographic boundaries constrain microbial diversity gradients in the South Pacific Ocean. Proceedings of the National Academy of Sciences, 115, E8266–E8275. 10.1073/pnas.1719335115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusch, T. B. H. , Dierking, J. , Andersson, H. C. , Bonsdorff, E. , Carstensen, J. , Casini, M. , Czajkowski, M. , Hasler, B. , Hinsby, K. , Hyytiäinen, K. , Johannesson, K. , Jomaa, S. , Jormalainen, V. , Kuosa, H. , Kurland, S. , Laikre, L. , MacKenzie, B. R. , Margonski, P. , Melzner, F. , Oesterwind, D. , Ojaveer, H. , Refsgaard, J. C. , Sandström, A. , Schwarz, G. , Tonderski, K. , Winder, M. , & Zandersen, M. (2018). The Baltic Sea as a time machine for the future coastal ocean. Science Advances, 4, eaar8195 10.1126/sciadv.aar8195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode, K. H. , & Nehring, D. (1979). Ausgewählte Methoden für die Analyse chemischer Inhaltsstoffe in Meer ‐ und Brackwasser. Geodätisch Geophysikalische Veröffentlichungen, 27, 1–68. [Google Scholar]
- Roy, T. , Bopp, L. , Gehlen, M. , Schneider, B. , Cadule, P. , Frölicher, T. L. , Segschneider, J. , Tjiputra, J. , Heinze, C. , & Joos, F. (2011). Regional impacts of climate change and atmospheric CO2 on future ocean carbon uptake: A multimodel linear feedback analysis. Journal of Climate, 24, 2300–2318. 10.1175/2010JCLI3787.1 [DOI] [Google Scholar]
- Sellner, K. G. (1997). Physiology, ecology, and toxic properties of marine cyanobacteria blooms. Limnology and Oceanography, 42, 1089–1104. 10.4319/lo.1997.42.5_part_2.1089 [DOI] [Google Scholar]
- Sheridan, C. , Steinberg, D. , & Kling, G. (2002). The microbial and metazoan community associated with colonies of Trichodesmium spp.: A quantitative survey. Journal of Plankton Research, 24, 913–922. 10.1093/plankt/24.9.913 [DOI] [Google Scholar]
- Sherwood, O. A. , Lehmann, M. F. , Schubert, C. J. , Scott, D. B. , & McCarthy, M. D. (2011). Nutrient regime shift in the western North Atlantic indicated by compound‐specific δ15N of deep‐sea gorgonian corals. Proceedings of the National Academy of Sciences, 108, 1011–1015. 10.1073/pnas.1004904108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stal, L. J. , Albertano, P. , Bergman, B. , von Bröckel, K. , Gallon, J. R. , Hayes, P. K. , Sivonen, K. , & Walsby, A. E. (2003). BASIC: Baltic Sea cyanobacteria. An investigation of the structure and dynamics of water blooms of cyanobacteria in the Baltic Sea‐responses to a changing environment. Continental Shelf Research, 23, 1695–1714. 10.1016/j.csr.2003.06.001 [DOI] [Google Scholar]
- Stal, L. J. , Staal, M. , & Villbrandt, M. (1999). Nutrient control of cyanobacterial blooms in the Baltic Sea. Aquatic Microbial Ecology, 18, 165–173. 10.3354/ame018165 [DOI] [Google Scholar]
- Steffan, S. A. , Chikaraishi, Y. , Currie, C. R. , Horn, H. , Gaines‐Day, H. R. , Pauli, J. N. , Zalapa, J. E. , & Ohkouchi, N. (2015). Microbes are trophic analogs of animals. Proceedings of the National Academy of Sciences, 112, 15119–15124. 10.1073/pnas.1508782112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg, D. K. , & Landry, M. R. (2017). Zooplankton and the ocean carbon cycle. Annual Review of Marine Science, 9, 413–444. 10.1146/annurev-marine-010814-015924 [DOI] [PubMed] [Google Scholar]
- Steinberg, D. K. , & Saba, G. K. (2008). Nitrogen consumption and metabolism in marine zooplankton In D. G., Capone, D. A., Bronk, M. R., Mulholland & Carpenter E. J. (Eds.), Nitrogen in the marine environment (pp. 1135–1196). Amsterdam: Elsevier Academic Press; 10.1016/B978-0-12-372522-6.00026-8 [DOI] [Google Scholar]
- Suikkanen, S. , Pulina, S. , Engström‐Öst, J. , Lehtiniemi, M. , Lehtinen, S. , & Brutemark, A. (2013). Climate change and eutrophication induced shifts in Northern Summer Plankton communities. PLoS ONE, 8, e66475 10.1371/journal.pone.0066475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson, R. E. , & Fine, I. V. (2003). Estimating mixed layer depth from oceanic profile data. Journal of Atmospheric and Oceanic Technology, 20, 319–329. [DOI] [Google Scholar]
- Vahtera, E. , Conley, D. J. , Gustafsson, B. G. , Kuosa, H. , Pitkänen, H. , Savchuk, O. P. , Tamminen, T. , Viitasalo, M. , Voss, M. , Wasmund, N. , & Wulff, F. (2007). Internal ecosystem feedbacks enhance nitrogen‐fixing cyanobacteria blooms and complicate management in the Baltic Sea. AMBIO: A Journal of the Human Environment, 36, 186–194. 10.1579/0044-7447(2007)36[186:IEFENC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Vahtera, E. , Laanemets, J. , Pavelson, J. , Huttunen, M. , & Kononen, K. (2005). Effect of upwelling on the pelagic environment and bloom‐forming cyanobacteria in the western Gulf of Finland, Baltic Sea. Journal of Marine Systems, 58, 67–82. 10.1016/j.jmarsys.2005.07.001 [DOI] [Google Scholar]
- Voss, M. , Dippner, J. W. , Humborg, C. , Hürdler, J. , Korth, F. , Neumann, T. , Schernewski, G. , & Venohr, M. (2011). History and scenarios of future development of Baltic Sea eutrophication. Estuarine, Coastal and Shelf Science, 92, 307–322. 10.1016/j.ecss.2010.12.037 [DOI] [Google Scholar]
- Walve, J. , & Larsson, U. (1999). Carbon, nitrogen and phosphorus stoichiometry of crustacean zooplankton in the Baltic Sea: Implications for nutrient recycling. Journal of Plankton Research, 21, 2309–2321. 10.1093/plankt/21.12.2309 [DOI] [Google Scholar]
- Walworth, N. G. , Fu, F.‐X. , Lee, M. D. , Cai, X. , Saito, M. A. , Webb, E. A. , & Hutchins, D. A. (2018). Nutrient‐colimited Trichodesmium as a nitrogen source or sink in a future ocean. Applied and Environmental Microbiology, 84, e02137‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannicke, N. , Korth, F. , Liskow, I. , & Voss, M. (2013). Incorporation of diazotrophic fixed N2 by mesozooplankton—Case studies in the southern Baltic Sea. Journal of Marine Systems, 117–118, 1–13. 10.1016/j.jmarsys.2013.03.005 [DOI] [Google Scholar]
- Wasmund, N. (1997). Occurence of cyanobacterial blooms in the Baltic Sea in relation to environmental conditions. Internationale Revue der gesamten Hydrobiologie und Hydrographie, 82, 169–184. 10.1002/(ISSN)1522-2632 [DOI] [Google Scholar]
- Wasmund, N. , Nausch, G. , & Voss, M. (2012). Upwelling events may cause cyanobacteria blooms in the Baltic Sea. Journal of Marine Systems, 90, 67–76. 10.1016/j.jmarsys.2011.09.001 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


