Abstract
The past decade has seen significant advances in understanding of the pathogenesis and progression of lung disease in cystic fibrosis (CF). Pulmonary inflammation, infection, and structural lung damage manifest very early in life and are prevalent among preschool children and infants, often in the absence of symptoms or signs. Early childhood represents a pivotal period amenable to intervention strategies that could delay or prevent the onset of lung damage and alter the longer-term clinical trajectory for individuals with CF. This review summarizes what we have learned about early lung disease in children with CF and discusses the implications for future clinical practice and research.
Keywords: cystic fibrosis, early lung disease, newborn screening, early intervention
Cystic fibrosis (CF) is a life-limiting, autosomal recessive disorder affecting approximately 70,000 people worldwide and 30,000 in the United States (1, 2). The genetic defect in CF leads to dysfunction in the protein product, the cystic fibrosis transmembrane regulator (CFTR). Over the past decade, a considerable body of research has established that irreversible, progressive lung disease develops very early in life in CF as a consequence of this basic defect and is likely the precursor of the severe bronchiectasis in adulthood associated with respiratory morbidity and early mortality. Early life events are likely to determine the progression, severity, and disease burden later in life, alerting the clinician to intervene early. Improved understanding of the early pathophysiology is also revealing a number of potential therapeutic targets with growing awareness that introducing new treatments early in life will be important to enhance outcomes in the future.
The exact mechanisms linking the basic defect to organ damage, including irreversible damage to the lungs, is unclear. Espoused theories include volume depletion of the airway surface liquid due to sodium (and water) hyperabsorption and abnormalities in salt homeostasis (2). The earliest postmortem studies in human infants identified relatively normal airways but abnormal mucus glands (3, 4). Recent data obtained from animal models, notably the CF pig model (reviewed by Stoltz and colleagues [5]), have highlighted a number of defects identifiable at birth that may be relevant to human infants with CF. These include congenital airway abnormalities, increased acidity of the airway surface liquid that results in inhibition of the function of antimicrobial peptides (6), and failure of mucus to detach from submucosal gland ducts (5, 7). These basic defects lead to the clinical consequences of infection, inflammation, functional abnormalities, and lung structural damage.
Understanding lung disease in early life in terms of lung structure (8–10), physiology (11–13), infection (8), and inflammation (14, 15) informs potential intervention strategies in routine clinical practice. These in turn are dependent on proactive pulmonary surveillance and an ability to sensitively detect early lung disease. Data from intensive CF early surveillance programs, such as those developed by the Australian Respiratory Early Surveillance Program for Cystic Fibrosis (10, 16) and the London Cystic Fibrosis Collaboration (17), have provided significant insights into the biological mechanisms and natural history of lung disease in early life (18, 19). Collectively, these insights inform clinicians of the importance of early childhood in the establishment of future lung disease, the role of early surveillance with sensitive methods of disease detection, and clinical and research directions. The aim of this review is to summarize these findings in the areas of lung inflammation and infection, structure, and function in early life, and to discuss implications for clinical practice and research.
Pulmonary Inflammation
Lung disease in CF is characterized by intense, neutrophil-dominated inflammation identified within the first few weeks of life, even in asymptomatic, culture-negative infants (14, 15, 20, 21). The presence of pulmonary inflammation in infancy and the preschool years is associated with worse nutritional status (22), the presence of organisms cultured from the lower respiratory airways (23), bronchiectasis on chest computed tomography (CT) imaging (8, 10, 24), and lung function abnormalities (16, 25). Although infection is a key contributor to the inflammatory milieu (23), the increase in pulmonary inflammation with age during the preschool years appears to be relatively independent of current or past infection status detected by bronchoalveolar lavage (BAL) (14). The role of neutrophil-derived serine proteases and reactive oxygen species in the evolution of early lung disease is recognized (26). Free neutrophil elastase activity is detectable in BAL in up to 30% of infants and predicts CT scan–diagnosed bronchiectasis by age 3 years (8, 27). Pulmonary infection in early life is associated with increased oxidative loss of glutathione and biomarkers of oxidative stress (26). These studies suggest the serine antiprotease host defense mechanisms are overwhelmed in CF, creating the potential for enzymatic degradation of the lung structural integrity (8, 20). Therapies targeting oxidative stress pathways (e.g., antimyeloperoxidase agents) may boost antioxidant defense and potentially slow the onset and progression of lung disease in CF. An expanding body of research is characterizing the impact of CFTR dysfunction on numerous immune pathways in the etiology of pulmonary inflammation (reviewed in [28]) and identifying potential therapeutic strategies for reducing inflammation and infection, including CFTR modulation. Clinical translation of these novel research findings into trials of tolerable antiinflammatory agents that effectively modulate the immune response has yet to materialize and presents a unique challenge in young children. However, modulation of pulmonary inflammation is a desirable and promising therapeutic avenue in the prevention of future lung disease.
Infection
Respiratory infections in CF are typically believed to be caused by bacteria such as Staphylococcus aureus, Haemophilus influenzae, and Pseudomonas aeruginosa. S. aureus is commonly cultured shortly after diagnosis and in up to 30% of infants during the first 6 months of life (1, 8). When cultured from the lower respiratory tract in infants and young children, these organisms are associated with pulmonary inflammation even in the absence of clinical symptoms or signs. The prevalence of infection increases throughout the preschool years (1), with S. aureus being the most common bacterium identified by standard culture. Aspergillus species and Streptococcus pneumoniae are also associated with pulmonary inflammation in young children with CF (23), but the role of these organisms in the development and progression of structural lung disease and lung function decline in CF remains unknown.
P. aeruginosa is a significant pathogen in terms of clinical decline and increased mortality (29), although the role of this organism in establishing or exacerbating early lung disease remains unclear. Initial infection with P. aeruginosa occurs early in life, with median age of first detection at around 2 years and as early as 3 months of age (24, 30). Recent data suggest that the environment, in addition to host and bacterial factors, plays a part in acquiring infection with this organism (31). Residence in a regional versus a metropolitan area is a significant risk factor for the first acquisition of P. aeruginosa (32). Temperature, rainfall, dew point, latitude, longitude, and elevation of residence have all been implicated in the first acquisition of P. aeruginosa, as has exposure to particulate environmental pollution (33, 34). Delaying the onset of first acquisition of infection with P. aeruginosa by environmental manipulation is an intervention that warrants further study.
Successful eradication of lower respiratory P. aeruginosa infection (detected in BAL fluid) is achievable in the majority of preschool children and infants (30, 35) and is associated with reduced prevalence of chronic P. aeruginosa infection in later life (36). Detection of P. aeruginosa in upper airway cultures is a poor predictor of lower respiratory infection, and P. aeruginosa serology neither improves the ability to predict first acquisition (37) nor has significant clinical value as an independent noninvasive marker of lower respiratory infection (38). The site of culture of P. aeruginosa does not seem to influence clinical outcomes (39), and it appears appropriate to treat P. aeruginosa when detected in upper airway samples. The role of P. aeruginosa as a causal factor in early lung disease or as a driver of disease progression is uncertain. Although detection and eradication of P. aeruginosa during the preschool years may prevent the morbidity and mortality associated with chronic P. aeruginosa infection, it does not appear sufficient to prevent the development of structural lung disease (39) or lower lung function. High rates of bronchiectasis were observed despite eradication treatment in an Australasian multicenter study (39). In other cohorts of children diagnosed either clinically or after newborn screening, prior eradication of P. aeruginosa was associated with ongoing increased pulmonary inflammation after eradication (30) and lower lung function measured by spirometry through preschool and school age, compared with those never infected with this organism (40, 41). These data suggest functional, structural, and inflammatory changes occur despite aggressive treatment and eradication of P. aeruginosa and also before its detection via airway cultures. It is therefore possible that P. aeruginosa infection may occur in those who already have worse lung disease or disease susceptibility.
When cultured, the eradication of S. aureus (methicillin-sensitive or -resistant organisms) or H. influenzae is not usually attempted, even though both are associated with lung function decline (41). Whether doing so is feasible or impacts on subsequent lung function decline or attenuates lung damage is worthy of further study, but such attempts at eradication need to be considered in the context of how this might impact the lung microbiota.
Whether the load of an organism present in the airways, as opposed to the presence of the organism per se, is important in CF lung disease is not known. The use of culture-independent techniques for microbiological analysis of airway specimens has to a certain extent altered our understanding of infecting and colonizing organisms in CF lung disease, such that infection is now understood to be polymicrobial, even though the microbiota might be dominated by a single, or a few, pathogens. Numerous studies, using both extended microbiological culture and molecular analysis techniques, have reported the frequent presence of obligate and facultative anaerobes in CF airway specimens, and there are now cross-sectional descriptions of the airway microbiota at various stages of CF lung disease. What remains to be elucidated, however, is the evolution of the lower airway microbiota in CF and its role in disease pathogenesis and progression.
Published longitudinal studies of infants and young children with CF have so far mainly explored the upper airway microbiota. Differences are apparent in the nasopharyngeal microbiota of subjects with CF and healthy control subjects from early infancy, before exposure to antibiotics. Two recent prospective cohort studies have described an increased relative abundance of Staphylococcus early in life (42, 43). In the study by Prevaes and colleagues (2016) of 324 samples from 20 infants, S. aureus, Streptococcus mitis, Corynebacterium accolens, and bacilli were more abundant than in samples from control subjects, and antibiotic use was associated with decreased total bacterial density and increased colonization with gram-negative bacteria (42). Mika and colleagues (2016) reported that in 461 samples from 30 infants, the relative abundance of Staphylococcae was increased and the relative abundance of Corynebacteriaceae and Pasteurellaceae was reduced. Antibiotic treatment was associated with a decrease in S. aureus and increase in coagulase-negative Staphylococci (43), again highlighting the potential for significant alterations in the microbiota associated with treatment. The composition of very early lower airway microbiota in infants with CF and its development over time is currently being explored (44). It is likely that treatment of traditional CF pathogens, including attempts at eradicating them in early life, will have impact on the microbiota and its constituency, and this needs to be considered in any adoption of such strategies.
Structural Lung Disease
Structural damage to the lung is the endpoint of the pathophysiological injury incurred through infection and inflammation, but due to difficulty in its assessment in its early stages it has not hitherto been the primary focus for clinicians. Many studies now identify infancy and the early preschool years as a critical period when structural changes in the CF lung begin (8, 9, 18, 45). Advances in CT technology, the advent of low-dose radiation acquisition protocols, and improved algorithms for acquisition of chest CT images have both reduced the radiation risk to the young child and markedly improved the sensitivity with which lung disease can be detected (46). Recent studies suggest that bronchiectasis, defined as a bronchus-to-arterial ratio of greater than 1.0, is present in many asymptomatic infants shortly after diagnosis (8). Although these radiological abnormalities are mild in comparison to the end-stage lung findings that predict survival in CF (47), the fact that they are present, persist, and progress in association with the presence of lower respiratory infection and neutrophil-dominated pulmonary inflammation (8, 10, 24) indicates that these early changes are the likely precursors of the bronchiectasis identified routinely in adolescent and adult patients with CF.
Bronchiectasis predates abnormalities detectable using traditional methods of disease detection, such as spirometry or chest radiograph (8, 10, 24, 45). CT scan–diagnosed bronchiectasis can be detected in up to 30 to 40% of children with CF aged between 3 and 4 years old (10, 24, 48) and in up to 80% of children by the age of 5 years (39, 49, 50). Bronchiectasis persists from one scan to the next within a 12-month period in three-fourths of individuals and increases in extent in 60% (24).
By definition, bronchiectasis is irreversible, and there is no evidence that this is not the case in early CF. Therefore, failure to prevent its development through current interventions might indicate a fundamental failure of treatment. Currently, there is no evidence that early identification of bronchiectasis will change or improve outcomes or whether prevention through treating upstream targets, such as infection or inflammation, is possible. Air trapping, defined as hyperlucent areas on expiratory chest CT scan, and bronchial wall thickening are seen more commonly than bronchiectasis. Although interobserver agreement when reporting bronchial wall thickening is poor (10), air trapping is more reliably identified, with excellent interobserver agreement (10, 24). The pathogenesis of early air trapping is unclear, although it may represent the earliest airway changes in CF, as suggested by the significant associations between air trapping and functional abnormalities reported in some of the earliest physiological studies done in infants with CF (51–53). More recent studies demonstrating poor correlation between end-expiratory volumes and gas trapping on CT scan (54) suggest other potential etiologies, including regional hypoperfusion (55). Air trapping may result from airway developmental abnormalities (56), severe bronchial wall thickening, or mucus impaction leading to airway closure. Some of these etiologies for air trapping, for example congenital tracheomalacia, might not be amenable to CF therapies, whereas others, such as inflammation and abnormal mucus, might be amenable. Air trapping on CT scan in infancy is a risk factor for bronchiectasis at 3 years of age (8, 27), and further study of the temporal relationship and associations between air trapping and irreversible, progressive bronchiectasis is indicated. New quantitative scoring systems designed for use in early mild lung disease in CF will significantly enhance the study of the pathogenesis of lung structural abnormalities and interventions designed to prevent them (57).
Lung Function
Lung function is diminished shortly after diagnosis of CF in infants after newborn screening as a group (13, 17) and is characterized by reduced forced expiratory flows and volumes and elevated functional residual capacity and lung clearance index (LCI), suggesting airway obstruction, air trapping, and ventilation inhomogeneity, respectively (13, 17, 53, 58). However, the majority of these infants have lung function within the normal range (17). Longitudinal surveillance suggests that lung function, including infant spirometry and multiple-breath washout, worsens with the presence of pulmonary inflammation and infection (16, 41, 59) but that those infants who remain free of pulmonary infections during infancy experience lung growth similar to that of healthy infants (41).
In older children with CF, the multiple-breath washout technique has been advocated as a potential surrogate for chest CT imaging for the detection of bronchiectasis (27, 46). Indeed, few school-age children with CF who had bronchiectasis had a normal LCI (49, 50). The negative and positive predictive values for the LCI were excellent in these children when using bronchiectasis identified on chest CT scan as the gold standard. In infants, LCI was associated with lower respiratory infection with P. aeruginosa and pulmonary inflammation (58) but not with early bronchiectasis (53), although indices of ventilation inhomogeneity were associated with the presence of air trapping on chest CT scan. More recently, we have reported the relationships between LCI and chest CT scan scored using the Perth-Rotterdam Annotated Grid Morphometric Analysis for CF (PRAGMA) algorithms in infants, children, and adolescents (60). In preschool and school-age children, the LCI had a good positive predictive value (83–86%) but a poor negative predictive value (50–55%) to detect the presence of bronchiectasis. It is likely then that LCI may be a useful surveillance tool to monitor structural lung disease in preschool and school-age children with CF. In infancy, LCI reflected air trapping better than it did CT-determined bronchiectasis (60). Clearly, more longitudinal studies from diagnosis are required, as interpretation of cross-sectional studies of lung function is limited.
Implications for Clinical Practice: A New Treatment Paradigm for CF
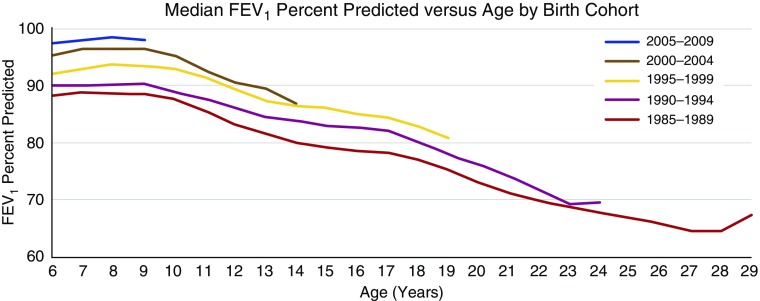
Epidemiological data demonstrate improving survival rates in CF, and FEV1 remains within normal ranges during childhood often until late adolescence; however, as shown in Figure 1, the slope of the decline in lung function through later childhood is unchanged.
Figure 1.
Median FEV1 has improved over the past two decades in those with cystic fibrosis (CF) by greater than 10% in 2009 (upper curve) compared with 1989 (lower curve). When first measured in school-age children with CF, median FEV1 is usually within the normal range (>80% predicted), where it remains throughout childhood (1). This indicates that factors such as treatment during the preschool years must have also improved. Despite this generational improvement in lung function, a large majority of children have underlying bronchiectasis, indicative of irreversible airway damage occurring irrespective of relatively preserved FEV1.
Despite FEV1 in the normal range, almost 80% of children with CF have evidence of bronchiectasis by the time they reach school age (39, 50). These data indicate that although we have been successful in achieving better lung function in modern cohorts of children with CF, largely through improved standard CF care, we have failed to prevent irreversible structural lung disease. The roots of structural lung disease in CF lie in early childhood and the developing lung. Therefore, the new treatment paradigm must be to attempt to prevent the onset and/or delay the progression of bronchiectasis, inflammation, and lung function decline (using more sensitive pulmonary function tests than spirometry) during the preschool years. If we are to see future improvements in longevity and quality of life, we should aim for both normal lung structure and function by the time children reach adolescence and early adulthood.
Current management of CF lung disease in most centers after diagnosis is weighted toward tertiary and quaternary prevention strategies (Table 1). The advent of newborn screening for CF, together with knowledge of the early onset and prevalence of lung disease, provides us with opportunities to develop secondary prevention strategies (Table 1). The next target for treatment arising from newborn screening is to develop genuine primary prevention strategies for structural lung disease, inflammation, and lung function in CF.
Table 1.
Strategies Associated with Screening Programs
| Screening Level | Definition |
|---|---|
| Primary prevention | Primary prevention strategies intend to avoid the development of disease. |
| Secondary prevention | Secondary prevention strategies attempt to diagnose and treat an existing disease in its early stages before it results in significant morbidity. |
| Tertiary prevention | Treatments aim to reduce the negative impact of established disease by restoring function and reducing disease-related complications. |
| Quaternary prevention | This term describes the set of health activities that mitigate or avoid the consequences of unnecessary or excessive interventions. |
Clinical Care and Future Strategies
An important question is, how should we respond to these data obtained mainly from observational studies? In addition to considering future therapeutic research strategies (61), are there any changes to current clinical practice that we can make immediately? Although we advocate for appropriately designed studies for all interventions in CF (62), it will not be possible to await the result of all those intervention studies, even if feasible, before striving to improve outcomes further for those with CF. On the basis of logic and experience, certain initiatives can be introduced to improve clinical care of infants and young children with CF, even if direct evidence of benefit is unavailable yet in this age group.
Knowledge that lung disease develops during the first few years of life obliges an improved and responsive process of CF education and support for parents and families after diagnosis. Early onset of lung disease, often in the absence of signs and symptoms, and the importance of surveillance and regular multidisciplinary CF care in the early years, should be central tenets of information for parents (63). Parents faced with potentially intensive surveillance and treatment in their “healthy-looking” child may benefit from psychosocial support to mitigate therapeutic ambivalence (64) and barriers to adherence that jeopardize early CF care.
Attitudinal change within CF clinics is important with increased attention to the importance of the early years in the establishment of lung disease. Accordingly, development of policies and guidelines that lead to a more unified proactive approach to care in asymptomatic and apparently healthy young children, rather than acceptance of a casual or reactive approach, are advocated. Measures including multiple-breath washout tests, CT imaging of the chest, and upper and lower airway microbiological sampling are available in the clinical sphere. The choice and timing of measures will be dependent on local expertise and acceptance of the challenges and limitations inherent to each modality.
Detection of lung abnormalities in early life makes a persuasive argument for intervention, even if evidence for specific treatments is scarce. A multidisciplinary review of the child’s progress and management, access to CF care, modifiable psychosocial risk factors, home treatment, and adherence may be undertaken. Therapeutic options may include antibiotics for CF pathogens detected in both symptomatic and asymptomatic individuals. Eradication of P. aeruginosa detected in respiratory cultures is considered standard clinical practice. The mucolytic and antiinflammatory actions of macrolides (65) and antibiotics targeting eradication of S. aureus and H. influenzae, even in the absence of current symptoms, may be considerations in the clinical discussion and response if such interventions are tolerated and can be shown to diminish inflammation without negatively impacting the lung microbiota (23). As upper airway cultures correlate poorly with those obtained from the lower airway (66), and in the context of organisms that are routinely cultured from the upper airways even in health (67), bronchoscopy and BAL are indicated when response to empirical treatment is suboptimal. There is rationale and support for introducing mucolytic agents to aid mucociliary clearance (68), with a small number of studies suggesting a reduction in pulmonary inflammation (69), exacerbations (70), lung function (71, 72), and CT abnormalities (73), although the increased burden and risks associated with administration of these treatments and maintaining equipment hygiene, and the potential risks associated with poor cleaning procedures, should be considered. Nutritional strategies aiming for rapid regain of birth weight percentiles (74) and normalizing levels of fat-soluble vitamins, especially vitamin D (75), are important. Increased frequency of clinical review and lower threshold for admissions for intensive multidisciplinary therapy are rational responses (68).
Potential future strategies include moving routine treatments into the preschool era and assessing their efficacy in this specific context, continuing to study CFTR modulators in preschool children and their role when introduced as soon as possible after diagnosis, and further developing international networks to conduct such studies. Large observational and therapeutic trials are being conducted worldwide, but with the availability of novel therapies that may change the course of this illness, the international CF community needs to work together to continue to develop the optimal outcome measures to reduce sample size requirements, to prioritize the most important studies to conduct in this finite population, and to carefully consider postapproval (phase 4) studies to look at long-term safety and efficacy of new therapies.
Predictors of Disease Severity and Targeted Treatment
The increase in survival in CF (76) has come at the cost of an increase in treatment burden through intensive use of antibiotics and physical and nutritional therapies. Although using therapies earlier and more intensively seems an appropriate response to current evidence, there are other potentially deleterious consequences, such as treatment-related side effects (77) and escalating health costs (78). Currently, treatments are recommended for the majority of patients, as our ability to predict an individual’s risk of progression is poor due to the weak genotype–phenotype correlations in CF, lack of disease predictors, and limited number of prospective longitudinal studies. As new, potentially disease-modifying treatments are likely to be extremely expensive and may have as-yet-unknown rare or long-term side effects, there is a desire to target those who have the most to gain to improve cost effectiveness and risk–benefit profiles, a strategy that should, ideally, apply to all therapies. Therefore, biological and psychosocial/nongenetic predictors of disease severity and progression are required urgently to inform clinical screening algorithms that aim to identify high-risk individuals from diagnosis. If we are to direct treatment to the very young, noninvasive biomarkers are required in infancy that predict the onset, severity, and progression of infection, inflammation, or bronchiectasis so that treatment can be tailored toward those with the greatest need (61). Longitudinal studies are beginning to identify the interplay between infection, inflammation, and bronchiectasis (27), but identifying better predictors should remain a crucial aspect of future research.
Monitoring Disease Progression and Effects of Therapy
For early intervention to succeed, tools are required to monitor disease progression and the effect of treatments, alongside the development of therapeutic interventions through clinical trials. Sensitive, reproducible outcome measures that can quantify and track early lung abnormalities in infants and young children are required. Significant advances in the last 10 years have led to the development of a range of techniques available to assess lung structure, inflammation, infection, and function in early life, and some of these have been used in clinical trials of interventions to prevent lung disease (79–81).
The advantages and limitations of techniques used in early intervention studies, which may have clinical potential in very young children, have been summarized (82). Chest CT scan and the multiple-breath washout were considered the best understood modalities at the time of the review, and since then both have been chosen as endpoints in investigator- and industry-sponsored clinical trials (79–81). The main advantages of CT are availability, access, and a well-validated, sensitive scoring technique (57). The main disadvantages are the need for anesthesia in very young children and radiation exposure. The risk of performing chest CT imaging requires consideration, as radiation doses should always be as low as reasonably achievable. Cumulative radiation doses provide risk akin to a single larger exposure to radiation. Despite this, it is estimated that current low-dose protocols are associated only with a very small, if not negligible, cancer risk (83). The estimated risk may be minimal in the context of a clinical trial with limited numbers of measurements (84). LCI is relatively simple, noninvasive, and accessible, although the relationship between changes in LCI and infection, inflammation, and progressive structural damage is unclear.
In addition, other potential tools are showing promise. These include magnetic resonance imaging, metabolomic biomarkers of inflammation (85), and oxidative stress (that can be measured in BAL, exhaled breath condensate, blood, and urine).
Improved algorithms and sequences have enhanced resolution of structural details using magnetic resonance imaging (86); however, these outcomes still need to be validated against chest CT imaging in young children (87). Magnetic resonance imaging might also offer additional insights, including measures of ventilation, inflammation, and perfusion (88). Clinical hurdles include the lack of standardized platforms for image acquisition and analysis and the relatively unpleasant environment of most scanners for very young children, such that sedation or anesthesia is still required.
The biggest challenge facing the clinical monitoring of young children with CF is how to detect progressive lung disease when symptoms are infrequent or the result of intercurrent viral infections that might not influence disease progression. In this context, current imaging modalities are unlikely to provide an answer in most situations: CT is not an appropriate tool for frequent assessments, and magnetic resonance imaging is expensive and there are likely to be access issues in most centers. The LCI and noninvasive markers of inflammation hold promise, but the relationships of these markers to underlying pathobiology during an exacerbation are not yet fully understood and need to be studied further.
Conclusions
Lung disease starts early in CF. As current approaches fail to prevent the majority of children with CF having established bronchiectasis by 5 years of age, a shift of focus is required to try to prevent this early disease progression, with future studies designed to modify the early disease trajectory targeting pulmonary inflammation, minimizing lung function decline, modifying the infectious environment, and aiming to prevent lung damage before it becomes irreversible. Maximizing the benefits and opportunities provided by newborn screening programs through earlier introduction and implementation of new CF treatment paradigms is important if we are to improve longevity and quality of life for the next generation of people with CF.
Footnotes
CME will be available for this article at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201606-1107CI on December 2, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: on behalf of the Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST-CF)
References
- 1.Cystic Fibrosis Foundation. Cystic Fibrosis Foundation Patient Registry Annual Data Report 2009. 2011 [accessed 2017 April 29]. Available from: www.cff.org.
- 2.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352:1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 3.Bedrossian CW, Greenberg SD, Singer DB, Hansen JJ, Rosenberg HS. The lung in cystic fibrosis: a quantitative study including prevalence of pathologic findings among different age groups. Hum Pathol. 1976;7:195–204. doi: 10.1016/s0046-8177(76)80023-8. [DOI] [PubMed] [Google Scholar]
- 4.Sturgess J, Imrie J. Quantitative evaluation of the development of tracheal submucosal glands in infants with cystic fibrosis and control infants. Am J Pathol. 1982;106:303–311. [PMC free article] [PubMed] [Google Scholar]
- 5.Stoltz DA, Meyerholz DK, Welsh MJ. Origins of cystic fibrosis lung disease. N Engl J Med. 2015;372:1574–1575. doi: 10.1056/NEJMc1502191. [DOI] [PubMed] [Google Scholar]
- 6.Shah VS, Meyerholz DK, Tang XX, Reznikov L, Abou Alaiwa M, Ernst SE, Karp PH, Wohlford-Lenane CL, Heilmann KP, Leidinger MR, et al. Airway acidification initiates host defense abnormalities in cystic fibrosis mice. Science. 2016;351:503–507. doi: 10.1126/science.aad5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoegger MJ, Fischer AJ, McMenimen JD, Ostedgaard LS, Tucker AJ, Awadalla MA, Moninger TO, Michalski AS, Hoffman EA, Zabner J, et al. Impaired mucus detachment disrupts mucociliary transport in a piglet model of cystic fibrosis. Science. 2014;345:818–822. doi: 10.1126/science.1255825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sly PD, Brennan S, Gangell C, de Klerk N, Murray C, Mott L, Stick SM, Robinson PJ, Robertson CF, Ranganathan SC Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST-CF) Lung disease at diagnosis in infants with cystic fibrosis detected by newborn screening. Am J Respir Crit Care Med. 2009;180:146–152. doi: 10.1164/rccm.200901-0069OC. [DOI] [PubMed] [Google Scholar]
- 9.Martínez TM, Llapur CJ, Williams TH, Coates C, Gunderman R, Cohen MD, Howenstine MS, Saba O, Coxson HO, Tepper RS. High-resolution computed tomography imaging of airway disease in infants with cystic fibrosis. Am J Respir Crit Care Med. 2005;172:1133–1138. doi: 10.1164/rccm.200412-1665OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stick SM, Brennan S, Murray C, Douglas T, von Ungern-Sternberg BS, Garratt LW, Gangell CL, De Klerk N, Linnane B, Ranganathan S, et al. Bronchiectasis in infants and preschool children diagnosed with cystic fibrosis after newborn screening. J Pediatr. 2009;155:623–6288.e1. doi: 10.1016/j.jpeds.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Ranganathan SC, Dezateux C, Bush A, Carr SB, Castle RA, Madge S, Price J, Stroobant J, Wade A, Wallis C, et al. London Collaborative Cystic Fibrosis Group. Airway function in infants newly diagnosed with cystic fibrosis. Lancet. 2001;358:1964–1965. doi: 10.1016/s0140-6736(01)06970-7. [DOI] [PubMed] [Google Scholar]
- 12.Ranganathan SC, Stocks J, Dezateux C, Bush A, Wade A, Carr S, Castle R, Dinwiddie R, Hoo AF, Lum S, et al. The evolution of airway function in early childhood following clinical diagnosis of cystic fibrosis. Am J Respir Crit Care Med. 2004;169:928–933. doi: 10.1164/rccm.200309-1344OC. [DOI] [PubMed] [Google Scholar]
- 13.Linnane BM, Hall GL, Nolan G, Brennan S, Stick SM, Sly PD, Robertson CF, Robinson PJ, Franklin PJ, Turner SW, et al. AREST-CF. Lung function in infants with cystic fibrosis diagnosed by newborn screening. Am J Respir Crit Care Med. 2008;178:1238–1244. doi: 10.1164/rccm.200804-551OC. [DOI] [PubMed] [Google Scholar]
- 14.Armstrong DS, Grimwood K, Carlin JB, Carzino R, Gutièrrez JP, Hull J, Olinsky A, Phelan EM, Robertson CF, Phelan PD. Lower airway inflammation in infants and young children with cystic fibrosis. Am J Respir Crit Care Med. 1997;156:1197–1204. doi: 10.1164/ajrccm.156.4.96-11058. [DOI] [PubMed] [Google Scholar]
- 15.Khan TZ, Wagener JS, Bost T, Martinez J, Accurso FJ, Riches DW. Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med. 1995;151:1075–1082. doi: 10.1164/ajrccm/151.4.1075. [DOI] [PubMed] [Google Scholar]
- 16.Pillarisetti N, Williamson E, Linnane B, Skoric B, Robertson CF, Robinson P, Massie J, Hall GL, Sly P, Stick S, et al. Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST CF) Infection, inflammation, and lung function decline in infants with cystic fibrosis. Am J Respir Crit Care Med. 2011;184:75–81. doi: 10.1164/rccm.201011-1892OC. [DOI] [PubMed] [Google Scholar]
- 17.Hoo AF, Thia LP, Nguyen TT, Bush A, Chudleigh J, Lum S, Ahmed D, Balfour Lynn I, Carr SB, Chavasse RJ, et al. London Cystic Fibrosis Collaboration. Lung function is abnormal in 3-month-old infants with cystic fibrosis diagnosed by newborn screening. Thorax. 2012;67:874–881. doi: 10.1136/thoraxjnl-2012-201747. [DOI] [PubMed] [Google Scholar]
- 18.VanDevanter DR, Kahle JS, O’Sullivan AK, Sikirica S, Hodgkins PS. Cystic fibrosis in young children: a review of disease manifestation, progression, and response to early treatment. J Cyst Fibros. 2016;15:147–157. doi: 10.1016/j.jcf.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Bush A, Sly PD. Evolution of cystic fibrosis lung function in the early years. Curr Opin Pulm Med. 2015;21:602–608. doi: 10.1097/MCP.0000000000000209. [DOI] [PubMed] [Google Scholar]
- 20.Armstrong DS, Grimwood K, Carzino R, Carlin JB, Olinsky A, Phelan PD. Lower respiratory infection and inflammation in infants with newly diagnosed cystic fibrosis. BMJ. 1995;310:1571–1572. doi: 10.1136/bmj.310.6994.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thursfield RM, Bush A, Alton EW, Davies JC. Airway inflammation is present by 4 months in CF infants diagnosed on newborn screening. Pediatr Pulmonol. 2012;47:352. [Google Scholar]
- 22.Ranganathan SC, Parsons F, Gangell C, Brennan S, Stick SM, Sly PD Australian Respiratory Early Surveillance Team for Cystic Fibrosis. Evolution of pulmonary inflammation and nutritional status in infants and young children with cystic fibrosis. Thorax. 2011;66:408–413. doi: 10.1136/thx.2010.139493. [DOI] [PubMed] [Google Scholar]
- 23.Gangell C, Gard S, Douglas T, Park J, de Klerk N, Keil T, Brennan S, Ranganathan S, Robins-Browne R, Sly PD AREST CF. Inflammatory responses to individual microorganisms in the lungs of children with cystic fibrosis. Clin Infect Dis. 2011;53:425–432. doi: 10.1093/cid/cir399. [DOI] [PubMed] [Google Scholar]
- 24.Mott LS, Park J, Murray CP, Gangell CL, de Klerk NH, Robinson PJ, Robertson CF, Ranganathan SC, Sly PD, Stick SM AREST CF. Progression of early structural lung disease in young children with cystic fibrosis assessed using CT. Thorax. 2012;67:509–516. doi: 10.1136/thoraxjnl-2011-200912. [DOI] [PubMed] [Google Scholar]
- 25.Brennan S, Hall GL, Horak F, Moeller A, Pitrez PM, Franzmann A, Turner S, de Klerk N, Franklin P, Winfield KR, et al. Correlation of forced oscillation technique in preschool children with cystic fibrosis with pulmonary inflammation. Thorax. 2005;60:159–163. doi: 10.1136/thx.2004.026419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kettle AJ, Turner R, Gangell CL, Harwood DT, Khalilova IS, Chapman AL, Winterbourn CC, Sly PD AREST CF Investigators. Oxidation contributes to low glutathione in the airways of children with cystic fibrosis. Eur Respir J. 2014;44:122–129. doi: 10.1183/09031936.00170213. [DOI] [PubMed] [Google Scholar]
- 27.Sly PD, Gangell CL, Chen L, Ware RS, Ranganathan S, Mott LS, Murray CP, Stick SM AREST CF Investigators. Risk factors for bronchiectasis in children with cystic fibrosis. N Engl J Med. 2013;368:1963–1970. doi: 10.1056/NEJMoa1301725. [DOI] [PubMed] [Google Scholar]
- 28.Cantin AM, Hartl D, Konstan MW, Chmiel JF. Inflammation in cystic fibrosis lung disease: pathogenesis and therapy. J Cyst Fibros. 2015;14:419–430. doi: 10.1016/j.jcf.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 29.Emerson J, Rosenfeld M, McNamara S, Ramsey B, Gibson RL. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol. 2002;34:91–100. doi: 10.1002/ppul.10127. [DOI] [PubMed] [Google Scholar]
- 30.Douglas TA, Brennan S, Gard S, Berry L, Gangell C, Stick SM, Clements BS, Sly PD. Acquisition and eradication of P. aeruginosa in young children with cystic fibrosis. Eur Respir J. 2009;33:305–311. doi: 10.1183/09031936.00043108. [DOI] [PubMed] [Google Scholar]
- 31.Psoter KJ, Rosenfeld M, De Roos AJ, Mayer JD, Wakefield J. Differential geographical risk of initial Pseudomonas aeruginosa acquisition in young US children with cystic fibrosis. Am J Epidemiol. 2014;179:1503–1513. doi: 10.1093/aje/kwu077. [DOI] [PubMed] [Google Scholar]
- 32.Ranganathan SC, Skoric B, Ramsay KA, Carzino R, Gibson AM, Hart E, Harrison J, Bell SC, Kidd TJ Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST-CF) Geographical differences in first acquisition of Pseudomonas aeruginosa in cystic fibrosis. Ann Am Thorac Soc. 2013;10:108–114. doi: 10.1513/AnnalsATS.201209-077OC. [DOI] [PubMed] [Google Scholar]
- 33.Psoter KJ, De Roos AJ, Wakefield J, Mayer JD, Bryan M, Rosenfeld M. Association of meteorological and geographical factors and risk of initial Pseudomonas aeruginosa acquisition in young children with cystic fibrosis. Epidemiol Infect. 2016;155:1075–1083. doi: 10.1017/S0950268815002411. [DOI] [PubMed] [Google Scholar]
- 34.Psoter KJ, De Roos AJ, Mayer JD, Kaufman JD, Wakefield J, Rosenfeld M. Fine particulate matter exposure and initial Pseudomonas aeruginosa acquisition in cystic fibrosis. Ann Am Thorac Soc. 2015;12:385–391. doi: 10.1513/AnnalsATS.201408-400OC. [DOI] [PubMed] [Google Scholar]
- 35.Kidd TJ, Ramsay KA, Vidmar S, Carlin JB, Bell SC, Wainwright CE, Grimwood K. ACFBAL Study Investigators. Pseudomonas aeruginosa genotypes acquired by children with cystic fibrosis by age 5-years. J Cyst Fibros. 2015;14:361–369. doi: 10.1016/j.jcf.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 36.Frederiksen B, Koch C, Høiby N. Antibiotic treatment of initial colonization with Pseudomonas aeruginosa postpones chronic infection and prevents deterioration of pulmonary function in cystic fibrosis. Pediatr Pulmonol. 1997;23:330–335. doi: 10.1002/(sici)1099-0496(199705)23:5<330::aid-ppul4>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 37.Daines C, VanDeVanter D, Khan U, Emerson J, Heltshe S, McNamara S, Anstead M, Langkamp M, Doring G, Ratjen F, et al. EPIC Investigators. Serology as a diagnostic tool for predicting initial Pseudomonas aeruginosa acquisition in children with cystic fibrosis. J Cyst Fibros. 2014;13:542–549. doi: 10.1016/j.jcf.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 38.Douglas TA, Brennan S, Berry L, Winfield K, Wainwright CE, Grimwood K, Stick SM, Sly PD members of AREST CF and ACFBAL Trial. Value of serology in predicting Pseudomonas aeruginosa infection in young children with cystic fibrosis. Thorax. 2010;65:985–990. doi: 10.1136/thx.2009.132845. [DOI] [PubMed] [Google Scholar]
- 39.Wainwright CE, Vidmar S, Armstrong DS, Byrnes CA, Carlin JB, Cheney J, Cooper PJ, Grimwood K, Moodie M, Robertson CF, et al. ACFBAL Study Investigators. Effect of bronchoalveolar lavage-directed therapy on Pseudomonas aeruginosa infection and structural lung injury in children with cystic fibrosis: a randomized trial. JAMA. 2011;306:163–171. doi: 10.1001/jama.2011.954. [DOI] [PubMed] [Google Scholar]
- 40.Kozlowska WJ, Bush A, Wade A, Aurora P, Carr SB, Castle RA, Hoo AF, Lum S, Price J, Ranganathan S, et al. London Cystic Fibrosis Collaboration. Lung function from infancy to the preschool years after clinical diagnosis of cystic fibrosis. Am J Respir Crit Care Med. 2008;178:42–49. doi: 10.1164/rccm.200710-1599OC. [DOI] [PubMed] [Google Scholar]
- 41.Ramsey KA, Ranganathan S, Park J, Skoric B, Adams AM, Simpson SJ, Robins-Browne RM, Franklin PJ, de Klerk NH, Sly PD, et al. AREST CF. Early respiratory infection is associated with reduced spirometry in children with cystic fibrosis. Am J Respir Crit Care Med. 2014;190:1111–1116. doi: 10.1164/rccm.201407-1277OC. [DOI] [PubMed] [Google Scholar]
- 42.Prevaes SM, de Winter-de Groot KM, Janssens HM, de Steenhuijsen Piters WA, Tramper-Stranders GA, Wyllie AL, Hasrat R, Tiddens HA, van Westreenen M, van der Ent CK, et al. Development of the nasopharyngeal microbiota in infants with cystic fibrosis. Am J Respir Crit Care Med. 2016;193:504–515. doi: 10.1164/rccm.201509-1759OC. [DOI] [PubMed] [Google Scholar]
- 43.Mika M, Korten I, Qi W, Regamey N, Frey U, Casaulta C, Latzin P, Hilty M SCILD study group. The nasal microbiota in infants with cystic fibrosis in the first year of life: a prospective cohort study. Lancet Respir Med. 2016;4:627–635. doi: 10.1016/S2213-2600(16)30081-9. [DOI] [PubMed] [Google Scholar]
- 44.Frayman KB, Armstrong DS, Carzino R, Ferkol T, Grimwood K, Storch GA, Teo SM, Wylie KM, Ranganathan SC.The lower airway microbiota in early cystic fibrosis lung disease: a longitudinal analysis. Thorax [online ahead of print] 9 Mar 2017; DOI: 10.1136/thoraxjnl-2016-209279. [DOI] [PubMed]
- 45.Brody AS. Early morphologic changes in the lungs of asymptomatic infants and young children with cystic fibrosis. J Pediatr. 2004;144:145–146. doi: 10.1016/j.jpeds.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 46.Simpson SJ, Mott LS, Esther CR, Jr, Stick SM, Hall GL. Novel end points for clinical trials in young children with cystic fibrosis. Expert Rev Respir Med. 2013;7:231–243. doi: 10.1586/ers.13.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Loeve M, Krestin GP, Rosenfeld M, de Bruijne M, Stick SM, Tiddens HA. Chest computed tomography: a validated surrogate endpoint of cystic fibrosis lung disease? Eur Respir J. 2013;42:844–857. doi: 10.1183/09031936.00051512. [DOI] [PubMed] [Google Scholar]
- 48.Mott LS, Gangell CL, Murray CP, Stick SM, Sly PD, Arest CF. Bronchiectasis in an asymptomatic infant with cystic fibrosis diagnosed following newborn screening. J Cyst Fibros. 2009;8:285–857. doi: 10.1016/j.jcf.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 49.Gustafsson PM, De Jong PA, Tiddens HA, Lindblad A. Multiple-breath inert gas washout and spirometry versus structural lung disease in cystic fibrosis. Thorax. 2008;63:129–134. doi: 10.1136/thx.2007.077784. [DOI] [PubMed] [Google Scholar]
- 50.Owens CM, Aurora P, Stanojevic S, Bush A, Wade A, Oliver C, Calder A, Price J, Carr SB, Shankar A, et al. London Cystic Fibrosis Collaboration. Lung Clearance Index and HRCT are complementary markers of lung abnormalities in young children with CF. Thorax. 2011;66:481–488. doi: 10.1136/thx.2010.150375. [DOI] [PubMed] [Google Scholar]
- 51.Gappa M, Ranganathan SC, Stocks J. Lung function testing in infants with cystic fibrosis: lessons from the past and future directions. Pediatr Pulmonol. 2001;32:228–245. doi: 10.1002/ppul.1113. [DOI] [PubMed] [Google Scholar]
- 52.Phelan PD, Gracey M, Williams HE, Anderson CM. Ventilatory function in infants with cystic fibrosis: physiological assessment of halation therapy. Arch Dis Child. 1969;44:393–400. doi: 10.1136/adc.44.235.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hall GL, Logie KM, Parsons F, Schulzke SM, Nolan G, Murray C, Ranganathan S, Robinson P, Sly PD, Stick SM, et al. AREST CF Investigators. Air trapping on chest CT is associated with worse ventilation distribution in infants with cystic fibrosis diagnosed following newborn screening. PLoS One. 2011;6:e23932. doi: 10.1371/journal.pone.0023932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosenow TM, Mok C, Ramsay KA, Murray C, Hall GL, Stick SM. End-expiratory lung volume is not associated with gas trapping on CT in early cystic fibrosis lung disease [abstract] Am J Respir Crit Care Med. 2016;193:A5591. [Google Scholar]
- 55.Wielpütz MO, Puderbach M, Kopp-Schneider A, Stahl M, Fritzsching E, Sommerburg O, Ley S, Sumkauskaite M, Biederer J, Kauczor HU, et al. Magnetic resonance imaging detects changes in structure and perfusion, and response to therapy in early cystic fibrosis lung disease. Am J Respir Crit Care Med. 2014;189:956–965. doi: 10.1164/rccm.201309-1659OC. [DOI] [PubMed] [Google Scholar]
- 56.Adam RJ, Michalski AS, Bauer C, Abou Alaiwa MH, Gross TJ, Awadalla MS, Bouzek DC, Gansemer ND, Taft PJ, Hoegger MJ, et al. Air trapping and airflow obstruction in newborn cystic fibrosis piglets. Am J Respir Crit Care Med. 2013;188:1434–1441. doi: 10.1164/rccm.201307-1268OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosenow T, Oudraad MC, Murray CP, Turkovic L, Kuo W, de Bruijne M, Ranganathan SC, Tiddens HA, Stick SM Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST CF) PRAGMA-CF: a quantitative structural lung disease computed tomography outcome in young children with cystic fibrosis. Am J Respir Crit Care Med. 2015;191:1158–1165. doi: 10.1164/rccm.201501-0061OC. [DOI] [PubMed] [Google Scholar]
- 58.Belessis Y, Dixon B, Hawkins G, Pereira J, Peat J, MacDonald R, Field P, Numa A, Morton J, Lui K, et al. Early cystic fibrosis lung disease detected by bronchoalveolar lavage and lung clearance index. Am J Respir Crit Care Med. 2012;185:862–873. doi: 10.1164/rccm.201109-1631OC. [DOI] [PubMed] [Google Scholar]
- 59.Simpson SJ, Ranganathan S, Park J, Turkovic L, Robins-Browne RM, Skoric B, Ramsey KA, Rosenow T, Banton GL, Berry L, et al. AREST CF. Progressive ventilation inhomogeneity in infants with cystic fibrosis after pulmonary infection. Eur Respir J. 2015;46:1680–1690. doi: 10.1183/13993003.00622-2015. [DOI] [PubMed] [Google Scholar]
- 60.Ramsey KA, Rosenow T, Turkovic L, Skoric B, Banton G, Adams AM, Simpson SJ, Murray C, Ranganathan SC, Stick SM, et al. AREST CF. Lung clearance index and structural lung disease on computed tomography in early cystic fibrosis. Am J Respir Crit Care Med. 2016;193:60–67. doi: 10.1164/rccm.201507-1409OC. [DOI] [PubMed] [Google Scholar]
- 61.Pittman JE, Cutting G, Davis SD, Ferkol T, Boucher R. Cystic fibrosis: NHLBI Workshop on the Primary Prevention of Chronic Lung Diseases. Ann Am Thorac Soc. 2014;11:S161–S168. doi: 10.1513/AnnalsATS.201312-444LD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stick SM, Sly PD. Exciting new clinical trials in cystic fibrosis: infants need not apply. Am J Respir Crit Care Med. 2011;183:1577–1578. doi: 10.1164/rccm.201102-0251ED. [DOI] [PubMed] [Google Scholar]
- 63.Jessup M, Douglas T, Priddis L, Branch-Smith C, Shields L, Arest CF AREST-CF. Parental experience of information and education processes following diagnosis of their infant with cystic fibrosis via newborn screening. J Pediatr Nurs. 2016;31:e233–e241. doi: 10.1016/j.pedn.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 64.Branch-Smith C, Pooley J, Shields L, Stick S, Douglas T ARESTCF. What do parents experience and how do they cope with the AREST-CF early surveillance program for infants and children with cystic fibrosis? J Cyst Fibros. 2013;12:S3. doi: 10.1016/j.jcf.2020.10.001. [DOI] [PubMed] [Google Scholar]
- 65.Saiman L, Anstead M, Mayer-Hamblett N, Lands LC, Kloster M, Hocevar-Trnka J, Goss CH, Rose LM, Burns JL, Marshall BC, et al. AZ0004 Azithromycin Study Group. Effect of azithromycin on pulmonary function in patients with cystic fibrosis uninfected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA. 2010;303:1707–1715. doi: 10.1001/jama.2010.563. [DOI] [PubMed] [Google Scholar]
- 66.Rosenfeld M, Emerson J, Accurso F, Armstrong D, Castile R, Grimwood K, Hiatt P, McCoy K, McNamara S, Ramsey B, et al. Diagnostic accuracy of oropharyngeal cultures in infants and young children with cystic fibrosis. Pediatr Pulmonol. 1999;28:321–328. doi: 10.1002/(sici)1099-0496(199911)28:5<321::aid-ppul3>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 67.Rosenfeld M, Bernardo-Ocampo C, Emerson J, Genatossio A, Burns J, Gibson R. Prevalence of cystic fibrosis pathogens in the oropharynx of healthy children and implications for cystic fibrosis care. J Cyst Fibros. 2012;11:456–457. doi: 10.1016/j.jcf.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 68.Padman R, McColley SA, Miller DP, Konstan MW, Morgan WJ, Schechter MS, Ren CL, Wagener JS Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis. Infant care patterns at epidemiologic study of cystic fibrosis sites that achieve superior childhood lung function. Pediatrics. 2007;119:e531–e537. doi: 10.1542/peds.2006-1414. [DOI] [PubMed] [Google Scholar]
- 69.Paul K, Rietschel E, Ballmann M, Griese M, Worlitzsch D, Shute J, Chen C, Schink T, Döring G, van Koningsbruggen S, et al. Bronchoalveolar Lavage for the Evaluation of Antiinflammatory Treatment Study Group. Effect of treatment with dornase alpha on airway inflammation in patients with cystic fibrosis. Am J Respir Crit Care Med. 2004;169:719–725. doi: 10.1164/rccm.200307-959OC. [DOI] [PubMed] [Google Scholar]
- 70.Quan JM, Tiddens HA, Sy JP, McKenzie SG, Montgomery MD, Robinson PJ, Wohl ME, Konstan MW Pulmozyme Early Intervention Trial Study Group. A two-year randomized, placebo-controlled trial of dornase alfa in young patients with cystic fibrosis with mild lung function abnormalities. J Pediatr. 2001;139:813–820. doi: 10.1067/mpd.2001.118570. [DOI] [PubMed] [Google Scholar]
- 71.Amin R, Subbarao P, Jabar A, Balkovec S, Jensen R, Kerrigan S, Gustafsson P, Ratjen F. Hypertonic saline improves the LCI in paediatric patients with CF with normal lung function. Thorax. 2010;65:379–383. doi: 10.1136/thx.2009.125831. [DOI] [PubMed] [Google Scholar]
- 72.Amin R, Subbarao P, Lou W, Jabar A, Balkovec S, Jensen R, Kerrigan S, Gustafsson P, Ratjen F. The effect of dornase alfa on ventilation inhomogeneity in patients with cystic fibrosis. Eur Respir J. 2011;37:806–812. doi: 10.1183/09031936.00072510. [DOI] [PubMed] [Google Scholar]
- 73.Nasr SZ, Kuhns LR, Brown RW, Hurwitz ME, Sanders GM, Strouse PJ. Use of computerized tomography and chest x-rays in evaluating efficacy of aerosolized recombinant human DNase in cystic fibrosis patients younger than age 5 years: a preliminary study. Pediatr Pulmonol. 2001;31:377–382. doi: 10.1002/ppul.1061. [DOI] [PubMed] [Google Scholar]
- 74.Lai HJ, Shoff SM, Farrell PM, Wisconsin Cystic Fibrosis Neonatal Screening G Wisconsin Cystic Fibrosis Neonatal Screening Group. Recovery of birth weight z score within 2 years of diagnosis is positively associated with pulmonary status at 6 years of age in children with cystic fibrosis. Pediatrics. 2009;123:714–722. doi: 10.1542/peds.2007-3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Douglas T, Park J, Ranganathan S, Hart E, Carzino R, Skoric B, Garratt L, Ebdon A, Sly PD, Stick S. Vitamin D deficiency is associated with increased risk of lower respiratory infection with S. aureus among infants & preschool children with CF [abstract] Pediatr Pulmonol. 2012;47:A264. [Google Scholar]
- 76.Dodge JA, Lewis PA, Stanton M, Wilsher J. Cystic fibrosis mortality and survival in the UK: 1947-2003. Eur Respir J. 2007;29:522–526. doi: 10.1183/09031936.00099506. [DOI] [PubMed] [Google Scholar]
- 77.Al-Aloul M, Miller H, Alapati S, Stockton PA, Ledson MJ, Walshaw MJ. Renal impairment in cystic fibrosis patients due to repeated intravenous aminoglycoside use. Pediatr Pulmonol. 2005;39:15–20. doi: 10.1002/ppul.20138. [DOI] [PubMed] [Google Scholar]
- 78.van Gool K, Norman R, Delatycki MB, Hall J, Massie J. Understanding the costs of care for cystic fibrosis: an analysis by age and health state. Value Health. 2013;16:345–355. doi: 10.1016/j.jval.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 79.Queensland Children’s Medical Research Institute. Prevention of bronchiectasis in infants with CF; 2016 [accessed 2016 Jun 1]. Available from: https://clinicaltrials.gov/ct2/show/NCT01270074.
- 80.CF Therapeutics Development Network Coordinating Center. Inhaled study of inhaled saline in cystic fibrosis; 2016 [accessed 2016 Jun 1]. Available from: https://clinicaltrials.gov/ct2/show/NCT00709280.
- 81.Vertex Pharmaceuticals Incorporated. A study to evaluate efficacy and safety of ivacaftor in subjects with cystic fibrosis who have a specified CFTR gating mutation; 2016 [accessed 2016 Jun 1]. Available from: https://clinicaltrials.gov/ct2/show/NCT02742519.
- 82.Stick S, Tiddens H, Aurora P, Gustafsson P, Ranganathan S, Robinson P, Rosenfeld M, Sly P, Ratjen F. Early intervention studies in infants and preschool children with cystic fibrosis: are we ready? Eur Respir J. 2013;42:527–538. doi: 10.1183/09031936.00108212. [DOI] [PubMed] [Google Scholar]
- 83.Rosenow T, Oudraad MC, Murray CP, Turkovic L, Kuo W, de Bruijne M, Ranganathan SC, Tiddens HA, Stick SM. Reply: excess risk of cancer from computed tomography scan is small but not so low as to be incalculable. Am J Respir Crit Care Med. 2015;192:1397–1399. doi: 10.1164/rccm.201508-1574LE. [DOI] [PubMed] [Google Scholar]
- 84.Kuo W, Ciet P, Tiddens HA, Zhang W, Guillerman RP, van Straten M. Monitoring cystic fibrosis lung disease by computed tomography: radiation risk in perspective. Am J Respir Crit Care Med. 2014;189:1328–1336. doi: 10.1164/rccm.201311-2099CI. [DOI] [PubMed] [Google Scholar]
- 85.Esther CR, Jr, Turkovic L, Rosenow T, Muhlebach MS, Boucher RC, Ranganathan S, Stick SM AREST CF. Metabolomic biomarkers predictive of early structural lung disease in cystic fibrosis. Eur Respir J. 2016;48:1612–1621. doi: 10.1183/13993003.00524-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wielpütz MO, Eichinger M, Puderbach M. Magnetic resonance imaging of cystic fibrosis lung disease. J Thorac Imaging. 2013;28:151–159. doi: 10.1097/RTI.0b013e31828d40d4. [DOI] [PubMed] [Google Scholar]
- 87.Ciet P, Serra G, Bertolo S, Spronk S, Ros M, Fraioli F, Quattrucci S, Assael MB, Catalano C, Pomerri F, et al. Assessment of CF lung disease using motion corrected PROPELLER MRI: a comparison with CT. Eur Radiol. 2016;26:780–787. doi: 10.1007/s00330-015-3850-9. [DOI] [PubMed] [Google Scholar]
- 88.Tiddens HA, Stick SM, Wild JM, Ciet P, Parker GJ, Koch A, Vogel-Claussen J. Respiratory tract exacerbations revisited: ventilation, inflammation, perfusion, and structure (VIPS) monitoring to redefine treatment. Pediatr Pulmonol. 2015;50:S57–S65. doi: 10.1002/ppul.23266. [DOI] [PubMed] [Google Scholar]