Abstract
Rationale: Intensive care unit (ICU) capacity strain refers to the potential limits placed on an ICU’s ability to provide high-quality care for all patients who may need it at a given time. Few studies have investigated how fluctuations in ICU capacity strain might influence care outside the ICU.
Objectives: To determine whether ICU capacity strain is associated with initial level of inpatient care and outcomes for emergency department (ED) patients hospitalized for sepsis.
Methods: We performed a retrospective cohort study of patients with sepsis admitted from the ED to a medical ward or ICU at three hospitals within the University of Pennsylvania Health System between 2012 and 2015. Patients were excluded if they required life support therapies, defined as invasive or noninvasive ventilatory support or vasopressors, at the time of admission. The exposures were four measures of ICU capacity strain at the time of the ED disposition decision: ICU occupancy, ICU turnover, ICU census acuity, and ward occupancy. The primary outcome was the decision to admit to a ward or to an ICU. Secondary analyses assessed the association of ICU capacity strain with in-hospital outcomes, including mortality.
Results: Among 77,142 hospital admissions from the ED, 3,067 patients met the study’s eligibility criteria. The ICU capacity strain metrics varied between and within study hospitals over time. In unadjusted analyses, ICU occupancy, ICU turnover, ICU census acuity, and ward occupancy were all negatively associated with ICU admission. In the fully adjusted model including patient-level covariates, only ICU occupancy remained associated with ICU admission (odds ratio, 0.87; 95% confidence interval, 0.79–0.96; P = 0.005), such that a 10% increase in ICU occupancy (e.g., one additional patient in a 10-bed ICU) was associated with a 13% decrease in the odds of ICU admission. Among the subset of patients admitted initially from the ED to a medical ward, ICU occupancy at the time of admission was associated with increased odds of hospital mortality (odds ratio, 1.61; 95% confidence interval, 1.21–2.14; P = 0.001).
Conclusions: The odds that patients in the ED with sepsis who do not require life support therapies will be admitted to the ICU are reduced when those ICUs experience high occupancy but not high levels of other previously explored measures of capacity strain. Patients with sepsis admitted to the wards during times of high ICU occupancy had increased odds of hospital mortality.
Keywords: intensive care unit capacity strain, ICU occupancy, sepsis
Intensive care unit (ICU) capacity strain refers to the potential limits—owing to occupancy, turnover, acuity, and other factors—placed on an ICU’s ability to provide high-quality care for all patients who may need it at a given time (1, 2). Prior research has demonstrated that ICU capacity strain varies markedly over time (3, 4) and influences ICU decision making (3, 5, 6) and processes of care (7, 8) but has weak, if any, associations with hospital mortality for ICU patients (3, 9). A smaller number of studies have investigated how fluctuations in ICU capacity strain might influence care outside the ICU (10–12). Researchers in one recent study reported that a fully occupied ICU was associated with reduced ICU admissions for patients in the emergency department (ED), but they examined capacity as full occupancy compared with anything less than full, potentially limiting an understanding of the association across the range of a continuous measure of occupancy (10).
Sepsis is the most expensive condition treated in U.S. hospitals and one of the most common causes of ICU admission and in-hospital death (13–16). We sought to determine, among patients who are admitted from the ED and do not require life support therapies at the time of admission, 1) whether ICU capacity strain is associated with ICU versus ward admission decisions, 2) which capacity strain metrics account for such associations, and 3) whether the severity of capacity strain is associated with patient outcomes.
Methods
Study Design and Study Sites
We performed a retrospective cohort study of patients with sepsis admitted from the ED to a medical ward or a medical ICU at three hospitals within the University of Pennsylvania Health System. Each of these hospitals has one ED and one medical ICU. (Table E1 in the online supplement describes study hospital characteristics.)
Study Population
Patients were eligible for inclusion if they 1) were admitted from the ED to a medical ward or medical ICU at one of the study hospitals between January 1, 2012, and September 30, 2015; 2) were at least 18 years of age at the time of hospital admission; and 3) had a clinical diagnosis of sepsis in the ED, defined as suspicion for infection (e.g., at least one microbiologic culture order written in the ED and at least one antibiotic or antifungal medication order written in the ED) and a Quick Sequential [Sepsis-related] Organ Failure Assessment (qSOFA) score of 2 or 3 (see Appendix E1) (15).
Patients were excluded if they were admitted initially to nonmedical wards or ICUs or were admitted directly to the hospital (i.e., not through the ED). To restrict the patient population to those not requiring life support therapies at the time of admission, whose ED disposition decision may not be discretionary, patients were excluded if vasopressors were ordered in the ED or if they required ventilatory assistance (invasive mechanical ventilation or noninvasive bilevel positive airway pressure [BiPAP]) at the time of admission (see Appendix E2). Finally, patients were excluded if they had any Do-Not-Resuscitate (DNR) status at the time of admission (see Appendix E3). Patients admitted to step-down units (SDUs) were excluded from the primary analysis because at the study hospitals, these units are designed primarily for cardiology admissions and do not routinely admit general medicine or cardiology patients with sepsis. A sensitivity analysis included patients admitted to SDUs as ward admissions.
Exposures
The primary study exposures were four capacity strain metrics previously associated with ICU processes of care and outcomes:
-
1.
ICU occupancy (defined as the percentage of medical ICU beds occupied)
-
2.
ICU turnover (defined as the percentage of medical ICU bed capacity newly admitted to the medical ICU in the prior 24 h)
-
3.
ICU census acuity (defined as the mean Sequential [Sepsis-related] Organ Failure Assessment [SOFA] score of all patients currently admitted to the medical ICU)
-
4.
Ward occupancy (17) (defined as the percentage of medical ward beds occupied) (see Appendix E4 for additional details)
Values of capacity strain metrics were assigned to study patients on the basis of the most recently calculated strain metric score before the time of each patient’s final ED disposition decision (a time point recorded in the electronic health record). ICU occupancy, ICU turnover, and ward occupancy were calculated once every hour throughout the study period. SOFA scores and ICU census acuity were calculated once every calendar day. SDU occupancy (defined as the percentage of SDU beds occupied) was calculated once every hour throughout the study period and was included as an additional strain metric in the SDU sensitivity analysis model. Sensitivity analyses were also performed with ICU occupancy modeled as the number of open ICU beds (to account for the fact that percentage ICU occupancy represents different numbers of open beds, depending on the bed capacity of a particular ICU) and to evaluate for any additional contributions from total time in the ED and the duration of time from ED disposition decision to inpatient admission.
Outcomes
The primary outcome was the ED disposition decision, defined as the initial inpatient level of care after leaving the ED (i.e., medical ward or ICU). Secondary outcomes included hospital mortality (with hospice discharge considered an in-hospital death), hospital length of stay (LOS), and discharge to home. Additional sensitivity analyses examined all eligible patients, regardless of DNR status, and models stratified on the basis of hospital, SOFA score, and qSOFA score (to evaluate whether certain patient subgroups might be differentially affected by capacity strain).
Adjustment Variables
Covariates selected a priori included age, sex, race, admission SOFA score, Comorbidity Point Score version 2 (COPS2, a comorbidity burden score based on 12 months of International Classification of Diseases diagnosis coding [18–20]), hospital, and academic year.
Statistical Analysis
Missingness of exposure, outcome, and adjustment variables was assessed and found to be sufficiently low (≤1.5% missingness for any individual variable, 2.7% of study patients with any missingness) that a complete case analysis could be performed without requiring imputation. Because all exposures and outcomes were in-hospital and contemporary to a given hospital admission, we considered each hospital admission, including multiple admissions of the same patient during the study period, to be unique patients for the purposes of our analysis; a sensitivity analysis considered only the first admission for each unique patient in the study population. Capacity strain metrics were tested first in unadjusted models and then in combination in the fully adjusted model that included all covariates. ICU occupancy, ICU turnover, ward occupancy, and SDU occupancy were scaled such that odds ratios (ORs) and 95% confidence intervals (CIs) represent the change in odds of the outcome associated with a 10% change in the tested strain metric. Binary outcomes were assessed with logistic regression models; the secondary outcome of hospital LOS was assessed using a Cox proportional hazards model with deaths considered as censoring events. Locally weighted scatterplot smoothing (LOWESS) plots were used to assess the linearity of the relationship between strain metrics and ED disposition decision. Model diagnostics were assessed by examining Pearson residuals. P values less than 0.05 were considered statistically significant. All analyses were conducted using Stata version 14.2 software (StataCorp LP). The study protocol was approved by the institutional review board of the University of Pennsylvania (Philadelphia, PA).
Results
Patient Characteristics
A total of 77,142 patients were admitted to a medical ward or ICU across the three study hospitals during the study period. Of these, 20,247 met criteria of suspicion for infection in the ED. Patients were excluded on the basis of requiring mechanical ventilation or BiPAP at the time of admission (n = 609), requiring vasopressors at the time of admission (n = 347), requiring both mechanical ventilation or BiPAP and vasopressors at the time of admission (n = 113), direct hospital admissions (i.e., from clinic or another hospital and not through the ED) or missing or corrupt ED date/time data (n = 560), and DNR status at the time of admission (n = 28). Among the remaining 18,590 patients with suspicion for infection, 3,151 (16.9%) patients met qSOFA criteria for sepsis, and 84 (2.7%) patients were excluded owing to one or more missing covariates or outcomes, leaving 3,067 patient admissions included in the final analyses (representing 2,720 unique patients, 29.1% of whom were admitted more than once during the study period).
Among these 3,067 patients with sepsis, the mean age was 60.8 years; 1,534 (50.0%) patients were male; 1,526 (49.8%) were black; and 2,988 (97.4%) were non-Hispanic. A total of 2,680 (87.4%) patients had a qSOFA score of 2, and 387 (12.6%) had a qSOFA score of 3. A total of 1,107 (36.1%) patients were admitted to the ICU. Median hospital LOS was 5.1 days (IQR, 2.9–9.0), and 290 (9.5%) patients died in the hospital or were discharged to hospice. Table 1 describes characteristics of the study population, and Table E2 describes patient characteristics by ED disposition decision.
Table 1.
Patient characteristics
| Characteristics | Patients with Sepsis (n = 3,067) |
|---|---|
| Hospital, n (%) | |
| A | 2,108 (68.7) |
| B | 388 (12.7) |
| C | 571 (18.6) |
| Age, yr, mean (SD) | 60.8 (17.6) |
| Male sex, n (%) | 1,534 (50.0) |
| Race, n (%) | |
| White | 1,344 (43.8) |
| Black | 1,526 (49.8) |
| Asian | 104 (3.4) |
| Other* | 93 (3.0) |
| Non-Hispanic ethnicity, n (%) | 2,988 (97.4) |
| qSOFA† score, n (%) | |
| 2 points | 2,680 (87.4) |
| 3 points | 387 (12.6) |
| COPS2 score, mean (SD) | 124 (75) |
| Admitted to the ICU, n (%) | 1,107 (36.1) |
| Hospital LOS, d, median (IQR) | 5.1 (2.9–9.0) |
| Hospital mortality, n (%) | 290 (9.5) |
Definition of abbreviations: COPS2 = Comorbidity Point Score version 2; ICU = intensive care unit; IQR = interquartile range; LOS = length of stay; qSOFA = Quick Sequential (Sepsis-related) Organ Failure Assessment; SD = standard deviation
Includes Hawaiian/Pacific Islander, American Indian/Native American, and self-reported race.
Inclusion criteria restricted patients to those with qSOFA scores less than or equal to 2.
ICU Capacity Strain Metrics
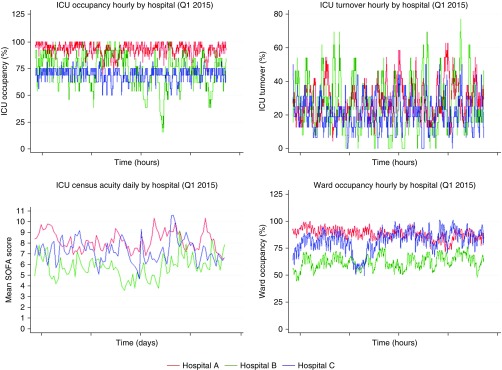
ICU occupancy was derived from 17,192 hospital admissions that included at least one medical ICU or cardiac care unit stay. ICU turnover and ICU census acuity were derived from 13,428 hospital admissions that included at least one medical ICU stay. (See Appendix E4 for additional ICU capacity strain metric calculation details.) Ward occupancy was derived from 156,569 hospital admissions that included at least one medical ward stay. Table 2 presents descriptive statistics of ICU capacity strain metrics by study hospital over the duration of the study period. Figure 1 illustrates that ICU capacity strain metrics varied on an hourly (ICU occupancy, ICU turnover, ward occupancy) and daily (ICU census acuity) level both within and among study hospitals over the duration of the study period. Summary statistics and variability of ICU capacity strain metrics remained similar when restricting to the ED disposition decision times of the study population (Figure E1, Table E3).
Table 2.
Intensive care unit capacity strain metrics, by hospital across the study period
| Hospital | Percent ICU Occupancy, Median (IQR) | Percent ICU Turnover, Median (IQR) | ICU Census Acuity, Mean SOFA Score (SD) | Percent Ward Occupancy, Median (IQR) |
|---|---|---|---|---|
| A | 91.7 (88.9–97.2) | 20.8 (16.7–29.2) | 8.1 (1.2) | 88.5 (84.6–92.5) |
| B | 69.2 (61.5–84.6) | 23.1 (15.4–30.8) | 5.9 (1.4) | 59.7 (53.5–66.7) |
| C | 75.0 (68.8–81.3) | 18.8 (12.5–25.0) | 6.0 (1.3) | 80.2 (72.5–85.7) |
Definition of abbreviations: ICU = intensive care unit; IQR = interquartile range; SOFA = Sequential (Sepsis-related) Organ Failure Assessment; SD = standard deviation
ICU occupancy, ICU turnover, and ward occupancy are calculated at the level of the hour over the study period. ICU census acuity is calculated at the level of the day over the study period.
Figure 1.
Intensive care unit (ICU) capacity strain metric variability, by hospital. The degree of ICU capacity strain metrics (vertical axes) is plotted on an hourly (for ICU occupancy, ICU turnover, and ward occupancy) or daily (for ICU census acuity) basis for the three study hospitals, represented by different colors, over a representative 3-month period during the study period. The figure demonstrates that the ICU capacity strain metrics varied on a granular level, both within and among study hospitals, over the duration of the study period. SOFA = Sequential (Sepsis-related) Organ Failure Assessment.
Unadjusted Analyses
Table 3 presents results from unadjusted analyses and the fully adjusted model. In the unadjusted analysis, ICU occupancy at the time of the ED disposition decision was associated with reduced odds of ICU admission (OR, 0.80; 95% CI, 0.76–0.84; P < 0.001), such that a 10% increase in ICU occupancy (e.g., one additional patient in a 10-bed ICU or two additional patients in a 20-bed ICU) was associated with a 20% decrease in the odds of ICU admission. In a LOWESS plot of unadjusted ICU occupancy and the likelihood of ICU admission, there was a strong negative linear relationship at ICU occupancies greater than approximately 70% (which applied during >75% of the disposition decisions for the study population). In a plot of ICU occupancy instead modeled as the number of open ICU beds, this relationship persisted between zero and approximately five open ICU beds (which applied during >83% of the ED disposition decisions for the study population). Figure 2 shows LOWESS plots of ICU occupancy and open ICU beds against ICU admission.
Table 3.
Association of intensive care unit capacity strain and intensive care unit admission
| Variables* | Unadjusted |
Adjusted |
||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| ICU occupancy† | 0.80 (0.76–0.84) | <0.001‡ | 0.87 (0.79–0.96) | 0.005‡ |
| ICU turnover† | 0.93 (0.86–0.99) | 0.03‡ | 1.08 (0.99–1.17) | 0.08 |
| ICU census acuity§ | 0.86 (0.82–0.91) | <0.001‡ | 1.00 (0.93–1.07) | 0.93 |
| Ward occupancy† | 0.94 (0.88–1.00) | 0.04‡ | 1.05 (0.93–1.18) | 0.44 |
| Age | 1.01 (1.00–1.01) | 0.001‡ | 1.00 (1.00–1.01) | 0.37 |
| Male sex | 1.37 (1.18–1.59) | <0.001‡ | 1.20 (1.02–1.42) | 0.03‡ |
| Race (reference, white) | ||||
| Black | 1.43 (1.23–1.67) | <0.001‡ | 1.17 (0.98–1.39) | 0.09 |
| Asian | 1.19 (0.78–1.80) | 0.42 | 1.27 (0.81–2.01) | 0.30 |
| Other | 1.13 (0.72–1.75) | 0.60 | 1.01 (0.62–1.65) | 0.96 |
| SOFA score | 1.31 (1.27–1.35) | <0.001‡ | 1.31 (1.26–1.35) | <0.001‡ |
| COPS2 score║ | 1.05 (1.04–1.06) | <0.001‡ | 1.04 (1.03–1.05) | <0.001‡ |
| Hospital (reference, A) | ||||
| B | 1.16 (0.92–1.46) | 0.22 | 1.21 (0.76–1.95) | 0.41 |
| C | 3.30 (2.73–4.00) | <0.001‡ | 3.31 (2.42–4.53) | <0.001‡ |
Definition of abbreviations: CI = confidence interval; COPS2 = Comorbidity Point Score version 2; ICU = intensive care unit; OR = odds ratio; SOFA = Sequential (Sepsis-related) Organ Failure Assessment.
Academic year covariate not shown.
ORs reflect a 10% increase in ICU occupancy, ICU turnover, and ward occupancy.
P < 0.05.
ORs reflect a 1-point increase in the mean SOFA score.
ORs reflect a 10-point increase in the COPS2 score.
Figure 2.
Locally weighted scatterplot smoothing plots of the percent intensive care unit (ICU) occupancy and open ICU beds against ICU admission. Among patients presenting to the emergency department with sepsis who do not receive life support therapies, the likelihood of ICU (vs. ward) admission (vertical axis) is plotted against ICU occupancy, represented as (A) percent ICU occupancy and (B) the number of open ICU beds, with a full ICU represented on the leftmost point on each horizontal axis. The figure demonstrates that beginning at approximately 70% occupancy and five open ICU beds, increasing ICU occupancy at time of the emergency department disposition decision is linearly associated with a reduced likelihood of ICU admission. (The plots were restricted to ICU occupancy ≥56.25% accounting for 95% of the study population and ≤10 open ICU beds accounting for 99% of the study population.)
In the unadjusted analyses, ICU turnover (OR, 0.93; 95% CI, 0.86–0.99; P = 0.03), ICU census acuity (OR, 0.86; 95% CI, 0.82–0.91; P < 0.001), and ward occupancy (OR, 0.94; 95% CI, 0.88–1.00; P = 0.04) were also associated with reduced odds of ICU admission. In LOWESS plots against the likelihood of ICU admission, ICU turnover demonstrated a consistently negative but nonlinear relationship with an inflection point at approximately 20% ICU turnover; ICU census acuity demonstrated a negative linear relationship; and ward occupancy demonstrated no relationship (Figure E2).
Association of ICU Capacity Strain and ICU Disposition Decision
In the fully adjusted model, the association of ICU occupancy with ICU admission persisted (OR, 0.87; 95% CI, 0.79–0.96; P = 0.005), such that a 10% increase in ICU occupancy was associated with a 13% decrease in the odds of ICU admission. This relationship likewise persisted when ICU occupancy was instead modeled as the number of open ICU beds and treated as both a continuous variable and an ordinal variable (zero, one, or two or more open ICU beds) (Table E4).
ICU turnover (OR, 1.08; 95% CI, 0.99–1.17; P = 0.08), ICU census acuity (OR, 1.00; 95% CI, 0.93–1.07; P = 0.93), and ward occupancy (OR, 1.05; 95% CI, 0.93–1.18; P = 0.44) were no longer associated with ICU admission after adjustment for all other strain metrics and covariates. Among patient-level covariates, index patient SOFA score (OR, 1.31 per 1-point SOFA score increase; 95% CI, 1.26–1.35; P < 0.001), COPS2 score (OR, 1.04 per 10-point COPS2 score increase; 95% CI, 1.03–1.05; P < 0.001), and age (OR, 1.20; 95% CI, 1.02–1.42; P = 0.03) remained significantly associated with ICU admission. Presentation to hospital C, which admits a greater proportion of its patients with sepsis to the ICU overall, was an independent predictor of ICU admission. Model diagnostics assessed by examining Pearson residuals revealed no outliers and good model performance. Variance inflation factors suggested collinearity between strain metrics, but iteratively excluding ICU turnover, ICU census acuity, and ward occupancy from the model yielded similar results, and excluding ICU occupancy yielded a nonpredictive model (Table E5). In sensitivity analyses, longer total time in the ED and longer duration of time from ED disposition decision to inpatient admission were each independently associated with reduced odds of ICU admission, and inclusion of either in the full model attenuated the ICU occupancy point estimate and strengthened a previously null ward occupancy OR (Table E6).
Association of ICU Occupancy and In-Hospital Outcomes
Analyses of the relationship between ICU capacity strain and hospital outcomes were conducted for ICU occupancy because it was the only ICU capacity strain metric associated with ICU admission in the fully adjusted model. Among the subset of patients admitted initially from the ED to a medical ward, ICU occupancy at the time of admission was associated with increased odds of hospital mortality (OR, 1.62; 95% CI, 1.28–2.07; P < 0.001), longer hospital LOS (hazard ratio, 0.94; 95% CI, 0.91–0.97; P < 0.001), and decreased odds of discharge to home (OR, 0.76; 95% CI, 0.67–0.86; P < 0.001) in unadjusted analyses. Among these ward patients, the association of ICU occupancy with hospital mortality (OR, 1.61; 95% CI, 1.21–2.14; P = 0.001) and with discharge to home (OR, 0.79; 95% CI, 0.67–0.92; P = 0.003) persisted after adjustment for patient-level covariates, whereas the association of ICU occupancy with hospital LOS was no longer statistically significant after adjustment. In contrast, for the overall study population and among the subset of patients admitted initially from the ED to the ICU, ICU occupancy at the time of admission was not associated with hospital mortality or discharge to home; of note, among patients admitted initially from the ED to the ICU, high ICU occupancy was associated with longer hospital LOS (hazard ratio, 0.92; 95% CI, 0.87–0.99; P = 0.02). (Hazard ratios <1 indicate a reduced likelihood of discharge and are therefore interpreted as an association with a longer LOS.) Table 4 presents results from unadjusted analyses and adjusted models assessing the association of ICU occupancy and in-hospital outcomes. Figure 3 plots the association between ICU occupancy and hospital mortality among patients with sepsis admitted to the ward, adjusted for patient-level covariates.
Table 4.
Association of intensive care unit occupancy and in-hospital outcomes
| Model | Patient Group | Hospital Mortality* |
Hospital LOS† |
Discharge to Home* |
|||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | HR (95% CI) | P Value | OR (95% CI) | P Value | ||
| Unadjusted | All patients | 1.03 (0.94–1.13) | 0.47 | 0.97 (0.95–1.00) | 0.05 | 0.96 (0.90–1.03) | 0.23 |
| ICU patients | 1.01 (0.90–1.13) | 0.87 | 0.94 (0.89–0.98) | 0.007‡ | 0.98 (0.90–1.07) | 0.68 | |
| Ward patients | 1.62 (1.28–2.07) | <0.001‡ | 0.94 (0.91–0.97) | <0.001‡ | 0.76 (0.67–0.86) | <0.001‡ | |
| Adjusted | All patients | 1.00 (0.88–1.13) | 0.998 | 0.98 (0.95–1.02) | 0.39 | 1.00 (0.91–1.09) | 0.95 |
| ICU patients | 0.98 (0.84–1.14) | 0.77 | 0.92 (0.87–0.99) | 0.02‡ | 0.99 (0.87–1.12) | 0.82 | |
| Ward patients | 1.61 (1.21–2.14) | 0.001‡ | 0.96 (0.92–1.00) | 0.06 | 0.79 (0.67–0.92) | 0.003‡ | |
Definition of abbreviations: CI = confidence interval; HR = hazard ratio; ICU = intensive care unit; LOS = length of stay; OR = odds ratio.
Logistic regression models.
Cox proportional hazards models censoring on death. HRs less than 1 indicate a reduced likelihood of discharge and are therefore interpreted as an association with a longer LOS.
P < 0.05.
ORs and HRs reflect a 10% increase in ICU occupancy.
Figure 3.
Association of intensive care unit (ICU) occupancy and hospital mortality among patients with sepsis admitted to the ward. Among patients presenting to the emergency department with sepsis who do not receive life support therapies and are admitted initially to a medical ward, observed hospital mortality (vertical axis) is plotted against the ICU occupancy (in 5% bucketed increments) at the time of the emergency department disposition decision. The figure demonstrates that ICU occupancy (between 50% and 100%) appears linearly associated with increased hospital mortality.
SDU Admissions
At the three study hospitals during the study period, 384 patients were admitted from the ED to an SDU and met all other inclusion criteria. When these patients were included as ward admissions in the full model, the association of ICU occupancy with ICU admission remained (OR, 0.87; 95% CI, 0.79–0.94; P = 0.001), and the newly included SDU occupancy metric was not predictive of ICU admission (OR, 1.02; 95% CI, 0.94–1.10; P = 0.61). (Table E7 displays the SDU sensitivity analysis model compared with the primary analysis model.)
Sensitivity Analyses
In additional sensitivity analyses, the association of ICU capacity strain metrics with ED disposition decision and the association of ICU occupancy with in-hospital outcomes remained similar when all patients were included, regardless of DNR status (Tables E8 and E9) and when only the first admission for each unique patient during the study period was included (Tables E10 and E11). When stratified by hospital rather than including hospital as a covariate, the point estimates for the association of ICU occupancy with ICU admission remained in the same direction as in the primary pooled model, but they lost statistical significance at the lower-volume hospitals B and C (Table E12). Similarly, the association between ICU occupancy and hospital mortality among patients admitted initially from the ED to a medical ward, stratified by hospital, showed consistent point estimates across facilities but loss of statistical significance at hospitals B and C (Table E13). Within the ICU and ward cohorts, further stratification based on qSOFA and SOFA scores did not reveal a pattern of effect modification between ICU occupancy and hospital mortality (Table E14).
Discussion
This study of the relationships between measures of ICU capacity strain and the disposition and outcomes of patients presenting to the ED with sepsis who do not receive life support therapies yields several important findings. First, ICU occupancy—a continuous variable treated as both percent ICU occupancy and the number of open ICU beds—was strongly negatively associated with the odds of ICU admission. This finding, robust to adjustment from other strain metrics and patient-level covariates and consistent across multiple sensitivity analyses, reinforces the findings of a prior single-center study showing that a completely full (vs. not full) ICU was associated with reduced ICU admission for all ED patients in whom ICU admission was requested (10). Our three-hospital study expands on these findings by treating strain metrics as continuous variables and ICU occupancy as both percent occupancy and the number of open beds.
Second, we found that ICU turnover, ICU census acuity, and ward occupancy were associated with ICU admission in unadjusted analyses, but these associations disappeared after adjustment for other strain metrics. This reinforces the importance of the ICU occupancy metric.
Third, this study illustrates that many ICU admissions may be highly discretionary, independent of clinical characteristics of the patient being triaged. It is well established that access to and use of ICU resources for patients with similar severities of illness for many diseases vary dramatically between hospitals (21). This study shows that the probability that patients with sepsis will be admitted to an ICU also varies dramatically within hospitals according to ICU bed availability, controlling for patient-level clinical characteristics.
Fourth, among patients with sepsis admitted initially to a medical ward, ICU occupancy was associated with increased odds of hospital mortality and decreased odds of discharge to home. In contrast, these associations were not found for all study patients or for patients admitted initially to the ICU.
There are several potential explanations for these findings. Sicker patients may be sent to wards during times of high ICU occupancy, thus “shunting” expected and observed worse outcomes to the wards that were not adequately captured by our risk adjustment. This explanation is supported by the fact that associations of ICU occupancy with all three outcomes among ward patients move toward their null values after adjustment for available patient characteristics; more granular characterization of these patients might have revealed truly null associations. The next possibility is that ICUs are better able to care for these sicker patients with sepsis. This explanation is supported by the fact that the association of ICU occupancy with mortality observed among patients sent to the wards is absent among those sent to ICUs. However, this finding could still also be consistent with incomplete risk adjustment—if more granular characterization of patients led to null associations with outcomes among all patients regardless of disposition, this would suggest that wards may be similarly capable of caring for these patients who might be considered to have discretionary indications for ICU admission. The findings in this study importantly cannot distinguish between these two related but different explanations. Finally, ED care may deteriorate during times of high ICU occupancy in ways that differentially affect patients awaiting ward beds (10), or ward care is adequate for these patients during times of low ICU occupancy but deteriorates during times of high ICU occupancy, perhaps because wards must then accommodate more and sicker patients with all illnesses and admission origins (22). Given the very different policy implications of these explanations, it will be important for future work to attempt to tease them apart.
Strengths and Limitations
The main strength of this study is the analysis of multiple strain metrics as continuous variables based on granular data from three diverse hospitals. The findings of this study should be interpreted in the context of a number of important limitations, however. First, this study evaluated patients from three hospitals in a single health system. Future studies would benefit from the addition of more hospitals from more geographically and organizationally diverse health systems and better take into account organizational differences between facilities. Second, our calculation of ward occupancy pooled all medical ward beds and did not take into account numerous granular physical ward– and service line–specific characteristics that may influence ward capacity strain (17). Third, the nature of the specialty SDUs at the study hospitals did not allow adequate study of medical SDUs in general. Fourth, the categorization of DNR status as an existing status (an exclusion criterion) versus a change in status (an outcome event that might be influenced by ICU capacity strain [6, 11]) is imperfect (see Appendix E3) and may be missing important nuances about how capacity strain and care limitations are linked. Finally, although we included multiple capacity strain metrics, the broader concept of capacity strain for a hospital or health system is certainly more complex. Further work is needed to better understand which and to what extent other factors (such as the acuity of other patients in the ED, not included in this study) are contributing to the overall strain felt by triaging and admitting clinicians and how composite strain models may change because of facility-specific factors.
Conclusions
Patients with sepsis not requiring life support therapies have a reduced likelihood of being admitted from the ED to the ICU when the ICU is crowded. Furthermore, such patients with sepsis who are admitted from the ED to the ward during times of ICU crowding have higher hospital mortality. This finding merits further investigation to determine whether it suggests the superiority of ICUs in caring for such patients or is instead due to residual confounding by severity of illness, such that sepsis outcomes on the ward rival those in the ICU.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Sarah J. Ratcliffe, Ph.D. (Department of Public Health Sciences, University of Virginia School of Medicine), for her statistical guidance and Barry D. Fuchs, M.D., M.S. (Division of Pulmonary, Allergy, and Critical Care, Hospital of the University of Pennsylvania), for his clinical and operational insights.
Footnotes
Supported by National Institutes of Health (NIH) grant T32HL098054 (G.L.A.), the Leonard Davis Institute of Health Economics (G.L.A., R.K., and G.E.W.), NIH grant K23GM112018 (V.X.L.), NIH grant R01HL136719 (G.L.A., V.X.L., M.K.D., B.B., G.J.E., and S.D.H.), The Permanente Medical Group, Inc. (G.J.E.), and the Gordon and Betty Moore Foundation (G.J.E.).
Author Contributions: G.L.A.: had full access to the data and takes responsibility for the complete manuscript; V.X.L., N.B.G., M.K.D., G.J.E., and S.D.H.: contributed to study design, data analysis and interpretation, and manuscript writing. R.K. and G.E.W.: contributed to data analysis and interpretation and manuscript writing; and B.B.: contributed to study design, data acquisition, data analysis and interpretation, and manuscript writing.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Halpern SD. ICU capacity strain and the quality and allocation of critical care. Curr Opin Crit Care. 2011;17:648–657. doi: 10.1097/MCC.0b013e32834c7a53. [DOI] [PubMed] [Google Scholar]
- 2.Kerlin MP, Harhay MO, Vranas KC, Cooney E, Ratcliffe SJ, Halpern SD. Objective factors associated with physicians’ and nurses’ perceptions of intensive care unit capacity strain. Ann Am Thorac Soc. 2014;11:167–172. doi: 10.1513/AnnalsATS.201306-141OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wagner J, Gabler NB, Ratcliffe SJ, Brown SE, Strom BL, Halpern SD. Outcomes among patients discharged from busy intensive care units. Ann Intern Med. 2013;159:447–455. doi: 10.7326/0003-4819-159-7-201310010-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wunsch H, Wagner J, Herlim M, Chong DH, Kramer AA, Halpern SD. ICU occupancy and mechanical ventilator use in the United States. Crit Care Med. 2013;41:2712–2719. doi: 10.1097/CCM.0b013e318298a139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKenzie MS, Auriemma CL, Olenik J, Cooney E, Gabler NB, Halpern SD. An observational study of decision making by medical intensivists. Crit Care Med. 2015;43:1660–1668. doi: 10.1097/CCM.0000000000001084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hua M, Halpern SD, Gabler NB, Wunsch H. Effect of ICU strain on timing of limitations in life-sustaining therapy and on death. Intensive Care Med. 2016;42:987–994. doi: 10.1007/s00134-016-4240-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weissman GE, Gabler NB, Brown SE, Halpern SD. Intensive care unit capacity strain and adherence to prophylaxis guidelines. J Crit Care. 2015;30:1303–1309. doi: 10.1016/j.jcrc.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown SE, Rey MM, Pardo D, Weinreb S, Ratcliffe SJ, Gabler NB, et al. The allocation of intensivists’ rounding time under conditions of intensive care unit capacity strain. Am J Respir Crit Care Med. 2014;190:831–834. doi: 10.1164/rccm.201406-1127LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gabler NB, Ratcliffe SJ, Wagner J, Asch DA, Rubenfeld GD, Angus DC, et al. Mortality among patients admitted to strained intensive care units. Am J Respir Crit Care Med. 2013;188:800–806. doi: 10.1164/rccm.201304-0622OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathews KS, Durst MS, Vargas-Torres C, Olson AD, Mazumdar M, Richardson LD. Effect of emergency department and ICU occupancy on admission decisions and outcomes for critically ill patients. Crit Care Med. 2018;46:720–727. doi: 10.1097/CCM.0000000000002993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stelfox HT, Hemmelgarn BR, Bagshaw SM, Gao S, Doig CJ, Nijssen-Jordan C, et al. Intensive care unit bed availability and outcomes for hospitalized patients with sudden clinical deterioration. Arch Intern Med. 2012;172:467–474. doi: 10.1001/archinternmed.2011.2315. [DOI] [PubMed] [Google Scholar]
- 12.Robert R, Coudroy R, Ragot S, Lesieur O, Runge I, Souday V, et al. Influence of ICU-bed availability on ICU admission decisions. Ann Intensive Care. 2015;5:55. doi: 10.1186/s13613-015-0099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torio CM, Moore BJ. National inpatient hospital costs: the most expensive conditions by payer, 2013. HCUP Statistical Brief #204. Rockville, MD: Agency for Healthcare Research and Quality; May 2016. [PubMed]
- 14.Strehlow MC, Emond SD, Shapiro NI, Pelletier AJ, Camargo CA., Jr National study of emergency department visits for sepsis, 1992 to 2001. Ann Emerg Med. 2006;48:326–331.e3. doi: 10.1016/j.annemergmed.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, Rea TD, Scherag A, et al. Assessment of clinical criteria for sepsis: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:762–774. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tiru B, DiNino EK, Orenstein A, Mailloux PT, Pesaturo A, Gupta A, et al. The economic and humanistic burden of severe sepsis. Pharmacoeconomics. 2015;33:925–937. doi: 10.1007/s40273-015-0282-y. [DOI] [PubMed] [Google Scholar]
- 17.Kohn R, Bayes B, Ratcliffe SJ, Halpern SD, Kerlin MP. Ward capacity strain: defining a new construct based on ED boarding time and ICU transfers [abstract] Am J Respir Crit Care Med. 2017;195:A7085. [Google Scholar]
- 18.Escobar GJ, Gardner MN, Greene JD, Draper D, Kipnis P. Risk-adjusting hospital mortality using a comprehensive electronic record in an integrated health care delivery system. Med Care. 2013;51:446–453. doi: 10.1097/MLR.0b013e3182881c8e. [DOI] [PubMed] [Google Scholar]
- 19.Escobar GJ, Greene JD, Scheirer P, Gardner MN, Draper D, Kipnis P. Risk-adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases. Med Care. 2008;46:232–239. doi: 10.1097/MLR.0b013e3181589bb6. [DOI] [PubMed] [Google Scholar]
- 20.Liu V, Kipnis P, Gould MK, Escobar GJ. Length of stay predictions: improvements through the use of automated laboratory and comorbidity variables. Med Care. 2010;48:739–744. doi: 10.1097/MLR.0b013e3181e359f3. [DOI] [PubMed] [Google Scholar]
- 21.Seymour CW, Iwashyna TJ, Ehlenbach WJ, Wunsch H, Cooke CR. Hospital-level variation in the use of intensive care. Health Serv Res. 2012;47:2060–2080. doi: 10.1111/j.1475-6773.2012.01402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Volchenboum SL, Mayampurath A, Göksu-Gürsoy G, Edelson DP, Howell MD, Churpek MM. Association between in-hospital critical illness events and outcomes in patients on the same ward. JAMA. 2016;316:2674–2675. doi: 10.1001/jama.2016.15505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.