Abstract
Patient-centered outcomes research (PCOR) represents a paradigm shift in research methods aimed to create the body of evidence that supports clinical practice and informs health care decisions. PCOR integrates patients and other key stakeholders including family members, policy makers, clinicians, and patient advocates and advocacy groups as research partners throughout all stages of the research process. The importance of PCOR has received increased recognition, yet there is little evidence available to help guide researchers interested in the design and conduct of PCOR. In May 2014, we convened a workshop to identify key issues related to designing, conducting, and disseminating findings from PCOR studies. Workshop participants included a diverse group of patients, patient advocates, clinicians (physicians, nurses, psychologists, and advanced practice providers), researchers, administrators, and funders within and beyond the pulmonary, critical care, and sleep medicine communities. Participants identified important issues and considerations to address when undertaking PCOR. In this report, we summarize the results of this workshop to inform members of the pulmonary, sleep, and critical care community interested in participating in PCOR. Key findings include the following: 1) requirements for research to be considered PCOR; 2) the potential significant impact of PCOR on patients, clinicians, and researchers; 3) guiding principles and practical strategies to form successful patient-centered research partnerships, conduct PCOR, and disseminate study results to a broad audience of stakeholders; 4) benefits and challenges of PCOR for researchers; and 5) resources available within the American Thoracic Society to help with the conduct of PCOR.
Keywords: patient-centered outcomes research, pulmonary medicine, critical care, sleep medicine
Contents
Overview
Introduction
Methods
Results
Definition and Impact of PCOR
Creating Patient-centered Research Partnerships: Identifying Roles, Incorporating Various Perspectives, and Preparing Research Teams to Work Together
Extending Reach through Innovative and Alternative Methods of Disseminating and Implementing Results
Benefits to Engaging in PCOR for Researchers
Challenges and Lessons Learned
ATS Resources to Support PCOR
Conclusions
Overview
In this workshop report, we summarize key issues related to the design, conduct, and dissemination of high-quality patient-centered outcomes research in pulmonary, critical care, and sleep medicine. Our main findings include the following:
-
•
Patient-centered outcomes research (PCOR) is characterized by the presence of all the following: 1) research questions and outcomes must be informed by patients’ priorities, choices, values, and perspectives; 2) patient research partners or patient advocates must be integrated into the research team throughout the conception, design, and conduct of the study as well as the interpretation and dissemination of the research findings; and 3) patient research partners or advocates should play a role that is equal to that of other research team members and should have the same decision-making authority.
-
•
Well-designed PCOR has the potential to produce significant and immediate impact on the lives of the patients and stakeholders.
-
•
Collaborative relationships between researchers and stakeholders can be enhanced by adhering to a few general guiding principles detailed in this report. The development of patient-centered research partnerships requires clearly defined roles for investigators, patient research partners, and other stakeholders as well as a plan for how best to balance the perspectives of each of these groups to maintain equipoise. A key step in preparing these partnerships is to ensure inclusivity of the diverse perspectives of people living with a disease, paying special attention to minimize disparities in representation.
-
•
Innovative dissemination and implementation strategies can extend the reach of results beyond presentations at scientific meetings and publications in scientific journals.
-
•
Building and maintaining stakeholder research partnerships can result in significant benefits for researchers, but can also pose significant challenges. Sharing lessons learned from prior projects can improve subsequent PCOR studies.
-
•
The American Thoracic Society provides resources that can be utilized to enhance PCOR projects, including the Public Advisory Round Table and the Patient and Family Education Committee.
Introduction
Patient-centered outcomes research (PCOR) represents a transformational change in how evidence is generated to guide clinical practice and assist patients with informed decision making. Traditionally, researchers conceptualized and conducted research in isolation from patients, clinicians, and other stakeholders and disseminated the findings primarily through the peer-reviewed literature and scientific meetings. Without patient input, these studies may not produce findings relevant to the people they were designed to help. In addition, without a broad dissemination framework, even successful trial results may not have been transmitted to patients in accessible ways or led to effective implementation into practice.
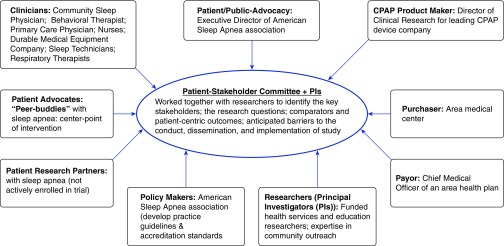
PCOR addresses these concerns by integrating voices and perspectives of patients, family members, clinicians, and other stakeholders throughout the research process and broadly disseminates study results with the goal of increasing the relevance, impact, and uptake of findings. In this way, PCOR identifies research priorities and outcomes that matter most to patients and other stakeholders, answers stakeholder-generated questions using methods designed to produce evidence with direct clinical relevance (including comparative effectiveness strategies) (1), and disseminates results in a way that makes them available, understandable, and actionable to patients and families. Relevant stakeholders differ depending on the project, but may include patient research partners, patient advocates (such as family members, community health care workers, or navigators) or advocacy groups, clinicians, health care payers and purchasers, funding bodies, policy makers, and representatives from industry, among others. For example, Figure 1 illustrates a diverse group of stakeholders and their roles in a peer-driven intervention designed to improve care delivery and coordination among individuals with sleep apnea (2).
Figure 1.
Diversity of stakeholders and their roles in a peer-driven intervention to improve care delivery and coordination among individuals with obstructive sleep apnea (2). CPAP = continuous positive airway pressure; PI = principal investigator.
PCOR has been a focus of researchers in the UK, Canada, and Europe for decades (see Table E1 in the online supplement). More recently, PCOR has gained significant attention in the United States with the implementation of the 2010 Affordable Care Act, which authorized the development of the Patient-centered Outcomes Research Institute (PCORI) (3, 4). PCORI aims to improve the quality and relevance of evidence available to help patients, families, clinicians, employers, insurers, and policy makers make informed health care decisions by supporting comparative effectiveness research and working to improve PCOR methodology (5, 6).
Recognizing the increasing need for high-quality PCOR in pulmonary, critical care, and sleep medicine, the overall goal of this workshop was to bring together patients and patient advocates, clinicians, researchers, and other stakeholders for a consensus workshop to identify key topics and issues related to the design, conduct, and dissemination of findings from PCOR studies. The results of the workshop are summarized in this report.
Methods
Workshop co-chairs (L.C.F., H.L.S., S.J.B., S.P., and E.K.K.) assembled a diverse group of 19 experts from the United States, Canada, UK, and Australia, including patients, patient advocates, clinicians, researchers, administrators, and funders. Participants brought a range of research, clinical, leadership, and personal experiences and expertise. Before the workshop, the co-chairs disseminated selected manuscripts to the attendees for review (7–9). These manuscripts were selected as background reading to familiarize participants with key themes such as engagement of patients in a variety of scientific endeavors including guideline development, participation in the peer review process, and in the design and conduct of research studies. Potential conflicts of interest were disclosed and managed in accordance with the policies and procedures of the American Thoracic Society (ATS). A full-day meeting was held on May 17, 2014, in conjunction with the International Conference of the American Thoracic Society.
The workshop opened with presentations on a series of topics (Table E2). Speakers integrated historical researcher and advocacy perspectives from the UK and United States into their presentations to provide context. In addition, the role of patient advocacy was highlighted in a presentation by Teresa Barnes, a representative from the ATS Public Advisory Roundtable and Vice President for Patient Advocacy and Outreach at the Coalition for Pulmonary Fibrosis. After this, a panel discussion was held for additional discussion and integration of the concepts presented. Based on this, co-chairs suggested topic areas in need of additional development or discussion. The afternoon was spent in facilitated discussion to further develop the concepts, insights, and practical considerations related to PCOR. Results from this discussion were used to identify and prioritize themes. The workshop was audio-recorded and co-chairs took notes. Both were reviewed by the co-chairs to identify additional themes, which were further refined during subsequent teleconferences. Co-chairs drafted an initial report that was reviewed by all workshop attendees. We also circulated an e-mail survey to gather the practical experiences of workshop attendees, and responses were integrated into the manuscript. All workshop attendees revised and approved the final manuscript for submission.
Results
Definition and Impact of PCOR
Defining patient-centered outcomes research
Table 1 summarizes several definitions of PCOR. Workshop attendees agreed these definitions include common necessary components for research to be considered “patient-centered.” These factors should be considered across the timeline of a research project from inception through implementation. First, patient interests and preferences should inform the research questions and selection of outcomes to ensure that research will generate evidence with the potential to impact patients’ lives. Patient-centered outcomes should be those determined to be important and relevant from the perspective of patients. They may be patient-reported or collected by other means. It is important to note that not all patient-reported outcomes are patient-centered (e.g., a patient survey that encompasses issues that clinicians, not necessarily patients, feel are important). Second, patients should be engaged as part of the team throughout the conception, design, and conduct of the study to ensure relevance and utility. Third, patient research partners or advocates should play a role in the project equivalent to that of other team members and should contribute equally to collaborative decisions. Fourth, patient research partners should be included in the dissemination of findings and products, including the creation of plain language summaries. This is a key step to ensure that the findings are accessible, understandable, and actionable to broad audiences. Level of involvement may vary during different phases of the research based on interests, expertise, and focus of work.
Table 1.
Published definitions of patient-centered outcomes research and related concepts
| Organization | Definition |
|---|---|
| Patient-centered Outcomes Research Institute (United States) | “Patient-centered Outcomes Research (PCOR) helps people and their caregivers communicate and make informed health care decisions, allowing their voices to be heard in assessing the value of health care options… PCOR: 1) assesses the benefits and harms of preventive, diagnostic, therapeutic, palliative, or health delivery systems interventions to inform decision making, highlighting comparisons and outcomes that matter to people; 2) is inclusive of an individual’s preferences, autonomy, and needs, focusing on outcomes that people notice and care about such as survival, function, symptoms, and health-related quality of life; 3) incorporates a wide variety of settings and diversity of participants to address individual differences and barriers to implementation and dissemination; and 4) investigates (or may investigate) optimizing outcomes while addressing burden to individuals, availability of services, technology, and personnel, and other stakeholders perspectives” (38) |
| Agency for Healthcare Research and Quality (United States) | “PCOR is comparative clinical effectiveness research on the impact on health outcomes of two or more preventative, diagnostic, treatment, or health care delivery approaches” [adapted from Section 6301(a) of the Patient Protection and Affordable Care Act of 2010] (39) |
| Canadian Institutes of Health Research | “Patient-oriented research refers to a continuum of research that engages patients as partners, focuses on patient-identified priorities and improves patient outcomes. This research, conducted by multidisciplinary teams in partnership with relevant stakeholders, aims to apply the knowledge generated to improve health care systems and practices” (40) |
| National Health Service, National Institute for Health Research, INVOLVE Coordinating Center (UK) | “…public involvement in research as research being carried out ‘with’ or ‘by’ members of the public rather than ‘to’, ‘about’ or ‘for’ them. This includes, for example, working with research funders to prioritize research, offering advice as members of a project steering group, commenting on and developing research materials and undertaking interviews with research participants. When using the term ‘public’ we include patients, potential patients, carers and people who use health and social care services as well as people from organizations that represent people who use services” (11) |
| National Health and Medical Research Council and Consumers Health Forum of Australia | “…opportunities to engage consumers and community members will depend on the type of research being undertaken…Consumers and community members can be, and are, involved at various levels of research activity and the institutions in which research is conducted. ‘Levels of research activity’ include planning, seeking funding, conducting the research, and communicating the outcomes. Consumers and community members should advise research institutions and researchers on their consumer and community perspectives and lived experiences …” (10) |
Tables 2 and 3 give examples of PCOR conducted by workshop attendees, primarily in the fields of pulmonary, sleep, and critical care medicine, and highlight the patient-centered outcomes and diverse mechanisms of stakeholder engagement used by each study.
Table 2.
Examples of patient-centered outcomes research in pulmonary, critical care, and sleep medicine
| Study Name | Study Team | Project Aims | Patient-centered Outcomes | Mechanisms of Stakeholder Engagement |
|---|---|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; HR-QOL = health-related quality of life; PROMIS = Patient-reported Outcomes Measurement Information System.
Table 3.
Additional relevant patient-centered outcomes research example (outside of pulmonary, critical care, and sleep)
| Study Name | Study Team | Project Aims | Patient-centered Outcomes | Mechanisms of Stakeholder Engagement |
|---|---|---|---|---|
|
|
|
|
Definition of abbreviations: PRO = patient-reported outcomes; PROMIS = Patient-Reported Outcomes Measurement Information System.
Potential impact of PCOR
The potential impact of PCOR was a central theme identified by workshop attendees. Well-designed PCOR facilitates the conduct of relevant studies that can improve the day-to-day lives of patients and stakeholders. The integration of patients, family, patient advocates, clinicians, and other stakeholders within the team allows these groups to inform the research agenda to ensure that their priorities are recognized. The significance of PCOR is likely not limited to those directly involved in research activities; it can also extend to the larger population of patients represented and to clinicians seeking ways to improve the lives of patients through evidence-based practices. Increasing the relevance of research may in turn lead to increased public and government support for research. Although evidence to support these claims is not yet available in the literature, anecdotal accounts regarding current PCOR efforts support the potential promise of stakeholder-engaged research.
Creating Patient-centered Research Partnerships: Identifying Roles, Incorporating Various Perspectives, and Preparing Research Teams to Work Together
Workshop participants agreed on several guiding principles for developing productive partnerships between researchers and stakeholders. These principles are summarized in Table 4 and described in more detail below.
Table 4.
Proposed general principles of researcher–stakeholder collaboration to conduct successful patient-centered outcomes research in pulmonary, critical care, and sleep medicine
| • Collectively decide on appropriate roles for stakeholder involvement (10, 11) |
| ○ Empower patient research partners and stakeholders to choose their role and level of involvement |
| • Ensure diverse voices are represented |
| ○ Take into account issues such as sex, educational status, SES, race/ethnicity, age, etc., and attempt to elicit input from patients from a variety of backgrounds |
| ○ Consider all potential relevant stakeholders, including family members |
| • Understand perspectives may differ across members of the research team |
| ○ Patient research partners: Provide perspective based on their own experiences, and identify issues meaningful to them and their community |
| ○ Investigators: Provide necessary scientific expertise and research experience; may need to prioritize funding considerations |
| ○ Other stakeholders (clinicians, policy makers, payers): Bring unique viewpoints |
| • Develop a plan to balance the perspectives of each stakeholder group to achieve consensus |
| ○ Encourage a group mentality |
| ○ Identify, discuss, and address potential conflicts of interests |
| ○ Understand that recommendations are nonbinding to promote sharing of ideas |
| • Prepare and train all stakeholders for their various roles |
| ○ Consider systematic training to develop knowledge, methodology, and skills needed to work together effectively (10, 11, 28) |
| ○ Tailor materials to individual roles: e.g., research “guidebook” for patient research partners; training for researchers in collaborative decision making, etc. |
| • Foster collaborative spirit from the outset |
| ○ Ensure that all members’ time and expertise are both valued and appropriately acknowledged, including possible remuneration (10, 11, 29) |
| ○ Demonstrate respect for each other’s needs and viewpoints |
| ○ Start with “big picture” framing to help align team members |
| ○ Encourage open communication for stakeholders with PI and study staff at any time |
| ○ Establish procedures for collaborative discussion and/or decision making |
| • Voting procedure |
| • Modified Robert’s Rules of Order (30) |
| • Quorum required for decisions |
| • Extend reach through innovative and alternative methods of disseminating and implementing results |
| ○ Plain language summaries of research findings to accompany scientific reports |
| ○ Publicize through local television campaigns or social media |
| ○ Summarize findings in community blogs or newsletters |
| • Learn from researchers who have successfully completed PCOR projects |
| ○ Help to identify common challenges and opportunities to improve the quality of future work |
Definition of abbreviations: PCOR = patient-centered outcomes research; PI = principal investigator; SES = socioeconomic status.
Collectively decide on appropriate roles for stakeholder involvement
Clearly defining roles and gaining an understanding of the role that each individual would like to serve can help facilitate productive patient-centered research partnerships (10, 11). Although the roles of research scientists tend to be well defined, there are a variety of potential roles for patient research partners and other stakeholders to take on throughout the research process, including identifying and prioritizing research questions, facilitating recruitment and retention of study participants, developing interventions and data collection strategies, and disseminating findings. It is important to acknowledge both the benefits and burdens of participating on the team, including anticipated time commitments (11). Patient and family research partners may have limited time and capacity to develop skills and participate in all roles. Not all stakeholders will have the same interests, and there may be differences across individuals as to their level of engagement with various aspects of the project (11). To ensure the inclusion of diverse perspectives, patient research partners should be empowered to be involved based on their comfort, functional, and educational levels. Activities to facilitate these roles include formulation of governing boards, participation in discussion boards/online forums, and helping develop questions for focus groups and surveys (12).
Ensure diverse patient voices are represented
Ensuring diversity in terms of age, sex, race, ethnicity, socioeconomic status, education, and sexual orientation, among others, helps to increase the inclusion of unique ideas. People living with the same disease can vary in their perspectives, health care knowledge, willingness, and physical or psychological ability to contribute. For example, patients with advanced education and training are often included as research partners and provide valuable input, yet their perspectives may not reflect those of the average patient. For this reason, it is important to include more than one participant from different groups, and to encourage patient research partners to reach out to other members of their community when appropriate, to attain broader perspectives (10, 11).
Several strategies were identified to elicit input from patients from diverse backgrounds, including underserved patients and groups, as well as others who may be challenging to engage. Community engagement is a potentially powerful way to embrace different perspectives while ensuring appropriate cultural sensitivity and equity within groups (13–15). Peer-engagement principles, including peer-support models, can be useful in all steps of PCOR (16–18). Novel technologies may be useful to reach broader audiences, including web-based methods, social media, video conferences, and mobile technologies such as crowd sourcing (19). However, newer technologies may exclude those less willing or able to use them (20). One-on-one interviews offer the opportunity to include those dealing with serious illness and/or limited mobility (21). These approaches allow researchers to illuminate a range of perspectives with scientific rigor. Such rigor should not be at odds with accepted methodological principles (21).
It is important to consider all potential relevant stakeholders. A significant burden of many illnesses falls on family members, including relatives, friends, and other caregivers. They often bear the burden of caregiving, and their ability to provide this care can be limited by the impact on their own psychological and physical health (22, 23). Individuals with severe illness or other causes for cognitive impairment may not be able to engage in PCOR during certain phases of their illness. Family members can bring the patient perspective, as well as their own perspective, of how the illness impacts the family. Moreover, there is increasing awareness of the importance of family outcomes and the value this contributes to patient outcomes (24).
Understand perspectives may differ across members of the research team
Patient research partners add value to the research process by relaying their first-hand experiences in living with illness and interacting with the health care system, and by identifying issues that are meaningful to them and their community. Investigators bring a deep understanding of the scientific approach, along with a variety of methodological and scientific expertise. These skills enable the group to create rigorous research that can be replicated and applied by others. Stakeholders can also bring important perspectives to the PCOR process. Clinicians bring perspectives and insights on health, disease, and clinical practice to the process. Nurses, respiratory therapists, technicians, and others can provide perspectives about care processes and treatment adherence. Payers are often interested in improving process efficiency and reducing fragmented care. Partnering for strong involvement with public advocacy and policy makers can lead to faster translation of the research findings into health policy with robust dissemination and sustainable implementation. Frameworks for stakeholder definitions and roles have been described (25, 26).
Develop a plan to balance the perspectives of each stakeholder group to achieve consensus
It is important to balance the agendas of all groups including patient research partners/family members, researchers, clinicians, funders, and other stakeholders. Although there are challenges to ensuring that everyone at the table has an equal voice, we note that PCOR represents a philosophy and methodological approach that can be integrated into most clinical research projects. Efforts must be made to inform, educate, empower, and engage participants to balance the needs of everyone though a group mentality to help maintain equipoise. As not all participants will come with a similar understanding of the scientific process including mitigation of bias, it is important to identify, discuss, and address potential conflicts of interest (27). Similarly, it can be helpful to develop an understanding with individuals that not all ideas or suggestions will be part of the final product. Being transparent about this from the beginning can help frame expectations.
Prepare and train stakeholders for their various roles
Patient research partners and other stakeholders need to develop a common language and understanding of terminology, methods, and ethics to work together effectively. Researchers also need to understand how to tailor roles and clarify expectations for different stakeholders based on their willingness, interest, and resources. One key strategy recommended was systematic training, analogous to “career development,” of all participants to develop the knowledge, methodology, and skills needed to work together effectively (10, 11, 28). This approach acknowledges the diversity of stakeholder backgrounds, experiences, and perspectives, and ensures a common understanding of the goals and process of working together.
Patient research partners may benefit from a video or written “guidebook” to the research project including a glossary of research terms. We anticipate these materials would need to be tailored to a specific project based on the roles and responsibilities involved. Researchers may benefit from additional training in engagement of patient research partners and other stakeholders, consensus methods, PCOR study designs and outcome measures, and analytic approaches that enable a patient-centered and collaborative approach. It is not a realistic or necessary goal to train all participants to have a high level of methodological or content knowledge. Nonscientist team members understand that researchers bring insight into both content issues and the feasibility of various research designs and approaches. It is the responsibility of researchers to explain such insights in ways that are understandable by nonscientist team members.
Foster a collaborative spirit from the outset
The process of developing and prioritizing research questions with stakeholders can be challenging. Investigators must prioritize development of a collaborative spirit. Although some disagreement among the group is expected, care should be taken by participants to ensure that all voices and perspectives are acknowledged as being valuable. Researchers also must ensure that the essential role of patient research partners and other stakeholders is appropriately acknowledged. Although policies may vary by funding organizations, compensation for time and effort is increasingly included in the budget for all members of the research team. For example, in the United States, PCORI expects applicants to include a plan and budget for compensating patients, caregivers, and advocacy organizations engaged as partners (29). This approach is also advocated by the National Health and Medical Research Council of Australia and the National Institute for Health Research in the UK (10, 11).
All members of the team must be committed to listen to one another, work collaboratively, and display patience with one another throughout the process. Patient research partners should be welcomed within partnerships to articulate their experiences and perspectives, while having a willingness to become familiar with terminology, methods, and ethical considerations of research (10). Researchers must show a genuine interest in individuals, demonstrate respect for all viewpoints, and provide explanations of scientific methods as needed (10). It can be helpful for researchers to interview patient research partners to learn more about their interests and use one-on-one coaching to bolster confidence about speaking up during meetings. Starting with a “big picture” framing of the research question can help align team members. In addition, researchers should encourage open lines of communication for stakeholders at any time.
Procedures should be established at the outset to foster collaborative decision making with a prespecified process of adjudication, including 1) defining a voting procedure that is fair to all participants; 2) using references such as the most recently revised Robert’s Rules of Order (30),which describes parliamentary procedures and is commonly used to guide proceedings for a variety of organizations within the United States; 3) deciding what constitutes a quorum for approving decisions such as the one-person, one-vote Delphi consensus method (31, 32); and 4) explaining that recommendations made by stakeholders are nonbinding and that researchers and stakeholders may choose which to follow.
Extending Reach through Innovative and Alternative Methods of Disseminating and Implementing Results
Dissemination of research findings in traditional venues alone is not enough to ensure that findings reach the individuals to whom they matter most—patients and families. Examples of effective ways in which researchers might partner with patients in dissemination include 1) writing plain language summaries of findings that accompany research reports; 2) participating in local media campaigns; 3) utilizing social media and other online outlets to publicize results; 4) summarizing findings for community resources, including website blogs or newsletters; and 5) returning results back to the study participants through infographics, videos, or other media (10, 11). Patient research partners should be invited to participate in the development of all study information and acknowledged as coauthors, including scientific publications, interim reports to the funder, press releases, and study information posted on the Internet.
Benefits to Engaging in PCOR for Researchers
Workshop participants noted that the process of PCOR was often rewarding to researchers in several ways. Early involvement of patient research partners and other stakeholders helps prioritize research questions and increases confidence that the topic is important to patients. Developing strong, collaborative relationships helps guide the research and increases its potential for success. Eliciting input on patient-valued aspects of care can lead to important revelations, which in the setting of flexibility in approach can move research from the theoretical to the practical to help create studies that address issues important to patients. Patient research partners and families are often grateful for the opportunity to participate, which leads to positive feelings for investigators regarding the commitment of the biomedical community and the value of research in facilitating new discoveries. Extra work may be involved when including patient research partners and other stakeholders, but there is a quid pro quo if the ideas generated are novel and meaningful and the results more relevant to patients.
Challenges and Lessons Learned
We acknowledge that although there are many potential rewards to creating partnerships with patients and other stakeholders, building and maintaining these partnerships can pose significant challenges.
Individuals bring a variety of perspectives, incentives, experiences, and approaches that need to be considered. These partnerships benefit from the development of trust over time, but often must be created de novo. A major challenge faced by PCOR teams includes practical aspects of working with stakeholders. For example, there are no generally accepted standards for collaborating with community and patient stakeholders. Agreements that are developed are often vague and can lack accountability. Preliminary guidelines are emerging around patient engagement in research (33) and how to identify metrics of success (34, 35).
Engaging patients in the design of studies can require more “lead-time” and may increase the cost of preparing research proposals. A challenge for researchers is the need to prioritize funding considerations to support a specific project while balancing the desire to actively involve patient research partners and stakeholders within the demands of a research setting. At times, these competing priorities can be at odds with the desires of individuals. These concerns may be mitigated at some centers by the formation of a standing patient advisory board that regularly meets to review research proposals in their initial stages across a range of disciplines or disease states.
PCOR must be rigorously conceived and conducted using validated methods. It is equally important to keep projects realistic despite broader aspirations. This can be accomplished by preparing stakeholders early in the process for the scope of the project. To reduce the risk of attrition, it is often helpful to offer multiple ways to participate to reduce time and travel burdens.
Not all individuals are well suited for the role of research partner, and there should be ongoing review of whether the individual and study are a good fit. There may be considerable emotional, physical, and time burdens placed on patient research partners, most of whom already are busy fulfilling multiple roles at work, home, and in their social lives. One must be vigilant about ensuring that expectations and time commitments are realistic for people who are not funded researchers or employees of the research program. Although many patient research partners benefit greatly and enjoy participating as research partners, this role can run the risk of strengthening identity as a patient, which may be in direct contrast with their desire for life to be normal. Sharing lessons across communities of researchers can be helpful to prepare for challenges that arise.
ATS Resources to Support PCOR
One of the key resources within the ATS that can help support PCOR is the ATS Public Advisory Roundtable (ATS PAR), which is a collaboration of the ATS and 15 patient interest organizations representing various lung and airway disorders to advance their shared educational, research, patient care, and advocacy goals (36). The ATS PAR has helped incorporate patients and advocates into all aspects of the ATS, including the society governance and the ATS International Conference. The PAR can help link researchers with patient research partners who can participate in all aspects of research and can lead to the creation of lasting partnerships. Many other patient advocacy groups also have shared interests with the ATS as well. Other ATS resources that can support PCOR include the Patient and Family Education Committee (PFEC), which performs ongoing assessments of ATS patient/public educational resources to ensure that they are relevant, current, health literate, and cost-effective (37). In its work, the PFEC also explores ways to ensure that the patient and family perspective is incorporated into educational activities for the fields of pulmonary, critical care, and sleep medicine.
Conclusions
PCOR is an important new approach to conducting research that could meaningfully improve the lives of patients and their families. It emphasizes the need to engage patient research partners and other stakeholders in all phases of a research project. An important principle is to ensure inclusion of diverse perspectives of multiple stakeholders on the team and to minimize disparities. Successful PCOR requires clear understanding and communication about roles and perspectives. Additional education and training for researchers and stakeholders on effective collaboration strategies are important to developing successful PCOR programs, and should be tailored to specific programs. Creating partnerships between researchers, patients, and other stakeholders brings both benefits and challenges. Innovative dissemination and implementation strategies may extend the reach and timely uptake of study findings to help maximize the desired impact on the lives of patients.
Supplementary Material
Acknowledgments
This workshop report was prepared by an ad hoc subcommittee of the Behavioral Sciences and Health Services Research and Nursing Assemblies.
Members of the subcommittee are as follows:
Erin K. Kross, M.D. (Chair)
Laura C. Feemster, M.D., M.S. (Co-Chair)
Howard L. Saft, M.D., M.S.H.S. (Co-Chair)
Susan J. Bartlett, Ph.D. (Co-Chair)
Sairam Parthasarathy, M.D. (Co-Chair)
David H. Au, M.D., M.S.
Teresa Barnes, B.A.
Peter Calverley, M.D.
Linda L. Chlan, Ph.D., R.N.
Colin R. Cooke, M.D., M.Sc.
Christopher E. Cox, M.D., M.H.A., M.P.H.
J. Randall Curtis, M.D., M.P.H.
David H. Hickam, M.D., M.P.H.
Jerry A. Krishnan, M.D., Ph.D.
Richard A. Mularski, M.D., M.S.H.S., M.C.R.
Lynn F. Reinke, Ph.D., A.R.N.P.
Eileen Rubin, J.D.
Smita Shah, Mb.ChB., M.C.H.
Donald R. Sullivan, M.D., M.A., M.C.R.
Acknowledgment
The authors thank John Walsh (COPD Foundation) for contributions to the workshop and for leadership in the pulmonary field on the importance of patients in the research endeavor.
Footnotes
Supported by the American Thoracic Society.
This Official Workshop Report of the American Thoracic Society was approved May 2018.
The views expressed here are those of the authors and do not necessarily reflect the position or policy of the U.S. government or of the Department of Veterans Affairs.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures: H.L.S. served as a consultant for Medtronic. S.P. received research support from Philips Respironics, Niveus Medical, and Younes Sleep Technologies; served as a speaker for Merck; served on an advisory committee for Bayer; served as a consultant for Nightbalance; received travel support from Philips Respironics and Vapotherm; received royalties from UpToDate; holds a patent for a home breathing device (Patent #UA 14-018 U.S.S.N. 61/884; PTAS 502570970). T.B. received travel support from Amgen, Biogen, Boehringer Ingelheim, miRagen, and Promedior; served as a consultant for New Amsterdam Sciences and Patara Pharma. P.C. served as a speaker, on an advisory committee, and received research support from AstraZeneca, Boehringer Ingelheim, and GlaxoSmithKline; served as a speaker for Novartis, Recipharm, and Takeda; served as a consultant for Boehringer Ingelheim and Takeda; received research support from Recipharm. D.H.H. is an employee of the Patient-Centered Outcomes Research Institute (PCORI). D.H.A. served on a data and safety monitoring board for Novartis; served as a consultant for Gilead. L.L.C. received royalties from Springer Publishing. L.F.R. received royalties from UpToDate. J.A.K. served as a consultant for Adelphi Values, CVS/Caremark, eMAX Health, and UpToDate; served on a data and safety monitoring board for Sanofi. L.C.F., S.J.B., J.R.C., R.A.M., C.R.C., C.E.C., E.R., S.S., D.R.S., and E.K.K. reported no relationships with relevant commercial interests.
Contributor Information
Collaborators: on behalf of the American Thoracic Society Behavioral Sciences and Health Services Research Assembly and Nursing Assembly
References
- 1.Carson SS, Goss CH, Patel SR, Anzueto A, Au DH, Elborn S, et al. American Thoracic Society Comparative Effectiveness Research Working Group. An official American Thoracic Society research statement: comparative effectiveness research in pulmonary, critical care, and sleep medicine. Am J Respir Crit Care Med. 2013;188:1253–1261. doi: 10.1164/rccm.201310-1790ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patient-centered Outcomes Research Institute (PCORI) PCORI funded projects: sample engagement plans [2015 June 11; accessed 2017 Sept 15]Available from: http://www.pcori.org/sites/default/files/PCORI-Sample-Engagement-Plans.pdf
- 3.Patient Protection and Affordable Care Act. Section 6301 and Section 10602, Public Law 111–148, 124 stat. 119, H.R. 3590. 2010.
- 4.Daugherty SE, Wahba S, Fleurence R PCORnet PPRN Consortium. Patient-powered research networks: building capacity for conducting patient-centered clinical outcomes research. J Am Med Inform Assoc. 2014;21:583–586. doi: 10.1136/amiajnl-2014-002758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patient-centered Outcomes Research Institute (PCORI) Washington, D.C: PCORI; 2017[accessed 2017 Sept 19]. Available from: https://www.pcori.org/ [Google Scholar]
- 6.Washington AE, Lipstein SH. The Patient-centered Outcomes Research Institute—promoting better information, decisions, and health. N Engl J Med. 2011;365:e31. doi: 10.1056/NEJMp1109407. [DOI] [PubMed] [Google Scholar]
- 7.Kelson M, Akl EA, Bastian H, Cluzeau F, Curtis JR, Guyatt G, et al. ATS/ERS Ad Hoc Committee on Integrating and Coordinating Efforts in COPD Guideline Development. Integrating values and consumer involvement in guidelines with the patient at the center: article 8 in integrating and coordinating efforts in COPD guideline development: an official ATS/ERS Workshop Report. Proc Am Thorac Soc. 2012;9:262–268. doi: 10.1513/pats.201208-061ST. [DOI] [PubMed] [Google Scholar]
- 8.Fleurence RL, Forsythe LP, Lauer M, Rotter J, Ioannidis JP, Beal A, et al. Engaging patients and stakeholders in research proposal review: the Patient-centered Outcomes Research Institute. Ann Intern Med. 2014;161:122–130. doi: 10.7326/M13-2412. [DOI] [PubMed] [Google Scholar]
- 9.Bartlett SJ, Barnes T, McIvor RA. Integrating patients into meaningful real-world research. Ann Am Thorac Soc. 2014;11(Suppl 2):S112–S117. doi: 10.1513/AnnalsATS.201309-327RM. [DOI] [PubMed] [Google Scholar]
- 10.National Health and Medical Research Council, Consumers Health Forum of Australia Statement on consumer and community involvement in health and medical research[2016. Sept; accessed 2017 Aug 27]. Available from: https://www.nhmrc.gov.au/_files_nhmrc/file/publications/16298_nhmrc_-_statement_on_consumer_and_community_involvement_in_health_and_medical_research-accessible.pdf.
- 11.National Institute for Health Research Briefing notes for researchers: involving the public in NHS, public health and social care research [2012; accessed 2017 Aug 28]Available from: http://www.invo.org.uk/posttypepublication/involve-briefing-notes-for-researchers/
- 12.Domecq JP, Prutsky G, Elraiyah T, Wang Z, Nabhan M, Shippee N, et al. Patient engagement in research: a systematic review. BMC Health Serv Res. 2014;14:89. doi: 10.1186/1472-6963-14-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rush CL, Darling M, Elliott MG, Febus-Sampayo I, Kuo C, Muñoz J, et al. Engaging Latina cancer survivors, their caregivers, and community partners in a randomized controlled trial: Nueva Vida intervention. Qual Life Res. 2015;24:1107–1118. doi: 10.1007/s11136-014-0847-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Page-Reeves J, Regino L, Murray-Krezan C, Bleecker M, Erhardt E, Burge M, et al. A comparative effectiveness study of two culturally competent models of diabetes self-management programming for Latinos from low-income households. BMC Endocr Disord. 2017;17:46. doi: 10.1186/s12902-017-0192-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darling M, Gonzalez F, Graves K, Sheppard VB, Hurtado-de-Mendoza A, Leventhal KG, et al. Practical tips for establishing partnerships with academic researchers: a resource guide for community-based organizations. Prog Community Health Partnersh. 2015;9:203–212. doi: 10.1353/cpr.2015.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mikkelsen ME, Jackson JC, Hopkins RO, Thompson C, Andrews A, Netzer G, et al. Peer support as a novel strategy to mitigate post-intensive care syndrome. AACN Adv Crit Care. 2016;27:221–229. doi: 10.4037/aacnacc2016667. [DOI] [PubMed] [Google Scholar]
- 17.Newbronner L, Chamberlain R, Borthwick R, Baxter M, Sanderson D. London: Health Foundation; 2013. Sustaining and spreading self-management and support: lessons from co-creating health phase 2. [Google Scholar]
- 18.Parthasarathy S, Wendel C, Haynes PL, Atwood C, Kuna S. A pilot study of CPAP adherence promotion by peer buddies with sleep apnea. J Clin Sleep Med. 2013;9:543–550. doi: 10.5664/jcsm.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hogg WE. Crowdsourcing and patient engagement in research. Can Fam Physician. 2015;61:283–284. [PMC free article] [PubMed] [Google Scholar]
- 20.Vogel MM, Combs SE, Kessel KA. mHealth and application technology supporting clinical trials: today’s limitations and future perspective of smartRCTs. Front Oncol. 2017;7:37. doi: 10.3389/fonc.2017.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patient-centered Outcomes Research Institute Research methodology [2017; accessed 2017 Sept 19]Available from: www.pcori.org/research-results/research-methodology
- 22.Davidson JE, Jones C, Bienvenu OJ. Family response to critical illness: postintensive care syndrome-family. Crit Care Med. 2012;40:618–624. doi: 10.1097/CCM.0b013e318236ebf9. [DOI] [PubMed] [Google Scholar]
- 23.Cameron JI, Herridge MS, Tansey CM, McAndrews MP, Cheung AM. Well-being in informal caregivers of survivors of acute respiratory distress syndrome. Crit Care Med. 2006;34:81–86. doi: 10.1097/01.ccm.0000190428.71765.31. [DOI] [PubMed] [Google Scholar]
- 24.Kross EK. The importance of caregiver outcomes after critical illness. Crit Care Med. 2015;43:1149–1150. doi: 10.1097/CCM.0000000000000910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Concannon TW, Meissner P, Grunbaum JA, McElwee N, Guise JM, Santa J, et al. A new taxonomy for stakeholder engagement in patient-centered outcomes research. J Gen Intern Med. 2012;27:985–991. doi: 10.1007/s11606-012-2037-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patient-centered Outcomes Research Institute PCORI’s stakeholders [2014; accessed 2017 Sept 19]Available from: www.pcori.org/funding-opportunities/what-we-mean-engagement/pcoris-stakeholders
- 27.Schünemann HJ, Osborne M, Moss J, Manthous C, Wagner G, Sicilian L, et al. ATS Ethics and Conflict of Interest Committee and the Documents Development and Implementation Committee. An official American Thoracic Society Policy statement: managing conflict of interest in professional societies. Am J Respir Crit Care Med. 2009;180:564–580. doi: 10.1164/rccm.200901-0126ST. [DOI] [PubMed] [Google Scholar]
- 28.Concannon TW, Fuster M, Saunders T, Patel K, Wong JB, Leslie LK, et al. A systematic review of stakeholder engagement in comparative effectiveness and patient-centered outcomes research. J Gen Intern Med. 2014;29:1692–1701. doi: 10.1007/s11606-014-2878-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patient-centered Outcomes Research Institute Financial compensation of patients, caregivers, and patient/caregiver organizations engaged in PCORI-funded research as engaged research partners [2015; accessed 2017 Oct 25]Available from: https://www.pcori.org/sites/default/files/PCORI-Compensation-Framework-for-Engaged-Research-Partners.pdf
- 30.Robert HM, III, Honemann DH, Balch TJ. Robert’s rules of order, newly revised. Cambridge: MA: Da Capo Press; 2011. [Google Scholar]
- 31.Brown B. Delphi process: a methodology used for the elicitation of opinions of experts. Santa Monica: CA: RAND Corporation; 1968. [Google Scholar]
- 32.Krishnan JA, Lindenauer PK, Au DH, Carson SS, Lee TA, McBurnie MA, et al. COPD Outcomes-based Network for Clinical Effectiveness and Research Translation. Stakeholder priorities for comparative effectiveness research in chronic obstructive pulmonary disease: a workshop report. Am J Respir Crit Care Med. 2013;187:320–326. doi: 10.1164/rccm.201206-0994WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirwan JR, de Wit M, Frank L, Haywood KL, Salek S, Brace-McDonnell S, et al. Emerging guidelines for patient engagement in research. Value Health. 2017;20:481–486. doi: 10.1016/j.jval.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Forsythe L, Heckert A, Margolis MK, Schrandt S, Frank L. Methods and impact of engagement in research, from theory to practice and back again: early findings from the Patient-centered Outcomes Research Institute. Qual Life Res. 2018;27:17–31. doi: 10.1007/s11136-017-1581-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brett J, Staniszewska S, Mockford C, Herron-Marx S, Hughes J, Tysall C, et al. A systematic review of the impact of patient and public involvement on service users, researchers and communities. Patient. 2014;7:387–395. doi: 10.1007/s40271-014-0065-0. [DOI] [PubMed] [Google Scholar]
- 36.American Thoracic Society. Public Advisory Roundtable[2017. accessed 2017 Sept 18]. Available from: https://www.thoracic.org/patients/par/
- 37.American Thoracic Society. Committees: Patient and Family Education Committee[2017. accessed 2017 Oct 25]. Available from: https://www.thoracic.org/members/committees/committeeDetail.php?id=a0I40000001iBQSEA2
- 38. Patient-centered Outcomes Research Institute. Patient-centered outcomes research [2013; accessed 2017 Oct 25]. Available from: https://www.pcori.org/research-results/patient-centered-outcomes-research.
- 39.Department of Health and Human Services Part I. Overview information: scaling established clinical decision support to facilitate the dissemination and implementation of patient-centered outcomes research findings (r18) [2017; accessed 2017 Sept 19]Available from: https://grants.nih.gov/grants/guide/pa-files/PA-16-283.html
- 40.Canadian Institutes of Health Research. Strategy for patient-oriented research[2017. accessed 2017 Oct 25]. Available from: http://www.cihr-irsc.gc.ca/e/41204.html
- 41.Fakhri S, Engelberg RA, Downey L, Nielsen EL, Paul S, Lahdya AZ, et al. Factors affecting patients’ preferences for and actual discussions about end-of-life care. J Pain Symptom Manage. 2016;52:386–394. doi: 10.1016/j.jpainsymman.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cox CE, Hough CL, Carson SS, White DB, Kahn JM, Olsen MK, et al. Effects of a telephone- and web-based coping skills training program compared with an education program for survivors of critical illness and their family members: a randomized clinical trial. Am J Respir Crit Care Med. 2018;197:66–78. doi: 10.1164/rccm.201704-0720OC. [DOI] [PubMed] [Google Scholar]
- 43.COPD Foundation RELIANCE Study [2017; accessed 2017 Sept 19]Available from: https://www.copdfoundation.org/Research/Other-Studies/Reliance.aspx
- 44.Bingham CO, III, Bartlett SJ, Merkel PA, Mielenz TJ, Pilkonis PA, Edmundson L, et al. Using patient-reported outcomes and PROMIS in research and clinical applications: experiences from the PCORI pilot projects. Qual Life Res. 2016;25:2109–2116. doi: 10.1007/s11136-016-1246-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.