Abstract
Chronic psychosocial adversity induces vulnerability to mental illnesses. Animal studies demonstrate that this may be mediated by dopaminergic dysfunction. We therefore investigated whether long-term exposure to psychosocial adversity was associated with dopamine dysfunction and its relationship to psychological and physiological responses to acute stress. Using 3,4-dihydroxy-6-[18F]-fluoro-l-phenylalanine ([18F]-DOPA) positron emission tomography (PET), we compared dopamine synthesis capacity in n = 17 human participants with high cumulative exposure to psychosocial adversity with n = 17 age- and sex-matched participants with low cumulative exposure. The PET scan took place 2 hr after the induction of acute psychosocial stress using the Montréal Imaging Stress Task to induce acute psychosocial stress. We found that dopamine synthesis correlated with subjective threat and physiological response to acute psychosocial stress in the low exposure group. Long-term exposure to psychosocial adversity was associated with dampened striatal dopaminergic function (p=0.03, d = 0.80) and that psychosocial adversity blunted physiological yet potentiated subjective responses to acute psychosocial stress. Future studies should investigate the roles of these changes in vulnerability to mental illnesses.
Research organism: Human
Introduction
Chronic psychosocial adversity increases the risk of mental illnesses including schizophrenia and depression (van Os et al., 2010; Howes and Murray, 2014; Parker, 1983). These adverse factors include developmental psychological trauma (Bendall et al., 2008) and adult life events (situations or occurrences that bring about a negative change in personal circumstances and involve threat) (Beards et al., 2013; Brown and Birley, 1968). Several lines of evidence indicate a potential causative component to these relationships as these risk exposures demonstrate dose-response relationships (Janssen et al., 2004; Morgan and Fisher, 2007; Pedersen and Mortensen, 2001). Reverse causality in the form of recall bias does not appear to be driving these associations (Cutajar et al., 2010), and the cessation of stressor reduces the risk of illness (Kelleher et al., 2013). However, we lack a precise mechanistic understanding of how exposure to these risk factors induces vulnerability to mental illness, and why these exposures all increase risk. Understanding this is important to identify targets for prevention and novel treatment. One common component underlying these factors is exposure to psychosocial stress (Howes and Murray, 2014) via activation of the hypothalamic-pituitary-adrenal (HPA) axis and the sympathetic nervous system as part of the normal biological stress response (Taylor, 2010).
The striatum is functionally connected to the threat detection system (Haber, 2014). Animal research has demonstrated that acute stressors including aversive stimuli induce a pronounced activation of the dopamine system in terms of dopamine neuron population activity (i.e. the numbers of neurons firing) and with regard to amphetamine-induced behaviours (Valenti et al., 2011). Long-lasting changes in dopamine function occur after single stress exposures, including altered responsivity to future stimulation (Holly and Miczek, 2016) in a manner similar to that induced by drugs of abuse (Saal et al., 2003) and such that stress plays a powerful role in the initiation, escalation, and relapse to drug abuse via dopaminergic mechanisms (Koob and Volkow, 2016).
In humans, childhood sexual abuse is associated with elevated urinary dopamine metabolites in childhood (De Bellis et al., 1994) and acute psychosocial stressors induce greater dopamine release in people with low self-reported maternal care (Pruessner et al., 2004). Stress-induced elevations in cortisol levels have been directly correlated with amphetamine-induced dopamine release (Wand et al., 2007) on the one hand, while corticotrophin-releasing hormone administration results in dopamine release on the other (Payer et al., 2017). In terms of long-term exposure, using fMRI in humans, there is evidence that institutional neglect is associated with reduced striatal reward function, which is mediated by the dopamine system (Mehta et al., 2010), and similar findings have been observed in prospective cohorts following childhood adversity (Dillon et al., 2009) suggestive of a possible causal relationship between childhood adversity and alterations of the dopamine system. Furthermore, maltreatment-associated reduced striatal function is associated with adverse outcomes including disrupted attachment (Takiguchi et al., 2015) and depression (Hanson et al., 2015). One study (Oswald et al., 2014) found positive associations between childhood trauma and amphetamine-induced dopamine release, which may be due to the phenomenon of cross-sensitization. Furthermore, within people who are at ultra-high clinical risk of psychosis raised dopamine synthesis capacity has been reported in those patients with high levels of childhood adversity (Egerton et al., 2016). In light of these findings, we wanted to examine the relationships between the autonomic, endocrine and subjective threat responses to an acute psychosocial stressor and dopaminergic function.
The relationships between neurobiological pathways and stress-induced physiological and subjective responses have yet to be fully elucidated in humans. Studies of psychosocial stressors and dopamine function have typically investigated risk factors in isolation, despite the fact that the risk factors cluster together and may share common underlying mechanisms (Hjern et al., 2004; Morgan and Fisher, 2007; Wicks et al., 2005). Since associations between one exposure and outcomes remain after controlling for the other exposures (Schäfer and Fisher, 2011), it is likely that additive effects and/or synergistic effects operate between risk factors (Morgan et al., 2014; Guloksuz et al., 2015; Lataster et al., 2012; Morgan et al., 2008; Schäfer and Fisher, 2011). Furthermore, there is evidence that childhood trauma increases risk of psychopathology in response to adult stressors (McLaughlin et al., 2010). It is also likely that ethnic minority status can increase the risk of psychopathology through social isolation, experiences of discrimination, victimisation and social defeat, which are all considered stressors (Morgan et al., 2010; Bécares et al., 2009; Cooper et al., 2008; Cooper et al., 2017; Selten and Cantor-Graae, 2005; Selten et al., 2012; Sharpley et al., 2001; Tidey and Miczek, 1996). Cognitive models propose that minority status is associated with greater levels of social threat (Combs et al., 2002; Morgan and Fisher, 2007). Indeed we have found evidence that black minority ethnic status is associated with greater amygdalar activation to out-group (i.e. white faces) than vice versa, neurobiological correlates of these theories/findings (McCutcheon et al., 2018) (McCutcheon et al., 2018). Taken with findings that experiences of racism are correlated with amygdala activation to white faces in black individuals (Greer et al., 2012), this suggests that ethnic minority status is associated with functional alterations in the brain circuits involved in threat processing. As minority ethnicity status has been found to be a chronic stressor and increase the risk of mental illness via chronic stress rather than a genetic component (Akdeniz et al., 2014), we included minority ethnic status as a stressor. The combined effect of the risk factors on dopamine function in humans is unknown. Furthermore, previous studies (Egerton et al., 2016; Oswald et al., 2014; Pruessner et al., 2004) in humans have investigated childhood factors alone. Given animal evidence that exposure to mild stressors potentiate dopaminergic activity whilst severe chronic stressors is associated with dopaminergic blunting (Holly and Miczek, 2016), a key outstanding question remains - what is the effect of chronic adversity, across both child and adult stages of life, on dopaminergic function? We therefore aimed to investigate the effects of exposures to multiple psychosocial risk factors for psychosis on dopaminergic function and the acute stress response. Given the findings of dopaminergic dysfunction associated with childhood maltreatment presented above, we hypothesised that healthy humans with a high cumulative exposure to psychosocial stressors would have altered striatal dopamine synthesis, compared to humans with a low exposure. We also sought examine the relationship between dopaminergic function and the subjective threat and physiological responses to acute psychosocial stress using the Montréal Imaging Stress Task (MIST), a validated stress task involving mental arithmetic under negative social appraisal (Dedovic et al., 2005; Lederbogen et al., 2011). We sought to measure salivary α-amylase, secreted from the parotid gland in response to adrenergic activity and a marker of stress-induced adrenergic activity (van Stegeren et al., 2006) which is associated with a faster increase during psychosocial stress than salivary cortisol (Maruyama et al., 2012), and mean arterial pressure (MAP), the product of cardiac output and total peripheral resistance, reflecting organ perfusion and providing a physiological measure of sympathetic activation.
Results
Participant characteristics and scan parameters
Seventeen HA participants were recruited to the study. All reported high levels of psychological stress exposure in childhood and adulthood (Table 1). Seventeen LA participants were recruited and, as expected, had significantly lower levels of childhood and adult stressors than the HA group (Table 1). Clinical rating scales are reported in Table 1. HA scored significantly higher than LA on subclinical measures of depressive symptoms (BDI), the degree to which previous stressors were having an impact on their lives in the week prior to scanning (BIE) and aberrant salience (ASI).
Table 1. Sample characteristics and scan parameters.
| Sample characteristic | LA ( = 17) | HA ( = 17) | pa | ||
|---|---|---|---|---|---|
| Age, years [mean(SD)] | 27.6 | (7.8) | 29.2 | (7.2) | 0.54 |
| Sex, n | nine female, eight male | eight female, nine male | 1.00 | ||
| Ethnicity, n | 17 WB | 4 BA, 1BB, 4 BC, 6 ME, 1 OE, 1 WB | <0.001 | ||
| Childhood Adversity | |||||
| CTQ [mean(SD)] | 3.8 | (5.2) | 15.3 | (16.1) | 0.01 |
| Parental loss (parental separation with loss of parental contact and/or death and/or going into foster care and/or being adopted) during childhood, n | 0 | 13 | <0.001 | ||
| Childhood sexual abuse | 0 | 6 | 0.02 | ||
| Adult Adversity | |||||
| Number of adverse life events over last 6 months [mean(SD)] | 0.5 (0.9) | 2.6 (1.9) | 0.001 | ||
| Life events score over last 6 months [mean(SD)] | 15.1 (37.0) | 72.3 (55.7) | <0.01 | ||
| Clinical Scores | |||||
| BDI [mean(SD)] | 2.7 (3.8) | 6.5 (5.6) | 0.03 | ||
| BAI [mean(SD)] | 4.8 (6.7) | 9.7 (10.2) | 0.11 | ||
| IES-6 [mean(SD)] | 1.7 (2.3) | 7.7 (7.6) | 0.01 | ||
| O-LIFE [mean(SD)] | 7.2 (6.5) | 13.1 (9.5) | 0.07 | ||
| ASI [mean(SD)] | 5.7 (5.8) | 11.6 (7.5) | 0.02 | ||
| Current Drug Usec,d | |||||
| Tobacco cigarette smokers in last 3 months (n) | three user, 14 non-users | four users, 13 non-users | 1.00 | ||
| Tobacco use in whole sample (cigarettes/day) [mean(SD)] | .4 | (1.5) | 1.7 | (3.6) | 0.19 |
| Alcohol use in last 3 months (n) | 15 users, two non-users | 14 users, three non-users | 1.00 | ||
| Alcohol use (UK alcohol units/week) [mean(SD)] | 10.2 | (9.0) | 7.0 | (8.9) | 0.30 |
| Scan parameter | |||||
| Injected dose (MBq) [mean(SD)] | 143.4 | (7.7) | 142.9 | (7.7) | 0.85 |
| Specific activity (MBq/µmol) [mean(SD)] | 35.3 | (6.7) | 41.4 | (15.4) | 0.14 |
| Whole striatal volume (mm3) [mean(SD)] | 16,842 | (5094) | 15,741 | (4,601) | 0.54 |
| Associative striatal volume (mm3) [mean(SD)] | 10,460 | (3202) | 9771 | (2885) | 0.54 |
| Limbic striatal volume (mm3) [mean(SD)] | 2005 | (610) | 1897 | (547) | 0.61 |
| Sensorimotor striatal volume (mm3) [mean(SD)] | 4375 | (1314) | 4072 | (1189) | 0.51 |
Abbreviations: ASI, Aberrant Salience Inventory; BA, black African; BAI; Beck Anxiety Inventory; BB, black British; BC, black Caribbean; BDI, Beck Depression Inventory; CTQ, Childhood Trauma Questionnaire; IES-6, Brief Impact of Events Scale;, mixed ethnicity; OE, other ethnicity; O-LIFE, Oxford-Liverpool Inventory of Feelings and Experiences; SEAT, Social Environment Assessment Tool; WB, White British.
a Independent-samples t-tests for variables with normal data distributions; Mann-Whitney U tests for variables with non-normal data distributions; χ2-tests for dichotomous variables.
bGroups were compared on a dichotomised ethnicity variable (white British vs ethnic minority).
c 1 UK alcohol unit = 10 mL (~7.88 g) alcohol.
There was no significant group difference in the amount of radioactivity injected or specific activity (Table 1). There was no significant difference in whole striatal or subdivision volumes between the groups (Table 1).
Striatal dopaminergic function
Kicer was significantly reduced in HA relative to LA in the whole striatum (t32 = 2.27, p=0.03; Figure 1). Secondary analysis in each striatal subdivision showed that this reduction reached significance in the limbic and associative subdivisions (Table 2).
Figure 1. Striatal dopamine synthesis capacity in Low Adversity (LA, n = 17) and High Adversity participants (HA, n = 17).
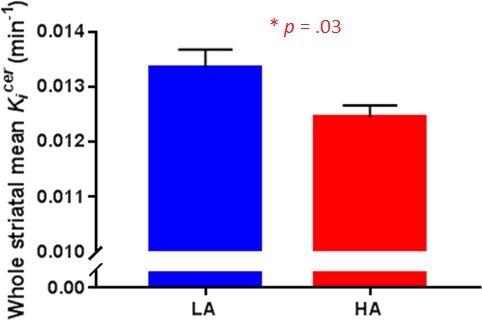
Dopamine synthesis capacity was significantly reduced in HA compared with LA (t32 = 2.27, p=0.03). Error bars indicate standard errors.
Table 2. [18F]-DOPA Kicer (min−1) by group.
| VOI | LA ( = 17) | HA ( = 17) | Group comparisonsa | Effect size | |||
|---|---|---|---|---|---|---|---|
| Mean | (SD) | Mean | (SD) | tdf | p | (Cohen’s D) | |
| STR | 0.0133 | (0.0014) | 0.0124 | (0.0013) | 2.2732 | 0.03 | 0.80 |
| AST | 0.0133 | (0.0011) | 0.0124 | (0.0010) | 2.2832 | 0.03 | 0.81 |
| LST | 0.0140 | (0.0015) | 0.0128 | (0.0010) | 2.6932 | 0.01 | 0.95 |
| SMST | 0.0132 | (0.0013) | 0.0125 | (0.0011) | 1.1732 | 0.10 | 0.41 |
Abbreviations: AST, associative striatum; LST, limbic striatum; Kicer, influx rate constant; SMST, sensorimotor striatum; STR, whole striatum; VOI, volume of interest.
a Independent-samples t-tests.
As the amount of smoking differed in the groups and heavy smoking can influence dopamine function (Bloomfield et al., 2014; Salokangas et al., 2000), we performed an ANCOVA to examine whether smoking was influencing our findings. When co-varying for amount of current cigarette use, the group difference remained significant in the limbic striatum only (F1,30 = 5.2, p=0.029, η2p=0.15).
Psychosocial stress-induced effects
There were no differences between the groups on subjective or biological measures of stress response at baseline (Table 3) apart from a statistical trend (p=0.07, d = 0.67) towards greater amylase concentrations in the HA group compared to the LA group. Response to psychosocial stress is shown in Table 4 and Figure 2. The HA group showed a heightened subjective response to psychosocial stress evidenced by Threatened (p=0.04, d = 0.86) compared to the LA group. By contrast, the HA showed a blunted physiological response to psychosocial stress, as evidenced by an attenuated increase in mean arterial blood pressure (MAP), p=0.03, d = 0.81 and trend for a lower increase in cortisol (p=0.06, d = 0.69). Some participants had a negative area under the curve due a reduction in salivary cortisol levels associated with the task.
Table 3. Baseline stress reactivity in Low Adversity (LA) and High Adversity (HA) groups at prior to acute psychosocial stress challenge.
| Measure | LA ( = 17) | HA ( = 17) | Group comparisons | Effect size | |||
|---|---|---|---|---|---|---|---|
| Mean | (SD) | Mean | (SD) | tdf | P | (Cohen’s D) | |
| Threatened (mm) | 6.75 | (15.52) | 4.58 | (6.00) | 0.4626 | 0.65 | 0.18 |
| Cortisol (U/mL) | 3.93 | (2.74) | 5.12 | (3.54) | 1.0830 | 0.29 | 0.38 |
| Amylase (U/mL) | 178.42 | (173.83) | 92.79 | (53.13) | 1.8330 | 0.07 | 0.67 |
| MAP (mmHg) | 89.67 | (9.45) | 90.38 | (9.63) | 0.2129 | 0.84 | 0.07 |
Abbreviations: HA, high adversity; LA, low adversity; MAP, mean arterial pressure.
Table 4. Acute response to psychosocial stress challenge in Low Adversity (LA) and High Adversity (HA) groups.
| Measure | LA ( = 17) | HA ( = 17) | Group comparisons | Effect size | |||
|---|---|---|---|---|---|---|---|
| Mean | (SD) | Mean | (SD) | tdf | p | (Cohen’s D) | |
| Threatened (AUC) | 191.25 | (587.99) | 780.83 | (764.33) | 2.3126 | 0.04 | 0.86 |
| Cortisol (AUC) | 122.34 | (156.49) | 11.75 | (166.14) | 1.9430 | 0.06 | 0.69 |
| Amylase (AUC) | 1616.67 | (5750.66) | 1015.84 | (2740.24) | 0.3730 | 0.72 | 0.13 |
| MAP (AUC) | 153.30 | (90.04) | 79.31 | (92.09) | 2.2629 | 0.03 | 0.81 |
Abbreviations: HA, high adversity; LA, low adversity; MAP, mean arterial pressure.
Figure 2. The High Adversity group showed a heightened subjective response and a blunted physiological response.
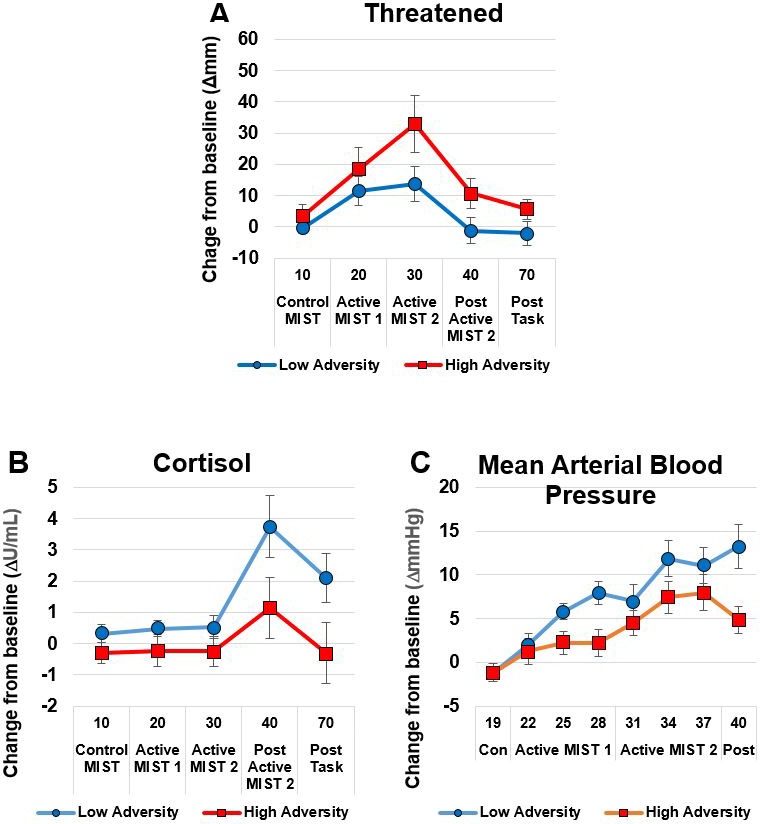
Panel A shows subjective Threatened responses; Panels B (Cortisol) and C (Mean Arterial Blood Pressure) show physiological response. Data show mean (+ /- SEM).
The relationships between physiological and subjective measures
We conducted correlations between the primary outcome of interest (dopamine synthesis capacity in the whole striatum) and variables showing differences in response to acute psychosocial stress (AUC for threatened, cortisol and mean arterial blood pressure). Extreme bivariate outliers (Cook’s distance >1) were removed (n = 7 from threat, n = 2 from cortisol, n = 5 for MAP). In the low adversity group, striatal dopamine synthesis capacity correlated with psychosocial stress-induced threat (r = 0.73, p=0.001, Figure 3A) and mean arterial blood pressure (r = −0.62, p=0.013, Figure 3B). There were no correlations between striatal dopamine synthesis capacity and measures of acute psychosocial response in the high adversity group.
Figure 3. Correlations between striatal dopamine synthesis capacity and acute response to psychosocial stress.
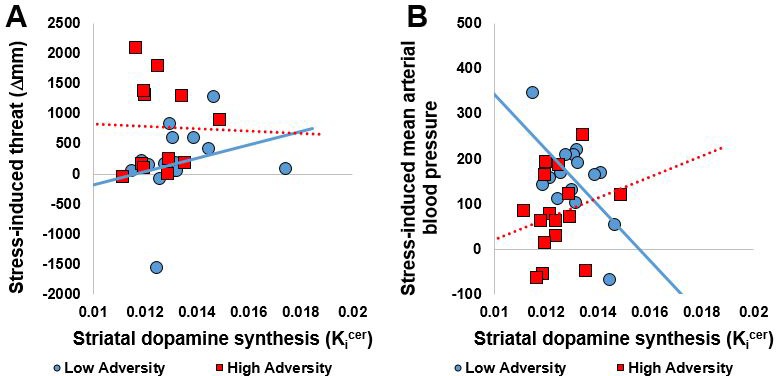
(A) Whole striatal dopamine synthesis capacity was positively correlated with stress-induced threat in the Low Adversity group (r = 0.73, p=0.001) but not the High Adversity group (r = −0.03, p=0.934). (B). Whole striatal dopamine synthesis capacity was negatively correlated with stress-induced threat and mean arterial blood pressure in the Low Adversity group (r = −0.62, p=0.013) but not the High Adversity group (r = 0.23, p=0.395). Extreme bivariate outliers have been removed from the figures.
As we found that dopamine synthesis capacity is correlated with both subjective and physiological response to stress in LA, we performed a regression analysis to identify which subregion is the best predictor for the physiological measures. Regression analyses did not identify which striatal subregion was the best predictor of the physiological measures (p>0.08).
Discussion
Our main finding is that chronic exposure to psychosocial stressors is associated with significantly reduced striatal dopamine synthesis capacity, particularly in the limbic (ventral) striatum. In addition, we found evidence that striatal dopamine synthesis capacity correlated with both the physiological and subjective responses to an acute psychosocial stressor. Chronic stress exposure is associated with a dissociation between physiological and psychological acute stress responses in the form of an attenuated stress-induced increase in blood pressure alongside a potentiated stress-induced subjective response. These findings support our hypothesis that high cumulative exposure to psychosocial adversity would be associated with altered dopamine synthesis capacity.
Interpretation of findings
Acute stress is associated with increased dopaminergic and autonomic output in animals and humans (Imperato et al., 1989; Wand et al., 2007) and recent human evidence indicates that corticotrophin-releasing hormone administration results in dopamine release (Payer et al., 2017). Animal research indicates that dopaminergic neurons are strongly excited by acute stress and aversive stimuli during adulthood (Brischoux et al., 2009; Cohen et al., 2012; Wenzel et al., 2015; Zweifel et al., 2011). Long-lasting neuroadaptive changes on VTA dopamine neurons have been observed after a single stress exposure, demonstrating that acute stress can alter VTA dopamine neuron responsivity to future stimulation (Saal et al., 2003). Exposure to a single acute stressor can also promote long-lasting neuroplastic changes in VTA dopamine neurons in a manner similar to exposure to recreational drugs (Dong et al., 2004; Graziane et al., 2013; Niehaus et al., 2010; Saal et al., 2003). Our results extend these findings and are consistent with evidence from animal models whereby subcortical dopamine transmission is blunted in response to multiple stressors in adulthood (Chrapusta et al., 1997; Gresch et al., 1994).
Whilst stress exposure in animals during the juvenile period and adolescence has a very different effect from to chronic stress in adulthood, our findings are also broadly consistent with developmental stress models (Brake et al., 2004; Meaney et al., 2002). Likewise, early maternal deprivation models in the very early juvenile period (from post-natal day 5) have been associated with hypodopaminergic behaviours in later life including reduced or attenuated responses to acute stress, conditioned locomotion, locomotor activity, and dopamine agonist-induced locomotion with amphetamine, alongside potentiated dopamine antagonist-induced decreases in anticipatory responses (Matthews et al., 1996; Hall et al., 1999). Exposure to repeated longer-term stressors leads to decreases in striatal dopamine function including nucleus accumbens dopamine output (Mangiavacchi et al., 2001), reduced cocaine-induced nucleus accumbens dopamine release (Shimamoto et al., 2011; Holly and Miczek, 2016), and reduced striatal dopamine receptor availability (Meaney et al., 2002; Brake et al., 2004). One explanation for the group difference is that long-term exposure to psychosocial stress is associated with dopaminergic, particularly in the limbic striatum, autonomic and endocrine downregulation. There is evidence of regional specificity in the direction of effects of acute vs chronic stress exposure. Acute and repeated stress activates the entire dopamine system projecting to much of the striatum (Valenti et al., 2011), in particular the associative (dorsal) striatum where object salience is important, whereas in chronic stress-induced depression (Holly and Miczek, 2016), the blunting occurs primarily in the neurons projecting to the ventromedial striatum (Moreines et al., 2017), where reward-related variables are processed. These are therefore likely to be different systems mediating the dopamine stress response that varies with duration of stress exposure, and with the induction of anxiety (acute or repeated stress) vs. depression (chronic stress). Our findings are consistent with Koob's opponent process model, where acute stress activates the dopamine system, which upon chronic exposure leads to a compensatory downregulation (Koob et al., 1997).
However, this interpretation is not consistent with all findings (Butterfield et al., 1999; Tidey and Miczek, 1996). These discrepant findings are likely to reflect differences in the stress paradigm employed as mild stressors tend to potentiate dopamine function and severe/chronic stressors tend to reduce activity (Holly and Miczek, 2016), which could be consistent with an adaptive role for dopamine in response to mild stressors, but chronic uncontrollable stress hijacking this system. For example, maternal deprivation and isolation of neonatal rats was associated with increased dopamine release (Hall et al., 1999; Kosten et al., 2003), whilst unavoidable stress administered over one week and three weeks was associated with a decrease in nucleus accumbens dopamine output (Mangiavacchi et al., 2001). Likewise, rats under a 10-day episodic defeat paradigm had a sensitised dopamine response in the nucleus accumbens, whilst when under a 5-week continuous subordination paradigm they exhibited a suppressed dopamine response (Miczek et al., 2011).
Our findings are consistent with a fMRI study with found that adolescents who had suffered severe early life deprivation exhibited ventral striatal hyporesponsivity during anticipation of monetary reward (Mehta et al., 2010). Yet, findings of increased ventral striatal dopamine release in response to psychosocial stress in humans who reported insufficient early life maternal care (Pruessner et al., 2004), and positive associations between childhood adversity and amphetamine-induced dopamine release are not consistent with these results (Oswald et al., 2014). Since the HA group reported high levels of adverse psychosocial experiences throughout their lives, it is therefore possible that exposure to moderate stressors results in an initial sensitisation of dopaminergic function whereas repeated exposures to severe stressors result in a subsequent down-regulation. Alternatively, it is possible that early life stress is non-linearly related to the responsivity of the cortisol and dopamine systems (Del Giudice et al., 2011). Other factors such as type of stressor may also cause different dopaminergic, autonomic and endocrine effects as work to date has demonstrated that different schedules, intensities, or modalities of stressor presentation can result in dramatically different behavioural and physiological responses (Holly and Miczek, 2016) and these stressor-specific effects are appear highly region specific. Taken together with findings of stress-induced increases in dopaminergic measures (e.g. Fulford and Marsden, 1998; Hall et al., 1999; Kosten et al., 2003; Tidey and Miczek, 1996), the nature, intensity, and schedule of repeated stress may be critical, such that mild or intermittent stressors appear to potentiate basal VTA dopamine neuron activity and more severe or chronic uncontrollable stressors appear to reduce basal VTA dopamine activity, and the response to later stressors of a different nature is generally cross-sensitized (Holly and Miczek, 2016). An alternative explanation for the seemingly discrepant finding is that different parts of dopaminergic system undergo divergent responses to repeated stress (Brake et al., 2004). Additionally, differences in stress-induced dopaminergic outputs have even been observed within the same single neuron projections depending on the location of glutamatergic inputs (Finlay and Zigmond, 1997).
Whilst our measures of stress-induced change in endocrine and physiological function returned to basal levels, we cannot exclude the possibility that inducing acute psychosocial stress prior to PET scanning had an effect on the result. Few studies have measured dopamine synthesis in the period following an acute stressor. One study, for example, found that dopamine synthesis was reduced following restraint stress (Demarest et al., 1985) which may have been due to the activation of inhibitory feedback mechanisms. It therefore remains possible, albeit unlikely, that our findings are due to acute up-regulation of inhibitory feedback mechanisms including stress-induced dopamine release inhibiting dopamine synthesis via autoreceptors (Castro et al., 1996). Alternatively, since corticosteroids regulate tyrosine hydroxylase activity (the rate-limiting step in the dopamine synthesis pathway) (Meyer, 1985), and there is evidence that acute corticosteroids are associated with subsequent decreased striatal dopamine synthesis (Lindley et al., 1999), it is possible, therefore, that our findings are due to participants with chronic stress being sensitized to the acute stress-induced decreases in dopamine synthesis via corticosteroid mediated pathways.
Coherence between the emotional, endocrine and autonomic stress outcome systems has been assumed by some but has been questioned by others (Campbell and Ehlert, 2012; Mauss et al., 2005). Thus, our findings of divergent physiological and subjective emotional responses associated with chronic stressors in the current study are therefore of interest to the field. Repeated exposure to corticosteroids can lead to attenuation of HPA axis activity through negative feedback mechanisms (Karssen et al., 2005) and so it is not surprising that repeated exposure to stressors would be associated with a reduced autonomic response to acute stress, as has been found in adolescent survivors of developmental trauma (Gooding et al., 2016). Propranolol and dexamethasone attenuate the autonomic and endocrine responses to acute psychosocial stress but not the psychological response (Ali et al., 2017) indicating that these responses can be dissociated. Nonetheless, the mechanisms underlying the dissociation between these measures and subjective emotions are unclear and further research is needed to elucidate these dissociative mechanisms. One possibility is that this dissociation reflects a shift away from dopaminergic mechanisms to a more cortisol based stress response. Our findings of exposure to stressors being associated with autonomic effects are also important in the context of understanding the mechanisms underlying the well-established associations between cardiovascular disease and both depression (Penninx, 2017) and psychosis (Osborn et al., 2007).
Our findings of heightened stress-induced threat are consistent with the threat-anticipation model of delusion formation (Freeman, 2007) and evidence that elevated sensitivity to socio-environmental stress via enhanced threat anticipation in daily life may be important psychological processes underlying the association between childhood adversity and psychosis (Reininghaus et al., 2016). Given our findings of decreased dopamine synthesis capacity associated with high psychosocial stressors, it remains possible that repeated psychosocial stressors in childhood increase the risk of psychotic symptoms by hypodopaminergic or non-dopaminergic processes. However, Thompson et al. (2013) found that patients with comorbid schizophrenia and substance dependence (associated with a blunted dopamine system) had reduced amphetamine-induced dopamine release. Yet, despite a blunted dopamine response, this study found the classically described relationship between dopamine release and increase in psychotic symptoms, which may be due to D2 receptor super-sensitivity (Seeman and Seeman, 2014). Alternatively, as these participants were healthy it remains possible that these participants have resilience and so the observed findings may be due to adaptive allostatic down-regulations (McEwen, 1998).
Although the case–control design of this study is not able to confirm a causative relationship between psychosocial stress and dopamine dysfunction, these findings warrant further research into potential causative mechanisms. Our findings, particularly of relationships between cumulative stress exposure and the dopamine system, may be important for understanding how exposures to multiple stressors induce changes in the dopamine system, and how these relate to both vulnerability to and resilience against mental illnesses. It would be important to consider these findings in light of a putative role of the dopaminergic system in the pathophysiology anhedonia in depression (Nutt, 2006), the role of dopamine in social motivation (Love, 2014), and findings that striatal neurons can incorporate social reward into their computations (Báez-Mendoza et al., 2013; Schultz, 2016). Our findings may be highly relevant in terms of our understanding of addiction, as a history of exposure to aversive stimuli is strongly associated with later addictive behaviour, with both clinical and preclinical work demonstrating that stress plays a powerful role in the initiation, escalation, and relapse to drug abuse (Shaham et al., 2000; Sinha, 2007; Sinha, 2009).
Strengths and limitations
A strength of our study is that it specifically examined the effect of multiple psychosocial risk factors to examine their combined effect since these factors often cluster together in the general population (Hjern et al., 2004; Teicher et al., 2016; Wicks et al., 2005) and so it is not possible to disentangle the different types of psychosocial adversity due to lack of power as we are unable to directly contrast the effect of single risk factors with multiple exposures, or determine if risk factors have synergistic effects. Likewise, we did not co-vary for the different psychosocial risks as analysis of covariance is suboptimal at adjusting for factors when groups differ significantly in their covariates (Miller and Chapman, 2001). We chose to recruit participants with high levels of stress exposure in early development and adulthood, because early developmental stressors increase the risk of psychopathology following adult stressors (McLaughlin et al., 2010). However, a potential limitation of our combination of early developmental and adult stressors is that it may confound the early life (i.e. likely programming) effects with the later in life acute stressors which occur after developmental sensitive periods.
The PET scan was conducted following exposure to acute psychosocial stress exposure. We sought to reduce the length of time between task and PET scan to reduce variance in time and to limit the variance associated with normal fluctuations in dopamine synthesis capacity that is to limit the temporal separation between the stress task and the PET scan. Whilst acute stress increases dopamine release (Pruessner et al., 2004), it is not clear if this has an acute effect on striatal dopamine synthesis capacity. Therefore, it is possible that the close temporal proximity of the stress task to the PET means that what was observed during the PET scan could have been influenced by the stress task. Thus, one possible explanation for our findings could be reduced change in dopamine synthesis capacity following stress in the high adversity group relative to the low stress group, which would be consistent with Koob’s model (Koob et al., 1997). However, we are not aware of evidence indicating that an acute stressor can alter the parameters that contribute to our index (i.e. the activity of aromatic acid decarboxylase) in the timeframe used in our study. Nonetheless, a controlled study comparing stress and no stress prior to PET to investigate the effects is needed to definitively determine if acute stress alters dopamine synthesis capacity. Given this, it is possible that an optimal experimental design would allow more time to pass between the stressor and the PET scan.
A further consideration is that our groups were not matched for ethnicity. Ethnicity is associated with differences in allele frequencies of dopaminergic genetic variants (Gelernter et al., 1993) and striatal D2/3 receptor availability (Wiers et al., 2018) and so we cannot exclude the possibility that this is driving our results. Nonetheless, the study by Wiers and colleagues (Wiers et al., 2018) found ethnicity was associated with dorsal (rather than ventral) striatal dopamine receptor effects and that the effects of ethnicity on dopamine receptor availability were not driven by variation in dopamine candidate genes suggesting that their results are influenced by socioeconomic factors and therefore psychosocial stressors per se. Since our effects were most pronounced in the ventral (rather than dorsal) striatum and we found relationships between psychosocial stressors and ventral striatal dopamine synthesis, it is therefore unlikely that our results can be explained by ethnicity. Our participants were of multiple ethnic groups and so we were unable to determine the effects of psychosocial stress associated with immigration accounting for ethnicity. Future studies of individual risk factors are needed to examine what type of risks may be driving our findings. Finally, as our study is cross-sectional we cannot rule out reverse causality and a longitudinal design is required to distinguish between the interpretations discussed above.
We cannot exclude the possibility that recall bias may be confounding our findings as the measures of psychosocial stress rely on self-report and it was therefore not possible to independently verify the histories of psychosocial stressors. As such, the assessment of childhood trauma may be liable to recall bias in depressed patients (Lewinsohn and Rosenbaum, 1987). However, measures of childhood trauma have been demonstrated to remain stable over time and to be independent of the current degree of abuse-related psychopathology (Paivio, 2001). Despite ongoing concerns that retrospective reporting overestimates associations between abuse and adult psychopathology (Gilbert et al., 2009), there is evidence that prospective and retrospective measures of abuse predict similar rates of mental illness (Scott et al., 2012), recall bias accounts for less than 1% of variance in measures of child abuse (Fergusson et al., 2011) and good reliability and validity has been reported for retrospective self-reports of early experiences obtained from individuals with psychotic disorder and so this is unlikely to be significantly confounding our results (Fisher et al., 2011). Nonetheless, difficulties remain in measuring and quantifying emotional neglect due in part to its highly subjective nature (Watson et al., 2014). We did not control for genetic influences, apart from excluding individuals with a family history of psychosis and so genetic differences between exposed and unexposed groups might contribute to our finding. Nonetheless, previous work in our laboratory (Stokes et al., 2013) on heritability of striatal dopamine synthesis capacity found that individual-specific environmental factors, rather than genetic factors, had the greatest effect on the limbic striatum which is consistent with the interpretation that the psychosocial exposures have contributed to our finding of striatal hypodopaminergia. A further limitation is that we did not account for menstrual cycle phase in our analysis, given effects on the HPA axis (Kirschbaum et al., 1999).
These findings show that long-term exposure to psychosocial stressors is associated with reduced striatal dopamine synthesis capacity, particularly in the limbic subdivision of the striatum, alongside a de-coupling of the acute stress response such that emotional responses are potentiated whilst cardiovascular and endocrine responses tended to be blunted. Further work is needed to understand what processes contribute to this decoupling and how this may contribute to the development of mental illness.
Materials and methods
This study was approved by the National Research Ethics Service (12/LO/1955) and the Administration of Radioactive Substances Advisory Committee (ARSAC). The study was conducted in accordance with the Helsinki Declaration. All participants provided informed written consent to participate after an oral and written explanation of the study.
Participant recruitment
We recruited two groups of healthy volunteers, one exposed to multiple risk factors (exposed high adversity group, ‘HA’)) and one not exposed (unexposed low adversity group, ‘LA’), from throughout the UK via public advertisement, newspaper advertisement and national media engagement. Responding individuals were then screened via telephone. LA ‘controls’ were individually matched to the HA group on the basis of age (+ /- 5 years) and sex. Inclusion criteria for all participants were: age 18–45 years, good physical health and capacity to give written informed consent. Exclusion criteria for all participants included: a personal history of psychiatric illness including substance abuse but not Nicotine Use Disorders; a history of psychotic illness in first degree relatives; evidence of an at risk mental state (Yung et al., 2005) and contraindications to PET including pregnancy, nursing mothers, severe obesity and previous clinical procedures involving exposure to significant ionizing radiation within the last year.
Additional inclusion criteria for the HA (exposed) group included at least one childhood stressor and at least two adult stressors. These were ascertained by structured clinical interview. Childhood trauma self-reports were triangulated with the Childhood Trauma Questionnaire (CTQ) (Bernstein and Fink, 1998) and the Childhood Experiences of Care and Abuse (CECA) (Bifulco et al., 2005).
Childhood stressors: Childhood (before age 16 years); History of childhood (before age 16 years) adversity defined as one or more of the following: parental loss (including separation with loss of parental contact and/or death and/or going into foster care and/or being adopted) and/or abuse (including physical, sexual abuse or neglect) and/or bullying (i.e. peer abuse) and/or major disaster and/or war and/or admission to hospital with life-threatening medical problem.
Adult stressor: Minority ethnic status; a significant life event defined as a bereavement, moving house, a change in job or financial circumstances, a new family member being born, a breakdown of a significant relationship, and/or unemployment within the last six months.
Additional inclusion criteria for the LA group included no exposure to the childhood factors listed above, ethnic majority status and no significant adverse life events in the last 6 months.
Psychosocial assessments
Assessments included the Beck Depression Inventory (BDI; Beck et al., 1996) Beck Anxiety Inventory (BAI) (Beck et al., 1988), Aberrant Salience Inventory (Cicero et al., 2010), the CTQ and an adapted bullying questionnaire (Olweus, 1996). Detailed histories of life events over the preceding 6 months were obtained via the List of Threatening Events (Brugha and Cragg, 1990), and a life events score then calculated from these events based on the Holmes and Rahe life events stress scale (Holmes and Rahe, 1967); Brief Impact of Events Scale (IES-6) (Thoresen et al., 2010).
PET scans
Participants were asked to fast for 5 hr and to refrain from smoking tobacco for 2 hr before imaging. On the day of the PET scan, urine drug screen (Monitect HC12, Branan Medical Corporation, Irvine, California) confirmed no recent drug use, and a negative urinary pregnancy test was required in all female participants. Head position was marked and monitored via laser crosshairs and video camera, and minimized using a head-strap. We used a Siemens Biograph 6 TruePoint PET-CT scanner (Siemens Healthcare, Erlangen, Germany). A computed tomography (CT) scan (effective dose = 0.36 mSv) was acquired for attenuation and model-based scatter correction prior to each PET scan. A target dose of approximately 150 MBq of [18F]-DOPA was administered by bolus intravenous injection at the start of PET imaging. Emission data were acquired in list mode for 95 min, reconstructed in a 128 × 128 matrix with 2.6x zoom via filter back projection with a three dimensional 5 mm full-width half-maximum Gaussian image filter and re-binned into 32 timeframes (comprising eight 15 s frames, three 60 s frames, five 120 s frames, and sixteen 300 s frames).
Image analysis
To correct for head movement in the scanner, non-attenuation-corrected dynamic images were denoised using a level 2, order 64 Battle-Lemarie wavelet filter. Nonattenuation-corrected images were used for the realignment algorithm as they include greater scalp signal, improving re-alignment compared with attenuated-corrected images (Turkheimer et al., 1999). Frames were realigned to a single ‘reference’ frame, acquired 10 min post-injection, employing a mutual information algorithm (Studholme et al., 1996). The transformation parameters were then applied to the corresponding attenuated-corrected dynamic images. The realigned frames were then summated, creating a movement-corrected dynamic image, which was used in the analysis. The cerebellar reference region (Kumakura and Cumming, 2009) was defined using a probabilistic atlas (Martinez et al., 2003), and as previously described, regions of interest (ROI) in the whole striatum and its functional sub-divisions (Haber, 2014) were delineated to create an ROI map (Egerton et al., 2010). SPM8 (http://www.fil.ion.ucl.ac.uk/spm) was then used to normalize the ROI map together with the tracer-specific ([18F]-DOPA) template (Egerton et al., 2016; Howes et al., 2009) to each individual PET summation image. This nonlinear transformation procedure allowed ROIs to be automatically placed on individual [18F]-DOPA PET dynamic images. The influx rate constants (Kicer, written as Ki in some previous publications (Howes et al., 2013) for the entire striatal ROI and the functional subdivisions bilaterally were calculated compared with uptake in the reference region using a graphical approach adapted for a reference tissue input function (Egerton et al., 2016).
Psychosocial stress paradigm
We induced psychosocial stress using the Montreal Imaging Stress Task (MIST) (Pruessner et al., 2004) 2 hr before PET scanning. The rationale for conducting the PET scan on the same day as the stress task was to reduce the variance in the time between the measures. Participants were aware that one of the measures would be inducing psychosocial stress; however, they were only told after the MIST was completed that this was the task to induce psychosocial stress, and they were then debriefed. They were told beforehand that they could stop the experimental procedures at any time. During the MIST, participants were asked to solve mental arithmetic problems first under a control condition during which no time constraint or feedback were present, and subsequently under the experimental condition where time and difficulty were automatically adjusted to result in a 30–40% error rate. During the experimental condition, we continuously made participants aware of their suboptimal performance via a visual performance bar and scripted verbal negative feedback delivered approximately every 1 min, where a confederate researcher reminded participants that they were performing much worse than average. The MIST was administered using pairs of researchers who were balanced for sex and ethnicity (i.e. male and female researchers, white British and minority ethnicities). There were 2 × 4 min blocks of control MIST control followed by a brief rest, and then 2 pairs of 2 × 4 min blocks of the experimental version including feedback and brief rest. We assessed subjective threat assessed before the task, at the end of control condition, after each 10 min paired block of MIST, and twice upon completion of the task at 30 min and 60 min after starting the experimental task (see Figure 4). We used visual analogue scales to measure subjective threat. Salivary cortisol and α-amylase (Engert et al., 2011) samples were taken at the same time points as the visual analogue scales. Heart rate and blood pressure recordings were taken at 3 min intervals during the control MIST and two experimental MIST conditions, with four readings in each of these three conditions.
Figure 4. Experimental procedures.
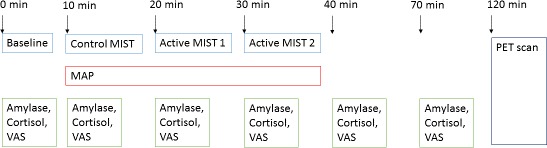
MIST, Montreal Imaging Stress Test; MAP, Mean Arterial Blood Pressure; VAS, visual analogue scale.
Power calculation
In a study of test-retest reliability of [18F]-DOPA PET (Egerton et al., 2010) striatal Kicer had an intraclass correlation coefficient of approximately 0.9 [mean (SD) Kicer = 0.01417 (0.00127)min−1 (test) and 0.01381 (0.00127)min−1 (re-test)]. Previous [18F]-DOPA uptake work has found an effect size of 1.25 in patients with schizophrenia (Howes et al.) which compares well with previous studies: 1.89 (Meyer-Lindenberg et al., 2002), 1.57 (McGowan et al., 2004). On the basis that large effect sizes are observed in disorders of dopamine function, this study was powered to anticipate an effect size of d = 1.00 when comparing differences between HA and LA groups. Therefore, to achieve a power of 0.8, with an effect size of 1.0, a = 0.05, using independent t-tests, n = 17 participants would be required per group.
Statistical analysis
Normality of distribution and homogeneity of variance were assessed using Kolmogorov–Smirnov and Levene’s tests respectively, and diagnostic plots. The primary analysis was for Group (HA/LA) differences in striatal dopamine synthesis capacity. The primary region of interest was the whole striatum. Exploratory analyses were performed in the functional subdivisions of the striatum. Independent samples t-tests were used for normally distributed data, Mann–Whitney U-tests for non-normally distributed data, and the χ2 test for dichotomous variables. Where the assumption of homogeneity of variance was violated for independent samples t-tests, p values were adjusted to assume unequal variance. Group differences in acute response to psychosocial stress were investigated by calculating the Area Under the Curve (AUC) using the trapezoid method. The AUC was calculated from the last measurement taken during the control MIST condition up until the last available post-task measurement. For subjective ratings (VAS), salivary cortisol and amylase, AUC was calculated from readings taken from control MIST (10 min), Active MIST 1 (20 min), Active MIST 2 (30 min), Post Active MIST 2 (40 min) and Post Task (70 min). For blood pressure (MAP), AUC was calculated from Control MIST (19 min), Active MIST 1 (22 minutes, 25 min, 28 min), Active MIST 2 (31 minutes, 34 min, 37 min), and Post Active MIST 2 (40 min). Prior to calculating the AUC, data were corrected for baseline performance by subtracting baseline scores from all subsequent time points. Independent sample t-tests were used to compare groups at baseline, to compare response to acute psychosocial stress using AUC. Pearson correlational analyses were conducted separately in each of the groups among variables showing group differences. Bivariate outliers (Cook’s distance >1) were excluded prior to correlational analyses.
Acknowledgements
We are very grateful to Yvonne Lewis and everyone at Imanova (now Invicro), Professor Vivette Glover, Drs David Bonsall and Dr Lucia Valmaggia for their assistance with this study. This work was funded by a Medical Research Grant to Professor Howes. Dr Bloomfield is funded by a UCL Excellence Fellowship and supported by the National Institute for Health Research University College London Hospitals Biomedical Research Centre.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
Michael AP Bloomfield, Email: m.bloomfield@ucl.ac.uk.
Oliver Howes, Email: oliver.howes@kcl.ac.uk.
Christian Büchel, University Medical Center Hamburg-Eppendorf, Germany.
Christian Büchel, University Medical Center Hamburg-Eppendorf, Germany.
Funding Information
This paper was supported by the following grants:
Medical Research Council MC-A656-5QD30 to Oliver Howes.
National Institute for Health Research to Michael AP Bloomfield.
National Institute for Health Research University College London Hospitals Biomedical Research Centre to Michael AP Bloomfield.
Wellcome Trust 094849/Z/10/Z to Oliver Howes.
University College London to Michael AP Bloomfield.
National Institute for Health Research Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London to Oliver Howes.
Additional information
Competing interests
No competing interests declared.
Professor Howes has received investigator-initiated research funding from and/or participated in advisory/speaker meetings organised by Astra-Zeneca, Autifony, BMS, Eli Lilly, Heptares, Jansenn, Lundbeck, Lyden-Delta, Otsuka, Servier, Sunovion, Rand and Roche. Neither Professor Howes nor his family have been employed by or have holdings/a financial stake in any biomedical company.
Author contributions
Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing—original draft, Project administration, Writing—review and editing.
Investigation, Writing—review and editing.
Methodology, Writing—review and editing.
Formal analysis, Writing—review and editing.
Conceptualization, Resources, Formal analysis, Supervision, Funding acquisition, Investigation, Methodology, Writing—original draft, Writing—review and editing.
Ethics
Human subjects: This study was approved by the National Research Ethics Service (12/LO/1955) and the Administration of Radioactive Substances Advisory Committee (ARSAC). The study was conducted in accordance with the Helsinki Declaration. All participants provided informed written consent to participate after an oral and written explanation of the study.
Additional files
Data availability
The raw data from this study are available on written request to the Chief Investigator. This restriction is due to sensitive data on human research participants. Processed data files for Figures 1 and 2, and table 2 are provided in Source data 1.
References
- Akdeniz C, Tost H, Streit F, Haddad L, Wüst S, Schäfer A, Schneider M, Rietschel M, Kirsch P, Meyer-Lindenberg A. Neuroimaging evidence for a role of neural social stress processing in ethnic minority-associated environmental risk. JAMA Psychiatry. 2014;71:672–680. doi: 10.1001/jamapsychiatry.2014.35. [DOI] [PubMed] [Google Scholar]
- Ali N, Nitschke JP, Cooperman C, Pruessner JC. Suppressing the endocrine and autonomic stress systems does not impact the emotional stress experience after psychosocial stress. Psychoneuroendocrinology. 2017;78:125–130. doi: 10.1016/j.psyneuen.2017.01.015. [DOI] [PubMed] [Google Scholar]
- Báez-Mendoza R, Harris CJ, Schultz W. Activity of striatal neurons reflects social action and own reward. PNAS. 2013;110:16634–16639. doi: 10.1073/pnas.1211342110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beards S, Gayer-Anderson C, Borges S, Dewey ME, Fisher HL, Morgan C. Life events and psychosis: a review and meta-analysis. Schizophrenia Bulletin. 2013;39:740–747. doi: 10.1093/schbul/sbt065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bécares L, Stafford M, Nazroo J. Fear of racism, employment and expected organizational racism: their association with health. The European Journal of Public Health. 2009;19:504–510. doi: 10.1093/eurpub/ckp071. [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. Journal of Consulting and Clinical Psychology. 1988;56:893–897. doi: 10.1037/0022-006X.56.6.893. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio: Psychological Corporation; 1996. [Google Scholar]
- Bendall S, Jackson HJ, Hulbert CA, McGorry PD. Childhood trauma and psychotic disorders: a systematic, critical review of the evidence. Schizophrenia Bulletin. 2008;34:568–579. doi: 10.1093/schbul/sbm121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein DP, Fink L. Childhood Trauma Questionnaire: A Retrospective Self-Report Manual. San Antonio: The Psychological Corporation; 1998. [Google Scholar]
- Bifulco A, Bernazzani O, Moran PM, Jacobs C. The childhood experience of care and abuse questionnaire (CECA.Q): validation in a community series. British Journal of Clinical Psychology. 2005;44:563–581. doi: 10.1348/014466505X35344. [DOI] [PubMed] [Google Scholar]
- Bloomfield MA, Pepper F, Egerton A, Demjaha A, Tomasi G, Mouchlianitis E, Maximen L, Veronese M, Turkheimer F, Selvaraj S, Howes OD. Dopamine function in cigarette smokers: an [¹⁸F]-DOPA PET study. Neuropsychopharmacology. 2014;39:2397–2404. doi: 10.1038/npp.2014.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brake WG, Zhang TY, Diorio J, Meaney MJ, Gratton A. Influence of early postnatal rearing conditions on mesocorticolimbic dopamine and behavioural responses to psychostimulants and stressors in adult rats. European Journal of Neuroscience. 2004;19:1863–1874. doi: 10.1111/j.1460-9568.2004.03286.x. [DOI] [PubMed] [Google Scholar]
- Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. PNAS. 2009;106:4894–4899. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GW, Birley JL. Crises and life changes and the onset of schizophrenia. Journal of Health and Social Behavior. 1968;9:203–214. doi: 10.2307/2948405. [DOI] [PubMed] [Google Scholar]
- Brugha TS, Cragg D. The list of threatening experiences: the reliability and validity of a brief life events questionnaire. Acta Psychiatrica Scandinavica. 1990;82:77–81. doi: 10.1111/j.1600-0447.1990.tb01360.x. [DOI] [PubMed] [Google Scholar]
- Butterfield MI, Panzer PG, Forneris CA. Victimization of women and its impact on assessment and treatment in the psychiatric emergency setting. Psychiatric Clinics of North America. 1999;22:875–896. doi: 10.1016/S0193-953X(05)70131-3. [DOI] [PubMed] [Google Scholar]
- Campbell J, Ehlert U. Acute psychosocial stress: does the emotional stress response correspond with physiological responses? Psychoneuroendocrinology. 2012;37:1111–1134. doi: 10.1016/j.psyneuen.2011.12.010. [DOI] [PubMed] [Google Scholar]
- Castro SL, Sved AF, Zigmond MJ. Increased neostriatal tyrosine hydroxylation during stress: role of extracellular dopamine and excitatory amino acids. Journal of Neurochemistry. 1996;66:824–833. doi: 10.1046/j.1471-4159.1996.66020824.x. [DOI] [PubMed] [Google Scholar]
- Chrapusta SJ, Wyatt RJ, Masserano JM. Effects of single and repeated footshock on dopamine release and metabolism in the brains of fischer rats. Journal of Neurochemistry. 1997;68:2024–2031. doi: 10.1046/j.1471-4159.1997.68052024.x. [DOI] [PubMed] [Google Scholar]
- Cicero DC, Kerns JG, McCarthy DM. The aberrant salience inventory: a new measure of psychosis proneness. Psychological Assessment. 2010;22:688–701. doi: 10.1037/a0019913. [DOI] [PubMed] [Google Scholar]
- Cohen JY, Haesler S, Vong L, Lowell BB, Uchida N. Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature. 2012;482:85–88. doi: 10.1038/nature10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs DR, Penn DL, Fenigstein A. Ethnic differences in subclinical paranoia: an expansion of norms of the paranoia scale. Cultural Diversity and Ethnic Minority Psychology. 2002;8:248–256. doi: 10.1037/1099-9809.8.3.248. [DOI] [PubMed] [Google Scholar]
- Cooper MA, McIntyre KE, Huhman KL. Activation of 5-HT1A autoreceptors in the dorsal raphe nucleus reduces the behavioral consequences of social defeat. Psychoneuroendocrinology. 2008;33:1236–1247. doi: 10.1016/j.psyneuen.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper SE, Kechner M, Caraballo-Pérez D, Kaska S, Robison AJ, Mazei-Robison MS. Comparison of chronic physical and emotional social defeat stress effects on mesocorticolimbic circuit activation and voluntary consumption of morphine. Scientific Reports. 2017;7:8445. doi: 10.1038/s41598-017-09106-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutajar MC, Mullen PE, Ogloff JR, Thomas SD, Wells DL, Spataro J. Schizophrenia and other psychotic disorders in a cohort of sexually abused children. Archives of General Psychiatry. 2010;67:1114–1119. doi: 10.1001/archgenpsychiatry.2010.147. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Lefter L, Trickett PK, Putnam FW. Urinary catecholamine excretion in sexually abused girls. Journal of the American Academy of Child & Adolescent Psychiatry. 1994;33:320–327. doi: 10.1097/00004583-199403000-00004. [DOI] [PubMed] [Google Scholar]
- Dedovic K, Renwick R, Mahani NK, Engert V, Lupien SJ, Pruessner JC. The Montreal imaging stress task: using functional imaging to investigate the effects of perceiving and processing psychosocial stress in the human brain. Journal of Psychiatry & Neuroscience : JPN. 2005;30:319–325. [PMC free article] [PubMed] [Google Scholar]
- Del Giudice M, Ellis BJ, Shirtcliff EA. The adaptive calibration model of stress responsivity. Neuroscience & Biobehavioral Reviews. 2011;35:1562–1592. doi: 10.1016/j.neubiorev.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarest KT, Moore KE, Riegle GD. Acute restraint stress decreases dopamine synthesis and turnover in the median eminence: a model for the study of the inhibitory neuronal influences on tuberoinfundibular dopaminergic neurons. Neuroendocrinology. 1985;41:437–444. doi: 10.1159/000124215. [DOI] [PubMed] [Google Scholar]
- Dillon DG, Holmes AJ, Birk JL, Brooks N, Lyons-Ruth K, Pizzagalli DA. Childhood adversity is associated with left basal ganglia dysfunction during reward anticipation in adulthood. Biological Psychiatry. 2009;66:206–213. doi: 10.1016/j.biopsych.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Saal D, Thomas M, Faust R, Bonci A, Robinson T, Malenka RC. Cocaine-induced potentiation of synaptic strength in dopamine neurons: behavioral correlates in GluRA(-/-) mice. PNAS. 2004;101:14282–14287. doi: 10.1073/pnas.0401553101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerton A, Demjaha A, McGuire P, Mehta MA, Howes OD. The test-retest reliability of 18F-DOPA PET in assessing striatal and extrastriatal presynaptic dopaminergic function. NeuroImage. 2010;50:524–531. doi: 10.1016/j.neuroimage.2009.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerton A, Valmaggia LR, Howes OD, Day F, Chaddock CA, Allen P, Winton-Brown TT, Bloomfield MAP, Bhattacharyya S, Chilcott J, Lappin JM, Murray RM, McGuire P. Adversity in childhood linked to elevated striatal dopamine function in adulthood. Schizophrenia Research. 2016;176:171–176. doi: 10.1016/j.schres.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engert V, Vogel S, Efanov SI, Duchesne A, Corbo V, Ali N, Pruessner JC. Investigation into the cross-correlation of salivary cortisol and alpha-amylase responses to psychological stress. Psychoneuroendocrinology. 2011;36:1294–1302. doi: 10.1016/j.psyneuen.2011.02.018. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Boden JM. Structural equation modeling of repeated retrospective reports of childhood maltreatment. International Journal of Methods in Psychiatric Research. 2011;20:93–104. doi: 10.1002/mpr.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay JM, Zigmond MJ. The effects of stress on central dopaminergic neurons: possible clinical implications. Neurochemical Research. 1997;22:1387–1394. doi: 10.1023/a:1022075324164. [DOI] [PubMed] [Google Scholar]
- Fisher HL, Craig TK, Fearon P, Morgan K, Dazzan P, Lappin J, Hutchinson G, Doody GA, Jones PB, McGuffin P, Murray RM, Leff J, Morgan C. Reliability and comparability of psychosis patients' retrospective reports of childhood abuse. Schizophrenia Bulletin. 2011;37:546–553. doi: 10.1093/schbul/sbp103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman D. Suspicious minds: the psychology of persecutory delusions. Clinical Psychology Review. 2007;27:425–457. doi: 10.1016/j.cpr.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Fulford AJ, Marsden CA. Effect of isolation-rearing on conditioned dopamine release in vivo in the nucleus accumbens of the rat. Journal of Neurochemistry. 1998;70:384–390. doi: 10.1046/j.1471-4159.1998.70010384.x. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Goldman D, Risch N. The A1 allele at the D2 dopamine receptor gene and alcoholism. A reappraisal. Jama. 1993;269:1673–1677. doi: 10.1001/jama.1993.03500130087038. [DOI] [PubMed] [Google Scholar]
- Gilbert R, Kemp A, Thoburn J, Sidebotham P, Radford L, Glaser D, MacMillan HL. Recognising and responding to child maltreatment. The Lancet. 2009;373:167–180. doi: 10.1016/S0140-6736(08)61707-9. [DOI] [PubMed] [Google Scholar]
- Gooding HC, Milliren CE, Austin SB, Sheridan MA, McLaughlin KA. Child abuse, resting blood pressure, and blood pressure reactivity to psychosocial stress. Journal of Pediatric Psychology. 2016;41:5–14. doi: 10.1093/jpepsy/jsv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziane NM, Polter AM, Briand LA, Pierce RC, Kauer JA. Kappa opioid receptors regulate stress-induced cocaine seeking and synaptic plasticity. Neuron. 2013;77:942–954. doi: 10.1016/j.neuron.2012.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer TM, Vendemia JM, Stancil M. Neural correlates of race-related social evaluations for african americans and white americans. Neuropsychology. 2012;26:704–712. doi: 10.1037/a0030035. [DOI] [PubMed] [Google Scholar]
- Gresch PJ, Sved AF, Zigmond MJ, Finlay JM. Stress-induced sensitization of dopamine and norepinephrine efflux in medial prefrontal cortex of the rat. Journal of Neurochemistry. 1994;63:575–583. doi: 10.1046/j.1471-4159.1994.63020575.x. [DOI] [PubMed] [Google Scholar]
- Guloksuz S, van Nierop M, Lieb R, van Winkel R, Wittchen HU, van Os J. Evidence that the presence of psychosis in non-psychotic disorder is environment-dependent and mediated by severity of non-psychotic psychopathology. Psychological Medicine. 2015;45:2389–2401. doi: 10.1017/S0033291715000380. [DOI] [PubMed] [Google Scholar]
- Haber SN. The place of dopamine in the cortico-basal ganglia circuit. Neuroscience. 2014;282:248–257. doi: 10.1016/j.neuroscience.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall FS, Wilkinson LS, Humby T, Robbins TW. Maternal deprivation of neonatal rats produces enduring changes in dopamine function. Synapse. 1999;32:37–43. doi: 10.1002/(SICI)1098-2396(199904)32:1<37::AID-SYN5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Hanson JL, Hariri AR, Williamson DE. Blunted ventral striatum development in adolescence reflects emotional neglect and predicts depressive symptoms. Biological Psychiatry. 2015;78:598–605. doi: 10.1016/j.biopsych.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjern A, Wicks S, Dalman C. Social adversity contributes to high morbidity in psychoses in immigrants--a national cohort study in two generations of swedish residents. Psychological Medicine. 2004;34:1025–1033. doi: 10.1017/S003329170300148X. [DOI] [PubMed] [Google Scholar]
- Holly EN, Miczek KA. Ventral tegmental area dopamine revisited: effects of acute and repeated stress. Psychopharmacology. 2016;233:163–186. doi: 10.1007/s00213-015-4151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes TH, Rahe RH. The social readjustment rating scale. Journal of Psychosomatic Research. 1967;11:213–218. doi: 10.1016/0022-3999(67)90010-4. [DOI] [PubMed] [Google Scholar]
- Howes OD, Montgomery AJ, Asselin MC, Murray RM, Valli I, Tabraham P, Bramon-Bosch E, Valmaggia L, Johns L, Broome M, McGuire PK, Grasby PM. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Archives of General Psychiatry. 2009;66:13–20. doi: 10.1001/archgenpsychiatry.2008.514. [DOI] [PubMed] [Google Scholar]
- Howes OD, Shotbolt P, Bloomfield M, Daalman K, Demjaha A, Diederen KM, Ibrahim K, Kim E, McGuire P, Kahn RS, Sommer IE. Dopaminergic function in the psychosis spectrum: an [18F]-DOPA imaging study in healthy individuals with auditory hallucinations. Schizophrenia Bulletin. 2013;39:807–814. doi: 10.1093/schbul/sbr195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Murray RM. Schizophrenia: an integrated sociodevelopmental-cognitive model. The Lancet. 2014;383:1677–1687. doi: 10.1016/S0140-6736(13)62036-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperato A, Puglisi-Allegra S, Casolini P, Zocchi A, Angelucci L. Stress-induced enhancement of dopamine and acetylcholine release in limbic structures: role of corticosterone. European Journal of Pharmacology. 1989;165:337–338. doi: 10.1016/0014-2999(89)90735-8. [DOI] [PubMed] [Google Scholar]
- Janssen I, Krabbendam L, Bak M, Hanssen M, Vollebergh W, de Graaf R, van Os J. Childhood abuse as a risk factor for psychotic experiences. Acta Psychiatrica Scandinavica. 2004;109:38–45. doi: 10.1046/j.0001-690X.2003.00217.x. [DOI] [PubMed] [Google Scholar]
- Karssen AM, Meijer OC, Berry A, Sanjuan Piñol R, de Kloet ER. Low doses of dexamethasone can produce a hypocorticosteroid state in the brain. Endocrinology. 2005;146:5587–5595. doi: 10.1210/en.2005-0501. [DOI] [PubMed] [Google Scholar]
- Kelleher I, Keeley H, Corcoran P, Ramsay H, Wasserman C, Carli V, Sarchiapone M, Hoven C, Wasserman D, Cannon M. Childhood trauma and psychosis in a prospective cohort study: cause, effect, and directionality. American Journal of Psychiatry. 2013;170:734–741. doi: 10.1176/appi.ajp.2012.12091169. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal Axis. Psychosomatic Medicine. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Koob GF, Caine SB, Parsons L, Markou A, Weiss F. Opponent process model and psychostimulant addiction. Pharmacology Biochemistry and Behavior. 1997;57:513–521. doi: 10.1016/S0091-3057(96)00438-8. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. The Lancet Psychiatry. 2016;3:760–773. doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TA, Zhang XY, Kehoe P. Chronic neonatal isolation stress enhances cocaine-induced increases in ventral striatal dopamine levels in rat pups. Developmental Brain Research. 2003;141:109–116. doi: 10.1016/S0165-3806(03)00003-8. [DOI] [PubMed] [Google Scholar]
- Kumakura Y, Cumming P. PET studies of cerebral levodopa metabolism: a review of clinical findings and modeling approaches. The Neuroscientist. 2009;15:635–650. doi: 10.1177/1073858409338217. [DOI] [PubMed] [Google Scholar]
- Lataster J, Myin-Germeys I, Lieb R, Wittchen HU, van Os J. Adversity and psychosis: a 10-year prospective study investigating synergism between early and recent adversity in psychosis. Acta Psychiatrica Scandinavica. 2012;125:388–399. doi: 10.1111/j.1600-0447.2011.01805.x. [DOI] [PubMed] [Google Scholar]
- Lederbogen F, Kirsch P, Haddad L, Streit F, Tost H, Schuch P, Wüst S, Pruessner JC, Rietschel M, Deuschle M, Meyer-Lindenberg A. City living and urban upbringing affect neural social stress processing in humans. Nature. 2011;474:498–501. doi: 10.1038/nature10190. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Rosenbaum M. Recall of parental behavior by acute depressives, remitted depressives, and nondepressives. Journal of Personality and Social Psychology. 1987;52:611–619. doi: 10.1037/0022-3514.52.3.611. [DOI] [PubMed] [Google Scholar]
- Lindley SE, Bengoechea TG, Schatzberg AF, Wong DL. Glucocorticoid effects on mesotelencephalic dopamine neurotransmission. Neuropsychopharmacology. 1999;21:399–407. doi: 10.1016/S0893-133X(98)00103-1. [DOI] [PubMed] [Google Scholar]
- Love TM. Oxytocin, motivation and the role of dopamine. Pharmacology Biochemistry and Behavior. 2014;119:49–60. doi: 10.1016/j.pbb.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiavacchi S, Masi F, Scheggi S, Leggio B, De Montis MG, Gambarana C. Long-term behavioral and neurochemical effects of chronic stress exposure in rats. Journal of Neurochemistry. 2001;79:1113–1121. doi: 10.1046/j.1471-4159.2001.00665.x. [DOI] [PubMed] [Google Scholar]
- Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y, Cooper T, Kegeles L, Zarahn E, Abi-Dargham A, Haber SN, Laruelle M. Imaging human mesolimbic dopamine transmission with positron emission tomography. part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. Journal of Cerebral Blood Flow & Metabolism. 2003;23:285–300. doi: 10.1097/01.WCB.0000048520.34839.1A. [DOI] [PubMed] [Google Scholar]
- Maruyama Y, Kawano A, Okamoto S, Ando T, Ishitobi Y, Tanaka Y, Inoue A, Imanaga J, Kanehisa M, Higuma H, Ninomiya T, Tsuru J, Hanada H, Akiyoshi J. Differences in salivary alpha-amylase and cortisol responsiveness following exposure to electrical stimulation versus the trier social stress tests. PLOS ONE. 2012;7:e39375. doi: 10.1371/journal.pone.0039375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews K, Hall FS, Wilkinson LS, Robbins TW. Retarded acquisition and reduced expression of conditioned locomotor activity in adult rats following repeated early maternal separation: effects of Prefeeding, d-amphetamine, dopamine antagonists and clonidine. Psychopharmacology. 1996;126:75–84. doi: 10.1007/BF02246414. [DOI] [PubMed] [Google Scholar]
- Mauss IB, Levenson RW, McCarter L, Wilhelm FH, Gross JJ. The tie that binds? coherence among emotion experience, behavior, and physiology. Emotion. 2005;5:175–190. doi: 10.1037/1528-3542.5.2.175. [DOI] [PubMed] [Google Scholar]
- McCutcheon R, Bloomfield MAP, Dahoun T, Quinlan M, Terbeck S, Mehta M, Howes O. Amygdala reactivity in ethnic minorities and its relationship to the social environment: an fMRI study. Psychological Medicine. 2018;48:1985–1992. doi: 10.1017/S0033291717003506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Stress, adaptation, and disease. allostasis and allostatic load. Annals of the New York Academy of Sciences. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- McGowan S, Lawrence AD, Sales T, Quested D, Grasby P. Presynaptic dopaminergic dysfunction in schizophrenia: a positron emission tomographic [18F]fluorodopa study. Archives of General Psychiatry. 2004;61:134–142. doi: 10.1001/archpsyc.61.2.134. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Kubzansky LD, Dunn EC, Waldinger R, Vaillant G, Koenen KC. Childhood social environment, emotional reactivity to stress, and mood and anxiety disorders across the life course. Depression and Anxiety. 2010;27:1087–1094. doi: 10.1002/da.20762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Brake W, Gratton A. Environmental regulation of the development of mesolimbic dopamine systems: a neurobiological mechanism for vulnerability to drug abuse? Psychoneuroendocrinology. 2002;27:127–138. doi: 10.1016/S0306-4530(01)00040-3. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Gore-Langton E, Golembo N, Colvert E, Williams SC, Sonuga-Barke E. Hyporesponsive reward anticipation in the basal ganglia following severe institutional deprivation early in life. Journal of Cognitive Neuroscience. 2010;22:2316–2325. doi: 10.1162/jocn.2009.21394. [DOI] [PubMed] [Google Scholar]
- Meyer JS. Biochemical effects of corticosteroids on neural tissues. Physiological Reviews. 1985;65:946–1020. doi: 10.1152/physrev.1985.65.4.946. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Miletich RS, Kohn PD, Esposito G, Carson RE, Quarantelli M, Weinberger DR, Berman KF. Reduced prefrontal activity predicts exaggerated striatal dopaminergic function in schizophrenia. Nature Neuroscience. 2002;5:267–271. doi: 10.1038/nn804. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Nikulina EM, Shimamoto A, Covington HE. Escalated or suppressed cocaine reward, tegmental BDNF, and accumbal dopamine caused by episodic versus continuous social stress in rats. Journal of Neuroscience. 2011;31:9848–9857. doi: 10.1523/JNEUROSCI.0637-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GA, Chapman JP. Misunderstanding analysis of covariance. Journal of Abnormal Psychology. 2001;110:40–48. doi: 10.1037/0021-843X.110.1.40. [DOI] [PubMed] [Google Scholar]
- Moreines JL, Owrutsky ZL, Grace AA. Involvement of infralimbic prefrontal cortex but not lateral habenula in dopamine attenuation after chronic mild stress. Neuropsychopharmacology. 2017;42:904–913. doi: 10.1038/npp.2016.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C, Kirkbride J, Hutchinson G, Craig T, Morgan K, Dazzan P, Boydell J, Doody GA, Jones PB, Murray RM, Leff J, Fearon P. Cumulative social disadvantage, ethnicity and first-episode psychosis: a case-control study. Psychological Medicine. 2008;38:1701–1715. doi: 10.1017/S0033291708004534. [DOI] [PubMed] [Google Scholar]
- Morgan C, Charalambides M, Hutchinson G, Murray RM. Migration, ethnicity, and psychosis: toward a sociodevelopmental model. Schizophrenia Bulletin. 2010;36:655–664. doi: 10.1093/schbul/sbq051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C, Reininghaus U, Fearon P, Hutchinson G, Morgan K, Dazzan P, Boydell J, Kirkbride JB, Doody GA, Jones PB, Murray RM, Craig T. Modelling the interplay between childhood and adult adversity in pathways to psychosis: initial evidence from the AESOP study. Psychological Medicine. 2014;44:407–419. doi: 10.1017/S0033291713000767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C, Fisher H. Environment and schizophrenia: environmental factors in schizophrenia: childhood trauma--a critical review. Schizophrenia Bulletin. 2007;33:3–10. doi: 10.1093/schbul/sbl053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehaus JL, Murali M, Kauer JA. Drugs of abuse and stress impair LTP at inhibitory synapses in the ventral tegmental area. European Journal of Neuroscience. 2010;32:108–117. doi: 10.1111/j.1460-9568.2010.07256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt DJ. The role of dopamine and norepinephrine in depression and antidepressant treatment. The Journal of Clinical Psychiatry. 2006;67 Suppl 6:3–8. [PubMed] [Google Scholar]
- Olweus DA. The Revised Olweus Bully/Victim Questionnaire. Bergen: University of Bergen; 1996. [DOI] [Google Scholar]
- Osborn DP, Levy G, Nazareth I, Petersen I, Islam A, King MB. Relative risk of cardiovascular and Cancer mortality in people with severe mental illness from the united kingdom's General Practice Rsearch Database. Archives of General Psychiatry. 2007;64:242–249. doi: 10.1001/archpsyc.64.2.242. [DOI] [PubMed] [Google Scholar]
- Oswald LM, Wand GS, Kuwabara H, Wong DF, Zhu S, Brasic JR. History of childhood adversity is positively associated with ventral striatal dopamine responses to amphetamine. Psychopharmacology. 2014;231:2417–2433. doi: 10.1007/s00213-013-3407-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paivio SC. Stability of retrospective self-reports of child abuse and neglect before and after therapy for child abuse issues. Child Abuse & Neglect. 2001;25:1053–1068. doi: 10.1016/S0145-2134(01)00256-3. [DOI] [PubMed] [Google Scholar]
- Parker G. Parental 'affectionless control' as an antecedent to adult depression. A risk factor delineated. Archives of General Psychiatry. 1983;40:956–960. doi: 10.1001/archpsyc.1983.01790080038005. [DOI] [PubMed] [Google Scholar]
- Payer D, Williams B, Mansouri E, Stevanovski S, Nakajima S, Le Foll B, Kish S, Houle S, Mizrahi R, George SR, George TP, Boileau I. Corticotropin-releasing hormone and dopamine release in healthy individuals. Psychoneuroendocrinology. 2017;76:192–196. doi: 10.1016/j.psyneuen.2016.11.034. [DOI] [PubMed] [Google Scholar]
- Pedersen CB, Mortensen PB. Evidence of a dose-response relationship between urbanicity during upbringing and schizophrenia risk. Archives of General Psychiatry. 2001;58:1039–1046. doi: 10.1001/archpsyc.58.11.1039. [DOI] [PubMed] [Google Scholar]
- Penninx BW. Depression and cardiovascular disease: epidemiological evidence on their linking mechanisms. Neuroscience & Biobehavioral Reviews. 2017;74:277–286. doi: 10.1016/j.neubiorev.2016.07.003. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Champagne F, Meaney MJ, Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C]raclopride. Journal of Neuroscience. 2004;24:2825–2831. doi: 10.1523/JNEUROSCI.3422-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reininghaus U, Gayer-Anderson C, Valmaggia L, Kempton MJ, Calem M, Onyejiaka A, Hubbard K, Dazzan P, Beards S, Fisher HL, Mills JG, McGuire P, Craig TK, Garety P, van Os J, Murray RM, Wykes T, Myin-Germeys I, Morgan C. Psychological processes underlying the association between childhood trauma and psychosis in daily life: an experience sampling study. Psychological Medicine. 2016;46:2799–2813. doi: 10.1017/S003329171600146X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/S0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Salokangas RK, Vilkman H, Ilonen T, Taiminen T, Bergman J, Haaparanta M, Solin O, Alanen A, Syvälahti E, Hietala J. High levels of dopamine activity in the basal ganglia of cigarette smokers. American Journal of Psychiatry. 2000;157:632–634. doi: 10.1176/appi.ajp.157.4.632. [DOI] [PubMed] [Google Scholar]
- Schäfer I, Fisher HL. Childhood trauma and psychosis - what is the evidence? Dialogues in Clinical Neuroscience. 2011;13:360–365. doi: 10.31887/DCNS.2011.13.2/ischaefer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Dopamine reward prediction-error signalling: a two-component response. Nature Reviews Neuroscience. 2016;17:183–195. doi: 10.1038/nrn.2015.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott KM, McLaughlin KA, Smith DA, Ellis PM. Childhood maltreatment and DSM-IV adult mental disorders: comparison of prospective and retrospective findings. British Journal of Psychiatry. 2012;200:469–475. doi: 10.1192/bjp.bp.111.103267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman MV, Seeman P. Is schizophrenia a dopamine supersensitivity psychotic reaction? Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2014;48:155–160. doi: 10.1016/j.pnpbp.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selten JP, Laan W, Kupka R, Smeets HM, van Os J. Risk of psychiatric treatment for mood disorders and psychotic disorders among migrants and Dutch nationals in Utrecht, The Netherlands. Social Psychiatry and Psychiatric Epidemiology. 2012;47:271–278. doi: 10.1007/s00127-010-0335-7. [DOI] [PubMed] [Google Scholar]
- Selten JP, Cantor-Graae E. Social defeat: risk factor for schizophrenia? British Journal of Psychiatry. 2005;187:101–102. doi: 10.1192/bjp.187.2.101. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Research Reviews. 2000;33:13–33. doi: 10.1016/S0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- Sharpley M, Hutchinson G, McKenzie K, Murray RM. Understanding the excess of psychosis among the African-Caribbean population in England. review of current hypotheses. The British Journal of Psychiatry. Supplement. 2001;40:s60–s68. doi: 10.1192/bjp.178.40.s60. [DOI] [PubMed] [Google Scholar]
- Shimamoto A, Debold JF, Holly EN, Miczek KA. Blunted accumbal dopamine response to cocaine following chronic social stress in female rats: exploring a link between depression and drug abuse. Psychopharmacology. 2011;218:271–279. doi: 10.1007/s00213-011-2364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. The role of stress in addiction relapse. Current Psychiatry Reports. 2007;9:388–395. doi: 10.1007/s11920-007-0050-6. [DOI] [PubMed] [Google Scholar]
- Sinha R. Modeling stress and drug craving in the laboratory: implications for addiction treatment development. Addiction Biology. 2009;14:84–98. doi: 10.1111/j.1369-1600.2008.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes PR, Shotbolt P, Mehta MA, Turkheimer E, Benecke A, Copeland C, Turkheimer FE, Lingford-Hughes AR, Howes OD. Nature or nurture? determining the heritability of human striatal dopamine function: an [18F]-DOPA PET study. Neuropsychopharmacology. 2013;38:485–491. doi: 10.1038/npp.2012.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studholme C, Hill DL, Hawkes DJ. Automated 3-D registration of MR and CT images of the head. Medical Image Analysis. 1996;1:163–175. doi: 10.1016/S1361-8415(96)80011-9. [DOI] [PubMed] [Google Scholar]
- Takiguchi S, Fujisawa TX, Mizushima S, Saito DN, Okamoto Y, Shimada K, Koizumi M, Kumazaki H, Jung M, Kosaka H, Hiratani M, Ohshima Y, Teicher MH, Tomoda A. Ventral striatum dysfunction in children and adolescents with reactive attachment disorder: functional MRI study. BJPsych Open. 2015;1:121–128. doi: 10.1192/bjpo.bp.115.001586. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Taylor SE. Mechanisms linking early life stress to adult health outcomes. PNAS. 2010;107:8507–8512. doi: 10.1073/pnas.1003890107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Samson JA, Anderson CM, Ohashi K. The effects of childhood maltreatment on brain structure, function and connectivity. Nature Reviews Neuroscience. 2016;17:652–666. doi: 10.1038/nrn.2016.111. [DOI] [PubMed] [Google Scholar]
- Thompson JL, Urban N, Slifstein M, Xu X, Kegeles LS, Girgis RR, Beckerman Y, Harkavy-Friedman JM, Gil R, Abi-Dargham A. Striatal dopamine release in schizophrenia comorbid with substance dependence. Molecular Psychiatry. 2013;18:909–915. doi: 10.1038/mp.2012.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoresen S, Tambs K, Hussain A, Heir T, Johansen VA, Bisson JI. Brief measure of posttraumatic stress reactions: impact of event Scale-6. Social Psychiatry and Psychiatric Epidemiology. 2010;45:405–412. doi: 10.1007/s00127-009-0073-x. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Miczek KA. Social defeat stress selectively alters mesocorticolimbic dopamine release: an in vivo microdialysis study. Brain Research. 1996;721:140–149. doi: 10.1016/0006-8993(96)00159-X. [DOI] [PubMed] [Google Scholar]
- Turkheimer FE, Brett M, Visvikis D, Cunningham VJ. Multiresolution analysis of emission tomography images in the wavelet domain. Journal of Cerebral Blood Flow & Metabolism. 1999;19:1189–1208. doi: 10.1097/00004647-199911000-00003. [DOI] [PubMed] [Google Scholar]
- Valenti O, Cifelli P, Gill KM, Grace AA. Antipsychotic drugs rapidly induce dopamine neuron depolarization block in a developmental rat model of schizophrenia. Journal of Neuroscience. 2011;31:12330–12338. doi: 10.1523/JNEUROSCI.2808-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Os J, Kenis G, Rutten BP. The environment and schizophrenia. Nature. 2010;468:203–212. doi: 10.1038/nature09563. [DOI] [PubMed] [Google Scholar]
- van Stegeren A, Rohleder N, Everaerd W, Wolf OT. Salivary alpha amylase as marker for adrenergic activity during stress: effect of betablockade. Psychoneuroendocrinology. 2006;31:137–141. doi: 10.1016/j.psyneuen.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Wand GS, Oswald LM, McCaul ME, Wong DF, Johnson E, Zhou Y, Kuwabara H, Kumar A. Association of amphetamine-induced striatal dopamine release and cortisol responses to psychological stress. Neuropsychopharmacology. 2007;32:2310–2320. doi: 10.1038/sj.npp.1301373. [DOI] [PubMed] [Google Scholar]
- Watson S, Gallagher P, Dougall D, Porter R, Moncrieff J, Ferrier IN, Young AH. Childhood trauma in bipolar disorder. Australian & New Zealand Journal of Psychiatry. 2014;48:564–570. doi: 10.1177/0004867413516681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel JM, Rauscher NA, Cheer JF, Oleson EB. A role for phasic dopamine release within the nucleus accumbens in encoding aversion: a review of the neurochemical literature. ACS Chemical Neuroscience. 2015;6:16–26. doi: 10.1021/cn500255p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicks S, Hjern A, Gunnell D, Lewis G, Dalman C. Social adversity in childhood and the risk of developing psychosis: a national cohort study. American Journal of Psychiatry. 2005;162:1652–1657. doi: 10.1176/appi.ajp.162.9.1652. [DOI] [PubMed] [Google Scholar]
- Wiers CE, Towb PC, Hodgkinson CA, Shen P-H, Freeman C, Miller G, Lindgren E, Shokri-Kojori E, Demiral Ş B, Kim SW, Tomasi D, Sun H, Wang G-J, Goldman D, Volkow ND. Association of genetic ancestry with striatal dopamine D2/D3 receptor availability. Molecular Psychiatry. 2018;23:1711–1716. doi: 10.1038/mp.2017.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung AR, Yuen HP, McGorry PD, Phillips LJ, Kelly D, Dell'Olio M, Francey SM, Cosgrave EM, Killackey E, Stanford C, Godfrey K, Buckby J. Mapping the onset of psychosis: the comprehensive assessment of At-Risk mental states. Australian & New Zealand Journal of Psychiatry. 2005;39:964–971. doi: 10.1080/j.1440-1614.2005.01714.x. [DOI] [PubMed] [Google Scholar]
- Zweifel LS, Fadok JP, Argilli E, Garelick MG, Jones GL, Dickerson TM, Allen JM, Mizumori SJ, Bonci A, Palmiter RD. Activation of dopamine neurons is critical for aversive conditioning and prevention of generalized anxiety. Nature Neuroscience. 2011;14:620–626. doi: 10.1038/nn.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]


