Abstract
Rationale: Characteristics and outcomes of lung cancer in patients with idiopathic pulmonary fibrosis (IPF) in the United States remain understudied.
Objectives: To determine the tumor characteristics and survival of patients with IPF with non–small cell lung cancer (NSCLC) using U.S. population–based data.
Methods: We selected Medicare beneficiaries from the Surveillance, Epidemiology, and End Results registry with histologically confirmed NSCLC diagnosed between 2007 and 2011. IPF was identified using two validated claims-based algorithms. We compared tumor characteristics and used logistic and Cox regression to compare rates of stage-appropriate therapy and of overall and lung cancer–specific survival in those with IPF and without IPF.
Results: A total of 54,453 patients with NSCLC were included. Those with IPF were more likely to be diagnosed at an earlier stage (P < 0.01) and to have squamous histology (46% vs. 35%; P < 0.01) and lower-lobe tumors (38% vs. 28%; P < 0.01) than those without IPF. Patients with IPF and stages I–II disease had odds of receiving stage-appropriate therapy similar to patients without IPF who had stages I–II disease (odds ratio [OR], 1.13; 95% confidence interval [CI], 0.89–1.43); however, those with advanced disease were less likely to be treated (OR, 0.82; 95% CI, 0.68–0.99). Overall and lung cancer–specific survival were worse in patients with IPF (respectively, hazard ratio [HR], 1.35; 95% CI, 1.26–1.45; and HR, 1.21; 95% CI, 1.10–1.32).
Conclusions: NSCLC has a unique presentation in patients with IPF and is associated with poorer prognosis. Further research is needed to identify optimal treatment strategies in this population.
Keywords: lung neoplasms, idiopathic pulmonary fibrosis, outcome assessment
Lung cancer is the leading cause of cancer death in the United States (1). Tobacco smoking is the main risk factor, but chronic lung diseases such as idiopathic pulmonary fibrosis (IPF) convey an additional independent risk (2–4). The prevalence of lung cancer in cohorts of IPF has been reported to exceed 10–30% (5–15), whereas the lifetime prevalence of lung cancer in the U.S. population is approximately 6% (1). Thus, patients with IPF have a high burden of lung cancer morbidity and mortality.
Despite an increased burden of disease, the characteristics, treatments, and outcomes of lung cancer in patients with IPF remain understudied in the U.S. population. Smaller studies have reported a higher incidence of squamous cell histology and a greater predominance of peripheral, lower-lobe lesions near areas of lung fibrosis (16–18). In terms of prognosis, Tomassetti and colleagues found that noncancer mortality exceeded lung cancer–specific mortality in an Italian cohort; 43% of patients with IPF and lung cancer died of acute exacerbations of IPF, and lung cancer treatment–related deaths exceeded mortality due to lung cancer progression (17% vs. 13%, respectively) (8). However, many of these studies were conducted in single centers and may lack generalizability. In this study, we aimed to determine the cancer characteristics, treatment patterns, and survival of patients with IPF and non–small cell lung cancer (NSCLC) relative to those without IPF using population-based data from the Surveillance, Epidemiology, and End Results (SEER)-Medicare database.
Methods
Data Source
Study patients were identified using the SEER-Medicare database, a large, population-based source of longitudinal data that includes demographic and clinical information for Medicare beneficiaries with cancer from several U.S. regions. The SEER program has been expanded to cover 28% of the U.S. population, with 94% of eligible patients older than 65 years of age linked to Medicare claims (19).
From SEER-Medicare, we included all patients older than 65 years of age with stages I–IV NSCLC who were diagnosed between 2007 and 2011. To identify patients with IPF and characterize comorbidities, only participants with at least 1 year of recorded Medicare data before cancer diagnosis were included. We excluded patients in health maintenance organizations and those without part B (outpatient) Medicare coverage, owing to lack of claims data.
Case Definition
To identify cases of IPF, we used the algorithm published by Raghu and colleagues (20) and subsequently validated (21): at least one claim for the International Statistical Classification of Diseases, Ninth Revision (ICD-9), code 516.3 and standard exclusionary criteria for alternate interstitial lung disease diagnoses (see Table E1 in the online supplement). In a sensitivity analysis, we used a modified algorithm recently validated by Ley and colleagues: at least two IPF claims of 516.3 and any computed tomographic (CT) imaging of the chest procedure code before the first diagnostic claim in combination with standard exclusion criteria for alternate interstitial lung disease diagnoses (22).
Study Variables
Using SEER data, we obtained sociodemographic information, including age, sex, race/ethnicity, and marital status. Socioeconomic status was categorized into quartiles according to census tract–level information provided by Medicare. Comorbidities were assessed using Medicare claims by applying the National Cancer Institute comorbidity index, which is a modified version of the Charlson comorbidity index with lung cancer–specific weights (23). The modified index accounts for the presence of acquired immunodeficiency syndrome, chronic obstructive pulmonary disease, liver disease, chronic kidney disease, dementia, myocardial infarction and congestive heart failure, peptic ulcer disease, paralysis, cerebrovascular disease, peripheral vascular disease, and rheumatologic conditions. Each comorbidity is weighted on the basis of probability of contributing to mortality within 1 year. To identify patients with severe lung impairment, we ascertained use of supplemental oxygen before treatment of lung cancer based on home health agency claims or the following codes: Healthcare Common Procedure Coding System codes E1390–E13901, E1405–E1406, K0738, E0424–E0425, E0430–E0435, E0439–E0446, and E0450 or ICD-9 code V46.2. Performance status is not directly provided by SEER-Medicare; therefore, we used home services on home health agency claims or nursing home residence (using an algorithm derived by Zuckerman and colleagues) as indicators of poor performance status (24).
NSCLC was staged according to the seventh edition of the American Joint Committee on Cancer staging manual using SEER data regarding tumor size, extension, location, and lymph node status (25). Cancer histology and grade were determined according to the International Classification of Diseases for Oncology, Third Edition, and morphology codes available in SEER (26).
The SEER registry routinely collects treatment data for 4 months after diagnosis, and therefore this time period was used to assess the initial course of therapy. We obtained data about major surgical resection and radiotherapy from the SEER registry and Medicare claims. Chemotherapy use was determined using Medicare claims based on validated algorithms because these data are not routinely reported by SEER (27). With these data, patients were coded as having received or not having received stage-appropriate treatment based on contemporaneous guidelines (28), defined as a minimum of surgical resection for stages I–II disease, chemoradiation or surgical resection for stage III disease, and chemotherapy for stage IV disease. Long-term outcomes included overall (all-cause mortality) and lung cancer–specific survival. Survival time was computed from the time of diagnosis to the event of interest or last follow-up (December 31, 2014). Underlying cause of death is provided by SEER on the basis of death certificate data.
Statistical Analysis
Tumor characteristics of patients with and without IPF were compared using the chi-square test for categorical variables and the Wilcoxon test for continuous variables. Logistic regression was used to compare the probability of stage-appropriate therapy after adjusting for demographics, comorbidity score, tumor characteristics, indicators of poor performance status, and supplemental oxygen use. The unadjusted overall and lung cancer–specific survival rates of patients with lung cancer with and without IPF were estimated using the Kaplan-Meier method and compared with the log-rank test. Adjusted survival was compared using the Cox proportional hazards model, adjusting for sociodemographic characteristics, comorbidity score, tumor characteristics, indicators of poor performance status, and supplemental oxygen use; results were stratified by receipt of stage-appropriate therapy.
All analyses were conducted using SAS statistical software (SAS Institute) with two-sided P values. The study was exempted from review by the institutional review board of the Icahn School of Medicine at Mount Sinai.
Results
Baseline Characteristics
A total of 54,453 patients with NSCLC were included in the final analysis (Figure 1). IPF was present in 855 subjects (2%) in the study population. Patients with lung cancer with IPF were older (median age, 76 vs. 74 yr), more frequently were men (60% vs. 52%), were more likely to be married (56% vs. 51%), and had higher income (28% vs. 24% in the highest income quartile) (Table 1). Comorbidities were also more prevalent among patients with IPF (71% with at least one comorbidity vs. 51% of control subjects with lung cancer) (Table 1). Although the proportions of those with poor performance status were similar (15% vs. 14%), patients with IPF more frequently had supplemental oxygen use (18% vs. 7%) (Table 1).
Figure 1.
Flow diagram of final study population. HMO = health maintenance organization; NSCLC = non–small cell lung cancer.
Table 1.
Characteristics of patients with non–small cell lung cancer, by presence of idiopathic pulmonary fibrosis
| Characteristic | Entire Cohort (N = 54,453) | No IPF (n = 53,598) | IPF (n = 855) |
|---|---|---|---|
| Age, yr, median (IQR) | 74 (69–80) | 74 (69–80) | 76 (71–81) |
| Male sex, n (%) | 28,621 (53) | 28,106 (52) | 515 (60) |
| Race/ethnicity, n (%) | |||
| White | 44,707 (82) | 43,988 (82) | 719 (84) |
| Black | 5,038 (9) | 4,979 (10) | 59 (7) |
| Hispanic | 2,004 (4) | 1,970 (4) | 34 (4) |
| Other | 2,704 (5) | 2,661 (5) | 43 (5) |
| Married, n (%) | 27,555 (51) | 27,078 (51) | 477 (56) |
| Median annual income quartile, n (%) | |||
| First (lowest) | 14,277 (26) | 14,091 (27) | 186 (22) |
| Second | 13,569 (25) | 13,343 (25) | 226 (27) |
| Third | 12,934 (24) | 12,730 (24) | 204 (24) |
| Fourth (highest) | 13,095 (24) | 12,861 (24) | 234 (28) |
| Comorbidity score, n (%) | |||
| 0 | 26,442 (49) | 26,191 (49) | 251 (29) |
| 1–2 | 20,079 (37) | 19,688 (37) | 391 (46) |
| >2 | 7,932 (15) | 7,719 (14) | 213 (25) |
| Indicators of poor functional status | |||
| Nursing home or home health services | 7,608 (14) | 7,480 (14) | 128 (15) |
| Supplemental oxygen | 4,085 (8) | 3,933 (7) | 152 (18) |
Definition of abbreviations: IPF = idiopathic pulmonary fibrosis; IQR = interquartile range.
In terms of NSCLC characteristics, patients with IPF were more likely to be diagnosed with stage I disease (31% vs. 25%; P < 0.01) and to be diagnosed with smaller tumors (median size, 31 vs. 35 mm; P < 0.01) (Table 2). In addition, patients with IPF had a greater proportion of squamous carcinoma (46% vs. 35%; P < 0.01) and lower-lobe tumors (38% vs. 28%; P < 0.01) (Table 2).
Table 2.
Non–small cell lung cancer features, by presence of idiopathic pulmonary fibrosis
| Tumor Characteristics | Entire Cohort (N = 54,453) | No IPF (n = 53,598) | IPF (n = 855) | P Value |
|---|---|---|---|---|
| Cancer stage, n (%) | ||||
| I | 13,591 (25) | 13,325 (25) | 266 (31) | <0.01 |
| II | 5,104 (9) | 5,018 (9) | 86 (10) | |
| IIIA | 7,003 (13) | 6,864 (13) | 139 (16) | |
| IIIB | 3,860 (7) | 3,799 (7) | 61 (8) | |
| IV | 24,895 (46) | 24,592 (46) | 303 (35) | |
| Tumor size, mm, median (IQR) | 35 (23–54) | 35 (23–54) | 31 (20–48) | <0.01 |
| Tumor size, mm, n (%) | ||||
| ≤20 | 9,497 (17) | 9,318 (17) | 179 (21) | <0.01 |
| 21–30 | 9,262 (17) | 9,107 (17) | 155 (18) | |
| 31–50 | 13,405 (25) | 13,202 (25) | 203 (24) | |
| 51–70 | 7,015 (13) | 6,932 (13) | 83 (10) | |
| ≥71 | 5,037 (9) | 4,974 (9) | 63 (7) | |
| Unknown | 10,237 (19) | 10,065 (19) | 172 (20) | |
| Histology, n (%) | ||||
| Adenocarcinoma | 29,959 (55) | 29,576 (55) | 383 (45) | <0.01 |
| Squamous cell carcinoma | 18,918 (35) | 18,521 (35) | 397 (46) | |
| Large cell carcinoma | 1,550 (3) | 1,530 (3) | 20 (2) | |
| Other histology | 4,026 (7) | 3,971 (7) | 55 (6) | |
| Tumor site, n (%) | ||||
| Upper lobe | 28,071 (52) | 27,701 (52) | 370 (43) | <0.01 |
| Middle lobe | 2,193 (4) | 2,159 (4) | 34 (4) | |
| Lower lobe | 15,346 (28) | 15,025 (28) | 321 (38) | |
| Other | 8,843 (16) | 8,713 (16) | 130 (15) |
Definition of abbreviations: IPF = idiopathic pulmonary fibrosis; IQR = interquartile range.
Probability of Stage-Appropriate Treatment
In unadjusted analysis, in lung cancer patients with and without IPF, there was no significant difference in the overall proportion of patients undergoing stage-appropriate therapy (48% vs. 49%; P = 0.33; Table E2; odds ratio [OR], 0.94; 95% confidence interval [CI], 0.82–1.07; Table 3). Similar results were obtained in analyses adjusted for age; sex; marital status; ethnicity; income; Charlson comorbidity; tumor size, histology, site, and stage; functional status; and pulmonary disability (OR, 0.90; 95% CI, 0.90–1.04) (Table 3). When stratified by stage and after controlling for confounders, there was no significant difference in treatment proportions among patients with lung cancer with early-stage (I–II) disease (OR, 1.13; 95% CI, 0.89–1.43); however, patients with IPF with advanced-stage (III–IV) disease were less likely to undergo treatment (OR, 0.82; 95% CI, 0.68–0.99) (Table 3).
Table 3.
Stage-appropriate therapy in patients with lung cancer with idiopathic pulmonary fibrosis compared with control subjects with lung cancer
| Analysis | Unadjusted OR (95% CI) | Adjusted OR* (95% CI) |
|---|---|---|
| Entire cohort | 0.94 (0.82–1.07) | 0.90 (0.78–1.04) |
| Stages I–II | 0.89 (0.72–1.11) | 1.13 (0.89–1.43) |
| Stages III–IV | 0.87 (0.73–1.04) | 0.82 (0.68–0.99) |
Definition of abbreviations: CI = confidence interval; OR = odds ratio.
Adjusted for age, sex, marital status, ethnicity, income, Charlson comorbidity index, tumor size, histology, site, stage, functional status, and pulmonary disability.
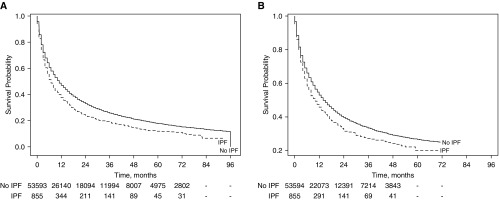
Survival
The median overall survival of patients with lung cancer with IPF was 7.0 ± 1.0 months compared with 11.0 ± 1.0 months in those without IPF (P < 0.01) (Figure 2A). Lung cancer accounted for most deaths in both groups (79% and 82% in the IPF and non-IPF groups, respectively; P = 0.09) (Table E3). In adjusted analysis, patients with IPF had significantly worse overall survival (hazard ratio [HR], 1.35; 95% CI, 1.26–1.45) (Table 4). Similarly, patients with IPF had worse overall survival in analyses stratified by type of treatment received (for patients who received stage-appropriate therapy, HR, 1.46; 95% CI, 1.31–1.64; for patients who did not receive stage-appropriate therapy, HR, 1.29; 95% CI, 1.18–1.43) (Table 4). Patients with IPF also had worse lung cancer–specific survival in adjusted analyses (HR, 1.32; 95% CI, 1.21–1.44) and in adjusted analysis stratified by treatment strategy (patients who received stage-appropriate therapy, HR, 1.48; 95% CI, 1.29–1.71; patients who did not receive stage-appropriate therapy, HR, 1.26; 95% CI, 1.12–1.41) (Table 4).
Figure 2.
Kaplan-Meier curves of (A) overall and (B) lung cancer–specific survival in patients with lung cancer with and without idiopathic pulmonary fibrosis (IPF).
Table 4.
Overall and lung cancer–specific survival based on presence of idiopathic pulmonary fibrosis
| Entire Cohort |
Received Stage-Appropriate Therapy |
Did Not Receive Stage-Appropriate Therapy |
||||
|---|---|---|---|---|---|---|
| No IPF | IPF | No IPF | IPF | No IPF | IPF | |
| No. of subjects | 53,598 | 855 | 26,358 | 406 | 27,240 | 449 |
| Overall survival | ||||||
| Unadjusted HR (95% CI) | 1 (Ref) | 1.26 (1.17–1.36) | 1 (Ref) | 1.31 (1.17–1.47) | 1 (Ref) | 1.22 (1.11–1.34) |
| Adjusted HR* (95% CI) | 1 (Ref) | 1.35 (1.26–1.45) | 1 (Ref) | 1.46 (1.31–1.64) | 1 (Ref) | 1.29 (1.18–1.43) |
| Lung cancer–specific survival | ||||||
| Unadjusted HR (95% CI) | 1 (Ref) | 1.21 (1.10–1.32) | 1 (Ref) | 1.26 (1.09–1.44) | 1 (Ref) | 1.17 (1.04–1.31) |
| Adjusted HR* (95% CI) | 1 (Ref) | 1.32 (1.21–1.44) | 1 (Ref) | 1.48 (1.29–1.71) | 1 (Ref) | 1.26 (1.12–1.41) |
Definition of abbreviations: CI = confidence interval; HR = hazard ratio; IPF = idiopathic pulmonary fibrosis; Ref = referent.
Adjusted for age; sex; marital status; ethnicity; income; Charlson comorbidity index; tumor size, histology, site, and stage; functional status; pulmonary disability; and stage-appropriate therapy.
Sensitivity Analysis
We repeated the analysis using the modified algorithm to identify patients with IPF (n = 155; 0.3%). Patients with IPF identified by this algorithm were older than those without IPF (76 vs. 74 yr) and had a greater burden of comorbidities and higher supplemental oxygen use (21% vs. 8%) (Table E4). There was an insufficient number of subjects with IPF and lung cancer to stratify the probability of treatment by stage. Adjusted analyses showed similar associations of IPF and worsened survival (Table E5).
Discussion
Patients with IPF represent a special lung cancer population at greater risk of poorer outcomes. In this study, we found that patients with IPF were more likely to be diagnosed at an early stage but had lower rates of treatment for advanced disease and worse long-term outcomes even after adjusting for differences in treatment, comorbidities, and functional status. The reasons for these findings are likely multifactorial and potentially include poorer tolerability of treatments, the negative impact of competing risks, and/or more aggressive cancer behavior.
The prevalence of IPF in lung cancer cannot be ascertained from coding data alone, because the estimate differs on the basis of the code abstraction strategy used. Using the modified coding algorithm, 0.3% of patients with lung cancer had a diagnosis of IPF. This strategy has higher specificity but reduced sensitivity (with a 65% false-negative rate) (22). We found that approximately 2% of patients with lung cancer had a diagnosis of IPF using the traditional coding algorithm. These findings are consistent with previous cohorts of patients with lung cancer (29–35). In comparison, Raghu and colleagues reported a prevalence of 0.5% in the general population using a similar claims-based algorithm (20). Thus, our findings suggest that IPF is substantially overrepresented in the lung cancer population. However, the SEER-Medicare registry does not include data on tobacco exposures, and IPF is associated with higher rates of smoking (36, 37), a factor that may explain our findings. Other possible factors that may explain a potential association between IPF and lung cancer include chronic inflammation and repair leading to malignant changes; likewise, dysregulated fibroblast proliferation may play an essential role in supporting the tumor microenvironment (38).
There were several differences in the characteristics of lung cancer in patients with IPF. First, IPF was associated with an increased number of lower-lobe tumors and a predominant squamous histology, which are consistent with findings in prior studies (6–9, 12, 39). A plausible explanation is that given the higher risks associated with lung biopsies, patients with IPF and more indolent–appearing subsolid lesions (which are more likely to be adenocarcinoma) were undersampled (40, 41). Second, tumors were diagnosed at an earlier stage and were of smaller size in patients with IPF. Patients with IPF with advanced tumors may be less likely to undergo tissue sampling or undergo less invasive staging evaluations, leading to a more favorable stage distribution relative to patients without IPF. It is also possible that tumors in these patients are detected earlier as a result of increased imaging surveillance as part of the management of their underlying lung disease. The frequent use of CT in this population may lead to overdiagnosis, a phenomenon that may explain the increased lung cancer rate in patients with IPF as well as the higher prevalence of early-stage tumors. However, the increased incidence of squamous cell carcinomas and the higher lung cancer mortality among patients with IPF argue against a high rate of overdiagnosis.
Lung cancer mortality is substantial in patients with IPF, accounting for 8–13% of deaths (4, 5, 11, 17, 42–44). Our study shows that IPF is associated with increased mortality in those who receive stage-appropriate treatment, which may be a result of treatment-related complications. Prior reports have shown increased complications after radiation therapy (45), chemotherapy (46–48), and surgery (16, 30, 49–55). Given the impact of IPF-related mortality and the higher lung cancer–related mortality, there is a clear need for comprehensive studies evaluating the role of treatment in this population. Although prospective clinical trials would provide the best evidence regarding optimal management, challenges in recruiting and randomizing these patients and high costs may make these studies unlikely. Thus, alternative approaches, such as comparative effectiveness studies using observational data and/or modeling approaches, may be necessary to assess the optimal management of patients with IPF with lung cancer.
Increased efforts in lung cancer prevention remain an important aspect of the management of patients with IPF. Potential interventions include smoking cessation and treatment of the underlying IPF, which have been associated with reduced risk of lung cancer development (7, 56). Lung cancer screening has been shown to reduce lung cancer mortality by 20% in high-risk smokers (57), and its use has recently been advocated to reduce lung cancer mortality in the IPF population (58). However, many patients with IPF may not be eligible for resection, given their advanced lung disease, and they often experience increased complications from surgery, chemotherapy, and radiation (45, 46). Therefore, the harms of lung cancer screening may exceed any potential additive benefits.
Our study has several limitations. First, the algorithm employed by Raghu and colleagues has a positive predictive value of 54% (21). However, some uncertainty may arise from making the diagnosis of IPF in clinical practice. Although a diagnosis of IPF may require histologic confirmation, many patients are too ill to undergo lung biopsy. Conversely, as many as 35% of patients with atypical CT findings actually have IPF (59). Moreover, our sensitivity analysis using a more specific (but less sensitive) coding algorithm yielded similar results. Second, SEER-Medicare data do not provide detailed information on lung function or performance status; thus, we were not able to fully characterize the severity of IPF. However, we used validated methods of ascertaining poor functional status and use of oxygen, two markers of disease severity. Third, we were unable to ascertain from claims data the extent to which patient preferences played a role in the decision not to undergo stage-appropriate therapy. Similarly, we are unable to determine the role of treatment of IPF with antifibrinolytic therapy in lung cancer outcomes, because these drugs were approved for use in 2014, after our study period of 2007–2011. The most recent SEER-Medicare database includes cancer cases diagnosed up to 2015, which would not provide a sufficiently large cohort of treated patients with IPF who were diagnosed 1 year before their lung cancer diagnosis to conduct an adequately powered analysis. Despite these limitations, our study’s main strengths are the use of a large cancer registry that is representative of the U.S. population and the availability of long-term survival outcomes.
In summary, we found that patients with IPF and lung cancer have a greater proportion of early-stage disease of squamous histology in a lower-lobe distribution. This atypical distribution may need to be incorporated into risk models for lung nodule management in patients with IPF. In addition, our data suggest that IPF is an independent risk factor for increased lung cancer and overall mortality. Further research is needed to identify the best strategies to control and treat lung cancer in this highly affected population.
Supplementary Material
Footnotes
Supported by the Stony Wold-Herbert Fund Inc. Research Fellowship Award.
Author Contributions: Conception and design, manuscript writing, and final approval: all authors; and collection, assembly, analysis, and interpretation of data: all authors
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Noone AM, Howlader N, Krapcho M, Miller D, Brest A, Yu M, et al. SEER cancer statistics review, 1975–2015. Bethesda, MD: National Cancer Institute; Apr 2018 [accessed 2019 Jan]. Available from: https://seer.cancer.gov/csr/1975_2015/
- 2.Hubbard R, Venn A, Lewis S, Britton J. Lung cancer and cryptogenic fibrosing alveolitis: a population-based cohort study. Am J Respir Crit Care Med. 2000;161:5–8. doi: 10.1164/ajrccm.161.1.9906062. [DOI] [PubMed] [Google Scholar]
- 3.Le Jeune I, Gribbin J, West J, Smith C, Cullinan P, Hubbard R. The incidence of cancer in patients with idiopathic pulmonary fibrosis and sarcoidosis in the UK. Respir Med. 2007;101:2534–2540. doi: 10.1016/j.rmed.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 4.Turner-Warwick M, Lebowitz M, Burrows B, Johnson A. Cryptogenic fibrosing alveolitis and lung cancer. Thorax. 1980;35:496–499. doi: 10.1136/thx.35.7.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kreuter M, Ehlers-Tenenbaum S, Palmowski K, Bruhwyler J, Oltmanns U, Muley T, et al. Impact of comorbidities on mortality in patients with idiopathic pulmonary fibrosis. PLoS One. 2016;11:e0151425. doi: 10.1371/journal.pone.0151425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee HJ, Im JG, Ahn JM, Yeon KM. Lung cancer in patients with idiopathic pulmonary fibrosis: CT findings. J Comput Assist Tomogr. 1996;20:979–982. doi: 10.1097/00004728-199611000-00020. [DOI] [PubMed] [Google Scholar]
- 7.Ozawa Y, Suda T, Naito T, Enomoto N, Hashimoto D, Fujisawa T, et al. Cumulative incidence of and predictive factors for lung cancer in IPF. Respirology. 2009;14:723–728. doi: 10.1111/j.1440-1843.2009.01547.x. [DOI] [PubMed] [Google Scholar]
- 8.Tomassetti S, Gurioli C, Ryu JH, Decker PA, Ravaglia C, Tantalocco P, et al. The impact of lung cancer on survival of idiopathic pulmonary fibrosis. Chest. 2015;147:157–164. doi: 10.1378/chest.14-0359. [DOI] [PubMed] [Google Scholar]
- 9.Park J, Kim DS, Shim TS, Lim CM, Koh Y, Lee SD, et al. Lung cancer in patients with idiopathic pulmonary fibrosis. Eur Respir J. 2001;17:1216–1219. doi: 10.1183/09031936.01.99055301. [DOI] [PubMed] [Google Scholar]
- 10.Nagai A, Chiyotani A, Nakadate T, Konno K. Lung cancer in patients with idiopathic pulmonary fibrosis. Tohoku J Exp Med. 1992;167:231–237. doi: 10.1620/tjem.167.231. [DOI] [PubMed] [Google Scholar]
- 11.Hamada K, Nagai S, Tanaka S, Handa T, Shigematsu M, Nagao T, et al. Significance of pulmonary arterial pressure and diffusion capacity of the lung as prognosticator in patients with idiopathic pulmonary fibrosis. Chest. 2007;131:650–656. doi: 10.1378/chest.06-1466. [DOI] [PubMed] [Google Scholar]
- 12.Matsushita H, Tanaka S, Saiki Y, Hara M, Nakata K, Tanimura S, et al. Lung cancer associated with usual interstitial pneumonia. Pathol Int. 1995;45:925–932. doi: 10.1111/j.1440-1827.1995.tb03417.x. [DOI] [PubMed] [Google Scholar]
- 13.Qunn L, Takemura T, Ikushima S, Ando T, Yanagawa T, Akiyama O, et al. Hyperplastic epithelial foci in honeycomb lesions in idiopathic pulmonary fibrosis. Virchows Arch. 2002;441:271–278. doi: 10.1007/s00428-002-0618-9. [DOI] [PubMed] [Google Scholar]
- 14.Hironaka M, Fukayama M. Pulmonary fibrosis and lung carcinoma: a comparative study of metaplastic epithelia in honeycombed areas of usual interstitial pneumonia with or without lung carcinoma. Pathol Int. 1999;49:1060–1066. doi: 10.1046/j.1440-1827.1999.00989.x. [DOI] [PubMed] [Google Scholar]
- 15.Raghu G, Amatto VC, Behr J, Stowasser S. Comorbidities in idiopathic pulmonary fibrosis patients: a systematic literature review. Eur Respir J. 2015;46:1113–1130. doi: 10.1183/13993003.02316-2014. [DOI] [PubMed] [Google Scholar]
- 16.Lee T, Park JY, Lee HY, Cho YJ, Yoon HI, Lee JH, et al. Lung cancer in patients with idiopathic pulmonary fibrosis: clinical characteristics and impact on survival. Respir Med. 2014;108:1549–1555. doi: 10.1016/j.rmed.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 17.Kreuter M, Ehlers-Tenenbaum S, Schaaf M, Oltmanns U, Palmowski K, Hoffmann H, et al. Treatment and outcome of lung cancer in idiopathic interstitial pneumonias. Sarcoidosis Vasc Diffuse Lung Dis. 2015;31:266–274. [PubMed] [Google Scholar]
- 18.Yoon JH, Nouraie M, Chen X, Zou RH, Sellares J, Veraldi KL, et al. Characteristics of lung cancer among patients with idiopathic pulmonary fibrosis and interstitial lung disease - analysis of institutional and population data. Respir Res. 2018;19:195. doi: 10.1186/s12931-018-0899-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8) Suppl:IV-3–IV-18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 20.Raghu G, Chen SY, Yeh WS, Maroni B, Li Q, Lee YC, et al. Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: incidence, prevalence, and survival, 2001-11. Lancet Respir Med. 2014;2:566–572. doi: 10.1016/S2213-2600(14)70101-8. [DOI] [PubMed] [Google Scholar]
- 21.Esposito DB, Lanes S, Donneyong M, Holick CN, Lasky JA, Lederer D, et al. Idiopathic pulmonary fibrosis in United States automated claims: incidence, prevalence, and algorithm validation. Am J Respir Crit Care Med. 2015;192:1200–1207. doi: 10.1164/rccm.201504-0818OC. [DOI] [PubMed] [Google Scholar]
- 22.Ley B, Urbania T, Husson G, Vittinghoff E, Brush DR, Eisner MD, et al. Code-based diagnostic algorithms for idiopathic pulmonary fibrosis: case validation and improvement. Ann Am Thorac Soc. 2017;14:880–887. doi: 10.1513/AnnalsATS.201610-764OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klabunde CN, Legler JM, Warren JL, Baldwin LM, Schrag D. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol. 2007;17:584–590. doi: 10.1016/j.annepidem.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 24.Zuckerman IH, Sato M, Hsu VD, Hernandez JJ. Validation of a method for identifying nursing home admissions using administrative claims. BMC Health Serv Res. 2007;7:202. doi: 10.1186/1472-6963-7-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging manual. 7th ed. New York: Springer; 2010. [Google Scholar]
- 26.et al. In: International Classification of Diseases for Oncology: ICD-O. 3rd ed. Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Paaarkin DM, editors; Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 27.Warren JL, Harlan LC, Fahey A, Virnig BA, Freeman JL, Klabunde CN, et al. Utility of the SEER-Medicare data to identify chemotherapy use. Med Care. 2002;40(8) Suppl:IV-55–IV-61. doi: 10.1097/01.MLR.0000020944.17670.D7. [DOI] [PubMed] [Google Scholar]
- 28.Puggina A, Broumas A, Ricciardi W, Boccia S. Cost-effectiveness of screening for lung cancer with low-dose computed tomography: a systematic literature review. Eur J Public Health. 2016;26:168–175. doi: 10.1093/eurpub/ckv158. [DOI] [PubMed] [Google Scholar]
- 29.Goto T, Maeshima A, Oyamada Y, Kato R. Idiopathic pulmonary fibrosis as a prognostic factor in non-small cell lung cancer. Int J Clin Oncol. 2014;19:266–273. doi: 10.1007/s10147-013-0566-1. [DOI] [PubMed] [Google Scholar]
- 30.Chiyo M, Sekine Y, Iwata T, Tatsumi K, Yasufuku K, Iyoda A, et al. Impact of interstitial lung disease on surgical morbidity and mortality for lung cancer: analyses of short-term and long-term outcomes. J Thorac Cardiovasc Surg. 2003;126:1141–1146. doi: 10.1016/s0022-5223(03)00791-8. [DOI] [PubMed] [Google Scholar]
- 31.Kawasaki H, Nagai K, Yokose T, Yoshida J, Nishimura M, Takahashi K, et al. Clinicopathological characteristics of surgically resected lung cancer associated with idiopathic pulmonary fibrosis. J Surg Oncol. 2001;76:53–57. doi: 10.1002/1096-9098(200101)76:1<53::aid-jso1009>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 32.Miyazaki K, Satoh H, Kurishima K, Nakamura R, Ishikawa H, Kagohashi K, et al. Impact of interstitial lung disease on survival for patients with non-small cell lung cancer. Anticancer Res. 2009;29:2671–2674. [PubMed] [Google Scholar]
- 33.Omori T, Tajiri M, Baba T, Ogura T, Iwasawa T, Okudela K, et al. Pulmonary resection for lung cancer in patients with idiopathic interstitial pneumonia. Ann Thorac Surg. 2015;100:954–960. doi: 10.1016/j.athoracsur.2015.03.094. [DOI] [PubMed] [Google Scholar]
- 34.Voltolini L, Bongiolatti S, Luzzi L, Bargagli E, Fossi A, Ghiribelli C, et al. Impact of interstitial lung disease on short-term and long-term survival of patients undergoing surgery for non-small-cell lung cancer: analysis of risk factors. Eur J Cardiothorac Surg. 2013;43:e17–e23. doi: 10.1093/ejcts/ezs560. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe A, Higami T, Ohori S, Koyanagi T, Nakashima S, Mawatari T. Is lung cancer resection indicated in patients with idiopathic pulmonary fibrosis? J Thorac Cardiovasc Surg. 2008;136:1357–1363.e2. doi: 10.1016/j.jtcvs.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 36.Taskar V, Coultas D. Exposures and idiopathic lung disease. Semin Respir Crit Care Med. 2008;29:670–679. doi: 10.1055/s-0028-1101277. [DOI] [PubMed] [Google Scholar]
- 37.Baumgartner KB, Samet JM, Stidley CA, Colby TV, Waldron JA. Cigarette smoking: a risk factor for idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1997;155:242–248. doi: 10.1164/ajrccm.155.1.9001319. [DOI] [PubMed] [Google Scholar]
- 38.Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16:582–598. doi: 10.1038/nrc.2016.73. [DOI] [PubMed] [Google Scholar]
- 39.Kawai T, Yakumaru K, Suzuki M, Kageyama K. Diffuse interstitial pulmonary fibrosis and lung cancer. Acta Pathol Jpn. 1987;37:11–19. doi: 10.1111/j.1440-1827.1987.tb03130.x. [DOI] [PubMed] [Google Scholar]
- 40.Kim EA, Johkoh T, Lee KS, Han J, Fujimoto K, Sadohara J, et al. Quantification of ground-glass opacity on high-resolution CT of small peripheral adenocarcinoma of the lung: pathologic and prognostic implications. AJR Am J Roentgenol. 2001;177:1417–1422. doi: 10.2214/ajr.177.6.1771417. [DOI] [PubMed] [Google Scholar]
- 41.Yip R, Wolf A, Tam K, Taioli E, Olkin I, Flores RM, et al. Outcomes of lung cancers manifesting as nonsolid nodules. Lung Cancer. 2016;97:35–42. doi: 10.1016/j.lungcan.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 42.Harris JM, Johnston ID, Rudd R, Taylor AJ, Cullinan P. Cryptogenic fibrosing alveolitis and lung cancer: the BTS study. Thorax. 2010;65:70–76. doi: 10.1136/thx.2009.121962. [DOI] [PubMed] [Google Scholar]
- 43.Lee SH, Kim DS, Kim YW, Chung MP, Uh ST, Park CS, et al. Association between occupational dust exposure and prognosis of idiopathic pulmonary fibrosis: a Korean national survey. Chest. 2015;147:465–474. doi: 10.1378/chest.14-0994. [DOI] [PubMed] [Google Scholar]
- 44.Jeon K, Chung MP, Lee KS, Chung MJ, Han J, Koh WJ, et al. Prognostic factors and causes of death in Korean patients with idiopathic pulmonary fibrosis. Respir Med. 2006;100:451–457. doi: 10.1016/j.rmed.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 45.Ozawa Y, Akahori D, Koda K, Abe T, Hasegawa H, Matsui T, et al. Distinctive impact of pre-existing interstitial lung disease on the risk of chemotherapy-related lung injury in patients with lung cancer. Cancer Chemother Pharmacol. 2016;77:1031–1038. doi: 10.1007/s00280-016-3025-7. [DOI] [PubMed] [Google Scholar]
- 46.Ozawa Y, Abe T, Omae M, Matsui T, Kato M, Hasegawa H, et al. Impact of preexisting interstitial lung disease on acute, extensive radiation pneumonitis: retrospective analysis of patients with lung cancer. PLoS One. 2015;10:e0140437. doi: 10.1371/journal.pone.0140437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Danson S, Blackhall F, Hulse P, Ranson M. Interstitial lung disease in lung cancer: separating disease progression from treatment effects. Drug Saf. 2005;28:103–113. doi: 10.2165/00002018-200528020-00002. [DOI] [PubMed] [Google Scholar]
- 48.Enomoto Y, Kenmotsu H, Watanabe N, Baba T, Murakami H, Yoh K, et al. Efficacy and safety of combined carboplatin, paclitaxel, and bevacizumab for patients with advanced non-squamous non-small cell lung cancer with pre-existing interstitial lung disease: a retrospective multi-institutional study. Anticancer Res. 2015;35:4259–4263. [PubMed] [Google Scholar]
- 49.Sato T, Teramukai S, Kondo H, Watanabe A, Ebina M, Kishi K, et al. Japanese Association for Chest Surgery. Impact and predictors of acute exacerbation of interstitial lung diseases after pulmonary resection for lung cancer. J Thorac Cardiovasc Surg. 2014;147:1604–1611.e3. doi: 10.1016/j.jtcvs.2013.09.050. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki H, Sekine Y, Yoshida S, Suzuki M, Shibuya K, Yonemori Y, et al. Risk of acute exacerbation of interstitial pneumonia after pulmonary resection for lung cancer in patients with idiopathic pulmonary fibrosis based on preoperative high-resolution computed tomography. Surg Today. 2011;41:914–921. doi: 10.1007/s00595-010-4384-z. [DOI] [PubMed] [Google Scholar]
- 51.Kumar P, Goldstraw P, Yamada K, Nicholson AG, Wells AU, Hansell DM, et al. Pulmonary fibrosis and lung cancer: risk and benefit analysis of pulmonary resection. J Thorac Cardiovasc Surg. 2003;125:1321–1327. doi: 10.1016/s0022-5223(03)00028-x. [DOI] [PubMed] [Google Scholar]
- 52.Lococo F, Rapicetta C, Carbonelli C, Paci M, Sgarbi G. Which is the best surgical resection in NSCLC patients with idiopathic pulmonary fibrosis? Surgical and oncological considerations [letter] Ann Thorac Surg. 2016;101:835. doi: 10.1016/j.athoracsur.2015.09.049. [DOI] [PubMed] [Google Scholar]
- 53.Martinod E, Azorin JF, Sadoun D, Destable MD, Le Toumelin P, Longchampt E, et al. Surgical resection of lung cancer in patients with underlying interstitial lung disease. Ann Thorac Surg. 2002;74:1004–1007. doi: 10.1016/s0003-4975(02)03848-1. [DOI] [PubMed] [Google Scholar]
- 54.Saito Y, Kawai Y, Takahashi N, Ikeya T, Murai K, Kawabata Y, et al. Survival after surgery for pathologic stage IA non-small cell lung cancer associated with idiopathic pulmonary fibrosis. Ann Thorac Surg. 2011;92:1812–1817. doi: 10.1016/j.athoracsur.2011.06.055. [DOI] [PubMed] [Google Scholar]
- 55.Watanabe A, Miyajima M, Mishina T, Nakazawa J, Harada R, Kawaharada N, et al. Surgical treatment for primary lung cancer combined with idiopathic pulmonary fibrosis. Gen Thorac Cardiovasc Surg. 2013;61:254–261. doi: 10.1007/s11748-012-0180-6. [DOI] [PubMed] [Google Scholar]
- 56.Miura Y, Saito T, Tanaka T, Takoi H, Yatagai Y, Inomata M, et al. Reduced incidence of lung cancer in patients with idiopathic pulmonary fibrosis treated with pirfenidone. Respir Investig. 2018;56:72–79. doi: 10.1016/j.resinv.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 57.Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, et al. National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tzouvelekis A, Spagnolo P, Bonella F, Vancheri C, Tzilas V, Crestani B, et al. Patients with IPF and lung cancer: diagnosis and management. Lancet Respir Med. 2018;6:86–88. doi: 10.1016/S2213-2600(17)30478-2. [DOI] [PubMed] [Google Scholar]
- 59.Rossi G, Spagnolo P. Biopsy in idiopathic pulmonary fibrosis: back to the future. Expert Rev Respir Med. 2017;11:679–684. doi: 10.1080/17476348.2017.1351302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.