Abstract
The GATA family zinc finger transcription factors GATA4 and GATA6 are known to play important roles in the development of the pancreas. In mice both Gata4 and Gata6 are required for pancreatic development. In humans GATA6 haploinsufficiency can cause pancreatic agenesis and heart defects. Congenital heart defects are also common in patients with GATA4 mutations and deletions but the role of GATA4 in the developing human pancreas is unproven.
We report 5 patients with deletions (n=4) or mutations of the GATA4 gene who have diabetes and a variable exocrine phenotype. In four cases diabetes presented in the neonatal period (age at diagnosis 1-7 days). A de novo GATA4 missense mutation (p.N273K) was identified in a patient with complete absence of the pancreas confirmed at post mortem. This mutation affects a highly conserved residue located in the second zinc finger domain of the GATA4 protein. In vitro studies showed reduced DNA binding and transactivational activity of the mutant protein.
We show that GATA4 mutations/deletions are a cause of neonatal or childhood-onset diabetes with or without exocrine insufficiency. These results confirm a role for GATA4 in normal development of the human pancreas.
Introduction
The GATA family of transcription factors, including GATA4 and GATA6, plays important roles in the development of several endodermally and mesodermally derived organs. Heterozygous mutations in GATA6 had previously been associated with congenital heart malformations (1) and, more recently, they were found to be the major cause of pancreatic agenesis in humans (2). Further studies have shown that patients with GATA6 mutations have a variable spectrum of diabetes manifestations, ranging from complete pancreatic agenesis to adult-onset diabetes without exocrine insufficiency (3).
GATA4 is a zinc finger transcription factor closely related to GATA6. Mutations in GATA4 have been described in familial and sporadic cases of heart malformation (4; 5) although non-penetrance is common (6). More than 120 cases of chromosome rearrangements resulting in interstitial or terminal deletions of chromosome 8p including GATA4 have been reported. The phenotypes associated with these deletions include congenital heart malformations, dysmorphic features and mental retardation (reviewed in (6)).
Mouse studies have suggested a role for Gata4 in pancreatic development. Gata4-/- mice die prior to pancreas specification because of defective extra-embryonic tissues formation (7; 8), but investigation of Gata4-/- mice rescued from embryonic lethality show agenesis of the ventral pancreas (9). Gata4+/- mice are phenotypically normal (7; 8).
In human embryos GATA4 is detected in the area of the developing pancreas from 29-31dpc (CS12), at the same time of the early transcription factor PDX1, suggesting a role of GATA4 in human pancreas development (10). There is one report of a GATA4 missense mutation (p.R319W) in a child with pancreatic agenesis and an atrial septal defect (11). The proband’s father and sister were also heterozygous for the mutation which was shown to impair protein function in vitro. Both had a congenital heart defect but no diabetes, so a causal link between GATA4 and pancreatic agenesis could not be proven and evidence to confirm a key role for GATA4 in development of the human pancreas is lacking.
We investigated the role of GATA4 in human pancreatic development by studying three patients with diabetes and chromosome 8p deletions spanning the gene, and searching for deletions or intragenic GATA4 mutations in 186 patients with neonatal diabetes (NDM) of unknown aetiology.
Materials and Methods
Patient cohort
Patients were defined from 2 sources:
186 probands diagnosed with neonatal diabetes before 6 months in whom no genetic diagnosis had been established were selected from a cohort of 867 patients with NDM who were referred from 73 countries. The relevant clinical information was provided by the referring clinician.
A cohort of patients with diabetes and known chromosomal abnormalities (n=13).
GATA4 mutation analysis
We used a custom targeted next-generation sequencing panel test (12) to screen the coding sequence and exon-intron boundaries of GATA4 in the neonatal diabetes cohort. The assay is able to detect partial and whole gene deletions in addition to base substitutions and indels. In two cases there was insufficient DNA for targeted NGS and mutation testing was carried out by Sanger sequencing. We also sequenced the GATA4 pancreatic and endodermal enhancers described by Rojas et al. (13; 14). Primer sequences are available on request. Sequencing reactions were run on an ABI3730 capillary machine (Applied Biosystems) and analyzed using Mutation Surveyor v3.98 (SoftGenetics) (reference sequence NM_002052.3). Parent-proband relationships were confirmed using the PowerPlex kit (Promega).
Deletions of GATA4
Deletions of GATA4 were either cytogenetically visible rearrangements including chromosome 8p in patients from the known chromosomal abnormalities cohort or detected by reduced copy number on the targeted NGS assay in the neonatal cohort. The extent of small GATA4 deletions was determined using the Affymetrix (Santa Clara, CA) Genome-Wide Human SNP Array 6.0 containing nearly 1 million copy number probes. Genotyping and within-batch copy number calling was performed by Aros Applied Biotechnology (Aarhus, Denmark). We visualised regions of reduced copy number state within Affymetrix Chromosome Analysis Suite, and identified probes marking deletion breakpoints.
Functional studies
Mammalian expression vector pIRES(+)-GATA4 was used to perform DpnI-mediated site-directed mutagenesis to generate GATA4 N273K (c.819C>A) mutation. Two independent clones were created, sequenced, and assayed in transient transfection assays in quadruplicates on three independent experiments, as described (15). Lipofectamine 2000 was used to transfect HeLa cells with 0.15μg of pGL3-WNT2 promoter-Luciferase construct (16), 2ng pRL Renilla Luciferase reporter vector, in conjunction with different amounts of empty vector (pIRES-hrGFP), pIRES-GATA4 or pIRES-GATA4-N273K to reach 0.5μg total DNA using. Firefly and Renilla luciferase activity was assayed using Dual-Luciferase Reporter Assay System (Promega). Binding of nuclear lysates from cells expressing GATA4 and GATA4-N273K protein to P32-labeled oligonucleotides including binding sites for GATA4 was performed as described (15). Sequences of oligonucleotides used in this assay include a predicted GATA binding site in the pancreatic (P2) HNF4A proximal promoter. Specificity of retardation complex was assessed by preincubating nuclear extracts with 100-fold excess wild type or mutant unlabeled oligonucleotides, or GATA4 antiserum (GATA4 C-20, Santa Cruz, sc-1237). Immunoblot analysis was used to verify that the expression efficiencies for vectors encoding wild type and N273K GATA4 were similar.
Results
Molecular genetics
We report three patients with diabetes and a known chromosomal abnormality resulting in GATA4 haploinsufficiency. Cases 1 and 2 have terminal 8p deletions of 12.5 or 17 Mb, and the third patient has an unbalanced translocation resulting in a large (~15 Mb) terminal 8p deletion together with a duplication of the tip of chromosome 9 (see Figure 1). The possibility of an intragenic or regulatory GATA4 mutation on the non-deleted 8p allele or a GATA6 mutation was excluded by sequence analysis.
Figure 1.
Schematic diagram indicating the extent of the deletion on chromosome 8p in cases I-IV, and gene content (Hg19) of the minimal deleted interval.
We then tested 186 patients with NDM for intragenic mutations or deletions of GATA4 and found two mutations. A whole gene deletion in case 4 was detected by targeted next generation sequencing and shown to be a 1 Mb interstitial deletion (chr8:10796092-11934660) that includes 8 additional genes (Figure 1) by copy number analysis of genome wide SNPs. A novel heterozygous missense mutation (c.819C>A, p.N273K) which affects a highly conserved amino acid located in the second zinc finger of the GATA4 protein was found in case 5 (Figure 2). The mutation is predicted as likely to be pathogenic by ALAMUT (Interactive Biosoftware, Rouen, France). Mutations in GATA6, PDX1 and PTF1A had previously been excluded in both cases.
Figure 2.
Schematic diagram showing the genomic and protein position of the GATA4 missense mutation.
All 5 patients were born to unaffected parents and testing in 4 cases showed that the GATA4 deletion or missense mutation had arisen de novo (Table 1). Four of the 5 patients were diagnosed with diabetes during the first week of life giving an overall frequency of GATA4 mutations in our NDM cohort of 4/867 (0.5%).
Table 1.
Clinical details of the 5 cases described. MRCP= Magnetic resonance cholangiopancreatography, USS= Ultrasound Scan, CT= Computerised Tomography, MRI= Magnetic resonance imaging, PM= Post Mortem, AV= Atrio-Ventricular, ASD= Atrial Septal Defect, VSD= Ventricular Septal Defect
| Patient | Genetic defect | De novo | Status | Age at diagnosis | Birth weight/gestation | Current age | Pancreas-clinical | Pancreas-biochemistry (Faecal elastase) | Pancreas-imaging | Cardiac malformations | Neurocognitive abnormalities | Other abnormalities |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 8p22pter del [12.5 Mb] | Yes | Diabetic | 13 years | 2440 g/40 weeks | 17 years | No symptoms of exocrine insufficiency | 284.1 mcg/g | Pancreatic hypoplasia of body and tail (MRCP) | AV canal defect, pulmonary stenosis, mitral and tricuspid abnormalities | Global developmental delay; epilepsy | |
| 2 | 8p23.3-22del [17 Mb] | Yes | PNDM | 7 days | 1780 g/Gestational age not available | 1 year | No symptoms of exocrine insufficiency | N/A | Pancreatic imaging normal (USS and CT) | ASD, VSD, pulmonary stenosis | Psycho-motor retardation, normal brain MRI | |
| 3 | 8p23.3-22del [15 Mb] | Yes | TNDM | 1 day | 1810 g/38 weeks | 10 years | No symptoms of exocrine insufficiency | 132.2 mcg/g | Not possible to visualize pancreas (USS) | Small ASD, small VSD, pulmonary stenosis | Mental retardation. MRI brain: subependymal calcification | |
| 4 | 8p23.1del [1 Mb] | Not maternally inherited but no paternal sample available | TNDM | 2 days | 1680 g/36 weeks | 7 years | On pancreatic enzyme supplementation | 17 mcg/g | Pancreatic hypoplasia (MRI) | ASD, pulmonary stenosis | Mild dysphasia, visual-perception deficit and slight difficulty in hearing discrimination. | Mild hypospadias, mild liver enlargement and rise of liver enzymes |
| 5 | GATA4 c.819C>A; p.N273K | Yes | Pancreatic agenesis | 1 day | 1240 g/34 weeks | Deceased | N/A | N/A | Complete absence of pancreas (PM) | None | Abnormal white matter development | Died of multi-organ failure (Day 4) |
Functional analysis of the novel missense mutation
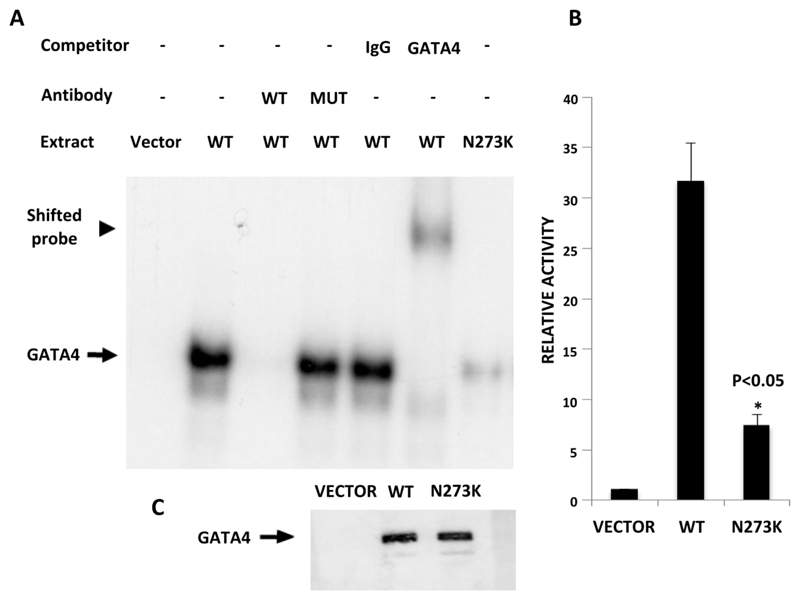
To test whether the p.N273K mutation truly affects GATA4 function, we examined the ability of wild-type and mutant proteins to bind and activate target sites. Electromobility shift assays showed that wild-type GATA4 exhibits high affinity binding to a GATA binding site in the pancreatic HNF4A promoter, and that the p.N273K mutation impairs this interaction (Figure 3A). Furthermore, transfected plasmids expressing GATA4 with the p.N273K mutation showed a 4.3-fold decrease in the ability to activate expression of a promoter that responds to GATA4 (16) (Figure 3B). These results indicate that the p.N273K mutation affects GATA4 transactivational activity by disrupting the ability of this protein to bind target recognition sequences.
Figure 3.
The p.N273K mutation disrupts GATA4 function. (A) Electrophoretic mobility shift assay showing that HELA cells transfected with wild type (WT) GATA4, but not the GATA4 p.N273K mutation, exhibit strong binding to a predicted GATA recognition sequence in the pancreatic (P2) HNF4A promoter. Binding disappears upon exposure to X100 excess wild type (WT) competitor oligonucleotides and supershifts with antiserum for GATA4, but is not affected by oligonucleotides with a mutation in the GATA consensus sequence (Mut), or by IgG. (B) A WNT2 promoter reporter construct that contains a high affinity GATA4 binding site shows transactivation by co-transfection with wild type GATA4. This is markedly decreased when co-transfecting the p.N273K plasmid. Control transfections were performed with an empty expression vector. P < 0.05 (Student’s t test). (C) Immunoblot analysis shows comparable expression efficiency for vectors encoding wild type and N273K GATA4.
Clinical characteristics: Pancreatic phenotype
The clinical characteristics are shown in Table 1. The endocrine phenotype was variable. Two patients had neonatal diabetes diagnosed in the first week of life which remitted temporarily but relapsed after 6 months and 7 years. Two patients have permanent diabetes diagnosed at 1 week or 13 years. The patient diagnosed at 13 years presented with an HbA1c of 114.2 mmol/mol (12.6%) following a 6 month history of polyuria and polydipsia, but was not ketotic. No pancreatic auto-antibodies were detected and her C-peptide production was preserved 3 years post diagnosis (stimulated C-peptide 3.8ng/mL). The fifth patient was born prematurely (34 weeks’ gestation), diagnosed with diabetes on the first day of life and died of multiple organ failure at four days of age. The 4 patients with neonatal diabetes had low birth weight (<3rd centile) consistent with insulin deficiency in utero. All 5 were treated with insulin.
The pancreatic exocrine phenotype was also variable. There was complete absence of the pancreas on post mortem examination of case 5. Case 4 has exocrine pancreatic insufficiency (faecal elastase=17 mcg/g) and is receiving exocrine supplementation. To assess subclinical exocrine insufficiency in the 3 patients not receiving exocrine supplementation we measured fecal elastase and performed pancreatic imaging. There was reduced pancreatic size in 2 patients and low fecal elastase in one (132 mcg/g; normal range>200 mcg/g).
Clinical characteristics: Extra-pancreatic phenotype
Congenital heart malformations were present in the 4 cases with GATA4 deletions and ranged from septal defects associated with pulmonary stenosis to atrio-ventricular canal defect but they were not seen in case 5 at post mortem. Developmental delay and neurocognitive defects were reported in all the 5 patients.
Discussion
Our study reports five patients with GATA4 mutations and a variable phenotype of transient or permanent diabetes diagnosed in neonates or during childhood. The exocrine pancreatic phenotype ranged from complete agenesis, to hypoplasia with subclinical exocrine insufficiency or normal exocrine function. Additional features included neurocognitive defects and congenital heart malformations. The variable phenotype was not correlated with the size of the deletion, consistent with the variable penetrance of GATA4 mutations/deletions reported in the literature. Both patients with pancreatic agenesis (this study and 11) have missense mutations rather than deletions, but the absence of diabetes in two heterozygous relatives suggests that a dominant-negative effect is unlikely (11). Our results indicate that GATA4 mutations/deletions are a rare cause of NDM, accounting for 0.5% of our international series (4/867 cases). The observation that post-zygotic GATA4 mutations in embryonic heart tissue can cause congenital heart defects (16) implies a similar mechanism for neonatal diabetes but is impossible to prove without access to pancreatic tissue.
The novel GATA4 missense p.N273K mutation is highly likely to be pathogenic because it affects a highly conserved amino acid within one of the zinc fingers, has arisen de novo and cell-based studies of the mutant protein demonstrated impaired DNA binding and reduced transactivational activity. A different mutation affecting the same residue (p.N273S) was previously reported in the heart tissue of a deceased patient with severe cardiac abnormalities (17), consistent with a pathogenic role of this mutation, albeit with inter-individual differences in the phenotypic expressivity.
The GATA4 deletions in our cohort ranged from 1 to 17 Mb with an overlapping interval that includes 8 additional genes. No mutations in these genes have previously been reported to cause NDM although previous reports describe an association between diabetes and this chromosome 8p region, with a possible critical region at 8p23 (18). Several candidate genes have been proposed, including PPP1R3B (19) and BLK (20), but not GATA4. BLK sequencing was included within the targeted next generation sequencing assay but no mutations found. Follow-up studies of individuals with 8p deletions and intragenic GATA4 mutations are required to ascertain the incidence of diabetes outside the neonatal period.
Conditional simultaneous knock-out of Gata4 and Gata6 in mouse pancreas causes pancreatic agenesis, whereas only mild early developmental defects are observed in mice in which only Gata6 or Gata4 are ablated, suggesting redundant roles for Gata4 and Gata6 in mouse pancreatic development (21; 22). Recent evidence also suggest that mouse pancreas development is sensitive to Gata4 and Gata6 dosage, as mice retaining only one Gata4 or Gata6 allele exhibit pancreatic developmental defects that are not observed with double homozygous mutant mice. These observations in mice should be contrasted with our results showing that in humans haploinsufficiency of either GATA6 (2; 3) or GATA4 can cause impaired pancreatic development resulting in a wide spectrum of diabetes manifestations. Similar discrepancies in human/mouse phenotypes have been observed in other endodermal transcription factors involved in pancreas development such as Hnf1a, Hnf1b and Hnf4a. Existing knowledge suggests that although the transcription factors that regulate pancreas development in humans and mice are very often the same, haploinsufficiency appears to cause much more severe manifestations during human as opposed to mouse pancreas development.
Intragenic GATA4 mutations or deletions causing GATA4 haploinsufficiency have been described in more than 140 patients with congenital heart malformations. In contrast, diabetes has only been reported in 6 patients with GATA4 mutations or deletions (this study and (11)), suggesting a lower penetrance of the pancreatic phenotype compared to the cardiac phenotype. The genetic and/or environmental factors underlying this phenotypic variability are not understood. Animal studies have shown that knock-out of one copy of Gata4 in mouse strains with different genetic background results in a marked difference in the cardiac phenotype observed: ranging from absence of pathogenic phenotype to complex congenital heart defects (23). This evidence suggests that additional genetic factors contribute to the penetrance of the cardiac phenotype. A similar mechanism in humans would explain the difference in the pancreatic and extra-pancreatic phenotypes observed in patients with GATA4 mutations or deletions. In our study we were not able to identify any variant in the coding or regulatory regions of GATA4 or GATA6 that could account for the presence of the pancreatic phenotype in our patients. Further studies will be needed to assess the possible contribution of other genes to the variable phenotype associated to mutations in this transcription factor.
In summary, our results show that GATA4 mutations are a rare cause of NDM and pancreatic agenesis. This is an important diagnosis to make since it confers an increased risk for future offspring being affected with NDM and/or congenital heart defects. Testing for intragenic mutations or deletions of GATA4 should therefore be considered in patients with neonatal or childhood onset diabetes and congenital heart defects in whom no GATA6 mutation has been identified. This study confirms a role for GATA4 in the development of the human pancreas.
Acknowledgements
The research leading to these results received funding from the European Community’s Seventh Framework Programme (FP7/2007-2013) under grant agreement number FP7-PEOPLE-ITN-2008 (Marie Curie Initial Training Networks, Biology of Liver and Pancreatic Development and Disease) and grant agreement number 223211 (Collaborative European Effort to Develop Diabetes Diagnostics, CEED3), Ministerio de Economía y Competitividad (SAF2011-27086), Diabetes UK (ref. 11/0004193) and the Wellcome Trust. S.E. and A.T.H. are employed as core members of staff within the National Institute for Health Research–funded Exeter Clinical Research Facility. S.E., J.F., and A.T.H. are Wellcome Trust Senior Investigators and A.T.H. is an NIHR Senior Investigator. M.B. and W.M. are supported by NCN grant 2011/01/M/NZ5/02815 and by Innovative Economy Operational Program – Activity 1.2 (the TEAM Program coordinated by the Foundation for Polish Science).
Footnotes
No potential conflicts of interest relevant to this article were reported.
C.S.-S. researched the clinical data, contributed to discussion and wrote the manuscript. E.D.F. researched the molecular genetics data, contributed to discussion and wrote the manuscript. H.L.A., S.E.F., G.P.N., I.W. and M.B. researched the molecular genetics data and reviewed the manuscript. C.E.T., J.A., J.C., G.D. and Z.M. researched the clinical data and reviewed the manuscript. M.B. and J.F. performed the functional studies, contributed to discussion and reviewed the manuscript. W.M. researched the clinical and molecular data, contributed to discussion and reviewed the manuscript. A.T.H. directed the research, contributed to discussion and reviewed the manuscript. S.E. directed the research, contributed to discussion and wrote the manuscript. S.E. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank the families who participated in this study.
References
- 1.Kodo K, Nishizawa T, Furutani M, Arai S, Yamamura E, Joo K, Takahashi T, Matsuoka R, Yamagishi H. GATA6 mutations cause human cardiac outflow tract defects by disrupting semaphorin-plexin signaling. Proc Natl Acad Sci U S A. 2009;106:13933–13938. doi: 10.1073/pnas.0904744106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lango Allen H, Flanagan SE, Shaw-Smith C, De Franco E, Akerman I, Caswell R, Consortium tIPA. Ferrer J, Hattersley A, Ellard S. GATA6 haploinsufficiency causes pancreatic agenesis in humans. Nature Genetics. 2011;1:20–22. doi: 10.1038/ng.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Franco E, Shaw-Smith C, Flanagan SE, Shepherd MH, Hattersley AT, Ellard S. GATA6 Mutations Cause a Broad Phenotypic Spectrum of Diabetes From Pancreatic Agenesis to Adult-Onset Diabetes Without Exocrine Insufficiency. Diabetes. 2013 Mar;62(3):993–7. doi: 10.2337/db12-0885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garg V, Kathiriya IS, Barnes R, Schluterman MK, King IN, Butler CA, Rothrock CR, Eapen RS, Hirayama-Yamada K, Joo K, Matsuoka R, et al. GATA4 mutations cause human congenital heart defects and reveal an interaction with TBX5. Nature. 2003;424:443–447. doi: 10.1038/nature01827. [DOI] [PubMed] [Google Scholar]
- 5.Nemer G, Fadlalah F, Usta J, Nemer M, Dbaibo G, Obeid M, Bitar F. A novel mutation in the GATA4 gene in patients with Tetralogy of Fallot. Hum Mutat. 2006;27:293–294. doi: 10.1002/humu.9410. [DOI] [PubMed] [Google Scholar]
- 6.Wat MJ, Shchelochkov OA, Holder AM, Breman AM, Dagli A, Bacino C, Scaglia F, Zori RT, Cheung SW, Scott DA, Kang SH. Chromosome 8p23.1 deletions as a cause of complex congenital heart defects and diaphragmatic hernia. Am J Med Genet A. 2009;149A:1661–1677. doi: 10.1002/ajmg.a.32896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuo CT, Morrisey EE, Anandappa R, Sigrist K, Lu MM, Parmacek MS, Soudais C, Leiden JM. GATA4 transcription factor is required for ventral morphogenesis and heart tube formation. Genes Dev. 1997;11:1048–1060. doi: 10.1101/gad.11.8.1048. [DOI] [PubMed] [Google Scholar]
- 8.Molkentin JD, Lin Q, Duncan SA, Olson EN. Requirement of the transcription factor GATA4 for heart tube formation and ventral morphogenesis. Genes Dev. 1997;11:1061–1072. doi: 10.1101/gad.11.8.1061. [DOI] [PubMed] [Google Scholar]
- 9.Watt AJ, Zhao R, Li J, Duncan SA. Development of the mammalian liver and ventral pancreas is dependent on GATA4. BMC Dev Biol. 2007;7:37. doi: 10.1186/1471-213X-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jennings RE, Berry AA, Kirkwood-Wilson R, Roberts NA, Hearn T, Salisbury RJ, Blaylock J, Hanley KP, Hanley NA. Development of the human pancreas from foregut to endocrine commitment. Diabetes. 2013 Oct;62(10):3514–22. doi: 10.2337/db12-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Amato E, Giacopelli F, Giannattasio A, D'Annunzio G, Bocciardi R, Musso M, Lorini R, Ravazzolo R. Genetic investigation in an Italian child with an unusual association of atrial septal defect, attributable to a new familial GATA4 gene mutation, and neonatal diabetes due to pancreatic agenesis. Diabet Med. 2010;27:1195–1200. doi: 10.1111/j.1464-5491.2010.03046.x. [DOI] [PubMed] [Google Scholar]
- 12.Ellard S, Lango Allen H, De Franco E, Flanagan SE, Hysenaj G, Colclough K, Houghton JA, Shepherd M, Hattersley AT, Weedon MN, Caswell R. Improved genetic testing for monogenic diabetes using targeted next-generation sequencing. Diabetologia. 2013 Sep;56(9):1958–63. doi: 10.1007/s00125-013-2962-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rojas A, Schachterle W, Xu SM, Black BL. An endoderm-specific transcriptional enhancer from the mouse Gata4 gene requires GATA and homeodomain protein-binding sites for function in vivo. Dev Dyn. 2009;238:2588–2598. doi: 10.1002/dvdy.22091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rojas A, Schachterle W, Xu SM, Martin F, Black BL. Direct transcriptional regulation of Gata4 during early endoderm specification is controlled by FoxA2 binding to an intronic enhancer. Dev Biol. 2010;346:346–355. doi: 10.1016/j.ydbio.2010.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boj SF, Parrizas M, Maestro MA, Ferrer J. A transcription factor regulatory circuit in differentiated pancreatic cells. Proc Natl Acad Sci U S A. 2001;98:14481–14486. doi: 10.1073/pnas.241349398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alexandrovich A, Arno M, Patient RK, Shah AM, Pizzey JA, Brewer AC. Wnt2 is a direct downstream target of GATA6 during early cardiogenesis. Mech Dev. 2006;123:297–311. doi: 10.1016/j.mod.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Reamon-Buettner SM, Borlak J. GATA4 zinc finger mutations as a molecular rationale for septation defects of the human heart. J Med Genet. 2005;42:e32. doi: 10.1136/jmg.2004.025395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pezzolesi MG, Nam M, Nagase T, Klupa T, Dunn JS, Mlynarski WM, Rich SS, Warram JH, Krolewski AS. Examination of candidate chromosomal regions for type 2 diabetes reveals a susceptibility locus on human chromosome 8p23.1. Diabetes. 2004;53:486–491. doi: 10.2337/diabetes.53.2.486. [DOI] [PubMed] [Google Scholar]
- 19.Dunn JS, Mlynarski WM, Pezzolesi MG, Borowiec M, Powers C, Krolewski AS, Doria A. Examination of PPP1R3B as a candidate gene for the type 2 diabetes and MODY loci on chromosome 8p23. Ann Hum Genet. 2006;70:587–593. doi: 10.1111/j.1469-1809.2005.00248.x. [DOI] [PubMed] [Google Scholar]
- 20.Borowiec M, Liew CW, Thompson R, Boonyasrisawat W, Hu J, Mlynarski WM, El Khattabi I, Kim SH, Marselli L, Rich SS, Krolewski AS, et al. Mutations at the BLK locus linked to maturity onset diabetes of the young and beta-cell dysfunction. Proc Natl Acad Sci U S A. 2009;106:14460–14465. doi: 10.1073/pnas.0906474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carrasco M, Delgado I, Soria B, Martin F, Rojas A. GATA4 and GATA6 control mouse pancreas organogenesis. J Clin Invest. 2012;122:3504–3515. doi: 10.1172/JCI63240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xuan S, Borok MJ, Decker KJ, Battle MA, Duncan SA, Hale MA, Macdonald RJ, Sussel L. Pancreas-specific deletion of mouse Gata4 and Gata6 causes pancreatic agenesis. J Clin Invest. 2012;122:3516–3528. doi: 10.1172/JCI63352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajagopal SK, Ma Q, Obler D, Shen J, Manichaikul A, Tomita-Mitchell A, Boardman K, Briggs C, Garg V, Srivastava D, Goldmuntz E, et al. Spectrum of heart disease associated with murine and human GATA4 mutation. J Mol Cell Cardiol. 2007;43:677–685. doi: 10.1016/j.yjmcc.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]