Abstract
Helicobacter pylori (H pylori) is known as one of the most common infectious pathogens, with high infection and recurrence rates worldwide. The prevalence of H pylori is up to 90% in developing countries, while the annual recurrence rate is much higher than that in developed countries. Recurrence can occur either by recrudescence or reinfection. Compared with reinfection, the time window for recrudescence is generally shorter, followed by the recurrence of H pylori–associated diseases in the short‐term. Many factors are involved in the H pylori reinfection, such as the prevalence of H pylori infection, living conditions and economic development, health conditions and so forth. Previous studies focused less on H pylori recrudescence. Therefore, the influencing factors for H pylori recrudescence needed further exploration. This study reviewed the recrudescence of H pylori infection and its influencing factors.
Keywords: colonization, Helicobacter pylori, influencing factors, recrudescence
1. INTRODUCTION
Helicobacter pylori (H pylori) is a microaerobic Gram‐negative bacterium that colonizes the human stomach and duodenum.1 It can cause lifelong infection without eradication. Many studies showed2, 3 that H pylori led to some important gastrointestinal diseases, such as chronic gastritis, peptic ulcer, gastric adenocarcinoma and mucosa‐associated lymphoid tissue lymphoma, and was associated with a variety of parenteral diseases such as idiopathic thrombocytopenic purpura. H pylori eradication significantly alleviated stomach inflammation, promoted ulcer healing and prevented gastric cancer.2 In 1994, H pylori was listed as Group I carcinogen. The 2015 Tokyo Global Consensus Report4 defines H pylori gastritis as an infectious disease and recommends eradication therapy for H pylori–infected individuals, except in the case of competing considerations. However, H pylori infection can still recur after eradication therapy. Recurrence can occur by either recrudescence or reinfection.5 Compared with reinfection, the time window for recrudescence is generally shorter. Recrudescence is generally considered as H pylori recurrence within 1 year after eradication, followed by the recurrence of H pylori–associated diseases in the short‐term.6, 7 Patients with short‐term recurrence suffer from the risk of recurrence of these diseases. The economic pressure, psychological burden and potential adverse drug reactions have increased dramatically. Therefore, exploring the factors related to H pylori recrudescence is important. In this study, the recrudescence of H pylori and its influencing factors were discussed.
2. DEFINITION AND DIAGNOSIS OF H PYLORI RECRUDESCENCE
Helicobacter pylori recurrence is generally divided into recrudescence and reinfection.5 Recrudescence is defined as the reappearance of the original infection following an initially false‐negative post‐eradication test result.2 A small amount of H pylori that has not been eradicated (H pylori was hidden in the deep part of the stomach or the gastric epithelial metaplasia of the duodenum) or is in dormancy (eg H pylori coccoid forms) is recolonized, reproduced and eventually detected. Therefore, the recrudescence strain is generally the original infectious strain. Reinfection is defined as infection with a new strain or a strain homologous to the original strain of H pylori.
So far, relevant studies have shown that recrudescence is generally considered as H pylori recurrence within 1 year after eradication.6, 7 Gisbert et al8 suggested that the cumulative annual recurrence rate after H pylori eradication was 5.3% after 1 year, 6.8% after 2 years, 7% after 3 years, 7.6% after 4 years and 9.3% after 5 years. The present study found that recurrence decreased with time and declined sharply after the first year. Kim et al9 performed a 2‐year follow‐up of patients with H pylori eradication. They found that, regardless of first‐line or second‐line therapy, the recurrence rate in the first year was significantly different (9.3% vs 4.5%) and the recurrence rate in the second year was similar (2.0% vs 2.9%). If the recurrence after H pylori eradication is reinfection, the annual reinfection rate should be stable.8, 9 However, the annual recurrence rate of H pylori increases at a steady rate, which is contrary to the conclusions of the aforementioned studies. It is generally believed that the recurrence of H pylori in the first year after eradication is mainly based on recrudescence. However, Raymond et al10 performed a strain typing study on three patients with repeated recurrence after H pylori eradication. The time interval between the first recrudescence of two patients was 2 years, and the recrudescence interval was 1‐3 years. One patient's first reinfection interval was up to 8 years and the reinfection interval was 1‐2 years. Accordingly, Raymond et al believed that the recurrence interval was not a reliable clinical marker for recrudescence. However, the number of specimens in the aforementioned study was extremely small, with some contingency in the conclusion. Further large‐sample H pylori strain typing follow‐up studies are needed to confirm this view.
To distinguish whether H pylori recurrence is recrudescence or reinfection, genotyping methods are used to judge the H pylori strain type before and after recurrence. H pylori genotyping methods include11 multi‐locus sequence typing (MLST), pulsed‐field gel electrophoresis (PFGE), random amplification of polymorphic DNA (RAPD), amplified fragment length polymorphism (AFLP), whole‐genome sequencing (WGS) and so on. Multi‐locus sequence typing analyses strain variation by polymerase chain reaction (PCR) amplification of multiple housekeeping genes (such as atpA, efp, mutY and so on) and determination of their nucleic acid sequences.12 Multi‐locus sequence typing has the advantages of high repeatability and high resolution and can provide more detailed information on human migration than human genetic analysis to a certain extent.12 However, MLST only reflects the variability of several housekeeping genes. Pulsed‐field gel electrophoresis is to detect some large fragments of linear DNA and is considered as the gold standard for bacterial typing.13 But it has not been widely used in H pylori typing. It is very crucial of restriction enzymes choice and enzyme digestion condition control, which still needs further exploration in exploration in H pylori typing.13 Random amplification of polymorphic DNA is a typing technique based on PCR that can perform polymorphism analysis on the entire unknown sequence genome. Even trace amounts of DNA can also be analysed. But there still exist some limitations. Random amplification of polymorphic DNA cannot provide any information about strain virulence factors and genetic evolution information.14 Meanwhile, it depends highly on the quality and quantity of the template.14 Amplified fragment length polymorphism is a molecular marker technology developed on the basis of PCR, which has the advantages of high repeatability and high resolution.15 However, it also has high‐quality requirements for DNA template and it is a non‐rapid detection method.15 Whole‐genome sequencing masters the entire genomic sequence of the microorganism. In theory, any microorganism can be typed with a resolution of a single base.13 However, at the same time, the experiment cost is large and the cycle is long. Therefore, the aforementioned gene detection methods have not been widely carried out clinically. In addition, some studies pointed out that even if the same H pylori strain was identified before and after recurrence, still the patient might be reinfected by the same strain in the environment.16 Therefore, how to quickly and effectively identify H pylori recurrence and recrudescence is a hot issue worthy of further study.
3. INFLUENCING FACTORS OF H PYLORI RECRUDESCENCE
The rates varied widely among countries and areas from a high of 21.3% to a low of 0.2%.5 In recent years, more attention has been paid to H pylori and its diseases at home and abroad. However, Hu et al17 performed a systematic review with meta‐analysis of H pylori recurrence rates worldwide. They suggested no change in the recurrence rates over the past 27 years.17 These findings showed that the studies on the prevention and treatment of H pylori recurrence might not have achieved great results.
The global annual recurrence rate of H pylori was 4.3%.17 The annual recurrence rate of H pylori in developing countries (13%) is much higher than that in developed countries (2.7%).18 Among studies on H pylori recurrence factors, reinfection studies are relatively mature. It is generally believed that reinfection mainly involves host factors such as total H pylori infection rate, personal hygiene habits and so on. The factors involved in H pylori recrudescence can be roughly divided into two categories (Figure 1)16: (1) false‐negative results of the review (mainly in vivo factors); and (2) small amounts of unkilled H pylori or dormant H pylori lurking in the human body (mainly in vitro factors). The influencing factors for H pylori recrudescence will be discussed next.
Figure 1.
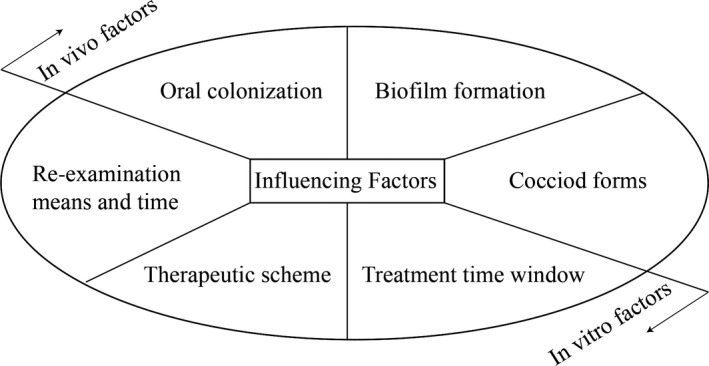
Influencing factors of Helicobacter pylori recrudescence. The factors involved in H pylori recrudescence can be roughly divided into two categories: (1) false‐negative results of the review (mainly in vivo factors); and (2) small amounts of unkilled H pylori or dormant H pylori lurking in the human body (mainly in vitro factors). H pylori itself is a major component of the in vivo factors including its oral colonization, biofilm formation and coccoid forms transformation. Re‐examination means and time, therapeutic scheme and treatment time window as in vitro intervention are also involved in H pylori recrudescence. Both in vivo and in vitro factors result in H pylori recrudescence
3.1. Selection of therapeutic scheme and treatment time window
Many studies have reported that the therapeutic scheme is closely related to H pylori recurrence within 1 year.8, 9 A therapeutic scheme with low H pylori‐eradication rate is temporary clearance rather than complete eradication, leading to H pylori recrudescence. Therefore, the rate of H pylori recrudescence negatively correlates with the eradication rate. The lower the H pylori‐eradication rate, the higher the H pylori recrudescence rate. Gisbert et al8 performed a prospective study involving 1000 patients, selecting two therapeutic schemes with low eradication rates (omeprazole plus amoxicillin, 32%; omeprazole plus amoxicillin and metronidazole, 56%) and two therapeutic schemes with high eradication rates (omeprazole plus clarithromycin and either amoxicillin or metronidazole, 85%; bismuth subcitrate, tetracycline chlorhydrate and metronidazole, 77%). The review results after 1 year showed that the former H pylori recrudescence rate was significantly higher than the latter (11.3% vs 4.7%, P = .006). A similar study was conducted in Korea, in which a standard triple therapy (eradication rate was 79.9%) and a barium‐containing quadruple therapy (eradication rate was 90.4%) were used for H pylori eradication.9 A follow‐up showed that H pylori recrudescence rates were 9.3% and 4.5% (P < .05) within 1 year.9 However, due to the geographical variation in H pylori resistance to antibiotics, even in the same treatment programme, H pylori‐eradication rates were different in different areas and countries.19, 20 Therefore, to increase the H pylori eradication and reduce its recurrence, different countries should use drugs as a first‐line treatment based on the epidemiological study of local H pylori antibiotic resistance.
In addition to therapeutic schemes, selecting an appropriate treatment time window is also important for the H pylori eradication. A meta‐analysis of China in 2017 (43 studies, 7686 patients) suggested that the 10‐day or 14‐day treatment of sputum quadruple therapy significantly improved H pylori eradication compared with its 7‐day therapy.21 Prolonging bismuth‐containing quadruple therapy from 10 to 14 days did not show better efficacy. Yuan et al22 analysed 59 studies based on a proton pump inhibitor (PPI) triple therapy and concluded that, regardless of the antibiotics type and its dose, an increase in the administration time of PPI triple therapy from 7 to 14 days significantly increased H pylori‐eradication rate (72.9% vs 81.9%, P < .05). Although the eradication rate increases with a prolonged administration time, the subsequent adverse drug reactions may also increase. Therefore, which H pylori therapeutic scheme to choose and how to choose the appropriate treatment time window to minimize H pylori recrudescence still need exploration. The direct relationship between the administration time for H pylori‐eradication therapy and H pylori recrudescence requires further clarification.
3.2. Re‐examination means and time
The detection methods for H pylori are of two types: invasive and non‐invasive. Invasive detection methods include rapid urease test, HE staining, Giemsa stain, bacterial culture and so on. The non‐invasive detection methods include a 13C‐urea breath test, 14C‐urea breath test, stool antigen test and so on. However, if only one of the aforementioned means is used to evaluate the efficacy, sensitivity and specificity are reduced. If a patient has used a PPI 2 months before re‐examination or an antibiotic 1 month before re‐examination, the breath test may be false negative because of the urease activity suppressed by these drugs. Especially, when the result is at a critical value, whether to use eradication therapy is difficult to determine.20 In addition, due to the drugs such as PPI, the H pylori distribution in the stomach changes (eg H pylori in the antrum is moved up to the corpus ventriculi). H pylori rejuvenates and multiplies due to the reduction or loss of drug efficacy after a period of eradication therapy. When H pylori is detected again, the result becomes positive again, which is H pylori recrudescence. Therefore, to reduce diagnostic error, it is best to use two or more different diagnostic techniques for H pylori recurrence detection.
In addition to H pylori detection methods, the evaluation time for H pylori eradication is also related to H pylori recrudescence. At present, the clinical evaluation of H pylori eradication is carried out at least 4 weeks after the therapy completion. Neil et al23 also believed that 1 month after eradication therapy was sufficient to evaluate the effect of H pylori eradication. Some studies16, 24 postponed the re‐examination means to 2 months to reduce the false‐negative rate. However, H pylori can be recrudesced within a few months after therapy. Ishizuka et al25 found that H pylori could reappear within 3 months after eradication therapy. When a patient is examined within 2‐3 months after eradication, it is difficult for the investigators to distinguish between eradication failure and recrudescence. Therefore, the selection of a time node for re‐examination influences the evaluation of H pylori recrudescence. However, a few studies currently define an evaluation period for H pylori eradication failure and recrudescence.
3.3. Helicobacter pylori oral colonization
Helicobacter pylori is mainly transmitted through multiple routes between humans, including faecal‐oral transmission, oral‐oral transmission, gastric‐oral transmission and iatrogenic transmission. It can also be transmitted to humans through water, environment and animals.26, 27 Therefore, H pylori in the oral cavity may play an important role in gastric H pylori transmission. Certain microaerobic environments are suitable for H pylori survival in the oral cavity, such as plaque, root canal and so on. Some studies have shown a link between oral H pylori and gastric H pylori (Table 1). Naviba et al28 included 23 studies (1861 patients) and found that the per cent of agreement between the dental plaque H pylori status and the gastric H pylori was estimated as 82%. Therefore, it is considered that oral H pylori and gastric H pylori have homology. Further, H pylori oral colonization may be one of the risk factors for gastric H pylori recrudescence. In addition, a meta‐analysis in 201129 showed that the prevalence of H pylori infection in the oral cavity in gastric H pylori–positive patients was significantly higher than that in gastric H pylori–negative patients (45% vs 23.9% OR 3.61, P < .0001). The eradication efficiency in stomach and oral cavity is 85.8% and 5.7%, respectively (OR 55.59, P < .00001). It shows that the clinical routine H pylori therapeutic scheme has a weak killing effect on oral H pylori. Bouziane et al30 performed a meta‐analysis (sample size: 298 cases) and evaluated the effect of periodontal therapy on the prevention of gastric H pylori recurrence. They found that, compared with the eradication therapy alone, the adjunction of periodontal therapy significantly reduced the relative risk of persistence of gastric H pylori by 63% (OR 0.37, P = .0004) in patients with gastric diseases. A recent prospective randomized trial in Thailand (sample size: 698 cases) also drew the same conclusion.31 After the eradication of gastric H pylori infection, the recurrence of gastric H pylori was significantly lower in the group receiving gastric H pylori treatment plus periodontal therapy than in that receiving gastric H pylori treatment alone (PP analysis: OR 0.69, P = .001; ITT analysis: OR 0.67, P = .001), while the eradication rates were not significantly different (PP analysis: OR 0.77, P = .078; ITT analysis: OR 0.87, P = .076). Therefore, oral H pylori may be one of the factors for H pylori recrudescence in the stomach.
Table 1.
The potential correlation between oral Helicobacter pylori and gastric H pylori
| Author | Type of study | sample size | Direction of study | Methods | Country | Index | Rate | P value | Summary of conclusion |
|---|---|---|---|---|---|---|---|---|---|
| Assumpcao et al44 | Cross‐sectional study | 99 | Genotype | RUT, PCR | Northern Brazil | Gene agreement rate | 89.0% | — | Significant association between oral H pylori and gastric H pylori |
| Ogunbodede et al45 | Cross‐sectional study | 66 | Colonization | culture, histological examination | Nigeria | Colonization correlation | — | .01 | The correlation (Spearman's) between gastric and oral H pylori colonization was significant |
| Roman‐Roman et al46 | Cross‐sectional study | 196 | Genotype | PCR, histological examination | Mexico | Gene agreement rate | 51.1% | — | H pylori might reach the stomach from oral cavity |
| Abadi et al47 | Cross‐sectional study | 132 | Colonization | PCR, culture | Iran | Prevalence of H pylori (oral H pylori vs gastric H pylori) | 100% vs 54.2% | .001 | Patients who previously infected with H pylori and cured were still carrying oral H pylori |
| Zou et al29 | Meta‐analysis | 1088 | Eradication | RUT, PCR, UBT, CLO test, histological examination | China | Eradication rate (gastric H pylori vs oral H pylori) | 85.8% vs5.7% | <.00001 | Oral H pylori was difficulty to eradication |
| Jia et al48 | Cohort study | 110 | Colonization | UBT | China | Prevalence of gastric H pylori (oral treatment vs no oral treatment) | 19.5% vs 84.3% | <.05 | Oral treatment was associated with lower gastric recurrence by H pylori |
| Zaric et al49 | Cohort study | 98 | Eradication | PCR | Serbia | Gastric H pylori‐eradication rate (oral treatment vs no oral treatment) | 77.3% vs 47.6% | .044 | Treated with the combined therapy exhibited successful eradication of gastric H pylori |
| Song et al50 | Cohort study | 431 | Eradication | UBT, HPS | China | Gastric H pylori‐eradication rate (oral treatment vs no oral treatment) | 94.7% vs 78.4% | .012 | Oral treatment might improve the eradication rate of gastric H pylori |
| Liu et al51 | Case‐control study | 443 | Colonization | RUT, PCR, histological examination | China | Prevalence of gastric H pylori (oral H pylori positive vs negative) | 80.1% vs 46.6% | <.01 | Oral H pylori showed concomitant stomach infection |
| Rasmussen et al52 | Cross‐sectional study | 78 | Colonization | Southern blotting | Brasil | Prevalence of oral H pylori (gastric H pylori positive vs negative) | 71.2% vs 50.0% | <.0001 | Oral H pylori showed a potential association with gastric reinfection |
| Anand et al53 | Case‐control study | 134 | Colonization | RUT, HPS, histological examination | India | Prevalence of gastric H pylori (oral H pylori positive vs negative) | 89.2% vs 71% | <.05 | H pylori in oral cavity was seldom eliminated by H pylori‐eradication therapy |
Abbreviations: CLO test, Campylobacter‐like organism test; HPS, H pylori antigen test; PCR, polymerase chain reaction; RUT, rapid urease test; UBT, urea breath test.
However, the conclusion may not be applicable to all populations because the studies involved a small sample size, some were limited to people of certain areas, and some involved different material parts of obtaining oral H pylori (such as dental plaque and saliva). Therefore, multi‐centre and large‐sample studies are needed to confirm the effect of oral H pylori treatment on reducing gastric H pylori recrudescence in the future.
3.4. H pylori coccoid forms
All living things have their own unique survival mechanisms in harsh environments, and H pylori is no exception. Morphologically, three forms of H pylori are presently considered to exist: spiral form, intermediate V‐ and U‐forms and coccoid form. Among them, the coccoid form is divided into two subtypes. Type A has irregular edges with a rough surface and is considered to be a dead form, while type B has a smoother surface, is smaller and is considered to be a dormant form.2, 32 A previous study has confirmed33 that coccoid H pylori exists in the stomach and duodenum, and the number in the duodenum is higher than that in the stomach. It indicated that the coccoid H pylori presence is related to the environment. When H pylori is exposed to harsh environments (use of antimicrobial agents, pH of the living environment, changes in oxygen content and so on), spiral H pylori can be converted into coccoid H pylori, which is the so‐called dormant form. Also, coccoid H pylori continues to maintain lower levels of metabolic activity, such as synthesis of urease and proteins, expression of virulence genes, and so on.34 However, coccoid H pylori cannot be recognized using traditional detection techniques or cultured and propagated in vitro. However, when the environment changes, it may be converted into the viable, culturable bacillary form that is spiral H pylori, and colonize and multiply in the stomach, resulting in H pylori recrudescence.35, 36 Coccoid H pylori were inoculated intragastrically in BALB/c mice. Cellini et al37 found that viable H pylori was isolated from the gastric mucosa after 2 weeks. She et al performed a similar study. When the gastric tissue mucosa was observed under an electron microscope 21 and 28 days after the last inoculation, spiral H pylori was found and an inflammatory reaction was observed in the pathology of stomach tissue. The aforementioned results confirmed that coccoid H pylori had the potential to transform into spiral H pylori and was the ‘seed’ of H pylori recrudescence.
A recent study found that coccoid H pylori failed to induce H pylori infection in mice. Boehnke et al38 allowed mice to freely drink water containing coccoid H pylori (109 cells/L) and found that either prolonged exposure of the mice to drinking water or shortened euthanasia did not result in H pylori colonization in the mouse stomach. Further studies conducted by the aforementioned investigators revealed that even if the mice were orally administered a high concentration of coccoid H pylori (four times the aforementioned dose) in drinking water for 2 weeks, the infection could not be induced. However, this study used the SS1 strain, which was different from the strains selected by Cellini and She (both from the H pylori strain in the stomach tissue of patients with ulcers). The contradictory conclusions might be related to the different transformation ability for different strains. However, no studies have been performed on the relationship between the spiral form of different H pylori strains and their spiral H pylori transformation ability.
3.5. H pylori biofilm formation
Biofilms can be defined as adherent aggregates of microorganisms encased with an extracellular polymeric substance. Growing evidence indicates that H pylori can also establish biofilms.39, 40 Moreover, a study found that the H pylori‐eradication rate in the N‐acetylcysteine (NAC)‐treated group (which can eliminate and prevent biofilm establishment) before the traditional eradication therapy was significantly higher than that in the NAC‐free group (65% vs 20%, P = .005).41 Hamidian et al42 also believed that NAC combined with triple eradication (amoxicillin, clarithromycin and omeprazole) could increase the eradication rate of the therapeutic scheme on H pylori (NAC group: 72.9%; placebo group: 60.9%, P = .005). Moreover, in the NAC pre‐treatment group, the H pylori load (colony‐forming units per gram of gastric tissue) decreased by about 1 log in C57BL mice compared with the untreated group.43 Therefore, it was speculated that H pylori present in the gastric mucosa under the biofilm might be one of the risk factors for recrudescence. In addition, scanning electron microscopy revealed that most of the H pylori under the biofilm was coccoid.44 After the conventional eradication treatment, the residual biofilm H pylori cannot be found by traditional detection methods even if the H pylori colony load under the biofilm is large, and it may become the ‘seed’ for future H pylori recrudescence. However, no relevant clinical study has confirmed the role of biofilms in H pylori recrudescence.
4. CONTROL AND PREVENTION OF H PYLORI RECRUDESCENCE
Helicobacter pylori recrudescence is one of the main causes for H pylori recurrence within 1 year after the eradication therapy. Many factors are involved in H pylori recrudescence, such as therapeutic scheme, treatment time window, re‐examination time and means, oral H pylori, H pylori coccoid forms, H pylori biofilm and so on. Therefore, to reduce the chance of H pylori recrudescence, a therapeutic scheme with high eradication rate or a suitable therapeutic scheme according to local antibiotic resistance, and a variety of re‐examination means should be chosen to reduce the false‐negative rate. In the meanwhile, strengthening patient education, improving patient compliance and increasing the H pylori‐eradication rate are necessary to avoid the limitations of drug selection and drug resistance in the second eradication. The relationship between H pylori oral colonization and H pylori recrudescence has not yet reached a consensus. However, an increasing number of studies have confirmed that oral nursing and periodontal treatment may reduce the H pylori infection and recrudescence rates in the stomach. Multi‐centre, large‐sample studies are required to increase the reliability of evidence in the future. The aim should be to disrupt the protection mechanism of H pylori in a harsh environment, prevent its transformation into coccoid forms and biofilm formation and improve the H pylori detection rate under the protection mechanism.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest concerning this study.
AUTHOR CONTRIBUTIONS
YS and JZ collected and read references. YS contributed to manuscript preparation. JZ revised the manuscript. All authors approved the final manuscript.
Sun Y, Zhang J. Helicobacter pylori recrudescence and its influencing factors. J Cell Mol Med. 2019;23:7919–7925. 10.1111/jcmm.14682
Funding information
The present study was supported by the grants of National Natural Science Foundation of China (grant no. 81400682) and Basic Public Welfare Research Project of Zhejiang Province (grant no. LGF18H030009).
REFERENCES
- 1. Zeng M, Mao XH, Li JX, et al. Efficacy, safety, and immunogenicity of an oral recombinant Helicobacter pylori vaccine in children in China: a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet. 2015;386:1457‐1464. [DOI] [PubMed] [Google Scholar]
- 2. Sarem M, Corti R. Role of Helicobacter pylori coccoid forms in infection and recrudescence. Gastroenterol Hepatol. 2016;39:28‐35. [DOI] [PubMed] [Google Scholar]
- 3. Wang X, Zhao X, Lu X, Li Y. Relationship between Helicobacter pylori infection and hematologic system diseases. Chin J Gastroenter Hepatol. 2018;27:134‐137. [Google Scholar]
- 4. Sugano K, Tack J, Kuipers EJ, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64:1353‐1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sjomina O, Pavlova J, Niv Y, Leja M. Epidemiology of Helicobacter pylori infection. Helicobacter. 2018;23(Suppl 1):e12514. [DOI] [PubMed] [Google Scholar]
- 6. Zhou LY, Song ZQ, Xue Y, Li X, Li YQ, Qian JM. Recurrence of Helicobacter pylori infection and the affecting factors: a follow‐up study. J Digest Dis. 2017;18:47‐55. [DOI] [PubMed] [Google Scholar]
- 7. Ryu KH, Yi SY, Na YJ, et al. Reinfection rate and endoscopic changes after successful eradication of Helicobacter pylori . World J Gastroenterol. 2010;16:251‐255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gisbert JP, Luna M, Gomez B, et al. Recurrence of Helicobacter pylori infection after several eradication therapies: long‐term follow‐up of 1000 patients. Aliment Pharm Therap. 2006;23:713‐719. [DOI] [PubMed] [Google Scholar]
- 9. Kim SY, Hyun JJ, Jung SW, Koo JS, Yim HJ, Lee SW. Helicobacter pylori recurrence after first‐ and second‐line eradication therapy in Korea: the problem of recrudescence or reinfection. Helicobacter. 2014;19:202‐206. [DOI] [PubMed] [Google Scholar]
- 10. Raymond J, Thiberge JM, Dauga C. Diagnosis of Helicobacter pylori recurrence: relapse or reinfection? Usefulness of molecular tools. Scand J Gastroenterol. 2016;51:672‐678. [DOI] [PubMed] [Google Scholar]
- 11. Wang J, Gu H. Research progress on genotyping of Helicobacter pylori . J Zhejiang Univ. 2018;47:97‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vale FF, Vadivelu J, Oleastro M, et al. Dormant phages of Helicobacter pylori reveal distinct populations in Europe. Sci Rep. 2015;5:14333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Salipante SJ, SenGupta DJ, Cummings LA, Land TA, Hoogestraat DR, Cookson BT. Application of whole‐genome sequencing for bacterial strain typing in molecular epidemiology. J Clin Microbiol. 2015;53:1072‐1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Han FC, Ng HC, Ho B. Stability of randomly amplified polymorphic DNA fingerprinting in genotyping clinical isolates of Helicobacter pylori . World J Gastroenterol. 2003;9:2021‐2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gibson JR, Slater E, Xerry J, Tompkins DS, Owen RJ. Use of an amplified‐fragment length polymorphism technique to fingerprint and differentiate isolates of Helicobacter pylori . J Clin Microbiol. 1998;36:2580‐2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morgan DR, Torres J, Sexton R, et al. Risk of recurrent Helicobacter pylori infection 1 year after initial eradication therapy in 7 Latin American communities. JAMA. 2013;309:578‐586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hu Y, Wan JH, Li XY, Zhu Y, Graham DY, Lu NH. Systematic review with meta‐analysis: the global recurrence rate of Helicobacter pylori . Aliment Pharmacol Ther. 2017;46:773‐779. [DOI] [PubMed] [Google Scholar]
- 18. Niv Y, Hazazi R. Helicobacter pylori recurrence in developed and developing countries: meta‐analysis of 13C‐urea breath test follow‐up after eradication. Helicobacter. 2008;13:56‐61. [DOI] [PubMed] [Google Scholar]
- 19. Zhang M. High antibiotic resistance rate: a difficult issue for Helicobacter pylori eradication treatment. World J Gastroenterol. 2015;21:13432‐13437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Greenberg ER, Anderson GL, Morgan DR, et al. 14‐day triple, 5‐day concomitant, and 10‐day sequential therapies for Helicobacter pylori infection in seven Latin American sites: a randomised trial. Lancet. 2011;378:507‐514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hu J. Review with meta‐analysis: 10 or 14 days bismuth‐based quadruple therapy for helicobacter pylori in China. Chongqing, China: Army Medical University; 2017. [Google Scholar]
- 22. Yuan Y, Ford AC, Khan KJ, et al. Optimum duration of regimens for Helicobacter pylori eradication. Cochrane Database Syst Rev. 2013;12:CD008337. [DOI] [PubMed] [Google Scholar]
- 23. Neil GA, Suchower LJ, Ronca PD, Skoglund ML. Time of Helicobacter pylori eradication assessment following treatment. Helicobacter. 1997;2:13‐20. [DOI] [PubMed] [Google Scholar]
- 24. Bruce MG, Bruden DL, Morris JM, et al. Reinfection after successful eradication of Helicobacter pylori in three different populations in Alaska. Epidemiol Infect. 2015;143:1236‐1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ishizuka J, Kato M, Sugiyama T, Asaka M. The appropriate time for the assessment of Helicobacter pylori eradication. Nihon Rinsho. 1999;57:111‐115. [PubMed] [Google Scholar]
- 26. Saeidi E, Sheikhshahrokh A. vacA genotype status of Helicobacter pylori isolated from foods with animal origin. Biomed Res Int. 2016;2016:8701067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ranjbar R, Khamesipour F, Jonaidi‐Jafari N, Rahimi E. Helicobacter pylori in bottled mineral water: genotyping and antimicrobial resistance properties. BMC Microbiol. 2016;16:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Navabi N, Aramon M, Mirzazadeh A. Does the presence of the Helicobacter pylori in the dental plaque associate with its gastric infection? A meta‐analysis and systematic review. Dent Res J. 2011;8:178‐182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zou QH, Li RQ. Helicobacter pylori in the oral cavity and gastric mucosa: a meta‐analysis. J Oral Pathol Med. 2011;40:317‐324. [DOI] [PubMed] [Google Scholar]
- 30. Bouziane A, Ahid S, Abouqal R, Ennibi O. Effect of periodontal therapy on prevention of gastric Helicobacter pylori recurrence: a systematic review and meta‐analysis. J Clin Periodontol. 2012;39:1166‐1173. [DOI] [PubMed] [Google Scholar]
- 31. Tongtawee T, Wattanawongdon W, Simawaranon T. Effects of periodontal therapy on eradication and recurrence of Helicobacter pylori infection after successful treatment. Jo Int Med Res. 2019;47:875‐883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reshetnyak VI, Reshetnyak TM. Significance of dormant forms of Helicobacter pylori in ulcerogenesis. World J Gastroenterol. 2017;23:4867‐4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Noach LA, Rolf TM, Tytgat GN. Electron microscopic study of association between Helicobacter pylori and gastric and duodenal mucosa. J Clin Pathol. 1994;47:699‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Poursina F, Faghri J, Moghim S, et al. Assessment of cagE and babA mRNA expression during morphological conversion of Helicobacter pylori from spiral to coccoid. Curr microbiol. 2013;66:406‐413. [DOI] [PubMed] [Google Scholar]
- 35. Hirukawa S, Sagara H, Kaneto S, et al. Characterization of morphological conversion of Helicobacter pylori under anaerobic conditions. Microbiol Immunol. 2018;62:221‐228. [DOI] [PubMed] [Google Scholar]
- 36. Cellini L, Allocati N, Angelucci D, et al. Coccoid Helicobacter pylori not culturable in vitro reverts in mice. Microbiol Immunol. 1994;38:843‐850. [DOI] [PubMed] [Google Scholar]
- 37. She FF, Lin JY, Liu JY, Huang C, Su DH. Virulence of water‐induced coccoid Helicobacter pylori and its experimental infection in mice. World J Gastroenterol. 2003;9:516‐520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Boehnke KF, Eaton KA, Fontaine C, et al. Reduced infectivity of waterborne viable but nonculturable Helicobacter pylori strain SS1 in mice. Helicobacter. 2017;22:e12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Attaran B, Falsafi T. Identification of factors associated with biofilm formation ability in the clinical isolates of Helicobacter pylori . Iran J Biotechnol. 2017;15:58‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Attaran B, Falsafi T, Moghaddam AN. Study of biofilm formation in C57Bl/6J mice by clinical isolates of Helicobacter pylori . Saudi J Gastroenterol. 2016;22:161‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cammarota G, Branca G, Ardito F, et al. Biofilm demolition and antibiotic treatment to eradicate resistant Helicobacter pylori: a clinical trial. Clin Gastroenterol Hepatol. 2010;8:817‐820.e3. [DOI] [PubMed] [Google Scholar]
- 42. Hamidian SM, Aletaha NS, Taslimi R, Montazeri M. An additive effect of oral N‐acetyl cysteine on eradication of Helicobacter pylori . J Pathog. 2015;2015:540271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Huynh HQ, Couper RT, Tran CD, Moore L, Kelso R, Butler RN. N‐acetylcysteine, a novel treatment for Helicobacter pylori infection. Dig Dis Sci. 2004;49:1853‐1861. [DOI] [PubMed] [Google Scholar]
- 44. Assumpcao MB, Martins LC, Melo Barbosa HP, et al. Helicobacter pylori in dental plaque and stomach of patients from Northern Brazil. World J Gastroenterol. 2010;16:3033‐3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ogunbodede EO, Lawal OO, Lamikanra A, Okeke IN, Rotimi O, Rasheed AA. Helicobacter pylori in the dental plaque and gastric mucosa of dyspeptic Nigerian patients. Trop Gastroenterol. 2002;23:127‐133. [PubMed] [Google Scholar]
- 46. Roman‐Roman A, Giono‐Cerezo S, Camorlinga‐Ponce M, Martinez‐Carrillo DN, Loaiza‐Loeza S, Fernandez‐Tilapa G. vacA genotypes of Helicobacter pylori in the oral cavity and stomach of patients with chronic gastritis and gastric ulcer. Enferm Infecc Microbio Clin. 2013;31:130‐135. [DOI] [PubMed] [Google Scholar]
- 47. Abadi AT, Mobarez AM, Teymournejad O, Karbalaei M. Concomitant colonization of Helicobacter pylori in dental plaque and gastric biopsy. J Pathog. 2014;2014:871601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jia CL, Jiang GS, Li CH, Li CR. Effect of dental plaque control on infection of Helicobacter pylori in gastric mucosa. J Periodontol. 2009;80:1606‐1609. [DOI] [PubMed] [Google Scholar]
- 49. Zaric S, Bojic B, Jankovic L, et al. Periodontal therapy improves gastric Helicobacter pylori eradication. J Dent Res. 2009;88:946‐950. [DOI] [PubMed] [Google Scholar]
- 50. Song HY, Li Y. Can eradication rate of gastric Helicobacter pylori be improved by killing oral Helicobacter pylori? World J Gastroenterol. 2013;19:6645‐6650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liu Y, Yue H, Li A, et al. An epidemiologic study on the correlation between oral Helicobacter pylori and gastric H. pylori . Curr Microbiol. 2009;58:449‐453. [DOI] [PubMed] [Google Scholar]
- 52. Rasmussen LT, Labio RW, Gatti LL, et al. Helicobacter pylori detection in gastric biopsies, saliva and dental plaque of Brazilian dyspeptic patients. Mem Inst Oswaldo Cruz. 2010;105:326‐330. [DOI] [PubMed] [Google Scholar]
- 53. Anand PS, Nandakumar K, Shenoy KT. Are dental plaque, poor oral hygiene, and periodontal disease associated with Helicobacter pylori infection? J Periodontol. 2006;77:692‐698. [DOI] [PubMed] [Google Scholar]


