Abstract
Dietary inorganic nitrate (nitrate) is a promising adjunctive treatment to reduce blood pressure and improve vascular function in hypertension. However, it remains unknown if the efficacy of nitrate is dependent upon an elevated blood pressure or altered by medication in patients with hypertension. Therefore, blood pressure and vascular function, measured by passive leg movement (PLM) and flow-mediated dilation (FMD), were assessed following 3 days of placebo (nitrate-free beetroot juice) and nitrate (nitrate-rich beetroot juice) administration in 13 patients (age: 53 ± 12 yr) with hypertension taking antihypertensive medications (study 1) and in 14 patients (49 ± 13 yr) with hypertension not taking antihypertensive medications (study 2). In study 1, plasma nitrite concentration was greater for nitrate than placebo (341 ± 118 vs. 308 ± 123 nmol/L, P < 0.05), yet blood pressure and vascular function were unaltered. In study 2, plasma nitrite concentration was greater for nitrate than placebo (340 ± 102 vs. 295 ± 93 nmol/L, P < 0.01). Systolic (136 ± 16 vs. 141 ± 19 mmHg), diastolic (84 ± 13 vs. 88 ± 12 mmHg), and mean (101 ± 12 vs. 106 ± 13 mmHg) blood pressures were lower (P < 0.05), whereas the PLM change in leg vascular conductance (6.0 ± 3.0 vs. 5.1 ± 2.6 mL·min−1·mmHg−1) and FMD (6.1 ± 2.4% vs. 4.1 ± 2.7%) were greater (P < 0.05) for nitrate than placebo. The changes in systolic blood pressure (r = −0.60) and FMD (r = −0.48) induced by nitrate were inversely correlated (P < 0.05) to the respective baseline values obtained in the placebo condition. Thus, the efficacy of nitrate to improve blood pressure and vascular function in hypertension appears to be dependent on the degree of blood pressure elevation and vascular dysfunction and not antihypertensive medication status, per se.
NEW & NOTEWORTHY Dietary nitrate (nitrate) is a promising intervention to improve blood pressure and vascular function in hypertension. We demonstrate that these beneficial effects of nitrate are inversely related to the baseline value in a continuous manner with no distinction between antihypertensive medication status. Thus, the efficacy of nitrate to improve blood pressure and vascular function in hypertension appears to be dependent on the degree of blood pressure elevation and vascular dysfunction and not antihypertensive mediation status.
Keywords: flow-mediated dilation, passive leg movement, vascular dysfunction
INTRODUCTION
For nearly a century, cardiovascular disease has been the leading cause of death in the United States and is currently the leading cause of death worldwide, for which hypertension is a leading risk factor (18, 27, 43a). Recently, the Systolic Blood Pressure Intervention Trial demonstrated that lowering systolic blood pressure (SBP) beyond the typical standard goal of 140 mmHg substantially lowers cardiovascular events and all-cause mortality (43a). Blood pressure, however, remains above the traditional standard goal in nearly half of all patients treated for hypertension (12). Patients with hypertension also display marked vascular dysfunction (22, 36, 39), which increases the risk for cardiac events and persists despite improvements in blood pressure status afforded by antihypertensive medications (29, 30). Thus, nonpharmaceutical therapies to improve blood pressure and vascular function are appealing for optimizing the treatment of hypertension and reducing clinical risk in these patients.
A primary culprit responsible for the vascular dysfunction and, potentially, elevated blood pressure in patients with hypertension is diminished nitric oxide (NO) bioavailability (11, 22, 36, 39). One nonpharmaceutical therapy garnering much clinical interest is dietary inorganic nitrate (nitrate) supplementation to increase NO bioavailability through the nitrate-nitrite-NO pathway (5, 42). Patients with uncontrolled hypertension (i.e., >140/90 mmHg) have an increased sensitivity to nitrate supplementation, likely because of an enhanced ability to reduce nitrite to NO (16, 25, 38). Consistent with this, nitrate supplementation improved both blood pressure and vascular function in patients with uncontrolled hypertension who were either drug naïve or suboptimally treated with antihypertensive medications (16, 25). Importantly, blood pressure and antihypertensive medications may influence the mechanisms involved in the reduction of nitrite to NO (16, 25, 35, 38). Thus, it remains to be determined if the efficacy of nitrate supplementation in patients with hypertension is reliant on an elevated blood pressure or is influenced by antihypertensive medications. This knowledge is critical for guiding the clinical use of nitrate supplementation as adjunctive treatment in hypertension.
Accordingly, the purpose of this study was to determine the influence of blood pressure and antihypertensive medication status in mediating the efficacy of nitrate supplementation in patients with hypertension. Vascular function was assessed using both passive leg movement (PLM), which is a robust NO-mediated assessment of microvascular function (8, 19, 32, 40, 41), and flow-mediated dilation (FMD), a conventional assessment of conduit artery function with important cardiovascular insight (9, 23, 29, 45). As nitrate supplementation in hypertension may mediate beneficial effects through alterations in oxidative stress (38), a comprehensive assessment of oxidative stress and antioxidant capacity was performed. We tested the hypotheses that nitrate supplementation would 1) increase plasma nitrate and nitrite concentrations, 2) lower blood pressure, and 3) improve vascular function in patients with hypertension independent of antihypertensive medication status.
METHODS
Experimental design.
The experimental protocol was approved by the Institutional Review Boards of the University of Utah and the Salt Lake City Veterans Affairs Medical Center. Written informed consent was attained after patients were informed of the experimental protocol and the potential risks of participation. Two separate double-blind, counterbalanced measures design studies were conducted to test the hypotheses. For study 1, 13 patients with diagnosed hypertension completed the experimental testing while taking their regularly prescribed antihypertensive medications. For study 2, 14 patients with diagnosed hypertension completed the experimental testing while not taking antihypertensive medications. Patients were included based on being 18–65 yr of age, having a body mass index <35, being clinically diagnosed with hypertension, having no clinically diagnosed secondary hypertension or heart failure, receiving stable antihypertensive medication therapy (>3 mo, if being treated for hypertension), and not taking ≥3 antihypertensive medications. The patients continued any nonhypertensive medications as normal. For study 2, the patients being treated for hypertension completed a 2-wk washout from antihypertensive medication with physician oversight before testing (13). Patient characteristics and antihypertensive medications are presented in Table 1. Testing was conducted at a similar time of day across visits for each patient. Patients were instructed to arrive at the laboratory having abstained from food and caffeine overnight (water allowed ad libitum) and from vigorous activity for 24 h.
Table 1.
Study 1 and study 2 patient characteristics and antihypertensive medications
| Study 1 | Study 2 | |
|---|---|---|
| Characteristics | ||
| Age, yr | 53 ± 12 | 49 ± 13 |
| Sex, men/women | 10/3 | 11/3 |
| Height, cm | 176 ± 7 | 176 ± 7 |
| Mass, kg | 80 ± 18 | 85 ± 20 |
| Body mass index, kg/m2 | 26 ± 4 | 27 ± 5 |
| Glucose, mg/dL | 83 ± 14 | 88 ± 23 |
| Sodium, mmol/L | 142 ± 2 | 142 ± 2 |
| Potassium, mmol/L | 4.0 ± 0.4 | 4.0 ± 0.4 |
| Chloride, mmol/L | 105 ± 2 | 105 ± 3 |
| Calcium, mg/dL | 9.5 ± 0.3 | 9.4 ± 0.4 |
| Creatinine, mg/dL | 0.9 ± 0.1 | 0.9 ± 0.1 |
| Total cholesterol, mg/dL | 188 ± 31 | 185 ± 36 |
| Triglycerides, mg/dL | 141 ± 64 | 125 ± 69 |
| HDL, mg/dL | 53 ± 15 | 52 ± 16 |
| LDL, mg/dL | 116 ± 30 | 117 ± 36 |
| Hemoglobin, g/dL | 15.5 ± 0.5 | 15.6 ± 0.6 |
| Hematocrit, % | 46 ± 2 | 48 ± 9 |
| Antihypertensive medications | ||
| ACEi or ARB | 10 (77) | 9 (64) |
| Beta receptor blocker | 2 (15) | 2 (14) |
| Calcium channel blockers | 2 (15) | 2 (14) |
| Diuretic | 5 (38) | 4 (29) |
| No therapy | 0 (0) | 2 (14) |
| Monotherapy | 10 (77) | 8 (57) |
| Dual therapy | 3 (23) | 4 (29) |
Data are means ± SD. Antihypertensive medications are presented as n (% of group). Patients were studied after a 2-wk washout from antihypertensive medications. ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Inorganic nitrate supplementation.
For the 2 mornings preceding and the morning of the experimental visits, patients consumed 70 mL of nitrate-rich beetroot concentrate containing 6.2 mmol inorganic nitrate (nitrate) or 70 mL of nitrate-depleted beetroot concentrate containing 0.006 mmol inorganic nitrate (placebo) (Beet It Sport, James White Drinks). The nitrate and placebo experimental visits were separated by a minimum of 7 days. Similar inorganic nitrate doses from beetroot have been utilized for patients with hypertension and have been demonstrated to induce robust increases in plasma nitrate and nitrite concentrations (25, 26, 44). Patients consumed the supplements ~1.5 h before experimental testing to allow time to achieve elevated plasma nitrate and nitrite concentrations (42). Throughout the study, patients were instructed to refrain from using antibacterial mouthwash to preserve the commensal oral bacteria requisite for the reduction of nitrate to nitrite (24). Additionally, patients were instructed to avoid nitrate-rich foods (e.g., leafy green vegetables and beets) throughout the study.
Resting blood pressure.
During each experimental visit, seated resting SBP, diastolic blood pressure (DBP), and mean arterial pressure (MAP) were measured by oscillometry (Tango M2, SunTech Medical). While in the seated position, patients were instrumented and allowed to relax, avoiding movement and conversation, for 5 min. Blood pressure was then measured in triplicate, with 1 min of recovery between measurements, and averaged to determine resting blood pressure (1).
Vascular function.
PLM was performed as previously described (8, 31, 41). Briefly, patients rested in an upright seated position for ~20 min before the start of data collection. The PLM protocol consisted of a 1-min baseline data acquisition followed by a 1-min bout of passive leg flexion and extension, performed by a member of the research team moving the lower leg through a 90° range of motion at 1 Hz (starting at a 180° angle of the knee), while the contralateral leg remained fully extended and supported. To avoid the startle reflex and active resistance to the PLM, patients were made aware that PLM would take place in ~1 min, but to minimize the chance of an anticipatory response, they were not informed of exactly when this movement would take place.
Brachial artery FMD was performed in accordance with current recommendations (21). Briefly, patients rested in the supine posture for ~20 min before the start of data collection and remained in this position throughout the FMD assessment. FMD was performed with a blood pressure cuff placed on the right arm, just distal to the elbow. Cuff inflation and deflation procedures were accomplished via a rapid inflation system (D.E. Hokanson Inc.). The FMD protocol consisted of 30 s of baseline data acquisition before inflation of the blood pressure cuff (to 250 mmHg for 5 min) and subsequent data acquisition for 2 min after cuff deflation.
Blood analyses and assays.
Venous blood samples were drawn from the antecubital region for blood assays. Plasma and serum samples were stored at −80°C until analysis. Plasma nitrate and nitrite concentrations were measured using a Sievers Nitric Oxide Analyzer (Sievers NOA 280i, Analytix Ltd). Lipid peroxidation was assessed by malondialdehyde levels (Bioxytech LPO-586). Endogenous antioxidant activity was assessed by superoxide dismutase (Cayman Chemical). High-sensitivity ELISA was used to determine plasma l-arginine (ALPCO), C-reactive protein (R&D Systems), interleukin 6 (R&D Systems), tumor necrosis factor alpha (R&D Systems), and protein carbonyl (Biocell Corp) levels.
Electron paramagnetic resonance spectroscopy and spin trapping.
Free radical levels were directly assessed by electron paramagnetic resonance (EPR) spectroscopy performed on whole blood. The EPR spectroscopy and spin trap methods have previously been described (3, 4). Briefly, 1.0 mL of venous blood was collected into a vacutainer containing 0.5 mL of the spin trap α-phenyl-tert-butylnitron (0.0140 mol/L). The α-phenyl-tert-butylnitron adduct was snap frozen in liquid nitrogen and stored at −80°C. After thawing, the α-phenyl-tert-butylnitron adduct (1,000 μL) was pipetted into a glass tube and extracted with 500 μL toluene. After centrifugation, 300 μL of the toluene/α-phenyl-tert-butylnitron extract was pipetted into a precision-bore quartz EPR sample tube (Wilmad). EPR spectroscopy was then performed at 21°C using an EMX X-band spectrometer (Bruker) and commercially available software (version 1.1b.51, Bruker Xenon System), which was also used to calculate the area under the curve of the EPR spectroscopy by double integration.
Data analysis.
Arterial blood velocity (Vmean) and vessel diameter measurements were performed in the common femoral artery and brachial artery for PLM and FMD, respectively, with Doppler ultrasound (Logiq e9, GE Medical Systems). An insonation angle of 60° and a maximal sample volume relative to the vessel were maintained throughout the protocols, and an angle-corrected, intensity-weighted area under the curve Vmean was automatically calculated using commercially available software (Logiq e9). Blood flow was calculated as: blood flow (mL/min) = Vmeanπ(diameter/2)2 × 60. During the PLM, beat-by-beat MAP was recorded by finger photoplethysmography with a Finometer (Finapres Medical Systems) using commercially available data acquisition software (AcqKnowledge, Biopac Systems). PLM leg blood flow and MAP were analyzed second-by-second and smoothed using a 3-s rolling average, which were then used to calculate leg vascular conductance (LVC) as: LVC (mL·min−1·mmHg−1) = leg blood flow/MAP. The peak change in LVC above baseline (ΔLVCpeak) was calculated as the peak LVC minus the baseline LVC. During the FMD, end-diastolic, ECG R-wave gated images were collected via video output from the Loqiq e9 for offline analysis of brachial artery diameter using automated edge-detection software (Medical Imaging Applications). FMD was quantified as the maximal percentage change in brachial artery diameter after cuff release. Shear rate was calculated as: shear rate (s−1) = 8Vmean/diameter. The reactive hyperemia was calculated as the total summed blood flow response after normalizing for baseline.
Statistical analysis.
Plasma nitrate and nitrite, blood pressure, and PLM and FMD variables were compared between nitrate and placebo using one-tailed Student’s paired tests based on a priori hypotheses. Blood assay and EPR variables were compared between nitrate and placebo using two-tailed Student’s paired t tests. The relationship between the nitrate-induced change in blood pressure, vascular function, and plasma l-arginine concentration and each variable’s respective placebo baseline value was assessed by Pearson correlation. Placebo baseline values were used in these correlations to focus on the effects of dietary inorganic nitrate and control for any additional nutritional effects of the supplements (e.g., antioxidant effect). For appropriate correlation analyses, data from the patients who completed study 1 and study 2 (n = 10) were averaged into a single data point to avoid the bias of repeated observations (6, 7). Power analyses indicated that 13 patients would achieve α = 0.05 and 1-β = 0.80 for changes in the outcome measures of 4.0 ± 5.4 mmHg (resting blood pressure), ΔLVCpeak of 1.0 ± 1.3 mL·min−1·mmHg−1 (PLM), and 1.0 ± 1.3% (FMD) (G*Power v3.1). Statistical significance was accepted at P < 0.05, and the results are presented as mean ± SD, P value, and 95% confidence interval for the difference of means (95% CI Δnitrate − placebo), unless otherwise specified.
RESULTS
Study 1
Blood analyses.
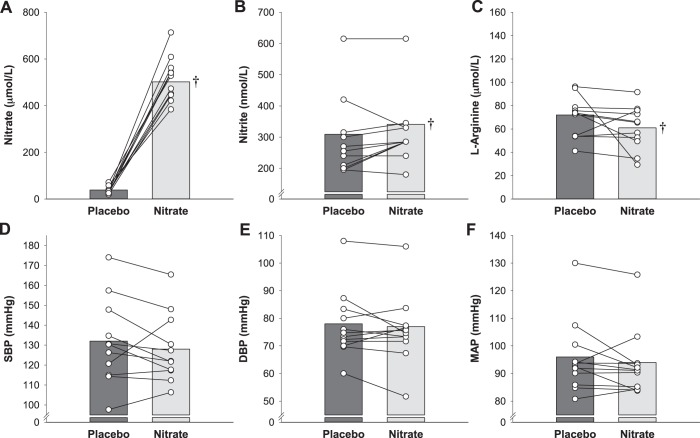
All patients complied with the intervention protocols, and the supplementations were well tolerated. Plasma nitrate, nitrite, and l-arginine concentration data are presented in Fig. 1. Both plasma nitrate (95% CI Δlower limit = 416 μmol/L, P < 0.001) and nitrite (95% CI Δlower limit = 6 nmol/L, P = 0.025) concentrations were greater, and l-arginine concentration lower (95% CI Δ = −23.2 to 1.6 µmol/L, P = 0.01), for the nitrate than placebo (Fig. 1). Plasma assay and EPR variables are presented in Table 2. Ferric-reducing ability of plasma was significantly lower for nitrate than placebo, but all other indices of oxidative stress and antioxidant capacity were unremarkable.
Fig. 1.
Effect of dietary inorganic nitrate (nitrate) supplementation on resting blood pressure and plasma nitrate, nitrite, and l-arginine concentrations in study 1. Plasma nitrate (A), nitrite (B), and l-arginine (C) concentrations and systolic blood pressure (SBP; D), diastolic blood pressure (DBP; E), and mean arterial pressure (MAP; F) for both placebo and nitrate conditions were measured in patients with hypertension taking antihypertensive medications. Individual (circles) and mean (bar) data are reported; n = 13 for all variables. †Different from placebo (P < 0.05).
Table 2.
Study 1 and study 2 blood assays and EPR spectroscopy
|
Study 1 |
Study 2 |
|||
|---|---|---|---|---|
| Placebo | Nitrate | Placebo | Nitrate | |
| cGMP, μmol/L | 3.75 ± 1.9 | 3.99 ± 2.1 | 5.15 ± 3.25 | 5.24 ± 3.08 |
| FRAP, mmol/L | 2.0 ± 0.3 | 1.8 ± 0.3† | 1.9 ± 0.3 | 1.9 ± 0.3 |
| MDA, μmol/L | 2.2 ± 0.7 | 2.2 ± 0.7 | 2.2 ± 0.8 | 2.2 ± 0.7 |
| SOD, U/mL | 1.6 ± 0.3 | 1.6 ± 0.4 | 1.7 ± 0.3 | 1.6 ± 0.3 |
| PC, nmol/mg | 0.07 ± 0.02 | 0.07 ± 0.02 | 0.07 ± 0.02 | 0.06 ± 0.02 |
| EPR, AU | 8.5 ± 7.8 | 13.0 ± 12.0 | 9.2 ± 7.3 | 8.7 ± 8.2 |
| CRP, U/mL | 2.1 ± 1.3 | 2.3 ± 1.4 | 2.4 ± 2.3 | 2.2 ± 1.1 |
| IL-6, pg/mL | 1.7 ± 0.8 | 1.4 ± 0.7 | 3.0 ± 2.7 | 1.7 ± 0.7 |
| TNF-α, pg/mL | 1.3 ± 0.3 | 1.3 ± 0.3 | 1.3 ± 0.4 | 1.2 ± 0.2 |
Data are means ± SD. Study 1: n = 13 for all variables (except FRAP, n = 12). Study 2: n = 14 for all variables. AU, arbitrary units; cGMP, cyclic guanosine monophosphate; CRP, C-reactive protein; EPR, electron paramagnetic resonance spectroscopy signal for free radicals; FRAP, ferric-reducing ability of plasma; IL-6, interleukin-6; MDA, malondialdehyde; PC, protein carbonyl; SOD, superoxide dismutase; TNF-α, tumor necrosis factor alpha.
Different from placebo within study (P < 0.05).
Blood pressure.
Blood pressure assessed before study enrollment during a routine clinical visit was 130 ± 8/76 ± 12 mmHg. SBP, DBP, and MAP collected as part of this study are presented in Table 3 and Fig. 1. There were no differences between nitrate and placebo for SBP (95% CI Δlower limit = 2.2 mmHg, P = 0.32), DBP (95% CI Δlower limit = 1.5 mmHg, P = 0.44), and MAP (95% CI Δlower limit = 1.3 mmHg, P = 0.31).
Table 3.
Study 1 and study 2 blood pressure and vascular function
|
Study 1 |
Study 2 |
|||
|---|---|---|---|---|
| Placebo | Nitrate | Placebo | Nitrate | |
| Blood pressure | ||||
| SBP, mmHg | 132 ± 20 | 129 ± 16 | 141 ± 19 | 136 ± 16† |
| DBP, mmHg | 78 ± 12 | 77 ± 12 | 88 ± 12 | 84 ± 13† |
| MAP, mmHg | 96 ± 12 | 94 ± 11 | 106 ± 13 | 101 ± 12† |
| PLM | ||||
| Baseline LVC, mL·min−1·mmHg−1 | 2.9 ± 0.9 | 3.2 ± 1.2 | 2.7 ± 1.0 | 3.1 ± 0.8 |
| ΔLVCpeak, mL·min−1·mmHg−1 | 6.0 ± 2.6 | 6.2 ± 2.5 | 5.1 ± 2.6 | 6.0 ± 3.0† |
| FMD | ||||
| Baseline diameter, mm | 4.6 ± 0.8 | 4.7 ± 0.9 | 4.7 ± 0.8 | 4.7 ± 0.9 |
| FMD, % | 3.6 ± 2.8 | 4.7 ± 2.3 | 4.1 ± 2.7 | 6.1 ± 2.4† |
| Total sum of shear, AU | 73,038 ± 63,974 | 64,810 ± 43,932 | 62,515 ± 51,417 | 69,871 ± 46,961 |
| Reactive hyperemia, mL | 605 ± 196 | 656 ± 310 | 574 ± 268 | 635 ± 239 |
Data are means ± SD. Study 1: n = 13 for all variables. Study 2: n = 14 for all variables. ΔLVCpeak, peak change in LVC above baseline; AU, arbitrary units; DBP, diastolic blood pressure; FMD, flow-mediated dilation; LVC, leg vascular conductance; MAP, mean arterial blood pressure; PLM, passive leg movement; SBP, systolic blood pressure.
Different from placebo within study (P < 0.05).
Vascular function.
Vascular function data are presented in Table 3 and Fig. 2. For the PLM assessment, baseline LVC was not different between nitrate and placebo (95% CI Δ = −0.2 to 0.9 mL·min−1·mmHg−1, P = 0.14). The PLM ΔLVCpeak was not different between nitrate and placebo (95% CI Δlower limit = −1.0 mL·min−1·mmHg−1, P = 0.76) (Fig. 2A). For the FMD assessment, baseline brachial artery diameter (95% CI Δ = −0.2 to 0.3 mm, P = 0.99), total sum of shear (95% CI Δ = −22,693 to 6,236 arbitrary units, P = 0.38), and reactive hyperemia (95% CI Δ = −76 to 179 mL, P = 0.39) were not different between nitrate and placebo. The FMD was not different between nitrate and placebo (95% CI Δlower limit = −0.6%, P = 0.27) (Fig. 2B).
Fig. 2.
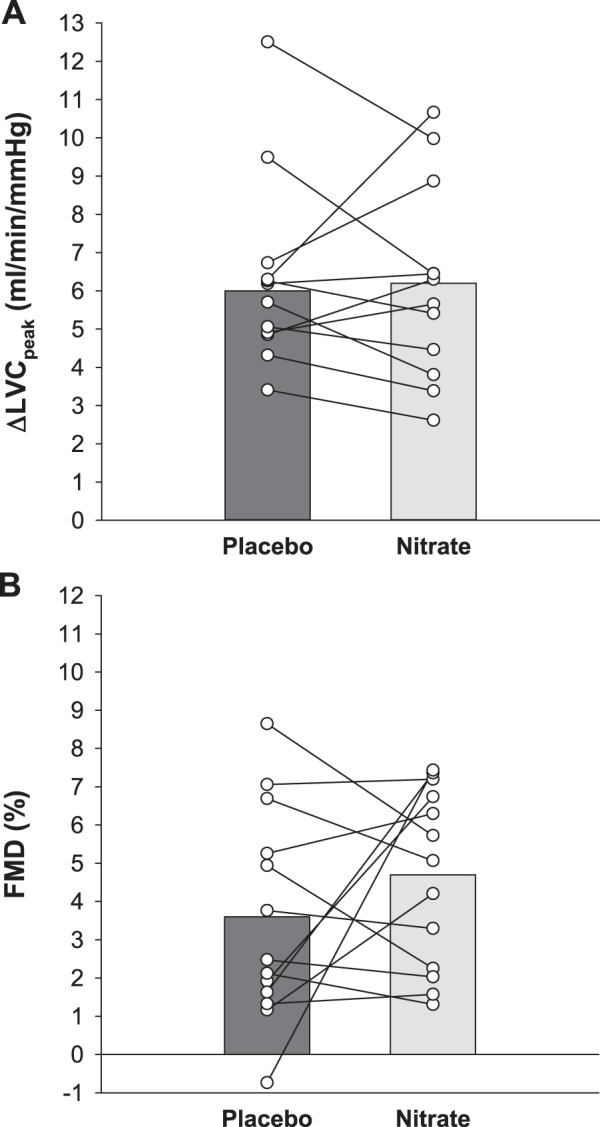
Effect of dietary inorganic nitrate (nitrate) supplementation on vascular function in study 1. Vascular function was assessed by alterations in leg vascular conductance (LVC) in response to passive leg movement (A) and by brachial artery flow-mediated dilation (FMD; B) for both placebo and nitrate conditions in patients with hypertension taking antihypertensive medications. Individual (circles) and mean (bar) data are reported; n = 13 for all variables. LVC, leg vascular conductance.
Study 2
Blood analyses.
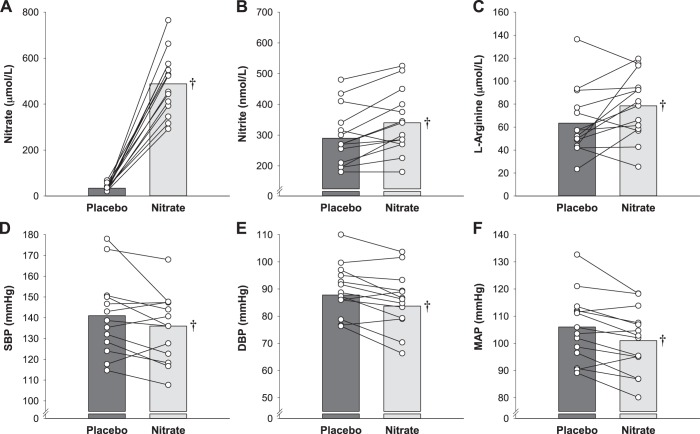
All patients complied with the intervention protocols and the supplementations were well tolerated. Plasma nitrate, nitrite, and l-arginine concentration data are presented in Fig. 3. Plasma nitrate (95% CI Δlower limit = 390 μmol/L, P < 0.001), nitrite (95% CI Δlower limit = 22 nmol/L, P < 0.001), and l-arginine (95% CI Δ = 0.5 to 26.7 µmol/L, P = 0.04) concentrations were greater for nitrate than placebo. Additional plasma assay and EPR variables are presented in Table 2. All indices of oxidative stress and antioxidant capacity were unremarkable.
Fig. 3.
Effect of dietary inorganic nitrate (nitrate) supplementation on resting blood pressure and plasma nitrate, nitrite, and l-arginine concentrations in study 2. Plasma nitrate (A), nitrite (B), and l-arginine (C) concentrations and systolic blood pressure (SBP; D), diastolic blood pressure (DBP; E), and mean arterial pressure (MAP; F) for both placebo and nitrate conditions were measured in patients with hypertension not taking antihypertensive medications. Individual (circles) and mean (bar) data are reported; n = 14 for all variables. †Different from placebo (P < 0.05).
Blood pressure.
Resting blood pressure before the antihypertensive medication washout was 126 ± 15/73 ± 13 mmHg. The blood pressure data are presented in Table 3 and Fig. 3. SBP (95% CI Δlower limit = −0.6 mmHg, P = 0.03), DBP (95% CI Δlower limit = −2.0 mmHg, P < 0.001), and MAP (95% CI Δlower limit = −2.2 mmHg, P < 0.001) were all lower following nitrate.
Vascular function.
Vascular function data are presented in Table 3 and Fig. 4. Baseline LVC was not different between nitrate and placebo for the PLM assessment (95% CI Δ = −0.2 to 0.9 mL·min−1·mmHg−1, P = 0.22). The PLM ΔLVCpeak was greater for nitrate than placebo (95% CI Δlower limit = 0.1 mL·min−1·mmHg−1, P = 0.028) (Fig. 4A). Baseline brachial artery diameter (95% CI Δ = −0.2 to 0.1 mm, P = 0.75), total sum of shear (95% CI Δ = −4,104 to 18,815 arbitrary units, P = 0.19), and reactive hyperemia (95% CI Δ = −50 to 172 mL, P = 0.26) were not different between nitrate and placebo for the FMD assessment. The FMD was greater for nitrate than placebo (95% CI Δlower limit = −1.1%, P < 0.001) (Fig. 4B).
Fig. 4.
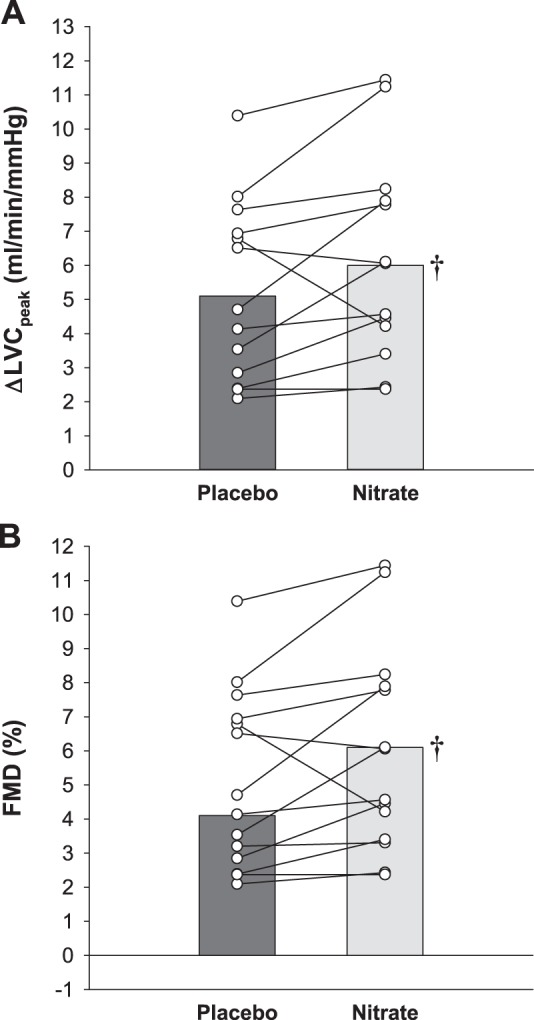
Effect of dietary inorganic nitrate (nitrate) supplementation on vascular function in study 2. Vascular function was assessed by alterations in leg vascular conductance (LVC) in response to passive leg movement (A) and by brachial artery flow-mediated dilation (FMD; B) for both placebo and nitrate conditions in patients with hypertension not taking antihypertensive medications. Individual (circles) and mean (bar) data are reported. n = 14 for all variables. †Different from placebo (P < 0.05). LVC, leg vascular conductance.
Correlation Outcomes
Across study 1 and study 2, the efficacy of nitrate to lower SBP (r = −0.60, P < 0.05), improve FMD (r = −0.48, P < 0.05), and augment l-arginine concentrations (r = −0.55, P < 0.05) was inversely correlated to the baseline value, assessed during the placebo treatment, of each variable (Fig. 5). There were no significant correlations between the nitrate-induced change and the respective baseline values for DBP, SBP, or PLM (P > 0.05).
Fig. 5.
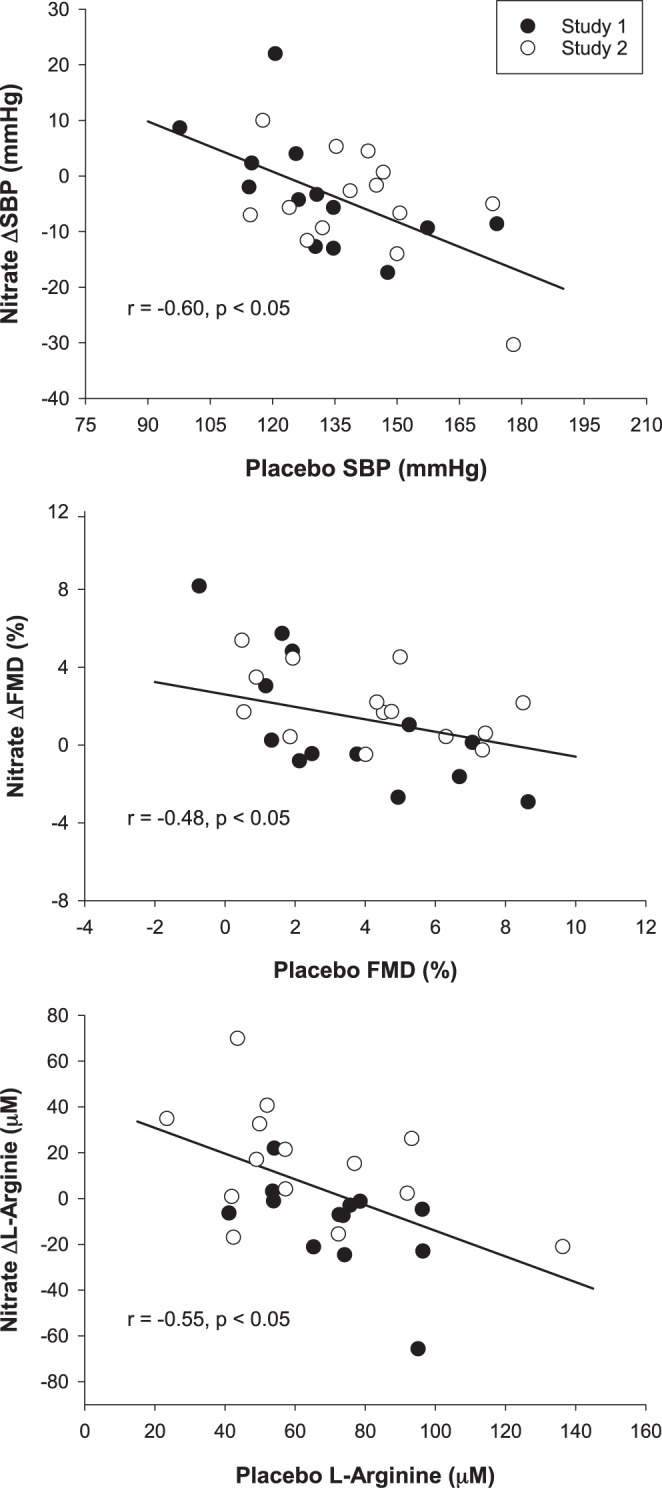
Correlations examining the determinants of the efficacy of dietary inorganic nitrate (nitrate) supplementation in patients with hypertension. The relationships between nitrate supplementation on systolic blood pressure (SBP; A), brachial artery flow-mediated dilation (FMD; B), and plasma l-arginine concentrations (C) and the initial (placebo) value for each variable. Study 1: patients with hypertension taking antihypertensive medications (n = 13). Study 2: patients with hypertension not taking antihypertensive medications (n = 14).
DISCUSSION
The purpose of this study was to determine the influence of blood pressure and antihypertensive medication status in mediating the efficacy of nitrate supplementation in patients with hypertension. Consistent with the first hypothesis, plasma nitrate and nitrite concentrations were augmented following nitrate supplementation independent of antihypertension medication status. However, in contrast to hypotheses 2 and 3, the beneficial effects of nitrate supplementation on blood pressure and vascular function were only observed in patients with hypertension not taking antihypertensive medications and not in patients with hypertension taking antihypertensive medications. Interestingly, this divergent influence of nitrate supplementation on blood pressure and vascular function was in line with alterations in plasma l-arginine concentration. Importantly, the beneficial effects of nitrate supplementation on SBP, brachial artery vascular function, and plasma l-arginine concentration were inversely related to the baseline value of each variable in a continuous manner with no clear distinction between antihypertensive medication status (Fig. 5). Thus, the efficacy of nitrate supplementation to improve blood pressure and vascular function in patients with hypertension appears to be dependent upon one’s initial degree of blood pressure elevation and vascular dysfunction and not antihypertensive mediation status, per se.
Nitrate supplementation and blood pressure in hypertension.
Hypertension is a leading risk factor for cardiovascular disease and all-cause mortality (18, 27, 43a), and lowering SBP beyond the typical standard goal of 140 mmHg substantially lowers cardiovascular events and all-cause mortality (43a). However, blood pressure remains elevated in nearly half of all patients treated for hypertension (12). The potential for nitrate supplementation to lower blood pressure makes this an attractive nonpharmaceutical adjunctive treatment for hypertension. In the current study, nitrate supplementation lowered blood pressure in the patients not taking antihypertensive medications but did not lower blood pressure in patients taking antihypertensive medications (Figs. 1 and 3). This evidence appears to support the notion that the efficacy of nitrate supplementation to lower blood pressure is limited when taking antihypertensive medications. However, upon further interrogation to determine if this finding was truly reflective of medication status, correlational analyses across studies were performed. These revealed that the effect of nitrate supplementation on SBP was inversely related to the starting SBP value in a continuous manner with no distinction between antihypertensive medication status. This evidence supports that nitrate supplementation will effectively lower SBP when a patient with hypertension presents with an elevated SBP, independent of antihypertensive medication status. Interestingly, no such relationship was evident for either DBP or MAP. The critical importance of SBP indicated by these relationships is consistent with evidence that SBP is more predictive of cardiovascular risk/events than DBP (14, 15, 43a). The current findings are consistent with previous studies that reported that nitrate supplementation lowered blood pressure in patients with hypertension who were drug naïve or taking antihypertensive medications and blood pressure was elevated (16, 25). Therefore, mounting evidence supports a clinically relevant effect of nitrate supplementation to lower blood pressure, and its associated risk, in patients with hypertension and an elevated blood pressure. Conversely, if blood pressure is well controlled, nitrate supplementation may not impart an additional reduction in hypertensive patients.
Nitrate supplementation and vascular function in hypertension.
Patients with hypertension display marked vascular dysfunction (22, 36, 39), that increases the risk for cardiac events and persists independent of improvements in blood pressure status afforded by antihypertensive medications (29, 30). Thus, in addition to lowering blood pressure as previously discussed, nitrate supplementation is also an attractive therapy to potentially improve vascular function in this patient cohort. In the current study, nitrate supplementation improved brachial artery vascular function in the patients with hypertension not taking antihypertensive medications but not in the patients with hypertension taking antihypertensive medications (Figs. 2 and 4). As with blood pressure, this evidence appears to support that the efficacy of nitrate supplementation to improve vascular function is limited when taking antihypertensive medications. However, as with SBP, the efficacy of nitrate supplementation on brachial artery vascular function was inversely related to the baseline function. The continuous relationship with no distinction between antihypertensive medication status supports that nitrate supplementation will improve brachial artery vascular function when a patient with hypertension presents with low brachial artery vascular function, independent of antihypertensive medication status. The findings of the current study are consistent with the nitrate-induced improvement in brachial artery vascular function observed in medically treated patients with hypertension presenting with an elevated blood pressure (6, 12, 18) but not in the current patients taking antihypertensive medications. Importantly, the greater PLM response supports an enhanced NO bioavailability and improved microvascular function with nitrate supplementation (8, 19, 32, 40, 41). Interestingly, no relationship was evident when comparing the placebo and nitrate PLM response across studies. The observed relationship differences for FMD and PLM may reflect distinct mechanistic effects of hypertension on arm versus leg and/or differences in conduit versus microvascular function (17, 33, 34, 43). The augmented FMD supports an improved conduit artery function with clinically relevant improvements in cardiovascular disease outcomes (9, 23, 29, 45). The findings of the current study support a clinically relevant effect of nitrate supplementation to improve vascular function and its associated risk in patients with hypertension and low vascular function.
Potential mechanisms of nitrate supplementation in hypertension.
In hypertension, the ability to reduce nitrite to NO is enhanced because of increased nitrite reductase activity, such as xanthine oxidoreductase (16, 25, 38). This results in patients with hypertension presenting with an elevated blood pressure having an increased sensitivity and therapeutic benefit from nitrate supplementation (16, 25, 38). This is consistent with the current findings of a therapeutic benefit from nitrate supplementation in the patients with hypertension presenting with an elevated blood pressure. The efficacy of nitrate supplementation to lower blood pressure and improve vascular function being dependent on basal blood pressure status may indicate that nitrite reductase activity in hypertension is influenced by blood pressure. Interestingly, nitrate supplementation altered plasma l-arginine concentrations, again in a manner dependent on the starting value. The alterations in plasma l-arginine concentration may result from alterations in arginase activity. In patients with hypertension, arginase activity is increased (28, 37), which would concomitantly decrease plasma l-arginine concentrations and NO bioavailability. Both antihypertensive medications and nitrate supplementation have been demonstrated to suppress arginase activity (2, 28, 37). Consistent with this, plasma l-arginine concentrations were only increased following nitrate supplementation in the patients with elevated blood pressure not taking antihypertensive medications. Nitrate supplementation has previously been demonstrated to increase plasma cGMP concentrations (25), but this did not occur in the current study. An alternative mechanistic explanation for the effects of nitrate supplementation may also be mediated through a decrease in oxidative stress, independent from alterations in blood pressure (38). Although there were no consistent alterations in our comprehensive assessment of oxidative stress or antioxidant capacity in the current study, our measures were systemic in nature. It is possible that the antioxidant effects may be occurring at the tissue or cellular level (38).
Experimental considerations.
Both acute and chronic nitrate supplementations have been used for studies in patients with hypertension (16, 25). Beneficial effects of nitrate supplementation on blood pressure and vascular function were demonstrated with chronic supplementation in patients with hypertension taking antihypertensive medications (25); however, in the current study, acute supplementation over 3 days had no effect on the current patients taking antihypertensive medications. Although we cannot rule out that these differences may be due to differences in acute versus chronic supplementation protocols, a recent study demonstrated that the effects of nitrate supplementation on blood pressure and vascular function may remain acute in nature (10). Indeed, chronic supplementation (7 days) did not impute any additional benefit in blood pressure or vascular function beyond a single acute dose in healthy participants (10). Thus, the differences in supplementation duration likely do not hinder these complimentary nitrate supplementation studies in hypertension. It should be appreciated that the current studies contained relatively small sample sizes and patients who contributed data to both studies. However, the counterbalanced measures design and appropriate statistical management of repeated measures across the two studies reduced measurement variability and bias. The potential lasting effects of the antihypertensive medications following the 2-wk washout cannot be ruled out. However, based on the half-life values for antihypertensive medications, it is likely that these were no longer effective following the washout period. It is recognized that the plasma nitrite samples may have been collected too early (i.e., 1.5 h after consumption) and, therefore, may not reflect peak concentrations. Importantly, plasma nitrite concentrations were elevated at this time and were likely maximized during the subsequent blood pressure and vascular function measurements. Although arginase activity was not directly measured in the current study, the alterations in plasma l-arginine concentrations with nitrate supplementation and medication are consistent with the previously demonstrated effects on arginase activity. Specifically, arginase activity would be expected to be high in patients with hypertension not taking antihypertensive medications (28, 37). Therefore, nitrate supplementation would be expected to l-arginine concentrations through the suppression of arginase activity (2). In contrast, arginase activity would be expected to be suppressed by the antihypertensive medications in patients with controlled hypertension (28, 37).
Perspectives.
The importance of adjunctive treatments in hypertension is highlighted by recent evidence that lowering SBP beyond the typical standard goal of 140 mmHg lowers cardiovascular events and all-cause mortality (43a) and the high prevalence of isolated SBP (14, 15, 43a). However, blood pressure remains elevated in nearly half of all patients treated for hypertension (12). The persistent vascular dysfunction present in hypertension, even with adequate antihypertensive therapy (29, 30), further increases the risk for cardiac events (22, 29, 30, 36, 39). Thus, mounting evidence supports a high clinical utility of nitrate supplementation as an adjunctive treatment for hypertension, particularly with the demonstration that the greatest effects in lowering SBP and improving brachial artery vascular function occur in those patients with the worst values, independent of antihypertensive medication status. The current findings offer a significant step toward identifying which patients with hypertension may consistently benefit from nitrate therapy. Additional large-scale studies over a longer period of supplementation are needed to robustly delineate the identified clinical indicators of the efficacy of nitrate and their potential integration to best utilize nitrate as an adjunctive treatment in patients with hypertension.
Conclusion.
The beneficial effects of nitrate supplementation on blood pressure and vascular function were only present in patients with hypertension not taking antihypertensive medication and not in patients with hypertension taking antihypertensive medications. This occurred despite greater plasma nitrate and nitrate concentrations in both cases. The divergent influence of nitrate supplementation on blood pressure and vascular function was in line with alterations in plasma l-arginine concentration. Importantly, the beneficial effects of nitrate supplementation on blood pressure, vascular function, and plasma l-arginine concentrations were inversely related to the initial value of each variable in a continuous manner with no clear distinction between antihypertensive medication status. Thus, the current findings demonstrate that the efficacy of nitrate supplementation to improve blood pressure and vascular function in patients with hypertension appears to be dependent on the degree of blood pressure elevation and vascular dysfunction and not antihypertensive mediation status, per se.
GRANTS
This study was supported by Veterans Affairs Rehabilitation Research and Development Career Development (IK2RX001215), Merit (E6910-R and E1697-R), Spire (E1433-P), Senior Research Career Scientist (E9275-L) awards, American Heart Association (14S-DG-18850039) and National Heart, Lung, and Blood Institute Grant HL-091830, and the Ruth L. Kirschstein National Research Service Award (1T32HL139451).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.M.B. and J.D.T. conceived and designed research; R.M.B., D.T.L.S., J.Z., V.R.R., R.S.R., and J.D.T. performed experiments; R.M.B., D.T.L.S., R.S.R., and J.D.T. analyzed data; R.M.B., D.T.L.S., R.S.R., and J.D.T. interpreted results of experiments; R.M.B., R.S.R., and J.D.T. prepared figures; R.M.B., R.S.R., and J.D.T. drafted manuscript; R.M.B., D.T.L.S., J.Z., V.R.R., R.S.R., and J.D.T. edited and revised manuscript; R.M.B., D.T.L.S., J.Z., R.S.R., and J.D.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank all of the participants for time and effort expended to complete this study.
REFERENCES
- 1.Ambrosius WT, Sink KM, Foy CG, Berlowitz DR, Cheung AK, Cushman WC, Fine LJ, Goff DC Jr, Johnson KC, Killeen AA, Lewis CE, Oparil S, Reboussin DM, Rocco MV, Snyder JK, Williamson JD, Wright JT Jr, Whelton PK; SPRINT Study Research Group . The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials 11: 532–546, 2014. doi: 10.1177/1740774514537404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashmore T, Fernandez BO, Branco-Price C, West JA, Cowburn AS, Heather LC, Griffin JL, Johnson RS, Feelisch M, Murray AJ. Dietary nitrate increases arginine availability and protects mitochondrial complex I and energetics in the hypoxic rat heart. J Physiol 592: 4715–4731, 2014. doi: 10.1113/jphysiol.2014.275263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailey DM, Davies B, Young IS, Jackson MJ, Davison GW, Isaacson R, Richardson RS. EPR spectroscopic detection of free radical outflow from an isolated muscle bed in exercising humans. J Appl Physiol (1985) 94: 1714–1718, 2003. doi: 10.1152/japplphysiol.01024.2002. [DOI] [PubMed] [Google Scholar]
- 4.Bailey DM, Young IS, McEneny J, Lawrenson L, Kim J, Barden J, Richardson RS. Regulation of free radical outflow from an isolated muscle bed in exercising humans. Am J Physiol Heart Circ Physiol 287: H1689–H1699, 2004. doi: 10.1152/ajpheart.00148.2004. [DOI] [PubMed] [Google Scholar]
- 5.Bailey SJ, Winyard P, Vanhatalo A, Blackwell JR, Dimenna FJ, Wilkerson DP, Tarr J, Benjamin N, Jones AM. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J Appl Physiol (1985) 107: 1144–1155, 2009. doi: 10.1152/japplphysiol.00722.2009. [DOI] [PubMed] [Google Scholar]
- 6.Bland JM, Altman DG. Calculating correlation coefficients with repeated observations: Part 1–correlation within subjects. BMJ 310: 446, 1995. doi: 10.1136/bmj.310.6977.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bland JM, Altman DG. Calculating correlation coefficients with repeated observations: Part 2–Correlation between subjects. BMJ 310: 633, 1995. doi: 10.1136/bmj.310.6980.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broxterman RM, Trinity JD, Gifford JR, Kwon OS, Kithas AC, Hydren JR, Nelson AD, Morgan DE, Jessop JE, Bledsoe AD, Richardson RS. Single passive leg movement assessment of vascular function: contribution of nitric oxide. J Appl Physiol (1985) 123: 1468–1476, 2017. doi: 10.1152/japplphysiol.00533.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broxterman RM, Witman MA, Trinity JD, Groot HJ, Rossman MJ, Park SY, Malenfant S, Gifford JR, Kwon OS, Park SH, Jarrett CL, Shields KL, Hydren JR, Bisconti AV, Owan T, Abraham A, Tandar A, Lui CY, Smith BR, Richardson RS. Strong relationship between vascular function in the coronary and brachial arteries. Hypertension 74: 208–215, 2019. doi: 10.1161/HYPERTENSIONAHA.119.12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burleigh M, Liddle L, Muggeridge DJ, Monaghan C, Sculthorpe N, Butcher J, Henriquez F, Easton C. Dietary nitrate supplementation alters the oral microbiome but does not improve the vascular responses to an acute nitrate dose. Nitric Oxide 89: 54–63, 2019. doi: 10.1016/j.niox.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 11.Dominiczak AF, Bohr DF. Nitric oxide and its putative role in hypertension. Hypertension 25: 1202–1211, 1995. doi: 10.1161/01.HYP.25.6.1202. [DOI] [PubMed] [Google Scholar]
- 12.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. JAMA 303: 2043–2050, 2010. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- 13.Ferdinand KC, Balavoine F, Besse B, Black HR, Desbrandes S, Dittrich HC, Nesbitt SD. Efficacy and safety of firibastat, a first-in-class brain aminopeptidase A inhibitor, in hypertensive overweight patients of multiple ethnic origins. Circulation 140: 138–146, 2019. doi: 10.1161/CIRCULATIONAHA.119.040070. [DOI] [PubMed] [Google Scholar]
- 14.Franklin SS. Cardiovascular risks related to increased diastolic, systolic and pulse pressure. An epidemiologist’s point of view. Pathol Biol (Paris) 47: 594–603, 1999. [PubMed] [Google Scholar]
- 15.Franklin SS, Jacobs MJ, Wong ND, L’Italien GJ, Lapuerta P. Predominance of isolated systolic hypertension among middle-aged and elderly US hypertensives: analysis based on National Health and Nutrition Examination Survey (NHANES) III. Hypertension 37: 869–874, 2001. doi: 10.1161/01.HYP.37.3.869. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh SM, Kapil V, Fuentes-Calvo I, Bubb KJ, Pearl V, Milsom AB, Khambata R, Maleki-Toyserkani S, Yousuf M, Benjamin N, Webb AJ, Caulfield MJ, Hobbs AJ, Ahluwalia A. Enhanced vasodilator activity of nitrite in hypertension: critical role for erythrocytic xanthine oxidoreductase and translational potential. Hypertension 61: 1091–1102, 2013. doi: 10.1161/HYPERTENSIONAHA.111.00933. [DOI] [PubMed] [Google Scholar]
- 17.Gifford JR, Richardson RS. CORP: ultrasound assessment of vascular function with the passive leg movement technique. J Appl Physiol (1985) 123: 1708–1720, 2017. doi: 10.1152/japplphysiol.00557.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenlund KJ, Giles WH, Keenan NL, Malarcher AM, Zheng ZJ, Croft JB. Heart diesease and stroke mortality in the twentieth century. Silent Victories: The History and Practice of Public Health in Twentieth Century America, edited by Ward JW, Warren C. Oxford, England: Oxford University Press, 2006, p. 381–400. [Google Scholar]
- 19.Groot HJ, Trinity JD, Layec G, Rossman MJ, Ives SJ, Morgan DE, Bledsoe A, Richardson RS. The role of nitric oxide in passive leg movement-induced vasodilatation with age: insight from alterations in femoral perfusion pressure. J Physiol 593: 3917–3928, 2015. doi: 10.1113/JP270195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension 55: 1075–1085, 2010. doi: 10.1161/HYPERTENSIONAHA.110.150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higashi Y, Sasaki S, Kurisu S, Yoshimizu A, Sasaki N, Matsuura H, Kajiyama G, Oshima T. Regular aerobic exercise augments endothelium-dependent vascular relaxation in normotensive as well as hypertensive subjects: role of endothelium-derived nitric oxide. Circulation 100: 1194–1202, 1999. doi: 10.1161/01.CIR.100.11.1194. [DOI] [PubMed] [Google Scholar]
- 23.Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging 26: 631–640, 2010. doi: 10.1007/s10554-010-9616-1. [DOI] [PubMed] [Google Scholar]
- 24.Kapil V, Haydar SM, Pearl V, Lundberg JO, Weitzberg E, Ahluwalia A. Physiological role for nitrate-reducing oral bacteria in blood pressure control. Free Radic Biol Med 55: 93–100, 2013. doi: 10.1016/j.freeradbiomed.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kapil V, Khambata RS, Robertson A, Caulfield MJ, Ahluwalia A. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: a randomized, phase 2, double-blind, placebo-controlled study. Hypertension 65: 320–327, 2015. doi: 10.1161/HYPERTENSIONAHA.114.04675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kapil V, Milsom AB, Okorie M, Maleki-Toyserkani S, Akram F, Rehman F, Arghandawi S, Pearl V, Benjamin N, Loukogeorgakis S, Macallister R, Hobbs AJ, Webb AJ, Ahluwalia A. Inorganic nitrate supplementation lowers blood pressure in humans: role for nitrite-derived NO. Hypertension 56: 274–281, 2010. [Erratum in Hypertension 56: e37–e39, 2010.] doi: 10.1161/HYPERTENSIONAHA.110.153536. [DOI] [PubMed] [Google Scholar]
- 27.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet 365: 217–223, 2005. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 28.Kosenko E, Tikhonova L, Suslikov A, Kaminsky Y. Impacts of lisinopril and lisinopril plus simvastatin on erythrocyte and plasma arginase, nitrite, and nitrate in hypertensive patients. J Clin Pharmacol 52: 102–109, 2012. doi: 10.1177/0091270010388647. [DOI] [PubMed] [Google Scholar]
- 29.Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation 111: 363–368, 2005. doi: 10.1161/01.CIR.0000153339.27064.14. [DOI] [PubMed] [Google Scholar]
- 30.Maruhashi T, Soga J, Fujimura N, Idei N, Mikami S, Iwamoto Y, Iwamoto A, Kajikawa M, Matsumoto T, Oda N, Kishimoto S, Matsui S, Hashimoto H, Aibara Y, Yusoff FBM, Hidaka T, Kihara Y, Chayama K, Noma K, Nakashima A, Goto C, Tomiyama H, Takase B, Kohro T, Suzuki T, Ishizu T, Ueda S, Yamazaki T, Furumoto T, Kario K, Inoue T, Koba S, Watanabe K, Takemoto Y, Hano T, Sata M, Ishibashi Y, Node K, Maemura K, Ohya Y, Furukawa T, Ito H, Ikeda H, Yamashina A, Higashi Y. Endothelial function is impaired in patients receiving antihypertensive drug treatment regardless of blood pressure level: FMD-J Study (Flow-Mediated Dilation Japan). Hypertension 70: 790–797, 2017. doi: 10.1161/HYPERTENSIONAHA.117.09612. [DOI] [PubMed] [Google Scholar]
- 31.McDaniel J, Hayman MA, Ives S, Fjeldstad AS, Trinity JD, Wray DW, Richardson RS. Attenuated exercise induced hyperaemia with age: mechanistic insight from passive limb movement. J Physiol 588: 4507–4517, 2010. doi: 10.1113/jphysiol.2010.198770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mortensen SP, Askew CD, Walker M, Nyberg M, Hellsten Y. The hyperaemic response to passive leg movement is dependent on nitric oxide: a new tool to evaluate endothelial nitric oxide function. J Physiol 590: 4391–4400, 2012. doi: 10.1113/jphysiol.2012.235952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishiyama SK, Wray DW, Richardson RS. Aging affects vascular structure and function in a limb-specific manner. J Appl Physiol (1985) 105: 1661–1670, 2008. doi: 10.1152/japplphysiol.90612.2008. [DOI] [PubMed] [Google Scholar]
- 34.Nishiyama SK, Wray DW, Richardson RS. Sex and limb-specific ischemic reperfusion and vascular reactivity. Am J Physiol Heart Circ Physiol 295: H1100–H1108, 2008. doi: 10.1152/ajpheart.00318.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohtahara A, Hisatome I, Yamamoto Y, Furuse M, Sonoyama K, Furuse Y, Hamada T, Katoh M, Watanabe M, Kinugawa T, Ogino K, Igawa O, Shimomura T, Murakami F, Yamamoto T, Shigemasa C. The release of the substrate for xanthine oxidase in hypertensive patients was suppressed by angiotensin converting enzyme inhibitors and alpha1-blockers. J Hypertens 19, Suppl: 575–582, 2001. doi: 10.1097/00004872-200103001-00009. [DOI] [PubMed] [Google Scholar]
- 36.Panza JA, Quyyumi AA, Brush JE Jr, Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med 323: 22–27, 1990. doi: 10.1056/NEJM199007053230105. [DOI] [PubMed] [Google Scholar]
- 37.Pernow J, Jung C. Arginase as a potential target in the treatment of cardiovascular disease: reversal of arginine steal? Cardiovasc Res 98: 334–343, 2013. doi: 10.1093/cvr/cvt036. [DOI] [PubMed] [Google Scholar]
- 38.Rizzi E, Amaral JH, Guimarães DA, Conde-Tella SO, Pinheiro LC, Gerlach RF, Castro MM, Tanus-Santos JE. Nitrite treatment downregulates vascular MMP-2 activity and inhibits vascular remodeling in hypertension independently of its antihypertensive effects. Free Radic Biol Med 130: 234–243, 2019. doi: 10.1016/j.freeradbiomed.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 39.Tang EH, Vanhoutte PM. Endothelial dysfunction: a strategic target in the treatment of hypertension? Pflugers Arch 459: 995–1004, 2010. doi: 10.1007/s00424-010-0786-4. [DOI] [PubMed] [Google Scholar]
- 40.Trinity JD, Groot HJ, Layec G, Rossman MJ, Ives SJ, Morgan DE, Gmelch BS, Bledsoe A, Richardson RS. Passive leg movement and nitric oxide-mediated vascular function: the impact of age. Am J Physiol Heart Circ Physiol 308: H672–H679, 2015. doi: 10.1152/ajpheart.00806.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trinity JD, Groot HJ, Layec G, Rossman MJ, Ives SJ, Runnels S, Gmelch B, Bledsoe A, Richardson RS. Nitric oxide and passive limb movement: a new approach to assess vascular function. J Physiol 590: 1413–1425, 2012. doi: 10.1113/jphysiol.2011.224741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Webb AJ, Patel N, Loukogeorgakis S, Okorie M, Aboud Z, Misra S, Rashid R, Miall P, Deanfield J, Benjamin N, MacAllister R, Hobbs AJ, Ahluwalia A. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension 51: 784–790, 2008. doi: 10.1161/HYPERTENSIONAHA.107.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wray DW, Uberoi A, Lawrenson L, Richardson RS. Heterogeneous limb vascular responsiveness to shear stimuli during dynamic exercise in humans. J Appl Physiol (1985) 99: 81–86, 2005. doi: 10.1152/japplphysiol.01285.2004. [DOI] [PubMed] [Google Scholar]
- 43a.Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC Jr, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT; SPRINT Research Group . A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 373: 2103–2116, 2015. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wylie LJ, Kelly J, Bailey SJ, Blackwell JR, Skiba PF, Winyard PG, Jeukendrup AE, Vanhatalo A, Jones AM. Beetroot juice and exercise: pharmacodynamic and dose-response relationships. J Appl Physiol (1985) 115: 325–336, 2013. doi: 10.1152/japplphysiol.00372.2013. [DOI] [PubMed] [Google Scholar]
- 45.Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation 120: 502–509, 2009. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]