Abstract
Age-related skeletal muscle atrophy is a very common and serious condition that remains poorly understood at the molecular level. Several lines of evidence have suggested that the tumor suppressor p53 may play a central, causative role in skeletal muscle aging, whereas other, apparently contradictory lines of evidence have suggested that p53 may be critical for normal skeletal muscle function. To help address these issues, we performed an aging study in male muscle-specific p53-knockout mice (p53 mKO mice), which have a lifelong absence of p53 expression in skeletal muscle fibers. We found that the absence of p53 expression in skeletal muscle fibers had no apparent deleterious or beneficial effects on skeletal muscle mass or function under basal conditions up to 6 mo of age, when mice are fully grown and exhibit peak muscle mass and function. Furthermore, at 22 and 25 mo of age, when age-related muscle weakness and atrophy are clearly evident in mice, p53 mKO mice demonstrated no improvement or worsening of skeletal muscle mass or function relative to littermate control mice. At advanced ages, p53 mKO mice began to die prematurely and had an increased incidence of osteosarcoma, precluding analyses of muscle mass and function in very old p53 mKO mice. In light of these results, we conclude that p53 expression in skeletal muscle fibers has minimal if any direct, cell autonomous effect on basal or age-related changes in skeletal muscle mass and function up to at least 22 mo of age.
NEW & NOTEWORTHY Previous studies implicated the transcriptional regulator p53 as a potential mediator of age-related skeletal muscle weakness and atrophy. We tested this hypothesis by investigating the effect of aging in muscle-specific p53-knockout mice. Our results strongly suggest that p53 activity within skeletal muscle fibers is not required for age-related skeletal muscle atrophy or weakness.
Keywords: aging, muscle atrophy, p53, sarcopenia, skeletal muscle
INTRODUCTION
Age-related skeletal muscle atrophy (also known as sarcopenia) significantly impairs the functional capacity, quality of life, and overall health of many older people. However, the molecular mechanisms of age-related skeletal muscle atrophy remain largely unknown, obscured by a lack of reliable nonmammalian model systems, the experimental difficulty of studying this slowly progressive condition in mammals, and the existence of thousands of age-associated molecular changes in skeletal muscle, many of which are likely epiphenomena. To date, we know of only one example of a protein that is required for the loss of skeletal muscle mass, quality, and strength during aging in mammals: the transcriptional regulator activating transcription factor 4 (ATF4) (9, 10). Conditional knockout mice that lack ATF4 in skeletal muscle fibers maintain peak muscle mass and strength into old age (10), indicating that ATF4-dependent signaling pathways within skeletal muscle fibers are critical for the development of age-related skeletal muscle atrophy and weakness. A major current challenge is to identify additional proteins that, like ATF4, play causal roles in age-related skeletal muscle atrophy and weakness.
The transcriptional regulator p53 was discovered in 1979, has been a subject of over 90,000 research articles, and has been suggested as a potential mediator of age-related skeletal muscle atrophy for many years. p53 is thought to be an important driver of age-related changes in most if not all cell types and tissues (20, 21), and transgenic mice with a lifelong increase of p53 activity in all cell types prematurely develop a wide range of aging-associated phenotypes, including skeletal muscle atrophy (22). Moreover, aging is associated with increased expression of p53 and canonical p53 target genes in skeletal muscle (13, 24, 25). Furthermore, in young adult mice p53 expression in skeletal muscle fibers induces skeletal muscle atrophy and is necessary for at least one acute form of skeletal muscle atrophy (immobilization-induced skeletal muscle atrophy) (14). Additionally, p53 and ATF4 have at least some similar functional effects within skeletal muscle fibers, as indicated by the finding that p53 and ATF4 mediate distinct and additive pathways to skeletal muscle atrophy during limb immobilization in young adult mice (14). These considerations suggest p53 as a candidate mediator of age-related skeletal muscle atrophy.
On the other hand, there is evidence that p53 has beneficial functions within skeletal muscle. For example, in skeletal muscle fibers p53 helps to maintain mitochondrial content and function (2), suggesting that p53 may actually be essential for normal muscle function. And, of course, p53 is immensely beneficial as a tumor suppressor, inasmuch as human patients and mouse models with loss-of-function mutations in the p53 gene have a dramatically increased incidence of cancer (6). Thus, to better understand the role of p53 in skeletal muscle aging, we investigated effects of aging in conditional knockout mice that constitutively lack p53 expression in skeletal muscle fibers.
MATERIALS AND METHODS
Mice and mouse protocols.
This study utilized two strains of mice, muscle-specific p53-knockout mice (p53 mKO mice) and their control littermates, that we described previously (14) and describe in greater detail in results. All mice in this study were males, littermates, and on a C57BL/6 background. Female mice were not studied because of cost considerations and economic limitations. Mice were housed (up to 5 mice per cage) in ventilated cages (Thoren Rack system, no. 9 size cages) at 21°C with 12:12-h light-dark cycles and ad libitum access to standard chow (Harlan-Teklad formula 7913) and water (filtered automatic watering system). The colony was confirmed to be specific pathogen free via routine biannual testing of sentinel mice for a wide range of pathogens including mouse hepatitis virus, Parvovirus (minute virus of mice, mouse parvovirus), Theiler’s murine encephalomyelitis virus, mouse rotovirus (EDIM), Sendai, Mycoplasma pulmonis, murine norovirus, pneumonia virus of mice, Reo3, Ectromelia, mouse adenovirus 1 and 2, lymphocytic choriomeningitis virus, pinworms, fur mites, ectoparasites, and endoparasites. The mouse housing room contained other strains of mice of both sexes. Forelimb grip strength was determined with a grip strength meter equipped with a triangular pull bar (Columbus Instruments), as described previously (8, 10, 16, 17). Mouse exercise capacity was determined as described previously (8): for 2 days, mice were acclimated to running on a motor-driven open treadmill with a shock grid (Columbus Instruments) for 5 min/day. During acclimation, the treadmill speed was set at 5 m/min and the treadmill incline was set at 0%. On the third day, exercise tolerance was tested, the shock grid was set at 0.2 mA, and the treadmill incline was set at 10%. For the first 5 min of testing, treadmill speed was set at 5 m/min. Every 2 min thereafter, the treadmill speed was increased by 2 m/min. Running was terminated when mice contacted the shock grid for 10 s. Body composition measurements were obtained with a Bruker Minispec LF 90II, as described previously (8). Cageside observations of all mice were made daily throughout the study. Aggressive male mice were separated from their cagemates and housed individually; there was no difference in aggression between the strains. In accordance with University of Iowa Office of Animal Research policy on humane end points, mice with tumors exceeding 2 cm in diameter were euthanized; all other mice were euthanized at the predetermined end points of the studies. Euthanasia was performed by subjecting animals to CO2 exposure (flow rate of 3 L/min) until breathing stopped for a period of 1 min, and euthanasia was confirmed by decapitation. Euthanasia methods were approved by the Panel on Euthanasia of the American Veterinary Medical Association. Blood glucose levels were obtained with an Accucheck Aviva glucose meter immediately after euthanasia, with blood samples gathered at the decapitation site. All animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Iowa.
Histological analysis of mouse skeletal muscle.
Hematoxylin and eosin (H&E) stains were performed by embedding and freezing skeletal muscles in tissue freezing medium (General Data Healthcare) and then preparing 10-μm sections from the midbelly of the muscle with a Microm HM 505E cryostat. For H&E stains, muscle sections were fixed in ice-cold zinc formalin for 15 min before staining with a DRS-601 automatic slide stainer (Sakura). For laminin staining, cryosections were blocked for 1 h at 25°C in 5% normal goat serum (NGS) and then incubated for 2 h at 25°C in 5% NGS containing a 1:2,000 dilution of anti-laminin (Sigma L9393). Sections were then rinsed with PBS, incubated for 1 h at 25°C in 5% NGS containing a 1:2,000 dilution of Alexa Fluor 568-conjugated anti-rabbit IgG, and then mounted in Vectashield (Vector Laboratories). Muscle sections were examined and photographed with a Nikon Eclipse Ti automated inverted microscope equipped with NIS-Elements BR digital imaging software. Image analysis was performed with ImageJ and MyoVision software (26), and muscle fiber diameter was determined with the lesser diameter (minimal Feret diameter) method, as recommended elsewhere (7).
Immunoblot analysis.
Skeletal muscles were snap-frozen in liquid nitrogen and homogenized in ice-cold homogenization buffer [50 mM HEPES, 4 mM EGTA, 10 mM EDTA, 15 mM sodium pyrophosphate, 100 mM β-glycerophosphate, Complete Mini protease inhibitor mixture (Roche), 25 mM sodium fluoride, 1% (vol/vol) Triton X-100, and PhosSTOP (Roche)] with a Precellys 24 (Bertin Technologies) homogenizer at 6,000 rpm for 30 s per cycle for 3 cycles. The muscle homogenate was rotated for 1 h at 4°C and then centrifuged at 16,000 g for 20 min at 4°C. An aliquot of the supernatant was used to determine protein concentration by the bicinchoninic acid method (Pierce), and another aliquot was mixed with 0.25 vol of sample buffer [250 mM Tris·HCl, pH 6.8, 10% SDS, 25% glycerol, 0.2% (wt/vol) bromophenol blue, and 5% (wt/vol) 2-mercaptoethanol] and heated at 95°C for 5 min. An equal amount of protein from each sample was subjected to SDS-PAGE and then transferred to 0.45-μm nitrocellulose membranes (Bio-Rad). Immunoblots were performed at 4°C for 16 h with a 1:500 dilution of rabbit monoclonal anti-p53 antibody (no. 32532; Cell Signaling Technologies). Bound antibodies were visualized by chemiluminescence (SuperSignal West Pico; Thermo Scientific) with horseradish peroxidase-conjugated anti-rabbit IgG (no. 7074; Cell Signaling Technologies). Membranes were stained with Ponceau S to confirm equal loading.
Quantitative real-time RT-PCR.
Extraction of skeletal muscle RNA was performed with TRIzol solution (Invitrogen) and purified with a TURBO DNA-free kit (Ambion) as described previously (12). Quantitative real-time RT-PCR (qPCR) was performed as previously described (12) with a High Capacity cDNA reverse transcription kit (Applied Biosystems). qPCR studies were performed with a 7500 Fast Real-time PCR System (Applied Biosystems) using p53, ATF4, p21, and Gadd45a TaqMan Gene Expression Assays (Applied Biosystems). All qPCR samples were run in triplicate, and the cycle threshold (Ct) values were averaged. For data analysis, the ΔΔCt method was utilized, with 36B4 mRNA serving as the invariant control.
Necropsies of 25-mo-old mice.
At necropsy, animals were comprehensively evaluated for tumors in a blinded manner (19) by a veterinary pathologist (D. K. Meyerholz). Suspected tumors were morphologically evaluated and confirmed by histopathological examination [i.e., fixed in 10% neutral buffered formalin for 5–7 days with routine processing, paraffin embedding, sectioning (4 μm), and histochemical staining with H&E].
Statistics.
Statistical analyses were performed with GraphPad Prism. The statistical tests and sample sizes are indicated in the figure legends.
RESULTS
Mice lacking p53 in skeletal muscle fibers demonstrate no apparent basal phenotype as fully grown, middle-aged adults.
p53 mKO mice are homozygous for a floxed p53 allele [exons 2–10 are flanked by LoxP sites (18)] and also carry the MCK (muscle creatine kinase)-Cre transgene (4), which excises the floxed p53 allele in skeletal muscle fibers, resulting in a constitutive loss of p53 mRNA and p53 protein in skeletal muscle (14). In a previous study, we found that 3-mo-old p53 mKO mice develop normally, have normal skeletal muscle mass and strength under basal conditions, and are partially resistant to immobilization-induced skeletal muscle atrophy (14). In the present study, we investigated whether p53 mKO mice might have an altered response to skeletal muscle aging.
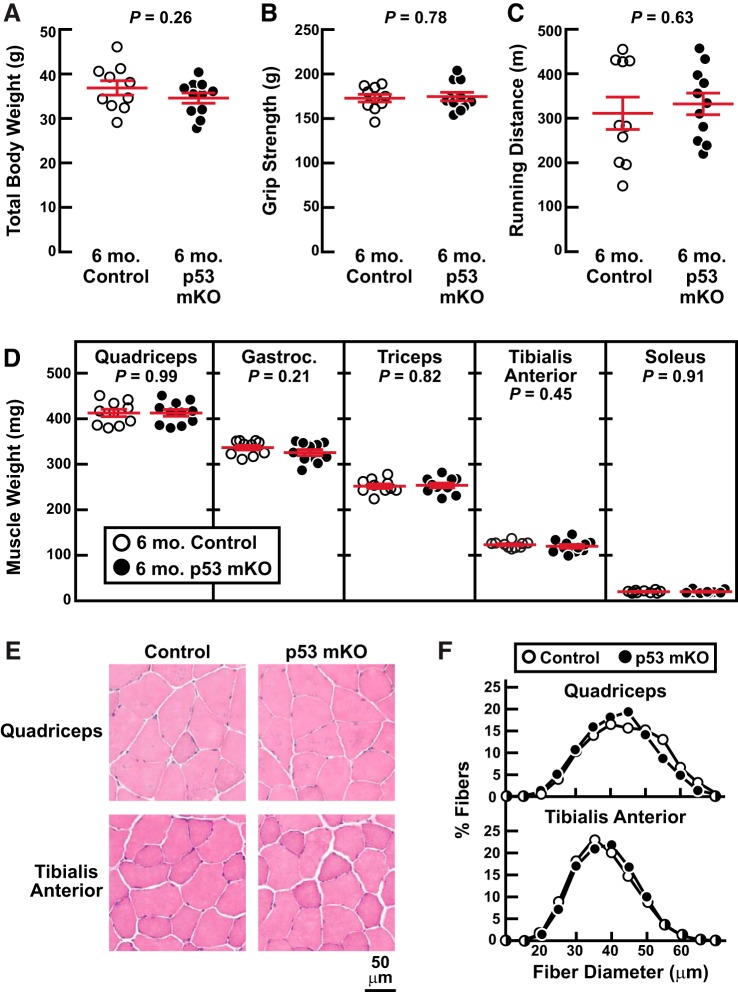
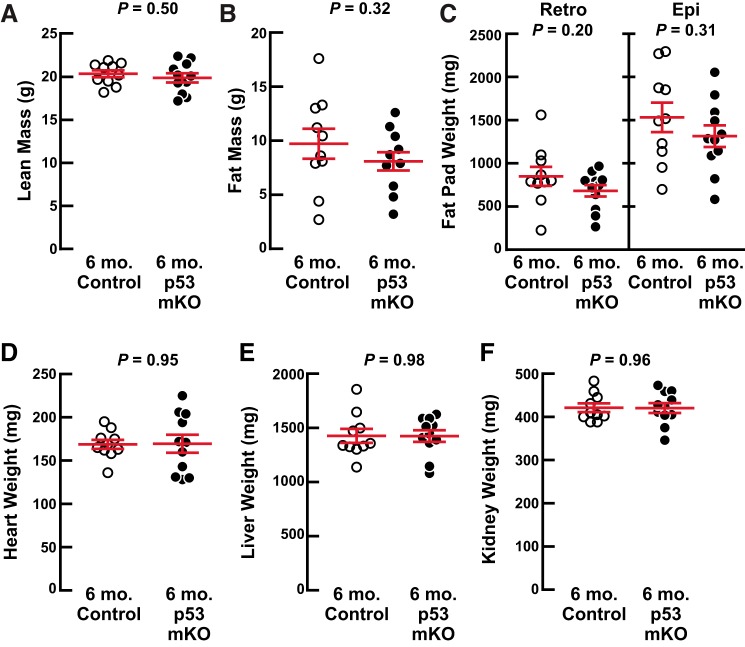
We began by phenotyping p53 mKO mice at 6 mo of age, when wild-type mice are fully grown and demonstrate peak muscle mass and strength. In these and all other studies described here, the p53 mKO mice were compared with littermate control mice that were also homozygous for the floxed p53 allele but lacked the MCK-Cre transgene and thus had uninterrupted p53 expression in skeletal muscle fibers (14). Figure 1 shows that 6-mo-old p53 mKO mice and littermate control mice possessed indistinguishable total body weights (Fig. 1A), grip strength (Fig. 1B), endurance exercise capacity (Fig. 1C), skeletal muscle weights (Fig. 1D), and skeletal muscle fiber size (Fig. 1, E and F). Furthermore, at 6 mo of age, p53 mKO mice had normal body composition by NMR (Fig. 2, A and B) as well as normal dissected weights of fat pads (Fig. 2C), heart (Fig. 2D), liver (Fig. 2E), and kidneys (Fig. 2F). These data indicate that a targeted and constitutive reduction of p53 expression in mouse skeletal muscle fibers has no apparent deleterious or beneficial effects up to 6 mo of age, when mice are fully grown and exhibit peak skeletal muscle mass and function.
Fig. 1.
Middle-aged muscle-specific p53-knockout mice (p53 mKO mice) have normal skeletal muscle mass and function. At 6 mo of age, p53 mKO mice and littermate control mice were assessed for total body weight (A), grip strength (B), endurance exercise capacity (C), dissected skeletal muscle weights (D), and skeletal muscle histology (E and F). In A–D, each data point represents 1 mouse and horizontal bars denote means ± SE. There were 10 control mice and 11 p53 mKO mice, and P values were determined with unpaired 2-tailed t tests. In D, data are weights of bilateral quadriceps femoris (quadriceps), gastrocnemius (gastroc), triceps brachii (triceps), tibialis anterior, and soleus muscles. In F, each distribution represents ≥3,700 fibers from 6 muscles.
Fig. 2.
Middle-aged muscle-specific p53-knockout mice (p53 mKO mice) have normal body composition. At 6 mo of age, p53 mKO mice and littermate control mice were assessed for lean (A) and fat (B) mass by NMR as well as dissected weights of bilateral retroperitoneal (retro) and epididymal (epi) fat pads (C), heart (D), liver (E), and bilateral kidneys (F). Each data point represents 1 mouse, and horizontal bars denote means ± SE. There were 10 control mice and 11 p53 mKO mice, and P values were determined with unpaired 2-tailed t tests.
p53 mKO mice demonstrate no apparent protection from age-related skeletal muscle atrophy and weakness up to 22 mo of age.
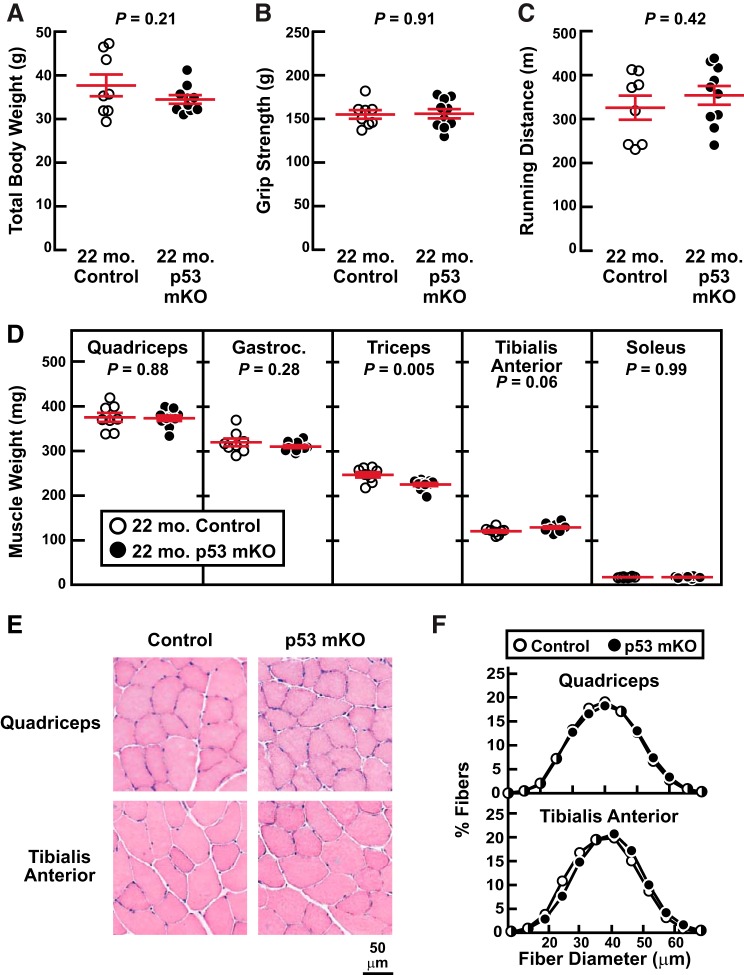
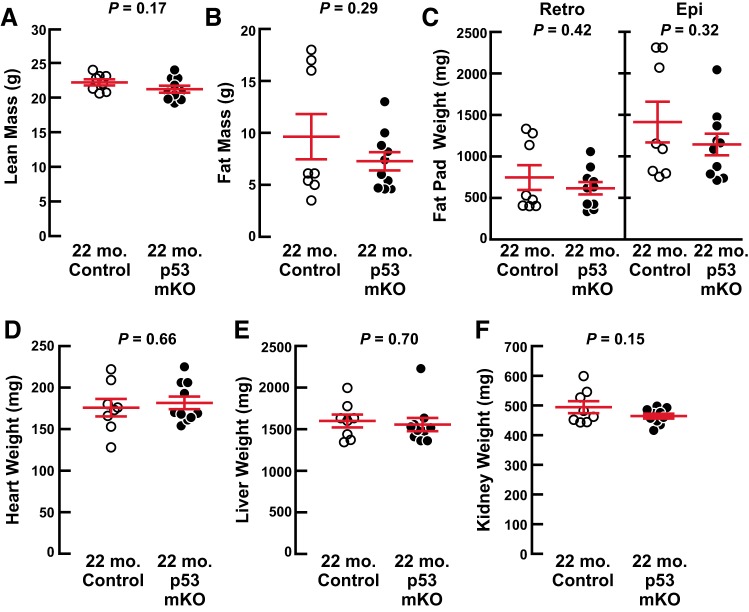
To investigate the effect of skeletal muscle p53 expression on age-related muscle weakness and atrophy, we compared p53 mKO mice and their littermate controls at 22 mo of age, when age-related muscle weakness and atrophy begin to be apparent in mice (10, 15). We found that 22-mo-old p53 mKO mice and littermate control mice possessed no differences in total body weight (Fig. 3A), grip strength (Fig. 3B), endurance exercise capacity (Fig. 3C), skeletal muscle weights (Fig. 3D), or skeletal muscle fiber size (Fig. 3, E and F). There were also no differences in body composition by NMR (Fig. 4, A and B) or dissected weights of fat pads (Fig. 4C), heart (Fig. 4D), liver (Fig. 4E). and kidneys (Fig. 4F). Thus, an absence of p53 expression in skeletal muscle fibers has no significant effect on skeletal muscle aging up to 22 mo of age, including no protective effect against age-related skeletal muscle atrophy and weakness.
Fig. 3.
Old muscle-specific p53-knockout mice (p53 mKO mice) have normal skeletal muscle mass and function. At 22 mo of age, p53 mKO mice and littermate control mice were assessed for total body weight (A), grip strength (B), endurance exercise capacity (C), dissected skeletal muscle weights (D), and skeletal muscle histology (E and F). In A–D, each data point represents 1 mouse, and horizontal bars denote means ± SE. There were 8 control mice and 10 p53 mKO mice, and P values were determined with unpaired 2-tailed t tests. In D, data are weights of bilateral quadriceps femoris (quadriceps), gastrocnemius (gastroc), triceps brachii (triceps), tibialis anterior, and soleus muscles. In F, each distribution represents >3,500 fibers from 6 muscles.
Fig. 4.
Old muscle-specific p53-knockout mice (p53 mKO mice) have normal body composition. At 22 mo of age, p53 mKO mice and littermate control mice were assessed for lean (A) and fat (B) mass by NMR as well as dissected weights of bilateral retroperitoneal (retro) and epididymal (epi) fat pads (C), heart (D), liver (E), and bilateral kidneys (F). Each data point represents 1 mouse, and horizontal bars denote means ± SE. There were 8 control mice and 10 p53 mKO mice, and P values were determined with unpaired 2-tailed t tests.
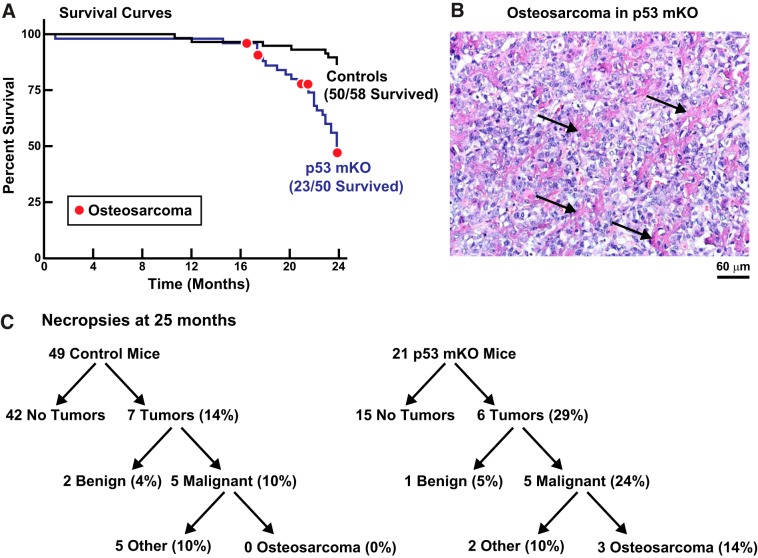
Old p53 mKO mice have an increased incidence of premature death and osteosarcoma.
In the aforementioned studies of 6- and 22-mo-old p53 mKO mice, all of the examined mice appeared healthy and, as the data demonstrate, were anatomically and functionally indistinguishable from control littermates. However, as p53 mKO mice approached old age, they began to die at an accelerating rate, and by 24 mo of age only 46% of p53 mKO mice were still living, compared with 86% of control littermates (Fig. 5A).
Fig. 5.
Old muscle-specific p53-knockout mice (p53 mKO mice) have an increased incidence of premature death, partly due to osteosarcoma. A: Kaplan–Meier survival curves for the first 24 mo of life of littermate control and p53 mKO mice. Red circles indicate mice that were euthanized because of the presence of leg tumors, all of which were histologically confirmed as osteosarcomas. The remaining deaths were spontaneous and unexplained. Sample sizes are indicated. B: representative hematoxylin and eosin stain of an osteosarcoma from an elderly p53 mKO mouse. Arrows indicate cords of osteoid produced by the tumor cells. C: results of comprehensive necropsies and tumor evaluations in 25-mo-old littermate control and p53 mKO mice. Sample sizes are indicated.
In most cases, the cause of death in old p53 mKO mice was spontaneous and unexplained; individual mice were simply found dead in their cages, with no apparent preceding illness or weight loss, no apparent tumors, and no apparent illness in their control littermates or p53 mKO littermates. Since the MCK-Cre transgene in p53 mKO mice also removes p53 expression from the heart (14), we performed detailed histopathological examinations of old p53 mKO and control littermate hearts but found no differences, including no evidence of excessive cardiac fibrosis or other structural pathology that might suggest an obvious cause of sudden cardiac death in p53 mKO mice. The absence of structural pathology was consistent with our finding of normal heart weights and endurance exercise capacity in p53 mKO mice but does not rule out other potential causes of sudden cardiac death, such as arrhythmia without structural pathology. We also considered the possibility that old p53 mKO mice might suffer from hypoglycemia or hyperglycemia; however, blood glucose levels were not different between the two genotypes at 22 mo of age (128 ± 6 mg/dL in control mice vs. 126 ± 4 mg/dL in p53 mKO mice; P = 0.76) or at 25 mo of age (130 ± 6 mg/dL in control mice vs. 122 ± 6 mg/dL in p53 mKO mice; P = 0.33).
Interestingly, some of the deaths in old p53 mKO mice were attributable to osteosarcomas (Fig. 5, A and B), which presented as leg tumors that necessitated euthanasia. Such tumors were never observed in control littermates. Because the very old p53 mKO mice were rapidly dying and no longer suitable for investigations of skeletal muscle aging, we euthanized all of the remaining p53 mKO and control littermate mice at 25 mo of age and subjected them to comprehensive necropsies and tumor evaluations (Fig. 5C). We found an equivalent incidence of nonosteosarcoma malignancies in control and p53 mKO mice (10% in both genotypes; Fig. 5C). However, there was a greatly increased incidence of osteosarcomas in p53 mKO mice (0% in control mice and 14% in p53 mKO mice; Fig. 5C). No other significant differences were found between the two genotypes at necropsy, which consisted of a careful gross anatomic examination and weighing of all tissues. Thus, at advanced ages, p53 mKO mice have a dramatically enhanced risk for osteosarcoma, as well as a markedly increased incidence of sudden death by unknown cause(s).
Healthy-appearing, tumor-free 25-mo-old p53 mKO mice demonstrate no apparent protection from age-related skeletal muscle atrophy and weakness.
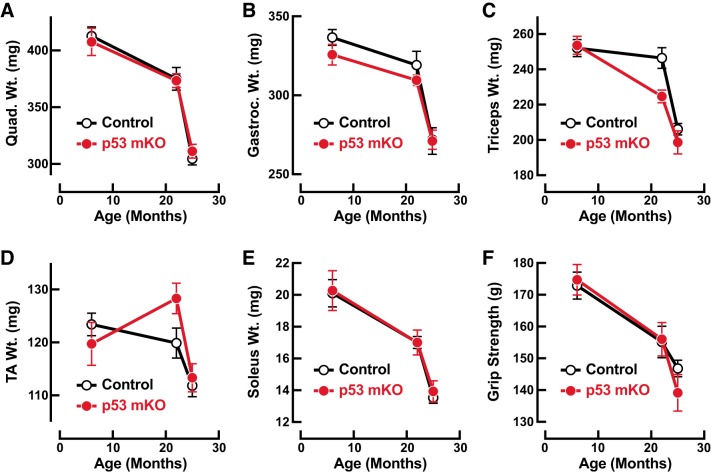
In our view, the increased mortality rate in very old p53 mKO mice precluded a highly rigorous analysis of skeletal muscle aging beyond 22 mo of age, since nearly any illness or severe stress can by itself trigger skeletal muscle atrophy. Nonetheless, and with that caveat in mind, we were interested in the possibility that healthy-appearing, tumor-free 25-mo-old p53 mKO mice might demonstrate some resistance to age-related muscle weakness and atrophy. To investigate this possibility, we assessed muscle mass and strength in a subset of 25-mo-old p53 mKO mice and littermate control mice that appeared grossly healthy at the time of necropsy and were subsequently confirmed to be tumor free via comprehensive necropsies and tumor evaluations (i.e., a subset of the mice from Fig. 5C). In that subset of 25-mo-old mice, p53 mKO mice and littermate control mice did not differ in total body weight (31.3 ± 0.7 g in control mice vs. 32.4 ± 1.4 g in p53 mKO mice; P = 0.45), skeletal muscle weight (Fig. 6, A–E), or grip strength (Fig. 6F). Moreover, when the data from those 25-mo-old mice were combined with data from 6- and 22-mo-old mice (i.e., data from Figs. 1 and 3), a clear and statistically significant age-related decline in muscle mass and strength was apparent in both genotypes, but again, there was no significant difference between p53 mKO mice and littermate control mice (Fig. 6).
Fig. 6.
Healthy-appearing, tumor-free 25-mo-old muscle-specific p53-knockout mice (p53 mKO mice) demonstrate no apparent protection from age-related skeletal muscle atrophy and weakness: effects of age and genotype on weights of bilateral quadriceps femoris muscles (quad; A), weights of bilateral gastrocnemius muscles (gastroc; B), weights of bilateral triceps brachii muscles (triceps; C), weights of bilateral tibialis anterior muscles (TA; D), weights of bilateral soleus muscles (E), and grip strength (F). Data points are means ± SE from the 6-mo-old p53 mKO mice and littermate control mice shown in Figs. 1 and 2 (10 or 11 mice per genotype), the 22-mo-old p53 mKO mice and littermate control mice shown in Figs. 3 and 4 (8–10 mice per genotype), and a subset of 25-mo-old p53 mKO mice and littermate control mice shown in Fig. 5C that appeared grossly healthy at the time of necropsy and were confirmed to be tumor free via comprehensive necropsies and tumor evaluations (40 control mice and 15 p53 mKO mice in A–E and 10 control mice and 8 p53 mKO mice in F). Two-way ANOVA revealed a significant effect of age on all of these parameters (P < 0.0001 for quad weight, gastroc weight, triceps weight, soleus weight, and grip strength; P < 0.001 for TA weight) but no significant effect of genotype on any of these parameters.
p53 is not required for age-related induction of p21 and Gadd45a mRNAs in skeletal muscle.
The hypothesis that p53 might be required for age-related muscle atrophy and weakness was based on several lines of circumstantial evidence. First, the Gadd45a and p21/Cdkn1a genes were two of the earliest identified examples of direct p53 target genes, and p21 and Gadd45a are two of the most highly induced mRNA transcripts in skeletal muscle during aging in both humans and mice (13, 24, 25). Second, expression of p21 or Gadd45a in skeletal muscle fibers is sufficient to induce muscle fiber atrophy (3, 5, 11, 14). Third, p53 is partially required for p21 expression in muscle fibers during immobilization-induced skeletal muscle atrophy in young adult mice (14). Fourth, p53 overexpression in muscle fibers induces atrophy, but only if p21 is present (14). However, it remained unknown whether p53 is required for age-related expression of p21 and Gadd45a in skeletal muscle.
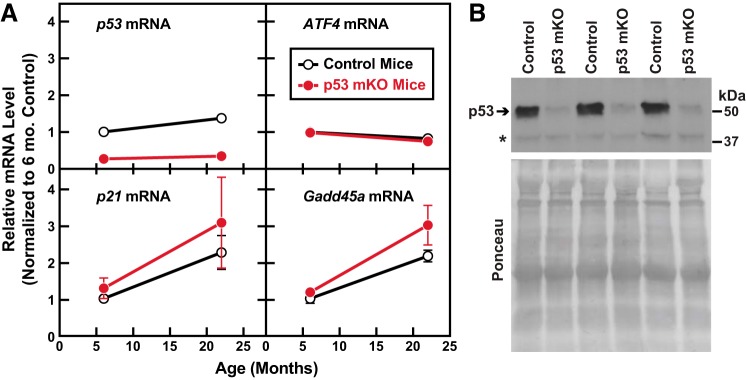
To test that hypothesis, we quantitated p21 and Gadd45a mRNAs, as well as p53 and ATF4 mRNAs, in the quadriceps muscles of 6- and 22-mo-old p53 mKO mice and littermate control mice. As expected, and as previously described (14), p53 mKO muscles contained reduced levels of p53 mRNA and p53 protein (Fig. 7). In addition, p53 mKO muscles contained normal levels of ATF4 mRNA (Fig. 7A), which encodes an independent transcriptional regulator of the p21 and Gadd45a genes in skeletal muscle and a bona fide mediator of age-related muscle atrophy and weakness (9, 10). Consistent with prior reports, the levels of p21 and Gadd45a mRNAs significantly increased with aging, but, interestingly, their levels were not reduced in p53 mKO muscles (Fig. 7A). Taken together, these data indicate that p53 expression in skeletal muscle fibers is not required for age-related induction of p21 and Gadd45a mRNAs in skeletal muscle. In addition, the unabated expression of ATF4, p21, and Gadd45a in p53 mKO muscles may potentially explain why p53 mKO muscles are not protected from age-related muscle atrophy and weakness.
Fig. 7.
p53 is not required for age-related induction of p21 and Gadd45a mRNAs in skeletal muscle. A: RNA from the quadriceps muscles of 6- and 22-mo-old muscle-specific p53-knockout mice (p53 mKO mice) and littermate control mice was subjected to quantitative real-time RT-PCR analysis to quantify levels of p53, ATF4, p21, and Gadd45a mRNAs. Data points are means ± SE from 5 or 6 mice per age and genotype. Two-way ANOVA revealed a significant effect of age on all 4 mRNAs and a significant effect of genotype on p53 mRNA (P < 0.0001) but not ATF4, p21, or Gadd45a mRNAs. B: protein from the gastrocnemius muscles of 22-mo-old p53 mKO mice and littermate control mice was subjected to immunoblot analysis of p53 protein. An equal amount of protein from each muscle (100 μg) was subjected to SDS-PAGE and immunoblot analysis with anti-p53 monoclonal IgG. Membranes were stained with Ponceau S to confirm equal loading. Asterisk denotes a nonspecific cross-reacting protein.
DISCUSSION
In the present study, we sought to better understand the role of p53 in skeletal muscle aging by investigating effects of aging on mice with a lifelong absence of p53 expression in skeletal muscle fibers. We found that an absence of p53 expression in skeletal muscle fibers has no apparent effect on basal or age-related changes in skeletal muscle mass and function up to at least 22 mo of age. Thus, although p53 activity in skeletal muscle fibers is sufficient to induce muscle fiber atrophy and required for skeletal muscle atrophy during limb immobilization (14), it does not appear to be required for age-related skeletal muscle atrophy or weakness, and, moreover, it does not appear to be required for the development or maintenance of normal basal skeletal muscle mass or function until at least old age.
The most prominent phenotype in p53 mKO mice was premature mortality at advanced age, resulting in a median life span of ~24 mo. Although the life span of p53 mKO mice is less than that of control littermates or wild-type mice, it is significantly longer than the median life span of global p53-knockout mice (4.5 mo) or heterozygous p53-knockout mice (18 mo) (6). In most p53 mKO mice that died prematurely, death was sudden and unrelated to cancer or any other overt signs of illness. In those mice the cause of death could not be determined, and additional studies will be needed to understand the etiology. One intriguing possibility is that, because of the absence of p53-mediated stress responses within muscle fibers and/or cardiac myocytes, p53 mKO mice may, with advanced age, begin to manifest metabolic disturbances that predispose to sudden death.
In other p53 mKO mice that died prematurely, the cause of death was osteosarcoma, which was present in ~1/7 of old p53 mKO mice but not in any littermate control mice. Although p53 is widely known as a tumor suppressor, particularly of osteosarcomas, the finding of osteosarcomas in p53 mKO mice was somewhat surprising because osteosarcomas arise from a cell type (osteoblast precursor cells) that is not thought to be targeted by MCK-Cre, and the cell types that are primarily if not exclusively targeted by MCK-Cre (skeletal muscle fibers and, to a lesser extent, cardiac myocytes) are terminally differentiated.
One trivial potential explanation for osteosarcomas in p53 mKO mice is that MCK-Cre has a low and previously undetected level of expression in osteoblast precursors, resulting in relatively rare Cre-mediated excision events that render some osteoblast precursors haploinsufficient for p53. In support of that hypothesis, osteosarcomas are the most frequent malignancy in mice that are globally haploinsufficient for p53 (6), and a targeted elimination of p53 in osteoblast precursors strongly predisposes mice to osteosarcoma (1, 23). A second related potential explanation for osteosarcomas in p53 mKO mice is that the p53 floxed allele used in this study is in fact mildly hypomorphic, thereby rendering all cells, including osteoblast precursors, relatively deficient in p53 activity; however, this explanation seems very unlikely, given that osteosarcomas were only observed in p53 mKO mice and not in control littermates, which were also homozygous for the exact same floxed p53 allele (6).
An alternative and much more interesting potential explanation for osteosarcomas in p53 mKO mice is that p53 expression in skeletal muscle fibers and/or heart has non-cell autonomous effects that somehow act on osteoblast precursors to prevent development of osteosarcoma. However, an extraordinary amount of attention has been given to p53 over the past 40 years, and to our knowledge a non-cell autonomous tumor suppressor function of p53 has not yet been described, so perhaps the first possibility mentioned (unexpected Cre-mediated excision of the floxed p53 allele in osteogenic precursor cells) is most likely. Further studies will be needed to resolve this issue, which seems important to either skeletal muscle biology (where MCK-Cre is a mainstay of molecular genetic investigations) or cancer biology (where a striated muscle-derived, osteosarcoma-suppressing mediator of p53 may be of interest).
The phenotype of aging p53 mKO mice stands in stark contrast to the phenotype of mice that lack ATF4 in skeletal muscle fibers [i.e., ATF4 mKO mice, where the same Cre driver used in p53 mKO mice, MCK-Cre, drives excision of floxed ATF4 alleles (10, 11, 14). Like p53 mKO mice, ATF4 mKO mice are resistant to immobilization-induced skeletal muscle atrophy as young adults (11, 14). However, in contrast to p53 mKO mice, ATF4 mKO mice are also resistant to age-related skeletal muscle atrophy and weakness (10) and do not exhibit premature mortality or increased tumor incidence. Thus, the existing data suggest that investigations of age-related skeletal muscle atrophy and weakness should be directed toward mechanisms that regulate and are regulated by ATF4, not p53.
The present study has several limitations. First, the premature mortality in very old p53 mKO mice precluded an analysis of skeletal muscle aging beyond 25 mo of age. Second, the muscle mass and strength data obtained from 25-mo-old p53 mKO mice may have been confounded by an underlying illness that we were unable to detect, even though the mice appeared outwardly healthy and were confirmed to be tumor free. Third, the present study only investigated male mice, and it remains possible that female p53 mKO mice could manifest a different phenotype during aging. Fourth, p53 mKO mice lack p53 expression in skeletal muscle fibers but not in other cell types where p53 may play an important role during skeletal muscle aging. Fifth, p53 mKO mice have a lifelong absence of p53 in skeletal muscle fibers, and it remains possible that acute inhibition of p53 expression in elderly skeletal muscle might generate a different phenotype.
In summary, these phenotypic investigations of p53’s role in skeletal muscle fibers during aging accentuate the critically important role of p53 as a tumor suppressor and also suggest the existence of other important effects of p53 on survival that may be unrelated to tumor suppression. In addition, these findings argue against the existence of important cell autonomous effects of skeletal muscle fiber p53 expression on muscle mass and function in the absence of immobilization or possibly other acute stress conditions. Finally, and most central to the original purpose of this study, the present results strongly suggest that p53 activity within skeletal muscle fibers is not a cause of age-related skeletal muscle weakness and atrophy.
GRANTS
This work was supported by the National Institutes of Health (Grants R01 AR-071762, R01 AG-060637, R44 AG-047684), the U.S. Department of Veterans Affairs (Grant I01BX00976), and the Fraternal Order of Eagles Diabetes Research Center at the University of Iowa.
DISCLOSURES
C. M. Adams, S. M. Ebert, and S. C. Bodine hold equity in Emmyon, Inc., where C. M. Adams and S. M. Ebert serve as officers and S. C. Bodine serves as a consultant. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
S.M.E., J.M.D., and C.M.A. conceived and designed research; S.M.E., J.M.D., D.K.M., S.A.B., A.A.-Z., A.D.D., Z.P.S., G.R.M., and C.M.A. performed experiments; S.M.E., J.M.D., D.K.M., K.C.T., and C.M.A. analyzed data; S.M.E., J.M.D., D.K.M., S.C.B., and C.M.A. interpreted results of experiments; S.M.E., J.M.D., and C.M.A. prepared figures; S.M.E., J.M.D., and C.M.A. drafted manuscript; S.M.E., J.M.D., S.C.B., and C.M.A. edited and revised manuscript; S.M.E., J.M.D., D.K.M., S.A.B., A.A.-Z., A.D.D., K.C.T., Z.P.S., G.R.M., S.C.B., and C.M.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. Peter Snyder and Daryl Granner for invaluable critical reading of the manuscript.
REFERENCES
- 1.Berman SD, Calo E, Landman AS, Danielian PS, Miller ES, West JC, Fonhoue BD, Caron A, Bronson R, Bouxsein ML, Mukherjee S, Lees JA. Metastatic osteosarcoma induced by inactivation of Rb and p53 in the osteoblast lineage. Proc Natl Acad Sci USA 105: 11851–11856, 2008. doi: 10.1073/pnas.0805462105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beyfuss K, Erlich AT, Triolo M, Hood DA. The role of p53 in determining mitochondrial adaptations to endurance training in skeletal muscle. Sci Rep 8: 14710, 2018. doi: 10.1038/s41598-018-32887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bongers KS, Fox DK, Ebert SM, Kunkel SD, Dyle MC, Bullard SA, Dierdorff JM, Adams CM. Skeletal muscle denervation causes skeletal muscle atrophy through a pathway that involves both Gadd45a and HDAC4. Am J Physiol Endocrinol Metab 305: E907–E915, 2013. doi: 10.1152/ajpendo.00380.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brüning JC, Michael MD, Winnay JN, Hayashi T, Hörsch D, Accili D, Goodyear LJ, Kahn CR. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol Cell 2: 559–569, 1998. doi: 10.1016/S1097-2765(00)80155-0. [DOI] [PubMed] [Google Scholar]
- 5.Bullard SA, Seo S, Schilling B, Dyle MC, Dierdorff JM, Ebert SM, DeLau AD, Gibson BW, Adams CM. Gadd45a protein promotes skeletal muscle atrophy by forming a complex with the protein kinase MEKK4. J Biol Chem 291: 17496–17509, 2016. doi: 10.1074/jbc.M116.740308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donehower LA, Lozano G. 20 years studying p53 functions in genetically engineered mice. Nat Rev Cancer 9: 831–841, 2009. doi: 10.1038/nrc2731. [DOI] [PubMed] [Google Scholar]
- 7.Dubowitz V, Sewry CA. Muscle Biopsy: a Practical Approach (3rd ed.). Philadelphia, PA: Saunders/Elsevier, 2007. [Google Scholar]
- 8.Dyle MC, Ebert SM, Cook DP, Kunkel SD, Fox DK, Bongers KS, Bullard SA, Dierdorff JM, Adams CM. Systems-based discovery of tomatidine as a natural small molecule inhibitor of skeletal muscle atrophy. J Biol Chem 289: 14913–14924, 2014. doi: 10.1074/jbc.M114.556241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ebert SM, Al-Zougbi A, Bodine SC, Adams CM. Skeletal muscle atrophy: discovery of mechanisms and potential therapies. Physiology (Bethesda) 34: 232–239, 2019. doi: 10.1152/physiol.00003.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ebert SM, Dyle MC, Bullard SA, Dierdorff JM, Murry DJ, Fox DK, Bongers KS, Lira VA, Meyerholz DK, Talley JJ, Adams CM. Identification and small molecule inhibition of an activating transcription factor 4 (ATF4)-dependent pathway to age-related skeletal muscle weakness and atrophy. J Biol Chem 290: 25497–25511, 2015. doi: 10.1074/jbc.M115.681445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ebert SM, Dyle MC, Kunkel SD, Bullard SA, Bongers KS, Fox DK, Dierdorff JM, Foster ED, Adams CM. Stress-induced skeletal muscle Gadd45a expression reprograms myonuclei and causes muscle atrophy. J Biol Chem 287: 27290–27301, 2012. doi: 10.1074/jbc.M112.374777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ebert SM, Monteys AM, Fox DK, Bongers KS, Shields BE, Malmberg SE, Davidson BL, Suneja M, Adams CM. The transcription factor ATF4 promotes skeletal myofiber atrophy during fasting. Mol Endocrinol 24: 790–799, 2010. doi: 10.1210/me.2009-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edwards MG, Anderson RM, Yuan M, Kendziorski CM, Weindruch R, Prolla TA. Gene expression profiling of aging reveals activation of a p53-mediated transcriptional program. BMC Genomics 8: 80, 2007. doi: 10.1186/1471-2164-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox DK, Ebert SM, Bongers KS, Dyle MC, Bullard SA, Dierdorff JM, Kunkel SD, Adams CM. p53 and ATF4 mediate distinct and additive pathways to skeletal muscle atrophy during limb immobilization. Am J Physiol Endocrinol Metab 307: E245–E261, 2014. doi: 10.1152/ajpendo.00010.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hwee DT, Baehr LM, Philp A, Baar K, Bodine SC. Maintenance of muscle mass and load-induced growth in Muscle RING Finger 1 null mice with age. Aging Cell 13: 92–101, 2014. doi: 10.1111/acel.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kunkel SD, Elmore CJ, Bongers KS, Ebert SM, Fox DK, Dyle MC, Bullard SA, Adams CM. Ursolic acid increases skeletal muscle and brown fat and decreases diet-induced obesity, glucose intolerance and fatty liver disease. PLoS One 7: e39332, 2012. doi: 10.1371/journal.pone.0039332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kunkel SD, Suneja M, Ebert SM, Bongers KS, Fox DK, Malmberg SE, Alipour F, Shields RK, Adams CM. mRNA expression signatures of human skeletal muscle atrophy identify a natural compound that increases muscle mass. Cell Metab 13: 627–638, 2011. doi: 10.1016/j.cmet.2011.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marino S, Vooijs M, van Der Gulden H, Jonkers J, Berns A. Induction of medulloblastomas in p53-null mutant mice by somatic inactivation of Rb in the external granular layer cells of the cerebellum. Genes Dev 14: 994–1004, 2000. [PMC free article] [PubMed] [Google Scholar]
- 19.Meyerholz DK, Beck AP. Principles and approaches for reproducible scoring of tissue stains in research. Lab Invest 98: 844–855, 2018. doi: 10.1038/s41374-018-0057-0. [DOI] [PubMed] [Google Scholar]
- 20.Ou HL, Schumacher B. DNA damage responses and p53 in the aging process. Blood 131: 488–495, 2018. doi: 10.1182/blood-2017-07-746396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rufini A, Tucci P, Celardo I, Melino G. Senescence and aging: the critical roles of p53. Oncogene 32: 5129–5143, 2013. doi: 10.1038/onc.2012.640. [DOI] [PubMed] [Google Scholar]
- 22.Tyner SD, Venkatachalam S, Choi J, Jones S, Ghebranious N, Igelmann H, Lu X, Soron G, Cooper B, Brayton C, Park SH, Thompson T, Karsenty G, Bradley A, Donehower LA. p53 mutant mice that display early ageing-associated phenotypes. Nature 415: 45–53, 2002. doi: 10.1038/415045a. [DOI] [PubMed] [Google Scholar]
- 23.Walkley CR, Qudsi R, Sankaran VG, Perry JA, Gostissa M, Roth SI, Rodda SJ, Snay E, Dunning P, Fahey FH, Alt FW, McMahon AP, Orkin SH. Conditional mouse osteosarcoma, dependent on p53 loss and potentiated by loss of Rb, mimics the human disease. Genes Dev 22: 1662–1676, 2008. doi: 10.1101/gad.1656808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Welle S, Brooks AI, Delehanty JM, Needler N, Bhatt K, Shah B, Thornton CA. Skeletal muscle gene expression profiles in 20-29 year old and 65-71 year old women. Exp Gerontol 39: 369–377, 2004. doi: 10.1016/j.exger.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 25.Welle S, Brooks AI, Delehanty JM, Needler N, Thornton CA. Gene expression profile of aging in human muscle. Physiol Genomics 14: 149–159, 2003. doi: 10.1152/physiolgenomics.00049.2003. [DOI] [PubMed] [Google Scholar]
- 26.Wen Y, Murach KA, Vechetti IJ Jr, Fry CS, Vickery C, Peterson CA, McCarthy JJ, Campbell KS. MyoVision: software for automated high-content analysis of skeletal muscle immunohistochemistry. J Appl Physiol (1985) 124: 40–51, 2018. doi: 10.1152/japplphysiol.00762.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]