Abstract
Volume-regulated anion channels (VRACs) encoded by the leucine-rich repeat containing 8 (LRRC8) gene family play critical roles in myriad cellular processes and might represent druggable targets. The dearth of pharmacological compounds available for studying VRAC physiology led us to perform a high-throughput screen of 1,184 of US Food and Drug Administration-approved drugs for novel VRAC modulators. We discovered the cysteinyl leukotriene receptor 1 (CysLT1R) antagonist, pranlukast, as a novel inhibitor of endogenous VRAC expressed in human embryonic kidney 293 (HEK293) cells. Pranlukast inhibits VRAC voltage-independently, reversibly, and dose-dependently with a maximal efficacy of only ~50%. The CysLT1R pathway has been implicated in activation of VRAC in other cell types, prompting us to test whether pranlukast requires the CysLT1R for inhibition of VRAC. Quantitative PCR analysis demonstrated that CYSLTR1 mRNA is virtually undetectable in HEK293 cells. Furthermore, the CysLT1R agonist leukotriene D4 had no effect on VRAC activity and failed to stimulate Gq-coupled receptor signaling. Heterologous expression of the CysLT1R reconstituted LTD4-CysLT1R- Gq-calcium signaling in HEK293 cells but had no effect on VRAC inhibition by pranlukast. Finally, we show the CysLT1R antagonist zafirlukast inhibits VRAC with an IC50 of ~17 µM and does so with full efficacy. Our data suggest that both pranlukast and zafirlukast are likely direct channel inhibitors that work independently of the CysLT1R. This study provides clarifying insights into the putative role of leukotriene signaling in modulation of VRAC and identifies two new chemical scaffolds that can be used for development of more potent and specific VRAC inhibitors.
Keywords: CysLT1R, HTS, LRRC8, VRAC
INTRODUCTION
The ability of a cell to regulate its volume in response to changes in swelling or shrinkage is an early evolutionary adaptation required for homeostasis (22, 38, 53, 54, 62). Cell swelling induced by hypo-osmotic shock activates a process known as regulatory volume decrease (RVD) that restores the cell to its normal volume. Volume-regulated anion channels (VRACs) enable RVD by mediating the efflux of chloride (Cl−) and small organic osmolytes (e.g., taurine) from the cell, which creates an outwardly directed osmotic driving force that promotes water efflux from the cell (22, 38, 53, 54, 62).
VRAC function has been studied extensively using electrophysiological techniques for nearly three decades (10, 21). VRACs are ubiquitously expressed in mammalian cells and have been implicated in diverse functions, in addition to cell volume regulation, such as cell proliferation (15, 52, 59), cell migration (40, 49), regulation of endothelial cell calcium signaling (43), release of excitatory amino acids in the brain (3, 17, 27, 66), and regulation of insulin secretion from pancreatic beta cells (5, 6, 30, 36, 56).
The identity of the genes encoding VRACs has been highly controversial (55), and only recently has the gene family been definitively established. In genome-wide RNA interference screens, the laboratories of Patapoutian and Jentsch found that the leucine-rich repeat containing 8A (LRRC8A) gene is essential for VRAC activity in mammalian cells (48, 60). Other gene family members include LRRC8B, LRRC8C, LRRC8D, and LRRC8E. The best available evidence suggests that VRACs are hexamers containing a LRRC8A subunit and at least one other LRRC8 subunit (13, 31–33, 60). The identification of the genes encoding VRAC creates unprecedented opportunities for studying the molecular and integrative physiology and therapeutic potential of these important channels.
Emerging evidence raises the intriguing possibility that VRAC might represent a druggable target in certain disease states [reviewed by Strange et al. (55)]. For example, several studies have suggested that VRAC expressed in astrocytes might be a therapeutic target in stroke (1, 42). Ischemic stroke leads to astrocyte swelling, VRAC activation, and release of glutamate, via VRAC, into the extracellular space. Glutamate release into synapses can activate NMDA receptors, triggering calcium influx and excitotoxic cell death (42). Genetic knockdown and knockout studies of LRRC8A in astrocytes abolish VRAC-dependent chloride current and glutamate release in cultures and in vivo (26, 66). In the latter study, astrocytic LRRC8A knockout reduced total infarct size, as well as improved neurological scores compared with wild-type (WT) mice following experimental stroke (66). These results are supported by pharmacology experiments using tamoxifen and 4-(2-butyl-6,7-dichloro-2-cyclopentyl-indan-1-on-5-yl) oxobutyric acid (DCPIB) to inhibit VRAC (7, 17, 34, 35, 46, 47, 69). However, the lack of specificity of these compounds raises concerns about potentially confounding off-target effects of the drugs (8, 11, 14, 18, 19). To fully establish the safety and efficacy of VRAC inhibition in stroke and other therapeutic areas, more potent and specific inhibitors of VRAC must be developed.
In this study, we performed a high throughput screen (HTS) with human embryonic kidney 293 (HEK293) cells for novel small-molecule modulators of VRAC. By interrogating a library of 1,184 US Food and Drug Administration-approved drugs, we identified the cysteinyl leukotriene receptor 1 (CysLT1R) antagonist, pranlukast, as a novel inhibitor of VRAC. Interestingly, CysLT1R has been implicated in the modulation of VRAC (23); however, we show that HEK293 cells do not functionally express the CysLT1 receptor. In addition, we show the structurally distinct CysLT1R inhibitor, zafirlukast, also inhibits VRAC. These studies provide clarifying insights into the putative role of CysLT1 receptor signaling in regulation of VRACs and identify CysLT1R antagonists as structurally novel small-molecule inhibitors of LRRC8 channels.
MATERIALS AND METHODS
Chemicals.
Pranlukast (Ono-1078) was purchased from Tocris (Bristol, UK). Zafirlukast was purchased from Sigma-Aldrich (St. Louis, MO). Leukotriene D4 (LTD4) in ethanol:H2O (95:5) was purchased from Cayman Chemical (Ann Arbor, MI). Ethanol was evaporated under nitrogen and LTD4 was resuspended in 100% DMSO. Fluo-8 AM was purchased from AAT Bioquest (Sunnyvale, CA). All salts were purchased from Sigma-Aldrich and were of the highest grade available.
Molecular biology.
The CysLT1R-pCMV6 plasmid was purchased from Origene Technologies. The YFP(H148Q/l152L) plasmid was generously provided by Dr. David Weaver (Vanderbilt University). We introduced an additional mutation (F46L) to increase yellow fluorescent protein (YFP) fluorescence emission and halide sensitivity (57) using a QuickChange Mutagenesis Kit (Agilent Technologies). The YFP(F46L/H148Q/I152L) cDNA was fully sequenced to ensure that the intended mutations were present and that no spurious mutations had occurred during the PCR amplification. We refer to YFP(F46L/H148Q/I152L) as Ozzy. LRRC8AKO-HEK293 cells were a kind gift from Dr. Rajan Sah (Washington University, St. Louis, MO). LRRC8A knockout was generated by a CRISPR-Cas9 mediated approach (61).
Quantitative real-time PCR.
For mRNA quantification of HEK293, HEK293-Ozzy, and THP-1 cells, total RNA was isolated using TRIzol (Life Technologies). FAM labeled Taqman probes against human LRRC8A (cat. no. 4331182), human LRRC8B (cat. no. 4331182), human LRRC8C (cat. no. 4331182), human LRRC8D (cat. no. 4351372), human LRRC8E (cat. no. 4331182), and human ACTB (cat. no. 4331182) were purchased from Applied Biosystems (Foster City, CA). qSTAR qPCR primer pairs against human CYSLTR1 (cat. no. HP209551) and GAPDH (cat. no. HP205798) were purchased from Origene Technologies. The SsoAdvanced Universal Probes Supermix was used as a qPCR reagent for LRRC8A-E and ACTB (Bio-Rad, Hercules, CA). The iTaq Universal SYBR Green One-Step Kit was used as a qPCR reagent for CYSLTR1 and GAPDH (Bio-Rad, Hercules, CA). Reactions containing 500 ng RNA were run from at least two biological replicates in duplicate in 10 µL reactions according to the manufacturer’s specifications using a Bio-Rad CFX96 instrument (Bio-Rad, Hercules, CA). ACTB and GAPDH were used as reference genes. Normalizations were done using the 2−ΔΔCT method.
Cell culture and transient transfection.
HEK293 cells were cultured in 75 cm2 flasks with Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, and 1% penicillin-streptomycin. HEK293 cells stably transfected with the YFP(F46L/H148Q/I152L) fluorophore (i.e., HEK293-Ozzy cells) were cultured in the same medium supplemented with 700 µg/mL G418 sulfate (Corning, Corning, NY). For patch-clamp experiments, HEK293 cells plated in 35-mm Nunclon dishes (Thermo Fisher Scientific, Rochester, NY) were co-transfected with 1 µg CysLT1R plasmid DNA and 0.5 µM green fluorescent protein (GFP; a transfection marker) using Lipofectamine LTX and studied the next day. For calcium imaging experiments, HEK293 cells were transfected with 8 µg CysLT1R plasmid DNA in T-75 flasks (Thermo Fisher Scientific, Rochester, NY), plated in 384-well plates (Corning; see below) the next day, and allowed to recover for an additional 24 h before experiments.
Patch-clamp electrophysiology.
The day of experiments, parental or transfected (see above) HEK293 cells were rinsed with divalent-free Hank’s balanced salt solution (HBSS), dissociated using 0.25% trypsin/1 mM EDTA for ~30-s, diluted with complete medium, plated on poly-l-lysine-coated round glass coverslips, and allowed to recover at 37°C in a 5% CO2 incubator for at least 1 h before experiments. Patch pipettes were pulled from Clark Custom 8520 Patch Glass (1.5 OD × 1.16 ID) (Harvard Apparatus, Holliston, MA) using a P-1000 Flaming/Brown Microelectrode puller (Sutter Instruments) to a resistance of 2–4 MΩ when filled with the following solution (in mM): 126 CsCl, 2 MgSO4, 20 HEPES, 1 EGTA, 2 Na2ATP, 0.5 GTP (pH 7.2 adjusted with CsOH, 275 mosM). The isotonic bath solution contained (in mM): 75 CsCl, 5 MgSO4, 1 calcium-d-gluconate, 12 HEPES, 8 Tris base, 5 glucose, 2 glutamine, and 95 sucrose (pH 7.4 adjusted with CsOH, 300 mosM). Hypotonic bath (250 mosM) was made by excluding sucrose from the isotonic bath.
Macroscopic currents were recorded under voltage-clamp conditions using an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA). Cells were voltage clamped at a holding potential of −30 mV and whole cell currents were elicited by a voltage ramp or step protocol. For voltage ramps, membrane potential was first stepped to −100 mV for 50 ms and then ramped over 1 s to +100 mV. This was followed by a step back to 0 mV for 4 s before this protocol was repeated. Step changes in membrane voltage were induced by stepping membrane voltage to −120 mV to +120 mV in 800 ms, 20 mV increments. Data were collected at 5 kHz and filtered at 1 kHz. Data acquisition and analysis were performed using pClamp 9.2 software suite (Molecular Devices). Percent inhibition of compounds was determined by normalizing data to currents inhibited by 10 µM DCPIB. Initial rate of swelling-induced VRAC activation was calculated by determining the linear slope of increasing current during the first 180 s following hypotonic solution addition.
Fluorescence reporter assay of VRAC function.
HEK293-Ozzy cells were dissociated using 0.25% trypsin-1 mM EDTA and plated at a density of 20,000 cells/well in clear bottomed, black-walled Corning PureCoat amine coated 384 well plates (Corning, Corning, NY) and cultured overnight at 37°C in a 5% CO2 incubator. The following day cells were washed with isotonic solution containing (in mM) 140 NaCl, 5 KCl, and 20 HEPES (pH 7.4, 310 mosM). Compounds from the FDA library were dissolved in hypotonic solution containing (in mM): 5 KCl, 20 HEPES, and 90 mannitol (pH 7.4, 120 mosM) and added simultaneously to all 384-wells at a final screening concentration of 10 µM. After 5 min allowed for cell swelling and VRAC activation, 100 mM NaI was added to all 384-wells simultaneously and Ozzy fluorescence was measured at 1 Hz using a Panoptic kinetic imaging plate reader (WaveFront Bioscience, Franklin, TN). Fluorescence values from each well were normalized to baseline readings (i.e., average of the first 5 readings before hypotonic solution is added). Percent fractional quenching was calculated by subtracting fluorescence readings obtained before and after NaI addition. Data were plotted with GraphPad Prism version 7.03 (GraphPad Software, San Diego, CA) to generate representative experiment traces and concentration-response curves using a nonlinear regression analysis.
Calcium imaging.
Parental HEK293 cells or HEK293 cells transiently transfected with CysLT1R (see above) were dissociated and plated in 384-well plates as described above. The next day, the cells are washed and incubated in assay buffer (HBSS + 20 mM HEPES) containing a final concentration of 2 µM Fluo-8 AM The cells were incubated with dye for 1 h at room temperature and washed with assay buffer. Compounds in assay buffer were added at 2X concentrations to reach final concentrations indicated in the figure legends. Fluo-8 fluorescence was measured using a Panoptic plate reader and normalized to baseline (average of the first 5 readings before solution addition).
Statistics.
All data are presented as means ± SE; n represents the number of cells in patch-clamp recordings, the number of plates in high throughput screens, or the number of wells in fluorescence-based assays. Statistical significance was determined by Student’s t test for unpaired means for two groups. For groups greater than two, one-way analysis of variance (ANOVA) and Kruskal-Wallis post hoc test were used.
RESULTS
Development of a VRAC reporter assay for HTS.
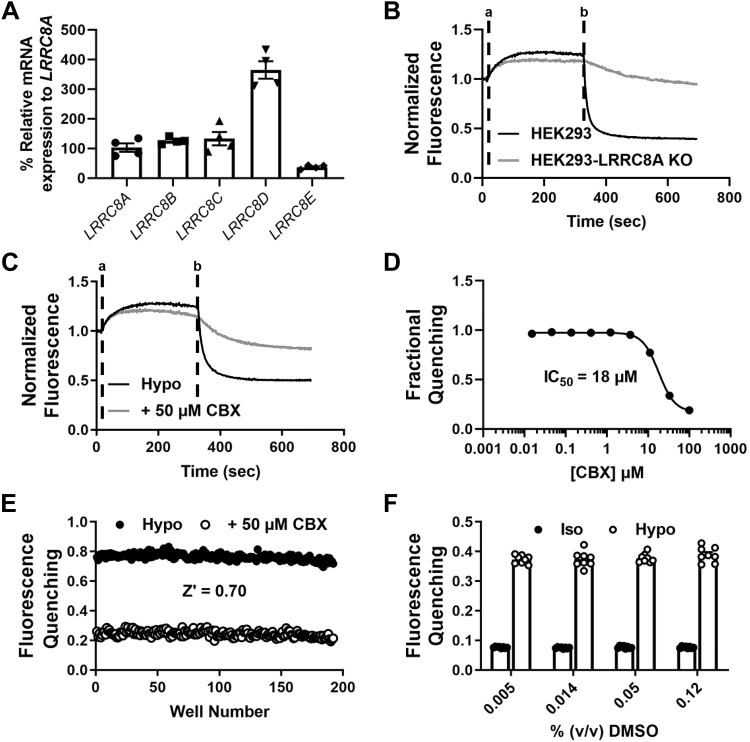
We developed a quantitative, fluorescence-based “quenching” assay that reports endogenous VRAC activity in HEK-239 cells to support an HTS campaign for novel channel modulators. qPCR analysis revealed expression of all LRRC8 gene family members, where LRRC8D was the predominant subunit expressed at the mRNA level (n = 4; Fig. 1A). The assay was adapted from those used to identify the LRRC8 gene family in genome-wide siRNA screens (48, 60). Stably transfected HEK293 cells expressing a halide-sensitive YFP mutant, termed Ozzy, were plated in 384-well plates, exposed to hypotonic media for 5 min to induce VRAC activation, and then treated with iodide-containing buffer to induce inwardly directed iodide flux through VRAC and quenching of the Ozzy fluorophore (Fig. 1B; black line). The degree of iodide-induced quenching was dramatically reduced in HEK293 cells lacking the essential VRAC subunit LRRC8A (Fig. 1B; gray line), indicating that most of the quenching signal reflects iodide flux through VRAC.
Fig. 1.
Ozzy assay development. A: PCR quantification of leucine-rich repeat containing 8 (LRRC8A–E) mRNA in Ozzy-human embryonic kidney 293 (HEK293) cells (n = 4). B: representative traces of Ozzy-quenching assay using HEK293 cells and HEK293-LRRC8A knockout (KO) cells. Hypotonic solutions were added at time “a” and NaI was added at time “b”. C: representative traces of an Ozzy-quenching assay using the known volume-regulated anion channel inhibitor carbenoxolone (CBX). Hypotonic solutions [hypotonic (hypo) bath; hypotonic bath + 50 μM CBX] were added at time “a” and NaI was added at time “b”. D: CBX concentration-response curve generated using Ozzy-quenching assay (mean ± SE IC50 = 18 ± 0.54 µM; n = 10). E: scatterplot of “checkerboard” analysis for Ozzy-quenching assay (mean ± SE Z′ = 0.70 ± 0.03, 8 plates over 3 days). F: effect of DMSO concentration in isotonic (Iso) and hypotonic bath solutions on Ozzy fluorescence quenching (n = 8).
We evaluated the assay’s ability to report inhibition of VRAC by the known inhibitor carbenoxolone [CBX; (4, 20, 67)]. CBX inhibited VRAC-mediated iodide quenching (e.g., Fig. 1C) in a dose-dependent manner with a mean ± SE IC50 of 18 ± 0.54 µM (n = 10; Fig. 1D), which is close to the value reported by others using electrophysiological techniques (4). Using CBX as a control inhibitor, we performed a so-called “checkerboard” assay to evaluate the uniformity and reproducibility of the assay. Every other well of a 384-well plate was treated with hypotonic media alone or hypotonic media containing 50 µM CBX. As shown in Fig. 1E, there was a clear separation of iodide-induced quenching observed in blocked versus unblocked population of wells (Fig. 1C). The mean ± SE Z′ value calculated from 8 plates over 3 days was 0.70 ± 0.03, indicating the assay is robust enough to support high-confidence hit-picking in a high-throughput screen. Finally, we found that the assay is tolerant to the small-molecule vehicle DMSO at concentrations ranging between 0.0046 and 0.12% (vol/vol) (Fig. 1F), ruling out the possibility of DMSO having direct, confounding effects at the screening concentration of 0.01% vol/vol.
Discovery and characterization of pranlukast as an inhibitor of VRAC.
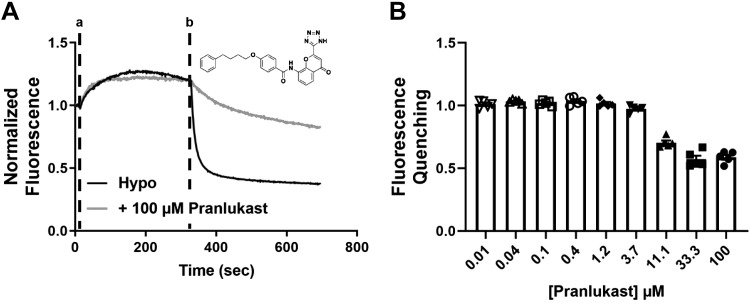
We screened 1,184 compounds from the SelleckChem FDA-approved drug collection for novel inhibitors of VRAC. The mean ± SE Z′ for the 6-plate run was 0.64 ± 0.01, indicating the assay performed well during the screen. The only reproducible inhibitor identified in the screen was pranlukast (Fig. 2A), an antagonist of the cysteinyl leukotriene receptor 1 (CysLT1) used for the treatment of asthma (64, 65). As shown in Fig. 2B, pranlukast inhibited VRAC-mediated iodide quenching dose-dependently and with partial efficacy (n = 5). We could not derive an IC50 because pranlukast does not fully inhibit the channel.
Fig. 2.
Discovery of pranlukast in a high-throughput screen of the US Food and Drug Administration library. A: representative traces of an Ozzy-quenching assay with pranlukast. Hypotonic solutions [hypotonic (hypo) bath; hypotonic bath + 100 μM pranlukast] were added at time “a” and NaI was added at time “b”. Inset: structure of pranlukast. B: dose-dependent inhibition of volume-regulated anion channel using Ozzy-quenching assay (n = 5).
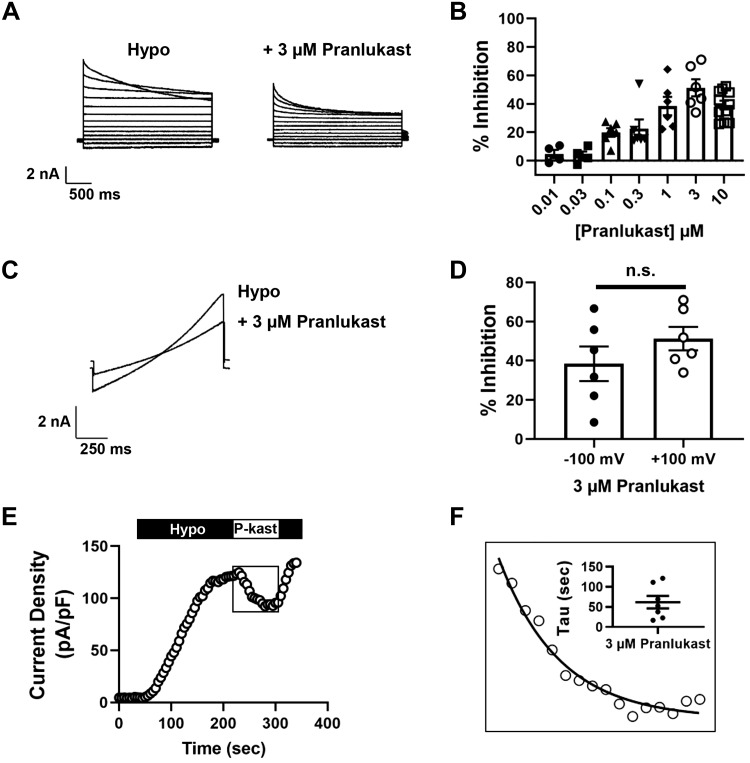
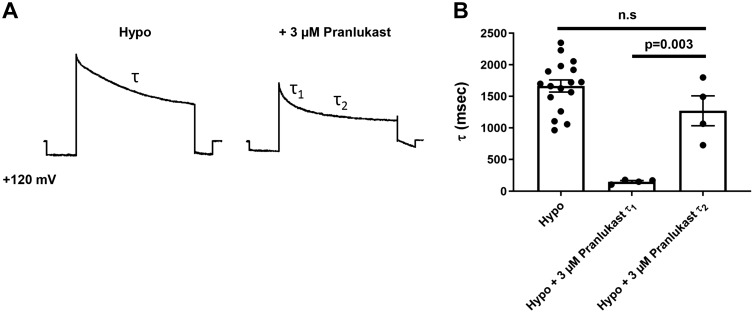
We confirmed and extended these observations using whole cell patch electrophysiology techniques to measure VRAC function and inhibitory characteristics of pranlukast. HEK293 cells were patch clamped with intracellular and extracellular solutions designed to isolate chloride currents under isotonic conditions (300 mosM) and then subjected to a 200–250 mosM hypotonic stimulus to induce cell swelling. As shown in Fig. 3A, cell swelling led to the activation of outwardly rectifying chloride current exhibiting time-dependent inactivation at positive potentials that is characteristic of VRAC (45). Bath application of 3 µM pranlukast, which is close to the solubility limit of pranlukast in these buffers, led to a reduction in VRAC current amplitude (Fig. 3A). Inhibition was dose-dependent with a mean ± SE maximal efficacy at 3 µM of 51 ± 6% (n ≥ 4; Fig. 3B). We evaluated the voltage-dependency of pranlukast inhibition in voltage-ramp experiments (Fig. 3C), which revealed that VRAC inhibition is voltage-independent (Fig. 3D). Pranlukast-dependent inhibition occurred with a mono-exponential time course (Fig. 3E) and mean ± SE time constant of 62 ± 16 s (n = 7; Fig. 3F) and was fully reversible (Fig. 3E). In control hypotonic buffer, inactivation of VRAC current at 120 mV is described by a single exponent with a mean ± SE time constant of 1.7 ± 0.1 s (n = 17; Fig. 4, A and B). In contrast, bath application of 3 µM pranlukast led to the appearance of a second, statistically significantly faster (P = 0.003) inactivation time constant (Fig. 4, A and B). There was no significant difference (P < 0.05) between the slower time constant in the presence of pranlukast and the single time constant measured in control buffer (Fig. 4, A and B). Taken together, these data are consistent with pranlukast being an authentic albeit partially efficacious inhibitor of VRAC activity.
Fig. 3.
Pranlukast inhibits volume-regulated anion channel (VRAC) currents. A: whole cell currents from human embryonic kidney 293 (HEK293) cells were measured using a step protocol from −120 to +120 mV, with 20 mV steps. Cells were exposed to hypotonic bath and hypotonic bath + 3 µM pranlukast. B: dose-dependent inhibition of VRAC with pranlukast from whole cell patch-clamp electrophysiology maximum efficacy at 3 µM = ~50%; n ≥ 4). C: whole cell currents from HEK293 cells were measured using a ramp protocol from −100 to +100 mV. Cells were exposed to hypotonic bath and hypotonic bath + 3 µM pranlukast. D: analysis of VRAC currents with 3 µM pranlukast at −100 and +100 mV by application of the voltage-ramp protocol. ns, Not significant. E: exemplar time course of VRAC activation under hypotonic solution exposure, 3 µM pranlukast (P-kast), and wash (hypo) treatment. F: 3 µM pranlukast-dependent current inhibition time constant (mean ± SE τ = 62 ± 16 s; n = 7).
Fig. 4.
Pranlukast-dependent volume-regulated anion channel (VRAC) inhibition induces a second inactivation time constant. A: whole cell currents from human embryonic kidney 293 (HEK293) cells exposed to hypotonic and hypotonic bath + 3 µM pranlukast were measured using a step protocol at +120 mV. B: quantification of inactivation time constants generated from exponential fit curves (P = 0.003; Hypo + 3 µM pranlukast τ1 vs. Hypo + 3 µM pranlukast τ2, one-way ANOVA and Kruskal-Wallis post hoc test; n ≥ 4).
Pranlukast inhibits VRAC independently of the CysLT1 receptor.
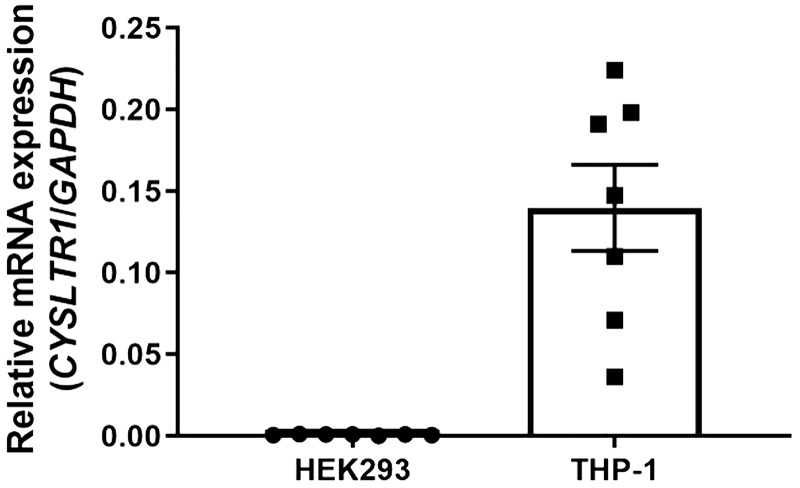
A previous study implicated leukotriene signaling and the CysLT1R pathway in VRAC modulation (23), raising the possibility that pranlukast inhibits VRAC indirectly via the CysLT1 receptor. We therefore systematically tested if pranlukast-dependent VRAC inhibition requires the expression and function of CysLT1R. Quantitative PCR was used to measure the abundance of CYSLTR1 mRNA expression in the same HEK293 cell line that was used for patch clamp experiments (Fig. 3) and the THP-1 monocytic cell line in which CysLT1R is known to be expressed (16, 24). Whereas abundant CYSLTR1 mRNA expression was detected in THP-1 cells, there was virtually no detectable mRNA expression present in HEK293 cells (n = 7; Fig. 5).
Fig. 5.
Cysteinyl leukotriene receptor 1 (CysLT1R) mRNA expression in human embryonic kidney 293 (HEK293) cells and THP-1 cells. qPCR quantification of CYSLTR1 mRNA in HEK293 and THP-1 cells (positive control) (n = 7).
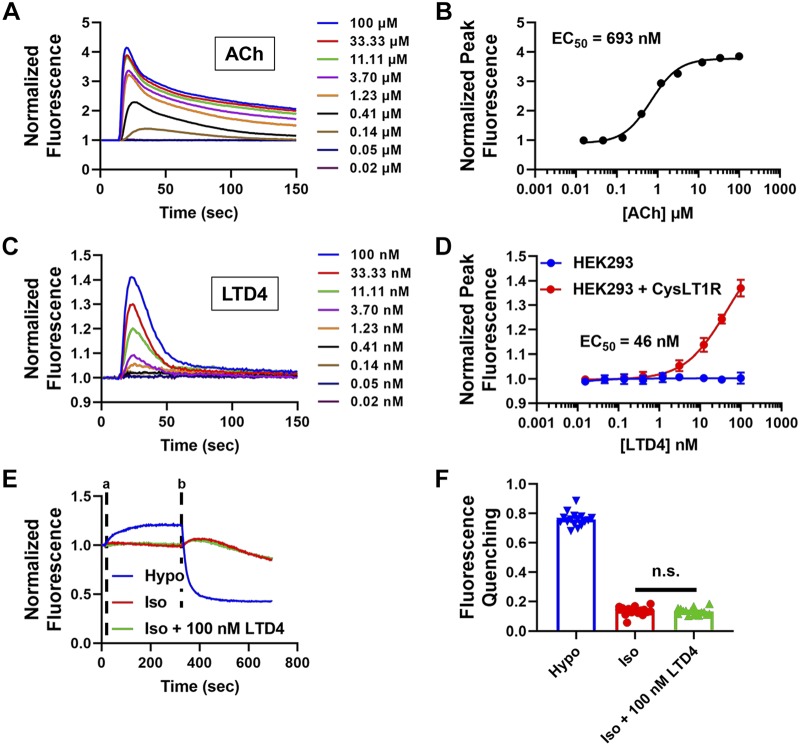
We determined if despite the low abundance of CYSLTR1 mRNA in HEK293 cells (Fig. 5) that there is enough receptor protein expression present to mediate effects of pranlukast on VRAC activity. CysLT1R is a Gq-coupled G protein-coupled receptor (GPCR) that leads to increases in intracellular calcium following receptor stimulation, enabling us to test for receptor function using a fluorescence calcium assay. We first confirmed that the Gq-PLC- phosphatidylinositol 4,5-29 bisphosphate (PIP2)-inositol trisphosphate (IP3)-calcium system is intact in HEK293 cells by taking advantage of the endogenous expression of the M3 muscarinic receptor, another Gq-coupled GPCR. HEK293 cells were plated in 384-well plates, loaded with the fluorescence indicator dye Fluo-8 AM, and then treated with escalating doses of acetylcholine (ACh). As shown in Fig. 6, A and B, ACh addition led to a dose-dependent increase in intracellular calcium, with a mean ± SE EC50 of 693 ± 0.05 nM (n = 5). In striking contrast, addition of the endogenous CysLT1R agonist LTD4 had no effect on intracellular calcium at doses up to 100 nM (n = 5; Fig. 6D; blue line). However, transfecting HEK293 cells with a plasmid encoding the CysLT1 receptor conferred sensitivity to LTD4 and led to a dose-dependent increase in intracellular calcium with LTD4 (Fig. 6, C and D; red line).
Fig. 6.
Cysteinyl leukotriene (CysLT1) receptors are not functionally expressed in human embryonic kidney 293 (HEK293) cells. A: representative traces of acetylcholine (ACh) concentration-response curve (CRC) in HEK-293 cells using Fluo-8 calcium assay. B: ACh CRC in HEK293 cells using Fluo-8 calcium assay (mean ± SE EC50 = 693 ± 0.05 nM, n = 5). C: representative traces of leukotriene D4 (LTD4) CRC in HEK293 cells transiently transfected with CysLT1R using Fluo-8 calcium assay (HEK293 cells alone did not show a response to LTD4). D: LTD4 CRC in HEK293 and HEK293 cells transiently transfected with CysLT1R (mean ± SE EC50 = 46 ± 19 nM in HEK293 + CysLT1R cells; n = 5) using Fluo-8 calcium assay. E: representative traces of HEK293 cells treated with hypotonic (Hypo), isotonic (Iso), or isotonic + 100 nM LTD4 solutions using Ozzy-quenching assay. Solutions are added at time “a” and NaI was added at time “b” (n = 16). F: Ozzy-quenching assay summary data from E. There was no significant difference (n.s.) between isotonic and isotonic + 100 nM LTD4 groups (one-way ANOVA and Kruskal-Wallis post hoc test).
It has been postulated that cell swelling leads to the synthesis of leukotrienes and potentiation of VRAC via the CysLT1 receptor (23). Therefore, as another test of CysLT1R functional expression, we tested if LTD4 activates VRAC in the absence of cell swelling. These experiments were performed using the Ozzy quenching assay. As shown in Fig. 6, E and F, 100 nM LTD4 in isotonic buffer failed to increase iodide-induced quenching compared with isotonic buffer alone, whereas hypotonic media led to robust fluorescence quenching. Taken together, the results of these experiments are consistent with the interpretation that HEK293 cells lack both CYSLTR1 mRNA and protein expression needed for receptor-mediated inhibition of VRAC by pranlukast.
Finally, we tested if heterologous expression of CysLT1R increases sensitivity of the VRAC current to inhibition by pranlukast. HEK293 cells were transfected with either GFP (control) or CysLT1R and GFP and patch clamped in the whole cell configuration. There was no statistically significant difference in VRAC inhibition by 1 µM pranlukast in HEK293 cells transfected with GFP (30.8 ± 4.5%; n = 4) or CysLT1R plasmid (30.4 ± 5.2%; n = 4). In addition, there was no difference in the initial rate of activation between HEK-293 cells transfected with GFP and those transfected with CysLT1R: 0.08 ± 0.009 and 0.08 ± 0.004 pA·pF−1·s−1, respectively.
VRAC is also inhibited by the structurally distinct CysLT1R antagonist zafirlukast.
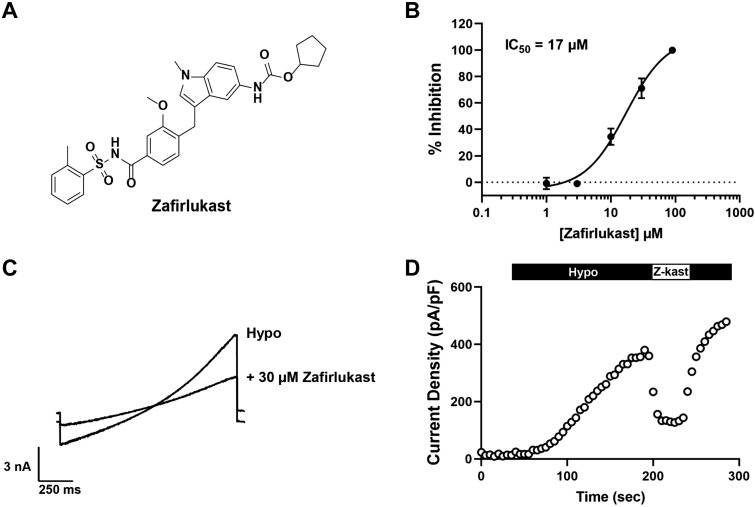
Holm et al. (23) reported that the CysLT1R antagonist, zafirlukast, which is structurally distinct from pranlukast (Fig. 7A), inhibits hypotonic-induced regulatory volume decrease (RVD) and taurine release from A549 lung epithelial cell line. Given that VRAC plays key roles in both of these physiological processes (55), we determined if zafirlukast inhibits VRAC in HEK293 cells. In fluorescence assays, zafirlukast inhibited iodide-induced Ozzy quenching dose-dependently with a mean ± SE IC50 of 17.0 ± 0.6 µM (n = 10). Similarly, patch clamp experiments revealed that zafirlukast acutely and reversibly inhibited swelling-induced VRAC activity with a mean ± SE IC50 of 17.2 ± 4.0 µM (n ≥ 4; Fig. 7, B–D). Importantly, however, and in contrast to pranlukast, zafirlukast fully inhibits VRAC at higher concentrations.
Fig. 7.
The cysteinyl leukotriene receptor 1 (CysLT1R) antagonist zafirlukast inhibits volume-regulated anion channel (VRAC). A: structure of zafirlukast. B: zafirlukast concentration-response curve generated from whole cell patch-clamp electrophysiology (mean ± SE IC50 = 17 ± 4 µM; n ≥ 4). C: whole cell currents from human embryonic kidney 293 (HEK293) cells were measured using a ramp protocol from −100 to +100 mV. Cells were exposed to hypotonic bath and hypotonic bath + 30 µM zafirlukast. D: exemplar time course of VRAC activation under hypotonic solution exposure, 30 µM zafirlukast (Z-kast), and wash (hypo) treatment.
DISCUSSION
Since the initial discovery of VRAC currents nearly 30 years ago (10, 21), studies aimed at understanding the physiological roles of this channel have relied heavily on nonspecific inhibitors such as DCPIB, DIDS, niflumic acid, and tamoxifen (18, 51). DCPIB is the best-in-class inhibitor with an IC50 of ~5 µM, and relatively little activity on other anion channels (18). However, recent studies have shown that DCPIB has promiscuous activity toward several other classes of transport proteins, including glutamate transporters, inward rectifier potassium channels, proton-potassium ATPase transporters, and connexin channels (8, 14, 19, 41). With the recent discovery of the LRRC8 gene family, genetic approaches have been used to uncover new and potentially druggable physiological and pathophysiological roles (see below) of VRAC, however compensatory changes in gene expression could potentially confound conclusions about the therapeutic potential of VRAC in certain disease settings. Therefore, fully understanding how acute pharmacological modulation of VRAC function in wild type animals might impact disease progression will require the development of potent, specific, and in vivo-active inhibitors and activators of the channel. This was the motivation behind the present work.
We took a molecular target-based HTS approach to discover new small-molecule modulators of VRAC. The functional assay was modeled after those employed by the Jentsch and Patapoutian groups to discover the LRRC8 gene family in whole-genome siRNA screens (48, 60). Target-based assays are typically developed around a cell line in which the molecular target of interest is massively overexpressed. While this approach is useful for increasing the signal-to-noise ratio of an assay, it can oversimplify the molecular complexity of a target protein in its native environment. For example, it appears that VRAC channels are hexamers composed of the essential LRRC8A subunit and at least one other subunit from the gene family (i.e., LRRC8B-E). The subunit composition and stoichiometry of native VRAC channels are currently unknown and may vary between cell types (55). To avoid biasing our assay toward an overexpressed target that might not represent the endogenously expressed channel, we developed the assay against the native VRAC expressed in HEK293 cells. Future studies will examine whether heterologously expressed VRACs of specific subunit composition (e.g., LRRC8A/C, LRRC8A/D, LRRC8A/D) exhibit unique pharmacological profiles.
We discovered pranlukast as a novel inhibitor of VRAC in a screen of 1,184 FDA-approved drugs. Pranlukast is a nanomolar-affinity antagonist of the CysLT1 receptor, a Gq-coupled receptor expressed in airway smooth muscle cells and other cell types (64, 65). CysLT1 receptor stimulation by the endogenous ligand LTD4 leads to airway constriction, inflammation, and airway damage associated with asthma (50). Pranlukast has been used clinically for decades to treat asthma and works as a competitive antagonist of LTD4 binding to CysLT1R and inhibition downstream of Gq-PLC-IP3-IP3 receptor-calcium signaling. Interestingly, LTD4 has also been shown to stimulate calcium signaling and augment cell volume regulation in Ehrlich ascites tumor cells (28, 44). While the effect of LTD4 on cell volume regulation appears to be independent of intracellular calcium and mediated primarily by cell volume-sensitive potassium channels (25), we systematically explored whether inhibition of VRAC by pranlukast is dependent on the expression and function of CysLT1R. We reasoned that if the inhibition of VRAC by pranlukast is dependent on the CysLT1R pathway, 1) the receptor must be expressed in HEK293 cells, which were used to discover pranlukast and characterize its effects on VRAC function, 2) the receptor should be functional such that its stimulation elicits Gq-coupled calcium signaling HEK293 cells, and 3) stimulation of the receptor should enhance VRAC activity.
Quantitative PCR analysis clearly showed that mRNA for CYSLTR1 is virtually undetectable in HEK293 cells, which is in striking contrast to the THP-1 monocytic cell line that was used as a positive control for CYSLTR1 expression. Our results agree with another study that found undetectable levels of CYSLTR1 mRNA expression in HEK293 cells (2). Although we showed that stimulation of the endogenously expressed Gq-coupled M3 muscarinic receptor with acetylcholine leads to a dose-dependent increase in intracellular calcium, LTD4 application was without effect on intracellular calcium in parental HEK293 cells. Transient transfection of the CysLT1 receptor was able to reconstitute Gq-coupled calcium signaling in HEK293 cells, but LTD4 had no effect on swelling-induced iodide quenching under these conditions. In addition, we observed clear VRAC inhibition in whole cell patch clamp experiments in which intracellular calcium was buffered to vanishingly low concentrations that would not support Gq-coupled calcium signaling. Finally, hypotonic cell swelling has been reported to induce the synthesis of leukotrienes in Ehrlich ascites tumor cells (37), raising the possibility that heterologous expression of the CysLT1R might increase the sensitivity of VRAC to inhibition by pranlukast. However, we found no evidence of this in the present study. Taken together, these data support the conclusion that inhibition of VRAC by pranlukast requires neither the expression nor function of the CysLT1 receptor in HEK293 cells.
We propose that pranlukast inhibits VRAC through direct interactions with the channel protein, for the following reasons. First, as noted above, pranlukast does not require its cognate receptor for VRAC inhibition. Second, the concentrations of pranlukast required to inhibit VRAC 0.1–10 µM are ~25–2,500-fold higher than that needed for inhibition of CysLT1R (IC50 = 4.3 nM) (39). These differences are even greater for zafirlukast (VRAC IC50 = 17 µM vs CysLT1R IC50 = 1.9 nM; >8,000-fold difference) indicating the binding sites for VRAC inhibition are fundamentally different and probably not located on the CysLT1 receptor. Third, pranlukast inhibits VRAC rapidly (τ ~1 min) and reversibly, as would be expected for a direct channel inhibitor. Fourth, pranlukast altered the kinetics VRAC inactivation at positive potentials. While it is formally possible that pranlukast-dependent changes in inactivation are mediated by some unknown signaling pathway or changes in cell membrane properties, for example, we feel it is more likely that this reflects interactions of the small molecule with the VRAC protein. Jentsch and colleagues showed that the inactivation properties of heterologously expressed VRAC currents are dependent on the subunit combination (60) and identified charged residues near the extracellular pore mouth that regulate the inactivation properties of the channel (58). Interestingly, these amino acids are near a residue in LRRC8A (i.e., arginine 103) that was recently proposed to comprise the biding site for DCPIB (33). Studies are underway to test the role of R103 and other pore-lining residues in VRAC inhibition by pranlukast and zafirlukast.
Although pranlukast and zafirlukast are used clinically to treat asthma, we believe it is unlikely that their therapeutic efficacy is related to inhibition of VRAC currents. Whole-plasma concentrations after a single dose range between 0.5 and 1.0 µM (9, 12, 29). However, >99% of both drugs is bound by serum protein, reducing the free fraction of drugs capable of engaging VRAC to <10 nM. This concentration is well below the dose required to inhibit VRAC.
The discovery of pranlukast and zafirlukast represents critical first steps in developing more potent and specific inhibitors of VRAC using medicinal chemistry approaches. Each drug represents a distinct chemical scaffold from which diverse analog libraries can be synthesized for testing. The fluorescence quenching assay described in this work will be ideal for testing compound libraries for analogs with improved potency and efficacy. Immediate chemical synthetic goals for pranlukast are improving VRAC inhibitory efficacy while maintaining potency. Goals for zafirlukast will be maintaining full inhibitor efficacy while improving potency. Both programs should strive toward eliminating activity toward CysLT1R. Studies of structure-activity relationships of pranlukast and zafirlukast could identify a common minimal pharmacophore required for VRAC inhibition and help synthesize structurally unique inhibitors with improved potency, efficacy, and selectivity.
In conclusion, the recent discovery of the LRRC8 gene family that encodes VRAC has renewed interest in developing the molecular pharmacology for this important channel. We provide proof-of-concept that the iodide-quenching assay can be used to discover new pharmacological modulators in high-throughput screens of small-molecule libraries. The development of potent, specific, and in vivo-active small-molecule tools will provide important new tools for probing the integrative physiology, druggability, and therapeutic potential of VRAC in stroke (66), pain (61), and metabolic regulation (30, 56, 63, 68).
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01 DK51610 (to K. Strange) and 1F31DK120225-01 (to E. E. Figueroa), by the Aspirnaut Program (to Julie K. Hudson and Billie G. Hudson), and institutional funds (to J. S. Denton).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.E.F., M.K., and J.SD conceived and designed research; E.E.F. and M.K. performed experiments; E.E.F. and M.K. analyzed data; E.E.F., M.K., K.S., and J.SD interpreted results of experiments; E.E.F. prepared figures; E.E.F., K.S., and J.SD drafted manuscript; E.E.F., K.S., and J.SD edited and revised manuscript; E.E.F., M.K., K.S., and J.SD approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Sujay Kharade and Trevion Williams for assistance early in the project and Dr. Rajan Sah (Washington Univ., St. Louis, MO) for the HEK293-LRRC8A−/− cell line.
REFERENCES
- 1.Akita T, Okada Y. Characteristics and roles of the volume-sensitive outwardly rectifying (VSOR) anion channel in the central nervous system. Neuroscience 275: 211–231, 2014. doi: 10.1016/j.neuroscience.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 2.Atwood BK, Lopez J, Wager-Miller J, Mackie K, Straiker A. Expression of G protein-coupled receptors and related proteins in HEK293, AtT20, BV2, and N18 cell lines as revealed by microarray analysis. BMC Genomics 12: 14, 2011. doi: 10.1186/1471-2164-12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basarsky TA, Feighan D, MacVicar BA. Glutamate release through volume-activated channels during spreading depression. J Neurosci 19: 6439–6445, 1999. doi: 10.1523/JNEUROSCI.19-15-06439.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benfenati V, Caprini M, Nicchia GP, Rossi A, Dovizio M, Cervetto C, Nobile M, Ferroni S. Carbenoxolone inhibits volume-regulated anion conductance in cultured rat cortical astroglia. Channels (Austin) 3: 323–336, 2009. doi: 10.4161/chan.3.5.9568. [DOI] [PubMed] [Google Scholar]
- 5.Best L, Brown PD, Sener A, Malaisse WJ. Electrical activity in pancreatic islet cells: the VRAC hypothesis. Islets 2: 59–64, 2010. doi: 10.4161/isl.2.2.11171. [DOI] [PubMed] [Google Scholar]
- 6.Best L, Sheader EA, Brown PD. A volume-activated anion conductance in insulin-secreting cells. Pflugers Arch 431: 363–370, 1996. doi: 10.1007/BF02207273. [DOI] [PubMed] [Google Scholar]
- 7.Boulos AS, Deshaies EM, Dalfino JC, Feustel PJ, Popp AJ, Drazin D. Tamoxifen as an effective neuroprotectant in an endovascular canine model of stroke. J Neurosurg 114: 1117–1126, 2011. doi: 10.3171/2010.8.JNS09352. [DOI] [PubMed] [Google Scholar]
- 8.Bowens NH, Dohare P, Kuo YH, Mongin AA. DCPIB, the proposed selective blocker of volume-regulated anion channels, inhibits several glutamate transport pathways in glial cells. Mol Pharmacol 83: 22–32, 2013. doi: 10.1124/mol.112.080457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brocks DR, Upward JW, Georgiou P, Stelman G, Doyle E, Allen E, Wyld P, Dennis MJ. The single and multiple dose pharmacokinetics of pranlukast in healthy volunteers. Eur J Clin Pharmacol 51: 303–308, 1996. doi: 10.1007/s002280050202. [DOI] [PubMed] [Google Scholar]
- 10.Cahalan MD, Lewis RS. Role of potassium and chloride channels in volume regulation by T lymphocytes. Soc Gen Physiol Ser 43: 281–301, 1988. [PubMed] [Google Scholar]
- 11.Decher N, Lang HJ, Nilius B, Brüggemann A, Busch AE, Steinmeyer K. DCPIB is a novel selective blocker of ICl,swell and prevents swelling-induced shortening of guinea-pig atrial action potential duration. Br J Pharmacol 134: 1467–1479, 2001. doi: 10.1038/sj.bjp.0704413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dekhuijzen PN, Koopmans PP. Pharmacokinetic profile of zafirlukast. Clin Pharmacokinet 41: 105–114, 2002. doi: 10.2165/00003088-200241020-00003. [DOI] [PubMed] [Google Scholar]
- 13.Deneka D, Sawicka M, Lam AKM, Paulino C, Dutzler R. Structure of a volume-regulated anion channel of the LRRC8 family. Nature 558: 254–259, 2018. doi: 10.1038/s41586-018-0134-y. [DOI] [PubMed] [Google Scholar]
- 14.Deng W, Mahajan R, Baumgarten CM, Logothetis DE. The ICl,swell inhibitor DCPIB blocks Kir channels that possess weak affinity for PIP2. Pflugers Arch 468: 817–824, 2016. doi: 10.1007/s00424-016-1794-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doroshenko P, Sabanov V, Doroshenko N. Cell cycle-related changes in regulatory volume decrease and volume-sensitive chloride conductance in mouse fibroblasts. J Cell Physiol 187: 65–72, 2001. doi:. [DOI] [PubMed] [Google Scholar]
- 16.Duroudier NP, Sayers I, Castagna CC, Fenech AG, Halapi E, Swan C, Hall IP. Functional polymorphism and differential regulation of CYSLTR1 transcription in human airway smooth muscle and monocytes. Cell Biochem Biophys 47: 119–129, 2007. doi: 10.1385/CBB:47:1:119. [DOI] [PubMed] [Google Scholar]
- 17.Feustel PJ, Jin Y, Kimelberg HK. Volume-regulated anion channels are the predominant contributors to release of excitatory amino acids in the ischemic cortical penumbra. Stroke 35: 1164–1168, 2004. doi: 10.1161/01.STR.0000124127.57946.a1. [DOI] [PubMed] [Google Scholar]
- 18.Friard J, Tauc M, Cougnon M, Compan V, Duranton C, Rubera I. Comparative effects of chloride channel inhibitors on LRRC8/VRAC-mediated chloride conductance. Front Pharmacol 8: 328, 2017. doi: 10.3389/fphar.2017.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujii T, Takahashi Y, Takeshima H, Saitoh C, Shimizu T, Takeguchi N, Sakai H. Inhibition of gastric H+,K+-ATPase by 4-(2-butyl-6,7-dichloro-2-cyclopentylindan-1-on-5-yl)oxybutyric acid (DCPIB), an inhibitor of volume-regulated anion channel. Eur J Pharmacol 765: 34–41, 2015. doi: 10.1016/j.ejphar.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 20.Gaitán-Peñas H, Gradogna A, Laparra-Cuervo L, Solsona C, Fernández-Dueñas V, Barrallo-Gimeno A, Ciruela F, Lakadamyali M, Pusch M, Estévez R. Investigation of LRRC8-mediated volume-regulated anion currents in Xenopus oocytes. Biophys J 111: 1429–1443, 2016. doi: 10.1016/j.bpj.2016.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hazama A, Okada Y. Ca2+ sensitivity of volume-regulatory K+ and Cl− channels in cultured human epithelial cells. J Physiol 402: 687–702, 1988. doi: 10.1113/jphysiol.1988.sp017229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffmann EK, Lambert IH, Pedersen SF. Physiology of cell volume regulation in vertebrates. Physiol Rev 89: 193–277, 2009. doi: 10.1152/physrev.00037.2007. [DOI] [PubMed] [Google Scholar]
- 23.Holm JB, Grygorczyk R, Lambert IH. Volume-sensitive release of organic osmolytes in the human lung epithelial cell line A549: role of the 5-lipoxygenase. Am J Physiol Cell Physiol 305: C48–C60, 2013. doi: 10.1152/ajpcell.00412.2012. [DOI] [PubMed] [Google Scholar]
- 24.Hoshino M, Izumi T, Shimizu T. Leukotriene D4 activates mitogen-activated protein kinase through a protein kinase Cα-Raf-1-dependent pathway in human monocytic leukemia THP-1 cells. J Biol Chem 273: 4878–4882, 1998. doi: 10.1074/jbc.273.9.4878. [DOI] [PubMed] [Google Scholar]
- 25.Hougaard C, Niemeyer MI, Hoffmann EK, Sepúlveda FV. K+ currents activated by leukotriene D4 or osmotic swelling in Ehrlich ascites tumour cells. Pflugers Arch 440: 283–294, 2000. doi: 10.1007/s004240000273. [DOI] [PubMed] [Google Scholar]
- 26.Hyzinski-García MC, Rudkouskaya A, Mongin AA. LRRC8A protein is indispensable for swelling-activated and ATP-induced release of excitatory amino acids in rat astrocytes. J Physiol 592: 4855–4862, 2014. doi: 10.1113/jphysiol.2014.278887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackson PS, Strange K. Volume-sensitive anion channels mediate swelling-activated inositol and taurine efflux. Am J Physiol Cell Physiol 265: C1489–C1500, 1993. doi: 10.1152/ajpcell.1993.265.6.C1489. [DOI] [PubMed] [Google Scholar]
- 28.Jørgensen NK, Lambert IH, Hoffmann EK. Role of LTD4 in the regulatory volume decrease response in Ehrlich ascites tumor cells. J Membr Biol 151: 159–173, 1996. doi: 10.1007/s002329900067. [DOI] [PubMed] [Google Scholar]
- 29.Kahnt AS, Rörsch F, Diehl O, Hofmann B, Lehmann C, Steinbrink SD, Angioni C, Geisslinger G, Grösch S, Steinhilber D, Maier TJ. Cysteinyl leukotriene-receptor-1 antagonists interfere with PGE2 synthesis by inhibiting mPGES-1 activity. Biochem Pharmacol 86: 286–296, 2013. doi: 10.1016/j.bcp.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Kang C, Xie L, Gunasekar SK, Mishra A, Zhang Y, Pai S, Gao Y, Kumar A, Norris AW, Stephens SB, Sah R. SWELL1 is a glucose sensor regulating β-cell excitability and systemic glycaemia. Nat Commun 9: 367, 2018. doi: 10.1038/s41467-017-02664-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kasuya G, Nakane T, Yokoyama T, Jia Y, Inoue M, Watanabe K, Nakamura R, Nishizawa T, Kusakizako T, Tsutsumi A, Yanagisawa H, Dohmae N, Hattori M, Ichijo H, Yan Z, Kikkawa M, Shirouzu M, Ishitani R, Nureki O. Cryo-EM structures of the human volume-regulated anion channel LRRC8. Nat Struct Mol Biol 25: 797–804, 2018. doi: 10.1038/s41594-018-0109-6. [DOI] [PubMed] [Google Scholar]
- 32.Kefauver JM, Saotome K, Dubin AE, Pallesen J, Cottrell CA, Cahalan SM, Qiu Z, Hong G, Crowley CS, Whitwam T, Lee WH, Ward AB, Patapoutian A. Structure of the human volume regulated anion channel. eLife 7: e38461, 2018. doi: 10.7554/eLife.38461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kern DM, Oh S, Hite RK, Brohawn SG. Cryo-EM structures of the DCPIB-inhibited volume-regulated anion channel LRRC8A in lipid nanodiscs. eLife 8: 8e42636, 2019. doi: 10.7554/eLife.42636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kimelberg HK, Feustel PJ, Jin Y, Paquette J, Boulos A, Keller RW Jr, Tranmer BI. Acute treatment with tamoxifen reduces ischemic damage following middle cerebral artery occlusion. Neuroreport 11: 2675–2679, 2000. doi: 10.1097/00001756-200008210-00014. [DOI] [PubMed] [Google Scholar]
- 35.Kimelberg HK, Jin Y, Charniga C, Feustel PJ. Neuroprotective activity of tamoxifen in permanent focal ischemia. J Neurosurg 99: 138–142, 2003. doi: 10.3171/jns.2003.99.1.0138. [DOI] [PubMed] [Google Scholar]
- 36.Kinard TA, Satin LS. An ATP-sensitive Cl− channel current that is activated by cell swelling, cAMP, and glyburide in insulin-secreting cells. Diabetes 44: 1461–1466, 1995. doi: 10.2337/diab.44.12.1461. [DOI] [PubMed] [Google Scholar]
- 37.Lambert IH, Hoffmann EK, Christensen P. Role of prostaglandins and leukotrienes in volume regulation by Ehrlich ascites tumor cells. J Membr Biol 98: 247–256, 1987. doi: 10.1007/BF01871187. [DOI] [PubMed] [Google Scholar]
- 38.Lang F, Busch GL, Ritter M, Völkl H, Waldegger S, Gulbins E, Häussinger D. Functional significance of cell volume regulatory mechanisms. Physiol Rev 78: 247–306, 1998. doi: 10.1152/physrev.1998.78.1.247. [DOI] [PubMed] [Google Scholar]
- 39.Lynch KR, O’Neill GP, Liu Q, Im DS, Sawyer N, Metters KM, Coulombe N, Abramovitz M, Figueroa DJ, Zeng Z, Connolly BM, Bai C, Austin CP, Chateauneuf A, Stocco R, Greig GM, Kargman S, Hooks SB, Hosfield E, Williams DL Jr, Ford-Hutchinson AW, Caskey CT, Evans JF. Characterization of the human cysteinyl leukotriene CysLT1 receptor. Nature 399: 789–793, 1999. doi: 10.1038/21658. [DOI] [PubMed] [Google Scholar]
- 40.Mao J, Wang L, Fan A, Wang J, Xu B, Jacob TJ, Chen L. Blockage of volume-activated chloride channels inhibits migration of nasopharyngeal carcinoma cells. Cell Physiol Biochem 19: 249–258, 2007. doi: 10.1159/000100644. [DOI] [PubMed] [Google Scholar]
- 41.Minieri L, Pivonkova H, Caprini M, Harantova L, Anderova M, Ferroni S. The inhibitor of volume-regulated anion channels DCPIB activates TREK potassium channels in cultured astrocytes. Br J Pharmacol 168: 1240–1254, 2013. doi: 10.1111/bph.12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mongin AA. Volume-regulated anion channel—a frenemy within the brain. Pflugers Arch 468: 421–441, 2016. doi: 10.1007/s00424-015-1765-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nilius B, Droogmans G. Ion channels and their functional role in vascular endothelium. Physiol Rev 81: 1415–1459, 2001. doi: 10.1152/physrev.2001.81.4.1415. [DOI] [PubMed] [Google Scholar]
- 44.Pedersen S, Hoffmann EK, Hougaard C, Jorgensen NK, Wybrandt GB, Lambert IH. Leukotriene D4-induced Ca2+ mobilization in Ehrlich ascites tumor cells. J Membr Biol 155: 61–73, 1997. doi: 10.1007/s002329900158. [DOI] [PubMed] [Google Scholar]
- 45.Pedersen SF, Okada Y, Nilius B. Biophysics and physiology of the volume-regulated anion channel (VRAC)/volume-sensitive outwardly rectifying anion channel (VSOR). Pflugers Arch 468: 371–383, 2016. doi: 10.1007/s00424-015-1781-6. [DOI] [PubMed] [Google Scholar]
- 46.Phillis JW, Song D, O’Regan MH. Inhibition by anion channel blockers of ischemia-evoked release of excitotoxic and other amino acids from rat cerebral cortex. Brain Res 758: 9–16, 1997. doi: 10.1016/S0006-8993(97)00155-8. [DOI] [PubMed] [Google Scholar]
- 47.Phillis JW, Song D, O’Regan MH. Tamoxifen, a chloride channel blocker, reduces glutamate and aspartate release from the ischemic cerebral cortex. Brain Res 780: 352–355, 1998. doi: 10.1016/S0006-8993(97)01352-8. [DOI] [PubMed] [Google Scholar]
- 48.Qiu Z, Dubin AE, Mathur J, Tu B, Reddy K, Miraglia LJ, Reinhardt J, Orth AP, Patapoutian A. SWELL1, a plasma membrane protein, is an essential component of volume-regulated anion channel. Cell 157: 447–458, 2014. doi: 10.1016/j.cell.2014.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ransom CB, O’Neal JT, Sontheimer H. Volume-activated chloride currents contribute to the resting conductance and invasive migration of human glioma cells. J Neurosci 21: 7674–7683, 2001. doi: 10.1523/JNEUROSCI.21-19-07674.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sasaki F, Yokomizo T. The leukotriene receptors as therapeutic targets of inflammatory diseases. Int Immunol 31: 607–615, 2019. doi: 10.1093/intimm/dxz044. [DOI] [PubMed] [Google Scholar]
- 51.Sato-Numata K, Numata T, Inoue R, Okada Y. Distinct pharmacological and molecular properties of the acid-sensitive outwardly rectifying (ASOR) anion channel from those of the volume-sensitive outwardly rectifying (VSOR) anion channel. Pflugers Arch 468: 795–803, 2016. doi: 10.1007/s00424-015-1786-1. [DOI] [PubMed] [Google Scholar]
- 52.Shen MR, Droogmans G, Eggermont J, Voets T, Ellory JC, Nilius B. Differential expression of volume-regulated anion channels during cell cycle progression of human cervical cancer cells. J Physiol 529: 385–394, 2000. doi: 10.1111/j.1469-7793.2000.00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Strange K. Cellular volume homeostasis. Adv Physiol Educ 28: 155–159, 2004. doi: 10.1152/advan.00034.2004. [DOI] [PubMed] [Google Scholar]
- 54.Strange K, Emma F, Jackson PS. Cellular and molecular physiology of volume-sensitive anion channels. Am J Physiol Cell Physiol 270: C711–C730, 1996. doi: 10.1152/ajpcell.1996.270.3.C711. [DOI] [PubMed] [Google Scholar]
- 55.Strange K, Yamada T, Denton JS. A 30-year journey from volume-regulated anion currents to molecular structure of the LRRC8 channel. J Gen Physiol 151: 100–117, 2019. doi: 10.1085/jgp.201812138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stuhlmann T, Planells-Cases R, Jentsch TJ. LRRC8/VRAC anion channels enhance β-cell glucose sensing and insulin secretion. Nat Commun 9: 1974, 2018. doi: 10.1038/s41467-018-04353-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tertyshnikova S, Dworetzky S, Bullen A, Natale J, Griffin C, Weaver D.. Cell-Based Assay for the Quantitative High Throughput Screening of Gamma-Aminobutyric Acid-Induced Halide Transport US Patent2006/0257934 A1. November 16, 2006.
- 58.Ullrich F, Reincke SM, Voss FK, Stauber T, Jentsch TJ. Inactivation and anion selectivity of volume-regulated anion channels (VRACs) depend on C-terminal residues of the first extracellular loop. J Biol Chem 291: 17040–17048, 2016. doi: 10.1074/jbc.M116.739342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Voets T, Szücs G, Droogmans G, Nilius B. Blockers of volume-activated Cl− currents inhibit endothelial cell proliferation. Pflugers Arch 431: 132–134, 1995. doi: 10.1007/BF00374387. [DOI] [PubMed] [Google Scholar]
- 60.Voss FK, Ullrich F, Münch J, Lazarow K, Lutter D, Mah N, Andrade-Navarro MA, von Kries JP, Stauber T, Jentsch TJ. Identification of LRRC8 heteromers as an essential component of the volume-regulated anion channel VRAC. Science 344: 634–638, 2014. doi: 10.1126/science.1252826. [DOI] [PubMed] [Google Scholar]
- 61.Wang R, Lu Y, Gunasekar S, Zhang Y, Benson CJ, Chapleau MW, Sah R, Abboud FM. The volume-regulated anion channel (LRRC8) in nodose neurons is sensitive to acidic pH. JCI Insight 2: e90632, 2017. doi: 10.1172/jci.insight.90632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wehner F, Olsen H, Tinel H, Kinne-Saffran E, Kinne RK. Cell volume regulation: osmolytes, osmolyte transport, and signal transduction. Rev Physiol Biochem Pharmacol 148: 1–80, 2003. doi: 10.1007/s10254-003-0009-x. [DOI] [PubMed] [Google Scholar]
- 63.Xie L, Zhang Y, Gunasekar SK, Mishra A, Cao L, Sah R. Induction of adipose and hepatic SWELL1 expression is required for maintaining systemic insulin-sensitivity in obesity. Channels (Austin) 11: 673–677, 2017. doi: 10.1080/19336950.2017.1373225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamaguchi T, Kohrogi H, Honda I, Kawano O, Sugimoto M, Araki S, Ando M. A novel leukotriene antagonist, ONO-1078, inhibits and reverses human bronchial contraction induced by leukotrienes C4 and D4 and antigen in vitro. Am Rev Respir Dis 146: 923–929, 1992. doi: 10.1164/ajrccm/146.4.923. [DOI] [PubMed] [Google Scholar]
- 65.Yamaguchi T, Kohrogi H, Honda I, Sugimoto M, Ando M, Araki S. [Preventive effect of a novel leukotrienes antagonist ONO-1078 on leukotriene C4- and D4-induced human bronchial smooth muscle contraction]. Arerugi 39: 1477–1483, 1990. [PubMed] [Google Scholar]
- 66.Yang J, Vitery MDC, Chen J, Osei-Owusu J, Chu J, Qiu Z. Glutamate-releasing SWELL1 channel in astrocytes modulates synaptic transmission and promotes brain damage in stroke. Neuron 102: 813–827.e6, 2019. doi: 10.1016/j.neuron.2019.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ye ZC, Oberheim N, Kettenmann H, Ransom BR. Pharmacological “cross-inhibition” of connexin hemichannels and swelling activated anion channels. Glia 57: 258–269, 2009. doi: 10.1002/glia.20754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang Y, Xie L, Gunasekar SK, Tong D, Mishra A, Gibson WJ, Wang C, Fidler T, Marthaler B, Klingelhutz A, Abel ED, Samuel I, Smith JK, Cao L, Sah R. SWELL1 is a regulator of adipocyte size, insulin signalling and glucose homeostasis. Nat Cell Biol 19: 504–517, 2017. [Erratum in Nat Cell Biol 19: 740; 873, 2017.] doi: 10.1038/ncb3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang Y, Zhang H, Feustel PJ, Kimelberg HK. DCPIB, a specific inhibitor of volume regulated anion channels (VRACs), reduces infarct size in MCAo and the release of glutamate in the ischemic cortical penumbra. Exp Neurol 210: 514–520, 2008. doi: 10.1016/j.expneurol.2007.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]