Abstract
We earlier established the mouse embryonic stem (ES) cell “GS-2” line expressing enhanced green fluorescent protein (EGFP) and have been routinely using it to understand the molecular regulation of differentiation into cardiomyocytes. During such studies, we made a serendipitous discovery that functional cardiomyocytes derived from ES cells stopped beating when exposed to blue light. We observed a gradual cessation of contractility within a few minutes, regardless of wavelength (nm) ranges tested: blue (~420–495), green (~510–575), and red (~600–700), with green light manifesting the strongest impact. Following shifting of cultures back into the incubator (darkness), cardiac clusters regained beatings within a few hours. The observed light-induced contractility-inhibition effect was intrinsic to cardiomyocytes and not due to interference from other cell types. Also, this was not influenced by any physicochemical parameters or intracellular EGFP expression. Interestingly, the light-induced cardiomyocyte contractility inhibition was accompanied by increased intracellular reactive oxygen species (ROS), which could be abolished in the presence of N-acetylcysteine (ROS quencher). Besides, the increased intracardiomyocyte ROS levels were incidental to the inhibition of calcium transients and suppression of mitochondrial activity, both being essential for sarcomere function. To the best of our knowledge, ours is the first report to demonstrate the monochromatic light-mediated inhibition of contractions of cardiomyocytes with no apparent loss of cell viability and contractility. Our findings have implications in cardiac cell biology context in terms of 1) mechanistic insights into light impact on cardiomyocyte contraction, 2) potential use in laser beam-guided (cardiac) microsurgery, photo-optics-dependent medical diagnostics, 3) transient cessation of hearts during coronary artery bypass grafting, and 4) functional preservation of hearts for transplantation.
Keywords: cardiomyocyte contractility, monochromatic light, reactive oxygen species
INTRODUCTION
The intrinsic fluorescence property of the green fluorescent protein (GFP) has been exploited in biological systems. It has revolutionized the field of live cell imaging in terms of intracellular events like differential gene expression (8), protein localization (11a), distribution of organelles (38), and the cell lineage tracing at the whole organism level (41). In view of its single excitation peak (488 nm) and greater fluorescence intensity (10, 48), the enhanced green fluorescent protein (EGFP), the humanized red-shifted variant of GFP, has indeed become one of the most widely used “reporter” genes for bioimaging (4).
Using the EGFP as a reporter gene, we earlier established EGFP-expressing transgenic “green” mouse (GU-2 and GU-3) lines and established a constitutively EGFP-expressing embryonic stem (ES) cell line (GS-2 line) (37). Using this cell line, we have been studying cell differentiation to functional neural (42) and cardiac (1) lineages. Because of the direct EGFP fluorescence (at 509 nm, following excitation at 488 nm), it has been possible to visualize GS-2 ES cell-derived differentiated cell types in vitro and in vivo (37). During one of our routine studies on cardiomyocyte differentiation of GS-2 ES cells, we made a serendipitous observation on the cessation of contractility of functional cardiomyocytes, following exposure to blue light. Because this finding has, so far, not been reported, we began investigating the underlying mechanisms.
In this regard, there have been a few reports on the effect of visible light exposure, especially blue light, on various cellular functions, such as proliferation (14, 16), differentiation (43, 50), and mitochondrial respiration (44, 49) involving a number of different cell types. However, only very few studies have looked at the light effect on cardiomyocyte contractility. In the chick heart cell aggregates, cessation of contractility, following exposure to ultraviolet light, was reported as being due to membrane depolarization in the cells (30). Contrastingly, low-energy visible light illumination was shown to accelerate the beating frequency in isolated chick hearts (15), also, in rat cardiomyocytes, which was accompanied by an increase in the intracellular calcium levels (24). In the human, a wavelength-specific change in heart rate variability was observed when healthy volunteers were exposed to monochromatic visible light (36). From the foregoing, it is interesting to investigate the phenomenon of light effect on cardiac contractility function. Because no detailed study investigating the effect of monochromatic visible light on cardiomyocyte contractility behavior has so far been reported, we investigated the possible mechanism(s) underlying our serendipitous observation on monochromatic visible light-induced inhibition of contractility of functional cardiomyocytes, derived from mouse ES cells.
MATERIALS AND METHODS
Culture and differentiation of mouse ES cells.
Two mouse ES cell lines were used in the present study. These included 1) the in-house-derived constitutive EGFP-expressing transgenic GS-2 ES cell line (37) and 2) the wild-type D3 ES cell line (13). Both cell lines were cultured as described previously (37, 45). Briefly, cells were cocultured on a monolayer of mitomycin C (mit C)-treated mouse embryonic fibroblasts (MEFs) on 0.1% gelatin (Sigma)-coated 35-mm dishes (Greiner). The cells were cultured in the ES cell culture medium containing DMEM, 15% ES cell-qualified fetal bovine serum (FBS), 100 µM nonessential amino acids (NEAA), 2 mM l-glutamine, 100 µM 2-mercaptoethanol, 100 U/mL penicillin, and 100 µg/mL streptomycin (all purchased from Thermo Fisher Scientific) supplemented with 1,000 U/mL leukemia inhibitory factor (Millipore). The ES cells were cultured until they attained confluency of 80–90%, after which colonies were trypsinized and passaged at a ratio of 1:6 or 1:8 on fresh mit C-treated MEFs.
For differentiation of ES cells, the hanging-drop method was used as previously described (37, 45). Briefly, the ES cell colonies were trypsinized and dispersed into a single cell suspension in ES cell differentiation medium containing DMEM, 20% heat-inactivated FBS, 100 µM NEAA, 2 mM l-glutamine, 100 µM 2-mercaptoethanol, 100 U/mL penicillin, and 100 µg/mL streptomycin. Embryoid bodies (EBs) were formed by aggregation of ~400 ES cells in a 20-µl drop for 48 h. Formed EBs were harvested and placed in suspension culture for an additional 72 h after which the EBs were transferred to 0.1% gelatin-coated 60-mm dishes or 24-well plates. Medium was changed every alternate day, and microscopic observations were made to identify the appearance of beating cardiomyocyte clusters in the EB outgrowth, as before (37).
Monochromatic light exposure experiments.
Individual beating cardiomyocyte clusters were identified in the EB outgrowths between days 10 and 12 of differentiation from four to five independent cultures for each experiment. Cardiomyocyte beatings were recorded on a temperature-controlled microscope stage of an Olympus IX-70 inverted microscope. Live videography was performed using the Olympus CellSens Software, and beating frequencies of cardiac clusters were determined by video playbacks and manual counting of the beats recorded per minute. For light exposure experiments, the beating pattern for each cluster was recorded for 2 min in white light to determine the baseline/initial beating frequency (Fig. 1). This was followed by exposure to blue, green, or red light using the fluorescein isothiocyanate, tetramethylrhodamine isothiocyanate, or Cy5 filters, respectively. To visualize the beating clusters, derived from GS-2 ES cells, white light of very low intensity was used while exposing them to green or red lights. A similar approach was used for beating clusters derived from the wild-type D3 ES cells during exposure to all three lights. Changes in beating frequency were monitored every minute until complete cessation of contractility. Culture dishes were then placed in the incubator (in the dark) and were taken out at 1, 2, 3, 6, 9, 12, and 24 h to observe the recovery of contractility in the beating clusters (Fig. 1).
Fig. 1.
Experimental design to examine the effect of monochromatic light on cardiomyocytes derived from embryonic stem (ES) cells and evaluation of the time kinetics of their cessation and recovery. Cardiomyocytes derived from ES cells were exposed to blue, green, or red light until complete cessation of beating was observed. Beatings were recorded every minute during monochromatic light exposure. Recovery of beating in the clusters following light exposure was monitored after 1, 2, 3, 6, 9, 12, and 24 h. The time frames indicated are not drawn to scale.
For exposure in Dulbecco’s phosphate-buffered saline (DPBS; Thermo Fisher Scientific), the cell culture medium was replaced with DPBS 15 min before monochromatic light exposure. After cessation of beating, DPBS was replaced with fresh cell culture medium, the dishes were placed back in the incubator, and the recovery of contractility was monitored as mentioned above. For assessment of cardiomyocyte contractility in the presence of N-acetylcysteine (NAC; Sigma), cell culture medium was replaced with culture medium containing 10 mM NAC for 15 min before monochromatic light exposure.
Cardiomyocyte enrichment.
On differentiation day 10, beating clusters from EB outgrowths were mechanically scooped out under an Olympus stereo zoom microscope using a glass pipette. The scooped beating clusters were then dispersed by collagenase II (Thermo Fisher Scientific) treatment for 15 min. The dispersed cardiomyocytes were then plated onto geltrex (Thermo Fisher Scientific)-coated 35-mm dishes.
Fluorescence localization studies.
Enriched cardiomyocytes derived from GS-2 ES cells were fixed in 4% paraformaldehyde, 1) before exposure to monochromatic light, 2) immediately after exposure to monochromatic light, or 3) after recovery of beating, for 15 min at room temperature. Fixed cells were then permeabilized using 0.2% Triton X-100 at 4°C for 5 min. Blocking buffer containing 1% BSA was added to the cells and incubated for 2–4 h. This was followed by incubation with cardiac troponin I antibody (1:200, catalog no. MAB1691, RRID:AB_11212281; Millipore) and anti-mouse Alexa Fluor 546 (catalog no. A-11003, RRID:AB_2534071; Thermo Fisher Scientific) antibody or with phalloidin-tetramethylrhodamine isothiocyanate (Sigma) for staining sarcomeric actin. The cells were then counterstained with DAPI (Sigma) and imaged using the Zeiss LSM880 microscope. All steps were followed by three washes with DPBS.
Quantification of reactive oxygen species.
Cells were washed two times with DPBS, and DMEM containing 10 µM dihydroethidium (DHE; Thermo Fisher Scientific) was added 10 min before monochromatic light exposure. Temporal changes in DHE fluorescence were recorded in beating clusters exposed to blue, green, or red light. The fluorescence intensity in the clusters before and after light exposure was measured using the ImageJ software to determine the fold change in DHE fluorescence.
Calcium imaging.
For calcium imaging, ~0.1 million enriched cardiomyocytes were plated on 35-mm dishes with glass bottoms (Greiner). Calcium transients in the contracting cardiomyocytes were visualized using the rhod 3 calcium imaging kit (Thermo Fisher Scientific) as per the manufacturer’s instructions. Briefly, 2 mL DMEM containing 10 µM of rhod 3-AM dye, 1× powerload concentrate, and 0.25 mM probenecid were added to each 35-mm dish, and the cells were incubated for 1 h at 37°C in the dark. The medium was then replaced with fresh DMEM containing 0.25 mM probenecid, and the cells were further incubated for 1 h before imaging on a Zeiss LSM880 microscope. Calcium transients were recorded for beating clusters before exposure to monochromatic light and after cessation of contractility, following light exposure. All captured videos were then analyzed using the ImageJ software to determine the fluorescence intensity changes in the cells.
Measurement of mitochondrial membrane potential.
Enriched cardiomyocytes (~0.1 million) cultured in a 35-mm dish with a glass bottom were stained with 1 µM tetramethylrhodamine methyl ester (TMRM) in DMEM for 30 min at 37°C in the dark. Cells were then washed three times with DPBS, and then fresh DMEM was added. The relative change in mitochondrial membrane potential (MMP), following exposure to monochromatic light, was measured using the Zeiss LSM880 microscope. The relative change in MMP was quantified by measuring the mean fluorescence intensity in the cells using the ImageJ software.
Measurement of cellular ATP.
EB outgrowths containing functional cardiomyocyte clusters were exposed to blue, green, or red light until complete cessation of beating was observed. The EB outgrowths were then trypsinized to obtain a single cell suspension. Total cellular ATP was quantified using the ATP bioluminescence assay kit HS II (Roche). Briefly, the beating clusters from 10 to 15 EB outgrowths in a single 35-mm dish were exposed to monochromatic light until they ceased to beat. Following cessation of beating, the cells were trypsinized, and one million cells were taken for quantification of cellular ATP. The cells were first lysed using the lysis reagent for 10–15 min on ice and then incubated with an equal volume of the luciferase reagent. The luminescence was then measured using the GloMax 96-well plate luminometer. All readings were normalized to the total number of cells analyzed.
Statistical analysis.
All values are presented as mean values ± SE. The statistical significance between the groups was determined by Student’s t-test or one-way ANOVA followed by Tukey’s honest significant difference post hoc test. Values were considered significant if the P value was <0.05. All statistical analyses were performed using GraphPad Prism Software, version 6.
RESULTS
Effect of blue light exposure on GS-2 ES cell-derived beating clusters.
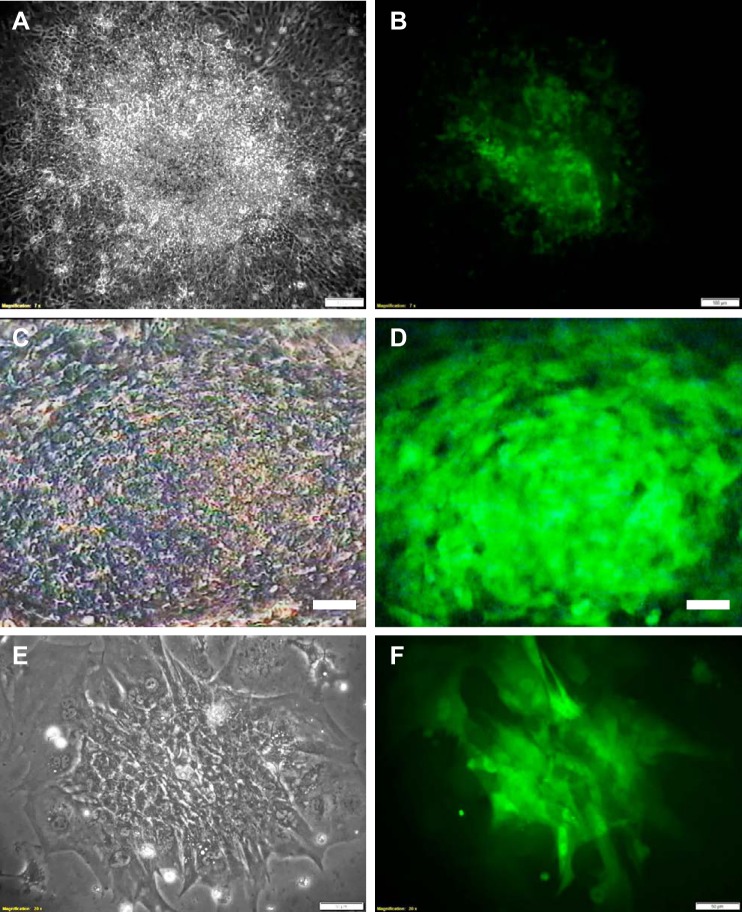
Our in-house-derived GS-2 ES cell line showed robust differentiation to functional cardiomyocytes. Multiple beating clusters were observed in the EB outgrowths from day 8 of differentiation (Fig. 2, A and B). The highest beating frequency (~80–90 beats/min) was observed in most of the clusters by day 10 of differentiation. Exposure of the beating clusters to blue light resulted in the complete cessation of their contractility within 15–20 min (Supplemental Video SV1A; Supplemental data available at https://doi.org/10.6084/m9.figshare.8792603). Following incubation in the dark, the clusters gradually regained their contractility within 1–2 h, with most of the clusters regaining 100% of their initial beating frequency within 24 h. Observations made in three independent experiments are summarized in Table 1. The cessation and recovery of beating in a single GS-2 ES cell-derived beating cluster (Fig. 2, C and D) is shown in Supplemental Video SV1B.
Fig. 2.
Cardiomyocytes differentiated from GS-2 embryonic stem (ES) cells. Multiple contracting cardiomyocyte clusters observed in a single EB outgrowth on differentiation day 10 (A and B), a single beating cluster (C and D), and enriched functional cardiomyocytes derived from EB outgrowths (E and F). A, C, and E show phase-contract images, and B, D, and F show their respective fluorescent images. Scale bar: 100 µm (A–D), and 50 µm (E and F).
Table 1.
Quantitative analysis of cessation and recovery of cardiomyocyte contractility in a cohort of beating clusters derived from GS-2 embryonic stem cells, following exposure to blue light
| Experiment | No. of Clusters Observed | No. of Clusters Ceased Beating (%) | Time Taken for Cessation, min | No. of Clusters Regained Beating (%) | Time Taken to Regain Beating, h |
|---|---|---|---|---|---|
| 1 | 7 | 7 (100) | 16.2 ± 1.5 | 6 (85.7) | 1–3 |
| 2 | 5 | 5 (100) | 13.4 ± 4 | 5 (100) | 1–2 |
| 3 | 4 | 3 (75) | 14.1 ± 3.5 | 3 (100) | 1–2 |
| Total/mean | 16 | 15 (93.75) | 14.57 ± 0.8 | 14 ( 93.33) | 1–3 |
Effect of monochromatic light exposure on cardiomyocyte contractility: involvement of wavelength, intracellular EGFP expression, and physicochemical parameters.
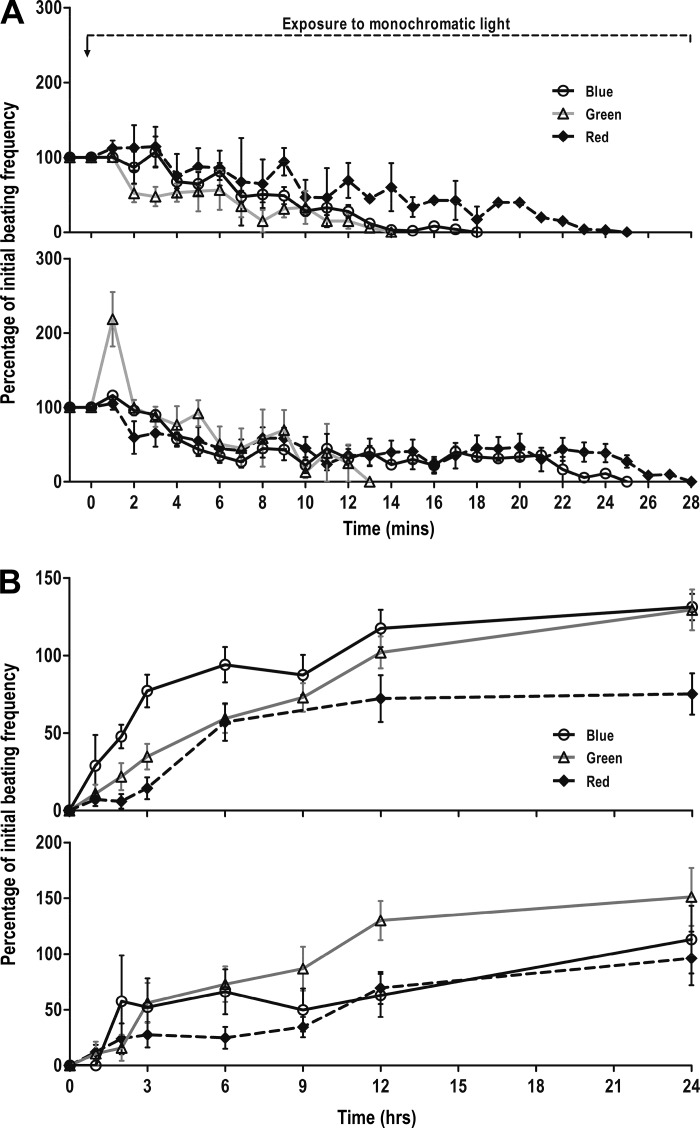
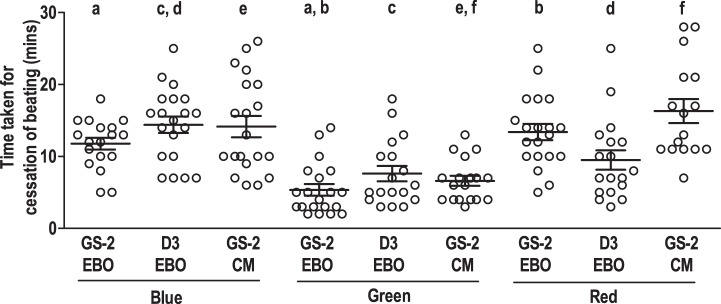
To understand the effect of various wavelengths of light on cardiomyocyte contractility, beating clusters derived from GS-2 ES cells were exposed to green and red lights (Supplemental Video SV2, A and B). Beating clusters exposed to either green or red light showed a similar cessation and recovery of contractility as was observed in the case of blue light (Fig. 3, A and B, top). A complete cessation of beating was observed in 85.7, 90.9, and 80% of clusters, following exposure to blue, green, and red lights, respectively. Interestingly, the time taken for cessation of beating, in the case of clusters exposed to green light, was significantly lower (5.4 ± 1.1 min) than clusters exposed to blue (11.8 ± 0.8 min) or red (13.4 ± 1.1 min) lights (Fig. 4). Following incubation in the dark, a complete recovery of beating was observed in all clusters that had ceased to contract following exposure to blue and green lights. However, only 75% of clusters regained contractility following exposure to red light. The clusters regained contractility by 1–2, 1–9, and 1–24 h following exposure to blue, green, and red lights, respectively, with almost all clusters recovering 100% of their initial beating frequency within 24 h.
Fig. 3.
Assessment of cessation and recovery of cardiomyocyte contractility in beating clusters and enriched cardiomyocytes derived from GS-2 embryonic stem (ES) cells, following exposure to three different lights. Time course of cessation (A) and recovery (B) profiles of cardiomyocyte contractility in beating clusters (top) and enriched cardiomyocytes (bottom) derived from GS-2 ES cells, following exposure to blue, green, or red lights. Values are shown as percentages of beating frequency with respect to initial beating frequency. Values represent means ± SE from 4 different experiments, with 4–5 clusters in each experiment and a total of 15–20 clusters in each group in A and B.
Fig. 4.
Time kinetics of cessation of cardiomyocyte contractility in beating clusters and enriched cardiomyocytes derived from embryonic stem (ES) cells, following exposure to monochromatic light. Time taken for cessation of cardiomyocyte contractility in beating clusters in intact EB outgrowths (EBO) derived from transgenic GS-2 ES cells, wild-type D3 ES cells, and enriched GS-2 ES cell-derived cardiomyocytes (CMs) following exposure to blue, green, and red light, respectively. Values represent means ± SE from 4 different experiments using 3–6 clusters in each experiment with a total of 14–20 clusters each group. Values with the same alphabet differ significantly: P < 0.0005 (f), 0.005 (a, b, c, and e), and 0.05 (d).
To understand whether intracellular EGFP expression had an influence on the monochromatic light-induced cessation of contractility, beating clusters derived from the wild-type D3 ES cells were exposed to blue, green, and red lights (Supplemental Video SV3, A–C). The time course of cessation and recovery was similar for beating clusters derived from D3 and GS-2 ES cells (Supplemental Fig. S1, A and B). In the case of D3 ES cell-derived beating clusters also, the time taken for cessation of beating was the least for green light exposure followed by red and blue light exposures (Supplemental Table S1). In addition to this, the time taken for cessation following exposure to any of the three lights was not significantly variable between beating clusters derived from either of the two cell lines (Fig. 4). Interestingly, however, the number of D3 ES cell-derived beating clusters that ceased to beat following blue and green light exposure was lower compared with GS-2 ES cell-derived beating clusters. Only 80, 75, and 75% of the D3 ES cell-derived beating clusters ceased to contract following exposure to blue, green, and red lights, respectively (Supplemental Table S1). Also, only 75, 94.4, and 94.4% of the D3 ES cell-derived beating clusters regained contractility following blue-, green-, and red light-mediated cessation, respectively. The time taken for recovery of beating in clusters following exposure to the three lights, however, was similar for both cell lines (Supplemental Table S1).
To understand the potential role of culture medium components and changes in extracellular pH in the monochromatic light-induced inhibition of cardiomyocyte contractility, beating clusters were exposed to blue, green, and red lights in DPBS. The time profile of cessation and recovery of contractility in GS-2 ES cell-derived beating clusters following exposure to the three lights in DPBS was similar to that observed in cell culture medium (Supplemental Fig. S2, A and B). The time taken for cessation of beating in the clusters was significantly lower following green light exposure when compared with blue and red light exposures (Supplemental Fig. S2C). Following incubation in the dark, all clusters that had ceased beating following exposure to monochromatic light, irrespective of the wavelength of the light used, regained contractility (Supplemental Table S2). The time taken for recovery of beating in clusters that ceased to contract following exposure to the three lights in DPBS was similar to that observed in the case of exposure in culture medium (Supplemental Table S2).
Effect of monochromatic light exposure on enriched cardiomyocytes.
The cessation and recovery of contractility in enriched cardiomyocytes derived from GS-2 ES cells was assessed following exposure to monochromatic light. Syncytia of functional cardiomyocytes derived from GS-2 ES cells were exposed to blue, green, or red light, and the time course of changes in beating frequency during monochromatic light exposure and recovery was evaluated (Figs. 2, E and F, and 3, A and B, bottom, and Supplemental Videos SV1C and SV4, A and B). Of the cardiomyocytes exposed to any of the three lights, 85–90% of the syncytia ceased to contract. The time take for cessation of contractility following exposure to blue, green, and red lights was 14.2 ± 1.4, 6.6 ± 0.7, and 16.3 ± 1.7 min, respectively. No significant difference was found in the cessation kinetics between intact beating clusters in EB outgrowth vis-à-vis syncytia of enriched cardiomyocytes when exposed to any of the three lights (Fig. 4).
Another striking observation was the beating behavior of enriched cardiomyocytes following exposure to green light. A rapid increase in the beating frequency was observed in almost all syncytia immediately following exposure to green light (Fig. 3A, bottom). This was followed by a gradual decrease in beating frequency in the cells similar to that observed in the case of intact beating clusters in the EB outgrowths.
While all cardiomyocytes that ceased to contract following exposure to green light regained contractility within 24 h, only 75% recovered contractility following blue and red light exposure. Also, the time taken for recovery of beating was longer for enriched cardiomyocytes exposed compared with intact beating clusters, wherein, enriched cardiomyocytes took 1–6, 1–9, and 3–12 h to recover beating following exposure to blue, green, and red lights, respectively.
Evaluation of the effect of monochromatic light on sarcomere integrity in enriched cardiomyocytes.
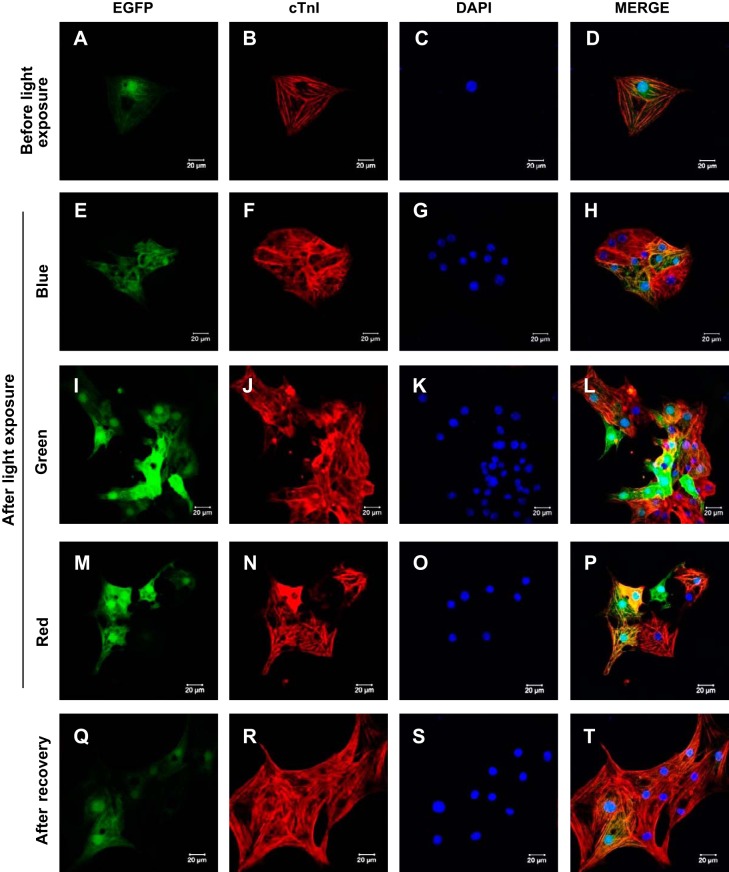
The sarcomere integrity of enriched cardiomyocytes that ceased to contract following exposure to blue, green, or red lights was evaluated by staining for sarcomere proteins (actin and troponin I). No striking differences were observed in the staining patterns of troponin I (Fig. 5, A–T) or actin (Supplemental Fig. S3, A–P) following exposure to any of the three lights when compared with unexposed cardiomyocytes or cardiomyocytes that regained beating.
Fig. 5.
Immunocytochemical assessment of sarcomere integrity in enriched cardiomyocytes derived from GS-2 embryonic stem (ES) cells, following exposure to monochromatic light. Enriched GS-2 ES cell-derived functional cardiomyocytes showing constitutive expression of enhanced green fluorescent protein (EGFP; A, E, I, M, and Q) and sarcomere organization evidenced by cardiac troponin I (cTnI) immunostaining (B, F, J, N, and R), counterstained with nuclear stain DAPI (C, G, K, O, and S) before exposure to monochromatic light (A–D), following exposure to blue (E–H), green (I–L), and red (M–P) lights, and after recovery of beating (Q–T). Scale bar: 20 µm.
Effect of monochromatic light on intracellular reactive oxygen species levels in beating clusters.
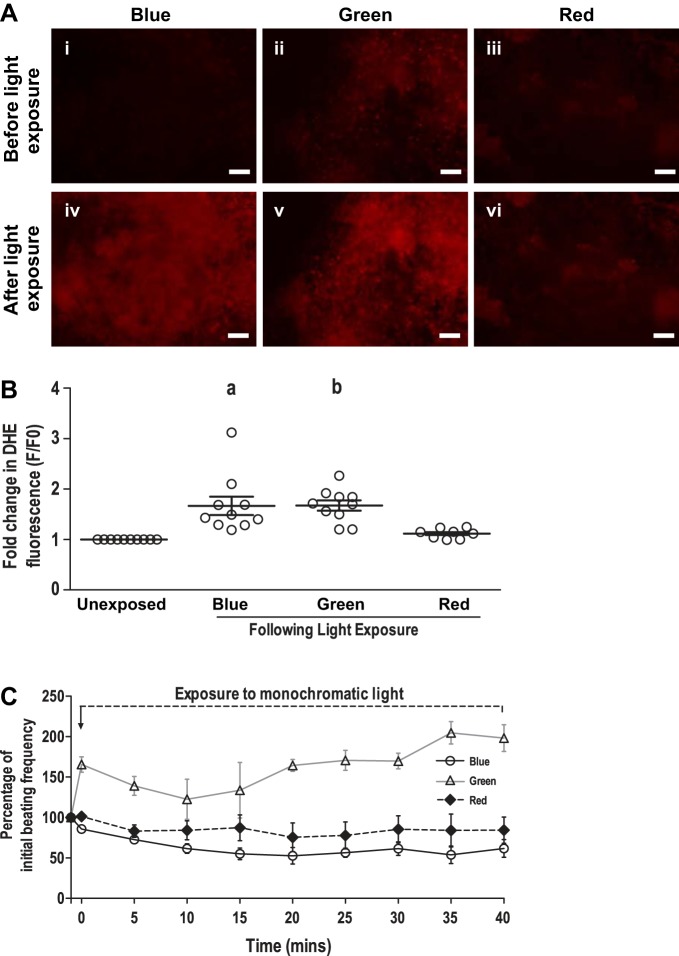
The effect of monochromatic light exposure on intracellular reactive oxygen species (ROS) levels in beating clusters derived from GS-2 ES cells was assessed. A significant increase in the intracellular ROS level was observed in beating clusters following exposure to blue and green lights but not following red light exposure, evidenced by an increase in DHE fluorescence in the clusters (Fig. 6, A and B). The increase in DHE fluorescence correlated to a decrease in beating frequency in the cardiomyocyte clusters in cases of blue and green light exposures. The role of light-induced ROS production in cessation of cardiomyocyte contractility was further validated by exposing beating clusters to blue and green lights in medium containing 10 mM NAC, a ROS quencher. As expected, the clusters did not cease to contract in medium supplemented with NAC, following exposure to blue or green lights (Fig. 6C). Interestingly, although red light exposure did not result in a significant increase in intracellular ROS levels, exposure of GS-2 ES cell-derived beating clusters to red light in cell culture medium supplemented with NAC prevented inhibition of cardiomyocyte contractility (Fig. 6C).
Fig. 6.
Assessment of intracellular reactive oxygen species (ROS) levels in beating cardiomyocyte clusters derived from GS-2 embryonic stem (ES) cells, following exposure to monochromatic light. A: representative images showing an intracellular ROS in beating cardiomyocyte clusters derived from GS-2 ES cells, following exposure to blue (i and iv), green (ii and v), and red (iii and vi) light evidenced by dihydroethidium (DHE) fluorescence. B: fold change in DHE fluorescence in cardiomyocyte beating clusters, following exposure to monochromatic light. C: time course of beating frequency in cardiomyocyte beating clusters derived from GS-2 ES cells, following exposure to blue, green, and red lights in medium supplemented with 10 mM N-acetylcysteine. Values in C are presented as percentages of initial beating frequency in C. Values are presented as means ± SE of 3 individual experiments with 3–4 clusters in each experiment and a total of 8–10 beating clusters in each group in B and C. aP < 0.01 and bP < 0.05. Scale bar: 100 µm.
Evaluation of effect of monochromatic light on calcium transients in enriched cardiomyocytes.
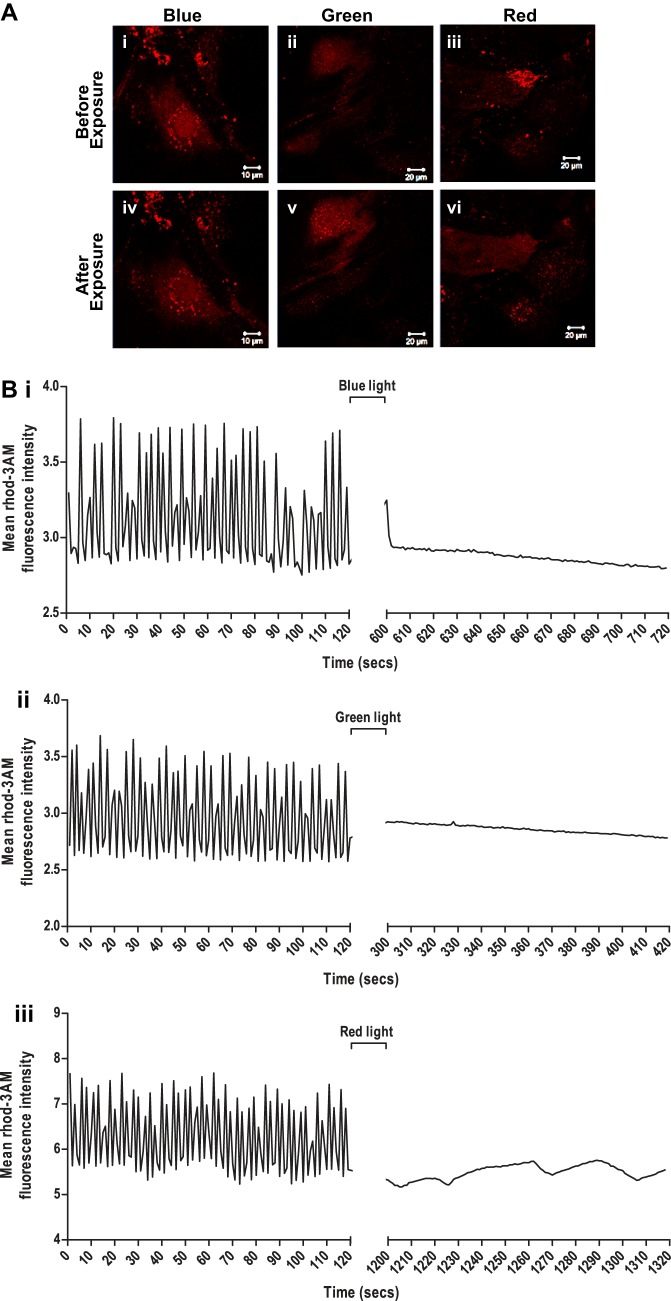
The effect of monochromatic light exposure on calcium transients in GS-2 ES cell-derived enriched cardiomyocytes was evaluated. Real-time videography showed efficient calcium cycling in unexposed cardiomyocytes as evidenced by the continuous increase and decrease in intracellular rhod 3-AM fluorescence with each contraction-relaxation cycle (Fig. 7, A and B). These transients were completely abolished in cardiomyocytes that ceased to contract following exposure to blue, green, and red lights (Fig. 7B). Interestingly, the basal rhod 3-AM fluorescence in enriched cardiomyocytes was higher following green light exposure but not following blue or red light exposure (Fig. 7B), indicating an increase in intracellular calcium levels in the cardiomyocytes following green light exposure.
Fig. 7.
Assessment of intracellular calcium transients in enriched cardiomyocytes derived from GS-2 embryonic stem (ES) cells, following exposure to monochromatic light. A: intracellular calcium in enriched cardiomyocytes derived from GS-2 ES cells before (i-iii) and following (iv-vi) exposure to blue (i and iv), green (ii and v), and red (iii and vi) light visualized by rhod 3-AM staining. Scale bar: 10 µm (i and iv) and 20 µm (ii, iii, v, and vi). B: quantification of intracellular calcium transient-enriched cardiomyocytes derived from GS-2 ES cells before and after exposure to blue (i), green (ii), and red (iii) light measured by changes in relative rhod 3-AM fluorescence intensity in the cells.
Effect of monochromatic light on mitochondrial membrane potential and ATP production.
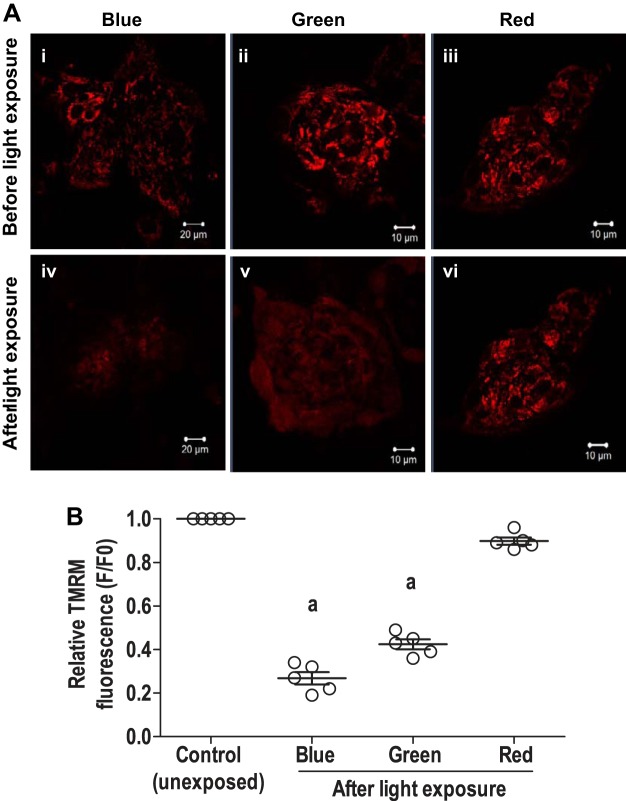
The mitochondrial activity in enriched cardiomyocytes derived from GS-2 ES cells following monochromatic light exposure was assessed by evaluating the relative changes in the MMP. A very significant loss of MMP was observed in enriched cardiomyocytes following exposure to blue and green lights (Fig. 8, A and B) as evidenced by loss of TMRM fluorescence in the cells. On the other hand, no significant changes were observed in TMRM fluorescence in cells following exposure to red light (Fig. 8, A and B). Furthermore, the loss of mitochondrial activity was assessed by measuring the total intracellular ATP content following monochromatic light exposure. Evaluation of total intracellular ATP content from whole EB outgrowths following exposure to blue, green, and red lights showed variable outcomes in the three individual experiments performed. Overall, a sharp decrease in the intracellular ATP levels was observed in the cardiomyocytes following exposure to blue and green lights, while no striking changes were observed following exposure to red light. The results of ATP estimation from four independent experiments are summarized in Supplemental Table S3.
Fig. 8.
Assessment of mitochondrial membrane potential (MMP) and intracellular ATP levels in cardiomyocytes derived from GS-2 embryonic stem (ES) cells, following exposure to monochromatic light. A: MMP in enriched cardiomyocytes derived from GS-2 ES cells before (i–iii) and following (iv–vi) exposure to blue (i and iv), green (ii and v), and red (iii and vi) light as evidenced by tetramethylrhodamine methyl ester (TMRM) staining. Scale bar: 20 µm (i and iv), 10 µm (ii, iii, v, vi). B: quantitative analysis of relative loss of MMP in enriched cardiomyocytes derived from GS2 ES cells, following exposure to blue, green, or red lights. Values are presented as means ± SE of 5 individual experiments in B. aP < 0.001.
DISCUSSION
Our study is the first to report on the detailed insights into the monochromatic light-mediated cessation of contractility in functional cardiomyocytes derived from mouse ES cells. We demonstrate a wavelength-independent and a reversible cessation of mouse ES cell-derived functional cardiomyocytes following exposure to monochromatic visible light, with the most striking effect observed with the green light. Interestingly, this phenomenon was, however, not observed following exposure to white light. Another unique aspect of our findings is the recovery of contractility in cardiomyocytes within a few hours, following incubation in the dark. This light-mediated cardiomyocyte contractility behavior that we observed could have implications in the development of optics-dependent techniques in the field of cardiology and medical diagnostics.
It is regarded that various extrinsic and/or intrinsic factors could influence the monochromatic light-mediated cessation of cardiomyocyte contractility. Among the extrinsic factors, intensity of light and physicochemical parameters, particularly temperature, pH, and medium composition, could play a critical role in the visible light-induced changes in cellular functions (33, 46). However, in our experiments, we observed that the cessation of cardiomyocyte contractility occurred despite exposure to the three wavelength lights in a temperature- and pH-regulated environment as well as in DPBS, which lack the presence of light-sensitive molecules (riboflavin, tryptophan, etc.) and have a stable pH, unlike the biocarbonate- and CO2-based culture medium. Interestingly, the above manifestation could be observed in the enriched cardiomyocytes as well. Therefore, our findings indicate that the extracellular environment did not affect the monochromatic light-mediated cessation of cardiomyocyte contractility and that the effect of monochromatic light was intrinsic to cardiomyocytes per se. We did not address the effect of intensity of light used for irradiation since the effect of light exposure does not appear to have any lasting effects on cell survival or its functionality. Of relevance in this context are a few reports on the effect of physicochemical factors, in a number of cell types, which include cardiomyocytes (23), dental pulp cells (26, 49), fibroblasts (33, 40), and neurons (39) that, contrary to our observations, have shown the extracellular environment to be a key mediator of light-induced intracellular changes. Interestingly, we also did not observe any correlation between the time taken for cessation of contractility and size of beating clusters (Supplemental Videos S1A and S2B). This was contrary to previous reports, wherein the size of cardiomyocyte clusters had a role to play in the cessation of cardiomyocyte contractility (30).
Moreover, we believe that the light-induced inhibition of cardiomyocyte contractility is not due to intracellular EGFP fluorescence, since we observed similar cessation and recovery both with the EGFP-expressing (GS-2) and wild-type (D3) ES cell lines. In contrast, a few reports showed that EGFP inhibit actin-myosin interactions in rat primary cardiomyocytes, thereby affecting their contractility (2, 31). Despite this, two key inferences could, therefore, be drawn from our observations. First, the intracellular transgenic EGFP expression in GS-2 ES cell-derived cardiomyocytes did not have any role in the visible light-induced cessation of contractility. Second, our findings are not only intrinsic to cardiomyocytes derived from GS-2 ES cells but could be extrapolated to cardiomyocytes differentiated from other ES cell lines as well. Of relevance in this context is our preliminary observation that the human induced pluripotent stem cell-derived cardiomyocytes also ceased to contract following exposure to blue light (data not shown), thereby indicating that our findings are not confined to murine cardiomyocytes, but it could be observed with those from others species, including humans.
It could be argued that the loss of cardiomyocyte contractility may arise as a consequence of the loss of sarcomere structure and/or function. Because we did not observe any change in either troponin I or sarcomeric actin staining in cardiomyocytes, following exposure to any of the three lights, we conclude that the loss of cardiomyocyte contractility is unlikely due to the loss of sarcomere structure. Nevertheless, the regulation of sarcomere function involves a complex interplay of various intracellular events, namely 1) entry of calcium ions into cells in response to changes in membrane potential, 2) calcium-induced calcium release from the sarcoplasmic reticulum (SR), 3) binding of calcium to the sarcomere, sliding of myosin chain over actin initiating the contraction of the cell, and 4) reuptake of calcium into the SR and mitochondria, resulting in the relaxation of the cardiomyocyte (6). These cellular events are in turn regulated by some key intrinsic factors like the ion channels, mitochondrial ATP production, etc. (5, 12, 17). Additionally, visible light is shown to directly or indirectly regulate these factors in various cell types, including cardiomyocytes. Hence, it is possible that the visible light-induced cessation of cardiomyocyte contractility that we observed may be due to the dysregulation of one of the aforementioned factors, which in turn lead to loss of sarcomere function.
An important factor influencing sarcomere function of cardiomyocytes is the cyclic (transient) changes in intracellular calcium levels via dynamic opening and closing of various cation channels in membrane, SR, and mitochondria. In our study, we observed a complete abolition of calcium transients in the GS-2 ES cell-derived enriched cardiomyocytes, following exposure to any of the three lights. Previous studies have shown the presence of light-sensitive cation channels, especially the transient receptor protein (TRP) family of ion channels, in murine cardiomyocytes (3, 32). Of significance is our observed increase in intracellular calcium levels following exposure of cells to green light. This increase could be due to activation of the TRPA1 and/or TRPV1 calcium channels (3, 7, 24). Because the opening and closing of ion channels is a rapid process, ranging from milliseconds to a few seconds (17), it is unlikely that the cessation of cardiomyocyte contractility observed in our study is due to their direct regulation by visible light. However, the green light-induced activation of the TRPV1 channels can be attributed to the rapid increase in beating frequency (Fig. 3A, bottom) and the increased basal rhod 3 fluorescence (Fig. 7B) in the enriched cardiomyocytes following light exposure.
In addition to inhibition of calcium transients, we also observed a significant temporal loss in the MMP in the cardiomyocytes following exposure to blue and green light, thereby indicating a loss of mitochondrial activity in these cells. We were able to validate this observation by observing a sharp decrease in cellular ATP levels in the EB outgrowths following cessation of cardiomyocyte contractility on exposure to blue or green lights. Consistent with our observation, a similar decrease in MMP and ATP production was reported in adipose-derived stem cells following exposure to blue and green lights (44). Of relevance here is the fact that, in cardiomyocytes, ATP has been shown to play a critical role in the ion channel activation, regulation of calcium uptake into the SR, and therefore the regulation of sarcomere function (5, 17). Interestingly, we observed no significant changes in the MMP or ATP levels following exposure of cardiomyocytes to red light. This is in contrast to previous reports where red light exposure resulted in an increase in both the MMP and ATP levels (11, 18, 44). Consolidating, our findings indicated that blue and green light, but not red light, exposure resulted in the cessation of cardiomyocyte contractility by inhibiting the mitochondrial activity.
Interestingly, we observed an inverse correlation between the beating frequency of cardiomyocytes and intracellular ROS levels, following exposure to blue and green light. We observed a significant increase in the ROS levels in the cardiomyocyte clusters that had ceased to contract following exposure to blue or green lights. Remarkably, with the green light exposure, the fold change observed was faster (4–5 min) than that with the blue light (15–20 min). Furthermore, supplementation of the culture medium with NAC abolished the effect of both blue- and green light-mediated inhibition of cardiomyocyte contractility. Our observations are consistent with previous reports that indicate a direct correlation between light-induced increase in ROS levels and modulation of cellular functions in a number of cell types. These include 1) proliferation in RPE cells (14), adipose-derived stem cells (ADSCs) (44), and fibroblasts (22), 2) osteogenic differentiation of ADSCs (43) and bone marrow-derived mesenchymal stem cells (50), and 3) mitochondrial respiration in adipose-derived stem cells (44), dental pulp stem cells (49), and astrocytes (21).
In fact, with the exception of a few studies that showed a positive correlation between ROS and cardiomyocyte contractility (28), most studies reported a ROS-mediated disruption of cardiomyocyte function (29, 51). In particular, barring a few reports showing an oxidative stress-independent mechanism (20), light-induced mitochondrial ROS formation has been identified as a major cause for the loss of MMP in various cell types such as cardiomyocytes (25), hepatocytes (19), RPE cells (27), and astrocytes (21). In fact, in the mouse, a direct correlation has been shown between elevated ROS levels, decrease in mitochondrial activity, and inhibition of cardiomyocyte contractility (47). These findings support our view on the involvement of light-induced increase in the intracellular ROS, especially mitochondrial and its consequence, leading to the cessation of cardiomyocyte contractility following exposure to blue or green lights.
Remarkably, although we observed that there was no significant increase in the total intracellular ROS levels in cardiomyocytes that ceased to contract following exposure to red light, the effect of red light exposure was nullified in the presence of NAC. We believe that the site of ROS production may be more relevant than the changes in total intracellular ROS since visible light has been shown to induce ROS at different cellular sources in a wavelength- and a cell type-dependent manner (25). In fact, multiple sources and sites for ROS production have been previously identified in functional cardiomyocytes, including mitochondria, NADPH oxidase, NOS, etc., which were shown to inhibit cardiomyocyte contractility (34, 52). In our study, because only the calcium transients were inhibited in the cardiomyocytes, following exposure to red light, it is possible that the small increase in intracellular ROS level directly inhibited calcium transients, consistent with the reported observation in cardiomyocytes (9, 24). We, therefore, believe that the different wavelengths of light could affect different sources and/or sites of ROS production in cardiomyocytes, consequently their contractility. While an in-depth understanding of the precise mechanism underlying red light-induced ROS-mediated cessation of cardiomyocyte contractility is necessary, such an experimental set up is beyond the scope of the current study.
Overall, the major conclusions that could be drawn from our study are: 1) monochromatic visible light exposure results in a reversible cessation in contractility of functional cardiomyocytes derived from mouse ES cells in a wavelength-independent manner, 2) monochromatic light-induced increase in ROS levels appears to be the major cause of inhibition of cardiomyocyte contractility following blue and green light exposure, 3) blue and green light, but not red light, exposure inhibits mitochondrial activity in functional cardiomyocytes, and 4) light-induced ROS and loss of mitochondrial activity inhibit intracellular calcium transients, thus affecting sarcomere function. Implications arising out of our findings are in the context of cardiac physiology and potential clinical cardiac function management strategies. First, the light effect phenomenon could be probed in modeling studies to achieve mechanistic insights into visible monochromatic light impact on cardiomyocyte contraction. Second, it could be exploited in laser beam-guided (cardiac) microsurgery, photo-optics-dependent medical diagnostics, and transient stopping of heart beats during coronary artery bypass grafting. Last, it could be explored for functional preservation of hearts before transplantation. In future, these exciting possibilities could be explored and researched.
GRANTS
This work was supported in part by Department of Biotechnology-Indian Institute of Science partnership program (No. 22-0307-0018-05-469), Government of India (P. B. Seshagiri), and National Heart, Lung, and Blood Institute Grant R01 HL136232 (M. Khan).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.S., D.S., and P.B.S. conceived and designed research; G.S. and D.S. performed experiments; D.S. and P.B.S. analyzed data; D.S. and P.B.S. interpreted results of experiments; D.S. prepared figures; D.S. drafted manuscript; G.S., M.K., and P.B.S. edited and revised manuscript; G.S., D.S., M.K., and P.B.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank S. Divya and K. N. Saima at the Bioimaging Facility, Division of Biological Sciences, Indian Institute of Science, for help in confocal imaging and Dr. Sandeep Eshwarappa, Indian Institute of Science, for help in carrying out ATP measurements.
REFERENCES
- 1.Abbey D, Seshagiri PB. Ascorbic acid-mediated enhanced cardiomyocyte differentiation of mouse ES-cells involves interplay of DNA methylation and multiple-signals. Differentiation 96: 1–14, 2017. doi: 10.1016/j.diff.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Agbulut O, Coirault C, Niederländer N, Huet A, Vicart P, Hagège A, Puceat M, Menasché P. GFP expression in muscle cells impairs actin-myosin interactions: implications for cell therapy. Nat Methods 3: 331, 2006. doi: 10.1038/nmeth0506-331. [DOI] [PubMed] [Google Scholar]
- 3.Andrei SR, Ghosh M, Sinharoy P, Dey S, Bratz IN, Damron DS. TRPA1 ion channel stimulation enhances cardiomyocyte contractile function via a CaMKII-dependent pathway. Channels (Austin) 11: 587–603, 2017. doi: 10.1080/19336950.2017.1365206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arpino JAJ, Rizkallah PJ, Jones DD. Crystal structure of enhanced green fluorescent protein to 1.35 Å resolution reveals alternative conformations for Glu222. PLoS One 7: e47132, 2012. doi: 10.1371/journal.pone.0047132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bers DM. Cardiac excitation-contraction coupling. Nature 415: 198–205, 2002. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 6.Biesiadecki BJ, Davis JP, Ziolo MT, Janssen PML. Tri-modal regulation of cardiac muscle relaxation; intracellular calcium decline, thin filament deactivation, and cross-bridge cycling kinetics. Biophys Rev 6: 273–289, 2014. doi: 10.1007/s12551-014-0143-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birkholz TR, Beane WS. The planarian TRPA1 homolog mediates extraocular behavioral responses to near-ultraviolet light. J Exp Biol 220: 2616–2625, 2017. doi: 10.1242/jeb.152298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC. Green fluorescent protein as a marker for gene expression. Science 263: 802–805, 1994. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- 9.Chaube R, Werstuck GH. Mitochondrial ROS versus ER ROS: which comes first in myocardial calcium dysregulation? Front Cardiovasc Med 3: 36, 2016. doi: 10.3389/fcvm.2016.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cormack BP, Valdivia RH, Falkow S. FACS-optimized mutants of the green fluorescent protein (GFP). Gene 173: 33–38, 1996. doi: 10.1016/0378-1119(95)00685-0. [DOI] [PubMed] [Google Scholar]
- 11.de Freitas LF, Hamblin MR. Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J Sel Top Quantum Electron 22: 7000417, 2016. doi: 10.1109/JSTQE.2016.2561201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11a.De Los Santos C, Chang CW, Mycek MA, Cardullo RA. FRAP, FLIM, and FRET: Detection and analysis of cellular dynamics on a molecular scale using fluorescence microscopy. Mol Reprod Dev 82: 587–604, 2015. doi: 10.1002/mrd.22501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dirksen RT. Sarcoplasmic reticulum-mitochondrial through-space coupling in skeletal muscle. Appl Physiol Nutr Metab 34: 389–395, 2009. doi: 10.1139/H09-044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doetschman TC, Eistetter H, Katz M, Schmidt W, Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol 87: 27–45, 1985. [PubMed] [Google Scholar]
- 14.Douthwright S, Sluder G. Live cell imaging: assessing the phototoxicity of 488 and 546 nm light and methods to alleviate it. J Cell Physiol 232: 2461–2468, 2017. doi: 10.1002/jcp.25588. [DOI] [PubMed] [Google Scholar]
- 15.Gimeno MA, Robets CM, Webb JL. Acceleration of rate of the early chick embryo heart by visible light. Nature 214: 1014–1016, 1967. doi: 10.1038/2141014a0. [DOI] [PubMed] [Google Scholar]
- 16.Gorgidze LA, Oshemkova SA, Vorobjev IA. Blue light inhibits mitosis in tissue culture cells. Biosci Rep 18: 215–224, 1998. doi: 10.1023/A:1020104914726. [DOI] [PubMed] [Google Scholar]
- 17.Grant AO. Cardiac ion channels. Circ Arrhythm Electrophysiol 2: 185–194, 2009. doi: 10.1161/CIRCEP.108.789081. [DOI] [PubMed] [Google Scholar]
- 18.Hamblin MR. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys 4: 337–361, 2017. doi: 10.3934/biophy.2017.3.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamblin MR. The role of nitric oxide in low level light therapy. Proc SPIE 6846: 684602, 2008. doi: 10.1117/12.764918. [DOI] [Google Scholar]
- 20.Jaiswal M, Haelterman NA, Sandoval H, Xiong B, Donti T, Kalsotra A, Yamamoto S, Cooper TA, Graham BH, Bellen HJ. Impaired mitochondrial energy production causes light- induced photoreceptor degeneration independent of oxidative stress. PLoS Biol 13: e1002197, 2015. doi: 10.1371/journal.pbio.1002197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jou MJ, Jou SB, Guo MJ, Wu HY, Peng TI. Mitochondrial reactive oxygen species generation and calcium increase induced by visible light in astrocytes. Ann NY Acad Sci 1011: 45–56, 2004. doi: 10.1196/annals.1293.005. [DOI] [PubMed] [Google Scholar]
- 22.Krassovka J, Borgschulze A, Sahlender B, Lögters T, Windolf J, Grotheer V. Blue light irradiation and its beneficial effect on Dupuytren’s fibroblasts. PLoS One 14: e0209833, 2019. doi: 10.1371/journal.pone.0209833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landeira-Fernandez AM, Castilho PC, Block BA. Thermal dependence of cardiac SR Ca2+-ATPase from fish and mammals. J Therm Biol 37: 217–223, 2012. doi: 10.1016/j.jtherbio.2012.01.003. [DOI] [Google Scholar]
- 24.Lavi R, Shainberg A, Friedmann H, Shneyvays V, Rickover O, Eichler M, Kaplan D, Lubart R. Low energy visible light induces reactive oxygen species generation and stimulates an increase of intracellular calcium concentration in cardiac cells. J Biol Chem 278: 40917–40922, 2003. doi: 10.1074/jbc.M303034200. [DOI] [PubMed] [Google Scholar]
- 25.Lavi R, Shainberg A, Shneyvays V, Hochauser E, Isaac A, Zinman T, Friedmann H, Lubart R. Detailed analysis of reactive oxygen species induced by visible light in various cell types. Lasers Surg Med 42: 473–480, 2010. doi: 10.1002/lsm.20919. [DOI] [PubMed] [Google Scholar]
- 26.Leprince J, Devaux J, Mullier T, Vreven J, Leloup G. Pulpal-temperature rise and polymerization efficiency of LED curing lights. Oper Dent 35: 220–230, 2010. doi: 10.2341/09-203-L. [DOI] [PubMed] [Google Scholar]
- 27.Marie M, Bigot K, Angebault C, Barrau C, Gondouin P, Pagan D, Fouquet S, Villette T, Sahel JA, Lenaers G, Picaud S. Light action spectrum on oxidative stress and mitochondrial damage in A2E-loaded retinal pigment epithelium cells. Cell Death Dis 9: 287, 2018. doi: 10.1038/s41419-018-0331-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morell M, Burgos JI, Gonano LA, Vila Petroff M. AMPK-dependent nitric oxide release provides contractile support during hyperosmotic stress. Basic Res Cardiol 113: 7, 2017. doi: 10.1007/s00395-017-0665-7. [DOI] [PubMed] [Google Scholar]
- 29.Moris D, Spartalis M, Spartalis E, Karachaliou GS, Karaolanis GI, Tsourouflis G, Tsilimigras DI, Tzatzaki E, Theocharis S. The role of reactive oxygen species in the pathophysiology of cardiovascular diseases and the clinical significance of myocardial redox. Ann Transl Med 5: 326, 2017. doi: 10.21037/atm.2017.06.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nathan RD, Pooler JP, DeHaan RL. Ultraviolet-induced alterations of beat rate and electrical properties of embryonic chick heart cell aggregates. J Gen Physiol 67: 27–44, 1976. doi: 10.1085/jgp.67.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishimura S, Nagai S, Sata M, Katoh M, Yamashita H, Saeki Y, Nagai R, Sugiura S. Expression of green fluorescent protein impairs the force-generating ability of isolated rat ventricular cardiomyocytes. Mol Cell Biochem 286: 59–65, 2006. doi: 10.1007/s11010-005-9090-6. [DOI] [PubMed] [Google Scholar]
- 32.Qi Y, Qi Z, Li Z, Wong CK, So C, Lo IC, Huang Y, Yao X, Tsang SY. Role of TRPV1 in the differentiation of mouse embryonic stem cells into cardiomyocytes. PLoS One 10: e0133211, 2015. doi: 10.1371/journal.pone.0133211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rotenberg S, Lewis JB, Lockwood PE, Tseng WY, Messer RL, Hsu SD, Omata Y, Wataha JC. Extracellular environment as one mediator of blue light-induced mitochondrial suppression. Dent Mater 22: 759–764, 2006. doi: 10.1016/j.dental.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 34.Santos CX, Anilkumar N, Zhang M, Brewer AC, Shah AM. Redox signaling in cardiac myocytes. Free Radic Biol Med 50: 777–793, 2011. doi: 10.1016/j.freeradbiomed.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schäfer A, Kratky KW. The effect of colored illumination on heart rate variability. Forsch Komplement Med 13: 167–173, 2006. doi: 10.1159/000092644. [DOI] [PubMed] [Google Scholar]
- 37.Singh G, Totiger TM, Seshagiri PB. Successful derivation of EGFP-transgenic embryonic stem cell line from a genetically non-permissive FVB/N mouse. Am J Stem Cell 1: 163–173, 2012. [PMC free article] [PubMed] [Google Scholar]
- 38.Stepanenko OV, Stepanenko OV, Shcherbakova DM, Kuznetsova IM, Turoverov KK, Verkhusha VV. Modern fluorescent proteins:from chromophore formation to novel intracellular applications. Biotechniques 51: 313–318, 2011. doi: 10.2144/000113765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stockley JH, Evans K, Matthey M, Volbracht K, Agathou S, Mukanowa J, Burrone J, Káradóttir RT. Surpassing light-induced cell damage in vitro with novel cell culture media. Sci Rep 7: 849, 2017. doi: 10.1038/s41598-017-00829-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stoien JD, Wang RJ. Effect of near-ultraviolet and visible light on mammalian cells in culture II. Formation of toxic photoproducts in tissue culture medium by blacklight. Proc Natl Acad Sci USA 71: 3961–3965, 1974. doi: 10.1073/pnas.71.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tekeli I, Aujard I, Trepat X, Jullien L, Raya A, Zalvidea D. Long-term in vivo single-cell lineage tracing of deep structures using three-photon activation. Light Sci Appl 5: e16084, 2016. doi: 10.1038/lsa.2016.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verma I, Rashid Z, Sikdar SK, Seshagiri PB. Efficient neural differentiation of mouse pluripotent stem cells in a serum-free medium and development of a novel strategy for enrichment of neural cells. Int J Dev Neurosci 61: 112–124, 2017. doi: 10.1016/j.ijdevneu.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y, Huang YY, Wang Y, Lyu P, Hamblin MR. Photobiomodulation (blue and green light) encourages osteoblastic-differentiation of human adipose-derived stem cells: role of intracellular calcium and light-gated ion channels. Sci Rep 6: 33719, 2016. doi: 10.1038/srep33719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Huang YY, Wang Y, Lyu P, Hamblin MR. Red (660 nm) or near-infrared (810 nm) photobiomodulation stimulates, while blue (415 nm), green (540 nm) light inhibits proliferation in human adipose-derived stem cells. Sci Rep 7: 7781, 2017. doi: 10.1038/s41598-017-07525-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wobus AM, Guan K, Yang HT, Boheler KR. Embryonic stem cells as a model to study cardiac, skeletal muscle, and vascular smooth muscle cell differentiation. Methods Mol Biol 185: 127–156, 2002. [DOI] [PubMed] [Google Scholar]
- 46.Wood JPM, Lascaratos G, Bron AJ, Osborne NN. The influence of visible light exposure on cultured RGC-5 cells. Mol Vis 14: 334–344, 2008. [PMC free article] [PubMed] [Google Scholar]
- 47.Xie YW, Kaminski PM, Wolin MS. Inhibition of rat cardiac muscle contraction and mitochondrial respiration by endogenous peroxynitrite formation during posthypoxic reoxygenation. Circ Res 82: 891–897, 1998. doi: 10.1161/01.RES.82.8.891. [DOI] [PubMed] [Google Scholar]
- 48.Yang TT, Cheng L, Kain SR. Optimized codon usage and chromophore mutations provide enhanced sensitivity with the green fluorescent protein. Nucleic Acids Res 24: 4592–4593, 1996. doi: 10.1093/nar/24.22.4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoshino F, Yoshida A. Effects of blue-light irradiation during dental treatment. Jpn Dent Sci Rev 54: 160–168, 2018. doi: 10.1016/j.jdsr.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yuan Y, Yan G, Gong R, Zhang L, Liu T, Feng C, Du W, Wang Y, Yang F, Li Y, Guo S, Ding F, Ma W, Idiiatullina E, Pavlov V, Han Z, Cai B, Yang L. Effects of blue light emitting diode irradiation on the proliferation, apoptosis and differentiation of bone marrow-derived mesenchymal stem cells. Cell Physiol Biochem 43: 237–246, 2017. doi: 10.1159/000480344. [DOI] [PubMed] [Google Scholar]
- 51.Zima AV, Blatter LA. Redox regulation of cardiac calcium channels and transporters. Cardiovasc Res 71: 310–321, 2006. doi: 10.1016/j.cardiores.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 52.Zinkevich NS, Gutterman DD. ROS-induced ROS release in vascular biology: redox-redox signaling. Am J Physiol Heart Circ Physiol 301: H647–H653, 2011. doi: 10.1152/ajpheart.01271.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]