Abstract
The α7β1-integrin is a transmembrane adhesion protein that connects laminin in the extracellular matrix (ECM) with actin in skeletal muscle fibers. The α7β1-integrin is highly expressed in skeletal muscle and is concentrated at costameres and myotendious junctions, providing the opportunity to transmit longitudinal and lateral forces across the membrane. Studies have demonstrated that α7-integrin subunit mRNA and protein are upregulated following eccentric contractions as a mechanism to reinforce load-bearing structures and resist injury with repeated bouts of exercise. It has been hypothesized for many years that the integrin can also promote protein turnover in a manner that can promote beneficial adaptations with resistance exercise training, including hypertrophy. This review provides basic information about integrin structure and activation and then explores its potential to serve as a critical mechanosensor and activator of muscle protein synthesis and growth. Overall, the hypothesis is proposed that the α7β1-integrin can contribute to mechanical-load induced skeletal muscle growth via an mammalian target of rapamycin complex 1-independent mechanism.
Keywords: hypertrophy, ILK, integrin, mTORC, skeletal muscle
INTEGRIN STRUCTURE AND ACTIVATION
Integrins are heterodimeric membrane proteins comprised of α- and β-subunits (6, 67). The integrin family consists of 18 unique α-subunits and 8 unique β-units that noncovalently associate into 24 known heterodimers, each expressed by a wide variety of mammalian cell types (6). The structure of each integrin subunit includes a large extracellular domain, a transmembrane domain, and a cytoplasmic domain. A complete description of integrin protein structure and conformational changes that occur upon activation can be found in other reviews (26, 76). The purpose of this review is to provide a brief introduction to integrin activation and then outline our current understanding of integrin expression and its involvement in the initiation of skeletal muscle adaptation in response to a mechanical stimulus in the form of contraction, exercise, or chronic loading.
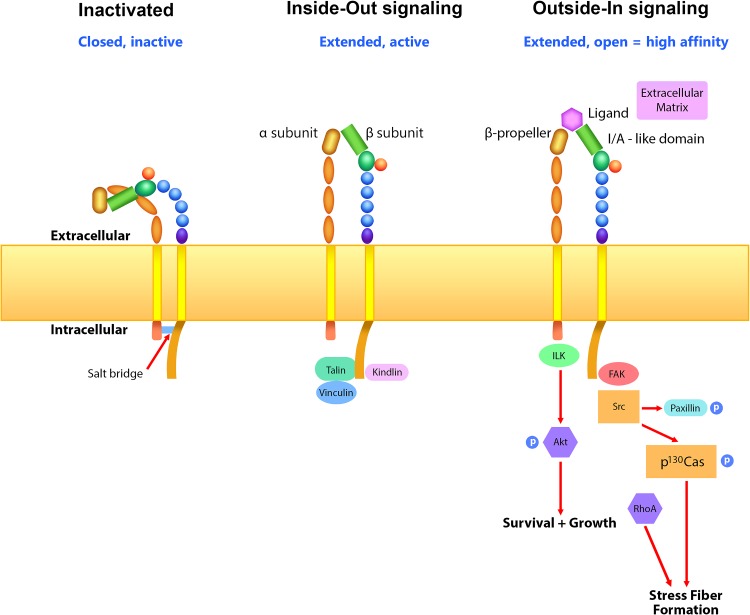
Integrins adhere the cell cytoskeleton to specific proteins within the extracellular matrix (ECM) for the purpose of stabilization/survival, migration, and proliferation. Integrins are bidirectional signaling molecules, allowing internal cues to regulate external adhesion (“Inside-Out” signaling) and relaying cues from the outside microenvironment to regulate cellular processes (“Outside-In” signaling). Integrins are initially transported from the endoplasmic reticulum to the plasma membrane with the extracellular domains of α- and β-subunits displaying a bent and inactivated or “closed” conformation, while the cytoplasmic domains are linked and stabilized by a salt bridge (28) (Fig. 1). In the case of “Inside-Out” signaling, the presence of intracellular force stimulates molecules such as talin and kindlin to attach to the β-subunit and destabilize the salt bridge, which promotes an extended and activated or “open” conformation allowing interaction with the ECM. The A or I domain (A/I domain) in the β-subunit, and in some cases the α-subunit, includes a metal ion-dependent adhesion site (MIDAS) that can serve as a ligand binding site (86). Following integrin activation, presentation of agonists in the external environment (laminin, collagen, fibronectin) facilitate further opening of the integrin extracellular domain and increased binding to the ECM. This high-affinity binding state then induces integrin clustering and assembly of associated cytoplasmic proteins at concentrated regions along the membrane, or focal adhesions (focal adhesion complex, or FAC), providing the basis for “Outside-In” molecular signaling. Due to the cooperative nature of “Inside-Out” and “Outside-In” conformational changes, endogenously and exogenously applied forces similarly impact integrin-mediated adhesion and intracellular signaling in a variety of mammalian cells.
Fig. 1.
Integrin activation. Following synthesis and localization at the membrane, the integrin will maintain a bent or “closed” conformation. Association of talin and vinculin, as well as kindlin and α-actinin, with the integrin in response to cytoskeletal tension destabilizes the salt bridge between the α- and β-cytoplasmic tails, allowing the extracellular domains to extend toward proteins within the extracellular matrix (ECM). “Inside-Out” signaling and increased cell-matrix interaction facilitate further opening of the space between α- and β-subunits, integrin clustering, focal adhesion complex formation, and initiation of signaling in response to mechanical strain (“Outside-In”) that results in stress fiber formation, survival, and growth. Studies suggest that focal adhesion kinase (FAK), paxillin, Src, p130Cas, and RhoA are important for stress fiber formation, whereas integrin-linked kinase (ILK)-Akt may facilitate survival and growth in response to mechanical strain.
IMPACT OF MECHANICAL STRAIN ON INTEGRIN-MEDIATED STRESS FIBER FORMATION AND SIGNALING
Talin is a large, 270 kDa scaffold protein, consisting of 1) a head region that binds to the NPxY motif on the β-subunit cytoplasmic domain, forcing the separation of α- and β-subunits, and 2) a long tail region that directly binds two F-actin sites and vinculin (11 sites) (120). Thus talin serves as a scaffold protein necessary for the initial stages of cell-matrix interactions. Talin-integrin interaction induces a conformational change in talin that exposes vinculin binding sites, allowing vinculin to further facilitate talin and F-actin interaction (40). Dependent on substrate stiffness, proteins other than talin may serve as primary F-actin binding proteins, such as kindlin, α-actinin, and the integrin-linked kinase (ILK)-PINCH-parvin complex (15, 40, 113, 138). Kindlin, for example, can bind a unique conserved NPxY sequence motif on the integrin β-subunit (membrane-distal site versus the membrane-proximal side required for talin) and can initiate integrin-mediated actin fibril formation independent of talin (124). Upon high-affinity ligand binding, activated integrins recruit numerous proteins to their short cytoplasmic tails. The precise composition of proteins that associate the nascent adhesions varies considerably based on cell type and environmental conditions, yet talin and vinculin are consistently represented and the majority also bind actin (25).
The application of force in the form of mechanical strain can allow prestressed, nascent adhesions to grow in size and form larger focal adhesion complexes via integrin clustering, F-actin bundling, and stress fiber formation (95, 120). Mechanical stretch on integrins can increase integrin-bound and autophosphoryated (Tyr397) focal adhesion kinase (pp125FAK or FAK), as well as stimulate Src family kinases to phosphorylate the docking protein p130Cas on multiple tyrosine residues (122). FAK can similarly phosphorylate paxillin, a key adaptor molecule containing a variety of interacting domains (LIM, SH2, SH3) (75). These events ensure recruitment of proteins to the maturing FAC that then induce strain-induced intracellular signaling. In addition to FAK, ILK can similarly bind to the integrin and facilitate phosphorylation of numerous downstream targets including Akt and GSK-3, as well as PINCH and other LIM-domain containing proteins, which allow for cell survival, migration, and spreading (7, 35, 59, 125, 126). ILK, although initially characterized as a serine/threonine kinase (59), appears to primarily serve as a scaffold protein, as mammalian ILK lacks catalytic activity (84, 139).
The signaling pathways that control stress fiber assembly can vary based on cell type (mobile versus nonmobile) and may or may not involve the integrin. However, the Ras homolog gene family, member A (RhoA), a small GTPase protein, appears to be universally important for actin assembly. RhoA can activate RhoA kinase (ROCK), a kinase that can inhibit the actin severing activity of cofilin, which ultimately prevents actin depolymerization and promotes stress fiber formation (61, 102, 61). The striated muscle activator of Rho signaling (STARS) protein, which is localized to I bands in cardiac and skeletal muscle, may be the primary mechanism for stress fiber formation in these cell types (2). Stress fiber formation in response to strain is important. Not only does it provide a means to resist force and protect against cytoskeletal disruption, it may provide a physical structure for transmission of mechanical signals to the nucleus for direct control of gene transcription and adaptation (29, 121).
INTEGRIN SPECIFICITY
In 1977, Richard Hynes reported the ability for fibronectin to induce the rearrangement of actin filaments (1), providing the first evidence for ECM-mediated control of cell function through a mechanism involving a yet unknown transmembrane protein. A fascinating history of the discovery of integrins is provided by Hynes in a personal account, including the eventual cloning of the fibronectin receptor (β1-integrin subunit), appropriately termed “integrin” to reflect its role in maintaining the cell-matrix interaction (68, 123). This personal perspective describes some of the challenges and delays faced in the identification of integrin subunits, including the complexity of integrin subunit specificity. Hynes and others revealed through their work that integrins can bind to multiple ECM ligands and more than one integrin may exist in the cell membrane. Integrin complexity, including the ability for multiple heterodimers to recognize and respond to dynamic changes in the ECM environment, continues to provide challenges to elucidating a precise role for integrins in a variety of cell types. Despite remaining questions, our current understanding is that β1-integrins, β2-integrins, and αv-containing integrins represent the majority of integrin heterodimers in vertebrates, with β1-integrins forming heterodimers with α-subunits 1–9 and v (6). Whereas α4β1- and α5β1-heterodimers primarily recognize fibronectin, α6β1- and α7β1-heterodimers exhibit preferential binding to laminin. An extensive review of the different integrin complexes and our current understanding of each is provided elsewhere (6).
Each cell in an organism possesses a specific integrin signature at the membrane that is dependent on factors such as development and tissue-specific microenvironmental cues, including ECM composition, elastic modulus, and topography. Whereas fibroblasts predominantly express α11-, α2-, α1-, and α5-integrin subunits, myogenic progenitor cells (satellite cells) (97, 98, 114) and skeletal muscle fibers (4, 5, 94, 129) predominantly express the α7-integrin subunit. A switch in integrin signature is observed in myoblasts during development, specifically during the stages of myoblast fusion and myotube formation, such that the fibronectin receptor α5β1 is downregulated to inhibit proliferation (14) and the laminin receptor α7β1 is upregulated to enhance differentiation (118). The switch in integrin expression is believed to occur in direct response to changes in the muscle cell ECM microenvironment during development, initially fibronectin rich to support myoblast proliferation and then changing to a laminin-rich environment to enhance differentiation (80). The α7β1-integrin is highly expressed in differentiated myotubes and is the major, if not exclusive, integrin receptor found in the adult myofiber across different species.
The α7β1-integrin preferentially binds laminin (LM-211 and LM-221) in the basal lamina and is concentrated at costameres (subsarcolemmal protein assembly between the membrane and Z bands), myotendinous (MTJ), and neuromuscular junctions (NMJ) (4, 22, 94, 105). Alternative splice variants exist for the α7-subunit, including the mutually exclusive X1 and X2 extracellular domains and the A and B cytoplasmic domains (30, 118, 148). Both A and B isoforms are expressed in skeletal muscle, whereas only the X2 isoform is expressed in adult skeletal muscle. While the A isoform is unique to adult skeletal muscle, B appears to be the predominant isoform present in the sarcolemma (22) and is also expressed in cardiac myocytes, intestinal cells, vascular smooth muscle cells, and neuronal cells (8, 30 129, 136, 143). Of the two β1-isoforms known to exist (A and D), the β1A-subunit is downregulated during myoblast differentiation and only the β1D-integrin subunit is expressed in mature myotubes (9, 128). Additionally, only the β1D-isoform is expressed in striated skeletal muscle and cardiomyocytes, where it is localized to costameres, MTJs, and NMJs (9, 128). These data suggest the α7BX2β1D-integrin is highly expressed and serves an important functional role in adult skeletal muscle.
THE α7β1-INTEGRIN AND NEUROMUSCULAR DISEASE
The dystrophin-glycoprotein (DGC) complex and the α7β1-integrin serve as the two primary laminin receptors in skeletal muscle, ultimately responsible for myofiber adhesion and cytoskeletal integrity. Genetic mutations in the components of either protein complex provide the basis for myopathies in humans and murine model systems, the majority of which are progressive in nature and exacerbated by mechanical strain during contraction (22, 62 98). Endogenous α7β1-integrin mRNA and protein are upregulated in skeletal muscle in humans and mice lacking the DGC (mdx mice) in an attempt to compensate for the absence of transmembrane adhesion (63). Talin and vinculin, which are necessary for integrin linkage to the cytoskeleton as described above, are similarly increased ~200% in mdx muscle at the MTJ, suggesting reinforcement of the FAC (85, 117). Muscle-specific overexpression of the α7BX2-integrin can ameliorate pathology (kyphosis, gait, contractures, NMJ, and MTJ structure) and extend the lifespan of mice with a severe form of dystrophy (mdx/utr−/−) (23, 24). The fact that a reciprocal increase in endogenous dystrophin protein does not occur in mice lacking the α7-integrin subunit suggests that the integrin and DGC complexes independently support adhesion and maintain muscle integrity. Together these studies provide irrefutable evidence that α7-integrin localization and FAC development at the fiber membrane are not only protective but necessary for muscle health.
DGC deficiency stimulates an increase in the synthesis and/or presence of numerous integrin-related focal adhesion proteins, and this complex may serve to initiate critical signaling pathways necessary for actin remodeling and cell survival. Hyperphosphorylation of FAK (Tyr577, Try397, and Tyr722), paxillin (Tyr118 and Tyr31), protein kinase-Cα (PKCα; Ser657), and the mitogen-activated kinase-activated protein kinase 2 (MAPKAPK2; Thr222) has been noted in mdx mice (117). Paxillin may be an important downstream effector of the integrin, as phosphorylation on Tyr31 has been shown to be necessary for myofibrillar protein organization and sarcomere formation in myotubes (117). In addition to these findings, we have reported that ILK expression and kinase activity are markedly increased in mdx/utr−/− and mdx/utr−/− muscle that overexpress the α7BX2-integrin (α7BX2-mdx/utr−/−) compared with wild-type (WT) and additional enhancement of the Akt (Ser473) phosphorylation-to-total Akt ratio is observed in α7BX2-mdx/utr−/− compared with mdx/utr−/−. In this study, we probed phosphorylation and total amounts of FAK at Tyr397, but the results were highly variable and thus not reported (unpublished observations). The α7-integrin subunit and ILK are preferentially coexpressed in glycolytic muscle fibers (96) and directly bind to each other through a critical tyrosine residue on the B cytoplasmic domain (21), providing additional support for an α7-integrin-ILK-Akt mediated pro-survival pathway.
While skeletal muscle-specific loss of ILK does not impact α7β1-integrin protein expression, widespread defects in FAC-associated protein localization at the MTJ and disruption of the actin cytoskeleton are readily apparent (51, 132). The progressive form of myopathy that develops and the increased susceptibility to mechanical strain-induced damage are highly reminiscent of the α7β1-integrin deficient mouse phenotype (22, 41, 98). All together, these studies point to ILK as an important downstream effector of the α7β1-integrin in skeletal muscle, and upregulation of the α7-integrin-ILK-Akt signaling pathway represents an important compensatory mechanism to stabilize and repair sarcomeric structure in the face of dystrophin deficiency.
THE α7β1-INTEGRIN AND PROTECTION FROM EXERCISE-INDUCED MYOFIBER DISRUPTION
Engagement in resistance exercise training can initiate an adaptive response in skeletal muscle that can provide protection from myofibril disruption, as well as increase myofiber size and enhance strength. The early cellular and molecular events responsible for training-induced adaptations have not been fully elucidated. However, an acute bout of (unaccustomed) exercise, particularly a session that incorporates eccentric or lengthening contractions, can cause localized membrane and myofibril disruption and subsequently elicit a stress response in an effort to facilitate repair.
We and others have demonstrated that an acute bout of eccentric exercise induces an increase in α7β1-integrin mRNA and protein in skeletal muscle of healthy humans and mice (20, 22, 34, 66). All isoforms of α7-integrin mRNA, including A, B, X1, and X2, are increased in muscle 3 h posteccentric exercise (30 min, downhill running) (22), and a 70% increase in α7B-integrin protein is observed by 24 h postexercise (20). Interestingly, only the α7-integrin subunit is responsive to exercise, as no change was observed in α5- and α6-mRNA and a decrease was observed in α4-mRNA (22). In young men, a more dramatic 3.8-fold increase in α7B-protein was reported at 24 h posteccentric exercise (15 sets of 10 repetitions, unilateral maximal contractions using an isokinetic dynamometer), including a reciprocal 3.9-fold increase in the β1D-subunit (34). The upregulation of the α7BX2-integrin subunit appears to be necessary for membrane repair and protection from subsequent myofiber disruption normally observed in healthy WT muscle postexercise, as α7-integrin knockout mice (α7-integrin−/−) are highly susceptible to muscle injury and compromised membrane integrity with a second (or repeat bout) of eccentric exercise (30 min, downhill running) 24 h later (22). We have also demonstrated that transgenic overexpression of the α7BX2-integrin in mouse muscle (muscle creatine kinase, MCK:α7BX2 integrin; α7Tg) can prevent decreases in membrane integrity and force, as well as mitigate an inflammatory response, following an acute bout of eccentric exercise (60 min, downhill running) (89). Given the localization and concentration of the integrin at the costamere, MTJ, and NMJ, it is assumed that the integrin serves as an anchor to resist forces present during contraction, ultimately preserving membrane, cytoskeletal, and sarcomeric structure.
The immediate upregulation of α7-integrin subunit mRNA and protein, as well as concomitant increases in talin and vinculin protein (48), are reminiscent of the response to DGC deficiency (85, 117). Loss of dystrophin and related dystrophin-associated proteins has been observed in rodent muscle in response to lengthening contractions (13), an observation that we have also noted in mouse muscle. Thus α7-integrin upregulation is likely a compensatory mechanism necessary to stabilize the cytoskeleton and prevent Z-band disruption posteccentric exercise. A question that remains to be addressed, however, is whether α7-integrin upregulation provides a mechanism for long-term reinforcement of Z bands and recruitment of unique signaling proteins to the costamere that facilitate additional benefits associated with resistance exercise training, including long-term structural and functional gains.
MECHANISMS DRIVING MECHANICAL LOAD-INDUCED PROTEIN SYNTHESIS AND SKELETAL MUSCLE HYPERTROPHY
Mammalian Target of Rapamycin Complex
The mammalian target of rapamycin (mTOR) is a critical regulator of protein synthesis and myofiber growth in response to anabolic stimuli, such as insulin, growth factors (IGF-1, IGF-2), certain amino acids, and mechanical force (54, 144). mTOR, a serine/threonine protein kinase, serves as a core component of two distinct multiprotein complexes, including mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2) (71, 115). Both complexes share association with DEP domain-containing mTOR-interacting protein (DEPTOR) and mammalian lethal with SEC13 protein 8 (mLST8), which serve to regulate mTOR kinase activity (144). The regulatory-associated protein of mTOR (RAPTOR) and the proline-rich Akt substrate of 40 kDa (PRAS40) specifically regulate mTORC1 activity, whereas rapamycin-insensitive companion of mTOR (RICTOR), stress-activated protein kinase-interacting protein 1 (mSIN1), and the RICTOR-binding proteins Protor-1/2 are unique to mTORC2 (111, 115). mTORC1 and mTORC2 can be distinguished based on sensitivity to rapamycin. FKBP12-rapamycin can allosterically interact with mTORC1 and destabilize RAPTOR-mTOR interaction, whereas rapamycin does not significantly impact mTORC2 (111). In general, the two complexes are responsive to different signals and exhibit specificity for different downstream effectors, with mTORC1 a primary coordinator of protein synthesis and autophagy (50, 90) and mTORC2 a primary regulator of cytoskeletal structure and cell survival (31, 71, 110).
mTORC complexes are localized to distinct subcellular compartments to allow spatial and temporal control of cell growth (11). mTORC1 translocation to the lysosomal membrane is observed in response to amino acids and mechanical stimulation in the form of eccentric contraction (73). This translocation event, which is controlled by the Rag GTPase and the GEF and lysosomal anchor for Rag (Ragulator), is important because it enhances proximity between mTOR and the GTP-bound form of Ras homolog enriched in brain (RHEB), which is required for mTOR activation. Following mTOR phosphorylation, RAPTOR binds a conserved TOR signaling (TOS) motif in downstream targets p70S6 kinase 1 (p70S6K) and the eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1) (108), facilitating phosphorylation events that increase capacity and efficiency of protein translation (90).
Several studies have now demonstrated a central role for mTORC1 in mechanical load-induced skeletal muscle growth (16, 36, 54, 79, 145). However, recent studies suggest an additional role for an mTORC1/RAPTOR-independent mechanism in skeletal muscle adaptation (109, 145). For example, Ogasawara et al. (109) recently demonstrated that while early hypertrophic signaling (<3 h postcontraction) was sensitive to rapamycin, later increases in protein synthesis (>6 h postcontraction) remained insensitive to rapamycin administration. Similarly, You et al. (145) reported that skeletal muscle-specific and conditional ablation of RAPTOR eliminates mTOR localization to the lysosome, p70S6K (Thr389) phosphorylation, and increased myofiber cross-sectional area (CSA) in response to high-intensity contraction and/or chronic mechanical loading in mouse plantaris muscle, yet phosphorylation of S6 (Ser235/236; Serine240/244) and 4E-BP1 (Thr36/45) and enhanced protein synthesis, as assessed by puromycin labeling, were not inhibited. These studies suggest a role for mTORC1-independent mechanisms in the regulation of protein turnover, an event that may be necessary for maintenance of beneficial structural and functional gains postexercise.
Mammalian Target of Rapamycin Complex 2
Minimal information exists regarding a role for mTORC2 in skeletal muscle, particularly in response to contraction and exercise. In nonmuscle cell types, endogenous mTORC2 is localized to the plasma membrane, outer mitochondrial membranes, and a subpopulation of endosomal vesicles (39). Similar to mTORC1, it is thought that partitioning of mTORC2 may allow for preferential activation of downstream targets, such as Akt (Ser473), serum- and glucocorticoid-regulated kinase 1 (SGK1; Ser422), protein kinase C (PKC; Ser657), and 4E-BP1 (multiple phosphorylation sites) (58, 71, 72, 77, 115, 116).
Unbiased proteomic screening experiments have demonstrated that ILK, presumably associated with integrins at the FAC, can directly bind RICTOR and facilitate mTORC2-mediated Akt phosphorylation and cell survival in cancer cells (101). In addition, early experiments in HeLa cells highlighted a role for RICTOR in PKC activation and cytoskeletal organization (115). Specifically, lentivirus knockdown of RICTOR reduced PKCα activity and induced reorganization of actin from the cell periphery to thick bundles in the cytoplasm with no clear connection to the remaining actin cytoskeleton. These studies suggest that an integrin-ILK-RICTOR complex may exist in a variety of mammalian cell types to ensure cytoskeleton integrity. However, whether this complex is augmented in skeletal muscle in response to mechanical load and the extent to which it serves a structural (protection against disruption) or functional (hypertrophic signaling) role in the context of loading are not known.
Hodson et al. (64), recently evaluated RICTOR localization in human skeletal muscle 1 and 3 h following an acute bout of resistance exercise (4 sets, unilateral leg extension at 70% RM until volitional failure, 2-min recovery between sets) in recreationally active young men. No change in mTOR-RICTOR colocalization was observed, yet complex formation may be dependent on training status, as well as the time course for evaluation (later time points are required). Conditional, muscle-specific RICTOR knockout mice exist (10, 77) that may be useful, but the effectiveness of this mouse model would depend on the extent of damage following application of a mechanical stimulus. Currently, RICTOR−/− mice do exhibit progressive myopathy or a negative phenotype with the application of aerobic exercise, but these mice have not yet been subjected to a load that would represent a significant challenge to muscle structure. A muscle-specific RICTOR transgenic mouse could also be generated to address a more precise role for mTORC2 in load-induced growth.
Serum Response Factor
Other mTORC1-independent mechanisms exist that may also contribute to load-induced growth. RhoA-mediated stress fiber formation can stimulate translocation of serum response factor (SRF) to the nucleus, where it can induce transcription of skeletal muscle-specific genes [skeletal α-actin, α-myosin heavy chain (MHC), myogenin] and initiate myoblast differentiation (134). Dominant negative mutants of the β1-integrin and inhibition of actin polymerization by cytochalasin D significantly reduce RhoA-induced gene expression in cardiac myocytes (135), suggesting an important role for the integrin in the regulation of muscle-specific protein expression. Functional overload can induce a RhoA protein expression at the sarcolemma in rat plantaris muscle at 3 days, as well as increase SRF protein in nuclear and cytoplasmic fractions in rooster anterior latissimus dorsi (ALD) muscle 7 days poststretch (42, 100). In humans, 8 wk of resistance exercise increased SRF, α-actin, and MHCIIa mRNA, as well as RhoA and nuclear SRF protein levels in quadriceps muscle (83). Induction of SRF loss in adult myofibers results in a progressive myopathy, characterized by an altered regenerative response and accumulation of fibrosis in mice (82). Loss of SRF also inhibits load-induced hypertrophy via lack of satellite cell fusion (57). However, the role of satellite cell fusion in load-induced growth is controversial (49, 99, 106) and the lack of growth response to mechanical load in this study likely reflects the requirement for satellite cells in very young animals (2 mo old). Thus the extent to which the integrin-RhoA-SRF signaling pathway contributes to load-induced growth in adult skeletal muscle remains questionable.
THE α7β1-INTEGRIN AND MECHANICAL LOAD-INDUCED SKELETAL MUSCLE HYPERTROPHY
The integrin is assumed to serve as a primary mechanosensor and regulator of mechanical load-induced growth, as depicted in nearly all illustrations presented in relevant reviews. However, our current understanding of the integrin role in growth is incomplete, which is also represented by the question marks that connect the integrin to mTORC signaling in illustrations of relevant reviews. Currently, minimal in vivo data exist to provide evidence of increased talin association with the integrin, integrin conformational changes, integrin clustering, or FAC assembly at the sarcolemma in response to mechanical load. FAK and ILK phosphorylation events have been used as indirect indicators of integrin activation, yet these correlational studies are not sufficient to confer a causal relationship between the integrin and load-induced growth. In this section, we will review the literature that examine a role for the integrin and integrin-associated proteins in load-induced skeletal muscle hypertrophy.
The creation of mice overexpressing the α7BX2-integrin subunit in skeletal muscle (MCK:α7BX2-integrin; α7Tg) provided the first opportunity to examine a direct role for the integrin in exercise-mediated adaptation (20, 89). The paradigm of acute downhill running (−20° decline, 17 m/min, 30–60 min) was originally chosen to discern a role in the protection from myofiber disruption consistently observed with muscle lengthening, a role that was outlined earlier in this review. Unexpected phenotypes were observed in α7Tg muscle following exercise, including an increase in the mean fiber CSA and accumulation of Pax7+ satellite cells and newly formed fibers (eMHC+) compared with WT muscle (89). The increase in satellite cell content and new fiber synthesis in the absence of disruption still cannot be explained by integrin overexpression given its restricted expression within the adult myofiber (MCK promoter). The increase in fiber CSA was similarly perplexing for two reasons. First, one would not expect fiber growth to occur with a single bout of exercise, particularly a type of exercise that includes an aerobic component. Second, our prior work demonstrated a decrease in mTOR signaling, including reductions in the phosphorylation of Akt (Ser473), mTOR (Ser2448), and p70S6K (Thr389), in α7Tg muscle immediately and 3 h postexercise (20). Therefore, if the α7-integrin were to increase protein synthesis (not assessed in this study) and fiber hypertrophy in the context of eccentric exercise, a mechanism other than mTORC1 signaling would likely be required.
The results from the first study provided the incentive to conduct an eccentric exercise training study to determine the full extent to which α7-integrin overexpression could induce skeletal muscle growth and other exercise-specific phenotypes (149). Downhill running (3×/wk; 4 wk) did not promote muscle growth in WT, yet resulted in a significant increase in the mean fiber CSA and percentage of large caliber fibers ranging >3,000 µm2 in α7Tg muscle. A significant increase in the fiber CSA was also observed in the sedentary state of α7Tg muscle compared with WT, yet mice were fasted for 24 h in this study before dissection, and this observation may simply reflect the ability for the integrin to prevent a decrease in CSA due to atrophy. This rationale is supported by the relatively low mean fiber CSA values recorded. Regardless, preferential enhancement of myofibrillar protein content and whole muscle CSA in α7Tg compared with WT provide additional evidence for integrin-mediated growth in response to load (149). Increases in mTOR signaling were observed in this training study, but the extent to which these late, long-term changes in signaling are meaningful is not known. Finally, enhanced vascular remodeling and arteriogenesis was also observed in α7Tg muscle following acute (7 days post exercise) and repeated bouts of eccentric exercise, an adaptation that may occur to ensure proper nutrient and oxygen delivery to hypertrophied fibers (65).
Surgical ablation of the gastrocnemius muscle and chronic loading of the compensatory plantaris muscle (and or soleus muscle) is traditionally used to discern the mechanistic basis for mechanical strain-induced growth. Myotenectomy (MTE) is a modified version of synergist ablation in which the gastrocnemius muscle is severed and the tendon is removed, resulting in loading of the remaining plantaris and soleus muscles, a procedure that can minimize damage while maximizing the potential for growth. Our laboratory recently completed a study in which α7Tg mice were subjected to MTE and then chronically loaded for 1 day (to evaluate signaling and growth) or 14 days (to evaluate growth) (unpublished observations). In this study, a preferential increase in muscle weight was observed in α7Tg muscle compared with WT at 1 and 14 days. The impact of α7-integrin overexpression on mechanical load-induced growth was only apparent in the glycolytic plantaris muscle and not the oxidative soleus muscle, appropriately reflecting preferential expression of MCK and transgene expression in type 2 muscle fibers (soleus was used as a control). Unlike downhill running, an increase in mean fiber CSA was not observed with chronic load in α7Tg muscle, likely the result of extensive fiber splitting that occurred in α7Tg mice compared with WT in response to this supraphysiological stimulus. mTORC1 signaling was elevated in both WT and α7Tg muscle 1 day postload, but no enhancement was observed and a trend toward a decrease was noted in α7Tg, consistent with our prior results (20). Interestingly, in light of findings reported by You et al. (145) described above, S6 (Ser235/236) phosphorylation was not suppressed and 4E-BP1 (Ser65) phosphorylation was increased in α7Tg muscle. The results from these studies support a role for the α7β1-integrin in the induction of load-induced growth, but the primary mechanisms driving integrin-mediated responses are not yet known.
MECHANISTIC BASIS FOR α7β1-INTEGRIN-MEDIATED SKELETAL MUSCLE GROWTH
Focal Adhesion Kinase
Mechanical stimulation can increase FAK activity in a variety of cells, suggesting a role for FAK in load-induced growth. In fact, FAK autophosphorylation has been used as a readout for integrin activation in response to mechanical load, yet we are not aware of any studies that demonstrate a direct linkage between the α7β1-integrin and FAK in skeletal muscle nor activation of FAK by the integrin.
Flück et al. (43) were the first to report an increase in FAK protein expression (~80%) and autophosphorylation (Tyr397, ~3-fold) in rooster anterior latissimus dorsi (ALD) muscle 1.5, 7, and 13 days following initiation of chronic stretch. FAK colocalized with vinculin at the sarcolemma, and paxillin protein content was similarly increased (~2-fold). FAK and paxillin protein expression as well as FAK phosphorylation (Tyr397) were also increased in rat soleus muscle 1 and 8 days following chronic overload induced by surgical ablation of the synergistic gastrocnemius muscle (43, 53). Subsequent studies using the hindlimb suspension-reload model demonstrated that FAK overexpression via transfection of pCMV-FAK plasmid can enhance p70S6K phosphorylation and activity (S411 at 6 h, T421/S424 and total S6K activity at 24 h) in the tibialis anterior (TA) muscle during the reload phase compared with contralateral controls receiving pCMV empty vector (78). This event was not dependent on Akt activity and did not elicit any change in downstream phosphorylation of 4E-BP1 (Thr37/46). In a follow up study, overexpression of FAK (pCMV-FAK plasmid), which was exclusively localized to the sarcolemma and sarcoplasm, led to a small (6%) increase in mean myofiber CSA in soleus muscle during normal weight bearing (38). Overexpression of FAK also facilitated a 16% increase in the mean fiber CSA 1 day following reload in FAK+ fibers compared with FAK− fibers, yet comparison to contralateral controls was not provided to discern the extent of growth that occurred in response to load. In addition, the impact of FAK overexpression in response load in healthy muscle (outside the context of unload-reload) has not been established nor have any changes in muscle weight or protein synthesis been reported. Overall, these data suggest that FAK and paxillin are responsive to chronic mechanical loading and correlate with muscle hypertrophy, yet evidence that FAK is the mechanosensitive target of the integrin is weak.
Study results also exist that contradict a role for FAK in load-induced growth. First, total FAK protein and FAK phosphorylation at Tyr397 are decreased 90 min following downhill running in rat soleus muscle compared with nonexercised controls (56). We have observed similar decreases in the plantaris muscle of WT mice 1 day following chronic mechanical loading (unpublished observations). Increases in FAK phosphorylation have been observed in the distal region of human muscle compared with the midbelly region following unilateral eccentric exercise training (3×/wk, 8 wk), yet no differences in the rates of protein synthesis were observed between sites (47). Finally, the finding that FAK can facilitate insulin-like growth factor I (IGF-I)-mediated myotube growth via inhibition of tuberous sclerosis complex 2 (TSC2) and activation of mTOR signaling is tempered by studies that demonstrate no requirement for IGF-I or systemic factors in load-induced muscle hypertrophy (119, 137). Thus additional studies are needed to demonstrate a definitive role for FAK as a primary mechanism for integrin-mediated growth in response to a mechanical stimulus.
Integrin-Linked Kinase
In the context of mechanical loading, cardiac-specific overexpression of a constitutively active form of ILK (Ser343D) in mice can induce myocardial cell hypertrophy (88), yet this event occurred independent of a significant increase in Akt phosphorylation and the extent to which this information translates to skeletal muscle is not known. ILK mRNA is robustly increased 3–7 days postload in mouse skeletal muscle (27), suggesting some potential for involvement. We have observed a drop in total ILK protein expression 1 day postload (unpublished observations), which may provide the rationale for increased transcription 3–7 days later (27). In addition, ILK protein expression appears to recover faster in α7Tg mice compared with WT by 14 days. It is tempting to speculate that temporary membrane disruption is responsible for ILK depletion and that integrin-mediated repair can restore the integrin-ILK complex and its potential involvement in training-induced skeletal muscle adaptation. Overall, ILK may contribute to integrin-mediated growth, but more evidence is needed.
Serum Response Factor and Unfolded Protein Response
A microarray analysis was recently performed using RNA extracted from skeletal muscle of WT and α7Tg mice under sedentary conditions and 3 h following an acute bout of downhill running exercise to elucidate some of the mechanisms driving integrin-mediated skeletal muscle growth (93). We did not observe any increase in SRF-mediated transcriptional products in the microarray, suggesting no involvement of SRF. Alternatively, KEGG pathway analysis detected an overrepresentation of genes related to protein processing in the endoplasmic reticulum (ER) pathway in α7Tg mice in the absence of exercise. Indeed, upregulation of heat shock protein mRNA, including Hsp40 and Hspa5/BiP, was verified, and decreased CHOP protein was detected in α7Tg mice suggesting a preferential unfolded protein response (UPR). We reasoned that a decrease in misfolded protein abundance would serve to increase protein quality and incorporation during myofibrillar protein synthesis. Using a shRNA knockdown approach in myotubes, however, several of the top differentially expressed genes related to protein processing in the ER were not directly regulated by the integrin, suggesting contribution of nonmyofiber cells (stem cells, fibroblasts) to the beneficial UPR observed. While interesting, it is unclear how this event may benefit muscle in the context of mechanical loading.
Other Potential Mechanisms
The microarray analysis detected differential expression of additional genes related to stress resistance and protein translation. Significant increases in angiogenin (Ang) and solute carrier family 7 member 5 (Slc7a5) mRNA, as well as a decrease in N-myc downregulated gene 2 (Ndrg2) mRNA, were detected in α7Tg muscle postexercise. These changes are particularly relevant to growth, as angiogenin is a ribonuclease that can increase translation of mRNAs with an internal ribosome entry site (IRES) and can serve as a transcription factor to increase ribosomal RNA (rRNA) during periods of stress (69, 112, 142). Slc7a5, or the L-type amino acid transporter 1 (LAT1), is enhanced in human and rat skeletal muscle postexercise (12, 91) and can activate mTOR activity via leucine uptake or leucine efflux from lysosomes (103, 140). Finally, Ndrg2 is a negative regulator of muscle protein synthesis (45, 46). Of these candidate genes, Slc7a5 and Ndrg2 were appropriately downregulated and upregulated, respectively, by integrin knockdown in myotubes. The fact Ndrg2 may be regulated by mTORC2 activity via SGK (107) suggests some potential for the integrin to regulate growth via an integrin-ILK-mTORC2-mediated pathway. In addition, Slc7a5 is a downstream transcriptional target of the Yes-associated protein (YAP), which can also induce muscle growth independent of mTORC1 (55).
The Yes-associated protein (YAP) is a transcriptional coactivator expressed in a variety of mammalian cells that is strongly associated with tissue growth in a manner that is dependent on cell attachment. YAP is normally retained in an inactive state in the cytoplasm and may be targeted for degradation via phosphorylation by the mammalian STE20-like protein kinase 1 (MST1/2) and subsequent phosphorylation of large tumor suppressor 1 kinases (LATS1/2), integral members of the Hippo signaling pathway (74). Ser112 phosphorylation (Ser127 in humans) by LATS1/2 is responsible for cytoplasmic retention, whereas Ser381 mediates recruitment of an E3 ubiquitin ligase that targets YAP for degradation (60, 74, 133). Upon inactivation of Hippo signaling or alternative mechanisms for dephosphorylation, YAP translocates to the nucleus where it serves as a transcriptional coactivator in association with TEA domain family members 1–4 (TEAD1–4) and other transcription factors (74). Downstream effectors of YAP include transcription of genes necessary for cell survival, stabilization and growth, including Runx (141), Smads (55), ankyrins (37), connective tissue growth factor, amphiregulin (146), c-myc (55), and Slc7a5 (60). Studies have demonstrated that cell attachment and actin stress fiber formation promotes YAP nuclear accumulation, while actin destabilization and detachment leads to phosphorylation and cytoplasmic retention via the Hippo pathway (37, 74, 130, 147. A recent study demonstrated that F-actin abundance and cytoskeleton integrity can promote YAP nuclear translocation and activity in a manner independent of contractility and Ser112 phosphorylation (32). Thus integrin-mediated stress fiber formation in response to a mechanical stimulus can promote YAP activity, regardless of phosphorylation status.
Goodman et al. (55) recently reported significant upregulation of total and phosphorylated YAP (Ser112) in response to chronic overload. The time course for stimulation was distinct from mTORC1, such that p70S6K phosphorylation (Ser389) was optimal 2 days postload and YAP increased in parallel with Akt phosphorylation (Thr308) 4–10 days postload. Transfection of YAP (unphosphorylated) in the tibialis anterior muscle resulted in a rapamycin-insensitive increase in myofiber CSA in the absence of a mechanical stimulus, which also resulted in an increase in c-Myc protein (55). These data support other studies that demonstrate the ability for YAP to directly promote an increase in muscle mass via interaction with TEAD transcription factors (133). In addition, we have observed higher total YAP expression in α7Tg skeletal muscle compared with WT skeletal muscle (unpublished observations), which is consistent with constitutively elevated expression of YAP in mdx muscle (where integrin expression is enhanced to compensate for loss of dystrophin) (70). Overall, these data suggest that the integrin may serve to promote cytoskeletal integrity, YAP expression and/or activity, and growth in a manner that does not require mTORC1 activity.
CLINICAL CONSIDERATIONS
Immobilization and bed rest following severe injury or disease can result in significant alterations in body composition, including loss of muscle mass and strength, as well as loss of bone density and an increase in fat and collagen accumulation (131). Several mechanisms appear to account for the decrease in muscle fiber size during disuse atrophy, including increased protein degradation and suppression of muscle protein synthesis (3, 18). Upregulation of forkhead box O (FoxO) transcription factors can increase gene expression of muscle-specific E3 ubiquitin ligases, MuRF1 and MAFbx/atrogin, as well as autophagy-related genes, LC3 and Bnip3 (19). Significant muscle sparing is observed in MuRF1−/− and MAFbx−/− mice following hindlimb unloading and denervation (17, 81). However, deletion of MuRF1 does not fully suppress the ubiquitin proteasome pathway (UPP) in mice, suggesting that E3 ligase-independent pathways contribute to atrophy (19). Reproducible reductions in the rate of muscle protein synthesis have been observed in human skeletal muscle with disuse (33). Thus loss of a mechanosensing pathway may contribute to significant declines in muscle mass observed following an extended period of inactivity.
We are only aware of one study that has examined α7-integrin subunit mRNA following a period of unloading in mice (104). In this study, α7-integrin mRNA was significantly decreased in rat gastrocnemius muscle following 2 wk of hindlimb suspension, which correlated with significant declines in 45S pre-rRNA abundance, muscle protein synthesis (myofibrillar, cytoplasmic, and mitochondrial fractions), and myofiber CSA (104). Reductions in the phosphorylation of Akt (Ser473) and total FAK were observed, but a significant decline in FAK phosphorylation was not. These results are consistent with data by Li et al. (87), demonstrating a decrease in total FAK, but no change in FAK phosphorylation, in human vastus lateralis muscle following 10 and 21 days of bedrest. The results for FAK phosphorylation are not consistent throughout the literature, however, as decreases or no change have been reported in other studies (33, 44, 52). Further support for a decline in α7-integrin -ILK complex formation with disuse is provided by a recent human study (92). In this study, decreases in ILK, RhoA, and actin cytoskeleton signaling were observed in young and old muscle via RNA sequencing following 5 days of bed rest. In addition, a significant decrease in α7-integrin mRNA (28%) was observed in old skeletal muscle following disuse.
One potential confounding factor in the evaluation of integrin and costamere-associated protein with disuse atrophy is the fact that specific integrin isoforms (α7B), FAK and paxillin are expressed in microvascular endothelial cells and vascular smooth muscle cells. Thus any decline in the total or phosphorylated amount of any of these proteins may occur as a result of capillary refraction, which can occur with disuse muscle atrophy (127). Thus lineage tracing or genetic manipulation of the integrin would provide the best approach to elucidating a role for a mechanosensing pathway in loss of muscle mass with long-term disuse.
CONCLUSIONS
The α7β1-integrin represents the predominant integrin heterodimer expressed in skeletal muscle. While our studies demonstrate an important role for the integrin in promoting skeletal muscle hypertrophy in response to a mechanical stimulus, the detailed intracellular signaling events responsible for adaptation remain unknown. In this review, we provide the suggestion that the α7β1-integrin does not promote myofiber growth via FAK, SRF, or mTORC1, the canonical integrin-mediated pathways highlighted in the current literature. Upregulation of the α7β1-integrin following an acute bout of unaccustomed eccentric exercise likely allows for ILK binding, reinforcement of the cytoskeleton at the costamere, and increased YAP activity, a mechanosensing mechanism that may or may not involve RICTOR and mTORC2 activity. We envision a two-step process following mechanical loading in which mTORC1 is activated by amino acid uptake or release from the lysosome to rapidly increase protein synthesis to establish homeostasis, then sustained maintenance and remodeling of muscle structure via an integrin-ILK-mTORC2-YAP-driven mechanism (Fig. 2). Further delineation of a role for the integrin in mTORC1-independent muscle growth following a mechanical stimulus will not only allow for enhanced sport performance but provide impactful knowledge toward mitigating loss of muscle mass in the context of disuse and aging.
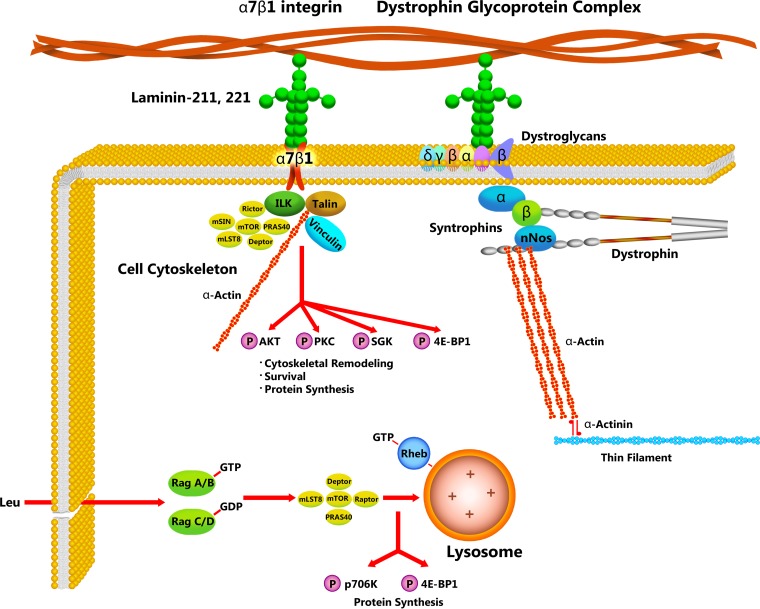
Fig. 2.
Hypothetical role for the α7β1-integrin in the induction of skeletal muscle hypertrophy in response to mechanical stimulation. Two mammalian target of rapamycin complexes (mTORC) exist in skeletal muscle: 1) one that can localize to the lysosomal membrane in response to increased amino acid concentration (via uptake through LAT1/Slc7a5 or lysosomal efflux) and includes Raptor (mTORC1), and 2) another that can localize to the plasma membrane and associate with Rictor, likely via direct association with the integrin-linked kinase (ILK) (mTORC2). Immediately following exercise (rapamycin-sensitive phase), mTORC1 is transported to the lysosome via Ragulators (Rag), where mTOR is activated by the GTP-bound form of Ras homolog enriched in brain (Rheb), allowing phosphorylation of p70S6K and eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1) and increased capacity and efficiency of protein translation. Activation of mTORC1 is essential for postexercise enhancement of myofiber hypertrophy. Studies demonstrate that the α7β1-integrin can also increase muscle hypertrophy in response to mechanical stimulation, yet it appears to facilitate this event independent of mTORC1. In this review, we propose that the α7β1-integrin tethers mTORC2 to the sarcolemma via association of Rictor with ILK in the hours following exercise (rapamycin insensitive phase) and that mTORC2 localization at the membrane allows for activation of downstream signaling pathways that promote survival (Akt), cytoskeletal remodeling, and stability (PKC; which may impact Yes-Associated Protein or YAP), and long-term enhancement of protein translation (SGK and 4E-BP1). nNOS, neuronal nitric oxide synthase.
GRANTS
This work was supported National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant R21-AR-065578 (to M. D. Boppart).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.D.B. prepared figures; M.D.B. and Z.S.M. drafted manuscript; M.D.B. and Z.S.M. edited and revised manuscript; M.D.B. and Z.S.M. approved final version of manuscript.
REFERENCES
- 1.Ali IU, Mautner V, Lanza R, Hynes RO. Restoration of normal morphology, adhesion and cytoskeleton in transformed cells by addition of a transformation-sensitive surface protein. Cell 11: 115–126, 1977. doi: 10.1016/0092-8674(77)90322-1. [DOI] [PubMed] [Google Scholar]
- 2.Arai A, Spencer JA, Olson EN. STARS, a striated muscle activator of Rho signaling and serum response factor-dependent transcription. J Biol Chem 277: 24453–24459, 2002. doi: 10.1074/jbc.M202216200. [DOI] [PubMed] [Google Scholar]
- 3.Atherton PJ, Greenhaff PL, Phillips SM, Bodine SC, Adams CM, Lang CH. Control of skeletal muscle atrophy in response to disuse: clinical/preclinical contentions and fallacies of evidence. Am J Physiol Endocrinol Metab 311: E594–E604, 2016. doi: 10.1152/ajpendo.00257.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bao ZZ, Lakonishok M, Kaufman S, Horwitz AF. α7 β1 integrin is a component of the myotendinous junction on skeletal muscle. J Cell Sci 106: 579–589, 1993. [DOI] [PubMed] [Google Scholar]
- 5.Baudoin C, Goumans MJ, Mummery C, Sonnenberg A. Knockout and knockin of the beta1 exon D define distinct roles for integrin splice variants in heart function and embryonic development. Genes Dev 12: 1202–1216, 1998. doi: 10.1101/gad.12.8.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res 339: 269–280, 2010. doi: 10.1007/s00441-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barreuther MF, Grabel LB. The role of phosphorylation in modulating β1 integrin localization. Exp Cell Res 222: 10–15, 1996. doi: 10.1006/excr.1996.0002. [DOI] [PubMed] [Google Scholar]
- 8.Basora N, Vachon PH, Herring-Gillam FE, Perreault N, Beaulieu JF. Relation between integrin alpha7Bbeta1 expression in human intestinal cells and enterocytic differentiation. Gastroenterology 113: 1510–1521, 1997. doi: 10.1053/gast.1997.v113.pm9352853. [DOI] [PubMed] [Google Scholar]
- 9.Belkin AM, Zhidkova NI, Balzac F, Altruda F, Tomatis D, Maier A, Tarone G, Koteliansky VE, Burridge K. Beta 1D integrin displaces the beta 1A isoform in striated muscles: localization at junctional structures and signaling potential in nonmuscle cells. J Cell Biol 132: 211–226, 1996. doi: 10.1083/jcb.132.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bentzinger CF, Romanino K, Cloëtta D, Lin S, Mascarenhas JB, Oliveri F, Xia J, Casanova E, Costa CF, Brink M, Zorzato F, Hall MN, Rüegg MA. Skeletal muscle-specific ablation of raptor, but not of rictor, causes metabolic changes and results in muscle dystrophy. Cell Metab 8: 411–424, 2008. doi: 10.1016/j.cmet.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Betz C, Hall MN. Where is mTOR and what is it doing there? J Cell Biol 203: 563–574, 2013. doi: 10.1083/jcb.201306041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biolo G, Maggi SP, Williams BD, Tipton KD, Wolfe RR. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am J Physiol Endocrinol Metab 268: E514–E520, 1995. doi: 10.1152/ajpendo.1995.268.3.E514. [DOI] [PubMed] [Google Scholar]
- 13.Biral D, Jakubiec-Puka A, Ciechomska I, Sandri M, Rossini K, Carraro U, Betto R. Loss of dystrophin and some dystrophin-associated proteins with concomitant signs of apoptosis in rat leg muscle overworked in extension. Acta Neuropathol 100: 618–626, 2000. doi: 10.1007/s004010000231. [DOI] [PubMed] [Google Scholar]
- 14.Blaschuk KL, Holland PC. The regulation of alpha 5 beta 1 integrin expression in human muscle cells. Dev Biol 164: 475–483, 1994. doi: 10.1006/dbio.1994.1217. [DOI] [PubMed] [Google Scholar]
- 15.Bledzka K, Bialkowska K, Sossey-Alaoui K, Vaynberg J, Pluskota E, Qin J, Plow EF. Kindlin-2 directly binds actin and regulates integrin outside-in signaling. J Cell Biol 213: 97–108, 2016. doi: 10.1083/jcb.201501006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3: 1014–1019, 2001. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- 17.Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294: 1704–1708, 2001. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 18.Bodine SC. Disuse-induced muscle wasting. Int J Biochem Cell Biol 45: 2200–2208, 2013. doi: 10.1016/j.biocel.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bodine SC, Baehr LM. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am J Physiol Endocrinol Metab 307: E469–E484, 2014. doi: 10.1152/ajpendo.00204.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boppart MD, Burkin DJ, Kaufman SJ. α7β1-Integrin regulates mechanotransduction and prevents skeletal muscle injury. Am J Physiol Cell Physiol 290: C1660–C1665, 2006. doi: 10.1152/ajpcell.00317.2005. [DOI] [PubMed] [Google Scholar]
- 21.Boppart MD, Burkin DJ, Kaufman SJ. Activation of AKT signaling promotes cell growth and survival in α7β1 integrin-mediated alleviation of muscular dystrophy. Biochim Biophys Acta 1812: 439–446, 2011. doi: 10.1016/j.bbadis.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boppart MD, Volker SE, Alexander N, Burkin DJ, Kaufman SJ. Exercise promotes α7 integrin gene transcription and protection of skeletal muscle. Am J Physiol Regul Integr Comp Physiol 295: R1623–R1630, 2008. doi: 10.1152/ajpregu.00089.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burkin DJ, Wallace GQ, Nicol KJ, Kaufman DJ, Kaufman SJ. Enhanced expression of the α 7 β 1 integrin reduces muscular dystrophy and restores viability in dystrophic mice. J Cell Biol 152: 1207–1218, 2001. doi: 10.1083/jcb.152.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burkin DJ, Wallace GQ, Milner DJ, Chaney EJ, Mulligan JA, Kaufman SJ. Transgenic expression of alpha7beta1 integrin maintains muscle integrity, increases regenerative capacity, promotes hypertrophy, and reduces cardiomyopathy in dystrophic mice. Am J Pathol 166: 253–263, 2005. doi: 10.1016/S0002-9440(10)62249-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu Rev Cell Dev Biol 12: 463–519, 1996. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- 26.Campbell ID, Humphries MJ. Integrin structure, activation, and interactions. Cold Spring Harb Perspect Biol 3: a004994, 2011. doi: 10.1101/cshperspect.a004994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaillou T, Lee JD, England JH, Esser KA, McCarthy JJ. Time course of gene expression during mouse skeletal muscle hypertrophy. J Appl Physiol (1985) 115: 1065–1074, 2013. doi: 10.1152/japplphysiol.00611.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheah M, Andrews MR. Integrin activation: implications for axon regeneration. Cells 7: 20, 2018. doi: 10.3390/cells7030020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cho S, Irianto J, Discher DE. Mechanosensing by the nucleus: from pathways to scaling relationships. J Cell Biol 216: 305–315, 2017. doi: 10.1083/jcb.201610042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collo G, Starr L, Quaranta V. A new isoform of the laminin receptor integrin alpha 7 beta 1 is developmentally regulated in skeletal muscle. J Biol Chem 268: 19019–19024, 1993. [PubMed] [Google Scholar]
- 31.Cybulski N, Hall MN. TOR complex 2: a signaling pathway of its own. Trends Biochem Sci 34: 620–627, 2009. doi: 10.1016/j.tibs.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 32.Das A, Fischer RS, Pan D, Waterman CM. YAP nuclear localization in the absence of cell-cell contact is mediated by a filamentous actin-dependent, myosin II-and phosphor-YAP-independent pathway during extracellular matrix mechanosensing. J Biol Chem 291: 6096–6110, 2016. doi: 10.1074/jbc.M115.708313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Boer MD, Selby A, Atherton P, Smith K, Seynnes OR, Maganaris CN, Maffulli N, Movin T, Narici MV, Rennie MJ. The temporal responses of protein synthesis, gene expression and cell signalling in human quadriceps muscle and patellar tendon to disuse. J Physiol 585: 241–251, 2007. doi: 10.1113/jphysiol.2007.142828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Lisio M, Farup J, Sukiennik RA, Clevenger N, Nallabelli J, Nelson B, Ryan K, Rahbek SK, de Paoli F, Vissing K, Boppart MD. The acute response of pericytes to muscle-damaging eccentric contraction and protein supplementation in human skeletal muscle. J Appl Physiol (1985) 119: 900–907, 2015. doi: 10.1152/japplphysiol.01112.2014. [DOI] [PubMed] [Google Scholar]
- 35.Delcommenne M, Tan C, Gray V, Rue L, Woodgett J, Dedhar S. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc Natl Acad Sci USA 95: 11211–11216, 1998. doi: 10.1073/pnas.95.19.11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drummond MJ, Fry CS, Glynn EL, Dreyer HC, Dhanani S, Timmerman KL, Volpi E, Rasmussen BB. Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J Physiol 587: 1535–1546, 2009. doi: 10.1113/jphysiol.2008.163816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature 474: 179–183, 2011. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 38.Durieux AC, D’Antona G, Desplanches D, Freyssenet D, Klossner S, Bottinelli R, Flück M. Focal adhesion kinase is a load-dependent governor of the slow contractile and oxidative muscle phenotype. J Physiol 587: 3703–3717, 2009. doi: 10.1113/jphysiol.2009.171355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ebner M, Sinkovics B, Szczygieł M, Ribeiro DW, Yudushkin I. Localization of mTORC2 activity inside cells. J Cell Biol 216: 343–353, 2017. doi: 10.1083/jcb.201610060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elosegui-Artola A, Oria R, Chen Y, Kosmalska A, Pérez-González C, Castro N, Zhu C, Trepat X, Roca-Cusachs P. Mechanical regulation of a molecular clutch defines force transmission and transduction in response to matrix rigidity. Nat Cell Biol 18: 540–548, 2016. doi: 10.1038/ncb3336. [DOI] [PubMed] [Google Scholar]
- 41.Flintoff-Dye NL, Welser J, Rooney J, Scowen P, Tamowski S, Hatton W, Burkin DJ. Role for the alpha7beta1 integrin in vascular development and integrity. Dev Dyn 234: 11–21, 2005. doi: 10.1002/dvdy.20462. [DOI] [PubMed] [Google Scholar]
- 42.Flück M, Carson JA, Schwartz RJ, Booth FW. SRF protein is upregulated during stretch-induced hypertrophy of rooster ALD muscle. J Appl Physiol (1985) 86: 1793–1799, 1999. doi: 10.1152/jappl.1999.86.6.1793. [DOI] [PubMed] [Google Scholar]
- 43.Flück M, Carson JA, Gordon SE, Ziemiecki A, Booth FW. Focal adhesion proteins FAK and paxillin increase in hypertrophied skeletal muscle. Am J Physiol Cell Physiol 277: C152–C162, 1999. doi: 10.1152/ajpcell.1999.277.1.C152. [DOI] [PubMed] [Google Scholar]
- 44.Flück M, Li R, Valdivieso P, Linnehan RM, Castells J, Tesch P, Gustafsson T. Early changes in costameric and mitochondrial protein expression with unloading are muscle specific. BioMed Res Int 2014: 519310, 2014. doi: 10.1155/2014/519310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Foletta VC, Prior MJ, Stupka N, Carey K, Segal DH, Jones S, Swinton C, Martin S, Cameron-Smith D, Walder KR. NDRG2, a novel regulator of myoblast proliferation, is regulated by anabolic and catabolic factors. J Physiol 587: 1619–1634, 2009. doi: 10.1113/jphysiol.2008.167882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Foletta VC, Brown EL, Cho Y, Snow RJ, Kralli A, Russell AP. Ndrg2 is a PGC-1α/ERRα target gene that controls protein synthesis and expression of contractile-type genes in C2C12 myotubes. Biochim Biophys Acta 1833: 3112–3123, 2013. doi: 10.1016/j.bbamcr.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 47.Franchi MV, Ruoss S, Valdivieso P, Mitchell KW, Smith K, Atherton PJ, Narici MV, Flück M. Regional regulation of focal adhesion kinase after concentric and eccentric loading is related to remodelling of human skeletal muscle. Acta Physiol (Oxf) 223: e13056, 2018. doi: 10.1111/apha.13056. [DOI] [PubMed] [Google Scholar]
- 48.Frenette J, Côté CH. Modulation of structural protein content of the myotendinous junction following eccentric contractions. Int J Sports Med 21: 313–320, 2000. doi: 10.1055/s-2000-3774. [DOI] [PubMed] [Google Scholar]
- 49.Fry CS, Lee JD, Jackson JR, Kirby TJ, Stasko SA, Liu H, Dupont-Versteegden EE, McCarthy JJ, Peterson CA. Regulation of the muscle fiber microenvironment by activated satellite cells during hypertrophy. FASEB J 28: 1654–1665, 2014. doi: 10.1096/fj.13-239426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ganley IG, Lam H, Wang J, Ding X, Chen S, Jiang X. ULK1.ATG13.FIP200 complex mediates mTOR signaling and is essential for autophagy. J Biol Chem 284: 12297–12305, 2009. doi: 10.1074/jbc.M900573200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gheyara AL, Vallejo-Illarramendi A, Zang K, Mei L, St-Arnaud R, Dedhar S, Reichardt LF. Deletion of integrin-linked kinase from skeletal muscles of mice resembles muscular dystrophy due to alpha 7 beta 1-integrin deficiency. Am J Pathol 171: 1966–1977, 2007. doi: 10.2353/ajpath.2007.070555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Glover EI, Phillips SM, Oates BR, Tang JE, Tarnopolsky MA, Selby A, Smith K, Rennie MJ. Immobilization induces anabolic resistance in human myofibrillar protein synthesis w ith low and high dose amino acid infusion. J Physiol 586: 6049–6061, 2008. doi: 10.1113/jphysiol.2008.160333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gordon SE, Flück M, Booth FW. Selected Contribution: Skeletal muscle focal adhesion kinase, paxillin, and serum response factor are loading dependent. J Appl Physiol (1985) 90: 1174–1183, 2001. doi: 10.1152/jappl.2001.90.3.1174. [DOI] [PubMed] [Google Scholar]
- 54.Goodman CA, Frey JW, Mabrey DM, Jacobs BL, Lincoln HC, You JS, Hornberger TA. The role of skeletal muscle mTOR in the regulation of mechanical load-induced growth. J Physiol 589: 5485–5501, 2011. doi: 10.1113/jphysiol.2011.218255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goodman CA, Dietz JM, Jacobs BL, McNally RM, You JS, Hornberger TA. Yes-associated protein is up-regulated by mechanical overload and is sufficient to induce skeletal muscle hypertrophy. FEBS Lett 589: 1491–1497, 2015. doi: 10.1016/j.febslet.2015.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Graham ZA, Gallagher PM, Cardozo CP. Focal adhesion kinase and its role in skeletal muscle. J Muscle Res Cell Motil 36: 305–315, 2015. doi: 10.1007/s10974-015-9415-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guerci A, Lahoute C, Hébrard S, Collard L, Graindorge D, Favier M, Cagnard N, Batonnet-Pichon S, Précigout G, Garcia L, Tuil D, Daegelen D, Sotiropoulos A. Srf-dependent paracrine signals produced by myofibers control satellite cell-mediated skeletal muscle hypertrophy. Cell Metab 15: 25–37, 2012. doi: 10.1016/j.cmet.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 58.Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, Brown M, Fitzgerald KJ, Sabatini DM. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell 11: 859–871, 2006. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 59.Hannigan GE, Leung-Hagesteijn C, Fitz-Gibbon L, Coppolino MG, Radeva G, Filmus J, Bell JC, Dedhar S. Regulation of cell adhesion and anchorage-dependent growth by a new beta 1-integrin-linked protein kinase. Nature 379: 91–96, 1996. doi: 10.1038/379091a0. [DOI] [PubMed] [Google Scholar]
- 60.Hansen CG, Ng YL, Lam WL, Plouffe SW, Guan KL. The Hippo pathway effectors YAP and TAZ promote cell growth by modulating amino acid signaling to mTORC1. Cell Res 25: 1299–1313, 2015. doi: 10.1038/cr.2015.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hayakawa K, Tatsumi H, Sokabe M. Actin filaments function as a tension sensor by tension-dependent binding of cofilin to the filament. J Cell Biol 195: 721–727, 2011. doi: 10.1083/jcb.201102039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hayashi YK, Chou FL, Engvall E, Ogawa M, Matsuda C, Hirabayashi S, Yokochi K, Ziober BL, Kramer RH, Kaufman SJ, Ozawa E, Goto Y, Nonaka I, Tsukahara T, Wang JZ, Hoffman EP, Arahata K. Mutations in the integrin alpha7 gene cause congenital myopathy. Nat Genet 19: 94–97, 1998. doi: 10.1038/ng0598-94. [DOI] [PubMed] [Google Scholar]
- 63.Hodges BL, Hayashi YK, Nonaka I, Wang W, Arahata K, Kaufman SJ. Altered expression of the alpha7beta1 integrin in human and murine muscular dystrophies. J Cell Sci 110: 2873–2881, 1997. [DOI] [PubMed] [Google Scholar]
- 64.Hodson N, McGlory C, Oikawa SY, Jeromson S, Song Z, Rüegg MA, Hamilton DL, Phillips SM, Philp A. Differential localization and anabolic responsiveness of mTOR complexes in human skeletal muscle in response to feeding and exercise. Am J Physiol Cell Physiol 313: C604–C611, 2017. doi: 10.1152/ajpcell.00176.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huntsman HD, Zachwieja N, Zou K, Ripchik P, Valero MC, De Lisio M, Boppart MD. Mesenchymal stem cells contribute to vascular growth in skeletal muscle in response to eccentric exercise. Am J Physiol Heart Circ Physiol 304: H72–H81, 2013. doi: 10.1152/ajpheart.00541.2012. [DOI] [PubMed] [Google Scholar]
- 66.Hyldahl RD, Nelson B, Xin L, Welling T, Groscost L, Hubal MJ, Chipkin S, Clarkson PM, Parcell AC. Extracellular matrix remodeling and its contribution to protective adaptation following lengthening contractions in human muscle. FASEB J 29: 2894–2904, 2015. doi: 10.1096/fj.14-266668. [DOI] [PubMed] [Google Scholar]
- 67.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell 110: 673–687, 2002. doi: 10.1016/S0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 68.Hynes RO. The emergence of integrins: a personal and historical perspective. Matrix Biol 23: 333–340, 2004. doi: 10.1016/j.matbio.2004.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ivanov P, Emara MM, Villen J, Gygi SP, Anderson P. Angiogenin-induced tRNA fragments inhibit translation initiation. Mol Cell 43: 613–623, 2011. doi: 10.1016/j.molcel.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Iyer SR, Shah SB, Ward CW, Stains JP, Spangenburg EE, Folker ES, Lovering RM. Differential YAP nuclear signaling in healthy and dystrophic skeletal muscle. Am J Physiol Cell Physiol 317: C48–C57, 2019. doi: 10.1152/ajpcell.00432.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jacinto E, Loewith R, Schmidt A, Lin S, Rüegg MA, Hall A, Hall MN. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol 6: 1122–1128, 2004. doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- 72.Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, Huang Q, Qin J, Su B. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell 127: 125–137, 2006. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 73.Jacobs BL, You JS, Frey JW, Goodman CA, Gundermann DM, Hornberger TA. Eccentric contractions increase the phosphorylation of tuberous sclerosis complex-2 (TSC2) and alter the targeting of TSC2 and the mechanistic target of rapamycin to the lysosome. J Physiol 591: 4611–4620, 2013. doi: 10.1113/jphysiol.2013.256339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Juan WC, Hong W. Targeting the Hippo signaling pathway for tissue regeneration and cancer therapy. Genes (Basel) 7: 55, 2016. doi: 10.3390/genes7090055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kadrmas JL, Beckerle MC. The LIM domain: from the cytoskeleton to the nucleus. Nat Rev Mol Cell Biol 5: 920–931, 2004. doi: 10.1038/nrm1499. [DOI] [PubMed] [Google Scholar]
- 76.Kim C, Ye F, Ginsberg MH. Regulation of integrin activation. Annu Rev Cell Dev Biol 27: 321–345, 2011. doi: 10.1146/annurev-cellbio-100109-104104. [DOI] [PubMed] [Google Scholar]
- 77.Kleinert M, Parker BL, Fritzen AM, Knudsen JR, Jensen TE, Kjøbsted R, Sylow L, Ruegg M, James DE, Richter EA. Mammalian target of rapamycin complex 2 regulates muscle glucose uptake during exercise in mice. J Physiol 595: 4845–4855, 2017. doi: 10.1113/JP274203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Klossner S, Durieux AC, Freyssenet D, Flueck M. Mechano-transduction to muscle protein synthesis is modulated by FAK. Eur J Appl Physiol 106: 389–398, 2009. doi: 10.1007/s00421-009-1032-7. [DOI] [PubMed] [Google Scholar]
- 79.Kubica N, Bolster DR, Farrell PA, Kimball SR, Jefferson LS. Resistance exercise increases muscle protein synthesis and translation of eukaryotic initiation factor 2Bepsilon mRNA in a mammalian target of rapamycin-dependent manner. J Biol Chem 280: 7570–7580, 2005. doi: 10.1074/jbc.M413732200. [DOI] [PubMed] [Google Scholar]
- 80.Kühl U, Ocalan M, Timpl R, von der Mark K. Role of laminin and fibronectin in selecting myogenic versus fibrogenic cells from skeletal muscle cells in vitro. Dev Biol 117: 628–635, 1986. doi: 10.1016/0012-1606(86)90331-3. [DOI] [PubMed] [Google Scholar]
- 81.Labeit S, Kohl CH, Witt CC, Labeit D, Jung J, Granzier H. Modulation of muscle atrophy, fatigue and MLC phosphorylation by MuRF1 as indicated by hindlimb suspension studies on MuRF1-KO mice. J Biomed Biotechnol 2010: 693741, 2010. doi: 10.1155/2010/693741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lahoute C, Sotiropoulos A, Favier M, Guillet-Deniau I, Charvet C, Ferry A, Butler-Browne G, Metzger D, Tuil D, Daegelen D. Premature aging in skeletal muscle lacking serum response factor. PLoS One 3: e3910, 2008. doi: 10.1371/journal.pone.0003910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lamon S, Wallace MA, Léger B, Russell AP. Regulation of STARS and its downstream targets suggest a novel pathway involved in human skeletal muscle hypertrophy and atrophy. J Physiol 587: 1795–1803, 2009. doi: 10.1113/jphysiol.2009.168674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lange A, Wickström SA, Jakobson M, Zent R, Sainio K, Fässler R. Integrin-linked kinase is an adaptor with essential functions during mouse development. Nature 461: 1002–1006, 2009. doi: 10.1038/nature08468. [DOI] [PubMed] [Google Scholar]
- 85.Law DJ, Allen DL, Tidball JG. Talin, vinculin and DRP (utrophin) concentrations are increased at mdx myotendinous junctions following onset of necrosis. J Cell Sci 107: 1477–1483, 1994. [DOI] [PubMed] [Google Scholar]
- 86.Lee JO, Bankston LA, Arnaout MA, Liddington RC. Two conformations of the integrin A-domain (I-domain): a pathway for activation? Structure 3: 1333–1340, 1995. doi: 10.1016/S0969-2126(01)00271-4. [DOI] [PubMed] [Google Scholar]
- 87.Li R, Narici MV, Erskine RM, Seynnes OR, Rittweger J, Pišot R, Šimunič B, Flück M. Costamere remodeling with muscle loading and unloading in healthy young men. J Anat 223: 525–536, 2013. doi: 10.1111/joa.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lu H, Fedak PW, Dai X, Du C, Zhou YQ, Henkelman M, Mongroo PS, Lau A, Yamabi H, Hinek A, Husain M, Hannigan G, Coles JG. Integrin-linked kinase expression is elevated in human cardiac hypertrophy and induces hypertrophy in transgenic mice. Circulation 114: 2271–2279, 2006. doi: 10.1161/CIRCULATIONAHA.106.642330. [DOI] [PubMed] [Google Scholar]
- 89.Lueders TN, Zou K, Huntsman HD, Meador B, Mahmassani Z, Abel M, Valero MC, Huey KA, Boppart MD. The α7β1-integrin accelerates fiber hypertrophy and myogenesis following a single bout of eccentric exercise. Am J Physiol Cell Physiol 301: C938–C946, 2011. doi: 10.1152/ajpcell.00515.2010. [DOI] [PubMed] [Google Scholar]
- 90.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol 10: 307–318, 2009. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- 91.MacKenzie MG, Hamilton DL, Murray JT, Taylor PM, Baar K. mVps34 is activated following high-resistance contractions. J Physiol 587: 253–260, 2009. doi: 10.1113/jphysiol.2008.159830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mahmassani ZS, Reidy PT, McKenzie AI, Stubben C, Howard MT, Drummond MJ. Age-dependent skeletal muscle transcriptome response to bed rest-induced atrophy. J Appl Physiol (1985) 126: 894–902, 2019. doi: 10.1152/japplphysiol.00811.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mahmassani ZS, Son K, Pincu Y, Munroe M, Drnevich J, Chen J, Boppart MD. α7β1-Integrin regulation of gene transcription in skeletal muscle following an acute bout of eccentric exercise. Am J Physiol Cell Physiol 312: C638–C650, 2017. doi: 10.1152/ajpcell.00106.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Martin PT, Kaufman SJ, Kramer RH, Sanes JR. Synaptic integrins in developing, adult, and mutant muscle: selective association of alpha1, alpha7A, and alpha7B integrins with the neuromuscular junction. Dev Biol 174: 125–139, 1996. doi: 10.1006/dbio.1996.0057. [DOI] [PubMed] [Google Scholar]
- 95.Martino F, Perestrelo AR, Vinarský V, Pagliari S, Forte G. Cellular mechanotransduction: from tension to function. Front Physiol 9: 824, 2018. doi: 10.3389/fphys.2018.00824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mathes S, Vanmunster M, Bloch W, Suhr F. Evidence for skeletal muscle fiber type-specific expressions of mechanosensors. Cell Mol Life Sci 76: 2987–3004, 2019. doi: 10.1096/fj.201701422R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mayer U. Integrins: redundant or important players in skeletal muscle? J Biol Chem 278: 14587–14590, 2003. doi: 10.1074/jbc.R200022200. [DOI] [PubMed] [Google Scholar]
- 98.Mayer U, Saher G, Fässler R, Bornemann A, Echtermeyer F, von der Mark H, Miosge N, Pöschl E, von der Mark K. Absence of integrin alpha 7 causes a novel form of muscular dystrophy. Nat Genet 17: 318–323, 1997. doi: 10.1038/ng1197-318. [DOI] [PubMed] [Google Scholar]
- 99.McCarthy JJ, Mula J, Miyazaki M, Erfani R, Garrison K, Farooqui AB, Srikuea R, Lawson BA, Grimes B, Keller C, Van Zant G, Campbell KS, Esser KA, Dupont-Versteegden EE, Peterson CA. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development 138: 3657–3666, 2011. doi: 10.1242/dev.068858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McClung JM, Lee WJ, Thompson RW, Lowe LL, Carson JA. RhoA induction by functional overload and nandrolone decanoate administration in rat skeletal muscle. Pflugers Arch 447: 345–355, 2003. doi: 10.1007/s00424-003-1151-7. [DOI] [PubMed] [Google Scholar]
- 101.McDonald PC, Fielding AB, Dedhar S. Integrin-linked kinase–essential roles in physiology and cancer biology. J Cell Sci 121: 3121–3132, 2008. doi: 10.1242/jcs.017996. [DOI] [PubMed] [Google Scholar]
- 102.McGough A, Pope B, Chiu W, Weeds A. Cofilin changes the twist of F-actin: implications for actin filament dynamics and cellular function. J Cell Biol 138: 771–781, 1997. doi: 10.1083/jcb.138.4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Milkereit R, Persaud A, Vanoaica L, Guetg A, Verrey F, Rotin D. LAPTM4b recruits the LAT1-4F2hc Leu transporter to lysosomes and promotes mTORC1 activation. Nat Commun 6: 7250, 2015. doi: 10.1038/ncomms8250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Miller BF, Hamilton KL, Majeed ZR, Abshire SM, Confides AL, Hayek AM, Hunt ER, Shipman P, Peelor FF 3rd, Butterfield TA, Dupont-Versteegden EE. Enhanced skeletal muscle regrowth and remodelling in massaged and contralateral non-massaged hindlimb. J Physiol 596: 83–103, 2018. doi: 10.1113/JP275089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Miosge N, Klenczar C, Herken R, Willem M, Mayer U. Organization of the myotendinous junction is dependent on the presence of alpha7beta1 integrin. Lab Invest 79: 1591–1599, 1999. [PubMed] [Google Scholar]
- 106.Murach KA, White SH, Wen Y, Ho A, Dupont-Versteegden EE, McCarthy JJ, Peterson CA. Differential requirement for satellite cells during overload-induced muscle hypertrophy in growing versus mature mice. Skelet Muscle 7: 14, 2017. doi: 10.1186/s13395-017-0132-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Murray JT, Campbell DG, Morrice N, Auld GC, Shpiro N, Marquez R, Peggie M, Bain J, Bloomberg GB, Grahammer F, Lang F, Wulff P, Kuhl D, Cohen P. Exploitation of KESTREL to identify NDRG family members as physiological substrates for SGK1 and GSK3. Biochem J 384: 477–488, 2004. doi: 10.1042/BJ20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nojima H, Tokunaga C, Eguchi S, Oshiro N, Hidayat S, Yoshino K, Hara K, Tanaka N, Avruch J, Yonezawa K. The mammalian target of rapamycin (mTOR) partner, raptor, binds the mTOR substrates p70 S6 kinase and 4E-BP1 through their TOR signaling (TOS) motif. J Biol Chem 278: 15461–15464, 2003. doi: 10.1074/jbc.C200665200. [DOI] [PubMed] [Google Scholar]
- 109.Ogasawara R, Suginohara T. Rapamycin-insensitive mechanistic target of rapamycin regulates basal and resistance exercise-induced muscle protein synthesis. FASEB J 32: 5824–5834, 2018. doi: 10.1096/fj.201701422R. [DOI] [PubMed] [Google Scholar]
- 110.Oh WJ, Jacinto E. mTOR complex 2 signaling and functions. Cell Cycle 10: 2305–2316, 2011. doi: 10.4161/cc.10.14.16586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pearce LR, Huang X, Boudeau J, Pawłowski R, Wullschleger S, Deak M, Ibrahim AF, Gourlay R, Magnuson MA, Alessi DR. Identification of Protor as a novel Rictor-binding component of mTOR complex-2. Biochem J 405: 513–522, 2007. doi: 10.1042/BJ20070540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pizzo E, Sarcinelli C, Sheng J, Fusco S, Formiggini F, Netti P, Yu W, D’Alessio G, Hu GF. Ribonuclease/angiogenin inhibitor 1 regulates stress-induced subcellular localization of angiogenin to control growth and survival. J Cell Sci 126: 4308–4319, 2013. doi: 10.1242/jcs.134551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Roca-Cusachs P, del Rio A, Puklin-Faucher E, Gauthier NC, Biais N, Sheetz MP. Integrin-dependent force transmission to the extracellular matrix by α-actinin triggers adhesion maturation. Proc Natl Acad Sci USA 110: E1361–E1370, 2013. doi: 10.1073/pnas.1220723110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sacco A, Doyonnas R, Kraft P, Vitorovic S, Blau HM. Self-renewal and expansion of single transplanted muscle stem cells. Nature 456: 502–506, 2008. doi: 10.1038/nature07384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol 14: 1296–1302, 2004. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 116.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307: 1098–1101, 2005. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 117.Sen S, Tewari M, Zajac A, Barton E, Sweeney HL, Discher DE. Upregulation of paxillin and focal adhesion signaling follows dystroglycan complex deletions and promotes a hypertensive state of differentiation. Eur J Cell Biol 90: 249–260, 2011. doi: 10.1016/j.ejcb.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Song WK, Wang W, Sato H, Bielser DA, Kaufman SJ. Expression of alpha 7 integrin cytoplasmic domains during skeletal muscle development: alternate forms, conformational change, and homologies with serine/threonine kinases and tyrosine phosphatases. J Cell Sci 106: 1139–1152, 1993. [DOI] [PubMed] [Google Scholar]
- 119.Spangenburg EE, Le Roith D, Ward CW, Bodine SC. A functional insulin-like growth factor receptor is not necessary for load-induced skeletal muscle hypertrophy. J Physiol 586: 283–291, 2008. doi: 10.1113/jphysiol.2007.141507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sun Z, Guo SS, Fässler R. Integrin-mediated mechanotransduction. J Cell Biol 215: 445–456, 2016. doi: 10.1083/jcb.201609037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tajik A, Zhang Y, Wei F, Sun J, Jia Q, Zhou W, Singh R, Khanna N, Belmont AS, Wang N. Transcription upregulation via force-induced direct stretching of chromatin. Nat Mater 15: 1287–1296, 2016. doi: 10.1038/nmat4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tamada M, Sheetz MP, Sawada Y. Activation of a signaling cascade by cytoskeleton stretch. Dev Cell 7: 709–718, 2004. doi: 10.1016/j.devcel.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 123.Tamkun JW, DeSimone DW, Fonda D, Patel RS, Buck C, Horwitz AF, Hynes RO. Structure of integrin, a glycoprotein involved in the transmembrane linkage between fibronectin and actin. Cell 46: 271–282, 1986. doi: 10.1016/0092-8674(86)90744-0. [DOI] [PubMed] [Google Scholar]
- 124.Theodosiou M, Widmaier M, Böttcher RT, Rognoni E, Veelders M, Bharadwaj M, Lambacher A, Austen K, Müller DJ, Zent R, Fässler R. Kindlin-2 cooperates with talin to activate integrins and induces cell spreading by directly binding paxillin. eLife 5: e10130, 2016. doi: 10.7554/eLife.10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tu Y, Li F, Goicoechea S, Wu C. The LIM-only protein PINCH directly interacts with integrin-linked kinase and is recruited to integrin-rich sites in spreading cells. Mol Cell Biol 19: 2425–2434, 1999. doi: 10.1128/MCB.19.3.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tu Y, Li F, Wu C. Nck-2, a novel Src homology2/3-containing adaptor protein that interacts with the LIM-only protein PINCH and components of growth factor receptor kinase-signaling pathways. Mol Biol Cell 9: 3367–3382, 1998. doi: 10.1091/mbc.9.12.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tyml K, Mathieu-Costello O. Structural and functional changes in the microvasculature of disused skeletal muscle. Front Biosci 6: D45–D52, 2001. doi: 10.2741/A592. [DOI] [PubMed] [Google Scholar]
- 128.van der Flier A, Kuikman I, Baudoin C, van der Neut R, Sonnenberg A. A novel beta 1 integrin isoform produced by alternative splicing: unique expression in cardiac and skeletal muscle. FEBS Lett 369: 340–344, 1995. doi: 10.1016/0014-5793(95)00814-P. [DOI] [PubMed] [Google Scholar]
- 129.Velling T, Collo G, Sorokin L, Durbeej M, Zhang H, Gullberg D. Distinct alpha 7A beta 1 and alpha 7B beta 1 integrin expression patterns during mouse development: alpha 7A is restricted to skeletal muscle but alpha 7B is expressed in striated muscle, vasculature, and nervous system. Dev Dyn 207: 355–371, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- 130.Wada K, Itoga K, Okano T, Yonemura S, Sasaki H. Hippo pathway regulation by cell morphology and stress fibers. Development 138: 3907–3914, 2011. doi: 10.1242/dev.070987. [DOI] [PubMed] [Google Scholar]
- 131.Wall BT, Dirks ML, van Loon LJ. Skeletal muscle atrophy during short-term disuse: implications for age-related sarcopenia. Ageing Res Rev 12: 898–906, 2013. doi: 10.1016/j.arr.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 132.Wang HV, Chang LW, Brixius K, Wickström SA, Montanez E, Thievessen I, Schwander M, Müller U, Bloch W, Mayer U, Fässler R. Integrin-linked kinase stabilizes myotendinous junctions and protects muscle from stress-induced damage. J Cell Biol 180: 1037–1049, 2008. doi: 10.1083/jcb.200707175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Watt KI, Turner BJ, Hagg A, Zhang X, Davey JR, Qian H, Beyer C, Winbanks CE, Harvey KF, Gregorevic P. The Hippo pathway effector YAP is a critical regulator of skeletal muscle fibre size. Nat Commun 6: 6048, 2015. doi: 10.1038/ncomms7048. [DOI] [PubMed] [Google Scholar]
- 134.Wei L, Zhou W, Croissant JD, Johansen FE, Prywes R, Balasubramanyam A, Schwartz RJ. RhoA signaling via serum response factor plays an obligatory role in myogenic differentiation. J Biol Chem 273: 30287–30294, 1998. doi: 10.1074/jbc.273.46.30287. [DOI] [PubMed] [Google Scholar]
- 135.Wei L, Wang L, Carson JA, Agan JE, Imanaka-Yoshida K, Schwartz RJ. beta1 integrin and organized actin filaments facilitate cardiomyocyte-specific RhoA-dependent activation of the skeletal alpha-actin promoter. FASEB J 15: 785–796, 2001. doi: 10.1096/fj.00-026com. [DOI] [PubMed] [Google Scholar]
- 136.Werner A, Willem M, Jones LL, Kreutzberg GW, Mayer U, Raivich G. Impaired axonal regeneration in alpha7 integrin-deficient mice. J Neurosci 20: 1822–1830, 2000. doi: 10.1523/JNEUROSCI.20-05-01822.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.West DW, Kujbida GW, Moore DR, Atherton P, Burd NA, Padzik JP, De Lisio M, Tang JE, Parise G, Rennie MJ, Baker SK, Phillips SM. Resistance exercise-induced increases in putative anabolic hormones do not enhance muscle protein synthesis or intracellular signalling in young men. J Physiol 587: 5239–5247, 2009. doi: 10.1113/jphysiol.2009.177220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wickström SA, Lange A, Montanez E, Fässler R. The ILK/PINCH/parvin complex: the kinase is dead, long live the pseudokinase! EMBO J 29: 281–291, 2010. doi: 10.1038/emboj.2009.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Widmaier M, Rognoni E, Radovanac K, Azimifar SB, Fässler R. Integrin-linked kinase at a glance. J Cell Sci 125: 1839–1843, 2012. doi: 10.1242/jcs.093864. [DOI] [PubMed] [Google Scholar]
- 140.Wolfson RL, Sabatini DM. The dawn of the age of amino acid sensors for the mTORC1 pathway. Cell Metab 26: 301–309, 2017. doi: 10.1016/j.cmet.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yagi R, Chen LF, Shigesada K, Murakami Y, Ito Y. A WW domain-containing yes-associated protein (YAP) is a novel transcriptional co-activator. EMBO J 18: 2551–2562, 1999. doi: 10.1093/emboj/18.9.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yamasaki S, Ivanov P, Hu GF, Anderson P. Angiogenin cleaves tRNA and promotes stress-induced translational repression. J Cell Biol 185: 35–42, 2009. doi: 10.1083/jcb.200811106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yao CC, Breuss J, Pytela R, Kramer RH. Functional expression of the alpha 7 integrin receptor in differentiated smooth muscle cells. J Cell Sci 110: 1477–1487, 1997. [DOI] [PubMed] [Google Scholar]
- 144.Yoon MS. mTOR as a key regulator in maintaining skeletal muscle mass. Front Physiol 8: 788, 2017. doi: 10.3389/fphys.2017.00788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.You JS, McNally RM, Jacobs BL, Privett RE, Gundermann DM, Lin KH, Steinert ND, Goodman CA, Hornberger TA. The role of raptor in the mechanical load-induced regulation of mTOR signaling, protein synthesis, and skeletal muscle hypertrophy. FASEB J 33: 4021–4034, 2019. doi: 10.1096/fj.201801653RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhang J, Ji JY, Yu M, Overholtzer M, Smolen GA, Wang R, Brugge JS, Dyson NJ, Haber DA. YAP-dependent induction of amphiregulin identifies a non-cell-autonomous component of the Hippo pathway. Nat Cell Biol 11: 1444–1450, 2009. doi: 10.1038/ncb1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhao B, Li L, Wang L, Wang CY, Yu J, Guan KL. Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev 26: 54–68, 2012. doi: 10.1101/gad.173435.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ziober BL, Vu MP, Waleh N, Crawford J, Lin CS, Kramer RH. Alternative extracellular and cytoplasmic domains of the integrin alpha 7 subunit are differentially expressed during development. J Biol Chem 268: 26773–26783, 1993. [PubMed] [Google Scholar]
- 149.Zou K, Meador BM, Johnson B, Huntsman HD, Mahmassani Z, Valero MC, Huey KA, Boppart MD. The α7β1-integrin increases muscle hypertrophy following multiple bouts of eccentric exercise. J Appl Physiol (1985) 111: 1134–1141, 2011. doi: 10.1152/japplphysiol.00081.2011. [DOI] [PubMed] [Google Scholar]