Abstract
Calponin 2 is an actin cytoskeleton-associated regulatory protein that inhibits the activity of myosin-ATPase and cytoskeleton dynamics. Recent studies have demonstrated that deletion of calponin 2 restricts the proinflammatory activation of macrophages in atherosclerosis and arthritis to attenuate the disease progression in mice. Here we demonstrate that the levels of calponin 2 vary among different macrophage populations, which may reflect their adaptation to specific tissue microenvironment corresponding to specific functional states. Interestingly, lung resident macrophages express significantly lower calponin 2 than peritoneal resident macrophages, which correlates with decreased substrate adhesion and reduced expression of proinflammatory cytokines and a proresolution phenotype. Deletion of calponin 2 in peritoneal macrophages also decreased substrate adhesion and downregulated the expression of proinflammatory cytokines. Providing the first line of defense against microbial invasion while receiving constant exposure to extrinsic antigens, lung macrophages need to maintain a necessary level of activity while limiting exaggerated inflammatory reaction. Therefore, their low level of calponin 2 may reflect an important physiological adaption. Downregulation of calponin 2 in macrophages may be targeted as a cytoskeleton-based novel mechanism, possibly via endoplasmic reticulum stress altering the processing and secretion of cytokines, to regulate immune response and promote quiescence for the treatment of inflammatory diseases.
Keywords: actin, adhesion, cytoskeleton, inflammation, macrophage
INTRODUCTION
Aberrant activation and polarization of macrophages are associated with a wide range of inflammatory and autoimmune diseases (57). Targeting macrophage functions is a promising direction for therapeutic interventions. Macrophage polarization toward proinflammatory (M1) or proresolution (M2) phenotype is directed by both soluble factors such as cytokines and growth factors and mechanical cues such as strain and stiffness of the extracellular matrix (63, 89). Macrophage activation also involves changes in cell morphology and cytoskeletal rearrangements leading to functional differentiations (61). Actin-dependent cell elasticity was recognized to determine phagocytosis and cytokine secretion by macrophages (71). To accentuate the role of cytoskeleton in macrophage function, directly altering macrophage cell shape was shown to initiate macrophage polarization in an actin-myosin contractility-dependent manner (64). Therefore, manipulating actin cytoskeleton to induce functional changes in macrophages may lead to innovative therapeutic approaches against the pathogenesis and progression of inflammatory diseases.
The study herein evaluates calponin 2 as a potential target to manipulate macrophage cytoskeleton. Calponins comprise a family of mechanoregulated actin filament-associated regulatory proteins that inhibit actin-activated myosin ATPase and motor function (1, 27, 102). Three isoforms are present in vertebrates: calponin 1, 2, and 3 are encoded by homologous genes Cnn1, Cnn2, and Cnn3, respectively (45, 55). Calponins regulate cell motility-related processes such as smooth muscle contractility (70, 99), cell proliferation (33), myogenesis (86), trophoblastic fusion (87), and vascular development (92), depending on their cell-type-specific spatiotemporal expression (44, 55, 82). Calponin 2 is the calponin isoform known to be expressed in myeloid leukocytes and regulate their motility-based cellular functions, including velocity of migration, diapedesis, phagocytosis, and spreading in adhesion culture (38, 73). Deletion of calponin 2 in macrophages markedly attenuates the development of inflammatory arthritis (37) and atherosclerosis (56) in mice, which positions calponin 2 as a promising target for correcting the aberrant mechanoregulation of macrophages in diseases.
Resident macrophages in different organs and tissues exhibit different immunophenotypes and function adept to particular tissue microenvironments (14, 22). To determine whether calponin 2 contributes to the tissue-specific adaptation and activation of macrophages in a mechanically dynamic tissue microenvironment (79, 93), the present study examined the role of calponin 2 in lung alveolar resident macrophages (referred to as lung macrophages hereafter). Lung macrophages are uniquely located at a boundary with the outside environment where they are constantly exposed to new antigens and must sustain tolerogenicity while maintaining capacity to initiate inflammation in response to infection (49, 51). To test whether and how calponin-mediated mechanical adaptations of lung macrophages contribute to their conditionally quiescent phenotype, we evaluated the expression and role of calponin 2 in the function of lung macrophages as compared with lavage-derived peritoneal resident macrophages (referred to as peritoneal macrophages hereafter). Because the peritoneal cavity is normally sterile with no direct interaction with the external environment, exposure of peritoneal macrophages to inflammatory stimuli provokes a rapid response (18, 100). Our results found a significantly lower level of calponin 2 in lung macrophages as compared with that in peritoneal macrophages. The reduced expression of calponin 2 in lung macrophages correlated with reduced expression of proinflammatory markers, similarly to the peritoneal macrophages isolated from Cnn2−/− mice. Potentially via decreased substrate adhesion and activation of macrophages and altering the processing and secretion of cytokines, regulation of calponin 2 provides a novel molecular mechanism for targeting mechanodependent activation of macrophages in search of new treatments for inflammatory diseases.
EXPERIMENTAL PROCEDURES
Genetically modified mice.
All animal studies were approved by the Institutional Animal Care and Use Committee of Wayne State University. The generation of Cnn2-floxed (Cnn2f/f) mice and induction of systemic Cnn2 knockout have been previously described (38). The colony of Cnn2−/− mice has been backcrossed with wild-type (WT) C57BL/6 mice for nine generations or more to ensure >99.8% of C57BL/6 genetic background. Genotypes were confirmed using PCR.
Isolation and culture of lung and peritoneal macrophages.
Lung macrophages were obtained by lung lavage as described before (8, 104) with minor modifications. Briefly, the trachea of a euthanized mouse was exposed and an incision was made to insert a blunted 21-G needle that was then tied with a sewing thread. Phosphate-buffered saline (PBS) containing 0.5 mM ethylenediaminetetraacetic acid (EDTA) was slowly infused using a 1-mL syringe and gently aspirated back. The infusion and aspiration were repeated eight times with fresh PBS to obtain a total of 8–9 mL of cell suspension.
Following the isolation of lung macrophages, peritoneal macrophages were isolated from the mouse by peritoneal lavage as described before (104). Briefly, the abdominal skin was retracted following an incision along the abdominal midline to expose peritoneal wall. A 21-G needle was carefully inserted into the peritoneal cavity to avoid puncturing the internal organs, and 10 mL PBS was injected using a 10-mL syringe, followed by an aspiration of 8–9 mL of cell suspension.
Subsequent flow cytometry analysis determined that ~90% of leukocytes in lung exudates and 30–40% of leukocytes in peritoneal exudates were F4/80+ macrophages.
SDS-PAGE and immunoblotting.
SDS-polyacrlamide gel electrophoresis (PAGE) and Western blotting were carried out as described before (32, 56). Macrophages from freshly isolated lung and peritoneal exudates were selected by a brief incubation on tissue culture plates to allow adherence, washed with PBS, and lysed rapidly with SDS-PAGE sample buffer. The protein extracts were heated and subjected to electrophoresis using 12% gels in Laemmli buffer system with an acrylamide:bisacrylamide ratio of 29:1. The first set of gels was fixed and stained with Coomassie Blue R-250 for densitometry quantification and normalization of protein input. Analytical sets of gels were subjected to a semidry transfer onto nitrocellulose membranes for immunoblotting. The membranes were blocked with 1% bovine serum albumin (BSA) in Tris-buffered saline with 0.1% Tween-20 and incubated with anti-calponin 2 antibodies RAH2 (70) and 1D11 (34) or antibodies to measure endoplasmic reticulum (ER) stress, anti-glucose-regulated protein 78 (anti-GRP78; Thermo Fisher Scientific, Waltham, MA), and anti-inositol-requiring enzyme-1α (IRE1α; Cell Signaling Technology, Danvers, MA). Alkaline phosphatase-labeled anti-rabbit or anti-mouse IgG secondary antibody (Santa Cruz Biotechnology, Dallas, TX) and 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium chromogenic substrate reaction were used for signal development. Similarly, spleen tissue of every mouse studied was examined with using RAH2 antibody Western blotting to confirm the genotype.
Flow cytometry.
Lung and peritoneal macrophages were washed in PBS and stained with 1 μL viability dye (Ghost Dye Red 710, Tonbo Biosciences, San Diego, CA) in 1 mL PBS at room temperature for 15 min. After being washed in FACS buffer (PBS containing 0.5% bovine serum albumin and 0.1% sodium azide), the cells were incubated with 10 μL FcR blocking reagent (Miltenyi Biotec, Auburn, CA) in 90 μL FACS buffer at 4°C for 10 min, washed again in FACS buffer, and stained with fluorophore-conjugated extracellular primary antibodies (Table 1) at 4°C in the dark for 30 min.
Table 1.
List of antibodies used for immunophenotyping
| Antigen | Fluorophore | Antibody | Company |
|---|---|---|---|
| Extracellular staining antibodies | |||
| CD45 | APC-Fire 750 | 30-F11 | Biolegend |
| F4/80 | PE-Cy7 | BM8 | Biolegend |
| CD206 | Brilliant Violet 421 | C068C2 | Biolegend |
| CD38 | Brilliant Violet 605 | 90/CD38 | BD Biosciences |
| Intracellular staining antibodies | |||
| iNOS | APC | CXNFT | eBioscience |
| EGR2 | PE | Erongr2 | eBioscience |
| IL-10 | Brilliant Violet 605 | JES5-16E3 | Biolegend |
| TNF | Brilliant Violet 421 | MP6-XT22 | Biolegend |
| PERK | PE | B-5 | Santa Cruz Biotechnology |
| CHOP | Alexa Fluor 488 | B-3 | Santa Cruz Biotechnology |
iNOS, inducible nitric oxide synthase; EGR2, early growth response protein 2; PERK, protein kinase RNA-like endoplasmic reticulum kinase; CHOP, CCAAT/enhancer-binding protein homologous protein.
After the extracellular staining, the cells were fixed and permeabilized using BD Cytofix/Cytoperm Kit (BD Biosciences) or Foxp3 Transcription Factor Staining Buffer Set (eBioscience, Thermo Fisher Scientific) for intracellular or nuclear staining. The cells were then incubated with fluorophore-conjugated intracellular primary antibodies (Table 1) and unlabeled rabbit anti-calponin 2 primary antibody RAH2 or unlabeled rabbit anti-LC3B primary antibody (Cell Signaling Technology) at 4°C in the dark for 30 min. Calponin 2 and LC3B staining required an additional 30-min incubation at 4°C with a FITC-conjugated anti-rabbit IgG (Sigma Aldrich, St. Louis, MO) or Alexa Fluor 647-conjugated anti-rabbit IgG (Thermo Fisher Scientific), respectively. Following the intracellular staining, the cells were washed and resuspended in FACS buffer for acquisition on the BD LSR II Flow Cytometer (BD Biosciences) at the Microscopy, Imaging and Cytometry Resources Core at Wayne State University, School of Medicine. The gating strategy consisted of identifying macrophages [cells (FSC vs. SSC) → singlets (FSC-area vs. FSC-height) → live (negative for a viability dye) → CD45+ → F4/80+] and quantifying the proteins of interest within the macrophage population.
Cell adhesion assay.
Lung and peritoneal macrophages freshly isolated from wild-type (WT) and Cnn2−/− mice (n = 3 each) were seeded in 12-well culture dishes with a density of 5 × 104 cells per well in 500 μL RPMI-1640 medium containing 10% FBS and incubated in 5% CO2 at 37°C for 5 or 35 min. At the indicated time points, the nonadherent cells were removed by gentle washing with prewarmed medium for three times and the adherent cells were fixed in the wells with 1% glutaraldehyde in the media for 30 min. Five micrographs were taken for each well with a Zeiss Axio Observer A1 phase contrast microscope using a ×5 objective lens (Carl Zeiss Microscopy, Jena, Germany). The cells per micrograph were counted using ImageJ software version 1.51s (NIH, Bethesda, MD).
Cytokine profiling.
As previously described (56), macrophages freshly isolated from WT and Cnn2−/− mice (n = 3 each) were incubated in lysis buffer (50 mM Tris·HCl, 150 mM NaCl, 0.5% NP-40, and 1 mM EDTA, pH 7.4) containing protease inhibitor cocktail (cat. no. I3911; Sigma-Aldrich) on ice for 30 min with intermittent vortexing. After a 10-min centrifugation at 4°C, total protein extracted in the clarified supernatant was sent to determine the concentration of multiple cytokines at a commercial service facility (Eve Technologies, Calgary, AB, Canada) using bead-based multiplex immunoassays.
Silica treatment.
Lung and peritoneal macrophages freshly isolated from WT and Cnn2−/− mice (n = 3 each) were washed in PBS; resuspended in RPMI-1640 medium containing 10% FBS, 2 mM l-glutamine, 100 U/mL penicillin, and 50 U/mL streptomycin; and plated in 12-well plates. After allowing the macrophages to adhere for 1 h, the nonattached cells were washed away and the media were supplemented with plain or green fluorescent 200-nm silica particles (Micromod, Rostock, Germany) to a final concentration of 100 μg/mL. Media without silica were used as a negative control. The macrophages were incubated in 5% CO2 at 37°C for 72 h and then collected after an incubation with 0.5 mM EDTA on ice for 15 min. After washing in PBS, the cells were subjected to staining for flow cytometry.
Immunofluorescence microscopy.
Lung and peritoneal exudates from WT and Cnn2−/− mice (n = 3 each) were incubated in high-glucose Dlbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS, 2 mM l-glutamine, 100 U/mL penicillin, and 50 U/mL streptomycin on coverslips for 1 h to select for macrophages and allow adherence for staining using anti-P62 (BioLegend, Pasadena, CA) or anti-LC3B (Cell Signaling Technology) primary antibodies and anti-mouse IgG conjugated to Alexa Fluor 488 (BioLegend) or anti-rabbit IgG conjugated to FITC (Sigma-Aldrich), respectively. Before visualization under a fluorescence microscope (Keyence, Itasca, IL), the coverslips were mounted with ProLong Gold antifade reagent containing DAPI for a costain of nuclei (Molecular Probes by Life Technologies, ThermoFisher Scientific).
Data analysis.
The densitometry analysis of SDS-gel and Western blot was performed using ImageJ software. The flow cytometry data were analyzed using FlowJo software version 10 (FlowJo, Ashland, OR). Statistical significance of comparisons was determined using Student’s t test with GraphPad Prism software version 7.0c (GraphPad Software, La Jolla, CA). The threshold for significance was set at P ≤ 0.05. The bar graphs represent means ± SE.
RESULTS
Lung macrophages express significantly lower levels of calponin 2 than that in peritoneal macrophages.
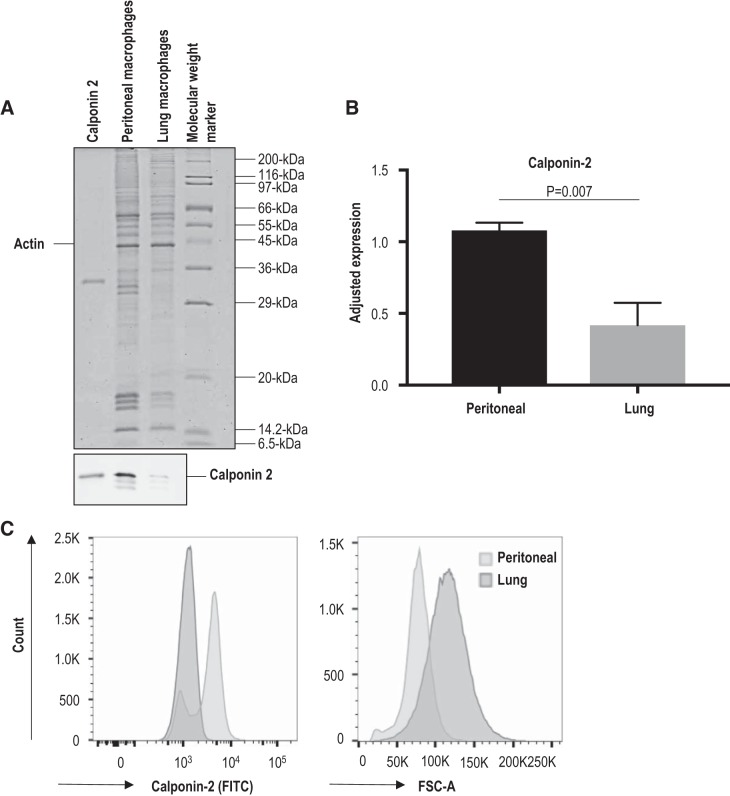
Expression of calponin 2 in lung and peritoneal macrophages was determined by immunoblotting (Fig. 1, A and B). Since expression of calponin 2 may vary with changes in actin cytoskeleton (32), densitometry analysis was normalized to the expression of histones that reflect the number of cells (Fig. 1B) in addition to actin or total protein (data not shown). All the quantification analyses detected significantly lower levels of calponin 2 in lung macrophages as compared with that in peritoneal macrophages. Flow cytometric analysis confirmed the significantly lower expression of calponin 2 in CD45+F4/80+ lung macrophages (Fig. 1C) with their fluorescent spectra entirely overlapping with that of a negative control (data not shown). Furthermore, freshly isolated lung macrophages are noticeably larger in size than peritoneal macrophages, as depicted by their rightward shift in flow cytometric forward scatter plot (Fig. 1C). Because larger cells possess more actin cytoskeletal networks, the downregulation of calponin 2 in lung macrophages implies that the calponin:actin ratio is further reduced in lung macrophages, indicating a mechanism that is known to affect the mechanics of actin filaments in vitro (42). These data indicate that the level and function of calponin 2 in lung macrophages are markedly lower than those in peritoneal macrophages.
Fig. 1.
Significantly lower level of calponin 2 in lung macrophages than in peritoneal macrophages. A: the levels of calponin 2 in lung and peritoneal macrophages isolated from wild-type mice (n = 4 mice) were determined by immunoblotting. The protein input was normalized by the level of actin. Purified mouse calponin 2 protein was used as a positive control. B: the results were quantified by densitometry analysis using ImageJ software. The level of calponin 2 expression was evaluated relative to the levels of histones that reflect the number of cells. Bars depict means ± SE. C: the expression of calponin 2 in CD45+F4/80+ macrophages was confirmed by flow cytometry using rabbit anti-calponin 2 antibody RAH2 and FITC-conjugated anti-rabbit IgG. The rightward shift in forward scatter area (FSC-A) demonstrates the larger size of freshly isolated lung macrophages when compared with that of peritoneal macrophages.
Lower level of calponin 2 leads to reduced adhesion and correlates with decreased expression of F4/80.
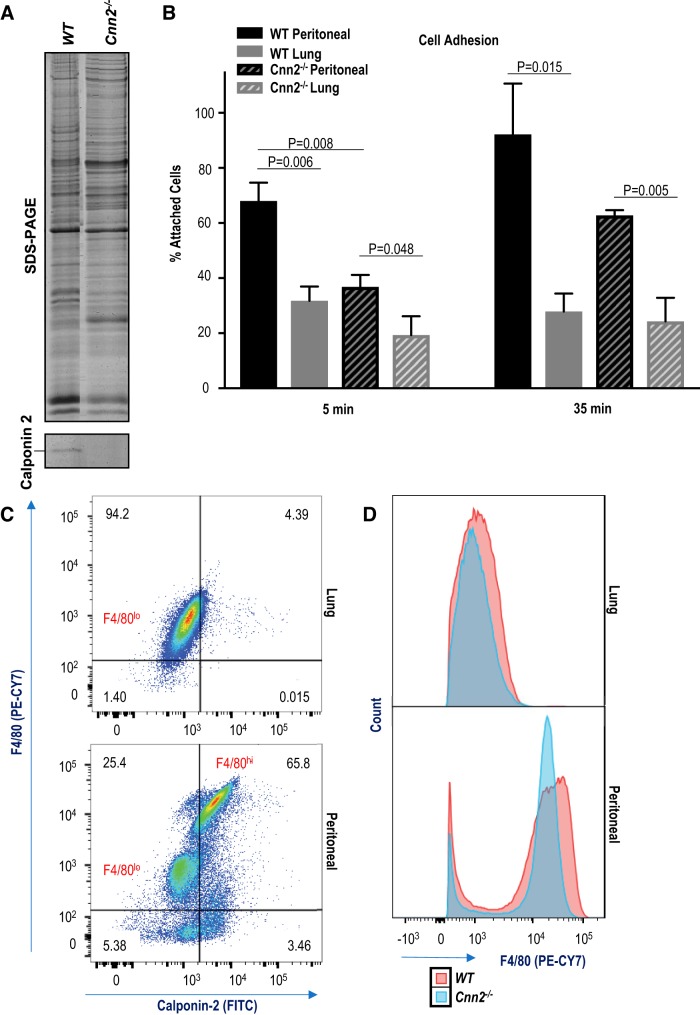
Calponin binds the key components of cytoskeleton, F-actin and myosin (16a), and regulates cell adhesion by inhibiting myosin motor-driven cytoskeletal dynamics and motility, akin to troponin in striated muscles (45, 67). Our previous studies have shown that reduction in calponin 2 results in decreased adhesion in prostate cancer cells (66), platelets (29), fibroblasts (35), and peritoneal macrophages (38, 56). Consistently, lung macrophages expressing lower levels of calponin 2 adhere less efficiently to culture substrate than WT peritoneal macrophages (Fig. 2B). We employed calponin 2 knockout mice (Fig. 2A) to isolate calponin 2-deficient (Cnn2−/−) macrophages and determine whether calponin 2 deficiency in peritoneal macrophages causes reduced adhesion as observed in lung macrophages. The results showed that Cnn2−/− peritoneal macrophages indeed exhibit decreased substrate adhesion in culture as compared with that of WT peritoneal macrophages (Fig. 2B). On the contrary, no significant differences in substrate adhesion were observed between WT and Cnn2−/− lung macrophages that have minimum difference in calponin 2 contents (Fig. 2B), suggesting that the functional effect of the significant downregulation of calponin 2 in WT lung macrophages is similar to that of knocking out the gene expression. Therefore, a threshold of calponin 2 may be required for effective cell adhesion and lung macrophages restrict the expression of calponin 2 to modulate cell adhesion.
Fig. 2.
Lower levels of calponin 2 lead to reduced substrate adhesion and correlate with decreased expression of F4/80. A: complete deletion of calponin 2 was reconfirmed in spleen harvested from Cnn2−/− mouse with a wild-type (WT) control. B: substrate adhesion of peritoneal and lung macrophages freshly isolated from WT and Cnn2−/− mice (n = 3 mice per group) was determined by direct counting 5 and 35 min after seeding. The data are graphed as the percentage of attached cells out of the total cells expected to attach based on the seeding density and proportion of macrophages of each sample. Bars depict means ± SE. C: the coexpression of F4/80 and calponin 2 in live CD45+F4/80+ macrophages was determined by flow cytometry using anti-F4/80 antibody directly conjugated to PE-CY7 and unlabeled anti-calponin 2 antibody RAH2 in conjunction with FITC-conjugated secondary antibody. The percentage of F4/80+Cnn2+ macrophages is indicated. D: the expression level of F4/80 was compared in WT and Cnn2−/− CD45+F4/80+ macrophages using flow cytometry. Histograms display the overlays of PE-CY7 fluorescent spectra for lung and peritoneal macrophages.
F4/80 is a cell surface glycoprotein extensively used as a marker of murine macrophages. The biological role of F4/80 in macrophages remains mysterious but may involve cell-cell and cell-matrix contacts (21). The expression of F4/80 is modulated by the state of macrophage activation (28, 53, 103) and increases upon culturing on and adherence to plastic culture dishes in vitro (30), cellular processes that rely on the functions of actin cytoskeleton (84) and are in part mediated by calponin 2 (32). Interestingly, our data demonstrate that F4/80hi peritoneal macrophages express calponin 2, while F4/80lo peritoneal macrophages do not (Fig. 2C). F4/80lo peritoneal macrophages are derived from monocytes constitutively entering the peritoneal cavity and serve as precursors to F4/80hi macrophages (3). Therefore, the phenotypic adaptation of newly arrived peritoneal macrophages to peritoneal microenvironment may involve upregulation of calponin 2. Consistent with the correlation between the expressions of calponin 2 and F4/80, Cnn2−/− peritoneal macrophages exhibit decreased expression of F4/80 (Fig. 2D).
Lung macrophages are both F4/80lo (14) and Cnn2lo (Fig. 2B), a phenotype that may correspond to their reduced substrate adherence (Fig. 2B). Other studies have shown that adhesion of lung macrophages directly correlates with their activation assessed by production of superoxide (83) and that lung macrophages display weaker adhesion in response to proinflammatory stimuli when compared with other myeloid cells, including blood monocytes (58). Hence, it is tempting to speculate that lung macrophages may employ the reduced expression of calponin 2 and subsequently F4/80 (32) to restrict their inflammatory response via decreased adhesion.
Lower levels of calponin 2 reduce the abundance of proinflammatory (CD38+iNOS+), but not proresolution (CD206+, Egr2+), macrophages.
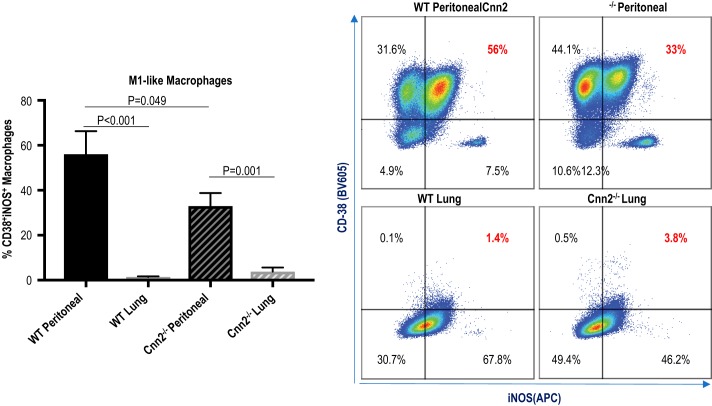
To determine whether the lower expression of calponin 2 in lung macrophages indeed restricts polarization toward proinflammatory phenotype, immunophenotyping of freshly isolated macrophages was performed using flow cytometry. The expression of the previously established proinflammatory M1 mediators inducible nitric oxide synthase (iNOS) (77) and cyclic ADP ribose hydrolase (CD38) (25, 41) was examined in live lung and peritoneal macrophages (gated as CD45+F4/80+ cells). iNOS is a classic M1 marker, while CD38 is a novel M1-like marker expressed in a lower proportion of M1 macrophages (41). The results demonstrate that the percentage of CD38+iNOS+ macrophages was significantly lower in lung macrophages than that in peritoneal macrophages (Fig. 3). Peritoneal macrophages isolated from Cnn2−/− mice also contained fewer CD38+iNOS+ macrophages than from WT mice (Fig. 3). When comparing WT lung macrophages and Cnn2−/− lung macrophages, no differences in expression of CD38 and iNOS were observed (Fig. 3), suggesting that the low levels of calponin 2 in WT lung macrophages are already below the threshold needed for macrophages to polarize toward M1-like phenotype.
Fig. 3.
Lower levels of calponin 2 correlate to reduced proportion of M1-like CD38+iNOS+ macrophages. Flow cytometric analysis was performed on peritoneal and lung macrophages freshly isolated from wild-type (WT) and Cnn2−/− mice (n = 4 mice per group) to determine the expression of M1-like markers CD-38 and inducible nitric oxide synthase (iNOS) in live CD45+F4/80+ cells. The percentages of CD38+iNOS+ macrophages are represented by bar graphs (means ± SE), and the expression pattern of CD38 and iNOS in macrophages is depicted by dot plots.
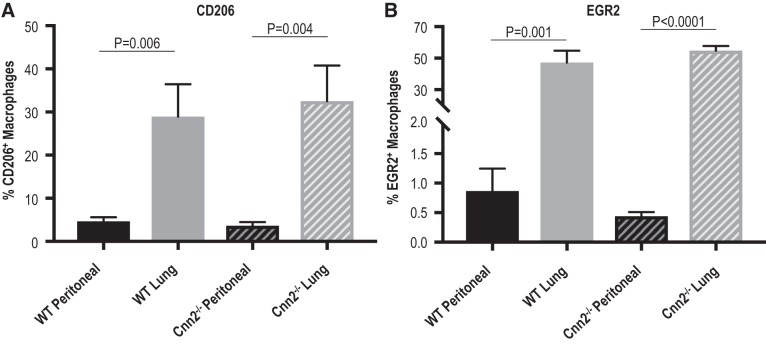
To assess whether calponin 2 affects macrophage polarization toward alternative proresolution phenotype, expression of M2 markers, mannose receptor (CD206) (81) and early growth response protein 2 (Egr2) (41), were evaluated in live lung and peritoneal macrophages isolated from WT and Cnn2−/− mice. Although the percentages of CD206+ and Egr2+ macrophages were higher among lung macrophages than peritoneal macrophages, Cnn2−/− did not have an effect (Fig. 4), indicating that the upregulation of M2 markers in lung macrophages is independent of calponin 2. Taken together, the immunophenotyping data demonstrate that the low expression of calponin 2 abates the proinflammatory M1-like phenotype but does not affect the M2-like phenotype in lung macrophages.
Fig. 4.
Higher expression of M2-like markers CD206 and early growth response protein 2 (EGR2) in lung versus peritoneal macrophages independent of calponin 2. Flow cytometric analysis was performed on peritoneal and lung macrophages freshly isolated from wild-type (WT) and Cnn2−/− mice (n = 3–5 mice per group) to determine the expression of M2-like markers CD-206 and EGR2 in live CD45+F4/80+ cells. A: the percentages of CD206+ macrophages are represented by bar graphs (means ± SE). B: the percentages of EGR2+ macrophages are represented by bar graphs (means ± SE).
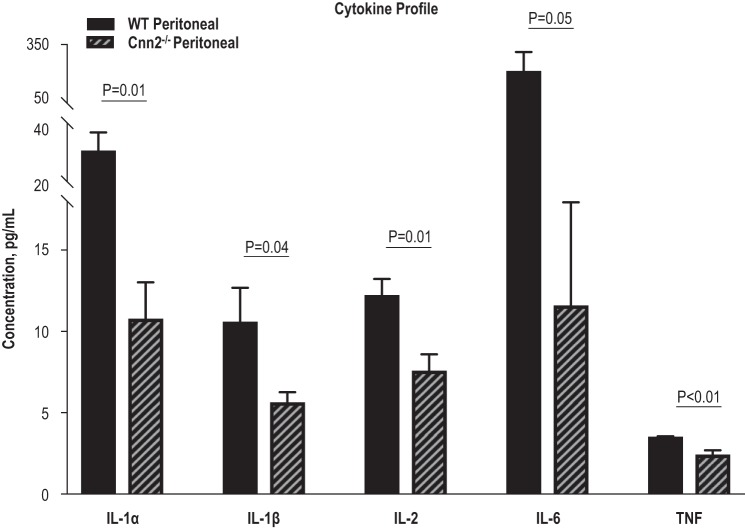
Deletion of calponin 2 results in decreased expression of IL-1α, IL-1β, IL-6, and TNF in peritoneal macrophages.
The lower abundance of proinflammatory peritoneal macrophages in Cnn2−/− mice than in WT mice suggested that the deletion of calponin 2 modulates macrophage activation, promoting quiescence. To further compare the activation of peritoneal macrophages in Cnn2−/− and WT mice, the intracellular protein concentrations of several cytokines were quantified using bead-based multiplex immunoassays. Consistent with their abated M1-like phenotype, Cnn2−/− peritoneal macrophages produced less of the proinflammatory cytokines IL-1α, IL-1β, IL-6, and TNF than WT macrophages (Fig. 5). Peritoneal macrophages are known to synthesize basal levels of proinflammatory cytokines (76), which can be quickly upregulated and secreted in response to pathogen-associated molecular patterns (PAMPs) or endogenous damage-associated molecular patterns (DAMPs), which the cells may encounter (50, 105). Our results demonstrate at the protein level that the deletion of calponin 2 decreases the basal levels of proinflammatory cytokines in peritoneal macrophages, potentially constraining the inflammatory responses.
Fig. 5.
Deletion of calponin 2 results in decreases of IL-1α, IL-1β, IL-6, and TNF in peritoneal macrophages. Total protein was extracted from peritoneal macrophages freshly isolated from wild-type (WT) and Cnn2−/− mice (n = 3 mice per group) and the levels of proinflammatory cytokines IL-1α, IL-1β, IL-6, and TNF were determined using bead-based multiplex immunoassays. Bars depict means ± SE.
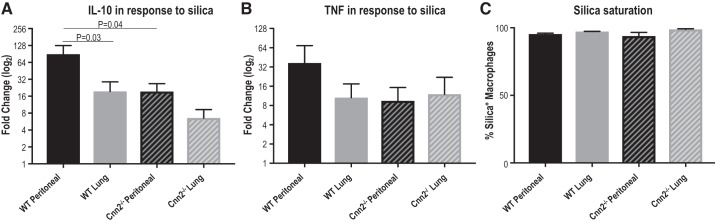
Reduction of calponin 2 attenuates inflammatory response of macrophages to silica.
To determine whether calponin 2 contributes to the inflammatory response of macrophages upon treatment with proinflammatory stimuli, mouse lung and peritoneal macrophages were cultured for 72 h with or without 100 μg/mL 200-nm silica particles. Crystalline silica is considered a DAMP (6, 9) that gets internalized by macrophages (23) and activates NF-κB (15, 47) and inflammasome (16, 19, 31) signaling. Silica treatment resulted in a positive fold change of IL-10 and TNF proteins in both lung and peritoneal macrophages isolated from WT or Cnn2−/− mice alike (Fig. 6, A and B). However, the silica-induced increase in IL-10 protein expression was significantly higher in WT peritoneal macrophages than WT lung macrophages or Cnn2−/− peritoneal macrophages (Fig. 6A). A similar trend in expression was also evident for TNF, although it did not reach the threshold of statistical significance (Fig. 6B). No differences in silica-induced expression of IL-10 or TNF were apparent between WT and Cnn2−/− lung macrophages (Fig. 6, A and B), as predicted based on their aforementioned functional and phenotypic similarities. Of note, the concurrent incubation with fluorescently labeled silica allowed us to confirm that the internalization of silica by all four macrophage groups reached saturation during the 72-h incubation (Fig. 6C) and, therefore, the effect of calponin 2 on the capacity of phagocytosis (38) is deemed to be a negligible factor in the observed response.
Fig. 6.
Lower levels of calponin 2 attenuate inflammatory response of macrophages to silica particles. Peritoneal and lung macrophages freshly isolated from wild-type (WT) and Cnn2−/− mice (n = 3 mice per group) were cultured for 72 h with or without 200-nm silica particles (100 μg/mL). A and B: the treated cells were subjected to flow cytometric analysis to examine the expression of IL-10 (A) and TNF (B) in live CD45+F4/80+ macrophages. The fold change in cytokine expression (the ratio of the difference between silica-stimulated and unstimulated cytokine expression over the unstimulated expression) is depicted by bar graphs (means ± SE). C: saturated silica phagocytosis under the experimental condition was confirmed by parallel incubation of the macrophages with green fluorescent silica particles. The percentage of the macrophages that contain silica is depicted by bar graphs (means ± SE).
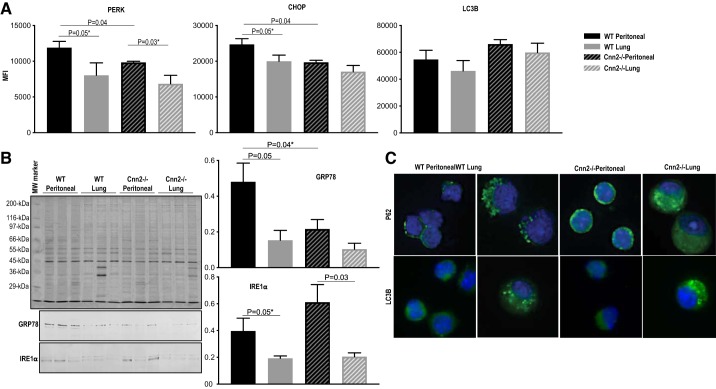
Calponin 2 participates in endoplasmic reticulum stress response.
Actin cytoskeleton dynamics are linked to ER stress responses (7, 40, 95, 97). A well-documented cross talk also exists between ER stress and inflammation (12, 24, 78). As an actin cytoskeleton-associated regulatory protein, calponin 2 may contribute to immune regulation by participating in the ER stress signaling. To test this possibility, ER stress markers PERK (protein kinase RNA-like endoplasmic reticulum kinase) and CHOP (CCAAT/enhancer-binding protein homologous protein) were assessed in macrophages isolated from WT and Cnn2−/− mice by flow cytometry using a previously described method (74, 101). Our data indicate that Cnn2−/− peritoneal macrophages exhibit significantly reduced expression of PERK and CHOP (Fig. 7A) when compared with WT peritoneal macrophages. Consistently, WT lung macrophages that are low in calponin 2 also downregulate PERK and CHOP (Fig. 7A). The expression of the additional ER stress markers GRP78 and IRE1α was further assessed by immunoblotting. GRP78 was lower in WT lung macrophages, Cnn2−/− peritoneal macrophages, and Cnn2−/− lung macrophages when compared with WT peritoneal macrophages (Fig. 7B). Conversely, IRE1α was lower in lung macrophages than peritoneal macrophages isolated from both WT and Cnn2−/− mice (Fig. 7B). While their lower expression in lung vs peritoneal macrophages indicates an intrinsic difference of the two populations, the differential expressions were independent of calponin 2.
Fig. 7.
Calponin 2 contributes to endoplasmic reticulum (ER) stress response of macrophages. A: flow cytometric analysis was performed on peritoneal and lung macrophages freshly isolated from wild-type (WT) and Cnn2−/− mice (n = 3 mice per group) to determine the expression of ER stress response markers PERK (protein kinase RNA-like endoplasmic reticulum kinase) and CHOP (CCAAT/enhancer-binding protein homologous protein) and the autophagy marker LC3B in live CD45+F4/80+ macrophages. The mean fluorescence intensity (MFI) is represented by bar graphs (means ± SE). *One-tailed Student’s t test. B: the levels of glucose-regulated protein 78 (GRP78) and inositol-requiring enzyme-1α (IRE1α) in lung and peritoneal macrophages isolated from WT and Cnn2−/− mice (n = 3 mice per group) were determined by immunoblotting. The protein input was normalized by the level of actin. C: immunofluorescence microscopic localization of P62 and LC3B (green) was performed on WT and Cnn2−/− lung and peritoneal macrophages. Nuclei were stained with DAPI (blue). Representative images were obtained using a ×40 objective.
ER stress can provoke recycling of macromolecules and organelles, a process known as autophagy (36, 85, 88). To determine whether autophagy is perturbed in the absence of calponin 2, expression of a classic autophagy marker LC3B was examined in WT and Cnn2−/− lung and peritoneal macrophages by flow cytometry. Macrophages deficient in calponin 2 (Cnn2−/− macrophages and lung macrophages) did not significantly differ from the high calponin 2 WT peritoneal macrophages in their expression of LC3B (Fig. 7A). Immunofluorescence microscopy also revealed no consistent difference in intracellular distribution patterns of LC3B among the four groups (Fig. 7C). However, p62, a target of autophagy that forms speckles in metabolically stressed cells (68), exhibited fewer punctate in Cnn2−/− macrophages than WT macrophages (Fig. 7C). The notion that calponin 2 may participate in ER stress/autophagy in macrophages merits further investigation.
DISCUSSION
Our present study revealed that decreases in calponin 2 attenuate proinflammatory activation of macrophages. Therefore, calponin 2 may be a novel target for regulating the mechanodependent activation of macrophages in the treatment of inflammatory diseases. The following observations may help to understand the underlying mechanisms.
Downregulation of calponin 2 as a mechanodependent adaptation of lung macrophages to maintain quiescence.
Tissue resident macrophages originate from either yolk sac, fetal liver, or a monocyte intermediate during distinct prenatal hematogenic waves with some input from adult hematopoiesis (2, 22). However, the phenotypic diversity of tissue resident macrophages cannot be explained solely by their developmental origin and largely depends on the programming cues from tissue microenvironment (2, 52, 80). Transfer of traceable yolk sac macrophages, fetal liver monocytes, or adult bone-marrow monocytes into an empty lung macrophage niche has been shown to generate transcriptionally near-identical and functional lung macrophages (96). Furthermore, adoptive transfer of tissue-resident peritoneal macrophages into lung induced expression of lung macrophage-specific genes and downregulated peritoneal macrophage-specific genes (26, 52). Therefore, the organ-tissue environment of the lung directs phenotypic and functional adaptations of macrophages.
The lung tissue is affected by mechanical dynamics associated with respiratory inflation and deflation (20). As a result, lung macrophages are subjected to a variety of mechanical forces (20) that have been shown to regulate macrophage activation and polarization (63, 75). As the first line of defense, lung macrophages must be on guard against the invading pathogens. Yet, most of the inhaled antigen gets expelled in the mucus or digested by lung macrophages in a manner that minimizes inflammatory response (54). It is not well understood how the mechanical cues in the lung tissue environment contribute to the quiescent phenotype of lung macrophages. Calponin 2 has been extensively demonstrated to function as a mechanoregulatory protein in the cytoskeleton (55). Findings in our present study indicate that the downregulation of calponin 2, a mechanoregulated actin-associated protein, may be a mechanodependent adaptation of lung macrophages for maintaining quiescence.
Expression of calponin 2 decreases during chronic cyclic stretching of lung alveolar cells in vitro due to the decrease in the total cytoskeletal tension that the cells sense over time (34), as the low-tension conditions have been shown to downregulate the expression of calponin 2 (32, 34, 43). Therefore, cyclic stretching of the lung during respiration may be a cause of the downregulation of calponin 2 observed in freshly isolated lung macrophages (Fig. 1).
The downregulation of calponin 2 is associated with the decreased adhesion (Fig. 2A) and reduced proinflammatory polarization of lung macrophages as compared with peritoneal macrophages (Fig. 3). Thus the downregulation of calponin 2 expression may be a novel mechanodependent mechanism to restrict activation and inflammatory polarization of macrophages. Of note, lung macrophages also display higher expression of proresolution M2 markers CD206 and EGR2 than peritoneal macrophages, a phenotype that is independent of calponin 2 (Fig. 4). This indicates that calponin 2 may contribute to inflammation by promoting M1-like polarization of macrophages and not by limiting M2-like polarization.
Deletion of calponin 2 attenuates proinflammatory polarization of peritoneal macrophages.
Our comparison of peritoneal macrophages from WT versus Cnn2−/− mice confirmed that calponin 2 indeed promotes proinflammatory polarization of macrophages, as its deletion results in decreased abundance of CD38+iNOS+ macrophages (Fig. 3) and has no effect on the expression of M2 markers CD206 and Egr2 in peritoneal macrophages (Fig. 4). Consistent with the downregulation of proinflammatory markers, peritoneal macrophages from Cnn2−/− mice exhibit lower expressions of IL-1α, IL-1β, IL-6, and TNF under baseline conditions (Fig. 5) with attenuated response to the inflammatory stimulation of silica (Fig. 6). These findings are consistent with our previous studies reporting that the calponin 2-null peritoneal macrophages and lipid-loaded foam cells have reduced expression of inflammatory cytokines and chemokines (56) and that myeloid cell-specific deletion of calponin 2 attenuates the severity of inflammatory arthritis (37) and atherosclerosis (56) in mice. It is also worth noting that Cnn2-low or -null macrophages remained responsive to silica stimulation (Fig. 6), reflecting a physiological modulation other than a loss of function.
Proinflammatory polarization of macrophages involves activation of ER stress signaling and autophagy (4, 10, 65). We hypothesized that the decrease of proinflammatory cytokines in macrophages from Cnn2−/− mice may involve dampened ER stress signaling. Our results demonstrate that the expression of ER stress markers is indeed lower in WT lung macrophages as well as in Cnn2−/− peritoneal macrophages when compared with WT peritoneal macrophages (Fig. 7A). Immunoblotting confirmed that the expression of GRP78 followed similar pattern as we saw with the flow cytometry experiments for PERK and CHOP, wherein low levels of calponin 2 in WT lung macrophages and Cnn2−/− peritoneal macrophages correlated with decreased ER stress as compared with WT peritoneal macrophages. The expression of IRE1α was lower in lung vs peritoneal macrophages independently of calponin 2 (Fig. 7B), indicating an intrinsic of the lung macrophages.
These data suggest that perturbed ER stress singling may be one of the molecular mechanisms by which downregulation of calponin 2 promotes macrophage quiescence. In the meantime, lung macrophages isolated from Cnn2−/− mice exhibit a similar state of activation to those isolated from WT mice (Figs. 3 and 4), presumably due to the below-threshold expression of calponin 2 in lung macrophages of WT mice.
Calponin 2 is a novel regulator of macrophage activation and polarization.
Actin cytoskeleton is a key mediator of macrophage activation and polarization (17, 64, 72). While macrophage adhesion (11), migration (46), and phagocytosis (62) clearly rely on the function of cytoskeleton, other mechanisms by which cytoskeletal dynamics affect macrophage activity have also been investigated (69, 91). Recent evidence reveals that rapid cytoskeletal rearrangements allow for reorganization and clustering of pattern recognition receptors (PRRs) on actin filaments-supported membrane protrusions that act as platforms for juxtaposing receptors and intracellular signaling machinery at the sites of contact with pathogens (39, 90, 91). Additionally, actin cytoskeleton physically interacts with and affects the activity of several inflammasomes (5, 48, 60, 69, 98), intracellular oligomers that process the proinflammatory cytokines IL-1β and IL-18 into their mature forms.
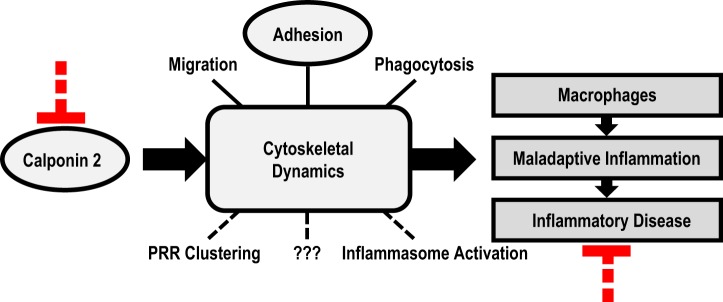
Considering that calponin 2 is an actin cytoskeleton-associated regulatory protein whose downregulation alters the function and polarization of macrophages and attenuates inflammatory arthritis and atherosclerosis, the results of the present study allow us to propose that altering cytoskeletal dynamics by targeting calponin 2 can be a novel approach for treating inflammatory diseases (Fig. 8). Supporting our hypothesis, previous studies have established the role of calponin 2 in cell migration (38, 66, 92, 94), adhesion (29, 35, 38, 56, 66), and phagocytosis (13, 38). Future studies may include the determination of whether calponin 2 regulates PRR clustering, inflammasome activation, or other elements of cytoskeletal dynamics in macrophages and how it can be targeted pharmaceutically to modulate inflammation. Ultimately, calponin 2 may provide a novel molecular target for specifically restricting M1 polarization of macrophages for the treatment of inflammatory diseases.
Fig. 8.
Calponin 2 as a novel target for modulating macrophage function in inflammatory responses. Calponin 2 is a cytoskeleton regulatory protein with a demonstrated function in macrophage migration, adhesion, and phagocytosis and potential roles in pattern recognition receptor (PRR) clustering, inflammasome activation, and other intracellular processes that may regulate the activation and polarization of macrophages implicated in the maladaptive activation in inflammatory diseases. Targeting calponin 2 presents a potentially novel strategy to modulate cytoskeleton-mediated functions and activation of macrophages for the treatment of inflammatory disease.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grants HL-086720, HL-127691, and HL-138007 (to J.-P. Jin).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.-P.J. conceived and designed research; O.P. and J.-J.S. performed experiments; O.P. and J.-J.S. analyzed data; O.P. and J.-P.J. interpreted results of experiments; O.P. and J.-P.J. prepared figures; O.P. and J.-P.J. drafted manuscript; O.P. and J.-P.J. edited and revised manuscript; O.P., J.-J.S., and J.-P.J. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Rong Liu and Dr. M. Moazzem Hossain for methodological contributions.
REFERENCES
- 1.Abe M, Takahashi K, Hiwada K. Effect of calponin on actin-activated myosin ATPase activity. J Biochem 108: 835–838, 1990. doi: 10.1093/oxfordjournals.jbchem.a123289. [DOI] [PubMed] [Google Scholar]
- 2.Amit I, Winter DR, Jung S. The role of the local environment and epigenetics in shaping macrophage identity and their effect on tissue homeostasis. Nat Immunol 17: 18–25, 2016. [Erratum in Nat Immunol 18: 246, 2017]. doi: 10.1038/ni.3325. [DOI] [PubMed] [Google Scholar]
- 3.Bain CC, Hawley CA, Garner H, Scott CL, Schridde A, Steers NJ, Mack M, Joshi A, Guilliams M, Mowat AM, Geissmann F, Jenkins SJ. Long-lived self-renewing bone marrow-derived macrophages displace embryo-derived cells to inhabit adult serous cavities. Nat Commun 7: ncomms11852, 2016. doi: 10.1038/ncomms11852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bronner DN, Abuaita BH, Chen X, Fitzgerald KA, Nuñez G, He Y, Yin XM, O’Riordan MX. Endoplasmic reticulum stress activates the inflammasome via NLRP3- and caspase-2-driven mitochondrial damage. Immunity 43: 451–462, 2015. doi: 10.1016/j.immuni.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burger D, Fickentscher C, de Moerloose P, Brandt KJ. F-actin dampens NLRP3 inflammasome activity via Flightless-I and LRRFIP2. Sci Rep 6: 29834, 2016. doi: 10.1038/srep29834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Busso N, So A. Microcrystals as DAMPs and their role in joint inflammation. Rheumatology (Oxford) 51: 1154–1160, 2012. doi: 10.1093/rheumatology/ker524. [DOI] [PubMed] [Google Scholar]
- 7.Chambers JE, Dalton LE, Clarke HJ, Malzer E, Dominicus CS, Patel V, Moorhead G, Ron D, Marciniak SJ. Actin dynamics tune the integrated stress response by regulating eukaryotic initiation factor 2α dephosphorylation. eLife 4: e04872, 2015. doi: 10.7554/eLife.04872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chavez-Santoscoy AV, Huntimer LM, Ramer-Tait AE, Wannemuehler M, Narasimhan B. Harvesting murine alveolar macrophages and evaluating cellular activation induced by polyanhydride nanoparticles. J Vis Exp (64): e3883, 2012. doi: 10.3791/3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol 10: 826–837, 2010. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen P, Cescon M, Bonaldo P. Autophagy-mediated regulation of macrophages and its applications for cancer. Autophagy 10: 192–200, 2014. doi: 10.4161/auto.26927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chong AS, Parish CR, Coombe DR. Evidence that the cytoskeleton plays a key role in cell adhesion. Immunol Cell Biol 65: 85–95, 1987. doi: 10.1038/icb.1987.10. [DOI] [PubMed] [Google Scholar]
- 12.Dandekar A, Mendez R, Zhang K. Cross talk between ER stress, oxidative stress, and inflammation in health and disease. Methods Mol Biol 1292: 205–214, 2015. doi: 10.1007/978-1-4939-2522-3_15. [DOI] [PubMed] [Google Scholar]
- 13.Das R, Ganapathy S, Settle M, Plow EF. Plasminogen promotes macrophage phagocytosis in mice. Blood 124: 679–688, 2014. doi: 10.1182/blood-2014-01-549659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol 14: 986–995, 2013. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Giuseppe M, Gambelli F, Hoyle GW, Lungarella G, Studer SM, Richards T, Yousem S, McCurry K, Dauber J, Kaminski N, Leikauf G, Ortiz LA. Systemic inhibition of NF-kappaB activation protects from silicosis. PLoS One 4: e5689, 2009. doi: 10.1371/journal.pone.0005689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dostert C, Pétrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 320: 674–677, 2008. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16a.EL-Mezgueldi M, Marston SB. The effects of smooth muscle calponin on the strong and weak myosin binding sites of F-actin. J Biol Chem 271: 28161–28167, 1996. doi: 10.1074/jbc.271.45.28161. [DOI] [PubMed] [Google Scholar]
- 17.Eswarappa SM, Pareek V, Chakravortty D. Role of actin cytoskeleton in LPS-induced NF-kappaB activation and nitric oxide production in murine macrophages. Innate Immun 14: 309–318, 2008. doi: 10.1177/1753425908096856. [DOI] [PubMed] [Google Scholar]
- 18.Fieren MW. The local inflammatory responses to infection of the peritoneal cavity in humans: their regulation by cytokines, macrophages, and other leukocytes. Mediators Inflamm 2012: 976241, 2012. doi: 10.1155/2012/976241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franchi L, Eigenbrod T, Núñez G. Cutting edge: TNF-alpha mediates sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation. J Immunol 183: 792–796, 2009. doi: 10.4049/jimmunol.0900173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia CS, Prota LF, Morales MM, Romero PV, Zin WA, Rocco PR. Understanding the mechanisms of lung mechanical stress. Braz J Med Biol Res 39: 697–706, 2006. doi: 10.1590/S0100-879X2006000600001. [DOI] [PubMed] [Google Scholar]
- 21.Gordon S, Hamann J, Lin HH, Stacey M. F4/80 and the related adhesion-GPCRs. Eur J Immunol 41: 2472–2476, 2011. doi: 10.1002/eji.201141715. [DOI] [PubMed] [Google Scholar]
- 22.Gordon S, Plüddemann A. Tissue macrophages: heterogeneity and functions. BMC Biol 15: 53, 2017. doi: 10.1186/s12915-017-0392-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gozal E, Ortiz LA, Zou X, Burow ME, Lasky JA, Friedman M. Silica-induced apoptosis in murine macrophage: involvement of tumor necrosis factor-alpha and nuclear factor-kappaB activation. Am J Respir Cell Mol Biol 27: 91–98, 2002. doi: 10.1165/ajrcmb.27.1.4790. [DOI] [PubMed] [Google Scholar]
- 24.Grootjans J, Kaser A, Kaufman RJ, Blumberg RS. The unfolded protein response in immunity and inflammation. Nat Rev Immunol 16: 469–484, 2016. doi: 10.1038/nri.2016.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guerau-De-Arellano M, Jablonski K, Amici SA, Gaudet A, Popovich PG, Webb L. miRNA regulation of macrophage inflammatory phenotype (Abstract) J Immunol 196, Suppl 1: 202.16, 2016. [Google Scholar]
- 26.Guth AM, Janssen WJ, Bosio CM, Crouch EC, Henson PM, Dow SW. Lung environment determines unique phenotype of alveolar macrophages. Am J Physiol Lung Cell Mol Physiol 296: L936–L946, 2009. doi: 10.1152/ajplung.90625.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haeberle JR. Calponin decreases the rate of cross-bridge cycling and increases maximum force production by smooth muscle myosin in an in vitro motility assay. J Biol Chem 269: 12424–12431, 1994. [PubMed] [Google Scholar]
- 28.Haidl ID, Jefferies WA. The macrophage cell surface glycoprotein F4/80 is a highly glycosylated proteoglycan. Eur J Immunol 26: 1139–1146, 1996. doi: 10.1002/eji.1830260527. [DOI] [PubMed] [Google Scholar]
- 29.Hines PC, Gao X, White JC, D’Agostino A, Jin JP. A novel role of h2-calponin in regulating whole blood thrombosis and platelet adhesion during physiologic flow. Physiol Rep 2: e12228, 2014. doi: 10.14814/phy2.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirsch S, Austyn JM, Gordon S. Expression of the macrophage-specific antigen F4/80 during differentiation of mouse bone marrow cells in culture. J Exp Med 154: 713–725, 1981. doi: 10.1084/jem.154.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol 9: 847–856, 2008. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hossain MM, Crish JF, Eckert RL, Lin JJ, Jin JP. h2-Calponin is regulated by mechanical tension and modifies the function of actin cytoskeleton. J Biol Chem 280: 42442–42453, 2005. doi: 10.1074/jbc.M509952200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hossain MM, Hwang DY, Huang QQ, Sasaki Y, Jin JP. Developmentally regulated expression of calponin isoforms and the effect of h2-calponin on cell proliferation. Am J Physiol Cell Physiol 284: C156–C167, 2003. doi: 10.1152/ajpcell.00233.2002. [DOI] [PubMed] [Google Scholar]
- 34.Hossain MM, Smith PG, Wu K, Jin JP. Cytoskeletal tension regulates both expression and degradation of h2-calponin in lung alveolar cells. Biochemistry 45: 15670–15683, 2006. doi: 10.1021/bi061718f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hossain MM, Zhao G, Woo MS, Wang JH, Jin JP. Deletion of calponin 2 in mouse fibroblasts increases myosin II-dependent cell traction force. Biochemistry 55: 6046–6055, 2016. doi: 10.1021/acs.biochem.6b00856. [DOI] [PubMed] [Google Scholar]
- 36.Høyer-Hansen M, Jäättelä M. Connecting endoplasmic reticulum stress to autophagy by unfolded protein response and calcium. Cell Death Differ 14: 1576–1582, 2007. doi: 10.1038/sj.cdd.4402200. [DOI] [PubMed] [Google Scholar]
- 37.Huang QQ, Hossain MM, Sun W, Xing L, Pope RM, Jin JP. Deletion of calponin 2 in macrophages attenuates the severity of inflammatory arthritis in mice. Am J Physiol Cell Physiol 311: C673–C685, 2016. doi: 10.1152/ajpcell.00331.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang QQ, Hossain MM, Wu K, Parai K, Pope RM, Jin JP. Role of H2-calponin in regulating macrophage motility and phagocytosis. J Biol Chem 283: 25887–25899, 2008. doi: 10.1074/jbc.M801163200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inoue M, Shinohara ML. Clustering of pattern recognition receptors for fungal detection. PLoS Pathog 10: e1003873, 2014. doi: 10.1371/journal.ppat.1003873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ishiwata-Kimata Y, Yamamoto YH, Takizawa K, Kohno K, Kimata Y. F-actin and a type-II myosin are required for efficient clustering of the ER stress sensor Ire1. Cell Struct Funct 38: 135–143, 2013. doi: 10.1247/csf.12033. [DOI] [PubMed] [Google Scholar]
- 41.Jablonski KA, Amici SA, Webb LM, Ruiz-Rosado JD, Popovich PG, Partida-Sanchez S, Guerau-de-Arellano M. Novel markers to delineate murine M1 and M2 macrophages. PLoS One 10: e0145342, 2015. doi: 10.1371/journal.pone.0145342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jensen MH, Morris EJ, Gallant CM, Morgan KG, Weitz DA, Moore JR. Mechanism of calponin stabilization of cross-linked actin networks. Biophys J 106: 793–800, 2014. doi: 10.1016/j.bpj.2013.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang WR, Cady G, Hossain MM, Huang QQ, Wang X, Jin JP. Mechanoregulation of h2-calponin gene expression and the role of Notch signaling. J Biol Chem 289: 1617–1628, 2014. doi: 10.1074/jbc.M113.498147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jin JP, Wu D, Gao J, Nigam R, Kwong S. Expression and purification of the h1 and h2 isoforms of calponin. Protein Expr Purif 31: 231–239, 2003. doi: 10.1016/S1046-5928(03)00185-2. [DOI] [PubMed] [Google Scholar]
- 45.Jin JP, Zhang Z, Bautista JA. Isoform diversity, regulation, and functional adaptation of troponin and calponin. Crit Rev Eukaryot Gene Expr 18: 93–124, 2008. doi: 10.1615/CritRevEukarGeneExpr.v18.i2.10. [DOI] [PubMed] [Google Scholar]
- 46.Jones GE. Cellular signaling in macrophage migration and chemotaxis. J Leukoc Biol 68: 593–602, 2000. doi: 10.1189/jlb.68.5.593. [DOI] [PubMed] [Google Scholar]
- 47.Kang JL, Pack IS, Hong SM, Lee HS, Castranova V. Silica induces nuclear factor-kappa B activation through tyrosine phosphorylation of I kappa B-alpha in RAW264.7 macrophages. Toxicol Appl Pharmacol 169: 59–65, 2000. doi: 10.1006/taap.2000.9039. [DOI] [PubMed] [Google Scholar]
- 48.Kim ML, Chae JJ, Park YH, De Nardo D, Stirzaker RA, Ko HJ, Tye H, Cengia L, DiRago L, Metcalf D, Roberts AW, Kastner DL, Lew AM, Lyras D, Kile BT, Croker BA, Masters SL. Aberrant actin depolymerization triggers the pyrin inflammasome and autoinflammatory disease that is dependent on IL-18, not IL-1β. J Exp Med 212: 927–938, 2015. doi: 10.1084/jem.20142384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kopf M, Schneider C, Nobs SP. The development and function of lung-resident macrophages and dendritic cells. Nat Immunol 16: 36–44, 2015. doi: 10.1038/ni.3052. [DOI] [PubMed] [Google Scholar]
- 50.Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol 30: 16–34, 2011. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- 51.Lambrecht BN. Alveolar macrophage in the driver’s seat. Immunity 24: 366–368, 2006. doi: 10.1016/j.immuni.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 52.Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, Jung S, Amit I. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell 159: 1312–1326, 2014. doi: 10.1016/j.cell.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leal RF, Milanski M, Ayrizono ML, Coope A, Rodrigues VS, Portovedo M, Oliveira LM, Fagundes JJ, Coy CS, Velloso LA. Toll-like receptor 4, F4/80 and pro-inflammatory cytokines in intestinal and mesenteric fat tissue of Crohn’s disease. Int J Clin Exp Med 6: 98–104, 2013. [PMC free article] [PubMed] [Google Scholar]
- 54.Lelkes E, Headley MB, Thornton EE, Looney MR, Krummel MF. The spatiotemporal cellular dynamics of lung immunity. Trends Immunol 35: 379–386, 2014. doi: 10.1016/j.it.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu R, Jin JP. Calponin isoforms CNN1, CNN2 and CNN3: regulators for actin cytoskeleton functions in smooth muscle and non-muscle cells. Gene 585: 143–153, 2016. doi: 10.1016/j.gene.2016.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu R, Jin JP. Deletion of calponin 2 in macrophages alters cytoskeleton-based functions and attenuates the development of atherosclerosis. J Mol Cell Cardiol 99: 87–99, 2016. doi: 10.1016/j.yjmcc.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu YC, Zou XB, Chai YF, Yao YM. Macrophage polarization in inflammatory diseases. Int J Biol Sci 10: 520–529, 2014. doi: 10.7150/ijbs.8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lundahl J, Halldén G, Sköld CM. Human blood monocytes, but not alveolar macrophages, reveal increased CD11b/CD18 expression and adhesion properties upon receptor-dependent activation. Eur Respir J 9: 1188–1194, 1996. doi: 10.1183/09031936.96.09061188. [DOI] [PubMed] [Google Scholar]
- 60.Man SM, Ekpenyong A, Tourlomousis P, Achouri S, Cammarota E, Hughes K, Rizzo A, Ng G, Wright JA, Cicuta P, Guck JR, Bryant CE. Actin polymerization as a key innate immune effector mechanism to control Salmonella infection. Proc Natl Acad Sci USA 111: 17588–17593, 2014. doi: 10.1073/pnas.1419925111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martin DK, Bootcov MR, Campbell TJ, French PW, Breit SN. Human macrophages contain a stretch-sensitive potassium channel that is activated by adherence and cytokines. J Membr Biol 147: 305–315, 1995. doi: 10.1007/BF00234528. [DOI] [PubMed] [Google Scholar]
- 62.May RC, Machesky LM. Phagocytosis and the actin cytoskeleton. J Cell Sci 114: 1061–1077, 2001. [DOI] [PubMed] [Google Scholar]
- 63.McWhorter FY, Davis CT, Liu WF. Physical and mechanical regulation of macrophage phenotype and function. Cell Mol Life Sci 72: 1303–1316, 2015. doi: 10.1007/s00018-014-1796-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McWhorter FY, Wang T, Nguyen P, Chung T, Liu WF. Modulation of macrophage phenotype by cell shape. Proc Natl Acad Sci USA 110: 17253–17258, 2013. doi: 10.1073/pnas.1308887110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Minton K. Stress-induced macrophage polarization. Nat Rev Immunol 17: 277, 2017. doi: 10.1038/nri.2017.41. [DOI] [PubMed] [Google Scholar]
- 66.Moazzem Hossain M, Wang X, Bergan RC, Jin JP. Diminished expression of h2-calponin in prostate cancer cells promotes cell proliferation, migration and the dependence of cell adhesion on substrate stiffness. FEBS Open Bio 4: 627–636, 2014. doi: 10.1016/j.fob.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morgan KG, Gangopadhyay SS. Invited review: cross-bridge regulation by thin filament-associated proteins. J Appl Physiol (1985) 91: 953–962, 2001. doi: 10.1152/jappl.2001.91.2.953. [DOI] [PubMed] [Google Scholar]
- 68.Moscat J, Diaz-Meco MT. To aggregate or not to aggregate? A new role for p62. EMBO Rep 10: 804, 2009. doi: 10.1038/embor.2009.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mostowy S, Shenoy AR. The cytoskeleton in cell-autonomous immunity: structural determinants of host defence. Nat Rev Immunol 15: 559–573, 2015. doi: 10.1038/nri3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nigam R, Jin JP, Triggle CR. h1- and h2-calponins are not essential for norepinephrine- or sodium fluoride-induced contraction of rat aortic smooth muscle. J Muscle Res Cell Motil 19: 695–703, 1998. doi: 10.1023/A:1005389300151. [DOI] [PubMed] [Google Scholar]
- 71.Patel NR, Bole M, Chen C, Hardin CC, Kho AT, Mih J, Deng L, Butler J, Tschumperlin D, Fredberg JJ, Krishnan R, Koziel H. Cell elasticity determines macrophage function. PLoS One 7: e41024, 2012. doi: 10.1371/journal.pone.0041024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pergola C, Schubert K, Pace S, Ziereisen J, Nikels F, Scherer O, Hüttel S, Zahler S, Vollmar AM, Weinigel C, Rummler S, Müller R, Raasch M, Mosig A, Koeberle A, Werz O. Modulation of actin dynamics as potential macrophage subtype-targeting anti-tumour strategy. Sci Rep 7: 41434, 2017. doi: 10.1038/srep41434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Plazyo O, Liu R, Moazzem Hossain M, Jin JP. Deletion of calponin 2 attenuates the development of calcific aortic valve disease in ApoE−/− mice. J Mol Cell Cardiol 121: 233–241, 2018. doi: 10.1016/j.yjmcc.2018.07.249. [DOI] [PubMed] [Google Scholar]
- 74.Popat A, Patel AA, Warnes G. A flow cytometric study of ER stress and autophagy. Cytometry A, 95: 672–682, 2019. doi: 10.1002/cyto.a.23665. [DOI] [PubMed] [Google Scholar]
- 75.Pugin J, Dunn I, Jolliet P, Tassaux D, Magnenat JL, Nicod LP, Chevrolet JC. Activation of human macrophages by mechanical ventilation in vitro. Am J Physiol Lung Cell Mol Physiol 275: L1040–L1050, 1998. doi: 10.1152/ajplung.1998.275.6.L1040. [DOI] [PubMed] [Google Scholar]
- 76.Rana N, Braun DP, House R, Gebel H, Rotman C, Dmowski WP. Basal and stimulated secretion of cytokines by peritoneal macrophages in women with endometriosis. Fertil Steril 65: 925–930, 1996. doi: 10.1016/S0015-0282(16)58262-4. [DOI] [PubMed] [Google Scholar]
- 77.Rath M, Müller I, Kropf P, Closs EI, Munder M. Metabolism via arginase or nitric oxide synthase: two competing arginine pathways in macrophages. Front Immunol 5: 532, 2014. doi: 10.3389/fimmu.2014.00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reverendo M, Mendes A, Argüello RJ, Gatti E, Pierre P. At the crossway of ER-stress and proinflammatory responses. FEBS J 286: 297–310, 2019. doi: 10.1111/febs.14391. [DOI] [PubMed] [Google Scholar]
- 79.Roan E, Waters CM. What do we know about mechanical strain in lung alveoli? Am J Physiol Lung Cell Mol Physiol 301: L625–L635, 2011. doi: 10.1152/ajplung.00105.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roberts AW, Lee BL, Deguine J, John S, Shlomchik MJ, Barton GM. Tissue-resident macrophages are locally programmed for silent clearance of apoptotic cells. Immunity 47: 913–927.e6, 2017. doi: 10.1016/j.immuni.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rőszer T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators Inflamm 2015: 816460, 2015. doi: 10.1155/2015/816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rozenblum GT, Gimona M. Calponins: adaptable modular regulators of the actin cytoskeleton. Int J Biochem Cell Biol 40: 1990–1995, 2008. doi: 10.1016/j.biocel.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 83.Ryer-Powder JE, Forman HJ. Adhering lung macrophages produce superoxide demonstrated with desferal-Mn(IV). Free Radic Biol Med 6: 513–518, 1989. doi: 10.1016/0891-5849(89)90044-0. [DOI] [PubMed] [Google Scholar]
- 84.Schwarz US, Gardel ML. United we stand: integrating the actin cytoskeleton and cell-matrix adhesions in cellular mechanotransduction. J Cell Sci 125: 3051–3060, 2012. doi: 10.1242/jcs.093716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Senft D, Ronai ZA. UPR, autophagy, and mitochondria crosstalk underlies the ER stress response. Trends Biochem Sci 40: 141–148, 2015. doi: 10.1016/j.tibs.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shibukawa Y, Yamazaki N, Daimon E, Wada Y. Rock-dependent calponin 3 phosphorylation regulates myoblast fusion. Exp Cell Res 319: 633–648, 2013. doi: 10.1016/j.yexcr.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 87.Shibukawa Y, Yamazaki N, Kumasawa K, Daimon E, Tajiri M, Okada Y, Ikawa M, Wada Y. Calponin 3 regulates actin cytoskeleton rearrangement in trophoblastic cell fusion. Mol Biol Cell 21: 3973–3984, 2010. doi: 10.1091/mbc.e10-03-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Song S, Tan J, Miao Y, Zhang Q. Crosstalk of ER stress-mediated autophagy and ER-phagy: Involvement of UPR and the core autophagy machinery. J Cell Physiol 233: 3867–3874, 2018. doi: 10.1002/jcp.26137. [DOI] [PubMed] [Google Scholar]
- 89.Sridharan R, Cameron AR, Kelly DJ, Kearney CJ, O’Brien FJ. Biomaterial based modulation of macrophage polarization: a review and suggested design principles. Mater Today 18: 313–325, 2015. doi: 10.1016/j.mattod.2015.01.019. [DOI] [Google Scholar]
- 90.St-Pierre J, Moreau F, Cornick S, Quach J, Begum S, Aracely Fernandez L, Gorman H, Chadee K. The macrophage cytoskeleton acts as a contact sensor upon interaction with Entamoeba histolytica to trigger IL-1β secretion. PLoS Pathog 13: e1006592, 2017. doi: 10.1371/journal.ppat.1006592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stow JL, Condon ND. The cell surface environment for pathogen recognition and entry. Clin Transl Immunology 5: e71, 2016. doi: 10.1038/cti.2016.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tang J, Hu G, Hanai J, Yadlapalli G, Lin Y, Zhang B, Galloway J, Bahary N, Sinha S, Thisse B, Thisse C, Jin JP, Zon LI, Sukhatme VP. A critical role for calponin 2 in vascular development. J Biol Chem 281: 6664–6672, 2006. doi: 10.1074/jbc.M506991200. [DOI] [PubMed] [Google Scholar]
- 93.Tschumperlin DJ, Boudreault F, Liu F. Recent advances and new opportunities in lung mechanobiology. J Biomech 43: 99–107, 2010. doi: 10.1016/j.jbiomech.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ulmer B, Hagenlocher C, Schmalholz S, Kurz S, Schweickert A, Kohl A, Roth L, Sela-Donenfeld D, Blum M. Calponin 2 acts as an effector of noncanonical Wnt-mediated cell polarization during neural crest cell migration. Cell Rep 3: 615–621, 2013. doi: 10.1016/j.celrep.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 95.Urra H, Henriquez DR, Cánovas J, Villarroel-Campos D, Carreras-Sureda A, Pulgar E, Molina E, Hazari YM, Limia CM, Alvarez-Rojas S, Figueroa R, Vidal RL, Rodriguez DA, Rivera CA, Court FA, Couve A, Qi L, Chevet E, Akai R, Iwawaki T, Concha ML, Glavic Á, Gonzalez-Billault C, Hetz C. IRE1α governs cytoskeleton remodelling and cell migration through a direct interaction with filamin A. Nat Cell Biol 20: 942–953, 2018. [Erratum in Nat Cell Biol 20: 1228, 2018]. doi: 10.1038/s41556-018-0141-0. [DOI] [PubMed] [Google Scholar]
- 96.van de Laar L, Saelens W, De Prijck S, Martens L, Scott CL, Van Isterdael G, Hoffmann E, Beyaert R, Saeys Y, Lambrecht BN, Guilliams M. Yolk sac macrophages, fetal liver, and adult monocytes can colonize an empty niche and develop into functional tissue-resident macrophages. Immunity 44: 755–768, 2016. doi: 10.1016/j.immuni.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 97.van Vliet AR, Giordano F, Gerlo S, Segura I, Van Eygen S, Molenberghs G, Rocha S, Houcine A, Derua R, Verfaillie T, Vangindertael J, De Keersmaecker H, Waelkens E, Tavernier J, Hofkens J, Annaert W, Carmeliet P, Samali A, Mizuno H, Agostinis P. The ER stress sensor PERK coordinates ER-plasma membrane contact site formation through interaction with filamin-A and F-actin remodeling. Mol Cell 65: 885–899.e6, 2017. doi: 10.1016/j.molcel.2017.01.020. [DOI] [PubMed] [Google Scholar]
- 98.Waite AL, Schaner P, Hu C, Richards N, Balci-Peynircioglu B, Hong A, Fox M, Gumucio DL. Pyrin and ASC co-localize to cellular sites that are rich in polymerizing actin. Exp Biol Med (Maywood) 234: 40–52, 2009. doi: 10.3181/0806-RM-184. [DOI] [PubMed] [Google Scholar]
- 99.Walsh MP. Calcium-dependent mechanisms of regulation of smooth muscle contraction. Biochem Cell Biol 69: 771–800, 1991. doi: 10.1139/o91-119. [DOI] [PubMed] [Google Scholar]
- 100.Wang J, Kubes P. A reservoir of mature cavity macrophages that can rapidly invade visceral organs to affect tissue repair. Cell 165: 668–678, 2016. doi: 10.1016/j.cell.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 101.Warnes G. Measurement of autophagy by flow cytometry. Curr Protoc Cytom 68: 9.45.1–9.45.10, 2014. doi: 10.1002/0471142956.cy0945s68. [DOI] [PubMed] [Google Scholar]
- 102.Winder SJ, Walsh MP. Smooth muscle calponin. Inhibition of actomyosin MgATPase and regulation by phosphorylation. J Biol Chem 265: 10148–10155, 1990. [PubMed] [Google Scholar]
- 103.Yu YR, O’Koren EG, Hotten DF, Kan MJ, Kopin D, Nelson ER, Que L, Gunn MD. A protocol for the comprehensive flow cytometric analysis of immune cells in normal and inflamed murine non-lymphoid tissues. PLoS One 11: e0150606, 2016. doi: 10.1371/journal.pone.0150606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang X, Goncalves R, Mosser DM. The isolation and characterization of murine macrophages. Curr Protoc Immunol. 83: 14.1.1–14.1.14, 2008. doi: 10.1002/0471142735.im1401s83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang X, Mosser DM. Macrophage activation by endogenous danger signals. J Pathol 214: 161–178, 2008. doi: 10.1002/path.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]