Abstract
Human pluripotent stem cells (hPSCs) are important resources for cell-based therapies and pharmaceutical applications. In order to realize the potential of hPSCs, it is critical to develop suitable technologies required for specific applications. Most hPSC technologies depend on cell culture, and are critically influenced by culture medium composition, extracellular matrices, handling methods, and culture platforms. This review summarizes the major technological advances in hPSC culture, and highlights the opportunities and challenges in future therapeutic applications.
Keywords: Human pluripotent stem cells, Human embryonic stem cells, Cell culture, Culture medium, Stem cell niche, Signal transduction, Embryoid bodies
Core tip: This review summarizes recent developments in cell culture systems for human pluripotent stem cells, including signal transduction requirements at different pluripotency stages, advances in extracellular matrices and handling methods, establishment of chemically defined conditions, and various cell culture platforms for specific purposes.
INTRODUCTION
Human pluripotent stem cells (hPSCs), including mainly human embryonic stem cells (hESCs) and induced pluripotent stem cells (hiPSCs), have the capacity to differentiate to all cell types in the human body[1-4]. Since they were first derived in 1998, hESCs have provided an unparalleled model to understand human embryogenesis, and has sparked a revolution in regenerative medicine[1,5]. Based on hESC culture conditions, hiPSCs were first derived in 2007, which again created an unprecedented opportunity to generate patient-specific hPSCs for disease modeling and therapeutic applications[3,4]. Because of their enormous potential, hPSCs have garnered vast interest in both basic research and clinical applications[6]. Unlike somatic cell types, pluripotent stem cells only transiently exist in the first few days of embryogenesis, and there is no natural environment that is capable of maintaining pluripotency in vivo for extended periods of time, so hPSCs in the lab are all artifacts of in vitro cell culture conditions[1]. It is commonly recognized that cell culture quality is a major limiting factor of hPSC applications. In the past 20 years, cell culture is a main research focus in the hPSC field, and many technological improvements have been made to realize the potential of hPSCs.
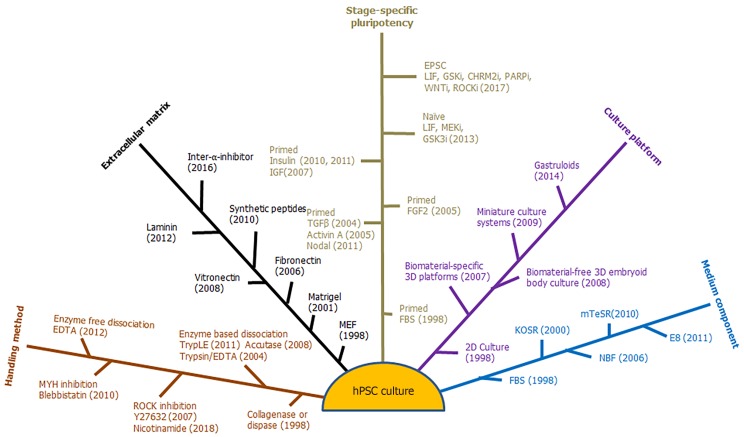
In order to maintain their pluripotency, hPSCs require proper combinations of extrinsic signal stimuli to establish a stem cell niche in cell culture systems[7]. Suitable methods and culture platforms are required to sustain cell survival and promote specific functions in various applications. hPSCs are traditionally cultured as a 2-dimensional (2D) monolayer on mouse embryonic fibroblast feeder cells (MEFs) in medium supplemented with either fetal bovine serum (FBS) or components extracted from serum[1,2]. This traditional culture is sufficient for hPSC maintenance and general characterization but cannot satisfy the needs of numerous potential applications, such as cell therapy and gene targeting. At the same time, new knowledge from basic research also leads to new questions and challenges for further technology development in cell culture[8-10]. This review will discuss five areas in hPSC culture development that includes: (1) Stage-specific signaling requirements; (2) Essential extracellular matrix; (3) Handling methods; (4) Defined culture composition; and (5) Culture platforms (Figure 1).
Figure 1.
Five areas in human pluripotent stem cell culture development. hPSC: Human pluripotent stem cell.
STAGE-SPECIFIC SIGNALING REQUIREMENTS FOR HPSC PLURIPOTENCY
After almost four decades of research, people have realized that mammalian PSCs could be maintained at distinctive developmental stages. hPSCs at each stage require a specific and different combination of growth factor stimulations. Three stages of pluripotency have been reported in hPSCs, including primed, naïve, and extended pluripotency. However, most hPSCs are derived and maintained as primed PSCs.
The pluripotency stages are defined according to the differentiation potential and developmental timing during mouse embryogenesis. In mouse embryogenesis, primed ESCs are derived from post-implantation epiblasts[11], and naïve ESCs come from the inner cell mass of preimplantation blastocysts[12,13], both of which show limited ability to contribute to the extraembryonic placental tissues in vivo. Recently, extended pluripotent stem cells (EPSCs) have been reported with extended ability to contribute to both extraembryonic and embryonic tissues[14,15]. Human and mouse PSCs share similar growth factor signal stimulations that are required to maintain PSCs at each specific stage. The hPSC pluripotency stages are determined according to signal requirements and corresponding gene expression.
Primed ESC stage - common hESC culture
Thomson et al[1] first established hESCs from the inner cell mass (ICM) of blastocysts in MEF feeder cell culture with FBS. In the following 20 years, researchers have endeavored to understand the essential signals from FBS and feeder cells that promote pluripotency (Table 1). Many cell culture systems have been established to maintain hESCs. FGF, TGF-β family, and insulin master the three essential signaling pathways for hPSC survival and pluripotency, and they have been combined together as the most common extrinsic stimuli to derive and maintain hESCs and hiPSCs[16-18]. FGF family members, with FGF2 as the main effector, activate the ERK pathway to promote cell survival, proliferation, and pluripotency[19-21]. TGF-β family members, including TGF-β, Activin, and Nodal, induce SMAD2/3-dependent transcription to sustain pluripotency[16,17,22]. Insulin is a promiscuous factor present in most hESC media, and is required for cell survival, metabolism, and pluripotency[17,18]. Insulin can be replaced by insulin-like growth factor (IGF), which activates the IGF receptor pathway to support hESCs[23]. Based on the hESC culture conditions, Tesar et al[11] derived primed mouse ESCs (mESCs) from post-implantation epiblasts, which are also called mouse epiblast stem cells (mEpiSCs). These primed mESCs exhibit similar growth factor preference as hESCs. If without specification, hESC culture condition usually refers to conditions that can sustain primed hPSCs and primed mPSCs.
Table 1.
Primed human pluripotent stem cell culture conditions
| Thomson et al[1] | Xu et al[53] | Xu et al[20] | Vallier et al[16] | Li et al[26] | Ludwig et al[30,80] | Liu et al[52] | Lu et al[33] | Yao et al[117] | Wang et al[23] | Chen et al[17] | Frank et al[118] | |
| FGF2 | - | + | + | + | + | + | + | + | + | + | + | + |
| TGF-β family | - | - | - | Activin | - | TGF-β | - | TGF-β | - | Activin | TGF-β/Nodal | TGF-β/Activin |
| Serum | FBS | KOSR | KOSR | XF | - | - | - | - | - | - | - | - |
| Other factors | - | - | Noggin | Insulin | FLT3 | Insulin | - | Insulin, BAFF, WNT3A | - | LR3-IGF1, HRG1β | Insulin | Dorsomorphin, IWP-2 |
| Common culture supplements | - | - | NEAA, BME, L-glutamine | BSA, L-glutamine, transferrin, BME, F12, Monothio-glycerol | BME, NEAA, L-glutamine | BSA, BME, transferrin, cholesterol, pipecolic acid, GABA | BME, N2, B27, L-glutamine | BME, transferrin, cholesterol, albumin, L-glutamine | BME, N2, B27, NEAA, BSA-Fraction-V, L-glutamine | BSA-Fraction-V, NEAA, Vc, transferrin, BME, trace elements A, B, C | Vc, transferrin, selenium, NaHCO3 | N2, B27, BSA, L-glutamine, NEAA, BME |
| Base medium | DMEM/F12 | DMEM | DMEM/F12 | IMDM | XVIVO-10 | DMEM/F12 | DMEM/F12 | DMEM/F12 | DMEM/F12 | DMEM/F12 | DMEM/F12 | DMEM/F12 |
| ECM | - | Matrigel | Matrigel | Matrigel | Matrigel | Matrigel | Fibronectin | Matrigel | Matrigel | Matrigel | Matrigel/ Vitronectin | Matrigel |
| Feeder | MEF | - | - | - | - | - | - | - | - | - | - | - |
| Markers | Surface markers[119]: SSEA3, SSEA4, TRA1-60, TRA1-81, CD24, CD57, CD90 | |||||||||||
| Nuclear markers: OCT4, NANOG, SOX2 | ||||||||||||
In addition to the growth factors ruled by the three mentioned pathways, other signaling pathways are also reported to support pluripotency. For example, FLT3, heregulin, heparin, heparan sulfate, S1P, and PDGF promote hESC pluripotency, and all are found to promote ERK pathway activation[24-29]. Beneficial effects are also observed with other factors such as pipecolic acid and GABA, but their molecular mechanisms remain unknown[30].
Besides the beneficial factors, some growth factor pathways need to be suppressed to maintain primed pluripotency in hESCs. Exogenous BMP signal from serum or serum products leads to the exit of self-renewal[31]. The inhibition of BMP pathway promotes hESC pluripotency even without addition of TGF-β in medium containing knockout serum replacement (KOSR)[20]. At the same time, WNT inhibition is also beneficial for cell pluripotency, while WNT activation leads to cell differentiation[32]. Multiple groups reported earlier that hESC pluripotency was promoted by WNT pathway activators, such as WNT3A, LiCl, and BIO[22,30,33], but those observations could be artifacts from differential culture background, coming from different medium composition. Presently, WNT activators are usually used to induce hPSC differentiation to mesoderm or neural crest lineages[34-36].
Based on the knowledge of hESC culture conditions, various primed stage hPSCs have been derived from different sources. In addition to the inner cell mass of embryos[1], hESCs are also derived from a single cell of an 8-cell blastomere without embryo destruction[37]. In recent years, patient-specific nuclear transfer-ESCs (hNT-ESCs) have been created through somatic cell nuclear transfer (SCNT) to caffeine-treated oocytes[38]. At the same time, hiPSCs are generated directly from somatic cells through somatic reprogramming by defined factors[3,4]. No matter the sources, the hPSCs derived under hESC conditions always reflect characteristics that resemble primed mouse ESCs.
Naïve hPSC stage
Immediately after the derivation of hESCs, researchers noticed that hESCs required a different growth factor regulation from preimplantation blastocyst-derived mESCs[39]. Smith and colleagues show that mouse ESCs can be maintained by leukemia inhibitory factor (LIF) in media containing FBS, by LIF and BMP4 in serum-free media, or by the dual inhibition of MEK and GSK3 (2i) in serum-free cultures[40-42]. These cells are called naïve mESCs in contrast with primed mESCs, because the naïve mESCs represent an earlier developmental stage. The naïve mESC conditions cannot be used to maintain primed hPSCs and mESCs. Examples include BMP4, ERK inhibitor, and GSK3 inhibitor, all of which induce the differentiation of primed hPSCs.
Naïve mESCs contribute to blastocyst chimeras more effectively, demonstrating a unique epigenetic signature with genome-wide DNA hypomethylation, and with the silent-X chromosome reactivated in female cells[43]. Compared to primed mESCs, naïve mESCs exhibit more genomic consistency and differentiation potentials. Naïve hPSCs could presumably gain similar advantages, yet hPSC pluripotency could not be maintained by mouse naïve conditions. It is reported that key genes such as ESRRB, KLF2, and BMP4 are not expressed in human naïve epiblasts[44]. By overexpressing KLF4, naïve hPSCs can be maintained under mouse 2i conditions with an additional PKA inhibitor[45]. This suggests that additional signal modulation is necessary to maintain hPSCs at the naïve stage.
In the past 5 years, major efforts have been devoted to developing culture conditions to maintain naïve hESCs. Six combinations of extrinsic stimuli are reported to maintain naïve hPSC, which are summarized in Table 2[46-51]. Similar to mouse culture, all naïve conditions require MEK and GSK3 inhibitors (2i), and LIF is applied in five of the six media. All the conditions include additional supplements besides base medium, such as KOSR, B27, N2, and TeSR1 supplements[52]. Interestingly, four conditions also contain FGF2 that is not required in mESC culture. At the same time, various additional small chemicals are used to modulate pathways, such as LCK/SRC, Raf, FGFR, HDAC, and PKC. These additional factors help induce gene expression that are beneficial to naïve pluripotency. Currently, there remains no consensus concerning which pathways are essential for the maintenance of naïve hPSCs. It is conceivable that a more unified culture system will be developed in the near future.
Table 2.
Naïve pluripotent stem cell and extended pluripotent stem cell culture conditions
|
Gafni et al[48] |
Chan et al[49] |
Valamehr et al[50] |
Ware et al[51] |
Theunissen et al[120] |
Guo et al[47] |
Ying et al[42] |
Yang et al[15] |
Yang et al[15] |
Yang et al[14] |
|
| Naive hESC | Naive hESC | Naive hESC | Naive hESC | Naive hESC | Naive hESC | Naive mESC | hEPSC | mEPSC | mEPSC | |
| MEK inhibitor | PD0325901 | PD0325901 | PD0325901 | PD0325901 | PD0325901 | PD0325901 | PD0325901 | - | - | PD0325901 |
| GSK3 inhibitor | CHIR 99021 | BIO | CHIR99021 | CHIR99021 | IM-12 | CHIR99021 | CHIR99021 | CHIR99021 | CHIR99021 | CHIR99021 |
| LIF | + | + | + | + | + | + | + | + | + | - |
| FGF2 | + | + | + | + | - | - | - | - | - | - |
| TGFβ | + | + | - | Activin | Activin | - | - | - | - | - |
| ROCK Inhibitor | Y27632 | Thiazovivin | Thiazovivin | Y27632 | Y27632 | Y27632 | - | Y27632 | - | - |
| Insulin | + | + | - | - | - | + | - | - | - | - |
| Other factors | JNKi; p38i; PKCi | Dorsomorphin | TGFi | TGFi; FGFRi; HDACi, STAT3i | B-RAFi; LCK/SRCi | PKCi | - | IWR1, dimethindene maleate, minocycline hydrochloride | Dimethindene maleate, minocycline hydrochloride | p38i, JNKi; SRCi; Tanki, WNTi |
| Base medium | KO DMEM | DMEM/F12 | DMEM/F12 | DMEM/F12 | DMEM/F12/ Neurobasal | DMEM/F12/ Neurobasal | DMEM/F12 | DMEM/F12/Neurobasal | DMEM/F12/Neurobasal | DMEM/F12 |
| Medium Supplement | KOSR, NEAA, AlbuMAX I, L-glutamine, BME | TeSR1 components | KOSR, NEAA, BME, L-glutamine | GlutaMAX, KOSR, NEAA, BME, sodium pyruvate | B27/N2, L-glutamine, NEsAA, BME, BSA | N2/B27, L-glutamine, BME, Vc, selenium, putrescine, proges-terone | N2/B27 | N2/B27, GlutaMAX, BME, BSA, KOSR | N2/B27, GlutaMAX, BME, BSA, KOSR | BME, GPS, NEAA, KOSR |
| Feeder | MEF optional | MEF | - | - | MEF | MEF | - | MEF | MEF | MEF |
| Coating | Gelatin/vitronectin | Matrigel | Matrigel/vitronectin | Matrigel | - | - | Gelatin | - | - | - |
| Markers | Surface markers[121]: CD7, CD77, CD75, CD130 | Oct4, Nanog, Rex1 | OCT4, KLF4, NANOG | Oct4, Nanog, Sox2, Sall4 | Oct4, Nanog, Sox2, Klf4, Rex1, Esrrb | |||||
| Nuclear markers[119]: OCT4, NANOG, SOX2, KLF4,5,17, TFCP2L1, DPPA3,5 | ||||||||||
mESC: Mouse embryonic stem cell; hEPSC: Human extended pluripotent stem cell; mEPSC: Mouse extended pluripotent stem cell.
It is important to point out that naïve pluripotency requires different maintenance signals, which sometimes have opposite effects on primed hPSCs. For example, the FGF and ERK pathway is inhibitory to naïve pluripotency, while it also promotes primed pluripotency. At the same time, BMP4 and WNT signals promote naïve hPSCs, but induce differentiation of primed hPSCs. Such phenomena demonstrate that hPSCs at each stage require distinctive signals for exiting pluripotency as well as for cell fate determination.
Extended pluripotency
Recently, two new mouse cell culture conditions have been reported to sustain ESCs with extended pluripotency. These cells can contribute to not only embryonic, but also extraembryonic lineages, giving them the name of extended pluripotent stem cells (EPSCs)[14,15]. Surprisingly, these two conditions only share GSK3 inhibition. Deng and colleagues showed that mouse EPSCs are maintained by the EPS-LCDM medium, which contains the combination of LIF, CHIR99021 (GSK inhibitor), dimethindene maleate (M2 muscarinic receptor inhibitor), and minocycline hydrochloride (PARP inhibitor)[15]. With the help of WNT inhibitor (IWR-endo-1) and ROCK inhibitor (Y27632), EPS-LCDM can be used to maintain human EPSCs. In contrast, Liu and colleagues reported a different formula to maintain EPSCs, which includes inhibitors of various pathways including MEK, GSK3, p38, JNK, SRC, and Tankyrase[14]. More work is necessary to determine whether the two EPSCs are at similar developmental stages, and what central regulation is shared by these two conditions.
Naïve hPSC and hEPSC cultures allow better cell survival after individualization than primed hPSCs, which is beneficial for applications such as gene targeting and expansion. They also provide alternative model systems to understand human embryogenesis, and more studies are needed to explore their potential. However, currently most studies and applications use primed hESC conditions, so the remainder of this review will focus on the technology development related to primed hPSCs.
CELL ADHESION FOR CELL SURVIVAL AND EXPANSION
In order to maintain hPSC pluripotency, a stem cell niche requires not only growth factor signals but also cell adhesion. Without the support of exogenous adhesion proteins, hPSCs either die or differentiate[17,53]. In original hESC derivation, cell adhesion signals are provided by MEF feeder cells and their secreted extracellular matrix (ECM)[1]. Matrigel was later found sufficient to support hESC survival and self-renewal without feeder cells[53]. Matrigel is isolated from mouse Engelbreth-Holm-Swarm teratocarcinoma cells, so it is not ideal for hPSCs that have applications in clinical therapies[54,55]. The establishment of feeder-free culture permits the possibility to optimize ECM and medium composition in parallel, and greatly accelerates technology development.
Matrigel is a mixture of mainly laminin and collagen, which can activate integrin signaling. Various integrin-activating ECM proteins and their recombinant derivatives can support hPSCs, which include laminin, vitronectin, fibronectin, and inter-α-inhibitor[33,56-59]. Recombinant vitronectin and laminin domains can be produced in bacteria or in a cell free system, and they are becoming popular choices for cell culture that needs defined recombinant ECM components[17,56,59].
Besides cell-ECM interaction, cell-cell interaction mediated by E-cadherin is another key component of hPSC niche. E-cadherins facilitate hPSC expansion in the form of colonies[60]. Recombinant E-cadherin is produced as a fusion protein that contains N-terminal E-cadherin and C-terminal IgG-Fc domains. This protein sustains hPSC survival and self-renewal in the absence of integrin-stimulating ECM[61]. When E-Cadherin and laminin are combined to create an artificial matrix, the clonal expansion of hPSCs is significantly improved in comparison to single-component matrices[62]. These data suggest that integrin- and E-cadherin-mediated adhesions provide the principle ECM cues in hPSC niche.
Based on hPSC niche composition, specific peptides have been identified to sustain hPSC culture[10,63]. The most popular peptides are RGD domain-based peptides, which activate integrin pathways. These peptides are sufficient for hPSC survival and expansion, and the efficiency is similar to that of matrigel and vitronectin[63,64]. Such peptides can be chemically produced in large scale, and are very attractive materials for tissue engineering and technology development. The recombinant proteins and peptides can be conjugated to various matrices to construct a synthetic ECM environment. With improved hPSC handling methods, hPSCs are more tolerant to various materials, which greatly expand the choices of ECM materials for different culture platforms and applications.
HANDLING METHODS
Compared to naïve hPSCs and hEPSCs, primed hPSCs are more prone to cell death after dissociation. Most hPSC culture manipulations involve cell dissociation or individualization. Efforts have been focused on promoting cell survival in hPSC handling methods that are essential for various applications.
In regular maintenance, hPSCs proliferate quickly and are usually passaged every 4-7 d. Traditionally, hPSCs are manually split, or dissociated with collagenase or dispase, and cells are collected as clumps[1,2]. Collagenase and dispase cause minimal disruption of hPSC niches, and cells survive well as aggregates of uneven sizes. Unfortunately, such a dissociation method is not suitable for gene targeting or other experiments that require individualized cells. After hPSCs are individualized with trypsin/EDTA[65], most cells die within 24 h after passaging, and fewer than 1% of hPSCs can survive clonally without exogenous intervention. After individualization, the loss of cell adhesion activates the Rho-associated protein kinase (ROCK) /Actinomyosin axis that leads to increased actin-myosin contractility and cell death. Cell survival of individualized cells is significantly improved by the inhibition of ROCK, myosin heavy chain (MYH), and actin proteins[66,67]. Vitamin B3 is sometimes used in hPSC expansion, and it was recently found that nicotinamide promotes cell survival by inhibiting ROCK activity[68]. Interestingly, even though caspase cascades are activated by dissociation, caspase inhibitors could not rescue cell survival. Researchers typically use ROCK inhibitors and the MYH inhibitor blebbistatin to facilitate single cell clonal formation and expansion[66,67,69].
Dissociation reagents are also important for the cell survival after dissociation. TrypLE and accutase are recombinant proteases that have gentler effects on cells than traditional trypsin/EDTA, which greatly improves cell survival after dissociation, whether or not ROCK inhibitors are present[70,71]. hPSCs can also be dissociated as small aggregates with enzyme-free EDTA/PBS or citric acid solutions[72,73]. After attaching to ECM coated surfaces, these small aggregates quickly re-establish colonies and can achieve good survival ratios even without ROCK inhibitors. This enzyme-free method does not digest the ECM, and does not need enzyme neutralization and removal. It provides some unique advantages in clonal hPSC expansion over enzyme-based dissociation methods[72].
Cryopreservation is the essential final stage of hPSC culture. It has long been problematic to efficiently revive hPSCs after cryopreservation. When hPSCs are harvested using dispase or collagenase, fewer than 5% of colonies could be recovered. In order to achieve good cryopreservation in these conditions, hPSCs need to be individualized, and later recovered in the presence of ROCK inhibitors or blebbistatin[74,75]. However, when enzyme-free PBS/EDTA is used to dissociate cells, hPSCs can be efficiently cryopreserved even without ROCK inhibitors[72].
With the emergence of new genome recombination technologies, increasingly hPSCs are being used in gene targeting that requires individualization and clonal expansion. For these purposes, TrypLE, accutase, and enzyme-free dissociation are usually used to harvest cells, and cells are often treated with ROCK inhibitor during electroporation and plating[76,77]. If antibiotics, such as puromycin and neomycin, are applied to select positive clones, ROCK inhibitor usually needs to be present to improve clonal hPSC expansion.
CULTURE CONSISTENCY, FROM SERUM TO CHEMICALLY DEFINED CONDITIONS
Even though growth factors and ECM are vital for hPSC maintenance, they are only a small part of cell culture components. Cell culture systems also include other equally important factors, such as water, nutrients, salts, vitamins, lipids, air, and temperature control[78]. Optimal culture composition is essential for cell survival, pluripotency, and therapeutic applications.
Most medium nutrients, salts, vitamins and water are provided through various basic media such as DMEM/F12. Additional medium components are usually supplied in the form of fetal bovine serum (FBS), KOSR, or defined mixture such as B27 supplement[52,79]. At the same time, MEF feeder cells are often used to provide unspecified beneficial factors[18]. FBS and KOSR contain undefined components of animal origins, and even B27 has bovine serum albumin (BSA) as a major component. These supplements pose major obstacles for hPSC applications. First, the exposure to animal cells and animal products leads to risk of immune rejection to hPSCs by contaminated animal components. This would make the hPSC product unsuitable for therapeutic applications. Second, secretion from feeder cells or serum components often lead to complications when trying to interpret a phenomenon or molecular mechanism. Third, the uncertainty of undefined composition also leads to complications in large-scale production which can cause a significant batch to batch variation. It is more desirable to have a robust culture system with defined composition while free of animal products.
Many defined cell culture systems have been developed to culture primed hPSCs. Individual labs and international collaboration have been involved in defining the components in cell culture media[16,23,26,33,80]. In 2010, the International Stem Cell Initiative systematically evaluated a few popular defined hPSC media, and mTeSR1 and STEMPRO demonstrated a more consistent ability to main hPSCs[18]. Besides FGF2, all these media contain BSA. Albumin is a principal serum protein, and makes up 3.5 to 5.0 g/dL in serum. Most cell culture contains albumin[81,82]. Even though BSA can be replaced by human serum albumin or recombinant albumin, the sheer amount of the albumin in medium significantly affects consistency which relies on the quality of albumin in each production batch. Chen et al[18] showed that the essential role of albumin is to block the toxicity of antioxidant 2-mercaptoethanol that is traditionally added into hPSC culture. When 2-mercaptoethanol is removed from medium, albumin is no longer required for hPSCs. Based on this finding, E8 medium was developed to sustain hPSC pluripotency with eight essential components[17]. E8 components include growth factors (insulin, FGF, and TGFβ), nutritional support (DMEM/F12 base medium), antioxidants (selenium, vitamin C, and transferrin) and pH modulator (NaHCO3). The E8 medium formula has a significantly simplified composition, which is a good platform to understand hPSC physiology. When albumin is no longer necessary, E8-based culture system can facilitate hPSC production for therapeutic applications, and hPSCs can be cultured in E8 medium for >50 passages without any signs of karyotypic abnormalities while maintaining their pluripotency. hESCs and hiPSCs maintained in E8 medium have been efficiently induced into many somatic cell types and tissues under adherent and suspension culture conditions[83-86].
When researchers use albumin-free media, many cellular treatments and manipulations need to be reevaluated. First, as a carrier protein, albumin is a strong adsorbent and blocker, so the concentrations of small chemicals used for cell manipulations need to be re-examined in albumin-free condition. Second, it is found that stem cells are more sensitive to suboptimal conditions or environmental changes in albumin-free medium, such as toxins, small chemical treatments, and medium acidosis[87].
Besides albumin, there are other factors that could also affect hPSC culture consistency. FGF2 is essential for pluripotency, but it is thermally unstable, which can result in precipitation and conformational changes of proteins, so high concentrations of FGF2 must be added in defined culture, and it needs to be replenished regularly. FGF2 can be stabilized by heparin, a specific point mutation, or slow release mechanism to help resolve this issue[28,88], and mutant forms of FGF2 that are stable against thermal denaturation have been established, such as K128N (Chen et al[89], 2016). It was also found that defined media produce different patterns of cellular metabolism compared to KOSR-containing media. Due to the lack of lipid components from KOSR, cells in E8 and TeSR demonstrate increased oxidative pentose phosphate pathway metabolism.
Although primed hPSCs can be maintained in E8 medium, there remains no similar albumin-free condition capable of naïve hPSC and EPSC maintenance. The media for the latter two hPSCs require albumin-containing supplements and additional small molecule modulators. This remains not only an interesting biological question but also a practical problem in the development of potential hPSC applications in the future.
CULTURE PLATFORMS FOR SPECIFIC PURPOSES
As discussed in previous sections, traditional hPSC culture systems are established on a 2D monolayer with suitable ECMs. However, conventional 2D monolayer culture does not accurately replicate the in vivo physiological environment, and often fails to meet the demands of research and therapeutic applications[90]. With the advances in culture medium, ECM, and handling methods, various culture platforms have been developed to utilize hPSCs beyond the usual 2D monolayer[91]. ROCK/MYH inhibitors promote cell survival and make hPSCs more tolerant to various treatments, which facilitates the fast development of hPSC culture platforms. We will briefly discuss biomaterial-free embryoid body culture as well as biomaterial-specific 2D and 3D platforms.
Biomaterial-free 3D embryoid body culture
When no ECM is supplemented to hPSCs, cells in suspension form embryoid bodies through E-cadherins. Many different methods have been developed to make embryoid bodies[92,93]. When hPSCs are harvested as individualized cells, ROCK inhibitor greatly promoted cell survival during the formation of embryoid bodies independent of the dissociation method.
The 3D suspension culture provides multiple advantages in large scale production, storage, and differentiation[94,95]. Clinical applications often require 107-1010 or more hPSCs. However, 2D culture cannot constantly produce uniform hPSCs in such large quantities. The embryoid bodies can be grown in stirred-suspension bioreactors, spinner flasks, or bag, greatly increasing cell culture capacity[96]. The suspension culture can now produce more than 1013 hPSCs[97]. Bioreactors provide a homogenous growth environment with real-time monitoring of oxygen level, medium acidosis, and metabolite concentrations[98]. The shear stress and slowed growth rate are common issues that need to be considered when cells are expanded.
The embryoid body structure mimics cell interaction in embryogenesis, and hPSCs can spontaneously differentiate to cell types of three germ layers in the absence of growth factors. Embryoid body can be used to evaluate pluripotency in vitro[99,100], and it has become an attractive alternative to a traditional teratoma assay[101]. The 3D culture can also be used for lineage-specific differentiation. In recent years, organoid is becoming a powerful model to understand embryogenesis and lineage-specific differentiation[102]. hPSC embryoid bodies can be adapted to organoid differentiation for specific cell types[103,104].
hPSCs in suspension display altered metabolic status and slower cell proliferation. Xu and colleagues utilized the altered hPSC physiology in suspension to develop the spheropreservation method[105]. In suspension, hPSC embryoid bodies can maintain cell viability and pluripotency at room temperature for several days. This allows the cells to be transported at room temperature without cryopreservation. It is a convenient way to transport cells without conventional methods that require either dry ice for frozen cells or a 37 °C container for live cell culture vessels.
Biomaterial-specific 3D platforms
3D scaffolds are increasingly used for hPSC maintenance and differentiation with the assistance of natural and synthetic materials[106]. These materials are usually biocompatible and biodegradable, and provide various biological signals and mechanical strength for specific applications. Many natural materials, such as hyaluronic acid (HA) and alginate, are functional for hPSCs maintenance, but they are difficult to control due to undefined polymer size and the potential to influence cellular signal transduction[107]. Synthetic polymers such as polyethylene glycol (PEG) are readily polymerized, and can be functionalized with specific ligands. This allows fine-tuning of the stem cell niche to meet the requirements of various applications. PEG-based hydrogel has been used to study the effect of N-cadherin peptide on mESC growth and neural differentiation[108], and also shown to successfully support the development of neural tube structure from single mESCs[109]. Many ligands have been discussed in previous sections. With the proper choice of biomaterials, hPSCs can be maintained, differentiated, and cryopreserved efficiently in 3D platforms[110].
Miniature culture systems
Besides large-scale production for therapeutic applications, new platforms have been developed as miniature culture systems that can be used for basic research and drug screening.
Microfluidic systems feature automatic operation, precise control of treatment parameters, as well as integrated functional modules. It has been used to interrogate the effect of cell patterning, physical factors, chemical factors as well as cell-cell and cell-ECM interactions in hPSCs. Microfluidic chips were designed for analysis of hPSC response to treatments as single colonies[111,112]. Similar systems have been used to understand how growth factor signals could impact cell fate determination[113,114], comparable to organ-on-a-chip platforms for cancer and somatic cells. Microfluidic devices are powerful tools for research in cellular function, cell fate determination as well as disease modeling.
Recently, gastruloid culture platforms were developed for pluripotent stem cells as a multicellular in vitro model of the gastrulating embryo. Generation of geometrically confined stem cell colonies significantly improves the reproducibility and quantitative analysis of differentiation. 2D micropatterned hESC colonies are generated on a surface coated with patterned ECM proteins or ligands[115]. The pattern can be precisely controlled in size, shape, and ligand. This platform not only improves the reproducibility of differentiation, but also provides a platform for microscopic imaging and screening. Recently, gastruloid culture has been used to study how geometric constrains could affect cell fate determination with specific spatial distribution[116]. An important advantage of this system compared to the embryoid bodies platform is the better control over cell number, being important when reproducing early developmental stages influenced by cell number and patterning.
CONCLUSION
In the past 20 years, hPSC culture technologies have evolved extensively on all fronts. We can expect to see hPSCs at all three embryonic stages efficiently maintained very soon. However, development of clinical therapies or disease models needs more than just pluripotent stem cells. The next great challenge is to efficiently differentiate or generate specific cell types from hPSCs in cell culture, which will require more complex signal transduction and medium composition for differentiation initiation, cell fate specification, and maturation. In addition, the cell culture platform will be involved in how differentiated cells could be used in various applications, which will require the infusion of bioengineering technologies and efficient cell handling methods. The principles of hPSC culture technology could be applied to differentiation and further applications. More exciting cell culture advances will eventually help to realize the great potential of hPSCs just as people imagined when the cells were first derived in 1998.
ACKNOWLEDGEMENTS
We thank Dr. Garry Wong for his comments and suggestions.
Footnotes
Conflict-of-interest statement: Dr. Chen has a patent 9644186 with royalties paid to Wisconsin Alumni Research Foundation. The other authors have no conflict of interest to declare.
Manuscript source: Invited manuscript
Peer-review started: March 28, 2019
First decision: April 16, 2019
Article in press: August 27, 2019
Specialty type: Cell and tissue engineering
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Grawish ME, Tanabe S S-Editor: Ma RY L-Editor: Wang TQ E-Editor: Wu YXJ
Contributor Information
Weiwei Liu, Centre of Reproduction, Development and Aging, Faculty of Health Sciences, University of Macau, Macau 999078, China; Institute of Translational Medicine, Faculty of Health Sciences, University of Macau, Macau 999078, China; Bioimaging and Stem Cell Core Facility, Faculty of Health Sciences, University of Macau, Macau 999078, China.
Chunhao Deng, Centre of Reproduction, Development and Aging, Faculty of Health Sciences, University of Macau, Macau 999078, China; Institute of Translational Medicine, Faculty of Health Sciences, University of Macau, Macau 999078, China.
Carlos Godoy-Parejo, Centre of Reproduction, Development and Aging, Faculty of Health Sciences, University of Macau, Macau 999078, China; Institute of Translational Medicine, Faculty of Health Sciences, University of Macau, Macau 999078, China.
Yumeng Zhang, Centre of Reproduction, Development and Aging, Faculty of Health Sciences, University of Macau, Macau 999078, China; Institute of Translational Medicine, Faculty of Health Sciences, University of Macau, Macau 999078, China.
Guokai Chen, Centre of Reproduction, Development and Aging, Faculty of Health Sciences, University of Macau, Macau 999078, China; Institute of Translational Medicine, Faculty of Health Sciences, University of Macau, Macau 999078, China. guokaichen@umac.mo.
References
- 1.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol. 2000;18:399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- 3.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 5.Castelvecchi D. Billion-star map of Milky Way set to transform astronomy. Nature. 2018;557:18. doi: 10.1038/d41586-018-04979-4. [DOI] [PubMed] [Google Scholar]
- 6.Yamanaka S. Induced pluripotent stem cells: past, present, and future. Cell Stem Cell. 2012;10:678–684. doi: 10.1016/j.stem.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Pera MF, Tam PP. Extrinsic regulation of pluripotent stem cells. Nature. 2010;465:713–720. doi: 10.1038/nature09228. [DOI] [PubMed] [Google Scholar]
- 8.Chen KG, Mallon BS, McKay RD, Robey PG. Human pluripotent stem cell culture: considerations for maintenance, expansion, and therapeutics. Cell Stem Cell. 2014;14:13–26. doi: 10.1016/j.stem.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ying QL, Smith A. The Art of Capturing Pluripotency: Creating the Right Culture. Stem Cell Reports. 2017;8:1457–1464. doi: 10.1016/j.stemcr.2017.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desai N, Rambhia P, Gishto A. Human embryonic stem cell cultivation: historical perspective and evolution of xeno-free culture systems. Reprod Biol Endocrinol. 2015;13:9. doi: 10.1186/s12958-015-0005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tesar PJ, Chenoweth JG, Brook FA, Davies TJ, Evans EP, Mack DL, Gardner RL, McKay RD. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448:196–199. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- 12.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 13.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang J, Ryan DJ, Wang W, Tsang JC, Lan G, Masaki H, Gao X, Antunes L, Yu Y, Zhu Z, Wang J, Kolodziejczyk AA, Campos LS, Wang C, Yang F, Zhong Z, Fu B, Eckersley-Maslin MA, Woods M, Tanaka Y, Chen X, Wilkinson AC, Bussell J, White J, Ramirez-Solis R, Reik W, Göttgens B, Teichmann SA, Tam PPL, Nakauchi H, Zou X, Lu L, Liu P. Establishment of mouse expanded potential stem cells. Nature. 2017;550:393–397. doi: 10.1038/nature24052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Y, Liu B, Xu J, Wang J, Wu J, Shi C, Xu Y, Dong J, Wang C, Lai W, Zhu J, Xiong L, Zhu D, Li X, Yang W, Yamauchi T, Sugawara A, Li Z, Sun F, Li X, Li C, He A, Du Y, Wang T, Zhao C, Li H, Chi X, Zhang H, Liu Y, Li C, Duo S, Yin M, Shen H, Belmonte JCI, Deng H. Derivation of Pluripotent Stem Cells with In Vivo Embryonic and Extraembryonic Potency. Cell. 2017;169:243–257.e25. doi: 10.1016/j.cell.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vallier L, Alexander M, Pedersen RA. Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J Cell Sci. 2005;118:4495–4509. doi: 10.1242/jcs.02553. [DOI] [PubMed] [Google Scholar]
- 17.Chen G, Gulbranson DR, Hou Z, Bolin JM, Ruotti V, Probasco MD, Smuga-Otto K, Howden SE, Diol NR, Propson NE, Wagner R, Lee GO, Antosiewicz-Bourget J, Teng JM, Thomson JA. Chemically defined conditions for human iPSC derivation and culture. Nat Methods. 2011;8:424–429. doi: 10.1038/nmeth.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.International Stem Cell Initiative Consortium. Akopian V, Andrews PW, Beil S, Benvenisty N, Brehm J, Christie M, Ford A, Fox V, Gokhale PJ, Healy L, Holm F, Hovatta O, Knowles BB, Ludwig TE, McKay RD, Miyazaki T, Nakatsuji N, Oh SK, Pera MF, Rossant J, Stacey GN, Suemori H. Comparison of defined culture systems for feeder cell free propagation of human embryonic stem cells. In Vitro Cell Dev Biol Anim. 2010;46:247–258. doi: 10.1007/s11626-010-9297-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lanner F, Rossant J. The role of FGF/Erk signaling in pluripotent cells. Development. 2010;137:3351–3360. doi: 10.1242/dev.050146. [DOI] [PubMed] [Google Scholar]
- 20.Xu RH, Peck RM, Li DS, Feng X, Ludwig T, Thomson JA. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nat Methods. 2005;2:185–190. doi: 10.1038/nmeth744. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Lin G, Martins-Taylor K, Zeng H, Xu RH. Inhibition of caspase-mediated anoikis is critical for basic fibroblast growth factor-sustained culture of human pluripotent stem cells. J Biol Chem. 2009;284:34054–34064. doi: 10.1074/jbc.M109.052290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- 23.Wang L, Schulz TC, Sherrer ES, Dauphin DS, Shin S, Nelson AM, Ware CB, Zhan M, Song CZ, Chen X, Brimble SN, McLean A, Galeano MJ, Uhl EW, D'Amour KA, Chesnut JD, Rao MS, Blau CA, Robins AJ. Self-renewal of human embryonic stem cells requires insulin-like growth factor-1 receptor and ERBB2 receptor signaling. Blood. 2007;110:4111–4119. doi: 10.1182/blood-2007-03-082586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Avery K, Avery S, Shepherd J, Heath PR, Moore H. Sphingosine-1-phosphate mediates transcriptional regulation of key targets associated with survival, proliferation, and pluripotency in human embryonic stem cells. Stem Cells Dev. 2008;17:1195–1205. doi: 10.1089/scd.2008.0063. [DOI] [PubMed] [Google Scholar]
- 25.Wong RC, Pera MF, Pébay A. Maintenance of human embryonic stem cells by sphingosine-1-phosphate and platelet-derived growth factor. Methods Mol Biol. 2012;874:167–175. doi: 10.1007/978-1-61779-800-9_13. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Powell S, Brunette E, Lebkowski J, Mandalam R. Expansion of human embryonic stem cells in defined serum-free medium devoid of animal-derived products. Biotechnol Bioeng. 2005;91:688–698. doi: 10.1002/bit.20536. [DOI] [PubMed] [Google Scholar]
- 27.Levenstein ME, Berggren WT, Lee JE, Conard KR, Llanas RA, Wagner RJ, Smith LM, Thomson JA. Secreted proteoglycans directly mediate human embryonic stem cell-basic fibroblast growth factor 2 interactions critical for proliferation. Stem Cells. 2008;26:3099–3107. doi: 10.1634/stemcells.2007-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen G, Gulbranson DR, Yu P, Hou Z, Thomson JA. Thermal stability of fibroblast growth factor protein is a determinant factor in regulating self-renewal, differentiation, and reprogramming in human pluripotent stem cells. Stem Cells. 2012;30:623–630. doi: 10.1002/stem.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pébay A, Wong RC, Pitson SM, Wolvetang EJ, Peh GS, Filipczyk A, Koh KL, Tellis I, Nguyen LT, Pera MF. Essential roles of sphingosine-1-phosphate and platelet-derived growth factor in the maintenance of human embryonic stem cells. Stem Cells. 2005;23:1541–1548. doi: 10.1634/stemcells.2004-0338. [DOI] [PubMed] [Google Scholar]
- 30.Ludwig TE, Bergendahl V, Levenstein ME, Yu J, Probasco MD, Thomson JA. Feeder-independent culture of human embryonic stem cells. Nat Methods. 2006;3:637–646. doi: 10.1038/nmeth902. [DOI] [PubMed] [Google Scholar]
- 31.Xu RH, Chen X, Li DS, Li R, Addicks GC, Glennon C, Zwaka TP, Thomson JA. BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nat Biotechnol. 2002;20:1261–1264. doi: 10.1038/nbt761. [DOI] [PubMed] [Google Scholar]
- 32.Davidson KC, Adams AM, Goodson JM, McDonald CE, Potter JC, Berndt JD, Biechele TL, Taylor RJ, Moon RT. Wnt/β-catenin signaling promotes differentiation, not self-renewal, of human embryonic stem cells and is repressed by Oct4. Proc Natl Acad Sci U S A. 2012;109:4485–4490. doi: 10.1073/pnas.1118777109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu J, Hou R, Booth CJ, Yang SH, Snyder M. Defined culture conditions of human embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103:5688–5693. doi: 10.1073/pnas.0601383103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davis LA, Zur Nieden NI. Mesodermal fate decisions of a stem cell: the Wnt switch. Cell Mol Life Sci. 2008;65:2658–2674. doi: 10.1007/s00018-008-8042-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elkouby YM, Frank D. 2010. [Google Scholar]
- 36.Moldes M, Zuo Y, Morrison RF, Silva D, Park BH, Liu J, Farmer SR. Peroxisome-proliferator-activated receptor gamma suppresses Wnt/beta-catenin signalling during adipogenesis. Biochem J. 2003;376:607–613. doi: 10.1042/BJ20030426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chung Y, Klimanskaya I, Becker S, Li T, Maserati M, Lu SJ, Zdravkovic T, Ilic D, Genbacev O, Fisher S, Krtolica A, Lanza R. Human embryonic stem cell lines generated without embryo destruction. Cell Stem Cell. 2008;2:113–117. doi: 10.1016/j.stem.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 38.Tachibana M, Amato P, Sparman M, Gutierrez NM, Tippner-Hedges R, Ma H, Kang E, Fulati A, Lee HS, Sritanaudomchai H, Masterson K, Larson J, Eaton D, Sadler-Fredd K, Battaglia D, Lee D, Wu D, Jensen J, Patton P, Gokhale S, Stouffer RL, Wolf D, Mitalipov S. Human embryonic stem cells derived by somatic cell nuclear transfer. Cell. 2013;153:1228–1238. doi: 10.1016/j.cell.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith AG. Embryo-derived stem cells: of mice and men. Annu Rev Cell Dev Biol. 2001;17:435–462. doi: 10.1146/annurev.cellbio.17.1.435. [DOI] [PubMed] [Google Scholar]
- 40.Smith AG, Heath JK, Donaldson DD, Wong GG, Moreau J, Stahl M, Rogers D. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature. 1988;336:688–690. doi: 10.1038/336688a0. [DOI] [PubMed] [Google Scholar]
- 41.Ying QL, Nichols J, Chambers I, Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281–292. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]
- 42.Ying QL, Wray J, Nichols J, Batlle-Morera L, Doble B, Woodgett J, Cohen P, Smith A. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martello G, Smith A. The nature of embryonic stem cells. Annu Rev Cell Dev Biol. 2014;30:647–675. doi: 10.1146/annurev-cellbio-100913-013116. [DOI] [PubMed] [Google Scholar]
- 44.Blakeley P, Fogarty NM, del Valle I, Wamaitha SE, Hu TX, Elder K, Snell P, Christie L, Robson P, Niakan KK. Defining the three cell lineages of the human blastocyst by single-cell RNA-seq. Development. 2015;142:3151–3165. doi: 10.1242/dev.123547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hanna J, Cheng AW, Saha K, Kim J, Lengner CJ, Soldner F, Cassady JP, Muffat J, Carey BW, Jaenisch R. Human embryonic stem cells with biological and epigenetic characteristics similar to those of mouse ESCs. Proc Natl Acad Sci USA. 2010;107:9222–9227. doi: 10.1073/pnas.1004584107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Theunissen TW, Friedli M, He Y, Planet E, O'Neil RC, Markoulaki S, Pontis J, Wang H, Iouranova A, Imbeault M, Duc J, Cohen MA, Wert KJ, Castanon R, Zhang Z, Huang Y, Nery JR, Drotar J, Lungjangwa T, Trono D, Ecker JR, Jaenisch R. Molecular Criteria for Defining the Naive Human Pluripotent State. Cell Stem Cell. 2016;19:502–515. doi: 10.1016/j.stem.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guo G, von Meyenn F, Santos F, Chen Y, Reik W, Bertone P, Smith A, Nichols J. Naive Pluripotent Stem Cells Derived Directly from Isolated Cells of the Human Inner Cell Mass. Stem Cell Reports. 2016;6:437–446. doi: 10.1016/j.stemcr.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gafni O, Weinberger L, Mansour AA, Manor YS, Chomsky E, Ben-Yosef D, Kalma Y, Viukov S, Maza I, Zviran A, Rais Y, Shipony Z, Mukamel Z, Krupalnik V, Zerbib M, Geula S, Caspi I, Schneir D, Shwartz T, Gilad S, Amann-Zalcenstein D, Benjamin S, Amit I, Tanay A, Massarwa R, Novershtern N, Hanna JH. Derivation of novel human ground state naive pluripotent stem cells. Nature. 2013;504:282–286. doi: 10.1038/nature12745. [DOI] [PubMed] [Google Scholar]
- 49.Chan YS, Göke J, Ng JH, Lu X, Gonzales KA, Tan CP, Tng WQ, Hong ZZ, Lim YS, Ng HH. Induction of a human pluripotent state with distinct regulatory circuitry that resembles preimplantation epiblast. Cell Stem Cell. 2013;13:663–675. doi: 10.1016/j.stem.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 50.Valamehr B, Robinson M, Abujarour R, Rezner B, Vranceanu F, Le T, Medcalf A, Lee TT, Fitch M, Robbins D, Flynn P. Platform for induction and maintenance of transgene-free hiPSCs resembling ground state pluripotent stem cells. Stem Cell Reports. 2014;2:366–381. doi: 10.1016/j.stemcr.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ware CB, Nelson AM, Mecham B, Hesson J, Zhou W, Jonlin EC, Jimenez-Caliani AJ, Deng X, Cavanaugh C, Cook S, Tesar PJ, Okada J, Margaretha L, Sperber H, Choi M, Blau CA, Treuting PM, Hawkins RD, Cirulli V, Ruohola-Baker H. Derivation of naive human embryonic stem cells. Proc Natl Acad Sci U S A. 2014;111:4484–4489. doi: 10.1073/pnas.1319738111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Y, Song Z, Zhao Y, Qin H, Cai J, Zhang H, Yu T, Jiang S, Wang G, Ding M, Deng H. A novel chemical-defined medium with bFGF and N2B27 supplements supports undifferentiated growth in human embryonic stem cells. Biochem Biophys Res Commun. 2006;346:131–139. doi: 10.1016/j.bbrc.2006.05.086. [DOI] [PubMed] [Google Scholar]
- 53.Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, Carpenter MK. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- 54.Kleinman HK, McGarvey ML, Hassell JR, Star VL, Cannon FB, Laurie GW, Martin GR. Basement membrane complexes with biological activity. Biochemistry. 1986;25:312–318. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- 55.Kleinman HK. Preparation of basement membrane components from EHS tumors. Curr Protoc Cell Biol. 2001;Chapter 10:Unit 10.2. doi: 10.1002/0471143030.cb1002s00. [DOI] [PubMed] [Google Scholar]
- 56.Braam SR, Zeinstra L, Litjens S, Ward-van Oostwaard D, van den Brink S, van Laake L, Lebrin F, Kats P, Hochstenbach R, Passier R, Sonnenberg A, Mummery CL. Recombinant vitronectin is a functionally defined substrate that supports human embryonic stem cell self-renewal via alphavbeta5 integrin. Stem Cells. 2008;26:2257–2265. doi: 10.1634/stemcells.2008-0291. [DOI] [PubMed] [Google Scholar]
- 57.Miyazaki T, Futaki S, Suemori H, Taniguchi Y, Yamada M, Kawasaki M, Hayashi M, Kumagai H, Nakatsuji N, Sekiguchi K, Kawase E. Laminin E8 fragments support efficient adhesion and expansion of dissociated human pluripotent stem cells. Nat Commun. 2012;3:1236. doi: 10.1038/ncomms2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pijuan-Galitó S, Tamm C, Schuster J, Sobol M, Forsberg L, Merry CL, Annerén C. Human serum-derived protein removes the need for coating in defined human pluripotent stem cell culture. Nat Commun. 2016;7:12170. doi: 10.1038/ncomms12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rodin S, Domogatskaya A, Ström S, Hansson EM, Chien KR, Inzunza J, Hovatta O, Tryggvason K. Long-term self-renewal of human pluripotent stem cells on human recombinant laminin-511. Nat Biotechnol. 2010;28:611–615. doi: 10.1038/nbt.1620. [DOI] [PubMed] [Google Scholar]
- 60.Li L, Bennett SA, Wang L. Role of E-cadherin and other cell adhesion molecules in survival and differentiation of human pluripotent stem cells. Cell Adh Migr. 2012;6:59–70. doi: 10.4161/cam.19583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nagaoka M, Si-Tayeb K, Akaike T, Duncan SA. Culture of human pluripotent stem cells using completely defined conditions on a recombinant E-cadherin substratum. BMC Dev Biol. 2010;10:60. doi: 10.1186/1471-213X-10-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rodin S, Antonsson L, Niaudet C, Simonson OE, Salmela E, Hansson EM, Domogatskaya A, Xiao Z, Damdimopoulou P, Sheikhi M, Inzunza J, Nilsson AS, Baker D, Kuiper R, Sun Y, Blennow E, Nordenskjöld M, Grinnemo KH, Kere J, Betsholtz C, Hovatta O, Tryggvason K. Clonal culturing of human embryonic stem cells on laminin-521/E-cadherin matrix in defined and xeno-free environment. Nat Commun. 2014;5:3195. doi: 10.1038/ncomms4195. [DOI] [PubMed] [Google Scholar]
- 63.Melkoumian Z, Weber JL, Weber DM, Fadeev AG, Zhou Y, Dolley-Sonneville P, Yang J, Qiu L, Priest CA, Shogbon C, Martin AW, Nelson J, West P, Beltzer JP, Pal S, Brandenberger R. Synthetic peptide-acrylate surfaces for long-term self-renewal and cardiomyocyte differentiation of human embryonic stem cells. Nat Biotechnol. 2010;28:606–610. doi: 10.1038/nbt.1629. [DOI] [PubMed] [Google Scholar]
- 64.Kolhar P, Kotamraju VR, Hikita ST, Clegg DO, Ruoslahti E. Synthetic surfaces for human embryonic stem cell culture. J Biotechnol. 2010;146:143–146. doi: 10.1016/j.jbiotec.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 65.Cowan CA, Klimanskaya I, McMahon J, Atienza J, Witmyer J, Zucker JP, Wang S, Morton CC, McMahon AP, Powers D, Melton DA. Derivation of embryonic stem-cell lines from human blastocysts. N Engl J Med. 2004;350:1353–1356. doi: 10.1056/NEJMsr040330. [DOI] [PubMed] [Google Scholar]
- 66.Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Nishikawa S, Muguruma K, Sasai Y. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- 67.Chen G, Hou Z, Gulbranson DR, Thomson JA. Actin-myosin contractility is responsible for the reduced viability of dissociated human embryonic stem cells. Cell Stem Cell. 2010;7:240–248. doi: 10.1016/j.stem.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meng Y, Ren Z, Xu F, Zhou X, Song C, Wang VY, Liu W, Lu L, Thomson JA, Chen G. Nicotinamide Promotes Cell Survival and Differentiation as Kinase Inhibitor in Human Pluripotent Stem Cells. Stem Cell Reports. 2018;11:1347–1356. doi: 10.1016/j.stemcr.2018.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Walker A, Su H, Conti MA, Harb N, Adelstein RS, Sato N. Non-muscle myosin II regulates survival threshold of pluripotent stem cells. Nat Commun. 2010;1:71. doi: 10.1038/ncomms1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.T'joen V, Declercq H, Cornelissen M. Expansion of human embryonic stem cells: a comparative study. Cell Prolif. 2011;44:462–476. doi: 10.1111/j.1365-2184.2011.00773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bajpai R, Lesperance J, Kim M, Terskikh AV. Efficient propagation of single cells Accutase-dissociated human embryonic stem cells. Mol Reprod Dev. 2008;75:818–827. doi: 10.1002/mrd.20809. [DOI] [PubMed] [Google Scholar]
- 72.Beers J, Gulbranson DR, George N, Siniscalchi LI, Jones J, Thomson JA, Chen G. Passaging and colony expansion of human pluripotent stem cells by enzyme-free dissociation in chemically defined culture conditions. Nat Protoc. 2012;7:2029–2040. doi: 10.1038/nprot.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nie Y, Walsh P, Clarke DL, Rowley JA, Fellner T. Scalable passaging of adherent human pluripotent stem cells. PLoS One. 2014;9:e88012. doi: 10.1371/journal.pone.0088012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Claassen DA, Desler MM, Rizzino A. ROCK inhibition enhances the recovery and growth of cryopreserved human embryonic stem cells and human induced pluripotent stem cells. Mol Reprod Dev. 2009;76:722–732. doi: 10.1002/mrd.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Martin-Ibañez R, Unger C, Strömberg A, Baker D, Canals JM, Hovatta O. Novel cryopreservation method for dissociated human embryonic stem cells in the presence of a ROCK inhibitor. Hum Reprod. 2008;23:2744–2754. doi: 10.1093/humrep/den316. [DOI] [PubMed] [Google Scholar]
- 76.Santos DP, Kiskinis E, Eggan K, Merkle FT. Comprehensive Protocols for CRISPR/Cas9-based Gene Editing in Human Pluripotent Stem Cells. Curr Protoc Stem Cell Biol. 2016;38:5B.6.1–5B.6.60. doi: 10.1002/cpsc.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zou J, Maeder ML, Mali P, Pruett-Miller SM, Thibodeau-Beganny S, Chou BK, Chen G, Ye Z, Park IH, Daley GQ, Porteus MH, Joung JK, Cheng L. Gene targeting of a disease-related gene in human induced pluripotent stem and embryonic stem cells. Cell Stem Cell. 2009;5:97–110. doi: 10.1016/j.stem.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yao T, Asayama Y. Animal-cell culture media: History, characteristics, and current issues. Reprod Med Biol. 2017;16:99–117. doi: 10.1002/rmb2.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Amit M, Carpenter MK, Inokuma MS, Chiu CP, Harris CP, Waknitz MA, Itskovitz-Eldor J, Thomson JA. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol. 2000;227:271–278. doi: 10.1006/dbio.2000.9912. [DOI] [PubMed] [Google Scholar]
- 80.Ludwig TE, Levenstein ME, Jones JM, Berggren WT, Mitchen ER, Frane JL, Crandall LJ, Daigh CA, Conard KR, Piekarczyk MS, Llanas RA, Thomson JA. Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol. 2006;24:185–187. doi: 10.1038/nbt1177. [DOI] [PubMed] [Google Scholar]
- 81.Lee P, Wu X. Review: modifications of human serum albumin and their binding effect. Curr Pharm Des. 2015;21:1862–1865. doi: 10.2174/1381612821666150302115025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Merlot AM, Kalinowski DS, Richardson DR. Unraveling the mysteries of serum albumin-more than just a serum protein. Front Physiol. 2014;5:299. doi: 10.3389/fphys.2014.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang Y, Chou BK, Dowey S, He C, Gerecht S, Cheng L. Scalable expansion of human induced pluripotent stem cells in the defined xeno-free E8 medium under adherent and suspension culture conditions. Stem Cell Res. 2013;11:1103–1116. doi: 10.1016/j.scr.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Uenishi G, Theisen D, Lee JH, Kumar A, Raymond M, Vodyanik M, Swanson S, Stewart R, Thomson J, Slukvin I. Tenascin C promotes hematoendothelial development and T lymphoid commitment from human pluripotent stem cells in chemically defined conditions. Stem Cell Reports. 2014;3:1073–1084. doi: 10.1016/j.stemcr.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Burridge PW, Holmström A, Wu JC. Chemically Defined Culture and Cardiomyocyte Differentiation of Human Pluripotent Stem Cells. Curr Protoc Hum Genet. 2015;87:21.3.1–21.315. doi: 10.1002/0471142905.hg2103s87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lindborg BA, Brekke JH, Vegoe AL, Ulrich CB, Haider KT, Subramaniam S, Venhuizen SL, Eide CR, Orchard PJ, Chen W, Wang Q, Pelaez F, Scott CM, Kokkoli E, Keirstead SA, Dutton JR, Tolar J, O'Brien TD. Rapid Induction of Cerebral Organoids From Human Induced Pluripotent Stem Cells Using a Chemically Defined Hydrogel and Defined Cell Culture Medium. Stem Cells Transl Med. 2016;5:970–979. doi: 10.5966/sctm.2015-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu W, Ren Z, Lu K, Song C, Cheung ECW, Zhou Z, Chen G. The Suppression of Medium Acidosis Improves the Maintenance and Differentiation of Human Pluripotent Stem Cells at High Density in Defined Cell Culture Medium. Int J Biol Sci. 2018;14:485–496. doi: 10.7150/ijbs.24681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lotz S, Goderie S, Tokas N, Hirsch SE, Ahmad F, Corneo B, Le S, Banerjee A, Kane RS, Stern JH, Temple S, Fasano CA. Sustained levels of FGF2 maintain undifferentiated stem cell cultures with biweekly feeding. PLoS One. 2013;8:e56289. doi: 10.1371/journal.pone.0056289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang H, Badur MG, Divakaruni AS, Parker SJ, Jäger C, Hiller K, Murphy AN, Metallo CM. Distinct Metabolic States Can Support Self-Renewal and Lipogenesis in Human Pluripotent Stem Cells under Different Culture Conditions. Cell Rep. 2016;16:1536–1547. doi: 10.1016/j.celrep.2016.06.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McKee C, Chaudhry GR. Advances and challenges in stem cell culture. Colloids Surf B Biointerfaces. 2017;159:62–77. doi: 10.1016/j.colsurfb.2017.07.051. [DOI] [PubMed] [Google Scholar]
- 91.Mirbagheri M, Adibnia V, Hughes BR, Waldman SD, Banquy X, Hwang DK. Advanced cell culture platforms: a growing quest for emulating natural tissues. Materials Horizons. 2019;6:45–71. [Google Scholar]
- 92.Pettinato G, Wen X, Zhang N. Engineering Strategies for the Formation of Embryoid Bodies from Human Pluripotent Stem Cells. Stem Cells Dev. 2015;24:1595–1609. doi: 10.1089/scd.2014.0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lin Y, Chen G. Embryoid body formation from human pluripotent stem cells in chemically defined E8 media, in StemBook. 2008: Cambridge (MA) [PubMed] [Google Scholar]
- 94.Adil MM, Schaffer DV. Expansion of human pluripotent stem cells. Curr Opin Chem Eng. 2017;15:24–35. [Google Scholar]
- 95.Langhans SA. Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front Pharmacol. 2018;9:6. doi: 10.3389/fphar.2018.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kropp C, Massai D, Zweigerdt R. Progress and challenges in large-scale expansion of human pluripotent stem cells. Process Biochem. 2017;59:244–254. [Google Scholar]
- 97.Li X, Ma R, Gu Q, Liang L, Wang L, Zhang Y, Wang X, Liu X, Li Z, Fang J, Wu J, Wang Y, Li W, Hu B, Wang L, Zhou Q, Hao J. A fully defined static suspension culture system for large-scale human embryonic stem cell production. Cell Death Dis. 2018;9:892. doi: 10.1038/s41419-018-0863-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kropp C, Kempf H, Halloin C, Robles-Diaz D, Franke A, Scheper T, Kinast K, Knorpp T, Joos TO, Haverich A, Martin U, Zweigerdt R, Olmer R. Impact of Feeding Strategies on the Scalable Expansion of Human Pluripotent Stem Cells in Single-Use Stirred Tank Bioreactors. Stem Cells Transl Med. 2016;5:1289–1301. doi: 10.5966/sctm.2015-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fergus J, Quintanilla R, Lakshmipathy U. Characterizing Pluripotent Stem Cells Using the TaqMan® hPSC Scorecard(TM) Panel. Methods Mol Biol. 2016;1307:25–37. doi: 10.1007/7651_2014_109. [DOI] [PubMed] [Google Scholar]
- 100.Müller FJ, Brändl B, Loring JF. Assessment of human pluripotent stem cells with PluriTest 2012. [PubMed] [Google Scholar]
- 101.Avior Y, Biancotti JC, Benvenisty N. TeratoScore: Assessing the Differentiation Potential of Human Pluripotent Stem Cells by Quantitative Expression Analysis of Teratomas. Stem Cell Reports. 2015;4:967–974. doi: 10.1016/j.stemcr.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Editorial. Method of the Year 2017: Organoids. Nature Methods. 2018;15(1):p. 1–1. [Google Scholar]
- 103.Mazerik J.N, Becker S, Sieving P.A. Sieving, 3-D retina organoids: Building platforms for therapies of the future. Cell Medicine. 2018;10 doi: 10.1177/2155179018773758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li Y, Muffat J, Omer A, Bosch I, Lancaster MA, Sur M, Gehrke L, Knoblich JA, Jaenisch R. Induction of Expansion and Folding in Human Cerebral Organoids. Cell Stem Cell. 2017;20:385–396.e3. doi: 10.1016/j.stem.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jiang B, Yan L, Miao Z, Li E, Wong KH, Xu RH. Spheroidal formation preserves human stem cells for prolonged time under ambient conditions for facile storage and transportation. Biomaterials. 2017;133:275–286. doi: 10.1016/j.biomaterials.2017.03.050. [DOI] [PubMed] [Google Scholar]
- 106.Kraehenbuehl TP, Langer R, Ferreira LS. Three-dimensional biomaterials for the study of human pluripotent stem cells. Nat Methods. 2011;8:731–736. doi: 10.1038/nmeth.1671. [DOI] [PubMed] [Google Scholar]
- 107.Gerecht S, Burdick JA, Ferreira LS, Townsend SA, Langer R, Vunjak-Novakovic G. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proc Natl Acad Sci USA. 2007;104:11298–11303. doi: 10.1073/pnas.0703723104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lim HJ, Mosley MC, Kurosu Y, Smith Callahan LA. Concentration dependent survival and neural differentiation of murine embryonic stem cells cultured on polyethylene glycol dimethacrylate hydrogels possessing a continuous concentration gradient of n-cadherin derived peptide His-Ala-Val-Asp-Lle. Acta Biomater. 2017;56:153–160. doi: 10.1016/j.actbio.2016.11.063. [DOI] [PubMed] [Google Scholar]
- 109.Ishihara K, Ranga A, Lutolf MP, Tanaka EM, Meinhardt A. Reconstitution of a Patterned Neural Tube from Single Mouse Embryonic Stem Cells. Methods Mol Biol. 2017;1597:43–55. doi: 10.1007/978-1-4939-6949-4_4. [DOI] [PubMed] [Google Scholar]
- 110.Serra M, Correia C, Malpique R, Brito C, Jensen J, Bjorquist P, Carrondo MJ, Alves PM. Microencapsulation technology: a powerful tool for integrating expansion and cryopreservation of human embryonic stem cells. PLoS One. 2011;6:e23212. doi: 10.1371/journal.pone.0023212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kamei K, Guo S, Yu ZT, Takahashi H, Gschweng E, Suh C, Wang X, Tang J, McLaughlin J, Witte ON, Lee KB, Tseng HR. An integrated microfluidic culture device for quantitative analysis of human embryonic stem cells. Lab Chip. 2009;9:555–563. doi: 10.1039/b809105f. [DOI] [PubMed] [Google Scholar]
- 112.Villa-Diaz LG, Torisawa YS, Uchida T, Ding J, Nogueira-de-Souza NC, O'Shea KS, Takayama S, Smith GD. Microfluidic culture of single human embryonic stem cell colonies. Lab Chip. 2009;9:1749–1755. doi: 10.1039/b820380f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sikorski DJ, Caron NJ, VanInsberghe M, Zahn H, Eaves CJ, Piret JM, Hansen CL. Clonal analysis of individual human embryonic stem cell differentiation patterns in microfluidic cultures. Biotechnol J. 2015;10:1546–1554. doi: 10.1002/biot.201500035. [DOI] [PubMed] [Google Scholar]
- 114.Zhang J, Wei X, Zeng R, Xu F, Li X. Stem cell culture and differentiation in microfluidic devices toward organ-on-a-chip. Future Sci OA. 2017;3:FSO187. doi: 10.4155/fsoa-2016-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Simunovic M, Brivanlou AH. Embryoids, organoids and gastruloids: new approaches to understanding embryogenesis. Development. 2017;144:976–985. doi: 10.1242/dev.143529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Warmflash A, Sorre B, Etoc F, Siggia ED, Brivanlou AH. A method to recapitulate early embryonic spatial patterning in human embryonic stem cells. Nat Methods. 2014;11:847–854. doi: 10.1038/nmeth.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yao S, Chen S, Clark J, Hao E, Beattie GM, Hayek A, Ding S. Long-term self-renewal and directed differentiation of human embryonic stem cells in chemically defined conditions. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:6907–6912. doi: 10.1073/pnas.0602280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Frank S, Zhang M, Scholer HR, Greber B. Small molecule-assisted, line-independent maintenance of human pluripotent stem cells in defined conditions. PloS One. 2012;7:e41958. doi: 10.1371/journal.pone.0041958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Collier AJ, Rugg-Gunn PJ. Identifying Human Naïve Pluripotent Stem Cells - Evaluating State-Specific Reporter Lines and Cell-Surface Markers. Bioessays. 2018;40:e1700239. doi: 10.1002/bies.201700239. [DOI] [PubMed] [Google Scholar]
- 120.Theunissen TW, Powell BE, Wang H, Mitalipova M, Faddah DA, Reddy J, Fan ZP, Maetzel D, Ganz K, Shi L, Lungjangwa T, Imsoonthornruksa S, Stelzer Y, Rangarajan S, D'Alessio A, Zhang J, Gao Q, Dawlaty MM, Young RA, Gray NS, Jaenisch R. Systematic identification of culture conditions for induction and maintenance of naive human pluripotency. Cell stem cell. 2014;15:524–526. doi: 10.1016/j.stem.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Collier AJ, Panula SP, Schell JP, Chovanec P, Plaza Reyes A, Petropoulos S, Corcoran AE, Walker R, Douagi I, Lanner F, Rugg-Gunn PJ. Comprehensive Cell Surface Protein Profiling Identifies Specific Markers of Human Naive and Primed Pluripotent States. Cell Stem Cell. 2017;20:874–890.e7. doi: 10.1016/j.stem.2017.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]