Abstract
Retinal degenerative disorders, such as diabetic retinopathy, retinitis pigmentosa, age-related macular degeneration or glaucoma, represent the most common causes of loss of vision and blindness. In spite of intensive research, treatment options to prevent, stop or cure these diseases are limited. Newer therapeutic approaches are offered by stem cell-based therapy. To date, various types of stem cells have been evaluated in a range of models. Among them, mesenchymal stem/stromal cells (MSCs) derived from bone marrow or adipose tissue and used as autologous cells have been proposed to have the potential to attenuate the negative manifestations of retinal diseases. MSCs delivered to the vicinity of the diseased retina can exert local anti-inflammatory and repair-promoting/regenerative effects on retinal cells. However, MSCs also produce numerous factors that could have negative impacts on retinal regeneration. The secretory activity of MSCs is strongly influenced by the cytokine environment. Therefore, the interactions among the molecules produced by the diseased retina, cytokines secreted by inflammatory cells and factors produced by MSCs will decide the development and propagation of retinal diseases. Here we discuss the interactions among cytokines and other factors in the environment of the diseased retina treated by MSCs, and we present results supporting immunoregulatory and trophic roles of molecules secreted in the vicinity of the retina during MSC-based therapy.
Keywords: Retina, Degenerative diseases, Stem cell therapy, Mesenchymal stem cells, Cytokines, Growth factors
Core tip: Cell-based therapy using autologous mesenchymal stem cells represents a perspective approach for the treatment of so far incurable degenerative retinal diseases. However, the therapeutic potential of mesenchymal stem cells strongly depends on the cytokine environment, where these cells are delivered. In this study, we discuss recent knowledge regarding the interactions and interplay among cytokines produced by cells of the diseased retina, inflammatory immune cells and therapeutically administered mesenchymal stem cells. We suggest that these interactions among cytokines and growth factors occurring in the microenvironment of the diseased retina could be critical for the outcome of the stem cell-based therapy for retinal disorders.
RETINAL DEGENERATIVE DISORDERS
The retina is a highly specialized tissue. This structure is composed of several layers of functionally different cell types that are inter-connected. Disease or a damage to any particular cell or layer has secondary effects on the surrounding cell types, and the progression of retinal damage results in retinal degenerative disorders. Inherited and age-related retinal degenerative disorders represent the most common cause of reduced vision and blindness. Among the most common retinal degenerative diseases are age-related macular degeneration, diabetic retinopathy, retinitis pigmentosa and glaucoma. These disorders have different etiology, various causes and starting mechanisms, and distinct retinal cell types are affected. However, they are all associated with chronic inflammation, immune cell infiltration and enhanced cytokine secretion.
Because all retinal degenerative diseases in the advanced stages are associated with a loss or a function damage of specialized retinal cells, their replacement or a support of the surviving cells would be the only effective approach to stop spreading of the disease or even return the visual function of the retina. Currently, a direct trans-plantation of healthy retinal explants is strongly limited. Therefore, the transfer of stem cells that can support survival and functioning of the remaining retinal cells, or even replace missing cells, offers a perspective approach to stop and treat retinal degenerative diseases. Recent experimental and preclinical data suggest a great potential of such cell therapies for the treatment of so far incurable ophthalmological diseases.
TYPES OF STEM CELLS FOR RETINA REGENERATION
Stem cells are characterized by their permanent growth in vitro and by their ability to differentiate or even transdifferentiate into other cell types. According to their origin, stem cells can be divided into embryonic stem cells and adult stem cells. The third type of stem cells is artificially prepared from any somatic cell by reprogramming its properties to a pluripotent state. For this intervention, genes associated with stemness are introduced into somatic cells, which gain some characteristics of stem cells. These cells, called induced pluripotent stem cells, have attracted a lot of attention as a possible source of autologous stem cells that could avoid immune rejection. However, these genetically modified cells have proved to be immunogenic even in an autologous host[1], and their often uncontrolled growth and the formation of teratomas limit their clinical potential. Similarly, the use of embryonic stem cells, which have a high differentiation potential and can be relatively easily differentiated into numerous different cell types, have ethical limitations associated with their origin. They are always used as allogeneic cells, and they often suffer from uncontrolled growth. In comparison with embryonic stem cells or induced pluripotent stem cells, adult stem cells have a lesser differentiation potential but can be obtained as autologous (patient’s own) cells, do not form teratomas or cancers and often fulfil the demands for use in regenerative medicine. Among the numerous types of adult stem cells, the highest potential have been proposed from mesenchymal stem/stromal cells (MSCs), which can be obtained relatively easily from the patient, propagated in vitro, if needed differentiated ex vivo and finally used as autologous therapeutic cells.
Numerous experimental studies have shown the beneficial effects of MSCs in the treatment of ophthalmological diseases. Intravitreal or subretinal transplantation of MSCs significantly delayed retinal degeneration and supported normal retinal functions[2-4]. In addition, in other types of eye diseases, such as experimental autoimmune uveitis[5,6], dry eye syndrome[7] or corneal epithelium damage[8,9], the therapeutic effects of MSCs on tissue regeneration have been demonstrated. The ability of MSCs to support interaction between retinal cells with neurons of optic nerve has been also documented. For example, Mead et al[10] showed that dental pulp stem cells promoted neuroprotection and axon regeneration after optic nerve injury. In other models, MSCs transplanted to the damaged area of the retina differentiated into retinal nerve cells[11] and promoted regeneration in a rat optic tract model[12].
MSCs
MSCs represent a heterogenous population of non-hematopoietic cells with multi-lineage differentiation potential. Originally, these cells were described as spindle shaped cells derived from bone marrow that adhere to plastic and form fibrocyte-like colonies[13]. For therapeutic purposes, MSCs are isolated mainly from the bone marrow or adipose tissue, but they can be obtained from nearly all tissues of the body. According to the International Society for Cellular Therapy, human MSCs are characterized by their ability to adhere to plastic in standard culture conditions, by their potential to differentiate into adipocytes, chondroblasts and osteoblasts, and by being positive for the surface markers CD105, CD73 and CD90 and negative for CD45, CD34, CD14, CD19 and CD11b[14]. MSCs from different sources (including bone marrow, adipose tissue, umbilical cord blood, etc) possess similar properties[15,16]. Under appropriate conditions, MSCs can be differentiated or even transdifferentiated into different cell types. It has been demonstrated that in the presence of selective chemicals or retinal cells, MSCs can differentiate into cells expressing retinal cell markers and characteristics[17-19]. We have shown that highly purified mouse bone marrow-derived cells fulfilling all the criteria proposed for MSCs and cultured in the presence of retinal cell extract and supernatant from activated T cells (to mimic the inflammatory environment of diseased retina) differentiated into cells expressing rhodopsin, S-antigen, recoverin, retinaldehyde binding protein, calbindin and retinal pigment epithelium (RPE) 65, which are the markers of specialized retinal cells[20]. The ability of MSCs to extensively proliferate, to be expanded in vitro and to differentiate into various cell types makes them attractive targets for regenerative and reparative medical applications. However, before clinical application these cells have to be precisely characterized and their preparation standardized, as it has been recently proposed[21].
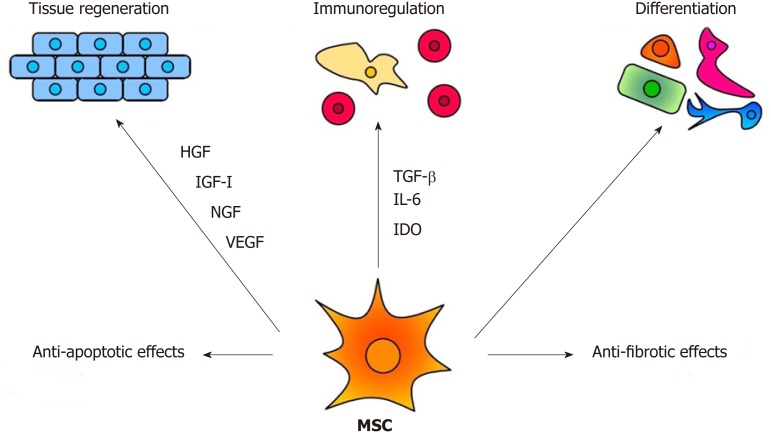
The therapeutic effects of MSCs are mediated by multiple mechanisms, as demonstrated in Figure 1. Among them, the immunomodulatory and secretory properties appear to be the most important. It has been shown that MSCs inhibit T and B cell functions, attenuate production of cytokines, decrease activity of cytotoxic T and NK cells and suppress transplantation, anti-cancer and inflammatory reactions[22,23]. On the other hand, MSCs are potent producers of numerous cytokines and growth factors[24,25]. It has been shown that mouse MSCs spontaneously produce transforming growth factor (TGF-β) and inducible interleukin-6 (IL-6), which are the basic cytokines regulating the development of anti-inflammatory regulatory T cells (Tregs) and pro-inflammatory Th17 cells[25]. The immunomodulatory properties make MSCs a promising tool for the treatment of harmful inflammatory reactions that accompany inherited retinal degenerative diseases and injuries. However, the spectrum and concentrations of immunoregulatory molecules produced by MSCs strongly depend on the cytokine environment[26,27]. This has to be taken into account when MSCs are delivered into the inflammatory environment of the diseased retina.
Figure 1.
The main mechanisms of the therapeutic effect of MSCs. MSC: Mesenchymal stem cell; HGF: Hepatocyte growth factor; IGF-1: Insulin-like growth factor-1; NGF: Nerve growth factor; VEGF: Vascular endothelial growth factor; TGF-β: Transforming growth factor-β; IDO: Indoleamine-2,3-dioxygenase; IL-6: Interleukin-6.
PRODUCTION OF CYTOKINES BY RETINAL CELLS
The retina is composed of a few layers of functionally different cell types that ensure visual acuity and internal homeostasis. The cells of the retina produce numerous cytokines and growth factors that support, in a paracrine mode, the survival of other retinal cells and contribute to the immune privilege of the eye[28]. However, from the very beginning of retinal disease or damage, the spectrum of produced cytokines is significantly changed, and the retina starts to produce elevated levels of molecules that are not produced or only minimally secreted in a steady-state.
It has been shown that increased levels of pro-inflammatory molecules, such as tumor necrosis factor (TNF-α), IL-6 or inducible nitric oxide synthase (iNOS), are found in the retina or aqueous humor of patients with degenerative retinal diseases or in animal models of retinal disorders[29-32]. All of these molecules have a wider spectrum of immunoregulatory activities but generally contribute to the development of a local inflammatory reaction. On the contrary, IL-10, IL-11 or TGF-β, which are also produced by retinal cells[32,33], have anti-inflammatory effects. Therefore, the balance between the production of pro- and anti-inflammatory molecules influences the extent of the inflammation and damage in the diseased retina.
The complexity of the action of individual cytokines is supported by the observation that another pro-inflammatory cytokine, IL-6, that is produced by several retinal cell types, increases the survival of retinal ganglion cells[34]. Eastlake et al[35] have shown that Müller glia cells produce numerous factors, such as granulocyte-growth factor, monocyte chemoattractant protein-1, platelet-derived growth factor-BB, vascular endothelial growth factor (VEGF) or TGF-β2 and that their production is increased in the gliotic retina. Recently, IL-33 produced by Müller cells of the retina was identified as a key regulator of inflammation and photoreceptor degeneration after retinal stress injury[36]. Thus, retinal microglia and RPE cells were proposed as the main sources of the majority of factors with immunomodulatory effects that play a pivotal role in the initiation and propagation of the neurodegenerative processes[37-39]. In addition to their high secretory potential, Müller glia were recently shown to be able to directly restore vision after de novo induction of genesis of rod photoreceptors in mammalian retinas[40]. It has been shown that cells of individual parts of the eye, such as the cornea, ciliary body or retina are able to inhibit the intraocular immune response and to contribute to the immune privilege of the eye[41]. In this respect, we observed that the explants of the mouse retina or the supernatants from the cultures of retinal explants inhibited the production of pro-inflammatory cytokines by activated spleen cells (unpublished results).
In addition to the production of immunoregulatory cytokines, such as TGF-β or IL-6, cells of the retina produce a number of molecules that function in the paracrine mode as growth and trophic factors. Brain-derived neurotrophic factor, ciliary neurotrophic factor, glial cell line-derived neurotrophic factor, nerve growth factor, neurotrophin-3 and basic fibroblast growth factor released by microglia and other retinal cell types have been shown to protect retinal cells and support the survival of photoreceptors[42]. On the contrary, pro-angiogenic factors, such as VEGF A-E, insulin-like growth factor-I (IGF-I), platelet-derived growth factor, placental growth factor, hepatocyte growth factor (HGF) and FGF-2, which can be produced by the retina and act paracrinely, are involved in neovascularization and rather worsen retinal diseases[43,44]. In the opposite way, the RPE cells produce retinal pigment epithelium-derived factor and trombospondin-1 which have been shown to counterbalance angiogenesis and inflammation[45].
Thus, the retina is a producer of numerous cytokines and factors, which act in a protective way, i.e. they inhibit the inflammatory reaction and support the survival and the growth of retinal cells. However, there are a number of cytokines and factors that attract pro-inflammatory cells, enhance inflammation, increase neovas-cularization and contribute to the damage of individual retinal layers. The identification of these factors and understanding of the mechanisms of the interplay among them will increase the efficacy of stem cell therapy for retinal degenerative disorders.
CYTOKINES PRODUCED BY INFLAMMATORY CELLS IN THE DISEASED RETINA
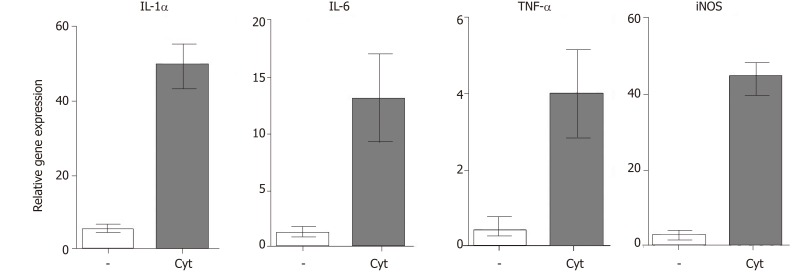
All retinal degenerative diseases are accompanied by a local cytokine disbalance, by increased production of chemokines and by infiltration with inflammatory cells. One of the first inflammatory cell populations detected in the diseased retina is macrophages and neutrophils. The accumulation of macrophages can be associated with elevated levels of vitreal granulocyte-macrophage colony-stimulating factor[46]. Macrophages are producers of numerous cytokines and factors, and their secretory profile depends on their polarization into M1 or M2 population. With the progression of the disease, increased concentrations of pro-inflammatory cytokines and chemokines, such as IL-1, IL-6, IL-8, TNF-α, interferon (IFN)-γ and monocyte chemoattractant protein-1 can be detected in vitreal liquid[47]. The deleterious role of these cytokines for the development and spreading of the disease was directly proved by the observation that intravitreal administration of IL-1β and TNF-α in mice induced vessel dilatation, bleading, retinal edema and microglia upregulation[48]. Kutty et al[49] showed that RPE cells exposed to IL-1, TNF-α and IFN-γ, which are secreted by lymphocytes or macrophages in the retina, decreased the expression of key genes involved in the visual cycle, epithelial morphology and phagocytosis. We observed that intravitreal administration of pro-inflammatory cytokines (such as IL-1α, TNF-α and IFN-γ) induced in the mouse retina an enhanced expression of genes for a large number of cytokines and pro-inflammatory molecules (Figure 2). With the progression of the retinal disease, the infiltration with cells of adaptive immunity can be detected[49,50]. Johnsen-Soriano et al[52] showed cytokine disbalance and significantly increased levels of Th1 cytokines IL-2 and IFN-γ and NO in the retina of diabetic rats. These pro-inflammatory molecules could contribute to the development of diabetic retinopathy. This concept is supported by the observation that transgenic mice expressing IFN-γ in the retina develop ocular inflammation and photoreceptor loss[53].
Figure 2.
Expression of genes for immunoregulatory molecules and growth factors in the retina after intravitreal injection of pro-inflammatory cytokines. Mice were injected intravitreally with pro-inflammatory cytokines IL-lα, IFN-γ and TNF-α (10 ng of each, in a total volume of 4 μL), and the retinas from the control and treated eyes were harvested after 72 h. The expression of genes for IL-lα, IL-6, TNF-α and iNOS was determined in the retinas by real-time PCR.
Thus, cytokines produced by cells infiltrating the diseased retina contribute to the development and spreading of retinal disease. They have additive and synergistic effects with cytokines produced by the diseased retina, and these interactions can further deteriorate or attenuate the disease progression.
CYTOKINES PRODUCED BY MSCS
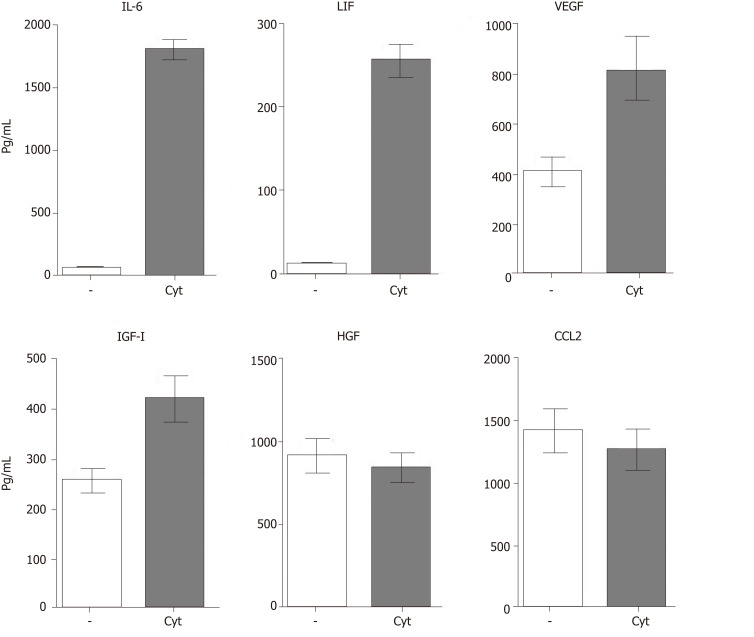
MSC-based therapy has been proposed and tested as a prospective treatment in various models of retinal degenerative diseases[2,3,54-56]. MSCs mediate their therapeutic effect by multiple mechanisms involving immunomodulation, production of trophic factors and a possible differentiation into other cell types. The paracrine trophic effects of molecules secreted by MSCs have recently been suggested as the primary mechanisms of MSC action. Some of these immunomodulatory and trophic factors are produced by MSCs spontaneously (such as HGF or CCL2), while others are secreted only after stimulation (for example IL-6 or LIF) or are secreted in lower quantities spontaneously and in increased levels after stimulation (VEGF or IGF-I) (Figure 3).
Figure 3.
Spontaneous and cytokine induced production of immunoregulatory cytokines and growth factors by mouse mesenchymal stem cells. Mouse mesenchymal stem cells (8 × 104 cells in 1 mL of culture medium) were cultured for 48 h unstimulated (-) or in the presence of 10 ng/mL of IL-1β and TNF-α (Cyt.) The concentrations of IL-6, LIF, VEGF, IGF-I, HGF and CCL2 in supernatants were determined by ELISA. IL-6: Interleukin-6; LIF: Leukemia inhibitory factor; VEGF: Vascular endothelial growth factor; IGF-I: Insulin-like growth factor-I; HGF: hepatocyte growth factor.
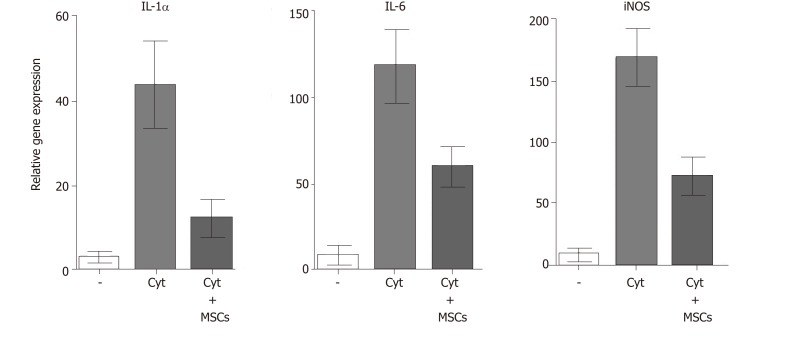
MSCs are well known as cells with a potent immunosuppressive potential. It has been demonstrated that MSCs inhibit the secretion of cytokines and have the ability to suppress transplantation, anti-cancer or inflammatory reactions[23,57]. Mathew et al[58] showed that intravitreal administration of MSCs decreased the intraocular level of inflammatory molecules TNF-α, IL-1β and IL-6 and rescued the retina from ischemic damage. We have shown that MSCs transferred into the damaged ocular surface significantly decreased infiltration with T lymphocytes and attenuated a local inflammatory reaction[8,59]. Furthermore, we observed that the cultivation of retinal explants with pro-inflammatory cytokines IL-1β, TNF-α and IFN-γ induced enhanced expression of genes for numerous cytokines and that this enhanced gene expression was attenuated in the presence of MSCs (Figure 4). The suppressive activity of MSCs could be profitable for the therapeutic inhibition of the inflammatory reaction in the diseased retina. Although different mechanisms can be responsible for the suppression, one of the most important effects could be the spontaneous production of TGF-β by MSCs. TGF-β is a strong negative regulator of immune reactions, and it is the main cytokine determining the development of Tregs which can indirectly contribute to immunosuppression mediated by MSCs[60]. However, in the presence of pro-inflammatory cytokines, MSCs also produce IL-6, which is a pro-inflammatory cytokine and attenuates the development of Tregs. This plasticity of MSCs should be kept in the mind when MSCs are delivered into the inflamed site with the aim to attenuate the inflammation.
Figure 4.
The expression of genes for pro-inflammatory molecules in stimulated retinal explants and suppression of the gene expression by MSCs. Retinal explants were cultured for 48 h untreated (-), stimulated with IL-lα, IFN-γ and TNF-α (Cyt.) or stimulated with the cytokines in cultures containing MSCs (3 × 104 in 1 mL). The expression of genes for IL-lα, IL-6 and iNOS in retinal explants was determined by real-time PCR. MSC: Mesenchymal stem cell; IL-6: Interleukin-6; IL-lα: Interleukin-lα; iNOS: Inducible nitrox oxide synthase; MSC: Mesenchymal stem cell.
In addition to the production of immunoregulatory cytokines, MSCs are also potent producers of numerous growth and trophic factors, such as HGF, LIF, VEGF, IGF-I, nerve growth factor, brain-derived neurotrophic factor, CDTF, glial cell line-derived neurotrophic factor or platelet-derived growth factor[56,61,62]. After the application of MSCs into the eye, the factors produced by MSCs support the survival and viability of various types of retinal cells[2,3]. Ezquer et al[63] showed that intravitreal injection of MSCs in diabetic mice increased intraocular levels of nerve growth factor, basic fibroblast growth factor and glial cell line-derived neurotrophic factor and triggered an effective cytoprotective microenvironment. The factors produced by MSCs have a wider spectrum of trophic effects. For example, it has been shown that HGF supports the growth and differentiation of numerous cell types[64], LIF is a highly pleiotropic factor promoting cell differentiation and tissue growth[65], and IGF-I is also a pleiotropic factor with multiple effects on cell differentiation, survival and growth[66].
The angiogenic effects of VEGF are also well known[67]. These molecules and numerous other growth and trophic factors produced by MSCs can contribute to the regeneration of the diseased retina. However, some of these factors have both positive and negative impacts on the diseased retinal tissue. For example, VEGF supports tissue healing by stimulating the formation of blood vessels, but inadequate neovascularization can contribute to the development of some types of retinal degeneration[67]. Thus, local concentrations of particular factors decide their effects, and the stage and extent of the disease are very important for the formation of the cytokine microenvironment and for therapeutic possibilities.
CONCLUSION
With the persistent absence of treatment protocols for retinal degenerative diseases, stem cell-based therapy offers a promising approach. Among the various stem cell types, MSCs provide several advantages. They can be prepared in a sufficient number as autologous stem cells and administered repeatedly, and there is no danger of uncontrolled cell growth or a formation of teratomas. Numerous experimental therapeutic protocols and clinical studies using intravitreal administration of MSCs have demonstrated the safety of this therapy without any harmful side effects[68,69].
Retinal degenerative diseases are associated with local inflammation, cytokine disbalance and a loss of specialized retinal cells, and MSCs can display therapeutic effects for all of these dysfunctions by multiple mechanisms. They are the producers of numerous growth and trophic factors that support the survival and growth of the remaining retinal cells. They also possess potent immunosuppressive properties to attenuate inflammatory reactions and have the ability to differentiate into many other cell types. It has been shown that in the presence of retinal cells, supernatant from cultures of retinal cells or in the presence of retinal cell extracts MSCs differentiate into cells expressing genes and markers typical for retinal cells[18,20,70].
While there is abundant data from various experimental models that demonstrate the positive effects of intraocularly administered MSCs on retinal healing, the results still have to be taken with a precaution. In some studies, human MSCs were injected intravitreally in rodents and their biocompatibility and positive therapeutic effects were described[71-73]. However, a recent study by Lohan et al[74] demonstrated that human MSCs administered into immunocompetent rats do not have the same therapeutic effects as rat MSCs and that human MSCs do not suppress the proliferation of rat T lymphocytes. Moreover, human MSCs are not activated by rat pro-inflammatory cytokines[74]. These interspecies incompatibilities have to be taken into account when the results from preclinical animal studies utilizing human MSCs are considered in the context of translation to clinical trials.
Another issue that deserves attention is the secretory profile of MSCs after their delivery into the inflammatory environment of the diseased retina. Such a secretory profile could be quite distinct from that in MSCs during their cultivation in vitro. We have shown in a mouse model that highly purified MSCs spontaneously produce significant levels of TGF-β but do not produce IL-6. However, in the presence of pro-inflammatory cytokines, such as IL-1β, TNF-α or IFN-γ, MSCs simultaneously secrete a significant amount of IL-6[25]. IL-6 is a pro-inflammatory cytokine, which together with TGF-β determine the development of the highly pro-inflammatory Th17 cells. A crucial role in the shift between inhibitory Tregs and pro-inflammatory Th17 cells is played by the ratio in concentrations of TGF-β and IL-6[25,75]. This plasticity in the secretory potential of MSCs represents limitations when cultured MSCs producing TGF-β are transferred into the inflammatory environment of the diseased retina. To prevent activation of pro-inflammatory cells, MSCs could be co-administrated with antibody anti-IL-6 or anti-IL-6 receptor, as this has been tested in patients with kidney allografts[76,77]. It has been shown that blocking of the IL-6 pathway can attenuate inflammation, support the activation of Tregs and enhance allograft survival. It can be proposed that such interventions into cytokine pathways or the regulation of cytokine interactions can significantly improve the efficiency of stem cell-based therapy for retinal diseases.
While there is still a debate about the origin and characterization of MSCs[21] and many unknown interactions among cytokines and other growth and trophic factors remain to be recognized, stem cell-based therapy represents a great promise and hope for the patients with visual problems and for the treatment of so far incurable retinal degenerative diseases.
Footnotes
Conflict-of-interest statement: The authors declare no conflicts of interest.
Manuscript source: Unsolicited manuscript
Peer-review started: May 27, 2019
First decision: July 31, 2019
Article in press: September 13, 2019
Specialty type: Cell and tissue engineering
Country of origin: Czech Republic
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bonartsev AP, Hassan AI, Cheng TH S-Editor: Dou Y L-Editor: Filipodia E-Editor: Xing YX
Contributor Information
Vladimir Holan, Department of Transplantation Immunology, Institute of Experimental Medicine of the Czech Academy of Sciences, Prague 14220, Czech Republic; Department of Cell Biology, Faculty of Science, Charles University, Prague 12843, Czech Republic. vladimir.holan@iem.cas.cz.
Barbora Hermankova, Department of Transplantation Immunology, Institute of Experimental Medicine of the Czech Academy of Sciences, Prague 14220, Czech Republic; Department of Cell Biology, Faculty of Science, Charles University, Prague 12843, Czech Republic.
Magdalena Krulova, Department of Transplantation Immunology, Institute of Experimental Medicine of the Czech Academy of Sciences, Prague 14220, Czech Republic; Department of Cell Biology, Faculty of Science, Charles University, Prague 12843, Czech Republic.
Alena Zajicova, Department of Transplantation Immunology, Institute of Experimental Medicine of the Czech Academy of Sciences, Prague 14220, Czech Republic.
References
- 1.Zhao T, Zhang ZN, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212–215. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
- 2.Arnhold S, Absenger Y, Klein H, Addicks K, Schraermeyer U. Transplantation of bone marrow-derived mesenchymal stem cells rescue photoreceptor cells in the dystrophic retina of the rhodopsin knockout mouse. Graefes Arch Clin Exp Ophthalmol. 2007;245:414–422. doi: 10.1007/s00417-006-0382-7. [DOI] [PubMed] [Google Scholar]
- 3.Inoue Y, Iriyama A, Ueno S, Takahashi H, Kondo M, Tamaki Y, Araie M, Yanagi Y. Subretinal transplantation of bone marrow mesenchymal stem cells delays retinal degeneration in the RCS rat model of retinal degeneration. Exp Eye Res. 2007;85:234–241. doi: 10.1016/j.exer.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Wang S, Lu B, Girman S, Duan J, McFarland T, Zhang QS, Grompe M, Adamus G, Appukuttan B, Lund R. Non-invasive stem cell therapy in a rat model for retinal degeneration and vascular pathology. PLoS One. 2010;5:e9200. doi: 10.1371/journal.pone.0009200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tasso R, Ilengo C, Quarto R, Cancedda R, Caspi RR, Pennesi G. Mesenchymal stem cells induce functionally active T-regulatory lymphocytes in a paracrine fashion and ameliorate experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 2012;53:786–793. doi: 10.1167/iovs.11-8211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao PT, Zhang LJ, Shao H, Bai LL, Yu B, Su C, Dong LJ, Liu X, Li XR, Zhang XM. Therapeutic effects of mesenchymal stem cells administered at later phase of recurrent experimental autoimmune uveitis. Int J Ophthalmol. 2016;9:1381–1389. doi: 10.18240/ijo.2016.10.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee MJ, Ko AY, Ko JH, Lee HJ, Kim MK, Wee WR, Khwarg SI, Oh JY. Mesenchymal stem/stromal cells protect the ocular surface by suppressing inflammation in an experimental dry eye. Mol Ther. 2015;23:139–146. doi: 10.1038/mt.2014.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holan V, Trosan P, Cejka C, Javorkova E, Zajicova A, Hermankova B, Chudickova M, Cejkova J. A Comparative Study of the Therapeutic Potential of Mesenchymal Stem Cells and Limbal Epithelial Stem Cells for Ocular Surface Reconstruction. Stem Cells Transl Med. 2015;4:1052–1063. doi: 10.5966/sctm.2015-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mittal SK, Omoto M, Amouzegar A, Sahu A, Rezazadeh A, Katikireddy KR, Shah DI, Sahu SK, Chauhan SK. Restoration of Corneal Transparency by Mesenchymal Stem Cells. Stem Cell Reports. 2016;7:583–590. doi: 10.1016/j.stemcr.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mead B, Logan A, Berry M, Leadbeater W, Scheven BA. Intravitreally transplanted dental pulp stem cells promote neuroprotection and axon regeneration of retinal ganglion cells after optic nerve injury. Invest Ophthalmol Vis Sci. 2013;54:7544–7556. doi: 10.1167/iovs.13-13045. [DOI] [PubMed] [Google Scholar]
- 11.Tomita M, Adachi Y, Yamada H, Takahashi K, Kiuchi K, Oyaizu H, Ikebukuro K, Kaneda H, Matsumura M, Ikehara S. Bone marrow-derived stem cells can differentiate into retinal cells in injured rat retina. Stem Cells. 2002;20:279–283. doi: 10.1634/stemcells.20-4-279. [DOI] [PubMed] [Google Scholar]
- 12.Zwart I, Hill AJ, Al-Allaf F, Shah M, Girdlestone J, Sanusi AB, Mehmet H, Navarrete R, Navarrete C, Jen LS. Umbilical cord blood mesenchymal stromal cells are neuroprotective and promote regeneration in a rat optic tract model. Exp Neurol. 2009;216:439–448. doi: 10.1016/j.expneurol.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 13.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 14.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 15.Musina RA, Bekchanova ES, Sukhikh GT. Comparison of mesenchymal stem cells obtained from different human tissues. Bull Exp Biol Med. 2005;139:504–509. doi: 10.1007/s10517-005-0331-1. [DOI] [PubMed] [Google Scholar]
- 16.Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 2012;21:2724–2752. doi: 10.1089/scd.2011.0722. [DOI] [PubMed] [Google Scholar]
- 17.Kicic A, Shen WY, Wilson AS, Constable IJ, Robertson T, Rakoczy PE. Differentiation of marrow stromal cells into photoreceptors in the rat eye. J Neurosci. 2003;23:7742–7749. doi: 10.1523/JNEUROSCI.23-21-07742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang C, Zhang J, Ao M, Li Y, Zhang C, Xu Y, Li X, Wang W. Combination of retinal pigment epithelium cell-conditioned medium and photoreceptor outer segments stimulate mesenchymal stem cell differentiation toward a functional retinal pigment epithelium cell phenotype. J Cell Biochem. 2012;113:590–598. doi: 10.1002/jcb.23383. [DOI] [PubMed] [Google Scholar]
- 19.Mathivanan I, Trepp C, Brunold C, Baerlocher G, Enzmann V. Retinal differentiation of human bone marrow-derived stem cells by co-culture with retinal pigment epithelium. in vitro Exp Cell Res. 2015;333:11–20. doi: 10.1016/j.yexcr.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Hermankova B, Kossl J, Javorkova E, Bohacova P, Hajkova M, Zajicova A, Krulova M, Holan V. The Identification of Interferon-γ as a Key Supportive Factor for Retinal Differentiation of Murine Mesenchymal Stem Cells. Stem Cells Dev. 2017;26:1399–1408. doi: 10.1089/scd.2017.0111. [DOI] [PubMed] [Google Scholar]
- 21.Sipp D, Robey PG, Turner L. Clear up this stem-cell mess. Nature. 2018;561:455–457. doi: 10.1038/d41586-018-06756-9. [DOI] [PubMed] [Google Scholar]
- 22.Le Blanc K, Ringdén O. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med. 2007;262:509–525. doi: 10.1111/j.1365-2796.2007.01844.x. [DOI] [PubMed] [Google Scholar]
- 23.Abumaree M, Al Jumah M, Pace RA, Kalionis B. Immunosuppressive properties of mesenchymal stem cells. Stem Cell Rev Rep. 2012;8:375–392. doi: 10.1007/s12015-011-9312-0. [DOI] [PubMed] [Google Scholar]
- 24.Oh JY, Kim MK, Shin MS, Wee WR, Lee JH. Cytokine secretion by human mesenchymal stem cells cocultured with damaged corneal epithelial cells. Cytokine. 2009;46:100–103. doi: 10.1016/j.cyto.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 25.Svobodova E, Krulova M, Zajicova A, Pokorna K, Prochazkova J, Trosan P, Holan V. The role of mouse mesenchymal stem cells in differentiation of naive T-cells into anti-inflammatory regulatory T-cell or proinflammatory helper T-cell 17 population. Stem Cells Dev. 2012;21:901–910. doi: 10.1089/scd.2011.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.English K, Barry FP, Field-Corbett CP, Mahon BP. IFN-gamma and TNF-alpha differentially regulate immunomodulation by murine mesenchymal stem cells. Immunol Lett. 2007;110:91–100. doi: 10.1016/j.imlet.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Holan V, Hermankova B, Bohacova P, Kossl J, Chudickova M, Hajkova M, Krulova M, Zajicova A, Javorkova E. Distinct Immunoregulatory Mechanisms in Mesenchymal Stem Cells: Role of the Cytokine Environment. Stem Cell Rev Rep. 2016;12:654–663. doi: 10.1007/s12015-016-9688-y. [DOI] [PubMed] [Google Scholar]
- 28.Forrester JV. Privilege revisited: an evaluation of the eye's defence mechanisms. Eye (Lond) 2009;23:756–766. doi: 10.1038/eye.2008.259. [DOI] [PubMed] [Google Scholar]
- 29.Chen KH, Wu CC, Roy S, Lee SM, Liu JH. Increased interleukin-6 in aqueous humor of neovascular glaucoma. Invest Ophthalmol Vis Sci. 1999;40:2627–2632. [PubMed] [Google Scholar]
- 30.Yuan L, Neufeld AH. Tumor necrosis factor-alpha: a potentially neurodestructive cytokine produced by glia in the human glaucomatous optic nerve head. Glia. 2000;32:42–50. [PubMed] [Google Scholar]
- 31.Neufeld AH, Kawai Si, Das S, Vora S, Gachie E, Connor JR, Manning PT. Loss of retinal ganglion cells following retinal ischemia: the role of inducible nitric oxide synthase. Exp Eye Res. 2002;75:521–528. doi: 10.1006/exer.2002.2042. [DOI] [PubMed] [Google Scholar]
- 32.Chua J, Vania M, Cheung CM, Ang M, Chee SP, Yang H, Li J, Wong TT. Expression profile of inflammatory cytokines in aqueous from glaucomatous eyes. Mol Vis. 2012;18:431–438. [PMC free article] [PubMed] [Google Scholar]
- 33.Nagineni CN, Kommineni VK, William A, Hooks JJ, Detrick B. IL-11 expression in retinal and corneal cells is regulated by interferon-gamma. Biochem Biophys Res Commun. 2010;391:287–292. doi: 10.1016/j.bbrc.2009.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sappington RM, Chan M, Calkins DJ. Interleukin-6 protects retinal ganglion cells from pressure-induced death. Invest Ophthalmol Vis Sci. 2006;47:2932–2942. doi: 10.1167/iovs.05-1407. [DOI] [PubMed] [Google Scholar]
- 35.Eastlake K, Banerjee PJ, Angbohang A, Charteris DG, Khaw PT, Limb GA. Müller glia as an important source of cytokines and inflammatory factors present in the gliotic retina during proliferative vitreoretinopathy. Glia. 2016;64:495–506. doi: 10.1002/glia.22942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xi H, Katschke KJ, Jr, Li Y, Truong T, Lee WP, Diehl L, Rangell L, Tao J, Arceo R, Eastham-Anderson J, Hackney JA, Iglesias A, Cote-Sierra J, Elstrott J, Weimer RM, van Lookeren Campagne M. IL-33 amplifies an innate immune response in the degenerating retina. J Exp Med. 2016;213:189–207. doi: 10.1084/jem.20150894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holtkamp GM, Kijlstra A, Peek R, de Vos AF. Retinal pigment epithelium-immune system interactions: cytokine production and cytokine-induced changes. Prog Retin Eye Res. 2001;20:29–48. doi: 10.1016/s1350-9462(00)00017-3. [DOI] [PubMed] [Google Scholar]
- 38.Madeira MH, Boia R, Santos PF, Ambrósio AF, Santiago AR. Contribution of microglia-mediated neuroinflammation to retinal degenerative diseases. Mediators Inflamm. 2015;2015:673090. doi: 10.1155/2015/673090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kiang L, Ross BX, Yao J, Shanmugam S, Andrews CA, Hansen S, Besirli CG, Zacks DN, Abcouwer SF. Vitreous Cytokine Expression and a Murine Model Suggest a Key Role of Microglia in the Inflammatory Response to Retinal Detachment. Invest Ophthalmol Vis Sci. 2018;59:3767–3778. doi: 10.1167/iovs.18-24489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yao K, Qiu S, Wang YV, Park SJH, Mohns EJ, Mehta B, Liu X, Chang B, Zenisek D, Crair MC, Demb JB, Chen B. Restoration of vision after de novo genesis of rod photoreceptors in mammalian retinas. Nature. 2018;560:484–488. doi: 10.1038/s41586-018-0425-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Forrester JV, Xu H. Good news-bad news: the Yin and Yang of immune privilege in the eye. Front Immunol. 2012;3:338. doi: 10.3389/fimmu.2012.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carwile ME, Culbert RB, Sturdivant RL, Kraft TW. Rod outer segment maintenance is enhanced in the presence of bFGF, CNTF and GDNF. Exp Eye Res. 1998;66:791–805. doi: 10.1006/exer.1998.0488. [DOI] [PubMed] [Google Scholar]
- 43.Simó R, Carrasco E, García-Ramírez M, Hernández C. Angiogenic and antiangiogenic factors in proliferative diabetic retinopathy. Curr Diabetes Rev. 2006;2:71–98. doi: 10.2174/157339906775473671. [DOI] [PubMed] [Google Scholar]
- 44.de Oliveira Dias JR, Rodrigues EB, Maia M, Magalhães O Jr, Penha FM, Farah ME. Cytokines in neovascular age-related macular degeneration: fundamentals of targeted combination therapy. Br J Ophthalmol. 2011;95:1631–1637. doi: 10.1136/bjo.2010.186361. [DOI] [PubMed] [Google Scholar]
- 45.Popescu M, Bogdan C, Pintea A, Rugină D, Ionescu C. Antiangiogenic cytokines as potential new therapeutic targets for resveratrol in diabetic retinopathy. Drug Des Devel Ther. 2018;12:1985–1996. doi: 10.2147/DDDT.S156941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang JC, Cao S, Wang A, To E, Law G, Gao J, Zhang D, Cui JZ, Matsubara JA. CFH Y402H polymorphism is associated with elevated vitreal GM-CSF and choroidal macrophages in the postmortem human eye. Mol Vis. 2015;21:264–272. [PMC free article] [PubMed] [Google Scholar]
- 47.Ambati J, Atkinson JP, Gelfand BD. Immunology of age-related macular degeneration. Nat Rev Immunol. 2013;13:438–451. doi: 10.1038/nri3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mugisho OO, Rupenthal ID, Squirrell DM, Bould SJ, Danesh-Meyer HV, Zhang J, Green CR, Acosta ML. Intravitreal pro-inflammatory cytokines in non-obese diabetic mice: Modelling signs of diabetic retinopathy. PLoS One. 2018;13:e0202156. doi: 10.1371/journal.pone.0202156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kutty RK, Samuel W, Boyce K, Cherukuri A, Duncan T, Jaworski C, Nagineni CN, Redmond TM. Proinflammatory cytokines decrease the expression of genes critical for RPE function. Mol Vis. 2016;22:1156–1168. [PMC free article] [PubMed] [Google Scholar]
- 50.Mahajan VB, Vallone JG, Lin JH, Mullins RF, Ko AC, Folk JC, Stone EM. T-cell infiltration in autosomal dominant neovascular inflammatory vitreoretinopathy. Mol Vis. 2010;16:1034–1040. [PMC free article] [PubMed] [Google Scholar]
- 51.He C, Lai P, Wang J, Zhou T, Huang Z, Zhou L, Liu X. TLR2/4 deficiency prevents oxygen-induced vascular degeneration and promotes revascularization by downregulating IL-17 in the retina. Sci Rep. 2016;6:27739. doi: 10.1038/srep27739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnsen-Soriano S, Sancho-Tello M, Arnal E, Navea A, Cervera E, Bosch-Morell F, Miranda M, Javier Romero F. IL-2 and IFN-gamma in the retina of diabetic rats. Graefes Arch Clin Exp Ophthalmol. 2010;248:985–990. doi: 10.1007/s00417-009-1289-x. [DOI] [PubMed] [Google Scholar]
- 53.Geiger K, Howes E, Gallina M, Huang XJ, Travis GH, Sarvetnick N. Transgenic mice expressing IFN-gamma in the retina develop inflammation of the eye and photoreceptor loss. Invest Ophthalmol Vis Sci. 1994;35:2667–2681. [PubMed] [Google Scholar]
- 54.Ding SLS, Kumar S, Mok PL. Cellular Reparative Mechanisms of Mesenchymal Stem Cells for Retinal Diseases. Int J Mol Sci. 2017:18. doi: 10.3390/ijms18081406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Holan V, Hermankova B, Kossl J. Perspectives of Stem Cell-Based Therapy for Age-Related Retinal Degenerative Diseases. Cell Transplant. 2017;26:1538–1541. doi: 10.1177/0963689717721227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park SS, Moisseiev E, Bauer G, Anderson JD, Grant MB, Zam A, Zawadzki RJ, Werner JS, Nolta JA. Advances in bone marrow stem cell therapy for retinal dysfunction. Prog Retin Eye Res. 2017;56:148–165. doi: 10.1016/j.preteyeres.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oh JY, Kim MK, Shin MS, Lee HJ, Ko JH, Wee WR, Lee JH. The anti-inflammatory and anti-angiogenic role of mesenchymal stem cells in corneal wound healing following chemical injury. Stem Cells. 2008;26:1047–1055. doi: 10.1634/stemcells.2007-0737. [DOI] [PubMed] [Google Scholar]
- 58.Mathew B, Poston JN, Dreixler JC, Torres L, Lopez J, Zelkha R, Balyasnikova I, Lesniak MS, Roth S. Bone-marrow mesenchymal stem-cell administration significantly improves outcome after retinal ischemia in rats. Graefes Arch Clin Exp Ophthalmol. 2017;255:1581–1592. doi: 10.1007/s00417-017-3690-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cejkova J, Trosan P, Cejka C, Lencova A, Zajicova A, Javorkova E, Kubinova S, Sykova E, Holan V. Suppression of alkali-induced oxidative injury in the cornea by mesenchymal stem cells growing on nanofiber scaffolds and transferred onto the damaged corneal surface. Exp Eye Res. 2013;116:312–323. doi: 10.1016/j.exer.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 60.Ma OK, Chan KH. Immunomodulation by mesenchymal stem cells: Interplay between mesenchymal stem cells and regulatory lymphocytes. World J Stem Cells. 2016;8:268–278. doi: 10.4252/wjsc.v8.i9.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meirelles Lda S, Fontes AM, Covas DT, Caplan AI. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009;20:419–427. doi: 10.1016/j.cytogfr.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 62.Kolomeyer AM, Zarbin MA. Trophic factors in the pathogenesis and therapy for retinal degenerative diseases. Surv Ophthalmol. 2014;59:134–165. doi: 10.1016/j.survophthal.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 63.Ezquer M, Urzua CA, Montecino S, Leal K, Conget P, Ezquer F. Intravitreal administration of multipotent mesenchymal stromal cells triggers a cytoprotective microenvironment in the retina of diabetic mice. Stem Cell Res Ther. 2016;7:42. doi: 10.1186/s13287-016-0299-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Conway K, Price P, Harding KG, Jiang WG. The molecular and clinical impact of hepatocyte growth factor, its receptor, activators, and inhibitors in wound healing. Wound Repair Regen. 2006;14:2–10. doi: 10.1111/j.1743-6109.2005.00081.x. [DOI] [PubMed] [Google Scholar]
- 65.Nicola NA, Babon JJ. Leukemia inhibitory factor (LIF) Cytokine Growth Factor Rev. 2015;26:533–544. doi: 10.1016/j.cytogfr.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mourkioti F, Rosenthal N. IGF-1, inflammation and stem cells: interactions during muscle regeneration. Trends Immunol. 2005;26:535–542. doi: 10.1016/j.it.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 67.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 68.Ng TK, Fortino VR, Pelaez D, Cheung HS. Progress of mesenchymal stem cell therapy for neural and retinal diseases. World J Stem Cells. 2014;6:111–119. doi: 10.4252/wjsc.v6.i2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Labrador-Velandia S, Alonso-Alonso ML, Alvarez-Sanchez S, González-Zamora J, Carretero-Barrio I, Pastor JC, Fernandez-Bueno I, Srivastava GK. Mesenchymal stem cell therapy in retinal and optic nerve diseases: An update of clinical trials. World J Stem Cells. 2016;8:376–383. doi: 10.4252/wjsc.v8.i11.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Salehi H, Amirpour N, Razavi S, Esfandiari E, Zavar R. Overview of retinal differentiation potential of mesenchymal stem cells: A promising approach for retinal cell therapy. Ann Anat. 2017;210:52–63. doi: 10.1016/j.aanat.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 71.Tzameret A, Sher I, Belkin M, Treves AJ, Meir A, Nagler A, Levkovitch-Verbin H, Rotenstreich Y, Solomon AS. Epiretinal transplantation of human bone marrow mesenchymal stem cells rescues retinal and vision function in a rat model of retinal degeneration. Stem Cell Res. 2015;15:387–394. doi: 10.1016/j.scr.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 72.Ji S, Lin S, Chen J, Huang X, Wei CC, Li Z, Tang S. Neuroprotection of Transplanting Human Umbilical Cord Mesenchymal Stem Cells in a Microbead Induced Ocular Hypertension Rat Model. Curr Eye Res. 2018;43:810–820. doi: 10.1080/02713683.2018.1440604. [DOI] [PubMed] [Google Scholar]
- 73.Labrador Velandia S, Di Lauro S, Alonso-Alonso ML, Tabera Bartolomé S, Srivastava GK, Pastor JC, Fernandez-Bueno I. Biocompatibility of intravitreal injection of human mesenchymal stem cells in immunocompetent rabbits. Graefes Arch Clin Exp Ophthalmol. 2018;256:125–134. doi: 10.1007/s00417-017-3842-3. [DOI] [PubMed] [Google Scholar]
- 74.Lohan P, Treacy O, Morcos M, Donohoe E, O'donoghue Y, Ryan AE, Elliman SJ, Ritter T, Griffin MD. Interspecies Incompatibilities Limit the Immunomodulatory Effect of Human Mesenchymal Stromal Cells in the Rat. Stem Cells. 2018;36:1210–1215. doi: 10.1002/stem.2840. [DOI] [PubMed] [Google Scholar]
- 75.Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, Ziegler SF, Littman DR. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Granofszky N, Farkas AM, Muckenhuber M, Mahr B, Unger L, Maschke S, Pilat N, Holly R, Wiletel M, Regele H, Wekerle T. Anti-Interleukin-6 Promotes Allogeneic Bone Marrow Engraftment and Prolonged Graft Survival in an Irradiation-Free Murine Transplant Model. Front Immunol. 2017;8:821. doi: 10.3389/fimmu.2017.00821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jordan SC, Choi J, Kim I, Wu G, Toyoda M, Shin B, Vo A. Interleukin-6, A Cytokine Critical to Mediation of Inflammation, Autoimmunity and Allograft Rejection: Therapeutic Implications of IL-6 Receptor Blockade. Transplantation. 2017;101:32–44. doi: 10.1097/TP.0000000000001452. [DOI] [PubMed] [Google Scholar]