Abstract
Acute anxiety impacts cognitive performance. Inhalation of air enriched with carbon dioxide (CO2) in healthy humans provides a novel experimental model of generalised anxiety, but has not previously been used to assess cognition. We used inhalation of 7.5% CO2 to induce acute anxiety and autonomic arousal in healthy volunteers during neuropsychological tasks of cognitive flexibility, emotional processing and spatial working memory in a single-blind, placebo-controlled, randomized, crossover, within-subjects study. In Experiment 1 (n = 44), participants made significantly more extra-dimensional shift errors on the Cambridge Neuropsychological Test Automated Battery (CANTAB) Intra-Extra Dimensional Set Shift task under CO2 inhalation compared with ‘normal’ air. Participants also had slower latencies when responding to positive words and made significantly more omission errors for negative words on the CANTAB Affective Go/No-go task. In Experiment 2 (n = 28), participants made significantly more total errors and had poorer heuristic search strategy on the CANTAB Spatial Working Memory task. In both experiments, CO2 inhalation significantly increased negative affect; state anxiety and fear; symptoms of panic; and systolic blood pressure/heart rate. Overall, CO2 inhalation produced robust anxiogenic effects and impaired fronto-executive functions of cognitive flexibility and working memory. Effects on emotional processing suggested a mood-congruent slowing in processing speed in the absence of a negative attentional bias. State-dependent effects of anxiety on cognitive-emotional interactions in the prefrontal cortex warrant further investigation.
Subject terms: Neuroscience, Psychology
Introduction
Emotion and cognition are closely integrated phenomena, such that emotions influence, and are influenced by, cognitive processes1–3. Executive functions heavily rely on the frontal lobes and are necessary for optimal selection, organisation and monitoring of actions for attaining goals4–6. However, negative emotional states, such as anxiety, increase arousal and corresponding autonomic responses and can also bias cognitive processes in favour of selectively prioritising negative information7,8. Anxiety in particular enhances vigilance when detecting emotionally-salient information in the environment, but disrupts working memory9,10. Although anxiety can mediate adaptive behaviour in response to threat, it can also impair core aspects of cognition through its profound influence on prefrontal executive functions11.
Deficits in executive functions have been found in anxiety disorders12–14. Acute anxiety impairs cognitive flexibility, the ability to adapt one’s behaviour in response to rapid changes in the environment15. Individuals with high-trait anxiety favour recently acquired responses, even when they are no longer relevant16. Reduced cognitive flexibility has been proposed to be undermined by interference from irrelevant stimuli, in which anxiety prioritises stimulus-driven (bottom-up) attention over and above goal-directed (top-down) attention16–18. Emotionally-salient cues are known to bias attention, such that high-trait anxious individuals will selectively attend to negative information that matches and exacerbates their emotional state19. One measure of emotional processing is the Affective Go/No-go task20,21. Using this task, patients with depression have shown to make more omission errors when responding to happy than to sad words and respond more quickly to sad targets22. Patients with depression have also shown an inability to shift their attention from one affective valence to another, further supporting mood-congruent processing of negative stimuli20. In healthy volunteers, findings support an affective bias for positive information, as shown by faster responses for happy faces23.
Effects of acute anxiety on working memory have been mixed, with some studies showing anxiety-inducing impairments, e.g., see refs. 24–27. Discrepant findings are likely due to different paradigms being used for manipulating emotional states, including variations in delivery and length of delay between the acute stressor and cognitive assessment28. Negative affect has been hypothesized to selectively deplete processing resources required for adequate working memory performance27,29,30. Classic findings demonstrate that anxiety improves performance on simpler and well-rehearsed tasks, but impair performance on tasks that require complex, flexible thinking31. More generally, behavioural performance improves with low levels of arousal, but decreases with higher levels through deleterious effects on cognitive processes such as working memory.
One safe, reliable and robust method for inducing acute anxiety and autonomic arousal is through the inhalation of a gas mixture with an enhanced concentration of carbon dioxide32,33. Air enriched with CO2 has previously shown to evoke anxiety-related symptoms in healthy volunteers34–36 and in patients with anxiety disorders37,38. Acute administration of the benzodiazepine agonist lorazepam and chronic administration of the selective serotonin re-uptake inhibitor (SSRI) paroxetine has been shown to attenuate the effects of CO2 inhalation on state anxiety in healthy volunteers39, thus providing an experimental model of generalised anxiety40. However, the effects of CO2 inhalation on cognitive performance are less well characterised. Only one laboratory study has used an emotional antisaccade task to show that inhalation of 7.5% CO2 selectively increases attention to threat in healthy humans41.
The aim of our study was to characterise impairments in fronto-executive functions in healthy humans using an experimental manipulation analogous to generalised anxiety. We report two experiments investigating the effects of 7.5% CO2 inhalation on cognitive flexibility, emotional processing and spatial working memory in healthy human volunteers. Tasks were selected based on previous studies showing prefrontal and amygdalar dysfunction in highly anxious individuals and in patients with generalised anxiety disorder (GAD)42,43. We hypothesized that compared with a ‘normal air’ control condition; CO2 inhalation would impair cognitive flexibility and spatial working memory and induce mood-congruent processing of negative information. We further hypothesized, consistent with the literature34,39–41, that CO2 inhalation would increase negative affect; state anxiety and fear; symptoms of panic; and cardiovascular measures associated with somatic anxiety.
Materials and methods
Participants
Seventy-two healthy volunteers were recruited via mailing lists, posted flyers and from the Behavioural and Clinical Neuroscience Institute research database. Inclusion criteria were no current or past medical, psychiatric or neurological conditions or substance abuse. Exclusion criteria were pregnancy, currently smoking and having a first-degree relative diagnosed with a panic disorder. Participants were free of regular medication intake, but use of the oral contraceptive pill was accepted. These criteria were screened using the Mini-International Neuropsychiatric Inventory44; and by telephone interview. Invited participants were asked to abstain from alcohol consumption 36 h prior to the experiment as well as caffeinated drinks from the midnight before testing. All participants provided written informed consent.
Gas mixture
Air enriched with CO2 (7.5% CO2, 21% O2, 71.5% N2) was used to evoke somatic anxiety34 and was stored in 10 L cylinders compressed with 200 bar. The air condition (control) consisted of approximately 0.0016% CO2, 21% O2 and 78% N2.
Neuropsychological measures
We tested cognitive tasks in two cohorts of healthy participants. Participants performed parallel versions of cognitive tasks from the computerised Cambridge Neuropsychological Test Automated Battery (CANTAB; www.cambridgecogntion.com) in the two gas inhalation sessions. Tasks were performed over two experiments given the limited time window to safely assess cognitive performance during the CO2 challenge. Inhalation of CO2 for up to 20 min is the maximal inhalation duration known to be safely tolerated without serious side effects (e.g., see refs. 34,37,39–41).
Experiment 1
CANTAB Intra-dimensional/Extra-dimensional Set-shifting task (IDED)
The CANTAB IDED task45 is a measure of rule acquisition and reversal. It features visual discrimination, attentional set formation, maintenance of attention, set shifting and flexibility. Two artificial dimensions are used: colour-filled shapes and white lines. Simple stimuli are made of just one of these dimensions, whereas compound stimuli are made of both, namely white lines overlying colour-filled shapes. Participants must use feedback to work out a rule that determines which stimulus is correct. After six correct responses, the stimuli and/or rule changes. Initially the task will involve simple stimuli which are made up of just one of the dimensions. Later on, compound stimuli are used. The shifts in rule are firstly ‘intra-dimensional’ and then secondly ‘extra-dimensional.’ Outcome measures include the total errors made; errors made in the critical stages of intra-dimensional set shift; errors made in the critical stages of extra-dimensional set shift; and the number of errors made prior to the extra-dimensional shift of the task.
CANTAB Affective Go/No-go task (AGN)
The CANTAB AGN task20 is a measure of information-processing biases for positive and negative stimuli. The task consists of several blocks, each of which presents a series of words from two of three different affective categories: positive (e.g. joyful), negative (e.g. burden) or neutral (e.g. pause). The participant is given a target category and is asked to select a word when it matches this category. Outcome measures include errors of commission (an incorrect response to a distractor stimulus on ‘No/go’ trials) and omission (no response to a target stimulus on ‘Go’ trials) and latency (speed of response).
Experiment 2
CANTAB Spatial Working Memory task (SWM)
The CANTAB SWM46 is a measure of ability to retain spatial information and to manipulate remembered items in working memory. It is a self-ordered task, which also assesses heuristic strategy. A number of coloured boxes are first shown on the screen. Participants are instructed to find a blue token in each box, using a process of elimination, and to use them to fill up an empty column on the right side of the screen. The colour and position of the boxes are changed from trial to trial (with the number of boxes increasing). Outcome measures include total errors (selecting boxes that have already been found to be empty and revisiting boxes which have already been found to contain a token) and strategy (a predetermined sequence by beginning with a specific box and then, once a blue token has been found, to return to that box to start the new search sequence).
Questionnaire measures
Administered trait measures included Spielberger’s Trait-Anxiety Inventory [STAI-T; ref. 47], Beck’s Depression Inventory [BDI; ref. 48] and the Intolerance of Uncertainty Scale [IUS; ref. 49]. The STAI-T is a 20-item self-report measure of trait anxiety, with higher scores indicating higher anxiety levels (range 20–80); the BDI is a 21-item self-report measure of depression, with higher scores indicating higher levels of depression severity (range 0–63); and the IUS is a 12-item self-report measure of responses to uncertainty, ambiguous situations and the future (e.g. ‘When it’s time to act, uncertainty paralyses me’), with higher scores indicating less tolerance (range 12–60). State measures included the Negative Affect subscale of the Positive and Negative Affect Schedule [PANAS; ref. 50; a 10-item measure of current negative affect, with items such as irritability, distress and nervousness enabling a more comprehensive measure of negative emotionality induced by CO2 inhalation41; range 10–50]; the Acute Panic Inventory [API; ref. 51; a 17-item measure of the severity of symptoms that typically occur during spontaneous panic attacks, with scores ranging from 0 = symptom not experienced to 3 = severe experience of symptom; e.g. ‘Do you have rapid or difficulty breathing’; range 0–51]; and 10 cm visual analogue scales of state anxiety, fear and happiness (higher scores indicate a greater emotional response).
Procedure
This study received full ethical approval from the University of Cambridge Psychology Research Ethics committee (Pre.2013.98). This study was a single-blind, placebo-controlled, randomised, within-subject crossover design. All participants provided written informed consent.
For the gas sessions, the experimenter installed the mask used for inhaling the mixture on the participant’s face. Participants were asked to breathe through a soft silicon rubber nasal-oral mask, which was attached to a tube that led to a 100 L Douglas bag. Participants faced a screen, with the gas bag positioned behind it. In the control condition, participants breathed room air and pre-recorded sounds of gas being released from the cylinder were played in the background. In the CO2 condition, the valve of the gas bag was switched, such that the participant breathed the gas mixture from the Douglas bag. A cylinder with a compressed gas mixture was used to keep the bag filled. For safety reasons, the participant was accompanied by at least two researchers and a carbon dioxide safety monitor was used to monitor the CO2 concentration in the testing room throughout the entire experiment. Systolic blood pressure (mmHG) and heart rate (beats per minute) were measured using a digital blood pressure monitor.
Baseline trait (anxiety, depression, intolerance of uncertainty), panic (API) and cardiovascular (heart rate, blood pressure) measures were first collected in this fixed order pre-inhalation (5 min). Each gas session then comprised cognitive testing, state (panic, affect, mood) and cardiovascular (as above) measures, which lasted a maximum duration of 20 min. A 5–10-min break was given in between the inhalation sessions. Participants in Experiment 1 completed the CANTAB IDED and AGN tasks and participants in Experiment 2 completed the CANTAB SWM test. Gas administration was counterbalanced separately by gender using two orders (CO2/air, air/CO2) randomly generated across experiments. Participants were given a 5–10-min rest period between the inhalation sessions. After the two inhalation sessions, participants were debriefed about their gas administration order. All participants were paid £8/h and thanked for their time.
Statistical analyses
With alpha set at 0.05 and 80% power, an a priori power analysis based on a previous study41 found that 15 participants would be sufficient to detect a within-subjects effect of CO2 inhalation on negative affect (ηp2 = 0.31). Paired samples t-tests were used to investigate within-subject differences in cognitive performance under CO2 and ‘normal’ air inhalation. To test the effect of the gas manipulation on negative affect, panic symptoms and autonomic arousal, repeated-measures ANOVAs were used with ‘Time’ as the within-subjects factor with three levels (baseline/pre-inhalation, CO2, and air). Main effects were further compared between mean scores under CO2 and mean scores at baseline and under air separately, adjusting confidence intervals using Bonferroni correction for multiple comparisons. Paired samples t-tests were performed to investigate state anxiety, fear and happiness under CO2 and air. Correlational and regression analyses were used to examine potential associations between cognitive performance and baseline trait measures, the degree of anxiety/panic symptoms reported and cardiovascular effects experienced under CO2. Due to the high number of neuropsychological test outcome measures, the Benjamini-Hochberg procedure52 was applied at q < 0.05 to control for false discovery; significant p-values remained (all two-sided).
Results
Participant characteristics
Experiment 1 was composed of 22 males and 22 females with a mean age of 29.25 years (SD = 10.66) and Experiment 2 was composed of 13 males and 14 females with a mean age of 26.78 years (SD = 9.94). All participants had completed at least pre-University education at the time of testing with an average of 18.25 years in education (SD = 1.26) in Experiment 1 and 17.17 years in education (SD = 2.57) in Experiment 2. As expected, mean trait-anxiety and depression scores were within normal ranges for healthy adults (STAI-T: Experiment 1 = 34.98, SD = 6.24; Experiment 2 = 31.86, SD = 7.70; normative score = 33.0, SD = 9.4; ref. 53; and BDI: Experiment 1 = 4.63, SD = 4.75; Experiment 2 = 2.68, SD = 3.08; minimal range = 0–13). Furthermore, mean intolerance of uncertainty scores (Intolerance of Uncertainty Scale) were 27.51 (SD = 8.27) in Experiment 1 and 26.32 (SD = 7.38) in Experiment 2. Age, gender, years in education, trait-anxiety, depression and intolerance of uncertainty were not significantly different between experiments (all p’s > 0.06). In both samples, baseline (pre-inhalation) measures of trait- and state anxiety, negative affect and panic symptoms were not significantly different between the two gas administration orders (all p’s > 0.09). Furthermore, gas order did not interact with the effects of CO2 on measures of state anxiety and fear, negative affect and panic (all p’s between 0.07 and 0.75) in either experiment.
Negative affect and mood state
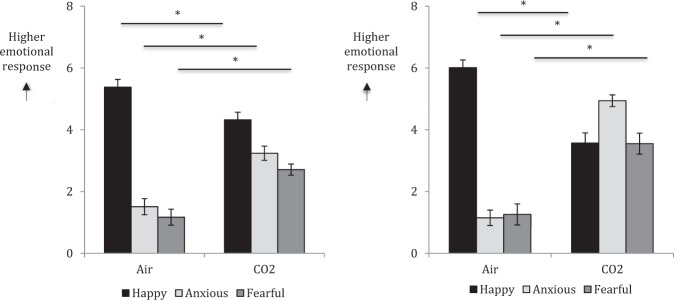
Means and standard deviations for negative affect at each time point are presented in Table 1. In Experiment 1, there was a main effect of Time, F(2, 40) = 9.40, p < 0.001, ηp2 = 0.32, such that negative affect was significantly higher under CO2 compared with baseline, p < 0.001 (mean difference = 2.81) and air, p = 0.003 (mean difference = 2.64). Similarly in Experiment 2, there was a main effect of Time, F(2, 26) = 17.79, p < 0.001, ηp2 = 0.56, with negative affect again being significantly higher under CO2 compared with baseline, p = 0.005 (mean difference = 5.34) and air, p < 0.001 (mean difference = 7.04). In both experiments, state anxiety and fear were significantly increased under CO2 compared with air (all p’s < 0.001), whereas state happiness was significantly decreased under CO2 compared with air (all p’s < 0.001) (Fig. 1). In both experiments, women gave significantly higher ratings for state fear than men (p = 0.03 and 0.007, respectively) during CO2 inhalation.
Table 1.
Means and standard deviations for measures of negative affect, panic symptoms, blood pressure and heart rate by experiment
| Experiment 1 | Experiment 2 | |
|---|---|---|
| Negative affect (NA) | ||
| Baseline | 12.00 (2.32) | 12.89 (2.36) |
| Air | 12.20 (3.91) | 11.39 (2.10) |
| CO2 | 14.84 (4.87)* | 18.43 (8.06)* |
| Panic symptoms (API) | ||
| Baseline | 1.60 (2.80) | 2.29 (2.99) |
| Air | 2.98 (3.67) | 2.86 (3.95) |
| CO2 | 10.18 (7.34)* | 17.54 (8.32)* |
| Systolic blood pressure | ||
| Baseline | 116.72 (12.86) | 119.22 (13.12) |
| Air | 114.07 (13.75) | 116.46 (11.27) |
| CO2 | 128.30 (17.91)* | 140.82 (21.54)* |
| Heart rate | ||
| Baseline | 70.45 (10.63) | 71.93 (10.29) |
| Air | 74.28 (11.72) | 73.25 (9.42) |
| CO2 | 76.98 (12.59)** | 91.89 (17.61)* |
NA Negative Affect Schedule subscale, API Acute Panic Inventory
* denotes significant difference between CO2 and baseline only; ** denotes significant difference between CO2 relative to baseline and air
Fig. 1.
Visual analogue scales of state happiness, anxiety and fear during air and CO2 inhalation in Experiment 1 (left) and Experiment 2 (right); in both experiments, state anxiety and fear were significantly increased under CO2, and state happiness was significantly decreased
Panic symptoms and autonomic arousal
Means and standard deviations for panic and arousal measures at each time point are presented in Table 1. In Experiment 1, there was a main effect of Time for the API, F(2, 40) = 41.35, p < 0.001, ηp2 = 0.68. As expected, mean panic symptoms were significantly higher under CO2 compared with baseline, p < 0.001 (mean difference = 8.57) and air, p < 0.001 (mean difference = 7.17). CO2 inhalation also increased cardiovascular measures of arousal. There were main effects of Time for both systolic blood pressure, F(2, 41) = 34.52, p < 0.001, ηp2 = 0.63 and heart rate, F(2, 41) = 10.47, p < 0.001, ηp2 = 0.34. Mean systolic blood pressure was significantly higher under CO2 compared with baseline, p < 0.001 (mean difference = 11.58) and air, p < 0.001 (mean difference = 14.23). However, mean heart rate under CO2 only significantly differed from mean heart rate at baseline, p < 0.001 (mean difference = 6.51).
In Experiment 2, there was a main effect of Time for the API, F(2, 26) = 50.67, p < 0.001, ηp2 = 0.80. Mean panic symptoms were significantly higher under CO2 compared with baseline, p < 0.001 (mean difference = 15.25) and air, p < 0.001 (mean difference = 14.68). Again, there were main effects of Time for systolic blood pressure, F(2, 25) = 27.08, p < 0.001, ηp2 = 0.68 and heart rate, F(2, 25) = 18.07, p < 0.001, ηp2 = 0.59. Mean systolic blood pressure was significantly higher under CO2 compared with baseline, p < 0.001 (mean difference = 21.48) and air, p < 0.001 (mean difference = 24.15). Mean heart rate was also significantly higher under CO2 compared with baseline, p < 0.001 (mean difference = 19.52) and air, p < 0.001 (mean difference = 18.04).
Cognitive measures
Experiment 1
Cognitive flexibility (CANTAB IDED)
A paired samples t-test revealed that participants (Fig. 2) made significantly more total errors under CO2 compared with air, t(43) = 2.69, p = 0.01, d = 0.41 (CO2 mean = 17.89, SD = 15.09; air mean = 13.00, SD = 9.70). Participants also made significantly more extra-dimensional shift errors under CO2 compared with air, t(43) = 2.63, p = 0.01, d = 0.40 (CO2 mean = 7.07, SD = 9.08; air mean = 3.77, SD = 5.30). Pre-ED errors were not significantly different, t(43) = 0.62, p = 0.54 (CO2 mean = 8.39, SD = 9.08; air mean = 7.39, SD = 7.14). Furthermore, the mean number of intra-dimensional shift errors under CO2 and air was not significantly different, t(43) = 1.86, p = 0.07 (CO2 mean = 0.75, SD = 0.84; air mean = 0.43, SD = 0.55.
Fig. 2.
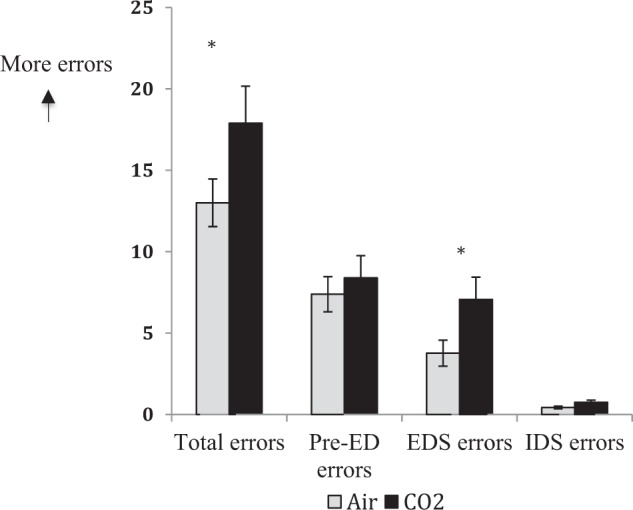
Participants made significantly more total errors and errors in the extra-dimensional stage of the CANTAB IDED task under CO2 compared with air; the number of errors made prior to the extra-dimensional stage and intra-dimensional shift errors were not significantly different (Experiment 1)
Affective bias (CANTAB AGN)
Commission errors were not significantly (Fig. 3) different, t(43) = 0.03, p = 0.98 (CO2 mean = 7.34, SD = 6.27; air mean = 7.34, SD = 6.21). However, participants made significantly more omission errors under CO2 compared with air, t(43) = 3.31, p = 0.002, d = 0.50 (mean CO2 = 6.95, SD = 5.85; mean air = 5.00, SD = 4.29). Follow-up paired samples t-tests revealed significantly more omission errors for negative words under CO2 compared with air, t(43) = 2.96, p = 0.005, d = 0.45 (mean CO2 = 3.41, SD = 3.65; mean air = 2.23, SD = 2.34), with mean omission errors for positive words not reaching significance, t(43) = 1.87, p = 0.07 (mean CO2 = 3.55, SD = 3.10; mean air = 2.77, SD = 2.55. Mean latencies for correct responses were significantly slower for positive words under CO2 compared with air, t(43) = 2.67, p = 0.01, d = 0.04 (mean CO2 = 591.65, SD = 65.33; mean air = 573.16, SD = 67.64), but not negative words, t(43) = 1.65, p = 0.11 (mean CO2 = 600.29, SD = 76.80; mean air = 586.80, SD = 66.45).
Fig. 3.
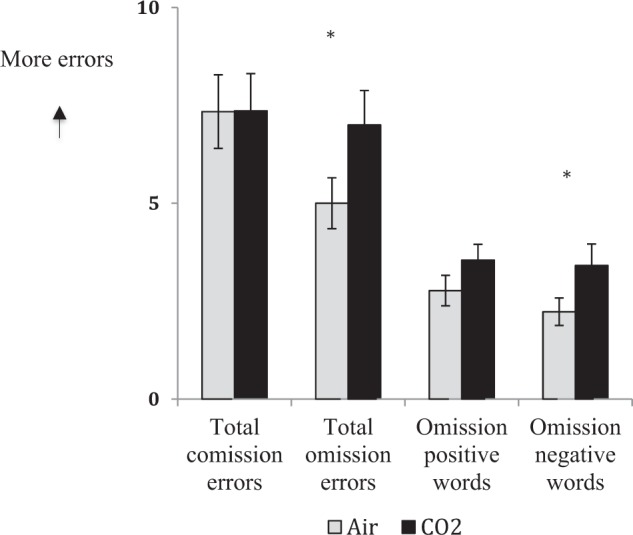
Participants made more total omission errors and omission errors for negative words on the CANTAB AGN task under under CO2 compared with air; total commission errors and omission errors for positive words were not significantly different (Experiment 1)
Experiment 2
Spatial working memory (CANTAB SWM)
Participants made significantly more total (Fig. 4) errors under CO2 compared with air, t(27) = 3.40, p = 0.002, d = 0.63 (CO2 mean = 33.89, SD = 21.97; air mean = 22.14, SD = 18.01). Follow-up within-subject comparisons at each level of difficulty revealed that participants made significantly more errors at the hardest task level (ten-box stage) under CO2 compared with air, t(27) = 3.19, p = 0.004, d = 0.60 (CO2 mean = 24.39, SD = 15.06; air mean = 15.11, SD = 11.45). Within-subject performance at lower levels of difficulty (eight-, six-, and four-box stages) was not significantly different (all p’s > 0.16). Participants showed an inferior heuristic search strategy under CO2 compared with air, t(27) = 2.38, p = 0.03, d = 0.45 (CO2 mean = 23.36, SD = 7.39; air mean = 21.50, SD = 6.73).
Fig. 4.
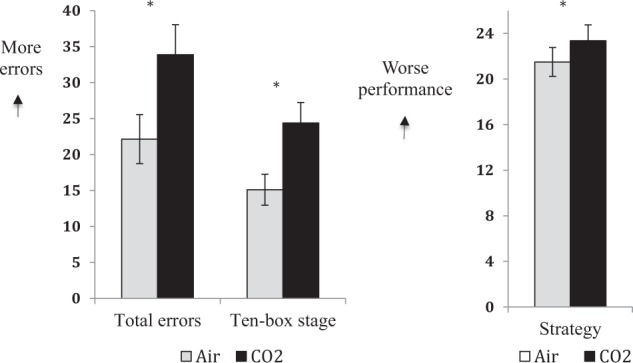
Participants made significantly more total errors and errors at the highest level of difficulty (ten-box stage) on the CANTAB SWM task under CO2 compared with air (left); participants also showed poorer search strategy under CO2 compared with air (right) (Experiment 2)
Whole sample analyses (performed separately by task)
Correlational analyses revealed that outcome measures from the IDED and SWM tasks were not significantly associated with symptoms of panic, state anxiety/fear or cardiovascular measures during CO2 inhalation (all p’s ≥ 0.05). However, on the AGN task, significant associations were found between total commission errors and negative affect (r = 0.47, p = 0.001), panic symptoms (r = 0.37, p = 0.01) and state fear (r = 0.32, p = 0.04), and total omission errors and negative affect (r = 0.41, p = 0.006) during CO2 inhalation. A regression analysis revealed that baseline intolerance of uncertainty significantly predicted omission errors for negative words under CO2, β = 0.32, t = 2.15, p = 0.04 (the model accounted for 32% of the variance and was significant, F(1, 42) = 4.62, p = 0.04).
Lastly, post hoc power analyses revealed that Experiment 1 achieved 74% power and Experiment 2 achieved 86% power based on key within-group effects of CO2 on IDED extra-dimensional shift errors and SWM errors made at the ten-box stage, respectively.
Discussion
We investigated the effects of experimentally induced acute anxiety and autonomic arousal on core fronto-executive functions in healthy humans as a model of those underlying generalised anxiety. As hypothesized, we found that compared with baseline and ‘normal’ air, inhalation of air enriched with 7.5% CO2 significantly increased negative affect, state anxiety/fear, symptoms of panic, and cardiovascular measures of systolic blood pressure and heart rate. Conversely and as expected, CO2 inhalation also significantly reduced state happiness. On the CANTAB IDED task, CO2 inhalation significantly increased the number of extra-dimensional, but not intra-dimensional, shift errors. Participants also made significantly more total errors and had an inferior heuristic search strategy on the CANTAB SWM task. Evidence of a mood-congruent processing bias was mixed: on the CANTAB AGN task, we found that participants responded more slowly to positive words, but made more omission errors for negative words. Correlational analyses revealed significant associations between impairments on the AGN task and negative affect, panic symptoms and state fear under CO2, suggesting that cognitive effects were influenced by emotional rather than cardiovascular changes. Throughout both experiments, the impact of CO2 was highly present during its inhalation, but disappeared immediately upon cessation, thereby demonstrating that anxiety induction was acute rather than chronic and with good reproducibility and safety.
Studies to date have shown evidence of anxiety-induced impairments on cognitive flexibility54–56. Participants in our study generally made very few, if any, intra-dimensional shift errors, indicating rather specific effects on attentional set shifting rather than discrimination learning per se57. Impaired extra-dimensional shifting is characteristic of adult patients with obsessive-compulsive disorder (OCD) and their unaffected first-degree relatives, suggesting that cognitive flexibility is a candidate endophenotype that exists even in the absence of clinically significant symptoms58. Our data raise the possibility that cognitive flexibility may also be impaired in generalised anxiety disorder (GAD), which might reflect specific dysfunction in prefrontal cortical regions. Indeed, poor attentional flexibility for extra-dimensional shifting is sensitive to frontal lobal injury59 and related to distinctly weakened functional connectivity between the caudate and the ventrolateral prefrontal cortex60. The effects of CO2 inhalation on emotional processing revealed slower responding to positive words, but significantly more omission errors for negative words. However, both total omission and commission errors were highly associated with negative emotions and panic under CO2 across the samples of each experiment separately. Healthy individuals are characterised by a positive attentional bias and have previously been shown to make more omission errors in response to sad stimuli than to happy stimuli, whereas patients with depression show the reverse pattern of responding20,22. Our data provide some evidence of an affective bias congruent with negative mood induction, such that healthy participants showed a slowing of response to positive words under CO2. Although CO2 inhalation is an emotional manipulation, an overall adaptive ‘healthy’ attentional bias may confer resilience against negative emotional processing. This may in part explain why less intolerance of uncertainty, a key construct impaired in GAD, OCD, panic and other emotional disorders, significantly predicted omission errors for negative words only (e.g. rejection of uncertainty/ambiguity protects against the processing of negative emotional information).
Theoretical and neurophysiological perspectives suggest that anxiety impairs working memory by competing for processing resources via modulation of prefrontal cortical network functioning29. As expected, we found that participants committed significantly more total errors in our spatial working memory task during CO2 inhalation compared with ‘normal’ air inhalation. Furthermore, this effect was driven by a significant difference at the hardest level of task difficulty (i.e. the ten-box stage). Participants under CO2 inhalation also exhibited a significantly worse ‘strategy’ score, meaning they more frequently started a new trial by searching for a token in a new box rather than following a more systematic search strategy46. Others have shown that increased cortisol or adrenergic activity impairs working memory61 and that alpha-1 receptor agonists can impair the spatial delayed response task in rhesus monkeys62. In general, alpha-1 activity tend to promote relatively automatic conditioned avoidance behaviours and habitual or ritualistic behaviours62,63.
Our data have important clinical implications. They generally contribute to the existing literature on the interaction between anxiety and cognitive processes using an experimental model that readily translates between animals and humans. More specifically, CO2 inhalation safely and quickly induces a maladaptive level of anxiety and arousal that impacts executive functioning similar to psychiatric disorders. As such, this model can be used to translate the efficacy of novel compounds from preclinical models to healthy human volunteers prior to clinical trials in patients (e.g. evaluation of new anxiolytic treatments such as beta-adrenergic agonists for anxiety disorders35). However, it should be noted that mood and panic symptoms in the present sample were below the values typically seen in clinical populations, making it difficult to generalise to clinical levels of anxiety. Others have suggested that higher concentrations of CO2 may provide a better model of panic disorder (e.g. 35%)38,64, although similar effects on panic-like symptoms have been observed using 7.5%34,37. Future work could investigate the potential differential effects of anxiety types (e.g. somatic vs. psychic; central vs. peripheral) on cognitive performance during CO2 inhalation as well as the neural circuitry implicated in the underlying mechanisms of anxiety when performing complex executive tasks (e.g. dorsolateral prefrontal cortex and lateral parietal cortex)65. Other research outside of the scope of the present study could disentangle psychological/emotional effects, interference from physical sensations and physiological changes in respiratory or autonomic function when interpreting cognitive changes. With respect to emotional processing, it has been previously shown that threat is associated with over-activation of the amygdala in highly anxious individuals41. Although our data showed that ratings of anxiety and fear significantly increased during CO2 inhalation (with ratings of happiness significantly decreasing), it is important to note that the stimuli used in the present study were generally negative rather than threatening. Achieving selective attentional effects may therefore require amygdala reactivity following anticipation or provocation of threat not induced by CO2 inhalation.
Limitations include single-blind administration of the gas manipulation (for safety reasons) and unequal sample sizes between experiments (although both achieved adequate power). Prior to debriefing, most participants reported that the CO2 inhalation session was a relatively intense and/or unpleasant experience, which may have increased demand characteristics of the air inhalation session, although sessions were counterbalanced to reduce this and gas administration order did not interact with key measures of subjective anxiety and panic. Finally, our sample mostly comprised young adults, which may not represent the wider population, but does capture a group in which anxiety disorders are increasingly prevalant. Although a gender difference was only found on subjective ratings of state fear during CO2 inhalation, more detailed investigation of their effects on cognitive/emotional responses to acute anxiety are warranted in larger samples.
Overall, the present study demonstrated state-dependent effects of acute anxiety and autonomic arousal on fronto-executive functions in healthy humans, including impaired cognitive flexibility and working memory. Effects on emotional processing showed a mood-congruent slowing of response in the absence of a negative attentional bias. Identification of resilience factors that protect against acute anxiety may help promote cognitive performance needed for achieving optimal behavioural performance. 7.5% CO2 inhalation in healthy humans also provides a robust model of generalised anxiety that could be used to test new drug therapies for its treatment.
Acknowledgements
This study was funded by a Wellcome Trust Senior Investigator Award to T.W.R. (104631/Z/14/Z/) and carried out in the Behavioural and Clinical Neuroscience Institute supported by a joint award from the Medical Research Council (G1000183) and Wellcome Trust (Strategic Award 093875/Z/10/Z). G.S. was funded by The Wallitt Foundation and Eton College, with support from the NIHR Cambridge Biomedical Research Centre (BRC) Mental Health theme. F.H. is supported by a Cambridge Trust Vice-Chanchellor’s Award and Fitzwilliam College scholarship and was previously supported by an Erasmus scholarship. B.J.S. receives funding from the NIHR Cambridge Biomedical Research Centre (BRC) Mental Health Theme.
Conflict of interest
B.J.S. consults for Cambridge Cognition, Peak and Mundipharma. T.W.R. consults for Cambridge Cognition, Mundipharma and Unilever and receives Royalties for CANTAB. The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: George Savulich, Frank H. Hezemans
References
- 1.Yiend, J., Mackintosh, B. & Savulich, G. in Cognitive Psychology (eds Braisby, N. & Gellatly, A) 507–545 (Oxford University Press, Oxford, 2012).
- 2.Taylor Tavares JV, Drevets WC, Sahakian BJ. Cognition in mania and depression. Psychol. Med. 2003;33:959–967. doi: 10.1017/S0033291703008432. [DOI] [PubMed] [Google Scholar]
- 3.Robinson, O. J., Roiser, J. P. & Sahakian, B. J. in Cognitive Impairment in Major Depressive Disorder: Clinical Relevance, Biological Substrates, and Treatment Opportunities (ed. McIntyre, R. S.) 69 (Cambridge University Press, Cambridge, 2016).
- 4.Fuster JM. Executive frontal functions. Exp. Brain Res. 2000;133:66–70. doi: 10.1007/s002210000401. [DOI] [PubMed] [Google Scholar]
- 5.Miller EK. The prefrontal cortex and cognitive control. Nat. Rev. Neurosci. 2000;1:59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- 6.Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci. Biobehav Rev. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Yiend, J. & Mackintosh, B. in Cognition, Emotion and Psychopathology (ed. Yiend, J.) 190–210 (Cambridge University Press, Cambridge, 2004).
- 8.Savulich G, Shergill S, Yiend J. Biased cognition in psychosis. J. Exp. Psychopathol. 2012;3:514–536. doi: 10.5127/jep.016711. [DOI] [Google Scholar]
- 9.Clarke R, Johnstone T. Prefrontal inhibition of threat processing reduces working memory interference. Front Hum. Neurosci. 2013;7:228. doi: 10.3389/fnhum.2013.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinson OJ, Vytal K, Cornwell BR, Grillon C. The impact of anxiety upon cognition: perspectives from human threat of shock studies. Front Hum. Neurosci. 2013;7:203. doi: 10.3389/fnhum.2013.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okon-Singer H, Hendler T, Pessoa L, Shakman AJ. The neurobiology of cognition-emotion interactions: fundamental questions and strategies for future research. Front Hum. Neurosci. 2015;9:58. doi: 10.3389/fnhum.2015.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gulpers B, Lugtenburg A, Zuidersma M, Verhey FRJ, Voshaar RCO. Anxiety disorders and figural fluency: a measure of executive function. J. Affect Disord. 2018;234:38–44. doi: 10.1016/j.jad.2018.02.038. [DOI] [PubMed] [Google Scholar]
- 13.Ajilchi B, Nejati V. Executive functions in students with depression, anxiety and stress symptoms. Basic Clin. Neurosci. 2017;8:223–232. doi: 10.18869/nirp.bcn.8.3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olley A, Malhi G, Sachdev P. Memory and executive functioning in obsessive-compulsive disorder: a selective review. J. Affect Disord. 2007;104:15–23. doi: 10.1016/j.jad.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 15.Klanker M, Feenstra M, Denys D. Dopaminergic control of cognitive flexibility in humans and animals. Front Neurosci. 2013;7:201. doi: 10.3389/fnins.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson CG, Nusbaum AT, Whitney P, Hinson JM. Trait anxiety impairs cognitive flexibility when overcoming a task acquired response and a preexisitng bias. PLoS ONE. 2018;13:e0204694. doi: 10.1371/journal.pone.0204694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eysenck MW, Derakshan N. New perspectives in attentional control theory. Personal. Individ. Differences. 2011;50:955–960. doi: 10.1016/j.paid.2010.08.019. [DOI] [Google Scholar]
- 18.Eysenck MW, Derakshan B, Santos R, Calvo MG. Anxiety and cognitive performance: attentional control theory. Emotion. 2007;7:336–353. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- 19.Yiend J. The effects of emotion on attention: a review of attentional processing of emotional information. Cognition Emot. 2010;24:3–47. doi: 10.1080/02699930903205698. [DOI] [Google Scholar]
- 20.Murphy FC, et al. Emotional bias and inhibitory control processes in mania and depression. Psychol. Med. 1999;29:1307–1321. doi: 10.1017/S0033291799001233. [DOI] [PubMed] [Google Scholar]
- 21.Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ. The neural basis of mood-congruent processing biases in depression. Arch. Gen. Psychiatry. 2002;59:597–604. doi: 10.1001/archpsyc.59.7.597. [DOI] [PubMed] [Google Scholar]
- 22.Erikson K, et al. Mood-congruent bias in affective go/no-go performance of unmedicated patients with major depressive disorder. Am. J. Psychiatry. 2005;162:2171–2173. doi: 10.1176/appi.ajp.162.11.2171. [DOI] [PubMed] [Google Scholar]
- 23.Schulz KP, et al. Does the emotional go/no-go task really measure behavioral inhibition? Convergence with measures of a non-emotional analog. Arch. Clin. Neuropsychol. 2007;22:151–160. doi: 10.1016/j.acn.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oei Everaerd, Elzinga, van Well Bermond. Psychosocial stress impairs working memory at high loads: an association with cortisol levels and memory retrieval. Stress. 2006;9:133–141. doi: 10.1080/10253890600965773. [DOI] [PubMed] [Google Scholar]
- 25.Schoofs D, Preuss D, Wolf OT. Psychosocial stress induces working memory impairments in an n-back paradigm. Psychoeuroendocrinology. 2008;33:643–653. doi: 10.1016/j.psyneuen.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Schoofs D, Wolf OT, Smeets T. Cold pressor stress impairs performance o working memory tasks requiring executive functions in healthy young men. Behav. Neursci. 2009;123:1066–1075. doi: 10.1037/a0016980. [DOI] [PubMed] [Google Scholar]
- 27.Shackman AJ, et al. Anxiety selectively disrupts visuospatial working memory. Emotion. 2006;6:40–61. doi: 10.1037/1528-3542.6.1.40. [DOI] [PubMed] [Google Scholar]
- 28.Smeets T, et al. Introducing the Masstrict Acute Stress Test (MAST): a quick and non-invasive approach to elicit robust autonomic and glucocorticoid stress responses. Psychoneuroendocrinology. 2012;37:1998–2008. doi: 10.1016/j.psyneuen.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 29.Eysenck MW, Calvo MG. Anxiety and performance: the processing efficiency theory. Cognition Emot. 1992;6:409–434. doi: 10.1080/02699939208409696. [DOI] [Google Scholar]
- 30.Lavie N, Hirst A, de Fockert JW, Viding E. Load theory of selective attention and cognitive control. J. Exp. Psychol. Gen. 2004;133:339–354. doi: 10.1037/0096-3445.133.3.339. [DOI] [PubMed] [Google Scholar]
- 31.Broadbent, D. E. Decision and Stress. (Academic Press, 1971).
- 32.Gorman JM, et al. Response to hyperventilation in a group of patients with panic disorder. Am. J. Psychiatry. 1984;141:857–861. doi: 10.1176/ajp.141.7.857. [DOI] [PubMed] [Google Scholar]
- 33.Van den Hout MA, Griez E. Panic symptoms after inhalation of carbon dioxide. Br. J. Psychiatry. 1984;144:503–507. doi: 10.1192/bjp.144.5.503. [DOI] [PubMed] [Google Scholar]
- 34.Bailey JE, Argyropoulos SV, Kendrick AH, Nutt DJ. Behavioural and cardiovascular effects of 7.5% CO2 inhalation in human volunteers. Depress Anxiety. 2005;21:18–25. doi: 10.1002/da.20048. [DOI] [PubMed] [Google Scholar]
- 35.Poma S, et al. Characterisation of a 7% carbon dioxide inhalation paradigm to evoke anxiety symptoms n healthy subjects. J. Psychopharmacol. 2005;19:494–503. doi: 10.1177/0269881105056533. [DOI] [PubMed] [Google Scholar]
- 36.Atwood AS, Catling JC, Kwong AS, Munafò MR. Effects of 7.5% cardon dioxide (CO2) inhalation and ethnicity on face memory. Physiol. Behav. 2015;147:97–101. doi: 10.1016/j.physbeh.2015.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seddon K, et al. Effects of 7.5% CO2 challenge in generalized anxiety disorder. J. Psychopharmacol. 2011;24:43–51. doi: 10.1177/0269881110364270. [DOI] [PubMed] [Google Scholar]
- 38.Perna G, Barbini B, Cocchi S, Bertani A, Gasperini M. 35% CO2 challenge in panic and mood disorders. J. Affect Disord. 1995;33:189–194. doi: 10.1016/0165-0327(94)00088-Q. [DOI] [PubMed] [Google Scholar]
- 39.Bailey JE, Kendrick A, Diaper A, Potokar JP, Nutt DJ. A validation of the 7.5% Behavioural and cardiovascular effects of 7.5% CO2 model of GAD using paroxetine and lorazepam in healthy volunteers. J. Psychopharmacol. 2007;21:42–49. doi: 10.1177/0269881106063889. [DOI] [PubMed] [Google Scholar]
- 40.Bailey JE, Dawson GR, Dourish CT, Nutt DJ. Validating the inhalation of 7.5% CO2 in healthy volunteers as a human experimental medicine: a model of generalized anxiety disorder (GAD) J. Psychopharmcol. 2011;25:1192–1198. doi: 10.1177/0269881111408455. [DOI] [PubMed] [Google Scholar]
- 41.Garner M, Atwood A, Baldwin DS, James A, Munafò MR. Inhalation of 7.5% carbon dioxide increases threat processing in humans. Neuropsychopharmacology. 2011;36:1557–1562. doi: 10.1038/npp.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bishop S, Duncan J, Brett M, Lawrence AD. Prefrontal cortical function and anxiety: controlling to threat-related stimuli. Nat. Neurosci. 2004;7:184–188. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- 43.Bishop SJ, Duncan J, Lawrence AD. State anxiety modulation of the amygdala response to unattended threat-related stimuli. J. Neurosci. 2004;24:10364–10368. doi: 10.1523/JNEUROSCI.2550-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lecrubier Y, et al. The Mini International Neuropsychiatric Inventory (MINI). A short diagnostic structured interview: reliability and validity according to CIDI. Eur. Psychiatry. 1998;12:224–231. doi: 10.1016/S0924-9338(97)83296-8. [DOI] [Google Scholar]
- 45.Downes JJ, et al. Impaired extra-dimensional shift performance in medicated and unmedicated Parkinson’s disease: evidence for a specific attentional dysfunction. Neuropsychologia. 1989;27:1329–1343. doi: 10.1016/0028-3932(89)90128-0. [DOI] [PubMed] [Google Scholar]
- 46.Owen AM, Downes JJ, Sahakian BJ, Polkey CE, Robbins TW. Planning and spatial working memory following frontal lobe lesion in man. Neuropsychologia. 1990;28:1021–1034. doi: 10.1016/0028-3932(90)90137-D. [DOI] [PubMed] [Google Scholar]
- 47.Spielberger CD, Gorsuch RL, Lushene PR, Vagg PR, Jacobs AG. Manual for the State-Trait Anxiety Inventory (Form Y). Palo Alto: Consulting Psychologists Press; 1983. [Google Scholar]
- 48.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch. Gen. Psychiatry. 1961;4:5614–5671. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 49.Carleton RN, Norton MPJ, Asmundson GJ. Earing the unknown: a short version of the intolerance of uncertainy scale. J. Anxiety Disord. 2007;21:105–117. doi: 10.1016/j.janxdis.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 50.Watson D, Clark LA, Tellegan A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J. Pers. Sco. Psychol. 1988;54:1063–1070. doi: 10.1037/0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 51.Dillon DJ, Gorman JM, Liebowitz MR, Fryer AJ, Klein DF. Measurement of lactate-induced panic and anxiety. Psychiatry Res. 1987;20:97–105. doi: 10.1016/0165-1781(87)90002-3. [DOI] [PubMed] [Google Scholar]
- 52.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful tool approach to multiple testing. J. R. Stat. Soc. B. 1995;57:289–300. [Google Scholar]
- 53.Del Carlo A, et al. Different measures of impulsivity in patients with anxiety disorders: a case control study. Psychiatry Res. 2012;197:231–236. doi: 10.1016/j.psychres.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 54.Plessow F, Fischer R, Kirschbaum C, Goschke T. Inflexibly focused under stress: acute psychosocial stress increases shielding of action goals at the expense of reduced cognitive flexibility with increasing time lag to stressor. J. Cogn. Neurosci. 2011;23:3218–3227. doi: 10.1162/jocn_a_00024. [DOI] [PubMed] [Google Scholar]
- 55.Alexander JK, Hillier A, Smith RM, Tivarus ME, Beversdorf DQ. Beta-adrenergic modulation of cognitive flexibility during stress. J. Cogn. Neuro. 2007;19:468–478. doi: 10.1162/jocn.2007.19.3.468. [DOI] [PubMed] [Google Scholar]
- 56.Laredo SA, et al. Effects of defeat stress on behavioral flexibility in males and females: modulation by the mu-opioid receptor. Eur. J. Neurosci. 2015;41:434–441. doi: 10.1111/ejn.12824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Robbins TW. Dissociating executive functions of the prefrontal cortex. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1996;35:1463–1470. doi: 10.1098/rstb.1996.0131. [DOI] [PubMed] [Google Scholar]
- 58.Chamberlain SR, et al. Impaired cognitive flexibility and motor inhibition in unaffected first-degree relatives of patients with obsessive-compulsive disorder. Am. J. Psychiatry. 2007;164:335–338. doi: 10.1176/ajp.2007.164.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Owen AM, Roberts AC, Polkey CE, Sahakian BJ, Robbins TW. Extra-dimensional versus intra-dimensional set shifting performance following frontal lobe excisions, temporal lobe excisions or amygalo-hyppocampectomy in man. Neuropsychologia. 1991;29:993–1006. doi: 10.1016/0028-3932(91)90063-E. [DOI] [PubMed] [Google Scholar]
- 60.Vaghi MM, et al. Specific frontostriatal circuits for impaired cognitive flexibility and goal-directed planning in obsessive-compulsive disorder: evidence from resting-state functional connectivity. Biol. Psychiatry. 2017;81:708–717. doi: 10.1016/j.biopsych.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Elzinga BM, Roelofs K. Cortisol-induced impairments of working memory require acute sympathetic activation. Behav. Neurosci. 2005;119:98–103. doi: 10.1037/0735-7044.119.1.98. [DOI] [PubMed] [Google Scholar]
- 62.Arnsten AFT. Stress signalling pathways that impair prefrontal cortex structure and function. Nat. Rev. Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smeets T, van Ruitenbeek P, Hartogsveid B. Quaedflieg CWEM. Stress-induced reliance on habitual behaviour is moderated by cortisol reactivity. Brain Cogn. 2018;S0278-2626:30046–0. doi: 10.1016/j.bandc.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 64.Colasanti A, et al. Carbon dioxide-induced emotion and respiratory symptoms in healthy volunteers. Neuropsychopharmacology. 2008;33:3013–3110. doi: 10.1038/npp.2008.31. [DOI] [PubMed] [Google Scholar]
- 65.Sheilds GS, Sazma MA, Yonelinas AP. The effects of acute stress on core executive functions: a meta-analysis and comparison with cortisol. Neurosci. Biobehav Rev. 2016;68:651–668. doi: 10.1016/j.neubiorev.2016.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]