Abstract
Autosomal-dominant polycystic kidney disease (ADPKD) induces a secretory phenotype, resulting in multiple fluid-filled cysts. We have previously demonstrated that VX-809, a corrector of the cystic fibrosis transmembrane conductance regulator (CFTR), reduces cyst growth. Here, we show that in normal mice CFTR is located within the cells and also at the apical and basolateral membranes. However, in polycystic kidney disease (pkd1)-knockout mice, CFTR was located at the plasma membrane, consistent with its role in cAMP-dependent fluid secretion. In cystic mice, VX-809 treatment increased CFTR levels at the apical membrane and reduced its association with the endoplasmic reticulum. Surprisingly, VX-809 treatment significantly increased CFTR's co-localization with the basolateral membrane in cystic mice. Na+/H+ exchanger 3 (NHE3) is present in pkd1-knockout and normal mice and in proximal tubule-derived, cultured pkd1-knockout cells. VX-809 increased the expression, activity, and apical plasma membrane localization of NHE3. Co-localization of epithelial sodium channel (ENaC) with the plasma membrane was reduced in cysts in pkd1-knockout mice, consistent with an inability of the cysts to absorb fluid. Interestingly, in the cystic mice, VX-809 treatment increased ENaC levels at the apical plasma membrane consistent with fluid absorption. Thus, VX-809 treatment of pkd1-null mouse kidneys significantly affected CFTR, NHE3, and ENaC, altering the cyst phenotype from one poised toward fluid secretion toward one more favorable for absorption. VX-809 also altered the location of CFTR but not of NHE3 or ENaC in normal mice. Given that VX-809 administration is safe, it may have potential utility for treating patients with ADPKD.
Keywords: chloride transport, cystic fibrosis transmembrane conductance regulator (CFTR), ion channel, kidney, protein targeting, renal physiology, sodium–proton exchange, sodium channel, epithelial sodium channel (ENaC)
Introduction
Autosomal dominant polycystic kidney disease (ADPKD)2 is a hereditary disorder that affects 1:1000 to 1:500 people. Mutations in the PKD1 or PKD2 gene, which encode polycystin 1 (PC1) and polycystin 2 (PC2), respectively, result in ADPKD (1–4). ADPKD is characterized by the formation of multiple large fluid-filled cysts that displace normal renal tissue and lead to end-stage renal disease by the 6th decade of life (5). Cyst growth involves cAMP-dependent fluid secretion into the cyst lumen (6), a reversal of the predominant function of the kidney, which is to reabsorb fluid. For example, a large fraction of the NaHCO3 and NaCl present in the glomerular filtrate is reabsorbed by the proximal tubules through the action of the sodium–proton exchanger NHE3 (7). In the collecting duct, fluid is absorbed via ENaC-dependent Na+ absorption (8). Thus, to secrete fluid, cysts that develop from the proximal tubules or collecting ducts minimize absorption and adopt a secretory phenotype.
In normal kidney function, proximal renal tubules are most often involved in the reabsorption of fluid filtered at the glomeruli (9). A large fraction of the NaHCO3 and NaCl present in the glomerular filtrate are reabsorbed by the proximal tubules through the action of NHE3 (7). Given its important role in renal transport, NHE3 activity is tightly regulated as follows: by phosphorylation, trafficking, Ca2+, and accessory regulatory proteins such as the PDZ domain-containing NHE regulatory factors PSD95/dlg/zonular occludens-1 (7). The remaining NaCl is absorbed later in the nephrons via a series of transport proteins, including the epithelial Na+ channel ENaC (8). These proteins lose their ability to absorb fluid in ADPKD, and the cystic cells acquire a secretory phenotype. The fluid is secreted into the cyst lumen via a cAMP-dependent mechanism (6, 10).
ENaC plays a role in the absorption of Na+ in the collecting duct (11), airway (12), and sweat duct (13). It is composed of three subunits (14). Patients with ADPKD have abnormal Na+ absorption, particularly in response to a high-salt diet (15), suggesting that absorption of Na+, particularly by ENaC, is disrupted by the cysts. In normal kidneys, the presence of ENaC in the apical membrane is controlled by a number of regulators, most notably serum and glucocorticoid-regulated kinase (SGK1) and neural precursor cell-expressed developmentally down-regulated protein (Nedd 4-2), which associate in a regulatory complex (16). SGK1 is induced by aldosterone, the major hormone that controls Na+ absorption in the kidney (17). Nedd4-2 is an E3 ubiquitin–protein ligase that induces the internalization and degradation of the α-ENaC subunit (18).
Insights into the role of ENaC in Na+ absorption in polycystic kidney disease have come from studies of another cystic disorder, autosomal recessive polycystic kidney disease (ARPKD). Interestingly, Germino and co-workers (19) have found that Nedd 4-2 is mislocalized in ARPKD, leading to up-regulation of ENaC activity and perhaps explaining the hypertension seen in these patients. In seeming contradiction, Veizis et al. (20) have reported that ENaC-mediated Na+ absorption is decreased in the collecting ducts of ARPKD BPK mice and that a reduction in Na+ absorption contributes to the accumulation of cyst fluid. Ilatovskaya et al. (21) may have resolved this apparent contradiction by showing that ENaC function is severely decreased in the PCK/CrljCrlPkhd1pck/CRL (PCK) rat model of ARPKD, when the rats are placed on a salt-restricted diet. Because the cysts get worse when the rats are on a restricted diet, it is clear that ablation of ENaC activity allows the cysts to accumulate fluid. In ARPKD, cysts form primarily from collecting ducts (19); thus, a reduction in the normally absorptive function of ENaC when rats are fed a salt-restricted diet is certainly consistent with increased fluid accumulation in the cyst lumen. Clearly, ENaC can play a dual role in PKD by contributing to hypertension by absorbing fluid when its protein levels are up-regulated and exacerbating cyst formation on a low-salt diet when its protein levels are reduced.
Patients with cystic fibrosis do not have overt defects in renal function (22), suggesting that the role of CFTR in normal renal physiology may be limited. However, given that CFTR is a cAMP-activated Cl− channel that drives fluid secretion in the airways (23) and is present in normal kidneys (24), it is not surprising that CFTR is involved in Cl− secretion into kidney cysts (25–27). Indeed, investigators have shown that in ADPKD, CFTR plays a role in cAMP-driven fluid secretion across epithelial cells (26, 27).
Several investigators have conducted in vivo studies to demonstrate the role of CFTR in ADPKD. For example, O'Sullivan et al. (28) showed that in a large family containing members with both CF and ADPKD, those members with both disorders have less severe kidney and liver disease. In contrast, Persu et al. (29) could not demonstrate any difference in kidney volumes or renal function in ADPKD patients bearing the F508-del CFTR compared with control patients with only ADPKD. Thus, the impact of CFTR on the progression of ADPKD is not entirely clear.
Despite these mixed results, many researchers have suggested that CFTR inhibitors might be used therapeutically in ADPKD to reduce fluid secretion (6, 30–33). For example, Verkman and co-workers (32) identified specific inhibitors of CFTR channel activity and applied them to ADPKD cysts. They screened small-molecule CFTR inhibitors of various chemical classes and found near-complete reduction of cyst growth with CFTRinh-172 and Ph-GlyH-101, without an effect on cell proliferation. Importantly, inhibitor treatment of pkd−/− mice, a model of ADPKD, slowed kidney enlargement and cyst expansion and preserved renal function (32).
CFTR is a member of the ATP-binding cassette family and is composed of two transmembrane domains, two nucleotide-binding domains, and a unique regulatory domain (34, 35). More than 1,900 mutations in CFTR cause cystic fibrosis. The most common mutation is ΔF508, found in NBD1, which affects about 90% of the CF patients (36) (http://www.genet.sickkids.on.ca/cftr). ΔF508-CFTR is a misfolded protein that is retained in the endoplasmic reticulum (ER) and degraded by proteasomes (37). Chemical correctors have been identified that act on ΔF508-CFTR either directly or indirectly to attenuate the deleterious effects of the disease (38–40). Among them, the corrector VX-809 rescues the trafficking of ΔF508-CFTR (41), although its clinical benefit when used alone is limited (42).
In a previously published report, we provided evidence that in a model of ADPKD, VX-809 reduces renal cyst size (43). We used the Pkd1fl/fl;Pax8rtTA;TetO-cre mouse model, in which doxycycline treatment leads to the expression of Cre and ablation of PC1 in renal tubular epithelial cells (44). When injected i.p. with doxycycline on postnatal day (PND)11, PND12, and PND13, these mice develop multiple large cysts and large polycystic kidneys before 3 weeks of age (44, 45). In sharp contrast, Pkd1fl/fl;Pax8rtTA;TetO-cre mice injected daily with VX-809 (30 mg/kg) from PND10 to PND20 show significantly less cyst growth and improved renal function when compared with DMSO-treated (43) (control) mice.
These results raise a conundrum. How can an inhibitor (such as the previously mentioned CFTRinh-172) and a corrector of CFTR both reduce cyst growth? In an interesting and well-done study, Li and Sheppard (33) used Madin-Darby canine kidney (MDCK) cells into which they transfected with WT or mutant ΔF508-CFTR to overexpress both the WT and mutant proteins. Setting aside potential problems with overexpression, they showed that the Vertex-derived corrector VRT-325, in combination with the potentiator VRT-532, reduced the cyst number by ∼30% and cyst volume by ∼50% when applied to untransfected MDCK cells (46); however, when transfected with additional WT CFTR, the cysts increased in size, suggesting that CFTR correctors might have a dual effect on absorption or secretion. MDCK cells are a model for ADPKD, forming cysts in 3D culture (47); thus, the observation that the corrector/potentiator combination reduces cyst growth in untransfected MDCK cells is identical to our published data demonstrating that another Vertex corrector, VX-809, reduces cyst growth in both PC1-null mice and mouse-derived proximal tubule cell models (43).
How the CFTR correctors can reduce cyst volume is not yet known but must involve the reduction in luminal fluid driven by absorption of NaCl out of the cyst. We questioned in this study whether the effect of CFTR correctors occurred via the combined efforts of CFTR, NHE3, and ENaC (14)
Results
VX-809 treatment of cystic kidneys
To begin to study the effect of VX-809, Pkd1fl/fl;Pax8rtTA;TetO-cre mice were injected i.p. with doxycycline (4 μg/g body weight) on postnatal day 11 (PND11), PND12, and PND13 to produce very rapid and aggressive cyst growth (44, 48). Four groups of animals were used: 1) mice injected only with DMSO, no doxycycline (ND); 2) mice injected with DMSO + VX-809 (ND + VX-809); 3) mice injected with doxycycline (D); 4) mice injected with doxycycline + VX809 (D + VX-809). Mice were injected with VX-809 from PND days 10–20 (30 mg/kg) (43). On PND21, the mice were euthanized.
Note that VX-809 reduces cyst area (Fig. 1), and as we have shown previously, it improves renal function (43). To determine how VX-809 reduces cyst size, we studied CFTR, NHE3, and ENaC using confocal microscopy to assess whether VX-809 reduces the size of the cysts by promoting an absorptive phenotype.
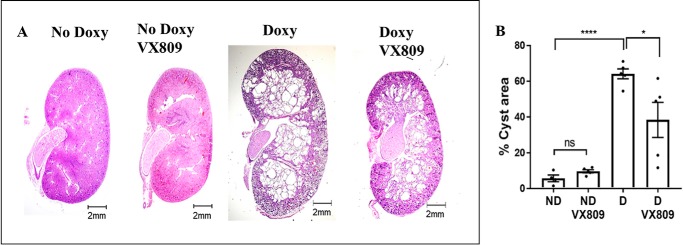
Figure 1.
VX-809 slows cyst growth in pkd1−/− mouse kidney. A, representative images of normal pkd1+/+ (no doxycycline) or pkd1−/− mouse kidney treated with doxycycline (doxy) to induce cyst formation and additionally treated with either DMSO or VX-809. Scale bar is 2 mm. B, measurement of % cyst area = cyst/total kidney cross-sectional area (43). Legends = mice injected only with DMSO, no doxycycline (ND); mice injected with DMSO + VX-809 (ND + VX-809); mice injected with doxycycline (D); mice injected with doxycycline + VX809 (D + VX-809). *, p < 0.05; ****, p < 0.0001; ns, not significant.
CFTR is present at the apical cell membrane
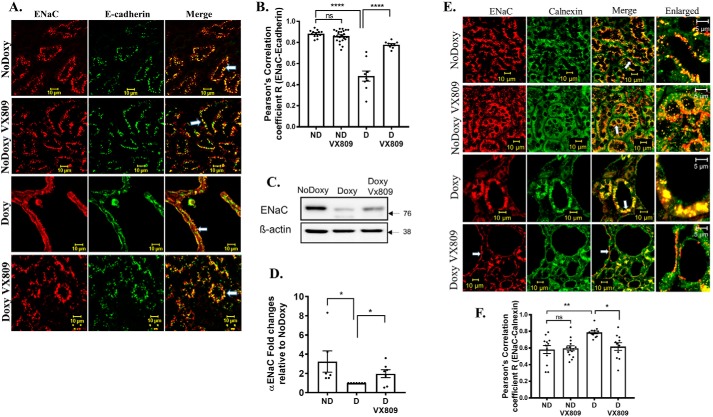
CFTR has been detected by PCR in the cortical and medullary collecting ducts of developing and adult kidneys (49, 50). VX-809 is known to affect the processing of the ΔF508 mutant CFTR protein (41) by stabilizing the interface between an unstable nucleotide-binding domain 1 and intracellular loop 4 (51). To determine whether VX-809 affects the location of WT CFTR in mouse kidneys, we performed confocal microscopy, focusing on both the cortical and medullary regions of the kidney (2). We conducted the studies in the Pkd1fl/fl;Pax8rtTA;TetO-cre mouse model as shown in Fig. 1 (43, 47, 52, 53). Please note that no staining was detected in a CFTR-null mouse model (see Fig. S1) verifying the antibodies are specific for CFTR. Fig. 2, A and C, shows that, as expected, CFTR co-localized with the plasma membrane marker E-cadherin in mouse kidneys induced to form cysts. This result is consistent with reports from others that WT CFTR is present in the apical cell membrane of cysts in ADPKD kidneys (26). Interestingly, VX-809 treatment significantly increased the co-localization of CFTR with E-cadherin by ∼20%. In normal mice where cysts are not induced with doxycycline, CFTR co-localization with E-cadherin is less than cystic kidneys but did not increase significantly when normal kidneys are treated with VX-809.
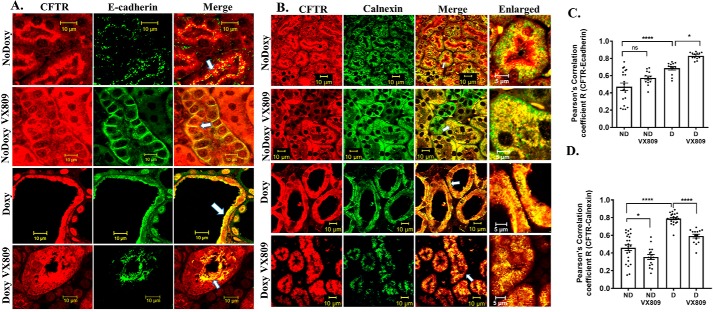
Figure 2.
A and C, CFTR and E-cadherin co-localization. Representative confocal images of normal pkd1+/+ (no doxycycline) or pkd1−/− mouse kidney treated with doxy to induce cyst formation and additionally treated with either DMSO or VX-809. The figure depicts sections stained for CFTR (red) or E-cadherin (green) or the merged image (yellow) that denotes co-localization. Scale bar is 10 μm. C, Pearson's correlation coefficient, R. Note: CFTR co-localization with the apical membrane marker E-cadherin increased by about 30% following VX-809 treatment. B and D, CFTR and calnexin co-localization. B, figure depicts sections stained for CFTR (red), calnexin (green), or the merged image (yellow) that denotes co-localization. Enlarged represents a portion of the merged panel enlarged at the arrow. Scale bar is 10 μm. D, summary of Pearson's correlation coefficient, R. Note: CFTR co-localization with the ER marker calnexin decreased with VX-809 treatment in cystic kidneys. In this and all subsequent figures, data represent results from the kidneys of three animals from each treatment category. 4–5 images were obtained from both cortical and medullary regions of each kidney, and Pearson's coefficients were determined. Statistical significance was determined by an ANOVA followed by a multiple comparisons test using GraphPad software. Each dot in the graph represents a single measurement within a confocal image followed by a calculation of the Pearson's coefficient. Statistical significance is as follows: *, p < 0.05; ****, p < 0.0001; ns, not significant.
It has been reported that a portion of the cell's E-cadherin is mis-processed in ADPKD, causing a decrease in the overall level of the protein, but it still retains its association with the apical membrane (54). Thus, the data we provide here suggest that co-localization of CFTR with E-cadherin is an indication that CFTR is indeed located at the apical plasma membrane both in cystic and normal mouse kidneys.
CFTR is present in the ER
VX-809 is known to affect CFTR trafficking out of the ER (41); therefore, to take this exploration one step further, we asked whether CFTR in the mouse kidney also co-localizes with the ER markers calnexin (Fig. 2, B and D) and KDEL (Fig. S2). Indeed, we found that CFTR does co-localize with calnexin and KDEL in the cystic kidney. The Pearson's coefficients for the co-localization of CFTR with calnexin and KDEL were high, indicating that CFTR is strongly associated within the ER in cystic kidneys. Treatment with VX-809 did indeed lower the co-localization, but there was still significant localization of CFTR in the ER after treatment. In normal mice, CFTR is co-localized with calnexin, but the co-localization is significantly less than that observed in cystic kidneys. Co-localization with calnexin is also reduced in normal kidneys treated with VX-809.
CFTR is present in the Golgi
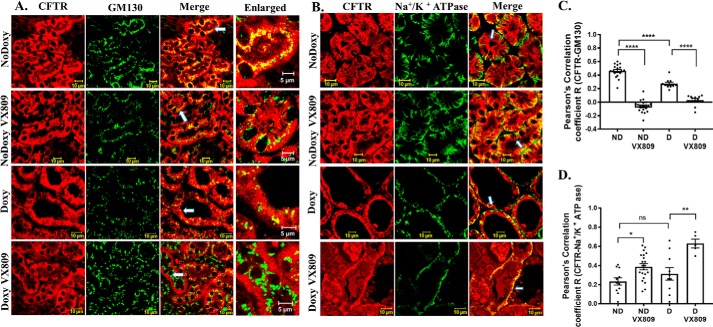
CFTR co-localizes with the cis-Golgi marker GM130 in both cystic and normal kidneys. VX-809 reduces co-localization in both cystic and normal kidneys treated with VX-809 (Fig. 3, A and C). The co-localization with GM130 is significantly higher in normal kidneys indicative of its normal presence in this organelle.
Figure 3.
A and C, CFTR and GM130 co-localization. Representative confocal images of normal pkd1+/+ (no doxycycline) or pkd1−/− mouse kidney treated with doxy to induce cyst formation and additionally treated with either DMSO or VX-809 (A). The figures depict sections stained for CFTR (red), GM130 (green), or the merged image (yellow) that denotes co-localization. Enlarged represents a portion of the merged panel enlarged at the arrow. Scale bar is 10 μm. C, summary of Pearson's correlation coefficient, R. Note: CFTR did not show significant co-localization with the cis-Golgi marker in VX-809–treated animals. B and D, CFTR and Na+/K+-ATPase co-localization. B, figure depicts sections stained for CFTR (red), Na+/K+-ATPase (green), or the merged image (yellow) that denotes co-localization. Scale bar is 10 μm. D, summary of Pearson's correlation coefficient, R. Note: CFTR co-localization with the basolateral membrane marker Na+/K+-ATPase significantly increased following VX-809 treatment. *, p < 0.05; **, p < 0.01; ****, p < 0.0001; ns, not significant.
CFTR strongly co-localizes at the basolateral membrane after VX-809 treatment
We next asked whether CFTR also co-localizes with Na+/K+-ATPase, a marker of the basolateral membrane (Fig. 3, B and D), and we found an interesting greater than 2-fold increase in the co-localization of CFTR with Na+/K+-ATPase after VX-809 treatment in cystic kidneys (Fig. 3, B and D), suggesting that VX-809 increases the primary localization of CFTR to the basolateral membrane. Importantly, the co-localization induced by VX-809 to the basolateral membrane also occurs in normal mice.
Relative presence of CFTR at the basolateral membrane increases with VX-809
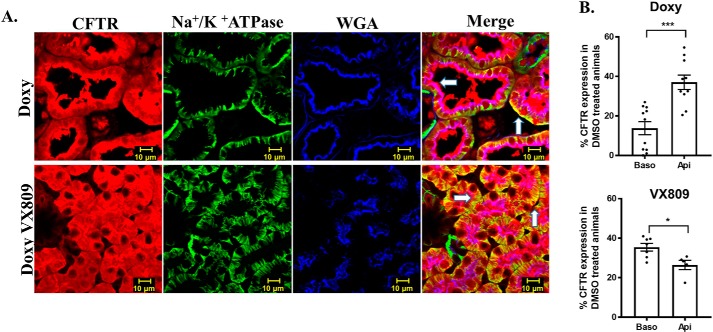
To explore the relative localization of CFTR with apical and basolateral markers, we conducted a triple stain of CFTR with wheat germ agglutinin (WGA), another apical marker (55), and the Na+/K+-ATPase (Fig. 4). One can readily see that CFTR co-localizes with WGA confirming the apical localization we observed when we co-localized CFTR with E-cadherin. Next, we measured the % CFTR in the apical versus basolateral membrane by measuring the CFTR that co-localized with either WGA or Na+/K+-ATPase and dividing that by the total CFTR staining. As can be seen (Fig. 4) in the cystic, doxy-treated animals, the % of CFTR that overlaps with WGA is greater than that which overlaps with the Na+/K+-ATPase. Importantly, following treatment with VX-809, the percentages are reversed with more CFTR overlapping with the Na+/K+-ATPase. These data show clearly that the presence of CFTR in the basolateral membrane increases following VX-809 treatment.
Figure 4.
CFTR and Na+/K+-ATPase and WGA co-localization. A, confocal laser-scanning triple-label immunofluorescence microscopy images of pkd1−/− mouse kidneys stained for CFTR (red), Na+/K+-ATPase, WGA (blue), and the merged image. Scale bar is 10 μm. Yellow denotes co-localization of CFTR with Na+/K+-ATPase, and purple denotes co-localization of CFTR with WGA. B, summary of the % co-localization with either Na+/K+-ATPase or WGA compared with the total CFTR staining. % CFTR was calculated by the total red fluorescence intensity compared with the green (Baso, basolateral) or blue (Api, apical). VX-809 treatment increased CFTR at the basolateral membrane. *, p < 0.05; ***, p < 0.001.
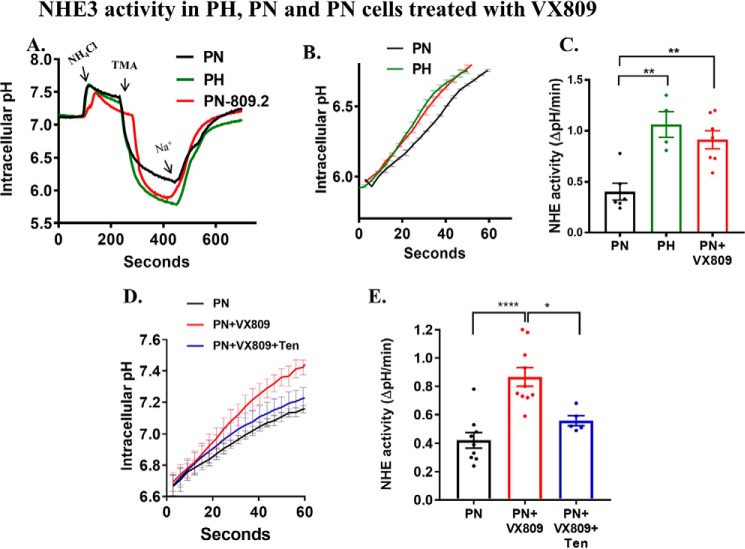
VX-809 increases the activity of NHE3
NHE3 transports protons out of the cell in exchange for Na+ (56); thus, its activity can be measured by removing Na+ and then determining the rate of change in intracellular pH (using a pH-sensitive dye) as Na+ is added back to the bath solution (57, 58). To measure NHE3 activity, we utilized the previously characterized model ADPKD cell line (59, 60) (PN = pkd1 knockout; PH = pkd1 heterozygote) isolated from single parental clones obtained from a pkdfl/− mouse that had been manufactured in the ImmortoMouse containing the H-2Kb-tsA58 gene. The pkd1-null cells (PN) stably express the Cre recombinase, but the control cells (PH) do not. Both PN and PH cells are from the original clone, which is heterozygous for the expression of PC1 (59, 60). PN and PH cells are of proximal tubule origin (60). Using a transport assay in PH (PC1-containing) and PN (PC1-null) cells (Fig. 5A) clearly revealed an initial change in pH in both cell types, resulting from the reintroduction of Na+, but the change was ∼2-fold greater in PH cells than in PN cells (Fig. 5B), indicating higher NHE activity in the PH cells than in the PC1-null cells. Given that NHE3 activity results in the absorption of Na+, a reduction in NHE activity is consistent with a cystic phenotype (61). Importantly, VX-809 causes a 2-fold increase in the rate of change in intracellular pH when Na+ is reintroduced (Fig. 5C), indicating an increase in NHE3 activity that is more consistent with an absorptive phenotype. To determine whether the measured activity is generated by NHE3 rather than other NHE family members, we added a specific inhibitor of NHE3, tenapanor, to the apical solution. Although several other NHE3 inhibitors are also available (62–64), tenapanor does not affect NHE1 or NHE2 at concentrations up to 30 μm (65). Tenapanor reduced the rate of change in intracellular pH in the VX-809–treated cells (Fig. 5, D and E) to that observed in the untreated cells, demonstrating that the increase in NHE activity induced by VX-809 is generated by NHE3.
Figure 5.
NHE3 activity in PN and PH cells. Na+/H+ exchange activity in PN cells was determined fluorometrically using the intracellular pH-sensitive dye BCECF-AM (10 μm). Cells were first exposed to ammonium chloride, followed by TMA to lower sodium in the extracellular solution (see “under Materials and methods” for details). At ∼400 s, sodium was abruptly added back to the apical solution. The resulting rapid increase in pH was used to assess NHE3 activity in PH and PN cells before and after treatment with VX-809 (A). The initial rate of increase in pH over the first 60 s (the initial rate of change) was used to quantify NHE3 activity as shown in B. Summary data are shown in C. Note that PH cells have higher NHE3 activity than PN cells. VX-809 increased NHE3 activity closer to normal levels. The initial rate of increase in pH was determined over the first 60 s (D) in PN cells or cells treated with VX-809 and NHE3 inhibitor (tenapanor). Summary data are shown in E. Note that VX-809 treatment increased NHE3 activity when compared with untreated PN cells. Tenapanor decreased the NHE3 activity to that observed in untreated PN cells. Initial rates of Na+-dependent intracellular alkalinization were calculated starting at pHi 5.6 over the initial 60 s of Na+ exposure (linear phase of pHi change) and expressed as ΔpH/ΔT in an individual experiment. The mean ± S.E. was determined from at least three experiments (n = 3). *, p < 0.05; **, p < 0.01; ****, p < 0.0001.
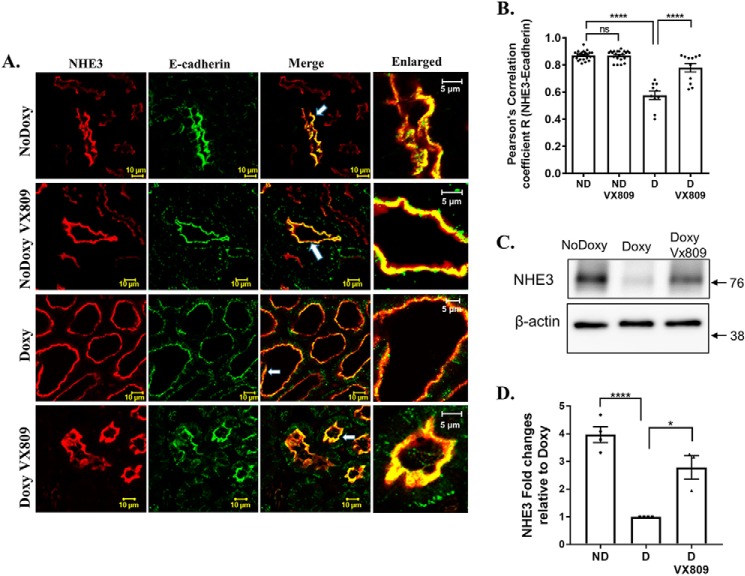
VX-809 increases NHE3 at the plasma membrane
To determine whether VX-809 alters the localization of NHE3, we utilized confocal microscopy. (Fig. 6, A and B) shows that in the cystic epithelium we saw the least co-localization between NHE3 and E-cadherin, a marker of the plasma membrane that is localized to the apical membrane and inter-epithelial cell junctions (66). In contrast, co-localization between NHE3 and E-cadherin was increased in the VX-809–treated animals, reaching levels found in the normal mice not treated with doxycycline indicating that VX-809 increased NHE3 at the plasma membrane. VX-809 had no effect on NHE3 in normal kidneys.
Figure 6.
A and B, NHE3 and E-cadherin co-localization. Representative confocal images of normal pkd1+/+ (no doxycycline) or pkd1−/− mouse kidney treated with doxy to induce cyst formation and additionally treated with either DMSO or VX-809. A, figure depicts sections stained for NHE3 (red), E-cadherin (green), or the merged image (yellow) that denotes co-localization. Scale bar is 10 μm. B, summary of Pearson's correlation coefficient, R. NHE3 co-localization with the membrane marker E-cadherin increased by about 50% following VX-809 treatment. C and D, NHE3 expression in pkd1+/+ (no doxycycline) and pkd1−/− (doxycycline) mice. C, Western blotting showing the expression of NHE3 in pkd1+/+ (no doxy) mice, pkd1−/− (doxy) mice, and pkd1−/− mice treated with VX-809 (doxy VX-809). D, columns represent averages ± S.E. of NHE3 expression. Data were analyzed by ANOVA followed by a multiple comparison test compared with doxycycline-treated animals. The experiment was repeated 3–4 times as depicted by the dots. *, p < 0.05; ****, p < 0.0001; ns, not significant. The NHE3 expression was higher in pkd1+/+ mice than in pkd1−/− mice. VX-809 treatment increased the NHE3 expression in pkd1−/− mice.
To study NHE3 further, we assayed for protein expression (Fig. 6, C and D). Treating the mice with doxycycline to induce cyst formation reduced the NHE3 protein in their kidneys consistent with the lesser activity found in cystic untreated kidneys, and treatment with VX-809 restored the protein toward normal levels again consistent with the increased activity noted in Fig. 5.
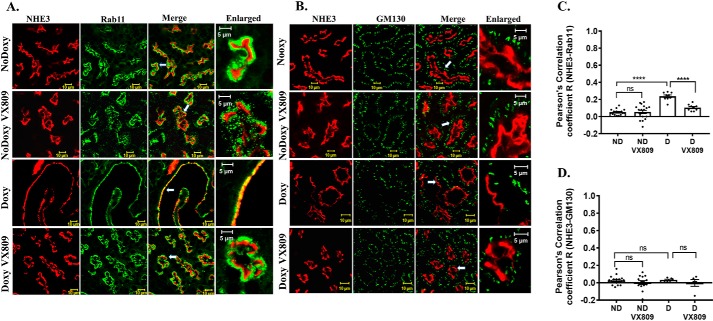
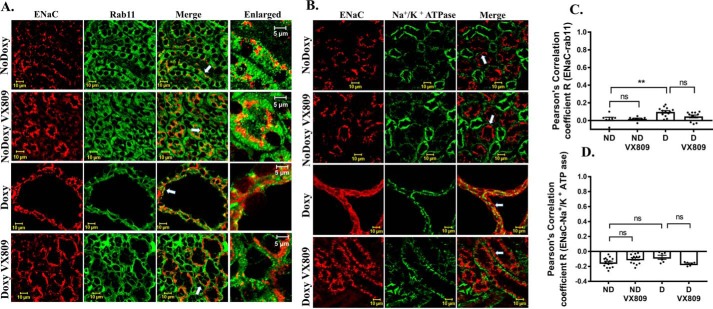
NHE3 co-localizes with the endocytic vesicle marker, Rab11, in cystic kidneys
It is well-known that the regulation of NHE3 occurs via changes in the rate of its endocytosis and exocytosis (67). Thus, to test the degree to which NHE3 localizes with endocytic vesicles, we determined its co-localization with Rab11 (Fig. 7, A and C), a GTPase that is associated with vesicles in the trans-Golgi network and recycling endosomes (68). In the doxy-treated animals, we saw a significant overlap between NHE3 and Rab11, indicating that in the cysts, NHE3 resides within the endosomal compartment. In striking contrast, after VX-809 treatment, there was little co-localization of NHE3 with Rab11. Even less co-localization of Rab11 and NHE3 occurs in the normal kidney, which is unaffected by VX-809. These findings clearly indicate that VX-809 promotes the movement of NHE3 to the luminal membrane. Further corroborating this finding, Fig. 7, B–D, shows that NHE3 does not co-localize with GM130, a marker for the cis-Golgi.
Figure 7.
A and C, NHE3 and Rab11 co-localization. Representative confocal images of normal pkd1+/+ (no doxycycline) or pkd1−/− mouse kidney treated with doxy to induce cyst formation and additionally treated with either DMSO or VX-809. A, figure depicts sections stained for NHE3 (red) or Rab11 (green) or the merged image (yellow) that denotes co-localization. Enlarged represents a portion of the merged panel enlarged at the arrow. Scale bar is 10 μm. C, summary of Pearson's correlation coefficient, R. Note: NHE3 co-localization with the endosomal marker Rab11 decreased by about 50% following VX-809 treatment in the cystic kidneys. B and D, lack of NHE3 and GM130 co-localization. B, figure depicts sections stained for NHE3 (red) or GM130 (green) or the merged image (yellow) that denotes co-localization. Enlarged represents a portion of the merged panel enlarged at the arrow. Scale bar is 10 μm. D, summary of Pearson's correlation coefficient, R. Note that NHE3 does not co-localize with the cis-Golgi marker GM130. ****, p < 0.0001; ns, not significant.
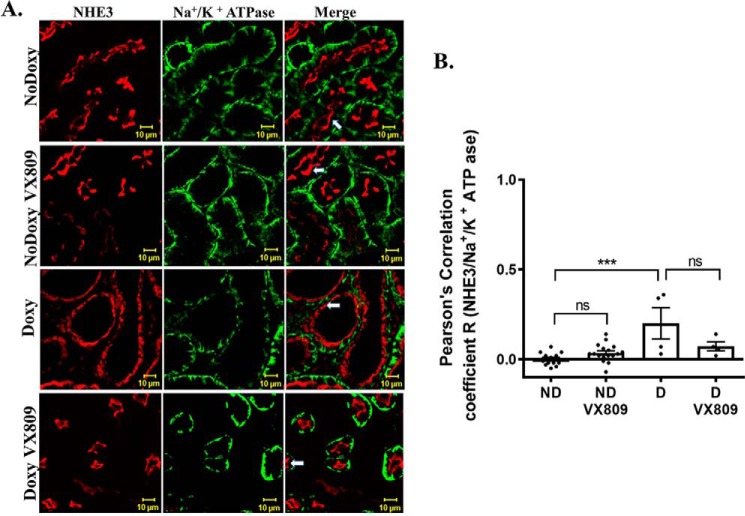
NHE3 does not co-localize with the Na+/K+-ATPase
Next, we asked whether NHE3 is localized to the basolateral or apical area of the cell. To address this question, we looked for co-localization of NHE3 with Na+/K+-ATPase (Fig. 8), which is typically found at the basolateral cell membrane (69). We observed that NHE3 and Na+/K+-ATPase are in two distinctive and separate locations in the cysts in both the doxy-treated mice and the doxy-VX-809–treated and in normal mice The Pearson's coefficient shows that there was no significant overlap between the two proteins.
Figure 8.
Lack of NHE3 and Na+/K+-ATPase co-localization. Representative confocal images of normal pkd1+/+ (no doxycycline) or pkd1−/− mouse kidney treated with doxy to induce cyst formation and additionally treated with either DMSO or VX-809. A, figure depicts sections stained for NHE3 (red) or Na+/K+-ATPase (green) or the merged image (yellow) that denotes co-localization. B, summary of Pearson's correlation coefficient, R. Note that NHE3 does not co-localize with the Na+/K+-ATPase in either the presence or absence of VX-809. ***, p < 0.001; ns, not significant.
Taken together, the data demonstrate that after VX-809 treatment, NHE3 is located primarily at the apical plasma membrane in the mouse kidney; typically, NHE3 is located at the plasma membrane in the proximal tubule (70) and functions in this location to exchange Na+ and H+. Thus, an increase in the apical location of NHE3 after VX-809 treatment is entirely consistent with the increase in NHE3 activity noted in Fig. 5.
α-ENaC apical membrane localization is restored by VX-809
As mentioned above, to adopt a secretory phenotype, the collecting duct must minimize its normal tendency to absorb fluid. This indeed happens with VX-809 treatment (Fig. 9, A and B). As expected, co-localization of α-ENaC with the plasma membrane marker E-cadherin was diminished in mouse kidneys induced to form medullary cysts (doxy). Fig. 9, A and B, also shows that VX-809 caused an almost doubling of the co-localization of α-ENaC with E-cadherin, consistent with a key role being played by α-ENaC absorption in shrinking the cysts following VX-809 treatment. Importantly, after VX-809 treatment, the co-localization of ENaC with E-cadherin resembled that found in normal noninduced kidneys. VX-809 had no effect on ENaC in normal kidneys.
Figure 9.
A and B, ENaC and E-cadherin co-localization. Representative confocal images of normal pkd1+/+ (no doxycycline) or pkd1−/− mouse kidney treated with doxy to induce cyst formation and additionally treated with either DMSO or VX-809. A, figure depicts sections stained for ENaC (red), E-cadherin (green), or the merged image (yellow) that denotes co-localization. Scale bar is 10 μm. B, summary of Pearson's correlation coefficient, R. Note: ENaC co-localization with the apical membrane marker E-cadherin increased by about 30% following VX-809 treatment. C, Western blotting of ENaC. Kidneys were treated as specified. No doxycycline (normal kidneys), doxycycline (PC1-null), and doxycycline + VX-809. D, summary data. n = 6–7. ANOVA followed by multiple comparisons. Note that ENaC protein expression dropped dramatically following induction of cysts and increased to near-normal values following VX-809 application. E and F, ENaC is found in the ER. E, figure depicts sections stained for ENaC (red) or calnexin (green) or the merged image (yellow) that denotes co-localization. Enlarged represents a portion of the merged panel enlarged at the arrow. Scale bar is 10 μm. F, summary of Pearson's correlation coefficient, R. Note: ENaC had significantly higher co-localization with the ER marker in cystic kidneys, which was reduced by VX-809-treatment. *, p < 0.05; **, p < 0.01; ****, p < 0.0001; ns, not significant.
α-ENaC association with the ER is restored by VX-809
We found that in cystic kidneys there is a dramatic reduction in the steady-state levels of α-ENaC (Fig. 9, C and D). To take this one-step further, we asked whether α-ENaC co-localizes with calnexin, an indication that it is present in the ER (Fig. 9, E and F). Indeed α-ENaC is present in the ER in normal kidneys but is higher in the cystic kidneys. Levels are restored to normal following VX-809 treatments. VX-809 has no effect on the co-localization of ENaC with calnexin in normal kidneys.
α-ENaC does not associate either with Rab11-positive endosomes or the basolateral membrane
Unlike what we observed for NHE3, ENaC does not co-localize the Rab11 (Fig. 10, A–C). Also, consistent with its role in Na+ absorption (14), α-ENaC does not co-localize with the basolateral membrane marker Na+/K+-ATPase, either before or after VX-809 treatment (Fig. 10, B–D) in cystic or normal kidneys.
Figure 10.
A and C, lack of ENaC and Rab11 co-localization. Representative confocal images of normal pkd1+/+ (no doxycycline) or pkd1−/− mouse kidney treated with doxy to induce cyst formation and additionally treated with either DMSO or VX-809. A, figures depict sections stained for ENaC (red) or Rab11 (green) or the merged image (yellow) that denotes co-localization. Enlarged represents a portion of the merged panel enlarged at the arrow. Scale bar is 10 μm. C, summary of Pearson's correlation coefficient, R. Note: ENaC did not show significant co-localization with the endosomal marker in either control or VX-809–treated animals. B and D, lack of ENaC and Na+/K+-ATPase co-localization. B, figure depicts sections stained for ENaC (red) or Na+/K+-ATPase (green) or the merged image (yellow) that denotes co-localization. Scale bar is 10 μm. D, summary of Pearson's correlation coefficient, R. Note: ENaC did not show significant co-localization with the basolateral marker in either control or VX-809–treated animals. **, p < 0.01; ns, not significant.
Discussion
VX-809 is a drug approved for use in CF patients to rescue folding mutations (41). Here, we show that treatment of PC1-null mouse kidneys with VX-809 has unexpected effects on key transport proteins, altering the phenotype of the cystic kidneys from one that is poised toward fluid secretion toward one more favorable for absorption. The secretory phenotype is consistent with ADPKD, whereas the absorptive phenotype promotes a reduction in cysts even in the presence of malfunctioning polycystins. Interestingly, under normal circumstances, CFTR plays a role in secretion in tissues such as the airways and gastrointestinal tract (23). In its role in Cl− secretion, CFTR is present at the apical cell membrane. Also under normal conditions, CFTR also plays a role in fluid absorption in the sweat ducts (71). In the sweat ducts, NaCl absorption (72) occurs via a combination of ENaC and CFTR, with CFTR present at both the apical and basolateral membranes. Thus, CFTR can clearly play a role in either secretion or absorption, depending on the physiological state of the tissue.
VX-809 increases CFTR at the apical membrane, but most strongly at the basolateral membrane
The classic site of action of CFTR in secretory epithelia, as elucidated by studies of cystic fibrosis, is at the apical cell membrane (23). CFTR is present in the luminal membrane of the cysts in ADPKD, and in a cystic high-cAMP environment, it actively moves Cl− into the cyst lumen (25).
How CFTR traffics to the apical membrane has been studied extensively. Early studies showed that the PDZ-interacting domain of CFTR is required for the polarization of CFTR to the apical plasma membrane in human airway and kidney epithelial cells and that the binding of CFTR to NHERF1 plays a role in this process (73). A subsequent study showed that the C-terminal cytoplasmic tail of CFTR, tagged with GFP, is localized to the apical plasma membrane, indicating again that an apical localization signal is present in this domain (74). Interestingly, co-expression of the C-terminal construct with full-length WT CFTR causes a redistribution of CFTR from the apical to the basolateral membranes, indicating that both proteins interact with the same target at the apical membrane. Two different mechanisms would explain these results. The first is that CFTR moves directly from the Golgi to the apical plasma membrane, driven by an apical localization signal. The second is that CFTR randomly moves to both the apical and basolateral cell membranes; in this case, to establish a location tilted toward the apical cell membrane would require that basolateral CFTR either be rapidly degraded or be transposed to the apical cell membrane.
Bidaud-Meynard et al. (55) have demonstrated that in airway cells basolateral CFTR delivery occurs via both biosynthetic (∼35%) and endocytic (∼65%) pathways and that basolateral channels are retrieved via basolateral–to–apical transcytosis, enhancing CFTR apical expression by 2-fold and suppressing its degradation. Given that CFTR has been observed in the basolateral membranes of both secretory and absorptive epithelia, the observation that VX-809 increases the localization of CFTR at the basolateral membrane indicates that VX-809 increases the stability of WT CFTR at the basolateral membrane.
Although not as well-studied, it is not unusual for CFTR to be present and functional at the basolateral membrane even in airway cells that primarily secrete Cl− (75). For example, Farmen et al. (76) showed when they overexpressed CFTR by infecting human airway cells isolated from CF patients with adenovirus containing WT-CFTR that CFTR expression in the basolateral membrane increased. To drive CFTR expression, they utilized two different promoters: a cytomegalovirus (CMV) promoter and a cytokeratin-18 (K18) promoter. They found that the CMV promoter produced 50-fold more transgene expression, but interestingly, the K18 promoter generated more Cl− current. They made the important observation that the CMV-driven CFTR expression increased the CFTR in the basolateral membrane, thereby reducing or shunting the Cl− secretion. In this study, VX-809 was administered to mice that already had a normal copy of CFTR causing an overall increase in CFTR protein levels in the kidney by 60% (43) leading to a 2-fold of CFTR in the basolateral membrane of cystic kidneys. Such an increase in CFTR in the basolateral cell membrane of the cysts as we show here would certainly reduce secretion and promote absorption.
The data of Farmen et al. (76) demonstrate that CFTR can be present in both the apical and basolateral membranes even in human airway cells, which are classically thought to secrete Cl−. In MDCK cells, newly-synthesized CFTR traffics randomly to both the apical and basolateral membranes but is stabilized in the apical membrane to increase its localization there (77). As it turns out, kidney cells like airway cells have a similar flexibility in expressing CFTR at both apical and basolateral membranes.
Another classic site of action of CFTR is in the sweat duct, where it participates in the absorption of Cl− (78). Here, CFTR is present at both the apical and basolateral membranes (79). Patients with cystic fibrosis have elevated sweat Cl− because of a failure of the sweat duct to reabsorb NaCl back into the body (78). The most common CF mutation, ΔF508, produces a defective protein that is trapped in the ER and degraded (80). Thus, it does not function in either the apical or basolateral membranes in the sweat duct, causing elevated sweat Cl−. VX-809 was developed to improve the processing and trafficking of ΔF508 (41). Because ΔF508 also affects the ability of CFTR to function as well as its localization, an effective treatment for CF must include a potentiator such as VX-770 (ivacaftor) to stimulate channel function (41) as well as a corrector such as VX-809 (lumacaftor) to rescue the defective protein processing. Patients given a combination of both compounds (Orkambi®) show a modest reduction in sweat Cl− (81). Recently, a triple combination has been developed that includes VX-659 (a novel compound), tezacaftor (VX-661), and ivacaftor (VX-770) (82, 83). This combination has resulted in a reduction in sweat Cl− of ∼40–50%, a remarkable effect. Although not studied directly in sweat duct cells, the triple combination substantially improved the trafficking and processing of ΔF508 in vitro, suggesting that the reduction in sweat Cl− resulted from a rescued processing, trafficking, and function of ΔF508 in the sweat duct in both apical and basolateral membranes.
NHE3 is expressed in a nonfunctional state in ADPKD cysts
In the proximal tubule, NHE3 is located in the microvilli (84) and is also found in recycling endosomes (85). We show here that NHE3 is expressed in the cysts of PC1-null mice, with some co-localization with the membrane marker cadherin. However, it is primarily located within a Rab11 compartment and is not associated with the cis-Golgi marker GM130 or with Na+/K+-ATPase, a marker for the basolateral cell membrane. In these cells, Rab11 is localized to the trans-Golgi network, post-Golgi vesicles, and apical recycling endosomes (68). Thus, in PC1 mice, NHE3 is expressed in the cysts but mainly within the endocytic compartment, consistent with the lack of NHE3 activity that we noted in the PC1-null (PN) cells. In the gastrointestinal tract, a lack of NHE3 function is associated with diarrhea (58). Thus, a lack of NHE3 function is clearly associated with a cystic phenotype.
In the normal kidney, NHE3 is present in the microvilli, along with other transporters (70). A number of regulators (86) decrease the activity of NHE3 by removing it from the microvilli. For example, as shown in a rat model of hypertension, NHE3 moves to specialized domains at the base of the microvilli (probably involving lipid rafts), where it is present in a nonfunctional state (84). NHE3's location in the microvilli is stabilized by the Na+/H+ exchange regulatory factor 1 (NHERF1), which binds to NHE3 and anchors it to the cytoskeleton in the microvilli via the actin-associated protein ezrin. Thus, any disruption in this interaction increases the internalization of NHE3 and the failure of NHE3 to recycle back to the microvilli (86), reducing their ability to absorb Na+. As can be observed from the confocal images presented here in the PC1-null mouse kidney, the cyst cells that contain NHE3 are thin and most likely have defective microvilli. Thus, our finding that NHE3 is co-localized with Rab11 most likely indicates aberrant trafficking of NHE3 to microvilli in the cysts.
Our data show that some NHE3 co-localizes with the plasma membrane marker E-cadherin, despite the lack of NHE3 activity obtained by the transport assay. cAMP inhibits NHE3 activity via its interaction with NHERF1 (87). Thus, it is likely that in the ADPKD cysts, in which cAMP is abnormally high (47), the activity of NHE3, even if present at the plasma membrane, would be reduced.
As mentioned above, NHE3 in cysts is located within the Rab11 compartment. The trafficking of NHE3 to the plasma membrane is normally regulated by intracellular Ca2+ (88, 89). Elevated intracellular Ca2+ causes the internalization of NHE3 from the plasma membrane as a means of reducing its ability to absorb Na+. Ca2+ regulation of NHE3 is thought to occur via a Ca2+-dependent release of NHE3 from NHERF3, leading to the internalization of NHE3. We have shown here that the release of Ca2+ from the ER is elevated in PN (PC1-null) mouse-derived proximal tubule cells. Thus, the abnormally high ER Ca2+ release in ADPKD might be another factor driving NHE3 internalization in the cyst cells.
VX-809 increases NHE3 expression at the plasma membrane and its activity
After treatment with VX-809, both the activity and co-localization of NHE3 with the membrane marker E-cadherin increase, and both are consistent with an absorptive phenotype. How can VX-809 promote the trafficking of NHE3 to the plasma membrane? Interestingly, CFTR forms a stable complex with both NHERF1 and NHERF2 that regulates not only the activity of NHE3 but also its expression level in the luminal membrane (90–92). The net effect is that CFTR enhances the ability of cAMP to inhibit NHE3 activity. Thus, the presence of CFTR in the luminal membrane of the cyst, combined with elevated cAMP, should cause a profound combined reduction in NHE3 activity. Our previous data showed that VX-809 lowers resting cAMP levels in PN cells by approximately half and also reduces ER Ca2+ release in response to thapsigargin (43). These VX-809–induced changes would be expected to remove a powerful inhibitory effect on NHE3 and increase its activity, as we have observed here.
VX-809 increases ENaC at the apical membrane, but not at the basolateral membrane
Interestingly, both WT CFTR (93) and ENaC (94) are inefficiently processed and to a large extent degraded. CFTR is profoundly dependent upon its interactions with several chaperones for its processing out of the ER (95), namely HSC70/HSP70, assisted by the co-chaperones HDJ-2 and HDJ-1 (96) and HSP90 (97). Furthermore, we have shown that HSP27 is up-regulated in ΔF508-CFTR–containing cells and plays a role in CFTR's degradation, along with HSP40 (98), and that reducing HSP27 restores the function of ΔF508, as do correctors.
Processing of ENaC is also critically dependent upon chaperones (94). For example, HSC70 decreases and HSP70 increases the function and surface expression of ENaC (99). The same chaperones can participate in the forward process and in degradation, depending upon their interaction with regulatory factors (100). In our published data, we have shown that treatment of cyst cells with VX-809 reduces the levels of HSP27, HSP70 (HSPA1A/HSPA1), and HSP90, three stress-responsive HSPs (101, 102).
We show here that induction of the cysts in the mice is associated with a profound drop in ENaC protein expression, possibly suggesting that ENaC levels are reduced by enhanced degradation. Thus, it is possible that VX-809, most likely by altering a proteostatic network of proteins, enhances the protein expression of ENaC to allow more ENaC to be present at the apical cell membrane and thereby promote fluid absorption.
VX-809 affects the co-localization of CFTR in the basolateral membrane of normal mice
Although VX-809 is used in patients with CF, the effect of VX-809 on transport proteins in the kidney has not been explored. Here, we observed that VX-809 decreases co-localization with a marker of the Golgi and increases the co-localization of CFTR with the basolateral membrane without any effect on NHE3 or ENaC. Given that patients with cystic fibrosis do not have major changes in renal function, it is unclear whether the observed changes in CFTR will affect patients taking the drug.
Conclusions
It has been suggested that in cyst epithelia, the Na+/K+-ATPase is mis-localized (103); our observation that neither NHE3 nor α-ENaC co-localizes with Na+/K+-ATPase, in contrast to CFTR's co-localization with this basolateral marker, is good evidence that the cysts maintain their ability to maintain the location of proteins in the apical versus basolateral membrane.
NHE3 primarily functions in the proximal tubule (70) and ENaC in the collecting duct (8). Given that VX-809 reduces cyst growth throughout the kidney (43), we suggest that in those cysts that arise from the proximal tubule or from the more distal segments of the kidney, VX-809 will induce CFTR to pair up with NHE3 in the former and ENaC in the latter, respectively, to absorb the fluid (25).
Thus, our results indicate that VX-809, by rearranging key transport proteins already present in the cystic epithelium, promotes an absorptive phenotype.
Materials and methods
Mouse experiments
All animal use complied with the guiding principles of The Johns Hopkins University Institutional Animal Care and Use Committee, and the protocols for this work were approved by the Committee. Pkd1fl/fl;Pax8rtTA;TetO-cre mice on a C57BL/6 background (104) were provided by the Baltimore PKD Center. Mouse experiments were conducted as described previously (43). Mice of both sexes were used in this study. They were generally fed a diet containing low fiber (5%), protein (20%), and fat (5–10%). The pelleted feed was supplied as regular, breeder, certified, irradiated, or autoclavable. Mice were supplied with food and water ad libitum; water was supplied using automatic waterers. The mice were maintained at 30–70% relative humidity and a temperature of 18–26 °C, with at least 10 room-air changes per h. The mice were housed in standard shoebox cages without filter tops; the cages were provided with bedding composed of paper, wood shavings, wood chips, or corncobs, which was routinely changed once or twice per week.
NHE3 transport assay
Monolayers of PN cells were grown on polycarbonate membranes (0.4-μm pore size) attached to plastic coverslips (filter slips) for 6 days. Approximately 16 h after treatment with VX-809, Na+/H+ exchange activity (NHE3) was determined with the intracellular pH (pHi)-sensitive dye BCECF-AM (30-min loading). The slides were placed in a fluorometer (Photon Technology International, Lawrenceville, NJ), after sequential acidification with NH4Cl (40 mm NH4Cl in 98 mm Na+ solution) and TMA+ solution (130 mm tetramethylammonium chloride (TMA), 5 mm KCl, 2 mm CaCl2, 1 mm MgSO4, 1 mm NaH2PO4, 25 mm glucose, and 20 mm HEPES, pH 7.4), cells were switched to Na+ solution (130 mm NaCl instead of TMA-Cl) for Na+-dependent pHi recovery. At the end of each experiment, the fluorescence ratio was calibrated to the pHi value using the high-potassium/nigericin method (105). Initial rates of Na+-dependent intracellular alkalinization were calculated, starting at pHi 5.6, over the initial 1-min Na+ exposure (linear phase of pHi change) and expressed as ΔpH/ΔT in an individual experiment. The mean ± S.E. was determined from at least three experiments.
Confocal microscopy
Mouse tissues were fixed with paraformaldehyde and embedded in paraffin blocks. Deparaffinized tissue sections were blocked with 4% serum for 1 h at 25 °C; antigen retrieval was accomplished by heat mediation in a citrate buffer (DAKO catalog no. S1699). Samples were incubated with primary antibody: anti-E-cadherin, R&D Systems catalog no. AF748; anti-Na+/K+-ATPase, Abcam catalog no. ab7671; anti-NHE3, Novus catalog no. NBP182574; anti-Rab11, BD Biosciences catalog no. 610656; anti-CFTR-596 antibody, Jack Riordan, University of North Carolina, Chapel Hill, or anti-GM130, BD catalog no. 610823; KDEL, catalog no. ab176333; calnexin, catalog no. NBP1-37774, all at 5 μg/ml in blocking buffer for 16 h at 4 °C. Anti-rabbit AlexaFluor 594- and AlexaFluor 488-conjugated anti-goat secondary antibodies were used at a 1:250 dilution for 1 h to label the proteins. Images were captured using a Zeiss LSM 700 or 880 confocal microscope with a ×63 oil-immersion objective (National Institutes of Health Grants S10OD016374 and. S10OD023548). For quantification of co-localization, Pearson's correlation coefficients were calculated using Zen 2012 software.
Western blotting: tissue lysates
The dissected tissue of interest was washed briefly with chilled 1× PBS to remove any contaminating blood and flash-frozen using liquid nitrogen. To prevent protein degradation, the frozen tissue samples were ground, using a mortar and pestle, while submerged in liquid nitrogen. In general, 1 ml of Nonidet P-40 (150 mm Tris-HCl (pH 7.4) with 50 mm NaCl, 1% Nonidet P-40, and Halt protease inhibitor) (Thermo Scientific, catalog no. 78438) buffer was used for approximately every 20 mg of tissue. Samples were rotated in the buffer for 1 h at 4 °C, and the lysates were then centrifuged at 10,000 × g for 10 min at 4 °C to pellet insoluble material, and the supernatants were collected. Protein concentrations were measured with protein assay (Bio-Rad, catalog no. 50-0006), and the supernatants were then denatured in 2× Laemmli buffer at 37 °C for 20 min and run on 4–15% TGX precast gels (Bio-Rad catalog no. 4561084) before transfer to a polyvinylidene fluoride membrane (Bio-Rad). The membranes were incubated with primary antibody overnight at 4 °C and then washed with TBS/Tween 20 buffer. A horseradish peroxidase–conjugated secondary antibody from GE Healthcare (NA934V; 1:10,000) was incubated with the membranes for 1 h, and then ECL Prime was used for band detection on the membranes. β-Actin was used as the loading control.
Data availability
All data generated or analyzed during this study will be made available by the corresponding author upon reasonable request. All data generated or analyzed during this study are included. No data sets were generated.
Author contributions
M. K. Y. and L. C. resources; M. K. Y. and L. C. data curation; M. K. Y. and L. C. validation; M. K. Y., C. C., and L. C. investigation; M. K. Y. and L. C. visualization; M. K. Y., B. C., and L. C. methodology; L. C. conceptualization; L. C. formal analysis; L. C. supervision; L. C. funding acquisition; L. C. writing-original draft; L. C. project administration; L. C. writing-review and editing.
Supplementary Material
Acknowledgments
We appreciate Deborah McClellan, Ph.D., for editing the manuscript. The functional assay for NHE3 was supported by the Hopkins Conte Digestive Diseases Basic and Translational Research Core Center and National Institutes of Health NIDDK Grant P30DK089502. We also thank the Yale Center for the Study of Polycystic Kidney Disease for providing the PN and PH cells and the Baltimore PKD Center for providing the mouse model. We thank William B. Guggino, Ph.D., for scientific discussions and help with the manuscript. We also thank Mark Donowitz, M.D., for the discussions on NHE3 physiology and functional assays.
This work was supported in part by Polycystic Kidney Disease Foundation Grant 238G19a. The authors have declared a conflict of interest. L. C. has license and consulting agreements with RA Capital. These did not influence the experiments conducted in this manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains Figs. S1 and S2.
- ADPKD
- autosomal-dominant polycystic kidney disease
- doxy
- doxycycline
- ENaC
- epithelial sodium channel
- CF
- cystic fibrosis
- CFTR
- cystic fibrosis transmembrane conductance regulator
- NHE3
- Na+/H+ exchanger 3
- ANOVA
- analysis of variance
- WGA
- wheat germ agglutinin
- TMA
- tetramethylammonium
- ER
- endoplasmic reticulum
- BCECF-AM
- 2′,7′-bis(carboxyethyl)-5,6-carboxyfluorescein-acetoxymethyl ester
- ARPKD
- autosomal recessive polycystic kidney disease
- PND
- postnatal day
- MDCK
- Madin-Darby canine kidney
- CMV
- cytomegalovirus
- K18
- cytokeratin-18.
References
- 1. Al-Bhalal L., and Akhtar M. (2005) Molecular basis of autosomal dominant polycystic kidney disease. Adv. Anat. Pathol. 12, 126–133 10.1097/01.pap.0000163959.29032.1f [DOI] [PubMed] [Google Scholar]
- 2. Calvet J. P., and Grantham J. J. (2001) The genetics and physiology of polycystic kidney disease. Semin. Nephrol. 21, 107–123 10.1053/snep.2001.20929 [DOI] [PubMed] [Google Scholar]
- 3. Burn T. C., Connors T. D., Dackowski W. R., Petry L. R., Van Raay T. J., Millholland J. M., Venet M., Miller G., Hakim R. M., and Landes G. M. (1995) Analysis of the genomic sequence for the autosomal dominant polycystic kidney disease (PKD1) gene predicts the presence of a leucine-rich repeat. The American PKD1 Consortium (APKD1 Consortium). Hum. Mol. Genet. 4, 575–582 10.1093/hmg/4.4.575 [DOI] [PubMed] [Google Scholar]
- 4. Mochizuki T., Wu G., Hayashi T., Xenophontos S. L., Veldhuisen B., Saris J. J., Reynolds D. M., Cai Y., Gabow P. A., Pierides A., Kimberling W. J., Breuning M. H., Deltas C. C., Peters D. J., and Somlo S. (1996) PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science 272, 1339–1342 10.1126/science.272.5266.1339 [DOI] [PubMed] [Google Scholar]
- 5. Harris P. C., and Torres V. E. (2006) Understanding pathogenic mechanisms in polycystic kidney disease provides clues for therapy. Curr. Opin. Nephrol. Hypertens. 15, 456–463 10.1097/01.mnh.0000232888.65895.e7 [DOI] [PubMed] [Google Scholar]
- 6. Hanaoka K., and Guggino W. B. (2000) cAMP regulates cell proliferation and cyst formation in autosomal polycystic kidney disease cells. J. Am. Soc. Nephrol. 11, 1179–1187 [DOI] [PubMed] [Google Scholar]
- 7. Moe O. W. (1999) Acute regulation of proximal tubule apical membrane Na/H exchanger NHE-3: role of phosphorylation, protein trafficking, and regulatory factors. J. Am. Soc. Nephrol. 10, 2412–2425 [DOI] [PubMed] [Google Scholar]
- 8. Barbry P., and Hofman P. (1997) Molecular biology of Na+ absorption. Am. J. Physiol. 273, G571–G585 10.1152/ajpgi.1997.273.3.G571 [DOI] [PubMed] [Google Scholar]
- 9. Koeppen B. M., and Stanton B. A. (2012) Renal Physiology E-Book: Mosby Physiology Monograph Series, Elsevier Science Publishing Co., Inc., New York [Google Scholar]
- 10. Grantham J. J., Geiser J. L., and Evan A. P. (1987) Cyst formation and growth in autosomal dominant polycystic kidney disease. Kidney Int. 31, 1145–1152 10.1038/ki.1987.121 [DOI] [PubMed] [Google Scholar]
- 11. McDonough A. A. (1998) Can ENaC regulate ICF as well as ECF volume? Focus on “osmotic pressure regulates αβγ-rENaC expressed in Xenopus oocytes” (editorial; comment). Am. J. Physiol. 275, C1179–C1181 10.1152/ajpcell.1998.275.5.C1179 [DOI] [PubMed] [Google Scholar]
- 12. Talbot C. L., Bosworth D. G., Briley E. L., Fenstermacher D. A., Boucher R. C., Gabriel S. E., and Barker P. M. (1999) Quantitation and localization of ENaC subunit expression in fetal, newborn, and adult mouse lung. Am. J. Respir. Cell Mol. Biol. 20, 398–406 10.1165/ajrcmb.20.3.3283 [DOI] [PubMed] [Google Scholar]
- 13. Reddy M. M., and Quinton P. M. (2003) Functional interaction of CFTR and ENaC in sweat glands. Pflugers Arch. 445, 499–503 10.1007/s00424-002-0959-x [DOI] [PubMed] [Google Scholar]
- 14. Alvarez de la Rosa D., Canessa C. M., Fyfe G. K., and Zhang P. (2000) Structure and regulation of amiloride-sensitive sodium channels. Annu. Rev. Physiol. 62, 573–594 10.1146/annurev.physiol.62.1.573 [DOI] [PubMed] [Google Scholar]
- 15. Graffe C. C., Bech J. N., Lauridsen T. G., and Pedersen E. B. (2012) Urinary excretion of AQP2 and ENaC in autosomal dominant polycystic kidney disease during basal conditions and after a hypertonic saline infusion. Am. J. Physiol. Renal Physiol. 302, F917–F927 10.1152/ajprenal.00616.2011 [DOI] [PubMed] [Google Scholar]
- 16. Rossier B. C. (2014) Epithelial sodium channel (ENaC) and the control of blood pressure. Curr. Opin. Pharmacol. 15, 33–46 10.1016/j.coph.2013.11.010 [DOI] [PubMed] [Google Scholar]
- 17. Chen S. Y., Bhargava A., Mastroberardino L., Meijer O. C., Wang J., Buse P., Firestone G. L., Verrey F., and Pearce D. (1999) Epithelial sodium channel regulated by aldosterone-induced protein sgk. Proc. Natl. Acad. Sci. U.S.A. 96, 2514–2519 10.1073/pnas.96.5.2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhou R., Patel S. V., and Snyder P. M. (2007) Nedd4-2 catalyzes ubiquitination and degradation of cell surface ENaC. J. Biol. Chem. 282, 20207–20212 10.1074/jbc.M611329200 [DOI] [PubMed] [Google Scholar]
- 19. Kaimori J. Y., Lin C. C., Outeda P., Garcia-Gonzalez M. A., Menezes L. F., Hartung E. A., Li A., Wu G., Fujita H., Sato Y., Nakanuma Y., Yamamoto S., Ichimaru N., Takahara S., Isaka Y., et al. (2017) NEDD4-family E3 ligase dysfunction due to PKHD1/Pkhd1 defects suggests a mechanistic model for ARPKD pathobiology. Sci. Rep. 7, 7733 10.1038/s41598-017-08284-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Veizis E. I., Carlin C. R., and Cotton C. U. (2004) Decreased amiloride-sensitive Na+ absorption in collecting duct principal cells isolated from BPK ARPKD mice. Am. J. Physiol. Renal Physiol. 286, F244–F254 10.1152/ajprenal.00169.2003 [DOI] [PubMed] [Google Scholar]
- 21. Ilatovskaya D. V., Levchenko V., Pavlov T. S., Isaeva E., Klemens C. A., Johnson J., Liu P., Kriegel A. J., and Staruschenko A. (2019) Salt-deficient diet exacerbates cystogenesis in ARPKD via epithelial sodium channel (ENaC). EBioMedicine 40, 663–674 10.1016/j.ebiom.2019.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cohen-Cymberknoh M., Shoseyov D., and Kerem E. (2011) Managing cystic fibrosis: strategies that increase life expectancy and improve quality of life. Am. J. Respir. Crit. Care Med. 183, 1463–1471 10.1164/rccm.201009-1478CI [DOI] [PubMed] [Google Scholar]
- 23. Fuller C. M., and Benos D. J. (1992) Cftr! Am. J. Physiol. 263, C267–C286 10.1152/ajpcell.1992.263.2.C267 [DOI] [PubMed] [Google Scholar]
- 24. Morales M. M., Nascimento D. S., Capella M. A., Lopes A. G., and Guggino W. B. (2001) Arginine vasopressin regulates CFTR and ClC-2 mRNA expression in rat kidney cortex and medulla. Pflugers Arch. 443, 202–211 10.1007/s004240100671 [DOI] [PubMed] [Google Scholar]
- 25. Ikeda M., Fong P., Cheng J., Boletta A., Qian F., Zhang X. M., Cai H., Germino G. G., and Guggino W. B. (2006) A regulatory role of polycystin-1 on cystic fibrosis transmembrane conductance regulator plasma membrane expression. Cell. Physiol. Biochem. 18, 9–20 10.1159/000095133 [DOI] [PubMed] [Google Scholar]
- 26. Hanaoka K., Devuyst O., Schwiebert E. M., Wilson P. D., and Guggino W. B. (1996) A role for CFTR in human autosomal dominant polycystic kidney disease. Am. J. Physiol. 270, C389–C399 10.1152/ajpcell.1996.270.1.C389 [DOI] [PubMed] [Google Scholar]
- 27. Sullivan L. P., Wallace D. P., and Grantham J. J. (1998) Epithelial transport in polycystic kidney disease. Physiol. Rev. 78, 1165–1191 10.1152/physrev.1998.78.4.1165 [DOI] [PubMed] [Google Scholar]
- 28. O'Sullivan D. A., Torres V. E., Gabow P. A., Thibodeau S. N., King B. F., and Bergstralh E. J. (1998) Cystic fibrosis and the phenotypic expression of autosomal dominant polycystic kidney disease. Am. J. Kidney Dis. 32, 976–983 10.1016/S0272-6386(98)70072-1 [DOI] [PubMed] [Google Scholar]
- 29. Persu A., Devuyst O., Lannoy N., Materne R., Brosnahan G., Gabow P. A., Pirson Y., and Verellen-Dumoulin C. (2000) CF gene and cystic fibrosis transmembrane conductance regulator expression in autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 11, 2285–2296 [DOI] [PubMed] [Google Scholar]
- 30. Tradtrantip L., Sonawane N. D., Namkung W., and Verkman A. S. (2009) Nanomolar potency pyrimido-pyrrolo-quinoxalinedione CFTR inhibitor reduces cyst size in a polycystic kidney disease model. J. Med. Chem. 52, 6447–6455 10.1021/jm9009873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Verkman A. S., Lukacs G. L., and Galietta L. J. (2006) CFTR chloride channel drug discovery–inhibitors as antidiarrheals and activators for therapy of cystic fibrosis. Curr. Pharm. Des. 12, 2235–2247 10.2174/138161206777585148 [DOI] [PubMed] [Google Scholar]
- 32. Yang B., Sonawane N. D., Zhao D., Somlo S., and Verkman A. S. (2008) Small-molecule CFTR inhibitors slow cyst growth in polycystic kidney disease. J. Am. Soc. Nephrol. 19, 1300–1310 10.1681/ASN.2007070828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li H., and Sheppard D. N. (2009) Therapeutic potential of cystic fibrosis transmembrane conductance regulator (CFTR) inhibitors in polycystic kidney disease. Biodrugs 23, 203–216 10.2165/11313570-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 34. Lewis H. A., Buchanan S. G., Burley S. K., Conners K., Dickey M., Dorwart M., Fowler R., Gao X., Guggino W. B., Hendrickson W. A., Hunt J. F., Kearins M. C., Lorimer D., Maloney P. C., Post K. W., et al. (2004) Structure of nucleotide-binding domain 1 of the cystic fibrosis transmembrane conductance regulator. EMBO J. 23, 282–293 10.1038/sj.emboj.7600040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Riordan J. R. (2008) CFTR function and prospects for therapy. Annu. Rev. Biochem. 77, 701–726 10.1146/annurev.biochem.75.103004.142532 [DOI] [PubMed] [Google Scholar]
- 36. Riordan J. R., Rommens J. M., Kerem B., Alon N., Rozmahel R., Grzelczak Z., Zielenski J., Lok S., Plavsic N., and Chou J. L. (1989) Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 245, 1066–1073 10.1126/science.2475911 [DOI] [PubMed] [Google Scholar]
- 37. Jensen T. J., Loo M. A., Pind S., Williams D. B., Goldberg A. L., and Riordan J. R. (1995) Multiple proteolytic systems, including the proteasome, contribute to CFTR processing. Cell 83, 129–135 10.1016/0092-8674(95)90241-4 [DOI] [PubMed] [Google Scholar]
- 38. Pedemonte N., Lukacs G. L., Du K., Caci E., Zegarra-Moran O., Galietta L. J., and Verkman A. S. (2005) Small-molecule correctors of defective ΔF508-CFTR cellular processing identified by high-throughput screening. J. Clin. Invest. 115, 2564–2571 10.1172/JCI24898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Accurso F. J., Rowe S. M., Clancy J. P., Boyle M. P., Dunitz J. M., Durie P. R., Sagel S. D., Hornick D. B., Konstan M. W., Donaldson S. H., Moss R. B., Pilewski J. M., Rubenstein R. C., Uluer A. Z., Aitken M. L., et al. (2010) Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N. Engl. J. Med. 363, 1991–2003 10.1056/NEJMoa0909825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Amaral M. D., and Farinha C. M. (2013) Rescuing mutant CFTR: a multi-task approach to a better outcome in treating cystic fibrosis. Curr. Pharm. Des. 19, 3497–3508 10.2174/13816128113199990318 [DOI] [PubMed] [Google Scholar]
- 41. Van Goor F., Hadida S., Grootenhuis P. D., Burton B., Stack J. H., Straley K. S., Decker C. J., Miller M., McCartney J., Olson E. R., Wine J. J., Frizzell R. A., Ashlock M., and Negulescu P. A. (2011) Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc. Natl. Acad. Sci. U.S.A. 108, 18843–18848 10.1073/pnas.1105787108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Clancy J. P., Rowe S. M., Accurso F. J., Aitken M. L., Amin R. S., Ashlock M. A., Ballmann M., Boyle M. P., Bronsveld I., Campbell P. W., De Boeck K., Donaldson S. H., Dorkin H. L., Dunitz J. M., Durie P. R., et al. (2012) Results of a phase IIa study of VX-809, an investigational CFTR corrector compound, in subjects with cystic fibrosis homozygous for the F508del-CFTR mutation. Thorax 67, 12–18 10.1136/thoraxjnl-2011-200393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yanda M. K., Liu Q., and Cebotaru L. (2018) A potential strategy for reducing cysts in autosomal dominant polycystic kidney disease with a CFTR corrector. J. Biol. Chem. 293, 11513–11526 10.1074/jbc.RA118.001846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Traykova-Brauch M., Schönig K., Greiner O., Miloud T., Jauch A., Bode M., Felsher D. W., Glick A. B., Kwiatkowski D. J., Bujard H., Horst J., von Knebel Doeberitz M., Niggli F. K., Kriz W., Gröne H. J., and Koesters R. (2008) An efficient and versatile system for acute and chronic modulation of renal tubular function in transgenic mice. Nat. Med. 14, 979–984 10.1038/nm.1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Piontek K. B., Huso D. L., Grinberg A., Liu L., Bedja D., Zhao H., Gabrielson K., Qian F., Mei C., Westphal H., and Germino G. G. (2004) A functional floxed allele of Pkd1 that can be conditionally inactivated in vivo. J. Am. Soc. Nephrol. 15, 3035–3043 10.1097/01.ASN.0000144204.01352.86 [DOI] [PubMed] [Google Scholar]
- 46. Li H., Yang W., Mendes F., Amaral M. D., and Sheppard D. N. (2012) Impact of the cystic fibrosis mutation F508del-CFTR on renal cyst formation and growth. Am. J. Physiol. Renal Physiol. 303, F1176–F1186 10.1152/ajprenal.00130.2012 [DOI] [PubMed] [Google Scholar]
- 47. Cebotaru L., Liu Q., Yanda M. K., Boinot C., Outeda P., Huso D. L., Watnick T., Guggino W. B., and Cebotaru V. (2016) Inhibition of histone deacetylase 6 activity reduces cyst growth in polycystic kidney disease. Kidney Int. 90, 90–99 10.1016/j.kint.2016.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Piontek K., Menezes L. F., Garcia-Gonzalez M. A., Huso D. L., and Germino G. G. (2007) A critical developmental switch defines the kinetics of kidney cyst formation after loss of Pkd1. Nat. Med. 13, 1490–1495 10.1038/nm1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Morales M. M., Carroll T. P., Morita T., Schwiebert E. M., Devuyst O., Wilson P. D., Lopes A. G., Stanton B. A., Dietz H. C., Cutting G. R., and Guggino W. B. (1996) Both the wild type and a functional isoform of CFTR are expressed in kidney. Am. J. Physiol. Renal Physiol. 270, F1038–F1048 10.1152/ajprenal.1996.270.6.F1038 [DOI] [PubMed] [Google Scholar]
- 50. Devuyst O., Burrow C. R., Schwiebert E. M., Guggino W. B., and Wilson P. D. (1996) Developmental regulation of CFTR expression during human nephrogenesis. Am. J. Physiol. 271, F723–F735 10.1152/ajprenal.1996.271.3.F723 [DOI] [PubMed] [Google Scholar]
- 51. Okiyoneda T., Veit G., Dekkers J. F., Bagdany M., Soya N., Xu H., Roldan A., Verkman A. S., Kurth M., Simon A., Hegedus T., Beekman J. M., and Lukacs G. L. (2013) Mechanism-based corrector combination restores ΔF508-CFTR folding and function. Nat. Chem. Biol. 9, 444–454 10.1038/nchembio.1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yanda M. K., Liu Q., Cebotaru V., Guggino W. B., and Cebotaru L. (2017) Histone deacetylase 6 inhibition reduces cysts by decreasing cAMP and Ca2+ in knock-out mouse models of polycystic kidney disease. J. Biol. Chem. 292, 17897–17908 10.1074/jbc.M117.803775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yanda M. K., Liu Q., and Cebotaru L. (2017) An inhibitor of histone deacetylase 6 activity, ACY-1215, reduces cAMP and cyst growth in polycystic kidney disease. Am. J. Physiol. Renal Physiol. 313, F997–F1004 10.1152/ajprenal.00186.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Charron A. J., Nakamura S., Bacallao R., and Wandinger-Ness A. (2000) Compromised cytoarchitecture and polarized trafficking in autosomal dominant polycystic kidney disease cells. J. Cell Biol. 149, 111–124 10.1083/jcb.149.1.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bidaud-Meynard A., Bossard F., Schnúr A., Fukuda R., Veit G., Xu H., and Lukacs G. L. (2019) Transcytosis maintains CFTR apical polarity in the face of constitutive and mutation-induced basolateral missorting. J. Cell Sci. 132, 1–16 10.1242/jcs.226886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chalumeau C., du Cheyron D., Defontaine N., Kellermann O., Paillard M., and Poggioli J. (2001) NHE3 activity and trafficking depend on the state of actin organization in proximal tubule. Am. J. Physiol. Renal Physiol. 280, F283–F290 10.1152/ajprenal.2001.280.2.F283 [DOI] [PubMed] [Google Scholar]
- 57. Lee-Kwon W., Kawano K., Choi J. W., Kim J. H., and Donowitz M. (2003) Lysophosphatidic acid stimulates brush border Na+/H+ exchanger 3 (NHE3) activity by increasing its exocytosis by an NHE3 kinase A regulatory protein-dependent mechanism. J. Biol. Chem. 278, 16494–16501 10.1074/jbc.M300580200 [DOI] [PubMed] [Google Scholar]
- 58. Janecke A. R., Heinz-Erian P., Yin J., Petersen B. S., Franke A., Lechner S., Fuchs I., Melancon S., Uhlig H. H., Travis S., Marinier E., Perisic V., Ristic N., Gerner P., Booth I. W., et al. (2015) Reduced sodium/proton exchanger NHE3 activity causes congenital sodium diarrhea. Hum. Mol. Genet. 24, 6614–6623 10.1093/hmg/ddv367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Joly D., Ishibe S., Nickel C., Yu Z., Somlo S., and Cantley L. G. (2006) The polycystin 1–C-terminal fragment stimulates ERK-dependent spreading of renal epithelial cells. J. Biol. Chem. 281, 26329–26339 10.1074/jbc.M601373200 [DOI] [PubMed] [Google Scholar]
- 60. Wei F., Karihaloo A., Yu Z., Marlier A., Seth P., Shibazaki S., Wang T., Sukhatme V. P., Somlo S., and Cantley L. G. (2008) Neutrophil gelatinase-associated lipocalin suppresses cyst growth by Pkd1 null cells in vitro and in vivo. Kidney Int. 74, 1310–1318 10.1038/ki.2008.395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hayashi H., Szászi K., Coady-Osberg N., Furuya W., Bretscher A. P., Orlowski J., and Grinstein S. (2004) Inhibition and redistribution of NHE3, the apical Na+/H+ exchanger, by Clostridium difficile toxin B. J. Gen. Physiol. 123, 491–504 10.1085/jgp.200308979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Schwark J. R., Jansen H. W., Lang H. J., Krick W., Burckhardt G., and Hropot M. (1998) S3226, a novel inhibitor of Na+/H+ exchanger subtype 3 in various cell types. Pflugers Arch. 436, 797–800 10.1007/s004240050704 [DOI] [PubMed] [Google Scholar]
- 63. Vallon V., Schwark J. R., Richter K., and Hropot M. (2000) Role of Na+/H+ exchanger NHE3 in nephron function: micropuncture studies with S3226, an inhibitor of NHE3. Am. J. Physiol. Renal Physiol. 278, F375–F379 10.1152/ajprenal.2000.278.3.F375 [DOI] [PubMed] [Google Scholar]
- 64. Rosenbaum D., Johansson S., Carlsson B., Spencer A., Stefansson B., Knutsson M., Jacobs J., and Charmot D. (2014) Tenapanor, a minimally absorbed NHE3 inhibitor, reduces dietary phosphorus absorption in healthy volunteers. J. Am. Soc. Nephrol. 25, 72A [Google Scholar]
- 65. Johansson S., Rosenbaum D. P., Knutsson M., and Leonsson-Zachrisson M. (2017) A phase 1 study of the safety, tolerability, pharmacodynamics, and pharmacokinetics of tenapanor in healthy Japanese volunteers. Clin. Exp. Nephrol. 21, 407–416 10.1007/s10157-016-1302-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Brüser L., and Bogdan S. (2017) Adherens junctions on the move-membrane trafficking of E-cadherin. Cold Spring Harb. Perspect. Biol. 9, a029140 10.1101/cshperspect.a029140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Donowitz M., Ming Tse C., and Fuster D. (2013) SLC9/NHE gene family, a plasma membrane and organellar family of Na+/H+ exchangers. Mol. Aspects Med. 34, 236–251 10.1016/j.mam.2012.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Welz T., Wellbourne-Wood J., and Kerkhoff E. (2014) Orchestration of cell surface proteins by Rab11. Trends Cell Biol. 24, 407–415 10.1016/j.tcb.2014.02.004 [DOI] [PubMed] [Google Scholar]
- 69. Rokaw M. D., Sarac E., Lechman E., West M., Angeski J., Johnson J. P., and Zeidel M. L. (1996) Chronic regulation of transepithelial Na+ transport by the rate of apical Na+ entry. Am. J. Physiol. 270, C600–C607 10.1152/ajpcell.1996.270.2.C600 [DOI] [PubMed] [Google Scholar]
- 70. Bobulescu I. A., and Moe O. W. (2009) Luminal Na+/H+ exchange in the proximal tubule. Pflugers Arch. 458, 5–21 10.1007/s00424-008-0595-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Reddy M. M., and Quinton P. M. (1987) Cl− permeability of sweat duct cell membranes: intracellular microelectrode analysis. Prog. Clin. Biol. Res. 254, 45–57 [PubMed] [Google Scholar]
- 72. Quinton P. M. (1999) Physiological basis of cystic fibrosis: a historical perspective. Physiol. Rev. 79, S3–S22 10.1152/physrev.1999.79.1.S3 [DOI] [PubMed] [Google Scholar]
- 73. Moyer B. D., Denton J., Karlson K. H., Reynolds D., Wang S., Mickle J. E., Milewski M., Cutting G. R., Guggino W. B., Li M., and Stanton B. A. (1999) A PDZ-interacting domain in CFTR is an apical membrane polarization signal. J. Clin. Invest. 104, 1353–1361 10.1172/JCI7453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Milewski M. I., Mickle J. E., Forrest J. K., Stafford D. M., Moyer B. D., Cheng J., Guggino W. B., Stanton B. A., and Cutting G. R. (2001) A PDZ-binding motif is essential but not sufficient to localize the C terminus of CFTR to the apical membrane. J. Cell Sci. 114, 719–726 [DOI] [PubMed] [Google Scholar]
- 75. Chambers L. A., Rollins B. M., and Tarran R. (2007) Liquid movement across the surface epithelium of large airways. Respir. Physiol. Neurobiol. 159, 256–270 10.1016/j.resp.2007.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Farmen S. L., Karp P. H., Ng P., Palmer D. J., Koehler D. R., Hu J., Beaudet A. L., Zabner J., and Welsh M. J. (2005) Gene transfer of CFTR to airway epithelia: low levels of expression are sufficient to correct Cl− transport and overexpression can generate basolateral CFTR. Am. J. Physiol. Lung Cell Mol. Physiol. 289, L1123–L1130 10.1152/ajplung.00049.2005 [DOI] [PubMed] [Google Scholar]
- 77. Swiatecka-Urban A., Duhaime M., Coutermarsh B., Karlson K. H., Collawn J., Milewski M., Cutting G. R., Guggino W. B., Langford G., and Stanton B. A. (2002) PDZ domain interaction controls the endocytic recycling of the cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 277, 40099–40105 10.1074/jbc.M206964200 [DOI] [PubMed] [Google Scholar]
- 78. Quinton P. M., and Reddy M. M. (1989) Cl− conductance and acid secretion in the human sweat duct. Ann. N.Y. Acad. Sci. 574, 438–446 10.1111/j.1749-6632.1989.tb25182.x [DOI] [PubMed] [Google Scholar]
- 79. Reddy M. M., and Quinton P. M. (1989) Localization of Cl− conductance in normal and Cl− impermeability in cystic fibrosis sweat duct epithelium. Am. J. Physiol. 257, C727–C735 10.1152/ajpcell.1989.257.4.C727 [DOI] [PubMed] [Google Scholar]
- 80. Gelman M. S., Kannegaard E. S., and Kopito R. R. (2002) A principal role for the proteasome in endoplasmic reticulum-associated degradation of misfolded intracellular cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 277, 11709–11714 10.1074/jbc.M111958200 [DOI] [PubMed] [Google Scholar]
- 81. Zhang W., Zhang X., Zhang Y. H., Strokes D. C., and Naren A. P. (2016) Lumacaftor/ivacaftor combination for CF patients homozygous for Phe508del-CFTR. Drugs Today 52, 229–237 10.1358/dot.2016.52.4.2467205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Davies J. C., Moskowitz S. M., Brown C., Horsley A., Mall M. A., McKone E. F., Plant B. J., Prais D., Ramsey B. W., Taylor-Cousar J. L., Tullis E., Uluer A., McKee C. M., Robertson S., Shilling R. A., et al. (2018) VX-659-tezacaftor-ivacaftor in patients with cystic fibrosis and one or two Phe508del alleles. N. Engl. J. Med. 379, 1599–1611 10.1056/NEJMoa1807119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Keating D., Marigowda G., Burr L., Daines C., Mall M. A., McKone E. F., Ramsey B. W., Rowe S. M., Sass L. A., Tullis E., McKee C. M., Moskowitz S. M., Robertson S., Savage J., Simard C., et al. (2018) VX-445–tezacaftor–ivacaftor in patients with cystic fibrosis and one or two Phe508del alleles. N. Engl. J. Med. 379, 1612–1620 10.1056/NEJMoa1807120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Yang L. E., Maunsbach A. B., Leong P. K., and McDonough A. A. (2004) Differential traffic of proximal tubule Na+ transporters during hypertension or PTH: NHE3 to base of microvilli vs. NaPi2 to endosomes. Am. J. Physiol. Renal Physiol. 287, F896–F906 10.1152/ajprenal.00160.2004 [DOI] [PubMed] [Google Scholar]
- 85. D'Souza S., Garcia-Cabado A., Yu F., Teter K., Lukacs G., Skorecki K., Moore H. P., Orlowski J., and Grinstein S. (1998) The epithelial sodium-hydrogen antiporter Na+/H+ exchanger 3 accumulates and is functional in recycling endosomes. J. Biol. Chem. 273, 2035–2043 10.1074/jbc.273.4.2035 [DOI] [PubMed] [Google Scholar]
- 86. Cha B., Tse M., Yun C., Kovbasnjuk O., Mohan S., Hubbard A., Arpin M., and Donowitz M. (2006) The NHE3 juxtamembrane cytoplasmic domain directly binds ezrin: dual role in NHE3 trafficking and mobility in the brush border. Mol. Biol. Cell 17, 2661–2673 10.1091/mbc.e05-09-0843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Yun C. H., Oh S., Zizak M., Steplock D., Tsao S., Tse C. M., Weinman E. J., and Donowitz M. (1997) cAMP-mediated inhibition of the epithelial brush border Na+/H+ exchanger, NHE3, requires an associated regulatory protein. Proc. Natl. Acad. Sci. U.S.A. 94, 3010–3015 10.1073/pnas.94.7.3010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lee-Kwon W., Kim J. H., Choi J. W., Kawano K., Cha B., Dartt D. A., Zoukhri D., and Donowitz M. (2003) Ca2+-dependent inhibition of NHE3 requires PKCα which binds to E3KARP to decrease surface NHE3 containing plasma membrane complexes. Am. J. Physiol. Cell Physiol. 285, C1527–C1536 10.1152/ajpcell.00017.2003 [DOI] [PubMed] [Google Scholar]
- 89. Kim J. H., Lee-Kwon W., Park J. B., Ryu S. H., Yun C. H., and Donowitz M. (2002) Ca2+-dependent inhibition of Na+/H+ exchanger 3 (NHE3) requires an NHE3–E3KARP–α-actinin-4 complex for oligomerization and endocytosis. J. Biol. Chem. 277, 23714–23724 10.1074/jbc.M200835200 [DOI] [PubMed] [Google Scholar]
- 90. Lee M. G., Ahn W., Lee J. A., Kim J. Y., Choi J. Y., Moe O. W., Milgram S. L., Muallem S., and Kim K. H. (2001) Coordination of pancreatic HCO3− secretion by protein–protein interaction between membrane transporters. JOP 2, 203–206 [PubMed] [Google Scholar]
- 91. Favia M., Fanelli T., Bagorda A., Di Sole F., Reshkin S. J., Suh P. G., Guerra L., and Casavola V. (2006) NHE3 inhibits PKA-dependent functional expression of CFTR by NHERF2 PDZ interactions. Biochem. Biophys. Res. Commun. 347, 452–459 10.1016/j.bbrc.2006.06.112 [DOI] [PubMed] [Google Scholar]
- 92. Bagorda A., Guerra L., Di Sole F., Hemle-Kolb C., Cardone R. A., Fanelli T., Reshkin S. J., Gisler S. M., Murer H., and Casavola V. (2002) Reciprocal protein kinase A regulatory interactions between cystic fibrosis transmembrane conductance regulator and Na+/H+ exchanger isoform 3 in a renal polarized epithelial cell model. J. Biol. Chem. 277, 21480–21488 10.1074/jbc.M112245200 [DOI] [PubMed] [Google Scholar]
- 93. Riordan J. R. (2005) Assembly of functional CFTR chloride channels. Annu. Rev. Physiol. 67, 701–718 10.1146/annurev.physiol.67.032003.154107 [DOI] [PubMed] [Google Scholar]
- 94. Buck T. M., Kolb A. R., Boyd C. R., Kleyman T. R., and Brodsky J. L. (2010) The endoplasmic reticulum–associated degradation of the epithelial sodium channel requires a unique complement of molecular chaperones. Mol. Biol. Cell 21, 1047–1058 10.1091/mbc.e09-11-0944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Amaral M. D. (2004) CFTR and chaperones: processing and degradation. J. Mol. Neurosci. 23, 41–48 10.1385/JMN:23:1-2:041 [DOI] [PubMed] [Google Scholar]
- 96. Meacham G. C., Lu Z., King S., Sorscher E., Tousson A., and Cyr D. M. (1999) The Hdj-2/Hsc70 chaperone pair facilitates early steps in CFTR biogenesis. EMBO J. 18, 1492–1505 10.1093/emboj/18.6.1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Loo M. A., Jensen T. J., Cui L., Hou Y., Chang X. B., and Riordan J. R. (1998) Perturbation of Hsp90 interaction with nascent CFTR prevents its maturation and accelerates its degradation by the proteasome. EMBO J. 17, 6879–6887 10.1093/emboj/17.23.6879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lopes-Pacheco M., Boinot C., Sabirzhanova I., Morales M. M., Guggino W. B., and Cebotaru L. (2015) Combination of correctors rescue ΔF508-CFTR by reducing its association with Hsp40 and Hsp27. J. Biol. Chem. 290, 25636–25645 10.1074/jbc.M115.671925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Goldfarb S. B., Kashlan O. B., Watkins J. N., Suaud L., Yan W., Kleyman T. R., and Rubenstein R. C. (2006) Differential effects of Hsc70 and Hsp70 on the intracellular trafficking and functional expression of epithelial sodium channels. Proc. Natl. Acad. Sci. U.S.A. 103, 5817–5822 10.1073/pnas.0507903103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Farinha C. M., Nogueira P., Mendes F., Penque D., and Amaral M. D. (2002) The human DnaJ homologue (Hdj)-1/heat-shock protein (Hsp) 40 co-chaperone is required for the in vivo stabilization of the cystic fibrosis transmembrane conductance regulator by Hsp70. Biochem. J. 366, 797–806 10.1042/bj20011717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Borkan S. C., and Gullans S. R. (2002) Molecular chaperones in the kidney. Annu. Rev. Physiol. 64, 503–527 10.1146/annurev.physiol.64.081501.155819 [DOI] [PubMed] [Google Scholar]
- 102. Seit-Nebi A. S., and Gusev N. B. (2010) Versatility of the small heat shock protein HSPB6 (Hsp20). Cell Stress Chaperones 15, 233–236 10.1007/s12192-009-0141-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Wilson P. D. (2011) Apico-basal polarity in polycystic kidney disease epithelia. Biochim. Biophys. Acta 1812, 1239–1248 10.1016/j.bbadis.2011.05.008 [DOI] [PubMed] [Google Scholar]
- 104. Ma M., Tian X., Igarashi P., Pazour G. J., and Somlo S. (2013) Loss of cilia suppresses cyst growth in genetic models of autosomal dominant polycystic kidney disease. Nat. Genet. 45, 1004–1012 10.1038/ng.2715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Levine S. A., Nath S. K., Yun C. H., Yip J. W., Montrose M., Donowitz M., and Tse C. M. (1995) Separate C-terminal domains of the epithelial specific brush border Na+/H+ exchanger isoform NHE3 are involved in stimulation and inhibition by protein kinases/growth factors. J. Biol. Chem. 270, 13716–13725 10.1074/jbc.270.23.13716 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study will be made available by the corresponding author upon reasonable request. All data generated or analyzed during this study are included. No data sets were generated.