Abstract
An estimated 5.4 million cases of nonmelanoma skin cancer are reported in the United States at an associated cost of $4.8 billion. ΔNp63α, a proto-oncogene in the p53 family of transcription factors, is overexpressed in squamous cell carcinoma (SCC) and associated with poor prognosis and survival. ΔNp63α elicits its tumorigenic effects in part by promoting cellular proliferation and cell survival. Despite its importance in SCC, the upstream regulation of ΔNp63α is poorly understood. In this study, we identify TIP60 as a novel upstream regulator of ΔNp63α. Using a combination of overexpression, silencing, stable expression, and pharmacological approaches in multiple cell lines, we showed that TIP60 up-regulates ΔNp63α expression. Utilizing cycloheximide treatment, we showed that TIP60 catalytic activity is required for stabilization of ΔNp63α protein levels. We further showed that TIP60 coexpression inhibits ΔNp63α ubiquitination and proteasomal degradation. Stabilization of ΔNp63α protein was further associated with TIP60-mediated acetylation. Finally, we demonstrated that TIP60-mediated regulation of ΔNp63α increases cellular proliferation by promoting G2/M progression through MTS assays and flow cytometry. Taken together, our findings provide evidence that TIP60 may contribute to SCC progression by increasing ΔNp63α protein levels, thereby promoting cellular proliferation.
Keywords: p63, acetyltransferase, cancer, cell proliferation, cell cycle, ubiquitylation (ubiquitination), non-melanoma skin cancer, squamous cell carcinoma, TIP60
Introduction
Dysregulation of the cell cycle underlies the uncontrolled cellular proliferation that arises in cancer. Cell cycle arrest typically occurs at either G1/S or G2/M phases in response to cellular stressors such as DNA damage, allowing cells to repair the damage or undergo apoptosis (1). Improper passage through these checkpoints causes cells to accumulate genomic mutations and proliferate uncontrollably, driving cancer cell development (2). p53 family transcription factors are major regulators of the cell cycle, and alterations in their expression can lead to malignant cellular transformation (3, 4).
The p63 gene is a p53 family member that encodes six isoforms because of differential promoter usage and C-terminal alternative splicing (5). ΔNp63α is the most abundantly expressed p63 isoform, found predominately in the basal stratum of the skin (6). ΔNp63α expression is critical for development of the epidermis, as it governs cellular proliferation and differentiation of the suprabasal layers (7). This is evident in p63-null mice, which are born without a stratified epidermis and lack epithelium-derived structures such as hair, teeth, and limbs (8). Although ΔNp63α is not mutated in cancer, its levels are elevated in non-melanoma skin cancers (NMSC)2 such as basal cell carcinoma and squamous cell carcinoma (SCC) (9). ΔNp63α is overexpressed in 85% of all SCC, including head and neck, esophagus, lung, and cervical SCC, and promotes cell cycle progression and cellular proliferation (3, 9–11). This is in part due to its direct transcriptional repression of p21Cip1/Waf1, a cyclin-dependent kinase (CDK) inhibitor that promotes both G1/S and G2/M cell cycle arrest (12–15).
Tat-interacting protein 60 kDa (TIP60) is a histone acetyltransferase originally discovered as an interacting partner of the HIV-1 Tat protein (16). TIP60 is a member of the MYST (MOZ, Ybf2/Sas3, SAS2, and TIP60) family of acetyltransferases, which are characterized by a conserved catalytic MYST domain that transfers an acetyl group from an acetyl-CoA to an ϵ-amino group of a substrate lysine (17). Although loss of TIP60 expression has been demonstrated to promote melanoma progression (18), a role for TIP60 in NMSC has yet to be established. Given that TIP60 is known to regulate other p53 family members (19–22) and plays a role in G2/M progression (23), we investigated whether TIP60 regulates ΔNp63α to affect cellular proliferation.
In this study, we identified a novel mechanism regulating ΔNp63α expression and its role in proliferation. TIP60 up-regulates ΔNp63α by increasing its transcript levels and by promoting its protein stability. ΔNp63α was also determined to be a novel substrate for acetylation by TIP60. This finding is significant because the TIP60–ΔNp63α signaling axis advances cellular G2/M progression by repressing p21Cip1/Waf1 expression, thereby promoting cancer cell proliferation.
Results
TIP60 increases ΔNp63α expression
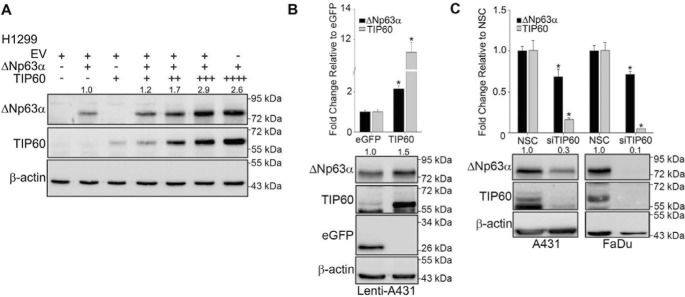
Because TIP60 is known to promote p53 expression (20), we investigated whether TIP60 also regulates ΔNp63α levels. ΔNp63α was cotransfected with increasing concentrations of TIP60 in p63-null H1299 cells, a non-small-cell lung cancer cell line. We observed a dose-dependent increase in ΔNp63α protein levels with increasing levels of TIP60 (Fig. 1A). Because this experiment utilized ectopic ΔNp63α, we wanted to next determine whether TIP60 also regulates endogenous ΔNp63α protein and transcript levels. We generated cell lines stably expressing TIP60 (lenti-TIP60) or eGFP (lenti-eGFP) as a control by viral transduction in the SCC cell line A431. Relative to eGFP, cells stably expressing TIP60 showed an increase in both endogenous ΔNp63α transcript (Fig. 1B, top panel) and protein levels (Fig. 1B, bottom panel). Conversely, silencing endogenous TIP60 using siRNA in A431 or FaDu (another SCC cell line) cells significantly decreased both ΔNp63α transcript (Fig. 1C, top panel) and protein levels (Fig. 1C, bottom panel) relative to the non-silencing control (NSC). Taken together, these experiments demonstrate that TIP60 positively regulates ΔNp63α expression.
Figure 1.
TIP60 increases ΔNp63α expression. A, H1299 cells were transfected with ΔNp63α alone or with increasing concentrations of TIP60 as indicated. TIP60 and ΔNp63α protein levels were assessed by immunoblot. The -fold change in protein level, normalized to β-actin relative to the ΔNp63α-alone condition, is listed above each band. B, lenti-A431-eGFP and A431-TIP60 stable cells were generated and monitored for ΔNp63α expression. TaqMan-based real-time PCR was performed to detect the transcript levels of ΔNp63α and TIP60 (top panel). Protein levels were confirmed using immunoblot analyses (bottom panel). C, A431 and FaDu cells were transfected with either NSC or siRNA targeting TIP60 (siTIP60). 48 h post-transfection, TaqMan-based real-time PCR was performed to detect transcript levels as in B (top panel). Whole-cell extracts were subjected to immunoblot analysis as in A (bottom panel). The -fold change in protein level, normalized to β-actin relative to the NSC-alone condition, is listed above each band. Error bars represent 1 S.D. *, p ≤ 0.05 compared with control cells in all panels.
Inhibition of TIP60 reduces ΔNp63α expression
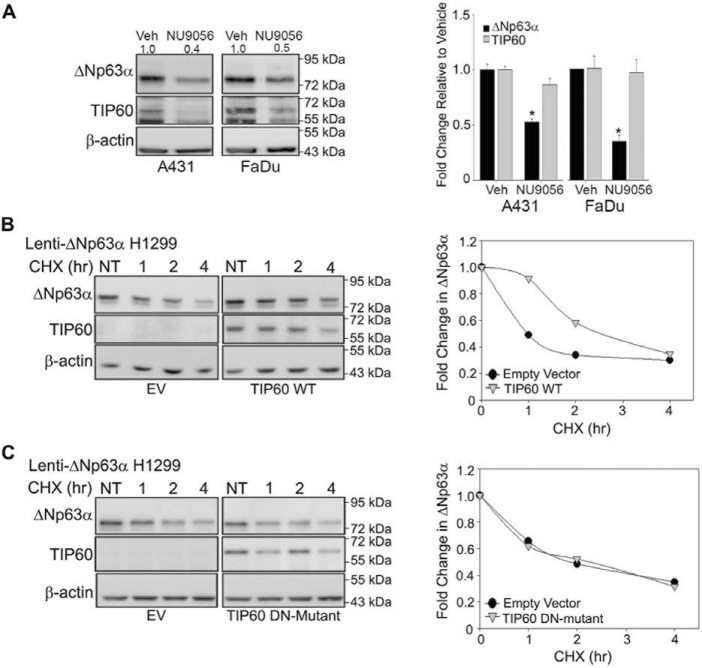
TIP60 catalytic activity promotes the transcription of multiple genes both directly and indirectly (24). To assess the effects of pharmacological inhibition of endogenous TIP60 acetyltransferase activity on ΔNp63α transcript levels, A431 and FaDu cells were treated with a TIP60-selective inhibitor (100 μm NU9056) (25, 26) for 24 h. NU9056 treatment did not affect TIP60 mRNA levels but reduced TIP60 protein levels, consistent with previous studies showing that TIP60 autoacetylation confers protein stability (27, 28). Inhibiting TIP60 activity with NU9056 reduced both ΔNp63α protein (Fig. 2A, left panel) and transcript (Fig. 2A, right panel) levels relative to DMSO vehicle controls. These results demonstrate that pharmacological inhibition of TIP60 activity is effective in reducing ΔNp63α expression.
Figure 2.
TIP60 inhibition reduces ΔNp63α expression. TIP60 catalytic activity is important for regulation of ΔNp63α transcript levels and protein stability. A, A431 cells treated with vehicle (Veh, DMSO) or with the TIP60-specific inhibitor NU9056 (100 μm) for 24 h. The changes in protein expression and transcript levels of ΔNp63α and TIP60 were measured by immunoblot analyses (left panel) and TaqMan-based real-time PCR (right panel), respectively (*p ≤ 0.05). Error bars indicate 1 S.D. B and C, H1299-ΔNp63α stable cells were transfected with TIP60 WT (B) or TIP60 DN mutant (C), followed by treatment with 100 μg/ml CHX for 1, 2, or 4 h, along with an NT control. Expression of ΔNp63α and TIP60 was confirmed via immunoblot analysis (left panels). The -fold change in ΔNp63α protein levels, normalized to β-actin and relative to the NT condition, is plotted in the right panels.
TIP60 is known to regulate the stability of various nonhistone proteins (24). To determine whether TIP60 positively regulates ΔNp63α protein half-life in a catalytically dependent manner, we studied the effects of a WT and a TIP60 dominant negative (DN) mutant on ΔNp63α stability. The TIP60 DN mutant contains two mutations (Q324E/G327E) that impair TIP60 acetyltransferase activity by interfering with acetyl-CoA binding to the catalytic site (28). Lenti-ΔNp63α stable H1299 cells were transfected with empty vector (EV), TIP60 WT, or the TIP60 DN mutant. 24 h post-transfection, the cells were treated with 100 μg/ml of the ribosomal inhibitor cycloheximide (CHX) and harvested 1, 2, and 4 h post-treatment. We then assessed ΔNp63α protein levels compared with nontreated (NT) controls. Quantitation of ΔNp63α protein levels by immunoblot analysis showed that TIP60 WT increased the half-life of exogenous ΔNp63α (Fig. 2B). However, no change in ΔNp63α protein stability was found with coexpression with the TIP60 DN mutant following CHX treatment (Fig. 2C). These observations demonstrate that ΔNp63α protein stability depends on TIP60 catalytic activity.
TIP60 inhibits ΔNp63α ubiquitination and proteasomal degradation
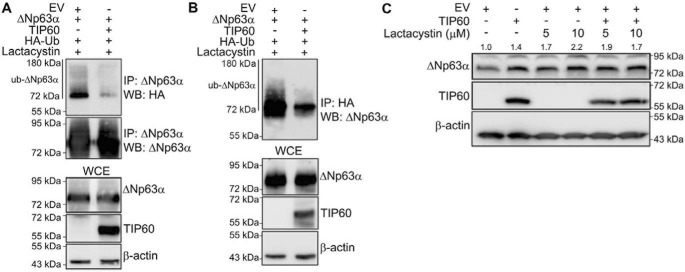
ΔNp63α protein stability depends on ubiquitin-mediated proteasomal degradation (29–32). To test whether TIP60 inhibits the ubiquitination of ΔNp63α, we cotransfected H1299 cells with expression plasmids encoding ΔNp63α and HA-ubiquitin in the presence and absence of TIP60. Immunoprecipitation of either ΔNp63α (Fig. 3A) or total HA-ubiquitinated protein complexes (Fig. 3B) from whole-cell extracts was performed, followed by immunoblotting with HA-specific (Fig. 3A) or p63-specific (Fig. 3B) antibodies, respectively. We observed that ubiquitinated ΔNp63α levels were reduced upon coexpression with TIP60 under both conditions (Fig. 3, A and B). To determine whether TIP60 also prevents the subsequent proteasomal degradation of ΔNp63α, we transfected lenti-ΔNp63α stable H1299 cells with either EV or TIP60 and/or treated them with the selective irreversible proteasome inhibitor lactacystin (Fig. 3C). As shown earlier (Fig. 1A), TIP60 increased ΔNp63α protein levels in the absence of lactacystin treatment (Fig. 3C, compare lane 2 with lane 1). Cells treated with increasing concentrations of lactacystin also had increased ΔNp63α protein levels (Fig. 3C, compare lanes 3 and 4 with lane 1). A combination of TIP60 overexpression followed by lactacystin treatment did not demonstrate a further increase in ΔNp63α protein levels (Fig. 3C, lanes 5 and 6) compared with each of those conditions alone. Taken together, these data provide evidence that TIP60 increases ΔNp63α protein stability by inhibiting its ubiquitination and subsequent proteasomal degradation.
Figure 3.
TIP60 reduces ΔNp63α ubiquitination and proteasomal degradation. H1299 cells were transfected with ΔNp63α and HA-ubiquitin (HA-Ub) and either EV or TIP60. 24 h post-transfection, cells were treated with 20 μm lactacystin for 4 h. A and B, whole-cell extracts were subjected to immunoprecipitation (IP) using anti-p63 (A) or anti-HA (B) antibodies. The bottom three blots show immunoblots for ΔNp63α, TIP60, and β-actin of the whole-cell extract (WCE) used for IP. WB, Western blot. C, lenti-ΔNp63α H1299 cells were transfected with either EV or TIP60 and treated with the indicated concentrations of lactacystin for 4 h. ΔNp63α and TIP60 were detected by immunoblot with anti-p63 and anti-TIP60 antibodies. The -fold change in protein level, normalized to β-actin relative to EV, is listed above each band.
TIP60 acetylates ΔNp63α
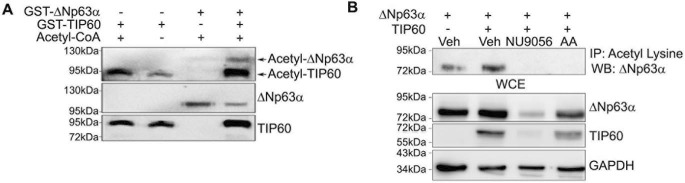
Because acetylation of nonhistone proteins has been demonstrated to reduce their ubiquitination and proteasomal degradation (33), we wanted to determine whether TIP60 directly acetylates ΔNp63α. We performed an in vitro acetylation assay using recombinant GST-ΔNp63α and GST-TIP60. Because TIP60 undergoes autoacetylation, it served as a positive control (34). As expected, we observed increased autoacetylation of TIP60 in the presence of acetyl-CoA (Fig. 4A, lane 1) relative to the low-background TIP60 acetylation observed in the absence of acetyl-CoA (Fig. 4A, lane 2). No background acetylation of GST-ΔNp63α was observed with acetyl-CoA (Fig. 4A, lane 3). However, acetylated GST-ΔNp63α was clearly observed with addition of TIP60 (Fig. 4A, lane 4), demonstrating that TIP60 directly acetylates ΔNp63α in vitro. To rule out the possibility that the observed acetylation of GST-ΔNp63α is not due to acetylation of the GST tag, we performed an in vitro reaction with GST and TIP60 and did not observe any acetylation of GST (data not shown). Finally, to confirm that ΔNp63α acetylation in cells is mediated by TIP60, H1299 cells were transfected with ΔNp63α with and without TIP60 and in the presence or absence of 100 μm NU9056 or 50 μm anacardic acid (a general acetyltransferase inhibitor). Immunoprecipitation from whole-cell extracts using anti-acetyl lysine antibody showed that TIP60 increased acetylation of ΔNp63α (Fig. 4B, compare lanes 1 and 2), as shown in Fig. 4A, but this acetylation was blocked by both inhibitors (Fig. 4B, lanes 3 and 4). Taken together, these data indicate that ΔNp63α is a novel TIP60 substrate.
Figure 4.
TIP60 acetylates ΔNp63α. A, an in vitro acetylation assay was carried out using recombinant TIP60-GST with GST-ΔNp63α. Immunoblot analysis was performed to detect proteins using anti-acetyl lysine, anti-TIP60, and anti-p63 antibodies. B, H1299 cells were transfected with ΔNp63α in the presence or absence of TIP60 as indicated and treated with 100 μm NU9056 or 50 μm anacardic acid for 24 h prior to immunoprecipitation with an anti-acetyl lysine antibody. GAPDH was used as a loading control. The bottom three panels show immunoblots for ΔNp63α, TIP60, and GAPDH for the whole-cell extract (WCE) used for IP. Veh, vehicle.
TIP60 regulation of ΔNp63α expression levels promotes SCC proliferation
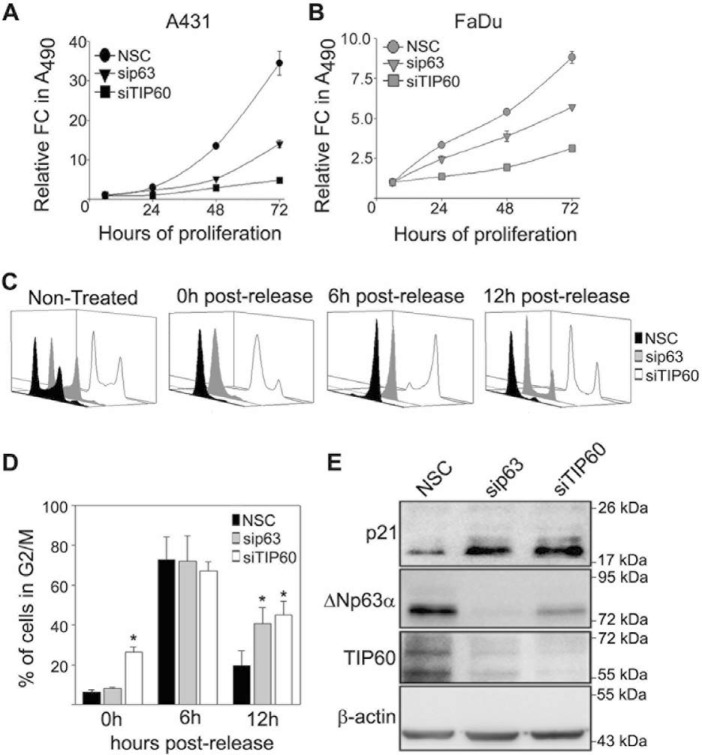
Elevated ΔNp63α levels have been shown to increase cellular proliferation (35–37). Because we demonstrated that TIP60 up-regulates ΔNp63α (Fig. 1), we wanted to determine whether silencing TIP60 or ΔNp63α in A431 and FaDu cells affects cellular proliferation. Silencing of either ΔNp63α or TIP60 reduced proliferation of both A431 (Fig. 5A) and FaDu cells (Fig. 5B) compared with control cells, as measured by MTS assay. As both TIP60 and ΔNp63α have been linked to cell cycle progression (3, 23, 38), we next investigated the effects of TIP60 and ΔNp63α silencing on the cell cycle. A431 cells transfected with NSC, ΔNp63α siRNA, or TIP60 siRNA were treated with a double thymidine block to synchronize cells in G1/S phase. 0 h, 6 h, and 12 h post-release from double thymidine block, cell cycle progression was monitored using flow cytometry. As expected, thymidine treatment synchronized cells transfected with NSC in G1/S (Fig. 5C, compare 0 h post-release with Non-treated). All three conditions showed accumulation in G2/M 6 h post-thymidine release, confirming progression through the cell cycle. 12 h post-thymidine release, silencing either TIP60 or ΔNp63α increased the proportion of cells in G2/M compared with NSC, as shown in Fig. 5C and quantitated in Fig. 5D. Because ΔNp63α is known to directly inhibit the expression of p21Cip1/Waf1 (15), an inhibitor of G2/M progression (39), we next examined whether silencing TIP60 also up-regulated p21Cip1/Waf1 levels. TIP60 silencing reduced ΔNp63α levels, consistent with Fig. 1C. Moreover, silencing either ΔNp63α or TIP60 increased p21Cip1/Waf1 levels 12 h post-thymidine release (Fig. 5E). Interestingly, we observed that silencing p63 also reduced TIP60 levels. Overall, these results show that inhibition of p21Cip1/Waf1 by ΔNp63α and TIP60 correlates with G2/M cell cycle progression.
Figure 5.
ΔNp63α and TIP60 silencing inhibits SCC proliferation. A and B, A431 (A) and FaDu (B) cells were transfected with NSC, sip63, or siTIP60 as indicated, and cell proliferation was measured by MTS cell titer. The y axis represents the -fold change of each condition relative to t = 6 h. Error bars represent standard deviation from the mean. C, cell cycle profiles of A431 cells transfected with NSC, sip63, or siTIP60 at the indicated time points after release following a double thymidine block and analyzed using flow cytometry. D, quantitation of the total percentage of cells progressing through G2/M 0, 6, and 12 h post-thymidine release under each condition shown in C. Error bars represent 1 S.E. of the mean from three independent experiments. *, p ≤ 0.05). E, immunoblot analysis of p21, ΔNp63α, and TIP60 of A431 cells (C) was performed with the indicated antibodies 12 h after release from double thymidine block.
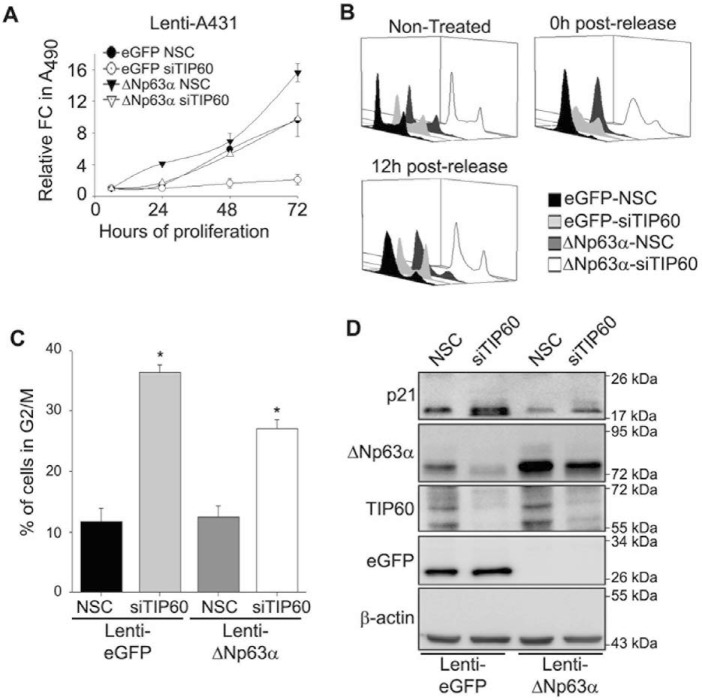
To determine whether TIP60-mediated cell proliferation occurs through ΔNp63α, we silenced TIP60 in lenti-eGFP and lenti-ΔNp63α stable A431 cells and monitored cell proliferation at different time points as indicated (Fig. 6A). As expected, lenti-ΔNp63α stable cells transfected with NSC (Fig. 6A, closed triangles) showed increased cell proliferation compared with lenti-eGFP stable cells transfected with NSC (Fig. 6A, closed circles). Although silencing TIP60 in lenti-eGFP A431 cells dramatically reduced cell proliferation (Fig. 6A, open circles), stable overexpression of ΔNp63α (lenti-ΔNp63α) rescued the effects of TIP60 silencing on cell proliferation (Fig. 6A, open triangles). Because we demonstrated that both TIP60 and ΔNp63α promote G2/M progression (Fig. 5, B and C), we next wanted to determine whether TIP60 promotes G2/M progression via up-regulation of ΔNp63α. We silenced TIP60 in lenti-eGFP and lenti-ΔNp63α stable A431 cells, followed by synchronization with double thymidine block as described in Fig. 5. Cell cycle progression was monitored using flow cytometry 0 h and 12 h post-release from double thymidine block. Thymidine treatment synchronized cells in G1/S under all conditions (Fig. 6B, compare 0 h post-release with Non-treated). As expected, silencing TIP60 in lenti-eGFP cells increased the proportion of cells in G2/M relative to NSC controls 12 h post-thymidine release (Fig. 6, B and C), consistent with Fig. 5. Stable overexpression of ΔNp63α in cells transfected with siTIP60 partially restored progression through G2/M phase compared with lenti-eGFP-siTIP60 12 h post-thymidine release (Fig. 6, B and C, ΔNp63α-siTIP60 versus eGFP-siTIP60 control). Additionally, the increase in p21Cip1/Waf1 protein levels resulting from TIP60 silencing in lenti-EGFP controls was abrogated in lenti-ΔNp63α cells (Fig. 6D). Taken together, these results indicate that TIP60-mediated G2/M progression occurs via regulation of ΔNp63α expression.
Figure 6.
ΔNp63α partially rescues SCC proliferation following TIP60 silencing. A, A431-eGFP and A431-ΔNp63α stable pool cells were transfected with NSC or siTIP60 as indicated, and cell proliferation was measured by MTS cell titer. The y axis represents the -fold change of each condition relative to t = 6 h. Error bars represent standard deviation from the mean. FC, -fold change. B, cell cycle profiles of A431-eGFP and A431-ΔNp63α stable pool cells transfected with NSC or siTIP60 at the indicated time points after release following a double thymidine block and analyzed using flow cytometry. C, quantitation of the total percentage of cells progressing through G2/M phase 12 h post-thymidine release under each condition shown in B. Error bars represent the standard deviation from the mean from three independent experiments (*, p ≤ 0.05). D, immunoblot analysis of p21, ΔNp63α, eGFP, and TIP60 of B was performed with the indicated antibodies 12 h post-synchronization.
Discussion
The proto-oncogene ΔNp63α is overexpressed in SCC and basal cell carcinoma and promotes cancer cell proliferation by driving cell cycle progression (3, 10). In this study, we identified TIP60 as a novel positive regulator of ΔNp63α transcript and protein levels (Fig. 1). We show that TIP60 prevents the ubiquitination and proteasomal degradation of ΔNp63α, which is associated with direct acetylation of ΔNp63α. Moreover, TIP60 up-regulation of ΔNp63α resulted in increased cellular proliferation and cell cycle progression.
We observed that NU9056 treatment reduced TIP60 protein levels (Fig. 2A), which is consistent with previous reports that TIP60 autoacetylation is important for its stability (27, 28). This indicates that TIP60 may regulate ΔNp63α transcript levels either directly or indirectly. Because TIP60 is not a transcription factor, it is likely that TIP60 regulates the transcription of ΔNp63α either by acetylating an unidentified transcription factor upstream of ΔNp63α or by its well-studied role in histone acetylation, leading to increased transcription. Moreover, the ability of TIP60 to regulate both the transcription and protein stability of its substrates is well-documented. For example, TIP60 is known to promote the stability of the c-MYC transcription factor (40). Interestingly, TIP60 has been shown to form a complex with c-MYB, resulting in down-regulation of c-MYC transcription (41). Moreover, TIP60 has been demonstrated to regulate p21 mRNA levels and protein stability in a p53-dependent context (20, 42). The regulation of ΔNp63α mRNA and protein levels evidenced by our study is consistent with these observations.
We have also further elaborated a role for TIP60 acetyltransferase activity regarding regulation of ΔNp63α protein levels. Interestingly, this finding appears to contrast the effect of TIP60 acetylation of p53. Although TIP60 acetylates p53 to regulate its DNA-binding capability following DNA damage (21, 22), TIP60 promotes p53 protein stability by preventing its MDM2-mediated degradation in a catalytically independent manner (20). We observed that TIP60 acetyltransferase activity is critical for ΔNp63α protein stability (Fig. 2, B and C). Moreover, we found that TIP60 inhibits ΔNp63α ubiquitination and proteasomal degradation (Fig. 3) and directly acetylates ΔNp63α (Fig. 4). Acetylation of lysine residues targeted by E3 ligases is well-known to inhibit the addition of ubiquitin moieties and subsequent proteasomal degradation of nonhistone proteins (33). These results indicate that TIP60 may stabilize ΔNp63α protein levels via acetylation. Still, we cannot rule out the possibility that TIP60 may also inhibit the activity of an E3 ligase that targets ΔNp63α for degradation. Future investigation will determine whether direct acetylation of ΔNp63α by TIP60 regulates ΔNp63α protein stability.
Several studies focus on the ability of TIP60 to promote p53 protein levels and inhibit cell proliferation in the context of cellular stress and DNA damage (20–22, 43–45). Interestingly, TIP60 has been demonstrated to increase cell proliferation in the absence of such stressors. For example, TIP60 forms a tertiary complex with MDM2 and TAp73β under physiological conditions, inhibiting TAp73β transactivation capability and promoting cellular growth (19). Moreover, TIP60 has been found to drive prostate cancer proliferation by increasing levels of c-MYC and androgen receptor (24). This apparent contradiction is perhaps due to levels of p53 under physiological conditions being relatively low and increasing following cellular stress; however, levels of ΔNp63α are high under physiological conditions and decrease following cellular stress (46). In most types of SCC, p53 is commonly mutated and no longer induces growth arrest or apoptosis (47). Therefore, TIP60 could be expected to increase rather than inhibit SCC proliferation under physiological conditions. Consistent with this hypothesis, we found that silencing TIP60 in the SCC cell lines A431 and FaDu reduces ΔNp63α expression and cell proliferation (Fig. 5, A and B). It is therefore important that future studies examine the role of TIP60-mediated ΔNp63α regulation in the presence of commonly used SCC therapeutic agents that cause DNA damage. Interestingly, knockdown of ΔNp63α in A431 cells also led to a reduction in TIP60 expression, indicating the possibility of a negative feedback loop. ΔNp63α may directly induce TIP60 by binding to the TIP60 promoter, although no TP63 binding sites could be identified in the TIP60 promoter region using JASPAR or publicly available ChIP sequencing datasets (48–50). Rather, if a feedback mechanism exists, then ΔNp63α likely regulates a factor upstream of TIP60. ΔNp63α increases cellular proliferation by promoting G2/M progression in part by inhibiting the expression of the p21Cip1/Waf1 gene (3, 12–15, 39), but the role of TIP60 in regulating the cell cycle is less defined. Doyon et al. (23) demonstrated that silencing TIP60 results in accumulation of cells in G2/M by an unknown mechanism. Although TIP60 has been reported previously to directly stabilize p21Cip1/Waf1 (42), we found that silencing TIP60, like ΔNp63α, reduced G2/M progression while simultaneously increasing p21Cip1/Waf1 levels (Fig. 5, C–E). This result indicates that TIP60 promotes G2/M progression by inhibiting p21Cip1/Waf1 expression in a ΔNp63α-dependent manner. This is interesting, considering acetylation of histones by TIP60 has been demonstrated to be inhibited during G2 phase because of phosphorylation of serine residues 86 and 90 by the Cyclin-B1–CDK-1 complex (51). Although required for G2 progression and mitotic entry, the Cyclin-B1–CDK1 complex must be degraded for mitotic exit (52, 53), illustrating that this would be a transient effect. Moreover, phosphorylation of these sites does not inhibit the ability of TIP60 to modify and regulate nonhistone proteins (54, 55).
We confirmed that rescuing expression of ΔNp63α following TIP60 silencing partially restores A431 cell proliferation (Fig. 6A), rescues G2/M progression, and suppresses p21Cip1/Waf1 expression (Fig. 6, B–D). These results indicate that TIP60 acetylation of ΔNp63α may not significantly influence the ability of ΔNp63α to regulate cellular proliferation. Acetylation may therefore be important for ΔNp63α protein stabilization but not its ability to regulate the p21Cip1/Waf1 promoter. These observations are consistent with the fact that TIP60 catalytic activity is not required for it to influence p53 and TAp73β regulation of cell proliferation (19, 21, 22). However, we cannot rule out the possibility that another acetyltransferase may partially compensate for the silencing of TIP60 expression in our experiments or that rescuing ΔNp63α acetylation would result in a more significant rescue of cell proliferation and G2/M progression.
The observations of this study are further supported by previous reports that treatment with a general acetyltransferase inhibitor known to target TIP60 activity, curcumin, reduces SCC tumor growth in mouse models (56–59). Adding clinical relevance to our findings, curcumin has been utilized in clinical trials to treat various cancers (60). Moreover, specific pharmacological inhibitors of TIP60, including NU9056, have been demonstrated to reduce proliferation of cancer cells (25, 26, 61).
Our findings provide evidence that ΔNp63α is a downstream target of TIP60, providing critical insight into the pro-tumorigenic role of ΔNp63α in NMSC. Down-regulation of TIP60 expression and inhibition of activity disrupt this signaling axis to impede NMSC growth. In conclusion, TIP60 may prove to be a beneficial target for therapeutic agents designed to treat diseases in which ΔNp63α expression is elevated.
Experimental procedures
Cell lines, reagents, and plasmids
Human non-small-cell lung carcinoma H1299 and human epidermoid carcinoma A431 cell lines were purchased from the ATCC (Manassas, VA). The human hypopharyngeal cancer FaDu cell line was obtained from Dr. Weiwen Long (Wright State University, Dayton, OH). The three cell lines were maintained in Dulbecco's modified Eagle's medium supplemented with 8% fetal bovine serum, 250 units penicillin, and 250 μg of streptomycin. Cycloheximide, sodium butyrate, and anacardic acid were purchased from Sigma-Aldrich (St. Louis, MO). NU9056 was purchased from ApexBio (Houston, TX). TIP60 expression plasmids (WT and DN mutant) were a generous gift from Dr. Edward Seto (George Washington University, Washington, DC), and the hemagglutinin-tagged ubiquitin plasmid (HA-Ub) was obtained from Dr. Steven Berberich (Wright State University). The expression plasmid encoding ΔNp63α was described earlier (62). The pLB2V5 lentiviral vector was obtained from Dr. Thomas Brown (Wright State University). The lenti-eGFP and lenti-ΔNp63α A431 and H1299 stable cells were generated as described earlier. Lenti-TIP60 stable cells were generated as follows. The TIP60 cDNA-containing lentiviral expression plasmid was constructed by amplifying the TIP60 cDNA sequence from pCDNA3.1-TIP60 and then cloning it into the pLB2V5 vector. The primers used for amplifying the TIP60 cDNA and incorporating the necessary 5′ EcoR1 and 3′ Xho1 cut sites were as follows: sense, 5′-CACCTGAATTCATGGCGGAGGTGGGGGAGATAA-3′; antisense, 5′-CGAACTCGAGTCACCACTTCCCCCTCTTGCTCCA-3′. The lenti-eGFP, ΔNp63α, and TIP60 stable cell lines were generated by infecting parental H1299 and A431 cells as described earlier (63). 72 h post-infection, cells were selected in blasticidin (3 μg/ml for A431, 10 μg/ml for H1299) to obtain stable expression of eGFP, TIP60, or ΔNp63α.
Silencing and overexpression
p63 and TIP60 silencing studies conducted in A431 and FaDu cell lines were performed by two rounds of siRNA transfection using Lipofectamine RNAiMax according to the manufacturer's instructions (Invitrogen). Allstars NSC, p63, and TIP60 siRNA were purchased from Qiagen (Valencia, CA). Target sequences for p63 were described earlier, and the TIP60 target sequence was CACCACATTGCCTGTCCTCTA (63). Overexpression performed in H1299 cells utilized lipid-based transfection using Lipofectamine 2000 (Invitrogen) as described earlier (63).
Immunoblot analysis
Whole-cell extracts were prepared by lysing cells in phosphatase inhibitor–containing buffer (50 mm Tris-HCl (pH 8), 120 mm NaCl, 5 mm EGTA, 1 mm EDTA, 5 mm sodium pyrophosphate, 10 mm NaF, 30 mm p-nitrophenyl phosphate, 1 mm benzamidine, 0.1% NP-40, 1% Triton X-100, and 0.2 mm PMSF) supplemented with protease inhibitor mixture (Sigma-Aldrich). Immunoblot analysis was performed as described previously (63).
Antibodies
p63 antibodies used were as follows: pan-p63 (4A4) and pan-p63 (N2C1) (Genetex, Irvine, CA). TIP60 (C7), GFP (FL), and β-actin were purchased from Santa Cruz Biotechnology (Dallas, TX). Anti-acetyl lysine (9441) and p21 (12D1) were purchased from Cell Signaling Technology (Danvers, MA), and HA (H3663) was purchased from Sigma-Aldrich. Appropriate HRP-conjugated secondary antibodies (Promega, Madison, WI) were used for chemiluminescence detection by Western Lightning Plus-ECL chemiluminescence substrate (PerkinElmer Life Sciences).
Quantitative RT-PCR
Total RNA extraction was performed using the eZNA RNA isolation kit (Omega Bio-Tek, Norcross, GA) according to the manufacturer's instructions. cDNA was synthesized using the TaqMan reverse transcription kit (Applied Biosystems, Foster City, CA) from 1 μg of total RNA. Quantitative RT-PCR was performed on the QuantStudio 7 Flex using gene-specific assays on demand targeted to GAPDH (4325792), p63 (Hs00978340_m1), and TIP60 (Hs00197310_m1) (Applied Biosystems), with each sample run in triplicate. Analysis was carried out using the comparative ΔΔCt method (64).
Ubiquitination assay
H1299 cells were transfected with the indicated plasmids. Cells were lysed using a high-salt lysis buffer (300 mm NaCl, 100 mm Tris (pH 8.0), 0.2 mm EDTA, 0.1% NP40, and 10% glycerol). For immunoprecipitation, whole-cell extracts were precleared with protein A beads (Santa Cruz Biotechnology), followed by overnight rotation with 1 μg of primary antibody. Immunoprecipitated protein complexes were captured with protein A beads and subjected to immunoblot analysis.
Immunoprecipitation assays for detection of acetyl ΔNp63α
Cells were treated for 6 h with 30 mm sodium butyrate prior to immunoprecipitation. Whole-cell extracts were precleared with protein A beads (Santa Cruz Biotechnology), followed by overnight rotation with 1 μg of anti-acetyl lysine antibody. Immunoprecipitated proteins were captured with protein A beads and subjected to immunoblot analysis with p63-specific or TIP60-specific antibody.
Recombinant protein preparation
The recombinant GST-TIP60 plasmid was obtained from Dr. Samisubbu R. Naidu (Indiana University, Indianapolis, IN), and the recombinant GST-ΔNp63α plasmid cloned into a pGEX 6p1 vector was generously donated by Dr. S. Basu (Queen Mary University, London, UK). Recombinant proteins were expressed in Escherichia coli and induced using 1 mm isopropyl 1-thio-β-d-galactopyranoside (Sigma-Aldrich) at 16 °C overnight. Proteins were purified by GST affinity chromatography using GSTrap FF columns (GE Healthcare) according to the manufacturer's instructions.
In vitro acetylation assay
100 ng of purified GST-TIP60 was incubated with 250 ng of GST-ΔNp63α and 100 μm acetyl-CoA (Sigma-Aldrich) in 1× histone acetyltransferase reaction buffer (50 mm Tris-HCl (pH 8.0), 150 mm NaCl, 10 mm sodium butyrate, and 10% glycerol) at 30 °C for 2 h. Acetylated and total protein levels were confirmed via immunoblot analysis.
Cell proliferation assays
24 h post-siRNA transfections, cells were harvested and reseeded in a 96-well flat-bottom culture dish at 2500/well for MTS assays. Proliferation was measured using the Cell Titer 96 AQueous One Solution cell proliferation assay (MTS, Promega) at the indicated time points according to the manufacturer's instructions.
Double thymidine block
A double thymidine block was performed using a standard protocol to arrest cells in G1/S phase as described earlier (65). Briefly, cells were seeded and allowed to reach 40% confluency. The medium was removed, replaced with fresh medium containing 2 mm thymidine, and cells were incubated for 19 h. Following incubation, cells were washed with PBS three times, and the medium was replaced. After 9 h, the medium was replaced with fresh medium containing 2 mm thymidine for 16 h. Cells were then washed with PBS as before, fresh media was added to each condition, and cells were harvested at the indicated time points.
Flow cytometry analysis
A431 cells were transfected with the indicated siRNA and synchronized by double thymidine block as described above. Cells were washed in PBS, fixed in 70% ethanol, and incubated at −20 °C for 16 h. Fixed cells were then suspended in 500 μl of PBS containing 50 μg/ml propidium iodide and 100 μm RNase. Cells were then gated and analyzed using an Accuri C6 flow cytometer (BD Biosciences). Histograms were generated using the FCS express 4 software (De Novo Software, Glendale, CA).
Statistical analysis
Independent-sample two-tailed t tests for equal variance were performed to test for significant differences between experimental groups and controls. Differences were considered statistically significant at p ≤ 0.05.
Author contributions
A. J. S., J. Z., and M. P. K. conceptualization; A. J. S. and M. P. K. resources; A. J. S., J. Z., and M. P. C. formal analysis; A. J. S. and M. P. K. funding acquisition; A. J. S., J. Z., M. P. C., A. H., N. D., and M. P. K. investigation; A. J. S., J. Z., M. P. C., A. H., N. D., and M. P. K. methodology; A. J. S., J. Z., M. P.C., N. D., and M. P. K. writing-original draft; A. J. S. and M. P. K. project administration; A. J. S. and M. P. K. writing-review and editing; J. Z., M. P. C., A. H., and N. D. validation.
This work was supported by NCI, National Institutes of Health Grant 1R01CA154715 (to M. P. K.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- NMSC
- non-melanoma skin cancer
- SCC
- squamous cell carcinoma
- CDK
- cyclin-dependent kinase
- eGFP
- enhanced GFP
- NSC
- non-silencing control
- DN
- dominant negative
- EV
- empty vector
- CHX
- cycloheximide
- NT
- non-treated
- MTS
- [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt]
- cDNA
- complementary DNA
- IP
- immunoprecipitation.
References
- 1. Diaz-Moralli S., Tarrado-Castellarnau M., Miranda A., and Cascante M. (2013) Targeting cell cycle regulation in cancer therapy. Pharmacol. Ther. 138, 255–271 10.1016/j.pharmthera.2013.01.011 [DOI] [PubMed] [Google Scholar]
- 2. Williams G. H., and Stoeber K. (2012) The cell cycle and cancer. J. Pathol. 226, 352–364 10.1002/path.3022 [DOI] [PubMed] [Google Scholar]
- 3. Allocati N., Di Ilio C., and De Laurenzi V. (2012) p63/p73 in the control of cell cycle and cell death. Exp. Cell Res. 318, 1285–1290 10.1016/j.yexcr.2012.01.023 [DOI] [PubMed] [Google Scholar]
- 4. Chen J. (2016) The cell-cycle arrest and apoptotic functions of p53 in tumor initiation and progression. Cold Spring Harb. Perspect. Med. 6, a026104 10.1101/cshperspect.a026104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yang A., Kaghad M., Wang Y., Gillett E., Fleming M. D., Dötsch V., Andrews N. C., Caput D., and McKeon F. (1998) p63, a p53 homolog at 3q27–29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol. Cell 2, 305–316 10.1016/S1097-2765(00)80275-0 [DOI] [PubMed] [Google Scholar]
- 6. Candi E., Rufini A., Terrinoni A., Dinsdale D., Ranalli M., Paradisi A., De Laurenzi V., Spagnoli L. G., Catani M. V., Ramadan S., Knight R. A., and Melino G. (2006) Differential roles of p63 isoforms in epidermal development: selective genetic complementation in p63 null mice. Cell Death Differ. 13, 1037–1047 10.1038/sj.cdd.4401926 [DOI] [PubMed] [Google Scholar]
- 7. Vanbokhoven H., Melino G., Candi E., and Declercq W. (2011) p63, a story of mice and men. J. Invest. Dermatol. 131, 1196–1207 10.1038/jid.2011.84 [DOI] [PubMed] [Google Scholar]
- 8. Mills A. A., Zheng B., Wang X. J., Vogel H., Roop D. R., and Bradley A. (1999) p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature 398, 708–713 10.1038/19531 [DOI] [PubMed] [Google Scholar]
- 9. Soares E., and Zhou H. (2018) Master regulatory role of p63 in epidermal development and disease. Cell. Mol. Life Sci. 75, 1179–1190 10.1007/s00018-017-2701-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bergholz J., and Xiao Z. X. (2012) Role of p63 in development, tumorigenesis and cancer progression. Cancer Microenviron. 5, 311–322 10.1007/s12307-012-0116-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leonard M. K., Kommagani R., Payal V., Mayo L. D., Shamma H. N., and Kadakia M. P. (2011) ΔNp63α regulates keratinocyte proliferation by controlling PTEN expression and localization. Cell Death Differ. 18, 1924–1933 10.1038/cdd.2011.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fischer M., Quaas M., Steiner L., and Engeland K. (2016) The p53-p21-DREAM-CDE/CHR pathway regulates G2/M cell cycle genes. Nucleic Acids Res. 44, 164–174 10.1093/nar/gkv927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Niculescu A. B. 3rd, Chen X., Smeets M., Hengst L., Prives C., and Reed S. I. (1998) Effects of p21(Cip1/Waf1) at both the G1/S and the G2/M cell cycle transitions: pRb is a critical determinant in blocking DNA replication and in preventing endoreduplication. Mol. Cell. Biol. 18, 629–643 10.1128/MCB.18.1.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schavolt K. L., and Pietenpol J. A. (2007) p53 and ΔNp63α differentially bind and regulate target genes involved in cell cycle arrest, DNA repair and apoptosis. Oncogene 26, 6125–6132 10.1038/sj.onc.1210441 [DOI] [PubMed] [Google Scholar]
- 15. Westfall M. D., Mays D. J., Sniezek J. C., and Pietenpol J. A. (2003) The ΔNp63α phosphoprotein binds the p21 and 14-3-3 σ promoters in vivo and has transcriptional repressor activity that is reduced by Hay-Wells syndrome-derived mutations. Mol. Cell. Biol. 23, 2264–2276 10.1128/MCB.23.7.2264-2276.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kamine J., Elangovan B., Subramanian T., Coleman D., and Chinnadurai G. (1996) Identification of a cellular protein that specifically interacts with the essential cysteine region of the HIV-1 Tat transactivator. Virology 216, 357–366 10.1006/viro.1996.0071 [DOI] [PubMed] [Google Scholar]
- 17. Sapountzi V., and Côté J. (2011) MYST-family histone acetyltransferases: beyond chromatin. Cell Mol. Life Sci. 68, 1147–1156 10.1007/s00018-010-0599-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen G., Cheng Y., Tang Y., Martinka M., and Li G. (2012) Role of Tip60 in human melanoma cell migration, metastasis, and patient survival. J. Invest. Dermatol. 132, 2632–2641 10.1038/jid.2012.193 [DOI] [PubMed] [Google Scholar]
- 19. Kim J. W., Song P. I., Jeong M. H., An J. H., Lee S. Y., Jang S. M., Song K. H., Armstrong C. A., and Choi K. H. (2008) TIP60 represses transcriptional activity of p73β via an MDM2-bridged ternary complex. J. Biol. Chem. 283, 20077–20086 10.1074/jbc.M800161200 [DOI] [PubMed] [Google Scholar]
- 20. Legube G., Linares L. K., Tyteca S., Caron C., Scheffner M., Chevillard-Briet M., and Trouche D. (2004) Role of the histone acetyl transferase Tip60 in the p53 pathway. J. Biol. Chem. 279, 44825–44833 10.1074/jbc.M407478200 [DOI] [PubMed] [Google Scholar]
- 21. Sykes S. M., Mellert H. S., Holbert M. A., Li K., Marmorstein R., Lane W. S., and McMahon S. B. (2006) Acetylation of the p53 DNA-binding domain regulates apoptosis induction. Mol. Cell 24, 841–851 10.1016/j.molcel.2006.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tang Y., Luo J., Zhang W., and Gu W. (2006) Tip60-dependent acetylation of p53 modulates the decision between cell-cycle arrest and apoptosis. Mol. Cell 24, 827–839 10.1016/j.molcel.2006.11.021 [DOI] [PubMed] [Google Scholar]
- 23. Doyon Y., Cayrou C., Ullah M., Landry A. J., Côté V., Selleck W., Lane W. S., Tan S., Yang X. J., and Côté J. (2006) ING tumor suppressor proteins are critical regulators of chromatin acetylation required for genome expression and perpetuation. Mol. Cell 21, 51–64 10.1016/j.molcel.2005.12.007 [DOI] [PubMed] [Google Scholar]
- 24. Sapountzi V., Logan I. R., and Robson C. N. (2006) Cellular functions of TIP60. Int. J. Biochem. Cell Biol. 38, 1496–1509 10.1016/j.biocel.2006.03.003 [DOI] [PubMed] [Google Scholar]
- 25. Brown J. A., Bourke E., Eriksson L. A., and Kerin M. J. (2016) Targeting cancer using KAT inhibitors to mimic lethal knockouts. Biochem. Soc. Trans. 44, 979–986 10.1042/BST20160081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Coffey K., Blackburn T. J., Cook S., Golding B. T., Griffin R. J., Hardcastle I. R., Hewitt L., Huberman K., McNeill H. V., Newell D. R., Roche C., Ryan-Munden C. A., Watson A., and Robson C. N. (2012) Characterisation of a Tip60 specific inhibitor, NU9056, in prostate cancer. PLoS ONE 7, e45539 10.1371/journal.pone.0045539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yi J., Huang X., Yang Y., Zhu W. G., Gu W., and Luo J. (2014) Regulation of histone acetyltransferase TIP60 function by histone deacetylase 3. J. Biol. Chem. 289, 33878–33886 10.1074/jbc.M114.575266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Peng L., Ling H., Yuan Z., Fang B., Bloom G., Fukasawa K., Koomen J., Chen J., Lane W. S., and Seto E. (2012) SIRT1 negatively regulates the activities, functions, and protein levels of hMOF and TIP60. Mol. Cell. Biol. 32, 2823–2836 10.1128/MCB.00496-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Armstrong S. R., Wu H., Wang B., Abuetabh Y., Sergi C., and Leng R. P. (2016) The regulation of tumor suppressor p63 by the ubiquitin-proteasome system. Int. J. Mol. Sci. 17, E2041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bakkers J., Camacho-Carvajal M., Nowak M., Kramer C., Danger B., and Hammerschmidt M. (2005) Destabilization of ΔNp63α by Nedd4-mediated ubiquitination and Ubc9-mediated sumoylation, and its implications on dorsoventral patterning of the zebrafish embryo. Cell Cycle 4, 790–800 10.4161/cc.4.6.1694 [DOI] [PubMed] [Google Scholar]
- 31. Jung Y. S., Qian Y., Yan W., and Chen X. (2013) Pirh2 E3 ubiquitin ligase modulates keratinocyte differentiation through p63. J. Invest. Dermatol. 133, 1178–1187 10.1038/jid.2012.466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rossi M., Aqeilan R. I., Neale M., Candi E., Salomoni P., Knight R. A., Croce C. M., and Melino G. (2006) The E3 ubiquitin ligase Itch controls the protein stability of p63. Proc. Natl. Acad. Sci. U.S.A. 103, 12753–12758 10.1073/pnas.0603449103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Caron C., Boyault C., and Khochbin S. (2005) Regulatory cross-talk between lysine acetylation and ubiquitination: role in the control of protein stability. BioEssays 27, 408–415 10.1002/bies.20210 [DOI] [PubMed] [Google Scholar]
- 34. Wang J., and Chen J. (2010) SIRT1 regulates autoacetylation and histone acetyltransferase activity of TIP60. J. Biol. Chem. 285, 11458–11464 10.1074/jbc.M109.087585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lo Muzio L., Santarelli A., Caltabiano R., Rubini C., Pieramici T., Trevisiol L., Carinci F., Leonardi R., De Lillo A., Lanzafame S., Bufo P., and Piattelli A. (2005) p63 overexpression associates with poor prognosis in head and neck squamous cell carcinoma. Hum. Pathol. 36, 187–194 10.1016/j.humpath.2004.12.003 [DOI] [PubMed] [Google Scholar]
- 36. Reis-Filho J. S., Torio B., Albergaria A., and Schmitt F. C. (2002) p63 expression in normal skin and usual cutaneous carcinomas. J. Cutan. Pathol. 29, 517–523 10.1034/j.1600-0560.2002.290902.x [DOI] [PubMed] [Google Scholar]
- 37. Sniezek J. C., Matheny K. E., Westfall M. D., and Pietenpol J. A. (2004) Dominant negative p63 isoform expression in head and neck squamous cell carcinoma. Laryngoscope 114, 2063–2072 10.1097/01.mlg.0000149437.35855.4b [DOI] [PubMed] [Google Scholar]
- 38. Lau C. P., Ng P. K., Li M. S., Tsui S. K., Huang L., and Kumta S. M. (2013) p63 regulates cell proliferation and cell cycle progression-associated genes in stromal cells of giant cell tumor of the bone. Int. J. Oncol. 42, 437–443 10.3892/ijo.2012.1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Abbas T., and Dutta A. (2009) p21 in cancer: intricate networks and multiple activities. Nat. Rev. Cancer 9, 400–414 10.1038/nrc2657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Patel J. H., Du Y., Ard P. G., Phillips C., Carella B., Chen C. J., Rakowski C., Chatterjee C., Lieberman P. M., Lane W. S., Blobel G. A., and McMahon S. B. (2004) The c-MYC oncoprotein is a substrate of the acetyltransferases hGCN5/PCAF and TIP60. Mol. Cell. Biol. 24, 10826–10834 10.1128/MCB.24.24.10826-10834.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhao H., Jin S., and Gewirtz A. M. (2012) The histone acetyltransferase TIP60 interacts with c-Myb and inactivates its transcriptional activity in human leukemia. J. Biol. Chem. 287, 925–934 10.1074/jbc.M111.279950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lee M. S., Seo J., Choi D. Y., Lee E. W., Ko A., Ha N. C., Yoon J. B., Lee H. W., Kim K. P., and Song J. (2013) Stabilization of p21 (Cip1/WAF1) following Tip60-dependent acetylation is required for p21-mediated DNA damage response. Cell Death Differ. 20, 620–629 10.1038/cdd.2012.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bassi C., Li Y. T., Khu K., Mateo F., Baniasadi P. S., Elia A., Mason J., Stambolic V., Pujana M. A., Mak T. W., and Gorrini C. (2016) The acetyltransferase Tip60 contributes to mammary tumorigenesis by modulating DNA repair. Cell Death Differ. 23, 1198–1208 10.1038/cdd.2015.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lakhter A. J., Kanginakudru S., Warren S., Touloukian C. E., Boissy R. E., and Naidu S. R. (2013) Impaired PIASy-Tip60 signaling weakens activation of p53 in melanoma. Melanoma Res. 23, 213–217 10.1097/CMR.0b013e328361056d [DOI] [PubMed] [Google Scholar]
- 45. Liu N., Wang J., Wang J., Wang R., Liu Z., Yu Y., and Lu H. (2013) ING5 is a Tip60 cofactor that acetylates p53 in response to DNA damage. Cancer Res. 73, 3749–3760 10.1158/0008-5472.CAN-12-3684 [DOI] [PubMed] [Google Scholar]
- 46. Pflaum J., Schlosser S., and Müller M. (2014) p53 family and cellular stress responses in cancer. Front. Oncol. 4, 285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chen A. C., Halliday G. M., and Damian D. L. (2013) Non-melanoma skin cancer: carcinogenesis and chemoprevention. Pathology 45, 331–341 10.1097/PAT.0b013e32835f515c [DOI] [PubMed] [Google Scholar]
- 48. Oti M., Kouwenhoven E. N., and Zhou H. (2015) Genome-wide p63-regulated gene expression in differentiating epidermal keratinocytes. Genomics Data 5, 159–163 10.1016/j.gdata.2015.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kouwenhoven E. N., Oti M., Niehues H., van Heeringen S. J., Schalkwijk J., Stunnenberg H. G., van Bokhoven H., and Zhou H. (2015) Transcription factor p63 bookmarks and regulates dynamic enhancers during epidermal differentiation. EMBO Rep. 16, 863–878 10.15252/embr.201439941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Khan A., Fornes O., Stigliani A., Gheorghe M., Castro-Mondragon J. A., van der Lee R., Bessy A., Chèneby J., Kulkarni S. R., Tan G., Baranasic D., Arenillas D. J., Sandelin A., Vandepoele K., Lenhard B., et al. (2018) JASPAR 2018: update of the open-access database of transcription factor binding profiles and its web framework. Nucleic Acids Res. 46, D260–D266 10.1093/nar/gkx1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lemercier C., Legube G., Caron C., Louwagie M., Garin J., Trouche D., and Khochbin S. (2003) Tip60 acetyltransferase activity is controlled by phosphorylation. J. Biol. Chem. 278, 4713–4718 10.1074/jbc.M211811200 [DOI] [PubMed] [Google Scholar]
- 52. Chang D. C., Xu N., and Luo K. Q. (2003) Degradation of cyclin B is required for the onset of anaphase in Mammalian cells. J. Biol. Chem. 278, 37865–37873 10.1074/jbc.M306376200 [DOI] [PubMed] [Google Scholar]
- 53. Clijsters L., van Zon W., Riet B. T., Voets E., Boekhout M., Ogink J., Rumpf-Kienzl C., and Wolthuis R. M. (2014) Inefficient degradation of cyclin B1 re-activates the spindle checkpoint right after sister chromatid disjunction. Cell Cycle 13, 2370–2378 10.4161/cc.29336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Brauns-Schubert P., Schubert F., Wissler M., Weiss M., Schlicher L., Bessler S., Safavi M., Miething C., Borner C., Brummer T., and Maurer U. (2018) CDK9-mediated phosphorylation controls the interaction of TIP60 with the transcriptional machinery. EMBO Rep. 19, 244–256 10.15252/embr.201744311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Charvet C., Wissler M., Brauns-Schubert P., Wang S. J., Tang Y., Sigloch F. C., Mellert H., Brandenburg M., Lindner S. E., Breit B., Green D. R., McMahon S. B., Borner C., Gu W., and Maurer U. (2011) Phosphorylation of Tip60 by GSK-3 determines the induction of PUMA and apoptosis by p53. Mol. Cell 42, 584–596 10.1016/j.molcel.2011.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. LoTempio M. M., Veena M. S., Steele H. L., Ramamurthy B., Ramalingam T. S., Cohen A. N., Chakrabarti R., Srivatsan E. S., and Wang M. B. (2005) Curcumin suppresses growth of head and neck squamous cell carcinoma. Clin. Cancer Res. 11, 6994–7002 10.1158/1078-0432.CCR-05-0301 [DOI] [PubMed] [Google Scholar]
- 57. Sonavane K., Phillips J., Ekshyyan O., Moore-Medlin T., Roberts Gill J., Rong X., Lakshmaiah R. R., Abreo F., Boudreaux D., Clifford J. L., and Nathan C. A. (2012) Topical curcumin-based cream is equivalent to dietary curcumin in a skin cancer model. J. Skin Cancer 2012, 147863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wu J., Wang J., Li M., Yang Y., Wang B., and Zheng Y. G. (2011) Small molecule inhibitors of histone acetyltransferase Tip60. Bioorg. Chem. 39, 53–58 10.1016/j.bioorg.2010.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhu X., and Zhu R. (2018) Curcumin suppresses the progression of laryngeal squamous cell carcinoma through the upregulation of miR-145 and inhibition of the PI3K/Akt/mTOR pathway. OncoTargets Ther. 11, 3521–3531 10.2147/OTT.S159236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Manzo F., Tambaro F. P., Mai A., and Altucci L. (2009) Histone acetyltransferase inhibitors and preclinical studies. Expert Opin. Ther. Pat. 19, 761–774 10.1517/13543770902895727 [DOI] [PubMed] [Google Scholar]
- 61. Gao C., Bourke E., Scobie M., Famme M. A., Koolmeister T., Helleday T., Eriksson L. A., Lowndes N. F., and Brown J. A. (2014) Rational design and validation of a Tip60 histone acetyltransferase inhibitor. Sci. Rep. 4, 5372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Caserta T. M., Kommagani R., Yuan Z., Robbins D. J., Mercer C. A., and Kadakia M. P. (2006) p63 overexpression induces the expression of Sonic Hedgehog. Mol. Cancer Res. 4, 759–768 10.1158/1541-7786.MCR-05-0149 [DOI] [PubMed] [Google Scholar]
- 63. Kommagani R., Leonard M. K., Lewis S., Romano R. A., Sinha S., and Kadakia M. P. (2009) Regulation of VDR by ΔNp63α is associated with inhibition of cell invasion. J. Cell Sci. 122, 2828–2835 10.1242/jcs.049619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pfaffl M. W. (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45 10.1093/nar/29.9.e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bostock C. J., Prescott D. M., and Kirkpatrick J. B. (1971) An evaluation of the double thymidine block for synchronizing mammalian cells at the G1-S border. Exp. Cell Res. 68, 163–168 10.1016/0014-4827(71)90599-4 [DOI] [PubMed] [Google Scholar]