Abstract
Fermented foods represent a significant portion of human diets with several beneficial effects. Foods produced by bacterial fermentation are enriched in short-chain fatty acids (SCFAs), which are functional products of dietary fibers via gut microbial fermentation. In addition to energy sources, SCFAs also act as signaling molecules via G-protein coupled receptors such as FFAR2 and FFAR3. Hence, dietary SCFAs in fermented foods may have a direct influence on metabolic functions. However, the detailed mechanism by dietary SCFAs remains unclear. Here, we show that dietary SCFAs protected against high-fat diet-induced obesity in mice in parallel with increased plasma SCFAs without changing cecal SCFA or gut microbial composition. Dietary SCFAs suppressed hepatic weight and lipid synthesis. These effects were abolished in FFAR3-deficient mice but not FFAR2-deficient. Thus, SCFAs supplementation improved hepatic metabolic functions via FFAR3 without influencing intestinal environment. These findings could help to promote the development of functional foods using SCFAs.
Subject terms: Endocrine system and metabolic diseases, Fat metabolism, Nutritional supplements, Molecular biology
Introduction
Dietary fiber is considered to be an essential healthy component of diet, and has been demonstrated to reduce the risk of metabolic diseases such as obesity and diabetes1,2. These benefits are mainly attributed to the influence of dietary fibers on increasing the levels of short-chain fatty acids (SCFAs), i.e., acetate, propionate, and butyrate, in the colon through gut microbial fermentation3,4. Various fermented foods made by bacterial fermentation, including cheese, butter, alcoholic beverages, pickles, sauerkraut, soy sauce, and yoghurt, are also highly enriched in SCFAs5–7; vinegar and alcoholic beverages contain acetate, cheese contains propionate and butyrate, and butter contains butyrate8–11. Fermented food products have also been reported to enhance nutrition, improve health, and prevent diseases, including cardio-metabolic disease and type 2 diabetes, on a global level12–14. However, the detailed metabolic benefits of fermented foods and the underlying mechanisms have not been completely elucidated to date.
SCFAs can not only be used for the de novo synthesis of lipids and glucose as the main energy sources for the host15,16 but also influence host physiological functions. Hence, SCFAs have been proposed to play an important role in the prevention and treatment of metabolic diseases. Despite some contradictory reports17,18, the metabolic benefits of SCFAs are widely accepted and supported with several lines of evidence19–24. Indeed, SCFAs were shown to improve metabolic function in mice and humans, demonstrating a direct causal relationship between the fermentation of dietary fibers and SCFAs25–28. SCFAs could also protect against diet-induced obesity and insulin resistance via systemic effects29–31. However, acetate was also reported to promote obesity via hyperphagia and insulin secretion in rodents32, and propionate impaired the action of insulin via glucagon secretion17.
Recent studies have shown that these metabolic benefits by SCFAs are exerted via their receptors, such as the G protein-coupled receptor GPR41/FFAR3 and GPR43/FFAR2. They are free fatty acids receptors with Gi/o coupling for FFAR3 and dual coupling through the Gi/o and Gq families for FFAR2. These receptors are activated by SCFAs with EC50 values of μM order33,34. Therefore, SCFAs exert not only intestinal effects but also systemic effects through circulating in the plasma35. FFAR3 regulates neural activity, glucose homeostasis, and lipid metabolism via secretion of catecholamine, endocrine hormones, and gut hormones36,37, whereas FFAR2 regulates glucose homeostasis and lipid metabolism via insulin action, the inflammatory response, and gut hormone secretion38–40. With oral administration, nutrients such as SCFAs are almost completely absorbed in the small intestine25. However, the detailed mechanisms underlying the difference in the metabolic benefits conferred by the direct intake of dietary SCFAs (such as through fermented foods) and those provided by gut microbes producing SCFAs from dietary fibers in the colon remain unclear.
To clarify these effects, in this study, we investigated the SCFAs-mediated systemic effects via circulating plasma upon dietary SCFA intake in a mouse model of high-fat diet (HFD)-induced obesity. We confirmed that dietary SCFA intake such as fermented foods mainly causes improved hepatic metabolic conditions via FFAR3 in HFD-induced obese mice.
Results
Dietary SCFA intake increases plasma SCFA levels
We firstly investigated changes in incorporated SCFAs level following SCFAs feeding in a mouse model of HFD-induced obesity. In this experiment, 7-week-old mice were fed HFDs containing 5% SCFAs (acetate, propionate, and butyrate, respectively), 5% cellulose or normal HFD as control for 4 weeks (Supplementary Table S1). Feeding of diets supplemented with each SCFA significantly increased the corresponding SCFA levels compared with those of the cellulose-supplemented and control HFD-fed mice (Supplementary Fig. S1A), whereas there were no changes in the cecal SCFAs levels (Supplementary Fig. S1B). Moreover, 16S rRNA gene amplicon sequencing confirmed that the SCFA diets did not alter the relative abundance of the major phyla constituting the gut microbiota (Supplementary Fig. S1C) or the gut microbiota composition, as indicated by principal coordinates analysis with reference to taxonomic datasets (Supplementary Fig. S1D).
Dietary SCFA intake exerts metabolic benefits
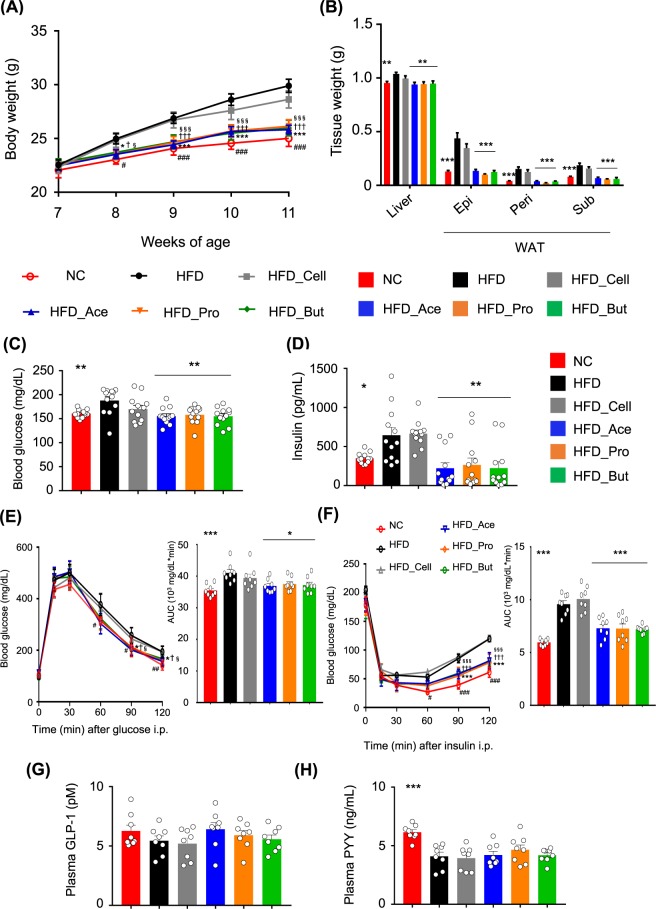
We next investigated changes in metabolic parameters following SCFAs feeding. HFD feeding to adult mice for 4 weeks can sufficiently induce obesity and related metabolic disturbances as compared to that in normal chow-fed mice (Fig. 1A–F)41–43. Body weights in the SCFAs-fed mice were significantly lower than those in the cellulose-fed and control mice during growth (Fig. 1A). The white adipose tissue (WAT) mass and liver weights (Fig. 1B), blood glucose levels (Fig. 1C), and plasma insulin levels (Fig. 1D) were also significantly lower in the SCFA groups than those in the cellulose-fed and control mice at 11 weeks of age. Furthermore, HFD-induced insulin resistance and impaired glucose tolerance, as determined by the glucose tolerance test (GTT) and insulin tolerance test (ITT), respectively, were significantly attenuated in SCFA-fed mice as compared to those in cellulose-fed and control mice (Fig. 1E,F). However, the plasma levels of the gut hormones glucagon like peptide-1 (GLP-1) and peptide YY (PYY) were similar among all groups, although plasma GLP-1 tended to be slightly higher in the SCFA-fed groups (Fig. 1G,H). Food intake was also similar among all groups (Supplementary Fig. S2).
Figure 1.
Short-chain fatty acid (SCFA) supplementation exerts metabolic benefits. Body weight changes (A), liver and white adipose tissue (WAT) weights (B), blood glucose (C), plasma insulin (D), glucose tolerance test (GTT) (E), insulin tolerance test (ITT) (F), GLP-1 (G), and PYY levels (H) measured after 4 weeks of normal chow (NC) or high-fat diet (HFD) feeding supplemented with 5% SCFAs. All data are presented as the means ± SEM (n = 8–12). Dunnett’s test; ***P < 0.001, **P < 0.01, and *P < 0.05, compared with HFD. ###P < 0.001, ##P < 0.01, and #P < 0.05 (HFD vs. NC), ***P < 0.001 and *P < 0.05 (HFD vs. Ace), †††P < 0.001 and †P < 0.05 (HFD vs. Pro), §§§P < 0.001 and §P < 0.05 (HFD vs. But) (A,E,F). Epi: epididymal tissue, Peri: perirenal tissue, Sub: subcutaneous tissue.
Dietary SCFA intake improves hepatic metabolic conditions
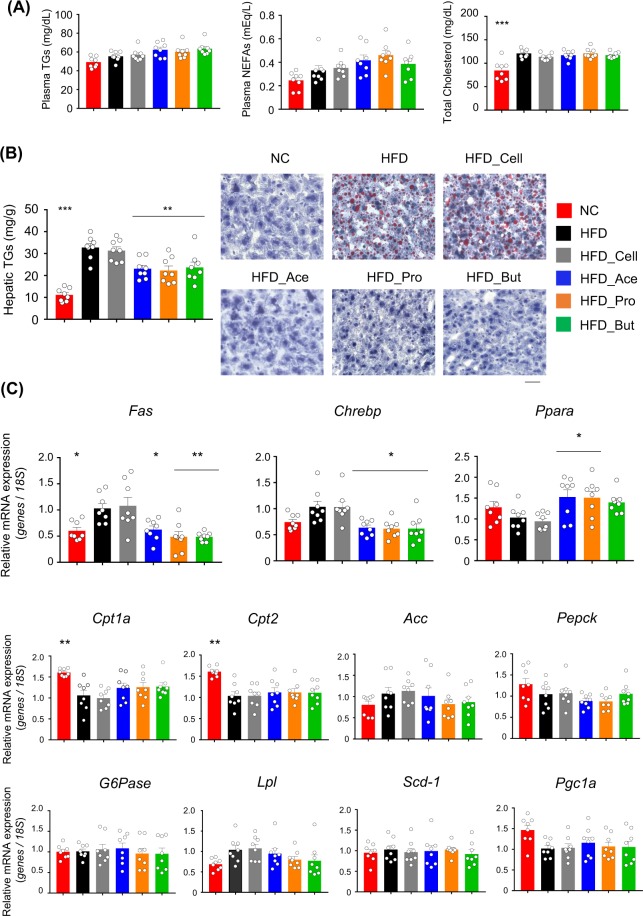
We further examined changes in lipid metabolism. Although plasma triglycerides (TGs), non-esterified fatty acids (NEFAs), and total cholesterol levels were comparable between each SCFAs-fed group and the cellulose-fed or control group (Fig. 2A), hepatic TG levels in each group of SCFA-fed mice were significantly lower than those in cellulose-fed and control mice (Fig. 2B). We also investigated the expression profiles of hepatic genes related to energy metabolism. The expression levels of Fas and Chrebp—which are related to fatty acid synthesis—decreased, while that of Ppara—as a key regulator of lipid metabolism with acetate and propionate feeding—was increased in the SCFAs-fed mice compared to those of cellulose-fed and control mice (Fig. 2C). However, these changes in genes related to energy metabolism were not observed in the WAT or muscle (Supplementary Figs S3 and 4). Thus, dietary SCFA intake suppressed the HFD-induced accumulation of hepatic TGs via changing hepatic lipid metabolism but not the WAT and muscle metabolism.
Figure 2.
Dietary short-chain fatty acid (SCFA) intake improves hepatic metabolic conditions. Plasma triglycerides, non-esterified fatty acids (NEFAs), and total cholesterol concentrations (A), hepatic triglyceride contents and histology of hepatocytes based on hematoxylin-eosin (H&E) oil red O stanning. Scale bar, 50 μm (B), and mRNA expression levels of hepatic energy metabolism-related genes (C) measured after 4 weeks of normal chow (NC) or high-fat diet (HFD) feeding supplemented with 5% SCFAs. All data are presented as the means ± SEM (n = 8). Dunnett’s test; ***P < 0.001, **P < 0.01, and *P < 0.05, compared with HFD.
FFAR3 deficiency abolishes the metabolic benefits of dietary SCFA intake
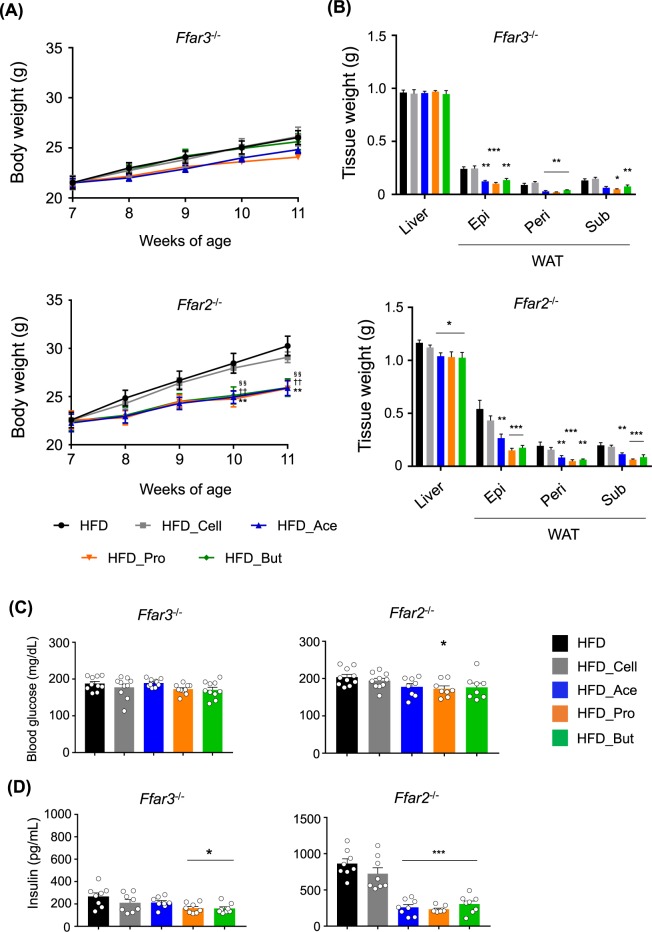
SCFAs exhibit various physiological functions related to energy regulation13. Therefore, we next investigated the roles of the SCFA receptors FFAR2 and FFAR3 in the observed metabolic improvement by increased plasma SCFAs from dietary SCFA intake using SCFA receptor-deficient mice. Before HFD feeding, initial body and tissue weights, and blood glucose, as well as hepatic lipid metabolic related genes such as Fas, Chrebp, and Ppara were similar among three groups (Supplementary Fig. S5). Although food intake was comparable among the three groups (Supplementary Fig. S2), the suppression of HFD-induced weight gain by SCFAs supplementation was attenuated in Ffar3−/− mice, especially with butyrate feeding, whereas the effects in Ffar2−/− mice were comparable with those in wild-type mice (Fig. 3A). Moreover, although the HFD-induced WAT mass increase was suppressed by the SCFAs in both Ffar3−/− and Ffar2−/− mice, similar to the wild-type mice, the suppression of HFD-induced liver weight gain by SCFAs feeding was abolished in the Ffar3−/− mice but not in the Ffar2−/− mice (Fig. 3B). The changes in blood glucose by SCFAs feeding were also completely abolished in Ffar3−/− mice and were attenuated in Ffar2−/− mice (Fig. 3C). Although basal plasma insulin levels in HFD-fed mice were lower in Ffar3−/− mice and higher in Ffar2−/− mice, the SCFAs suppressed these changes in both Ffar2−/− and Ffar3−/− mice, similar to the effects observed in wild-type mice (Fig. 3D). These results indicated that the functions of FFAR3 in the liver are partially related to the metabolic benefits from dietary SCFA intake.
Figure 3.
FFAR3 deficiency abolishes dietary short-chain fatty acid (SCFA) intake-induced metabolic benefits. Body weight changes (A), liver and white adipose tissue (WAT) weights (B), blood glucose (C), and plasma insulin (D) levels in Ffar3−/− (n = 10) and Ffar2−/− (n = 8–10) mice measured after 4 weeks of high-fat diet (HFD) feeding supplemented with 5% SCFAs. All data are presented as the means ± SEM. Dunnett’s test; ***P < 0.001, **P < 0.01, and *P < 0.05, compared with HFD. **P < 0.01 (HFD vs. Ace), ††P < 0.01 (HFD vs. Pro), §§P < 0.01 (HFD vs. But) (A). Epi: epididymal tissue, Peri: perirenal tissue, Sub: subcutaneous tissue.
FFAR3 deficiency abolishes the SCFAs-induced expression changes of hepatic lipid metabolism-related genes
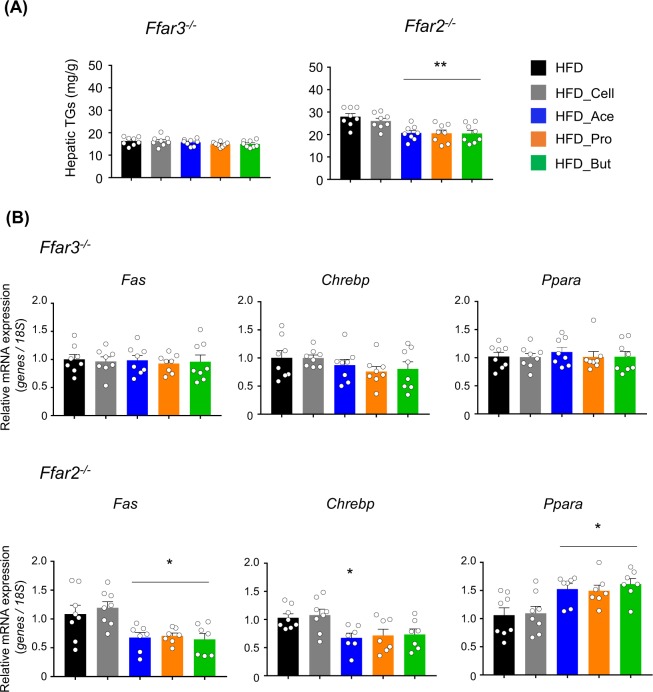
We next examined the influence of SCFAs on liver function in the SCFA receptor-deficient mice. Although the basal hepatic TG levels in HFD-fed mice were relatively lower in Ffar3−/− mice, SCFAs feeding did not suppress hepatic TG accumulation, whereas this increase was suppressed in Ffar2−/− mice as well as in wild-type mice (Fig. 4A). Moreover, the SCFAs-induced changes in hepatic lipid metabolism-related genes such as Fas, Chrebp, and Ppara were also abolished in Ffar3−/− mice but not in Ffar2−/− mice (Fig. 4B). Thus, dietary SCFA intake improves hepatic lipid metabolism via FFAR3.
Figure 4.
FFAR3 deficiency abolishes dietary short-chain fatty acid (SCFA) intake-induced expression change of hepatic lipid metabolism-related genes. Hepatic triglycerides contents (A) and mRNA expression levels of hepatic lipid metabolism-related genes (B) in Ffar3−/− and Ffar2−/− mice (n = 7–8) after 4 weeks of high-fat diet (HFD) feeding supplemented with 5% SCFAs. All data are presented as the means ± SEM. Dunnett’s test; **P < 0.01 and *P < 0.05, compared with HFD.
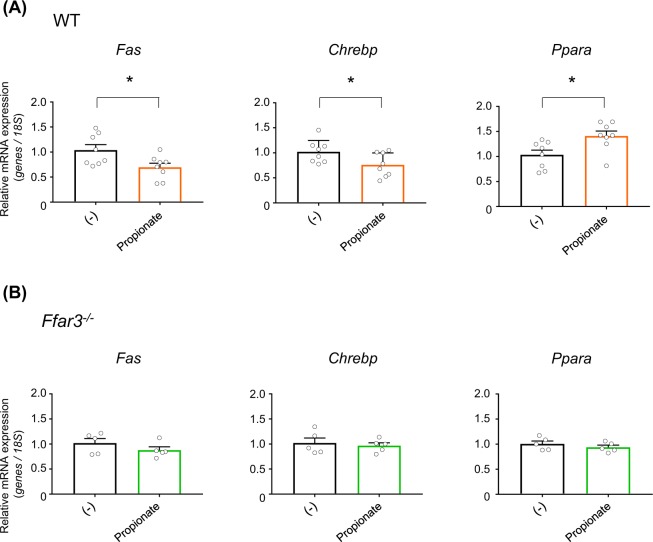
Finally, we investigated whether SCFAs influence hepatic lipid metabolism under direct stimulation rather than as a secondary long-term effect. Intraperitoneal administration of propionate, as the most potent agonist for FFAR3, transiently increases plasma propionate levels37. Similar to the effects of long-term dietary SCFAs feeding, acute propionate injection decreased the expression levels of hepatic Fas and Chrebp, and increased the expression level of hepatic Ppara in wild-type mice (Fig. 5A) without changing the body weight, liver weight, blood glucose, plasma TGs, and hepatic TGs (Supplementary Fig. S6). By contrast, the effects of acute propionate administration were almost completely abolished in Ffar3−/− mice (Fig. 5B). These results confirmed that dietary SCFA intake improves hepatic metabolic conditions via plasma SCFA-stimulated FFAR3.
Figure 5.
FFAR3 deficiency abolishes acute short-chain fatty acid (SCFA) injection-induced expression changes in hepatic lipid metabolism-related genes. mRNA expression levels of hepatic lipid metabolism-related genes in wild-type (WT) (n = 8) (A) and Ffar3−/− (B) mice (n = 5) at 24 h after intraperitoneal phosphate buffered saline (for control group) or propionate administration (1 g/kg body weight) under high-fat diet (HFD) feeding. All data are presented as the means ± SEM. Student’s t-test; *P < 0.05, compared with (−).
Discussion
Dietary fiber and its gut microbial metabolite SCFAs are well known to exert metabolic benefits to the host, and the mechanisms have been extensively examined. However, the underlying mechanism of the metabolic benefits provided by dietary SCFA intake, such as with fermented food enriched in SCFAs, are less well understood. Here, we demonstrate that dietary SCFA intake increases plasma SCFA levels to active FFAR3 and improves hepatic metabolic conditions without changing the intestinal environment.
Indeed, dietary SCFA intake increased plasma SCFAs levels but not cecal SCFAs levels without changing the gut microbial compositions in HFD-fed mice. Consequently, dietary SCFA intake dramatically suppressed the HFD-induced liver and WAT weight gains, without influencing gut hormones. This result indicates that direct intake of dietary SCFAs such as consumption of fermented foods also exhibits metabolic benefits. Although these effects appear to be similar to those of dietary fiber, the mechanism may differ to some degree. For example, dietary SCFA intake did not sufficiently change plasma gut hormone levels, although plasma GLP-1 tended to be slightly higher in the SCFA-fed groups, because almost all SCFAs are absorbed in the small intestine and they are not readily transfered to the colon25, whereas dietary fiber intake produces SCFAs via fermentation by gut microbiota mainly in the colon. L-cells producing GLP-1 and PYY are localize mainly in the distal ileum and colon. Therefore, direct SCFA intake might mainly exert systemic effects via the plasma SCFAs rather than via distal intestinal SCFAs. Further investigation is needed to test this hypothesis and clarify the difference between the effects of direct SCFA intake and gut microbiota-produced SCFAs.
We found that dietary SCFA intake suppressed the HFD-induced liver weight gain and hepatic TGs accumulation along with a change in hepatic lipid metabolism-related genes, and these effects were abolished by FFAR3 deficiency but not FFAR2 deficiency. Similarly, a previous study showed that dietary SCFA intake suppressed the synthesis of hepatic fatty acids44. Hence, we concluded that dietary SCFA intake improves hepatic metabolic conditions via FFAR3. However, since FFAR3 is barely expressed in the liver36, it is more likely that plasma SCFAs indirectly influence hepatic lipid metabolic-related genes such as Fas and Ppara in the liver via other FFAR3-expressing tissues. Given the high expression of FFAR3 in the peripheral nerves36, FFAR3-mediated neural activity might explain the observed improvement of hepatic metabolic conditions by SCFAs. The FFAR3-FFAR2 heteromer and species difference influence different intracellular signaling pathways45,46 and therefore, it might also exert other physiological effects. To further clarify this FFAR3-mediated molecular mechanism, further experiments are needed with tissue-specific FFAR3-deficient mice.
SCFAs are known to influence insulin actions via their receptors47,48. Indeed, the plasma insulin levels in HFD-fed control and cellulose-supplemented mice were already drastically lower at baseline in Ffar3−/− mice compared with those of wild-type mice. Hence, this difference might partially explain why the protection of dietary SCFA intake against HFD-induced obesity and hyperglycaemia was abolished in the Ffar3−/− mice. However, acute SCFAs intraperitoneal administration in Ffar3−/− mice also abolished the changes of SCFAs-induced hepatic lipid metabolism-related genes observed in wild-type mice. Accordingly, improvement of hepatic metabolic conditions by SCFAs appears to be due, at least in part, to the direct effects via FFAR3. Moreover, the protective effect of dietary SCFA intake against HFD-induced WAT weight gain was not abolished in both Ffar3−/− and Ffar2−/− mice. This indicates that other SCFA receptors beside FFAR2 and FFAR3, SCFA-mediated bioactivities, or the confounding effects of interactions between FFAR2 and FFAR3 might be related to the SCFA-mediated suppression of adipose fat accumulation. To further clarify this SCFA and receptor-mediated molecular mechanism, further experiments are also needed with FFAR2/FFAR3-double-deficient mice.
Overall, we showed that dietary intake of the three major SCFAs, acetate, propionate, and butyrate, protected against HFD-induced obesity, and improved hepatic metabolic conditions via FFAR3 in mice. These findings demonstrate that SCFAs themselves, in addition to the dietary fiber and/or gut microbiota, have anti-obesity effects and could be used to improve metabolic conditions. Thus, we have provided novel insight into the mechanism underlying the beneficial effects of fermented foods. Accordingly, these results may guide the development of functional foods for the prevention of metabolic disorders such as obesity and type 2 diabetes mellitus.
Methods
Mice, diet, and experimental design
Male C57BL/6J mice were purchased from Japan SLC (Shizuoka, Japan) and maintained under a strict 12-h light/dark cycle in a conventional animal room at 23.0 °C and 40–70% relative humidity. The mice were acclimated to the laboratory conditions on the CLEA Rodent Diet (CE-2, CLEA Japan, Inc., Tokyo, Japan) for 1 week prior to the treatment. The 7-week-old C57BL/6J mice were placed on a D12492 diet (HFD: 60% of calories from fat; Research diets, New Brunswick, NJ, USA) or an HFD containing 5% cellulose, acetate, propionate, or butyrate for 4 weeks (n = 12 per group). The compositions of the diets are given in Supplementary Table S1. These diets were adjusted so that the final percentages of protein, fat, and carbohydrates were almost equal in all groups. In all experiments, the 7-week-old mice were divided into six groups of similar average body weight (n = 8–10 per group). The generation of Ffar3−/− and Ffar2−/− mice was described previously36,40.
The body weights were measured once a week. Food intake was calculated as the average of daily food intake (g/day per mouse) for 4 weeks. All mice were then sacrificed under deep isoflurane-induced anaesthesia, and the liver, caecum, epididymal, perirenal, and subcutaneous adipose tissues were harvested and weighed. Blood was collected from the inferior vena cava using heparinised tubes and plasma was separated by immediate centrifugation (7,000 × g, 5 min, 4 °C). For intraperitoneal phosphate buffered saline (for control group) or propionate administration (sodium propionate, Wako, 1 g/kg body weight) as previous described36, 7-week-old wild-type and Ffar3−/− mice on C57BL/6J background were sacrificed and collected samples at 24 h after propionate administration under HFD feeding. All tissues and plasma were stored at −80 °C until further processing. All experimental procedures involving mice were performed in 2017–2019 according to protocols approved by the Committee on the Ethics of Animal Experiments of the Tokyo University of Agriculture and Technology (Permit No. 28-87). All efforts were made to minimise suffering.
Plasma biochemical analyses
The blood glucose concentration was measured using One Touch Ultra Test Strips (One Touch® Ultra®, Life Scan, Milpitas, CA, USA). The concentrations of plasma total cholesterol (Lab Assay™ Cholesterol, Wako, Tokyo, Japan), NEFAs (Lab Assay™ NEFA, Wako, Tokyo, Japan), plasma and hepatic TG (Lab Assay™ Triglyceride, Wako, Tokyo, Japan), plasma PYY (Mouse/Rat PYY ELISA Kit, Wako, Tokyo, Japan), GLP-1 (GLP-1 Active ELISA Kit, Merck Millipore, Darmstadt, Germany), and insulin (Insulin ELISA KIT (RTU), Shibayagi, Gunma, Japan) were measured following the manufacturer’s instructions. For plasma GLP-1 measurement, the plasma sample was treated with a dipeptidyl peptidase IV inhibitor (Merck Millipore, Darmstadt, Germany) to prevent the degradation of active GLP-1. SCFA levels in the plasma and caecum were determined following a modification of a previously described protocol49. Herein, the SCFA-containing ether layers were collected and pooled for gas chromatography-mass spectrometry analysis using a GCMS-QP2010 Ultra system (Shimadzu, Kyoto, Japan). The calibration curves for SCFAs were constructed, and the concentration of each SCFA in the samples was evaluated over a specified concentration range.
GTT and ITT
For GTT, 16-h fasted mice were given 2.0 mg of glucose per gram of body weight intraperitoneally (i.p.). For ITT, 3-h fasted mice were given human insulin (0.75mUg−1, i.p., Sigma). The blood glucose concentration was measured before injection and monitored at 15, 30, 60, 90 and 120 min after injection.
Quantification of hepatic TG
The liver contents were weighed and stored at −80 °C until further processing. Hepatic triacylglycerol contents were measured following a modification of a previously described protocol40. Briefly, liver homogenates were subjected to crude lipid extraction using a mixture of chloroform/methanol/0.45 M acetic acid. Subsequently, 3 volumes of mixture were added and shaken overnight (4 °C). After centrifugation at 1,500 × g for 10 min, the organic layer was collected, dried, and resuspended in isopropyl alcohol. Measurements were conducted using the Lab Assay™ Triglyceride (Wako, Tokyo, Japan).
Hepatic histology
Livers were embedded in OCT compounds (SAKURA, Finetek, Japan) and sectioned at 10 μm. Hematoxylin–eosin and oil red O (Sigma) staining were based on a previously described protocol with modification40, and examined with fluorescence microscope (All-in-One Fluorescence Microscope BZ-X700, KEYENCE, Osaka, Japan).
Analysis of gut microbiota by 16S rRNA gene sequencing
Faecal DNA was extracted using Fast DNA® SPIN Kit (MP Biomedicals, Santa Ana, CA, USA), and the V4 region of the 16S rRNA gene was amplified using dual-indexed primers. The amplicons were then sequenced using an Illumina MiSeq with a MiSeq Reagent kit V3 (Illumina, San Diego, CA, USA). Paired-end sequencing was carried out using the Illumina MiSeq platform. Processing and quality filtering of the reads were performed with Quantitative Insights into Microbial Ecology (QIIME) (v1.9.1) software, and the chimera-free sequences were aligned with the SILVA database (http://www.arb-silva.de) at a 97% identity threshold.
Real-time polymerase chain reaction (RT-PCR)
RT-PCR was conducted following a modification of a previously described protocol40. RT-PCR was completed with SYBR Premix Ex Taq II (TaKaRa, Shiga, JAPAN) and the Step OneTM Real-time PCR system (Applied Biosystems, Foster City, CA, USA). The ∆∆CT method was used to determine the relative expression levels with the mRNA levels of the housekeeping 18S gene as reference. The primer sequences are shown in Supplementary Table S2.
Statistical analysis
All values are presented as the mean ± SEM. The statistical significance of differences between groups was determined using a two-tailed unpaired Student’s t-test (two groups) or two-tailed one-way analysis of variance, followed by Dunnett’s post hoc test (≥three groups). To estimate the required sample size for each experiment, a priori power analysis was performed using the G*Power software ver3.1 (Franz Faul, Universiät Kiel, Germany; http://www.gpower.hhu.de/). Sample size used in the present study will achieve 95% actual power to detect an effect of each experiment assuming a one-way ANOVA with a 0.05 significance level. Except for the power analysis, all experimental data analyses were performed using GraphPad Prism 7.0 (Graphpad Software, San Diego, CA, USA).
Supplementary information
Acknowledgements
This work was partially supported by the AMED-CREST (JP18gm1010007 to I.K.) and JST-OPERA (JPMJOP1833 to I.K.).
Author contributions
H.S. performed the experiments, interpreted data, and wrote the paper; Y.M. performed the experiments; C.U. performed the experiments; R.M. performed the experiments; S.T. performed the experiments and interpreted data; R.O.-K. performed the experiments and interpreted data; I.K. supervised the project, interpreted data, and wrote the paper; I.K. had primary responsibility for the final content. All authors read and approved the final manuscript.
Data availability
The raw 16S rRNA sequence data have been deposited into the DNA Data Bank of Japan (DDBJ) database under accession no. DRA008619 [https://ddbj.nig.ac.jp/DRASearch/submission?acc = DRA008619].
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-019-53242-x.
References
- 1.Li X, Guo J, Ji K, Zhang P. Bamboo shoot fiber prevents obesity in mice by modulating the gut microbiota. Sci. Rep. 2016;6:32953. doi: 10.1038/srep32953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miyamoto J, et al. Barley beta-glucan improves metabolic condition via short-chain fatty acids produced by gut microbial fermentation in high fat diet fed mice. PLoS One. 2018;13:e0196579. doi: 10.1371/journal.pone.0196579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chambers ES, Preston T, Frost G, Morrison DJ. Role of gut microbiota-generated short-chain fatty acids in metabolic and cardiovascular health. Curr. Nutr. Rep. 2018;7:198–206. doi: 10.1007/s13668-018-0248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernandes J, Su W, Rahat-Rozenbloom S, Wolever TM, Comelli EM. Adiposity, gut microbiota and faecal short chain fatty acids are linked in adult humans. Nutr. Diabetes. 2014;4:e121. doi: 10.1038/nutd.2014.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolfe BE, Dutton RJ. Fermented foods as experimentally tractable microbial ecosystems. Cell. 2015;161:49–55. doi: 10.1016/j.cell.2015.02.034. [DOI] [PubMed] [Google Scholar]
- 6.Sieuwerts S, et al. Unraveling microbial interactions in food fermentations: from classical to genomics approaches. Appl. Environ. Microbiol. 2008;74:4997–5007. doi: 10.1128/AEM.00113-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montel MC, et al. Traditional cheeses: rich and diverse microbiota with associated benefits. Int. J. Food Microbiol. 2014;177:136–154. doi: 10.1016/j.ijfoodmicro.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 8.Li S, et al. Microbial diversity and their roles in the vinegar fermentation process. Appl. Microbiol. Biotechnol. 2015;99:4997–5024. doi: 10.1007/s00253-015-6659-1. [DOI] [PubMed] [Google Scholar]
- 9.Cameleyre M, Lytra G, Tempere S, Barbe JC. 2-Methylbutyl acetate in wines: Enantiomeric distribution and sensory impact on red wine fruity aroma. Food Chem. 2017;237:364–371. doi: 10.1016/j.foodchem.2017.05.093. [DOI] [PubMed] [Google Scholar]
- 10.Pandey A, Srivastava S, Rai P, Duke M. Cheese whey to biohydrogen and useful organic acids: A non-pathogenic microbial treatment by L. acidophilus. Sci. Rep. 2019;9:8320. doi: 10.1038/s41598-019-42752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalo P, Kemppinen A, Ollilainen V. Determination of triacylglycerols in butterfat by normal-phase HPLC and electrospray-tandem mass spectrometry. Lipids. 2009;44:169–195. doi: 10.1007/s11745-008-3247-5. [DOI] [PubMed] [Google Scholar]
- 12.Neumann, U. et al. Bioavailability and safety of nutrients from the microalgae Chlorella vulgaris, Nannochloropsis oceanica and Phaeodactylum tricornutum in C57BL/6 mice. Nutrients10 (2018). [DOI] [PMC free article] [PubMed]
- 13.Koh A, De, Vadder F, Kovatcheva-Datchary P, Bäckhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165:1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 14.van H, et al. Impact of microbial transformation of food on health - from fermented foods to fermentation in the gastro-intestinal tract. Curr. Opin. Biotechnol. 2011;22:211–219. doi: 10.1016/j.copbio.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Cani PD, et al. Microbial regulation of organismal energy homeostasis. Nat. Metab. 2019;1:34–46. doi: 10.1038/s42255-018-0017-4. [DOI] [PubMed] [Google Scholar]
- 16.Hu J, Lin S, Zheng B, Cheung PCK. Short-chain fatty acids in control of energy metabolism. Crit. Rev. Food Sci. Nutr. 2018;58:1243–1249. doi: 10.1080/10408398.2016.1245650. [DOI] [PubMed] [Google Scholar]
- 17.Tirosh A, et al. The short-chain fatty acid propionate increases glucagon and FABP4 production, impairing insulin action in mice and humans. Sci. Transl. Med. 2019;11:eaav0120. doi: 10.1126/scitranslmed.aav0120. [DOI] [PubMed] [Google Scholar]
- 18.Perry RJ, et al. Acetate mediates a microbiome-brain-beta-cell axis to promote metabolic syndrome. Nature. 2016;534:213–217. doi: 10.1038/nature18309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015;11:577–591. doi: 10.1038/nrendo.2015.128. [DOI] [PubMed] [Google Scholar]
- 20.Byrne CS, Chambers ES, Morrison DJ, Frost G. The role of short chain fatty acids in appetite regulation and energy homeostasis. Int. J. Obes. 2015;39:1331–1338. doi: 10.1038/ijo.2015.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishitsuji K, et al. Analysis of the gut microbiome and plasma short-chain fatty acid profiles in a spontaneous mouse model of metabolic syndrome. Sci. Rep. 2017;7:15876. doi: 10.1038/s41598-017-16189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanna S, et al. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat. Genet. 2019;51:600–605. doi: 10.1038/s41588-019-0350-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cornejo PI, et al. Importance of gut microbiota in obesity. Eur. J. Clin. Nutr. 2019;72(Suppl 1):26–37. doi: 10.1038/s41430-018-0306-8. [DOI] [PubMed] [Google Scholar]
- 24.Lu Y, et al. Short chain fatty acids prevent high-fat-diet-induced obesity in mice by regulating G protein-coupled receptors and gut microbiota. Sci. Rep. 2016;6:37589. doi: 10.1038/srep37589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chambers ES, et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut. 2015;64:1744–1754. doi: 10.1136/gutjnl-2014-307913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freeland KR, Wilson C, Wolever TM. Adaptation of colonic fermentation and glucagon-like peptide-1 secretion with increased wheat fibre intake for 1 year in hyperinsulinaemic human subjects. Br. J. Nutr. 2010;103:82–90. doi: 10.1017/S0007114509991462. [DOI] [PubMed] [Google Scholar]
- 27.Zmora N, Suez J, Elinav E. You are what you eat: diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019;16:35–56. doi: 10.1038/s41575-018-0061-2. [DOI] [PubMed] [Google Scholar]
- 28.He B, et al. Transmissible microbial and metabolomic remodeling by soluble dietary fiber improves metabolic homeostasis. Sci. Rep. 2015;5:10604. doi: 10.1038/srep10604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin HV, et al. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS One. 2012;7:e35240. doi: 10.1371/journal.pone.0035240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De VF, et al. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156:84–96. doi: 10.1016/j.cell.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 31.Donohoe DR, et al. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13:517–526. doi: 10.1016/j.cmet.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rachel JP, et al. Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature. 2016;534:213–217. doi: 10.1038/nature18309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Le PE, et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 2003;278:25481–25489. doi: 10.1074/jbc.M301403200. [DOI] [PubMed] [Google Scholar]
- 34.Brown AJ, et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 2003;278:11312–11319. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- 35.Lee WJ, Hase K. Gut microbiota-generated metabolites in animal health and disease. Nat. Chem. Biol. 2014;10:416–424. doi: 10.1038/nchembio.1535. [DOI] [PubMed] [Google Scholar]
- 36.Kimura I, et al. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41(GPR41) Proc. Natl. Acad. Sci. USA. 2011;108:8030–8035. doi: 10.1073/pnas.1016088108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Samuel BS, et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc. Natl. Acad. Sci. USA. 2008;105:16767–16772. doi: 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tolhurst G, et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61:364–371. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ge H, et al. Activation of G protein-coupled receptor 43 in adipocytes leads to inhibition of lipolysis and suppression of plasma free fatty acids. Endocrinology. 2008;149:4519–4526. doi: 10.1210/en.2008-0059. [DOI] [PubMed] [Google Scholar]
- 40.Kimura I, et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat. Commun. 2013;4:1829. doi: 10.1038/ncomms2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watanabe H, et al. Dietary Mung Bean Protein Reduces Hepatic Steatosis, Fibrosis, and Inflammation in Male Mice with Diet-Induced, Nonalcoholic Fatty Liver Disease. J. Nutr. 2017;147:52–60. doi: 10.3945/jn.116.231662. [DOI] [PubMed] [Google Scholar]
- 42.Watanabe K, et al. Dietary soybean protein ameliorates high-fat diet-induced obesity by modifying the gut microbiota-dependent biotransformation of bile acids. PLoS One. 2018;13:e0202083. doi: 10.1371/journal.pone.0202083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fleissner CK, et al. Absence of intestinal microbiota does not protect mice from diet-induced obesity. Br. J. Nutr. 2010;104:919–929. doi: 10.1017/S0007114510001303. [DOI] [PubMed] [Google Scholar]
- 44.den, Besten G, et al. Short-chain fatty acids protect against high-fat diet-induced obesity via a PPARγ-dependent switch from lipogenesis to fat oxidation. Diabetes. 2015;64:2398–2408. doi: 10.2337/db14-1213. [DOI] [PubMed] [Google Scholar]
- 45.Ang Z, et al. Human and mouse monocytes display distinct signalling and cytokine profiles upon stimulation with FFAR2/FFAR3 short-chain fatty acid receptor agonists. Sci. Rep. 2016;6:34145. doi: 10.1038/srep34145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ang Z, Xiong D, Wu M, Ding JL. FFAR2-FFAR3 receptor heteromerization modulates short-chain fatty acid sensing. FASEB J. 2018;32:289–303. doi: 10.1096/fj.201700252RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tang C, et al. Loss of FFA2 and FFA3 increases insulin secretion and improves glucose tolerance in type 2 diabetes. Nat. Med. 2015;21:173–177. doi: 10.1038/nm.3779. [DOI] [PubMed] [Google Scholar]
- 48.McNelis JC, et al. GPR43 potentiates β-cell function in obesity. Diabetes. 2015;64:3203–3217. doi: 10.2337/db14-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakajima A, et al. The short chain fatty acid receptor GPR43 regulates inflammatory signals in adipose tissue M2-type macrophages. PLoS One. 2017;12:e0179696. doi: 10.1371/journal.pone.0179696. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw 16S rRNA sequence data have been deposited into the DNA Data Bank of Japan (DDBJ) database under accession no. DRA008619 [https://ddbj.nig.ac.jp/DRASearch/submission?acc = DRA008619].