Abstract
Fertilization of an egg by multiple sperm, polyspermy, is lethal to most sexually reproducing species. To combat the entry of additional sperm into already fertilized eggs, organisms have developed various polyspermy blocks. One such barrier, the fast polyspermy block, uses a fertilization-activated depolarization of the egg membrane to electrically inhibit supernumerary sperm from entering the egg. The fast block is commonly used by eggs of oviparous animals with external fertilization. In this review we discuss the history of the fast block discovery, as well as general features shared by all organisms that use this polyspermy block. Given the diversity of habitats of external fertilizers, the fine details of the fast block-signaling pathways differ drastically between species, including the identity of the depolarizing ions. We highlight the known molecular mediators of these signaling pathways in amphibians and echinoderms, with a fine focus on ion channels that signal these fertilization-evoked depolarizations. We also discuss the investigation for a fast polyspermy block in mammals and teleost fish, and we outline potential fast block triggers. Since the first electrical recordings made on eggs in the 1950s, the fields of developmental biology and electrophysiology have substantially matured, and yet we are only now beginning to discern the intricate molecular mechanisms regulating the fast block to polyspermy.
Keywords: Fast block, polyspermy, ion channels, amphibian, echinoderm
Introduction
Fertilization of an egg by multiple sperm, a condition known as polyspermy, is catastrophic to embryonic development in most sexually reproducing species. Polyspermy induces the formation of multipolar spindle assemblies that disrupt mitotic division, as well as the inheritance of an unviable number of chromosomes (Bianchi and Wright 2016; Snook et al. 2011). While a few physiologically polyspermic species exist, including various reptiles and birds (Iwao 2012; Mizushima 2017), preventing polyspermy is generally necessary to ensure proper embryonic development. Accordingly, eggs have various mechanisms to prevent entry of more than one sperm; the two most common are referred to as the fast and slow blocks (Jaffe and Gould 1985; Wong and Wessel 2006).
The fast polyspermy block is an electrical barrier to sperm entry and is created by a fertilization-induced depolarization of the egg membrane within seconds of gamete unification (Jaffe 1976). To date, the fast block has only been documented in eggs from oviparous organisms with external fertilization, where the sperm-to-egg ratio is elevated at the time of fertilization (Cross and Elinson 1980; Jaffe 1976; Jaffe et al. 1983b; Miyazaki and Igusa 1981). This fertilization-induced depolarization persists until the enactment of the slow block, which occurs tens of seconds to minutes after fertilization, to ensure that nascent zygotes have uninterrupted protection from polyspermy (Jaffe and Gould 1985; Wong and Wessel 2006). The slow polyspermy block occurs ubiquitously in sexual reproducers (Jaffe and Gould 1985; Wong and Wessel 2006). In internal fertilizers, where it takes much longer for sperm to reach the egg (Cummins and Yanagimachi 1982; Suarez 1987), the slow block is the initial polyspermy barrier that sperm encounter at the egg (Jaffe and Gould 1985; Wong and Wessel 2006). The slow block broadly encompasses two post-fertilization events that physically prevent supernumerary sperm from entering an already fertilized egg: 1) an extracellular matrix (ECM) block, whereby the exocytosis of cortical granules from the egg transform the ECM into a barrier that sperm cannot bind to or penetrate, and 2) a membrane fusion block that removes sperm receptors from the egg membrane to block gamete unification (Bianchi and Wright 2016; Jaffe and Gould 1985; Wong and Wessel 2006). The slow block has been reviewed in depth previously (Bianchi and Wright 2016; Jaffe and Gould 1985; Runft et al. 2002; Stricker 1999; Wong and Wessel 2006); here we focus on the fast polyspermy block.
Remarkably, both a fast and slow polyspermy block were proposed by Ernest Just 100 years ago based on his own sand dollar fertilization experiments (Just 1919). Just specifically observed the lifting of the egg envelope in response to cortical granule exocytosis 30 seconds after insemination (Just 1919). Because numerous sperm reached the egg in this short time, he predicted that a more immediate barrier would be necessary to inhibit polyspermy (Just 1919).
The seminal discoveries of Ernest Just inspired the next generation of developmental biologists to study early embryonic development in marine organisms. In the 1950s, several independent groups laid the groundwork for uncovering the fast polyspermy block by making electrical recordings from various types of eggs during fertilization or artificial activation, including starfish (Albert et al. 1956), toads (Maeno 1959), and sea urchins (Hiramoto 1958). Although these fertilization-associated depolarizations seemed to be a shared feature in eggs from oviparous species, it would be another 20 years before scientists would uncover its physiological significance. Elucidating the role of these depolarizations required that scientists be able to control the membrane potential of the egg rather than simply make passive recordings. Creation of the voltage clamp (Cole 1949; Hodgkin et al. 1949; Marmont 1949) would ultimately enable the discovery of the fast block. In 1976, Laurinda Jaffe voltage clamped sea urchin eggs and demonstrated that the polarization of their membranes dictated whether sperm could enter (Jaffe 1976). Following this initial characterization of the fast block in sea urchins, similar studies expanded the list of organisms that use the fast polyspermy block to include, echinoderms (Miyazaki and Hirai 1979; Whitaker and Steinhardt 1983), ascidians (Goudeau et al. 1994), amphibians (Charbonneau et al. 1983; Cross and Elinson 1980; Iwao 1989), algae (Brawley 1991), and marine worms (Kline et al. 1985).
Diversity amongst the species that employ the fast block and environmental conditions at the site and time of fertilization has given rise to differing fast block signaling pathways. While the molecular mechanisms vary between species, three characteristics are shared amongst eggs that undergo the fast block (Nuccitelli and Grey 1984). First, fertilization induces a depolarization of the egg plasma membrane, referred to as the fertilization potential, which persists for at least one minute (Grey et al. 1982; Jaffe 1976). Second, sperm can bind, but not enter, eggs that are clamped at their fertilization potential voltage (Jaffe 1976; Lynn et al. 1988; Miyazaki and Hirai 1979). Lastly, eggs clamped at a more negative potential can be penetrated by multiple sperm (Jaffe 1976; Miyazaki and Hirai 1979).
This review focuses on the ion channels and signaling pathways that mediate the fast block. We pay particular attention to the signaling events induced by fertilization to depolarize eggs from amphibians and echinoderms; animals that have long been studied by developmental biologists. Additionally, we discuss the unknown parts of the fast block-signaling pathway. Finally, we highlight studies demonstrating that an electrical polyspermy block does not occur in mammals or teleost fish.
The fast polyspermy block in diverse organisms
An electrical, fast polyspermy block has been investigated in diverse organisms. Below we discuss experiments exploring whether amphibians, echinoderms, mammals, and teleost fish use a fertilization-evoked depolarization as a polyspermy block. To investigate these fast block pathways, ion channel biologists often use inhibitors to acutely disrupt a signaling pathway. Inhibitor application not only allows for precise temporal control of channel blockade, but it also allows scientists to observe changes in the absence of compensating pathways that could obscure findings. Moreover, use of inhibitors allows for the study of species where genetic manipulations are not feasible, such as making a knockout of the allotetraploid Xenopus laevis or reducing activity of an essential channel. We focus on studies that used multiple inhibitors to robustly substantiate observations and verify that the results observed were not due to off target effects.
Amphibians
The class Amphibia is comprised of three orders: Anura (frogs and toads), Urodela (newts and salamanders), and Gymnophiona (caecilians) (Pough 2007). While the fast block has been investigated in many anurans and urodeles, limited research has been conducted on gymnophionans likely due to their elusive existence. Intriguingly, some urodeles are physiologically polyspermic with internal fertilization (Iwao 2012), and their eggs do not depolarize at fertilization (Charbonneau et al. 1983; Iwao 1985). Yet, a necessity for monospermic fertilization and a fast polyspermy block exists in at least one urodele species: the clouded salamander Hynobius nebulosus (Iwao 1989). Anurans, by contrast, are physiologically monospermic, and the fast block has been studied in diverse anuran species, including the American toad Bufo americanus (Cross and Elinson 1980), the northern leopard frog Rana pipiens (Cross 1981; Cross and Elinson 1980), and the African clawed frog Xenopus laevis (Busa et al. 1985; Grey et al. 1982; Jaffe et al. 1983a; Webb and Nuccitelli 1985). Here we focus on the fast block in X. laevis, the species for which we have elucidated the most complete signaling pathway.
The fast block signaling pathway in X. laevis can be described in three phases. First, a small and brief step current is observed (Glahn and Nuccitelli 2003). Next, the egg abruptly depolarizes from rest at −20 mV to +5 mV, and this depolarization persists for several minutes (Cross and Elinson 1980; Grey et al. 1982; Wozniak et al. 2018,b; Wozniak et al. 2018,c). Finally, the membrane repolarizes back to rest over a period of several minutes (Peres and Mancinelli 1985). To uncover the ionic currents that mediate the fast block in X. laevis, fertilization-evoked depolarizations were monitored in artificial pond water with varying ionic compositions, as well as in the presence of various channel inhibitors (Glahn and Nuccitelli 2003; Grey et al. 1982; Webb and Nuccitelli 1985).
The step current observed in the initial phase of the fast block is speculated to arise from the earliest moments of sperm-egg contact (Glahn and Nuccitelli 2003). This step current is not observed as a distinct change in membrane polarization of the egg measured during whole cell recordings; rather, it is only apparent as a minimal current on a millisecond timescale during voltage clamp recordings (Glahn and Nuccitelli 2003). Thus, this current could reflect a change in membrane capacitation that results from sperm-egg membrane fusion. Alternatively, it could be the result of an ionic conductance.
In X. laevis, as well as other amphibians with a fast block, a fertilization-activated efflux of Cl− from the egg depolarizes the membrane (Fig. 1) (Charbonneau et al. 1983; Iwao 1989). Cl− currents are often responsible for hyperpolarizing excitable cells. However, amphibians generally fertilize in freshwater, which is more dilute than the intracellular milieu. Thus, when fertilization signals the opening of Cl− permeant channels, these anions leave the egg to thereby make the membrane potential more positive.
Figure 1. The fast block in X. laevis.
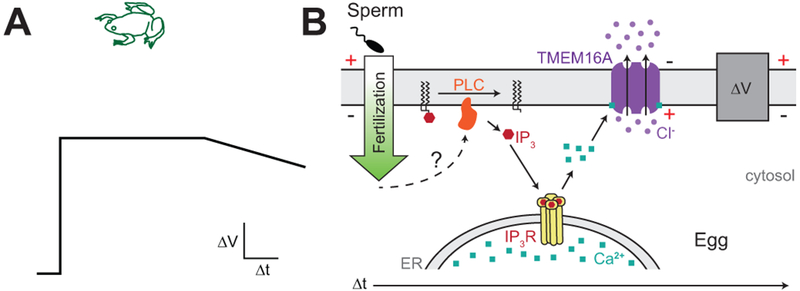
A) Schematic of a depolarization recorded during fertilization of an egg from the African clawed frog, Xenopus laevis. B) Signaling mechanisms of the fast block in X. laevis eggs. Fertilization in frog eggs signals activation of PLC, which cleaves phosphatidylinositol 4,5-bisphosphate (PIP2) into IP3 and diacylglycerol (DAG). IP3 evokes Ca2+ release from the ER, and this Ca2+ opens the TMEM16A channel to induce Cl− efflux and membrane depolarization. After a prolonged depolarization, the membrane potential repolarized back to rest via unknown ionic movement (adapted from Wozniak et al. 2018c).
Robust experimental findings collected by recordings X. laevis eggs during fertilization in differing concentrations of extracellular Cl− support a prominent role of a Cl− efflux in the fast block (Grey et al. 1982; Webb and Nuccitelli 1985). Fertilizations in solutions with limited extracellular Cl− led to larger than normal depolarizations due to a larger driving force; whereas fertilizations in higher extracellular Cl− resulted in smaller depolarizations (Grey et al. 1982; Webb and Nuccitelli 1985). Additional experiments replaced extracellular Cl− with other halides to further substantiate that a Cl− current mediates the fast polyspermy block. Typically, Cl− conducting ion channels can pass other halide anions (Wright and Diamond 1977), whereby these channels in X. laevis gametes preferentially pass I− > Br− > Cl− (Qu and Hartzell 2000). Because halides such as I− and Br− are not abundant in the egg, enriching the extracellular solution with these anions creates a chemical gradient that supports their influx into the cell. Compared to depolarizations recorded in typical solutions where the dominant extracellular anion was Cl−, fertilization evoked no change in the membrane potential of eggs inseminated in Br− containing solutions and induced hyperpolarizations in eggs inseminated in I− containing solutions (Grey et al. 1982). Fertilization in the presence of I− or Br− also increased polyspermy (Grey et al. 1982). Together these data reveal that fertilization opens a Cl− channel in X. laevis eggs to depolarize the membrane for the fast block.
In addition to a Cl− efflux, an increase in intracellular Ca2+ is required for fertilization to depolarize X. laevis eggs (Grey et al. 1982; Kline 1988). This necessity for increased cytosolic Ca2+ was demonstrated with experiments recording the membrane potential during fertilization of X. laevis eggs loaded with the Ca2+ chelator BAPTA (Kline 1988); BAPTA binds Ca2+ with high affinity and thereby quenches an elevation of this cation (Tsien 1980). In these BAPTA-loaded eggs, fertilization failed to evoke a depolarization and caused an increased incidence of polyspermy (Kline 1988). Additional experiments demonstrated that application of a Ca2+ ionophore, a lipid-soluble compound that transports Ca2+ across the plasma membrane, to eggs increased intracellular Ca2+ and evoked a depolarization in the absence of sperm (Grey et al. 1982). Together these results demonstrate that an increase in intracellular Ca2+ is necessary and sufficient to evoke the fast block in X. laevis eggs. These findings gave rise to the hypothesis that a Ca2+-activated Cl− channel (CaCC) mediates the depolarization in X. laevis eggs (Hartzell et al. 2009).
The molecular identity of the CaCC responsible for the fast block in X. laevis was recently identified as transmembrane protein 16a (TMEM16A) (Wozniak et al. 2018b). Using proteomics and RNA sequencing datasets, two candidate CaCC were found in fertilization-competent X. laevis eggs, TMEM16A and bestrophin 2a (BEST2A) (Session et al. 2016; Wozniak et al. 2018b; Wuhr et al. 2014). Insemination of eggs in the presence of multiple TMEM16A-specific inhibitors, each diminished or abolished fertilization-evoked depolarizations and increased polyspermy (Wozniak et al. 2018b). These data reveal that fertilization opens TMEM16A in X. laevis eggs to depolarize the egg membrane (Fig. 1B). Moreover, these data represent the first molecular identity of an ion channel mediating the fast block.
Theoretically, fertilization could increase intracellular Ca2+ to activate TMEM16A by either evoking Ca2+ entry from the external milieu or Ca2+ release from an intracellular store. To determine the importance of extracellular Ca2+ mediating the fast polyspermy block, X. laevis eggs were fertilized in the presence of broad-spectrum Ca2+ channel inhibitors that block the Ca2+-permeant channels present in these eggs (Wozniak et al. 2018c). However, fertilization evoked normal depolarizations in the presence of Ca2+ channel inhibitors, thereby demonstrating that Ca2+ entry is not required for the fast block. Conversely, fertilization failed to evoke any depolarization in eggs inseminated in the presence of inhibitors of either the inositol 1,4,5-trisphosphate receptor (IP3R) or phospholipase C (PLC); these fertilization conditions also increased polyspermy (Wozniak et al. 2018c). Together, these data indicate that PLC-induces elevated IP3 levels in X. laevis eggs to induce Ca2+ release from the ER, which opens TMEM16A to depolarize the membrane for the fast block (Fig. 1B).
The next step in uncovering how fertilization signals the fast block in X. laevis will be to uncover the pathway upstream of PLC activation. We do not yet know the identity of the PLC that signals the fast block or how it is activated by fertilization. Intriguingly, the slow polyspermy block in X. laevis also requires activation of a PLC (Sato et al. 2000). It is possible that the same signaling mechanisms are employed to activate the fast and slow blocks in X. laevis. In mammals, the slow block is activated by a sperm-derived PLCζ (Hachem et al. 2017; Nozawa et al. 2018; Saunders et al. 2002). Although PLCζ is also expressed in pufferfish eggs and testes from the teleost fish medaka (Coward et al. 2011; Ito et al. 2008), a gene encoding PLCζ has not been annotated in genomes for X. laevis or any other frog (Hammond et al. 2017; Hellsten et al. 2010; Session et al. 2016; Sun et al. 2015). To date, however, no one has directly tested which PLC isoform mediates the fast polyspermy block in X. laevis.
The ion channels and signaling mechanisms that repolarize the X. laevis egg following the fast block are not yet known. Immature X. laevis oocytes have a K+ leak current (Bauer et al. 1996) that could slowly repolarize egg if the channel is maintained in the plasma membrane during maturation. Equipped with the X. laevis egg proteome (Wuhr et al. 2014), identifying and targeting putative channels is possible. Overall, there is still much to learn about how fertilization initiates the fast block in X. laevis; and yet, we have the most complete understanding of the fast block-signaling pathway in this species.
Echinoderms
The fast polyspermy block has been studied in various echinoderms, including sea urchins (Jaffe 1976; Whitaker and Steinhardt 1983), starfish (Miyazaki and Hirai 1979; Moccia et al. 2004), and sand dollars (Steinhardt et al. 1971). Many similarities between the fast block in starfish and sea urchins have been established, such as the importance of voltage-gated Ca2+ channels in the initial depolarization and the coinciding elevation of intracellular Ca2+ visible in fluorescence imaging experiments, referred to as the cortical flash (Chun et al. 2014; Moccia et al. 2004; Ramos and Wessel 2013; Shen and Buck 1993; Wozniak et al. 2018a), and the maximum polarization of their fertilization potentials (Miyazaki and Hirai 1979; Whitaker and Steinhardt 1983). Here we will focus on the fast block in sea urchins.
Due to their natural abundance, ease in gamete collection, and readily achieved fertilization in the laboratory setting, sea urchins are arguably the most widely studied group of organisms for fast block experiments. Accordingly, their fast blocks have been studied in at least 14 different species (Nuccitelli and Grey 1984); yet, ubiquitous use of the fast block by all species of sea urchin eggs has been contested (Dale 2014; Dale and DeFelice 2011). Differences in the presence or absence of a fertilization-evoked depolarization could arise from poor egg quality post-impalement with electrodes, different fertilization temperatures, fertilizing sperm-to-egg ratios, and artificial seawater composition (Chambers and de Armendi 1979; Schmidt et al. 1982).
Over the years, fast blocks recordings from various sea urchin species have differed in their overall shapes (Chambers and de Armendi 1979; Jaffe 1976; Steinhardt et al. 1971; Uehara and Katou 1972); however, these recordings share some basic the features. First, sperm-egg contact activates a small depolarizing shift in the membrane potential which rests at −70 mV. Next, the egg rapidly depolarizes to a much higher potential of approximately +25 mV. Finally, minutes following sperm-egg contact, the egg slowly repolarizes to its resting potential. To uncover the identity of the ions that carry the currents responsible for the sea urchin fast block, electrophysiology recordings have been made during fertilization in varying ionic conditions, as well as in the presence of broad-spectrum ion channel inhibitors (Chambers and de Armendi 1979; Steinhardt et al. 1972).
The fast block is initiated by a step current that enables the membrane potential of the egg to reach the threshold required for voltage-gated channel activation (Lynn et al. 1988; Uehara and Katou 1972). Two lines of experimentation reveal that the step current is triggered by sperm attachment to the egg. First, experiments in L. variegatus report that even without entry, sperm binding to eggs evokes a step current (Chambers 1989). Second, attachment of two separate sperm to L. variegatus eggs led to sequential and additive step currents during a voltage clamp experiment (Lynn et al. 1988). Likely due to its brevity, the ions and the channel responsible for the step depolarization have yet to be determined (Lynn et al. 1988; Uehara and Katou 1972).
Robust experimental findings demonstrate that the steep depolarization of the membrane potential for the fast block is mediated by a voltage-gated Ca2+ channel (Chambers and de Armendi 1979; McCulloh et al. 2000; Swann et al. 1992). Decreasing extracellular Ca2+ concentrations leads to smaller overall shifts in the membrane potential in L. variegatus eggs thereby revealing that Ca2+ conducts this depolarizing current (Chambers and de Armendi 1979; Okamoto et al. 1977). A role for a voltage-gated channel in this depolarization is supported by the finding that small injections of current can activate this depolarization in unfertilized L. variegatus and L. pictus eggs (Chambers and de Armendi 1979; Swann et al. 1992). Notably, the depolarization can only be triggered if the resting potential of the egg is more negative than −40 mV (McCulloh et al. 2000; Nuccitelli and Grey 1984), likely because this voltage-gated Ca2+ channel will be inactivated at higher potentials.
Finally, the membrane repolarization following the fast block in sea urchin eggs is K+ driven (Steinhardt et al. 1972). Fertilization in high K+, or with K+ channel-inhibitors, led to prolonged depolarizations in L. pictus eggs (Steinhardt et al. 1972). Interestingly, the K+ current that repolarizes unfertilized eggs acts much more quickly than in a fertilized egg (on the order of ~2 seconds in unfertilized compared to 10 minutes following fertilization) (Chambers and de Armendi 1979). Unless the K+ channel that repolarizes the membrane of an unfertilized egg is removed from the membrane following fertilization, we propose that the channel is modified to slow its kinetics. For example, perhaps fertilization induces phosphorylation, changes in intracellular pH or membrane lipid composition, or increases the presence of a pore-blocking substance that slows the repolarization.
Although many experiments characterizing the fast block in sea urchin eggs have been performed, there is still much to learn including the molecular identities of the ion channels and signaling pathways that mediate the fast block. Newly available genomes (Sea Urchin Genome Sequencing et al. 2006), transcriptomes (Tu et al. 2014; Tu et al. 2012), and tools (Cary et al. 2018) for multiple sea urchin species will surely aid these future studies.
Mammals
It is generally believed that eggs from viviparous animals do not use the fast block to polyspermy (Jaffe and Gould 1985; Wong and Wessel 2006). This theory is based on several pieces of data demonstrating that mammalian eggs do not depolarize at fertilization. First, recordings made on mouse eggs during in vitro fertilization measured only small oscillations of the membrane potential, but never large depolarizations (Jaffe et al. 1983b). Second, rabbit egg recordings revealed a slow depolarization of only a few millivolts upon fertilization; the relatively small change in membrane potential over a prolonged period suggests that this electrical change is not a polyspermy block (McCulloh et al. 1983). Third, hamster eggs failed to depolarize upon in vitro fertilization; by contrast, fertilization evoked recurring hyperpolarizations in these eggs (Miyazaki and Igusa 1981). Finally, sperm successfully entered hamster eggs voltage-clamped at potentials ranging from −120 to +20 mV, thereby revealing that sperm entry into hamster eggs is not regulated by the egg membrane potential (Miyazaki and Igusa 1982). While it remains possible that some mammalian eggs depolarize at fertilization, we hypothesize that human eggs do not use the fast block. Given that the sperm-to-egg ratio is much lower during the internal fertilization of mammalian eggs (Cummins and Yanagimachi 1982; Suarez 1987), perhaps the slow block is sufficient to ensure monospermic fertilizations.
Teleost Fish
Most fish are physiologically monospermic, as well as oviparous with external fertilization, thus they require immediate polyspermy prevention mechanisms. Eggs from the fish medaka depolarize at fertilization; yet, this depolarization does not inhibit sperm entry (Nuccitelli 1980). The anatomy of teleost fish eggs may bypass the need for the fast block, whereby the ECM, called chorion, provides a protective barrier for the egg that is impenetrable to sperm, except at one or more micropyles (Jaffe and Gould 1985). Given this physical barrier to sperm covers a majority of the egg membrane, we suspect that most teleost fish do not require a fast block.
Concluding remarks
How fertilization triggers a depolarization remains an outstanding question. Three hypotheses, however, have been proposed to initiate the slow block (Runft et al. 2002). We believe that these three possible mechanisms may also apply to the fast block: First, the contact hypothesis proposes that sperm activate a receptor on the egg, and the ensuing receptor-mediated second messenger signaling then triggers the depolarization. Second, the membrane addition hypothesis proposes that fusion of the sperm-egg membranes introduces an active component from the sperm into the egg membrane. Finally, the content hypothesis predicts that a soluble sperm factor is released into the egg upon fusion to induce the fast block. Because the fast block pathways differ greatly in amphibians and echinoderms, it is possible that they use different models of activation. Continued advancements in developmental biology and electrophysiological tools, such as the discovery of novel inhibitors for specific pathway components and creating transgenic eggs, will allow for us to resolve these unanswered questions.
Acknowledgements
We thank Maiwase Tembo and Rachel E. Bainbridge for critical review of this manuscript. Research in our laboratory during the writing of this manuscript was supported by National Institutes of Health grant R01GM125638 to A.E.C., and the Margaret A. Oweida Predoctoral Fellowship to K.L.W.
Grant information:
Grant sponsor 1: National Institutes of Health, R01GM125638 to A.E.C.
Grant sponsor 2: Margaret A. Oweida Predoctoral Fellowship to K.L.W.
Footnotes
The authors declare no conflict of interest.
References
- Albert T, Monroy A, Kao CY, Grundfest H. 1956. Membrane Potential and resistance of the starfish egg before and after fertilization. Biol Bull (Woods Hole, Mass) 111:152–177. [Google Scholar]
- Bauer CK, Falk T, Schwarz JR. 1996. An endogenous inactivating inward-rectifying potassium current in oocytes of Xenopus laevis. Pflugers Arch 432(5):812–820. [DOI] [PubMed] [Google Scholar]
- Bianchi E, Wright GJ. 2016. Sperm Meets Egg: The Genetics of Mammalian Fertilization. Annu Rev Genet 50:93–111. [DOI] [PubMed] [Google Scholar]
- Brawley SH. 1991. The fast block against polyspermy in fucoid algae is an electrical block. Dev Biol 144(1):94–106. [DOI] [PubMed] [Google Scholar]
- Busa WB, Ferguson JE, Joseph SK, Williamson JR, Nuccitelli R. 1985. Activation of frog (Xenopus laevis) eggs by inositol trisphosphate. I. Characterization of Ca2+ release from intracellular stores. J Cell Biol 101(2):677–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cary GA, Cameron RA, Hinman VF. 2018. EchinoBase: Tools for Echinoderm Genome Analyses. Methods Mol Biol 1757:349–369. [DOI] [PubMed] [Google Scholar]
- Chambers EL. 1989. Fertilization in voltage-clamped sea urchin eggs Mechanisms of egg activation: Springer; p 1–18. [Google Scholar]
- Chambers EL, de Armendi J. 1979. Membrane potential, action potential and activation potential of eggs of the sea urchin, Lytechinus variegatus. Exp Cell Res 122(1):203–218. [DOI] [PubMed] [Google Scholar]
- Charbonneau M, Moreau M, Picheral B, Vilain JP, Guerrier P. 1983. Fertilization of amphibian eggs: a comparison of electrical responses between anurans and urodeles. Dev Biol 98(2):304–318. [DOI] [PubMed] [Google Scholar]
- Chun JT, Limatola N, Vasilev F, Santella L. 2014. Early events of fertilization in sea urchin eggs are sensitive to actin-binding organic molecules. Biochemical and biophysical research communications 450(3):1166–1174. [DOI] [PubMed] [Google Scholar]
- Cole KS. 1949. Dynamic electrical characteristics of the squid axon membrane. Archives des sciences physiologiques 3(2):253–258. [Google Scholar]
- Coward K, Ponting CP, Zhang N, Young C, Huang CJ, Chou CM, Kashir J, Fissore RA, Parrington J. 2011. Identification and functional analysis of an ovarian form of the egg activation factor phospholipase C zeta (PLCzeta) in pufferfish. Molecular reproduction and development 78(1):48–56. [DOI] [PubMed] [Google Scholar]
- Cross NL. 1981. Initiation of the activation potential by an increase in intracellular calcium in eggs of the frog, Rana pipiens. Dev Biol 85(2):380–384. [DOI] [PubMed] [Google Scholar]
- Cross NL, Elinson RP. 1980. A fast block to polyspermy in frogs mediated by changes in the membrane potential. Dev Biol 75(1):187–198. [DOI] [PubMed] [Google Scholar]
- Cummins J, Yanagimachi R. 1982. Sperm-egg ratios and the site of the acrosome reaction during in vivo fertilization in the hamster. Gamete research 5(3):239–256. [Google Scholar]
- Dale B 2014. Is the idea of a fast block to polyspermy based on artifact? Biochemical and biophysical research communications 450(3):1159–1165. [DOI] [PubMed] [Google Scholar]
- Dale B, DeFelice L. 2011. Polyspermy prevention: facts and artifacts? Journal of assisted reproduction and genetics 28(3):199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn D, Nuccitelli R. 2003. Voltage-clamp study of the activation currents and fast block to polyspermy in the egg of Xenopus laevis. Dev Growth Differ 45(2):187–197. [DOI] [PubMed] [Google Scholar]
- Goudeau H, Depresle Y, Rosa A, Goudeau M. 1994. Evidence by a voltage clamp study of an electrically mediated block to polyspermy in the egg of the ascidian Phallusia mammillata. Developmental biology 166(2):489–501. [DOI] [PubMed] [Google Scholar]
- Grey RD, Bastiani MJ, Webb DJ, Schertel ER. 1982. An electrical block is required to prevent polyspermy in eggs fertilized by natural mating of Xenopus laevis. Dev Biol 89(2):475–484. [DOI] [PubMed] [Google Scholar]
- Hachem A, Godwin J, Ruas M, Lee HC, Buitrago MF, Ardestani G, Bassett A, Fox S, Navarrete F, de Sutter P, Heindryckx B, Fissore R, Parrington J. 2017. PLCzeta is the physiological trigger of the Ca2+ oscillations that induce embryogenesis in mammals but offspring can be conceived in its absence Development (Cambridge, England: ). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond SA, Warren RL, Vandervalk BP, Kucuk E, Khan H, Gibb EA, Pandoh P, Kirk H, Zhao Y, Jones M, Mungall AJ, Coope R, Pleasance S, Moore RA, Holt RA, Round JM, Ohora S, Walle BV, Veldhoen N, Helbing CC, Birol I. 2017. The North American bullfrog draft genome provides insight into hormonal regulation of long noncoding RNA. Nat Commun 8(1):1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell HC, Yu K, Xiao Q, Chien LT, Qu Z. 2009. Anoctamin/TMEM16 family members are Ca2+-activated Cl− channels. J Physiol 587(Pt 10):2127–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellsten U, Harland RM, Gilchrist MJ, Hendrix D, Jurka J, Kapitonov V, Ovcharenko I, Putnam NH, Shu S, Taher L, Blitz IL, Blumberg B, Dichmann DS, Dubchak I, Amaya E, Detter JC, Fletcher R, Gerhard DS, Goodstein D, Graves T, Grigoriev IV, Grimwood J, Kawashima T, Lindquist E, Lucas SM, Mead PE, Mitros T, Ogino H, Ohta Y, Poliakov AV, Pollet N, Robert J, Salamov A, Sater AK, Schmutz J, Terry A, Vize PD, Warren WC, Wells D, Wills A, Wilson RK, Zimmerman LB, Zorn AM, Grainger R, Grammer T, Khokha MK, Richardson PM, Rokhsar DS. 2010. The genome of the Western clawed frog Xenopus tropicalis. Science 328(5978):633–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramoto Y 1958. Changes in electrical properties upon fertilization in the sea urchin egg. Experimental Cell Research 16:421–424. [DOI] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF, Katz B. 1949. Ionic currents underlying activity in the giant axon of the squid. Arch Sci Physiol 3:129–150. [Google Scholar]
- Ito M, Shikano T, Oda S, Horiguchi T, Tanimoto S, Awaji T, Mitani H, Miyazaki S. 2008. Difference in Ca2+ oscillation-inducing activity and nuclear translocation ability of PLCZ1, an egg-activating sperm factor candidate, between mouse, rat, human, and medaka fish. Biol Reprod 78(6):1081–1090. [DOI] [PubMed] [Google Scholar]
- Iwao Y 1985. The membrane potential changes of amphibian eggs during species- and cross-fertilization. Developmental biology 111(1):26–34. [Google Scholar]
- Iwao Y 1989. An electrically mediated block to polyspermy in the primitive urodele Hynobius nebulosus and phylogenetic comparison with other amphibians. Developmental biology 134(2):438–445. [DOI] [PubMed] [Google Scholar]
- Iwao Y 2012. Egg activation in physiological polyspermy. Reproduction 144(1):11–22. [DOI] [PubMed] [Google Scholar]
- Jaffe LA. 1976. Fast block to polyspermy in sea urchin eggs is electrically mediated. Nature 261(5555):68–71. [DOI] [PubMed] [Google Scholar]
- Jaffe LA, Cross NL, Picheral B. 1983a. Studies of the voltage-dependent polyspermy block using cross-species fertilization of amphibians. Dev Biol 98(2):319–326. [DOI] [PubMed] [Google Scholar]
- Jaffe LA, Gould M. 1985. Polyspermy-preventing mechanisms In: Metz CB, Monroy A, editors. Biology of Fertilization. New York: Academic Press; p 223–250. [Google Scholar]
- Jaffe LA, Sharp AP, Wolf DP. 1983b. Absence of an electrical polyspermy block in the mouse. Developmental biology 96(2):317–323. [DOI] [PubMed] [Google Scholar]
- Just E 1919. The Fertilization Reaction in Echinarachnius Parma. Biological Bulletin 36(1):1–10. [Google Scholar]
- Kline D 1988. Calcium-dependent events at fertilization of the frog egg: injection of a calcium buffer blocks ion channel opening, exocytosis, and formation of pronuclei. Dev Biol 126(2):346–361. [DOI] [PubMed] [Google Scholar]
- Kline D, Jaffe LA, Tucker RP. 1985. Fertilization potential and polyspermy prevention in the egg of the nemertean, Cerebratulus lacteus. The Journal of experimental zoology 236(1):45–52. [DOI] [PubMed] [Google Scholar]
- Lynn JW, McCulloh DH, Chambers EL. 1988. Voltage clamp studies of fertilization in sea urchin eggs. II. Current patterns in relation to sperm entry, nonentry, and activation. Dev Biol 128(2):305–323. [DOI] [PubMed] [Google Scholar]
- Maeno T 1959. Electrical characteristics and activation potential of Bufo eggs. J Gen Physiol 43:139–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmont G 1949. Studies on the axon membrane; a new method. J Cell Comp Physiol 34(3):351–382. [DOI] [PubMed] [Google Scholar]
- McCulloh DH, Ivonnet PI, Landowne D, Chambers EL. 2000. Calcium influx mediates the voltage-dependence of sperm entry into sea urchin eggs. Developmental biology 223(2):449–462. [DOI] [PubMed] [Google Scholar]
- McCulloh DH, Rexroad CE Jr., Levitan H. 1983. Insemination of rabbit eggs is associated with slow depolarization and repetitive diphasic membrane potentials. Developmental biology 95(2):372–377. [DOI] [PubMed] [Google Scholar]
- Miyazaki S, Hirai S. 1979. Fast polyspermy block and activation potential. Correlated changes during oocyte maturation of a starfish. Dev Biol 70(2):327–340. [DOI] [PubMed] [Google Scholar]
- Miyazaki S, Igusa Y. 1981. Fertilization potential in golden hamster eggs consists of recurring hyperpolarizations. Nature 290(5808):702–704. [DOI] [PubMed] [Google Scholar]
- Miyazaki S, Igusa Y. 1982. Ca-mediated activation of a K current at fertilization of golden hamster eggs. Proc Natl Acad Sci U S A 79(3):931–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima S 2017. Fertilization 2: Polyspermic Fertilization. Advances in experimental medicine and biology 1001:105–123. [DOI] [PubMed] [Google Scholar]
- Moccia F, Lim D, Kyozuka K, Santella L. 2004. NAADP triggers the fertilization potential in starfish oocytes. Cell Calcium 36(6):515–524. [DOI] [PubMed] [Google Scholar]
- Nozawa K, Satouh Y, Fujimoto T, Oji A, Ikawa M. 2018. Sperm-borne phospholipase C zeta-1 ensures monospermic fertilization in mice. Sci Rep 8(1):1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuccitelli R 1980. The fertilization potential is not necessary for the block to polyspermy or the activation of development in the medaka egg. Developmental biology 76(2):499–504. [DOI] [PubMed] [Google Scholar]
- Nuccitelli R, Grey RD. 1984. Controversy over the fast, partial, temporary block to polyspermy in sea urchins: a reevaluation. Dev Biol 103(1):1–17. [DOI] [PubMed] [Google Scholar]
- Okamoto H, Takahashi K, Yamashita N. 1977. Ionic currents through the membrane of the mammalian oocyte and their comparison with those in the tunicate and sea urchin. The Journal of physiology 267(2):465–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peres A, Mancinelli E. 1985. Sodium conductance and the activation potential in Xenopus laevis eggs. Pflugers Archiv : European journal of physiology 405(1):29–36. [DOI] [PubMed] [Google Scholar]
- Pough FH. 2007. Amphibian biology and husbandry. ILAR journal 48(3):203–213. [DOI] [PubMed] [Google Scholar]
- Qu Z, Hartzell HC. 2000. Anion permeation in Ca(2+)-activated Cl(-) channels. The Journal of general physiology 116(6):825–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos I, Wessel GM. 2013. Calcium pathway machinery at fertilization in echinoderms. Cell calcium 53(1):16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runft LL, Jaffe LA, Mehlmann LM. 2002. Egg activation at fertilization: where it all begins. Dev Biol 245(2):237–254. [DOI] [PubMed] [Google Scholar]
- Sato K, Tokmakov AA, Iwasaki T, Fukami Y. 2000. Tyrosine kinase-dependent activation of phospholipase Cgamma is required for calcium transient in Xenopus egg fertilization. Dev Biol 224(2):453–469. [DOI] [PubMed] [Google Scholar]
- Saunders CM, Larman MG, Parrington J, Cox LJ, Royse J, Blayney LM, Swann K, Lai FA. 2002. PLC zeta: a sperm-specific trigger of Ca(2+) oscillations in eggs and embryo development. Development 129(15):3533–3544. [DOI] [PubMed] [Google Scholar]
- Schmidt T, Patton C, Epel D. 1982. Is there a role for the Ca2+ influx during fertilization of the sea urchin egg? Developmental biology 90(2):284–290. [DOI] [PubMed] [Google Scholar]
- Sea Urchin Genome Sequencing C, Sodergren E, Weinstock GM, Davidson EH, Cameron RA, Gibbs RA, Angerer RC, Angerer LM, Arnone MI, Burgess DR, Burke RD, Coffman JA, Dean M, Elphick MR, Ettensohn CA, Foltz KR, Hamdoun A, Hynes RO, Klein WH, Marzluff W, McClay DR, Morris RL, Mushegian A, Rast JP, Smith LC, Thorndyke MC, Vacquier VD, Wessel GM, Wray G, Zhang L, Elsik CG, Ermolaeva O, Hlavina W, Hofmann G, Kitts P, Landrum MJ, Mackey AJ, Maglott D, Panopoulou G, Poustka AJ, Pruitt K, Sapojnikov V, Song X, Souvorov A, Solovyev V, Wei Z, Whittaker CA, Worley K, Durbin KJ, Shen Y, Fedrigo O, Garfield D, Haygood R, Primus A, Satija R, Severson T, Gonzalez-Garay ML, Jackson AR, Milosavljevic A, Tong M, Killian CE, Livingston BT, Wilt FH, Adams N, Belle R, Carbonneau S, Cheung R, Cormier P, Cosson B, Croce J, Fernandez-Guerra A, Geneviere AM, Goel M, Kelkar H, Morales J, Mulner-Lorillon O, Robertson AJ, Goldstone JV, Cole B, Epel D, Gold B, Hahn ME, Howard-Ashby M, Scally M, Stegeman JJ, Allgood EL, Cool J, Judkins KM, McCafferty SS, Musante AM, Obar RA, Rawson AP, Rossetti BJ, Gibbons IR, Hoffman MP, Leone A, Istrail S, Materna SC, Samanta MP, Stolc V, Tongprasit W, Tu Q, Bergeron KF, Brandhorst BP, Whittle J, Berney K, Bottjer DJ, Calestani C, Peterson K, Chow E, Yuan QA, Elhaik E, Graur D, Reese JT, Bosdet I, Heesun S, Marra MA, Schein J, Anderson MK, Brockton V, Buckley KM, Cohen AH, Fugmann SD, Hibino T, Loza-Coll M, Majeske AJ, Messier C, Nair SV, Pancer Z, Terwilliger DP, Agca C, Arboleda E, Chen N, Churcher AM, Hallbook F, Humphrey GW, Idris MM, Kiyama T, Liang S, Mellott D, Mu X, Murray G, Olinski RP, Raible F, Rowe M, Taylor JS, Tessmar-Raible K, Wang D, Wilson KH, Yaguchi S, Gaasterland T, Galindo BE, Gunaratne HJ, Juliano C, Kinukawa M, Moy GW, Neill AT, Nomura M, Raisch M, Reade A, Roux MM, Song JL, Su YH, Townley IK, Voronina E, Wong JL, Amore G, Branno M, Brown ER, Cavalieri V, Duboc V, Duloquin L, Flytzanis C, Gache C, Lapraz F, Lepage T, Locascio A, Martinez P, Matassi G, Matranga V, Range R, Rizzo F, Rottinger E, Beane W, Bradham C, Byrum C, Glenn T, Hussain S, Manning G, Miranda E, Thomason R, Walton K, Wikramanayke A, Wu SY, Xu R, Brown CT, Chen L, Gray RF, Lee PY, Nam J, Oliveri P, Smith J, Muzny D, Bell S, Chacko J, Cree A, Curry S, Davis C, Dinh H, Dugan-Rocha S, Fowler J, Gill R, Hamilton C, Hernandez J, Hines S, Hume J, Jackson L, Jolivet A, Kovar C, Lee S, Lewis L, Miner G, Morgan M, Nazareth LV, Okwuonu G, Parker D, Pu LL, Thorn R, Wright R. 2006. The genome of the sea urchin Strongylocentrotus purpuratus. Science 314(5801):941–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Session AM, Uno Y, Kwon T, Chapman JA, Toyoda A, Takahashi S, Fukui A, Hikosaka A, Suzuki A, Kondo M, van Heeringen SJ, Quigley I, Heinz S, Ogino H, Ochi H, Hellsten U, Lyons JB, Simakov O, Putnam N, Stites J, Kuroki Y, Tanaka T, Michiue T, Watanabe M, Bogdanovic O, Lister R, Georgiou G, Paranjpe SS, van Kruijsbergen I, Shu S, Carlson J, Kinoshita T, Ohta Y, Mawaribuchi S, Jenkins J, Grimwood J, Schmutz J, Mitros T, Mozaffari SV, Suzuki Y, Haramoto Y, Yamamoto TS, Takagi C, Heald R, Miller K, Haudenschild C, Kitzman J, Nakayama T, Izutsu Y, Robert J, Fortriede J, Burns K, Lotay V, Karimi K, Yasuoka Y, Dichmann DS, Flajnik MF, Houston DW, Shendure J, DuPasquier L, Vize PD, Zorn AM, Ito M, Marcotte EM, Wallingford JB, Ito Y, Asashima M, Ueno N, Matsuda Y, Veenstra GJ, Fujiyama A, Harland RM, Taira M, Rokhsar DS. 2016. Genome evolution in the allotetraploid frog Xenopus laevis. Nature 538(7625):336–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen SS, Buck WR. 1993. Sources of calcium in sea urchin eggs during the fertilization response. Developmental biology 157(1):157–169. [DOI] [PubMed] [Google Scholar]
- Snook RR, Hosken DJ, Karr TL. 2011. The biology and evolution of polyspermy: insights from cellular and functional studies of sperm and centrosomal behavior in the fertilized egg. Reproduction (Cambridge, England) 142(6):779–792. [DOI] [PubMed] [Google Scholar]
- Steinhardt RA, Lundin L, Mazia D. 1971. Bioelectric responses of the echinoderm egg to fertilization. Proceedings of the National Academy of Sciences of the United States of America 68(10):2426–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhardt RA, Shen S, Mazia D. 1972. Membrane potential, membrane resistance and an energy requirement for the development of potassium conductance in the fertilization reaction of echinoderm eggs. Exp Cell Res 72(1):195–203. [DOI] [PubMed] [Google Scholar]
- Stricker SA. 1999. Comparative biology of calcium signaling during fertilization and egg activation in animals. Dev Biol 211(2):157–176. [DOI] [PubMed] [Google Scholar]
- Suarez SS. 1987. Sperm transport and motility in the mouse oviduct: observations in situ. Biol Reprod 36(1):203–210. [DOI] [PubMed] [Google Scholar]
- Sun YB, Xiong ZJ, Xiang XY, Liu SP, Zhou WW, Tu XL, Zhong L, Wang L, Wu DD, Zhang BL, Zhu CL, Yang MM, Chen HM, Li F, Zhou L, Feng SH, Huang C, Zhang GJ, Irwin D, Hillis DM, Murphy RW, Yang HM, Che J, Wang J, Zhang YP. 2015. Whole-genome sequence of the Tibetan frog Nanorana parkeri and the comparative evolution of tetrapod genomes. Proc Natl Acad Sci U S A 112(11):E1257–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann K, McCulloh DH, McDougall A, Chambers EL, Whitaker M. 1992. Sperm-induced currents at fertilization in sea urchin eggs injected with EGTA and neomycin. Dev Biol 151(2):552–563. [DOI] [PubMed] [Google Scholar]
- Tsien RY. 1980. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry 19(11):2396–2404. [DOI] [PubMed] [Google Scholar]
- Tu Q, Cameron RA, Davidson EH. 2014. Quantitative developmental transcriptomes of the sea urchin Strongylocentrotus purpuratus. Dev Biol 385(2):160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Q, Cameron RA, Worley KC, Gibbs RA, Davidson EH. 2012. Gene structure in the sea urchin Strongylocentrotus purpuratus based on transcriptome analysis. Genome Res 22(10):2079–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara Katou. 1972. Changes of the membrane potential at the time of fertilization in the sea urhcin egg with special reference to the fertilization wave. Development, Growth, and Differentiation 14(2):175–184. [DOI] [PubMed] [Google Scholar]
- Webb DJ, Nuccitelli R. 1985. Fertilization potential and electrical properties of the Xenopus laevis egg. Dev Biol 107(2):395–406. [DOI] [PubMed] [Google Scholar]
- Whitaker MJ, Steinhardt RA. 1983. Evidence in support of the hypothesis of an electrically mediated fast block to polyspermy in sea urchin eggs. Developmental biology 95(1):244–248. [DOI] [PubMed] [Google Scholar]
- Wong JL, Wessel GM. 2006. Defending the zygote: search for the ancestral animal block to polyspermy. Curr Top Dev Biol 72:1–151. [DOI] [PubMed] [Google Scholar]
- Wozniak KL, Luque GM, Ahn SH. 2018a. When sperm meets egg: the spark of new life. Molecular reproduction and development 85(1):5. [Google Scholar]
- Wozniak KL, Phelps WA, Tembo M, Lee MT, Carlson AE. 2018b. The TMEM16A channel mediates the fast polyspermy block in Xenopus laevis. J Gen Physiol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak KL, Tembo M, Phelps WA, Lee MT, Carlson AE. 2018c. PLC and IP3-evoked Ca(2+) release initiate the fast block to polyspermy in Xenopus laevis eggs. J Gen Physiol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright EM, Diamond JM. 1977. Anion selectivity in biological systems. Physiol Rev 57(1):109–156. [DOI] [PubMed] [Google Scholar]
- Wuhr M, Freeman RM Jr., Presler M, Horb ME, Peshkin L, Gygi SP, Kirschner MW. 2014. Deep proteomics of the Xenopus laevis egg using an mRNA-derived reference database. Curr Biol 24(13):1467–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]


