Abstract
Background:
As the prevalence of non-alcoholic fatty liver disease (NAFLD) escalates, understanding its potential impact on the development of chronic kidney disease (CKD) is needed.
Objective:
To determine the longitudinal association of NAFLD with the development of advanced CKD in the United States.
Methods:
A retrospective cohort analysis of the Truven Health MarketScan Database (2006–2015) was conducted. We used Cox proportional hazards models to compare the risk of developing CKD stages 3–5 in NAFLD versus non-NAFLD patients, identified by ICD-9 codes, after 1:3 propensity score (PS) matching.
Results:
In a cohort of 262,619 newly diagnosed NAFLD patients and 769,878 PS (1:3) - matched non-NAFLD patients, we identified 5766 and 8655 new advanced (stage 3–5) CKD cases, respectively. The crude CKD incidence rate was 8.2 and 5.5 per 1,000 person-years in NAFLD and non-NAFLD groups, respectively. In multivariable Cox model, NAFLD patients had a 41% increased risk of developing advanced CKD compared to non-NAFLD patients [adjusted hazard ratio (aHR), 1.41; 95% confidence interval (CI), 1.36–1.46]. In the sensitivity analysis adjusting for time-varying covariates after NAFLD diagnosis, NAFLD persisted as a significant CKD risk factor (aHR, 1.58; 95% CI, 1.52–1.66) and the association remained significant when stratified by age, gender, and pre-existing comorbidities. The risk of CKD increased in NAFLD with compensated cirrhosis (aHR, 1.47; 95% CI, 1.36–1.59) and decompensated cirrhosis (aHR, 2.28; 95% CI, 2.12–2.46).
Conclusion:
NAFLD was independently associated with an increased risk of advanced CKD development suggesting renal function screening and regular monitoring are needed in this population.
Keywords: non-alcoholic fatty liver disease (NAFLD), chronic kidney disease (CKD), cohort, cirrhosis
Graphical Abstract
INTRODUCTION
Nonalcoholic fatty acid liver disease (NAFLD) has become the leading cause of chronic liver disease in the United States (US) and its prevalence is increasing rapidly due to the global epidemics of obesity and type 2 diabetes mellitus. (1) The disease spectrum of NAFLD ranges from simple steatosis to non-alcohol steatohepatitis, liver fibrosis, cirrhosis, and hepatocellular carcinoma. (1) As such, NALFD is now on the trajectory to become the most common indication for liver transplantation in the U.S. (2)
Over the past decade, accumulating evidence has also suggested that NAFLD may not only affect the liver, but also may increase the risk for extra-hepatic manifestations, including type 2 diabetes mellitus, cardiovascular disease, and chronic kidney disease (CKD). (3–6) CKD represents a significant health burden in the Western adult population, affecting over 25% of individuals older than 65 years old. (7) Recent data suggest that the number of deaths from CKD has doubled in the past two decades. (8) Furthermore, the incidence of simultaneous liver-kidney transplantation has increased substantially over the last five years. (9) An analysis of the United Network Organ Sharing (UNOS) database revealed that 35% of patients transplanted for NAFLD-related cirrhosis progressed to stage 3–4 CKD within two years of transplantation compared to only 10% of patients transplanted for other etiologies. (10)
Cross sectional studies have indicated that the prevalence of CKD ranged from 20% to 55% among patients with NAFLD compared with 5% to 30% among those without NAFLD, (11,12) but conflicting results have been reported in regards to whether NAFLD is an independent predictor for prevalent CKD. (13,14) A recent result from analysis of National Health and Nutrition Examination Survey (NHANES) data indicated that NAFLD was associated with an increased prevalence of CKD stages 1–3a but not with CKD stages 3b-5 among US adults. (14) The findings from cohort studies conducted outside of the US examining NAFLD as a risk factor for incident CKD have also been limited by single study center design and highly selective populations (male, diabetic only, Asian). (15–19) In addition, as reported in a recent meta-analysis of eight observational studies from Asia and Europe, NAFLD was found to be a significant predictor for incident CKD only among the Asian populations but not for the European cohorts. (20)
Due to lack of evidence from a US cohort study with a longitudinal follow-up, it is unclear whether NAFLD is associated with the development of CKD among the general US population. Thus, we aimed to determine the incidence of CKD among newly identified NAFLD patients compared to non-NAFLD patients and to identify risk factors associated with the development of CKD among NAFLD patients in the U.S using real-world data obtained from a large nationwide insurance database.
METHODS
Data source
We conducted a retrospective cohort analysis using the Truven Health Analytic MarketScan Commercial and Medicare Supplemental databases (2006 January −2015 August prior to the implementation of ICD-10 codes). This 10-year nationwide administrative claims database contains longitudinal person-level information of diagnoses, procedures, and prescriptions for over 160 million persons in the commercial dataset and 12 million persons in the Medicare Supplement database. The University of Florida Institutional review board granted approval for this study.
Study population
We identified patients with newly diagnosed NAFLD (no NAFLD diagnosis identified during the 12 months preceding first diagnosis date) using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes [571.8, 571.9]. (21) A person was determined to have NAFLD if they had one inpatient or two outpatient diagnoses of NAFLD on separate days within one calendar year. The index date was the date of the first NAFLD diagnosis. Our control group was developed by selecting 10 non-NAFLD patients who were matched to each NAFLD person on age, gender and calendar year. The index date for each person within the control group was randomly selected from one of their medical service dates. Patients were included if they were 18 years old and had to be continuously enrolled in the health plan for one year prior to the selected index date. We excluded persons who were diagnosed with CKD prior to their index date or had not been enrolled for at least one continuous year prior to the index date.
To adjust for the baseline differences in risk factors for CKD between NAFLD and non-NAFLD groups, we matched 3 non-NAFLD individuals (from the pool of the 10 non-NAFLD controls above) to each NAFLD individual using a calculated propensity score (PS). The PS was developed from a logistic regression model using baseline variables including age and gender as well as medical conditions reported in the literature as being associated with CKD which included diabetes, hypertension, obesity, hyperlipidemia, coronary artery disease, peripheral vascular disease, cerebrovascular disease, heart failure, and chronic obstructive pulmonary disease (COPD), which were identified by one inpatient or outpatient ICD-9-CM codes (Supporting Table S1). Other variables included medications known to affect renal function including angiotensin-converting-enzyme inhibitors (ACEI) and angiotensin II receptor blockers (ARB). To avoid detection bias and to balance healthcare utilization between the two groups, we included the mean number of outpatient visits and inpatient visits prior to the index date in the PS matching.
Study outcomes
The primary outcome was a diagnosis of CKD stages 3–5 identified by the ICD-9-CM codes of 585.3, 585.4, 585.5, and 585.6. (22) As such, the diagnosis of CKD was based on each person having at least one inpatient or two separate outpatient claims for CKD on different days within one year. Incident CKD was defined as the first date that CKD was diagnosed and was thus labeled as the date of outcome. Follow-up started from the index date and continued until the first CKD diagnosis date, withdrawal from insurance, or the study end date of 31 August 2015, whichever came first. To avoid a temporal relationship between NAFLD and CKD diagnosis, we performed a sensitivity analysis where we excluded CKD which developed within six months following a NAFLD diagnosis.
For determination of the CKD diagnosis, we also performed a sensitivity analysis using the ICD-9-CM code of 585.9 (unspecified CKD) in addition to 585.3, 585.4, 585.5, and 585.6 (CKD stages 3–5). In addition, to control for the impact of other liver disease, we also conducted a sensitivity analysis which included hepatitis C virus infection, hepatitis B virus infection, and alcoholic liver disease. Finally, we performed subgroup analyses as previous studies have suggested that there was an effect modification on the rate of CKD among certain subpopulations. (15–20) Therefore, we stratified the NAFLD cohort by age group, gender, diabetes, hypertension, and cirrhosis status.
Statistical Analysis
The t-test for continuous variables and chi-square tests for categorical variables were used to compare the baseline characteristics between NAFLD and non-NAFLD cohorts. To determine statistically significant differences between the cohorts following PS matching, we used the standardized difference of 0.2 as the threshold for a difference in between the groups. (23) The number of CKD events and person-time of observations were determined for each group and subsequently used to calculate the incidence rates of CKD (number of events per 1,000 person-years). A Cox proportional hazards regression model was used to determine the risk of developing CKD between the NAFLD and non-NAFLD cohorts. Furthermore, a Cox proportional hazards regression model using time-dependent covariates was conducted for the sensitivity analysis. The covariates that were adjusted for in the regression models included cirrhosis, decompensated cirrhosis (DCC), and hepatocellular carcinoma (HCC), in addition to the covariates we adjusted for in PS matching. Of note, previous studies have suggested that incorporating effect mediators or variables that were strongly associated with NAFLD but weakly associated with CKD into a matching schema can lead to unsuccessful matching and increased variance. (24–27) Thus, we did not match for the presence of advanced liver disease which many consider to be an effect mediator of NAFLD rather than a confounder but did control for it in the regression models. All study analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Patient characteristics
Between January 2006 - August 2015 (Supporting Figure S1), 262,661 NAFLD patients and 2,626,610 non-NAFLD patients met our study inclusion criteria while after PS matching, we identified 262,619 NAFLD patients and 769,878 non-NAFLD patients. The baseline demographic characteristics, comorbid conditions, and medication use in the NAFLD and the control cohorts before and after PS matching are shown in Table 1. In the PS-matched groups, patient demographic characteristics, including age (mean age: 51), gender (53% male), and several comorbid conditions (e.g., hypertension, diabetes mellitus) were comparable between two groups. All variables between the two cohorts were within the threshold of acceptable balance (standardized difference < 0.20). (23) The presence of advanced liver disease (cirrhosis, DCC, and HCC), not included in PS matching but adjusted in regression models, was more prevalent in the NAFLD cohort compared to the non-NAFLD cohort (Table 1).
Table 1.
Baseline characteristics before and after propensity score (PS) matching
| Patient Characteristics | Before PS Matching | After PS Matching† | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NAFLD | Non-NAFLD* | STD‡ | NAFLD cohort | Non-NAFLD cohort | STD‡ | ||||||
| n= 262,661 | n= 2,626,610 | n=262,619 | n= 769,878 | ||||||||
| Propensity score adjusted variables | Mean age, years (± SD) | 51 | (± 12.4) | 50.8 | (± 12.4) | 0.00 | 51 | (± 12.4) | 52 | (± 11.9) | −0.12 |
| Gender, n (%), male | 123,548 | 53.0 | 1,391,130 | 53.0 | 0.00 | 139,188 | 53.0 | 402,646 | 52.3 | 0.01 | |
| Comorbidities, n (%) | |||||||||||
| Hypertension | 128,257 | 48.8 | 747,040 | 28.4 | 0.40 | 128,158 | 48.8 | 380,320 | 49.4 | −0.01 | |
| Hyperlipidemia | 121,098 | 46.1 | 747,324 | 28.5 | 0.35 | 121,067 | 46.1 | 361,073 | 46.9 | −0.01 | |
| Diabetes mellitus | 70,316 | 26.8 | 294,364 | 11.2 | 0.35 | 70,382 | 26.8 | 187,080 | 24.3 | 0.05 | |
| Obesity | 42,091 | 16.0 | 126,450 | 4.8 | 0.31 | 42,019 | 16.0 | 102,394 | 13.3 | 0.07 | |
| COPD | 38,400 | 14.6 | 226,302 | 8.6 | 0.17 | 38,342 | 14.6 | 106,243 | 13.8 | 0.02 | |
| Heart Failure | 9,092 | 3.5 | 49,220 | 1.9 | 0.09 | 9,192 | 3.5 | 24,636 | 3.2 | 0.01 | |
| Peripheral vascular disease | 10,921 | 4.2 | 64,037 | 2.4 | 0.09 | 11,030 | 4.2 | 31,565 | 4.1 | 0.00 | |
| Cerebrovascular disease | 11,668 | 4.4 | 78,097 | 3.0 | 0.07 | 11,555 | 4.4 | 34,645 | 4.5 | 0.00 | |
| Coronary artery disease | 24,216 | 9.2 | 148,838 | 5.7 | 0.12 | 24,161 | 9.2 | 67,749 | 8.8 | 0.01 | |
| Baseline medication use, n (%) | |||||||||||
| ACEI | 55,440 | 21.1 | 350,859 | 13.4 | 0.19 | 55,413 | 21.1 | 158,595 | 20.6 | 0.01 | |
| ARB | 38,578 | 14.7 | 214,937 | 8.2 | 0.18 | 38,605 | 14.7 | 106,243 | 13.8 | 0.02 | |
| Mean outpatient visits, (± SD) | 18 | (± 17.0) | 12.0 | (± 13.8) | 0.35 | 17 | (± 12.4) | 15 | (± 16.6) | 0.18 | |
| Mean inpatient visits, (± SD) | 0.2 | (± 0.7) | 0.1 | (± 0.4) | 0.16 | 0.2 | (± 0.7) | 0.2 | (± 0.6) | 0.04 | |
| Propensity score unadjusted variables† | Liver severity, n (%) | ||||||||||
| Cirrhosis | 7,726 | 2.9 | 3,951 | 0.2 | 0.17 | 7,616 | 2.9 | 1,540 | 0.2 | 0.16 | |
| Decompensated cirrhosis | 7,436 | 2.8 | 9,115 | 0.4 | 0.15 | 7,353 | 2.8 | 4,619 | 0.6 | 0.13 | |
| Hepatocellular carcinoma | 785 | 0.3 | 843 | 0.03 | 0.50 | 788 | 0.3 | 770 | 0.1 | 0.05 | |
Abbreviations: PS propensity score, COPD chronic obstructive pulmonary disease, ACEI angiotensin-converting-enzyme inhibitors, ARB angiotensin II receptor blockers.
10 controls without NAFLD were matched on age, sex, and index date with each NAFLD subject.
Propensity Score matching did not include liver-related comorbidities, instead these covariates were adjusted as covariates in the Cox regression model.
The standardized difference in % is difference in means or proportions divided by standard error; imbalance defined as absolute value greater than 0.20 (small effect size)
CKD risk for among propensity score matched NAFLD and non-NAFLD cohorts
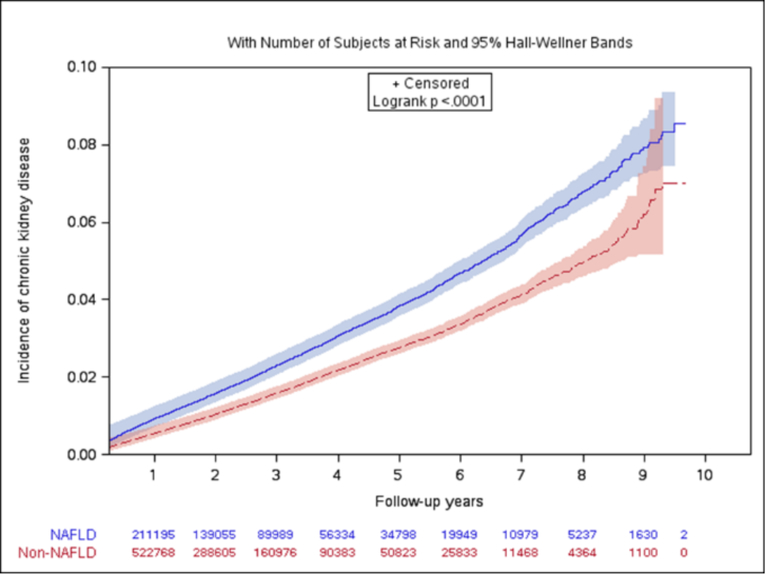
There were 5766 new CKD cases in the NAFLD cohort (n= 262,619) and 8655 new CKD cases in non-NAFLD cohort (n=769,878). The CKD cumulative incidence was consistently higher in patients with NAFLD compared to those without NAFLD (Figure 1). The CKD crude incidence rate was 8.2 per 1,000 person-years in the NAFLD cohort compared to 5.5 per 1,000 person-years in the non-NAFLD cohort (Table 2). The Cox proportional hazards regression model indicated that NAFLD patients had a 41% [adjusted hazard ratio (aHR), 1.41; 95% confidence interval (CI), 1.36–1.46] increased risk for the development of CKD, following adjustment for demographics, baseline covariates, and the use of ACEI and ARB compared to non-NAFLD patients.
Figure 1.
Cumulative incidence of chronic kidney disease by NAFLD status
Table 2.
Incidence rate and hazard ratio for chronic kidney disease (CKD) in NAFLD compared to non- NAFLD patients, primary analysis and subanalyses by age and gender
| NAFLD Status | No of patients | Person years | No of events | Crude incidence per 1000 person-years | Adjusted HR of CKD (95% CI) | P-value*** | ||
|---|---|---|---|---|---|---|---|---|
| Baseline covariates adjusted* | Baseline and time-varying covariates adjusted** | |||||||
| Primary analysis | NAFLD | 262,619 | 707,310 | 5,766 | 8.2 | 1.41 (1.36, 1.46) | 1.58 (1.52, 1.66) | |
| Non-NAFLD | 769,878 | 1,572,889 | 8,655 | 5.5 | Reference | Reference | ||
| Age | ||||||||
| 18–49 | NAFLD | 113,293 | 303,861 | 798 | 2.6 | 1.49 (1.35, 1.65) | 1.66 (1.47, 1.88) | <0.001 |
| Non-NAFLD | 317,052 | 651,841 | 915 | 1.4 | Reference | Reference | ||
| 50–59 | NAFLD | 87,991 | 249,790 | 1,930 | 7.7 | 1.48 (1.39, 1.57) | 1.60 (1.47, 1.73) | |
| Non-NAFLD | 260,349 | 576,143 | 2,489 | 4.3 | Reference | Reference | ||
| >= 60 | NAFLD | 61,228 | 153,443 | 3,031 | 19.8 | 1.31 (1.25, 1.37) | 1.56 (1.47, 1.67) | |
| Non-NAFLD | 183,558 | 369,791 | 5,241 | 14.2 | Reference | Reference | ||
| Gender | ||||||||
| Male | NAFLD | 123,527 | 328,401 | 3,074 | 9.4 | 1.58 (1.50, 1.66) | 1.70 (1.59, 1.81) | 0.001 |
| Non-NAFLD | 366,955 | 746,076 | 3,989 | 5.3 | Reference | Reference | ||
| Female | NAFLD | 139,064 | 378,858 | 2,690 | 7.1 | 1.33 (1.26, 1.40) | 1.47 (1.38, 1.58) | |
| Non-NAFLD | 401,258 | 817,245 | 4,313 | 5.3 | Reference | Reference | ||
Abbreviations: NAFLD Non-alcoholic fatty liver disease, CKD chronic kidney disease, CI confidence interval, HR hazard ratio
Adjusted for age, gender, diabetes, hypertension, obesity, hyperlipidemia, coronary artery disease, peripheral vascular disease, cerebrovascular disease, heart failure, and chronic obstructive pulmonary disease, angiotensin-converting-enzyme inhibitors, and angiotensin II receptor blockers, mean number of outpatient visits, and mean number of inpatient visits, cirrhosis, decompensated cirrhosis, and hepatocellular carcinoma.
Time varying covariates include age, gender, diabetes, hypertension, obesity, hyperlipidemia, coronary artery disease, peripheral vascular disease, cerebrovascular disease, heart failure, and chronic obstructive pulmonary disease, angiotensin-converting-enzyme inhibitors, and angiotensin II receptor blockers, cirrhosis, decompensated cirrhosis, and hepatocellular carcinoma.
P-value for interaction
The results of our sensitivity analysis using the Cox regression model with time-dependent covariates which took the change of comorbidities and medication use during follow-up into consideration, indicated that NAFLD patients had a 58% increased risk of CKD (aHR, 1.58; 95% CI, 1.52–1.66). After stratifying by age groups, the association of NAFLD and CKD was more significant among young adults (age 18–49; aHR, 1.49; 95% CI, 1.35–1.65) than the elderly population (age >= 60; aHR, 1.31; 95% CI, 1.25–1.37) (P interaction<0.001). This association was also more significant in males (aHR, 1.58; 95% CI, 1.50–1.66) than females (aHR, 1.33; 95% CI, 1.26–1.40) (P interaction=0.001). Thus, NAFLD appeared to be a risk for CKD in both males and females and in patients of different age groups.
In the sensitivity analyses where we included patients with a CKD diagnosis that occurred ≥ 6 months after the index date (aHR, 1.30; 95% CI, 1.25–1.36) as well as for those who had a diagnosis of unspecified CKD in addition to a diagnosis of CKD stages 3–5 (aHR, 1.28; 95% CI, 1.25–1.32), our results remained consistent with the primary analysis (Table 3). In another sensitive analysis where we adjusted for hepatitis C virus infection, hepatitis B virus infection, and alcoholic liver disease in addition to the covariates adjusted in the primary analysis, our results remained consistent with the primary analysis (aHR, 1.43; 95% CI, 1.38–1.48).
Table 3.
Incidence rate and hazard ratio for chronic kidney disease (CKD) in NAFLD compared to non-NAFLD patients in the sensitivity analyses.
| NAFLD Status | No of patients | Person years | No of events | Crude Incidence per 1000 person-years | Baseline covariates adjusted HR of CKD (95% CI)* | |
|---|---|---|---|---|---|---|
| Patients with CKD diagnosis ≥ 6 months after baseline | NAFLD | 261,158 | 707,016 | 4302 | 6.1 | 1.30 (1.25, 1.36) |
| Non-NAFLD | 765,552 | 1,567,022 | 6290 | 4.0 | Reference | |
| CKD diagnosis with 585.9 (unspecified CKD) ICD9 codes in addition to CKD stages 3–5 | NAFLD | 262,619 | 706,226 | 10615 | 15.0 | 1.28 (1.25, 1.32) |
| Non-NAFLD | 769,878 | 1,551,269 | 16364 | 10.5 | Reference | |
| A regression model controlling for HBV, HCV, and alcohol liver disease in addition to variables adjusted in primary analysis | NAFLD | 262,619 | 707,310 | 5766 | 8.2 | 1.43 (1.38, 1.48) |
| Non-NAFLD | 769,878 | 1,572,889 | 8655 | 5.5 | Reference |
Abbreviations: NAFLD Non-alcoholic fatty liver disease, CKD chronic kidney disease, CI confidence interval, HR hazard ratio
Adjusted for age, gender, diabetes, hypertension, obesity, hyperlipidemia, coronary artery disease, peripheral vascular disease, cerebrovascular disease, heart failure, and chronic obstructive pulmonary disease , angiotensin-converting-enzyme inhibitors, and angiotensin II receptor blockers, mean number of outpatient visits, and mean number of inpatient visits, cirrhosis, decompensated cirrhosis, and hepatocellular carcinoma.
Subgroup analysis
Table 4 shows that in the subgroup analysis stratified by presence of hypertension, hyperlipidemia, diabetes, obesity, and cirrhosis (including compensated and decompensated cirrhosis) at baseline, NAFLD was consistently associated with an increased risk of CKD compared with non-NAFLD patients, regardless of the presence of the above co-morbid conditions. For example, although NAFLD patients with hypertension had a three times higher crude incidence of CKD compared to NAFLD patients without hypertension (12.1 per 1000 person-years vs. 4.7 per 1000 person-years), the association between NAFLD and incident CKD was stronger in patients without hypertension (aHR,1.82; 95% CI, 1.70–1.94) compared to those with hypertension (aHR, 1.28; 95% CI, 1.23–1.33).
Table 4.
Incidence rate and hazard ratio for chronic kidney disease (CKD) in NAFLD compared to non-NAFLD patients in predefined subgroups at baseline
| Study population | NAFLD Status | No. of Patients | Person-years | CKD events | Crude Incidence of CKD (per 1,000 person-years) | Baseline covariates adjusted HR of CKD (95% CI)* | P-value** |
|---|---|---|---|---|---|---|---|
| Hypertension | NAFLD | 128,112 | 332,740 | 4,010 | 12.1 | 1.28 (1.23, 1.33) | <0.001 |
| Non-NAFLD | 365,885 | 713,929 | 6,481 | 9.1 | Reference | ||
| Non-hypertension | NAFLD | 134,370 | 374,267 | 1,749 | 4.7 | 1.82 (1.70, 1.94) | |
| Non-NAFLD | 398,161 | 808,240 | 2,041 | 2.5 | Reference | ||
| Hyperlipidemia | NAFLD | 120,954 | 317,513 | 2,825 | 8.9 | 1.28 (1.22, 1.34) | <0.001 |
| Non-NAFLD | 347,468 | 683,110 | 4,590 | 6.7 | Reference | ||
| Non-hyperlipidemia | NAFLD | 141,506 | 389,505 | 2,933 | 7.5 | 1.57 (1.50, 1.66) | |
| Non-NAFLD | 418,128 | 878,664 | 4,242 | 4.8 | Reference | ||
| Diabetes | NAFLD | 70,140 | 181,679 | 2,977 | 16.4 | 1.17 (1.11, 1.22) | <0.001 |
| Non-NAFLD | 194227 | 378,702 | 5,140 | 13.6 | Reference | ||
| Non-diabetes | NAFLD | 192,304 | 525,282 | 2,784 | 5.3 | 1.79 (1.70, 1.88) | |
| Non-NAFLD | 573,935 | 1,179,699 | 3,690 | 3.1 | Reference | ||
| Obesity | NAFLD | 41,695 | 96,048 | 716 | 7.5 | 1.08 (0.98, 1.19) | <0.001 |
| Non-NAFLD | 101,515 | 174,882 | 1,193 | 6.8 | Reference | ||
| Non-obesity | NAFLD | 220,552 | 610,486 | 5,048 | 8.3 | 1.44 (1.39, 1.50) | |
| Non-NAFLD | 660,040 | 1,378,129 | 7,731 | 5.6 | Reference | ||
| Cirrhosis | NAFLD | 3,951 | 9,723 | 325 | 33.4 | 1.48 (1.21, 1.81) | 0.277 |
| Non-NAFLD | 3,951 | 6,557 | 143 | 21.8 | Reference | ||
| Non-cirrhosis | NAFLD | 254,896 | 688,588 | 5,081 | 7.4 | 1.41 (1.36, 1.46) | |
| Non-NAFLD | 748,916 | 1,523,273 | 8,233 | 5.4 | Reference |
Abbreviations: NAFLD Non-alcoholic fatty liver disease, CKD chronic kidney disease, CI confidence interval, HR hazard ratio
Adjusted for age, gender, diabetes, hypertension, obesity, hyperlipidemia, coronary artery disease, peripheral vascular disease, cerebrovascular disease, heart failure, and chronic obstructive pulmonary disease, angiotensin-converting-enzyme inhibitors, and angiotensin II receptor blockers, mean number of outpatient visits, and mean number of inpatient visits, cirrhosis, decompensated cirrhosis, and hepatocellular carcinoma.
P-value for interaction
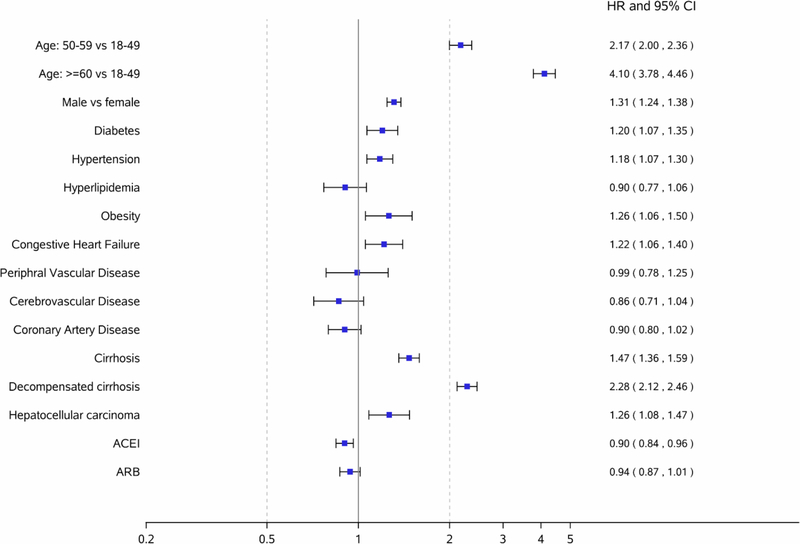
Risk factors associated with CKD among NAFLD patients
Figure 2 shows risk factors for CKD among NAFLD patients. Factors associated with an increased risk of developing CKD in NAFLD patients included the following: age >= 60 years (aHR, 4.10; 95% CI, 3.78– 4.46), male (aHR, 1.31 ; 95% CI, 1.24– 1.38), diabetes (aHR, 1.20; 95% CI, 1.07–1.35), hypertension (aHR, 1.18; 95% CI, 1.07– 1.30), obesity (aHR, 1.26; 95% CI, 1.06–1.50), congestive heart failure (aHR, 1.22; 95% CI, 1.06–1.40), compensated cirrhosis (aHR, 1.47; 95% CI,1.36–1.59), DCC (aHR, 2.28; 95% CI, 2.12–2.46), and HCC (aHR, 1.26; 95% CI, 1.08–1.47).
Figure 2.
Factors associated with an increased risk of chronic kidney disease among NAFLD patients
DISCUSSION
Our findings from this large retrospective PS-matched nationwide cohort study provide evidence that there is an increased risk of CKD among adults with NAFLD. We found that the crude incidence rate among the NAFLD cohort was 1.5 times higher compared to those without NAFLD (8.2 vs 5.5 per 1, person year). In fact, following our regression analysis, adults with NAFLD were found to have a 41% increased risk for the development of CKD compared to those without NAFLD. Furthermore, after adjustment for the time varying covariates, the risk of CKD among adults with NAFLD increased to 58%. This 17% increase in the risk for CKD is attributed to the additional adjustment for the risk factors known to be associated with CKD development occurring after the index NAFLD diagnosis. In addition, the association between NAFLD and incident CKD remained consistently significant in our other subgroup analyses that were stratified by age and gender, as well as across subgroups of patients with and without the presence of comorbidities including hypertension, hyperlipidemia, diabetes, and cirrhosis.
However, researchers conducted a recent prospective cohort study in Caucasian men from Finland (n=2338) and found no relationship between NAFLD (defined as elevated serum gamma-glutamyltransferase) and CKD. (28) Investigators suggested the reason NAFLD was not associated with CKD was probably due to a small sample size and low event rate although they had a long follow-up duration (over 20 years). (28) Another explanation may be related to how they diagnosed NAFLD, as elevated liver enzymes are not liver disease specific but are rather only used to suggest there may be liver damage, and not all NAFLD patients have elevated liver enzymes. (29)
In a meta-analysis of results from European populations, the association was not reported as statistically significant due to a small sample size (n=3), heterogeneity, and lack of high-quality studies with full adjustment for covariates. (20) Rather, our results corroborate findings from a recent cohort study and meta-analysis conducted for Asian populations, which suggested that NAFLD was associated with an almost 40% increased risk of CKD. (19, 20) Our study identified 14,421 incident CKD cases, significantly more than the previous studies, which lends itself to more robust subgroup and sensitivity analyses among NAFLD patients (n=262,619; 53% male). For example, we excluded patients who had a diagnosis of CKD within 6 months after a NAFLD diagnosis in a sensitivity analysis to avoid concurrent diagnosis of CKD and NAFLD, yet we found that the association remained significant. Thus, our findings confirm that NAFLD is an independent risk factor for CKD in a much larger sample size of US general population, providing further support for the evidence.
In addition, we also identified additional risk factors of CKD among NAFLD patients (based on exclusively among NAFLD patients). We found the risk of CKD increased as the severity of NAFLD increased, as indicated by the diagnosis of compensated cirrhosis (aHR, 1.47; 95% CI,1.36–1.59) and DCC (aHR, 2.28; 95% CI, 2.12–2.46). In addition to liver severity, we also identified age>=60, congestive heart failure, diabetes, hypertension, and obesity as key factors associated with increased risk of CKD in NAFLD patients.
The pathophysiologic mechanisms linking NAFLD and CKD are not yet completely understood, though several plausible mechanisms have been suggested. (30, 31) Some investigators have postulated that NAFLD may exacerbate systemic and hepatic insulin resistance, causing atherogenic dyslipidemia, and releasing a variety of pro-inflammatory, pro-coagulant, pro-oxidant, and pro-fibrogenic mediators which results in the development and progression of CKD. (32–33) Until there is further understanding of the pathophysiological mechanism of NAFLD and CKD, treatment to prevent or reverse the damage cannot be recommended, though improved NAFLD histology associated with 1-year lifestyle modification was recently shown to be associated with improved kidney function in a randomized controlled trial. (34) Additional studies in this area are undoubtedly warranted to provide guidance for CKD screening and prevention for the growing population of persons with NAFLD in the US and globally.
There are several strengths of our study. First, to overcome the limitations that arise by using retrospective data, PS matching and time-varying Cox proportional hazard models were used to balance the groups such as what might occur with true randomization. This methodological strength provides further evidence of the strong relationship between NAFLD and CKD. Secondly, we had a large sample size (262,619 NAFLD patients) and our cohort was representative of the general population in the US especially since we used one of the largest US database of patient data, which includes nearly 20 million individuals annually, 77 large employers, and more than 100 health plans. A recent meta-analysis combined results from 8 non-US observational studies included 32,842 NAFLD patients. (20) Lastly, we controlled for the known comorbidities and medications associated with renal functioning as a means of isolating the effects of NAFLD as well as conducting several sensitivity and subgroup analyses to access the robustness of our results and NAFLD still remained as a risk factor for developing CKD.
Our study also had several limitations. First, we did not have data on laboratory/radiology results (e.g., liver enzymes, ultrasound) to corroborate ICD-9 coding so the diagnosis of NAFLD was dependent on ICD-9 codes which could lead to underdiagnoses of NAFLD. A prior study has validated the use of billing ICD 9 codes for the diagnosis of NAFLD definition, with a positive predictive value of 89%, (21) and this approach is commonly used in retrospective claims data analyses. However, the validation study also reported a negative predictive value of 36%, which suggests that some NAFLD patients remain unrecognized and underdiagnosed in routine practice. (21) Thus, we may have underestimated the risk of CKD in NAFLD patients because it is possible that patients in the control group (non-NAFLD) might have as yet undiagnosed NAFLD. In addition, the study lacked estimated glomerular filtration rate for accurate staging of the severity of CKD. It is possible that incomplete, missing, or miscoded claims may impact the study findings; however, coding errors are likely distributed evenly between the NAFLD and non-NAFLD groups. In fact, the incidence rates of CKD stages 3–5 in our population were comparable with the rates reported from a prospective observational cohort study (4.3~10.4 per 1000 person-years). (35) Second, claims data also have limitations stemming from the nature of administrative claims for payment purposes. For example, compared to a previous study in ultrasound-proven NAFLD patients, we found that the prevalence of obesity at NAFLD diagnosis was significantly lower in our study (16.0% vs 67.5%) although the prevalence of diabetes mellitus was similar (26.8% vs 26.3%). (36) This is likely due to the low sensitivity of ICD-9 codes for obesity (25.2%), which means that we captured only a portion of obese patients; however, it is unlikely that this undercoding of obesity would alter our results because it is not expected to distribute differently between our NAFLD and control cohorts. (37) Third, though we adjusted for as many confounders as available and known to be associated with CKD as well as mean total number of outpatient visits and inpatient visits; however, there may have been some unmeasured confounders that were not reported and thus unavailable (e.g., smoking). Lastly, detection bias may be introduced by differential screening frequencies for kidney diseases between NAFLD and non-NAFLD patients.
In conclusion, our results from our large nationwide cohort study of PS-matched NAFLD and non-NAFLD patients from the US general population provide evidence that a diagnosis of NAFLD conferred a significantly increased risk of CKD, with the association between NAFLD and CKD development remaining significant in sensitivity and subgroup analyses. Our findings suggest that NAFLD patients should be screened for CKD, to be monitored regularly for renal function, and to receive early interventions to potentially decrease the risk of CKD, especially those are at high risk of developing incident CKD such as older or male patients and those with diabetes, hypertension, heart failure, and cirrhosis. Further research is needed to investigate the underlying mechanism of this association and to determine interventions necessary to treat patients with NAFLD at high risk for developing CKD.
Supplementary Material
Financial Support:
Research reported in this publication was supported in part by the National Institute on Drug Abuse of the National Institutes of Health under award number K01DA045618 (to HP).
Abbreviations:
- HR
hazard ratio
- CI
confidence interval
- ACEI
angiotensin-converting-enzyme inhibitors
- ARB
angiotensin II receptor blockers
Footnotes
Financial Disclosure
Nguyen: Research support: NCI, Gilead, Pfizer, Janssen, BK Kee Foundation; Advisory board/consulting: Novartis, Gilead, Janssen, Bayer, Eisai, Lab of Advanced Medicine, Exact Sciences.
Conflict of Interest
Others declare no conflicts of interest that pertain to this study.
References
- 1.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328–357. [DOI] [PubMed] [Google Scholar]
- 2.Singal AK, Hasanin M, Kaif M, Wiesner R, Kuo YF. Nonalcoholic Steatohepatitis is the Most Rapidly Growing Indication for Simultaneous Liver Kidney Transplantation in the United States. Transplantation 2016;100:607–612. [DOI] [PubMed] [Google Scholar]
- 3.Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol 2013;10:330–344. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong MJ, Adams LA, Canbay A, Syn WK. Extrahepatic complications of nonalcoholic fatty liver disease. Hepatology 2014;59:1174–1197. [DOI] [PubMed] [Google Scholar]
- 5.Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med 2010;363:1341–1350. [DOI] [PubMed] [Google Scholar]
- 6.Targher G, Chonchol MB, Byrne CD. CKD and nonalcoholic fatty liver disease. Am J Kidney Dis 2014;64:638–652. [DOI] [PubMed] [Google Scholar]
- 7.Marcuccilli M, Chonchol M. NAFLD and Chronic Kidney Disease. Int J Mol Sci 2016;17:562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murray CJ, Atkinson C, Bhalla K, Birbeck G, Burstein R, Chou D, Dellavalle R, et al. The state of US health, 1990–2010: burden of diseases, injuries, and risk factors. JAMA 2013;310:591–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol 2013;10:686–690. [DOI] [PubMed] [Google Scholar]
- 10.Singal AK, Salameh H, Kuo YF, Wiesner RH. Evolving frequency and outcomes of simultaneous liver kidney transplants based on liver disease etiology. Transplantation 2014;98:216–221. [DOI] [PubMed] [Google Scholar]
- 11.Adams LA, Anstee QM, Tilg H, Targher G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut 2017;66:1138–1153. [DOI] [PubMed] [Google Scholar]
- 12.Ahn AL, Choi JK, Kim MN, Kim SA, Oh EJ, Kweon HJ, Cho DY. Non-alcoholic Fatty Liver Disease and Chronic Kidney Disease in Koreans Aged 50 Years or Older. Korean J Fam Med 2013;34:199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sirota JC, McFann K, Targher G, Chonchol M, Jalal DI. Association between nonalcoholic liver disease and chronic kidney disease: an ultrasound analysis from NHANES 1988–1994. Am J Nephrol 2012;36:466–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paik J, Golabi P, Younossi, Mishra A, Trimble G, Younossi ZM. Chronic kidney disease is independently associated with increased mortality in patients with nonalcoholic fatty liver disease. Liver Int 2019;39(2):342–352. [DOI] [PubMed] [Google Scholar]
- 15.Targher G, Chonchol M, Bertolini L, Rodella S, Zenari L, Lippi G, Franchini M, et al. Increased risk of CKD among type 2 diabetics with nonalcoholic fatty liver disease. J Am Soc Nephrol 2008;19:1564–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Targher G, Mantovani A, Pichiri I, Mingolla L, Cavalieri V, Mantovani W, Pancheri S, et al. Nonalcoholic fatty liver disease is independently associated with an increased incidence of chronic kidney disease in patients with type 1 diabetes. Diabetes Care 2014;37:1729–1736. [DOI] [PubMed] [Google Scholar]
- 17.Chang Y, Ryu S, Sung E, Woo HY, Oh E, Cha K, Jung E, et al. Nonalcoholic fatty liver disease predicts chronic kidney disease in nonhypertensive and nondiabetic Korean men. Metabolism 2008;57:569–576. [DOI] [PubMed] [Google Scholar]
- 18.Ryu S, Chang Y, Kim DI, Kim WS, Suh BS. gamma-Glutamyltransferase as a predictor of chronic kidney disease in nonhypertensive and nondiabetic Korean men. Clin Chem 2007;53:71–77. [DOI] [PubMed] [Google Scholar]
- 19.Sinn DH, Kang D, Jang HR, Gu S, Cho SJ, Paik SW, Ryu S, et al. Development of chronic kidney disease in patients with non-alcoholic fatty liver disease: A cohort study. J Hepatol 2017;67:1274–1280. [DOI] [PubMed] [Google Scholar]
- 20.Mantovani A, Zaza G, Byrne CD, Lonardo A, Zoppini G, Bonora E, Targher G. Nonalcoholic fatty liver disease increases risk of incident chronic kidney disease: A systematic review and meta-analysis. Metabolism 2018;79:64–76. [DOI] [PubMed] [Google Scholar]
- 21.Corey KE, Kartoun U, Zheng H, Shaw SY. Development and Validation of an Algorithm to Identify Nonalcoholic Fatty Liver Disease in the Electronic Medical Record. Dig Dis Sci 2016;61:913–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ronksley PE, Tonelli M, Quan H, Manns BJ, James MT, Clement FM, Samuel S, et al. Validating a case definition for chronic kidney disease using administrative data. Nephrol Dial Transplant 2012;27:1826–1831. [DOI] [PubMed] [Google Scholar]
- 23.D’Agostino RB Jr., Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998;17:2265–2281. [DOI] [PubMed] [Google Scholar]
- 24.Austin PC, Grootendorst P, Anderson GM. A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: a Monte Carlo study. Stat Med 2007;26:734–753. [DOI] [PubMed] [Google Scholar]
- 25.Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Sturmer T. Variable selection for propensity score models. Am J Epidemiol 2006;163:1149–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patrick AR, Schneeweiss S, Brookhart MA, Glynn RJ, Rothman KJ, Avorn J, Sturmer T. The implications of propensity score variable selection strategies in pharmacoepidemiology: an empirical illustration. Pharmacoepidemiol Drug Saf 2011;20:551–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park H, Chen C, Wang W, Henry L, Cook RL, Nelson DR. Chronic hepatitis C virus (HCV) increases the risk of chronic kidney disease (CKD) while effective HCV treatment decreases the incidence of CKD. Hepatology 2018;67(2):492–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kunutsor SK, Laukkanen JA. Gamma-glutamyltransferase and risk of chronic kidney disease: A prospective cohort study. Clin Chim Acta 2017;473:39–44. [DOI] [PubMed] [Google Scholar]
- 29.Younossi ZM, Loomba R, Anstee QM, Rinella ME, Bugianesi E, Marchesini G, Newuschwander-Tetri BA, et al. Diagnositc modalities for nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, and associated fibrosis. Hepatology 2018;68(1):349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.James MT, Hemmelgarn BR, Tonelli M. Early recognition and prevention of chronic kidney disease. Lancet 2010;375:1296–1309. [DOI] [PubMed] [Google Scholar]
- 31.Yki-Jarvinen H Liver fat in the pathogenesis of insulin resistance and type 2 diabetes. Dig Dis 2010;28:203–209. [DOI] [PubMed] [Google Scholar]
- 32.Guebre-Egziabher F, Alix PM, Koppe L, Pelletier CC, Kalbacher E, Fouque D, Soulage CO. Ectopic lipid accumulation: A potential cause for metabolic disturbances and a contributor to the alteration of kidney function. Biochimie 2013;95:1971–1979. [DOI] [PubMed] [Google Scholar]
- 33.Targher G, Byrne C. Non-alcoholic fatty liver disease: an emerginig driving force in chronic kidney disease. Nat Rev Nephrol 2017;13:297–310. [DOI] [PubMed] [Google Scholar]
- 34.Vilar-Gomez E, Calzadilla-Bertot L, Friedman SL, Gra-Oramas B, Gonzalez-Fabian L, Villa-Jimenez O, Lazo-Del Vallin S, et al. Improvement in liver histology due to lifestyle modification is independently associated with improved kidney function in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther 2017;45:332–344. [DOI] [PubMed] [Google Scholar]
- 35.Bash LD, Coresh J, Kottgen A, Parekh RS, Fulop T, Wang Y, et al. Defining incident chronic kidney disease in the research setting: The ARIC Study. Am J Epidemiol. 2009;170:414–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–31. [DOI] [PubMed] [Google Scholar]
- 37.Ammann EM, Kalsekar I, Yoo A, Johnston SS. Validation of body mass index (BMI)-related ICD-9-CM and ICD-10-CM administrative diagnosis codes recorded in US claims data. Pharmacoepidemiol Drug Saf. 2018;27:1092–100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.