Abstract
Dihydroartemisinin (DHA)-piperaquine is being evaluated as intermittent preventive therapy for malaria, but dosing has not been optimized for children. We assessed exposure to DHA and piperaquine in Ugandan children at two ages during infancy. Intensive sampling was performed in 32 children at 32 weeks of age, 31 children at 104 weeks, and 30 female adult controls. Compared to adults, DHA area under the concentration time curve (AUC0–8hr) was 52% higher at 32 weeks and comparable at 104 weeks. Compared to adults, piperaquine AUC0–21d was 35% lower at 32 weeks and 53% lower at 104 weeks. Terminal piperaquine concentrations on Days 7, 14, and 21 were lower in children compared to adults and lower at 104 compared to 32 weeks. Piperaquine exposure was lower in young children compared to adults, and lower at 104 compared to 32 weeks of age, suggesting a need for age-based DHA-piperaquine dose optimization for chemoprevention.
Keywords: malaria, pharmacokinetics, pediatric, prevention, anti-infective, global health
INTRODUCTION
Despite the worldwide effort to decrease the incidence of malaria, in 2017 there were an estimated 219 million new cases of malaria and 435,000 deaths, with 93% of these deaths occurring in Africa, primarily due to Plasmodium falciparum (1). Children under five years of age are the most vulnerable population, accounting for an estimated 61% of deaths in 2017 (1). Pregnant women are another vulnerable population, with up to 41% of women exhibiting evidence of placental malaria in parts of Africa (2). Placental malaria increases risks for low birth weight and infant mortality (3).
To reduce the burden of malaria, intermittent preventive treatment (IPT), with standard treatment doses of antimalarials administered at regular intervals, has been endorsed for high risk groups. IPT with monthly sulfadoxine-pyrimethamine (SP) is the standard of care in malaria-endemic regions for pregnant women during the second and third trimester, although drug resistance limits the antimalarial efficacy of SP (4). Seasonal malaria chemoprevention, with SP/amodiaquine administered monthly during the transmission season, is endorsed for use in children in regions of West and Central Africa with highly seasonal malaria and relatively low levels of drug resistance (5). However, there is no specific recommendation for chemoprevention in children in most of Africa, where malaria transmission occurs year-round and drug resistance limits the efficacy of SP/amodiaquine. Our group and others are evaluating IPT with dihydroartemisinin (DHA)-piperaquine, an artemisinin-based combination therapy that is well suited for chemoprevention due to the long (~3 weeks) half-life of piperaquine (6, 7). Recent trials showed DHA-piperaquine offered significant reductions in risks of maternal and placental malaria compared to IPT with SP (8, 9). Similarly, in young children (10) and schoolchildren (11) monthly DHA-piperaquine offered potent preventive efficacy.
Despite data demonstrating DHA-piperaquine efficacy for IPT, there is an absence of pharmacokinetic (PK) and pharmacodynamic (PD) information for target populations. In particular, optimized IPT dosing strategies for children are lacking. DHA, which is short-acting, is metabolized by UDP-glucuronyltranferase (UGT)(12). Piperaquine is metabolized by cytochrome p450 (CYP) 3A4/2C8 (13). Physiological changes can alter DHA-piperaquine metabolism and pharmacokinetics. We previously reported that pregnant women exhibit a reduction in exposure to both DHA and piperaquine compared to non-pregnant controls (14), attributable to induction of both UGT and CYP metabolism. These data support refined DHA-piperaquine dosing recommendations for pregnant women (15).
In children, metabolism is also distinct from that in adults, in that maturation of CYP3A4/2C8 and UGT occurs gradually during the first year (16, 17) and first 6 months (18) of life, respectively. Specifically, CYP3A4 levels only approach about 50% of adult levels at 6 months of age (19), but then mature rapidly (20). Owing to these distinctions, we hypothesized that young children exhibit significant differences in DHA-piperaquine disposition compared to adults.
In the context of treatment, limited studies have evaluated the PK of DHA-piperaquine in young children, mostly with a sparse sampling design; it is uncertain if these results, obtained from ill children, are representative of those in healthy children receiving IPT (21). Therefore, to inform IPT dosing guidelines for young children, we carried out intensive PK studies of DHA-piperaquine administered as IPT at two time points during the first two years of life in Ugandan children.
RESULTS
Study profile
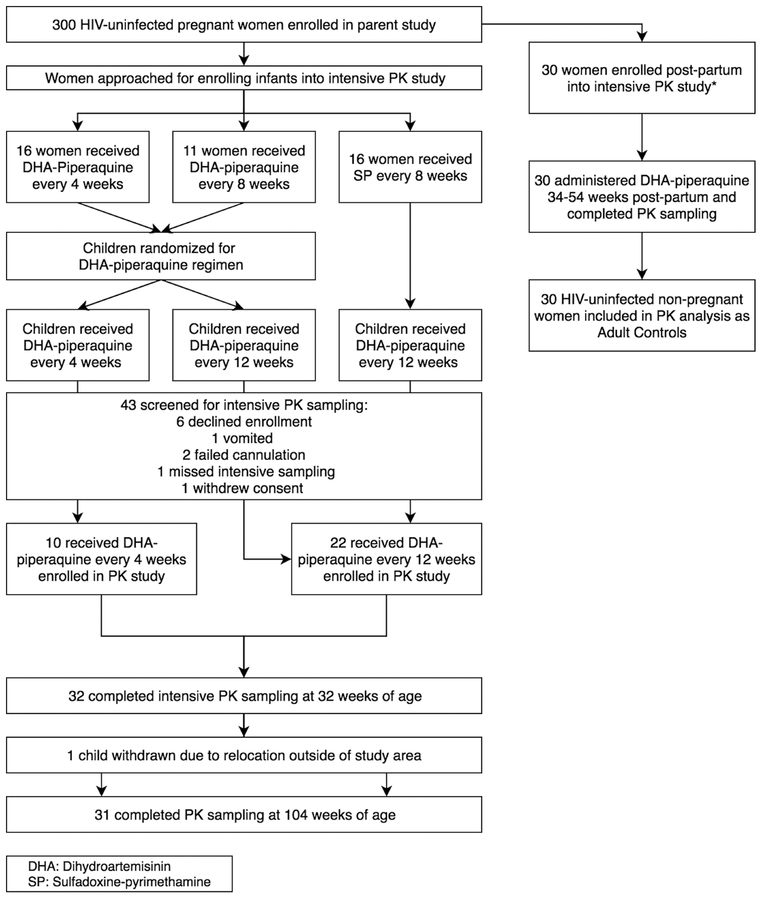
Screening and enrollment of study participants is summarized in Figure 1, and baseline characteristics of study subjects are in Table 1. Adult female controls underwent intensive PK assessments at a median time of 39 weeks post-partum.
Figure 1.
Enrollment and completion of intensive PK studies from trials evaluating DHA- piperaquine as IPTi for malaria; DP denotes DHA-piperaquine; SP, sulfadoxine-pyrimethamine; PK, pharmacokinetics; DHA, dihydroartemisinin; PQ, piperaquine. *Adult data previously reported (14).
Table 1.
Baseline characteristics of participants at time of PK study enrollment. Data represent median (range).
| Children 32 Weeks, n=32 | Children 104 Weeks, n=31 | HIV-Uninfected Adults, n=30 | |
|---|---|---|---|
| Age (yr) | 0.61 | 2 | 24 (19, 32) |
| Female | 14 | 14 | 30 |
| Male | 18 | 17 | |
| Weight (kg) | 7.53 (6.01, 9.03) | 10.55 (8.37, 12.86) | 52.9 (38.5, 72.9) |
| Height (cm) | 68 (62, 71) | 83 (77, 89) | 162 (148, 174) |
| Weeks post-partum | 38.5 (34, 54) |
Pharmacokinetics of DHA
Comparison of infants and adults
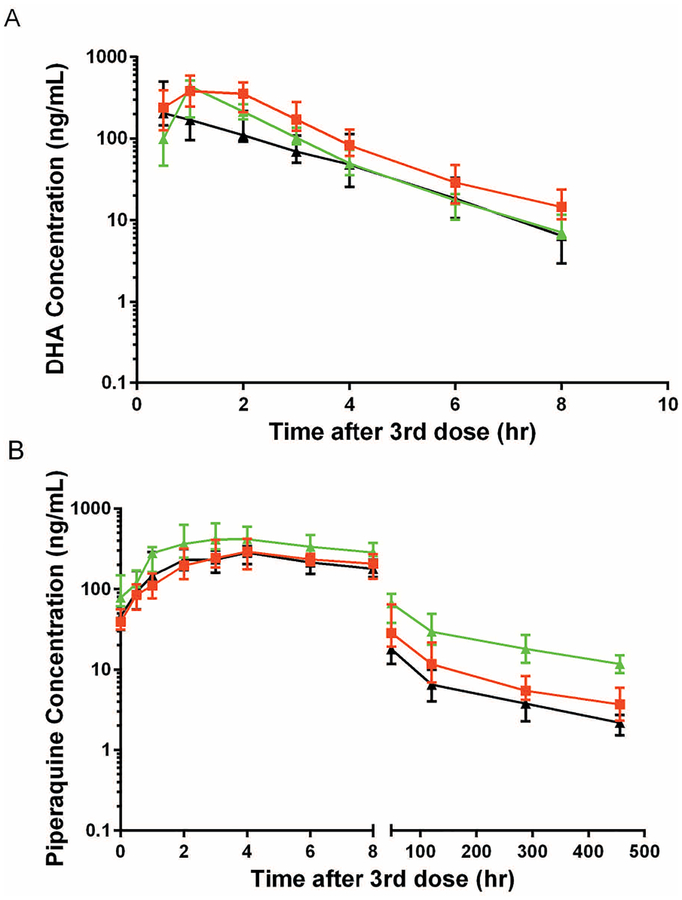
Drug exposure in 32 infants at 32 weeks of age and 31 infants at 104 weeks of age was compared to that in adult controls (Table 2; Figure 2a). Compared to adults, DHA exposure was higher in the 32-week-old infants, with the AUC0–8hr and Cmax 32% (p=0.016) and 52% (p<0.0001) higher, respectively. Likewise, the DHA C8hr terminal concentration was 97% higher at 32 weeks compared to that in adults (p=0.0001). In contrast, DHA half-life was not significantly different at 32 weeks compared to that in adults. No significant difference in DHA exposure was found between infants at 104 weeks of age and adults for Cmax, AUC0–8hr, half-life or C8hr terminal concentration.
Table 2.
Impact of infancy on the pharmacokinetics of DHA
| Children | Ratio | ||||||
|---|---|---|---|---|---|---|---|
| 32 weeks | 104 weeks | Adults | 104 weeks / 32 weeks | ||||
| n=32 | n=31 | n=29 | Paired (n=31) | All subjects | 32wk/adults | 104wk/adults | |
| Cmax, ng/ML | 480 (402, 574) | 355 (264, 478) | 363 (304, 432) | 0.737 (0.24) | 0.740 (0.12) | 1.32 (0.016) | 0.978 (0.89) |
| Tmax, hr | 1.07 (0.99, 2.03) | 0.57 (0.53, 2.00) | 1.03 (1.00, 1.98) | 0.533 (0.052) | 0.533 (0.037) | 1.04 (0.72) | 0.553 (0.015) |
| t1/2, hr* | 1.72 (1.48, 2.00) | 1.49 (1.24, 1.79) | 1.49 (1.37, 1.62) | 0.855 (0.098) | 0.866 (0.078) | 1.15 (0.20) | 1.00 (0.35) |
| AUC0–8hr, hr·ng/mL | 1150 (978, 1344) | 762 (623, 932) | 754 (662, 860) | 0.662 (0.0045) | 0.663 (0.0011) | 1.52 (<0.0001) | 1.01 (0.71) |
| C8hr, ng/mL | 14.7 (11.6, 18.6) | 6.85 (4.99, 9.39) | 7.45 (5.96, 9.31) | 0.460 (0.0004) | 0.466 (0.0001) | 1.97 (0.0001) | 0.919 (0.50) |
Cmax, maximal concentration; Tmax, time to reach maximal concentration; t1/2, drug elimination half-life; AUC, area under concentration-time curve; C8hr, DHA concentrations at 8hr post 3rd dose. Data are presented as geometric means (95% confidence interval) except for Tmax, which is reported as median (interquartile range). P-values are calculated using the signed rank test for paired analysis and rank sum test for unpaired. Significance level: alpha = 0.05.
n=30 for 32 weeks, 29 for paired analysis, and 28 for adults.
Figure 2.
Plasma concentration-time profile of DHA (A) and piperaquine (B) in children aged 104 weeks (black line) and 32 weeks (red line) and postpartum women (green line). Data is reported as median (IQR).
Comparison of infants at 32 and 104 weeks of age
To evaluate the impact of childhood development on DHA pharmacokinetics, data from 32 infants aged 32 weeks and 31 of the same infants aged 104 weeks were compared (Table 2; Figure 2a). By unpaired analysis (permitting a comparison using all of the results), DHA exposure was lower in infants at 104 weeks of age compared to that at 32 weeks of age, with the AUC0–8hr and C8hr terminal concentration lower by 34% (p=0.0011) and 53%, respectively. By paired analysis (comparing the same 31 infants studied at 32 weeks and 104 weeks of age), the impact of age on each PK parameter was nearly identical to that estimated by unpaired analysis (Table 2). When comparing infants receiving DHA-piperaquine every 4 weeks to those receiving treatment every 12 weeks, there was no difference in PK parameters at either 32 or 104 weeks of age (data not shown).
Pharmacokinetics of piperaquine
Capillary and venous concentration correlation
The median capillary plasma piperaquine concentration 24 hours post-3rd dose was 54.5 (range 17.7, 188) ng/mL; the corresponding venous concentration was 58.9 (16.2, 250) ng/mL. For 30 adult controls, the median capillary and venous plasma piperaquine concentrations 24 hours post-3rd dose were 115 (26.4, 292) ng/mL and 104 (24.8, 251) ng/mL, respectively. The correlation equation for simultaneous venous and capillary plasma measurements 24 hours post-3rd dose for infants was lnCcap=0.773*lnCvenous+0.895 (n=63), r2=0.614, and for adults was lnCcap=0.975*lnCvenous+0.320 (n=30), r2=0.877.
Comparison of infants and adults
Drug exposure for piperaquine was compared in the same infants studied for DHA (Table 3; Figure 2b). Compared to adults, piperaquine exposure was lower in the 32-week-old infants, with the Cmax and AUC0–21d 31% (p=0.0034) and 35% (p=0.0002) lower, respectively. Likewise, terminal concentrations of piperaquine on days 7, 14, and 21 were 35% to 42% lower in infants at 32 weeks compared to adults (p=0.0002 for each). Piperaquine exposure was lower still in infants at 104-weeks of age, with the Cmax and AUC0–21d 38% (p=0.0002) and 53% (p<0.0001) lower than in adults, respectively. Notably, terminal concentrations of piperaquine on days 7, 14, and 21 were 51% to 60% lower in infants at 104 weeks of age compared to those in adults (p<0.0001). As dose varied in children, we utilized weight normalized Dose/AUC (uncorrected for baseline concentration) to estimate changes in drug clearance and found geometric mean values (95% CI) of 1.87 (1.62, 2.16), 2.13 (1.91, 2.38) and 1.39 (1.20, 1.60) L/hr/kg for children at 32 weeks, children at 104 weeks, and adult controls, respectively (p<0.005 comparing 32 and 104 weeks to adults).
Table 3.
Impact of infancy on the pharmacokinetics of piperaquine
| Children | Ratio | ||||||
|---|---|---|---|---|---|---|---|
| 32 weeks | 104 weeks | Adults | 104 weeks /32 weeks | ||||
| n=32 | n=31 | n=30 | Paired (n=31) | All subjects | 32wk/adults | 104wk/adults | |
| Cmax, ng/mL | 345 (279, 427) | 309 (266, 359) | 499 (393, 633) | 0.939 (0.46) | 0.896 (0.69) | 0.691 (0.0034) | 0.619 (0.0002) |
| Tmax, hr | 4.03 (3.06, 6.01) | 4.02 (2.03, 6.05) | 3.06 (2.07, 4.03) | 0.998 (0.38) | 0.998 (0.22) | 1.32 (0.0063) | 1.31 (0.40) |
| *t 1/2, hr | 174 (149, 203) | 177 (148, 212) | 208 (187, 232) | 1.01 (0.47) | 1.02 (0.96) | 0.837 (0.090) | 0.851 (0.11) |
| AUC0–21d, hr·μg/mL | 11.4 (9.77, 13.2) | 8.24 (7.28, 9.33) | 17.6 (15.1, 20.7) | 0.741 (0.0020) | 0.723 (0.0033) | 0.648 (0.0002) | 0.468 (<0.0001) |
| C7d, ng/mL | 22.6 (18.8, 27.2) | 16.3 (13.6, 19.6) | 39.0 (32.3, 47.2) | 0.732 (0.013) | 0.721 (0.013) | 0.579 (0.0002) | 0.418 (<0.0001) |
| C14d, ng/mL | 13.9 (12.0, 16.0) | 9.10 (7.68, 10.8) | 22.6 (18.7, 27.3) | 0.654 (0.0005) | 0.655 (0.0006) | 0.615 (0.0002) | 0.403 (<0.0001) |
| C21d, ng/mL | 9.42 (8.18, 10.8) | 7.09 (6.03, 8.34) | 14.5 (12.2, 17.1) | 0.754 (0.011) | 0.753 (0.0046) | 0.650 (0.0002) | 0.489 (<0.0001) |
Abbreviations as in Table 2. AUC was calculated using piperaquine concentrations from venous plasma, including conversion of capillary to venous concentrations; C7d, C14d, and C21d are actual capillary plasma concentrations at day 7, 14, and 21 post 3rd dose. Data are presented as geometric means (95% confidence interval) except for Tmax, which is reported as median (interquartile range). P-values are calculated using the signed rank test for paired analysis and the rank sum test for unpaired. Significance level: alpha = 0.05.
n=24 for 32 weeks, 22 for 104 weeks, 16 for paired analysis, 29 for adults.
Comparison of infants at 32 and 104 weeks of age
To evaluate the impact of childhood development on piperaquine pharmacokinetics, data from 32 infants aged 32 weeks and 31 infants aged 104 weeks were compared (Table 3; Figure 3a). Based on unpaired analysis, piperaquine exposure was lower in infants at 104 weeks of age compared to 32 weeks of age, with AUC0–21d lower by 28% (p=0.003). Terminal concentrations of piperaquine on days 7, 14, and 21 were also significantly lower (25% to 35%) in infants at 104 weeks compared to 32 weeks (Table 3). In contrast, the Cmax and half-life were not different between infants at 32 and 104 weeks of age. By paired analysis (comparing the same 31 infants studied at 32 weeks and 104 weeks of age), the impact of age on each PK parameter was nearly identical to that estimated by unpaired analysis (Table 3). When comparing infants receiving DHA-piperaquine every 4 weeks to those receiving treatment every 12 weeks, PK parameters were unchanged at both 32 and 104 weeks, except for a 33% lower terminal concentration on day 14 in infants receiving the drug every 12 weeks (at the time of the 32-week evaluation, p=0.012, data not shown). Weight normalized Dose/AUC comparing ages 32 and 104 weeks yielded geometric mean values (95% CI) of 1.87 (1.62, 2.16) and 2.13 (1.91, 2.38) L/hr/kg for the two groups (p=0.20)
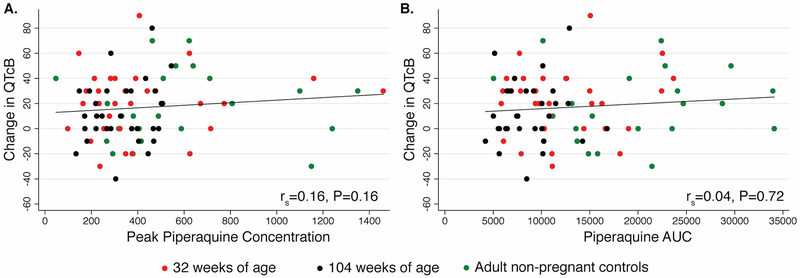
Figure 3.
Correlation of changes in the QTcB interval and pharmacokinetic exposure of piperaquine. AUC denotes area under the concentration vs. time curve to 21 days.
Correlation of piperaquine terminal concentrations and AUC
Measurements of piperaquine at 7-, 14-, or 21-days post-treatment may be used to monitor piperaquine concentrations during clinical studies, since intensive PK sampling is often not practical. Therefore, we sought to determine if terminal concentrations were predictive of overall piperaquine exposure. Piperaquine day 7, 14, and 21 concentrations were highly correlated with AUC0– 21d (Pearson r = 0.698 (p<0.0001), 0.582 (P<0.0001), and 0.366 (p=0.003) at 7, 14, and 21 days, respectively).
ECG findings
No adverse events suggestive of cardiotoxicity were observed for infants or adults. Using Bazett’s formula, at 32 weeks of age, the QTcB was ≤450 msec for 97% (29/30; range 375–472 msec) of infants pre-treatment, and 73% (22/30; range 386–482 msec) post-treatment. At 104 weeks, all pre- and post-treatment QTcB intervals were ≤450 msec (Figure 3 and Table S1). Bazett’s formula was shown to be less sensitive to heart rate changes than Fridericia’s formula (Figure S1). However, results were also analyzed by Fridericia’s formula where all pre-treatment and post-treatment QTcF values were ≤450 msec. There were no significant differences in pre- or post-treatment QTcB or QTcF between 32 and 104 weeks of age.
There were mean 21 msec and 14 msec increases in QTcB interval between ECGs pre-dose and 3–4 h following the third dose at 32 weeks (p<0.001) and 104 weeks of age(p=0.004), respectively. The difference between changes in QTcB at 32 and 104 weeks of age was not statistically significant (p=0.28), and both changes were similar to that observed in adults (17 msec; p=0.67 and p=0.52, respectively). Similar differences were seen using the Fridericia’s correction. There was no correlation between piperaquine Cmax or AUC0–21d and QTcB (Figure 3).
DISCUSSION
DHA-piperaquine is a promising regimen for IPT, but optimal dosing in young children is uncertain. We performed an intensive PK analysis of both components of this regimen in infants at 32 and 104 weeks of age receiving DHA-piperaquine as IPT. Compared to adults, infants had either higher or comparable exposure to DHA, but lower exposure to piperaquine, with the difference more pronounced at 104 compared to 32 weeks of age. Since the protective benefits of IPT are largely attributed to piperaquine exposure, these results suggest that young children, especially those about 2 years of age, may have been underdosed in prior IPT studies, leading to inadequate protection.
Our results for DHA-piperaquine exposure in infancy are comparable to prior reports, although most prior studies were in the context of treatment for uncomplicated malaria rather than IPT in healthy children (21). Specifically, in Ugandan children less than 2 years of age treated for malaria with DHA-piperaquine, exposure to piperaquine was ~33% lower compared to that in children 2–10 years of age (22–25). A pooled analysis reported that children less than 5 years of age were at the highest risk of receiving suboptimal DHA-piperaquine doses and at the greatest risk for reinfection after therapy (26). These results informed new guidelines for treatment, but our study evaluated dosing based on older guidelines (27).
The impact of childhood development on drug metabolism has been documented for many drugs (17, 28, 29). Phase I metabolism, including CYP3A4 and CYP2C8 metabolism of piperaquine, is characterized by structural changes of the drug via oxidation, reduction, or hydrolysis. Phase II metabolism, including UGT metabolism of DHA, involves conjugation with a more water-soluble moiety via glucuronidation, acetylation, or sulfation. For DHA, infants had ~30% greater AUC0–8hr and ~50% greater peak concentration compared to adults, at 32 weeks of age, but by 104 weeks of age AUC0–8hr and peak concentrations were similar to those in adults, suggesting a maturation of metabolism over time. DHA is metabolized by UGT1A9 and UGT2B7 (12), with UGTs present in both the gastrointestinal tract and liver (30, 31); thus, metabolism may impact both bioavailability and elimination. In the neonate, glucuronidation is deficient (32), and these conjugative enzymes mature over the first 6 months of age (16, 18). Our results are consistent with an increase in DHA metabolism as children mature to 104 weeks and UGT function approaches adult levels. Other UGT-metabolized drugs, such as morphine, also metabolized by UGT2B7, have demonstrated a similar pattern (31). Conversion of morphine to its glucuronide metabolites increased ~6 fold from the time of birth to 6 months of age (18). Likewise, two studies investigated lorazepam, a UGT substrate, and found elimination in the newborn is slow compared to adults with a half-life 3–4 fold longer than in adults (33, 34).
In contrast to results for DHA, the AUC0–21d of piperaquine was ~30% and ~50% lower at 32 and 104 weeks of age, compared to levels in adults, respectively, with ~30% lower peak concentrations at both ages. Likewise, terminal concentrations were 35–60% lower at 32 and 104 weeks compared to adult values. Our results suggest that CYP450 metabolism is greater than expected in young children when compared to adults, as shown for other CYP450 substrates (35, 36). CYP450 enzymes mature to adult levels over the first 12 months of life (16). Metabolic maturation over time in infancy has been demonstrated for many CYP3A4 substrates, including alfentanil, sirolimus, and nevirapine(29, 37, 38). However, infants and older children can exhibit higher clearances compared to adults, reducing PK exposure and necessitating higher doses (per kg) of some drugs (39, 40), which may even exceed maximum adult dose recommendations (40). A trend toward increasing weight normalized Dose/AUC for children 32 to 104 weeks of age is consistent with increasing hepatic enzyme maturation.
In addition to alterations in PK, implementing the former treatment nomogram for chemoprevention, based on weight bands, may have contributed to the differences in drug exposure between 32 and 104 weeks. Children at 32 weeks fell toward the middle of their weight band while children at 104 weeks approached the higher end of their weight band, potentially contributing to lower exposure at 104 weeks. Specifically, for children at 32 weeks, 100% received 20/160 mg DHA-piperaquine, while at 104 weeks, 74% received 20/160 mg DHA-piperaquine and 26% received 30/240 mg DHA-piperaquine. For treatment of malaria, the WHO has recently revised its DHA-piperaquine guidelines for young children (41), based largely on prior PK and PD studies (22–25) (26). These modified treatment guidelines, if utilized for chemoprevention, would compensate in part for the reductions observed in this study. However, to fully compensate, metabolic maturation during infancy must also inform optimized chemoprevention dosing guidelines.
The magnitude of piperaquine exposure required for effective IPT is not well defined. In pregnant Ugandan women receiving IPT with DHA-piperaquine, a plasma concentration of 13.9 ng/mL provided 99% protection from parasitemia. This target concentration is best maintained throughout the IPT dosing interval to minimize risk of placental malaria (15). In Thai adults receiving IPT with DHA-piperaquine, trough piperaquine concentrations exceeding 31 ng/mL were required to protect fully against malaria. For children in the present study, piperaquine concentrations on days 14 and 21 were often below the 13.9 ng/mL target established for pregnant women although the target for children may not be the same and is currently under investigation. Terminal concentrations on day 7, 14, and 21 were strongly correlated with AUCs, underscoring the value of these measurements as surrogates for overall exposure.
The lower AUCs seen in children, compared to adults, and suggest that higher mg/kg dosing of DHA-piperaquine for IPT are appropriate in young children, especially because young children will have decreased antimalarial immunity compared to older children and adults. Recently, an exposure-response evaluation of DHA-piperaquine as seasonal malaria chemoprevention in young children in Burkina Faso revealed that higher doses for DHA-piperaquine than currently used, are necessary to adequately protect against seasonal malaria (42), underscoring the importance of our findings. We plan to use sparse longitudinal sampling in a larger cohort of children over the first 2 years of life to address the impact of covariates, including allometric scaling and age-dependent enzyme maturation on piperaquine exposure and outcomes. These models should permit simulations for optimizing DHA-piperaquine chemoprevention regimens for young children.
As we previously reported in pregnant women (14), we did not observe a significant correlation between piperaquine exposure and QT interval, which may be due to the relatively low peak concentrations seen in these children compared to those reported previously in adults (43, 44). Prior studies demonstrated correlations between peak piperaquine levels and QTcF (43, 44), including one study in Cambodian male adults who received a compressed high dose two-day regimen, with peak piperaquine concentrations of ~ 900 ng/mL and a mean increase in QTcF of 46 msec. In our study of children, standard dosing of DHA-piperaquine was associated with a modest, but insignificant, QTcB prolongation of 21 and 14 msec at 32 and 104 weeks of age, respectively, a change similar to the 17 msec change in QTcF we previously reported for pregnant women (14).
Higher doses for children <20 kg are recommended by the WHO, and adverse cardiac events have not been detected (45). If these guidelines are adopted for chemoprevention, we do not expect the safety profile to be impacted, as peak piperaquine concentrations estimated at 32 and 104 weeks were <350 ng/ml, values much lower than previously associated with clinically relevant QTc prolongation (44).
This study had limitations. Data for infants receiving DHA-piperaquine monthly and every 3 months were merged; this was considered acceptable since the majority of piperaquine is eliminated within 30 days. Our sub-analysis, which compared children receiving the two regimens, showed no difference in PK parameters (data not shown). Our adult controls were postpartum women; they might differ from other adults. The study included venous and capillary measures of DHA-piperaquine and a correlation estimate based on simultaneous venous and capillary sampling at 24 hours post-final dose. Our correlation between venous and capillary measures is imperfect since the correlation may be different for terminal concentrations of DHA-piperaquine than for our 24 hours post-dose correlation. Finally, children received crushed tablets and adults, whole tablets. However, dissolution studies have shown that this variation does not alter the dissolution profiles; as piperaquine absorption is slow (Tmax ~ 5 h), the rate of dissolution is not expected to influence absorption (46).
In summary, infants at 32 and 104 weeks of age receiving IPT with DHA- piperaquine exhibited lower piperaquine exposure than adults, with this decrease most pronounced at 104 weeks. These differences are attributed to both developmental maturation of piperaquine metabolism and limitations of current chemoprevention dosing strategies. Studies evaluating higher doses of DHA-piperaquine for IPT are warranted.
METHODS
Study Area and Patients
This study occurred between August, 2015 and May, 2017 in Tororo, Uganda, a region with historically high rates of malaria transmission (47). Eligible participants included a) children born to mothers enrolled in a randomized trial that evaluated DHA-piperaquine as IPT during pregnancy (48) and b) as adult controls, women enrolled in the same study evaluated after delivery, and studied when at least 12 weeks post-partum. All study subjects were HIV uninfected.
The trial was registered at http://ClinicalTrials.gov (). Protocols and procedures were approved by the Uganda National Council of Science and Technology and institutional review boards of Makerere University and the University of California, San Francisco. Written informed consent was obtained from adult study participants, and, for children, their parents or guardians.
Study Design
Infants born to mothers enrolled in the parent trial were separately consented and enrolled prior to or at 32 weeks of age for intensive PK evaluations at both 32 and 104 weeks of age. Mothers had been randomized at time of enrollment in the parent trial to receive IPTp during the second and third trimesters; specifically, SP every 8 weeks, DHA-piperaquine every 8 weeks, or DHA-piperaquine every 4 weeks was initiated at either 16 or 20 weeks gestational age (Figure 1)(48).
Infants born to mothers randomized to SP in the parent study received DHA-piperaquine every 12 weeks; infants born to mothers randomized to either DHA-piperaquine regimen were randomized to DHA-piperaquine every 4 weeks or every 12 weeks. Treatment was initiated in all children at 8 weeks of age. To eliminate a need for unblinding, intensive PK studies were scheduled when all infants (regardless of regimen) received DHA-piperaquine, i.e. at 32 and 104 weeks of age. Adult controls were mothers enrolled in the parent trial who were at least 12 weeks post-partum and consented separately for an intensive PK evaluation around a single treatment of DHA-piperaquine (120/960 mg given daily for 3 consecutive days with or without food, using full strength 40 mg/320 mg tablets, Duo-Cotexin, Holley-Cotec, Beijing, China).
DHA-piperaquine dosing in children consisted of half-strength tablets (20/160 mg DHA-piperaquine) given daily for 3 consecutive days without food. Children ≤5.9 kg received a 1/2 tablet, 6.0–10.9 kg 1 tablet, 11.0–14.9 kg 1 1/2 tablets, 15.0–19.9 kg 2 tablets, 20.0–23.9 kg 2 1/2 tablets, and 24.0–25.9 kg 3 tablets; dosing was based on prior WHO treatment guidelines presently being used for chemoprevention clinical trials. Current treatment guidelines for uncomplicated malaria have been updated (41). All doses for children and adult controls were directly observed in the clinic by study nurses, who administered crushed tablets (in water) to the children or intact tablets to the adults.
For both children and adults, intensive PK sampling occurred before and after the third daily dose of DHA-piperaquine. Venous samples were collected pre-dose, and 0.5, 1, 2, 3, 4, 6, 8, and 24 hours post-dose to determine DHA and piperaquine concentrations. Capillary samples were collected at 24 hours and on days 4, 7, 14, and 21 post-dose to determine piperaquine concentrations. Samples were centrifuged within 60 minutes at 2,000g for 10 minutes, and the plasma supernatant was collected and stored at −80°C prior to drug quantitation. Capillary and venous samples collected concurrently 24 hours post-dose were used to determine correlations between capillary and venous piperaquine concentration results.
High performance liquid chromatography tandem mass spectrometry was utilized, as previously described, to determine the concentrations of DHA and piperaquine (49, 50). For DHA, the calibration range was 0.5–200 ng/ml, the lower limit of quantification (LLOQ) was 0.5 ng/ml, and the coefficient of variation (CV%) was <10% for quality control (QC) concentrations. For piperaquine, the original method was modified to expand the calibration range. The overall calibration range was 0.5–1000 ng/ml; the LLOQ was 0.5 ng/mL and the CV was <10% for QC concentrations. The primary outcomes of the study were plasma PK parameters for DHA and piperaquine, which included the maximal concentration (Cmax), time to Cmax (Tmax), elimination half-life (t1/2), and area-under-the-plasma concentration versus time curve to 8 hours for DHA (AUC0–8 hr) and to 21 days for piperaquine (AUC0–21d). Terminal concentrations were also determined and included the concentration of DHA at 8 hours (C8hr) and piperaquine at days 7, 14 and 21 (C7d, C14d, C21d). Non-compartmental analysis was performed using WinNonlin® 6.4 (Certara L.P., Princeton, NJ, USA) using the linear up-log down trapezoidal rule and first order input. Results that fell below the LLOQ were treated as missing data except for the pre-dose drug concentration before the third dose, which was set at 0 if below the LLOQ.
Linear regression was used to determine the correlation between capillary and venous plasma piperaquine concentration results after natural log transformation of the data using STATA SE 12.1 (StataCorp, College Station, TX, USA). Piperaquine capillary concentration results were converted to predicted venous values using the generated correlation equation. The converted concentrations were used for the estimation of AUC0–21d. C7d, C14d, and C21d piperaquine concentrations were presented as non-adjusted capillary concentrations.
Electrocardiogram (ECG) monitoring
For safety assessments, 12 lead ECGs were performed before the first dose and 3 – 4 hours following the third dose in all study participants. Calipers were used to measure QT and RR intervals, and the corrected QT interval (QTc) was calculated using Bazett’s formula (QTcB, /) and Fridericia’s formula (QTcF, /).
Statistical Analysis
Data analysis was completed using STATA® version SE12.1 (StataCorp, College Station, TX, USA). Power calculations were based on observed mean AUC and standard deviations from our prior studies. At least 30 subjects for each study group were required to detect a difference in mean AUC between groups of 29.5% for piperaquine and 31% for DHA with a significance level (alpha) of 0.05 and 80% power using a two-sided two-sample t-test (CV for AUC DHA=38% and piperaquine=35%). To determine PK parameter values, a Wilcoxon signed-rank test was used for paired analysis in children and rank sum tests were used for unpaired analysis between adults and children and between children in the two age groups. Statistical significance was considered a two-sided adjusted p-value <0.017 for comparison between 3 groups. Geometric means (GM) or medians were reported as appropriate. For ECG analyses, pre-treatment and post-treatment comparisons of QTc intervals were performed using paired t-tests, and comparisons between groups were performed using unpaired t-tests. Correlations between changes in the QTcF interval and piperaquine exposure were assessed using Pearson’s correlation (Rs).
To evaluate whether maternal DHA-piperaquine exposure (via IPTp) and/or or infant sex were associated with piperaquine exposure at 32 or 104 weeks, generalized estimating equations with robust standard errors were used and marginal estimates reported. In these analyses, evidence for effect modification was assessed between maternal IPTp, infant sex, and piperaquine exposure in infancy, and results are reported from stratified analysis where significant interaction (P<0.20) was noted.
Supplementary Material
STUDY HIGHLIGHTS.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
DHA‐piperaquine is being studied for malaria chemoprevention in young children, but the differences between adults and children, and the impact of age on DHA‐piperaquine exposure is not fully understood.
WHAT QUESTION DID THIS STUDY ADDRESS?
This study is first to evaluate the intensive pharmacokinetics of DHA-piperaquine in young children when used as intermittent preventative therapy (IPT). This study also explored the impact of enzyme maturation on DHA-piperaquine exposure.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
By comparing DHA and piperaquine exposure between children and adults, children at 104 weeks of age have significantly reduced DHA exposure compared to those at 32 weeks of age, and that both ages have significantly reduced piperaquine exposure compared to adults.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
Our results suggest that the study of modified dosing strategies of DHA-piperaquine in children, including using the updated weight-based treatment dosing for DHA‐piperaquine when given as IPT, is urgently needed. Further research is also needed to better characterize the impact of enzyme maturation on DHA-piperaquine pharmacokinetics.
ACKNOWLEDGEMENTS
We thank Catherine Tugaineyo, Bridget Nzarubara, and Tamara Clark for their administrative support, Florence Marzan and David Gingrich for generation of drug levels, and the children and mothers who participated in the study.
Funding Information: This work was supported by the National Institutes of Health (4P01HD059454 and R01AI117001), including a grant to the UCSF Center for AIDS Research (P30AI022763).
Footnotes
CONFLICT OF INTEREST: The authors declared no competing interests for this work.
REFERENCES
- (1).World Health Organization. (2018). World Malaria Report 2018 (https://www.who.int/malaria/media/world-malaria-report-2018/en/, 2018).
- (2).Walker PG, ter Kuile FO, Garske T, Menendez C & Ghani AC Estimated risk of placental infection and low birthweight attributable to Plasmodium falciparum malaria in Africa in 2010: a modelling study. Lancet Glob Health 2, e460–7 (2014). [DOI] [PubMed] [Google Scholar]
- (3).Steketee RW, Nahlen BL, Parise ME & Menendez C The burden of malaria in pregnancy in malaria-endemic areas. Am J Trop Med Hyg 64, 28–35 (2001). [DOI] [PubMed] [Google Scholar]
- (4).World Health Organization. Intermittent preventive treatment in pregnancy (IPTp). <https://www.who.int/malaria/areas/preventive_therapies/pregnancy/en/> (2018). Accessed January 31 2019.
- (5).World Health Organization. Seasonal malaria chemoprevention (SMC). <https://www.who.int/malaria/areas/preventive_therapies/children/en/> (2017). Accessed January 31 2019. [Google Scholar]
- (6).Four Artemisinin-Based Combinations Study Group. A head-to-head comparison of four artemisinin-based combinations for treating uncomplicated malaria in African children: a randomized trial. PLoS Med 8, e1001119 (2011). doi: 10.1371/journal.pmed.1001119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).White NJ Intermittent presumptive treatment for malaria. PLoS Med 2, e3 (2005). doi: 10.1371/journal.pmed.0020003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Desai M et al. Intermittent screening and treatment or intermittent preventive treatment with dihydroartemisinin-piperaquine versus intermittent preventive treatment with sulfadoxine-pyrimethamine for the control of malaria during pregnancy in western Kenya: an open-label, three-group, randomised controlled superiority trial. Lancet (London, England) 386, 2507–19 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Kakuru A et al. Dihydroartemisinin-Piperaquine for the Prevention of Malaria in Pregnancy. The New England journal of medicine 374, 928–39 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Bigira V et al. Protective efficacy and safety of three antimalarial regimens for the prevention of malaria in young Ugandan children: a randomized controlled trial. PLoS Med 11, e1001689 (2014). doi: 10.1371/journal.pmed.1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Nankabirwa JI et al. Impact of intermittent preventive treatment with dihydroartemisinin-piperaquine on malaria in Ugandan schoolchildren: a randomized, placebo-controlled trial. Clinical infectious diseases : an official publication of theInfectious Diseases Society of America 58, 1404–12 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Ilett KF et al. Glucuronidation of dihydroartemisinin in vivo and by human liver microsomes and expressed UDP-glucuronosyltransferases. Drug Metab Dispos 30, 1005–12 (2002). [DOI] [PubMed] [Google Scholar]
- (13).Lee TM et al. In vitro metabolism of piperaquine is primarily mediated by CYP3A4. Xenobiotica; the fate of foreign compounds in biological systems 42, 1088–95 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Kajubi R et al. Antiretroviral Therapy With Efavirenz Accentuates Pregnancy-Associated Reduction of Dihydroartemisinin-Piperaquine Exposure During Malaria Chemoprevention. Clinical pharmacology and therapeutics 102, 520–8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Savic RM et al. Intermittent Preventive Treatment for Malaria in Pregnancy: Optimization of Target Concentrations of Dihydroartemisinin-Piperaquine. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 67, 1079–88 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Bartelink IH, Rademaker CM, Schobben AF & van den Anker JN Guidelines on paediatric dosing on the basis of developmental physiology and pharmacokinetic considerations. Clin Pharmacokinet 45, 1077–97 (2006). [DOI] [PubMed] [Google Scholar]
- (17).Wagner J & Abdel-Rahman SM Pediatric pharmacokinetics. Pediatrics in review 34, 258–69 (2013). [DOI] [PubMed] [Google Scholar]
- (18).Bouwmeester NJ, Anderson BJ, Tibboel D & Holford NH Developmental pharmacokinetics of morphine and its metabolites in neonates, infants and young children. Br J Anaesth 92, 208–17 (2004). [DOI] [PubMed] [Google Scholar]
- (19).de Wildt SN, Kearns GL, Leeder JS & van den Anker JN Cytochrome P450 3A - Ontogeny and drug disposition. Clinical Pharmacokinetics 37, 485–505 (1999). [DOI] [PubMed] [Google Scholar]
- (20).Ince I et al. A Novel Maturation Function for Clearance of the Cytochrome P450 3A Substrate Midazolam from Preterm Neonates to Adults. Clinical Pharmacokinetics 52, 555–65 (2013). [DOI] [PubMed] [Google Scholar]
- (21).Pukrittayakamee S et al. A study of the factors affecting the metabolic clearance of quinine in malaria. Eur J Clin Pharmacol 52, 487–93 (1997). [DOI] [PubMed] [Google Scholar]
- (22).Creek DJ et al. Pharmacokinetic predictors for recurrent malaria after dihydroartemisinin-piperaquine treatment of uncomplicated malaria in ugandan infants. The Journal of infectious diseases 207, 1646–54 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Sambol NC et al. Population Pharmacokinetics of Piperaquine in Young Ugandan Children Treated With Dihydroartemisinin-Piperaquine for Uncomplicated Malaria. Clinical pharmacology and therapeutics 98, 87–95 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Zongo I et al. Efficacy and day 7 plasma piperaquine concentrations in African children treated for uncomplicated malaria with dihydroartemisinin-piperaquine. PLoS One 9, e103200 (2014). 10.1371/journal.pone.0103200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Tarning J et al. Population pharmacokinetics and pharmacodynamics of piperaquine in children with uncomplicated falciparum malaria. Clinical pharmacology and therapeutics 91, 497–505 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Sundell K et al. Variable piperaquine exposure significantly impacts protective efficacy of monthly dihydroartemisinin-piperaquine for the prevention of malaria in Ugandan children. Malaria journal 14, 368 (2015). doi: 10.1186/s12936-015-0908-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).World Health Organization. (2013). Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: Recommendations for a public health approach (Geneva, Switzerland: http://www.who.int/hiv/pub/guidelines/arv2013/en/index.html, 2013). [PubMed] [Google Scholar]
- (28).Anderson BJ & Holford NH Understanding dosing: children are small adults, neonates are immature children. Arch Dis Child 98, 737–44 (2013). [DOI] [PubMed] [Google Scholar]
- (29).Ginsberg G et al. Evaluation of child/adult pharmacokinetic differences from a database derived from the therapeutic drug literature. Toxicol Sci 66, 185–200 (2002). [DOI] [PubMed] [Google Scholar]
- (30).Yang G et al. Glucuronidation: driving factors and their impact on glucuronide disposition. Drug Metab Rev 49, 105–38 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Ohno S & Nakajin S Determination of mRNA expression of human UDP-glucuronosyltransferases and application for localization in various human tissues by real-time reverse transcriptase-polymerase chain reaction. Drug Metab Dispos 37, 32–40 (2009). [DOI] [PubMed] [Google Scholar]
- (32).Alcorn J & McNamara PJ Ontogeny of hepatic and renal systemic clearance pathways in infants: part I. Clin Pharmacokinet 41, 959–98 (2002). [DOI] [PubMed] [Google Scholar]
- (33).Cummings AJ & Whitelaw AG A study of conjugation and drug elimination in the human neonate. Br J Clin Pharmacol 12, 511–5 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).McDermott CA, Kowalczyk AL, Schnitzler ER, Mangurten HH, Rodvold KA & Metrick S Pharmacokinetics of lorazepam in critically ill neonates with seizures. J Pediatr 120, 479–83 (1992). [DOI] [PubMed] [Google Scholar]
- (35).Yang J et al. Population Pharmacokinetics of Lopinavir/Ritonavir: Changes Across Formulations and Human Development From Infancy Through Adulthood. J Clin Pharmacol 58, 1604–17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Blanco JG, Harrison PL, Evans WE & Relling MV Human cytochrome P450 maximal activities in pediatric versus adult liver. Drug Metab Dispos 28, 379–82 (2000). [PubMed] [Google Scholar]
- (37).Lau E et al. Nevirapine Pharmacokinetics and Safety in Neonates Receiving Combination Antiretroviral Therapy for Prevention of Vertical HIV Transmission. J Acquir Immune Defic Syndr 74, 493–8 (2017). [DOI] [PubMed] [Google Scholar]
- (38).Mizuno T et al. Developmental pharmacokinetics of sirolimus: Implications for precision dosing in neonates and infants with complicated vascular anomalies. Pediatr Blood Cancer 64, (2017). [DOI] [PubMed] [Google Scholar]
- (39).Cooney GF, Habucky K & Hoppu K Cyclosporin pharmacokinetics in paediatric transplant recipients. Clin Pharmacokinet 32, 481–95 (1997). [DOI] [PubMed] [Google Scholar]
- (40).Floren LC et al. Nelfinavir pharmacokinetics in stable human immunodeficiency virus-positive children: Pediatric AIDS Clinical Trials Group Protocol 377. Pediatrics 112, e220–7 (2003). [DOI] [PubMed] [Google Scholar]
- (41).World Health Organization. (2015). Guidelines for the Treatment of Malaria - Third Edition (Geneva, Switzerland, 2015). [Google Scholar]
- (42).Chotsiri P et al. Optimal dosing of dihydroartemisinin-piperaquine for seasonal malaria chemoprevention in young children. Nat Commun 10, 480 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Funck-Brentano C et al. Effects of Dihydroartemisinin-Piperaquine Phosphate and Artemether-Lumefantrine on QTc Interval Prolongation. Sci Rep 9, 777 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Manning J et al. Randomized, double-blind, placebo-controlled clinical trial of a two-day regimen of dihydroartemisinin-piperaquine for malaria prevention halted for concern over prolonged corrected QT interval. Antimicrob Agents Chemother 58, 6056–67 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Chan XHS, Win YN, Mawer LJ, Tan JY, Brugada J & White NJ Risk of sudden unexplained death after use of dihydroartemisinin-piperaquine for malaria: a systematic review and Bayesian meta-analysis. Lancet Infect Dis 18, 913–23 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).European Medicines Agency. (2011). Human medicine European public assessment report (EPAR): Eurartesim. (Report no. 739355/2011) 1–129 (2011).
- (47).Kamya MR et al. Malaria transmission, infection, and disease at three sites with varied transmission intensity in Uganda: implications for malaria control. Am J Trop Med Hyg 92, 903–12 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Jagannathan P et al. Dihydroartemisinin-piperaquine for intermittent preventive treatment of malaria during pregnancy and risk of malaria in early childhood: A randomized controlled trial. PLoS Med 15, e1002606 (2018). doi: 10.1371/journal.pmed.1002606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Huang L, Olson A, Gingrich D & Aweeka FT Determination of artemether and dihydroartemisinin in human plasma with a new hydrogen peroxide stabilization method. Bioanalysis 5, 1501–6 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Kjellin LL, Dorsey G, Rosenthal PJ, Aweeka F & Huang L Determination of the antimalarial drug piperaquine in small volume pediatric plasma samples by LC-MS/MS. Bioanalysis 6, 3081–9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.