Abstract
Genetic variation may differentially modify drug and placebo treatment effects in randomized trials (RCTs). In asthma, although lung function and asthma control improvements are commonplace with placebo, pharmacogenomics of placebo versus drug response remains unexamined. In a GWAS of subjective and objective outcomes with placebo treatment in Childhood Asthma Management Program (CAMP) of nedocromil/budesonide versus placebo (N=604), effect estimates for lead SNPs were compared across arms. The coughing/wheezing lead SNP, rs2392165 (β=0.94; P=1.10E-07) mapped to BBS9, a gene implicated in lung development which contains a lung function expression quantitative trait locus (eQTL). The effect was attenuated with budesonide (Pinteraction=1.48E-07), but not nedocromil (Pinteraction=0.06). The lead FVC SNP, rs12930749 (β=−5.80; P=1.47E-06), mapped to KIAA0556, a locus genome-wide associated with respiratory diseases. The rs12930749 effect was attenuated with budesonide (Pinteraction=1.32E-02) and nedocromil (Pinteraction=1.09E-02). Pharmacogenomic analysis revealed differential effects with placebo and drug treatment that could potentially guide precision drug development in asthma.
Introduction
Drug efficacy in randomized clinical trials (RCT) is determined by subtracting treatment response in the placebo arm from response in the study arm. This simple calculation is based on the assumption that responses in the drug and placebo treatment arms are additive (1). However, a growing number of studies have identified genetic variants that differentially modify outcomes in both the drug and placebo treatment arms of RCTs (2–7). While these findings challenge the additivity assumption (8), they can also guide development of precision therapies and lead to identification of genetic subpopulations that may benefit from active or placebo treatments.
In RCTs, the term placebo was originally used to describe the inert tablet or intervention employed as a control (9). However, more recent definitions of placebo have broadened the term to include everything but the active treatment, i.e., the physical characteristics of the treatment (pill color, inhaler), attitude (warm, caring, confident) and physical appearance (white coat, stethoscope) of the clinician, and the information to which the patient is exposed regarding the study drug (potential side- and adverse effects). Although lacking in pharmacological activity, placebo treatments can improve outcomes in clinical trials for a wide range of conditions and diseases (10–12). This variability in response to placebo treatment is attributed to regression to the mean, the natural history of the disease, variations in treatment adherence, and non-specific effects of placebo treatment or placebo effects that are heavily influenced by expectations of the patient. Response to placebo treatments can be positive as well as negative, termed nocebo effects (13). Nocebo effects are commonly observed with the occurrence of side- and adverse effects that are induced in the placebo arm by negative patient expectations (14). Placebo research has been prevalent in conditions like pain, depression, and irritable bowel syndrome where determination of treatment response relies on subjective measures that are more susceptible to non-specific placebo effects. Building on strong neuroimaging evidence that response to placebo treatment induces signaling in dopaminergic and opioid pathways (15), recent studies are finding evidence that the placebo response may be also influenced by genomic variations in placebo response pathway proteins, the placebome (16).
Asthma is a chronic inflammatory disease of the airways, which in the U.S. affects over 25 million people of which 8.4% are children (17). Studies designed to examine expectancy and placebo effects in asthma indicate that placebo treatment has a clear influence on subjective study outcomes such as asthma control, which includes coughing and wheezing (18, 19). Although placebo effects among patients with asthma are stronger in patient-reported outcomes, there remains considerable heterogeneity in both objective and subjective outcomes in placebo treatment arms and many asthma trials are unsuccessful because drugs with a compelling mechanism of action fail to demonstrate greater efficacy than placebo (20, 21). Hence, asthma trials provide the unique opportunity to compare pharmacogenomic effects, in objective and subjective outcomes, across placebo and drug treatments.
The Childhood Asthma Management Program (CAMP) was a randomized double-blinded, placebo-controlled study of nedocromil or budesonide compared to placebo for asthma over four years in children between the ages of 5 and 12 (22). To examine placebo pharmacogenomics in an asthma RCT, we conducted a genome-wide association study (GWAS) of response to placebo treatment in CAMP. We hypothesized that, as in previously reported studies (3, 5, 6, 23, 24), association between lead SNPs in the placebo arm would be attenuated in the drug treatment arms of CAMP. Our primary subjective outcome was a patient-reported coughing and wheezing measure, and our primary objective outcome was change in forced vital capacity (FVC), a measure of lung function for which there was no difference between drug and placebo in the original CAMP trial. Single nucleotide polymorphisms (SNPs) with the most significant associations in the placebo arm were examined in the budesonide and nedocromil drug treatment arms.
Results
With the exception of sex, demographic, and baseline characteristics of the 604 white participants from the Childhood Asthma Management Program (CAMP) randomized to the three treatment arms – placebo (N=250), budesonide (N=175), or nedocromil (N=179) – did not differ by treatment arm (Table 1). Although there were differences in the proportion of females across the treatment arms, (43%), budesonide (43%) and nedocromil (32%), adjusting for sex and age did not modify the results.
Table 1.
Demographics and baseline characteristics of white CAMP participants (N=604) in the three treatment arms.
| Characteristics | Placebo (N=250) |
Budesonide (N=175) |
Nedocromil (N=179) |
|
|---|---|---|---|---|
| Age, years (SD) | 8.8 (2.2) | 8.8 (2.1) | 9.0 (2.2) | |
| Height, cm (SD) | 132.8 (14.1) | 133.1 (13.5) | 134.1 (13.9) | |
| Female, N (%) | 107 (42.8) | 76 (43.4) | 57 (31.8) | |
| Asthma Severity N (%) | Mild | 127 (50.8) | 78 (44.6) | 87 (48.6) |
| Moderate | 123 (49.2) | 97 (55.4) | 92 (51.4) | |
| Coughing/wheezing at baseline (SD) | 4.9 (1.1) | 5.0 (1.1) | 4.9 (1.1) | |
| FVC before bronchodilator use (% of predicted) | 105.3 (12.7) | 105.4 (13.7) | 103.1 (12.9) | |
| FVC after bronchodilator use (% of predicted) | 107.1 (12.1) | 108.2 (13.6) | 105.6 (12.3) | |
GWAS of subjective outcome (cough/wheeze)
Genome-wide suggestive (P<1.0E-05) SNPs for the subjective outcome are represented in the Manhattan plot in Figure 1. In the quantile-quantile (Q-Q) plot, no inflation of data was observed based on a lambda of 1.00 (Figure S1). None of the SNP associations in the coughing/wheezing subjective outcome reached genome-wide significance (P<5.0E-08). The lead SNP, rs2392165 (β=0.94; P=1.10E-07), mapped to BBS9, a gene whose expression is associated with FVC and is implicated in the development of the lungs and ciliogenesis. Cilia are hair-like micro-organelles that direct mucus and debris cephalad in the respiratory tract; they are often impaired in asthma and other respiratory diseases (25), and are sensitive to exposure to ciliomodulatory drugs like budesonide (26–29). Variants in BBS9 were previously identified as lung cis-expression quantitative trait loci (eQTL) in three independent cohorts (30). Based on clumping, the other genome-wide suggestive loci were reduced to representative SNPs and mapped to proximal genes (Table S1).
Figure 1.
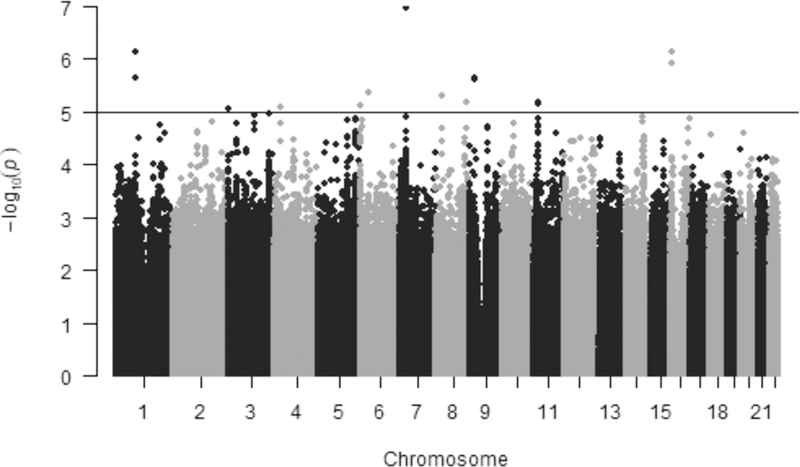
Manhattan plot for GWAS of coughing/wheezing by self-report in the placebo treatment arm of CAMP. Results are plotted as minus log-transformed P-values from coughing/wheezing model controlled for age, sex and clinic site. Even numbered chromosomes are in grey and odd numbered chromosomes in black. The black line delineates SNPs that exceed the genome-wide significance threshold of P<1.0E-05.
There was no difference in coughing/wheezing by rs2392165 genotype at baseline (Figure 2). However, compared to the A allele, in the placebo arm, the minor (G) allele was associated with less improvement in coughing/wheezing at the end of the trial (Figure 2). In the budesonide arm, this effect was reversed (Table S1), such that the G allele was associated with greater improvement in coughing/wheezing (β=−0.53; P=0.02). This difference was evident in a statistically significant SNP-by-budesonide treatment interaction (Pinteraction= 1.48E-07). Furthermore, comparison of coughing/wheezing between the placebo and budesonide treatment arms among G-allele homozygotes (P=2.61E-04) or G-allele carriers (P= 1.68E-06) indicated that G/G and G/A participants benefited from budesonide treatment compared to placebo. Although the effect was also attenuated in the nedocromil arm, the direction of the effect was similar to that observed with placebo (β=0.47; P=0.04) but the SNP-by-nedocromil treatment interaction (Pinteraction=0.06) was not significant.
Figure 2.
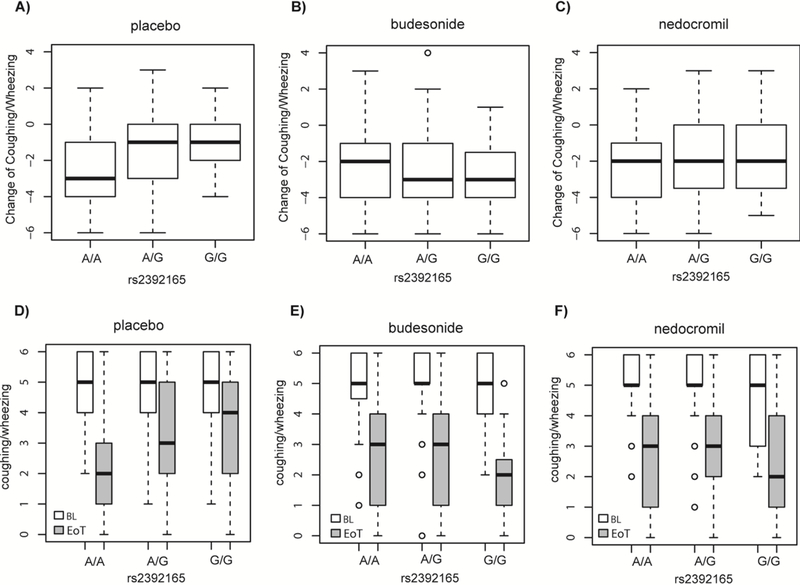
Change in coughing/wheezing frequency stratified by rs2392165 (BBS9) genotype in the A) placebo; B) budesonide; and C) nedocromil treatment arms. Baseline (BL) and end of treatment (EoT) coughing/wheezing stratified by rs2392165 genotype in the D) placebo; E) budesonide; and F) nedocromil treatment arms. Box plots denote the median (dark line) and interquartile ranges, circles represent outliers.
GWAS of objective outcome (FVC)
In the Q-Q plot of the placebo arm GWAS of FVC, no inflation of data was observed based on a lambda of 1.01 (Figure S2). The two lead genome-wide suggestive SNPs, rs12930749 and rs12933955 were in high LD and had the same β estimate and P-value (β=−5.80; P=1.50E-07). Both SNPs mapped to an intronic region in katanin-interacting protein, KIAA0556. KIAA0556 encodes an evolutionarily conserved ciliary protein that regulates the stability of microtubules and is associated with Joubert’s Syndrome a rare autosomal recessive genetic disorder that affects balance and coordination via effects on the cerebellum (31). In total, 95 other SNPs in LD (r2>0.5) with the lead SNPs mapped to the KIAA0556 region on chromosome 16 (Figure S3 and Table S2). This region is downstream of two interleukin receptors, IL-4R and IL-21R, which influence allergic response and immune dysfunction (32–34). In GWAS of the UK Biobank, KIAA0556 was found to be associated with 38 reported traits including asthma, respiratory diseases, eczema and eosinophil counts (Table S3 and Figure S4). The lead SNP is also an eQTL for the IL21R (Table S4). Based on clumping, the other genome-wide suggestive loci were reduced to representative SNPs (Table S2) and mapped to proximal genes, several of which have plausible links to asthma and lung function.
In the placebo treatment arm, improvement in FVC decreased with increasing numbers of rs12930749 minor G-alleles (β=−5.80; P=1.47E-06). In comparison to the placebo arm, the magnitude of the rs12930749 effect was modestly attenuated with budesonide (β=0.01; P=0.46; Pinteraction=1.32E-02) and nedocromil (β=−0.23; P=0.89; Pinteraction=1.09E-02) randomized treatment (Figure 4 and Table S2).
Figure 4.
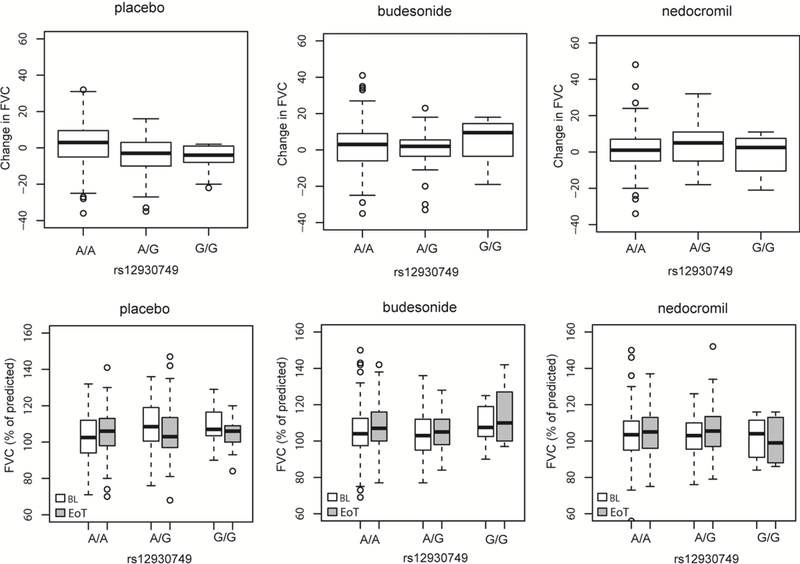
Change in FVC (% of predicted) stratified by rs12930749 (KIAA0556) genotype in the three CAMP treatment arms A) placebo; B) budesonide; and C) nedocromil treatment arms. Baseline (BL) and end of treatment (EoT) FVC stratified by rs12930749 KIAA0556 genotype in the D) placebo; E) budesonide; and F) nedocromil treatment arms. The mean is denoted by the dark black lines bisecting the boxes which represent the standard deviations; the range is indicated by the dashed lines with outliers as the circles. Box plots denote the median (dark line) and interquartile ranges, circles represent outliers.
Discussion
Here we report genome-wide suggestive associations with clinical improvement in subjective (coughing/wheezing) and objective (FVC) outcomes in the placebo arm of the CAMP RCT of asthma. For the subjective outcome, the lead SNP rs2392165 mapped to BBS9, which was implicated in lung development and is a lung eQTL. For the objective outcome, FVC, the lead SNP, rs12930749, mapped to KIAA0556, a gene associated with asthma and several traits related to respiratory function in the UK Biobank. Both BBS9 and KIAA0556 are involved in ciliary function and development. Ciliary function is important for respiratory function and is perturbed in asthma. Importantly, budesonide has in vitro and in vivo ciliary modulatory activity (26–29). When we examined the association of the lead placebo arm SNPs with both objective and subjective outcomes in the study arms, we found statistically significant SNP-by-treatment interaction effects such that the effect estimates were attenuated in the budesonide and nedocromil drug treatment arms. The pharmacogenetic interaction effects reported here are novel and build on recent studies that challenge an important assumption in RCT methodology, viz., that placebo and drug treatment effects are simply additive (8).
Improvements in clinical outcomes in the placebo arm can result from a complex interplay of regression to the mean, natural history of the disease, and placebo effects guided by patient expectations. In the active treatment arm, double-blinding allows us to examine the drug effect in the context of all these other factors present in the placebo arm. Although genetic variation can further add to this complexity, pharmacogenomic effects are seldom compared in the placebo and drug treatment arms of RCTs (35). The genetic association signals identified here for coughing/wheezing and for FVC have links to lung and ciliary function through plausible biological processes that might influence the subset of patients whose outcomes varied with randomized allocation to placebo in CAMP. However, without a no-treatment control -- a limitation of this study -- we cannot determine definitively whether natural history or placebo effects contributed to the outcomes observed in the placebo GWAS. Yet, the attenuation of these effects in the drug treatment arms is striking and suggests that the possibility that processes active in the placebo arm were different in the drug treatment arms. In particular, the genetic subset of patients in this study who benefited from budesonide, a drug with ciliomodulatory effects, did not experience any changes with randomized placebo, suggesting this group may be targeted for a precision validation trial. Given the potential pharmacogenomic effects reported here, replication, to determine if these findings are biologically relevant or attributed to chance, will need to account for the types of asthma treatments used by patients that might induce pharmacogenomic interactions (with drug or placebo) in RCTs as well as observational studies.
Subjective outcomes in asthma are known to be more prone to placebo effects (36). Furthermore, subjective outcomes in children are often difficult to ascertain, as parents and care-givers usually report on the child’s qualitative symptoms, a phenomenon termed placebo-by-proxy (37). Although we observed no genome-wide significant associations with outcomes in the placebo arm, since CAMP was a double-blinded study, one might expect placebo effects, if any existed, to be similar across all treatment arms. The statistically significant differences (Pinteractions) between the control and active treatment arms reported here are, therefore, hypothesis-generating. Many early studies of the role of expectation in shaping subjective responses suggest that active treatments can elicit taste or smell cues that can modify patient’s expectations and create a bias towards better outcomes in the drug treatment arm that are unrelated to the pharmacological effects of the drug and nocebo effects in the placebo arm. This pharmacological clinical activity bias has also been attributed to disease-drug interactions (38). One hypothesis as to why coughing/wheezing increased in the placebo arm with increasing numbers of rs2392165 G-alleles (a potential nocebo effect), but was decreased among the same groups allocated to budesonide, could be that absence of expected side-effects led to a belief on the part of the parent or child that they were not enrolled in the active treatment arms, resulting in worse outcomes. The CAMP study was not, however, designed to examine subjective effects on asthma. This design feature, along with the small size of this study, and lack of replication are important limitations of this study. Hence, replication of these findings in a study that explicitly examines subjective outcomes, such as the asthma trial by Wise and colleagues (18), is warranted. Finally, given the complexity of subjective and objective outcomes in asthma trials, it is not possible to attribute the effects reported here to a few genes, as they are much more likely the result of multiple genetic loci.
In summary, these findings suggest the potential for genetic loci to influence differentially subjective and objective outcomes in both the placebo and drug treatment arms of an asthma RCT. With further validation using retrospective studies, these findings may guide the identification of subpopulations that could be targeted for personalized asthma treatment and tested in prospective precision asthma RCTs paving the way for a broader systems pharmacogenomic approach to the design and interpretation of RCTs.
Methods
Study Population
The Childhood Asthma Management Program (CAMP), a randomized double-blinded, placebo-controlled study of nedocromil, (a mast cell stabilizer), and budesonide, (an inhaled glucocorticoid) used in the management of asthma, compared to matched placebo-nedocromil or placebo-budesonide over four years in children between the ages of 5 and 12 with mild-to-moderate asthma (22). The children were randomly assigned to receive 200 μg of budesonide, 8 mg of nedocromil, or placebo twice daily and treated for four to six years. All children used albuterol for asthma symptoms as needed. Of the 1,041 children enrolled in CAMP, 968 children contributed DNA samples. To minimize heterogeneity in population structure this analysis was performed among the white subjects, the largest homogenous group in CAMP. Hence, we included 604 genotyped children in our analysis based on available genotype data. The CAMP trial was approved by the Institutional Review Boards of Brigham and Women’s Hospital and the other participating centers.
Genotyping
CAMP genotyping procedures have been previously described (39). Briefly, CAMP participant samples were genotyped on the Human Hap550v3 or Infinium HD Human610-Quad BeadChip (Illumina, San Diego, Calif). Stringent quality-control was conducted for the genome-wide SNP genotypic data using PLINK (version 1.9) (40, 41). SNPs were mapped to the hg18 reference genome. As quality control steps for conducting the GWAS, we used PLINK to extract SNPs with minor allele frequency (MAF) >0.05 and with a Hardy-Weinberg equilibrium (HWE) P>1E-08. We also checked gender and ‘missingness’ across SNPs and individuals and found no instances of missingness larger than 10%. The HRC r1.1 reference panel was used for imputation of non-genotyped and missing SNPs; the genome coordinates build was GRCh37 (42).
Outcome measures
The composite measure of asthma control (change in coughing and wheezing by self-report) was a continuous variable that assessed the difference between baseline and the end of the study at 48 months. Although respiratory symptoms are commonly used to assess the impact of treatment interventions, at the time that this randomized trial was conducted, validated measures of asthma control were not available and minimal clinically important differences were not established for the coughing/wheezing instrument used in the current study (43). Missing values at the end of the trial were imputed by the last observation carried forward. Negative beta estimates represent an improvement in symptoms associated with the minor allele. Change in forced vital capacity (percentage of predicted FVC before bronchodilator use) was a priori selected as the primary objective outcome because in the original CAMP trial, there was no difference in change in FVC between the placebo and budesonide arms suggesting these arms would have similar gene-outcome association profiles (22). FVC was determined by spirometry, after administration of a short-acting bronchodilator (beta-2 agonist), to be the volume of air exhaled in 6 seconds or more after maximal inhalation. Positive beta estimates represent an improvement in lung function associated with the minor allele.
Statistical Analysis
Both our subjective and objective outcomes were quantitative traits, hence we performed multiple linear regression analyses using additive minor allele-encoded gene dosage models as implemented in PLINK. We controlled for age, sex, and clinical site. SNPs were considered to be genome-wide suggestive or significant if they were associated at thresholds of P<1.0E-05 or P<5.0E-08, respectively. Our goal was to determine first if there were loci associated with outcomes in the placebo treatment arm. We then examined the association of all SNPs with P<1.0E-05, after excluding SNPs in high linkage disequilibrium (LD) by clumping, with the outcomes in the two study treatment arms. SNPsnap was used to clump groups of SNPs every 250mB and r2 of 0.5 (44). A plot of -(log10 transformed P-values) on the chromosome 16 region near the most significant FVC SNP was created using LocusZoom (45). The Open Target Platform was used to mine GWAS data on the lead SNPs in the UK Biobank (46). To test differences between placebo and drug treatment outcome effect estimates, we included SNP-by-treatment interaction terms in the models.
Supplementary Material
Figure 3.
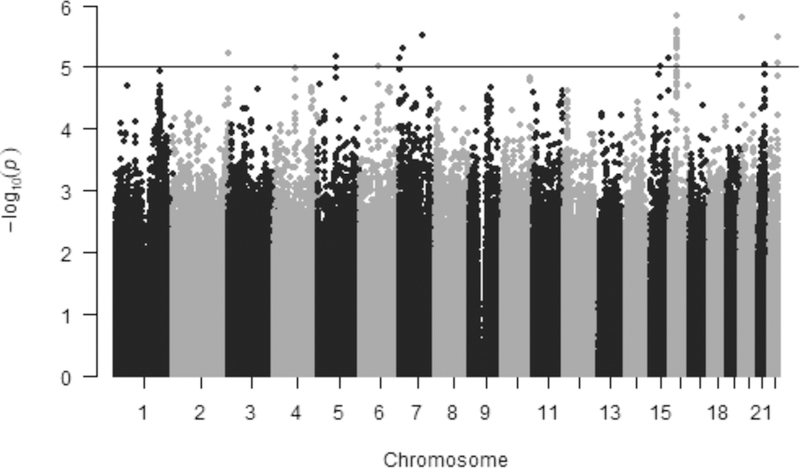
Manhattan plot for GWAS of FVC in the placebo treatment arm of CAMP. Results are plotted as minus log-transformed P-values from the genotype association FVC model controlled for age, sex and clinic site. Even numbered chromosomes are in grey and odd numbered chromosomes in black. The black line delineates SNPs that exceed the genome-wide significance threshold of 1.0E-05.
Study Highlights.
o. What is the current knowledge on the topic?
There is some evidence that genetic variation can influence outcomes in the placebo control arm of randomized clinical trials. Interestingly, the genetic associations observed in the placebo arm have been found to be attenuated in the active study treatment arm, suggesting that gene-drug/placebo effect modification can account for null findings in some studies.
o. What question did this study address?
This study examines gene-drug/placebo interaction effects in subjective and objective outcomes in a large, randomized, placebo-controlled trial of asthma among adolescents, CAMP.
o. What does this study add to our knowledge?
This study supports the previous data that challenge the simple hypothesis that placebo and drug effects are additive and the difference between them is equivalent to the efficacy of the drug being evaluated in a clinical trial.
o. How might this change clinical pharmacology or translational science?
These findings suggest that it may be possible to use pharmacogenetic analysis of placebo and active treatment arms to identify subpopulations that benefit from study drug. Hitherto, these differential effects were masked by differential pharmacogenomic effects in active and control arms that, in some studies, cancelled each other out, leaving trialists to conclude a trial was null, when, in fact, it was not.
Acknowledgments
Funding: KTH is supported by a grant from the National Heart, Lung, and Blood Institute (NHLBI) of the U.S. National Institutes of Health (NIH) (K01HL130625). J.L was supported by grants from the NIH (HG007690, HL119145, and GM107618) and the American Heart Association (D700382). D.C.C.-C. was funded by a grant from the NHLBI (K01 HL127265).
In the past three years, Edwin K. Silverman has received grant and travel support from GlaxoSmithKline.
Footnotes
Conflict of Interest: All other authors declared no competing interests for this work.
References
- (1).Coleshill MJ, Sharpe L, Colloca L, Zachariae R & Colagiuri B. Placebo and active treatment additivity in placebo analgesia: Research to date and future directions. Int Rev Neurobiol 139, 407–41 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Hall KT et al. Comt and alpha-tocopherol effects in cancer prevention: Gene-supplement interactions in two randomized clinical trials. J Natl Cancer Inst, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Hall KT et al. Genetic variation at the coronary artery disease risk locus gucy1a3 modifies cardiovascular disease prevention effects of aspirin. Eur Heart J, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Hall KT, Loscalzo J & Kaptchuk TJ Systems pharmacogenomics - gene, disease, drug and placebo interactions: A case study in comt. Pharmacogenomics 20, 529–51 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Hall KT et al. Polymorphisms in catechol-o-methyltransferase modify treatment effects of aspirin on risk of cardiovascular disease. Arterioscler Thromb Vasc Biol 34, 2160–7 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Chan JM et al. Selenium- or vitamin e-related gene variants, interaction with supplementation, and risk of high-grade prostate cancer in select. Cancer Epidemiol Biomarkers Prev 25, 1050–8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Tardif JC et al. Pharmacogenomic determinants of the cardiovascular effects of dalcetrapib. Circ Cardiovasc Genet 8, 372–82 (2015). [DOI] [PubMed] [Google Scholar]
- (8).Hall KT & Loscalzo J. Drug-placebo additivity in randomized clinical trials. Clinical Pharmacology & Therapeutics in press, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Kaptchuk TJ & Miller FG Placebo effects in medicine. N Engl J Med 373, 8–9 (2015). [DOI] [PubMed] [Google Scholar]
- (10).Patel SM et al. The placebo effect in irritable bowel syndrome trials: A meta-analysis. Neurogastroenterol Motil 17, 332–40 (2005). [DOI] [PubMed] [Google Scholar]
- (11).Finniss DG, Kaptchuk TJ, Miller F & Benedetti F. Biological, clinical, and ethical advances of placebo effects. Lancet 375, 686–95 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Brunoni AR, Lopes M, Kaptchuk TJ & Fregni F. Placebo response of non-pharmacological and pharmacological trials in major depression: A systematic review and meta-analysis. PLoS One 4, e4824 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Howick J, Webster R, Kirby N & Hood K. Rapid overview of systematic reviews of nocebo effects reported by patients taking placebos in clinical trials. Trials 19, 674 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Colloca L. Nocebo effects can make you feel pain. Science 358, 44 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Wager TD & Atlas LY The neuroscience of placebo effects: Connecting context, learning and health. Nat Rev Neurosci 16, 403–18 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Wang RS, Hall KT, Giulianini F, Passow D, Kaptchuk TJ & Loscalzo J. Network analysis of the genomic basis of the placebo effect. JCI Insight 2, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Prevention, C.f.D.C.a. Most recent national asthma data. <https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm> (2017).
- (18).Wise RA et al. Randomized trial of the effect of drug presentation on asthma outcomes: The american lung association asthma clinical research centers. J Allergy Clin Immunol 124, 436–44, 44e1–8 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Wechsler ME et al. Active albuterol or placebo, sham acupuncture, or no intervention in asthma. The New England journal of medicine 365, 119–26 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Limited V. Clinical efficacy, safety and tolerability of vr475 1 mg/2 ml twice daily given for 52 weeks compared to placebo. <https://www.clinicaltrialsregister.eu/ctr-search/trial/2015-000353-20/results> (2018). Accessed August 8, 2019 2019.
- (21).Busse WW et al. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-il-17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respir Crit Care Med 188, 1294–302 (2013). [DOI] [PubMed] [Google Scholar]
- (22).Childhood Asthma Management Program Research, G. et al. Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med 343, 1054–63 (2000). [DOI] [PubMed] [Google Scholar]
- (23).Hall KT et al. Genetic variation in catechol-o-methyltransferase modifies effects of clonidine treatment in chronic fatigue syndrome. Pharmacogenomics J 16, 454–60 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Chasman DI et al. Polymorphism in the apolipoprotein(a) gene, plasma lipoprotein(a), cardiovascular disease, and low-dose aspirin therapy. Atherosclerosis 203, 371–6 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Sharma S, Chhabra D, Kho AT, Hayden LP, Tantisira KG & Weiss ST The genomic origins of asthma. Thorax 69, 481–7 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Jiao J, Meng N & Zhang L. The effect of topical corticosteroids, topical antihistamines, and preservatives on human ciliary beat frequency. ORL J Otorhinolaryngol Relat Spec 76, 127–36 (2014). [DOI] [PubMed] [Google Scholar]
- (27).Pappova L, Joskova M, Kazimierova I, Sutovska M & Franova S. Combination therapy with budesonide and salmeterol in experimental allergic inflammation. Adv Exp Med Biol 935, 25–34 (2016). [DOI] [PubMed] [Google Scholar]
- (28).Laitinen LA, Laitinen A & Haahtela T. A comparative study of the effects of an inhaled corticosteroid, budesonide, and a beta 2-agonist, terbutaline, on airway inflammation in newly diagnosed asthma: A randomized, double-blind, parallel-group controlled trial. J Allergy Clin Immunol 90, 32–42 (1992). [DOI] [PubMed] [Google Scholar]
- (29).Khan NA et al. Identification of drugs that restore primary cilium expression in cancer cells. Oncotarget 7, 9975–92 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Hao K. et al. Lung eqtls to help reveal the molecular underpinnings of asthma. PLoS Genet 8, e1003029 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Sanders AA et al. Kiaa0556 is a novel ciliary basal body component mutated in joubert syndrome. Genome Biol 16, 293 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Maes T, Joos GF & Brusselle GG Targeting interleukin-4 in asthma: Lost in translation? Am J Respir Cell Mol Biol 47, 261–70 (2012). [DOI] [PubMed] [Google Scholar]
- (33).Foster PS & Mattes J. Il-21 comes of age. Immunol Cell Biol 87, 359–60 (2009). [DOI] [PubMed] [Google Scholar]
- (34).Halwani R, Sultana A, Vazquez-Tello A, Jamhawi A, Al-Masri AA & Al-Muhsen S. Th-17 regulatory cytokines il-21, il-23, and il-6 enhance neutrophil production of il-17 cytokines during asthma. J Asthma 54, 893–904 (2017). [DOI] [PubMed] [Google Scholar]
- (35).Hall KT, Loscalzo J & Kaptchuk T. Systems pharmacogenomics: Gene, disease, drug and placebo interactions -- a case study in comt. Pharmacogenomics in press, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Dutile S, Kaptchuk TJ & Wechsler ME The placebo effect in asthma. Curr Allergy Asthma Rep 14, 456 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Grelotti DJ & Kaptchuk TJ Placebo by proxy. BMJ 343, d4345 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Kirsch I. Are drug and placebo effects in depression additive? Biol Psychiatry 47, 733–5 (2000). [DOI] [PubMed] [Google Scholar]
- (39).Forno E. et al. Genome-wide association study of the age of onset of childhood asthma. J Allergy Clin Immunol 130, 83–90 e4 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Purcell S. et al. Plink: A tool set for whole-genome association and population-based linkage analyses. American journal of human genetics 81, 559–75 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM & Lee JJ Second-generation plink: Rising to the challenge of larger and richer datasets. Gigascience 4, 7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Das S. et al. Next-generation genotype imputation service and methods. Nat Genet 48, 1284–7 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Krishnan JA et al. Asthma outcomes: Symptoms. J Allergy Clin Immunol 129, S124–35 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Pers TH, Timshel P & Hirschhorn JN Snpsnap: A web-based tool for identification and annotation of matched snps. Bioinformatics 31, 418–20 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Pruim RJ et al. Locuszoom: Regional visualization of genome-wide association scan results. Bioinformatics 26, 2336–7 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Carvalho-Silva D. et al. Open targets platform: New developments and updates two years on. Nucleic Acids Res 47, D1056–D65 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


