Abstract
Background:
The use of transcranial Direct Current Stimulation (tDCS) to study anatomical and physiological dynamics and circuits supporting cognition and executive functions in particular has dramatically increased in recent years. However, its mechanisms of action remain only partially understood.
Objective:
In this study we assess the cognitive and physiological effects of anodal tDCS to the DLPFC on executive function in order to understand (1) the role of DLPFC laterality, (2) the physiological dynamics sustaining the modulation of executive function by tDCS, and (3) the impact of state-dependent dynamics.
Methods:
In a randomized, placebo-controlled, cross-over study, we applied anodal tDCS targeting the left vs. right DLPFC vs. sham in 20 healthy individuals (10 males, 10 females). Immediately before and after tDCS, subjects performed the Flanker Task while we measured behavioral (reaction time and accuracy) and neurophysiological (ERP) responses. Specifically, the amplitude of N200, P300, ERN and Pe is compared before and after stimulation.
Results:
Anodal tDCS to the left DLPFC lead to a significant improvement in reaction time, an increase in P300 amplitude and a decrease in N200 amplitude in a state-dependent manner: baseline ERP amplitudes conditioned the effects of tDCS.
Conclusion:
Given the role of these ERPs in conflict-related tasks, we speculate that tDCS is modulating the subconstructs of selective attention, conflict monitoring and response inhibition. These findings contribute to a further understanding of the role of left DLPFC in the modulation of executive function, and shed light into the mechanisms of action and the state dependent nature of tDCS.
Keywords: tDCS, EEG, ERP, executive function, state dependency
Introduction
Executive function is the subset of high-order cognitive capacities that sustains adaptive goal-directed behavior and thought. These functions are essential for successful adaptation to all environments, including professional and social [1]. Neural circuits underlying executive function include cortical, subcortical and cerebellar nodes, with the prefrontal cortex (PFC) and its different functional subdivisions as a central processing hub [2, 3].
The Eriksen Flanker Task (EFT) [4] (Figure 1) is a well-established experimental paradigm to assess three executive subconstructs: sustained attention, conflict monitoring and response inhibition (Figure 1). The circuits involved in the EFT include the dorsolateral prefrontal cortex (DLPFC) and the anterior cingulate cortex as critical hubs related to sustained attention, conflict monitoring and response inhibition [5]. Human electrophysiological studies assessing event related potentials (ERPs) with electroencephalography (EEG) have established relevant signatures of executive function during the EFT. Specifically, the N200, P300 and ERN/Pe (Error Related Negativity/Positivity) have been identified as the main ERPs characterizing the inhibitory and attentional functions in conflict tasks [6-9].
Figure 1. Experimental task design scheme.
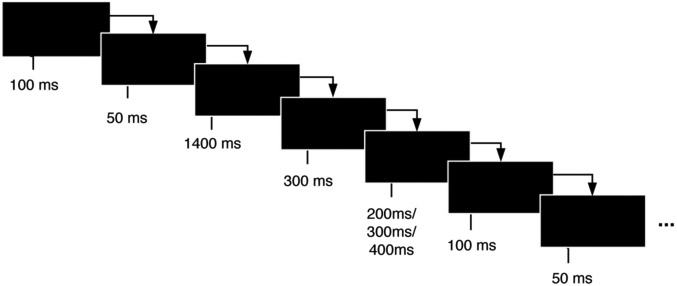
In the Flanker task, subjects must attend and respond to the direction of a central arrow that is surrounded (“flanked”) by distracting stimuli. Trials are classified as congruent, in which the central arrow points to the same direction as the flanker arrows, and incongruent trials, in which the central arrow points to the opposite direction of the flanker arrows. Subjects are instructed to press the left or right arrow buttons depending on the direction to which the central arrow is pointing at, ignoring the flanker arrows. In this study, the task consisted of 140 trials in two blocks of 70, with 2 congruent trials for each incongruent trial, in order to build a tendency towards congruent responses and thus increase the difficulty of conflict detection in incongruent trials. The experiment was coded and performed using E-Prime software (version 2.0.10.356-SP2). The onset of each target stimulus at the EEG data was synchronized by sending a TTL pulse from E-Prime to the EEG system with a time resolution of 2 ms. The flanker arrows were presented alone for 100 ms and then joined by the target arrow for 50 ms. Stimulus presentation was followed by a black screen for 1400 ms. If the participant did not respond by the response deadline (600 ms), a screen reading ‘TOO SLOW!’ was presented for 300 ms. If a response was made before the deadline, the black screen remained on screen for the 300 ms interval. Each trial ended with presentation of an additional black screen for a randomly chosen duration (200, 300 or 400 ms). Thus, trial duration varied between 2050–2250 ms. Participants were instructed to react as accurately and as fast as possible. Each subject had a different, fully random sequence of congruent and incongruent trials. The task had a total duration of 10 min, with a 1-minute training before the task started.
Important anatomical, physiological and cognitive questions about executive function remain. Anatomically, we still lack a clear understanding of the role of DLPFC laterality in executive function. Physiologically, the specific dynamics that sustain adaptive processing and that are disrupted in dysexecutive individuals also remain poorly understood. The mechanisms by which neuromodulation affects circuits, including state-dependent properties, are even greater unknowns. These are important questions for basic cognitive neuroscience, and represent critical knowledge gaps for the rational development of clinical neuroscience tools such as biomarkers or novel therapeutics. The pathological disruption of executive functions is broadly known as dysexecutive syndrome [10]. Its relevance crosses the boundaries of neurology and psychiatry, as it is a significant cause of morbidity and mortality in conditions as diverse as major depressive disorder, post-traumatic stress disorder, schizophrenia, attention deficit and hyperactivity disorder, traumatic brain injury, epilepsy, and neurodegenerative dementias and movement disorders.
Transcranial Direct Current Stimulation (tDCS) is emerging as a promising tool in human neuroscience research and for the treatment of neuropsychiatric disorders, in particular dysexecutive syndromes [11, 12]. Most previous studies evaluating the effects of tDCS in the EFT used only behavioral outcomes [13-15]. To our knowledge, the current study is the first one that combines behavioral and electrophysiological data (ERPs) to assess the lateralized effects of anodal tDCS to the DLPFC during the EFT.
In this study we aimed to assess the cognitive and physiological effects of anodal tDCS to the DLPFC on executive function to better understand (1) lateralization of executive functions in DLPFC, (2) the physiological signatures associated with adaptive tDCS modulation of cognition and (3) the state-dependent dynamics inherent to these circuits, by which baseline neurophysiology may condition the modulatory capacity of tDCS. In a cross-over, randomized, double-blind, placebo-controlled design, we tested 20 healthy individuals during 3 experimental visits, and compared the effect of anodal tDCS targeting the left DLPFC vs. right DLPFC vs. sham (Figure 2). Immediately before and after tDCS, subjects performed the EFT task while we measured behavioral (reaction time and accuracy) and neurophysiological (ERPs) responses.
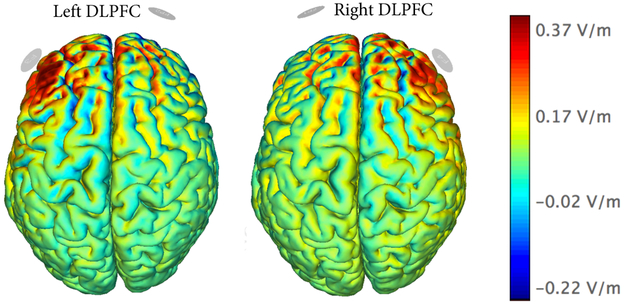
Figure 2. Electrical field model.
Modeling of the normal component of the electrical field (V/m) created by the montage targeting the left DLPFC (Anodal F3, Cathodal Fp2) and right DLPFC (Anodal F4, Cathodal Fp1). The modeling is based on a finite element model described in [35].
Materials and Methods
Participants
The study was approved by the local ethics committee of the Massachusetts General Hospital. Twenty healthy subjects (10 males and 10 females) participated in the present study, each engaging in 3 separate visits for a total of 60 experimental sessions. The age range was 18-53 years old (mean=32.6 and SD=15.6). Two subjects were excluded from the analysis because of extreme movement artifacts or due to behavioral or ERP outliers. The outlier criteria for behavioral measures consisted on discarding subjects who had more than 3 SD between the mean of each session at baseline (before stimulation) or more than 3 SD between the mean before and after stimulation at the Sham condition. Thus, the final study sample consists of 18 healthy adults: 9 males and 9 females.
Experimental Design
Each subject performed 3 experimental sessions corresponding to Sham, anodal tDCS stimulation targeting the left DLPFC (Left stimulation) and anodal tDCS stimulation targeting the right DLPFC (Right stimulation). Sessions were spaced by at least three day to avoid after-effects of tDCS. The order of stimulation administration (Sham, Left or Right) was randomized across subjects to avoid any confounding effects due to task proficiency. Before and after each tDCS session, each subject performed the Eriksen Flanker task (Figure 1). The accuracy of correct/incorrect responses and the RT for each stimulus were measured while also recording EEG data during the task for the extraction of attentional and inhibition-related ERPs (N200, P300, ERN and Pe).
tDCS stimulation
For each session, 2mA of anodal stimulation was applied for 30 minutes targeting the Right or Left DLPFC with Ag/AgCl electrodes (contact area 3.14 cm2) using the hybrid tDCS-EEG Starstim® system (Neuroelectrics, USA). The duration of the ramp up and down at the beginning and the end of the stimulation was set to 15 seconds. The impedance was controlled by the device, normally ranging below 10 Ω. During the stimulation period the subject was instructed to sit and relax with eyes open. The anodal electrode was placed on the scalp at the F4 (for Right DLPFC stimulation) or F3 (for Left DLPFC stimulation) positions, according to the international 10-20 EEG system. The cathode was placed in the contralateral supraorbital region (Fp1 or Fp2). For the sham condition, the electrodes were placed at the same positions but the current was applied only for the 15-second ramp up phase at the beginning and the end of a 30-minute sham-stimulation period, to simulate the potential experience of local tingling sensation that real stimulation produces but without sustained effect on cortical activity.
EEG recording/processing
EEG was recorded from seven positions (Fp1, Fp2, F3, Fz, F4, P3 and P4, according to the international 10-10 EEG positioning system) with the same 3.14 cm2 electrodes made of Ag/AgCl used for stimulation, at a sampling frequency of 500 samples/second. EEG data was referenced to the right mastoid. Independent component analysis (ICA) was utilized to remove activity associated with blinks, eye movements, and other artifacts. Data was filtered to a frequency band of 1-20Hz to remove non-neural physiological activity (skin/sweat potentials) and noise from electrical outlets. Trials were epoched within a time frame of 200 ms before and 800 ms after the stimulus onset. Epochs were detrended and normalized by dividing them by the standard deviation of each epoch. The mean of the pre-stimulus 200ms-baseline was then subtracted from the entire ERP waveform for each epoch to eliminate any voltage offset. After rejecting trials that had at least a sample above +/−150uV, the remaining trials were averaged for each time point and stimulation condition.
Statistical analysis
RT of single trials was introduced into a Generalized Linear Model with Mixed Effects (GLMM) with a Gamma distribution, modeled using the glmer function of the lme4 package in R software. We have previously shown that the gamma distribution is particularly well-suited to modeling reaction times during conflict tasks [17]. Two random effects were included in the model; a random slope for each step of TrialNumber (ranging from 1 to 280 within a session), which accounted for practice effects within each session, and a random intercept for each Subject ID to account for baseline differences between subjects. Trial Type (incongruent vs congruent), TimePoint (PREPOST stimulation), StimType (Left DLPFC, Right DLPFC, Sham), and the interactions between them were included as fixed effects. Only trials with correct responses were included in the RT analysis.
The Akaike Information Criterion (AIC) was used to assess the complexity added by each factor to the GLMM models. By convention, a factor was included in the model if it did not increase the model’s AIC by more than 5 points and it had a significant effect [18]. For three-way interactions (TimePoint*StimType*TrialType), multiple pairwise post-hoc tests were conducted for each level of TrialType, with correction for multiple comparisons using the ‘mvt’ method from the lsmeans package in R. Coefficients were considered significant when p<0.05 (confidence interval of 95%).
Accuracy (percentage of correct responses) was also modeled using a generalized logistic regression model with mixed effects and a binomial distribution. RT and the interaction between StimType, TimePoint and TrialType were included as fixed factors, while the Subject ID was included as a random effect. In this case, Trial Number was not included as a random factor as it did not meet the AIC criterion.
Only incongruent trials with correct responses were included in the N200 and P300 analysis, and only trials with incorrect responses were included in the ERN/Pe analysis. After visual inspection of the grand average waveforms, mean ERP amplitude of each single trial was calculated in a specific time-window centered in the peak latency of the grand average waveforms (see Figure 4 for specific time windows for each ERP). Mean amplitudes were then introduced into a linear model with mixed effects and a normal distribution (normality of the data was confirmed with a Shapiro-Wilk’s test). The interaction between StimType and TimePoint was included as a fixed factor, and the Subject ID as random factor. Trial Number was not included as it did not meet the AIC criterion. Given that the highest amplitude changes were observed in the frontal positions, the ERP analysis was focused on the average of F3, Fz and F4 positions.
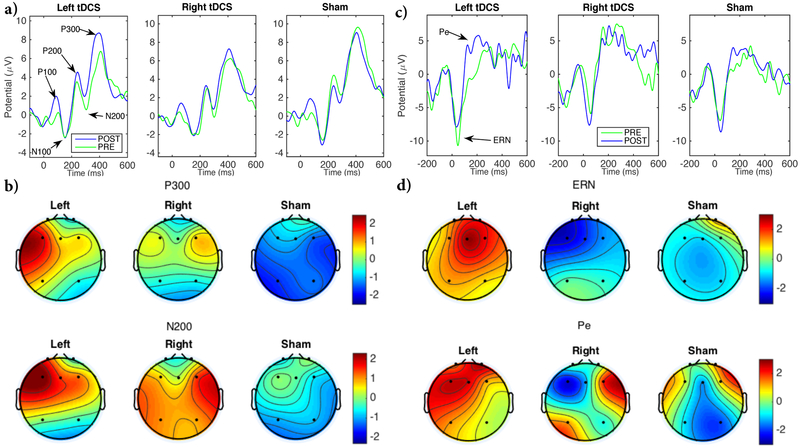
Figure 4. ERP results.
a) Grand average ERPs time-locked to incongruent stimuli before and after Left, Right and Sham. Waveforms correspond to the average of F3, Fz and F4 positions. b) Scalp topographies of POST-PRE difference of P300 (top) and N200 (bottom) for Left, Right and Sham stimulation (μV). Averaging time window for P300 = [350, 470] ms and N200 = [240, 360] ms. c) Grand average ERPs time-locked to incorrect responses before and after Left, Right and Sham. Waveforms correspond to the average of F3, Fz and F4 positions. d) Scalp topographies of POST-PRE difference of ERN (top) and Pe (bottom) for Left, Right and Sham stimulation (μV). Averaging time window for ERN = [0, 120] ms and Pe = [100, 300] ms. See Figure S2 for ERP waveforms for congruent trials, Figures S3 and S4 for ERP waveforms at each individual electrode, and Table S3 for the full LMM parameters for ERPs.
The trial-by-trial correlation of N200 and P300 amplitude with RT was also calculated with a linear model with mixed effects, with the Subject ID as a random factor.
State dependencies were also calculated for the metrics that showed significant changes after tDCS (RT, P300 and N200 amplitude). The mean of these metrics was computed separately for real tDCS (Left and Right) and Sham. Beta coefficients were calculated with a linear model with mixed effects, with the absolute difference from PRE to POST stimulation as the dependent variable, the mean of the same (or another) variable at baseline as fixed factor, and the Subject ID as random factor. P values were corrected for multiple comparisons using the False Discovery Rate (FDR) method. State dependencies were considered significant when p<0.05.
Results
Reaction time
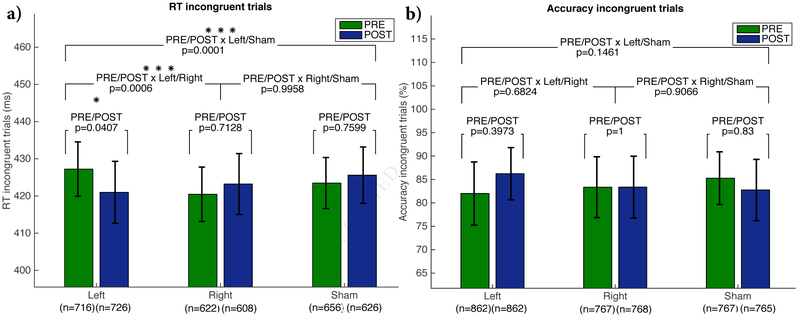
The interaction between StimType (Left DLPFC/Right DLPFC/Sham), TimePoint (PRE/POST stimulation) and TrialType (Incongruent/Congruent) showed a significant effect for Left-sided stimulation compared to Sham (β=10.82ms, CI=[7.06, 14.58], p=0.001), suggesting that the effect of Left-sided stimulation is significantly different for congruent versus incongruent trials. After post-hoc tests and corrections (Tables S1 and S2), Left stimulation compared to Sham (β=−8.37ms, CI=[−13.21, −3.52], p=0.0001) and compared to Right stimulation β=9.00ms, CI=[3.02, 14.99], p=0.0006) led to a significant decrease in reaction time for incongruent trials only (Figure 3a). For congruent trials there were no significant changes for any of the stimulation conditions (Figure S1).
Figure 3. Behavioral results.
a) Mean reaction time and b) accuracy as percentage of correct responses for incongruent trials, including post-hoc tests with ‘mvt’ correction. Error bars indicate confidence intervals. Significance indicates (*) = p<0.05, (**) = p<0.01, (***) = p<0.001. Numbers in parentheses indicate n values. See Figure S1 for statistical results for congruent trials, and Tables S1 and S2 for the full GLMM parameters for reaction time and accuracy.
Accuracy
Similarly, the interaction between StimType, TimePoint and TrialType showed a significant effect for Left stimulation (β=0.34, CI=[0.16, 0.72], p=0.005), suggesting its effect is significantly different for congruent versus incongruent trials. However, after post-hoc tests and corrections, none of the stimulation conditions met the prespecified threshold for significant change (Figures 3b and S1b).
Event Related Potentials: mechanisms of tDCS modulation
As previously described [19], the first three peaks that appeared in both incongruent (Figure 4a) and congruent trials (Figure S2) correspond to P100 (around 100ms), N100 (around 180ms) and P200 (around 250-280ms). P100 and N100 correspond to visual evoked potentials, while P200 reflects the spontaneous orienting of attention allocation associated with the onset of the flanker stimuli. These are not directly related to conflict processing and therefore they were not included in the analysis.
As expected, incongruent trials presented an additional N200 around 280 ms after the target onset and a P300 around 400 ms, as shown in Figure 4a. Figure 4b shows the topographic maps of the POST-PRE difference of N200 and P300 amplitude (see ERPs of each individual electrode in Figure S3). Note that the largest amplitude changes for N200 and P300 occurred mostly around the area of the anodal stimulation electrode that matched the laterality of the stimulation (F3 and F4).
Similar to behavioral results, the interaction between StimType and TimePoint reveals a significant N200 amplitude decrease after Left-sided stimulation compared to Sham (β=1.53uV, CI=[0.03, 3.02], p=0.046). Rightsided stimulation also decreased N200 amplitude, but its effect was not significantly different from Sham (β=1.03uV, CI=[−0.52, 2.57], p=0.193).
P300 appeared in incongruent trials with a latency of about 400 ms after the onset of the target stimuli. The interaction between StimType and TimePoint led to a significant P300 amplitude increase also for Left-sided stimulation compared to Sham (β=2.57uV, CI=[1.00, 4.15], p=0.001). Right-sided stimulation increased P300 as well, but its effect was not significant compared to Sham (β=1.51uV, CI=[−0.11, 3.13], p=0.067).
ERN appeared with a latency of 50 ms after the incorrect response and Pe with a latency of 200 ms after the incorrect responses (Figures 4c and 4d). ERN and Pe did not experience any significant changes for any of the stimulation conditions (see Figure S4 and Table S3 for individual electrodes and LMM parameters).
Event Related Potentials: cross-sectional correlation with reaction time
The amplitude of N200 and P300 were each found to be significantly correlated with reaction time for incongruent trials, i.e., the greater the P300 amplitude, the faster the reaction time (β=−0.27mμV, CI=[−0.43, −0.09], p=0.002) and the smaller the N200, the faster the reaction time (β=−0.19ms/μV, CI=[−0.37, −0.01], p=0.036). Reaction time was also a significant predictor of the P300 (β=−0.009μV/ms, CI=[−0.01, −0.003], p=0.003) and the N200 (β=−0.006μV/ms, CI=[−0.01, −0.0001], p=0.044), .
State dependencies
Table 1 shows the effect of baseline (PRE-tDCS) variables on the change of the same (or other) variables after stimulation (see Materials and Methods for further details). Figure S5 shows the scatter plots of the significant predictors.
Table 1. State dependencies.
Estimates, confidence intervals and FDR-corrected p values for each comparison.
| Real tDCS | Sham | ||||||
|---|---|---|---|---|---|---|---|
| Predictor variable at baseline |
Change
in dependent variable after tDCS |
Estimate | Confidence interval |
FDR
corrected p values |
Estimate | Confidence interval |
FDR
corrected p values |
| RTPRE | ΔRTPOST-PRE | 0.17 ms/μV | [−0.05, 0.38] | 0.1933 | −0.002 mμV | [−0.30, 0.30] | 0.9880 |
| P300 PRE | TΔRTPOST-PRE | 2.13 ms/μV | [0.39, 3.92] | 0.0407* | −0.33 mμV | [−3.50, 2.83] | 0.9408 |
| N200PRE | ΔRTPOST-PRE | 1.19 ms/μV | [−0.52, 2.92] | 0.2152 | −5.12 mμV | [−5.12, 0.76] | 0.6630 |
| RTPRE | ΔP300POST-PRE | −0.01 | [−0.06, 0.02] | 0.5874 | −0.03 | [−0.09, 0.02] | 0.6630 |
| P300PRE | ΔP300POST-PRE | −0.62 | [−1.08, −0.16] | 0.0297* | −0.36 | [−0.98, 0.25] | 0.6630 |
| N200PRE | ΔP300POST-PRE | −0.55 | [−0.94, −0.12] | 0.0297* | −0.06 | [−0.73, 0.60] | 0.9408 |
| RTPRE | ΔN200POST-PRE | −0.01 | [−0.05, 0.03] | 0.6053 | −0.01 | [−0.05, 0.02] | 0.8222 |
| P300 PRE | ΔN200POST-PRE | −0.30 | [−0.63, 0.02] | 0.1220 | −0.18 | [−0.61, 0.25] | 0.8222 |
| N200PRE | ΔN200POST-PRE | −0.42 | [−0.75, −0.11] | 0.0297* | −0.08 | [−0.54, 0.36] | 0.9408 |
P300 amplitude (β=−0.62, CI=[−1.08, −0.16], p=0.0297) and N200 amplitude (β=−0.42, CI=[−0.75, −0.11], p=0.0297) before stimulation are significant predictors of the change in their own amplitudes after stimulation, i.e., the modulatory effect of tDCS depends on the electrophysiological state before the intervention. Specifically, the larger the P300 peak at baseline, the smaller the P300 increase after tDCS. Similarly, the smaller the N200 peak at baseline, the smaller the N200 decrease after tDCS. The baseline N200 also predicted the change in P300 (β=−0.55, CI=[−0.94, −0.12], p=0.0297), i.e., the greater the amplitude of the N200 at baseline, the greater the increase in P300, but baseline P300 did not predict the change in N200.
Importantly, baseline physiological states not only predict tDCS changes in physiology, but also predict changes in behavior. Baseline P300 amplitude is a significant predictor of the change in its amplitude as well as a predictor of the change in reaction time after tDCS (β=2.13mμV, CI=[0.39, 3.92], p=0.0407). However, reaction time is not a significant predictor of the change in behavioral or electrophysiological outcomes, suggesting that physiological baseline is a better reflection of brain state than is behavioral baseline. As expected, Sham did not show any significant state dependencies, discarding any potential effects due to regression to the mean [20].
Discussion
To the best of our knowledge, this is the first study to assess the lateralized effects of anodal tDCS to the left and right DLPFC with cognitive (task) and physiological (EEG) measures of executive function, and the additional aim of quantifying state-dependent dynamics.
Cognitive Effects: anodal tDCS to the DLPFC improves behavioral outcomes in the EFT with a predilection for left-sided modulation
Our results confirm that anodal tDCS targeting the DLPFC can improve cognitive performance in the EFT. Specifically, we found that anodal tDCS targeting the left DLPFC results in a significant reduction in RT in incongruent trials, compared to a non-significant change after sham or anodal tDCS targeting the right DLPFC.
Although there were no statistically significant changes in accuracy, it is still worth noting that there is no tradeoff between the effects of tDCS on RT and accuracy, i.e., RT is improved after left-sided stimulation, but not at the expense of worsening accuracy. Similar effects have recently been observed from deep brain stimulation [21], with EEG changes lateralized to left DLPFC, suggesting multiple ways of tapping into these cortico-striatal circuits of executive function.
Our findings suggest a left lateralization of the DLPFC functions needed to complete the EFT, particularly for the more difficult incongruent trials. These results are in line with the work by [14], which also showed a speed up in RTs for anodal tDCS targeting the left DLPFC relative to sham in the EFT (without explicitly addressing the role of laterality or the impact on neurophysiology). That said, RT and accuracy are compound scores that reflect the efficiency of distinct serial and parallel computations (i.e. cognitive operations) needed to complete the task. While behavioral outcomes alone may not easily dissect the impact of tDCS on specific operations, physiological measures could provide greater insights.
Physiological effects: the improvement in executive function is correlated with a decrease in N200 and increase in P300 amplitudes
The improved RT of incongruent trials is associated with a decrease in N200 and increase in P300 amplitudes, also selectively for Left anodal tDCS. The N200 and P300 are exclusive signatures of incongruent trials, hence establishing a parallelism between the behavioral and physiological effects: anodal tDCS to the left (but not right) DLPFC modulates incongruent trials’ behavioral outcomes and its direct physiological underpinnings.
According to the conflict monitoring model, larger N200 amplitudes in the EFT indicate that individuals are attending more to task-irrelevant (flanker) information than task-relevant (target stimulus) information [6]. From a signal detection theory perspective, the N200 amplitude is a measure of noise (external variables) and effort (internal variables), so reducing it means better signal-to-noise ratio and more efortless and efficient use of cognitive resources available for signal detection (due to easier psychometric properties or more proficient processing strategies). Perceptual and attentional deficits have been found to produce substantially greater N200 amplitudes [22]. Given that the psychometric properties of the task did not change, we interpret the decrease in N200 amplitude after left-sided stimulation as a decrease of the disruption caused by the flankers due to improved selective attention, which reduced the effort required to complete the task.
Unlike N200, P300 is not a measure of attentional effort. Instead, larger P300 amplitudes have been proven to be related to better ability of conflict post-processing and subsequent behavioral inhibition of the incorrect prepotent responses [23-25]. A number of previous studies have found reduced P300 amplitudes in populations with attention and inhibition deficits compared to healthy controls [25-27]. According to literature, we interpret the increase in P300 amplitude after left-sided stimulation as an improvement in post-conflict resolution, leading to improved response inhibition. This could be a separate yet synergistic mechanism by which tDCS was able to improve behavioral outcomes: in addition to improving selective attention, i.e., effective suppression of conflicting, noisy information (captured in the N200), it also improved the ability to inhibit the prepotent response (hence the change in the P300 as well). Improvement in the two related but different (possibly sequential) cognitive computations would translate into faster accurate responses.
The N200 and P300 results are in line with previous studies assessing tDCS effects on these ERPs in other tasks. [28] found a significant increase in P300 amplitude in the n-back task after anodal tDCS at the left DLPFC in healthy subjects, which was also correlated with a decrease in RT. Another study found decreased N200 amplitudes after anodal tDCS at the left DLPFC in a visual working memory task with patients with affective disorders [29]. Similarly, [30] also showed a decrease in N200 and an increase in P300 amplitudes in a Go/Nogo task after anodal tDCS at the right DLPFC in patients with food craving.
Physiological biomarkers: cross-sectional trial-by-trial correlation of ERP amplitude and RT
Different models have associated N200 and P300 with specific cognitive operations [6, 23]. One may hypothesize from these models that these ERPs may be correlated with processing efficiency, and hence have value as a potential biomarker for cognitive performance. To empirically test this hypothesis, and to support the interpretation of the observed tDCS effects, we used our own dataset to assess if the amplitude of the N200 and P300 on a trial by trial basis was indeed associated cross-sectionally with behavioral performance in the EFT. Indeed, the significant correlation of N200 and P300 with RT for incongruent trials confirms the hypothesis that the amplitude of these ERPs is an indicator of processing efficiency, thus highlighting its value as a potential biomarker for cognitive performance.
State-dependency: baseline physiological signatures predict the tDCS modulation of physiology and behavior
Our results indicate that the effect of tDCS depends on the baseline electrophysiological state before the intervention. Specifically, these findings imply that tDCS leads to greater modulation of physiology and behavior in subjects with baseline physiological signatures indicative of less adaptive processing, as they allow greater range of modulation (e.g. low baseline P300 amplitude allows greater increase in its amplitude by tDCS). This is in line with similar studies that found that TMS leads to greater facilitation of less active neural populations [31].
These effects can be explained by the principle of state-dependency, a phenomenon whereby the response of a system to an external stimulus is affected not only by the properties of that stimulus (e.g. tDCS parameters) but also by the internal state of the system. Since we are trying to modulate a moving target (circuit neurophysiological processes) with highly inter- and intra-subject dynamic properties, the baseline state is likely to be significantly different across subjects and sessions, highlighting the importance of comparing before- versus after-stimulation outcomes as opposed to only assessing during or after-stimulation. Furthermore, this highlights the need to take into account activity during stimulation, which has been shown to have a systematic influence on neuromodulation outcomes [32].
The state-dependent characteristics of tDCS have important implications for treatment development: clinical trials, and neuroscience studies alike, would benefit from controlling patients’ baseline state in order to minimize response variability. Furthermore, state-dependency could be used to maximize the effects of tDCS by manipulating the subject’s initial state before or during the intervention in order to achieve optimal outcomes. State dependencies are not limited to tDCS but are also common to other neuromodulation techniques [33], as well as pharmacological and behavioral interventions [34] that try to impact a highly dynamic target such as brain physiology.
Limitations
One of the limitations is the small sample size, accompanied by the fact that overall performance levels were too accurate, leaving few incorrect responses to detect significant changes in ERN/Pe amplitudes after tDCS with appropriate power. This study was designed administering tDCS at rest, but combining tDCS with an online task (the EFT or another) may lead to different, possibly stronger and more specific, modulatory effects and cognitive results. These results have been validated with the Flanker task in a healthy population, and are limited to shortterm effects for one session of 30 minutes of stimulation, but they should be replicated with larger healthy and clinical populations, and possibly with other tasks and repeated stimulation sessions.
Conclusions and significance
We found that anodal tDCS targeting the left DLPFC (but not right DLPFC or sham) modulates executive function during the EFT, as shown by a reduction in reaction time for incongruent trials and a non-significant improvement in accuracy. The same anatomical specificity (i.e. anodal Left DLPFC) is observed for the modulation of physiological signatures of incongruent trials: reduction of N200 and increase of P300 amplitudes. We interpret these results as an optimization of both signal processing (i.e. selective attention) and response inhibition (i.e. cognitive control) by tDCS in the context of conflicting stimuli and cognitive demands (i.e. incongruent trials). Indeed, we confirmed that the amplitudes of the P300 and the N200 were correlated with RT on a trial-by-trial basis, empirically supporting our mechanistic interpretation and suggesting value in these ERPs as biomarkers of executive function efficacy. Last, we describe that the effect size of the tDCS-induced changes in neurophysiology and behavior is dependent on the physiological state before the intervention, shedding light on state-dependent dynamics of tDCS modulation of executive function.
Supplementary Material
Highlights.
Anodal tDCS to the left DLPFC leads to faster reaction times in the Flanker task
Cognitive improvement is correlated with changes in N200 and P300 amplitudes
Baseline physiology predicts cognitive and physiological effects of tDCS
N200 and P300 amplitudes correlate with reaction time on a trial by trial basis
Acknowledgements
This research was partly supported by NIH grants (RO1 MH112737, R21 DA042271, R21 AG056958 and R21 MH115280) to JAC and grants from NIH (UH3 NS100548) and the OneMind Institute to ASW.
Footnotes
Conflicts of Interest
LDV is an employee at Neuroelectrics and a PhD student in the Camprodon Lab. GR is a co-founder of Neuroelectrics, a company that manufactures tDCS and EEG technology. AW has patent applications pending related to cognitive enhancement through brain stimulation and new methods of transcranial electrical stimulation. JAC is a member of the scientific advisory board for Apex Neuroscience Inc. The remaining authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Diamond A Executive Functions. Annual Review of Psychology 2013;64(1):135–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].MacDonald AW, Cohen JD, Stenger VA, Carter CS. Dissociating the Role of the Dorsolateral Prefrontal and Anterior Cingulate Cortex in Cognitive Control. Science (New York, NY) 2000;288(5472):1835. [DOI] [PubMed] [Google Scholar]
- [3].Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual review of neuroscience 2001;24:167–202. [DOI] [PubMed] [Google Scholar]
- [4].Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception & Psychophysics 1974;16(1):143–9. [Google Scholar]
- [5].Fan J, Flombaum JI, McCandliss BD, Thomas KM, Posner MI. Cognitive and brain consequences of conflict. Neuroimage 2003;18(1):42–57. [DOI] [PubMed] [Google Scholar]
- [6].Danielmeier C, Wessel JR, Steinhauser M, Ullsperger M. Modulation of the error-related negativity by response conflict. Psychophysiology 2009;46(6):1288–98. [DOI] [PubMed] [Google Scholar]
- [7].Clayson PE, Larson MJ. Conflict adaptation and sequential trial effects: support for the conflict monitoring theory. Neuropsychologia 2011;49(7):1953–61. [DOI] [PubMed] [Google Scholar]
- [8].Larson MJ, Clayson PE, Clawson A. Making sense of all the conflict: A theoretical review and critique of conflict-related ERPs. International Journal of Psychophysiology 2014;93(3):283–97. [DOI] [PubMed] [Google Scholar]
- [9].Falkenstein M, Hoormann J, Christ S, Hohnsbein J. ERP components on reaction errors and their functional significance: a tutorial. Biol Psychol 2000;51(2-3):87–107. [DOI] [PubMed] [Google Scholar]
- [10].Daffner KR, Searl MM. The dysexecutive syndromes In: Goldenberg G, Miller BL, editors. Neuropsychology and behavioral neurology., New York, NY: Elsevier; 2008, p. 249–67. [DOI] [PubMed] [Google Scholar]
- [11].Philip NS, Nelson BG, Frohlich F, Lim KO, Widge AS, Carpenter LL. Low-Intensity Transcranial Current Stimulation in Psychiatry. The American journal of psychiatry 2017;174(7):628–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sarkis RA, Kaur N, Camprodon JA. Transcranial Direct Current Stimulation (tDCS): Modulation of Executive Function in Health and Disease. Current Behavioral Neuroscience Reports 2014;1(2):74–85. [Google Scholar]
- [13].Breitling C, Zaehle T, Dannhauer M, Bonath B, Tegelbeckers J, Flechtner H-H, et al. Improving Interference Control in ADHD Patients with Transcranial Direct Current Stimulation (tDCS). Frontiers in cellular neuroscience 2016;10(March):72-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Karuza EA, Balewski ZZ, Hamilton RH, Medaglia JD, Tardiff N, Thompson-Schill SL. Mapping the Parameter Space of tDCS and Cognitive Control via Manipulation of Current Polarity and Intensity. Front Hum Neurosci 2016;10:665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gbadeyan O, McMahon K, Steinhauser M, Meinzer M. Stimulation of Dorsolateral Prefrontal Cortex Enhances Adaptive Cognitive Control: A High-Definition Transcranial Direct Current Stimulation Study. The Journal of Neuroscience 2016;36(50): 12530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bikson M, Grossman P, Thomas C, Zannou AL, Jiang J, Adnan T, et al. Safety of Transcranial Direct Current Stimulation: Evidence Based Update 2016. Brain Stimul 2016;9(5):641–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Widge AS, Ellard KK, Paulk AC, Basu I, Yousefi A, Zorowitz S, et al. Treating refractory mental illness with closed-loop brain stimulation: Progress towards a patient-specific transdiagnostic approach. Experimental neurology 2017;287(Pt4):461–72. [DOI] [PubMed] [Google Scholar]
- [18].Tremblay A, Newman AJ. Modeling nonlinear relationships in ERP data using mixed-effects regression with R examples. Psychophysiology 2015;52(1):124–39. [DOI] [PubMed] [Google Scholar]
- [19].Kopp B, Rist F, Mattler U. N200 in the Flanker task as a neurobehavioral tool for investigating executive control. Psychophysiology 1996;33(3):282–94. [DOI] [PubMed] [Google Scholar]
- [20].Barnett AG, Dobson AJ, van der Pols JC. Regression to the mean: what it is and how to deal with it. International Journal of Epidemiology 2004;34(1):215–20. [DOI] [PubMed] [Google Scholar]
- [21].AS W, S Z, I B, AC P, SS C, EN E, et al. Deep brain stimulation of the internal capsule enhances human cognitive control and prefrontal cortex function. Nature communications 2019;Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yeung N, Ralph J, Nieuwenhuis S. Drink alcohol and dim the lights: the impact of cognitive deficits on medial frontal cortex function. Cogn Affect Behav Neurosci 2007;7(4):347–55. [DOI] [PubMed] [Google Scholar]
- [23].Clayson PE, Larson MJ. Effects of repetition priming on electrophysiological and behavioral indices of conflict adaptation and cognitive control. Psychophysiology 2011;48(12):1621–30. [DOI] [PubMed] [Google Scholar]
- [24].Enriquez-Geppert S, Konrad C, Pantev C, Huster RJ. Conflict and inhibition differentially affect the N200/P300 complex in a combined go/nogo and stop-signal task. Neuroimage 2010;51(2):877–87. [DOI] [PubMed] [Google Scholar]
- [25].Polich J Updating P300: An Integrative Theory of P3a and P3b. Clin Neurophysiol 2009;118(10):2128–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ford JM. Schizophrenia: the broken P300 and beyond. Psychophysiology 1999;36(6):667–82. [PubMed] [Google Scholar]
- [27].Szuromi B, Czobor P, Komlosi S, Bitter I. P300 deficits in adults with attention deficit hyperactivity disorder: a metaanalysis. Psychol Med 2011;41(7):1529–38. [DOI] [PubMed] [Google Scholar]
- [28].Keeser D, Meindl T, Bor J, Palm U, Pogarell O, Mulert C, et al. Prefrontal Transcranial Direct Current Stimulation Changes Connectivity of Resting-State Networks during fMRI. The Journal of Neuroscience 2011;31(43):15284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Powell TY, Boonstra TW, Martin DM, Loo CK, Breakspear M. Modulation of cortical activity by transcranial direct current stimulation in patients with affective disorder. PLoS One 2014;9(6):e98503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lapenta OM, Sierve KD, de Macedo EC, Fregni F, Boggio PS. Transcranial direct current stimulation modulates ERP-indexed inhibitory control and reduces food consumption. Appetite 2014;83:42–8. [DOI] [PubMed] [Google Scholar]
- [31].Cattaneo Z, Silvanto J. Time course of the state-dependent effect of transcranial magnetic stimulation in the TMS-adaptation paradigm. Neurosci Lett 2008;443(2):82–5. [DOI] [PubMed] [Google Scholar]
- [32].Nozari N, Woodard K, Thompson-Schill SL. Consequences of cathodal stimulation for behavior: When does it help and when does it hurt performance? PLoS ONE 2014;9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Silvanto J, Pascual-Leone A. State-Dependency of Transcranial Magnetic Stimulation. Brain topography 2008;21(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Overton DA. State Dependent Learning and Drug Discriminations In: Iversen LL, Iversen SD, Snyder SH, editors. Drugs, Neurotransmitters, and Behavior, Boston, MA: Springer US; 1984, p. 59–127. [Google Scholar]
- [35].Ruffini G, Fox MD, Ripolles O, Miranda PC, Pascual-Leone A. Optimization of multifocal transcranial current stimulation for weighted cortical pattern targeting from realistic modeling of electric fields. Neuroimage 2014;89:216–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.