Abstract
Background
Neonatal seizures are associated with adverse neurologic sequelae including epilepsy in childhood. Here, we aim to determine whether levels of cytokines in neonates with brain injury are associated with acute symptomatic seizures or remote epilepsy.
Methods
This is a cohort study of term newborns with encephalopathy at UCSF between 10/1993 and 1/2000 who had dried blood spots. Maternal, perinatal/postnatal, neuroimaging, and epilepsy variables were abstracted by chart review. Logistic regression was used to compare levels of cytokines with acute seizures and the development of epilepsy.
Results
In a cohort of 26 newborns with neonatal encephalopathy at risk for hypoxic ischemic encephalopathy with blood spots for analysis, diffuse alterations in both pro- and anti-inflammatory cytokine levels were observed between those with (11/28, 39%) and without acute symptomatic seizures. Seventeen of the 26 (63%) patients had more than two years of follow-up and 4/17 (24%) developed epilepsy. Higher levels of pro-inflammatory cytokines IL-6 and TNF-α, within the IL-1β pathway were significantly associated with epilepsy.
Conclusions
Elevations in pro-inflammatory cytokines in the IL-1β pathway were associated with later onset of epilepsy. Larger cohort studies are needed to confirm the predictive value of these circulating biomarkers.
Introduction
Neonatal seizures are an indicator of neurologic dysfunction with an incidence of 2.8 to 4.4/1000 live births. (1, 2) Neonates with seizures are at high risk for a range of adverse neurologic sequelae compared to those without seizures, with up to 25% developing remote epilepsy. (3, 4) Risk factors for epilepsy include severity of encephalopathy, severity and type of brain injury, abnormal EEG background, and seizure frequency. (3–5) Together these variables can identify a high-risk group with an approximately 50% chance of developing epilepsy. (3–5) Additional predictors of epilepsy, however are needed in order to improve stratification, to better inform families, and to guide therapeutic studies that can alter epilepsy outcomes. (6, 7)
Alterations in levels of inflammatory cytokines, in particular the IL-1β pathway may serve as biomarkers of neurologic disease. These molecules are secreted by activated neuroglia often within an hour of an inciting central nervous system insult, including status epilepticus, stroke, and infection. (8–10) IL-1β activates its endogenous receptor with resultant increases in neuronal excitability. (11–13) After an initial CNS insult, ongoing inflammation may alter neuronal plasticity with network reorganization through several transcriptionally regulated effects, with potential for aberrant and epileptogenic circuits. (14–18) Activation of the pathway enhances the permeability of an already dysfunctional blood-brain barrier, allowing for movement and detection of these proteins into the peripheral circulation, raising the possibility of their utility as a biomarker of disease. (19, 20)
In this study, we aimed to determine if levels of cytokines in neonates with brain injury are associated with acute symptomatic seizures and the development of epilepsy in childhood. We hypothesize that changes in a diffuse set of neonatal cytokines will be associated with acute seizures, though only increases in cytokines within the pro-inflammatory IL-1β pathway will be associated with remote epilepsy.
Methods
Subjects
This is a nested cohort study within a longitudinal investigation of term and near-term newborns at risk of neonatal encephalopathy. (21, 22) As previously reported, newborns were recruited who were admitted to the Intensive Care Nursery at UCSF and a nearby county hospital between 10/1993 and 1/2000 and had any of the following: 1) umbilical artery pH < 7.1; 2) umbilical artery base deficit > 10; 3) Apgar score ≤ 5 at 5 min of life; or 4) overt neonatal encephalopathy as assessed by a neonatologist. This cohort was assembled before the adoption of therapeutic hypothermia. Neonates were excluded if there was evidence of major congenital malformations, congenital metabolic diseases, or perinatal or intrauterine infection. The original cohort enrolled 125 neonates, 62 of which had cytokine levels evaluated from dried blood spots. Here, we aimed to evaluate cytokine levels in term neonates with high risk of brain injury. We applied additional exclusion criteria to the study base, excluding neonates: < 37 weeks gestational age; without both clinical and physiologic evidence of hypoxic-ischemia (and therefore not meeting our institutional therapeutic hypothermia criteria) (23); deceased during the birth admission; and, dried blood spots collected < 24 or > 120 hours after birth to allow for evaluation of relevant cytokines at peak levels after an acute neurologic injury. (21, 24, 25) Epilepsy classification was restricted to those with at least two years of follow-up to allow for the development of epilepsy. (3, 4)
Measurements
Cytokines
Cytokine levels were previously evaluated, and levels reported in an investigation evaluating their association with Magnetic Resonance Spectroscopy (MRS) and development. (21) In brief, dried blood spots were obtained from heel-stick blood as apart of California’s newborn screening program. Dried blood spots were analyzed by recycling immunoaffinity chromatography. (26) Twenty-five microliter samples were injected into serially connected microcolumns, each containing a different immobilized capture antibody (IL-1, IL-6, IL-8, IL-9, IL-12, IL-13, and TNF-α). (22) Analytes were released by treatment with acidic buffer and measured by laser-induced fluorescence.
Clinical data
Trained research assistants prospectively abstracted demographics and birth delivery data. (21) Encephalopathy scores were determined by expert review. (21, 22) Magnetic resonance imaging (MRI) injury scores were determined by combining scores for abnormalities in the deep gray nuclei and white matter. (27) Two authors (ALN, HCG) reviewed charts for encephalopathy etiology, presence of clinical and/or electrographic neonatal acute symptomatic seizures and epilepsy as defined by the International League Against Epilepsy 2014 criteria. (28)
Data Analysis
Analyses were performed using Stata 15.1 software (Stata Corp, College Station, Texas). The chi-square test was used to compare categorical variables, the Whitney Rank Sum test was used to compare ordinal variables, and the t-test was used to compare continuous variables with acute seizures or epilepsy. For the analyses of cytokines, linear regression was used to compare levels with continuous variables, the Kruskal-Wallis test was used with ordinal variables, and t-tests or analysis of variance were used for comparisons categorical predictors and outcome variables. We performed validation using sensitivity analyses with nonparametric tests. The area under the receiver operator curve (AUC) for cytokine levels and epilepsy were estimated using non-parametric methods.
Rv3.2.0 (R Foundation for Statistical Computing, Vienna, Austria) was used for clustering methods, using the gplots package. For each patient, cytokine values were scaled by subtracting the mean cytokine value across all patients from each particular patient cytokine level. This value was then divided by the standard deviation of the particular cytokine. Hierarchical agglomerative clustering algorithms were used to evaluate clusters of patients within the plasma cytokine determinants. Data were standardized as Z-scores by subtracting mean and divided by standard deviation for each cytokine. Pearson’s correlation similarity metric and Ward linkage function were used for hierarchical agglomerative cluster analysis. For each clustering algorithm, dendrograms, heatmaps and bubblemaps were created to visualize the clusters and scaled cytokine values. Dark blue colors on the heatmaps represent low cytokine values, white colors represent average values, and red represent high values. Larger bubbles on bubble maps correspond to higher cytokine values.
The protocol was approved by the Committee for Human Research at the University of California, San Francisco and voluntary informed consent was obtained from parents or legal guardians.
Results
Cohort characteristics
From a study base of 62 neonates with encephalopathy, we excluded seven (11%) neonates < 37 weeks gestational age, ten (16%) infants without clinical and physiologic evidence of hypoxia-ischemia, two (3%) deceased during the birth admission, and 10 (16%) infants with dried blood spots collected < 24 or > 120 hours after birth. Seven (11%) of 62 infants had missing clinical details on birth history and follow-up, leaving 26 infants for evaluation for acute symptomatic seizures. Among these 26 infants, 22 (85%) had radiologic evidence consistent with hypoxia-ischemia, three (12%) had radiologic evidence of ischemic or hemorrhagic stroke, and one (4%) had laboratory confirmed meningitis.
Acute seizures
Fifteen (58%) of 26 neonates had acute symptomatic seizures. Seizure onset was within 48 hours of life in all subjects. Five neonates had rare (< 7) seizures, two had frequent recurrent seizures (≥7), and two had status epilepticus. The remaining six had greater than one seizure, but the precise burden could not be determined by chart review. Neonates with acute seizures had higher encephalopathy scores and a trend for higher MRI injury scores compared to those without acute seizures (Table 1). The groups did not differ on other demographic, clinical, and maternal risk factors.
Table 1:
Clinical characteristics and MRI findings among a cohort of neonates with encephalopathy.
| Acute Seizures (n=15) | No Acute Seizures (n=11) | p-value | Epilepsy (n=4) | No Epilepsy (n=13) | p-value | |
|---|---|---|---|---|---|---|
| Male, n (%) | 10 (67) | 7 (63) | 0.87 | 2 (50) | 9 (69) | 0.48 |
| Average GA (wk)a | 39.4 (1.5) | 40.1 (1.2) | 0.21 | 39.9 (1.4) | 39.1 (1.2) | 0.33 |
| Birth Weight (g) a | 3337 (129) | 3175 (151) | 0.42 | 3375 (172) | 3289 (120) | 0.73 |
| Race, n (%) | ||||||
| American Indian | 1 (7) | 0 | 1 (25) | 0 | ||
| Asian/PI | 2 (13) | 3 (33) | 0.23 | 1 (25) | 3 (23) | 0.28 |
| Black | 4 (27) | 0 | 1 (25) | 3 (23) | ||
| White | 8 (53) | 6 (67) | 1 (25) | 7 (54) | ||
| Hispanic, n (%) | 0 | 2 | 0.09 | 0 | 0 | - |
| Encephalopathy | ||||||
| Etiology b | ||||||
| HIE/NE | 11 (73) | 11 (100) | 0.17 | 3 (75%) | 11 (84%) | 0.14 |
| Stroke | 3 (20) | 0 | 0 | 2 (16%) | ||
| Meningitis | 1 (7) | 0 | 1 (25%) | 0 | ||
| Encephalopathy Scorea (21, 22) | 5 (4–6) | 3 (2–4) | <0.001 | 6 (4–6) | 4 (4–6) | 0.13 |
| MRI Injury Score c (27) | 5 (2–9) | 2 (0–6) | 0.07 | 6 (5–8) | 3 (2–6) | 0.15 |
| Acute Seizures | Not applicable | 4 (100%) | 8 (62%) | 0.26 | ||
| Maternal Risk Factors | ||||||
| Age (years) a | 30.5 (8.4) | 30.7 (6.1) | 0.97 | 25.4 (6.4) | 30.8 (6.6) | 0.17 |
| Steroid treatment, n (%) | 1 (7) | 0 | 0.38 | 0 | 1 (8) | 0.57 |
| Antibiotic, n (%) | 5 (33) | 4 (36) | 0.87 | 3 (75) | 3 (23) | 0.06 |
| Fever, n (%) | 2 (13) | 3 (27) | 0.49 | 1 (25) | 1 (8) | 0.10 |
: mean (95% CI) or
median (interquartile range).
Radiologic evidence of HIE (including gray or white matter injury), radiologic evidence of ischemic or hemorrhagic stroke, and laboratory evidence of meningitis.
Abbreviations: GA: Gestational age; HIE/NE: Hypoxic-ischemic encephalopathy/ Neonatal encephalopathy; IQR: Interquartile Range; wk: week.
Remote Epilepsy
Seventeen (65%) of 26 neonates had more than two years of follow-up data for review to evaluate for epilepsy onset after the neonatal period. Four of 17 (24%) developed epilepsy, all of whom had a history of acute symptomatic seizures. There were no significant differences in demographic, clinical, and maternal risk factors between those with and without remote epilepsy (Table 1).
Cytokine analyses
Cytokine levels were evaluated at 2.5 days of life (95% confidence interval: 2.1 to 2.9). There were no significant differences in timing of DBS collection between those with and without acute symptomatic seizure or between those with and without remote epilepsy. Cytokine levels did not vary by sex, race, gestational age, maternal age, encephalopathy etiology, encephalopathy score, or MRI severity score.
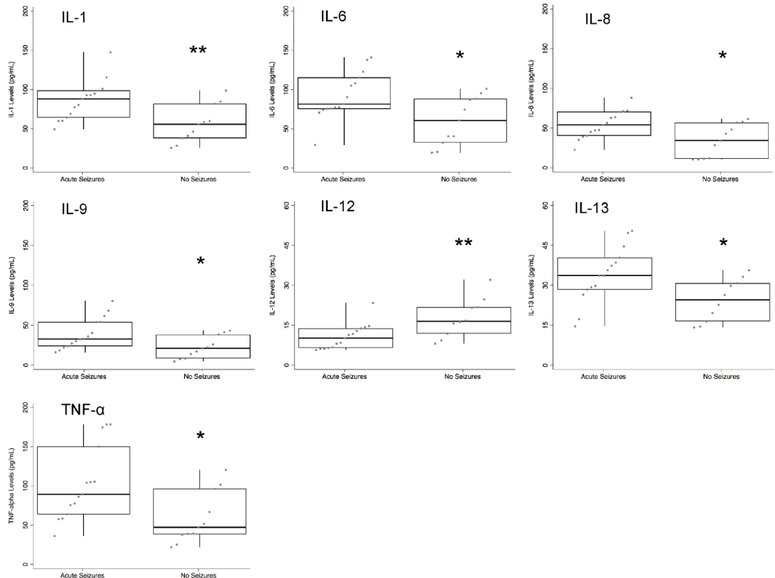
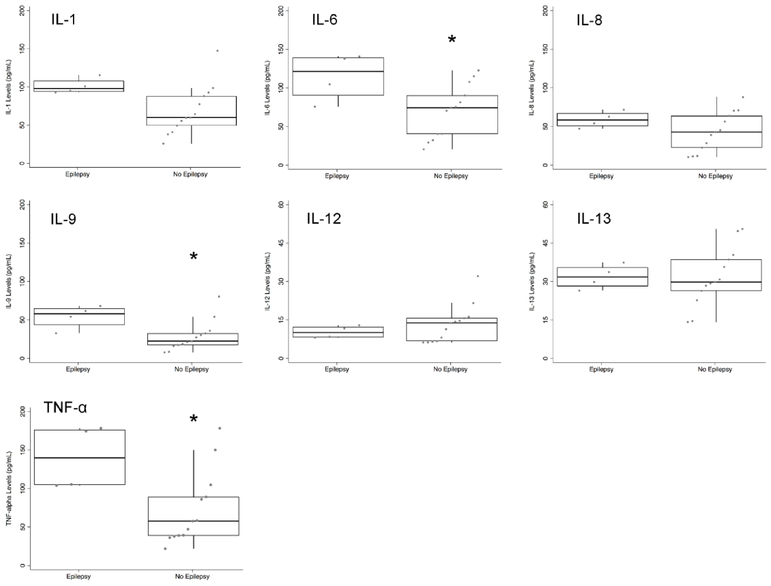
Neonates with acute symptomatic seizures had broad difference in cytokines levels in comparison to those without acute seizures (Figure 1). Those with acute seizures had higher levels of IL-1, 6, 8, 9, 13, and TNF-α, as well as lower levels of IL-12 compared to those without acute seizures. In contrast, neonates who developed epilepsy had higher levels of cytokines specific to the IL-1β pathway, including IL-6 and TNF-α, as well as IL-9 (Figure 2). There was trend for higher IL-1 levels (combined α- and β- subunits) in those who developed epilepsy (p=0.07).
Figure 1:
Neonatal cytokine levels in children with acute symptomatic/early seizures (n=151) compared to those without seizures (n=11). Levels are plotted from 0–200 pg/mL, except for IL-12 and IL-13, which are plotted from 0–60 pg/mL. * p < 0.05, ** p< 0.01.
Figure 2:
Neonatal cytokine levels in children with epilepsy (n=4) compared to those without epilepsy (n=13). Levels are plotted from 0–200 pg/mL, except for IL-12 and IL-13, which are plotted from 0–60 pg/mL. * p<0.05.
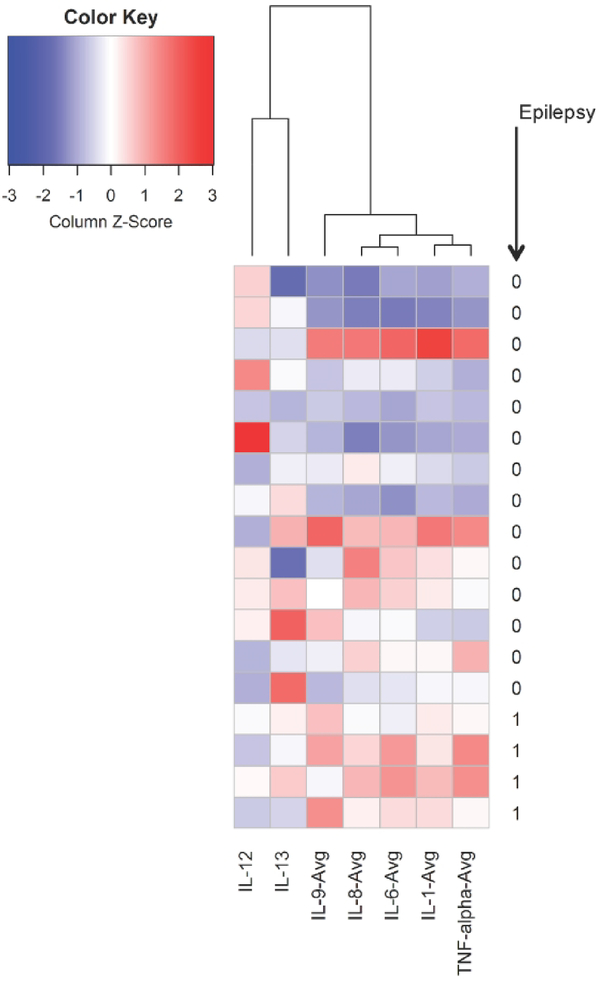
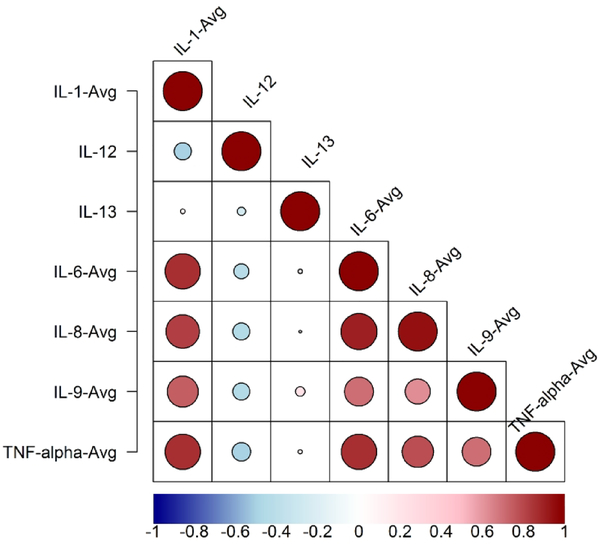
We evaluated whether cytokine levels can discriminate between those with and without remote epilepsy with the receiver operator curve characteristics. The AUC for cytokines within the pro-inflammatory IL-1β pathway ranged from 0.87–0.88, demonstrating a strong effect and superior performance to IL-12 and IL-13 (Table 2). Hierarchical agglomerative clustering algorithms revealed difference in patterns of cytokine level changes in neonates with and without later onset of epilepsy (Figure 3). Dendrograms (Figure 3) and bubble/heatmaps (Figure 4) confirmed correlations of cytokines within the pro-inflammatory IL-1β pathway (IL-1, IL-6, IL-8, TNF-α). IL-12 and IL-13, which are involved in disparate signaling pathways, were not correlated with the other cytokines measured.
Table 2:
Area under the receiver operating characteristic (AUC) coefficients for cytokine levels based upon development of remote epilepsy.
| Receiver Operator Curve Area under the Curve (95% confidence interval) | |
|---|---|
| IL-1 | 0.88 (0.72–1) |
| IL-6 | 0.85 (0.63–1) |
| IL-8 | 0.71 (0.45–0.97) |
| IL-9 | 0.88 (0.71–1) |
| IL-12 | 0.41 (0.15–0.68) |
| IL-13 | 0.53 (0.24–0.81) |
| TNF-α | 0.87 (0.69–1) |
Figure 3:
Dendrogram and heatmap of neonatal cytokines levels in 26 neonates with neonatal encephalopathy using hierarchical agglomerative clustering. Children with epilepsy are denoted with “1” and children without epilepsy are denoted with “0”. Dark blue colors represent low cytokine values, white colors represent average values, and red represent high values.
Figure 4:
Correlations among cytokine levels in 26 neonates with neonatal encephalopathy. Larger bubbles and darker red coloring indicates a higher correlation coefficient whereas smaller bubbles and dark blue coloring indicate lower correlation coefficients.
Discussion
Here, we evaluated the association of inflammatory cytokines with acute symptomatic seizures and development of remote epilepsy in a cohort of neonates with encephalopathy. Acute symptomatic seizures in neonates with encephalopathy were associated with broad changes in cytokines in both pro- and anti-inflammatory cytokine levels. However, only increased levels of pro-inflammatory cytokines in the IL-1β pathway, and to a lesser extend IL-9 were associated with later development of epilepsy.
This group previously reported that in this cohort of neonates with encephalopathy, elevations in IL-1, IL-6, and IL-8 on dried blood spots were correlated with elevated lactate peaks on MRS. (21) Similarly, “The Extremely Low Gestational Age Newborn Study” (ELGAN) study evaluated several cytokines in premature infants born less than 28 weeks gestation using analyses of dried blood spots. (25) Changes in cytokine levels in several pro- and anti-inflammatory pathways were associated with the presence of intraventricular hemorrhage and/or white matter injury. (29) As neonatal brain injury is a risk factor for the development of acute symptomatic seizures, (3, 4) it is not surprising that we found acute symptomatic seizures after neonatal brain injury were associated with alterations in a number of pro- and anti-inflammatory cytokine pathways. Our findings corroborate data from a cohort of 13 neonates with hypoxic-ischemic encephalopathy, where serum levels of IL-1 receptor antagonist (an inhibitor of the IL-1 pathway) were lower and levels of IL-8 were higher among neonates with seizures compared to controls. (24) Though, in all clinical studies evaluating cytokines levels in neonates to date, the development of remote epilepsy was not assessed.
Our finding that neonatal elevations of cytokines within the IL-1β pathway are associated with the development of epilepsy in childhood is novel. This pathway, however has been associated with epilepsy in several other clinical populations including adults with traumatic brain injury and post-traumatic epilepsy. (30, 31) Therefore, this pathway may serve as a marker of epilepsy across several types of brain injury with the potential for understanding mechanisms of epileptogenesis. (32) Cytokines in the IL-1β pathway modulate the excitatory NMDA receptor and the inhibitory GABA receptor on neurons and astrocytes, resulting in lower seizure thresholds, which can lead to a positive feedback loop with perpetuation of seizure activity and brain inflammation. (11–13, 33, 34) After an initial brain injury, this ongoing inflammation can alter neuronal plasticity with network reorganization through several transcriptionally regulated effects, with potential for aberrant and epileptogenic circuits. (14–18)
In addition to differences in the IL-1β pathway we also found that increased levels of IL-9 were associated with later development of epilepsy. The role of IL-9 in neurologic injury is less understood, with an emerging role in encephalomyelitis and multiple sclerosis. (35) Further investigations are needed to validate this finding.
Limitations of our study include our small sample size, which did not allow us to evaluate for potential confounding by brain injury severity on cytokine levels and later development of epilepsy. The etiology of neonatal encephalopathy however, was similar to that observed in larger cohorts of term neonates. While the prevalence of acute symptomatic seizures in neonates with encephalopathy is higher than that of contemporary cohorts (58%), it is consistent with the prevalence of acute symptomatic seizures in neonates before therapeutic hypothermia was adopted. (36) In this cohort, the evaluation of acute symptomatic seizures was not standardized. During the time period in which the cohort was assembled, EEG was not consistently used to evaluate for subclinical seizures in the neonatal period. While this could result in misclassification of neonates without ‘acute symptomatic seizure’, this would reduce our ability to detect an association of cytokine levels with seizures. The prevalence of epilepsy (24%) in this cohort is similar to that observed in larger cohort studies. (37) Owing to the retrospective cohort design, several patients were lost to follow-up for evaluation of epilepsy. While this could introduce bias, we anticipate cases of epilepsy were unlikely to be misclassified owing to referral patterns within our catchment area.
Here, we used dried blood spots to measure cytokine levels. This sampling results in increased measurement variability compared to measurement of cytokine levels in serum or plasma likely owing to the use of capillary blood, sample spread and activity of leukocytes during sample storage without early centrifugation. (38, 39) We would anticipate the use of serum or plasma would decrease the spread of cytokine levels by group and therefore strengthen the observed associations. Here, measurement of IL-1 levels included both the IL-1α and β- subunits. The IL-1β subunit is responsible for inflammation after a neurologic injury, whereas the α-subunit is largely skin derived and would not be expected to change after brain injury. (40) Levels of IL-1α and IL-1β are similar in circulation; therefore inclusion of IL-1α in measurements would dilute the association of IL-1β with our outcomes. If subunit composition analysis of IL-1β were possible, we hypothesize that the trend for increased IL-1 levels associated with epilepsy would have a larger effect size and become statistically significant.
Our findings support the utility of circulating cytokines as a predictor of epilepsy after neonatal brain injury. Larger, prospective studies in contemporary cohorts of neonates are necessary to validate these findings and evaluate for interactions between changes in cytokines levels the IL-1β pathway with clinical factors known to increase risk of remote epilepsy such as brain injury and acute seizure severity.
Article Impact Answers.
In a cohort study of term neonates with encephalopathy, we evaluated the association of seven cytokines with acute symptomatic seizures and development of remote epilepsy.
Acute symptomatic seizures were associated with diffuse alterations in both pro- and anti-inflammatory cytokine levels.
However, only increased levels of pro-inflammatory cytokines in the IL-1β pathway, and to a lesser extend IL-9 were associated with later development of epilepsy.
These findings support the utility of circulating cytokines as a predictor of epilepsy after neonatal brain injury.
Acknowledgements
The authors thank Dr. Agnes Bartha for providing the groundwork for this dataset.
Statement of financial support: Supported by National Institutes of Health Grants RR 01271, NS 35902, NS 40117, and the American Academy of Neurology Clinical Research Training Fellowship in Epilepsy.
Footnotes
Disclosures statement: The authors declare no conflicts of interest.
Disclosures
Dr. Adam Numis: The author declares no conflicts of interest.
Dr. Xutao Deng: The author declares no conflicts of interest.
Dr. Audrey Foster-Barber: The author declares no conflicts of interest.
Dr. Elizabeth E Rogers: The author declares no conflicts of interest.
Dr. A James Barkovich: The author declares no conflicts of interest.
Dr. Donna M Ferriero: The author declares no conflicts of interest.
Dr. Hannah C Glass: The author declares no conflicts of interest.
Category of Study: Clinical
References
- 1.Ronen GM, Penney S, Andrews W 1999. The epidemiology of clinical neonatal seizures in Newfoundland: a population-based study. J Pediatr 134:71–75. [DOI] [PubMed] [Google Scholar]
- 2.Lanska MJ, Lanska DJ, Baumann RJ, Kryscio RJ 1995. A population-based study of neonatal seizures in Fayette County, Kentucky. Neurology 45:724–732. [DOI] [PubMed] [Google Scholar]
- 3.Fox CK, Glass HC, Sidney S, Smith SE, Fullerton HJ 2016. Neonatal seizures triple the risk of a remote seizure after perinatal ischemic stroke. Neurology 86:2179–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glass HC, Hong KJ, Rogers EE, et al. 2011. Risk factors for epilepsy in children with neonatal encephalopathy. Pediatr Res 70:535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glass HC, Numis AL, Gano D, Bali V, Rogers EE 2018. Outcomes After Acute Symptomatic Seizures in Children Admitted to a Neonatal Neurocritical Care Service. Pediatr Neurol 84:39–45. [DOI] [PubMed] [Google Scholar]
- 6.Sillanpaa M, Camfield P, Camfield C 1995. Predicting long-term outcome of childhood epilepsy in Nova Scotia, Canada, and Turku, Finland. Validation of a simple scoring system. Arch Neurol 52:589–592. [DOI] [PubMed] [Google Scholar]
- 7.Ronen GM, Buckley D, Penney S, Streiner DL 2007. Long-term prognosis in children with neonatal seizures: a population-based study. Neurology 69:1816–1822. [DOI] [PubMed] [Google Scholar]
- 8.Vezzani A, Friedman A, Dingledine RJ 2013. The role of inflammation in epileptogenesis. Neuropharmacology 69:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Simoni MG, Perego C, Ravizza T, et al. 2000. Inflammatory cytokines and related genes are induced in the rat hippocampus by limbic status epilepticus. Eur J Neurosci 12:2623–2633. [DOI] [PubMed] [Google Scholar]
- 10.Rooker S, Jander S, Van Reempts J, et al. 2006. Spatiotemporal pattern of neuroinflammation after impact-acceleration closed head injury in the rat. Mediators Inflamm 2006:90123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang S, Cheng Q, Malik S, Yang J 2000. Interleukin-1beta inhibits gamma-aminobutyric acid type A (GABA(A)) receptor current in cultured hippocampal neurons. J Pharmacol Exp Ther 292:497–504. [PubMed] [Google Scholar]
- 12.Lai AY, Swayze RD, El-Husseini A, Song C 2006. Interleukin-1 beta modulates AMPA receptor expression and phosphorylation in hippocampal neurons. J Neuroimmunol 175:97–106. [DOI] [PubMed] [Google Scholar]
- 13.Roseti C, van Vliet EA, Cifelli P, et al. 2015. GABAA currents are decreased by IL-1beta in epileptogenic tissue of patients with temporal lobe epilepsy: implications for ictogenesis. Neurobiol Dis 82:311–320. [DOI] [PubMed] [Google Scholar]
- 14.Pugazhenthi S, Zhang Y, Bouchard R, Mahaffey G 2013. Induction of an inflammatory loop by interleukin-1beta and tumor necrosis factor-alpha involves NF-kB and STAT-1 in differentiated human neuroprogenitor cells. PLoS One 8:e69585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ross FM, Allan SM, Rothwell NJ, Verkhratsky A 2003. A dual role for interleukin-1 in LTP in mouse hippocampal slices. J Neuroimmunol 144:61–67. [DOI] [PubMed] [Google Scholar]
- 16.del Rey A, Balschun D, Wetzel W, Randolf A, Besedovsky HO 2013. A cytokine network involving brain-borne IL-1beta, IL-1ra, IL-18, IL-6, and TNFalpha operates during long-term potentiation and learning. Brain Behav Immun 33:15–23. [DOI] [PubMed] [Google Scholar]
- 17.Yin P, Li Z, Wang YY, et al. 2013. Neonatal immune challenge exacerbates seizure-induced hippocampus-dependent memory impairment in adult rats. Epilepsy Behav 27:9–17. [DOI] [PubMed] [Google Scholar]
- 18.Balosso S, Maroso M, Sanchez-Alavez M, et al. 2008. A novel non-transcriptional pathway mediates the proconvulsive effects of interleukin-1beta. Brain 131:3256–3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qin LJ, Gu YT, Zhang H, Xue YX 2009. Bradykinin-induced blood-tumor barrier opening is mediated by tumor necrosis factor-alpha. Neurosci Lett 450:172–175. [DOI] [PubMed] [Google Scholar]
- 20.Didier N, Romero IA, Creminon C, Wijkhuisen A, Grassi J, Mabondzo A 2003. Secretion of interleukin-1beta by astrocytes mediates endothelin-1 and tumour necrosis factor-alpha effects on human brain microvascular endothelial cell permeability. J Neurochem 86:246–254. [DOI] [PubMed] [Google Scholar]
- 21.Bartha AI, Foster-Barber A, Miller SP, et al. 2004. Neonatal encephalopathy: association of cytokines with MR spectroscopy and outcome. Pediatr Res 56:960–966. [DOI] [PubMed] [Google Scholar]
- 22.Foster-Barber A, Ferriero DM 2002. Neonatal encephalopathy in the term infant: neuroimaging and inflammatory cytokines. Ment Retard Dev Disabil Res Rev 8:20–24. [DOI] [PubMed] [Google Scholar]
- 23.Bonifacio SL, Glass HC, Vanderpluym J, et al. 2011. Perinatal events and early magnetic resonance imaging in therapeutic hypothermia. J Pediatr 158:360–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Youn YA, Kim SJ, Sung IK, Chung SY, Kim YH, Lee IG 2012. Serial examination of serum IL-8, IL-10 and IL-1Ra levels is significant in neonatal seizures induced by hypoxic-ischaemic encephalopathy. Scand J Immunol 76:286–293. [DOI] [PubMed] [Google Scholar]
- 25.O’Shea TM, Allred EN, Kuban KC, et al. 2012. Elevated concentrations of inflammation-related proteins in postnatal blood predict severe developmental delay at 2 years of age in extremely preterm infants. J Pediatr 160:395–401 e394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phillips TM, Krum JM 1998. Recycling immunoaffinity chromatography for multiple analyte analysis in biological samples. J Chromatogr B Biomed Sci Appl 715:55–63. [DOI] [PubMed] [Google Scholar]
- 27.Barkovich AJ, Hajnal BL, Vigneron D, et al. 1998. Prediction of neuromotor outcome in perinatal asphyxia: evaluation of MR scoring systems. AJNR Am J Neuroradiol 19:143–149. [PMC free article] [PubMed] [Google Scholar]
- 28.Hajnal BL, Sahebkar-Moghaddam F, Barnwell AJ, Barkovich AJ, Ferriero DM 1999. Early prediction of neurologic outcome after perinatal depression. Pediatr Neurol 21:788–793. [DOI] [PubMed] [Google Scholar]
- 29.Leviton A, Allred EN, Dammann O, et al. 2013. Systemic inflammation, intraventricular hemorrhage, and white matter injury. J Child Neurol 28:1637–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diamond ML, Ritter AC, Failla MD, et al. 2014. IL-1beta associations with posttraumatic epilepsy development: a genetics and biomarker cohort study. Epilepsia 55:1109–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gallentine WB, Shinnar S, Hesdorffer DC, et al. 2017. Plasma cytokines associated with febrile status epilepticus in children: A potential biomarker for acute hippocampal injury. Epilepsia 58:1102–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vezzani A, Aronica E, Mazarati A, Pittman QJ 2013. Epilepsy and brain inflammation. Exp Neurol 244:11–21. [DOI] [PubMed] [Google Scholar]
- 33.Chiavegato A, Zurolo E, Losi G, Aronica E, Carmignoto G 2014. The inflammatory molecules IL-1beta and HMGB1 can rapidly enhance focal seizure generation in a brain slice model of temporal lobe epilepsy. Front Cell Neurosci 8:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tao AF, Xu ZH, Chen B, et al. 2015. The Pro-inflammatory Cytokine Interleukin-1beta is a Key Regulatory Factor for the Postictal Suppression in Mice. CNS Neurosci Ther 21:642–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ding X, Cao F, Cui L, Ciric B, Zhang GX, Rostami A 2015. IL-9 signaling affects central nervous system resident cells during inflammatory stimuli. Exp Mol Pathol 99:570–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orbach SA, Bonifacio SL, Kuzniewicz MW, Glass HC 2014. Lower incidence of seizure among neonates treated with therapeutic hypothermia. J Child Neurol 29:1502–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pisani F, Facini C, Pavlidis E, Spagnoli C, Boylan G 2015. Epilepsy after neonatal seizures: literature review. Eur J Paediatr Neurol 19:6–14. [DOI] [PubMed] [Google Scholar]
- 38.Skogstrand K, Ekelund CK, Thorsen P, et al. 2008. Effects of blood sample handling procedures on measurable inflammatory markers in plasma, serum and dried blood spot samples. J Immunol Methods 336:78–84. [DOI] [PubMed] [Google Scholar]
- 39.Schiffer JM, Maniatis P, Garza I, et al. 2013. Quantitative assessment of anthrax vaccine immunogenicity using the dried blood spot matrix. Biologicals 41:98–103. [DOI] [PubMed] [Google Scholar]
- 40.Feldmeyer L, Werner S, French LE, Beer HD 2010. Interleukin-1, inflammasomes and the skin. Eur J Cell Biol 89:638–644. [DOI] [PubMed] [Google Scholar]