Abstract
Background
Preterm very low birth weight (VLBW) infants experience physiologic maturation and transitions off therapies from 32–35 weeks postmenstrual age (PMA), which may impact episodic bradycardia and oxygen desaturation. We sought to characterize bradycardias and desaturations from 32–35 weeks PMA and test whether events at 32 weeks PMA are associated with NICU length of stay.
Methods
For 265 VLBW infants from 32–35 weeks PMA, we quantified the number and duration of bradycardias (HR <100 for ≥ 4s) and desaturations (SpO2 <80% for ≥ 10s) and compared events around discontinuation of CPAP, caffeine, and supplemental oxygen. We modeled associations between clinical variables, bradycardias and desaturations at 32 weeks PMA, and discharge PMA.
Results
Desaturations decreased from 60 to 41 per day at 32 and 35 weeks respectively (p<0.01). Duration of desaturations and number and duration of bradycardias decreased to a smaller extent (p<0.05), and there was a non-significant trend toward increased desaturations after stopping CPAP and caffeine. Controlling for clinical variables, longer duration of bradycardias and desaturations at 32 weeks PMA was associated with later discharge PMA.
Conclusion
Delayed recovery from bradycardias and desaturations at 32 weeks PMA, perhaps reflecting less physiologic resilience, is associated with prolonged NICU stay for VLBW infants.
Background
Episodes of bradycardia and oxygen desaturation, sometimes associated with apnea, are common among preterm very low birth weight (VLBW) infants in the Neonatal Intensive Care Unit (NICU)(1–3). Clinicians administer respiratory support and caffeine to infants early in the NICU course to reduce the frequency and severity of these events, and as infants’ lung disease and control of breathing improve, therapies are weaned and discontinued(4). In some infants, clinically significant bradycardias and desaturations persist as they approach term corrected age and are otherwise ready for discharge home(5), and a more complete understanding of these events might lead to strategies to reduce NICU length of stay (LOS).
Bradycardias and desaturations attract clinical attention when they trigger bedside monitor alarms, and event quantitation typically relies on nursing documentation that underrepresents their frequency and severity(6,7). Intermittent hypoxemia is associated with adverse outcomes (8,9), and a more reliable method for quantitation of events is required to accurately describe their natural history and clinical associations as well as the impact of adding or withdrawing therapies. Our group previously developed an automated algorithm that analyzes NICU bedside monitor waveforms and vital signs for central apnea and periodic breathing(1,10,11). More recently we reported on bradycardia and desaturation events in the first 4 weeks following birth, irrespective of apnea, and their association with chronic respiratory morbidity and other adverse outcomes(2). The natural extension of this work was to study these events closer to the end of the NICU stay.
Between 32 and 35 weeks PMA, most preterm VLBW infants experience physiologic maturation, which would be expected to reduce the frequency and severity of apnea spells. However, during this same period, many infants transition off CPAP, caffeine and supplemental oxygen which might at least transiently increase obstructive or central apnea with associated decline in heart rate or oxygen saturation. We sought to quantitate number and duration of bradycardia and desaturation events from 32–35 weeks PMA, including around the time of transition off therapies, and to test the hypothesis that more or longer events at 32 weeks PMA are associated with discharge at later PMA.
Methods
Cohort
The study population was drawn from all VLBW infants 23–33 weeks’ gestation admitted to the University of Virginia NICU between January 2009 and March 2014 who were discharged home and who did not have chromosomal syndromes or congenital anomalies that could impact oxygenation. We excluded infants with < 7 days of bedside monitor vital sign data available in the first four weeks after birth in order to exclude those who were transferred to our unit at later ages for procedures. We also excluded infants with less than 48 hours of cumulative monitor data available in the 32nd and 35th week PMA since those were the principal periods of interest for the analyses.
The University of Virginia NICU is a Level IV facility (Vermont Oxford Network Type C). This study was approved by the University of Virginia Institutional Review Board with waiver of consent. Algorithm analysis of bradycardia and desaturation events was performed after patients were discharged and not available to clinicians for decisions about patient care.
Clinical Data Collection
Study data included demographic and clinical features that were routinely extracted from the medical record into a relational clinical database (NeoData, Isoprime, Chicago IL). Clinical variables potentially impacting NICU LOS based both on the literature(12) and on our prior work were recorded, including diagnosis of septicemia (positive blood culture and at least 5 days of antibiotics) and necrotizing enterocolitis (NEC Bell’s stage 2–3), and days on ventilator. Ventilatory support at 32 weeks PMA was incorporated into a Respiratory Acuity Score (RAS), which we previously published as being associated with BPD and other outcomes(2). Briefly, mode of support at 32 0/7 weeks PMA is assigned a value which is multiplied by the mean FiO2 that day to arrive at the RAS. We also recorded, for each infant, the PMA at which CPAP, caffeine, and supplemental oxygen were discontinued. In the years of the study, CPAP was generally continued until a VLBW infant was > 32 weeks PMA and on <40% supplemental oxygen. Caffeine was administered to all infants <32 weeks gestation at birth and discontinued when an infant was greater than 32 weeks PMA, off CPAP and not having significant apneic spells. Target SpO2 range for VLBW infants on supplemental FiO2 was 88–95%.
Bedside Monitor Data Collection
Heart rate (HR) from electrocardiogram and SpO2 from pulse oximetry were collected from continuous bedside monitoring devices using BedMaster (Excel Medical, Jupiter FL). HR and SpO2 readings were sampled every 2 seconds (0.5 Hz). SpO2 is monitored with Masimo technology, with an averaging time of 8 seconds.
Using the monitor data available for each infant between PMA 32 and 35 weeks (and excluding incontrovertible artifact of HR and SpO2 values of zero), we employed an automated algorithm to identify bradycardia and desaturation events, as we have previously published(2). Bradycardia was defined as HR < 100 for ≥ 4 seconds, and desaturation as SpO2 < 80% for ≥ 10 seconds. Events were joined if the value rose above then fell back below the threshold in <4 seconds for bradycardia and <10 seconds for desaturation events. We quantified the number and duration of events, normalized to amount of available data. For the figures and for modeling, median number and duration of events at 32 weeks PMA represents all days with data available from 32 0/7 to 32 6/7, and at 35 weeks PMA represents all days from 35 0/7 to 35 6/7.
Statistical analyses
Data are presented as median and interquartile range unless otherwise noted. Number and duration of events across PMAs and after discontinuing therapies were compared with Wilcoxon rank-sum tests. Impact of event number and duration at 32 weeks PMA on discharge PMA was first analyzed with univariate linear regression modeling. We then performed multiple regression analysis to control for demographic and clinical variables shown in univariate analysis to impact NICU LOS. For both the univariate and adjusted methods, we tested the significance of event predictors using T-tests of coefficients, and the variability explained by each model with adjusted R2. Analyses were performed in R version 3.4.1 with statistical significance considered as p < 0.05.
Results
Demographics
During the 5-year study period, 628 VLBW infants were admitted to the UVA NICU. Of these, 363 were excluded; 72 died or had major chromosomal or congenital anomalies that could impact oxygenation, 111 were transferred to an outside hospital or another unit, and 180 had insufficient bedside monitor data available. Reasons for insufficient monitor data included transfer to our unit at a late age or technical issues with the data collection system.
The study cohort included 265 VLBW infants with median gestational age 27 weeks. Table 1 shows demographic and clinical features for the entire cohort and for three birth weight subgroups. Median PMA at discharge home was 37 weeks and 83 infants (31%) were discharged on supplemental oxygen. Of the 47 infants (18%) discharged at or beyond 40 weeks PMA, 27 had discharge delayed due to respiratory causes.
Table 1:
Demographic and clinical variables by birth weight category
| <750 grams | 750–999 grams | 1000–1499 grams | All <1500 grams | |
|---|---|---|---|---|
| TOTAL | 60 (23%) | 84 (32%) | 121 (46%) | 265 (100%) |
| Demographics | ||||
| Female | 32 (53%) | 30 (36%) | 68 (56%) | 130 (49%) |
| Gestational age weeks | 24 (23,25) | 26 (25,28) | 29 (28,31) | 27 (25,29) |
| Birth weight grams | 650 (580,720) | 860 (810,942) | 1230 (1120,1375) | 961 (770,1200) |
| Clinical variables | ||||
| Apgar 1 minute | 3 (1,6) | 5 (3,7) | 6 (4,8) | 6 (2,7) |
| Apgar 5 minutes | 7 (4,8) | 7 (6,8) | 8 (7,8) | 7 (6,8) |
| Septicemia | 13 (22%) | 21 (25%) | 11 (9%) | 45 (17%) |
| Necrotizing enterocolitis | 1 (2%) | 10 (12%) | 4 (3%) | 15 (6%) |
| Respiratory Acuity Score at 32w | 1.18 (0.86,1.68) | 0.94 (0.67,1.3) | 0.31 (0,0.84) | 0.84 (0.22,1.16) |
| Therapy transition times | ||||
| CPAP discontinuation PMA | 34 (32,36) | 33 (31,35) | 31 (30,32) | 32 (31,34) |
| Caffeine discontinuation PMA | 35 (34,37) | 34 (33,36) | 32 (32,33) | 33 (32,35) |
| Room air PMA | 35 (33,37) | 34 (33,36) | 32 (31,33) | 33 (31,35) |
| Discharge home | ||||
| Discharge PMA | 39 (38,40) | 38 (36,39) | 36 (36,37) | 37 (36,39) |
| Discharged >= 40 weeks PMA | 22 (37%) | 19 (23%) | 6 (5%) | 47 (18%) |
| Discharged with oxygen | 35 (58%) | 34 (40%) | 14 (12%) | 83 (31%) |
Data shown as n (%) or median (25th, 75th percentile). CPAP continuous positive airway pressure; PMA postmenstrual age
Frequency and duration of bradycardia and desaturation events from 32–35 weeks PMA
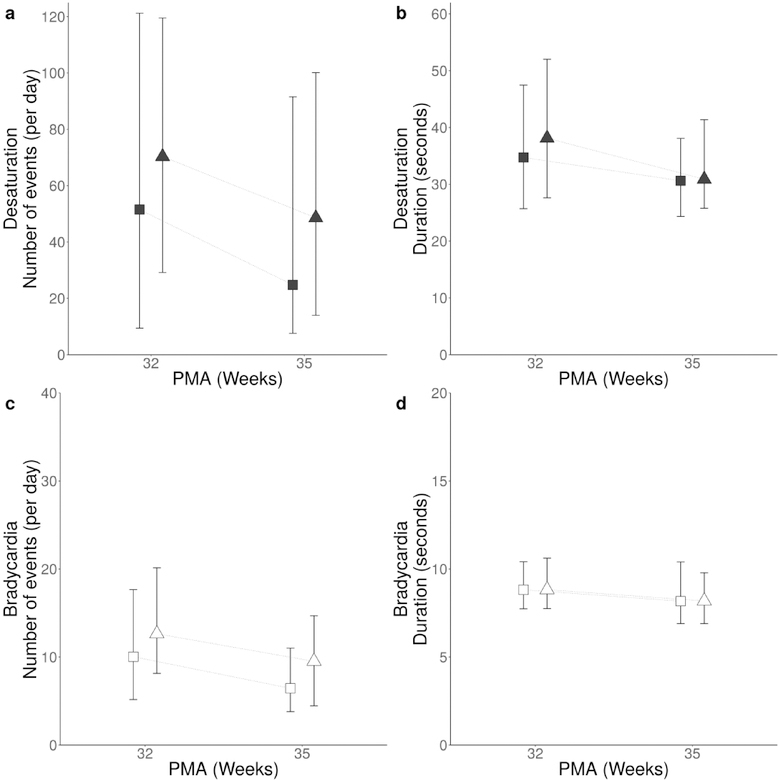
Percent of time with HR and SpO2 data available from 32–35 weeks PMA for the 265 infants was 87%, yielding > 17 infant years of data with 81,947 bradycardia and 454,040 desaturation events analyzed. At the 32 week time point (from 32 0/7 to 32 6/7 weeks) all 265 infants had at least one bradycardia and one desaturation event per day (median), and 97% and 93% of infants had at least 10 bradycardias and desaturations, respectively. Figure 1 shows that desaturations were approximately 6-fold more frequent and 4-fold longer in duration compared to bradycardias. There was a decrease in number of desaturations over time, from median 60 events per day at 32 weeks to 41 events per day at 35 weeks (from 35 0/7 to 35 6/7)(p < 0.05), with wide variability between infants. The decrease in number of bradycardias from 32–35 weeks was statistically significant but small (12 per day at 32 weeks and 8 per day at 35 weeks, p < 0.05). Male infants experienced more events than females: median 11 versus 9 bradycardias per day and 72 versus 41 desaturations per day (p<0.05). Duration of events was slightly lower at 35 versus 32 weeks PMA (bradycardia 9 vs. 8 seconds p< 0.05, desaturation 36 vs 31 seconds, p < 0.05).
Figure 1: Number and duration of bradycardia and desaturation events at 32 and 35 weeks PMA by sex.
Median daily number and duration of desaturations at 32 weeks PMA (32 0/7 to 32 6/7) and 35 weeks PMA (35 0/7 to 35 6/7) are shown as filled symbols (panels A and B); triangles for males, squares for females. Bradycardia number and duration at 32 and 35 weeks PMA are shown as open symbols (panels C and D). Error bars indicate interquartile range.
Impact of discontinuing CPAP, caffeine, and supplemental oxygen on number of events
Supplemental Figure S1 shows the relationship between the number and duration of bradycardia and desaturation events at 32w PMA and the PMA at which CPAP, caffeine, and supplemental oxygen were discontinued. There was an association between more desaturations and longer bradycardias and desaturations at 32w PMA and later discontinuation of these therapies.
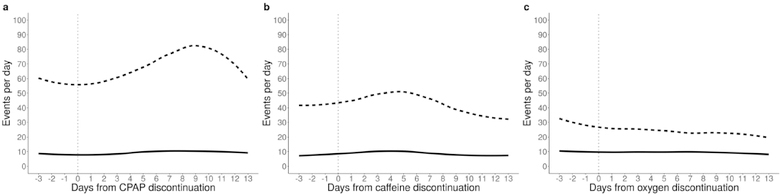
For infants who came off CPAP, caffeine, and supplemental oxygen between 32 and 35 weeks PMA (n=105, 170, and 85 respectively), we analyzed number of bradycardia and desaturation events before and after discontinuation. Figure 2 shows that bradycardia events did not change (median 8–10 events per day before and after). For desaturations, there was a non-significant, transient trend toward increased events in the week after discontinuing CPAP and caffeine, but not after stopping supplemental oxygen. Desaturations peaked 9 days after coming off CPAP, with median 68 events per day (interquartile range, IQR, 21,132). This was slightly higher but not significantly different compared to the number of desaturations the day that CPAP was discontinued (median 59, IQR 14, 97, p=0.34). The small increase in desaturations 5 days following caffeine discontinuation was also not significantly different compared to the day of discontinuation (p=0.32).
Figure 2: Trends in bradycardia and desaturation events after CPAP, caffeine, and supplemental oxygen discontinuation.
Median number of bradycardias (solid lines) and desaturations (dashed lines) are shown from 3 days before until 13 days after discontinuation of CPAP (panel A), caffeine (panel B), and supplemental oxygen (panel C).
Association of bradycardia and desaturation events at 32 weeks PMA with length of stay
Using linear regression modeling, we evaluated the association between discharge PMA, clinical variables, and bradycardia and desaturation characteristics at 32 weeks PMA. Table 2 shows results of univariate (top) and multivariate (bottom) analyses, including p values as well as beta coefficient indicating the direction and the R2 value the strength of the association. In univariate analysis, number and duration of desaturations and duration of bradycardias were associated with later discharge PMA, but number of bradycardias was not (p=0.684). Multiple clinical variables were associated with later discharge PMA in univariate analysis, but sex was not (p=0.109). The clinical variable with the strongest association with late NICU discharge was Respiratory Acuity Score at 32 0/7 weeks PMA with R2 of 0.336, compared to the next strongest variable of gestational age with R2 of 0.189. A combined clinical model with GA, birth weight, sex, 1 and 5 minute Apgar scores, 32w Respiratory Acuity Score, and diagnosis of severe IVH, NEC, and septicemia had R2 of 0.411. In multivariate analysis adjusting for all clinical variables, duration of bradycardia and desaturation events at 32 weeks PMA (but not number of events) contributed significantly to identifying infants with later discharge PMA (p<0.01, R2 0.428). This association of longer events at 32 weeks PMA (32 0/7 through 32 6/7) and longer NICU stay held true when analyses were repeated without the 47 infants discharged at ≥40 weeks PMA.
Table 2:
Univariate and multiple regression modeling showing association of clinical variables and bradycardia and desaturation characteristics at 32 weeks PMA with discharge PMA
| Beta value | p value | R2 | |
|---|---|---|---|
| BD characteristics (univariate) | |||
| Number bradycardias | NS | 0.684 | NS |
| Duration bradycardias | 0.187 | < 0.001 | 0.045 |
| Number desaturations | 0.011 | < 0.001 | 0.09 |
| Duration desaturations | 0.066 | < 0.001 | 0.254 |
| Clinical variables (univariate) | |||
| Sex (Male) | NS | 0.109 | NS |
| Gestational age weeks | −0.396 | < 0.001 | 0.189 |
| Birth weight grams | −3.636 | < 0.001 | 0.179 |
| Apgar1 minute | −0.272 | < 0.001 | 0.092 |
| Apgar5 minutes | −0.323 | < 0.001 | 0.073 |
| Septicemia | 1.339 | < 0.001 | 0.043 |
| Necrotizing enterocolitis | 1.949 | 0.002 | 0.034 |
| Respiratory acuity score | 1.482 | < 0.001 | 0.336 |
| All clinical variables | NA | NA | 0.411 |
| BD characteristics (adjusted for all clinical) | |||
| Number bradycardias | NS | 0.678 | NS |
| Duration bradycardias | 0.12 | 0.003 | 0.428 |
| Number desaturations | NS | 0.813 | NS |
| Duration desaturations | 0.024 | 0.004 | 0.428 |
| NS non significant; BD bradycardia desaturation | |||
Beta value sign indicates the direction and R2 the strength of the association
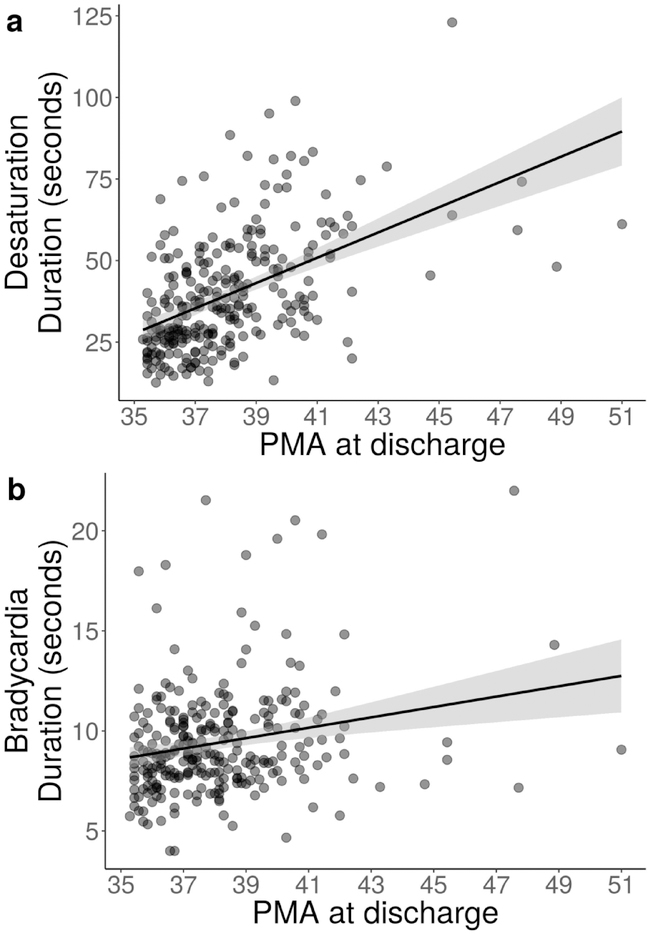
Figure 3 shows the association between average duration of bradycardia and desaturation events at 32 weeks PMA and discharge PMA. For every one second increase in mean bradycardia duration, NICU LOS increased by 1.3 days, and for every one second increase in mean desaturation duration, NICU LOS increased by 0.5 days.
Figure 3: Association between duration of bradycardia and desaturation events at 32 weeks and discharge PMA.
Scatter plot, with one point for each of the 265 infants, demonstrating a positive linear relationship between PMA at discharge and average duration of desaturations (panel A) and bradycardias (panel B) at 32 weeks PMA. The solid line indicates the fitted linear model and grey shading indicates the 95% confidence interval around the trend.
Discussion
In a large cohort of VLBW infants with continuous cardiorespiratory monitor data available from 32 to 35 weeks PMA, we quantified bradycardia and desaturation events and their impact on NICU length of stay. The main finding is that events decreased slightly in both number and duration over this period and, after adjusting for demographic and clinical variables, duration (but not number) of bradycardia and desaturation events at 32 weeks PMA was associated with later PMA at discharge.
As VLBW infants approach term corrected age, occurrence of cardiorespiratory events is impacted by competing variables. On the one hand, the physiologic maturation taking place would be expected to decrease number and severity of events. On the other hand, respiratory and apnea therapies are being withdrawn, which may lead to increased events. Overall, we found a modest decrease in number of desaturations and a small decrease in number of bradycardias from 32 to 35 weeks PMA suggesting maturation is occurring allowing infants to “outgrow” these spells. Duration of events decreased to an even smaller extent suggesting that recovery time is slower to mature After discontinuation of CPAP, caffeine, and supplemental oxygen we did not find a significant change in events, in contrast to our prior report of a marginal, transient increase in central apnea with associated bradycardia and desaturation after stopping caffeine (13). A limitation of the analyses is that we could not account for the many clinical variables that might impact heart rate deceleration and oxygen desaturation events with or without central or obstructive apnea during this PMA time frame. These include initiation of oral feeding, gastroesophageal reflux, administration of immunizations, performance of ophthalmologic examinations, thermal challenge during incubator weaning, physiologic nadir of anemia of prematurity(14), and other clinical stressors. It is notable that some infants continued to experience a large number of events of SpO2 <80% lasting at least 10 seconds even at 35 weeks PMA, highlighting the ongoing challenge of maintaining target oxygen saturations in preterm infants as they approach term corrected age.
Resilience, a term we use to refer to the ability to recover more quickly from cardiorespiratory events either spontaneously or with clinician intervention, may indicate a more stable physiologic state with advancing age. It is not surprising then that shorter duration of events at 32 weeks is associated with shorter length of stay, since many preterm infants spend extra time in the NICU simply due to bradycardias and desaturations, especially when they are deep or prolonged, or when they require caregiver intervention rather than self-resolving. Some of these events are due to immature control of breathing but they also may be impacted by chronic lung disease which often prolongs NICU stay. We found that a high Respiratory Acuity Score at 32 weeks PMA, factoring in not only FiO2 but also level of ventilatory support, was strongly associated with prolonged NICU stay, and duration of bradycardia and desaturation events increased the association. We speculate that prediction of prolonged NICU LOS through analysis of cardiorespiratory events at 32 weeks might identify a subset of infants that would benefit from longer duration of therapies such as caffeine, and this in turn might allow earlier discharge from the NICU. (15)
Our study is robust in that we analyzed >17 infant-years of HR and SpO2 data on a large number of VLBW infants, but several limitations deserve consideration. The analyses did not address central apnea, which we have previously studied(1,6,10) or obstructive apnea, which is difficult to quantify and contributes a great deal to episodes of bradycardia and desaturation. Also, in developing our algorithms we selected a relatively high threshold for bradycardia (<100 beats/minute) and a low threshold for desaturation (<80%) compared to other studies(3,16). Finally, we could not account for all the variables that might influence these events, nor did we carry the analyses through to discharge home. This area is ripe for further investigation since hypoxemia may impact multiple outcomes and since spells of bradycardia and desaturation are often the final factor delaying NICU discharge.
Conclusions
Longer duration of bradycardia and desaturation events at 32 weeks PMA, perhaps reflecting less physiologic resilience, is associated with prolonged NICU stay. Identification of infants with long events at this maturational stage might allow advanced planning and interventions to facilitate earlier safe discharge home from the NICU.
Supplementary Material
Acknowledgments
Financial support: NIH HD072071, HD064488
Footnotes
Conflicts/disclosures related to this work: none
References
- 1.Fairchild K, Mohr M, Paget-Brown A, Tabacaru C, Lake D, Delos J, et al. Clinical associations of immature breathing in preterm infants: part 1-central apnea. Pediatr Res. 2016. March 9;80(1):21–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fairchild KD, Nagraj VP, Sullivan BA, Moorman JR, Lake DE. Oxygen desaturations in the early neonatal period predict development of bronchopulmonary dysplasia. Pediatr Res. 2018. October 29; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin RJ, DiFiore JM Di, Macfarlane PM, Wilson CG. Physiologic basis for intermittent hypoxic episodes in preterm infants. Adv Exp Med Biol. 2012;758:351–8. [DOI] [PubMed] [Google Scholar]
- 4.Tabacaru CR, Jang SY, Patel M, Davalian F, Zanelli S, Fairchild KD. Impact of Caffeine Boluses and Caffeine Discontinuation on Apnea and Hypoxemia in Preterm Infants. J Caffeine Res. 2017. September 1;7(3):103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eichenwald EC, Aina A, Stark AR. Apnea frequently persists beyond term gestation in infants delivered at 24 to 28 weeks. Pediatrics. 1997. September 1;100(3):354–9. [DOI] [PubMed] [Google Scholar]
- 6.Vergales B, Paget-Brown AAO, Lee H, Guin LE, Smoot TJ, Rusin CG, et al. Accurate Automated Apnea Analysis in Preterm Infants. Am J Perinatol. 2014. February;31(2):157–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brockmann PE, Wiechers C, Pantalitschka T, Diebold J, Vagedes J, Poets CF. Under-recognition of alarms in a neonatal intensive care unit. Arch Dis Child Fetal Neonatal Ed. 2013. November;98(6):F524–7. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt B, Whyte RK, Asztalos EV, Moddemann D, Poets C, Rabi Y, et al. Effects of targeting higher vs lower arterial oxygen saturations on death or disability in extremely preterm infants: a randomized clinical trial. JAMA. 2013. May 22;309(20):2111–20. [DOI] [PubMed] [Google Scholar]
- 9.Poets CF, Roberts RS, Schmidt B, Whyte RK, Asztalos EV., Bader D, et al. Association Between Intermittent Hypoxemia or Bradycardia and Late Death or Disability in Extremely Preterm Infants. JAMA. 2015. August 11;314(6):595–603. [DOI] [PubMed] [Google Scholar]
- 10.Lee H, Rusin CG, Lake DE, Clark MT, Guin L, Smoot TJ, et al. A new algorithm for detecting central apnea in neonates. Physiol Meas. 2011. January;33(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel M, Mohr M, Lake D, Delos J, Moorman JRR, Sinkin RARA, et al. Clinical Associations with Immature Breathing in Preterm Infants. Part 2: Periodic Breathing. Pediatr Res. 2016. July 22;80(1):28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seaton SE, Barker L, Jenkins D, Draper ES, Abrams KR, Manktelow BN. What factors predict length of stay in a neonatal unit: a systematic review. BMJ Open. 2016. October 18;6(10):e010466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tabacaru CR, Jang SY, Patel M, Davalian F, Zanelli S, Fairchild KD. Impact of Caffeine Boluses and Caffeine Discontinuation on Apnea and Hypoxemia in Preterm Infants. J Caffeine Res. 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zagol K, Lake DE, Vergales B, Moorman ME, Paget-Brown A, Lee H, et al. Anemia, apnea of prematurity, and blood transfusions. J Pediatr. Elsevier; 2012. September;161(3):417–421.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rhein LM, Dobson NR, Darnall R a, Corwin MJ, Heeren TC, Poets CF, et al. Effects of caffeine on intermittent hypoxia in infants born prematurely: a randomized clinical trial. JAMA Pediatr. 2014. March;168(3):250–7. [DOI] [PubMed] [Google Scholar]
- 16.Esquer C, Claure N, D’Ugard C, Wada Y, Bancalari E. Mechanisms of hypoxemia episodes in spontaneously breathing preterm infants after mechanical ventilation. Neonatology. 2008. January;94(2):100–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.