Abstract
People with hemiparesis after stroke appear to recover 70–80% of the difference between their baseline and the maximum Upper Extremity Fugl–Meyer (UEFM) score, a phenomenon called Proportional Recovery (PR). Two recent commentaries explained that PR should be expected because of mathematical coupling between the baseline and change score. Here we ask: if mathematical coupling encourages PR, why do a fraction of stroke patients (the “non-fitters”) not exhibit PR? At the neuroanatomical level of analysis, this question was answered by Byblow et al. – non-fitters lack corticospinal tract (CST) integrity at baseline – but here we address the mathematical and behavioral causes. We first derive a new interpretation of the slope of PR: it is the average probability of scoring across remaining scale items at follow-up. PR therefore breaks when enough test items are discretely more difficult for a patient at follow-up, flattening the slope of recovery. For the UEFM, we show that non-fitters are most unlikely to recover the ability to score on the test items related to wrist/hand dexterity, shoulder flexion without bending the elbow, and finger-to-nose movement , supporting the finding that non-fitters lack CST integrity. However, we also show that a subset of non-fitters respond better to robotic movement training in the chronic phase of stroke. These persons are just able to move the arm out of the flexion synergy and pick up small blocks, both markers of CST integrity. Non-fitters therefore raise interesting questions about the CST function and the basis for response to intensive movement training.
1. Introduction
The Proportional Recovery (PR) Rule was first observed in the context of upper extremity (UE) movement using the Fugl-Meyer (FM) Score 1, a widely used impairment-based measure in stroke rehabilitation research, and then confirmed in larger samples (e.g. 2–4). Samples of patients were evaluated at one week and a follow-up (typically three or six months) and most (the so-called “fitters”) recovered on average 70–80% (depending on the study) of the difference between their baseline and UEFM score and the maximum UEFM score (Figure 1A). The rule is:
| (1) |
where is the mean change in score for all subjects with a baseline score of So, T is the maximum score (66 for the UEFM), and m ≈ 0.7 ― 0.8 is the slope. Other subjects, termed “non-fitters”, achieved substantially less recovery than that predicted from the PR Rule (Figure 1A). The PR Rule has subsequently been shown for measures of lower limb impairment, visuospatial neglect, aphasia, and success at reaching pellets in a rodent stroke model (reviewed in 5), leading to the possibility that it reflects a fundamental biological mechanism of recovery.
Figure 1.
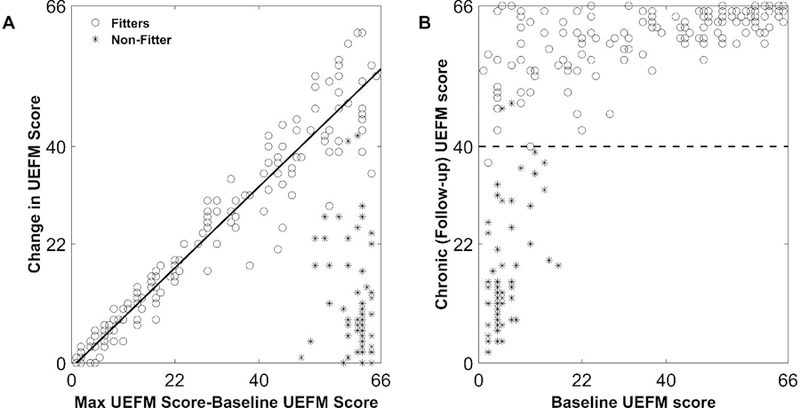
Proportional recovery of the upper extremity Fugl-Meyer (UEFM) score after stroke. These plots combine data that we digitized from reported data of 214 subjects enrolled in two studies 2,3.Baseline scores were measured within 2 weeks post stroke and follow-up at 6 months (chronic stage) A: For “fitters” (o), the average change in UEFM score is proportional to how many points a patient has left to recover (i.e. the maximum UEFM score of 66 minus the baseline UEFM score). “Non-fitters” (*) show decreased recovery. B: By plotting the same data another way (i.e. showing the relationship between chronic (follow-up) UEFM score and baseline UEFM score), it is apparent that 99% of patients with a chronic UEFM score less than 40 (dashed line) in the chronic stage are non-fitters.
Predictions of stroke recovery are multifactorial and highly variable 6, so the finding of such a regularity, especially one driven by a single impairment-based input and present for a wide variety of outcome measures, is striking and even mathematically suspicious, or in the words of Hawe et al. 5, “ too good to be true”. Indeed, Hawe et al. 5 and Hope et al. 7 recently used statistical analysis and simulation to demonstrate that a PR-like relationship arises even if outcomes are unpredictable and recovery is random. Mathematical coupling between and So (since ), high variability of So compared to Sf, and ceiling effects all contribute to PR. These phenomena are known in statistical theory and difficult to correct 5.
But if mathematical coupling encourages PR, then why does PR not arise for a particular subset of stroke patients – the “non-fitters” – who recover less than that predicted by PR? At the neuroanatomical level of analysis, this question has already been elegantly answered: lack of corticospinal tract (CST) integrity strongly predicts non-fitters 3,4,8–11. The first to demonstrate this were Byblow et al. 3 who used motor evoked potentials (MEPs) and found that individuals who at baseline evaluation did not have an MEP response (i.e. who were “MEP-”) were highly likely to be the non-fitters. Stinear et al. 4 confirmed this predictive value of MEP in a larger sample.
Here, we sought to gain mathematical and behavioral insight into the rule breakers of PR by analyzing the rule in terms of the way the UEFM score is constructed – as a sum of subscores on a series of test items – and determining how the ability to score on those items at follow-up mathematically influences PR. We also queried the effect of rehabilitation for fitters and non-fitters by analyzing data from robotic UE training.
2. Preliminary Observations
We digitized reported data from 214 subjects enrolled in two studies 2,3 for analysis here. The inclusion criteria for both studies were (see Table S1 for more detail): (1) first ever ischemic stroke: anterior circulation2 /monohemispheric3 (2) assessments within 2 weeks of stroke (3) no severe cognitive or communication impairment (4) no severe mobility and daily activity impairment (premorbid Barthel Index of ≥ 192 ) (5) no homonymous hemianopia or blindness, no visuospatial neglect or complete somatosensory loss3 (6) no evidence for cerebellar stroke. Only 7% out of 4082 consecutive stroke inpatients met the above criteria in the Winters et al.2 study (36% were unwilling to participate, died, or trasferred to other centers). However, in a latter study, Stinear et al. 4 conformed the same result for a wider stroke population which included people with recurrent stroke and intracerebral hemorrhage.
The second preliminary observation has to do with identifying non-fitters. It is not possible to reliably identify non-fitters using their baseline UEFM alone 2,10–12. Individuals with lower initial UEFM are not necessarily the non-fitters, as can be seen in Figure 1A. However, importantly for our analysis here, it is possible to identify non-fitters when they reach the chronic stage of recovery using an UEFM threshold of about 40. 99% of patients with a chronic UEFM score less than 40 in the chronic stage are non-fitters, as seen in Figure 1B. That is, fitters nearly always recover to an UEFM greater than 40. We will use this observation below in several analyses.
3. Interpreting the PR Rule in Terms of Scale Construction
We first analyze PR for scales that are calculated by summing subscores on a set of items (such as the UEFM score). We show that PR arises robustly for such scales (as expected because of mathematical coupling 5,7), and that the slope has a probabilistic interpretation related to scale construction. We also show that the local slope is not constant for previously reported UEFM PR data, consistent with this probabilistic interpretation. Finally, PR is broken (producing non-fitters) when a substantial number of scale items are discretely more difficult to score on at follow-up for some patients. Finally, we identify the specific UEFM test items that non-fitters have the lowest probability of being able to perform.
Constructing an Outcome Measure
Assume we evaluate recovery at two time points (baseline and follow-up) using a measure that is composed by adding subscores on a set of items. Assume each item can be scored 0 (cannot perform) or 1 (performs well). Then, T is the maximum total score a patient can achieve on the T measure. Assume a patient has achieved a score So at baseline by scoring a 1 on items 1 to So. Then, there are T―So items left for him/her to score 1 on at the follow-up, assuming patients don’t regress on items (i.e. they will score a 1 on any item at follow-up that they scored 1 on at baseline).
Now, assume that at the follow-up the subscore for each remaining item i is a random variable ti that takes the value 1 with probability pi and the value 0 with probability 1 ― pi. We assume that the pi are functions of (T―So) (the number of remaining items = the number of points the patient has left to achieve following the baseline assessment, if items are scored 0 and 1). For example, we expect that patients who are better recovered at baseline will have a higher probability of scoring well on the remaining items at the follow-up. We will call (T―So) the “item recovery probability function” (IRPF) since pi is the probability of scoring 1 on the remaining item given a score of i So at baseline. Then, the increase in score at follow-up is also a random variable:
| (2) |
Now, imagine designing an outcome measure – what should one design the IRPF’s look like? A common strategy in scale design is to observe a sample of patients at baseline and then follow them; this appears to be how the UEFM scale was developed 13. Based on observations and some theoretical basis for a patient’s evolving capabilities (such as a progression out of abnormal synergies for the UEFM scale 13), one then designs a set of test items such that there is a finite probability that some fraction of subjects will be able to do each item at follow-up. Ideally, these probabilities will vary across items, creating a range of items from easy to difficult. Indeed, in one formal approach to scale development, Rasch Analysis, scales are designed so that the probabilities of achieving items are spaced across the range of ability measured over a sample of recovered subjects 14. When Rasch analysis is applied to the UEFM scale, it shows that the UEFM items span a range of difficulties and can be rank-ordered in terms of those difficulties 15.
Figure 2A shows a set of theoretical IRPFs with the number of test items T = 33, matching the UEFM scale. The lower curves represent more difficult items. The probabilities increase with increasing baseline score (i.e. decreasing remaining items to score on), capturing the intuition that patients who are better recovered at baseline have a higher chance of scoring on items at follow-up.
Figure 2.
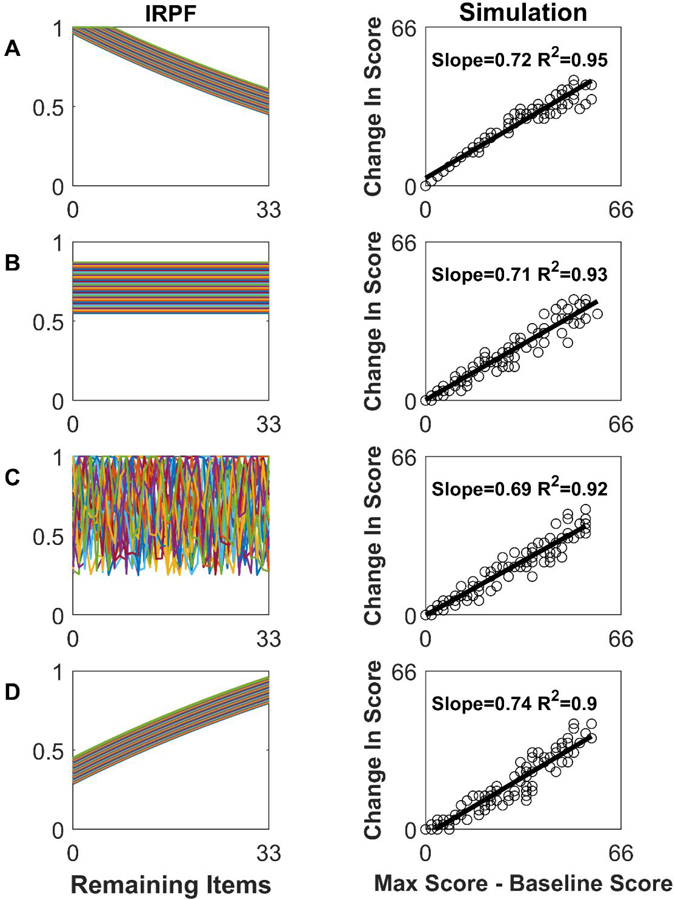
Proportional recovery arises robustly from simulations using a variety of item recovery probability functions (IRPF’s). Left column: Examples of IRPFs, the functions that define the probability of scoring on an item at follow-up given the number of remaining items left to score on. Each line is the IRPF for a different item, for a test composed of 33 items scored 0 or 2. Right column: Recovery results from simulating 120 patients. As can be seen, a variety of IRPFs produce a PR-like relationship, which is expected given mathematical coupling.
Simulating Recovery
Given a set of IRPFs, we can simulate recovery. To produce a total score equal to that possible with the UEFM scale (i.e. 66 pts), we assumed the 33 items could be scored a 0 or a 2. We used a uniform initial distribution of baseline scores Sofor 120 patients. Their predicted recovery exhibited a PR-like behavior (Figure 2A, right column).
How dependent is the PR-like behavior on the shape of the IRPFs? We ran the simulation with several types of IRPFs: pi = a random constant that varied with item number i but not So (Figure 2B); = random number that varied i with and So (Figure 2C); and pi= min (i.e. nonlinear decay in recovery probability) (Figure 2D). In each case, we obtained a PR-like rule (Figure 2 right column), as would be expected due to mathematical coupling.
Bending PR: The Local Slope
We can gain insight into why PR rises robustly for a wide range of IRPFs by estimating the slope of the PR curve. Returning to the case when only scores of 0 and 1 are possible, the mean increase in score from Equation (2) is:
| (3) |
is the average item recovery probability across the remaining items for which the subject has not yet scored 1. Equation 3 potentially restates the PR Rule of Equation 1, but proportionality will hold exactly only if is constant across So. If is not constant, then the local slope of the relationship is:
| (4) |
Equation 4 can be solved assuming a constant m, resulting in a family of functions shifted by an arbitrary constant. Thus, an infinite number of curves produce an exact PR rule. Can we gain insight into scales that would not be expected to produce an exact PR?
First, note that if the IRPFs vary smoothly across So, will also vary smoothly. In this case, even if is not constant, one still gets a PR-like relationship, with the local slope varying according to Equation 4 (Figure 3).
Figure 3.
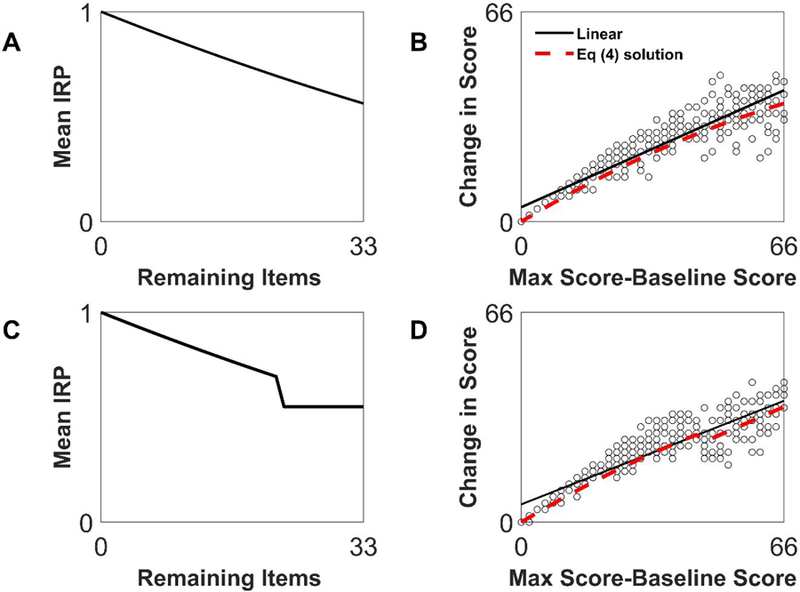
Bending and breaking proportional recovery. Shown is the relationship of average item recovery probability function (IRPF, left column) and recovery (right column). In the top row, the average IRPF is smooth but not constant, and produces a nonlinear relationship that is nevertheless well fit by a linear regression (solid line, R2=0.88). The red dashed line shows the predicted fit from Equation 4. In the bottom row, the average IRPF is discontinuous, but the resulting recovery relationship does not look discontinuous, but rather just somewhat non-linear, and again is still well fit by linear regression (R2=0.83), although the local slope varies as Equation 4 (red dashed line).
If is (first-order) unsmooth, then jumps, causing the instantaneous slope (So) to jump (Figure 3C). But, slope discontinuities are masked by the stochastic nature of the IRPFs (Figure 3D).
It is well established in statistics that it is difficult to discern local slope variations with noisy data and/or a limited sample size. Further, global linear regression, which is the only method that appears to have been applied to detect PR, is ill-suited for detecting non-linearity 16. We therefore suggest that the proportionality of PR, besides arising from mathematical coupling, is also an approximation that arises partly because of the smoothness of the average IRPF, and partly because global linear regression cannot detect local slope variations.
We used local linear regression to estimate if the local slope of PR varies for different impairment levels.The local slope is the slope of a line fitted to a subset of the data, where the subset of data is selected as the data points (i.e. patients) that fall in a window of size 20 along the x-axis. We shifted the window along the x-axis to estimate the slope for different data subsets.
Figure 4 shows that the local slope estimate varies from 0.0 to 1.0, in a similar pattern for two different data sets, and is not constant as would be expected for exact proportionality across impairment levels (Equation 3). Note that the local slope estimated in the windows over impairment level of 46–66 (i.e. initial UFEM<20) is smaller (Figure 4C). This is consistent with the expectation that average recovery probability will decrease with decreasing initial score So– i.e. subjects with lower baseline scores have a lower probability of scoring on remaining items at follow-up (resulting in a lower local slope). Also, the global regression slope closely matches the local slope for the patients who are less impaired at baseline – these subjects have smaller recovery variance (partly due to a ceiling effect), which drives the global regression.
Figure 4.
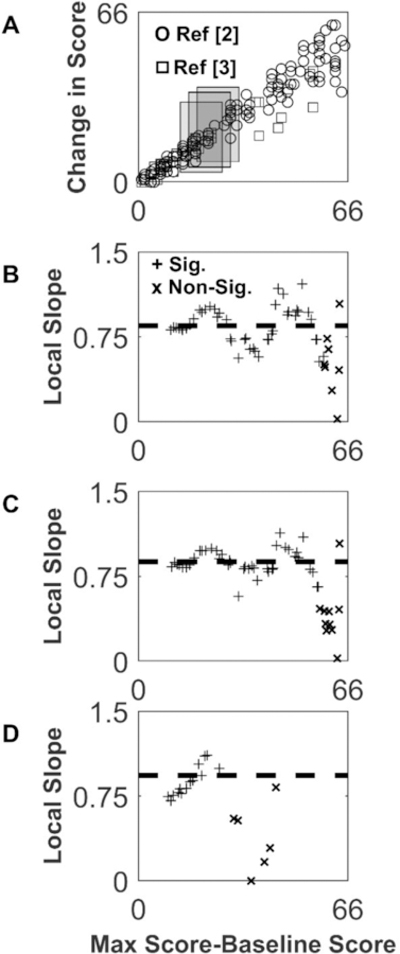
Estimating the local slope of recovery using local linear regression. A: UEFM recovery data from 2 (circles) and 3 (squares) shown with sample windows (20 UEFM points wide) used to create the local estimate of slope (grey rectangles). For the data points (i.e. subjects) falling within each window, we performed a regression to estimate the slope for those data points alone (i.e. the “local slope”). B: Local slope estimated with the linear regression over each window for the data combined from 2,3. C: Local slope from 2 alone, with window size of 20 UEFM points. D: Local slope from 3 alone, with a window size of 20 UEFM points. Plus signs show points for which the p-value of the local regression < 0.05, and “x” where the p > 0.05. Horizontal dashed line shows slope from global regression. Note that the global regression slope result is dictated by the data from the individuals with higher baseline scores because their data is less variable.
Breaking PR: Non-Fitters
What are the mathematical conditions needed to create non-fitters who break the PR rule, given the various statistical factors working positively toward creating an appearance of PR? Working with our simulator, we found that if we set recovery probabilities for a substantial number of items to a low value for a specific set of subjects with a baseline score below some score (i.e. we made a set of items extremely difficult for those low-level subjects, Figure 5A and C), then those subjects recovered poorly in the simulation, and they appeared as non-fitters (Figure 5B and D).
Figure 5.
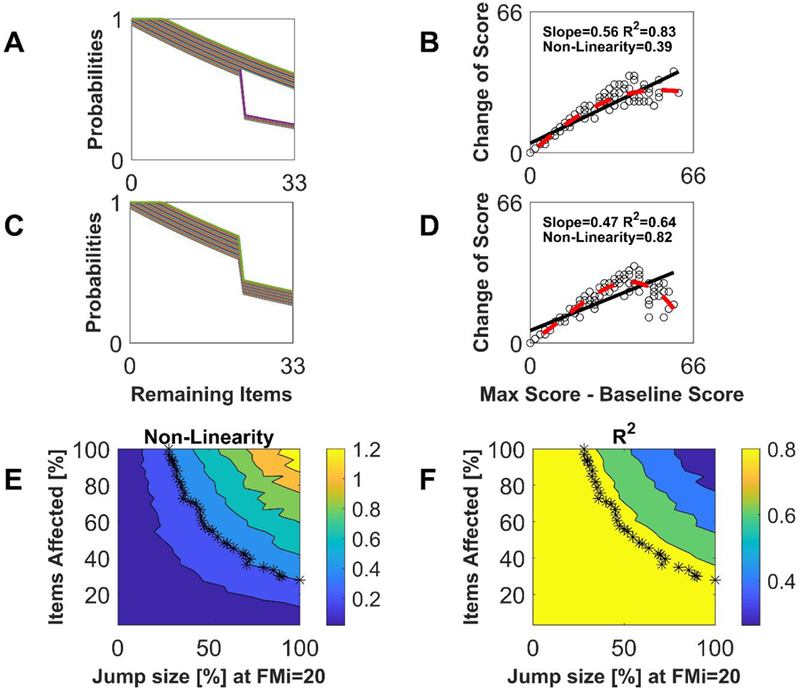
Relationship between item recovery probability function (IRPF) nonlinearities and nonfitters. A great enough jump nonlinearity in a great enough number of the IRPFs creates a nonlinearity in the PR-type relationship, even though the nonlinearities are smoothed by the summation associated with calculating a total score. A: Sample IRPFs with 50% jump nonlinearities in 30% of the items. B: PR plot resulting from IRPFs in A. The nonlinearity measure was quantified as where g(x) is the best-fit 3rd-order polynomial (dashed line) and f(x) is the linear PR model (solid line). Nonlinearity started becoming visually apparent with NL ≥ 0.4. C: Sample IRPFs with 40% jump nonlinearities in all items. D: PR plot resulting from IRPFs in C. E: Results from simulations where P% of the 33 IRPFs, starting with the most difficult items, exhibited a jump decrease by D% at So = 20, varying P and D. F: R2 values for the same simulations as in E. The asterisks in E and F show simulation runs where L = 0.4, the value where we judged non-linearity to be visually apparent. In panel F one can see that simulations with NL=0.4 always produced R2 values greater than 0.8 – i.e. R2 is a poor indicator of nonlinearity.
To quantify how substantial the IRPF discontinuities have to be to produce a visible non-linearity, we simulated the situation were P% of the IRPFs, starting with the most difficult items, exhibited a jump decrease by D% at So = 20, varying P and D (Figure 5). Large values of D mean those items are nearly impossible for a subset of patients to score on at follow-up. We analyzed the R2 and nonlinearity of the resulting PR-like rule, where we defined nonlinearity NL as the root mean square deviation of a third-degree polynomial fit to the data away from a linear fit 17,18. Nonlinearity became visually obvious when NL approached ~0.4 (Figure 5B).
Achieving an NL=0.4 required at least ~50% jumps in at least ~50% of the items (Figure 5B and E). R2 values were always above 0.8 for simulations with NL = 0.4 (Figure 5F), confirming that linear regression statistics were poor at signaling the emerging nonlinearity 16.
Very Difficult UEFM Items for Non-Fitters
Given that very difficult items can “break” PR, what are the very difficult items for the non-fitters of the UEFM at follow-up? As observed above, 99% of patients with a UEFM score less than 40 in the chronic stage are non-fitters. We used UEFM measurements from 319 chronic stroke patients reported in several studies 19–26 to rank-order items from low to high probability of scoring on that item (either a 1 or 2), using the estimated probabilities at UEFM = 40 to create the rank ordering. We found that individuals with chronic UEFM < 40 (i.e. non-fitters) had the lowest probabilities on the items that required distal coordinated movement (Figure 6). These “very difficult” items tested the ability to move distal joints (wrist circumduction, wrist stability, forearm pronation/supination, wrist flexion/extension, Grasp A – i.e. “pretend you are holding the handle of a brief case”, and Grasp B – i.e. abducting the thumb to grasp a piece of paper;), to actively flex the shoulder through the extreme of its range of motion (shoulder flexion from 90180 degrees) without bending the elbow, and to make repeated high speed targeted reaching movements without vision (i.e. the finger-to-nose movement item of the UEFM test). The two remaining wrist/hand items were easy/medium, but the easy one only required mass flexion of the hand, and the medium one required flexion of the wrist, without requiring control over other joints or active extension of the fingers or wrist. That is, “in-synergy” UEFM items were relatively easy for these non-fitters to achieve, in contrast to the items requiring distal coordinated movement (Figure 6).
Figure 6.
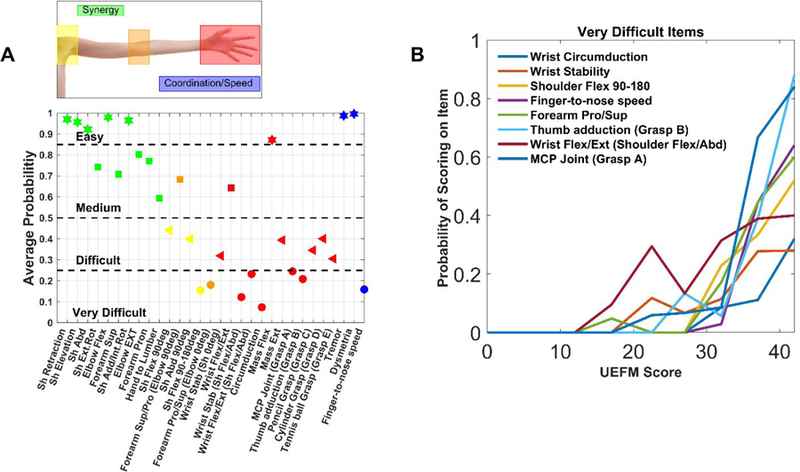
Analysis of difficulty on scoring on items of the UEFM assessment. A: Average probability of scoring either a 1 or 2 on each UEFM item for non-fitters taken as a group (i.e. subjects with follow-up UEFM scores <40). The three reflex test items were excluded as all recorded subjects scored a 1 or 2 on these items. We categorized item difficulty based on the probability of scoring on the item, We also highlight the type of movement tested: synergistic movement (green), shoulder (yellow), elbow (orange), wrist + hand (red), and UE tremor/coordination/speed (blue). B: Probability of scoring on the very difficult items as a function of chronic UEFM score. Items are listed from most difficult to easiest in the legend, top to bottom.
Summary
To summarize, we derived a new interpretation of the slope of PR: it is the average probability of scoring across remaining scale items at follow-up. The proportionality of PR, besides arising from mathematical coupling, is also an approximation that arises because of the smoothness of the average IRPF, and because global linear regression cannot detect local slope variations. The local slope estimate varies substantially in a consistent pattern for two different published UEFM recovery data sets – a result not predicted by mathematical coupling, but understandable from the scale construction perspective. Finally, what is required to overcome these mathematical phenomena that encourage the appearance of PR is that a patient must exhibit discretely lower probability of scoring on a substantial subset of test items at follow-up. We showed that UEFM non-fitters are most unlikely to score on test items related to wrist/hand dexterity, extreme active shoulder range of motion, and finger-to-nose speed at follow-up. This is consistent with the idea that non-fitters lack sufficient CST, since CST is thought to be the neural substrate for these types of hand and arm movements 27,28.
4. How Do Non-Fitters Respond to Rehabilitation?
One possible implication of PR is that rehabilitation therapy does not matter because patients follow the PR prediction despite varying amounts of therapy 3,29. However, Hawe et al. 5 highlighted that there is substantial variability in UEFM recovery even for the fitters. The number of fitters displaying ≈70% recovery is near chance levels. Non-fitters have even greater variability of recovery (Figure 1), leading us to hypothesize that they are particularly responsive to the varying amounts of movement training they receive during rehabilitation.
To test this hypothesis, we returned to an analysis of UEFM response to robotic UE therapy in the chronic phase of stroke 30. We grouped patients based on UEFM scores at study entry that fell into three zones: [0–21], [22–40], or [41–66]. Interestingly, these groups correspond to poorly recovered non-fitters, better recovered non-fitters, and fitters, respectively in the acute phase studies (Figure 7A, data taken from 2,3). Here we make the assumption that people who qualified for a robotic therapy study in the chronic phase and had an UEFM score < 40 would have been classified as non-fitters had they been tracked from the acute phase. It may be that there is a different pattern of upper extremity recovery that has not yet been identified for the type of patient who would not have qualified for PR studies, but then qualified for a chronic robotic therapy study (see Supplemental Material). However, in examining the inclusion/exclusion criterion for the chronic robotic therapy studies, we found they were similar to the PR studies (Table S1, Supplemental Material). Where the criteria deviated (such as not requiring a first-ever anterior circulation stroke), a recent study examining a broader, more clinically relevant sample of patients with stroke, including those with intracerebral hemorrhage and previous stroke found the same pattern of upper extremity PR (the pattern of fitters and non-fitters) and the same predictive value of MEP 4. Thus, we feel the assumption that participants with chronic UEFM score < 40 would have been classified as non-fitters is reasonable, although it should be verified in future research.
Figure 7.
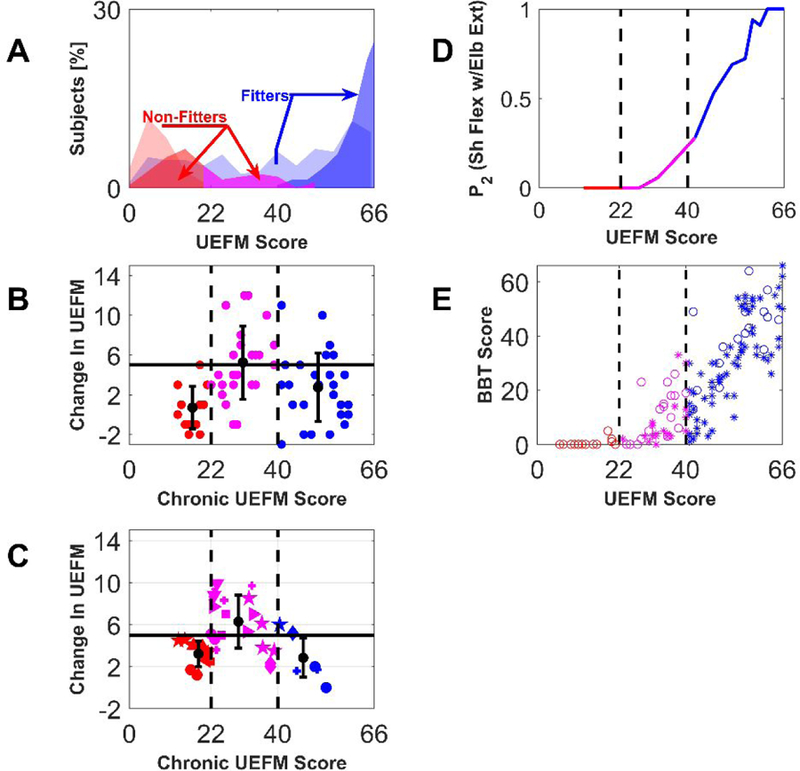
Analysis of a special group of non-fitters who respond better to intensive UE movement training provided in the chronic phase of stroke. A: Distribution of patients (% out of total 214 patients reported in 2,3) at baseline (light colors) and follow-up (dark colors), “fitters” (blue) and “non-fitters” (red-poor recovery, magenta – better recovered) B: Change in UEFM score after UE robotic movement training as reported in our previous work 30. The betterrecovered non-fitters showed an increased UEFM response to robotic movement training. (ANOVA, p < 0.001, with follow-up t-tests, Group 1–2 comparison p = 0.001, Group 2–3 comparison p = 0.015; Group 1–3 comparison p=0.03).C: Mean change in UEFM score reported for control and experimental groups in 18 robotic rehabilitation clinical trials with chronic stroke patients (studies 21,32,41–48,33–40taken from the systematic review 31). Treatment groups included conventional therapy, functional electrical stimulation, and robot-assisted arm training. Matching symbols indicate groups from the same studies. For B and C, the horizontal solid line shows the MCID for the UEFM test, the vertical dashed lines denote how we subdivided the subjects into three UEFM zones. The groups with mean starting UEFM score in the middle zone again had a significantly higher mean change in UEFM score (ANOVA, p < 0.001, with follow-up t-tests, Group 1–2 comparison p < 0.001, Group 2–3 comparison p = 0.0013; Group 1–3 comparison p=0.56).). D: Estimated probability to score ‘2’ in the UEFM test item testing shoulder flexion from 0–90˚ with the elbow fully extended, plotted as a function of UEFM score. As described in the text, this is based on data from 319 subjects from several studies 19–26. Subjects with UEFM in the range [22–40] (i.e. the rehabilitation responsive non-fitters) are the ones just able to perform this out-of-flexion-synergy movement. E: Box and Blocks Test score as a function of the UEFM based on data from several studies 23,26,51. Subjects with UEFM in the range [22–40] also are the ones predicted to just able to grasp and release blocks on the BBT. Here, we identified the underlying relationship between motor performance on the UEFM and the BBT in order to estimate BBT scores from UEFM scores.
For each group we calculated the mean reported change in UEFM score due to robotic rehabilitation. Non-fitters with mean baseline UEFM scores in the range [22–40] enefited significantly more from rehabilitation delivered in the chronic phase after stroke (Figure 7B, ANOVA, p < 0.001, with follow-up t-tests, Group 1–2 comparison p = 0.001, Group 2–3 comparison p = 0.015; Group 1–3 comparison p=0.03).
We sought to test this finding using data from a larger number of studies. We therefore analyzed the rehabilitation response of non-fitters using results from a set of randomized controlled trials of robotic and conventional rehabilitation therapy reported in a systematic review 31. We considered the studies that enrolled persons in the chronic phase after stroke, leaving 18 trials (i.e. 21,32,41–48,33–40). We sorted results from these studies into groups with reported mean UEFM scores at study entry that fell into three zones: [0–21], [22–40], or [41–66]. These groups again correspond to poorly recovered non-fitters, better recovered non-fitters, and fitters, respectively (Figure 7A, data taken from 2,3).
For each group we calculated the mean reported change in UEFM score due to rehabilitation of any kind (i.e. robot or control therapy).
Participants with mean baseline UEFM scores in the range [22–40] (i.e. the better-recovered non-fitters) again benefited significantly more from intensive movement practice in the chronic phase after stroke (Figure 7C, ANOVA, p < 0.001, with follow-up t-tests, Group 1–2 comparison p < 0.001, Group 2–3 comparison p = 0.0013; Group 1–3 comparison p=0.56).
Note that the distribution of patients becomes increasingly bimodal as recovery progresses, with the better recovered fitters in the “bottom of the well” of this distribution (Figure 7A). Thus, the better-recovered non-fitters are somewhat scarce: they are ~12% of all stroke patients enrolled in the PR trial data we scanned, and 45% of the non-fitters.
As described above, the lowest probability UEFM items for non-fitters are the ones that require hand and arm dexterity, consistent with lack of CST integrity. But perhaps these better-recovered, rehabilitation responsive non-fitters have return of CST integrity. From the perspective of abnormal synergies, a litmus test of CST integrity is the ability to flex the shoulder to 90 degrees while keeping the elbow fully extended 49,50– i.e. the test of “out-of-flexion-synergy” movement on the UEFM. We plotted the probability of scoring a “2” on the test as a function of UEFM score, again for 319 chronic stroke patients in 19–26. Figure 7D shows that this probability becomes non-zero in the UEFM range of better recovered non-fitters, consistent some return of CST function.
Return of CST function should also be associated with return of hand function. The UEFM coarsely grades hand impairment and does not test hand function, so we turned to the Box and Blocks Test (BBT). The BBT requires individuals to pick up small blocks and place them over a divider, and is thus a functional measure designed to be sensitive to the return of dexterity. We used published data from 140 subjects 23,26,51 with both BBT and FM scores to define the underlying relationship between these two motor scales. Using this relationship we found that the better recovered non-fitters would be expected to exhibit a small amount of hand dexterity on the BBT (Fig. 7E). Thus, the putative rehabilitation-responsive non-fitters, who are known to be MEP- at baseline due to the work of Byblow et al., appear to be the patients in the chronic stage who can begin to move the arm out of synergy and pick up small blocks, both markers of some CST integrity.
Discussion -- Point of View and Directions for Research
Mathematical coupling, as well as other statistical factors identified here, encourage the appearance of PR, and yet a fraction of stroke patients, the non-fitters, do not exhibit PR. We showed that the local slope of PR relates to test item difficulties. Thus, PR breaks when a patient has a discretely lower probability of scoring on a substantial fraction of test items at follow-up. For the UEFM, the lowest probability items are the ones that test wrist/hand dexterity, extreme active shoulder flexion, and high speed finger-to-nose movement, consistent with the finding that non-fitters lack CST integrity 3,4,8–11.
Winters et al.2 found that lack of finger extension within 72 hours post-stroke was the best behavioral predictor of non-fitters. The present results highlight that non-fitters continue to do poorly on tests of hand dexterity, but also arm coordination, and this is why they overcome the influence of mathematical coupling.
However, better-recovered non-fitters appear to exhibit significantly enhanced UEFM response to robotic UE movement training, given the assumption that people who qualified for a robotic therapy study in the chronic phase and had an UEFM score < 40 would have been classified as non-fitters had they been tracked from the acute phase (see Supplemental Material for an analysis of this assumption). A related result was recently noted in an analysis of reaching rehabilitation in a rat stroke model in the subacute phase (n = 123 rats) 52. A subset of rats classified as non-fitters responded better to reaching practice. Fitters recovered the ability to recover food pellets regardless of whether they performed reaching practice. Thus, intensive movement practice mattered for the non-fitters but not the fitters.
In the context of the UEFM, what could explain the heightened responsiveness of better-recovered non-fitters to movement practice? One possibility is that the UEFM scale is non-linear, making it easier to score larger gains when starting from the middle range of the UEFM. Our analysis of the UEFM scale does not support this – it is more difficult to score on items when one starts from the middle range (see Figure 4). Further, the UEFM has a very small floor effect and the ceiling effect is also small 53,54 and unlikely to constrain recovery for people with scores in the range 40–50, since the UEFM can go up to 66 and is linearly related to BBT in the range 40–50 (and beyond see Figure 7E). Yet subjects in this range of 40–50 nonetheless showed relatively decreased rehabilitation response (see Figure 7B and C). Another possibility is that individuals in the chronic phase of stroke experience a decay in their UEFM over time due to disuse of the UE. Thus, when people in the chronic phase of stroke were enrolled in the movement training studies analyzed here, they may have simply overcame that decay. Studies that tracked motor function after stroke for years have indeed sometimes found a decay in UEFM or other UE motor scores 55,56, but at other times have not 57,58. Neither of these alternate explanations account for the related finding from the rodent study, since the outcome measure there was not the UEFM (it was pellets retrieved) and the rats received rehabilitation soon after the induced stroke, not in the chronic stage.
Do a subset of non-fitters therefore defy the expectation of a poorer response to UE rehabilitative movement training that has been associated with higher amounts of CST damage 59–61. Perhaps these non-fitters rely on cortical plasticity to make use of residual pathways 10 such as brainstem pathways 49,62,63 . Another possibility is that these subjects have some residual CST integrity. TMS-based assays of CST integrity might miss detecting small amounts of residual CST early after stroke, or, alternatively, some level of neural reorganization might need time to occur and allow a small amount of residual CST to become functional. Our findings regarding the emergence of out-of-flexion-synergy arm movement and hand manipulation in the rehabilitation-responsive, non-fitter population support this idea, as these are behavioral markers of CST integrity. Byblow et al. found that 6 of the 11 initially MEP-participants recovered MEPs at later evaluations 3. Birchenall et al. 64 also found recovery of MEP. In the Byblow et al. study, MEP-participants also showed some recovery if FA of the CST was small, suggesting residual CST without initial MEP. This finding is supported by a later study of Buch et al. 12 but not that of Doughty et al. 65. Stinear et al. 4 also showed that MEP-patients had greater UEFM recovery if they had lower lesion load to the CST, consistent with the results reported by Feng et al. 11.
We recently found that chronic stroke patients did not respond to robotic hand movement training if they lacked somatosensory integrity 66. Somatosensory integrity was indeed the strongest predictor of rehabilitation response in this context, stronger than CST integrity 67. This highlights the fact that damage to other tracts and systems play a key role in determining response to intensive hand movement training. Somatosensory integrity might be required to effectively carry the feedback necessary to drive CST-related plasticity. In this scenario, perhaps it is the initially MEP- but bettered-recovered and rehabilitation-responsive non-fitters who have this somatosensory integrity.
If a small amount of CST, initially unresponsive to TMS, serves as the basis for an increased response to movement training, it is unclear why people with presumably a greater amount CST integrity – the lower level fitters (with chronic UEFM in the range 40–50) – have a relatively reduced response to intensive movement training. Perhaps fitters make progress through spontaneous limb use, making any applied training appear less effective. In contrast, the special population of non-fitters might need to learn to make use of their small amounts of residual CST by performing especially large amounts of dedicated, practice-based exploration 68,69. Work from our laboratory on robotic exoskeleton training is consistent with this idea, in that it found that people in the chronic phase of stroke who were MEP+, but with smaller rather than larger MEP, responded better to UE robotic movement training 70. In any case, an important direction for future research is to determine how best to identify this putative special class of non-fitters as early as possible after stroke, since they may be the best candidates for intensive movement training, robotic or otherwise, if one is interested in selecting candidates who will have the largest UEFM change from the training.
Supplementary Material
Footnotes
The Authors declare that there is no conflict of interest.
Contributor Information
Merav R. Senesh, Department of Mechanical and Aerospace Engineering University of California at Irvine.
David J. Reinkensmeyer, Department of Mechanical and Aerospace Engineering, Department of Anatomy and Neurobiology, Department of Biomedical Engineering, Department of Physical Medicine and Rehabilitation, University of California at Irvine, 4200 Engineering Gateway Irvine, CA 92697-3975
References
- 1.Prabhakaran S, Zarahn E, Riley C, et al. Inter-individual variability in the capacity for motor recovery after ischemic stroke. Neurorehabil Neural Repair 2008;22(1):64–71. doi: 10.1177/1545968307305302 [DOI] [PubMed] [Google Scholar]
- 2.Winters C, van Wegen EEH, Daffertshofer A, Kwakkel G. Generalizability of the proportional recovery model for the upper extremity after an ischemic stroke. Neurorehabil Neural Repair 2015;29(7):614–622. doi: 10.1177/1545968314562115 [DOI] [PubMed] [Google Scholar]
- 3.Byblow WD, Stinear CM, Barber PA, Petoe MA, Ackerley SJ. Proportional recovery after stroke depends on corticomotor integrity. Ann Neurol 2015;78(6):848–859. doi: 10.1002/ana.24472 [DOI] [PubMed] [Google Scholar]
- 4.Stinear CM, Byblow WD, Ackerley SJ, Smith M, Borges VM, Barber PA. Proportional motor recovery after stroke: implications for trial design. Stroke 2017;48(3):795–798. doi: 10.1161/STROKEAHA.116.016020 [DOI] [PubMed] [Google Scholar]
- 5.Hawe RL, Scott SH, Dukelow SP. Taking proportional out of stroke recovery. Stroke 50(1):204–211. doi: 10.1161/STROKEAHA.118.023006 [DOI] [PubMed] [Google Scholar]
- 6.Boyd LA, Hayward KS, Ward NS, et al. Biomarkers of stroke recovery: Consensus-based core recommendations from the Stroke Recovery and Rehabilitation Roundtable. Int J Stroke 2017;12(5):480–493. doi: 10.1177/1747493017714176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hope TMH, Friston K, Price CJ, Leff AP, Rotshtein P, Bowman H. Recovery after stroke: not so Proportional After all? Brain 2019;142(1):15–22. doi: 10.1371/journal.pone.0147697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koch P, Schulz R, Hummel FC. Structural connectivity analyses in motor recovery research after stroke. Ann Clin Transl Neurol 2016;19(3):233–244. doi: 10.1002/acn3.278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Landsittel D, Lioutas V, Kautz S. Corticospinal tract lesion load - a potential imaging biomarker for stroke motor outcomes. Ann Neurol 2015;78(6):860–870. doi: 10.1002/ana.24510.Corticospinal [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zarahn E, Alon L, Ryan SL, et al. Prediction of motor recovery using initial impairment and fMRI 48 h poststroke. Cereb Cortex 2018;12:2712–2721. doi: 10.1093/cercor/bhr047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng W, Wang J, Chhatbar PY, et al. Corticospinal tract lesion load: an imaging biomarker for stroke motor outcomes. Ann Neurol 2015;78(6):860–870. doi: 10.1002/ana.24510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buch ER, Rizk S, Nicolo P, Cohen LG, Schnider A, Guggisberg AG. Predicting motor improvement after stroke with clinical assessment and diffusion tensor imaging. Neurology 2016;86(20):1924–1925. doi: 10.1212/WNL.0000000000002675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med 1975;7(1):13–31. [PubMed] [Google Scholar]
- 14.McDowell I Measuring Health: A Guide to Rating Scales and Questionnaires: 3rd ed. New York, NY: Oxford University Press; 2006. doi: 10.1179/108331900786166731 [DOI] [Google Scholar]
- 15.Woodbury ML, Velozo CA, Richards LG, Duncan PW, Studenski S, Lai S-M. Dimensionality and construct validity of the Fugl-Meyer assessment of the upper extremity. Arch Phys Med Rehabil 2007;88(6):715–723. doi: 10.1016/j.apmr.2007.02.036 [DOI] [PubMed] [Google Scholar]
- 16.Anscombe FJ. Graphs in statistical analysis. Am Stat 1973;27(1):17–21. doi: 10.1080/00031305.1973.10478966 [DOI] [Google Scholar]
- 17.Emancipator K, Kroll MH. A quantitative measure of nonlinearity. Clin Chem 1993;39(5):766–772. doi: 10.1002/prca.201090041 [DOI] [PubMed] [Google Scholar]
- 18.Kroll MH, Emancipator K. A theoretical evaluation of linearity. Clin Chem 1993;39(3):405–413. [PubMed] [Google Scholar]
- 19.Dodakian L, McKenzie AL, Le V, et al. A home-based telerehabilitation program for patients with stroke. Neurorehabil Neural Repair 2017;31(10–11):923–933. doi: 10.1177/1545968317733818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taheri H, Rowe JB, Gardner D, Chan V, Reinkensmeyer DJ, Wolbrecht ET. Robot-assisted guitar hero for finger rehabilitation after stroke. In: Proc. 34th Annual International Conference of the IEEE EMBS, San Diego, CA USA ; 2012:3911–3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Housman SJ, Scott KM, Reinkensmeyer DJ. A randomized controlled trial of gravity-supported, computer-enhanced arm exercise for individuals with severe hemiparesis. Neurorehabil Neural Repair 2009:505–514. [DOI] [PubMed] [Google Scholar]
- 22.Milot M-H, Spencer SJ, Chan V, et al. A crossover pilot study evaluating the functional outcomes of two different types of robotic movement training in chronic stroke survivors using the arm exoskeleton BONES. J Neuroeng Rehabil 2013;10:112. doi: 10.1186/1743-0003-10-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reinkensmeyer DJ, Wolbrecht ET, Chan V, Chou C, Cramer SC, Bobrow JE. Comparison of 3D, assist-as-needed robotic arm/hand movement training provided with Pneu-WREX to conventional table top therapy following chronic stroke. Am J Phys Med Rehabil Acad Physiatr 2012;91(11 0 3):S232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Secoli R, Milot MH, Rosati G, Reinkensmeyer DJ. Effect of visual distraction and auditory feedback on patient effort during robot-assisted movement training after stroke. J Neuroeng Rehabil 2011;8(1):1–10. doi: 10.1186/1743-0003-8-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taheri H, Rowe JB, Gardner D, et al. Design and preliminary evaluation of the FINGER rehabilitation robot: controlling challenge and quantifying finger individuation during musical computer game play. J Neuroeng Rehabil 2014;11:10. doi: 10.1186/1743-0003-11-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolbrecht ET, Rowe JB, Chan V, Ingemanson ML, Cramer SC, Reinkensmeyer DJ. Finger strength, individuation, and their interaction: Relationship to hand function and corticospinal tract injury after stroke. Clin Neurophysiol 2018;129(4):797–808. doi: 10.1016/j.clinph.2018.01.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lemon RN. Descending pathways in motor control. Annu Rev Neurosci 2008;31(1):195–218. doi: 10.1146/annurev.neuro.31.060407.125547 [DOI] [PubMed] [Google Scholar]
- 28.Devanne H, Cohen LG, Kouchtir-Devanne N, Capaday C. Integrated motor cortical control of task-related muscles during pointing in humans. J Neurophysiol 2002;87(6):3006–3017. doi: 10.1152/jn.2002.87.6.3006 [DOI] [PubMed] [Google Scholar]
- 29.Krakauer JW, Carmichael ST, Corbett D, Wittenberg GF. Getting neurorehabilitation right. Neurorehabil Neural Repair 2012;26(8):923–931. doi: 10.1177/1545968312440745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.See J, Dodakian L, Chou C, et al. A standardized approach to the Fugl-Meyer assessment and its implications for clinical trials. Neurorehabil Neural Repair 2013;27(8):732–741. doi: 10.1177/1545968313491000 [DOI] [PubMed] [Google Scholar]
- 31.Mehrholz J, Pohl M, Platz T, Kugler J, Elsner B. Electromechanical and robot-assisted arm training for improving activities of daily living , arm function , and arm muscle strength after stroke ( Review ). Cochrane Database Syst Rev 2018;(9). doi:10.1002/14651858.CD006876.pub5 .10.1002/14651858.CD006876.pub5www.cochranelibrary.com. www.cochranelibrary.com [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ang KK, Guan C, Phua KS, et al. Brain-computer interface-based robotic end effector system for wrist and hand rehabilitation: results of a three-armed randomized controlled trial for chronic stroke. Front Neuroeng 2014;7(July):1–9. doi: 10.3389/fneng.2014.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Conroy SS, Whitall J, Dipietro L, et al. Effect of gravity on robot-assisted motor training after chronic stroke: A randomized trial. Arch Phys Med Rehabil 2011;92(11):1754–1761. doi: 10.1016/j.apmr.2011.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daly JJ, Hogan N, Perepezko EM, et al. Response to upper-limb robotics and functional neuromuscular. J Rehabil Res Dev 2005;42(6):723. doi: 10.1682/JRRD.2005.02.0048 [DOI] [PubMed] [Google Scholar]
- 35.Hsieh Y, Wu C, Liao W, Lin K, Wu K, Lee C. Effects of treatment intensity in upper limb robotassisted therapy for chronic stroke. Neurorehabil Neural Repair 2011;25(6):503–511. doi: 10.1177/1545968310394871 [DOI] [PubMed] [Google Scholar]
- 36.Klamroth-Marganska V, Blanco J, Campen K, et al. Three-dimensional, task-specific robot therapy of the arm after stroke: a multicentre, parallel-group randomised trial. Lancet Neurol 2014;13(2):159–166. doi: 10.1016/S1474-4422(13)70305-3 [DOI] [PubMed] [Google Scholar]
- 37.van der Lee JH, Wagenaar RC, Lankhorst GJ, Vogelaar TW, Devillé WL, Bouter LM. Forced use of the upper extremity in chronic stroke patients. Stroke 1999:2369–2375. doi: 10.1161/01.STR.30.11.2369 [DOI] [PubMed] [Google Scholar]
- 38.Hsieh YW, Lin KC, Horng YS, Wu CY, Wu TC, Ku FL. Sequential combination of robot-assisted therapy and constraint-induced therapy in stroke rehabilitation: A randomized controlled trial. J Neurol 2014;261(5):1037–1045. doi: 10.1007/s00415-014-7345-4 [DOI] [PubMed] [Google Scholar]
- 39.Hwang CH, Seong JW, Son D-S. Individual finger synchronized robot-assisted hand rehabilitation in subacute to chronic stroke: a prospective randomized clinical trial of efficacy. Clin Rehabil 2012;26(8):696–704. doi: 10.1177/0269215511431473 [DOI] [PubMed] [Google Scholar]
- 40.Lo AC, Guarino PD, Richards LG, et al. Robot-assisted therapy for long-term upper-limb impairment after stroke. N Engl J Med 2010;362(19):1772–1783. doi: 10.1056/NEJMoa0911341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burgar CG, Lum PS, Shor PC, Machiel Van der Loos HF. Development of robots for rehabilitation therapy: the Palo Alto VA/Stanford experience. J Rehabil Res Dev 2000;37(6):663–673. [PubMed] [Google Scholar]
- 42.McCabe J, Monkiewicz M, Holcomb J, Pundik S, Daly JJ. Comparison of robotics, functional electrical stimulation, and motor learning methods for treatment of persistent upper extremity dysfunction after stroke: A randomized controlled trial. Arch Phys Med Rehabil 2015;96(6):981–990. doi: 10.1016/j.apmr.2014.10.022 [DOI] [PubMed] [Google Scholar]
- 43.Susanto EA, Tong RK, Ockenfeld C, Ho NS. Efficacy of robot-assisted fingers training in chronic stroke survivors: a pilot randomized-controlled trial. J Neuroeng Rehabil 2015;12(1):42. doi: 10.1186/s12984-015-0033-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Timmermans AA, Lemmens RJ, Monfrance M, et al. Effects of task-oriented robot training on arm function, activity, and quality of life in chronic stroke patients: A randomized controlled trial. J Neuroeng Rehabil 2014;11(1). doi: 10.1186/1743-0003-11-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Volpe BT, Lynch D, Rykman-Berland A, et al. Intensive sensorimotor arm training mediated by therapist or robot improves hemiparesis in patients with chronic stroke. Neurorehabil Neural Repair 2008;22(3):305–310. doi: 10.1177/1545968307311102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu C, Yang C-L, Chuang L, Lin H, Chen K, Huang W. Effect of therapist-based versus robotassisted bilateral arm training on motor control, functional performance, and quality of life after chronic stroke: a clinical trial. Phys Ther 2012;92(8):1006–1016. [DOI] [PubMed] [Google Scholar]
- 47.Brokaw EB, Nichols D, Holley RJ, Lum PS. Robotic therapy provides a stimulus for upper limb motor recovery after stroke that is complementary to and distinct from conventional therapy. Neurorehabil Neural Repair 2014;28(4):367–376. doi: 10.1177/1545968313510974 [DOI] [PubMed] [Google Scholar]
- 48.Valles KB, Montes S, Madrigal MDJ, Burciaga A, Martínez ME, Johnson MJ. Technologyassisted stroke rehabilitation in Mexico : a pilot randomized trial comparing traditional therapy to circuit training in a Robot / technology-assisted therapy gym. J Neuroeng Rehabil 2016;13(83):1–15. doi: 10.1186/s12984-016-0190-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McPherson JG, Chen A, Ellis MD, Yao J, Heckman CJ, Dewald JPA. Progressive recruitment of contralesional cortico-reticulospinal pathways drives motor impairment post stroke. J Physiol 2018;596(7):1211–1225. doi: 10.1113/JP274968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McMorland AJC, Runnalls KD, Byblow WD. A neuroanatomical framework for upper limb synergies after stroke. Front Hum Neurosci 2015;9:82. doi: 10.3389/fnhum.2015.00082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Platz T, Pinkowski C, van Wijck F, Kim I-H, di Bella P, Johnson G. Reliability and validity of arm function assessment with standardized guidelines for the Fugl-Meyer Test, Action Research Arm Test and Box and Block Test: a multicentre study. Clin Rehabil 2005;19(4):404–411. doi: 10.1191/0269215505cr832oa [DOI] [PubMed] [Google Scholar]
- 52.Jeffers MS, Karthikeyan S, Gomez-Smith M, et al. Does stroke rehabilitation really matter? part B: an algorithm for prescribing an effective intensity of rehabilitation. Neurorehabil Neural Repair 2018;32(1):73–83. doi: 10.1177/1545968317753074 [DOI] [PubMed] [Google Scholar]
- 53.Lin J-H, Hsu M-J, Sheu C-F, et al. Psychometric comparisons of 4 measures for assessing upperextremity function in people with stroke. Phys Ther 2009;89(8):840–850. doi: 10.2522/ptj.20080285 [DOI] [PubMed] [Google Scholar]
- 54.Hsueh I-P, Hsu M-J, Sheu C-F, Lee S, Hsieh C-L, Lin J-H. Psychometric comparisons of 2 versions of the Fugl-Meyer Motor Scale and 2 versions of the Stroke Rehabilitation Assessment of Movement. Neurorehabil Neural Repair 2008;22(6):737–744. doi: 10.1177/1545968308315999 [DOI] [PubMed] [Google Scholar]
- 55.Löfgren B, Nyberg L, Mattsson M, Gustafson Y. Three years after in-patient stroke rehabilitation: a follow-up study. Cerebrovasc Dis 1999;9(3):163–170. doi: 10.1159/000015948 [DOI] [PubMed] [Google Scholar]
- 56.Meyer S, Verheyden G, Brinkmann N, et al. Functional and motor outcome 5 years after stroke is equivalent to outcome at 2 months. Stroke 2015;46(6):1613–1619. doi: 10.1161/STROKEAHA.115.009421 [DOI] [PubMed] [Google Scholar]
- 57.Broeks JG, Lankhorst GJ, Rumping K, Prevo AJ. The long-term outcome of arm function after stroke: results of a follow-up study. Disabil Rehabil 1999;21(8):357–364. http://www.ncbi.nlm.nih.gov/pubmed/10503976. Accessed February 8, 2019. [DOI] [PubMed] [Google Scholar]
- 58.Feys H, De Weerdt W, Verbeke G, et al. Early and repetitive stimulation of the arm can substantially improve the long-term outcome after stroke: a 5-year follow-up study of a randomized trial. Stroke 2004;35(4):924–929. doi: 10.1161/01.STR.0000121645.44752.f7 [DOI] [PubMed] [Google Scholar]
- 59.Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain 2007;130:170–180. doi: 10.1093/brain/awl333 [DOI] [PubMed] [Google Scholar]
- 60.Riley JD, Le V, Der-Yeghiaian L, et al. Anatomy of stroke injury predicts gains From therapy. Stroke 2011;42(2):421–426. doi: 10.1161/STROKEAHA.110.599340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cassidy JM, Tran G, Quinlan EB, Cramer SC. Neuroimaging Identifies Patients Most Likely to Respond to a Restorative Stroke Therapy. Stroke 2018;49(2):433–438. doi: 10.1161/STROKEAHA.117.018844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baker SN. The primate reticulospinal tract, hand function and functional recovery. J Physiol 2011;589(Pt 23):5603–5612. doi: 10.1113/jphysiol.2011.215160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Riddle CN, Edgley SA, Baker SN. Direct and indirect connections with upper limb motoneurons from the primate reticulospinal tract. J Neurosci 2009;29(15):4993–4999. doi: 10.1523/JNEUROSCI.3720-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Birchenall J, Térémetz M, Roca P, et al. Individual recovery profiles of manual dexterity, and relation to corticospinal lesion load and excitability after stroke –a longitudinal pilot study. Neurophysiol Clin October 2018. doi: 10.1016/J.NEUCLI.2018.10.065 [DOI] [PubMed] [Google Scholar]
- 65.Doughty C, Wang J, Feng W, Hackney D, Pani E, Schlaug G. Detection and predictive value of fractional anisotropy changes of the corticospinal tract in the acute phase of a stroke. Stroke 2016;47(6):1520–1526. doi: 10.1161/STROKEAHA.115.012088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rowe JB, Chan V, Ingemanson ML, Cramer SC, Wolbrecht ET, Reinkensmeyer DJ. Robotic Assistance for Training Finger Movement Using a Hebbian Model: A Randomized Controlled Trial. Neurorehabil Neural Repair 2017;31(8). doi: 10.1177/1545968317721975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ingemanson ML, Rowe JR, Chan V, Wolbrecht ET, Reinkensmeyer DJ, Cramer SC. Somatosensory system integrity explains differences in treatment response after stroke. Neurology February 2019: 10.1212/WNL.0000000000007041. doi: 10.1212/WNL.0000000000007041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reinkensmeyer DJ, Guigon E, Maier MA. A computational model of use-dependent motor recovery following stroke: optimizing corticospinal activations via reinforcement learning can explain residual capacity and other strength recovery dynamics. Neural Networks 2012;29–30:60–69. doi: 10.1016/j.neunet.2012.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Norman SL, Lobo-Prat J, Reinkensmeyer DJ. How do strength and coordination recovery interact after stroke? A computational model for informing robotic training. In: 2017 International Conference on Rehabilitation Robotics (ICORR). Vol 2017 IEEE; 2017:181–186. doi: 10.1109/ICORR.2017.8009243 [DOI] [PubMed] [Google Scholar]
- 70.Milot M-H, Spencer SJ, Chan V, et al. Corticospinal excitability as a predictor of functional gains at the affected upper limb following robotic training in chronic stroke survivors. Neurorehabil Neural Repair 2014;28(9). doi: 10.1177/1545968314527351 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


