Abstract
Transcranial electrical stimulation (tES) aims to alter brain function non-invasively by applying current to electrodes on the scalp. Decades of research and technological advancement are associated with a growing diversity of tES methods and the associated nomenclature for describing these methods. Whether intended to produce a specific response so the brain can be studied or lead to a more enduring change in behavior (e.g. for treatment), the motivations for using tES have themselves influenced the evolution of nomenclature, leading to some scientific, clinical, and public confusion. This ambiguity arises from (i) the infinite parameter space available in designing tES methods of application and (ii) varied naming conventions based upon the intended effects and/or methods of application. Here, we compile a cohesive nomenclature for contemporary tES technologies that respects existing and historical norms, while incorporating insight and classifications based on state-of-the-art findings. We consolidate and clarify existing terminology conventions, but do not aim to create new nomenclature. The presented nomenclature aims to balance adopting broad definitions that encourage flexibility and innovation in research approaches, against classification specificity that minimizes ambiguity about protocols but can hinder progress. Constructive research around tES classification, such as transcranial direct current stimulation (tDCS), should allow some variations in protocol but also distinguish from approaches that bear so little resemblance that their safety and efficacy should not be compared directly. The proposed framework includes terms in contemporary use across peer-reviewed publications, including relatively new nomenclature introduced in the past decade, such as transcranial alternating current stimulation (tACS) and transcranial pulsed current stimulation (tPCS), as well as terms with long historical use such as electroconvulsive therapy (ECT). We also define commonly used terms-of-the-trade including electrode, lead, anode, and cathode, whose prior use, in varied contexts, can also be a source of confusion. This comprehensive clarification of nomenclature and associated preliminary proposals for standardized terminology can support the development of consensus on efficacy, safety, and regulatory standards.
Keywords: Transcranial electrical stimulation (tES), terminology, nomenclature, classification, brain stimulation
1. Scope and Approach
The motivations for this classification document are multifold. There is a need to develop and implement standard language describing transcranial electrical (or electric) stimulation (tES) devices and methods in order to foster the advancement of clinical trials, regulation, and informed medical treatment. Such a consensus is currently lacking, reflecting a dearth of definitions for even extensively tested and apparently straightforward techniques like “tDCS” or for terms like “electrode” which are ubiquitous yet not well defined in the context of tES. A consensus on definitions helps inform clinicians and researchers on how to control tES delivery features relevant for safety and efficacy [1]. The historical lack of standardization in nomenclature has been identified as one potential impediment to the broader adoption of tES [2]. The ongoing advancement of tES science and clinical trials would be facilitated by consensus on protocols across groups based around a common nomenclature. Namely, when group A describes using what they term technique X, and group B describes the safety and efficacy of approach X, it should be clear by nomenclature if they are, in fact, discussing comparable tES techniques. Similarly, regulatory agencies and ultimately patients rely on classification to make informed decisions.
We present the first comprehensive analysis of contemporary tES nomenclature. Our approach is explicitly limited to the explanation of terminology used contemporaneously in tES publications, and thus not to suggest creation, revision, or embargo of terminology. Nonetheless, we provide context to terminology that may be ambiguous or specious. The compiled classifications consider features of stimulation such as indication for use, electrical waveform, electrode montage, and treatment schedules as relevant to define specific approaches. This document is specific to tES methods and does not address electrical stimulation using invasive electrodes, transcranial magnetic stimulation (TMS), transcranial ultrasound, or transcranial photonic stimulation. Terminology here is explicit to human tES use only, as animal models may adopt varied naming conventions. For a review of historical nomenclature that is uncommon in contemporary peer-reviewed publications (such as electrosleep) see Guleyupoglu et al [2].
The outcomes of tES are not simply dependent on the nomenclature used but on the complete details of the administered dose [3], any combined task, subject state, clinical population being treated, inclusion/exclusion criteria, methods of assessment, as well as specific medical and subject factors [4, 5]. Therefore, each study or clinical trial should also be evaluated based on integrating all these factors. For this reason, the use of any nomenclature does not reduce the need to fully report the dose used [3] as explained below (Section 2). Classification remains inevitable for practical purposes (i.e. there is a natural tendency to group and name technologies), and useful when applied rationally and consistently. The development of definitions may be guided by bridging across variations of a technique that theoretically inform each other. For example, two studies of “tDCS” with distinct electrode positions may result in different outcomes but, they may have a similarity in the general approach that allows these studies to closely inform each other and future efforts on “tDCS”. Conversely, a study that claims to examine “tDCS” but in fact used an unrelated and incompatible protocol will produce outcomes not relevant to the broader understanding of “tDCS”. Though each classification developed here encompasses a range of related techniques that presumably inform each other, even when minor variations exist there may be differences in safety or efficacy.
There are two approaches [2] to defining classification of tES:
Physical: Method of stimulation application (dose), such as current waveform shape (e.g. direct current, alternating current) and amplitude, electrode montage and timing of application (see Section 2); and/or
- Intended Use: Empirical or perceived outcome / site/ target of stimulation, which can span several non-exclusive categories:
- hypothesized mechanism of action on the body (e.g. “excitability modulation”, “network synchronization”, “functional connectivity” changes); and/or
- hypothesized anatomical target (e.g. “transorbital”, “deep”), which reflects the region of interest rather than the (only) region influenced; and/or
- expected outcomes/medical indication (e.g. neurorehabilitation)—this could be the primary outcome of interest in a given clinical trial, rather than the main/only outcome.
While a definition based strictly on physical method of application (e.g. DOSE as defined by [3]) reduces ambiguity), in practice most classifications of tES imply, to some degree, the expected mechanisms of action, nominal anatomical target, outcome of interest, or a combination of these. This is the case even when the technique name seems to derive purely from a physical dose definition. For example, tACS indicates sinusoidal rather than any biphasic ac waveform, and tACS further suggests low intensities. Thus, use of tACS implies a more restricted parameter space than just “ac” which in engineering may be any current amplitude and can refer to non-sinusoidal waveforms as well. Most tES classifications adopt an approach combining parameters (dose), intended mechanism, target, indication and/or outcome. In some cases, even the components of the stimulation device involve intent in their naming, such as the terms “active” electrode (the electrode, which is presumed to produce the intended outcome) and “reference” electrode (the electrode, which is presumed to not directly produce the intended outcome).
Our approach defines terms as used in the current scientific literature (see glossary Table 1); we avoid new terminology. Nonetheless, inconsistent and ambiguous use of terms required us to constrain or refine classification (rather than try to develop definitions inclusive of all historical uses of a given term). In defining classifications for tES, there is a compromise between broad classification (which allows for needed dose exploration and optimization) and more restrictive classification that creates the least possible ambiguity. In general, we adopted broader definitions, even including dose ranges yet to be tested, while also describing “conventional” practices that are limited to the common current uses. This nomenclature guidance is intended neither as a safety nor an efficacy review. The inclusion or exclusion of a protocol in a classification or a “conventional” range does not imply any judgment of safety or efficacy.
Table 1:
Glossary of selected tES terms. These definitions should be understood as specific to usage in the tES literature only. Consult the text for a full definition.
| Term | Abbreviated Definition | Section |
|---|---|---|
| Dose | Electrode montage and waveform (including shape, pulse width, polarity, frequency, duration,and amplitude of current as well as number and frequency of sessions) | 2 |
| Electrode montage | Number, size, shape, and position of all electrodes | 2.1 |
| Electrode, Electrode assembly | The electrochemical electrode (metal or conductive-rubber), electrolyte (gel, fluid, cream), and supporting structures (sponge, holder) | 2.2, 2.3 |
| Electrolyte | Electrically conductive fluid, gel, or cream that fills the space between the skin and the metal/conductive-rubber electrochemical electrode | 2.4 |
| Active, Stimulating, Return, Reference electrode | Related to presumed importance (active, stimulation) or unimportance (return, reference) of an electrode for a given brain target or outcome | 2.5 |
| Resistance, impedance | Total resistance of all electrodes and body in tested path before session (static) or during session (dynamic) | 2.6 |
| Headgear | Non-conductive accessory used to fix electrodes in position | 2.7 |
| Lead | Insulated conductor connecting electrodes to stimulator | 2.8 |
| Anode (Electrode) | Electrode where current enters body | 2.9 |
| Cathode (Electrode) | Electrode where current exits body | 2.10 |
| Monophasic, Unidirectional, Biphasic, Multiphasic (Waveforms) | If waveform has a single polarity (Monophasic, Unidirectional) or alternating polarity (Biphasic, Multiphasic) | 2.11 |
| Unipolar, Monopolar, Bipolar, Bilateral, Unilateral (Electrode montages) | Related to positions of electrodes relative to nominal target; if a single (unipolar, monopolar) or two (bipolar, bilateral, lateralized) electrodes are considered important for a given outcome | 2.12 |
| Non-invasive electrical stimulation | Electrical stimulation with non-invasive device; tES is non-invasive | 2.13 |
| Stimulation duration / Session duration | Time period from initiation to end of current flow, may exclude any amplitude ramp-up and ramp-down | 2.14 |
| Repetitive | Multiple sessions of tES | 2.15 |
| 1×1 (Montage) | Only two electrodes | 2.16 |
| Limited output tES / tDCS | Stimulation meeting regulatory standards of limited output | 2.17 |
| Sham | Intended to not produce a given outcome while blinding subjects | 2.19 |
| Transcranial Electrical Stimulation (tES) | Non-invasive device intended to directly change brain function by passing electrical currents to the brain through at least one electrode on the scalp | 3 |
| Transcranial Direct Current Stimulation (tDCS) | tES with sustained direct current (dc) waveform | 3.1 |
| Dual tDCS | 1×1 symmetric bilateral tDCS | 3.1 |
| High-Definition tES / tDCS | tES with small electrodes, a center electrode surrounded by a ring of electrodes of opposite polarity | 3.1.1 |
| Transcranial Pulsed Current Stimulation (tPCS) | tES with pulsed waveform | 3.2 |
| Transcranial Electrical Stimulation (TES, capitalized “T”) | tPCS with few, low-frequency, suprathreshold pulses | 3.2.1 |
| Cranial Electrotherapy Stimulation (CES) | Low-intensity tPCS FDA cleared as CES | 3.2.2 |
| Electroconvulsive therapy (ECT) | High-intensity tPCS sufficient to produce seizure | 3.2.3 |
| (Slow) Oscillating transcranial Direct Current Stimulation (so-tDCS), transcranial Sinusoidal Direct Current Stimulation (ts-DCS) | tES using monophasic current stimulation where the amplitude of stimulation is slowly modulated | 3.3 |
| Transcranial Sinusoidal Direct Current Stimulation (ts-DCS) | o-tDCS where the waveform is a monophasic sinusoid | 3.3 |
| Transcranial Alternating Current Stimulation (tACS) | tES using sinusoidal current waveform | 3.4 |
| Interferential Stimulation, Temporal Interference Stimulation | Two sine waves, both at high but slightly different frequencies, applied via two pairs of electrodes | 3.4.1 |
| Repetitive transorbital alternating current stimulation (rtACS),transcorneal electrical stimulation (TcES),transscleral electrical stimulation (TsES) | Electrodes are positioned near the eye with the aim to inject current to the eyeball to reach the nervous tissue of the retina and brain | 3.5 |
| Transcranial Random Noise Stimulation (tRNS) | tES with a noise waveform | 3.6 |
Following conventions of use in the field, tES classifications are not simply literal – meaning, a classification is rarely the amalgamation of the physical meaning of each word, with nothing less and nothing more. The classifications here are therefore proper names. Thus, tES classifications are typically more restrictive based on both dose and intent than implied by the broadest technical interpretation of its name (e.g. tACS). For this reason, we respect capitalization norms for acronyms – notably the common use of a lower case “t”. While we follow common practices for capitalization of names of techniques, we do not endorse strict criteria for lower/upper case acronyms.
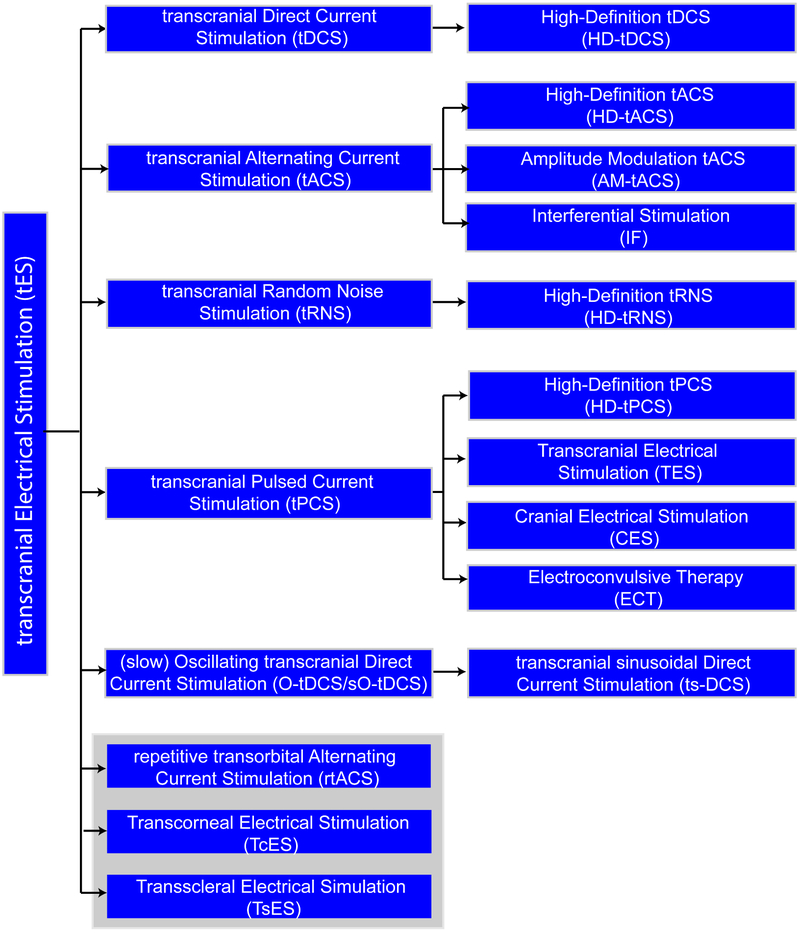
The classifications of tES defined here are not mutually exclusive since they may involve overlapping dose and/or mechanism of action. For example, a stimulation protocol defined by the intent to restore visual function (“transorbital”), may in fact be identical to a stimulation protocol using ac current defined by its waveform (“tACS”). In some cases, definitions are a dependent sub-class (e.g. HD-tDCS is a type of tDCS). Figure 1 summarizes tES definitions with their dependencies.
Figure 1:
Tree chart of transcranial electrical stimulation (tES) classification. The terms are organized here as defined in this consensus paper based on principles we developed including referencing both method of stimulation application (dose) and/or intended use, with reference to how terms are primarily used in tES literature rather than strict definition of each word. The categories are therefore not mutually exclusive (e.g. a technique may be both IF and HD-tACS) and a full report of stimulation dose (see Section 2) is always required in each publication for reproducibility. As explained in our classification approach, terms can reflect aspects of electrode montage, waveform, and/or intended outcome (e.g. if a technique is classified as rtACS or as tACS can depend on indication) – terms are defined here as commonly used in the literature without adding new qualifications or terminology. However, this organization of terminology into a classification tree is original to the present paper (e.g. ECT is typically not referred to as a type of tPCS). The grey box groups methods for techniques focused on vision rehabilitation.
Though tES produces current densities at the scalp that are higher than in the brain [6], such that outcomes can also derive from stimulation of cranial and peripheral nerves, here “tES” is adopted to encompass all forms of cranial non-invasive electrical stimulation where at least some action is presumed to derive from current flow to the brain (a “direct” change in brain function). Electrical stimulation approaches intended to only activate peripheral nerves (e.g. trigeminal nerve stimulation) are thus not in the scope of this document. This distinction can be controversial since there is expected overlap between these two categories. Yet, it allows this document to be focused on transcranial techniques. Approaches intended to stimulate the eye (e.g. transorbital stimulation) are included here.
The paper is organized by first defining dose (Section 2)—the terms used to describe the tES stimulation parameters, which should always be reported, and which can qualify or describe the type of tES. The paper then defines classification of transcranial electrical stimulation methods (Section 3).
2. Stimulation parameter (dose) reporting for tES reproducibility
According to the definition by Peterchev et al. [3], tES DOSE comprises all parameters of the stimulation device that affect the electromagnetic field generated in the body. Dose thus includes the stimulation waveform (e.g. ac/dc or pulse shape); its frequency, amplitude, and duration; the number of electrodes and their size and shape, as well as the number and frequency of stimulation sessions (see summary and examples in Table 2). To allow for the interpretation and reproduction of tES methods, it is critical to report the complete dose with all relevant parameters of stimulation (Table 2). Complete details of the electrode assembly must also be provided, including electrode material, coupling medium, electrode size (surface area), electrode thickness, and any relevant details on electrode single-use or re-use [7].
Table 2:
Parameters of tES dose and related factors (adapted from [3]).
| Stimulation Waveform Parameters‡ |
Examples* | Section |
|---|---|---|
| For transcranial Direct Current Stimulation (tDCS): current amplitude, duration, and details of ramp up/down. | Amplitude of 2 mA applied for 20 minutes duration, with 30 second linear ramp up/down. | 3.1 |
| For transcranial Alternating Current Stimulation (tACS): current amplitude, frequency, and offset (dc bias) or details of ramp up/down. | 10 Hz sinusoidal current with peak amplitude of 1 mA (peak to baseline), applied for 30 minutes, with no dc bias and a linear ramp up/down over 15 seconds. | 3.4, 2.11 |
| For transcranial Pulsed Current Stimulation: pulse shape, amplitude, duration, polarity, inter-pulse-interval (pulse repetition frequency), intertrain interval (duration between pulse trains), total number of pulses. | Monophasic rectangular pulse, 4 mA peak current, 1 ms pulse width, train of 100 pulses at 100 Hz frequency, 10 seconds between pulse trains, 2000 total pulses (20 pulse trains). | 3.2, 2.11 |
| For Electroconvulsive Therapy (ECT): pulse shape, directionality, amplitude, width, train frequency, train duration, number of sessions, and interval between sessions. | Rectangular current pulses with 800 mA amplitude, 1.0 ms width, and alternating polarity, delivered at 40 Hz (pulse-pairs per second) for 4 s. | 3.2.3 |
| For repeated sessions, duration of all sessions, interval between sessions and total number of sessions. | 20 minutes daily, 5 days per week (weekdays only), for 4 weeks. OR Repeated within 18–26 hours with 5 sessions completed in 5 days |
2 |
| If the dose is individually titrated for efficacy or safety: Describe the titration procedure method (formula) and how the final dose is determined. Ideally, report dose per subject, but at a minimum descriptive statistics on the dose across the whole population of subjects/patients participating in the study should be provided. Optionally, the dose applied at each iteration and the final dose applied may be reported. | Subject 1: 2.0 mA amplitude Subject 2: 1.5 mA amplitude OR Increase amplitude by 0.5 mA increments every 30 seconds until subject reports sensation. Experimentally resulting in amplitude range of 1.0 to 2.0 mA with an average amplitude of 1.5 mA and a SD of 0.5 mA. |
2 |
| Montage and Electrode Assembly (including conductive solution) | ||
| All electrode assembly components including electrode, conductive solution (electrolyte), and any supporting materials (e.g. sponge). If a well-defined manufacturer/model is used, it may be sufficient to report it, but to reduce ambiguity key features should be specified. The reporting of a unique product model may allow collection of manufacturing details not apparent to the researcher (e.g. product materials); however, basic electrode assembly description should still be provided to minimize ambiguity. | 7×5 cm sponge; sponge material – e.g. cellulose pocket area, 2 cm thick per sponge; 3×5 cm area conductive rubber electrode centered inside sponge pocket with 0.9% isotonic saline. Electrode Model Z and Gel Model Y by Company X. | 2.2, 2.3 |
| Electrode position on the scalp relative to a clearly defined (reproducible) system (e.g. 10–10, landmarks, imaging, or evoked neurophysiology). This must be specified for all electrodes. | Pad centered on F4 (EEG 10-10) and oriented orthogonal to vertex, OR The position labeled “F4” on Company X, Cap Y, OR Centered on motor “hot spot” as identified by TMS, OR Positioned on forehead with the bottom center of the pad directly above the eyebrow and centered on the eye. |
2.1 |
| Electrode composition, headgear, equipment, and subject preparation | ||
| Skin preparation techniques. | Gentle alcohol wipe, OR Abrasion, OR None. |
- |
| Head gear | Two 5.1 cm wide elastic fasteners made with hypoallergic rubber, affixed with 2 plastic joints.@ | 2.6 |
Complete characterization of waveform of electrode voltage (for voltage-controlled devices) or current (for current-controlled devices).
Examples are intended to illustrate how to apply/report parameters and are not intended to prescribe any specific or preferred implementation.
Researchers apply different forms of head-gear which may vary outcomes even if dose is maintained; for example, variations in pressure on scalp can influence adverse events such as pressure headache or erythema [8].
The classification (name) of an approach usually reflects only a subset of the dose parameters, and perhaps the intended outcome. For example, tDCS may be defined by only the waveform parameters (e.g. low amplitude dc), irrespective of electrode montage. Of course, knowledge of the electrode montage is needed to reproduce a given tDCS method. Therefore specifying only the classification of a method is insufficient to allow for reproducibility. Classifications may also reflect the process used to select the dose (e.g. subject titration, prior experience) and summary metrics (e.g. electrode current density or total charge), but this still does not reduce the need to report the complete final dose applied.
This section attempts to disambiguate terms that are used to describe technical aspects of tES methodology and specify the dose in a given protocol.
2.1. Electrode Montage / Configuration
Electrode Montage (or Configuration) typically refers to the number of electrodes (minimum 2), their respective size and shape, and the method by which they are fixed on the head. Electrode position on the scalp (and body for extracephalic electrodes) should be defined using any principally reproducible system, which can include EEG 10–10 (e.g. “electrode position C4”), anatomical landmarks (e.g. “supraorbital”), imaging, or evoked neurophysiology (e.g. “over the motor hotspot identified by TMS”). Montage should be specified for all electrodes. As defined here, electrode montage includes therefore all aspects of dose except waveform. However, in some publications electrode montage may be used interchangeably with dose. This is discouraged to the extent that it leads to ambiguity between dose, which includes waveform, and montage, which does not.
2.2. Electrode Assembly
The electrode assembly refers to all components that carry current between the connector-end of device lead wire and the scalp such as metal electrode, conducting rubber electrode, electrolyte, sponge, as well as materials used to shape these components or otherwise direct current flow (casing, sponge, rivets). The headgear used to position the electrodes on the body or scalp is typically distinct from the electrode assembly (e.g. non-conductive head-strap), but in some designs the components of the electrode assembly may be embedded into the headgear. In tES the term “electrode” (see Section 2.3) is commonly used to designate the entire electrode assembly.
2.3. Electrode
Physical electrode (not a term standard in the tES literature) refers to the material (or surface) where charge carried by electrons is converted to charge carried by ions. For tES, this is limited to the surface of the metal and/or conductive rubber in contact with the electrolyte (such a saline or gel). In tES, however, ELECTRODE is used to refer to the entire electrode assembly (see definition in Section 2.2). In electrochemistry, electrode refers only to the interface of the “physical electrode” metal/conductive-rubber (or other electron carrier) with saline/gel (or another electrolyte). In some forms of tES, especially tDCS, this physical electrode does not touch the skin for safety reasons, whereas in other forms of tES, such as ECT, the physical electrode may be pressed directly against the scalp depending on the device type. Reproducibility can be limited by ambiguities in referencing either the whole electrode assembly or just the physical electrode. For example, it should be made clear if the provided dimensions (e.g. 5×5 cm) refer to just the physical electrode (e.g. the conductive rubber or metal surface contacting the ionic medium) or to the overall electrode assembly (e.g. gel surface of sponges contacting the skin).
It is customary to discuss montage (placement) and waveform applied with respect to a specific electrode. For example, delivery of 1 mA to an electrode implies delivery of 1 mA through the electrode assembly and the electrode interface. Use of an electrode as an “anode” is physically correct and implies the electrode assembly functions as an anode. In most forms of tES, electrode size conventionally refers to the overall electrode-assembly surface area in contact with skin, unless otherwise indicated (see Electrolyte). Therefore, the convention in the literature of calling the entire electrode-assembly the “electrode” is manageable provided: (i) the distinction between the physical electrode and electrode assembly is clear; and (ii) overall details of the electrode assembly, including the electrode design, are explicit.
2.4. Electrolyte
The ELECTROLYTE is the component of the electrode assembly where charge is carried by ions. It is in contact with both the physical electrode and the skin, and also completes a circuit of electrical current flow. The electrolyte may be saline or another salt-containing solution [9], hydrogel, or fatty (oily) cream. To prevent spread, fluid electrolytes may be suspended in a porous material like a sponge and/or contained by a holding vessel like a cup. In some cases, such as with fatty creams, the electrolyte may be sufficiently viscous not to require a suspension. In some application, such as tDCS, the electrolyte is a barrier between the physical electrode and the skin such that the minimum distance between the physical electrode and the skin is the electrolyte thickness. This minimum distance may be determined by a non-conductive (e.g. plastic) separator or holder, by sponge thickness, or by the thickness of the paste. When the physical electrode is in direct contact with the skin, as in ECT, the electrolyte fills in any air gaps between electrode and skin surfaces.
Some studies have used water to saturate tES electrodes; in such cases the water contains ions and/or absorbs them from the skin. “Salt-free” gels and creams have also been evaluated for tES [10], but often have other chemical substitutes for supporting charge transfer.
The total surface area where the physical electrode and/or electrolyte interface with the skin is typically referred to in tES as the electrode size (e.g. “5×5 cm2 electrode” or “5 cm diameter disk electrode”). The surface area where the electrolyte interfaces with the physical electrode is typically different than where the electrolyte interfaces with the skin area.
2.5. “Active”/ “Stimulating”, “Return” / “Reference” (electrode)
The terms RETURN or REFERENCE electrodes have been typically used to describe an electrode that is presumed to be less relevant to the intervention outcomes of interest. For example, an electrode may be given this designation if it is not in proximity to brain regions of interest for a particular intended use. Similarly, the physiological activity of electrodes can be reduced for example by increasing the electrode size or using a ring of electrodes, which reduces the current density in the vicinity of these electrodes [11, 12]. However, all electrodes are functional in the engineering sense if they are used to carry current. Even if they are assumed to be unimportant to the hypothesis being tested, the configuration and polarity of these electrodes will affect current distribution in the brain and must therefore be explicitly reported. This applies to extra-cephalic electrodes as well, since they also affect the current flow in the brain [13, 14]. For voltage controlled stimulation [15], the term “reference” may also be used to define polarity in an engineering sense (e.g. “5 V relative to the reference electrode”). In all these scenarios, the configuration and position of the “return” electrode can influence current flow near/under the “active” electrode.
Analogously, the terms ACTIVE, STIMULATING, or TARGET ELECTRODE have been typically used to refer to the electrode presumed to be physiologically active in regard to the primary intervention outcome – or more specifically that the physiological or behavioral outcome of interest is due to current passing through these electrodes. In stimulation systems with multiple electrodes (three or more), there can be the ability to use some electrodes and not others for stimulation; for example, in a three-electrode system to pass +1 mA at one electrode, −1 mA at another electrode, and 0 mA (no current) at the third electrode. Electrodes without current are unused and, in this context, referred to as Inactive, any electrode with non-zero current considered ACTIVE. Such a situation is typical for implanted systems, where extra electrodes provide for programming flexibility. For those tES systems where electrodes are applied individually (one at a time), the placement of unused (inactive) electrodes would be generally unnecessary. Multi-channel (HD) tES systems that include head-gear embedded with an electrode array may operate using a selective sub-set of electrodes for stimulation (active electrode) with the remainder inactive [16-18], but this use of terminology is rare in the tES literature.
“Active”, “stimulating”, “target”, “return”, and “reference” are thus terms that relate to the “intent” of stimulation, or (less commonly) used casually with no specific functional implication. If these terms are used it should be with (i) the recognition that despite intent, the physiological actions of stimulation are exerted by a complex distribution of electrical current flow between the two (or more) electrodes, and (ii) the complete documentation of the stimulation dose (e.g. it is never appropriate to omit details of reference electrode size, placement, and materials). Generally, using objective engineering terminology such as “anode” and “cathode” (see definitions in Sections 2.8 and 2.9) can reduce the implication of intent or assumed physiological role of electrodes.
2.6. Resistance and Impedance
Resistance is a ubiquitous term in tES and considered important in pre-testing and monitoring of stimulation. When tES is current controlled, the voltage output of the stimulator (between two electrodes or between an electrode and a reference) is adjusted to maintain a controlled current. In the context of tES, the term RESISTANCE usually refers to this voltage at the output of the device divided by the applied current, per Ohm’s law. To measure resistance prior to stimulation, the stimulator applies a small test current and the resulting voltage is recorded. The resistance is then calculated through Ohm’s law by dividing the voltage by the test current.
The resistance measured is the sum of the resistance of the electrodes [19] and the body, including the skin and the skin–electrode interface. A high resistance may therefore reflect a high resistance of one (or more) electrodes, or the skin contact. An atypically high resistance can be a sign of a setup problem such as poor electrode contact or insufficient electrolyte. Therefore, when resistance is tested before stimulation, it helps the operator to identify suboptimal set-up and take corrective actions that could lower the resistance. Similarly, during stimulation an atypically high resistance may indicate non-ideal conditions at the electrode or skin. However, once stimulation begins there are less options by the operator to correct the setup, and in some cases, stimulation is aborted.
A subtle point is that resistance can change with the applied current and waveform. For this reason, the resistance measured before stimulation by the low-test current (which can be referred to as STATIC impedance) would be different than the resistance measured using application-specific currents during stimulation (which can be referred to as DYNAMIC impedance). Nonetheless, the test resistance before stimulation is considered a meaningful predictor of resistance during stimulation—a suboptimal set-up will usually result in atypical resistance already in the pre-stimulation test period. Still, because static resistance and dynamic resistance vary, and also conditions may change over time during a session, resistance during stimulation is monitored.
What qualifies as a “high” resistance is application specific. It is important to emphasize that a relatively low resistance is not a guarantee of optimal setup. Rather it is incumbent on the operator to employ best practices in electrode preparation and setup, and subject and device monitoring [20], with resistance measurement serving as a secondary marker. In addition to potentially indicating non-optimal electrode–skin contact, a high resistance would increase the stimulator output voltage required to provide a given current. If the required output voltage is too high, it may exceed the maximum (compliance) voltage of the tES device, which may result in current reduction or the device aborting stimulation [21].
As a technical note, the electrode and tissue are never simply “resistive” (either before or during stimulation). For example, the calculated “resistance” depends on the strength of the current, meaning that the resistance is nonlinear [22]. The term "impedance" refers to the broader relation between the applied current and the voltage needed to maintain this current flow. The impedance also includes frequency-specific responses (e.g. the response to sinusoids of varied frequency or brief pulses). Moreover, electrodes, tissues, and their interfaces have complex nonlinear impedances that may vary over time (i.e. are time-variant) [23-26]. However, “resistance” and “impedance” are often used interchangeably and nonspecifically in the tES literature, typically relying on how a given tES device tests and reports values. While across tES devices resistance/impedance is typically calculated in devices by dividing the peak voltage measured by the peak current applied, the static resistance (or impedance) reported by two different tES devices on the same electrode set-up may differ because tES devices do not use a consistent test current—different tES devices use various test current intensities or waveforms (e.g. dc vs brief pulses).
2.7. Headgear
All components that are used to position and hold the electrode assembly to the body are part of the HEADGEAR. As defined here, the headgear is primarily fabricated using non-conductive components (e.g. elastic or fabric). However, some conductive components like the electrode assembly and/or the lead wires may be (partially) integrated into the headgear. The headgear serves to hold these components in place, position them relative to the scalp, and/or facilitate set-up. In some applications, the electrodes are held in place by the operator, such as the plastic handles that support steel-disk electrodes in ECT. In such cases there is no head gear but details of how electrodes are supported should be provided.
2.8. Lead
The LEAD is a wire used to connect the electrode to the stimulator output. The wire is insulated except at the device (proximal) and electrode (distal) terminal. The distal terminal is typically connected to the electrode in a manner such that the material of the lead wire does not contact the electrolyte or skin. If the lead contacts the electrolyte, then the lead terminal becomes an electrode.
2.9. Anode / Anode Electrode / Anode Electrode Assembly
At the ANODE, positive current enters the body. For two-electrode systems the anode has a positive voltage relative to the cathode. If a current-controlled waveform applied to any given electrode changes polarity (for example if a biphasic sinusoid is applied such that the current direction to any given electrode changes direction), then the electrode may technically not be an anode for the entire waveform of stimulation. Thus, for tPCS biphasic pulses, the polarity of a specific (e.g. the initial) phase of the pulse should be specified (e.g. “anodic-first”). For this reason, “anode” is not used in biphasic stimulation. Rather, if the waveform is symmetric, polarity may be ignored (e.g. sinusoid with zero offset) as the electrodes are interchangeable in this sense. If the waveform is asymmetric, the polarity of the waveform should be specified relative to specific electrodes (e.g. 5 mA square pulse applied from electrode 1). In tDCS, electrode polarity does not change by definition. The separate terms of “anodal” and “anodic phase” are used to describe the hypothesized mechanism of stimulation or pulsed waveform detail, respectively (See Section 2.11).
2.10. Cathode / Cathode Electrode / Cathode Electrode Assembly
At the CATHODE, positive current exits the body. See also polarity notes in Anode definition above. The separate terms of “cathodal” and “cathodic phase” are used to describe the hypothesized mechanism of stimulation or pulsed waveform, respectively (See Section 2.11).
2.11. Monophasic, Unidirectional, Biphasic, Multiphasic (Waveform)
If during a session any given electrode functions always as either a cathode or an anode (meaning the current through each electrode is in a fixed direction, though the magnitude may change) then the stimulation is MONOPHASIC (Figure 2). Monophasic should not be confused with stimulation with only an anode or only a cathode, which is technically impossible since all stimulation involves at least one anode and one cathode. Unidirectional is used to indicate monophasic. A monophasic waveform must be defined relative to one electrode (e.g. “1 mA, 1 ms monophasic pulse with electrode A as anode and electrode B as cathode,” or “1 mA, 1 ms monophasic pulse from electrode A to electrode B”).
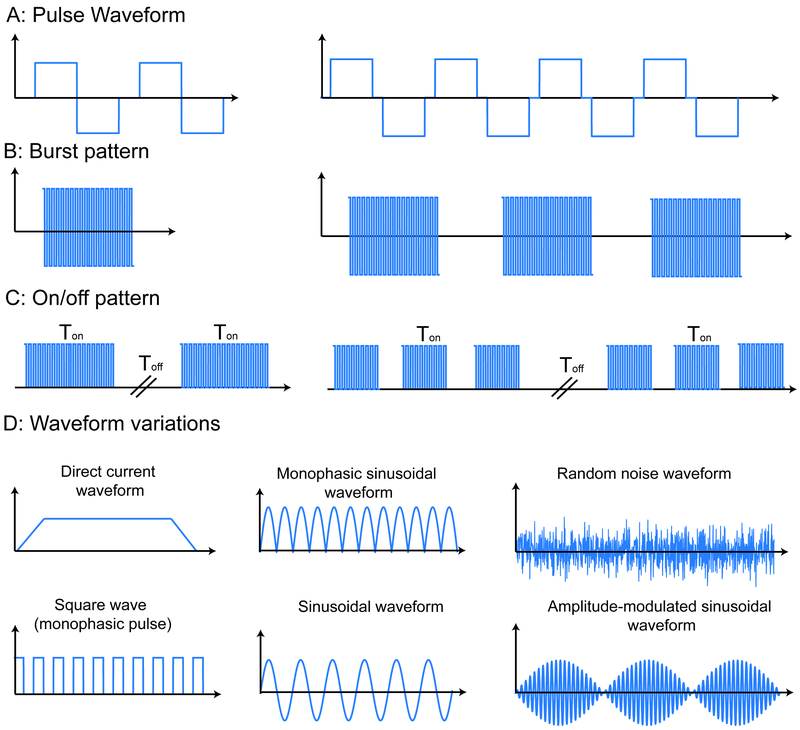
Figure 2:
Overview of terms used to describe waveform in tES. A–C address waveforms composed of rectangular pulses with expanding temporal scale, while D shows additional waveform types. A: The pulse train waveform is specified by parameters including the frequency, pulse shape, width, amplitude, and interphase delay as well as the pulse repetition frequency. B: The burst stimulation pattern includes the repetition time and number of pulses or cycles per burst; if no burst pattern is reported then the stimulation pattern is continuous. C: The on/off period (duty cycle) describes the time the stimulation pattern—continuous or burst—is active/inactive. D: Direct current has a fixed amplitude but may include ramp up/down and is, by definition, monophasic. Unless otherwise indicated, sinusoidal stimulation has a single frequency and is symmetric biphasic (no dc offset). Monophasic sinusoidal/pulse waveforms have single polarity. Amplitude-modulated sinewave is a high frequency sine modulated by a low-frequency envelope. There are various types of noise-based stimulation, conventionally with no dc offset.
If during a stimulation session any electrode changes from an anode to a cathode for any period of time, then the stimulation is MULTIPHASIC. Biphasic typically indicates stimulation with two phases (modes) that are alternated (e.g. anode and cathode switch) during stimulation (Figure 2). The importance of clarifying waveform polarity relative to electrodes can depend on the waveform. For example, for tACS the symmetry and continuous nature of the waveform suggests the waveform does not need to be defined respective to one electrode (e.g. it is sufficient to say “2 mA peak, 20 Hz sinewave current between electrodes A and B”). However, for asymmetric waveforms (e.g. two pulse phases with different amplitude and duration, Figure 2) it is important to specify waveform relative to one electrode (e.g. “leading pulse phase of 1 mA for 1 ms with electrode A as anode and electrode B as cathode, and a recovery phase of 2 mA for 0.5 ms with electrode B as anode and electrode A as cathode”).
Monophasic or biphasic pulses are typical waveforms used in some forms of tES like tPCS. Pulses are applied repetitively in a train, with the inverse of the time between pulses as the stimulation frequency. Unless otherwise specified, individual pulses are assumed to be rectangular. Individual pulses have a pulse duration (width) and amplitude. A waveform of pulses can be monophasic or biphasic. Monophasic waveforms have pulses of a single polarity (Figure 2), while biphasic waveforms have pulses that invert polarity, typically in paired opposite-polarity phases [19]. Wave types beside pulses typically take the form of a simple periodic waveform, such as a sinusoid (Figure 2). In the case that pulses are not evenly spaced in time, any burst patterns or on/off times should be reported (Figure 2).
When waveforms are monophasic, asymmetric biphasic, or symmetric biphasic but with importance of phase (e.g. phase order), then the polarity of the waveform needs to be defined with respect to the electrodes (e.g. “monophasic square wave with 5 V peak from electrode A to electrode B”). As noted (Section 2.8), in electrical stimulation an anode electrode always indicates an electrode where, a given moment in time, current (defined as flow of positive charge) enters the body and the cathode electrode indicates an electrode where current simultaneously exits the body [19]. Since in all electrical stimulation there is always an anode and a cathode present, the terms “anodal stimulation” can simply indicate that the nominal target is near the anode electrode [7]. Similarly, “cathodal stimulation” is also a statement of hypothesis indicating that the nominal target is near the cathode electrode (e.g. “cathodal stimulation of motor cortex” indicating that the cathode electrode is placed proximal to the motor cortex; see anodal/cathodal in tDCS (Section 3.1). Alternatively, in some cases like bilateral monophasic stimulation, “anodal” / ”cathodal” indicates the polarity of the waveform (e.g. “an electrode was placed on each mastoid with anodal right stimulation”). Finally, in some applications where biphasic stimulation is used (such that each electrode can alternate between anode and cathode), the terms “anodic phase” and “cathodic phase” will be used (e.g. a cathodic pulse phase is followed by an anodic pulse phase). In this sense, when brain stimulation is assumed to be driven by one phase, the terms “anodic stimulation” and “cathodic stimulation” are used (e.g. monopolar cathodic stimulation, where a cathodic activating phase is followed an anodic phase used for charge recovery) [19].
2.12. Unipolar, Monopolar, Bipolar, Bilateral, Unilateral (Electrode montage)
Conventionally, UNIPOLAR or MONOPOLAR indicate an electrode configuration with one relatively small electrode near a nominal target and another (e.g. “return”) electrode that is relatively large and/or some distance from the nominal target (e.g. extracephalic location). In contrast, a BIPOLAR montage indicates two electrodes of the same size and both relatively near the target and/or intentionally across the target [27]. While for invasive stimulation the use of unipolar/bipolar are well defined and related to stimulation outcomes, for tES these terms may reflect more the intent of stimulation than the resulting brain current flow patterns (see also “Active”, “Stimulating”, “Return” or “Reference” electrode). In tES, the rationale for unipolar/monopolar montage terminology is typically the assumption that an electrode position closer to the nominal target and/or a relatively smaller size electrode will play a key role in producing the intended outcomes compared to the other, farther and/or larger electrode. In tES, however, if and how a larger electrode reduces its relative potency depends on details of dose and the selected outcome measures [28-34]. For the case of 4×1 HD-tDCS, the polarity set by the center electrode and diffusion of return current to the four surrounding electrodes may produce a functionally unipolar current flow [35].
We emphasize that all tES must have an equal amount of current entering and exiting the brain. This includes montages with extracephalic electrodes, where current under the cephalic electrode is balanced by current through the inferior surface of the brain (and across deep and mid-brain structures). For example, in tDCS, the total magnitude of inward direct cortical current is equal to the total magnitude of outward direct cortical current. Thus, while some terminology such as cathodal-tDCS or anodal-tPCS suggest a unipolar mode of action, these are rather statements of hypothesized mechanisms of action based on proximity to the nominal physiological target.
Since all tES has (at least) two electrodes, the rationale for explicit “bipolar” montage terminology may relate the intention to stimulate two regions near both electrodes or a larger region spanning both electrodes. When electrodes are placed on the head, especially to target structures in both hemispheres, the montage may be referred to as BILATERAL. This typically symmetric electrode placements on each hemisphere [36]. When only two electrodes are used for bilateral montages, it is also bipolar. More electrodes (e.g. four [37]) can also be used in bilateral montages. Bifrontal typically indicates a symmetric bilateral montage on the scalp across frontal brain regions; bitemporal—across the scalp overlying temporal cortex of both hemispheres; and bifrontotemporal—an intermediate position between these two. For ECT, bifrontal and bitemporal further refer to specific electrode placements (see Section 3.2.2).
In summary, biphasic/monophasic refer to waveform (defined separately) and are independent of the bipolar/unipolar/bilateral electrode configuration (i.e. bilateral indicates placement of electrodes while biphasic indicates waveform). With monophasic waveforms, each electrode in a bipolar montage may be assumed to have distinct effects since it is either an anode or a cathode.
tDCS is by definition monophasic, with the anode and cathode (defined in Sections 2.8, 2.9) considered functionally distinct, thereby leading to specialized terminology. Bilateral tDCS is also called Dual-tDCS where both electrodes are considered “active”, which may be symmetric or not symmetric [38]. Lateralized tDCS typically refers to a symmetric bilateral bipolar (two electrode) montage with the intention to differentially modulate hemispheres [39-42]. Bihemispheric tDCS may be used interchangeably with bilateral tDCS when two electrodes (bipolar) are used [43-48]. Or bihemispheric tDCS can indicate the case when electrodes of the same polarity are placed on both hemispheres (e.g. two anodes, one on each hemisphere) and a third electrode of opposite polarity is placed elsewhere (e.g. extra-cephalically)—this can be further specified as Bihemispheric Anodal/Cathodal tDCS or referred to as BILATERAL BICEPHALIC tDCS.
Technically, any montage with an electrode on the contralateral supra-orbital (SO) region is non-symmetric bilateral (if only two electrodes are used, it is also bipolar), but is not typically referred to as such in publications as the So electrode (which can be anode or cathode) is considered the “return”. In such cases the term UNIHEMISPHERIC is used to indicate the relative asymmetry [49, 50]; but like many terms, this should be understood as a statement of functional hypothesis. Unilateral may indicate the nominal brain targets are in one hemisphere, which may be implemented in tES by placing all cephalic electrodes over one hemisphere, or more commonly using extracephalic electrodes [51, 52]. Terminology can easily get convoluted, for example when electrodes of the same polarity are placed on the same hemispheres (e.g. two anodes on the same hemisphere) and a third electrode of opposite polarity is placed elsewhere (e.g. extra-cephalically), the configuration can be referred to as UNILATERAL MULTIPLE MONOPOLAR [53]. Moreover, in some studies, “unilateral” (like “unihemispheric”) is used to refer to a montage with a contralateral supra-orbital (SO) position of the “return” electrode, especially when the goal is to contrast with symmetric bilateral bipolar montages [54-56]. A 4×1 HD-tDCS montage (defined in Section 3.1.1) can be used when the goal is to actually restrict current flow to one hemisphere [57-59].
In summary, tES current flow patterns are more diffuse and complex than with invasive stimulation. Many terms relating to electrode montage are indicative of presumed mechanisms of action (e.g. a nominal brain target and mechanism of neuromodulation) rather than the physics of current flow patterns.
2.13. Non-invasive (electrical stimulation)
Non-invasive medical procedures are typically defined as not breaking the skin or entering a body cavity. Non-invasive medical devices do not involve an invasive medical procedure. tES is thus non-invasive. While the current delivered by any form of tES (including ECT) crosses into the body and produces physiologic responses (including changing skin properties), this does not meet the standard for an invasive medical procedure/device any more than a stone used for massage (which transfers physical force into the body) or a heating blanket (transferring heat into the body).
2.14. Stimulation Duration / Session Duration
Stimulation duration refers to a limited (fixed) time period of administration of a set program of tES. In some uses, stimulation duration is defined as the time period from initiation to end of current flow, which may include amplitude ramp-up or ramp-down periods that are used to enhance tolerability. in typical uses, the duration of tES is limited to the period of time when tES is at the target maximal amplitude (e.g. 2 mA), thereby omitting ramp up/down periods. To avoid ambiguity, protocols should clearly define the content of “duration”. For example, one could state that “the overall duration of the current flow was 21 minutes including ramp-up and ramp-down periods for 30 s each” or “the overall duration of the stimulation was 20 minutes, with an additional 30 s ramp-up and 30 s ramp-down.”
When a waveform is defined as part of a classification, this conventionally refers to the waveform after ramp-up and before ramp-down, though typically assuming that during the ramp-up/ramp-down the waveform is the same but of increasing/decreasing peak amplitude (e.g. 10 minutes of 2 mA 10 Hz tACS starting/ending a ramp-up/down of 10 Hz ac for 30 s each). Some waveforms may have no ramp-up/down, especially those of very brief session duration (e.g. ECT, Section 3.2.3) or where the waveform is itself modulated (e.g. so-tDCS, Section 3.3).
Session duration may be defined in various ways. In some uses, it may be equivalent to stimulation duration, whereas in other uses it may also encompass the overall time of an experimental or clinical procedure, including subject set-up, instructions, application of electrodes, tests unrelated to tES, anesthesia administration, removal of electrodes, etc. Potentially, multiple tES classifications with specified durations could be applied within the duration of a single session.
2.15. Repetitive
The phrase REPETITIVE is uncommon in the context of tES classifications, and when used typically refers to multiple sessions. For example, repetitive transorbital alternating current stimulation (rtACS) is specific to multiple sessions of stimulation, with an intended outcome of neurorehabilitation that depends on multiple sessions. In other electrical stimulation applications, “repetitive” may alternatively be used when describing pulsed waveforms within a single session—this is the typical use in repetitive transcranial magnetic stimulation (rTMS) as it differentiates from single pulse TMS. But for tES, repetition of sessions is usually not incorporated in the classification, except for a few rare cases where specifically relevant. The schedule of multi-session tES will be described by the number of sessions and rate of repetition (e.g. “daily on weekdays for a total of 10 sessions over 2 weeks”). There is evidence that repeated sessions across days or within days can produce cumulative effects [60, 61]; however, describing a protocol with multiple (repeated) sessions (e.g. 2 sessions of tDCS per day) typically does not warrant new terminology.
2.16. 1×1 (montage)
The 1×1 MONTAGE refers to tES deployment with only two electrodes. For monophasic stimulation, like tDCS, this indicates one anode electrode and one cathode electrode.
2.17. Limited-Output tES
Limited-output tES was previously defined for the purposes of reconciling regulatory controls (following FDA conventions to reduce the regulatory burden for limited-output devices) with tES dose used in modern clinical trials [21]. Limited-output tES restricts dose including:
A maximum charge per phase that does not exceed Q, where Q = 20 + 28×t μC, where t is the phase duration expressed in ms and measured at 50% of the phase amplitude.
A maximum average current that does not exceed 10 mA.
A maximum primary phase duration that does not exceed 500 μs except as specified in (g).
The current is minimized when no stimulation is being applied.
A maximum current density that does not exceed an rms value of 2 mA/cm2 on the physical electrode surface.
A maximum average power density that does not exceed 0.25 W/cm2 on the physical electrode surface.
For devices using direct current or continuous sustained current passage greater than 1 s, or square wave, or rectified or bias sinusoidal, or pulses with > 25% duty cycle including all phases, if the maximum average current does not exceed 4 mA (average absolute value) then criteria (a), (c), and (d) are waived.
A maximum peak output current that does not exceed 30 mA (at any instant for all electrodes combined).
A maximum time per individual session that does not exceed 60 min.
A maximum total charge per session that does not exceed 6000 mC.
2.18. Limited-Voltage tDCS
LIMITED-VOLTAGE TRANSCRANIAL DIRECT CURRENT STIMULATION includes devices and protocols that meet all the criteria of (i) tDCS; (ii) Limited-Output tES; and (iii) maximum output below 20 V [22].
2.19. Sham
In tES studies, SHAM indicates a dose and ancillary procedural features (e.g. device appearance and sounds, application procedure) which are intended to serve as a control arm against an active condition, for example in testing the efficacy of a tES intervention (active condition against sham condition). Conventionally, tES sham is intended to produce experiences in the subjects that limits the ability of subjects to guess (greater than chance) which study arm they are participating in (supporting single blind experiments), while removing or reducing the aspect of stimulation that is thought to mediate the intended effects of tES [7]. For example, a common objective of tES sham is to replicate the scalp sensation of stimulation while minimizing the delivery of an electric field to the brain. A common sham approach is the “fade in and out” where the current is increased gradually (as typical in the active arm) but then ramped back down, thereby creating a transient sensation that is not expected to produce significant neuromodulation. The fade in and out can be applied at the start of the session [62, 63], at the end of the session, and/or at random intervals during the session [64]. Because current is applied, this is also referred to as “active sham”, which is not to be confused with “active control” when the same waveform is applied using a different electrode montage.
Additional approaches to sham, such as using two adjacent High-Definition electrodes, have been proposed [65]. It is important in any discussion about the appropriateness of a given sham condition [66, 67] to consider the explicit goals of the sham arm [63, 68]. It is also important to recognize that the effectiveness of a sham depends on the degree of sensation produced in the active arm, which in turn depends on the electrode design; thus, better electrode design that reduces sensation in the active arm can enable more reliable sham-controlled experiments.
3. Transcranial electrical stimulation (tES)
The term TRANSCRANIAL ELECTRICAL (OR ELECTRIC) STIMULATION (tES) is the preferred nomenclature for any non-invasive device intended to directly change brain function by passing low- or high-amplitude electrical currents, of any waveform, through at least one electrode on the scalp [1]. The total amount of current entering the body at one (or the sum of several) electrodes must be equal to the total current exiting the body at one (or the sum of several) electrodes – i.e. the total current in and out of the body must be equal at any instant. This is true when the current does not change polarity in monophasic stimulation or when current does change polarity in biphasic stimulation. For this reason, it is possible to describe the current strength, at any given instant or the peak amplitude over the course of a session as one number (e.g. 2 mA) rather than needing to specify independently the positive and negative current (e.g. +2 mA and −2 mA for the anode and cathode, respectively).
Though variants to tES as a global classification have been proposed, inspection of relevant historical [2] and modern literature confirms tES is the most conventional terminology [1]. “Non-invasive brain stimulation” (NIBS) and “transcranial brain stimulation” are not specific to electrical techniques. The alternative term “transcranial current stimulation” (first used in only 2008 [12]) is comparatively rare. While upper-case first letter, “TES”, may be used, it could be confused with the specific variant using supra-threshold single pulse waveforms [69].
The intended outcome of tES includes direct actions on the central nervous system (even if peripheral actions such cranial nerve stimulation, peripheral vascular effects, and/or muscle activation cannot be excluded). Specific intended outcome often appears, alongside dose characteristics, as part of tES classification. Devices that use any implanted electrodes, including intra-cranial or subcutaneous, should not be included in tES (regardless of whether such techniques result in current passage across the cranium).
3.1. Transcranial Direct Current Stimulation (tDCS)
TRANSCRANIAL DIRECT CURRENT STIMULATION (tDCS) is a tES technique in which the stimulus waveform is a sustained direct current (dc) applied to the head for the purpose of producing a direct change in brain function. The current amplitude of tDcS is limited with the intention to produce modulation of excitability and/or to change ongoing activity rather than to trigger directly action potentials (as the brain is active, tDcS will change the ongoing firing rate of neurons activated for other reasons [70]). The sustained waveform of tDCS reflects this intention. Though not required, when used, the lower-case “t” in tDCS emphasizes a proper name.
In any given session, tDCS uses a single current amplitude with minimal variation during the course of stimulation except for one ramp-up and one ramp-down period (typically a 10–30 s linear ramp). Since tDCS dose is defined as a waveform of a sustained direct current, only the amplitude (in mA), duration (in seconds or minutes), and ramp up/down details are needed to specify the waveform associated with each electrode (Table 2).
Trains of monophasic pulses are not tDCS, but rather transcranial Pulsed Current Stimulation, even when a dc offset is included. An oscillating tDCS (a monophasic square waveform), or a rectified or monophasic sinusoidal waveform are not included in tDCS as defined here (e.g. see oscillating transcranial Direct Current Stimulation, otDCS).
All practical tDCS devices produce an imperfect signal, such that a stimulator produces both a dc signal and some small superimposed noise. The level at which this fractional non-dc noise component no longer meets the definition of tDCS is unclear [71, 72] and, as a result, there are no clear noise thresholds based simply on output (e.g. noise amplitude of 1% or 0.1% of dc). Rather, the point at which a method is no longer considered tDCS may be: when the outcome of a noisy-tDCS source fails to reproduce the effects of a high quality tDCS source.
The terminology “anodal-tDCS” (a-tDCS) and “cathodal-tDCS” (c-tDCS), though common, should be used with caution. All tDCS methods involve at least one anode and one cathode (to complete a minimal circuit), and all current entering the cortex must exit (and also pass though intermediate brain regions). There is no pure unipolar tDCS (i.e. anodal or cathodal effects exerted under one electrode only), as may be implied by these terms. The terms “anodal” and “cathodal” in this context thus reflect the intended outcome of stimulation by that electrode and should be used and understood as only an expected outcome (or hypothesis). The extent to which anodal and cathodal sources produce net effects on excitation and inhibition, especially in the context of brain state, are complex. The preferred language should be “anode electrode over brain region X” [73] or “anode electrode at scalp coordinate Z defined by the EEG 10–20 system” rather than “anodal tDCS of brain region X” since the latter incorrectly implies anodic current delivered to just that brain region [74] and moreover over-simplistic intended outcomes. The terms “anodal” and “cathodal” in tDCS may be combined with terminologies related to electrode montage (such as unilateral, defined elsewhere) which are similarly an expression of hypothesized mechanisms. The terms “anode” and “cathode” (defined in Sections 2.9 and 2.10) are not ambiguous in electrical stimulation, as they indicate only if current enters or exits the scalp, respectively, at the electrode.
Dual-tDCS (bilateral tDCS [38]) indicates that both the anode electrode and cathode electrode are positioned to intentionally produce excitation and inhibition, respectively, typically symmetrically on the head [75-78], thereby explicitly leveraging the inherent mixed polarity of tDCS. However, we emphasize that both electrodes are active in all tDCS configurations. Less commonly used, Unihemispheric concurrent dual-site a-tDCS (a-tDCSUHCDS) uses two anode electrodes positioned over nominal targets (e.g. M1 and S1, or M1 and DLPFC, or M1 and V1) with two supraorbital cathode electrodes [79, 80]. Dual-site High-Definition transcranial direct current stimulation has been verified in computational models [81, 82].
The application of direct current dates back centuries to the earliest batteries [2, 83] with reported clinical trials from at least the 1960’s [84]. Interestingly, these efforts used a dose with less current (e.g. 0.3 mA) but significantly higher duration (e.g. hours), corresponding to higher net charge, than modern tDCS studies [84]. Such paradigms are outside the scope of conventional tDCS as described next.
CONVENTIONAL tDCS includes those protocols (e.g. waveform intensities and durations) that are commonly used in modern (post 2000) human trials including exploratory studies and clinical trials. Most conventional efforts used two electrodes (though some efforts used 3 or even 4 [85]) with current intensities spanning 1.0 to 2.5 mA (though 3–4 mA has been tested as well [86]). Conventional durations span 4 seconds (used only for transient changes [87]) to tens of minutes (typically 10–40 min used for durable changes [5]). Under this conventional dose and when proper technology and protocols are used [7], tDCS is well tolerated [6, 10, 88-90].
Conventional tDCS uses rectangular electrode assemblies of 5×5 cm to 5×7 cm skin–electrolyte contact area, though both smaller and larger electrode assemblies have been explored [11]. Conventional tDCS electrode assemblies use either metal or conductive rubber electrodes [91]. To provide safe, low-impedance contact with the skin, isotonic saline (saturated in a sponge) or other electrolytes such as gels and/or creams are used. The details of electrode assembly design (see Sections 2.1–2.4) are considered important for tolerability. For example, it is important to maintain a minimal distance between the electrode and skin, as well as the area of the electrode compared to the electrolyte–skin area.
3.1.1. High-Definition transcranial Electrical Stimulation (HD-tES), High-Definition Electrodes, High-Definition transcranial Direct Current Stimulation (HD-tDCS), 4×1 Montage
High-definition transcranial electrical stimulation (HD-tES) is conventionally defined as a tES montage using compact electrodes (e.g. < 5 cm2 total electrode–skin contact area, typically defined by a rigid gel holder) arranged in an array with a center electrode surrounded by a “ring” of electrodes, where the center and ring electrodes have opposite polarities. This design is intended to restrict current predominantly to the cortex circumscribed by the ring [92]. The 4×1 HD-tES montage comprises a center electrode surrounded by a ring of 4 electrodes of polarity opposite to that of the center electrode [59, 74]. The increased current density necessitates the use of specially designed electrodes [93] that are called High-Definition electrodes; stimulation at tDCS relevant intensities with other forms of small electrodes that are poorly designed can result in skin irritation.
Stimulation that meets the definition of both tDCS and High-Definition tES is called HD-tDCS [94, 95], including 4×1 HD-tDCS. Stimulation that meets the definition of both tACS and High-Definition tES is called HD-tACS [58, 82]. Current waveforms, intensities and durations used for HD-tES typically mirror those used with the corresponding pad-based technique. For example, like tDCS, HD-tDCS generally uses intensities of 1–2 mA [96-99] with a few using higher currents [100].
A montage with at least one HD electrode and other non-HD (pad) electrodes has been described as a hybrid-HD montage [101]. HD-tES/tDCS has also been used to describe an electrode surrounded by an annulus pad electrode [102]. HD-tDCS has been used to describe approaches that optimize stimulation strength or focality [103]. HD-tES has also been used to describe approaches targeting multiple brain regions [18, 58, 81, 82, 104]. HD-tDCS to two targets has been called Dual-site High-Definition transcranial direct current stimulation [81].
A feature of smaller electrodes is the potential to use a higher number of electrodes and/or electrodes in closer proximity; this in turn provides increased flexibility in montage design [95] and facilitates simultaneous recording of EEG during tES [105].
3.2. transcranial Pulsed Current Stimulation (tPCS)
Transcranial pulsed current stimulation (tPCS) is a form of tES where a train of pulses is applied. A wide variety of pulsed waveforms may be used (see Figure 2). Pulses may be monophasic or biphasic [106]. tPCS may or may not include a dc bias – a waveform with dc and pulsed components would be classified under tPCS, not tDCS.
Historically, the term “tPCS” is uncommon but has gained popularity in recent years [107-110] in alignment with terms such as tACS and tDCS. However, the use of devices delivering tPCS spans well over a century [2] with many variants that hold unique names (e.g. ECT, CES).
When monophasic tPCS is applied, it is reasonable to refer to one electrode as the anode and the other as the cathode. The terms “Anodal-tPCS” (a-tPCS [111]) and “cathodal-tPCS” (c-tPCS) may be used per convention, but as with a-tDCS and c-tDCS they should be used with caution and recognizing there is no pure unipolar tPCS, as may be implied by the terms. It is preferred to state this matter as follows: “the tPCS anode electrode is positioned over brain region X” and not “anodal tPCS of brain region X is applied” since the latter incorrectly implies current delivered to just that brain region [74].
If tPCS is biphasic and perfectly symmetric (e.g. biphasic simple square wave), then it may not be necessary to define current polarity per electrode or which electrode is the reference for the waveform (unless information on phase is relevant). However, if the waveform is asymmetric then, even if it is biphasic, the reference electrode (term used here in the mathematical sense) used to specify the waveform should be identified (e.g. +1 mA for 1 ms, and −0.1 mA for 10 ms through electrode 1, or +15 V for 3 ms and −5 V for 1 ms from electrode 1 relative to electrode 2).
tPCS is not restricted by pulse pattern or intensity (amplitude). Therefore, approaches as diverse as forms of electroconvulsive therapy (ECT; any tPCS that is intended to produce a seizure) and forms of cranial electrotherapy stimulation (CES; any tPCS that meets the FDA statutes) may be considered tPCS (Figure 1).
3.2.1. Transcranial Electrical Stimulation (TES with capital “T”)
A specific form of tPCS is one involving application of one or a few high-amplitude pulses with the goal of directly activating brain tissue which is referred to simply as TRANSCRANIAL ELECTRICAL (OR ELECTRIC) STIMULATION (TES). Examples of TES include application over the motor cortex to induce a motor response [92] or visual cortex to produce phosphenes. To reach motor threshold, TES typically uses a pulse amplitude in the hundreds of mA with durations of tens to hundreds of μs [92]. The “T” is conventionally capitalized. This form of TES is currently used mostly during intraoperative monitoring when the patient is anesthetized. Because it produces significant discomfort, TES is used rarely in awake subjects [92], sometimes as a comparison to TMS, since TES is understood to activate different neural populations than TMS [112, 113]. The electrode configurations used for TES are typically bipolar [92], though 4×1 HD has been tested as well [92]. TES uses too few or low-frequency pulses to produce a seizure, so it does not overlap with ECT (another form of suprathreshold tPCS).
3.2.2. Cranial Electrotherapy Stimulation (CES)
Cranial electrotherapy stimulation (CES) in modern use is derived from an FDA classification for a specific form of tES. CES is thus defined legally in the USA as any device which the FDA has designated CES. Per the FDA, CES is defined as “a device that applies electrical current to a patient's head to treat insomnia, depression, or anxiety” (21 CFR 882.5800). CES waveforms are a form of tPCS using high-frequency pulse trains with electrodes applied across the forehead, mastoids, or ear-lobes using clips [114].
3.2.3. Electroconvulsive therapy (ECT)
ELECTROCONVULSIVE THERAPY (ECT) involves the delivery of repetitive current stimuli to induce a therapeutic seizure [115]. Whereas older forms of ECT used sinusoidal currents, modern ECT relies on brief current pulses. Modern ECT is therefore a subclass of tPCS. In standard ECT devices, the stimulus pulses are current-controlled, rectangular, and monophasic with polarity alternating from pulse to pulse, forming pulse pairs of opposite polarity. Modern ECT is administered under general anesthesia and muscle relaxants. The seizure is typically determined by motor activity in a limb and by seizure activity in electroencephalography (EEG). Under current recommendations, the waveform is titrated relative to the individual’s seizure threshold [116]. Typical ECT parameters are amplitude of 800–900 mA (although lower currents have been used experimentally), pulse width of 0.25–1 ms, train duration ≤ 8 s, and train frequency of 10–120 Hz (pulse-pairs per second). The FDA limits the dose of ECT devices cleared in the USA to 576.0 mC and 101.4 J into a 220 Ω load, whereas in some other countries the limit is twice as high. Typical electrodes are either 5-cm-diameter stainless steel disks used with electrolyte gel or disposable adhesive conductive pads.
The term ECT is often used with modifiers that specify the delivery approach, especially electrode placement and pulse width. Standard electrode placements include right unilateral (RUL), bitemporal (BT), and bifrontal (BF) [117]. Note that “bilateral” includes both bitemporal and bifrontal, though before the introduction of bifrontal ECT, it was used to denote bitemporal placement. In some cases, other electrode configurations, such as left unilateral (LUL), are used. Conventionally, pulse width is classified as brief (≤ 0.5 ms, typically 0.5–1 ms) or ultrabrief (UB, < 0.5 ms, typically 0.25–0.3 ms). Over the years a wide variety of ECT paradigms have been explored, and there is ongoing research in alternative ECT parameters including individualized current amplitude, lower or higher current amplitude, unidirectional pulse trains, extended trains, and various electrode configurations [118-126].
3.3. (Slow) Oscillating transcranial Direct Current Stimulation (so-tDCS), transcranial Sinusoidal Direct Current Stimulation (ts-DCS)
(slow) Oscillatory transcranial direct current stimulation (o-tDCS/so-tDCS) is a form of tES using direct current stimulation where the amplitude of the stimulus is regularly modulated, but which remains monophasic (such that the polarity of stimulation is never inverted) and where the intensity remains limited with the intent to produce subthreshold modulation. The waveform is typically monophasic square, trapezoidal, or monophasic sinusoidal wave. o-tDCS and its variants conventionally use electrode montages adapted from tDCS. Slow oscillatory tDCS (so-tDCS) conventionally refers to a signal with a frequency below 1 Hz (e.g. 0.75 Hz) [127]. The on-off time of o-tDCS and its derivatives may be varied (e.g. 5 intervals with 1 minute gap [128]). so-tDCS may also be qualified as anodal or cathodal [127], though this infers a hypothesized anatomical target as discussed above (see anodal/cathodal in tDCS).
Transcranial sinusoidal direct current stimulation (ts-DCS) is a form of o-tDCS where the waveform is a monophasic (biased) sinusoid. so-tDCS may also be used to describe protocols with sinusoids when the frequency is low [127, 128]. ts-DCS frequencies and intensities span those used in tACS [129]. Slow oscillatory stimulation (SOS) or transcranial slow oscillatory stimulation may refer to variants of so-tDCS or ts-DCS [130, 131].
The distinction between modes of o-tDCS from tDCS (which in principle may be applied briefly and intermittently, e.g. 15 second on tDCS, 15 seconds off tDCS, repeated [132]) and from tPCS (where pulse duration can in principle be increased to hundreds of ms) is, as defined here, one of intended outcome. o-tDCS is expected to produce changes in part through the change in current (namely the neurophysiologic intended outcomes are assumed to reflect the non-static nature of current flow), and a sustained phase of stimulation (namely the neurophysiologic outcomes are assumed to reflect actions when the current is sustained). tPCS is presumably focused only on transient effects while tDCS only on sustained effects. We caution that this distinction of intention is subtle, subject to change/interpretation, and that the rationale for o-tDCS (as opposed to tPCS) is often not explicitly stated in o-tDCS publications. Nonetheless, based on current understanding and use of conventions, we categorize o-tDCS and its variants as a category that is district from tPCS or tDCS (Figure 1). We emphasize that all studies should report the dose applied regardless of the terminology used.
3.4. transcranial Alternating Current Stimulation (tACS)
Transcranial alternating current stimulation (tACS) is a form of tES involving application of sinusoidal current across the scalp to the brain [133-135]. The sinusoid may be biased (which should be specified relative to a reference electrode) but must have at least some biphasic components. A combination of sinusoids (summation) may be used. tACS is sub-convulsive as the applied intensities are at least an order of magnitude less compared to intensities produced by devices intended to induce seizures as part of the therapeutic outcome. Thus, ECT is not a form of tACS. As conventionally used in the neuromodulation literature, tACS does not include any waveform that is non-sinusoidal. While other waveforms may be “alternating current” in the engineering sense (i.e., current flow direction at the electrode (and therefore the brain) reverses direction), tACS classifies a specific method using only sinusoids.
Conventional intensities are typically limited to 1–2 mA or less [136]. Conventional tACS uses a single peak current amplitude with minimal variation during the course of stimulation, except for conventional ramp-up and ramp-down periods (typically 10–30 s linear). However, some forms of tACS intentionally modulate the amplitude: Amplitude Modulation tACS (AM-tACS) [137].
The interval from the initiation of current flow (start of ramp-up of sinusoid) to the end of current flow (end of sinusoid ramp-down) is a single tACS session; when specifying the duration of a tACS session it should be made clear if this is inclusive or excusive of the ramp-up and ramp-down period.
Conventional tACS methods are adapted from tDCS, such that electrode assemblies use either metal or conductive rubber electrodes with typical area of 5×5 cm to 5×7 cm skin–electrolyte contact area. Like in tDCS, electrolytes are more commonly saline (saturated in a sponge) but gels and creams have been used as well.
For tACS, the current frequency and amplitude and the duration of the stimulation are the major parameters that are known to shape the direction and duration of the after-effects. Conventionally, tACS employs frequencies or combinations of frequencies in the physiologic EEG range (below 200 Hz), in some cases with the intention to interact with or influence these oscillations [70, 138-142]. However, higher frequencies in the kHz range have also been studied [116, 143, 144]. When kHz frequencies are applied across multiple electrodes [145], with a slight difference in frequency across different electrode pairs, this is classified as Interferential Stimulation (defined separately in Section 3.4.1).
3.4.1. Interferential Stimulation / Temporal Interference Stimulation
Interferential stimulation or temporal interference stimulation (IF) is the simultaneous application of two (or more) sinewaves, both at high but slightly different frequencies via two (or more) pairs of electrodes. IF is therefore a subtype of tACS. The summation of two high-frequency (e.g. ~ 2 kHz) sine waves of slightly different frequencies results in a waveform that is a high-frequency carrier-wave (average of the two sine waves) modulated by a low frequency (e.g. 10 Hz) envelope oscillating at a "beat" frequency. This beat frequency is the difference of the frequencies of the two sinusoids (Figure 2D). There is a long-standing interest in IF [2] and recent work has focused on the possibility of stimulating deep targets [145].
3.5. Repetitive transorbital alternating current stimulation (rtACS), transcorneal electrical stimulation (TcES), transscleral electrical stimulation (TsES)
In REPETITIVE TRANSORBITAL ALTERNATING CURRENT STIMULATION (rtACS) stimulating electrodes are positioned near the eye (2–4 per orbit) [146-150] with the aim to inject current to the eyeball to reach the nervous tissue of the retina and brain. rtACS has been proposed to induce vision restoration by activating residual visual functions in patients with damage to the retina, optic nerve, or brain [151]. rtACS requires multiple sessions (typically at least 10); rtACS thus reflects a classification where the intended outcome (rehabilitation) forms the definition.
Transcorneal electrodes have the shapes of contact lenses [152-156]. Transcorneal hair-like DTL electrodes [157] directly contact the cornea [158-163] or the cornea and sclera [155]. Studies where electrodes are positioned on the scalp use the terminology rtACS, but those not positioned on the scalp use the terminologies TRANSCORNEAL ELECTRICAL STIMULATION (TcES) or TRANSSCLERAL ELECTRICAL STIMULATION (TsES).
3.6. transcranial Random Noise Stimulation (tRNS)
Transcranial random noise stimulation (tRNS) is a form of tES that was developed with the intent to desynchronize pathological cortical rhythms [164] but additional putative mechanisms, such as stochastic resonance [165, 166], may be relevant. Stimulation is conventionally multiphasic (current has both polarities) and various forms of noise may be applied. In typical examples, during tRNS a frequency spectrum between 0.1 Hz and 640 Hz (full spectrum) or 100–640 Hz (high frequency spectrum) is applied. During one embodiment of "random noise" stimulation, the random levels of current are generated by an internal random number generator 1280 times per second; the probability function of the random noise current stimulation follows a Gaussian or bell-shaped curve with zero mean and a variance, where 99% of all generated current levels are between ± 1 mA (when 1 mA stimulation amplitude is used). In the frequency domain, all coefficients of the random sequence have a similar size ("white noise"). tRNS is adapted from tDCS and tACS, such that electrodes and related application paradigms are the same as mentioned above.
Because there are many forms of “noise” (and the neurophysiologic effects are specific) it is important for dose reproduction (Section 2) to provide sufficient details on how the waveform was formulated to allow reproduction.
4. Summary
This consensus expert report provides the first comprehensive and integrated classification of terminology used in contemporary transcranial electrical stimulation. Such a list cannot be exhaustive and for each classification there can be publications that apply the term differently. Reflecting how terms are used, our approach to classification considers both the dose of stimulation (electrode configuration and stimulus waveform) and the intended outcomes of stimulation. No part of this paper should be taken as an endorsement of one approach over another, and, as emphasized, the use of terminology in a publication does not substitute the need to fully document dose [3]. Individual researchers may acquaint themselves with existing terminology and acronyms and adopt existing (as opposed to creating new) terminology as appropriate. Any analysis or review of tES technology, safety, or efficacy should clearly (re)define the dose range of any terminology used or reference a classification such as the one provided here.
This paper was motivated by the value of cataloging common terms used in the tES literature. Our explicit scope and approach (Section 1) was to explain standing usage rather than to propose modified or new terms or to ban specific usage of terms. Nevertheless, our classification draws attention to ambiguous terminology, particularly terms that rely heavily on speculative modes of action. To the extent the field as a whole will adopt (and enforce) clarified terminology, our review of existing conventions is an important contribution. However, an immediate remedy is available: publications should fully document dose [3] in a reproducible manner (cognizant of how basic terms are used; see Section 2 and Table 2). Only referencing a specific tES category (Section 3) fails to fully convey such information.
It is incumbent on the tES field (through societies, working groups, and conferences) to consider the development of revised classifications and standards, including if any terminology should be formally discouraged. Prospectively, this paper contributes to this process by cataloging standing nomenclature and conventions. Retrospectively, understanding and leveraging the historical publication record evidently requires understating terminology as used contemporaneously – the explicitly limited scope of the present paper.
Highlights:
Modern tES suffers from a diversity of ill-defined nomenclature.
A consistent and clear taxonomy for tES will support clear and reproducible research.
This review presents the first comprehensive nomenclature scheme for tES.
Acknowledgement
The contributions of A. V. Peterchev were supported by the NIH under grants R01MH091083, R21MH106772, and R01MH111889. The contributions of A. J. Woods were supported by the NIH under grants R01AG054077, K01AG050707, and R21MH112206. The contributions of M. Bikson were supported by the NIH under grants MH111896, NS101362, U54CA137788/ U54CA132378, and NS054783. The contributions of B. M. Hampstead were supported from the VA (IRX001534) and NIA (R01AG058724). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The City University of New York has inventions on tES with MB as inventor. MB has equity in Soterix Medical and serves on the scientific advisory boards of Boston Scientific and GlaxoSmithKline. AVP is inventor on patents and patent applications and has received research and travel support as well as patent royalties from Rogue Research, research and travel support, consulting fees, as well as equipment donation from Tal Medical / Neurex, research and patent application support from Magstim, as well as equipment loans from MagVenture, all related to technology for transcranial magnetic stimulation. CKL has received research equipment support from Soterix Medical, and honoraria and travel support from Mecta for teaching in an International ECT course. BWB is an inventor on tES patents and patent applications, has equity in Bodhi NeuroTech and serves as a consultant to eQuility. MAN is an advisor for Neuroelectrics. AA has received research equipment, honoraria and travel support from support from NeuroConn/NeuroCare. HK has received research equipment support from Soterix Medical Inc, as well as research support and a single-event travel support from Ybrain, Inc.
We wish to draw the attention of the Editor to the following facts which may be considered as potential conflicts of interest and to significant financial contributions to this work:
The City University of New York has inventions on tES with MB as inventor. MB has equity in Soterix Medical and serves on the scientific advisory boards of Boston Scientific and GlaxoSmithKline. AVP is inventor on patents and patent applications and has received research and travel support as well as patent royalties from Rogue Research, research and travel support, consulting fees, as well as equipment donation from Tal Medical / Neurex, research and patent application support from Magstim, as well as equipment loans from MagVenture, all related to technology for transcranial magnetic stimulation. CKL has received research equipment support from Soterix Medical, honoraria and travel support from Mecta for teaching in an International ECT course, and research funding and equipment loan from Brainsway. BWB is an inventor on tES patents and patent applications, has equity in Bodhi NeuroTech and serves as a consultant to eQuility. MAN is an advisor for Neuroelectrics. AA has received research equipment, honoraria and travel support from support from NeuroConn/NeuroCare. HK has received research equipment support from Soterix Medical Inc, as well as research support and a single-event travel support from Ybrain, Inc. WJT is an inventor on patents for electrical neuromodulation and ultrasonic neuromodulation. WJT is a co-founder and holds equity in IST, LLC. None of the remaining authors have potential conflicts of interest to be disclosed.
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
We understand that the Corresponding Author is the sole contact for the Editorial process (including Editorial Manager and direct communications with the office). He/she is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs. We confirm that we have provided a current, correct email address which is accessible by the Corresponding Author and which has been configured to accept email from (zesmaeilpour@ccny.cuny.edu):
References
- 1.Antal A, Alekseichuk I, Bikson M, Brockmöller J, Brunoni A, Chen R, et al. Low intensity transcranial electric stimulation: safety, ethical, legal regulatory and application guidelines. Clin Neurophysiol. 2017;128(9):1774–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guleyupoglu B, Schestatsky P, Edwards D, Fregni F, Bikson M. Classification of methods in transcranial electrical stimulation (tES) and evolving strategy from historical approaches to contemporary innovations. J Neurosci Methods. 2013;219(2):297–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peterchev AV, Wagner TA, Miranda PC, Nitsche MA, Paulus W, Lisanby SH, et al. Fundamentals of transcranial electric and magnetic stimulation dose: definition, selection, and reporting practices. Brain Stimul. 2012;5(4):435–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buch ER, Santarnecchi E, Antal A, Born J, Celnik PA, Classen J, et al. Effects of tDCS on motor learning and memory formation: a consensus and critical position paper. Clin Neurophysiol. 2017;128(4):589–603. [DOI] [PubMed] [Google Scholar]
- 5.Brunoni AR, Nitsche MA, Bolognini N, Bikson M, Wagner T, Merabet L, et al. Clinical research with transcranial direct current stimulation (tDCS): challenges and future directions. Brain Stimul. 2012;5(3):175–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bikson M, Grossman P, Thomas C, Zannou AL, Jiang J, Adnan T, et al. Safety of transcranial direct current stimulation: evidence based update 2016. Brain Stimul. 2016;9(5):641–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woods AJ, Antal A, Bikson M, Boggio PS, Brunoni AR, Celnik P, et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin Neurophysiol. 2016;127(2):1031–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woods AJ, Bryant V, Sacchetti D, Gervits F, Hamilton R. Effects of electrode drift in transcranial direct current stimulation. Brain Stimul. 2015;8(3):515–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dundas J, Thickbroom G, Mastaglia F. Perception of comfort during transcranial DC stimulation: effect of NaCl solution concentration applied to sponge electrodes. Clin Neurophysiol. 2007;118(5):1166–70. [DOI] [PubMed] [Google Scholar]
- 10.Minhas P, Bansal V, Patel J, Ho JS, Diaz J, Datta A, et al. Electrodes for high-definition transcutaneous DC stimulation for applications in drug delivery and electrotherapy, including tDCS. J Neurosci Methods. 2010;190(2):188–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nitsche MA, Doemkes S, Karakose T, Antal A, Liebetanz D, Lang N, et al. Shaping the effects of transcranial direct current stimulation of the human motor cortex. J Neurophysiol. 2007;97(4):3109–17. [DOI] [PubMed] [Google Scholar]
- 12.Datta A, Elwassif M, Battaglia F, Bikson M. Transcranial current stimulation focality using disc and ring electrode configurations: FEM analysis. J Neural Eng. 2008;5(2):163. [DOI] [PubMed] [Google Scholar]
- 13.Truong DQ, Hüber M, Xie X, Datta A, Rahman A, Parra LC, et al. Clinician accessible tools for GUI computational models of transcranial electrical stimulation: BONSAI and SPHERES. Brain Stimul. 2014;7(4):521–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bikson M, Datta A, Rahman A, Scaturro J. Electrode montages for tDCS and weak transcranial electrical stimulation: role of “return” electrode’s position and size. Clin Neurophysiol. 2010;121(12):1976–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silberstein SD, Mechtler LL, Kudrow DB, Calhoun AH, McClure C, Saper JR, et al. Non–Invasive Vagus Nerve Stimulation for the AC ute T reatment of Cluster Headache: Findings From the Randomized, Double-Blind, Sham-Controlled ACT1 Study. Headache. 2016;56(8):1317–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mendonca ME, Santana MB, Baptista AF, Datta A, Bikson M, Fregni F, et al. Transcranial DC stimulation in fibromyalgia: optimized cortical target supported by high-resolution computational models. J Pain. 2011;12(5):610–7. [DOI] [PubMed] [Google Scholar]
- 17.Gebodh N, Esmaeilpour Z, Adair D, Chelette K, Dmochowski J, Woods AJ, et al. Inherent physiological artifacts in EEG during tDCS. Neuroimage. 2019;185:408–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischer DB, Fried PJ, Ruffini G, Ripolles O, Salvador R, Banus J, et al. Multifocal tDCS targeting the resting state motor network increases cortical excitability beyond traditional tDCS targeting unilateral motor cortex. Neuroimage. [DOI] [PubMed] [Google Scholar]
- 19.Merrill DR, Bikson M, Jefferys JG. Electrical stimulation of excitable tissue: design of efficacious and safe protocols. J Neurosci Methods. 2005;141(2):171–98. [DOI] [PubMed] [Google Scholar]
- 20.Woods AJ, Antal A, Bikson M, Boggio PS, Brunoni AR, Celnik P, et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin Neurophysiol. 2016;127(2):1031–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bikson M, Paneri B, Mourdoukoutas A, Esmaeilpour Z, Badran BW, Azzam R, et al. Limited output transcranial electrical stimulation (LOTES-2017): Engineering principles, regulatory statutes, and industry standards for wellness, over-the-counter, or prescription devices with low risk. Brain Stimul. 2018;11(1):134–57. [DOI] [PubMed] [Google Scholar]
- 22.Hahn C, Rice J, Macuff S, Minhas P, Rahman A, Bikson M. Methods for extra-low voltage transcranial direct current stimulation: current and time dependent impedance decreases. Clin Neurophysiol. 2013;124(3):551–6. [DOI] [PubMed] [Google Scholar]
- 23.Pabst O, Martinsen ØG, Chua L. The non-linear electrical properties of human skin make it a generic memristor. Sci Rep. 2018;8(1):15806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Björklund S, Ruzgas T, Nowacka A, Dahi I, Topgaard D, Sparr E, et al. Skin membrane electrical impedance properties under the influence of a varying water gradient. Biophysical journal. 2013;104(12):2639–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalia YN, Guy RH. The electrical characteristics of human skin in vivo. J Pharm Res. 1995;12(11):1605–13. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto T, Yamamoto Y. Non-linear electrical properties of skin in the low frequency range. Med Biol Eng Comput. 1981;19(3):302. [DOI] [PubMed] [Google Scholar]
- 27.Rawji V, Ciocca M, Zacharia A, Soares D, Truong D, Bikson M, et al. tDCS changes in motor excitability are specific to orientation of current flow. Brain Stimul. 2018;11(2):289–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foerster AS, Rezaee Z, Paulus W, Nitsche MA, Dutta A. Effects of Cathode Location and the Size of Anode on Anodal Transcranial Direct Current Stimulation Over the Leg Motor Area in Healthy Humans. Front Neurosci. 2018;12:443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bikson M, Datta A, Rahman A, Scaturro J. Electrode montages for tDCS and weak transcranial electrical stimulation: role of "return" electrode's position and size. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2010;121(12):1976–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ho KA, Taylor JL, Chew T, Galvez V, Alonzo A, Bai S, et al. The Effect of Transcranial Direct Current Stimulation (tDCS) Electrode Size and Current Intensity on Motor Cortical Excitability: Evidence From Single and Repeated Sessions. Brain Stimul. 2016;9(1):1–7. [DOI] [PubMed] [Google Scholar]
- 31.Bastani A, Jaberzadeh S. a-tDCS differential modulation of corticospinal excitability: the effects of electrode size. Brain Stimul. 2013;6(6):932–7. [DOI] [PubMed] [Google Scholar]
- 32.Miranda PC, Faria P, Hallett M. What does the ratio of injected current to electrode area tell us about current density in the brain during tDCS? Clin Neurophysiol. 2009;120(6):1183–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nitsche MA, Doemkes S, Karakose T, Antal A, Liebetanz D, Lang N, et al. Shaping the effects of transcranial direct current stimulation of the human motor cortex. J Neurophysiol. 2007;97(4):3109–17. [DOI] [PubMed] [Google Scholar]
- 34.Deng ZD, Lisanby SH, Peterchev AV. Controlling stimulation strength and focality in electroconvulsive therapy via current amplitude and electrode size and spacing: comparison with magnetic seizure therapy. J ECT. 2013;29(4):325–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alam M, Truong DQ, Khadka N, Bikson M. Spatial and polarity precision of concentric high-definition transcranial direct current stimulation (HD-tDCS). Phys Med Biol. 2016;61(12):4506–21. [DOI] [PubMed] [Google Scholar]
- 36.Pahor A, Jausovec N. The Effects of Theta and Gamma tACS on Working Memory and Electrophysiology. Front Hum Neurosci. 2017;11:651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Di Lazzaro V, Dileone M, Capone F, Pellegrino G, Ranieri F, Musumeci G, et al. Immediate and late modulation of interhemipheric imbalance with bilateral transcranial direct current stimulation in acute stroke. Brain Stimul. 2014;7(6):841–8. [DOI] [PubMed] [Google Scholar]
- 38.Hadoush H, Al-Sharman A, Khalil H, Banihani SA, Al-Jarrah M. Sleep Quality, Depression, and Quality of Life After Bilateral Anodal Transcranial Direct Current Stimulation in Patients with Parkinson's Disease. Med Sci Monit Basic Res. 2018;24:198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zich C, Harty S, Kranczioch C, Mansfield KL, Sella F, Debener S, et al. Modulating hemispheric lateralization by brain stimulation yields gain in mental and physical activity. Sci Rep. 2017;7(1):13430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li LM, Leech R, Scott G, Malhotra P, Seemungal B, Sharp DJ. The effect of oppositional parietal transcranial direct current stimulation on lateralized brain functions. Eur J Neurosci. 2015;42(11):2904–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dambacher F, Schuhmann T, Lobbestael J, Arntz A, Brugman S, Sack AT. No Effects of Bilateral tDCS over Inferior Frontal Gyrus on Response Inhibition and Aggression. PLoS One. 2015;10(7):e0132170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rego GG, Lapenta OM, Marques LM, Costa TL, Leite J, Carvalho S, et al. Hemispheric dorsolateral prefrontal cortex lateralization in the regulation of empathy for pain. Neurosci Lett. 2015;594:12–6. [DOI] [PubMed] [Google Scholar]
- 43.Waters-Metenier S, Husain M, Wiestler T, Diedrichsen J. Bihemispheric transcranial direct current stimulation enhances effector-independent representations of motor synergy and sequence learning. J Neurosci. 2014;34(3):1037–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lindenberg R, Renga V, Zhu LL, Nair D, Schlaug G. Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients. Neurology. 2010;75(24):2176–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Waters S, Wiestler T, Diedrichsen J. Cooperation Not Competition: Bihemispheric tDCS and fMRI Show Role for Ipsilateral Hemisphere in Motor Learning. J Neurosci 2017;37(31):7500–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chhatbar PY, Ramakrishnan V, Kautz S, George MS, Adams RJ, Feng W. Transcranial Direct Current Stimulation Post-Stroke Upper Extremity Motor Recovery Studies Exhibit a Dose-Response Relationship. Brain Stimul. 2016;9(1):16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lindenberg R, Sieg MM, Meinzer M, Nachtigall L, Floel A. Neural correlates of unihemispheric and bihemispheric motor cortex stimulation in healthy young adults. Neuroimage. 2016;140:141–9. [DOI] [PubMed] [Google Scholar]
- 48.Montenegro RA, Midgley A, Massaferri R, Bernardes W, Okano AH, Farinatti P. Bihemispheric Motor Cortex Transcranial Direct Current Stimulation Improves Force Steadiness in Post-Stroke Hemiparetic Patients: A Randomized Crossover Controlled Trial. Front Hum Neurosci. 2016;10:426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fiori V, Nitsche M, Iasevoli L, Cucuzza G, Caltagirone C, Marangolo P. Differential effects of bihemispheric and unihemispheric transcranial direct current stimulation in young and elderly adults in verbal learning. Behav Brain Res. 2017;321:170–5. [DOI] [PubMed] [Google Scholar]
- 50.Lindenberg R, Nachtigall L, Meinzer M, Sieg MM, Floel A. Differential effects of dual and unihemispheric motor cortex stimulation in older adults. J Neurosci. 2013;33(21):9176–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Balzarotti S, Colombo B. Effects of Unilateral Transcranial Direct Current Stimulation of Left Prefrontal Cortex on Processing and Memory of Emotional Visual Stimuli. PLoS One. 2016;11(7):e0159555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paciello F, Podda MV, Rolesi R, Cocco S, Petrosini L, Troiani D, et al. Anodal transcranial direct current stimulation affects auditory cortex plasticity in normal-hearing and noise-exposed rats. Brain Stimul. 2018;11(5):1008–23. [DOI] [PubMed] [Google Scholar]
- 53.Nasseri P, Nitsche MA, Ekhtiari H. A framework for categorizing electrode montages in transcranial direct current stimulation. Front Hum Neurosci. 2015;9:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mordillo-Mateos L, Turpin-Fenoll L, Millan-Pascual J, Nunez-Perez N, Panyavin I, Gomez-Arguelles JM, et al. Effects of simultaneous bilateral tDCS of the human motor cortex. Brain Stimul. 2012;5(3):214–22. [DOI] [PubMed] [Google Scholar]
- 55.Sehm B, Kipping J, Schafer A, Villringer A, Ragert P. A Comparison between Uni- and Bilateral tDCS Effects on Functional Connectivity of the Human Motor Cortex. Front Hum Neurosci. 2013;7:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sehm B, Schafer A, Kipping J, Margulies D, Conde V, Taubert M, et al. Dynamic modulation of intrinsic functional connectivity by transcranial direct current stimulation. J Neurophysiol. 2012;108(12):3253–63. [DOI] [PubMed] [Google Scholar]
- 57.Heise KF, Kortzorg N, Saturnino GB, Fujiyama H, Cuypers K, Thielscher A, et al. Evaluation of a Modified High-Definition Electrode Montage for Transcranial Alternating Current Stimulation (tACS) of Pre-Central Areas. Brain Stimul. 2016;9(5):700–4. [DOI] [PubMed] [Google Scholar]
- 58.Helfrich RF, Knepper H, Nolte G, Struber D, Rach S, Herrmann CS, et al. Selective modulation of interhemispheric functional connectivity by HD-tACS shapes perception. PLoS Biol. 2014;12(12):e1002031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kuo HI, Bikson M, Datta A, Minhas P, Paulus W, Kuo MF, et al. Comparing cortical plasticity induced by conventional and high-definition 4 × 1 ring tDCS: a neurophysiological study. Brain Stimul. 2013;6(4):644–8. [DOI] [PubMed] [Google Scholar]
- 60.Monte-Silva K, Kuo M-F, Hessenthaler S, Fresnoza S, Liebetanz D, Paulus W, et al. Induction of late LTP-like plasticity in the human motor cortex by repeated non-invasive brain stimulation. Brain Stimul. 2013;6(3):424–32. [DOI] [PubMed] [Google Scholar]
- 61.Brunoni AR, Moffa AH, Fregni F, Palm U, Padberg F, Blumberger DM, et al. Transcranial direct current stimulation for acute major depressive episodes: meta-analysis of individual patient data. Br J Psychiatry. 2016;208(6):522–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ambrus GG, Al-Moyed H, Chaieb L, Sarp L, Antal A, Paulus W. The fade-in--short stimulation--fade out approach to sham tDCS--reliable at 1 mA for naive and experienced subjects, but not investigators. Brain Stimul. 2012;5(4):499–504. [DOI] [PubMed] [Google Scholar]
- 63.Bikson M, Brunoni AR, Charvet LE, Clark VP, Cohen LG, Deng ZD, et al. Rigor and reproducibility in research with transcranial electrical stimulation: An NIMH-sponsored workshop. Brain Stimul. 2018;11(3):465–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brunoni AR, Sampaio-Junior B, Moffa AH, Borrione L, Nogueira BS, Aparicio LV, et al. The Escitalopram versus Electric Current Therapy for Treating Depression Clinical Study (ELECT-TDCS): rationale and study design of a non-inferiority, triple-arm, placebo-controlled clinical trial. Sao Paulo Med J. 2015;133(3):252–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Richardson Jessica D. PF, Datta Abhishek, Truong Dennis, Bikson Marom, Fridriksson Julius. Toward Development of Sham Protocols for HighDefinition Transcranial Direct Current Stimulation (HD-tDCS). Neuroregulation. 2014;Vol. 1(1):62–72. [Google Scholar]
- 66.Turi Z, Csifcsak G, Boayue NM, Aslaksen P, Antal A, Paulus W, et al. Blinding is compromised for transcranial direct current stimulation at 1 mA for 20 min in young healthy adults. Eur J Neurosci. 2019. [DOI] [PubMed] [Google Scholar]
- 67.Fonteneau C, Mondino M, Arns M, Baeken C, Bikson M, Brunoni AR, et al. Sham tDCS: A hidden source of variability? Reflections for further blinded, controlled trials. Brain Stimul. 2019;12(3):668–673. [DOI] [PubMed] [Google Scholar]
- 68.Guarienti F, Caumo W, Shiozawa P, Cordeiro Q, Boggio PS, Bensenor IM, et al. Reducing transcranial direct current stimulation-induced erythema with skin pretreatment: considerations for sham-controlled clinical trials. Neuromodulation. 2015;18(4):261–5. [DOI] [PubMed] [Google Scholar]
- 69.Merton P, Morton H. Stimulation of the cerebral cortex in the intact human subject. Nature. 1980;285(5762):227. [DOI] [PubMed] [Google Scholar]
- 70.Reato D, Rahman A, Bikson M, Parra LC. Effects of weak transcranial alternating current stimulation on brain activity—a review of known mechanisms from animal studies. Front Hum Neurosci. 2013;7:687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Salimpour Y, Wei Z, Duy PQ, Anderson WS. Does transcranial direct current stimulation actually deliver DC stimulation? Brain Stimul. 2016;9(4):623–4. [DOI] [PubMed] [Google Scholar]
- 72.Chhatbar PY, Sawers JR, Feng W. Response to the Response to “Does tDCS Actually Deliver DC Stimulation?”. Brain Stimul. 2016;9(6):952–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Clemens B, Jung S, Mingoia G, Weyer D, Domahs F, Willmes K. Influence of anodal transcranial direct current stimulation (tDCS) over the right angular gyrus on brain activity during rest. PLoS One. 2014;9(4):e95984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Datta A, Bansal V, Diaz J, Patel J, Reato D, Bikson M. Gyri-precise head model of transcranial direct current stimulation: improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimul. 2009;2(4):201–7. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Klomjai W, Aneksan B, Pheungphrarattanatrai A, Chantanachai T, Choowong N, Bunleukhet S, et al. Effect of single-session dual-tDCS before physical therapy on lower-limb performance in sub-acute stroke patients: A randomized sham-controlled crossover study. Ann Phys Rehabil Med. 2018;61(5):286–91. [DOI] [PubMed] [Google Scholar]
- 76.Pingue V, Priori A, Malovini A, Pistarini C. Dual Transcranial Direct Current Stimulation for Poststroke Dysphagia: A Randomized Controlled Trial. Neurorehabil Neural Repair. 2018;32(6-7):635–44. [DOI] [PubMed] [Google Scholar]
- 77.Sarasso P, Ninghetto M, Salatino A, Ronga I, Bongiardina A, Iarrobino I, et al. Everything is (still) illuminated: Dual right cathodal-left anodal tDCS of PPC prevents fatigue on a visual detection task. Brain Stimul. 2019;12(1):187–9. [DOI] [PubMed] [Google Scholar]
- 78.Karok S, Witney AG. Enhanced motor learning following task-concurrent dual transcranial direct current stimulation. PLoS One. 2013;8(12):e85693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vaseghi B, Zoghi M, Jaberzadeh S. The effects of anodal-tDCS on corticospinal excitability enhancement and its after-effects: conventional vs. unihemispheric concurrent dual-site stimulation. Front Hum Neurosci. 2015;9:533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Achacheluee ST, Rahnama L, Karimi N, Abdollahi I, Arslan SA, Jaberzadeh S. The Effect of Unihemispheric Concurrent Dual-Site Transcranial Direct Current Stimulation of Primary Motor and Dorsolateral Prefrontal Cortices on Motor Function in Patients With Sub-Acute Stroke. Front Hum Neurosci. 2018;12:441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hill AT, Rogasch NC, Fitzgerald PB, Hoy KE. Effects of single versus dual-site High-Definition transcranial direct current stimulation (HD-tDCS) on cortical reactivity and working memory performance in healthy subjects. Brain Stimul. 2018;11(5):1033–43. [DOI] [PubMed] [Google Scholar]
- 82.Reinhart RMG, Nguyen JA. Working memory revived in older adults by synchronizing rhythmic brain circuits. Nat Neurosci. 2019;820–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Paulus W, Opitz A. Ohm’s law and tDCS over the centuries. Clin Neurophysiol. 2013;124(3):429–30. [DOI] [PubMed] [Google Scholar]
- 84.Esmaeilpour Z, Schestatsky P, Bikson M, Brunoni AR, Pellegrinelli A, Piovesan FX, et al. Notes on human trials of transcranial direct current stimulation between 1960 and 1998. Front Hum Neurosci. 2017;11:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sellers KK, Mellin JM, Lustenberger CM, Boyle MR, Lee WH, Peterchev AV, et al. Transcranial direct current stimulation (tDCS) of frontal cortex decreases performance on the WAIS-IV intelligence test. Behav Brain Res. 2015;290:32–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chhatbar PY, Chen R, Deardorff R, Dellenbach B, Kautz SA, George MS, et al. Safety and tolerability of transcranial direct current stimulation to stroke patients-a phase I current escalation study. Brain Stimul. 2017;10(3):553–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527(3):633–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fertonani A, Ferrari C, Miniussi C. What do you feel if I apply transcranial electric stimulation? Safety, sensations and secondary induced effects. Clin Neurophysiol. 2015;126(11):2181–8. [DOI] [PubMed] [Google Scholar]
- 89.Moliadze V, Andreas S, Lyzhko E, Schmanke T, Gurashvili T, Freitag CM, et al. Ten minutes of 1 mA transcranial direct current stimulation was well tolerated by children and adolescents: self-reports and resting state EEG analysis. Brain Res Bull. 2015;119:25–33. [DOI] [PubMed] [Google Scholar]
- 90.Paneri B, Adair D, Thomas C, Khadka N, Patel V, Tyler WJ, et al. Tolerability of repeated application of transcranial electrical stimulation with limited outputs to healthy subjects. Brain Stimul. 2016;9(5):740–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kronberg G, Bikson M, editors. Electrode assembly design for transcranial direct current stimulation: a FEM modeling study. Conf Proc IEEE Eng Med Biol Soc 2012. [DOI] [PubMed] [Google Scholar]
- 92.Edwards D, Cortes M, Datta A, Minhas P, Wassermann EM, Bikson M. Physiological and modeling evidence for focal transcranial electrical brain stimulation in humans: a basis for high-definition tDCS. Neuroimage. 2013;74:266–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Minhas P, Bansal V, Patel J, Ho JS, Diaz J, Datta A, et al. Electrodes for high-definition transcutaneous DC stimulation for applications in drug delivery and electrotherapy, including tDCS. J Neurosci Methods. 2010;190(2):188–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dmochowski JP, Datta A, Bikson M, Su Y, Parra LC. Optimized multi-electrode stimulation increases focality and intensity at target. J Neural Eng. 2011;8(4):046011. [DOI] [PubMed] [Google Scholar]
- 95.Dmochowski JP, Datta A, Huang Y, Richardson JD, Bikson M, Fridriksson J, et al. Targeted transcranial direct current stimulation for rehabilitation after stroke. Neuroimage. 2013;75:12–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Borckardt JJ, Bikson M, Frohman H, Reeves ST, Datta A, Bansal V, et al. A pilot study of the tolerability and effects of high-definition transcranial direct current stimulation (HD-tDCS) on pain perception. J Pain. 2012;13(2):112–20. [DOI] [PubMed] [Google Scholar]
- 97.Shekhawat GS, Sundram F, Bikson M, Truong D, De Ridder D, Stinear CM, et al. Intensity, Duration, and Location of High-Definition Transcranial Direct Current Stimulation for Tinnitus Relief. Neurorehabil Neural Repair. 2016;30(4):349–59. [DOI] [PubMed] [Google Scholar]
- 98.Nikolin S, Loo CK, Bai S, Dokos S, Martin DM. Focalised stimulation using high definition transcranial direct current stimulation (HD-tDCS) to investigate declarative verbal learning and memory functioning. Neuroimage. 2015;117:11–9. [DOI] [PubMed] [Google Scholar]
- 99.Nikolin S, Lauf S, Loo CK, Martin D. Effects of High-Definition Transcranial Direct Current Stimulation (HD-tDCS) of the Intraparietal Sulcus and Dorsolateral Prefrontal Cortex on Working Memory and Divided Attention. Front Integr Neurosci. 2018;12:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Reckow J, Rahman-Filipiak A, Garcia S, Schlaefflin S, Calhoun O, DaSilva AF, et al. Tolerability and blinding of 4×1 high-definition transcranial direct current stimulation (HD-tDCS) at two and three milliamps. Brain Stimul. 2018;11(5):991–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Truong DQ, Datta A, Xu J, Fregni F, Bikson M, editors. Prefrontal cortex transcranial direct current stimulation via a combined high definition and conventional electrode montage: a FEM modeling studying. Conf Proc IEEE Eng Med Biol Soc 2012. [Google Scholar]
- 102.Perceval G, Martin AK, Copland DA, Laine M, Meinzer M. High-definition tDCS of the temporoparietal cortex enhances access to newly learned words. Sci Rep. 2017;7(1):17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dmochowski JP, Bikson M, Datta A, Richardson J, Fridriksson J, Parra LC. On the role of electric field orientation in optimal design of transcranial current stimulation. Conf Proc IEEE Eng Med Biol Soc 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Turski CA, Kessler-Jones A, Chow C, Hermann B, Hsu D, Jones J, et al. Extended Multiple-Field High-Definition transcranial direct current stimulation (HD-tDCS) is well tolerated and safe in healthy adults. Restor Neurol Neurosci. 2017;35(6):631–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Roy A, Baxter B, He B. High-definition transcranial direct current stimulation induces both acute and persistent changes in broadband cortical synchronization: a simultaneous tDCS-EEG study. IEEE Trans Biomed Eng. 2014;61(7):1967–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jaberzadeh S, Bastani A, Zoghi M. Anodal transcranial pulsed current stimulation: a novel technique to enhance corticospinal excitability. Clinical Neurophysiology. 2014;125(2):344–51. [DOI] [PubMed] [Google Scholar]
- 107.Morales-Quezada L, Saavedra LC, Rozisky J, Hadlington L, Fregni F. Intensity-dependent effects of transcranial pulsed current stimulation on interhemispheric connectivity: a high-resolution qEEG, sham-controlled study. Neuroreport. 2014;25(13):1054–8. [DOI] [PubMed] [Google Scholar]
- 108.Saavedra LC, Morales-Quezada L, Doruk D, Rozinsky J, Coutinho L, Faria P, et al. QEEG indexed frontal connectivity effects of transcranial pulsed current stimulation (tPCS): a sham-controlled mechanistic trial. Neurosci Lett. 2014;577:61–5. [DOI] [PubMed] [Google Scholar]
- 109.Sours C, Alon G, Roys S, Gullapalli RP. Modulation of resting state functional connectivity of the motor network by transcranial pulsed current stimulation. Brain Connect. 2014;4(3):157–65. [DOI] [PubMed] [Google Scholar]
- 110.Fitzgerald PB. Transcranial pulsed current stimulation: a new way forward? Clin Neurophysiol. 2014;125(2):217–9. [DOI] [PubMed] [Google Scholar]
- 111.Jaberzadeh S, Bastani A, Zoghi M. Anodal transcranial pulsed current stimulation: A novel technique to enhance corticospinal excitability. Clin Neurophysiol. 2014;125(2):344–51. [DOI] [PubMed] [Google Scholar]
- 112.Amassian VE, Cracco RQ, Maccabee PJ. Focal stimulation of human cerebral cortex with the magnetic coil: a comparison with electrical stimulation. Electroencephalogr Clin Neurophysiol. 1989;74(6):401–16. [DOI] [PubMed] [Google Scholar]
- 113.Rothwell JC. Techniques and mechanisms of action of transcranial stimulation of the human motor cortex. J Neurosci Methods. 1997;74(2):113–22. [DOI] [PubMed] [Google Scholar]
- 114.Datta A, Dmochowski JP, Guleyupoglu B, Bikson M, Fregni F. Cranial electrotherapy stimulation and transcranial pulsed current stimulation: a computer based high-resolution modeling study. Neuroimage. 2013;65:280–7. [DOI] [PubMed] [Google Scholar]
- 115.Lisanby SH. Electroconvulsive therapy for depression. N Engl J Med. 2007;357(19):1939–45. [DOI] [PubMed] [Google Scholar]
- 116.Moliadze V, Atalay D, Antal A, Paulus W. Close to threshold transcranial electrical stimulation preferentially activates inhibitory networks before switching to excitation with higher intensities. Brain Stimul. 2012;5(4):505–11. [DOI] [PubMed] [Google Scholar]
- 117.APA. The practice of electroconvulsive therapy: recommendations for treatment, training, and privileging, Washington (DC): American Psychiatric Association (APA) Committee on Electroconvulsive Therapy, 2001. [Google Scholar]
- 118.Nahas Z, Short B, Burns C, Archer M, Schmidt M, Prudic J, et al. A feasibility study of a new method for electrically producing seizures in man: focal electrically administered seizure therapy [FEAST]. Brain Stimul. 2013;6(3):403–8. [DOI] [PubMed] [Google Scholar]
- 119.Chahine G, Short B, Spicer K, Schmidt M, Burns C, Atoui M, et al. Regional cerebral blood flow changes associated with focal electrically administered seizure therapy (FEAST). Brain Stimul. 2014;7(3):483–5. [DOI] [PubMed] [Google Scholar]
- 120.Peterchev AV, Rosa MA, Deng ZD, Prudic J, Lisanby SH. Electroconvulsive therapy stimulus parameters: rethinking dosage. J ECT. 2010;26(3):159–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Loo CK, Schweitzer I, Pratt C. Recent advances in optimizing electroconvulsive therapy. Aust N Z J Psychiatry. 2006;40(8):632–8. [DOI] [PubMed] [Google Scholar]
- 122.Sahlem GL, Short EB, Kerns S, Snipes J, DeVries W, Fox JB, et al. Expanded Safety and Efficacy Data for a New Method of Performing Electroconvulsive Therapy: Focal Electrically Administered Seizure Therapy. J ECT. 2016;32(3):197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mayur P, Harris A, Gangadhar B. 500-mA ECT--A Proof of Concept Report. J ECT. 2015;31(2):e23–6. [DOI] [PubMed] [Google Scholar]
- 124.Rosa MA, Abdo GL, Lisanby SH, Peterchev A. Seizure induction with low-amplitude-current (0.5 A) electroconvulsive therapy. J ECT. 2011;27(4):341–2. [DOI] [PubMed] [Google Scholar]
- 125.Loo CK, Bai S, Martin D, Galvez V, Dokos S. Revisiting frontoparietal montage in electroconvulsive therapy: clinical observations and computer modeling: a future treatment option for unilateral electroconvulsive therapy. J ECT. 2015;31(1):e7–e13. [DOI] [PubMed] [Google Scholar]
- 126.Loo CK, Katalinic N, Smith DJ, Ingram A, Dowling N, Martin D, et al. A randomized controlled trial of brief and ultrabrief pulse right unilateral electroconvulsive therapy. Int J Neuropsychopharmacol. 2014;18(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Groppa S, Bergmann TO, Siems C, Molle M, Marshall L, Siebner HR. Slow-oscillatory transcranial direct current stimulation can induce bidirectional shifts in motor cortical excitability in awake humans. Neuroscience. 2010;166(4):1219–25. [DOI] [PubMed] [Google Scholar]
- 128.Eggert T, Dorn H, Sauter C, Nitsche MA, Bajbouj M, Danker-Hopfe H. No effects of slow oscillatory transcranial direct current stimulation (tDCS) on sleep-dependent memory consolidation in healthy elderly subjects. Brain Stimul. 2013;6(6):938–45. [DOI] [PubMed] [Google Scholar]
- 129.Antal A, Boros K, Poreisz C, Chaieb L, Terney D, Paulus W. Comparatively weak after-effects of transcranial alternating current stimulation (tACS) on cortical excitability in humans. Brain Stimul. 2008;1(2):97–105. [DOI] [PubMed] [Google Scholar]
- 130.Westerberg CE, Florczak SM, Weintraub S, Mesulam MM, Marshall L, Zee PC, et al. Memory improvement via slow-oscillatory stimulation during sleep in older adults. Neurobiol Aging. 2015;36(9):2577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Binder S, Berg K, Gasca F, Lafon B, Parra LC, Born J, et al. Transcranial slow oscillation stimulation during sleep enhances memory consolidation in rats. Brain Stimul. 2014;7(4):508–15. [DOI] [PubMed] [Google Scholar]
- 132.Marshall L, Molle M, Hallschmid M, Born J. Transcranial direct current stimulation during sleep improves declarative memory. J Neurosci. 2004;24(44):9985–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Antal A, Boros K, Poreisz C, Chaieb L, Terney D, Paulus W. Comparatively weak after-effects of transcranial alternating current stimulation (tACS) on cortical excitability in humans. Brain Stimul. 2008;1(2):97–105. [DOI] [PubMed] [Google Scholar]
- 134.Mehta AR, Brittain J-S, Brown P. The selective influence of rhythmic cortical versus cerebellar transcranial stimulation on human physiological tremor. JNeurosci. 2014;34(22):7501–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kar K, Krekelberg B. Transcranial alternating current stimulation attenuates visual motion adaptation. J Neurosci. 2014;34(21):7334–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Moliadze V, Atalay D, Antal A, Paulus W. Close to threshold transcranial electrical stimulation preferentially activates inhibitory networks before switching to excitation with higher intensities. Brain Stimulation. 2012;5(4):606–11. [DOI] [PubMed] [Google Scholar]
- 137.Negahbani E, Kasten FH, Herrmann CS, Frohlich F. Targeting alpha-band oscillations in a cortical model with amplitude-modulated high-frequency transcranial electric stimulation. Neuroimage. 2018;173:3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mehta AR, Brittain JS, Brown P. The selective influence of rhythmic cortical versus cerebellar transcranial stimulation on human physiological tremor. J Neurosci. 2014;34(22):7501–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Helfrich RF, Schneider TR, Rach S, Trautmann-Lengsfeld SA, Engel AK, Herrmann CS. Entrainment of brain oscillations by transcranial alternating current stimulation. Curr Biol. 2014;24(3):333–9. [DOI] [PubMed] [Google Scholar]
- 140.Jausovec N, Jausovec K. Increasing working memory capacity with theta transcranial alternating current stimulation (tACS). Biol Psychol. 2014;96:42–7. [DOI] [PubMed] [Google Scholar]
- 141.Alekseichuk I, Turi Z, Amador de Lara G, Antal A, Paulus W. Spatial Working Memory in Humans Depends on Theta and High Gamma Synchronization in the Prefrontal Cortex. Curr Biol. 2016;26(12):1513–21. [DOI] [PubMed] [Google Scholar]
- 142.Lara GA, Alekseichuk I, Turi Z, Lehr A, Antal A, Paulus W. Perturbation of theta-gamma coupling at the temporal lobe hinders verbal declarative memory. Brain Stimul. 2018;11(3):509–17. [DOI] [PubMed] [Google Scholar]
- 143.Chaieb L, Wilpert EC, Reber TP, Fell J. Auditory beat stimulation and its effects on cognition and mood States. Front Psychiatry. 2015;6:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kunz P, Antal A, Hewitt M, Neef A, Opitz A, Paulus W. 5 kHz Transcranial Alternating Current Stimulation: Lack of Cortical Excitability Changes When Grouped in a Theta Burst Pattern. Front Hum Neurosci. 2016;10:683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Grossman N, Bono D, Dedic N, Kodandaramaiah SB, Rudenko A, Suk HJ, et al. Noninvasive Deep Brain Stimulation via Temporally Interfering Electric Fields. Cell. 2017;169(6):1029–41.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gall C, Fedorov AB, Ernst L, Borrmann A, Sabel BA. Repetitive transorbital alternating current stimulation in optic neuropathy. NeuroRehabilitation. 2010;27(4):335–41. [DOI] [PubMed] [Google Scholar]
- 147.Sabel BA, Fedorov AB, Naue N, Borrmann A, Herrmann C, Gall C. Non-invasive alternating current stimulation improves vision in optic neuropathy. Restor Neurol Neurosci. 2011;29(6):493–505. [DOI] [PubMed] [Google Scholar]
- 148.Gall C, Sgorzaly S, Schmidt S, Brandt S, Fedorov A, Sabel BA. Noninvasive transorbital alternating current stimulation improves subjective visual functioning and vision-related quality of life in optic neuropathy. Brain Stimul. 2011;4(4):175–88. [DOI] [PubMed] [Google Scholar]
- 149.Fedorov A, Jobke S, Bersnev V, Chibisova A, Chibisova Y, Gall C, et al. Restoration of vision after optic nerve lesions with noninvasive transorbital alternating current stimulation: a clinical observational study. Brain Stimul. 2011;4(4):189–201. [DOI] [PubMed] [Google Scholar]
- 150.Gall C, Schmidt S, Schittkowski MP, Antal A, Ambrus GG, Paulus W, et al. Alternating current stimulation for vision restoration after optic nerve damage: a randomized clinical trial. PLoS One. 2016;11(6):e0156134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bola M, Gall C, Moewes C, Fedorov A, Hinrichs H, Sabel BA. Brain functional connectivity network breakdown and restoration in blindness. Neurology. 2014:83(6):542–51. [DOI] [PubMed] [Google Scholar]
- 152.Morimoto T, Fukui T, Matsushita K, Okawa Y, Shimojyo H, Kusaka S, et al. Evaluation of residual retinal function by pupillary constrictions and phosphenes using transcorneal electrical stimulation in patients with retinal degeneration. Graefes Arch Clin Exp Ophthalmol. 2006;244(10):1283–92. [DOI] [PubMed] [Google Scholar]
- 153.Inomata K, Shinoda K, Ohde H, Tsunoda K, Hanazono G, Kimura I, et al. Transcorneal electrical stimulation of retina to treat longstanding retinal artery occlusion. Graefes Arch Clin Exp Ophthalmol. 2007;245(12):1773–80. [DOI] [PubMed] [Google Scholar]
- 154.Fujikado T, Morimoto T, Matsushita K, Shimojo H, Okawa Y, Tano Y. Effect of transcorneal electrical stimulation in patients with nonarteritic ischemic optic neuropathy or traumatic optic neuropathy. Jpn J Ophthalmol. 2006;50(3):266–73. [DOI] [PubMed] [Google Scholar]
- 155.Fujikado T, Morimoto T, Kanda H, Kusaka S, Nakauchi K, Ozawa M, et al. Evaluation of phosphenes elicited by extraocular stimulation in normals and by suprachoroidal-transretinal stimulation in patients with retinitis pigmentosa. Graefes Arch Clin Exp Ophthalmol. 2007;245(10):1411–9. [DOI] [PubMed] [Google Scholar]
- 156.Oono S, Kurimoto T, Kashimoto R, Tagami Y, Okamoto N, Mimura O. Transcorneal electrical stimulation improves visual function in eyes with branch retinal artery occlusion. Clin Ophthalmol. 2011;5:397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Dawson WW, Trick GL, Litzkow CA. Improved electrode for electroretinography. Invest Ophthalmol Vis Sci. 1979;18(9):988–91. [PubMed] [Google Scholar]
- 158.Schatz A, Pach J, Gosheva M, Naycheva L, Willmann G, Wilhelm B, et al. Transcorneal Electrical Stimulation for Patients With Retinitis Pigmentosa: A Prospective, Randomized, Sham-Controlled Follow-up Study Over 1 Year. Invest Ophthalmol Vis Sci. 2017;58(1):257–69. [DOI] [PubMed] [Google Scholar]
- 159.Ozeki N, Shinoda K, Ohde H, Ishida S, Tsubota K. Improvement of visual acuity after transcorneal electrical stimulation in case of Best vitelliform macular dystrophy. Graefes Arch Clin Exp Ophthalmol. 2013;251(7):1867–70. [DOI] [PubMed] [Google Scholar]
- 160.Naycheva L, Schatz A, Rock T, Willmann G, Messias A, Bartz-Schmidt KU, et al. Phosphene thresholds elicited by transcorneal electrical stimulation in healthy subjects and patients with retinal diseases. Invest Ophthalmol Vis Sci. 2012;53(12):7440–8. [DOI] [PubMed] [Google Scholar]
- 161.Xie J, Wang GJ, Yow L, C JC, Humayun MS, Weiland JD, et al. Modeling and percept of transcorneal electrical stimulation in humans. IEEE Trans Biomed Eng. 2011;58(7):1932–9. [DOI] [PubMed] [Google Scholar]
- 162.Schatz A, Rock T, Naycheva L, Willmann G, Wilhelm B, Peters T, et al. Transcorneal electrical stimulation for patients with retinitis pigmentosa: a prospective, randomized, sham-controlled exploratory study. Invest Ophthalmol Vis Sci. 2011;52(7):4485–96. [DOI] [PubMed] [Google Scholar]
- 163.Naycheva L, Schatz A, Willmann G, Bartz-Schmidt KU, Zrenner E, Rock T, et al. Transcorneal electrical stimulation in patients with retinal artery occlusion: a prospective, randomized, sham-controlled pilot study. Ophthalmol Ther. 2013;2(1):25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Terney D, Chaieb L, Moliadze V, Antal A, Paulus W. Increasing human brain excitability by transcranial high-frequency random noise stimulation. J Neurosci. 2008;28(52):14147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Stacey WC, Durand DM. Stochastic resonance improves signal detection in hippocampal CA1 neurons. J Neurophysiol. 2000;83(3):1394–402. [DOI] [PubMed] [Google Scholar]
- 166.Reed T, Cohen Kadosh R. Transcranial electrical stimulation (tES) mechanisms and its effects on cortical excitability and connectivity. J Inherit Metab Dis. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]