Abstract
Background
Roux-en-Y gastric bypass (RYGB) produces greater weight loss compared with a purely restrictive procedure such as laparoscopic adjustable gastric banding (LAGB).
Objective
The objective of this study was to quantify changes in hormones that regulate energy homeostasis and appetitive sensations before and after LAGB (n=18) and RYGB (n=38) in order to better understand the mechanisms underlying the greater weight loss after RYGB.
Methods
A standardized test meal was administered prior to surgery, at 6 months, and annually thereafter to year 2 after LAGB and year 4 after RYGB. Blood samples were obtained in the fasted state and 30, 60, 90, and 120 minutes post-meal.
Results
Progressive increases in fasting PYY were observed after RYGB together with increases in postprandial area under the curve (AUC) levels that were unchanged after LAGB. GLP-1 AUC increased only after RYGB. There was a weight loss-related increase in fasting ghrelin levels after LAGB that was unchanged one year after RYGB despite greater percentage weight loss; ghrelin subsequently increased at years 2-4 post-RYGB. HOMA-IR decreased after both procedures but correlated with weight loss only after LAGB, whereas leptin correlated with weight loss in both groups. Sweet cravings decreased after RYGB.
Conclusion
A number of weight loss-independent changes in the gut hormonal milieu likely act in concert to promote a decrease in insulin resistance and greater weight loss efficacy after RYGB. A progressive change in hormone levels over time may reflect gut enteroplasticity after RYGB. A decrease in sweet cravings specific to RYGB may further promote superior weight loss outcomes.
Keywords: Roux-en-Y gastric bypass, Adjustable Gastric Banding, metabolic surgery, Ghrelin, glp-1, PYY, insulin resistance, appetite, sweet cravings, Bariatric Surgery
INTRODUCTION
Bariatric surgery has proven to be the most effective long-term treatment for obesity. Laparoscopic Roux-en-Y gastric bypass (RYGB) has been considered the “gold standard” of bariatric procedures as it produces greater sustainable weight loss compared with other bariatric procedures [1]. While the laparoscopic adjustable gastric band (LAGB) procedure has lower complication rates and nutritional deficiencies, poorer outcomes and reoperation rates have resulted in this procedure falling out of favor. In a previous report, we presented the one-year results of a prospective study comparing circulating levels of gut hormones and metabolic outcomes between LAGB and RYGB [2]. In this current study, longer-term data were obtained annually on patients two years after LAGB and annually out to four years after RYGB. The objective was to gain insight into possible mechanisms underlying postoperative weight regulation and differences in weight loss and maintenance through the study of gut hormones and appetitive sensations.
METHODS
Protocol
The cohort described in this report consists of 56 subjects enrolled in an ongoing prospective study at Columbia University Medical Center who have undergone either the LAGB (n = 18) or RYGB (n = 38) procedure and have at least one year of follow-up data. The operations were performed as described [2] and the choice of procedure was based on the preference of surgeon and patient. Subjects were seen preoperatively at year 0, and at 0.5, 1, 2, 3, and 4 years after the procedure for measurement of body weight and venous blood collection. A liquid meal challenge (Optifast, Novartis, Minneapolis, MN, USA; 474 ml, 320 kcal, 50% carbohydrate, 35% protein and 15% fat) consumed within a 15 min period was administered at all visits and was well tolerated. Venous blood was drawn in a fasting state and 30, 60, 90 and 120 min post-prandial. After centrifugation at 4°C both serum and plasma were stored at −80°C.
Hormone Assays
Leptin, total ghrelin, insulin and glucose were measured as described earlier [3]. Total PYY was measured by ELISA (Millipore, MO, USA) with a sensitivity of 10 pg/ml. Total GLP-1 was measured by RIA after alcohol extraction according to manufacturer’s protocol (Millipore). Sensitivity of the assay is 3 pM and recovery in each assay was tested by parallel extraction of standards. An aliquot from a pool of plasma was included in each assay to ensure there was no change over time. All samples were assayed in duplicate.
Statistical Analysis
Area-under-the-curve (AUC) was computed using the trapezoidal rule. Insulin resistance was calculated using the Homeostasis Model Assessment (HOMA-IR) [4]. Ghrelin suppression was calculated as the percentage decrease in plasma ghrelin level at 30 min compared with baseline. Characteristics of surgical groups at baseline were compared with independent t-tests. Changes in longitudinal trends within- and between-groups over the observation period were estimated using linear mixed models for repeated measures. For each outcome variable, the fixed effects for type of procedure, time, the interaction of procedure with time, and the level of the outcome variable preoperatively entered as a continuous covariate were analyzed. An AR(1) covariance structure was used to model the within-subject autocorrelation among times. In some models, percentage weight loss as a continuous adjusting covariate was included. Model estimated means and standard errors are presented with P values for the fixed effect of procedure-by-time interaction and with P values for differences between surgical groups at a specific time, or within a surgical group between time, calculated from the differences in model estimated means and the method of simultaneous confidence intervals. Within-subject difference and percentage difference from baseline values were calculated. Spearman correlations were performed to assess the association between preoperative variables and postoperative outcomes, between change in variables and postoperative outcomes, and between final variable values and postoperative outcomes. No adjustments were made for multiple outcomes measured in the same individuals. P values < 0.05 were considered statistically significant while comparisons with P values between 0.10 and 0.05 were considered to show a trend worthy of interest given the exploratory nature of these analyses.
RESULTS
Study Subjects
There were 56 subjects enrolled in this cohort consisting of 12 males, 44 females (48% Hispanic, 52% non-Hispanic; 16% African American, 84% Caucasian). Eighteen subjects underwent LAGB and thirty-eight underwent RYGB. At year 2 there were 10 subjects (44% lost to follow-up) in the LAGB group. LAGB subjects were not analyzed past year 2. In the RYGB group the number of subjects at years 2, 3 and 4 were 25, 20, and 16, respectively. By year 4 data were available for 42% of RYGB subjects which was due to a 34% lost to follow-up rate with the remaining subjects still due for visits at the time of data analysis. To ensure there was no bias from the subjects lost to follow-up, weight and weight loss percentage were compared to the patients that remained enrolled and no differences were found between the two groups (P values: 0.4 - 0.9).
Mean age was similar in both groups: LAGB (46.9 ± 2.1y) and RYGB (43.8 ± 2.5y). Anthropometric characteristics are presented in Table 1. While baseline weight was similar between groups initial BMI was lower in the LAGB group. Significant weight loss was achieved in both groups, with twice as much percentage weight loss after RYGB than LAGB at each time point. Within each cohort, however, there was a wide range of weight loss outcomes.
Table 1:
Baseline characteristics and changes over time in body weight, glucose and plasma hormone levels after LAGB and RYGB
| LAGB | RYGB | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Year 0 n = 18 |
Year 0.5 n = 16 |
Year 1 n = 18 |
Year 2 n = 10 |
Year 0 n = 38 |
Year 0.5 n = 37 |
Year 1 n = 37 |
Year 2 n = 25 |
Year 3 n = 20 |
Year 4 n = 15 |
|
|
Wt/BMI | ||||||||||
| Wt (kg) | 120 ± 1 | 106 ± 1cf | 101 ± 1cf | 102 ± 2cf | 126 ± 1 | 94 ± 1c | 87 ± 1c | 86 ± 1c | 88 ± 1c | 89 ± 1c |
| BMI (kg/m2) | 41.8 ± 0.9e | 37.1 ± 0.9cd | 35.4 ± 0.9ce | 35.1 ± 1.0ce | 47.2 ± 0.7 | 35.6 ± 0.8c | 32.8 ± 0.8c | 32.3 ± 0.9c | 33.1 ± 1.0c | 33.3 ± 1.1c |
| WL (%) | n/a | 11.7 ± 1.2cf | 15.4 ± 1.2cf | 15.3 ± 1.4cf | n/a | 24.8 ± 0.8c | 30.5 ± 0.8c | 31.9 ± 0.9c | 30.0 ± 1.0c | 29.6 ± 1.1c |
| WL Range (%) | n/a | (−2 -to 23) | (−5 to 27) | (−1 to 25) | n/a | (15 to 33) | (14 to 45) | (15 to 45) | (14 to 41) | (14 to 41) |
|
Glucose (mg/dl) | ||||||||||
| Fasting | 106 ± 3 | 99 ± 4d | 97 ± 4d | 99 ± 6 | 116 ± 3 | 90 ± 4c | 87 ± 4c | 94 ± 5c | 97 ± 6b | 95 ± 6b |
| AUC x 103 | 19.1 ± 0.7 | 17.5 ± 0.7 | 17.4 ± 0.8d | 18.4 ± 1.1 | 22.2 ± 0.7 | 16.7 ± 0.8c | 16.6 ± 0.8c | 17.5 ± 1.0c | 18.4 ± 1.2b | 19.0 ± 1.3a |
|
Insulin (uIU/ml) | ||||||||||
| Fasting | 19 ± 1 | 12 ± 2be | 13 ± 2b | 11 ± 2b | 19 ± 1 | 9 ± 1c | 8 ± 1c | 6 ± 1c | 7 ± 2c | 6 ± 2c |
| AUC x 103 | 9.0 ± 0.7 | 6.6 ± 0.6b | 7.0 ± 0.7a | 6.0 ± 0.9b | 8.3 ± 0.5 | 5.7 ± 0.6c | 4.9 ± 0.6c | 5.1 ± 0.7c | 5.3 ± 0.9c | 5.0 ± 0.9c |
|
HOMA-IR | ||||||||||
| 5.4 ± 0.5 | 3.0 ± 0.6bd | 2.9 ± 0.6be | 1.5 ± 0.7c | 5.0 ± 0.3 | 1.7 ± 0.3c | 1.2 ± 0.3c | 1.0 ± 0.3c | 0.9 ± 0.4c | 0.7 ± 0.4c | |
|
PYY (pg/ml) | ||||||||||
| Fasting | 130 ± 12 | 113 ± 14 | 131 ± 14 | 115 ± 17 | 86 ± 11 | 114 ± 13c | 116 ± 13c | 128 ± 15c | 129 ± 19c | 188 ± 21c |
| AUC x 103 | 32.7 ± 3.4 | 43.8 ± 3.8a | 40.2 ± 3.9e | 33.3 ± 4.8f | 24.8 ± 4.2 | 52.4 ± 4.6c | 54.4 ± 4.6c | 59.5 ± 5.4c | 68.9 ± 6.6c | 65.0 ± 7.3c |
|
GLP-1 (pmol/l) | ||||||||||
| Fasting | 16 ± 1 | 13 ± 2 | 11 ± 2a | 15 ± 2 | 14 ± 2 | 16 ± 2 | 13 ± 2 | 15 ± 2 | 17 ± 3 | 17 ± 2 |
| AUC x 103 | 1.6 ± 0.1 | 1.5 ± 0.1e | 1.3 ± 0.2e | 1.8 ± 0.2 | 1.4 ± 0.2 | 2.5 ± 0.2c | 2.2 ± 0.2b | 2.3 ± 0.2b | 3.0 ± 0.2c | 2.4 ± 0.2b |
|
Ghrelin (pg/ml) | ||||||||||
| Fasting | 298 ± 23 | 310 ± 25 | 392 ± 27b | 417 ± 34b | 336 ± 22 | 324 ± 24 | 345 ± 26 | 440 ± 29b | 461 ± 34b | 487 ± 38b |
| AUC x 103 | 51.8 ± 4.3 | 51.5 ± 4.3 | 63.2 ± 4.5a | 69.6 ± 5.5a | 51.8 ± 3.0 | 53.3 ± 3.2 | 53.1 ± 3.4 | 61.1 ± 3.8a | 67.2 ± 4.6b | 72.2 ± 5.0b |
| Suppression (%) | 17 ± 2 | 18 ± 2 | 21 ± 2 | 22 ± 2 | 18 ± 2 | 21 ± 2 | 23 ± 2a | 29 ± 2c | 25 ± 3a | 22 ± 3 |
|
Leptin (ng/ml) | ||||||||||
| Fasting | 39 ± 2 | 26 ± 2cf | 25 ± 2cf | 25 ± 3cf | 42 ± 1 | 18 ± 1c | 15 ± 1c | 16 ± 2c | 22 ± 2c | 23 ± 2c |
Values presented are linear mixed model for repeated measures estimated within-subject mean ± SEM. P value compared with Year 0 within group:
P < 0.05,
P < 0.01,
P < 0.001.
P value compared with RYGB at same time point:
P < 0.05,
P < 0.01,
P < 0.001.
HOMA-IR units: (mmol x μIU x L−2). AUC x 103 are integrated over 0-120 min with the exception of AUC for GLP-1 which was determined from 0-60 min.
Glucose and Hormone Levels
At baseline, there were no significant differences between the LAGB and RYGB cohorts for any of the blood parameters tested (Table 1). Both cohorts included a similar number of patients with type 2 diabetes mellitus (22% LAGB, 26% RYGB, P = 0.75). Combined data from patients with and without type 2 diabetes mellitus are presented since changes in glycemic parameters and gut hormone levels followed a similar pattern when subjects with and without diabetes were analyzed separately (data not shown). Prominent differences between the groups were observed in postprandial glucose and insulin excursions, even though the differences were not readily apparent when AUC measurements over the 120-minute period were compared (Fig. 1). Postprandial glucose values were significantly lower post-RYGB at all time points out to 4 years compared to baseline values, whereas post-LAGB, postprandial glucose values returned towards baseline by year 2. Insulin levels at minutes 60 through 120 were reduced post-RYGB but after LAGB this reduction was less apparent. The opposite was observed at 30 min when insulin levels remained elevated after RYGB but were reduced after LAGB and the difference between procedures at 2 years was significant (P = 0.019). HOMA-IR also improved in both groups, however, at year 2 levels correlated with weight loss in the LAGB group (r = 0.562; P = 0.029) but not in the RYGB group (r = 0.104; P = 0.55).
Figure 1.
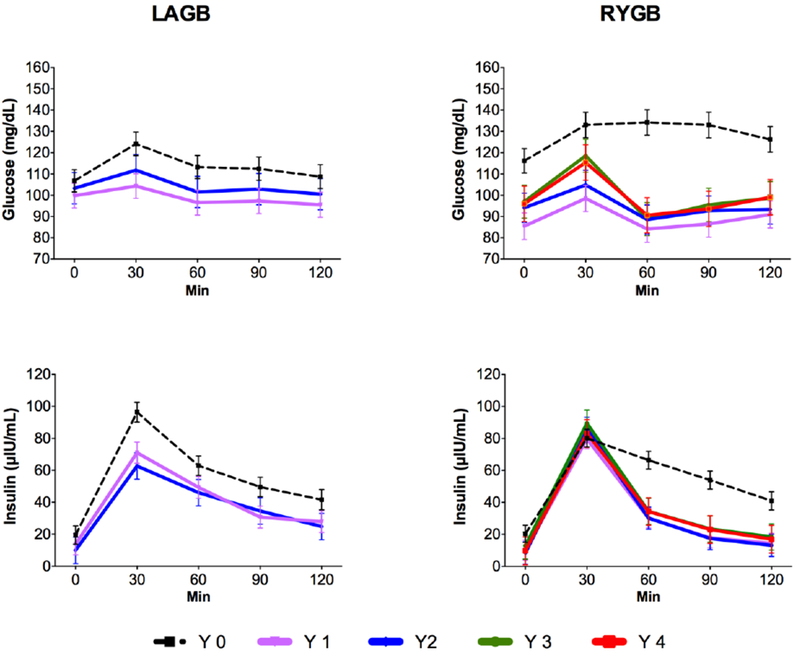
Fasting and postprandial glucose and insulin levels before and after LAGB and RYGB. Values are reported as mean ± SEM.
Despite twice the percentage weight loss, ghrelin levels did not change during the first year after RYGB. In contrast, significant increases in fasting ghrelin levels were observed starting at year 2, and remained elevated compared to baseline through year 4 (Table 1, Fig. 2). Interestingly, while significant increases in fasting and post-prandial AUC ghrelin occurred at year 2 following both procedures, only those of the LAGB cohort correlated to percentage weight loss (r = 0.915, P < 0.001 for LAGB; r = 0.171, P = 0.41 for RYGB). Postprandial suppression of ghrelin became more pronounced after RYGB (Table 1).
Figure 2.
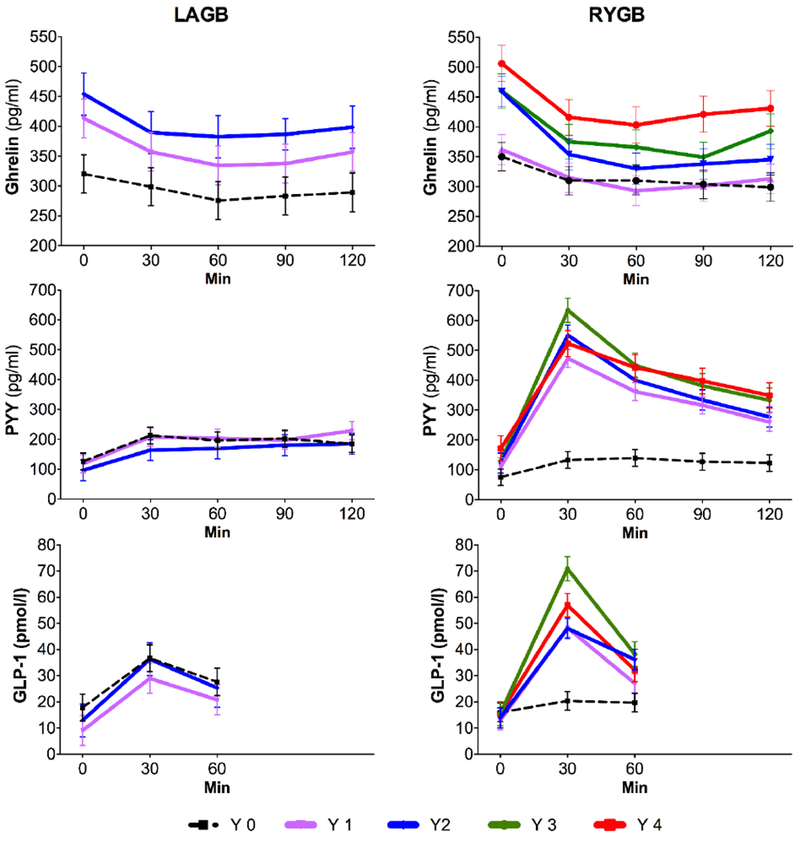
Fasting and postprandial gut hormone levels before and after LAGB and RYGB. Values are reported as mean ± SEM.
Fasting levels of PYY and GLP-1 remained relatively stable post-LAGB (Table 1, Fig. 2). Fasting GLP-1 also did not change after RYGB, whereas there were notable increases in PYY levels. Postprandial levels of both GLP-1 and PYY increased considerably only in the RYGB group and were maintained through year 4 (Fig. 2). Levels of PYY and GLP-1 did not correlate with percentage weight loss in either group.
Both cohorts had similar leptin levels at baseline and robust post-operative decreases in leptin that were maintained throughout the study (Table 1). In both cohorts and at all time-points, the percentage change in leptin negatively correlated with percentage weight loss (r values ranging from −0.6 to −0.8). The degree to which leptin levels decreased was greater post-RYGB at all time-points (P < 0.001) consistent with the greater degree of weight reduction.
Visual Analog Scale (VAS) Analysis
VAS ratings were used to assess hunger, fullness and sweet craving in the fasted state and at 30, 60, 90 and 120 min after consumption of the test meal. Both cohorts had similar baseline values and there were no notable post-operative changes in either fasted (data not shown) or post-prandial states in any of these measures in either group with the exception of sweet craving (Fig. 3). When asked “How much do you crave something sweet right now?” ranging from “not at all” to “extremely” the RYGB cohort demonstrated significant decreases in both fasting and post-prandial measures of sweet craving. There was no change in sweet cravings post-LAGB. While both cohorts had similar levels at baseline, sweet cravings were considerably different between groups postoperatively.
Figure 3.
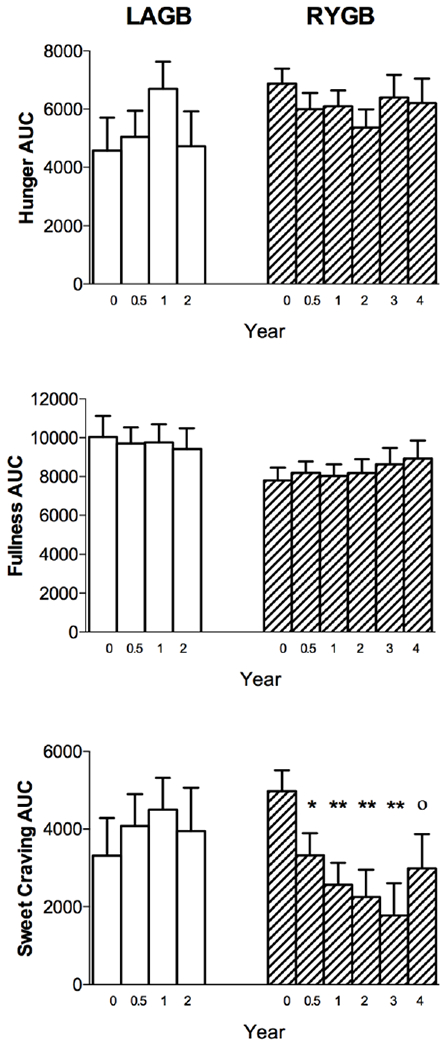
Visual analogue scale results of appetitive ratings. LAGB = open bars; RYGB = cross-hatched bars. Values are reported as mean AUC ± SEM. *P < 0.05; **P < 0.01; °P = 0.058 compared with preoperative within-group value.
DISCUSSION
In this study we have demonstrated the striking differences between long-term hormonal changes and weight loss that occur after LAGB and RYGB. Despite their similarities at baseline, patients exhibited twice as much weight loss after RYGB and maintained that weight loss through four years of follow-up. Since both procedures restrict nutrient flow, the more robust weight, hormonal, and metabolic changes after RYGB are likely consequences of the anatomical alterations specific to this operation [5]. The rerouting of nutrient delivery and chronically high gastric emptying rates after RYGB stimulate adaptive changes to the gut, including increased villus height and surface area [6], which possibly contributed to the profound changes observed in this study in nutrient-dependent release of gut hormones by an increased number of enteroendocrine cells [7]. The enteroplastic ability of the gut as it adapts to the changes in metabolic demands post-RYGB may serve to explain the most striking changes unique to the RYGB cohort such as the greater than two-fold increase in fasting PYY over time and in postprandial levels of PYY and GLP-1.
Changes in both GLP-1 and PYY may contribute to improvements in glucose homeostasis that are independent of weight loss after RYGB. For example, the increased secretion of GLP-1 may underlie the peak in insulin levels at 30 min which were significantly greater in the RYGB group. Certainly, activation of the GLP-1 receptor pathway has become a powerful tool for the treatment of both type 2 diabetes and obesity. It is unclear, however, whether the enhanced level of insulin post-RYGB is solely due to GLP-1 and/or other factors such as increased rate of glucose absorption. In rodent models of diet-induced obesity, PYY improved insulin sensitivity [8]. The increased secretion of PYY, in addition to greater weight loss, may contribute to greater improvements in insulin sensitivity after RYGB. In fact, changes in insulin sensitivity post-LAGB were dependent on weight loss while HOMA-IR post-RYGB was not correlated with weight loss.
Gut adaptations may also serve to explain the interesting changes in ghrelin levels post-RYGB that occur over the long-term. Ghrelin secretion is increased as a counter-regulatory response to a reduction in fat mass with caloric restriction [9]. As expected, ghrelin levels increased significantly within a year post-LAGB, and in direct correlation with weight loss [10, 11]. Despite the greater degree of weight loss post-RYGB, ghrelin levels did not increase within the one-year timeframe. By year 2, however, both fasting and post-prandial ghrelin levels increased and remained significantly greater than baseline through the end of the study. In contrast to the increase in ghrelin levels after LAGB, the later increases in ghrelin after RYGB were independent of any change in body weight. The cause of this apparent dissociation of weight change and ghrelin secretion after RYGB is unclear. It is also interesting to note that ghrelin suppression becomes more prominent after RYGB. The mechanisms of ghrelin regulation are not fully understood and multiple studies have reported increased, decreased, and unchanged post-operative levels of ghrelin, resulting in a lack of consensus on the expected trend of ghrelin parameters post-RYGB [12]. This prospective study long-term study shows that perhaps the postoperative timepoint at which patients are studied may explain some of the seemingly contradictory outcomes presented in various reports.
We sought to determine the effect of procedure type on appetitive sensations using VAS measurements. Intravenous administration of PYY to humans has been shown to both increase the sensation of satiety and decrease food intake [13] and the use of a GLP-1 receptor agonist for the treatment of obesity makes it likely that the increase of these endogenous peptides after RYGB would have similar effects on appetite. In contrast, intravenous administration of ghrelin in humans results in increased hunger and caloric consumption [9]. Contrary to our expectations, there were no changes in fasting or postprandial sensations of hunger or fullness or associations with hormone levels. Neither were baseline measures predictive of weight loss outcomes. However, a notable finding was the marked decrease in sweet cravings post-RYGB. Several studies have found that sweet cravings are either sustained or increased after LAGB or diet-induced weight loss, yet paradoxically decreased post-RYGB. Confirming these results, our LAGB cohort experienced no change in sweet cravings, while fasting and post-prandial measures of sweet cravings significantly decreased post-RYGB. Hormones implicated in the sensitivity to, and perceptions of, sweet taste include GLP-1, PYY, and leptin [14]. Increased levels of GLP-1 have been shown to enhance the ability to taste sweet flavors and increased levels of either GLP-1 or PYY heighten aversion to sweet taste and subsequently decrease sweet cravings [14, 15]. It is conceivable that the decreased craving for sweets in the RYGB cohort is a consequence of the post-operative increases in GLP-1 and PYY. Alternatively, leptin dampens the ability to taste sweet and suppresses overall intake of sweet foods [16]. A decrease in leptin levels, however, may not translate clinically to increases in sweet consumption as obesity-associated remodeling within the central nervous system may conceivably have altered responsivity of leptin receptor pathways [17].
The main strength of this study is the longitudinal comparison of a number of important appetitive hormones between two types of bariatric operations with different weight loss efficacy. An important limitation is that a solid meal stimulus might produce different results both in terms of hormone response and changes in appetitive sensations. It is also impossible to decipher whether the observed hormone changes are causal to changes in body weight. While it is possible that individuals who did not complete the study somehow differed than those who did, analysis of weight loss trajectories and baseline characteristics such as age, sex, weight and BMI did not reveal any differences between completers and non-completers. We also did not address a number of other factors that contribute to weight loss and maintenance such as physical activity, emotional and behavioral characteristics of our participants, other neuroendocrine modulators of energy balance and microbiota profiles. Frequency of band adjustments were also not formally assessed.
In summary, our results demonstrate an overall hormonal milieu that may provide greater weight loss efficacy after RYGB and even with an increase in ghrelin over time changes in GLP-1 and PYY may assist in the maintenance of weight loss. Important areas for further study should be geared toward a better understanding of sweet cravings and the variable response between individuals as such information could aid with the optimization of surgical outcomes in a greater number of patients.
Acknowledgments
Funding sources: NIH DK072011; NIH T32 DK07271; NCRR UL1 RR024156
CONFLICT OF INTEREST DISCLOSURE
This study was funded by NIH DK072011; NIH T32 DK07271; NCRR UL1 RR024156 Author 8 has patent 16046592 pending, and patent 20040039452 issued. Author 9 has received stock options from Digma Medical and is on the Scientific Advisory Board of Digma Medical and GI Dynamics. The remaining authors have nothing to disclose.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest Statement: AT, GF, DJM, BT, AT, IMC, LA have nothing to declare. MB has patent 16046592 pending, and patent 20040039452 issued. JK serves on the Scientific Advisory Board of Digma Medical and receives stock options, and receives financial compensation for serving on the Scientific Advisory Board of GI Dynamics.
ETHICAL STATEMENT
All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
CONSENT STATEMENT
Informed consent was obtained from all individual participants included in the study.
REFERENCES
- 1.Chang SH, et al. , The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003-2012. JAMA Surg, 2014. 149(3): p. 275–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korner J, et al. , Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes (Lond), 2009. 33(7): p. 786–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korner J, et al. , Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. J Clin Endocrinol Metab, 2005. 90(1): p. 359–65. [DOI] [PubMed] [Google Scholar]
- 4.Matthews DR, et al. , Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia, 1985. 28(7): p. 412–9. [DOI] [PubMed] [Google Scholar]
- 5.Seeley RJ, Chambers AP, and Sandoval DA, The role of gut adaptation in the potent effects of multiple bariatric surgeries on obesity and diabetes. Cell Metab, 2015. 21(3): p. 369–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.le Roux CW, et al. , Gut hypertrophy after gastric bypass is associated with increased glucagon-like peptide 2 and intestinal crypt cell proliferation. Ann Surg, 2010. 252(1): p. 50–6. [DOI] [PubMed] [Google Scholar]
- 7.Mumphrey MB, et al. , Roux-en-Y gastric bypass surgery increases number but not density of CCK-, GLP-1-, 5-HT-, and neurotensin-expressing enteroendocrine cells in rats. Neurogastroenterol Motil, 2013. 25(1): p. e70–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vrang N, et al. , PYY(3-36) reduces food intake and body weight and improves insulin sensitivity in rodent models of diet-induced obesity. Am J Physiol Regul Integr Comp Physiol, 2006. 291(2): p. R367–75. [DOI] [PubMed] [Google Scholar]
- 9.Wren AM, et al. , Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab, 2001. 86(12): p. 5992. [DOI] [PubMed] [Google Scholar]
- 10.Stoeckli R, et al. , Changes of body weight and plasma ghrelin levels after gastric banding and gastric bypass. Obes Res, 2004. 12(2): p. 346–50. [DOI] [PubMed] [Google Scholar]
- 11.Cummings DE, et al. , Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med, 2002. 346(21): p. 1623–30. [DOI] [PubMed] [Google Scholar]
- 12.Beckman LM, Beckman TR, and Earthman CP, Changes in gastrointestinal hormones and leptin after Roux-en-Y gastric bypass procedure: a review. J Am Diet Assoc, 2010. 110(4): p. 571–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Batterham RL, et al. , Inhibition of food intake in obese subjects by peptide YY3-36. N Engl J Med, 2003. 349(10): p. 941–8. [DOI] [PubMed] [Google Scholar]
- 14.Mathes CM and Spector AC, Food selection and taste changes in humans after Roux-en-Y gastric bypass surgery: a direct-measures approach. Physiol Behav, 2012. 107(4): p. 476–83. [DOI] [PubMed] [Google Scholar]
- 15.Bueter M, et al. , Alterations of sucrose preference after Roux-en-Y gastric bypass. Physiol Behav, 2011. 104(5): p. 709–21. [DOI] [PubMed] [Google Scholar]
- 16.Shigemura N, et al. , Leptin modulates behavioral responses to sweet substances by influencing peripheral taste structures. Endocrinology, 2004. 145(2): p. 839–47. [DOI] [PubMed] [Google Scholar]
- 17.Lee EB and Ahima RS, Alteration of hypothalamic cellular dynamics in obesity. J Clin Invest, 2012. 122(1): p. 22–5. [DOI] [PMC free article] [PubMed] [Google Scholar]


