Abstract
Objectives
Peptest is a new non‐invasive reflux diagnostic test based on lateral flow technology that containing two highly specific human pepsin monoclonal antibodies for detecting pepsin, a biomarker for reflux disease. The primary aim of this multicenter clinical study was to validate the efficacy of Peptest in patients diagnosed with gastroesophageal reflux and healthy controls in China.
Methods
Patients with suspected gastroesophageal reflux underwent an endoscopy and were classified into non‐erosive reflux disease and erosive esophagitis subgroups. A healthy control group was also recruited. All participants were given a reflux disease questionnaire—patients scoring greater than 12 and controls scoring zero. All participants provided a postprandial saliva sample and most patients gave an additional post‐symptom sample for pepsin analysis.
Results
Altogether 1032 participants aged between 19 and 78 years were recruited. They consisted of 488 patients with non‐erosive reflux disease, 221 with erosive esophagitis and 323 healthy controls. The number of postprandial and post‐symptom samples analyzed totaled 1031 and 692, respectively. The results across all centers showed an overall pepsin‐positive sensitivity of 85%, a specificity of 60%, a positive predictive value of 82%, a negative predictive value of 65% and a positive likelihood ratio of 2.12.
Conclusion
The sensitivity of Peptest was high, but the specificity achieved in some centers was low, resulting overall in only a moderate specificity. Further diagnostic investigative studies are warranted.
Keywords: diagnosis, gastroesophageal reflux, monoclonal antibodies, non‐invasive test, pepsin biomarker, salivary
1. INTRODUCTION
Gastroesophageal reflux disease (GERD) is increasing, affecting up to 17% of the population in Asian countries and up to 40% in Western countries.1 The condition becomes apparent when contents of the gastric juice, specifically pepsin, leave the stomach and backflow into the esophagus, often causing damage and a myriad of symptoms including heartburn.2 GERD can be categorized into two subsections, erosive esophagitis (EE) and non‐erosive reflux disease (NERD). EE affects only 30% of the GERD population but it is extremely serious. This subcategory causes damage and may lead to additional future complications including Barrett's esophagus and adenocarcinoma.1 Affecting up to 70% of patients with GERD, NERD is the most frequent form of reflux disease.3, 4 However, NERD is less severe than EE and it does not damage the esophageal muscle, causing the patient to suffer solely from troublesome symptoms.
Awareness and identification of patients in China presenting with GERD is increasing yearly. Currently, GERD is estimated to affect 20% of the Chinese population5 as Chinese physicians regularly recognize the prevalence of the symptoms of extra esophageal reflux. This increase in recognition is leading to the identification of laryngopharyngeal reflux (LPR) in the Chinese population.6
Pepsin is the key aggressive enzyme in the gastric juice and is a vital biomarker used in the diagnosis of GERD.7, 8, 9 . As pepsin is produced only in the stomach, its large size allows the enzyme to be detected.10, 11 Concentrations found within saliva samples are strong evidence that GERD and LPR are taking place.12, 13
The growth in familiarity with reflux disease has led to an explosion in patients demanding diagnosis and treatment. No new specific reflux diagnostics are available, leaving physicians reliant on old and invasive tests, including endoscopy. Other, more expensive invasive tests are slowly being introduced in China, including 24‐hour both single‐ and dual‐probe pH monitoring and 24‐hour impedance/pH testing.14
At present, the diagnosis of GERD uses syndrome‐based diagnoses, which are often invasive and lack specificity. Endoscopic examinations are expensive and are associated with many disadvantages, including low detection rates that account for only 2.95%‐4.1% of NERD diagnoses,15 which is low when the majority of patients are presenting with symptoms of NERD. Recently, a novel, non‐invasive test (Peptest, RD Biomed Limited, UK) has shown promising results for diagnosing patients with GERD. As pepsin is considered to be a biomarker for prior reflux events, the Peptest detects and measures pepsin in expectorated saliva and is frequently used in the Western world to complement the reflux disease questionnaire (RDQ). To validate the clinical benefits of Peptest in China a multicenter clinical study has been conducted.
2. PATIENTS AND METHODS
2.1. Participants
In total, 1032 participants aged between 19 and 78 years were involved in this study. These participants included 323 asymptomatic healthy control subjects, 488 patients with NERD and 221 patients with EE. Details of patients with GERD (both NERD and EE) and the healthy participants entered into the multicenter study are shown in Figure 1.
Figure 1.

Patients with gastroesophageal reflux disease (GERD) and healthy controls entered into the multicenter clinical study. EE, erosive esophagitis; NERD, non‐erosive reflux disease
2.2. Participant recruitment
Patients over the age of 18 years with suspected GERD undertook an endoscopic examination during a 6‐month period. According to the endoscopic results, the patients were classified into two subtypes: NERD and EE. All patients with NERD and EE completed the RDQ and those who scored less than 12 were excluded from the study. To be enrolled, participants had to present with persistent symptoms that had been experienced for up to 4 weeks prior to the start of the study. The first patient was recruited on 25 May 2015 and the final patient on 21 December 2016.
Healthy controls over the age of 18 were recruited through hospital advertisements. Once the participant signed up on the basis that they did not display any symptoms associated with reflux, they completed the RDQ and a reflux symptom index (RSI) questionnaire. All healthy participants had to score 0 in the RDQ and 9 or less in the RSI questionnaire, including scoring 0 in statements about symptoms of heartburn.
Participants were excluded if the criteria were not met and additional participants were excluded if they had taken gastric motility drugs within 7 days of entering the study or had experienced: (a) esophageal or gastric cancer; (b) dysphagia; (c) esophageal spasm and achalasia; (d) surgery of the stomach or esophagus; or (e) functional heartburn.
2.3. RDQ
All participants completed the RDQ16, 17 and healthy controls completed the RSI.18, 19
2.4. Sample collection
All participants were asked to provide a postprandial saliva sample, and the patients with EE and NERD were encouraged to provide an addition post‐symptom sample. All samples were collected following the standard procedure in 30‐mL collection tubes that contained 0.5 mL of 0.01 mmol/L citric acid to prevent pepsin auto‐digestion.20 The postprandial sample was collected 1 hour after the participants had consumed their main meal and the post‐symptom sample was collected within 15 minutes after experiencing gastroesophageal reflux symptoms. All samples were stored at 4°C prior to pepsin analysis.
2.5. Sample analysis
All collection tubes were centrifuged at 2100 ×g (4000 rpm) for 5 minutes, when a clear supernatant layer had been formed. If this layer was not visible the samples were centrifuged for a further 5 minutes at 2100 ×g, before 80 μL from the surface of the supernatant was drawn up into an automated pipette. The sample was transferred to an Eppendorf tube containing 240 μL of migration buffer (pH 8.2) before being vortexed mixed for 10 seconds. A second pipette was used to transfer 80 μL of the sample/migration buffer solution to the circular well of a lateral flow device (LFD) containing two unique human monoclonal antibodies: one to detect and the other to capture pepsin (Peptest, RD Biomed Limited, UK; Figure 2). Fifteen minutes after introducing the clinical sample for pepsin analysis to the well of the Peptest, the LFD was visualized for the presence of pepsin at the t line (test line) in the window of the LFD. The higher the intensity of the blue t line, the higher the concentration of pepsin present in the clinical sample. Pepsin concentrations visualized as ≤75 ng/mL were defined as weak positive and consequently treated as negative. Therefore, following saliva sample analysis, a pepsin concentration of 75 ng/mL and less was considered physiological and samples with a pepsin concentration above 75 ng/mL were considered pathological.
Figure 2.
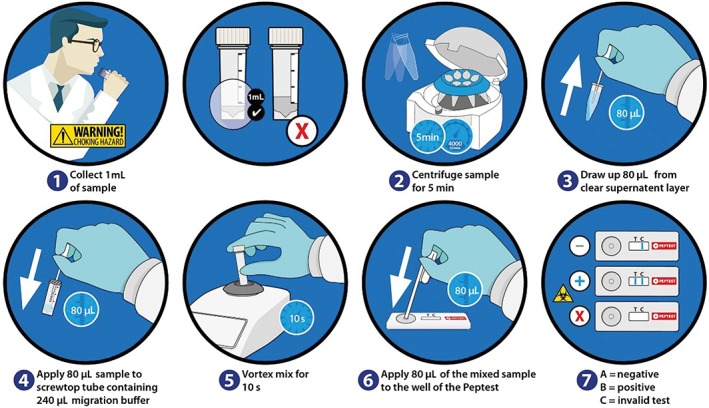
Collection and analysis of saliva samples using Peptest
The clinical study protocol was registered on the publicly accessible database http://clinicaltrials.gov PRS (http://register.clinicatrials.gov) with a clinical trial registration number of NCT02456779. The clinical study protocol was reviewed and approved by each of the nine clinical centers and all studies involving human participants were conducted in accordance with the ethical standards of each institute and as laid down in an appropriate version of the Declaration of Helsinki (as revised in Brazil, 2013). The ethical approval ID of the principle investigators Professor JYF's institute, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, was 2015‐050. Informed consent was obtained from all individual participants included in the study.
2.6. Statistical analysis
Continuous variables were expressed as mean ± standard deviation, whereas categorical variables were expressed as numbers and percentages. The statistical packages used were GraphPad Prism 7 and GraphPad Prism 8 (GraphPad Software, San Diego, CA, USA). To compare centers an unpaired t‐test was carried out to determine P values using the Pearson’s correlation test and a χ2 test and a one‐sample t‐test and Wilcoxon test performed when appropriate. P < 0.05 was considered statistically significant. A forest plot was used to demonstrate overall sensitivity and specificity among all nine centers using VassarStats (Vassar College, Ploughkeepsie, NY, USA).
3. RESULTS
The characteristics of all participants included in the present study are shown in Table 1.
Table 1.
Characteristics of patients diagnosed with non‐erosive reflux disease (NERD), erosive esophagitis (EE), and healthy controls (HC) (n = 1032)
| NERD | EE | HC | |
|---|---|---|---|
| Number | 488 | 221 | 323 |
| Mean age range (y) | 49 | 52.5 | 37 |
| Mean reflux disease questionnaire score | 20.9 | 20.3 | 0 |
| Smoking males | 64 | 58 | 17 |
3.1. Breakdown of patients with GERD into NERD and EE
Male and female patients diagnosed with GERD that was defined as NERD (n = 488) or EE (n = 221) and a healthy control group (n = 323) aged between 19 and 78 years were recruited from seven hospitals in Shanghai and two in Beijing.
The characteristics of study participants recruited from each hospital center is illustrated in Figure 1. The ratio of patients with NERD to EE was 1:0.45. There were noticeably more women presenting with NERD than men compared with EE. The total number of healthy controls recruited was 323 with a male:female split of 95 men to 228 women, a ratio of 0.4:1, as displayed in Table 2.
Table 2.
Number of male and female participants with non‐erosive reflux disease (NERD) and erosive esophagitis (EE) and healthy controls in each center
| Center | NERD | EE | Healthy | Total | ||||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | Male | Female | |
| 1 | 16 | 35 | 20 | 7 | 10 | 20 | 46 | 62 |
| 2 | 16 | 34 | 10 | 12 | 8 | 22 | 34 | 68 |
| 3 | 11 | 38 | 10 | 11 | 15 | 16 | 36 | 65 |
| 4 | 16 | 26 | 12 | 5 | 8 | 28 | 36 | 59 |
| 5 | 32 | 46 | 13 | 9 | 11 | 31 | 56 | 86 |
| 6 | 10 | 44 | 16 | 19 | 9 | 37 | 35 | 100 |
| 7 | 18 | 53 | 15 | 13 | 11 | 31 | 44 | 97 |
| 8 | 18 | 31 | 15 | 11 | 12 | 19 | 45 | 61 |
| 9 | 17 | 27 | 15 | 8 | 11 | 24 | 43 | 59 |
3.2. Age comparison across the hospital centers
The age range and average age breakdown for male and female patients with NERD and EE recruited for each of the nine hospitals in comparison with the healthy control population is shown in Table 3. Across the nine hospitals there was a close comparison in both age and sex, and neither age nor sex influenced the performance of Peptest. The average age of the patient groups was 49 years for NERD and 52 years for EE (P = 0.0035). Across all nine centers there was a tendency for male and female healthy controls to be younger than the patients, with an average of 37 years (P < 0.0001).
Table 3.
Age ranges and average age (y) in brackets of all patients and healthy controls in each center
| Center | NERD | EE | Healthy | |||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | |
| 1 | 28‐70 (54) | 24‐75 (52) | 31‐70 (54) | 47‐65 (57) | 24‐61 (36) | 23‐56 (33) |
| 2 | 28‐65 (49) | 24‐65 (49) | 27‐65 (58) | 33‐65 (57) | 27‐64 (43) | 24‐64 (34) |
| 3 | 33‐70 (53) | 26‐70 (52) | 32‐68 (56) | 25‐67 (57) | 27‐68 (46) | 26‐67 (43) |
| 4 | 20‐71 (43) | 26‐72 (49) | 25‐70 (46) | 39‐61 (53) | 19‐29 (22) | 19‐60 (26) |
| 5 | 21‐60 (37) | 23‐64 (39) | 27‐63 (45) | 29‐60 (47) | 25‐66 (38) | 22‐66 (37) |
| 6 | 37‐69 (54) | 28‐71 (53) | 30‐70 (51) | 29‐68 (56) | 25‐51 (34) | 24‐61 (41) |
| 7 | 26‐69 (49) | 32‐70 (58) | 20‐65 (44) | 21‐69 (53) | 25‐65 (35) | 23‐68 (41) |
| 8 | 23‐72 (49) | 21‐67 (49) | 23‐65 (48) | 29‐78 (64) | 28‐52 (39) | 23‐59 (40) |
| 9 | 23‐68 (47) | 20‐67 (47) | 29‐72 (48) | 31‐67 (50) | 22‐62 (36) | 21‐65 (38) |
EEE, erosive esophagitis; NERD, non‐erosive reflux disease
3.3. RDQ scores by patients and healthy control groups
At recruitment, all patients and control participants were asked to complete the RDQ. A score of zero at study entry was an inclusion criterion for all control participants (n = 323).
Most patients in the NERD group scored between 16 and 25 on the RDQ and the EE group most scored between 16 and 20. The mean RDQ scores across each of the nine hospital centers for both male and female patients with NERD and EE are displayed in Table 4. Although there was no significant difference in terms of RDQ severity across the nine hospital centers, there was a tendency for patients in both the NERD and EE groups recruited from centers three and five to have higher RDQ scores than the other centers. This observation was reflected in the higher Peptest sensitivity found at these two centers.
Table 4.
Mean reflux disease questionnaire (RDQ) scores across each center for male and female patients with non‐erosive reflux disease (NERD) and erosive esophagitis (EE)
| Center | Mean RDQ score | |||
|---|---|---|---|---|
| NERD | EE | |||
| Male | Female | Male | Female | |
| 1 | 20.31 | 21.60 | 20.10 | 17.48 |
| 2 | 18.69 | 19.54 | 18.40 | 15.84 |
| 3 | 24.36 | 23.47 | 23.20 | 21.82 |
| 4 | 28.06 | 21.50 | 18.25 | 23.40 |
| 5 | 23.50 | 22.82 | 23.92 | 23.56 |
| 6 | 17.80 | 19.36 | 19.19 | 19.63 |
| 7 | 20.78 | 24.74 | 20.33 | 21.69 |
| 8 | 17.06 | 19.29 | 17.87 | 17.27 |
| 9 | 18.06 | 20.11 | 19.33 | 22.25 |
The mean RDQ score for patients with NERD and EE at each of the nine centers is illustrated in Figure 3. There is no difference in the RDQ score between these two patient groups across all hospitals.
Figure 3.

Mean reflux disease questionnaire (RDQ) scores generated in each center for patients with  non‐erosive reflux disease (NERD) and
non‐erosive reflux disease (NERD) and  erosive esophagitis (EE)
erosive esophagitis (EE)
3.4. Collection of postprandial and post‐symptom saliva samples
All participants provided postprandial saliva samples, with the patients in the NERD and EE groups providing an additional sample that was taken after they had experienced symptoms. The distribution of the samples provided is shown in Table 5.
Table 5.
Sample distribution of patients diagnosed with gastroesophageal reflux disease (GERD) in non‐erosive reflux disease (NERD) and erosive esophagitis (EE) groups and healthy control subjects
| Sample type | GERD | Healthy controls | |
|---|---|---|---|
| NERD | EE | ||
| Post‐prandial | 488 | 220 | 323 |
| Post‐symptom | 478 | 214 | n/a |
n/a, not available.
The total number of saliva samples collected and analyzed for pepsin was 1723, of which most (n = 1031) were postprandial. The saliva sample collection was broken down even more by comparing the number of positive postprandial and post‐symptom samples in the NERD (n = 383/170) and the EE (n = 337/139) patient group, compared with the positive postprandial samples collected in the healthy controls (n = 130). We concluded from the pepsin sample analysis that 84% of the post‐symptom samples were pepsin‐positive, compared with 70% of the postprandial samples. The level of pepsin positivity in the postprandial healthy controls was 40%, and this compared favorably with other published studies. When comparing the postprandial pepsin‐positive and ‐negative samples, it was clear that some centers had higher positivity in their healthy controls. This was especially true for centers one and nine.
3.5. The influence of smoking
The number of patients with GERD (both NERD and EE) and healthy controls recruited across the nine hospital centers that currently smoke, had a history of smoking or were non‐smokers is shown in Table 6.
Table 6.
Number of patients with non‐erosive reflux disease (NERD) and erosive esophagitis (EE), and healthy controls (HC), and their relationship to smoking
| N (%) of participants | |||
|---|---|---|---|
| NERD | EE | HC | |
| Current smokers | 55 (11.3) | 47 (21.3) | 15 (4.7) |
| History of smoking | 9 (1.8) | 11 (5.0) | 2 (0.6) |
| Non‐smoker | 424 (86.9) | 163 (73.7) | 303 (93.8) |
| Failed to provide smoking information | 0 (0) | 0 (0) | 3 (0.9) |
There were far more current smokers in the patient groups than in the healthy group (P < 0.03). A higher number of the smokers in the NERD and EE patient groups were pepsin‐positive, demonstrating that a higher percentage of the patients were reflux‐positive, as detected by Peptest.
The total number of patients with GERD who were current smokers or who had a history of smoking was 122, which constitutes 17% of the total GERD population of 709 patients. The pepsin positivity analysis in the patient population with GERD had a sensitivity of 85%. The same analysis using only patients with GERD who smoked or had a history of smoking had a sensitivity of 90%. This small increase in pepsin (Peptest) sensitivity correlates with the current literature, suggesting that smoking makes individuals more susceptible to upper gastrointestinal disease and the treatment of these conditions less effective.
In contrast, the total number of healthy controls who smoked or had a history of smoking was 17, constituting 5% of the total healthy control population of 323 participants. The specificity of the healthy control group was 60%. The analysis of only the smoking/history of the smoking population reduced the specificity to 53%, which reflects the influence of smoking in otherwise healthy individuals that puts them at a greater risk of developing reflux disease than individuals that do not smoke.
3.6. Overall sensitivity and specificity
The data generated in the study can be further broken down to show the incidence of NERD, EE and combined sensitivities and specificities. For both men and women there was a similar pattern of Peptest positivity in both postprandial and post‐symptom samples, with some centers recording a higher pepsin sensitivity than others. However, overall Peptest positivity was higher in women (81%) than men (74%), with a combined sensitivity of 78%.
The combined breakdown for male and female data for all patients with NERD and EE from each hospital center is shown in Table 7. The female sensitivity results were slightly higher than that for male patients (86% vs 84% in the NERD group and 91% vs 79% in the EE group).
Table 7.
Breakdown of non‐erosive reflux disease (NERD) and erosive esophagitis (EE) and combined sensitivities and specificities for all samples provided by men and women (n = 1723) from each center
| Sensitivity | ||||
|---|---|---|---|---|
| Center number | NERD (%) | EE (%) | Overall (%) | Specificity (%) |
| 1 | 67 | 74 | 69 | 37 |
| 2 | 82 | 91 | 85 | 53 |
| 3 | 92 | 90 | 91 | 84 |
| 4 | 86 | 76 | 83 | 64 |
| 5 | 100 | 100 | 100 | 64 |
| 6 | 100 | 91 | 97 | 63 |
| 7 | 93 | 93 | 93 | 62 |
| 8 | 69 | 77 | 72 | 68 |
| 9 | 68 | 61 | 66 | 40 |
| Overall | 86 | 84 | 85 | 60 |
Peptest pepsin sensitivity across all nine hospitals for the combined male and female population (n = 1032) was 85%. The center with the highest sensitivity was center five (100%), with five centers reporting a sensitivity of 85% or greater, as displayed in Table 8.
Table 8.
Breakdown of the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and positive likelihood ratio for all men and women with gastroesophageal reflux disease (n = 1032) from each center
| Center number | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Positive likelihood |
|---|---|---|---|---|---|
| 1 | 69 | 37 | 74 | 31 | 1.09 |
| 2 | 85 | 53 | 81 | 59 | 1.82 |
| 3 | 91 | 84 | 93 | 81 | 5.67 |
| 4 | 83 | 64 | 79 | 70 | 2.30 |
| 5 | 100 | 64 | 87 | 100 | 2.80 |
| 6 | 97 | 63 | 83 | 91 | 2.61 |
| 7 | 93 | 62 | 85 | 79 | 2.44 |
| 8 | 72 | 68 | 84 | 50 | 2.23 |
| 9 | 66 | 40 | 68 | 38 | 1.09 |
| Overall | 85 | 60 | 82 | 65 | 2.12 |
The data from each center was further analyzed using a forest plot to represent the sensitivity and specificity in all nine hospital centers to check for overall conformity between centers (Figure 4). The statistical analysis showed there was no significant difference for sensitivity (two‐tailed P = 0.9995), based on a theoretical mean of 0.8396 with a standard deviation of discrepancy of 0.125.
Figure 4.

Forest plots showing sensitivity and specificity across all nine centers. CI, confidence interval
There was also no significant difference for specificity (two‐tailed P = 0.9992), based on a theoretical mean of 0.5942 with a standard deviation of discrepancy of 0.1441.
The overall summary for all patients with GERD and all healthy participants enrolled in the study is shown in Table 8. Peptest pepsin positivity was 85%, with a specificity of 60%, with an overall study positive predictive value (PPV) of 82%, a negative predictive value (NPV) of 65% and a positive likelihood ratio of 2.12.
4. DISCUSSION
Recently, pepsin in saliva has been recognized as a diagnostic biomarker in GERD and LPR, and it is proposed as a non‐invasive method to diagnose reflux disease in China.6, 21 Peptest (RD Biomed Limited), a rapid, non‐invasive reflux diagnostic tool that detects the presence of pepsin in the saliva of patients suspected of reflux disease, was introduced and validated in major hospitals in Shanghai (seven hospitals) and Beijing (two hospitals) with a total of 1032 patients and control subjects.22 All the patients recruited in the study were diagnosed with GERD and further subdivided into NERD and EE groups. A total of 323 asymptomatic healthy participants were also recruited into the study.
It was important to standardize the salivary pepsin method and collection times. Therefore, all healthy controls and patients were asked to provide a postprandial saliva sample and the patients with NERD and EE were encouraged to provide an additional saliva sample within 15 minutes of experiencing reflux symptoms.23, 24, 25 The pepsin sample analysis was done as described in the methods section.
The reflux questionnaire is commonly used in both Western countries and in China. In the current study all participants completed the RDQ16 and in addition the control participants completed the RSI.18 The control participants generated a score of zero when completing the RDQ, the NERD group scored between 16 and 25 on the RDQ, and the EE group scored between 16 and 20. Furthermore, hospitals that had higher RDQ scores reported higher Peptest sensitivity. The RSI questionnaire inclusion criterion for healthy controls was a score ≤9 out of 45. In addition, healthy controls were required to score 0 in the question on symptoms of heartburn.
When observing the combined postprandial and post‐symptom sensitivities and specificities provided by the male patients, the overall range of pepsin positivity varied between a sensitivity of 55% and 100% and a specificity of 45% to 80%. Similarly, in the female patients the overall breakdown for sensitivity was 62% to 100%, with specificity ranging from 33% to 88%. When combining the total number of samples provided by male and female patients, the overall pepsin positivity for sensitivity was 85% and 60% for specificity.
A higher number of female patients (n = 657) than male patients (n = 374) provided postprandial samples for pepsin analysis. Although the specificities were similar for both male (61%) and female (59%) patients, there was a tendency for the overall pepsin sensitivity to be higher in female patients (81%) than in the male patients (74%).
The combined male and female Peptest positivity was 78%, with some centers reporting higher sensitivity to pepsin in their postprandial samples. Four centers achieved sensitivities between 84% and 97% and five centers achieved sensitivity values ranging from 57% to 76%. Although there was no significant difference in terms of RDQ severity across the nine hospital centers, there was a tendency in some centers to have recruited NERD and EE patients with higher RDQ scores which was reflected in higher Peptest sensitivity at these centers. A similar pattern was observed in the post‐symptom pepsin sensitivity analysis, with four centers reporting higher pepsin sensitivities than the other centers. This indicates that patients with a higher RDQ at recruitment are more likely to have a higher Peptest pepsin sensitivity.
The end‐point of any validation study that introduces a new non‐invasive diagnostic test on the market is how it performs in terms of sensitivity and specificity. In the validation of Peptest the positive diagnosis of reflux disease, including the PPV and the NPV, were also analyzed.
In the current study the total number of saliva samples collected and analyzed for pepsin was 1723, in which a total of 1159 samples were pepsin positive. This included 553 positive samples in the NERD patient group and 476 positive samples in the EE patient group. There were 130 positive saliva samples in the healthy control group. A frequent question “When is the best time for patient saliva samples to be taken to record pepsin positivity?” was asked in the current study. There were 720 pepsin‐positive postprandial samples and 309 pepsin‐positive post‐symptom samples in the NERD and EE groups. This suggests the best time to collect saliva samples and subsequent pepsin analysis is 60 minutes postprandial.
How well did the overall results compare and agree with other studies evaluating Peptest in gastroenterology, Ear nose and throat (ENT) and respiratory patients? In the present study, 1032 patients with GERD and healthy controls were evaluated across nine centers in Shanghai and Beijing. The overall pepsin sensitivity was 85% and specificity 60% with a PPV of 82% and an NPV of 65%. The positive likelihood ratio was 2.12. Specificity and NPV were lower than expected. This was largely due to the poor selection of control participants in some centers, as previously discussed. However, the overall results in the current study are comparable with previously published studies.11, 23, 26, 27, 28, 29, 30, 31, 32, 33 Sensitivity generally ranged between 80% and 90% with specificity reported to be between 90% and 100%.
Did smoking influence the positive pepsin response to Peptest? Although there were more current smokers in the patient group than in the healthy control group the numbers were still low, with 17% of patients with GERD and 5% of healthy controls reporting that they smoked or had a history of smoking. The answer to the question was, yes, smoking had some influence on overall sensitivity and specificity. Sensitivity in patients with GERD increased and specificity in healthy controls decreased. One conclusion is that in future clinical studies patients and participants who smoke or have a history of smoking should be excluded, and smoking thus needs to be added to the exclusion criteria.
Forest plots were used to compare differences between centers in terms of the observed sensitivity and specificity of the results seen in Figure 4. The statistical analysis clearly showed there were no significant differences between centers, based on the Peptest showing that pepsin due to reflux was present.
Together, both pepsin and Peptest enable a rapid diagnosis of GERD in clinical samples. The easy‐to‐use, patient friendly, non‐invasive and rapid Peptest improves the accuracy of reflux diagnosis by complementing questionnaires and invasive diagnostic tests to tailor a more beneficial and appropriate course of medication.
The study also revealed that the prevalence of reflux disease in the Chinese population was much higher than previously reported. There are likely to be two main reasons for this: first, the fast‐growing westernized lifestyle in China and second, the possibility that reflux disease has been underdiagnosed due to a lack of effective and objective diagnostic tests.
The advantage of using Peptest over currently used invasive reflux diagnostic tests is that it enabled the rapid diagnosis of GERD in an easy‐to‐use, patient friendly test, improving the accuracy of reflux diagnosis and resulting in an overall pepsin sensitivity of 85% and specificity of 60%, which was comparable with international, peer‐reviewed Peptest clinical literature.
A limitation of the present study was the lack of quantification of the pepsin concentrations in the saliva samples. Most previously published studies have used the Peptest LFD, reader which allows the concentration of pepsin to be determined in the saliva sample in ng/mL. The reader was not used in the current study in China. This lack of quantification made it more difficult to differentiate between physiological and pathological reflux. We know from other studies that physiological reflux is common in healthy controls and studies have observed the presence of physiological pepsin in around 35% of a healthy control population.23, 24, 25 This is likely to be responsible for reports by some centers of higher pepsin levels in their healthy control groups than others. This differential was not observed in the patients with GERD presenting with mainly pathological pepsin levels and reflux.
The age ranges of the patient groups and the healthy control group were similar and there was only a small difference in the average age between the NERD group (49 years) and the EE group (52.5 years). Overall, when comparing all nine centers, there was a tendency for the control participants to be younger (37 years) than the patient groups. Our target recruitment aimed to use age‐matched control and patient groups. However, it proved difficult to recruit older control participants and one criticism and limitation of our present study could be the age differences between the control study groups and patient study groups.
In conclusion, Peptest is a rapid, non‐invasive reflux diagnostic tool that detects the presence of pepsin in the saliva of patients suspected of reflux. The study enrolled 1032 participants in total who received an endoscopy and a Peptest. The results showed that the sensitivity of the Peptest was high, but the specificity achieved in some centers was low, resulting overall in only a moderate specificity. Further diagnostic investigative studies are warranted.
CONFLICT OF INTEREST
The authors have no financial support to declare. PWD, RKL, and ADW are employees of RD Biomed Limited, UK.
Wang YF, Yang CQ, Chen YX, et al. Validation in China of a non‐invasive salivary pepsin biomarker containing two unique human pepsin monoclonal antibodies to diagnose gastroesophageal reflux disease. J Dig Dis. 2019;20:278–287. 10.1111/1751-2980.12783
Contributor Information
Peter W. Dettmar, Email: peter.dettmar@rdbiomed.com.
Jing‐Yuan Fang, Email: fangjingyuan_new@163.com.
REFERENCES
- 1. Lee SP, Sung IK, Kam JH, Lee SY, Park HS, Shim CS. The clinical features and predisposing factors of asymptomatic erosive esophagitis. Dig Dis Sci. 2016;61(12):3522‐3529. [DOI] [PubMed] [Google Scholar]
- 2. Minatsuki C, Yamamichi N, Shimamoto T, et al. Background factors of reflux esophagitis and non‐erosive reflux disease: a cross‐sectional study of 10,837 subjects in Japan. PLoS One. 2013;8(7):e69891. 10.1371/journal.pone.0069891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Bortoli N, Ottonello A, Zerbib F, Sifrim D, Gyawali CP, Savarino E. Between GERD and NERD: the relevance of weakly acidic reflux. Ann N Y Acad Sci. 2016;1380(1):218‐229. [DOI] [PubMed] [Google Scholar]
- 4. Woodland P, Al‐Zinaty M, Yazaki E, Sifrim D. In vivo evaluation of acid‐induced changes in oesophageal mucosa integrity and sensitivity in non‐erosive reflux disease. Gut. 2013;62(9):1256‐1261. [DOI] [PubMed] [Google Scholar]
- 5. Boeckxstaens G, El‐Serag HB, Smout AJ, Kahrilas PJ. Symptomatic reflux disease: the present, the past and the future. Gut. 2014;63(7):1185‐1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang J, Zhao Y, Ren J, Xu Y. Pepsin in saliva as a diagnostic biomarker in laryngopharyngeal reflux: a meta‐analysis. Eur Arch Otorhinolaryngol. 2018;275(3):671‐678. [DOI] [PubMed] [Google Scholar]
- 7. Dettmar PW, Castell DO, Heading RC, et al. Review article: reflux and its consequences — the laryngeal, pulmonary and oesophageal manifestations. Aliment Pharmacol Ther. 2011;33(Suppl):1‐71. [DOI] [PubMed] [Google Scholar]
- 8. Li YW, Sifrim D, Xie C, Chen M, Xiao YL. Relationship between salivary pepsin concentration and esophageal mucosal integrity in patients with gastroesophageal reflux disease. J Neurogastroenterol Motil. 2017;23(4):517‐525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Johnston N, Dettmar PW, Ondrey FG, Nanchal R, Lee SH, Bock JM. Pepsin: biomarker, mediator, and therapeutic target for reflux and aspiration. Ann N Y Acad Sci. 2018;1434(1):282‐289. [DOI] [PubMed] [Google Scholar]
- 10. Bardhan KD, Strugala V, Dettmar PW. Reflux revisited: advancing the role of pepsin. Int J Otolaryngol. 2012;2012:646901 10.1155/2012/646901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ocak E, Kubat G, Yorulmaz İ. Immunoserlogic pepsin detection in the saliva as a non‐invasive rapid diagnostic test for laryngopharyngeal reflux. Balkan Med J. 2015;32(1):46‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Johnston N, Dettmar PW, Strugala V, Allen JE, Chan WW. Laryngopharyngeal reflux and GERD. Ann N Y Acad Sci. 2013;1300:71‐79. [DOI] [PubMed] [Google Scholar]
- 13. Calvo‐Henríquez C, Ruano‐Ravina A, Vaamonde P, Martínez‐Capoccioni G, Martín‐Martín C. Is pepsin a reliable marker of laryngopharyngeal reflux? A systematic review. Otolaryngol Head Neck Surg. 2017;157(3):385‐391. [DOI] [PubMed] [Google Scholar]
- 14. Wang AJ, Liang MJ, Jiang AY, et al. Gastroesophageal and laryngopharyngeal reflux detected by 24‐hour combined impedance and pH monitoring in healthy Chinese volunteers. J Dig Dis. 2011;12(3):173‐180. [DOI] [PubMed] [Google Scholar]
- 15. Lin SR, Xu GM, Hu PJ, et al. The 2006 China Gastrosophageal reflux disease expert consensus [in Chinese]. Chin J Gastroenterol. 2007;12(3):233‐239. [Google Scholar]
- 16. Shaw M, Dent J, Beebe T, et al. The reflux disease questionnaire: a measure for assessment of treatment response in clinical trials. Health Qual Life Outcomes. 2008;6:31 10.1186/1477-7525-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harnik G. In the clinic: gastroesophageal reflux disease. Ann Intern Med. 2015;163(1):ITC1 10.7326/AITIC20507070. [DOI] [PubMed] [Google Scholar]
- 18. Mesallam TA, Stemple JC, Sobeih TM, Elluru RG. Reflux symptom index versus reflux finding score. Ann Otol Rhinol Laryngol. 2007;116(6):436‐440. [DOI] [PubMed] [Google Scholar]
- 19. Belafsky PC, Postma GN, Koufman JA. Validity and reliability of the reflux symptom index (RSI). J Voice. 2002;16(2):274‐277. [DOI] [PubMed] [Google Scholar]
- 20. Kim TH, Lee KJ, Yeo M, Kim DK, Cho SW. Pepsin detection in the sputum/ saliva for the diagnosis of gastroesophageal reflux disease in patients clinically suspected atypical gastroesophageal reflux disease symptoms. Digestion. 2008;77(3‐4):201‐206. [DOI] [PubMed] [Google Scholar]
- 21. Du X, Wang F, Hu Z, et al. The diagnostic value of pepsin detection in saliva for gastro‐esophageal reflux disease: a preliminary study from China. BMC Gastroenterol. 2017;17(1):107 10.1186/s12876-017-0667-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dettmar P, Fang JY, Yu XF, et al. The validation of Peptest: a new non‐invasive technology for the diagnosis of gastroesophageal reflux disease in China. UEG J. 2018;6(Suppl):A301. [Google Scholar]
- 23. Hayat JO, Gabieta‐Gomez S, Yazaki E, et al. Pepsin in saliva gastroesophageal reflux monitoring in 100 healthy symptomatic subjects and 65 patients with significant heartburn/regurgitation. UGE J. 2013;1(Suppl):A113. [Google Scholar]
- 24. Hayat JO, Gabieta S, Woodcock A, et al. Postprandial pepsin saliva in healthy subjects and patients with GERD. Relationship with postprandial reflux. Gastroenterology. 2014;146(Suppl):S‐751. [Google Scholar]
- 25. Hayat JO, Gabieta‐Somnez S, Yazaki E, et al. Pepsin in saliva for the diagnosis of gastro‐oesophageal reflux disease. Gut. 2015;64(3):373‐380. [DOI] [PubMed] [Google Scholar]
- 26. Bor S, Capangolu DS, Yildirim E, Vardar R, Woodcock A, Dettmar PW. The validation of peptest a new non‐invasive technology for the diagnosis of laryngopharangeal reflux (LPR). Gut. 2012;61(Suppl):A83. [Google Scholar]
- 27. de Bortoli N, Savarino E, Furnari M, et al. Use of a non‐invasive pepsin diagnostic test to detect GERD: correlation with MII‐pH in a series of suspected NERD patients. A pilot study. Dig Liver Dis. 2013;45(Suppl):S68‐S69. [Google Scholar]
- 28. Saritas Yuksel E, Hong SK, Strugala V, et al. Rapid salivary pepsin test: blinded assessment of test performance in gastroesophageal reflux disease. Laryngoscope. 2012;122(6):1312‐1316. [DOI] [PubMed] [Google Scholar]
- 29. de Bortoli N, Savarino E, Furnari M, et al. Use of a non‐invasive pepsin diagnostic test to detect GERD: correlation with MII‐pH evaluation in a series of suspected NERD patients. A pilot study. Gastroenterology. 2013;144(Suppl):S‐118. [Google Scholar]
- 30. Adkins C, Yadlapati R, Jaiyeola D, et al. Salivary pepsin concentrations are higher for patients with reflux associated laryngeal symptoms: a prospective pilot study. Gastroenterology. 2015;148(Suppl):S‐610. [Google Scholar]
- 31. Strugala V, Bardhan KD, McGlashan J, Dettmar PW. Differentiation between LPR and GORD with the use of a simple non‐invasive diagnostic test for reflux by detection of pepsin in expectorated saliva. Paper presented at the 15th British Academic Conference in Otolaryngology and ENT Expo, Liverpool, 8‐10 July 2015.
- 32. Strugala V, Dettmar PW, Bardhan KD. Optimisation of the peptest diagnostic test for detection of GORD using pepsin as a marker: an ideal primary care tool. Gut. 2015;64(Suppl):A492‐A493. [Google Scholar]
- 33. Gelardi M, Eplite A, Mezzina A, et al. Clinical‐diagnostic correlations in the laryngopharyngeal reflux (LPR). The role of Peptest™. Int J Open Acce Otolary. 2017;1(1):1‐8. [Google Scholar]


