Abstract
Primary prevention of type 1 diabetes (T1D) requires intervention in genetically at‐risk infants. The Global Platform for the Prevention of Autoimmune Diabetes (GPPAD) has established a screening program, GPPAD‐02, that identifies infants with a genetic high risk of T1D, enrolls these into primary prevention trials, and follows the children for beta‐cell autoantibodies and diabetes. Genetic testing is offered either at delivery, together with the regular newborn testing, or at a newborn health care visits before the age of 5 months in regions of Germany (Bavaria, Saxony, Lower Saxony), UK (Oxford), Poland (Warsaw), Belgium (Leuven), and Sweden (Region Skåne). Seven clinical centers will screen around 330 000 infants. Using a genetic score based on 46 T1D susceptibility single‐nucleotide polymorphisms (SNPs) or three SNPS and a first‐degree family history for T1D, infants with a high (>10%) genetic risk for developing multiple beta‐cell autoantibodies by the age of 6 years are identified. Screening from October 2017 to December 2018 was performed in 50 669 infants. The prevalence of high genetic risk for T1D in these infants was 1.1%. Infants with high genetic risk for T1D are followed up and offered to participate in a randomized controlled trial aiming to prevent beta‐cell autoimmunity and T1D by tolerance induction with oral insulin. The GPPAD‐02 study provides a unique path to primary prevention of beta‐cell autoimmunity in the general population. The eventual benefit to the community, if successful, will be a reduction in the number of children developing beta‐cell autoimmunity and T1D.
Keywords: beta‐cell autoantibodies, genetic risk for type 1 diabetes, type 1 diabetes
1. BACKGROUND
The primary prevention of common chronic disease is a major public health goal. Type 1 diabetes (T1D) is among the more frequent chronic diseases in childhood, and is increasing in incidence.1 T1D results from an immune‐mediated destruction of pancreatic islet beta‐cells resulting in insulin deficiency. This process is identified by circulating autoantibodies to beta‐cell antigens, indicative of a break in immunological self‐tolerance.2, 3 Neonates or infants who are at increased genetic risk for multiple beta‐cell autoantibodies and T1D can be identified using genetic markers and T1D family history.4 Risk stratification by such genetic markers is now sufficiently advanced to bring testing to a population‐based level and, thereby, facilitates the primary prevention of beta‐cell autoimmunity and T1D.5
Primary prevention of T1D has a strong rationale. There is a clear peak incidence period of beta‐cell autoantibody seroconversion between age 9 months and 3 years demonstrated in German,6 Finnish,7 and The Environmental Determinants of Diabetes in the young (TEDDY) studies.8 This provides a convenient study follow‐up time in order to execute controlled clinical trials. Insulin is a first autoantibody target.6, 8, 9 It is widely held that if infant tolerance to beta‐cell autoantigens could be enhanced, this could prevent or delay the onset of pre‐ or asymptomatic T1D (defined as loss of tolerance and multiple beta‐cell autoantibodies), and hence prevent or delay the clinical onset of diabetes.10 As a primary prevention, we have laid the foundation for autoantigen‐specific tolerance induction initiated prior to an autoimmune memory response. We have identified a dose of insulin that when administered orally on a daily basis to beta‐cell autoantibody negative children at increased genetic risk is safe (neither affecting plasma glucose levels nor causing an allergic reaction) and appears to engage the immune system in a manner that is consistent with immune‐mediated, tolerogenic protection.11 Therefore, the task is now to identify neonates or infants at increased genetic risk for T1D to execute the first insulin‐based primary prevention trial. The objective of the Global Platform for the Prevention of Autoimmune Diabetes (GPPAD)‐02 study protocol is to identify neonates or infants who have >10% risk for developing multiple beta‐cell autoantibodies by the age of 6 years, which is the risk we have defined as the requirement for participation in a primary prevention randomized controlled trial that tests the effects of daily oral insulin vs placebo on the incidence of beta‐cell autoantibody seroconversion. GPPAD will continue and likely improve screening for future primary prevention clinical trials.
2. STUDY ORGANIZATION
GPPAD is a network of collaborating investigators from seven clinical trial centers located in five European countries: Germany (Bavaria, Saxony, Lower Saxony); UK (Oxford); Poland (Warsaw); Belgium (Leuven), and Sweden (Region Skåne). GPPAD was created to allow a multidisciplinary approach to prevent T1D and is coordinated by a GPPAD coordinating center in Germany (Munich).
2.1. Identification of high genetic risk for T1D
T1D has a multifactorial etiology, which is determined by genetic and environmental factors.3 Lifetime risk in a European population is around 0.4%. A first‐degree family history of T1D is associated with a 5% risk for T1D.4 There are at least 50 regions of the genome where genetic variation is associated with T1D risk,12 the most important of these being in the HLA DR‐DQ region of chromosome 6. Certain HLA DR‐DQ genotypes confer markedly elevated risk for T1D. Notably, infants who are first‐degree relatives of patients and who have the HLA DR4‐DQ8 haplotype, and infants who have either the HLA DR3/DR4‐DQ8 or the DR4‐DQ8/DR4‐DQ8 genotype have a risk of around 5%.4, 13 Typing at additional T1D susceptibility regions can identify neonates or infants with risks that are 10% or more.5 We have recently developed a T1D genetic score that identified neonates or infants without family history of T1D who had a greater than 10% risk for pre‐symptomatic T1D, and a nearly 2‐fold higher risk than children identified by high‐risk HLA genotypes alone. Thus, family history and genetic markers can be used to identify neonates or infants with 25‐fold increased risk for T1D.5 Genetic risk in GPPAD‐02 is based on these newly developed risk scores derived from a total of 46 single‐nucleotide polymorphisms (SNPs) including SNPs that define HLA DR3, HLA DR4, and HLA DQ8 alleles as well as SNPs from HLA class I and non‐HLA T1D susceptibility genes, and from HLA class II protective alleles (Supplemental Table 1).
3. STUDY PROCEDURES
3.1. Testing for T1D risk
Newborn screening for genetic, endocrine, and metabolic disorders is routinely done within the first days after birth at obstetric clinics or pediatrician offices, using a few drops of blood from the heel onto filter paper cards, or venous blood taken from the back of the hand. Testing for T1D risk is offered to families together with the regular newborn screening as a supplemental test with separate consent (Belgium, Germany, Poland, UK). Alternatively, it is offered at delivery using cord blood (Sweden) or at a pediatric visit before the age of 5 months (Sweden, Germany). At least one and a maximum of two blood spots are collected for testing of T1D risk using a separate GPPAD‐02 filter paper card (Belgium, Germany, Sweden) or using the newborn screening filter paper card (UK, Poland). The dried blood spot filter paper cards are sent to the specialized newborn screening laboratories or the local clinical study center for subsequent retrieval. The screening target is 330 000 newborns or infants prior to age of 5 months.
3.2. Consent procedure
Parents or legal guardians of neonates or infants are asked whether they wish to participate in the study. A qualified physician, midwife, or study nurse performs the informed consent in accordance with country‐specific guidelines and ethical review board requirements, providing written and verbal information explaining the objectives of the GPPAD‐02 study and the opportunities of primary prevention and follow‐up for infants with high genetic risk. The families are told that if their child is found to have a high risk for T1D, they will be contacted and offered the possibility of participation in the prevention study with further informed consent. In Sweden, additional information on a clinical trial aiming to prevent celiac disease is given to children with intermediate risk for T1D and risk for celiac disease is also provided.
Parents or guardians are given sufficient time to read the informed consent material and have any questions answered. It is explained that participation in the project is voluntary and that consent can be withdrawn at any time without providing a reason and without disadvantages by doing so. Confidentiality and protection of the data of every participant is maintained throughout the entire study. Only the local study personnel have access to personal identifiers. The informed consent form must be signed and dated by at least one parent/guardian. The parent or guardian is given a copy of the informed consent.
3.3. Demographic data
Name, contact information of the parents, child's date of birth, gender, weight, date of blood collection, mother's date of birth, first‐degree family history of T1D are collected either by a separate questionnaire or by extracting the information from the regular newborn screening card. Only pseudonymized data are entered into a central database held at the GPPAD coordinating center, Helmholtz Zentrum Muenchen, Germany.
3.4. Genetic testing
DNA is extracted from a 3.2‐mm punch of one dried blood spot and tested for the 46 SNPs (Supplemental Table 1). DNA extraction and SNP typing are performed in a central genotyping lab (LGC group, TEDDINGTON, Middlesex, UK [https://www.lgcgroup.com]; Figure 1A).
Figure 1.
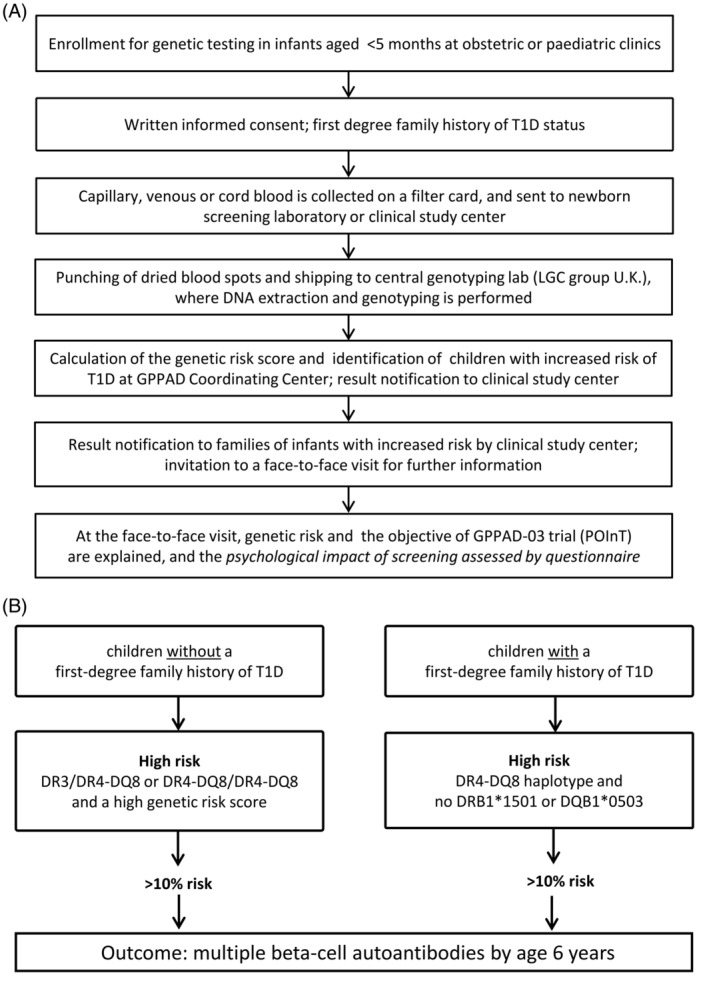
(A) GPPAD‐02 study design and (B) Definition of increased genetic risk
For sites where extra blood spots are collected for the GPPAD study (Germany, Sweden, Belgium), the remainder of the card is kept with the local GPPAD‐02 team for at least 3 months in case repeat testing is required. Thereafter, the card is destroyed or, if there is consent for it to be kept for further research, stored at −20°C or −80°C.
The genetic risk score is calculated as previously described,7 by multiplying the number of risk alleles (ie, 0, 1 or 2 for each single SNP) with the weight assigned to each SNP (Supplemental Table 1). The weighted contributions of all SNPs plus an additive constant for each of the two HLA class II categories: 3.15 for infants who have the HLA DR4‐DQ8/DR4‐DQ8 genotype, 3.98 for infants who have the HLA DR3/DR4‐DQ8 genotype, 3.12 for infants who have the HLA DR3/DR3 genotype, 2.08 for infants who have the HLA DR4‐DQ8/DRX genotype, 1.55 for infants who have the HLA DR3/DRX genotype are summed.
3.5. Definition of high genetic risk
For neonates or infants without a first‐degree family history of T1D, high genetic risk is defined as a DR3/DR4‐DQ8 or DR4‐DQ8/DR4‐DQ8 genotype, and a high genetic risk score >14.4. For neonates or infants with a first‐degree family history of T1D, high genetic risk is defined as having HLA DR4 and DQ8, and none of the protective alleles DRB1*1501 (SNP tag rs3129889) or DQB1*0503 (SNP tag rs1794265). Neonates or infants identified with high genetic risk are expected to have >10% risk for developing multiple beta‐cell autoantibodies by the age of 6 years.9 The estimated prevalence of neonates or infants with this high risk for T1D is ~1% (Figure 1B).
3.6. Result notification
Families of infants with a high risk for T1D are contacted by phone by an experienced physician/pediatrician or trained nurse from the local GPPAD‐02 team. The family is invited to a face‐to‐face consultation at the clinical study center, or alternatively at the primary care pediatrician's office. Dependent on the site‐specific arrangements, the family may receive a result letter confirming the date of the consultation, and a brochure, which explains the meaning of T1D risk to the family, describes T1D symptoms, and informs about trials to prevent disease initiation and progression. The face‐to‐face consultation visit is planned to take place before 5 months of age and includes all parents/guardians. The content of the brochure may also be discussed during the consultation (Figure 1A).
Families of children with an estimated risk that is less than 10% are not contacted unless there is a country‐specific follow‐up of children with intermediate risk of T1D or who have a high risk for celiac disease (Sweden), but are provided with a contact phone number if they want to inquire about the result.
3.7. Invitation to participate in prevention trial
Infants with high genetic risk for T1D are asked to participate in a randomized controlled trial aiming to prevent the initiation of beta‐cell autoimmunity and T1D (GPPAD‐03 POInT Trial; http://clinicaltrials.gov Identifier: NCT03364868). Families who decide not to participate in GPPAD‐03 (POInT) may be asked to participate in existing natural follow‐up studies aiming to prevent metabolic complications such as diabetic ketoacidosis through an early diagnosis of T1D program, and to investigate immunological alterations in the pathogenesis of the disease (ie, Fr1da in Bavaria, Fr1dolin in Lower Saxony). Participation in GPPAD‐03 (POInT) and Fr1da/Fr1dolin requires a separate consent (Figure 1). In Sweden, subjects at intermediate risk for T1D and high risk for celiac disease are asked to participate in a prevention study of celiac disease (http://clinicaltrials.gov Identifier: NCT03562221).
3.8. Psychological assessment
The psychological impact of the consultation in which the increased genetic risk for developing T1D is communicated is subsequently monitored using a standardized questionnaire (Patient Health Questionnaire) in Germany and Sweden. The questionnaire looks for evidence of depression, anxiety and burden, and is handed to the parents at the face‐to‐face consultation visit. A trained and experienced psychologist of the GPPAD‐02 team checks the questionnaire within 5 days. Families with elevated anxiety and depression levels (depression score > 5) are referred for consultation to a psychologist. In general, the parents are offered to contact the free telephone GPPAD‐02‐hotline with any questions or concerns.
3.9. Statistical analyses
Analyses that are planned as part of the study will be limited to descriptive statistics. These will include the number of infants consented and tested per month per region and country, the frequency of infants with and without a first‐degree relative with T1D, the proportion of children with high genetic risk, the proportion of families attending the consultation visit, the proportion of families with increased depression or anxiety and the distribution of scores from the Patient Health Questionnaire, and the proportion of families consenting to participate in the GPPAD‐03 prevention trial.
Comparisons of the genetic risk score between the study centers were performed using Kruskal‐Wallis test, one‐way analysis of variance with P‐values corrected by the Bonferroni adjustment and the comparison between boys and girls was performed using the Mann‐Whitney U test.
4. DATA SHARING
GPPAD is committed to open data sharing in compliance with all applicable European and GPPAD Consortium Member State, Data Protection and Privacy Protection laws, rules and regulations to facilitate scientific progress and patient benefit. GPPAD makes anonymized data of GPPAD‐02 available to the scientific community on a regular basis (https://www.gppad.org/en/data-sharing/).
5. PRELIMINARY RESULTS
From October 2017 to December 2018, 50 669 infants, including 891 (1.76%) with a first‐degree family history of T1D (FDR), have been screened for increased genetic risk of T1D (Figure 2). The T1D‐associated HLA risk genotypes DR3/DR4‐DQ8 or DR4‐DQ8/DR4‐DQ8 were present in 1263 (2.5%) children from the general population and the HLA DR4‐DQ8 haplotype was present in 250 (28.1%) FDR children (Table 1). Among the general population children with HLA DR3/DR4‐DQ8 or DR4‐DQ8/DR4‐DQ8 genotypes, 327 (0.7% of all screened) children had a genetic risk score >14.4 which is the threshold above which there is a >10% risk for developing multiple beta‐cell autoantibodies by the age of 6 years. Of the remainder, 631 (1.3% of all screened) children had a genetic risk score between 13.1 and 14.4, and 305 (0.6% or all screened) a genetic risk score below 13.1. In FDR children, 250 (28.1%) of 891 had a HLA DR4‐DQ8 haplotype and no protective allele associated with >10% risk to develop multiple beta‐cell autoantibodies by the age of 6 years (Table 1). The minor allele frequency of each SNP and the median genetic risk score are provided in Table 2. The median genetic risk score of all children was 10.59 (IQR: 9.76‐11.62) and of the eligible infants was 14.55 (IQR: 12.98‐15.02). The genetic risk score of screened children was higher in Sweden (Region Skåne) (median: 10.96, IQR: 9.86‐11.99, P < .01) and lower in Leuven, Belgium (median: 10.22, IQR: 9.59‐11.18, P < .01) when compared to the other study centers and was not significantly different between male (median: 10.59, IQR: 9.76‐11.64) and females (median: 10.58, IQR: 9.75‐11.61; P = .43).
Figure 2.
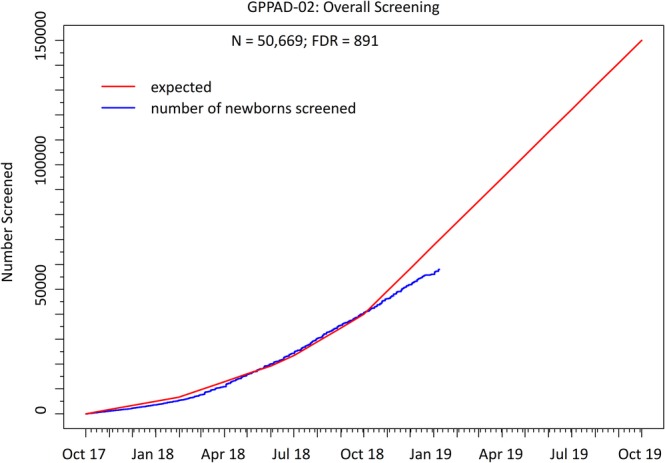
Cumulative number of newborns/infants screened in the GPPAD‐02 study. Numbers are shown from the start of the study in October 2017. The red line represents the expected numbers and the blue line shows the actual number of screened infants
Table 1.
First results from population‐based screening for type 1 diabetes genetic risk (GPPAD‐02)
| General population | ||||||
|---|---|---|---|---|---|---|
| Total | Germany | Poland | Sweden | Belgium | UK | |
| Total screened | 49 778 | 34 677 | 12 971 | 448 | 344 | 1338 |
| HLA DR3/DR4‐DQ8 or DR4‐DQ8/DR4‐DQ8 | 1263 (2.5%) | 885 (2.6%) | 300 (2.3%) | 29 (6.5%) | 3 (0.9%) | 46 (3.4%) |
| Risk score in HLA DR3/DR4‐DQ8 or DR4‐DQ8/DR4‐DQ8 | ||||||
| <13.1 | 305 (0.6%) | 214 (0.6%) | 73 (0.6%) | 5 (1.1%) | 1 (0.3%) | 12 (0.9%) |
| 13.1‐14.4 | 631 (1.3%) | 447 (1.3%) | 146 (1.1%) | 15 (3.4%) | 2 (0.6%) | 21 (1.6%) |
| >14.4a | 327 (0.7%) | 224 (0.7%) | 81 (0.6%) | 9 (2.0%) | 0 (0%) | 13 (1.0%) |
| HLA DR3/DR3b | 557 (1.1%) | 354 (1.0%) | 160 (1.2%) | 11 (2.5%) | 3 (0.9%) | 29 (2.2%) |
| First‐degree relatives | ||||||
|---|---|---|---|---|---|---|
| Total | Germany | Poland | Sweden | Belgium | UK | |
| Total screened | 891 | 709 | 149 | 20 | 5 | 8 |
| HLA DR4‐DQ8 and no DRB1*1501 or DQB1*0503a | 250 (28.1%) | 195 (27.5%) | 38 (25.5%) | 8 (40.0%) | 1 (20.0%) | 8 (100.0%) |
Eligible for POInT Trial (GPPAD‐03).
High risk for celiac disease.
Table 2.
Minor allele frequency of each SNP and the median genetic risk score
| SNP | Gene | Minor allele frequency | ||||||
|---|---|---|---|---|---|---|---|---|
| Dresden | Hannover | Leuven | Malmö | Munich | Oxford | Warsaw | ||
| rs17426593 | DR4 | 0.135 | 0.151 | 0.132 | 0.216 | 0.137 | 0.175 | 0.112 |
| rs2187668 | DR3 | 0.100 | 0.115 | 0.102 | 0.12 | 0.101 | 0.131 | 0.106 |
| rs7454108 | DQ8 | 0.090 | 0.099 | 0.075 | 0.14 | 0.090 | 0.091 | 0.079 |
| rs3129889 | DRB1*1501 | 0.125 | 0.121 | 0.091 | 0.127 | 0.121 | 0.114 | 0.121 |
| rs1794265 | DQB1*0503 | 0.025 | 0.034 | 0.031 | 0.024 | 0.032 | 0.030 | 0.021 |
| rs1264813 | HLA A 24 | 0.120 | 0.124 | 0.110 | 0.108 | 0.126 | 0.101 | 0.116 |
| rs2395029 | HLA B 5701 | 0.035 | 0.033 | 0.035 | 0.032 | 0.032 | 0.035 | 0.035 |
| rs2476601 | PTPN22 | 0.108 | 0.093 | 0.068 | 0.112 | 0.092 | 0.088 | 0.130 |
| rs2816316 | RGS1 | 0.178 | 0.181 | 0.195 | 0.198 | 0.177 | 0.180 | 0.182 |
| rs3024505 | IL10 | 0.164 | 0.166 | 0.140 | 0.154 | 0.161 | 0.145 | 0.168 |
| rs1990760 | IFIH1 | 0.385 | 0.405 | 0.399 | 0.376 | 0.402 | 0.424 | 0.354 |
| rs3087243 | CTLA4 | 0.425 | 0.444 | 0.451 | 0.412 | 0.437 | 0.459 | 0.369 |
| rs10517086 | C4orf52 | 0.283 | 0.287 | 0.293 | 0.278 | 0.286 | 0.279 | 0.285 |
| rs2069763 | IL2 | 0.360 | 0.350 | 0.305 | 0.397 | 0.338 | 0.318 | 0.357 |
| rs6897932 | IL7R | 0.246 | 0.250 | 0.277 | 0.290 | 0.243 | 0.248 | 0.254 |
| rs3757247 | BACH2 | 0.447 | 0.450 | 0.425 | 0.412 | 0.456 | 0.457 | 0.447 |
| rs9388489 | C6orf173 | 0.466 | 0.479 | 0.483 | 0.461 | 0.481 | 0.486 | 0.444 |
| rs6920220 | TNFAIP3 | 0.187 | 0.195 | 0.163 | 0.229 | 0.183 | 0.204 | 0.165 |
| rs1738074 | TAGAP | 0.383 | 0.389 | 0.451 | 0.425 | 0.393 | 0.445 | 0.344 |
| rs7804356 | SCAP2 | 0.225 | 0.234 | 0.244 | 0.231 | 0.230 | 0.231 | 0.189 |
| rs4948088 | COBL | 0.042 | 0.040 | 0.038 | 0.044 | 0.038 | 0.042 | 0.038 |
| rs7020673 | GLIS3 | 0.469 | 0.468 | 0.439 | 0.496 | 0.465 | 0.471 | 0.468 |
| rs12722495 | IL2RA | 0.100 | 0.094 | 0.074 | 0.099 | 0.099 | 0.084 | 0.092 |
| rs947474 | PRKCQ | 0.194 | 0.199 | 0.194 | 0.168 | 0.195 | 0.197 | 0.191 |
| rs10509540 | RNLS/C10orf59 | 0.267 | 0.267 | 0.267 | 0.302 | 0.267 | 0.259 | 0.266 |
| rs1004446 | INS | 0.357 | 0.358 | 0.385 | 0.332 | 0.350 | 0.380 | 0.351 |
| rs4763879 | CD69 | 0.381 | 0.364 | 0.332 | 0.370 | 0.368 | 0.355 | 0.380 |
| rs2292239 | ERBB3 | 0.309 | 0.305 | 0.275 | 0.341 | 0.309 | 0.324 | 0.300 |
| rs3184504 | SH2B3 | 0.496 | 0.477 | 0.442 | 0.448 | 0.483 | 0.403 | 0.496 |
| rs1465788 | ZFP36L1 | 0.265 | 0.272 | 0.244 | 0.258 | 0.267 | 0.282 | 0.257 |
| rs17574546 | RASGRP1 | 0.180 | 0.177 | 0.166 | 0.198 | 0.180 | 0.179 | 0.176 |
| rs3825932 | CTSH | 0.327 | 0.327 | 0.373 | 0.326 | 0.332 | 0.362 | 0.312 |
| rs12708716 | CLEC16A | 0.357 | 0.345 | 0.387 | 0.324 | 0.367 | 0.364 | 0.345 |
| rs4788084 | IL27 | 0.442 | 0.417 | 0.383 | 0.435 | 0.411 | 0.390 | 0.472 |
| rs7202877 | CTRB2 | 0.116 | 0.100 | 0.093 | 0.126 | 0.106 | 0.091 | 0.127 |
| rs2290400 | ORMDL3 | 0.494 | 0.489 | 0.448 | 0.463 | 0.488 | 0.491 | 0.468 |
| rs7221109 | CCR7 | 0.406 | 0.384 | 0.368 | 0.387 | 0.405 | 0.354 | 0.434 |
| rs45450798 | PTPN2 | 0.149 | 0.147 | 0.159 | 0.145 | 0.144 | 0.157 | 0.138 |
| rs763361 | CD226 | 0.482 | 0.477 | 0.500 | 0.480 | 0.488 | 0.499 | 0.444 |
| rs425105 | PRKD2 | 0.160 | 0.156 | 0.136 | 0.150 | 0.159 | 0.173 | 0.172 |
| rs2281808 | SIRPG | 0.327 | 0.313 | 0.316 | 0.324 | 0.319 | 0.318 | 0.333 |
| rs3788013 | UBASH3a | 0.410 | 0.409 | 0.386 | 0.418 | 0.412 | 0.423 | 0.388 |
| rs5753037 | RPS3AP51 | 0.353 | 0.361 | 0.366 | 0.361 | 0.353 | 0.339 | 0.324 |
| rs229541 | IL2B | 0.423 | 0.425 | 0.430 | 0.413 | 0.432 | 0.451 | 0.400 |
| rs5979785 | TLR8 | 0.266 | 0.287 | 0.296 | 0.263 | 0.283 | 0.299 | 0.232 |
| rs2664170 | GAB3 | 0.307 | 0.318 | 0.346 | 0.307 | 0.307 | 0.339 | 0.307 |
| Genetic risk score (median) | 10.58 | 10.65 | 10.22 | 10.96 | 10.54 | 10.56 | 10.57 | |
Abbreviation: SNPs, single‐nucleotide polymorphisms.
6. SIGNIFICANCE AND NOVELTY
The GPPAD‐02 study will provide a unique population‐based path to primary prevention of beta‐cell autoimmunity. Participation in GPPAD‐02 also provides a platform to enable assessment of interventions for secondary prevention of T1D if children develop beta‐cell autoantibodies in the course of the prevention or during natural follow‐up. GPPAD and the GPPAD‐02 study will, therefore, closely collaborate with INNODIA (http://www.innodia.eu), TrialNet (http://www.diabetestrialnet.org), and others for such opportunities. The eventual benefit to the community, if successful, will be a reduction in the number of children developing T1D.
Supporting information
Supplemental Table 1 List of SNPs determined GPPAD‐02 and risk score calculation.
ACKNOWLEDGEMENTS
The GPPAD‐02 study is financed by research grants from the Leona M. and Harry B. Helmsley Charitable Trust (Grant Numbers 2018PG‐T1D022, 2018PG‐T1D024, 2018PG‐T1D025). The GPPAD coordinating center is financed by the Leona M. and Harry B. Helmsley Charitable Trust (Grant Number 2018PG‐T1D022) as well as the Helmholtz Zentrum Munich German Research Center for Environmental Health and funding from the German Federal Ministry of Education and Research (BMBF) to the German Center for Diabetes Research (DZD e.V.)
Appendix A 1.
GPPAD STUDY GROUP
GPPAD‐Coordinating Center (CC)
Melanie Gündert1, Stefanie Arnolds1, Robin Assfalg1, Corinna Barz1, Karina Blasius1, Cigdem Gezginci1, Cordula Falk1, Joerg Hasford2, Florian Haupt1, Martin Heigermoser1, Bianca Höfelschweiger1, Verena Hoffmann1, Manja Jolink1, Nana Kwarteng1, Ramona Lickert1, Claudia Matzke1, Rebecca Niewöhner1, Michaela Ott1, Peter Ruile1, Marlon Scholz1, Katharina Schütte‐Borkovec1, Mira Taulien1, Lorena Wendel1, Katharina Wystub‐Lis1, José Maria Zapardiel Gonzalo1
1Institute of Diabetes Research, Helmholtz Zentrum München, Neuherberg, Germany
2Institut für Medizinische Informationsverarbeitung, Biometrie und Epidemiologie, Ludwig‐Maximilians‐Universität München, Munich, Germany
Belgium Clinical Center
Kristina Casteels, Goele Smeets, Hilde Morobé, Jasmin Paulus, Renka Van Heyste, Sophie Achten, Emma Larivière, Janne Houben, Lionel Marcelis, Luc Regal
Germany, Dresden Clinical Center
Ezio Bonifacio, Reinhard Berner, Uta Ceglarek, Petrina Delivani, Sevina Dietz, Yannick Fuchs, Gita Gemulla, Manja Gottschalk, Sophie Heinke, Angela Hommel, Anne Karasinsky, Susann Kowal, Fabian Lander, Robert Morgenstern, Katharina Nitzsche, Bianca Schlee, Marina Stopsack, Marc Weigelt, Pauline Wimberger, Marie‐Luise Zielmann, Nicole Zubizarreta
Germany, Hannover Clinical Center
Olga Kordonouri, Torben Biester, Thomas Danne, Nils Janzen, Ute Holtkamp, Karin Lange, Erika Marquardt, Frank Roloff, Kerstin Semler, Thekla von dem Berge
Germany, Munich Clinical Center
Anette G. Ziegler1,2, Peter Achenbach1, Melanie Bunk1, Anita Gavrisan1, Katharina Gestrich1, Willi Grätz1, Pascale Heim‐Ohmayer1, Melanie Herbst1, Julia Hirte1, Theresa Faure1, Anna Hofelich1, Evdokia Kalideri1, Cornelia Kraus1, Yvonne Kriesen1, Karin Lange1, Jasmin Ohli1, Claudia Ramminger1, Jennifer Schairer1, Christiane Winkler1, Susanne Wittich1, Stephanie Zillmer1
1Institute of Diabetes Research, Helmholtz Zentrum München, Neuherberg, Germany
2Forschergruppe Diabetes, Klinikum rechts der Isar, Technische Universität München, Medical faculty, Munich, Germany
Poland Clinical Center
Agnieszka Szypowska, Mariusz Ołtarzewski, Sylwia Dybkowska, Katarzyna Dżygało, Lidia Groele, Karolina Dłużniak‐Gołaska, Dorota Owczarek, Katarzyna Popko, Agnieszka Skrobot, Anna Taczanowska, Beata Zduńczyk
Sweden Clinical Center
Helena Elding Larsson, Markus Lundgren, Åke Lernmark, Daniel Agardh, Jeanette Åkerström Kördel, Carin Andrén Aronsson, Rasmus Bennet, Charlotte Brundin, Annika Fors, Lina Fransson, Berglind Jónsdóttir, Ida Jönsson, Zeliha Mestan, Anita Ramelius, Evelyn Tekum Amboh, Carina Törn
UK Clinical Center
Matthew Snape, John A Todd, Owen Bendor‐Samuel, James Bland, Edward Choi, Rachel Craik, Kimberly Davis, Arancha de la Horra, Yama Farooq, Clare Scudder, Ian Smith, Manu Vatish, Louise Willis, Tabitha Wishlade
Winkler C, Haupt F, Heigermoser M, et al. Identification of infants with increased type 1 diabetes genetic risk for enrollment into Primary Prevention Trials—GPPAD‐02 study design and first results. Pediatr Diabetes. 2019;20:720–727. 10.1111/pedi.12870
Funding information Leona M. and Harry B. Helmsley Charitable Trust, Grant/Award Number: 2018PG‐T1D022
REFERENCES
- 1. Patterson CC, Harjutsalo V, Rosenbauer J, et al. Trends and cyclical variation in the incidence of childhood type 1 diabetes in 26 European centres in the 25 year period 1989‐2013: a multicentre prospective registration study. Diabetologia. 2019;62(3):408‐417. [DOI] [PubMed] [Google Scholar]
- 2. Ziegler AG, Rewers M, Simell O, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA. 2013;309:2473‐2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ziegler AG, Nepom GT. Prediction and pathogenesis in type 1 diabetes. Immunity. 2010;32(4):468‐478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bonifacio E. Predicting type 1 diabetes using biomarkers. Diabetes Care. 2015;38(6):989‐996. [DOI] [PubMed] [Google Scholar]
- 5. Bonifacio E, Beyerlein A, Hippich M, et al. Genetic scores to stratify risk of developing multiple islet autoantibodies and type 1 diabetes: a prospective study in children. PLoS Med. 2018;15(4):e1002548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ziegler AG, Bonifacio E. Age‐related islet autoantibody incidence in offspring of patients with type 1 diabetes. Diabetologia. 2012;55:1937‐1943. [DOI] [PubMed] [Google Scholar]
- 7. Parikka V, Näntö‐Salonen K, Saarinen M, et al. Early seroconversion and rapidly increasing autoantibody concentrations predict prepubertal manifestation of type 1 diabetes in children at genetic risk. Diabetologia. 2012;55:1926‐1936. [DOI] [PubMed] [Google Scholar]
- 8. Krischer JP, Lynch KF, Schatz DA, et al. The six‐year incidence of diabetes associated autoantibodies in genetically at‐risk children: the TEDDY Study. Diabetologia. 2015;58(5):980‐987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ilonen J, Hammais A, Laine AP, et al. Patterns of b‐cell autoantibody appearance and genetic associations during the first years of life. Diabetes. 2013;62:3636‐3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Staeva TP, Chatenoud L, Insel R, Atkinson MA. Recent lessons learned from prevention and recent‐onset type 1 diabetes immunotherapy trials. Diabetes. 2013;62(1):9‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bonifacio E, Ziegler AG, Klingensmith G, et al. Effects of high dose oral insulin on immune responses in children at high risk for type 1 diabetes: the Pre‐POINT randomized clinical trial. JAMA. 2015;313(15):1541‐1549. [DOI] [PubMed] [Google Scholar]
- 12. Cooper JD, Smyth DJ, Smiles AM, et al. Meta‐analysis of genome‐wide association study data identifies additional type 1 diabetes risk loci. Nat Genet. 2008;40:1399‐1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lambert AP, Gillespie KM, Thomson G, et al. Absolute risk of childhood‐onset type 1 diabetes defined by human leukocyte antigen class II genotype: a population‐based study in the United Kingdom. J Clin Endocrinol Metab. 2004;89:4037‐4043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1 List of SNPs determined GPPAD‐02 and risk score calculation.


