Abstract
Objective
Relapse of AML after allogeneic hematopoietic stem cell transplantation (HSCT) has a poor prognosis, and standard of care therapy is lacking. Early (<6 months) relapse is associated with dismal outcome, while the majority of relapses occur early after transplantation. A more precise indication which patients could benefit from reinduction therapy is warranted.
Methods
We retrospectively analyzed outcomes of 83 patients with postallogeneic HSCT relapse. Patients were divided based on intention to treat (curative vs supportive care).
Results
Of the 50 patients treated with curative intent, 44% reached complete remission (CR) upon reinduction chemotherapy, and of these patients, 50% survived. Two survivors reached CR after immunotherapy (donor lymphocyte infusion (DLI), without reinduction chemotherapy). Sixty‐nine percent of the survivors had received high‐intensity cytarabine treatment, followed by immunologic consolidation. Relapse <3 months after transplantation was predictive for adverse survival (P = .004), but relapse <6 months was not. In fact, >50% of the survivors had a relapse <6 months.
Conclusion
We confirmed the dismal prognosis of postallogeneic HSCT relapse. Importantly, our data demonstrate that patients fit enough to receive high‐dose chemotherapy, even when relapse occurred <6 months, had the best chance to obtain durable remissions, in particular when immunologic consolidation was performed after reaching CR.
Keywords: acute myeloid leukemia, allogeneic hematopoietic stem cell transplantation, graft‐versus‐leukemia, outcome
1. INTRODUCTION
Allogeneic hematopoietic stem cell transplantation (HSCT) is the preferred treatment for patients with (MRD positive) intermediate‐risk or poor‐risk acute myeloid leukemia (AML) and high‐risk myelodysplastic syndrome (MDS). While transplant related mortality has decreased, the risk for relapse has not. Disease relapse is a common and important cause of the poor long‐term survival of allogeneic HSCT recipients with AML. Relapse accounts for 30%‐40% of deaths after allogeneic HSCT, depending on the type of the donor and disease status at transplant.1, 2 Prognosis of postallogeneic HSCT AML relapse is poor3, 4 and has hardly improved in the past decades. There is no standard of care for patients who relapse after allogeneic HSCT. Age (<37 year) and a longer time (>5 months) between allogeneic HSCT and relapse have been identified as favorable prognostic factors.3, 5, 6 Other factors such as clinical condition and personal considerations of the patient may be weighed in the decision to either give supportive care, offer low‐dose chemotherapy, or attempt high‐dose reinduction chemotherapy. In reality, curative treatment options are often limited, and in many cases, it can be more appropriate to refrain from intensive treatment regimens and opt for best supportive care.
Most AML relapses occur in the first 6 months after allogeneic HSCT, a period during which many patients still receive immunosuppressive therapy. Rapid tapering of immunosuppression can potentially lead to the initiation of a graft‐versus‐leukemia (GvL) effect and remission of leukemia.7, 8, 9 When patients relapse after cessation of immunosuppressive therapy or when tapering of immunosuppression does not result in remission, hypomethylating therapy such as azacitidine or decitabine may be used to induce remission. Hypomethylating agents have, apart from their direct anti‐leukemic effects, immunomodulatory properties,10, 11 for example through natural killer (NK) cells, potentially augmenting GvL responses. In a small group of patients with AML or MDS relapse after allogeneic HSCT, azacitidine induced complete remission, and about 50% of these patients had prolonged (>2 years) disease‐free survival.12 If the clinical condition of the patient allows, high‐dose chemotherapy can be tried in order to achieve complete remission.1, 13 The decision of physician and patient to treat or refrain from treatment is a difficult consideration between chance of cure with the risk of severe debilitating side effects and indivertible death in the setting of end of life care that is aimed at maximizing comfort.
Complete remission after reinduction chemotherapy can be consolidated by (re‐)inducing a GvL response, most often via donor lymphocyte infusions (DLI). DLI can be very effective in inducing lasting GvL responses after postallogeneic HSCT AML relapse.3, 14 However, in case of cord blood transplantation DLI is unavailable, and not all patients are eligible for DLI as it may re‐induce or exacerbate graft‐versus‐host disease (GvHD). In case of an early detected molecular or cytogenetic relapse, DLI can be performed without reinduction therapy. Alternatively, a second allogeneic HSCT with a new donor can be performed. DLI or a second allogeneic HSCT is most successful in female patients with a late relapse (>6 months) of cytogenetically favorable AML and when DLI is given once complete remission is achieved.1, 2, 3, 14
In this study, we retrospectively analyzed outcomes of patients with postallogeneic HSCT AML relapse, in whom the decision to offer curative or best supportive care therapy was based on factors described above, a per‐patient analysis of physical fitness and comorbidity, and individual considerations of the patient.
2. PATIENTS AND METHODS
2.1. Patients
The medical records of all 83 adult patients (>17 years of age) with myeloid malignancies who had received an allogeneic HSCT at the Amsterdam University Medical Centers between January 1, 2010, and December 31, 2016, and in whom disease relapse occurred before December 31, 2017, were reviewed. Eighty of these patients were diagnosed with AML, and three patients underwent allogeneic HSCT for chronic myelomonocytic leukemia (CMMoL) or high‐risk myelodysplastic syndrome (MDS with excess of blasts (EB) II). Patients were classified according to the WHO Classification of myeloid neoplasms and acute leukemia 2008 (hereafter WHO 2008 Classification).15 Risk was defined according to the risk group classification used in the then active HOVON 92, HOVON 102, and HOVON 132 trials (Table S1) in which most patients participated. The few patients not participating in these trials were classified and treated according these trials. This prognostic classification is based on known risk factors with respect to cytogenetic abnormalities and molecular alterations, white blood cell count (WBC), and attainment of early complete remission. The cytogenetic abnormalities and molecular alterations that were used for the risk assessment of these patients are in line with the prognostic risk groups of the European Leukemia Net 2010.16 Six patients received one or more prophylactic donor lymphocyte infusions (DLI) after allogeneic HSCT, before relapse. Immunologic consolidation with consecutive DLI was started at least 6 weeks after recovery form chemotherapy‐induced neutropenia. In accordance with the Declaration of Helsinki, written informed consent was obtained from all patients.
2.2. Statistical analysis
The collected data were coded and anonymously processed. The analyses were done using IBM SPSS Statistics 24.0.0.1 software. Patient characteristics were compared using Mann‐Whitney U tests in case of continuous variable, and the chi‐square test or Fisher's exact test were used to compare categorical variables. For the ordinal variable prognostic class, both Pearson's r and Kendall's tau were determined. Survival differences were evaluated using Kaplan‐Meier analysis and compared by using the log‐rank test.
3. RESULTS
3.1. Patient characteristics
Demographics of the 83 patients who had received an allogeneic HSCT for AML or high‐risk MDS between January 1, 2010 and December 31, 2016, and relapsed before December 31, 2017, are shown in Table 1. Three patients (4%) who had been initially classified as good risk had received an allogeneic HSCT because of relapsed AML. Four of the patients had an intracranial or leptomeningeal localization of their relapse, and no AML blasts in the bone marrow. Median time of follow‐up for survivors was 220 weeks, ranging from 49 to 391 weeks.
Table 1.
Patient characteristics
| Characteristic | All patients (N = 83) |
|---|---|
| Outcome—no. (%) | |
| Deceased | 70 (84) |
| Survive | 13 (16) |
| Sex—no. (%) | |
| Male | 46 (55) |
| Female | 37 (45) |
| Age at time of allogeneic HSCT—y | |
| Median (interquartile range) | 54 (47‐62) |
| Range | 18‐71 |
| Prognostic class at time of AML diagnosis—no. (%) | |
| Good risk | 3 (4) |
| Intermediate risk | 19 (23) |
| Poor risk | 35 (42) |
| Very poor risk | 26 (31) |
| WHO classification—no. (%) | |
| AML with recurrent genetic abnormalities | 33 (40) |
| AML with myelodysplasia‐related changes | 9 (11) |
| Therapy‐related myeloid neoplasms | 6 (7) |
| AML NOS | 32 (39) |
| Other | 3 (4) |
| HSCT donor—no. (%) | |
| MUD (10/10) | 53 (64) |
| SIB | 28 (34) |
| CB | 2 (2) |
| Conditioning regimen—no. (%) | |
| Myeloablative | 8 (10) |
| Reduced intensity | 71 (85) |
| FLAMSA | 4 (5) |
| Bone marrow blast count at time of relapse—% (N = 74) | |
| Median (interquartile range) | 25 (14‐52.25) |
| Range | 0†‐99 |
| Time between allogeneic HSCT and relapse—wk | |
| Median (interquartile range) | 21 (12‐32) |
| Range | 3‐187 |
| Very early vs late relapse—no. (%) | |
| Very early (<3 mo) | 23 (28) |
| Late (≥3 mo) | 60 (72) |
| Early vs late relapse—no. (%) | |
| Early (<6 mo) | 52 (63) |
| Late (≥6 mo) | 31 (37) |
| Intention to treat—no. (%) | |
| Curative | 50 (60) |
| Palliative (best supportive care) | 33 (40) |
Four patients had an isolated central nervous system relapse.
3.2. Curative treatment vs best supportive care
Patients were classified into two groups: patients who were treated with curative intent (CIT group) and patients who refrained from curative treatment and received best supportive care either at their own request or as advised by the treating physician (BSC group). Treatment with curative intent was considered in all patients that were fit enough according to the treating physician, in particular when relapse occurred more than 6 months after allogeneic HSCT. Despite the fact that relapse less than 6 months after allogeneic HSCT is considered a very poor prognostic factor, 11 patients with such an early relapse received reinduction chemotherapy, in most cases because of young age, excellent clinical condition, and very high motivation of the patient. The decision to treat or to refrain from curative treatment was always made after extensive elaboration between physicians and in close consultation with the patient and his/her family.
Fifty (60%) of the patients were treated with curative intent of which 13 (26%) were still alive at the end of follow‐up (16% of all patients in this study; Table 2). Patients in the CIT group were younger than BSC patients (median 53 vs 58 years, P = .043), had significantly lower bone marrow blast counts (median 21% vs 38%, P = .025), and had lower cytogenetical/molecular risk characteristics (r = .226; P = .040; Table 2). When excluding the relapses that were restricted to the central nervous system, no significant difference in bone marrow blast count was observed between the BSC and CIT groups (median 38% vs 24%, P = .082). No differences could be assessed for the WHO 2008 Classification as the subgroups were too small to properly evaluate. The time between allogeneic HSCT and relapse ranged from 3 to 63 weeks for the BSC group and 5‐187 weeks for the CIT group. Overall, prognosis was very poor, with a 2‐year survival of 17% for the whole group (Figure 1). Median time of survival was 16 weeks for the CIT group and only 3 weeks for the best supportive care group (Table 2).
Table 2.
Patient characteristics of best supportive care and curative‐intent groups
| Characteristic | Curative (n = 50) | Best supportive care (n = 33) | |
|---|---|---|---|
| Outcome—no. (%) | |||
| Deceased | 37 (74) | 33 (100) | |
| Survived | 13 (26) | 0 (0) | |
| Sex—no. (%) | |||
| Male | 29 (58) | 17 (48.5) | P = .561 |
| Female | 21 (42) | 16 (51.5) | |
| Age at time of allogeneic HSCT—y | |||
| Median (interquartile range) | 53 (39‐58) | 58 (50‐64) | P = .043 |
| Range | 18‐71 | 26‐68 | |
| Prognostic class at time of AML diagnosis—no. (%) | |||
| Good risk | 2 (4) | 1 (3) | Pearson's r = .226; P = .040 |
| Intermediate risk | 15 (30) | 4 (12) | |
| Poor risk | 21 (42) | 14 (42) | |
| Very poor risk | 12 (24) | 14 (42) | |
| WHO classification—no. (%) | |||
| AML with recurrent genetic abnormalities | 19 (38) | 14 (42) | |
| AML with myelodysplasia‐related changes | 5 (10) | 4 (12) | |
| Therapy‐related myeloid neoplasms | 3 (6) | 3 (9) | |
| AML NOS | 21 (42) | 11 (33) | |
| Other | 2 (4) | 1 (3) | |
| Transplantation type—no. (%) | |||
| MUD (10/10) | 31 (62) | 22 (67) | |
| SIB | 8 (36) | 10 (30) | |
| CB | 1 (2) | 1 (3) | |
| Conditioning regimen—no. (%) | |||
| Myeloablative | 7 (14) | 1 (3) | P = .137 |
| Reduced intensity or FLAMSA | 43 (86) | 32 (97) | |
| Bone marrow blast count at time of relapse—% (n = 74) | |||
| Median (interquartile range) | 21 (10‐50) | 38 (20‐67) | P = .025† |
| Range | 0‐74 | 10‐99 | |
| No data | 4 | 5 | |
| Time between allogeneic HSCT and relapse—wk | |||
| Median (interquartile range) | 21 (14‐47) | 21 (6‐28) | P = .047 |
| Range | 5‐187 | 3‐63 | |
| Early vs late relapse—no. (%) | |||
| Early (<3 mo) | 11 (22) | 12 (36) | P = .119 |
| Late (≥3 mo) | 39 (78) | 21 (64) | |
| Time between relapse and death—wk | |||
| Median (interquartile range) | 16 (6‐31) | 3 (1‐10) | P < .001 |
| Range | 2‐119 | 0‐37 | |
When n = 4 patients with isolated CNS relapse are excluded, the difference in bone marrow blast counts between the two groups is not significant.
Figure 1.
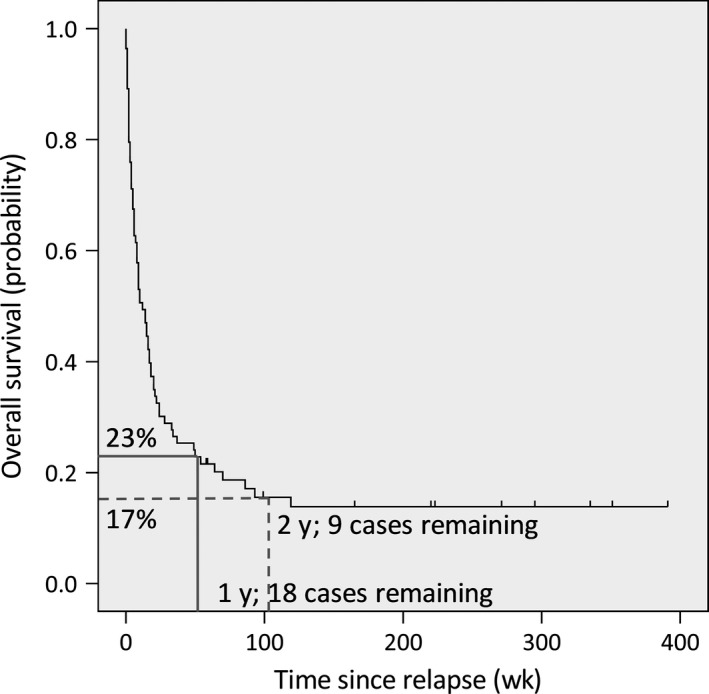
Overall survival. Survival curve of the entire patient cohort (n = 83). One‐year survival was 23%, and 2‐y survival was 17%
In the CIT group, two patients (4%) with low blast counts (8% and 10%, respectively) received DLI without reinduction therapy, in 23 patients (46%) immunosuppressants were tapered and stopped, 10 patients (20%) were treated with a hypomethylating agent, of whom 3 (6%) received this agent as single treatment, and 22 patients (44%) were treated with high‐dose chemotherapy. High‐dose chemotherapy consisted of high‐dose cytarabine in the majority of cases, and FLAG (fludarabine, cytarabine, G‐CSF) or other regimens in the other patients (Table S2). Four patients had an isolated central nervous system relapse with intracranial chloroma or leptomeningeal AML localization. These patients were in the CIT group and received intrathecal cytarabine, in some combined with systemic cytarabine, intrathecal methotrexate, or radiotherapy. Two of these patients attained complete remission and were alive at 6.5 and 7.5 years after their relapse. DLI is given as consolidation therapy to those patients that obtained complete remission. Most patients that did not receive consolidation with DLI had rapid progression of the disease and/or failing of reinduction therapy. For 2 patients, the donor was not (cord blood HSCT) or no longer available; the rest of the patients did not receive DLI because of GvHD.
3.3. Determinants of survival
The CIT group was then split into survivors and non‐survivors, as per end of follow‐up (December 31, 2017), in order to identify prognostic relevant differences between these two groups (Figure 2). In one patient, AML relapsed after she had obtained complete remission following reinduction therapy with curative intent (tapering of immunosuppressants followed by hypomethylating therapy). This patient was still alive but with active AML at the end of follow‐up and therefore evaluated in the survivor group (Table S2 , patient ID_030). There was no difference in age, type of transplantation, conditioning regimen, bone marrow blast percentage at time of relapse or the time between transplantation, and relapse between survivors and non‐survivors. However, survivors had more often received high‐dose reinduction chemotherapy (69% vs 35%, P = .035) and immunologic consolidation therapy (69% vs 22%, P = .003; Table 3). In addition, survivors had more often reached complete remission after reinduction therapy, whereas this was only the case for a minority of the non‐survivors (85% vs 30%, P = .001). Together, the CR rate after reinduction therapy was 22/50 (44%), of whom 10 survived without relapse (Figure 2), which is in line with published studies.3, 4, 12, 17 Five patients received immunologic consolidation therapy (four patients DLI, one patient second allogeneic HSCT), despite not obtaining complete remission, and two of them survived (one who received DLI and the patient who received 2nd allogeneic HSCT). Estimated median survival time of patients who did not reach complete remission upon reinduction therapy was 9 weeks vs 119 weeks for the patients that did (Figure 3A). Survival was significantly better for the patients who relapsed more than 3 months after allogeneic HSCT compared with those who relapsed within 3 months after allogeneic HSCT (P = .004; Figure 3B). When relapse within 6 months after allogeneic HSCT vs after 6 months was taken as a cutoff, no significant difference in survival was observed. In fact, 7 of the 12 disease‐free survivors had received reinduction therapy despite early (<6 months after allogeneic HSCT) relapse (Table S2). The size of the patient group did not allow for outcome analyses of molecular and cytogenetic risk groups.
Figure 2.
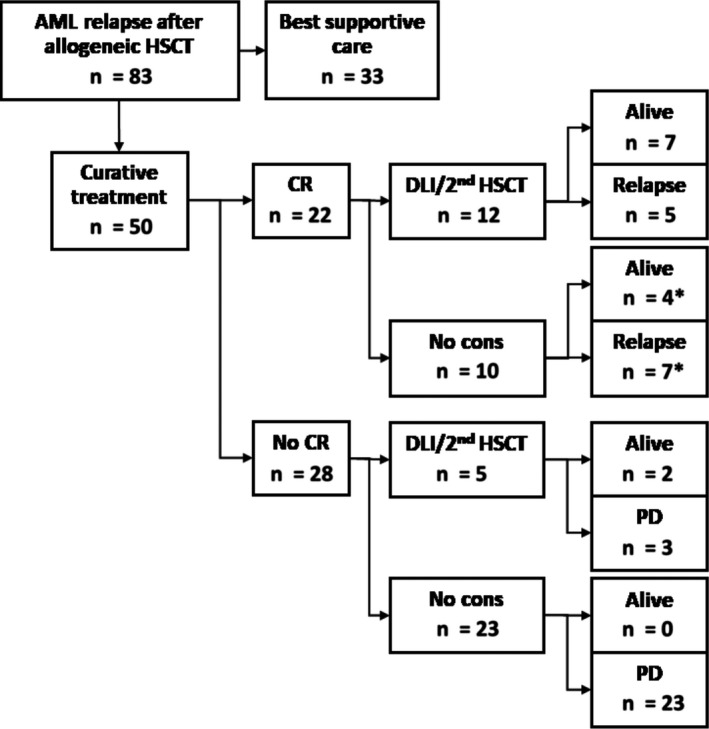
Curative‐intent group. Representation of patients in the curative‐intent treatment group divided into patients reaching complete remission or not. *Group containing one patient who was alive at the end of follow‐up despite relapse after re‐obtaining complete remission. CR, complete remission; No cons: no immunologic consolidation; PD, progressive disease
Table 3.
Patient characteristics of survivors and non‐survivors in the curative‐intent group
| Characteristic | Non‐survivors (n = 37) | Survivors (n = 13) | |
|---|---|---|---|
| Sex—no. (%) | |||
| Male | 21 (57) | 8 (62) | P = .764 |
| Female | 16 (43) | 5 (39) | |
| Age at time of allogeneic HSCT—y | |||
| Median (interquartile range) | 53 (39‐60) | 53 (36‐59) | P = .674 |
| Range | 18‐71 | 22‐62 | |
| Age at time of relapse—y | |||
| Median (interquartile range) | 53 (40‐60) | 53 (38‐60) | P = .707 |
| Range | 19‐74 | 22‐62 | |
| Bone marrow blast count at time of relapse—% (n = 46) | |||
| Median (interquartile range) | 20 (14‐51) | 22 (8‐49) | P = .745 |
| Range | 1‐72 | 0‐74 | |
| No data | 3 | 1 | |
| Reinduction therapy—no. (%) | |||
| No reinduction therapy | 1 (3) | 1 (8) | |
| Hypomethylating therapy only | 3 (8) | 0 (0) | |
| High‐dose chemotherapy | 13 (35) | 9 (69) | |
| Reduce/stop immunosuppressants | 20 (54) | 3 (23) | |
| Reinduction therapy—no. (%) | |||
| No/low‐intensity therapy | 24 (65) | 4 (31) | P = .035 |
| High‐intensity | 13 (35) | 9 (69) | |
| Outcome after reinduction therapy—no. (%) | |||
| CR | 11 (30) | 11 (85) | P = .001 |
| No CR | 26 (70) | 2 (15) | |
| Hypomethylating agents—no. (%) | |||
| Yes | 7 (19) | 3 (23) | P = .533 |
| No | 29 (81) | 10 (77) | |
| No data | 1 | ||
| Consolidation therapy | |||
| No consolidation therapy | 29 (78) | 4 (31) | P = .003 |
| Consolidation therapy | 8 (22) | 9† (69) | |
| Time between HSCT and relapse—wk | |||
| Median (interquartile range) | 21 (12‐46) | 21 (17‐59) | P = .479 |
| Range | 5‐142 | 11‐187 | |
| Early vs late relapse—no. (%) | |||
| Early (<3 mo) | 10 (27) | 1 (8) | P = .144 |
| Late (≥3 mo) | 27 (73) | 12 (92) | |
Two received a second allogeneic HSCT with a different donor, the other patients DLI.
Figure 3.
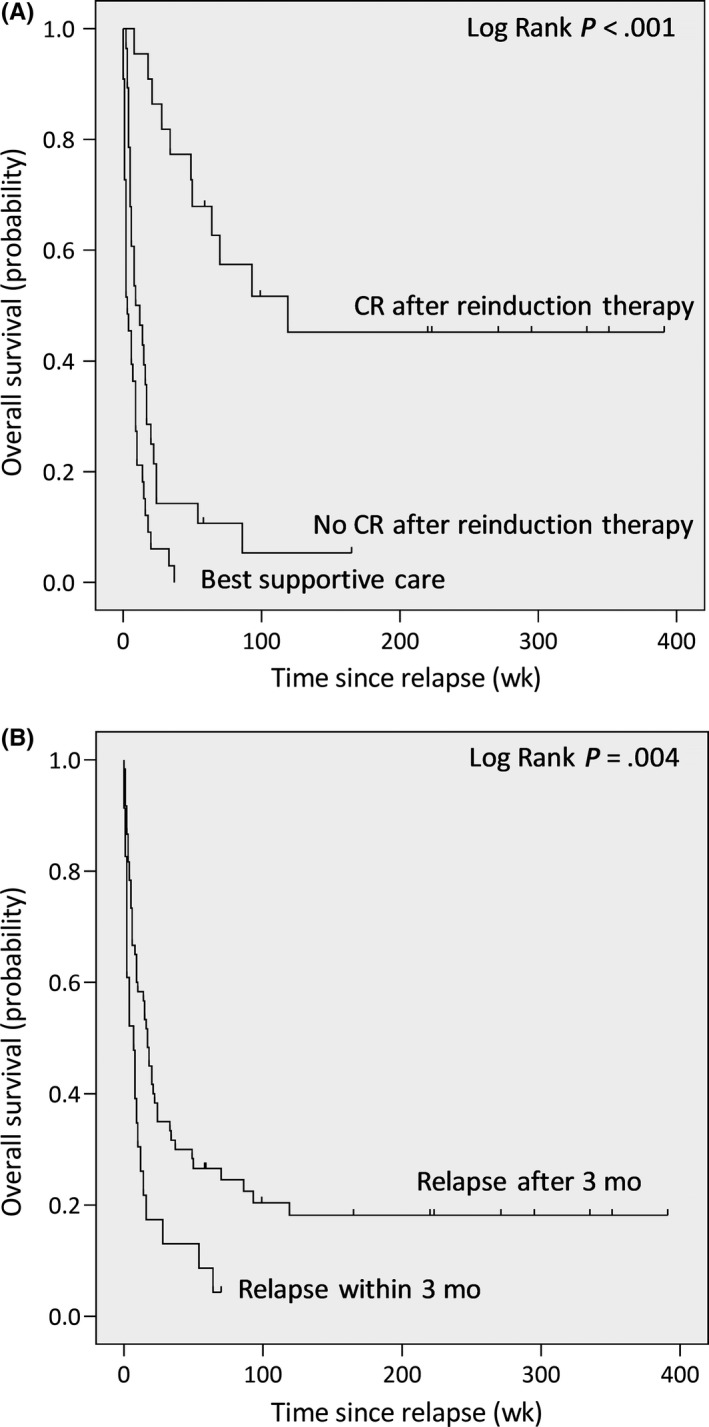
Survival of best supportive care and curative‐intent groups, and of early vs late relapse. A, The curative‐intent group was grouped into patients that did and did not reach complete remission (CR) after reinduction therapy. Patients treated with curative intent, who reached CR after reinduction therapy, have significantly better survival than the two other groups. B, Survival curves for patients with early vs late relapse with early relapse defined as relapse within 3 mo after allogeneic HSCT
4. DISCUSSION
Patients with relapsed AML or MDS after allogeneic HSCT have a dismal prognosis. It remains a challenge to identify those patients that may benefit from curative‐intent salvage therapy when best supportive care in reality is probably most appropriate for the majority of patients. Selection of patients for either best supportive care or curative‐intent therapy is generally based on the physician's assessment of the patient's chances of survival, his/her fitness, and the wish of the patient. Predictors for survival, as described in literature, including time from allogeneic HSCT to relapse, patient age and sex, cytogenetics, blast count at diagnosis of relapse, and remission status at immunologic consolidation (DLI or 2nd allogeneic HSCT) are also taken into consideration.3, 4, 12, 14, 18, 19, 20, 21, 22, 23 In this retrospective analysis of a non‐preselected patient group with relapse of AML or high‐risk MDS after allogeneic HSCT, we attempted to identify factors that should be taken into account when considering treatment options for individual patients. We could confirm the very poor prognosis of relapsed AML after allogeneic HSCT. The 2‐year survival of only 17% in patients with relapsed AML after allogeneic HSCT is in line with other studies (Figure 1).17, 18 Outcome was best for those patients who were fit enough to receive high‐dose reinduction therapy followed by immunologic consolidation therapy with DLI or 2nd allogeneic HSCT in complete remission. We confirmed previous reports showing that time between allogeneic HSCT and relapse was significantly correlated with survival, with very early relapses (<3 months) having a dismal prognosis.3, 4, 19, 20 Most importantly, we found that early relapse, between 3 and 6 months after allogeneic HSCT, did not have a worse prognosis compared with relapse >6 months after allogeneic HSCT. This finding is of clinical significance, as the majority of relapses occurs within 6 months after allogeneic HSCT. Given that 2nd relapses may occur late, but always sooner than the first relapse, it is important to have a sufficient period of follow‐up in order to identify the long‐term survivors. With a median time of follow‐up of over 4 years, which was longer than the initial time period between allogeneic HSCT and relapse in all but one patient, we are confident that the patients that we qualified as non‐relapse survivors are likely to be cured.
Previous analyses on survival after relapse focused on patients treated with curative intent only, inherently containing the risk of selection bias. In our study, we included all patients with relapsed AML or high‐risk MDS after allogeneic HSCT in our center. Comparison of the CIT and BSC groups, defined based on intention to treat, revealed that patients who according to their physicians did not qualify for curative‐intent therapy or choose to refrain from intensive therapy (the latter being a minority of cases) were a little older (53 [range 18‐71] vs 58 [26‐68] years, P = .04) and had higher rates of very poor‐risk AML at primary diagnosis (Table 2). Age was not significantly different between survivors and non‐survivors of patients treated with curative intent, while the curative‐intent group had a higher age range. Blast count at diagnosis of relapse was not significantly associated with outcome within the CIT patient group, and due to the size of the patient group, we could not correlate cytogenetics with survival. The absence of a correlation between survival and age or bone marrow blast count is in contrast to other studies3, 20 and may be related to the lower number of patients in our study. The majority of patients received reduced‐intensity conditioning (RIC). Nevertheless, survivor and non‐survivor groups in our analysis both contained young and old patients, with low and higher amounts of bone marrow blasts. Together, these data suggest that fitness rather than age, blast count, or time of relapse (except when <3 months after allogeneic HSCT) should be taken into account when considering patients for remission reinduction therapy.
We confirm previous reports demonstrating that re‐obtaining complete remission is an important prognostic determinant.3, 4, 20, 21, 22, 24 Only 2 of the 28 patients that did not reach complete remission after curative‐intent therapy survived (Figure 2 and Table 3), while 85% of the survivors had obtained complete remission after reinduction therapy. Remission can be induced using different strategies. The strength of the GvL effect is underlined by the observation that cessation of immunosuppressants alone (without subsequent hypomethylating agents or high‐dose chemotherapy) led to remission of AML in 5 out of 18 patients (28%). Cessation of immunosuppressants in combination with hypomethylating agents led to CR in two out of five patients. None of the five remaining patients receiving hypomethylating therapy (stand‐alone or in combination with other therapies) obtained complete remission. These results are in line with the 15%‐19% complete remission rates reported after hypomethylating therapy in literature.12, 25 In a direct (retrospective) comparison, high‐dose chemotherapy was more effective than reinduction therapy with hypomethylating agents, inducing complete remission in 40% vs 7% of patients, respectively.26 Also in our cohort, high‐dose therapy was most successful in inducing complete remission, with 75% of patients obtaining complete remission following high‐dose reinduction chemotherapy. It should be noted that the patients treated with these more intensive salvage therapies were the fittest patients with initially the best estimated prognosis, most likely contributing to the better outcome of these patients.
Equally important and challenging is the maintenance of complete remission. Of the 12 patients who after obtaining complete remission received immunological consolidation, most often DLI, and in one case second allogeneic HSCT, seven patients survived (58%). In contrast, only 30% of the patients who reached CR, but, due to varying reasons, could not proceed to immunologic consolidation, survived (Figure 2). AML relapse after allogeneic HSCT is associated with a dysregulation in immune function pathways such as HLA expression by AML blasts that may help AML cells evade donor immune responses.27 Immunologic consolidation can be effective when newly infused donor immune cells that are naive with regard to the patient's AML elicit a GvL response independent of HLA‐related antigen presentation, for example, to targets that are expressed on the membrane of AML blasts.28, 29 Thus, as also suggested by other studies, immunological consolidation is of importance for survival in this setting. GvHD occurred in some but not all surviving patients suggesting that immunologic consolidation can also be effective when it does not lead to GvHD.3
With new therapies for AML emerging, the arsenal to treat AML relapse after allogeneic HSCT is expanding. The Bcl2 inhibitor Venetoclax in combination with hypomethylating agents has shown promising results in patients with relapsed or refractory AML.30 A wide range of mutation‐targeting agents is available, most of them tyrosine‐kinase inhibitors directed against FLT3.31 Adoptive transfer of natural killer (NK) cells and T cells targeting AML‐specific antigens are under investigation, and chimeric antigen receptor (CAR) T cells and bispecific T‐cell engagers targeting CD33 and CD123 are being developed.13, 32 Prospective clinical trials are required to investigate the potential of these novel therapies in the treatment of AML relapse after allogeneic HSCT.
Taken together, while relapsed AML and MDS after allogeneic HSCT have a dismal prognosis, a subset of patients may benefit from curative‐intent therapy. Selection of candidates remains a challenge and should be based on patient's fitness as determined by the team of treating physicians and on the motivation of the patient. In patients deemed fit, reinduction therapy may offer a prospect for cure. Our data suggest that more patients might be eligible for intensive reinduction treatment than previously assumed as time between transplantation and relapse should only be taken into account in case of very early relapses, for example, <3 months after allogeneic HSCT, in which case outcome is dismal. Reinduction therapy that fails may prolong life expectancy but most often at the expense of quality of life. If complete remission is obtained, however, chances for cure improve significantly, in particular when immunologic consolidation therapy to reinvigorate or redirect GvL responses is given.
CONFLICT OF INTEREST
None.
Supporting information
ACKNOWLEDGEMENTS
GdJ is financially supported by the Dutch Cancer Society (KWF 2014‐6557). MDH is supported by a VIDI grant (NWO ZonMW 91715362) and a LSBR Fellowship (1438F).
de Jong G, Janssen JJWM, Biemond BJ, et al. Survival of early posthematopoietic stem cell transplantation relapse of myeloid malignancies. Eur J Haematol. 2019;103:491–499. 10.1111/ejh.13315
REFERENCES
- 1. Fathi AT, Bin CY. Treatment of relapse of acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation. Curr Hematol Malig Rep. 2014;9(2):186‐192. [DOI] [PubMed] [Google Scholar]
- 2. van den Brink M, Porter DL, Giralt S, et al. Relapse after allogeneic hematopoietic cell therapy. Biol Blood Marrow Transplant. 2010;16(1):S138‐S145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schmid C, Labopin M, Nagler A, et al. Donor lymphocyte infusion in the treatment of first hematological relapse after allogeneic stem‐cell transplantation in adults with acute myeloid leukemia: a retrospective risk factors analysis and comparison with other strategies by the EBMT Acute Leukem. J Clin Oncol. 2007;25(31):4938‐4945. [DOI] [PubMed] [Google Scholar]
- 4. Kharfan‐Dabaja MA, Labopin M, Polge E, et al. Association of second allogeneic hematopoietic cell transplant vs donor lymphocyte infusion with overall survival in patients with acute myeloid leukemia relapse. JAMA Oncol. 2018;4(9):1245‐1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schmid C, Labopin M, Nagler A, et al. Treatment, risk factors, and outcome of adults with relapsed AML after reduced intensity conditioning for allogeneic stem cell transplantation. Blood. 2012;119(6):1599‐1606. [DOI] [PubMed] [Google Scholar]
- 6. Warlick ED, DeFor T, Blazar BR, et al. Successful remission rates and survival after lymphodepleting chemotherapy for relapsed hematologic malignancies postallogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18(3):480‐486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Elmaagacli A, Beelen DW, Trenn G, Schmidt O, Nahler M, Schaefer UW. Induction of a graft‐versus‐leukemia reaction by cyclosporin A withdrawal as immunotherapy for leukemia relapsing after allogeneic bone marrow transplantation. Bone Marrow Transplant. 1999;23(8):771‐777. [DOI] [PubMed] [Google Scholar]
- 8. Gillissen MA, De Jong G, Levie SE, et al. AML relapse after rituximab treatment for GvHD: crucial role for B cells in GvL responses. Bone Marrow Transplant. 2016;51(9):1245‐1248. [DOI] [PubMed] [Google Scholar]
- 9. Yang J, Cai YU, Jiang JieLing, et al. Early tapering of immunosuppressive agents after HLA‐matched donor transplantation can improve the survival of patients with advanced acute myeloid leukemia. Ann Hematol. 2018;97(3):497‐507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kopp LM, Ray A, Denman CJ, et al. Decitabine has a biphasic effect on natural killer cell viability, phenotype, and function under proliferative conditions. Mol Immunol. 2013;54:296‐301. [DOI] [PubMed] [Google Scholar]
- 11. Cany J, Roeven M, Evert J, et al. Decitabine enhances targeting of AML cells by CD34 1 progenitor‐derived NK cells in NOD / SCID / IL2Rg null mice. Blood. 2018;131(2):202‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Craddock C, Labopin M, Robin M, et al. Clinical activity of azacitidine in patients who relapse after allogeneic stem cell transplantation for acute myeloid leukemia. Haematologica. 2016;101(7):879‐883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee CJ, Savani BN, Mohty M, et al. Post‐remission strategies for the prevention of relapse following allogeneic hematopoietic cell transplantation for high‐risk acute myeloid leukemia: expert review from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transpla. Bone Marrow Transplant. 2018;13(9):579. [DOI] [PubMed] [Google Scholar]
- 14. Ossenkoppele GJ, Janssen J, van de Loosdrecht AA. Risk factors for relapse after allogeneic transplantation in acute myeloid leukemia. Haematologica. 2016;101(1):20‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937‐951. [DOI] [PubMed] [Google Scholar]
- 16. Mrózek K, Marcucci G, Nicolet D, et al. Prognostic significance of the European LeukemiaNet standardized system for reporting cytogenetic and molecular alterations in adults with acute myeloid leukemia. J Clin Oncol. 2012;30(36):4515‐4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pollyea DA, Artz AS, Stock W, et al. Outcomes of patients with AML and MDS who relapse or progress after reduced intensity allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2007;40(11):1027‐1032. [DOI] [PubMed] [Google Scholar]
- 18. Mielcarek M, Storer BE, Flowers M, Storb R, Sandmaier BM, Martin PJ. Outcomes among patients with recurrent high‐risk hematologic malignancies after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2007;13(10):1160‐1168. [DOI] [PubMed] [Google Scholar]
- 19. Bejanyan N, Weisdorf DJ, Logan BR, et al. Survival of patients with acute myeloid leukemia relapsing after allogeneic hematopoietic cell transplantation: a center for international blood and marrow transplant research study. Biol Blood Marrow Transplant. 2015;21(3):454‐459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eapen M, Giralt SA, Horowitz MM, et al. Second transplant for acute and chronic leukemia relapsing after first HLA‐identical sibling transplant. Bone Marrow Transplant. 2004;34(8):721‐727. [DOI] [PubMed] [Google Scholar]
- 21. Christopeit M, Kuss O, Finke J, et al. Second allograft for hematologic relapse of acute leukemia after first allogeneic stem‐cell transplantation from related and unrelated donors: the role of donor change. J Clin Oncol. 2013;31(26):3259‐3271. [DOI] [PubMed] [Google Scholar]
- 22. Orti G, Sanz J, Bermudez A, et al. Outcome of second allogeneic hematopoietic cell transplantation after relapse of myeloid malignancies following allogeneic hematopoietic cell transplantation: a retrospective cohort on behalf of the Grupo Español de Trasplante Hematopoyetico. Biol Blood Marrow Transplant. 2016;22(3):584‐588. [DOI] [PubMed] [Google Scholar]
- 23. Lim A, Curley C, Fong CY, et al. Acute myeloid leukaemia relapsing after allogeneic haemopoietic stem cell transplantation: prognostic factors and impact of initial therapy of relapse. Intern Med J. 2018;48(3):276‐285. [DOI] [PubMed] [Google Scholar]
- 24. Schneidawind C, Hagmaier V, Faul C, Kanz L, Bethge W, Schneidawind D. Second allogeneic hematopoietic cell transplantation enables long‐term disease‐free survival in relapsed acute leukemia. Ann Hematol. 2018;97(12):2491‐2500. [DOI] [PubMed] [Google Scholar]
- 25. Sommer S, Cruijsen M, Claus R, et al. Decitabine in combination with donor lymphocyte infusions can induce remissions in relapsed myeloid malignancies with higher leukemic burden after allogeneic hematopoietic cell transplantation. Leuk Res. 2018;72:20‐26. [DOI] [PubMed] [Google Scholar]
- 26. Motabi IH, Ghobadi A, Liu J, et al. Chemotherapy versus hypomethylating agents for the treatment of relapsed acute myeloid leukemia and myelodysplastic syndrome after allogeneic stem cell transplant. Biol Blood Marrow Transplant. 2016;22(7):1324‐1329. [DOI] [PubMed] [Google Scholar]
- 27. Christopher MJ, Petti AA, Rettig MP, et al. Immune escape of relapsed AML cells after allogeneic transplantation. N Engl J Med. 2018;379(24):2330‐2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gillissen MA, Kedde M, De Jong G, et al. AML‐specific cytotoxic antibodies in patients with durable graft‐versus‐leukemia responses. Blood. 2018;131(1):131‐143. [DOI] [PubMed] [Google Scholar]
- 29. Gillissen MA, de Jong G, Kedde M, et al. Patient‐derived antibody recognizes a unique CD43 epitope expressed on all AML and has antileukemia activity in mice. Blood Adv. 2017;1(19):1551‐1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. DiNardo CD, Rausch CR, Benton C, et al. Clinical experience with the BCL2‐inhibitor venetoclax in combination therapy for relapsed and refractory acute myeloid leukemia and related myeloid malignancies. Am J Hematol. 2018;93(3):401‐407. [DOI] [PubMed] [Google Scholar]
- 31. Desplat V, Villacreces A, Guitart V. Targeting tyrosine kinases in acute myeloid leukemia: why, who and how? Int J Mol Sci. 2019;20(14):3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang H, Kaur G, Sankin AI, Chen F, Guan F, Zang X. Immune checkpoint blockade and CAR‐T cell therapy in hematologic malignancies. J Hematol Oncol. 2019;12(1):1‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


