Summary
Evidence for the health impact of obesity has largely focussed on adults. We estimated the population prevalence and prevalence ratio of obesity‐associated comorbidities in children and adolescents aged 5 to 18 years. Five databases were searched from inception to 14 January 2018. Population‐based observational studies reporting comorbidity prevalence by weight category (healthy weight/overweight/obese) in children and adolescents aged 5 to 18 years from any country were eligible. Comorbidity prevalence, stratified by weight category, was extracted and prevalence ratios (relative to healthy weight) estimated using random effects meta‐analyses. Of 9183 abstracts, 52 eligible studies (1 553 683 participants) reported prevalence of eight comorbidities or risk markers including diabetes and nonalcoholic fatty liver disease (NAFLD). Evidence for psychological comorbidities was lacking. Meta‐analyses suggested prevalence ratio for prediabetes (fasting glucose ≥ 100 mg/dL) for those with obesity relative to those of a healthy weight was 1.4 (95% confidence interval [CI], 1.2‐1.6) and for NAFLD 26.1 (9.4‐72.3). In the general population, children and adolescents with overweight/obesity have a higher prevalence of comorbidities relative to those of a healthy weight. This review provides clinicians with information when assessing children and researchers a foundation upon which to build a comprehensive dataset to understand the health consequences of childhood obesity.
Keywords: childhood obesity, comorbidities, population prevalence, systematic review
1. INTRODUCTION
Obesity is a major global epidemic impacting approximately 20% of the population and costing $2.0 trillion.1, 2 In adults, there is a range of known physical and psychological obesity‐related comorbidities including coronary heart disease, type 2 diabetes, hypertension, dyslipidaemia, pulmonary disorders, depression, and some cancers.3, 4 Studies in children have largely focused on the link to increased risk of obesity as adults.5, 6 However, there is increasing evidence that paediatric populations are also susceptible to a range of physical and psychological obesity‐related comorbidities, including type 2 diabetes and depression.7, 8, 9, 10, 11, 12, 13, 14
Given the extent of the global obesity epidemic, the impact on an individual's health, and the cost to health care systems and global economies, it is important to identify obesity‐related comorbidities in childhood to aid early identification and treatment.15
There is currently limited information comparing the relative frequency of obesity‐related comorbidities among children with overweight or obesity relative to healthy‐weight individuals. Previous systematic reviews have either not included children under 14 years,16 have included data from specific clinical populations,17 or were limited to specific countries.18 Furthermore, none of the previous reviews included meta‐analyses, instead solely providing narrative review. Thus, it is difficult to compare and interpret the prevalence of comorbidities between weight categories. We aimed to identify population‐based research that identified any obesity‐associated comorbidity in children and adolescents aged 5 to 18 years and to estimate the prevalence and prevalence ratio of each comorbidity using meta‐analyses.
2. METHODS
2.1. Search strategy and selection criteria
MOOSE guidelines for reporting systematic reviews of observational data were considered to structure the search, conduct, and report the review.19 The search strategy aimed to identify articles providing the prevalence of any type of obesity‐associated comorbidity reported in children and adolescents aged 5 to 18 years. A search was conducted of MEDLINE, MEDLINE in Process, EMBASE, PsycINFO, and Web of Science since the date of inception to January 2018. MESH and free‐text terms were grouped into four categories (prevalence, obesity, comorbidities, and childhood). We included free‐text terms of specific comorbidities, such as type 2 diabetes, depression, self‐esteem, and sleep disorders, to increase the specificity of the results, but the search was not limited to these. An example search strategy is provided in Data S2.
Manuscripts were eligible for inclusion if they reported observational study designs that provided prevalence (or data allowing its calculation) of comorbidities by weight category (healthy weight, overweight, and obese—based on body mass index [BMI] percentiles), in children and adolescents aged 5 to 18 years. Studies that included selected populations (eg, those with a history of illness in the family or identified from hospital clinics) and participants where obesity was a symptom of an underlying illness (eg, Prader‐Willi syndrome, Cushing syndrome, hypothyroidism, and Hashimoto disease) or a side effect from medication (eg, antidepressants and antipsychotics) were excluded. Non‐English articles were only eligible if English translations were available. Studies were eligible for inclusion in a meta‐analysis if there were at least two studies of the same comorbidity, in which the same definition was applied.
A sample of 693 (8%) of potentially eligible manuscripts were reviewed independently by two reviewers (V.S. and M.B.) over four rounds, each round indicating substantial agreement.20, 21 Disagreements were resolved through discussion. Full results of these analyses are provided in Data S3.
2.2. Data extraction and quality assessment
Details of authors, study design, setting, sample size, participant characteristics, and prevalence by weight category were extracted to bespoke data collection spreadsheets. Three authors were contacted to resolve queries regarding study design, method, and/or results; however, no additional information was provided. Study quality was assessed using the Joanna Briggs Institute's Critical Appraisal Checklist using simplistic scoring (0‐5—poor quality; 6‐10—high quality).22, 23 The checklist includes components for sample representativeness, reliability of measurement, and whether sufficient details of BMI classification and comorbidity definition were reported.
Comorbidities that were defined using different indicators (eg, fasting plasma glucose, homeostasis model assessment for insulin resistance [HOMA‐IR], and HbA1c as different indicators of diabetes) were reviewed separately and only grouped for discussion. Similarly, manuscripts presenting data using differing cut‐offs to define a comorbidity were separated in the analysis. Data were extracted by weight category (healthy weight: <85th percentile; overweight: ≥85th and <95th percentile; and obese: ≥95th percentile) to enable comparison between these subgroups.
2.3. Statistical methods
Crude prevalence of comorbidities within weight categories was estimated for all comorbidities and indicators.
For comorbidities that were reported in at least two studies, study‐specific prevalence ratios were plotted against the variance of the estimate in funnel plots, to illustrate variability between studies and assess publication bias through informal inspection. Combined estimates of prevalence ratios and 95% confidence intervals (CIs) were calculated using DerSimonian and Laird method for random effects models as programmed in the Comprehensive Meta‐Analysis software (Englewood, New Jersey).24 Forest plots for each comorbidity/indicator allowed side‐by‐side comparison of the studies. As part of the analysis, potential reasons for heterogeneity were identified, such as participant or sampling differences, country of study, sample size, gender, setting, and/or study design. The focus was directed by the availability of information reported in the articles, eg, if prevalence was provided by gender or age or if a number of the studies were conducted in the same country.
3. RESULTS
3.1. Descriptive analysis
The search identified 11 404 papers, of which 2221 were duplicates. The resulting 9183 abstracts were screened, resulting in 52 eligible manuscripts (1 553 683 participants) describing prevalence of obesity comorbidities within three weight categories in population‐based samples (Figure 1). Nineteen papers reported the prevalence of more than one comorbidity (or more than one population)25; these have been grouped into eight types of comorbidity: (1) hyperglycaemia, (2) dyslipidaemia, (3) hypertension, (4) nonalcoholic fatty liver disease (NAFLD), (5) cardiovascular risk, (6) pulmonary disorder, (7) psychological comorbidities, and (8) other comorbidities.
Figure 1.
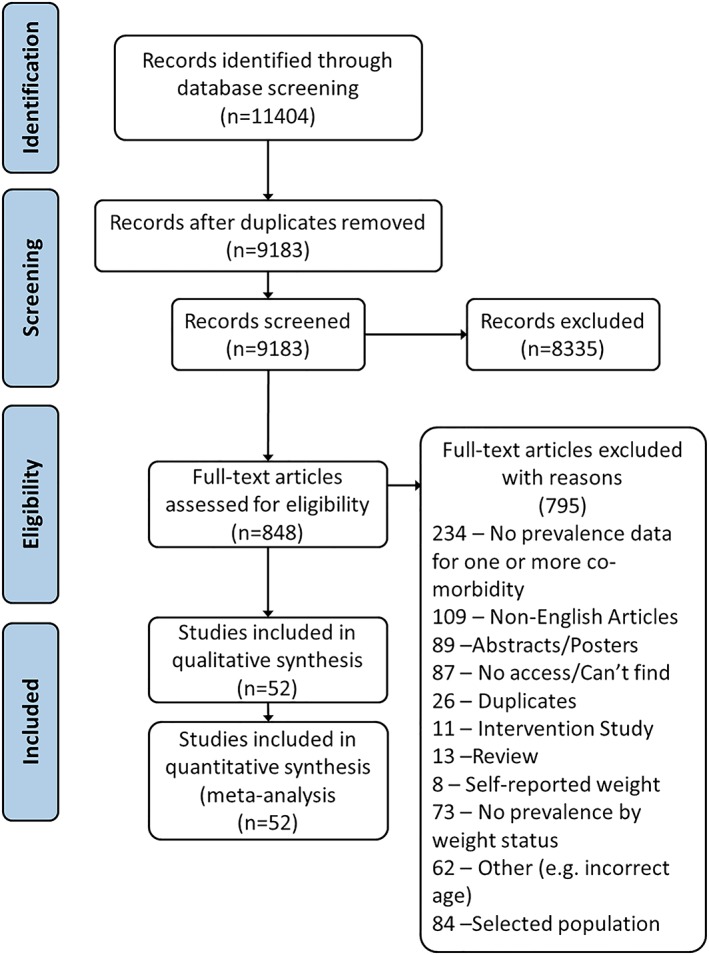
Systematic review flow chart [Colour figure can be viewed at http://wileyonlinelibrary.com]
Data were gathered from 20 countries across five continents, with the majority of studies based in Asia and Europe. While some studies provided separate estimates for children (≤13 y) and adolescents (≥13 y), the majority reported combined prevalence. This meant prevalence for each subgroup (children and adolescents) could not be estimated. Three papers reported assessing pubertal status, only Papoutsakis et al reported prevalence by pubertal status; however, they did not control for weight status; therefore, the impact of weight and pubertal status on prevalence could not be determined.26, 27, 28 The remaining papers provided data according to age; however, there was inconsistency in the cut‐offs applied, with cut‐offs including 10 to 11, 12 to 14, and 15 to 18; <12 and ≥12; and 8 to 11 and 12 to 14.29, 30, 31
In the majority of comparisons, prevalence was reported to be greatest in children with overweight or obesity compared with those of a healthy weight. For most, prevalence increased with increasing weight category. Crude prevalence estimates varied substantially between studies (Table 1). Data S1 provides a summary of studies reporting prevalence data, grouped by comorbidity (measure/test and cut‐off).
Table 1.
Summary of studies reporting prevalence data, grouped by comorbidity (measure/test and cut‐off)
| Comorbidity | Number of Studies | Total Sample Size | Overall Age Range | Prevalence (%) in Healthy Weight (n/N) | Prevalence (%) in Overweight (n/N) | Prevalence (%) in Obese (n/N) | Quality Score Range |
|---|---|---|---|---|---|---|---|
| Anxiety | 1 | 8460 | 10‐18 | 13.1 (806/6154) | 12.4 (106/858) | 9.7 (52/538) | 6 |
| Asthma | 3 | 4012 | 2‐22 | 10.9 (232/2134) | 15.9 (96/603) | 16.6 (133/803) | 6‐7 |
| Carotid‐intima media thickness | 1 | 575 | 11‐13 | 24.2 (76/314) | 36.9 (66/179) | 42.7 (35/82) | 7 |
| Depression | 1 | 8640 | 10‐18 | 30.1 (1853/6154) | 30.2 (259/858) | 30.9 (166/538) | 6 |
| Hyperglycaemia (OGTT) | 1 | 76 732 | 17‐17 | <1 (7/53 684) | <1 (4/9202) | 0.3 (8/2897) | 9 |
| Hyperglycaemia (FPG) | 10 | 21 855 | 6‐18 | 6.6 (806/12 152) | 9.7 (274/2821) | 10.5 (318/3021) | 4‐10 |
| Hyperglycaemia (insulin resistance) | 1 | 248 | 10‐12 | 2.2 (3/137) | 1.4 (1/74) | 10.8 (4/37) | 5 |
| Elevated uric acid | 1 | 2405 | 6‐12 | 14.5 (254/1753) | 28.3 (102/361) | 43.6 (127/291) | 7 |
| Exercise‐induced wheeze/cough | 1 | 903 | 7‐11 | 0.5 (4/755) | 36.9 (31/84) | 68.8 (44/64) | 7 |
| Flatfoot | 3 | 827 652 | 5‐19 | 13.5 (87 055/646 176) | 17.9 (15 323/85 805) | 21.8 (10 394/47 762) | 4‐10 |
| Gallstones | 1 | 510 816 | 10‐19 | 0.1 (215/301 549) | 0.2 (179/99 987) | 0.3 (372/109 280) | 9 |
| Low self‐esteem | 1 | 2491 | 9‐14 | 4.5 (86/1905) | 8.9 (32/359) | 14.5 (33/227) | 4 |
| High blood pressure (>90 percentile) | 24 | 136 144 | 1‐20 | 3.1 (2928/94 803) | 6.5 (1336/20 478) | 17.9 (3726/20 863) | 4‐9 |
| Low HDL cholesterol | 7 | 18 352 | 6‐18 | 8.1 (433/5316) | 15.7 (347/2215) | 20.3 (400/1968) | 4‐9 |
| High C‐reactive protein | 1 | 575 | 11‐13 | 2.6 (8/306) | 15.5 (24/155) | 32.3 (20/62) | 4 |
| High triglycerides | 7 | 15 405 | 2‐19 | 4.2 (236/5598) | 12.6 (213/1693) | 19.2 (383/1997) | 4‐9 |
| High LDL cholesterol | 2 | 1113 | 9‐15 | 3.9 (23/597) | 7.5 (22/294) | 12.2 (27/222) | 4‐5 |
| Lower back pain | 1 | 966 | 10‐16 | 46.7 (344/736) | 47.2 (84/178) | 53.9 (28/52) | 8 |
| Metabolic syndrome (Cook et al) | 2 | 2732 | 7‐18 | 0.9 (18/1886) | 1.8 (7/400) | 19.1 (85/446) | 7 |
| Metabolic syndrome (de Ferranti et al) | 1 | 2761 | 15‐19 | 0.3 (3/1049) | 1.4 (4/289) | 20.8 (75/360) | 7 |
| Metabolic syndrome (IDF) | 6 | 18 792 | 6‐18 | 0.7 (48/6970) | 6.7 (190/2830) | 20.4 (586/2868) | 4‐9 |
| Metabolic syndrome (NCEP) | 6 | 8705 | 6‐18 | 0.5 (28/5204) | 9.2 (148/1617) | 32.8 (598/1825) | 5‐10 |
| NAFLD (ultrasound) | 5 | 5305 | 6‐18 | 2.6 (90/3495) | 10.9 (115/1059) | 46.7 (309/662) | 4‐7 |
| NAFLD (elevated ALT) | 2 | 643 | 6‐18 | 0.6 (2/322) | 6.2 (8/130) | 25.7 (49/191) | 8‐9 |
| NAFLD (elevated AST) | 1 | 496 | 14‐17 | 4.3 (16/376) | 10.9 (10/92) | 17.9 (5/28) | 9 |
| Obstructive sleep apnoea | 1 | 966 | 10‐16 | 2.0 (15/736) | 3.4 (6/178) | 3.9 (2/52) | 8 |
| High total cholesterol | 4 | 4484 | 6‐16 | 12.4 (383/3086) | 11.6 (97/833) | 17.9 (101/565) | 4‐8 |
| Traumatic dental injuries | 2 | 2565 | 6‐13 | 12.8 (243/1892) | 12.1 (54/445) | 13.6 (31/228) | 8 |
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; FPG, fasting plasma glucose; HDL, high‐density lipoprotein; IDF, International Diabetes Federation; LDL, low‐density lipoprotein; NAFLD, nonalcoholic fatty liver disease; NCEP, National Cholesterol Education Program; OGTT, oral glucose tolerance test.
Figure 2 provides a summary of meta‐analyses of prevalence ratios for eligible comorbidities in children who were overweight or obese relative to children of a healthy weight (individual forest plots are in Data S4). Overall, results suggest that children with obesity have the greatest prevalence of comorbidities; I 2 statistics ranged greatly across comorbidities.
Figure 2.
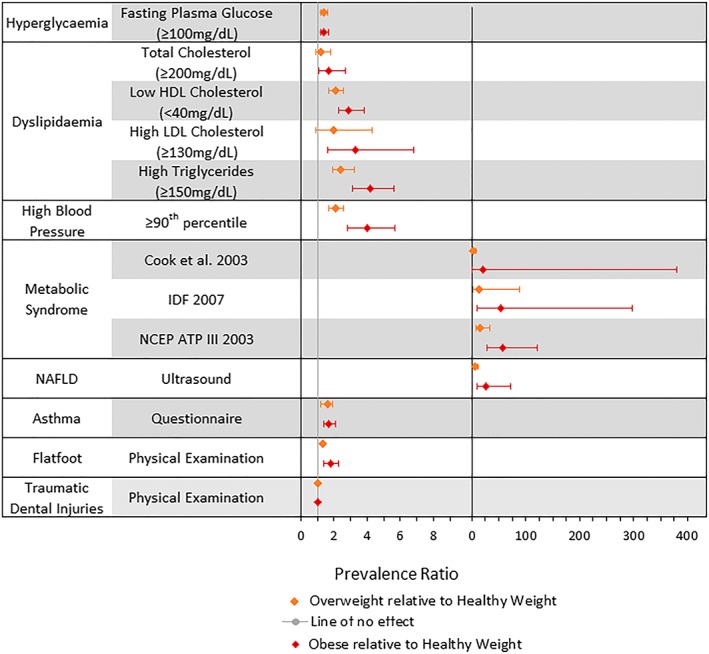
Average random effects estimates of prevalence ratios and 95% confidence intervals for the comorbidities/comorbidity indicators. Left‐hand side excludes metabolic syndrome and nonalcoholic fatty liver disease (NAFLD) because of differences in scale [Colour figure can be viewed at http://wileyonlinelibrary.com]
3.2. Hyperglycaemia
Twelve studies provided prevalence estimates for markers of hyperglycaemia (n = 98 835), the majority of which assessed levels of fasting glucose. While children and adolescents with obesity regularly had the highest prevalence of hyperglycaemia, patterns were inconsistent, and the magnitude of prevalence varied. Nine studies (21 855 participants) used fasting plasma glucose ≥ 100 mg/dL (≥5.6 mmol/L) to define hyperglycaemia and were included in a meta‐analysis. Prevalence ratios for elevated fasting plasma glucose were 1.4 (95% CI, 1.2‐1.6; I 2: 0.0%) for children and adolescents with overweight and 1.4 (95% CI, 1.2‐1.7; I 2: 23.4%) for those with obesity relative to those of healthy weight. Wang et al did not provide data controlling for pubertal status.26 Two of the remaining eight studies considered children aged 6 to 13 years; however, the reported prevalence ranged from 3.4% to 29% in the group with obesity.32, 33
3.3. Dyslipidaemia
Prevalence of dyslipidaemia was available from 19 studies, assessed by multiple markers (high‐density lipoprotein [HDL], low‐density lipoprotein [LDL], triglyceride [TG], and total cholesterol) in nine distinct populations, with 18 844 participants. Overall, dyslipidaemia was consistently greater in children with obesity, and there was an overall increase in prevalence with increasing weight category, but crude prevalence estimates varied from 1% to 23% in children and adolescents of healthy weight and 0% to 62% in those with obesity. Prevalence ratios for high total cholesterol (1.7 [95% CI, 1.1‐2.7; I 2: 48.6%]), low HDL (2.9 [95% CI, 2.1‐4.1; I 2: 78.6%]), high LDL (3.3 [95% CI, 1.6‐6.8; I 2: 35.6%]), and high TGs (4.2 [95% CI, 3.1‐5.6; I 2: 56.2%]) indicated consistently greater prevalence of dyslipidaemia in children and adolescents with obesity compared with those of a healthy weight. Analogous estimates of prevalence ratios for children with overweight were closer to one but mostly significant (see Table 1). Tandon et al and Minghelli et al considered similarly aged participants, 10‐ to 18‐ and 10‐ to 16‐year‐olds, respectively; however, the reported prevalence was considerably different, 61.5% and 0% in those with obesity, respectively.34, 35 Although it should be noted that Minghelli et al only recruited 52 participants with obesity, therefore, the prevalence may be a factor of the small group size.
3.4. Cardiovascular risk factors
Cardiovascular risk was investigated by one study using carotid‐intima media thickness and in 14 studies comparing the prevalence of metabolic syndrome according to four different definitions. Prevalence estimates were high overall in children/adolescents with overweight and obesity and were highest in children/adolescents with obesity (majority greater than 20%). Only studies reporting metabolic syndrome (n = 13, 28 922 participants) were included in the meta‐analysis. Compared with children and adolescents of a healthy weight, those with obesity had prevalence ratio 21.2 (95% CI, 1.2‐381.6; I 2: 94.3%), 53.9 (95% CI, 9.7‐297.9; I 2: 93.7%), and 58.0 (95% CI, 27.7‐121.3; I 2: 61.0%) for metabolic syndrome based on criteria proposed by Cook et al, International Diabetes Federation (IDF), and the National Cholesterol Education Program (NCEP), respectively.36, 37, 38 Prevalence ratios comparing children and adolescents with overweight to healthy‐weight children and adolescents were also high, although lower than for those with obesity (see Table 1).
3.5. Hypertension
Thirty‐one studies reporting prehypertension or hypertension were identified (n = 176 987 individuals). Crude prevalence rates were highest in children and adolescents with overweight or obesity. Studies used a variety of thresholds to define hypertension, including ≥90th to <95th percentile (prehypertension), ≥90th percentile, and ≥95th percentile. Individual analysis indicated similar prevalence ratios; therefore, the data were combined to calculate the prevalence of hypertension overall. In 29 studies, the prevalence ratio was 4.0 (95% CI, 2.8‐5.7; I 2: 95.2%) in children and adolescents with obesity compared with those of a healthy weight. Similarly, prevalence estimates were greater in children and adolescents with overweight than in those of a healthy weight, but lower than those with obesity (see Table 1). Dyson et al recruited participants from India, China and Mexico and provided prevalence data by country.25 Prevalence in those with obesity ranged from 23% (China) to 47% (India).
3.6. Nonalcoholic fatty liver disease
Eight studies provided prevalence of NAFLD by weight status (n = 6444). All suggested a higher prevalence in children and adolescents with obesity. Crude prevalence estimates in children and adolescents of a healthy weight ranged from 0% to 6.8% compared with 4.3% to 21.3% in those who were overweight and 17.9% to 62.0% in those with obesity. Five studies (n = 5305) that assessed NAFLD by ultrasound were eligible for inclusion in the meta‐analysis. Combined prevalence ratios for NAFLD were 26.1 (95% CI, 9.4‐72.2; I 2: 91.4%) for children and adolescents with obesity and 6.1 (95% CI, 3.3‐11.2; I 2: 67.9%) for those who were overweight. Two studies recruited children of similar ages, Adibi et al 6‐ to 18‐year‐olds and Alavian et al 7‐ to 18‐year‐olds. Both reported considerably higher prevalence in those with obesity, 54% and 32%, compared with the healthy‐weight group.39, 40
3.7. Pulmonary disorders
Five studies compared the prevalence of pulmonary disorders in children and adolescents by weight status (n = 5881), three were asthma studies (n = 4012).41, 42, 43 Meta‐analysis of the asthma studies indicated that children and adolescents with obesity and with overweight had, respectively, 1.7 (95% CI, 1.4‐2.1; I 2: 0.0%) and 1.6 (95% CI, 1.2‐1.9; I 2: 0.0%) times the prevalence of asthma compared with those of a healthy weight. These studies were relatively small, of moderate quality, and assessed asthma status using parent and/or self‐report. Exercise‐induced wheeze/cough was considered in one study (n = 903) in which prevalence in children with obesity was high (68.8%) compared with those of a healthy weight (0.5%).44 Although of moderate quality, status was based on self‐report and was not eligible for inclusion in the meta‐analysis. One study compared prevalence of obstructive sleep apnoea (n = 966) and found increasing prevalence as weight status increased.34
3.8. Psychological disorders
Two studies (n = 11 088) compared psychological comorbidities and met our eligibility criteria for inclusion into the review.29, 45 Zakeri et al (n = 8339) considered both depression and anxiety using the Global School‐based Health Survey (GSHS).46 Franklin et al measured low self‐esteem (n = 2749) using the Self‐perception Profile for Children. In contrast to other comorbidities, Zakeri et al found that the prevalence of neither depression nor anxiety differed by weight status. Franklin et al reported the highest prevalence of low self‐esteem in children and adolescents with obesity, though this was considered of poor quality according to the Joanna Briggs Institute's Critical Appraisal Tool.
3.9. Less established comorbidities
We identified studies of five less established comorbidities/indicators, including high C‐reactive protein, elevated uric acid, gallstones, low back pain, and traumatic dental injuries. Although there was considerable variation in reported prevalence, for most studies, prevalence was highest in children and adolescents with obesity. Two comorbidities, traumatic dental injuries and flatfoot, met criteria for inclusion in the meta‐analysis. The prevalence of traumatic dental injuries did not differ by weight status (n = 2565).47, 48 Children and adolescents with obesity had a 1.8 (95% CI, 1.4‐2.3; I 2: 71.5%) greater prevalence of flatfoot than those of a healthy weight.49, 50
4. DISCUSSION
Our systematic review identified 52 eligible studies involving 1 553 683 participants, reporting eight comorbidities or risk markers including diabetes, hypertension, dyslipidaemia, and NAFLD, which could be included in meta‐analysis. The results suggested that children and adolescents with obesity had a higher prevalence of the majority of the identified comorbidities, relative to those of healthy weight. The review indicated that there were small to large increases in prevalence between weight categories for most comorbidities. Meta‐analyses suggested children and adolescents with obesity were 1.4 times more likely to have prediabetes, 4.4 times more likely to have high blood pressure, 26.1 times more likely to have NAFLD, and 1.7 times more likely to have self‐reported asthma. Although the prevalence ratios for metabolic syndrome were high, the prevalence among healthy‐weight children and adolescents was very low (at most 1%) and the results are likely to be inflated because of obesity forming part of the criteria for diagnosis.36
Results from previous systematic reviews supported our findings to some degree.16, 17, 18 Additionally, we identified an increase in the risk of NAFLD and carotid‐intima media thickness in children and adolescents with obesity that had not been previously identified by some or all of the previous reviews. The prevalence of NAFLD is particularly concerning, as it is regarded as a silent disease with a propensity to fibrosis and cirrhosis.51
Other reviews identified other comorbidities, for example, Guh et al found a number of cancers associated with obesity (eg, colorectal, kidney, prostate, breast, and ovarian). However, the review of Guh et al was not limited to children and the estimates for cancer predominantly considered participants aged 40 years and older in whom cancers are more prevalent. Furthermore, cancers tend to have long latent periods and the ones identified are rare in childhood.52
Despite the current results suggesting a higher prevalence of comorbidities in those with obesity, the association between weight status and prevalence of comorbidities was often difficult to ascertain because of between‐study heterogeneity. In the current systematic review, there was a lack of consistency between studies in definitions and cut‐offs for comorbidities. For high blood pressure, three cut‐offs were identified (≥90th percentile, ≥90th and <95th percentile, and ≥95th percentile), and for low HDL cholesterol, both <40 and ≤40 mg/dL were used in studies. However, for both of these comorbidities, studies with different cut‐offs were combined into one group, since the prevalence ratios for each definition were comparable. For metabolic syndrome, however, it was not appropriate to combine definitions for analysis because of heterogeneity in prevalence ratios between different definitions. Currently, there is no consensus on the definition of metabolic syndrome, and different definitions resulted in heterogeneous population prevalence, limiting the ability to obtain a single estimate.53
There was also considerable variation in study sample sizes, which can influence the reported prevalence (smaller groups are subject to greater sampling variance, which would impact the accuracy of the prevalence ratio estimates). This was controlled for to some extent in the meta‐analysis, in which smaller studies were given less weight than larger studies.54
4.1. Strengths and limitations
To our knowledge, we have conducted the first international systematic review and meta‐analyses considering the prevalence of comorbidities in children and adolescents with overweight or obesity relative to those of a healthy weight. Our systematic review and meta‐analyses followed a predefined protocol, which explicitly detailed the inclusion and exclusion criteria for the studies and the steps undertaken in each stage. The search criteria included multiple observational study designs that enabled greater sensitivity in detecting relevant literature in order to develop an exhaustive list of obesity‐related comorbidities. This was a limitation of previous systematic reviews in the area.16, 17, 18 We conducted a sensitive review with scope to identify any type of comorbidity, and thus, we were not restricted to reporting prevalence rates of a confined set of comorbidities, which may have omitted key findings in important disease areas.
Some limitations are worthy of consideration. Firstly, it is recognised that pubertal stage is an important factor when determining the prevalence of comorbidities. O'Hara et al estimated the prevalence of cardiometabolic risk factors in children aged 3 to 19 years and reported a positive association between age and prevalence.55, 56 Despite this, the majority of studies in the current review did not control for age or pubertal status when providing prevalence estimates by each group. This meant prevalence data for growth or age subgroups could not be estimated. Further, papers that did provide data by age were inconsistent in the cut‐offs applied. Secondly, only a small number of articles pertaining to psychological comorbidities were identified and these used imperfect measures of these constructs. It is possible that other articles may have been identified through a more specific search. However, it may also be indicative of a lack of prevalence studies in this population. Thirdly, studies related to all psychological comorbidities and some physical, utilised self‐report questionnaires, some of which were not validated. This has the potential to hinder the accuracy of the data, because of self‐report bias and nonspecific tests, and reduced the generalisability of the results. That said, it should be noted that, at present, alternative screening methods for many of these comorbidities are not available. Finally, non‐English language articles were not included in the review, potentially excluding relevant articles and impacting the accuracy of prevalence estimates and the generalisability of the results. However, previous reviews have offered conflicting information about the impact of language restrictions on results, with some suggesting that translation was inefficient and did not yield appreciably different results.57, 58, 59, 60 All non‐English articles were retained in a separate Endnote folder for possible future research (available upon request).
Other evidence has reported positive associations between childhood and obesity‐associated comorbidity prevalence; many of which have severe implications if left unmanaged.61, 62, 63, 64, 65, 66 The results of the meta‐analysis highlight the need to address children and adolescents with obesity given the alarming presence of ill health that can be seen and which is likely to continue and exacerbate into adulthood. Research has indicated that the long‐term health consequences can be reduced if children with obesity go on to become healthy‐weight adults.67
The review addresses the limitations of previous systematic reviews and provides population prevalence estimates using global literature. This consolidation of child and adolescent obesity‐associated comorbidity literature provides clinicians with clear and concise information when assessing the health of children and provides researchers a foundation upon which to build a comprehensive dataset to understanding the health consequences of childhood obesity. Moreover, the review identifies gaps in existing research, such as the lack of data by age or pubertal status, which future research can address to improve the accuracy and generalisability of future prevalence estimates. Finally, given the reported prevalence and potential long‐term implications to health and well‐being, these data can also be used to support the need for development of effective obesity treatment and prevention strategies at a national and local level.
There are some recommendations for future research. Firstly, we recommend that studies measure and report data by pubertal status to better understand the impact of pubertal status on comorbidity prevalence. Secondly, additional research in general populations, stratified by weight category, is required to obtain an understanding of the impact of increased weight on the prevalence, severity, and reversibility of these comorbidities in children and adolescents. Thirdly, additional population‐based research is required to enable generalisability of the results beyond the study sample and to enhance between‐study homogeneity and enable more accurate population prevalence ratio estimates to be calculated.
CONFLICT OF INTEREST
No conflict of interest was declared.
Supporting information
Supplemental File 1:
Summary of studies reporting prevalence data, grouped by co‐morbidity (measure/test and cut‐off)
Supplemental File 2:
Search Strategy
Supplemental File 3:
Kappa Values
Supplemental File 4:
Forest Plots for the Prevalence Ratios by co‐morbidity
Sharma V, Coleman S, Nixon J, et al. A systematic review and meta‐analysis estimating the population prevalence of comorbidities in children and adolescents aged 5 to 18 years. Obesity Reviews. 2019;20:1341–1349. 10.1111/obr.12904
REFERENCES
- 1. Dobbs, R. , Sawers, C. , Thompson, F. , et al. Overcoming obesity: an initial economic analysis. http://www.mckinsey.com. [series online] 2014. Available from: http://www.mckinsey.com/~/media/McKinsey/dotcom/Insights/Economic%20Studies/How%20the%20world%20could%20better%20fight%20obesity/MGI_Overcoming_obesity_Full_report.ashx. Accessed October 13, 2015.
- 2. OCED . Obesity update 2017. https://www.oecd.org. [series online] 2017. Available from: https://www.oecd.org/els/health-systems/Obesity-Update-2017.pdf. Accessed November 6, 2017.
- 3. Andolfi C, Fisichella PM. Epidemiology of obesity and associated comorbidities. J Laparoendosc Adv Surg Tech A. 2018;28(8):919‐924. [DOI] [PubMed] [Google Scholar]
- 4. Elagizi A, Kachur S, Lavie CJ, et al. An overview and update on obesity and the obesity paradox in cardiovascular diseases. Prog Cardiovasc Dis. 2018;61(2):142‐150. [DOI] [PubMed] [Google Scholar]
- 5. Kumar S, Kelly AS. Review of childhood obesity. Mayo Clin Proc. 2017;92(2):251‐265. [DOI] [PubMed] [Google Scholar]
- 6. The NS, Suchindran C, North KE, Popkin BM, Gordon‐Larsen P. Association of adolescent obesity with risk of severe obesity in adulthood. JAMA. 2010;304(18):2042‐2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hannon TS, Rao G, Arslanian SA. Childhood obesity and type 2 diabetes mellitus. Pediatrics. 2005;116(2):473‐480. [DOI] [PubMed] [Google Scholar]
- 8. Loth KA, Mond J, Wall M, Neumark‐Sztainer D. Weight status and emotional well‐being. J Pediatr Psychol. 2011;36(2):216‐225. [DOI] [PubMed] [Google Scholar]
- 9. Maggio ABR, Martin XE, Gasser CS, et al. Medical and non‐medical complications among children and adolescents with excessive body weight. BMC Pediatr. 2014;14(1):232 10.1186/1471-2431-14-232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McCarthy MI. Genomics, type 2 diabetes, and obesity. NEJM. 2010;363(24):2339‐2350. 10.1056/NEJMra0906948 [DOI] [PubMed] [Google Scholar]
- 11. Nonalcoholic Steatohepatitis . National Institute of Diabetes and Digestive and Kidney Disease website. http://www.niddk.nih.gov/health-information/health-topics/liver-disease/nonalcoholic-steatohepatitis/Page Published 2015. Accessed January 5, 2017.
- 12. Poirier P, Giles TD, Bray GA, et al. Obesity and cardiovascular disease: pathophysiology, evaluation and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity and Metabolism. Circulation. 2006;113(6):898‐918. [DOI] [PubMed] [Google Scholar]
- 13. Strauss RS, Pollack HA. Social marginalisation of overweight children. Arch Pediatr Adolesc Med. 2003;157(8):746‐752. [DOI] [PubMed] [Google Scholar]
- 14. Wardle J, Cooke L. The impact of obesity on psychological well‐being. Best Pract Res Clin Endocrinol Metab. 2005;19(3):421‐440. [DOI] [PubMed] [Google Scholar]
- 15. NICE . Managing overweight and obesity among children and young people: lifestyle weight management services. http://www.nice.org.uk/guidance/ph47 Published October 2013. Accessed September 1, 2014.
- 16. Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co‐morbidities related to obesity and overweight: a systematic review and meta‐analysis. BMC Public Health. 2009;9(1):88 10.1186/1471-2458-9-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pulgaron ER. Childhood obesity: a review of increased risk for physical and psychological co‐morbidities. Clin Ther. 2013;35:18‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sanders RH, Han A, Baker JS, Cobley S. Childhood obesity and its physical and psychological co‐morbidities: a systematic review of Australian children and adolescents. Eur J Pediatr. 2015;174(6):715‐746. [DOI] [PubMed] [Google Scholar]
- 19. Stroup DF, Berlin JA, Morton SC, et al. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008‐2012. [DOI] [PubMed] [Google Scholar]
- 20. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159‐174. [PubMed] [Google Scholar]
- 21. Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med. 2005;37(5):360‐363. [PubMed] [Google Scholar]
- 22. Munn Z, Moola S, Riitano D, Lisy K. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int J Health Pol Manag. 2014;3(3):123‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gonzalez‐Serrano J, Serrano J, Lopez‐Pintor RM, Paredes VM, Casañas E, Hernández G. Prevalence of oral mucosal disorders in diabetes mellitus patients compared with a control group. J Diabetes Res. 2016;2016:1‐11. 10.1155/2016/5048967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Deeks JJ, Higgins JPT. Statistical algorithms in Review Manager 5. Research Gate [series online] Published February 2010. Available from https://www.researchgate.net/profile/Jonathan_Deeks2/publication/252093205_Statistical_Algorithms_in_Review_Manager_5/links/54d159b70cf28370d0e07f9e/Statistical-Algorithms-in-Review-Manager-5.pdf. Accessed June 21, 2015.
- 25. Dyson PA, Anthony D, Fenton B, Matthews DR, Stevens DE. Community Interventions for Health Collaboration. High rates of child hypertension associated with obesity: a community survey in China, India and Mexico. Paediatr Int Child Health. 2014;34(1):43‐49. [DOI] [PubMed] [Google Scholar]
- 26. Wang Q, Yin J, Xu L, et al. Prevalence of metabolic syndrome in a cohort of Chinese schoolchildren: comparison of two definitions and assessment of adipokines as components by factor analysis. BMC Public Health. 2013;13(1):249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Manios Y, Moschonis G, Kourlaba G, et al. Prevalence and independent predictors of insulin resistance in children from Crete, Greece: the children study. Diabet Med. 2008;25(1):65‐72. [DOI] [PubMed] [Google Scholar]
- 28. Papoutsakis C, Yannakoulia M, Ntalla I, Dedoussis GV. Metabolic syndrome in a Mediterranean pediatric cohort: prevalence using International Diabetes Federation–derived criteria and associations with adiponectin and leptin. Metabol Clin Exp. 2012;61:14‐145. [DOI] [PubMed] [Google Scholar]
- 29. Zakeri M, Sedaghat M, Motlagh ME, Tayari Ashtiani R, Ardalan G. BMI correlation with psychiatric problems among 10‐18 years Iranian students. Acta Med Iran. 2012;50(3):177‐184. [PubMed] [Google Scholar]
- 30. Messiah SE, Arheart KL, Luke B, Lipshultz SE, Miller TL. Relationship between body mass index and metabolic syndrome risk factors among US 8‐ to 14‐year‐olds, 1999 to 2002. J Pediatr. 2008;153(2):215‐221. [DOI] [PubMed] [Google Scholar]
- 31. Wiegand S, Keller KM, Robl M, et al. Obese boys at increased risk for nonalcoholic liver disease: evaluation of 16 390 overweight or obese children and adolescents. Int J Obes (Lond). 2010;34(10):1468‐1474. [DOI] [PubMed] [Google Scholar]
- 32. Chu NF, Pan WH. Prevalence of obesity and its comorbidities among schoolchildren in Taiwan. Asia Pac J Clin Nutr. 2007;16:601‐607. [PubMed] [Google Scholar]
- 33. Del‐Rio‐Navarro BE, Velazquez‐Monroy O, Lara‐Esqueda A, et al. Obesity and metabolic risks in children. Arch Med Res. 2008;39(2):215‐221. [DOI] [PubMed] [Google Scholar]
- 34. Minghelli B, Oliveira R, Nunes C. Association of obesity with chronic disease and musculoskeletal factors. Rev Assoc Med Bras. 2015;61(4):347‐354. [DOI] [PubMed] [Google Scholar]
- 35. Tandon N, Garg MK, Singh Y, Marwaha RK. Prevalence of metabolic syndrome among urban Indian adolescents and its relation with insulin resistance (HOMA‐IR). J Pediatr Endocrinol. 2013;26:1123‐1130. [DOI] [PubMed] [Google Scholar]
- 36. Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988‐1994. Arch Pediatr Adolesc Med. 2003;157(8):821‐827. [DOI] [PubMed] [Google Scholar]
- 37. Zimmet, P , Alberti, G , Shaw, J. et al. The IDF consensus worldwide definition of the metabolic syndrome. [series online]. Published 2006. Available from https://www.idf.org/webdata/docs/IDF_Meta_def_final.pdf. Accessed September 28, 2015.
- 38. Expert Panel . Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA. 2001;285:2486. [DOI] [PubMed] [Google Scholar]
- 39. Adibi A, Kelishadi R, Beihaghi A, Salehi H, Talaei M. Sonographic fatty liver in overweight and obese children, a cross sectional study in Isfahan. Endokrynol Pol. 2009;60:14‐19. [PubMed] [Google Scholar]
- 40. Alavian SM, Mohammad‐Alizadeh AH, Esna‐Ashari F, Ardalan G, Hajarizadeh B. Non‐alcoholic fatty liver disease prevalence among school‐aged children and adolescents in Iran and its association with biochemical and anthropometric measures. Liver Int. 2009;29(2):159‐163. [DOI] [PubMed] [Google Scholar]
- 41. Kwon HL, Ortiz B, Swaner R, et al. Childhood asthma and extreme values of body mass index: The Harlem Children's Zone Asthma Initiative. J Urban Health. 2006;83(3):421‐433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Noonan CW, Brown BD, Bentley B. Variability in childhood asthma and body mass index across Northern Plains American Indian communities. J Asthma. 2010;47:4986‐4500. [DOI] [PubMed] [Google Scholar]
- 43. Ribeiro‐Silva RDC, Oliveira‐Assis AM, Junqueira SB, et al. Food and nutrition insecurity: a marker of vulnerability to asthma symptoms. Public Health Nutr. 2013;17:14‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kajbaf TZ, Asar S, Alipoor MR. Relationship between obesity and asthma symptoms among children in Ahvaz, Iran: a cross sectional study. Ital J Pediatr. 2011;37(1):1 10.1186/1824-7288-37-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Franklin J, Denyer G, Steinbeck KS, Caterson ID, Hill AJ. Obesity and risk of low self‐esteem: a statewide survey of Australian children. Pediatrics. 2006;118(6):2481‐2487. [DOI] [PubMed] [Google Scholar]
- 46. World Health Organisation . Global school‐based student health survey. [series online] Available from http://www.who.int/ncds/surveillance/global-school-student-survey/datasets/en/. 2018. Accessed January 2, 2018.
- 47. Al‐Bajjali TT, Rajab LD. Traumatic dental injuries among 12‐year‐old Jordanian schoolchildren: an investigation on obesity and other risk factors. BMC Oral Health. 2014;14(1):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Basha S, Mohammad RN, Swamy HS, Sexena V. Association between traumatic dental injury, obesity, and socioeconomic status in 6‐ and 13‐year‐old schoolchildren. Soc Work Public Health. 2015;30(4):336‐344. [DOI] [PubMed] [Google Scholar]
- 49. Chen JP, Chung MJ, Wang MJ. Flatfoot prevalence and foot dimensions of 5‐ to 13‐year‐old children in Taiwan. Foot Ankle Int. 2009;30(4):326‐332. [DOI] [PubMed] [Google Scholar]
- 50. Tenenbaum S, Hershkovich O, Gordon B, et al. Flexible pes planus in adolescents: body mass index, body height, and gender—an epidemiological study. Foot Ankle Int. 2013;4:811‐817. [DOI] [PubMed] [Google Scholar]
- 51. El‐Zayadi AR. Hepatic steatosis: a benign disease or a silent killer. World J Gastroenterol. 2008;14(26):4120‐4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nadler DL, Zurbenko IG. Estimating cancer latency times using a Weibull model. Adv Epidemiology. 2014;2014:1‐8. 10.1155/2014/746769 [DOI] [Google Scholar]
- 53. Kassi E, Pervanidou P, Kaltsas G, Chrousos G. Metabolic syndrome: definitions and controversies. BMC Med. 2011;9(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Borenstein M, Hedges LV, Rothstein HR, Higgins J. Introduction to Meta‐Analysis. West Sussex, UK: John Wiley & Sons Ltd; 2009. [Google Scholar]
- 55. O'Hara V, Browne N, Fathima S, et al. Obesity cardiometabolic comorbidity prevalence in children in a rural weight‐management program. Glob Pediatr Health. 2017;4:1‐10. 10.1177/2333794X17729303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Li L, Perez A, Wu LT, Ranjit N, Brown HS, Kelder SH. Cardiometabolic risk factors among severely obese children and adolescents in the United States, 1999‐2012. Child Obes. 2016;12(1):12‐19. [DOI] [PubMed] [Google Scholar]
- 57. Gregoire G, Derderian F, Le Lorier J. Selecting the language of the publications included in a meta‐analysis: is there a Tower of Babel bias? J Clin Epidemiol. 1995;48(1):159‐163. [DOI] [PubMed] [Google Scholar]
- 58. Juni P, Holenstein F, Sterne J, Bartlett C, Egger M. Direction and impact of language bias in meta‐analyses of controlled trials: empirical study. Int J Epidemiol. 2002;31(1):115‐123. [DOI] [PubMed] [Google Scholar]
- 59. Bown MJ, Sutton AJ. Quality control in systematic reviews and meta‐analyses. Eur J Vasc Endovasc Surg. 2010;40(5):669‐677. [DOI] [PubMed] [Google Scholar]
- 60. Morrison A, Polisena J, Husereau D, et al. The effect of English‐language restriction on systematic review‐based meta‐analyses: a systematic review of empirical studies. Int J Technol Assess Health Care. 2012;28(2):138‐144. [DOI] [PubMed] [Google Scholar]
- 61. Sabin M, Crowne E, Shield JP. Childhood obesity and type 2 diabetes. Nurs Times. 2002;98(19):49‐50. [PubMed] [Google Scholar]
- 62. Narayan KMV, Boyle JP, Thompson TJ, Gregg EW, Williamson DF. Effect of BMI on lifetime risk for diabetes in the U.S. Diabet Med. 2007;30(6):1562‐1566. [DOI] [PubMed] [Google Scholar]
- 63. Abdullah A, Peeters A, De Courten M, Stoelwinder J. The magnitude of association between overweight and obesity and the risk of diabetes: a meta‐analysis of prospective cohort studies. Diabetes Res Clin Pract. 2010;89(3):309‐319. [DOI] [PubMed] [Google Scholar]
- 64. Reilly JJ, Kelly J. Long‐term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. Int J Obes (Lond). 2011;35(7):891‐898. [DOI] [PubMed] [Google Scholar]
- 65. Staimez LR, Weber MB, Narayan KM, Oza‐Frank R. A systematic review of overweight, obesity, and type 2 diabetes among Asian American subgroups. Curr Diabetes Rev. 2013;9(4):312‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kodama S, Horikawa C, Fujihara K, et al. Quantitative relationship between body weight gain in adulthood and incident type 2 diabetes: a meta‐analysis. Obes Rev. 2014;15(3):202‐214. [DOI] [PubMed] [Google Scholar]
- 67. Juonala M, Magnussen CG, Berenson GS, et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. NEJM. 2011;365(20):1876‐1885. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental File 1:
Summary of studies reporting prevalence data, grouped by co‐morbidity (measure/test and cut‐off)
Supplemental File 2:
Search Strategy
Supplemental File 3:
Kappa Values
Supplemental File 4:
Forest Plots for the Prevalence Ratios by co‐morbidity


