Abstract
Aim
To evaluate effects of a 0.454% stannous fluoride test toothpaste on dentine hypersensitivity (DH) applied by fingertip, then 3 days’ brushing, versus a sodium monofluorophosphate‐based control.
Materials and Methods
In three randomized clinical studies, DH was assessed using evaporative (Schiff scale) and tactile (Yeaple probe) stimuli. Participants applied toothpaste to two sensitive teeth by fingertip (60 s each); DH was re‐assessed, prior to brushing. Test treatment participants brushed their sensitive teeth, with all participants then brushing all teeth for ≥60 s, twice daily for 3 days. DH was re‐assessed. Data were analysed by study and then pooled.
Results
In two studies, test treatment significantly reduced DH versus control treatment after fingertip application and 3 days’ brushing (both measures). In one study, both treatments significantly reduced DH without between‐treatment differences. Mean Schiff differences (95% confidence intervals) for fingertip/3d were as follows: Study 1: −0.09 (−0.280, 0.092)/ −0.18 (−0.442, 0.072); Study 2: −0.72 (−0.839, −0.610)/ −1.02 (−1.150, −0.882); and Study 3: −0.26 (−0.387, −0.123)/ −0.92 (−1.055, −0.793). Pooled analysis indicated test treatment significantly reduced DH versus control (both timepoints, both measures). Toothpastes were generally well‐tolerated.
Conclusion
Studies indicated that single, fingertip application of a SnF2 toothpaste reduced DH versus a control. DH relief increased over 3 days.
Keywords: clinical study, controls, dentin sensitivity, tin fluoride
1. INTRODUCTION
Dentine hypersensitivity (DH) is defined as ‘pain derived from exposed dentine in response to chemical, thermal, tactile or osmotic stimuli’ (Addy, 1990). DH occurs when dentine tubules are patent following dentine exposure, most frequently from gingival recession or enamel wear (Rimondini, Baroni, & Carrassi, 1995; West, Lussi, Seong, & Hellwig, 2013), and stimuli cause fluid movement within tubules leading to pulpal nerve excitation and subsequent pain (Brännström, 1963). Dentine hypersensitivity can negatively impact oral health‐related quality of life due to frequent pain insults (Gibson et al., 2010).
Dentine hypersensitivity treatment focuses on two approaches: occluding dentine tubules or blocking impulse transmission in dentinal nerves. The former relies on ingredients that form solid deposits which occlude/partially occlude dentine tubules so external stimuli do not cause substantial shifts in fluid movement (Earl & Langford, 2013; Earl, Ward, & Langford, 2010). Such agents include strontium or stannous salts (Makin, 2013; Markowitz, 2009; Mason et al., 2010; West, Seong, & Davis, 2015), arginine plus insoluble calcium salts (Bae, Kim, & Myung, 2015; Hughes et al., 2010) or bioglasses (Gendreau, Barlow, & Mason, 2011; Pradeep & Sharma, 2010). This approach has potential to work from first application (Ayad et al., 2009; Creeth, Gallob, et al., 2017; Creeth, Goyal, et al., 2017; Fu et al., 2010; He, Barker, Qaqish, & Sharma, 2011; He, Chang, et al., 2011; He, Cheng, Biesbrock, Chang, & Sun, 2011; Mason et al., 2010; Nathoo et al., 2009; Parkinson et al., 2013; Sharma, Roy, Kakar, Greenspan, & Scott, 2011; West, Newcombe, et al., 2013). Blocking of impulse transmission has been achieved by potassium ions (Bae et al., 2015; Markowitz, 2009); however, repeated administration appears to be required before symptomatic relief occurs (West, Seong, & Davies, 2014).
Directly applying an occluding desensitizing toothpaste to reduce DH can be achieved through focused brushing or fingertip application. In the former, sensitive teeth are brushed first, followed by whole‐mouth brushing (Creeth, Gallob, et al., 2017; Creeth, Goyal, et al., 2017; He, Barker, et al., 2011; He, Chang, et al., 2011; He, Cheng, et al., 2011; Parkinson et al., 2013; Sharma et al., 2011). In the latter, toothpaste is gently massaged into sensitive teeth (Ayad et al., 2009; Fu et al., 2010; Mason et al., 2010; Nathoo et al., 2009; Schiff et al., 2009; West, Newcombe, et al., 2013), a technique frequently recommended by oral healthcare professionals for immediate DH symptom relief.
Stannous ions, most commonly used in toothpastes as stannous fluoride (SnF2), have been demonstrated to occlude dentine tubules in vitro (Burnett, 2013; Burnett, Wilson, & Lucas, 2013; Earl & Langford, 2013; Khan & Wilson, 2017). SnF2 toothpaste formulations have been used for several decades (Makin, 2013; Schiff, He, Sagel, & Baker, 2006) and are widely accepted as an effective DH treatment (Bae et al., 2015; West et al., 2015). Short‐term studies (up to 3 days) have overall been positive for SnF2 toothpastes applied using the focused brushing technique, with many demonstrating clinical efficacy versus a control toothpaste (Creeth, Gallob, et al., 2017; Creeth, Goyal, et al., 2017; He, Barker, et al., 2011; He, Chang, et al., 2011; He, Cheng, et al., 2011; Parkinson et al., 2016; Sharma et al., 2011). However, some DH studies with a SnF2 (Parkinson et al., 2016) or stannous chloride (Cepeda‐Bravo et al., 2014) toothpaste have not shown differences.
There appears to be no published assessment of DH relief from SnF2 toothpastes applied using a fingertip technique. The three clinical studies presented here addressed this question using an experimental SnF2 toothpaste incorporating an anhydrous base (to stabilize stannous ions against oxidation and hydrolysis) and the polyphosphate pentasodium triphosphate (to control stannous ions’ propensity to stain enamel (Addy, Moran, Griffiths, & Wills‐Wood, 1985). The objective was to determine whether this formulation could provide rapid, effective, symptomatic DH relief when applied by the fingertip technique after a single application, and also after 3 days’ twice‐daily application by focused brushing, compared to a conventional fluoride toothpaste. An exploratory, post hoc, pooled analysis was carried out combining all results to estimate overall efficacy across the three studies.
2. MATERIALS AND METHODS
These randomized, examiner‐blind, two‐treatment arm, parallel design studies were stratified by maximum baseline Schiff sensitivity score. Study 1 was conducted at two centres of a UK clinical research facility; Studies 2 and 3 were conducted at a UK dental school. The third study was performed after the first two studies had been completed. All studies were conducted in accordance with the Declaration of Helsinki, approved by independent research ethics committees before initiation and registered at ClinicalTrials.org: Study 1 (NCT02612064): North‐West Lancaster REC, #15/NW/0784; Study 2 (NCT02751450): NRES South West—Exeter REC, 16/SW/0006; and Study 3 (NCT02924350): NRES West Midlands—South Birmingham REC, 16/WM/0407.
2.1. Participants
Studies enrolled healthy participants aged 18–55 (Study 1) or 18–65 (Studies 2/3) years with no clinically significant or relevant abnormalities on oral examination. Participants had ≥20 natural teeth and a self‐reported history of DH between 6 months and 10 years. At screening, eligible participants had at least two non‐adjacent accessible teeth (incisors, canines or premolars) with dentine exposure at the cervical margin, a Modified Gingival Index (MGI) (Lobene, Weatherford, Ross, Lamm, & Menaker, 1986) score of 0 adjacent to the test area, no mobility, and a positive response to a qualifying evaporative (air) assessment. At baseline (Day 0), eligible participants had a minimum of two accessible, non‐adjacent teeth with signs of DH, determined by a qualifying tactile stimulus threshold of ≤20 g and a Schiff sensitivity score ≥2 (Schiff et al., 1994).
Exclusion criteria included pregnancy; breastfeeding; allergy/intolerance to study materials; any chronic debilitating disease that could affect study outcome; xerostomia; medication affecting pain perception; dental prophylaxis within 4 weeks, vital tooth bleaching within 8 weeks or scaling within 3 months of screening; periodontal disease; dental implants (test teeth only for Study 3); full coverage restorations; orthodontic brackets; caries; and sensitive teeth not expected to respond to treatment with over‐the‐counter toothpastes. Study 2 participants were ineligible for Study 3.
2.2. Procedures
At the screening visit, each participant provided written informed consent before their demographic characteristics, medical history and medication use were recorded. An oral soft tissue (OST) examination was conducted. Participants’ dentition was assessed sequentially for missing teeth and teeth excluded as per the criteria; evidence of dentine exposure; gingival health status (MGI); tooth mobility; sensitivity to an evaporative (air) stimulus (where a ‘yes’ response indicated sensitivity). Eligible participants were supplied with an acclimatization toothpaste (Signal® Family Protection, Unilever) and toothbrush (Aquafresh® Clean Control [Everyday Clean], GSK Consumer Healthcare) to use twice daily for 4–8 weeks between screening and baseline visits (acclimatization period). First toothpaste use was carried out under supervision.
Before subsequent study visits, participants refrained from brushing their teeth and using any other oral hygiene aids, and from taking analgesics for ≥8 hr, from eating and drinking for ≥4 hr and from excessive alcohol consumption for 24 hr. Sips of water were permitted, but not within 1 hr of the study visit. During the study, participants could not use any other dental products, apart from dental floss for impacted food removal, and refrained from any non‐emergency dental treatment.
At the baseline visit (Day 0), ongoing eligibility was assessed, any adverse events and changes to concomitant medications were recorded, and acclimatization toothpaste compliance was confirmed. Following an OST examination, sensitivity of eligible teeth identified at screening was evaluated. The examiner selected two non‐adjacent teeth, designated ‘test teeth’, from those that met the qualifying sensitivity assessments to be evaluated throughout the study.
Eligible participants were randomized to one of two toothpastes according to a schedule provided by the study sponsor's biostatistics department. Randomization was stratified by test teeth maximum baseline Schiff sensitivity score (2/3). The test toothpaste contained 0.454% SnF2 (1,100 ppm fluoride) and 5% pentasodium triphosphate in an anhydrous glycerin‐based formulation. The control toothpaste contained 0.76% sodium monofluorophosphate (1,000 ppm fluoride) (Colgate® Cavity Protection; Colgate‐Palmolive) in a conventional aqueous formulation. Study products were overwrapped to blind participants to identity. The dental examiner, study statistician, data management staff and other sponsor employees were blinded to toothpaste allocation.
To assess the effect of a single fingertip application of toothpaste, participants (under supervision) applied a pea‐sized amount of assigned study product to their fingertip and gently rubbed the toothpaste onto exposed dentine at the cervical margin of one of the two test teeth for 60 s. They repeated the procedure on the other test tooth. No rinsing was permitted. DH was then measured using evaporative (air) and tactile stimuli.
Prior to leaving the study centre, participants brushed (under supervision) with their assigned toothpaste. Test group participants used a focused brushing technique where they first brushed each of the two test teeth with a full ribbon of toothpaste on the toothbrush and then brushed their whole mouth for at least 60 s. Control group participants brushed their whole mouth with a full ribbon of toothpaste in their usual manner for at least 60 s. Participants could rinse with 5 ml tap water for up to 5 s. At home, participants followed their assigned brushing regimen for 3 days, twice daily and then returned to the study centre. Following confirmation of ongoing study compliance and an OST examination, final DH assessments were undertaken.
2.3. Assessments
In accordance with consensus guidelines (Holland, Narhi, Addy, Gangarosa, & Orchardson, 1997), two independent, stimulus‐based clinical measures were used to assess DH. Firstly, a tactile stimulus was administered using a constant‐pressure (Yeaple) probe (Polson, Caton, Yeaple, & Zander, 1980) to the exposed sensitive dentine. Testing began at 10 g of pressure and then increased in 10 g increments for each successive challenge until the tactile threshold was reached where the participant gave two consecutive ‘yes’ responses, indicating the stimulus caused pain/discomfort, at the same pressure setting. At baseline, a maximum force of 20 g was used. At subsequent visits, if no response was given by 80 g, the reading was recorded as >80 g.
After a minimum 5 min recovery period, evaporative sensitivity was assessed by directing air from 1 cm for 1 s from a dental air syringe onto the exposed dentine surface, with the test tooth surface isolated to prevent exposure of adjacent teeth or surrounding soft tissue. The examiner's assessment of the participant's response to the evaporative (air) stimulus was recorded using the Schiff sensitivity scale (0 = participant does not respond to air stimulus; 1 = participant responds to air stimulus but does not request discontinuation; 2 = participant responds to air stimulus and requests discontinuation or moves from stimulus; 3 = participant responds to air stimulus, considers stimulus to be painful and requests discontinuation of the stimulus) (Schiff et al., 1994). For logistical reasons, in Study 1, separate examiners performed the two assessments, and in Studies 2 and 3, the same examiner performed both assessments.
2.4. Safety
Spontaneously reported adverse events (AEs) and OST examination abnormalities were recorded from first use of acclimatization toothpaste until 5 days after last use of study toothpaste. The investigator graded each AE (mild, moderate, severe) and assessed whether they were treatment‐related. Treatment‐emergent AEs (TEAEs) were reported for the safety population, which included all randomized participants.
2.5. Data analysis
2.5.1. Sample size determination
Based on outcomes from previous studies (Goyal, Sufi, Qaqish, & Creeth, 2017; Parkinson et al., 2016), for Studies 1 and 2, sufficient participants were screened to ensure approximately 107 per group completed the study to give 80% power to detect a mean difference of 0.25 (standard deviation [SD] 0.6487) between treatment groups in change from baseline in Schiff sensitivity score after 3 days' use using a two‐sided t test of significance level 0.05. This represents a potentially clinically meaningful difference. Study 3 was powered based on Studies 1 and 2 outcomes; sufficient participants were screened to ensure approximately 92 evaluable participants per group completed the study to give 90% power to detect a mean between‐treatment difference of 0.25 units in Schiff sensitivity score (SD 0.5198) after 3 days’ use.
2.5.2. Efficacy analyses
All statistical analyses were conducted using SAS version 9.2 (SAS Institute Inc.).
2.5.3. Individual study analysis
The primary objective was to investigate the ability of the test treatment to reduce DH as elicited by evaporative (air) stimulus after 3 days' use, compared to the control treatment. Secondary objectives included this comparison after a tactile stimulus and comparison between treatments with both efficacy measures after a single fingertip application. Efficacy endpoints were change from baseline (mean of the two selected test teeth) for each efficacy measure at each timepoint. Efficacy analyses were performed on the intent‐to‐treat (ITT) population, defined as all randomized participants who provided at least one post‐baseline assessment of efficacy.
Change from baseline was evaluated by analysis of covariance (ANCOVA). For Study 1 Schiff sensitivity score data, treatment group and study site were factors and baseline Schiff sensitivity score was a covariate. For Study 1 tactile threshold data, treatment group, study site and baseline Schiff sensitivity score stratification value were factors with baseline tactile threshold as a covariate. Similar analyses were performed for Studies 2 and 3 without study site as a factor.
2.5.4. Pooled analysis
As an exploratory, post hoc analysis, data from all three studies were pooled and analysed based on the individual study ITT populations. Change from baseline in Schiff sensitivity scores at each timepoint was analysed using an ANCOVA with factors for treatment and study with baseline (Schiff sensitivity score) as a covariate. Tactile threshold data were analysed similarly except baseline Schiff stratification score was included as a factor and the covariate was baseline tactile threshold. The effects of age (median age ≤30 years/>30 years) and gender (male/female) were investigated by introducing these as factors in the model.
An experimental ‘responder’ analysis was performed on change in Schiff sensitivity score, whereby an individual with a Schiff sensitivity score reduction of ≥1 was considered a ‘responder’, otherwise a ‘non‐responder’. This was repeated for a Schiff sensitivity score reduction of ≥0.5. Analyses were conducted separately for each timepoint using logistic regression via PROC LOGISTIC. Factors were the same as for the ANCOVA model.
3. RESULTS
Details of participant study flow are shown in Figure 1. Treatment group demographic characteristics were similar across groups for the safety and ITT populations of all studies (Table 1). There were differences in baseline Schiff score distribution and mean participant age between studies, with Study 1 having both the highest proportion of those with baseline Schiff score 3, and the highest mean age. Study times (first participant enrolled/last participant completed) were as follows: Study 1: November 2015/June 2016; Study 2: February 2016/June 2016; and Study 3: November 2016/March 2017.
Figure 1.
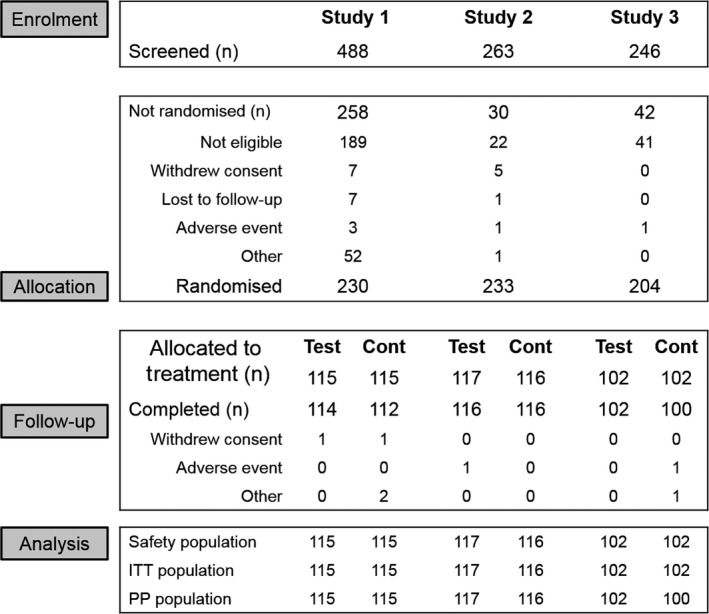
Participant disposition throughout study
Table 1.
Summary of baseline characteristics (safety population)
| Characteristic | Study 1 | Study 2 | Study 3 | |||
|---|---|---|---|---|---|---|
|
Test (n = 115) |
Control (n = 115) |
Test (n = 117) |
Control (n = 116) |
Test (n = 102) |
Control (n = 102) |
|
| Sex, n (%) | ||||||
| Male | 24 (20.9) | 23 (20.0) | 36 (30.8) | 31 (26.7) | 37 (36.3) | 28 (27.5) |
| Female | 91 (79.1) | 92 (80.0) | 81 (69.2) | 85 (73.3) | 65 (63.7) | 74 (72.5) |
| Age, years | ||||||
| Mean (SD) | 40.7 (8.62) | 39.9 (9.18) | 34.3 (12.92) | 32.8 (11.52) | 22.8 (6.61) | 22.2 (5.12) |
| Range | 20–55 | 18–55 | 18–64 | 18–64 | 18–55 | 18–51 |
| Race, n (%) | ||||||
| White | 112 (97.4) | 112 (97.4) | 93 (79.5) | 102 (87.9) | 85 (83.3) | 90 (88.2) |
| Black | 2 (1.7) | 0 | 4 (3.4) | 3 (2.6) | 5 (4.9) | 4 (3.9) |
| Asian | 1 (0.9) | 1 (0.9) | 20 (17.1) | 8 (6.9) | 11 (10.8) | 5 (4.9) |
| Other | 0 | 2 (1.7) | 0 | 3 (2.6) | 1 (1.0) | 3 (2.9) |
| Schiff strata 2, n (%) | 12 (10.4) | 11 (9.6) | 98 (83.8) | 98 (84.5) | 75 (73.5) | 76 (74.5) |
| Schiff strata 3, n (%) | 103 (89.6) | 104 (90.4) | 19 (16.2) | 18 (15.5) | 27 (26.5) | 26 (25.5) |
3.1. Efficacy
In Study 1, treatment efficacy was not influenced by study centre, so this interaction term was not included in the model.
In all studies, for Schiff sensitivity scores, both test and control groups showed a statistically significant decrease from baseline after both single fingertip application (‘immediate’ use) and 3 days' brushing (Table 2, Figure 2a). In Studies 2 and 3, at both timepoints, the test treatment reduced Schiff scores significantly more than did the control (Table 2). Differences at all timepoints were considered clinically relevant (above 0.25 units). However, differences in Schiff scores between test and control did not reach significance at either timepoint in Study 1.
Table 2.
Change from baseline and between‐treatment comparisons for change in Schiff sensitivity score and tactile threshold (intent‐to‐treat population)
| Test | Control | Test versus controlc | |
|---|---|---|---|
| Schiff sensitivity scorea | |||
| Study 1 | |||
| Baseline | 2.82 (0.031) | 2.80 (0.031) | |
| Immediate |
−0.41 (−0.54, −0.27) p < .0001 |
−0.31 (−0.44, −0.18) p < .0001 |
−0.09 (−0.280, 0.092) p = .3226 |
| Day 3 |
−0.96 (−1.14, −0.78) p < .0001 |
−0.77 (−0.95, −0.59) p < .0001 |
−0.18 (−0.442, 0.072) p = .1575 |
| Study 2 | |||
| Baseline | 2.12 (0.028) | 2.12 (0.028) | |
| Immediate |
−0.89 (−0.97, −0.81) p < .0001 |
−0.17 (−0.25, −0.09) p < .0001 |
−0.72 (−0.839, −0.610) p < .0001 |
| Day 3 |
−1.40 (−1.49, −1.30) p < .0001 |
−0.38 (−0.48, 0.28) p < .0001 |
−1.02 (−1.150, −0.882) p < .0001 |
| Study 3 | |||
| Baseline | 2.18 (0.033) | 2.18 (0.033) | |
| Immediate |
−0.63 (−0.73, −0.54) p < .0001 |
−0.38 (−0.47, 0.28) p < .0001 |
−0.26 (−0.387, −0.123) p = .0002 |
| Day 3 |
−1.37 (−1.46, −1.28) p < .0001 |
−0.45 (−0.54, 0.35) p < .0001 |
−0.92 (−1.055, −0.793) p < .0001 |
| Tactile threshold (g)b | |||
| Study 1 | |||
| Baseline | 10 (10, 20) | 10 (10, 20) | |
| Immediate | 0 (−5, 65)d | 0 (−10, 40)d | p = .3372 |
| Day 3 | 10 (−10, 80)d | 5 (−10, 80)d | p = .3719 |
| Study 2 | |||
| Baseline | 10.85 (0.229) | 10.69 (0.193) | |
| Immediate |
19.42 (17.16, 21.68) p < .0001 |
3.74 (1.47, 6.01) p = .0014 |
15.68 (12.476, 18.883) p < .0001 |
| Day 3 |
35.48 (32.40, 38.55) p < .0001 |
6.55 (3.47, 9.62) p < .0001 |
28.93 (24.576, 33.277) p < .0001 |
| Study 3 | |||
| Baseline | 12.35 (0.373) | 11.76 (0.346) | |
| Immediate |
15.19 (12.81, 17.56) p < .0001 |
8.05 (5.67, 10.43) p < .0001 |
7.14 (3.768, 10.506) p < .0001 |
| Day 3 |
32.87 (30.03, 35.71) p < .0001 |
10.37 (7.50, 13.24) p < .0001 |
22.50 (18.458, 26.541) p < .0001 |
Baseline values are raw mean scores (± standard error); post‐baseline values are adjusted mean changes from baseline (± 95% confidence interval).
Baseline values are raw median scores (minimum, maximum) for Study 1, raw mean scores (± standard error) for Studies 2 and 3; post‐baseline values are adjusted median change from baseline scores (minimum, maximum) for Study 1, adjusted mean change from baseline scores (± 95% confidence interval) for Studies 2 and 3.
Difference (95% confidence) p‐value from ANCOVA model: first‐named minus second‐named group. For Schiff sensitivity score, a negative difference favours the first‐named group. For Study 1 tactile threshold, p‐value is from a van Elteren nonparametric test. For tactile threshold (Studies 2 and 3 only), a positive difference favours the first‐named group.
The analysis of the statistical significance of change from baseline in Tactile threshold was not performed for Study 1.
Figure 2.
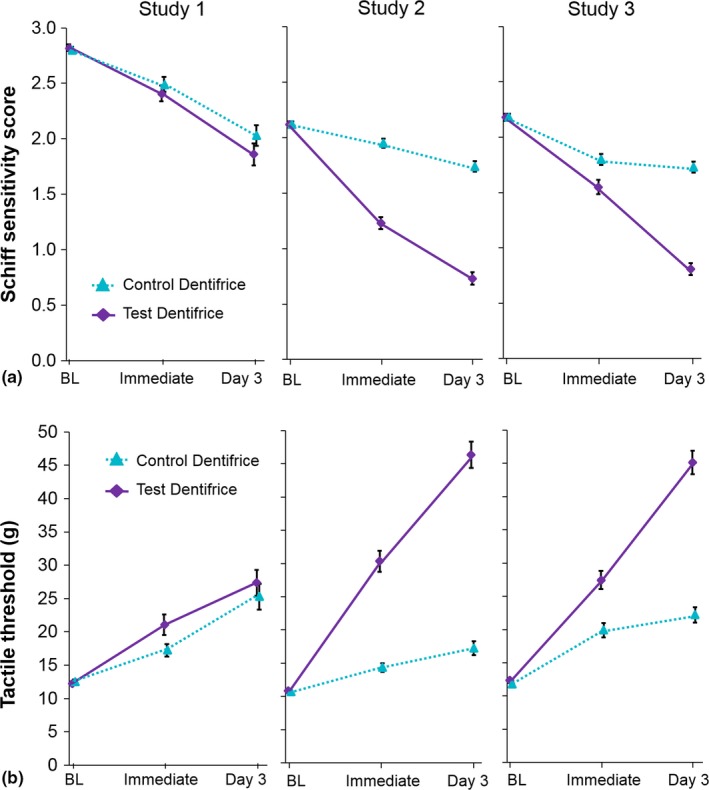
Mean (± standard error) a) Schiff sensitivity scores and b) tactile threshold (intent‐to‐treat population). Data offset for clarity; BL, baseline; tactile threshold values range from 0 to 80 g
There was a statistically significant increase from baseline in tactile threshold scores for both treatments in Studies 2 and 3 at both timepoints (Table 2, Figure 2b). For Study 1 tactile threshold data, evidence of departure from model assumptions meant a nonparametric van Elteren test was performed; differences from baseline were not significant. For Studies 2 and 3, the test treatment gave statistically significantly higher tactile threshold scores versus control following both immediate and 3 days’ use. As for the Schiff data, there were no significant differences between treatments in Study 1 (Table 2).
In all studies, for both treatments and measures, DH relief increased over time.
3.2. Pooled analysis
The post hoc pooled analysis demonstrated a statistically significantly greater decrease in Schiff sensitivity score and increase in tactile threshold for the test versus control treatment after both a single fingertip application and 3 days' use (Table 3; Figure 3), with degree of difference increasing over time (both measures). Allowing for the between‐study differences in efficacy as a function of age or gender showed almost identical results (Figure 3), indicating these did not meaningfully influence overall outcome.
Table 3.
Exploratory pooled analysis of Schiff sensitivity and tactile threshold scores (post hoc, intent‐to‐treat population)
| Test (n = 334) | Control (n = 333) | Test versus controlb | |
|---|---|---|---|
| Schiff sensitivity scorea | |||
| Immediate |
−0.65 (−0.71, −0.58) p <.0001 |
−0.28 (−0.34, −0.22) p <.0001 |
−0.36 (−0.45, −0.28) p <.0001 |
| Day 3 |
−1.24 (−1.32, −1.16) p <.0001 |
−0.53 (−0.61, −0.46) p <.0001 |
−0.70 (−0.81, −0.59) p <.0001 |
| Tactile threshold (g)a | |||
| Immediate |
14.47 (13.07, 15.87) p <.0001 |
5.41 (4.01, 6.81) p <.0001 |
9.06 (7.08, 11.04) p <.0001 |
| Day 3 |
27.66 (25.69, 29.63) p <.0001 |
9.85 (7.87, 11.83) p <.0001 |
17.81 (15.01, 20.60) p <.0001 |
Adjusted mean change from baseline, 95% confidence intervals and p‐value from ANCOVA model with treatment and study as factors (and Schiff sensitivity score for tactile threshold) and baseline Schiff sensitivity score or tactile threshold, as appropriate, as covariate
Negative difference favours the test toothpaste for the Schiff sensitivity score; positive difference favours the test toothpaste for the tactile threshold score
Figure 3.
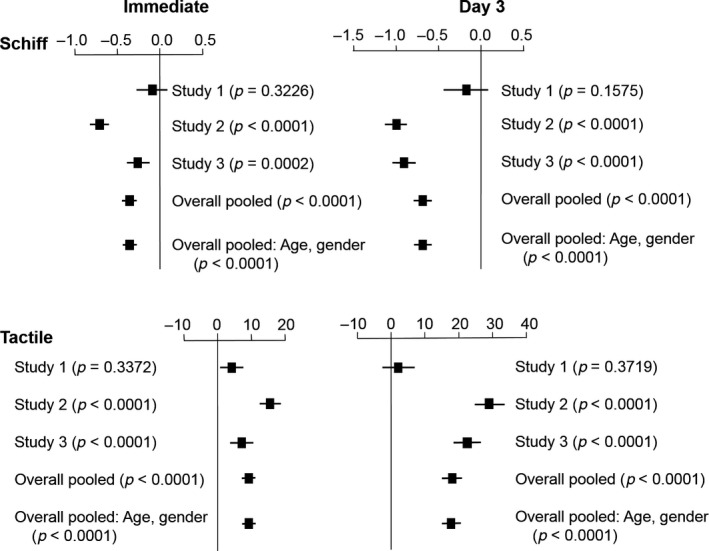
Schiff sensitivity score and tactile threshold mean between‐treatment difference and 95% CIs for each individual study and pooled analysis including age, gender and baseline score as factors (intent‐to‐treat population), immediately after fingertip application and on Day 3. In Study 1, mean and 95% CIs from ANCOVA, p‐value from nonparametric analysis
The experimental responder analysis (Table 4) found that after a single fingertip application, the odds ratio for likelihood of experiencing a decrease in Schiff sensitivity score of at least 1 for the test group versus the control was 4.4. The equivalent odds ratio for a 0.5‐point decrease was 3.2. After 3 days' use, odds ratios were 8.7 and 4.8, respectively. Differences were statistically significant at both timepoints, for both Schiff‐score changes.
Table 4.
Exploratory responder analysis for Schiff sensitivity score: pooled data (post hoc, intent‐to‐treat population)
| Responder rates | Odds ratio (95% CI)a | p‐value | ||
|---|---|---|---|---|
| Test | Control | Log odds (95%CI)b | ||
| 1‐point change in Schiff sensitivity score | ||||
| Immediate | 41.6% | 14.1% |
4.41 (3.02, 6.45) 1.48 (1.10, 1.86) |
p < .0001 |
| Day 3 | 75.9% | 27.1% |
8.69 (6.10, 12.38) 2.16 (1.80, 2.51) |
p < .0001 |
| 0.5‐point change in Schiff sensitivity score | ||||
| Immediate | 65.3% | 39.3% |
3.20 (2.29, 4.47) 1.16 (0.82, 1.49) |
p < .0001 |
| Day 3 | 84.9% | 56.4% |
4.78 (3.25, 7.04) 1.56 (1.17, 1.95) |
p < .0001 |
From logistic regression with factors for treatment and study with baseline Schiff sensitivity score as a covariate
Exponential (natural log odds and 95% confidence intervals)
3.3. Safety
No TEAE was considered treatment‐related. In Study 1, three test group participants reported four TEAEs; five control group participants reported six TEAEs. Four TEAEs were oral, in two test group participants and one control group participant. All TEAEs were mild (none serious) and resolved by study end. In Study 2, two test group participants reported two TEAEs; three control group participants reported three TEAEs. None were oral; four were mild and resolved by study end, one severe TEAE (prostate cancer) led to participant withdrew. In Study 3, nine test group participants reported nine TEAEs (one oral) and 11 control group participants reported 11 TEAEs (none oral); all were mild (none serious). One TEAE led to participant withdrawal (nasopharyngitis), all but one TEAE (gastric haemorrhage) resolved by study end.
4. DISCUSSION
This is the first known report of a SnF2‐based toothpaste reducing DH when applied directly by fingertip to hypersensitive teeth. This is important because it enables individuals with DH to achieve immediate relief using a gentle, controllable product‐application method. Using this technique, two of the three studies showed the experimental SnF2 toothpaste reduced DH after single application significantly more than the control toothpaste on both evaporative (air) and tactile assessments.
The post hoc pooled analysis of the three studies indicated that there were overall treatment differences, with the pooled benefit in Schiff score across the three studies of 0.36 units. This is considered clinically significant relief. A complementary perspective on the data was provided by the experimental responder analysis, which concluded the test group participants had an odds ratio of measurable DH relief several times higher than control group participants.
The fingertip application approach has been reported to be effective in clinical studies for two occlusion technologies: strontium acetate and arginine‐calcium carbonate (Ayad et al., 2009; Fu et al., 2010; Mason et al., 2010; Nathoo et al., 2009; Schiff et al., 2009). The current studies confirm that the test SnF2 toothpaste applied by fingertip can also provide immediate DH relief. The control toothpaste was not designed for DH treatment; however, the reduction in DH scores suggests that this toothpaste base can also provide some relief when massaged onto sensitive dentine. It is likely that abrasive particles and thickening agents within the formulation lodge at least temporarily in the tubule openings (West, Addy, & Hughes, 1998). The massaging action itself may also provide some minor relief (Ayad et al., 2009).
The fingertip application was followed by 3 days' twice‐daily brushing to gauge efficacy in a more conventional oral hygiene regimen. The test group used a focused‐brushing technique on the test teeth; those in the control group brushed without specifically treating sensitive areas. This approach was taken to follow previous studies (He, Barker, et al., 2011; He, Chang, et al., 2011; He, Cheng, et al., 2011; Sharma et al., 2011) on the premise that individuals follow manufacturers' instructions for a product. This treatment reduced DH relative to baseline after 3 days in all three studies, across both treatment groups. The post hoc pooled analysis showed DH relief was greater in the test group than the control, continuing to build over the 3 days to reach a Schiff score difference of 0.70, considered clinically significant relief. The experimental responder analysis reflected this conclusion, indicating a several‐fold higher odds ratio for experiencing relief among test group participants. This degree of effect is consistent with previous studies of this toothpaste when applied solely by toothbrushing: benefits were demonstrated after first use, increasing after 3 days (Creeth, Gallob, et al., 2017; Creeth, Goyal, et al., 2017).
The three different studies performed here gave a range of results. The third study, performed after the first two (which gave contrasting results), is helpful in reaching an overall conclusion regarding efficacy. Of potential relevance to the different results observed were differences in participant characteristics: in Study 1, almost all participants were in the higher Schiff stratum, while in Studies 2 and 3, the majority were in the lower stratum. Study 3 participants were generally younger than in Study 2, who in turn were generally younger than in Study 1.
The difference in average baseline Schiff score between studies is of particular interest, raising the question whether the lack of treatment difference observed in Study 1 is linked to the high proportion of participants with Schiff score 3 in that study. However, assessing the effect of baseline DH severity is confounded by the fact that this difference cannot be disentangled from other study‐to‐study differences. The pooled analysis considered differences in baseline DH severity, as well as differences in age and gender, and found none of these factors meaningfully affected the overall outcome.
The questions raised by these population differences regarding their influence on the analysis cannot be fully answered with available information; however, the authors believe the conclusion from the pooled analysis, indicating that clear differences exist between the effects of test and reference treatments, is appropriate.
To clarify whether baseline Schiff score influences the difference in efficacy between the test and control treatments, a further, separate study would be required, with sufficient participants in each baseline Schiff score stratum to permit a valid analysis.
Other factors may explain differences in results between studies. It is well known in pain studies that response to an inactive treatment can occur due to expectation of a treatment benefit (placebo effects) (Kirsch, 2013), to behavioural changes due to study participation (Hawthorne effects) (Benedetti, Carlino, & Piedimonte, 2016), or to the intrinsically episodic nature of DH, which may peak then resolve without intervention (West, Addy, Jackson, & Ridge, 1997). In addition, due to normal biological variation, between‐treatment differences shown in a single study are estimates that may be above or below the true difference. Although a range of control steps were taken to reduce their potential impact, including acclimatization and inclusion criteria specifying DH duration, all these factors could have influenced results.
In conclusion, the evidence from the three studies presented in this report shows that 0.454% SnF2 formulated into an anhydrous, polyphosphate‐containing base can reduce DH pain when applied once directly by fingertip to hypersensitive teeth, relative to a conventional fluoride toothpaste. DH relief increased over 3 days of twice‐daily brushing. This conclusion supports and extends previous studies of such toothpastes (Creeth, Gallob, et al., 2017; Creeth, Goyal, et al., 2017; Parkinson et al., 2013, 2016), which, taken together, demonstrate the experimental formulation's ability to provide short‐ and long‐term DH reduction.
CONFLICT OF INTEREST
J Creeth, P Gomez‐Pereira and F Sufi are employees of GSK Consumer Healthcare. Intertek Clinical Research Services (of whom R Maclure was an employee at the time, J Holt), the Clinical Trials Unit of Bristol Dental School and Hospital (J Seong, N Chapman, N West) and Syneos Health (of whom C Budhawant was an employee at the time) have all received funding from GSK Consumer Healthcare.
Clinical Relevance.
Scientific rationale for study: Stannous fluoride (SnF2) toothpastes have been shown in long‐term studies to reduce dentine hypersensitivity (DH). This investigation explored whether a SnF2 toothpaste applied by two focused methods—fingertip application alone or with focused brushing prior to whole‐mouth brushing—could reduce DH immediately and after short‐term use.
Principal findings: Across the three studies, the evidence showed the SnF2 toothpaste applied directly by fingertip reduced DH after a single use and, when applied by focused brushing, following 3 days’ use, versus a non‐sensitivity toothpaste.
Practical implications: When applied directly to affected teeth, toothpastes containing SnF2 can reduce DH immediately and with continued use.
ACKNOWLEDGEMENTS
The authors would like to thank study staff and participants as well as Nan Wang, formerly of GSK Consumer Healthcare, for help with statistical analysis and Eleanor Roberts, Beeline Science Communications, Ltd. (funded by GSK Consumer Healthcare), and Maria Davies, Bristol Dental School and Hospital, for help with manuscript preparation. Anonymized individual participant data and study documents can be requested for further research from http://www.clinicalstudydatarequest.com.
Creeth J, Maclure R, Seong J, et al. Three randomized studies of dentine hypersensitivity reduction after short‐term SnF2 toothpaste use. J Clin Periodontol. 2019;46:1105–1115. 10.1111/jcpe.13175
Funding information
This study was sponsored by GSK Consumer Healthcare.
REFERENCES
- Addy, M. (1990). Etiology and clinical implications of dentine hypersensitivity. Dental Clinics of North America, 34, 503–514. [PubMed] [Google Scholar]
- Addy, M. , Moran, J. , Griffiths, A. A. , & Wills‐Wood, N. J. (1985). Extrinsic tooth discoloration by metals and chlorhexidine. I. Surface protein denaturation or dietary precipitation? British Dental Journal, 159, 281–285. [DOI] [PubMed] [Google Scholar]
- Ayad, F. , Ayad, N. , Delgado, E. , Zhang, Y. P. , DeVizio, W. , Cummins, D. , & Mateo, L. R. (2009). Comparing the efficacy in providing instant relief of dentin hypersensitivity of a new toothpaste containing 8.0% arginine, calcium carbonate, and 1450 ppm fluoride to a benchmark desensitizing toothpaste containing 2% potassium ion and 1450 ppm fluoride, and to a control toothpaste with 1450 ppm fluoride: A 3‐day clinical study in Mississauga, Canada. Journal of Clinical Dentistry, 20, 115–122. [PubMed] [Google Scholar]
- Bae, J. H. , Kim, Y. K. , & Myung, S. K. (2015). Desensitizing toothpaste versus placebo for dentin hypersensitivity: A systematic review and meta‐analysis. Journal of Clinical Periodontology, 42, 131–141. 10.1111/jcpe.12347 [DOI] [PubMed] [Google Scholar]
- Benedetti, F. , Carlino, E. , & Piedimonte, A. (2016). Increasing uncertainty in CNS clinical trials: The role of placebo, nocebo, and Hawthorne effects. Lancet Neurology, 15, 736–747. 10.1016/S1474-4422(16)00066-1 [DOI] [PubMed] [Google Scholar]
- Brännström, M. (1963). A hydrodynamic mechanism in the transmission of pain producing stimuli through dentine In Anderson D. J. (Ed.). Sensory mechanisms in dentine. Proceedings of a symposium held at the Royal Society of Medicine, London, September 24th, 1962 (pp. 73–79). Oxford, UK: Pergamon Press. [Google Scholar]
- Burnett, G. (2013). The effect of an experimental anhydrous stannous fluoride dentifrice on the acid resistance of dentin smear layers. American Journal of Dentistry, 26, 15A–18A. [PubMed] [Google Scholar]
- Burnett, G. R. , Willson, R. J. , & Lucas, R. A. (2013). In vitro studies investigating the dentin tubule‐occlusion properties of an experimental anhydrous stannous fluoride dentifrice. American Journal of Dentistry, 26, 10A–14A. [PubMed] [Google Scholar]
- Cepeda‐Bravo, J. , Ayad, N. , Mateo, L. R. , Delgado, E. , Zhang, Y. P. , & Miller, S. (2014). Instant dentin hypersensitivity reduction efficacy of commercially available dentifrices. Journal of Dental Research, 93(Sp Iss B), abstract 1554. https://iadr.abstractarchives.com/abstract/43am-186025/instant-dentin-hypersensitivity-reduction-efficacy-ofcommercially-available-dentifrices [Google Scholar]
- Creeth, J. E. , Gallob, J. , Qaqish, J. , Sufi, F. , Patel, N. , & Goyal, C. R. (2017). Short‐term Efficacy Studies of an occluding dentifrice on dentinal hypersensitivity. Journal of Dental Research, 96(Sp Iss A), abstract 1543. https://iadr.abstractarchives.com/abstract/17iags-2635085/short-term-efficacy-studies-of-an-occludingdentifrice-on-dentinal-hypersensitivity [Google Scholar]
- Creeth, J. E. , Goyal, C. R. , Qaqish, J. , Sufi, F. , Gomez‐Pereira, P. , & Budhawant, C. (2017). Short‐term efficacy of an occluding toothpaste on dentinal hypersensitivity. International Dental Journal, 67(Suppl S1), abstract P122. [Google Scholar]
- Earl, J. S. , & Langford, R. M. (2013). Physical and chemical characterization of the surface layers formed on dentin following treatment with an experimental anhydrous stannous fluoride toothpaste. American Journal of Dentistry, 26, 19A–24A. [PubMed] [Google Scholar]
- Earl, J. S. , Ward, M. B. , & Langford, R. M. (2010). Investigation of dentinal tubule occlusion using FIB‐SEM milling and EDX. Journal of Clinical Dentistry, 21(2), 37–41. [PubMed] [Google Scholar]
- Fu, Y. , Li, X. , Que, K. , Wang, M. , Hu, D. , Mateo, L. R. , … Zhang, Y. P. (2010). Instant dentin hypersensitivity relief of a new desensitizing toothpaste containing 8.0% arginine, a high cleaning calcium carbonate system and 1450 ppm fluoride: A 3‐day clinical study in Chengdu, China. American Journal of Dentistry, 23(Spec No), 20A–27A. [PubMed] [Google Scholar]
- Gendreau, L. , Barlow, A. P. , & Mason, S. C. (2011). Overview of the clinical evidence for the use of NovaMin in providing relief from the pain of dentin hypersensitivity. Journal of Clinical Dentistry, 22, 90–95. [PubMed] [Google Scholar]
- Gibson, B. , Boiko, O. V. , Baker, S. , Robinson, P. G. , Barlow, A. , Player, T. , & Locker, D. (2010). The everyday impact of dentine sensitivity: Personal and functional aspects. Social Science and Dentistry, 1, 11–20. [Google Scholar]
- Goyal, C. , Sufi, F. , Qaqish, J. , & Creeth, J. (2017). Two‐week efficacy of an occluding toothpaste on dentinal hypersensitivity. Journal of Dental Research, 96(Spec Iss A), abstract 1544. https://iadr.abstractarchives.com/abstract/17iags-2639966/two-week-efficacy-of-an-occluding-dentifrice-ondentinal-hypersensitivity [Google Scholar]
- He, T. , Barker, M. L. , Qaqish, J. , & Sharma, N. (2011). Fast onset sensitivity relief of a 0.454% stannous fluoride toothpaste. Journal of Clinical Dentistry, 22, 46–50. [PubMed] [Google Scholar]
- He, T. , Chang, J. , Cheng, R. , Li, X. , Sun, L. , & Biesbrock, A. R. (2011). Clinical evaluation of the fast onset and sustained sensitivity relief of a 0.454% stannous fluoride toothpaste compared to an 8.0% arginine‐calcium carbonate‐sodium monofluorophosphate toothpaste. American Journal of Dentistry, 24, 336–340. [PubMed] [Google Scholar]
- He, T. , Cheng, R. , Biesbrock, A. P. , Chang, A. , & Sun, L. (2011). Rapid desensitizing efficacy of a stannous‐containing sodium fluoride toothpaste. Journal of Clinical Dentistry, 22, 40–45. [PubMed] [Google Scholar]
- Holland, G. R. , Narhi, M. N. , Addy, M. , Gangarosa, L. , & Orchardson, R. (1997). Guidelines for the design and conduct of clinical trials on DH. Journal of Clinical Periodontology, 24, 808–813. [DOI] [PubMed] [Google Scholar]
- Hughes, N. , Mason, S. , Jeffery, P. , Welton, H. , Tobin, M. , O’Shea, C. , & Browne, M. (2010). A comparative clinical study investigating the efficacy of a test toothpaste containing 8 % strontium acetate and 1040 ppm sodium fluoride versus a marketed control toothpaste containing 8 % arginine, calcium carbonate, and 1450 ppm sodium monofluorophosphate in reducing dentinal hypersensitivity. Journal of Clinical Dentistry, 21, 49–55. [PubMed] [Google Scholar]
- Khan, S. , & Wilson, R. (2017). Dentine permeability by an experimental formulation containing stannous fluoride. Journal of Dental Research, 96(Spec Iss A), abstract 2122. https://iadr.abstractarchives.com/abstract/17iags-2631820/dentine-permeability-by-an-experimentalformulation-containing-stannous-fluoride [Google Scholar]
- Kirsch, I. (2013). The placebo effect revisited: Lessons learned to date. Complementary Therapies in Medicine, 21, 102–104. 10.1016/j.ctim.2012.12.003 [DOI] [PubMed] [Google Scholar]
- Lobene, R. R. , Weatherford, T. , Ross, N. M. , Lamm, R. A. , & Menaker, L. (1986). A modified gingival index for use in clinical trials. Clinical and Preventative Dentistry, 8, 3–6. [PubMed] [Google Scholar]
- Makin, S. A. (2013). Stannous fluoride toothpastes. American Journal of Dentistry, 26(Spec no A), 3A–9A. [PubMed] [Google Scholar]
- Markowitz, K. (2009). The original desensitizers: Strontium and potassium salts. Journal of Clinical Dentistry, 20, 145–151. [PubMed] [Google Scholar]
- Mason, S. , Hughes, N. , Sufi, F. , Bannon, L. , Maggio, B. , & North, M. (2010). A comparative clinical study investigating the efficacy of a toothpaste containing 8% strontium acetate and 1040 ppm fluoride in a silica base and a control toothpaste containing 1450 ppm fluoride in a silica base to provide immediate relief of dentin hypersensitivity. Journal of Clinical Dentistry, 21(2), 42–48. [PubMed] [Google Scholar]
- Nathoo, S. , Delgado, E. , Zhang, Y. P. , DeVizio, W. , Cummins, D. , & Mateo, L. R. (2009). Comparing the efficacy in providing instant relief of dentin hypersensitivity of a new toothpaste containing 8.0% arginine, calcium carbonate, and 1450 ppm fluoride to a benchmark desensitizing toothpaste containing 2% potassium ion and 1450 ppm fluoride and to a control toothpaste with 1450 ppm fluoride: A three‐day clinical study in New Jersey, USA. Journal of Clinical Dentistry, 20, 123–130. [PubMed] [Google Scholar]
- Parkinson, C. R. , Hughes, N. N. , Hall, C. , Whelton, H. , Gallob, J. , & Mason, S. (2016). Three randomized clinical trials to assess the short‐term efficacy of anhydrous 0.454% w/w stannous fluoride toothpastes for the relief of dentin hypersensitivity. American Journal of Dentistry, 29, 25–32. [PubMed] [Google Scholar]
- Parkinson, C. , Hughes, N. , Jeffery, P. , Jain, R. , Kennedy, L. , Qaqish, J. , … Mason, S. (2013). The efficacy of an experimental toothpaste containing 0.454% w/w stannous fluoride in providing relief from the pain of dentin hypersensitivity: An 8‐week clinical study. American Journal of Dentistry, 26, 25A–31A. [PubMed] [Google Scholar]
- Polson, A. M. , Caton, J. G. , Yeaple, R. N. , & Zander, H. A. (1980). Histological determination of probe tip penetration into gingival sulcus of humans using an electronic pressure‐sensitive probe. Journal of Clinical Periodontology, 7, 479–488. 10.1111/j.1600-051X.1980.tb02154.x [DOI] [PubMed] [Google Scholar]
- Pradeep, A. R. , & Sharma, A. (2010). Comparison of clinical efficacy of a toothpaste containing calcium sodium phosphosilicate to a toothpaste containing potassium nitrate and to a placebo on dentinal hypersensitivity: A randomized clinical trial. Journal of Periodontology, 81, 1167–1173. 10.1902/jop.2010.100056 [DOI] [PubMed] [Google Scholar]
- Rimondini, L. , Baroni, C. , & Carrassi, A. (1995). Ultrastructure of hypersensitive and non‐sensitive dentine. A study on replica models. Journal of Clinical Periodontology, 22, 899–902. 10.1111/j.1600-051X.1995.tb01792.x [DOI] [PubMed] [Google Scholar]
- Schiff, T. , Delgado, E. , Zhang, Y. P. , Cummins, D. , DeVizio, W. , & Mateo, L. R. (2009). Dentin hypersensitivity: Effective treatment with an in‐office desensitizing paste containing 8% arginine and calcium carbonate. American Journal of Dentistry, 22(Spec no A), 3A–7A. [PubMed] [Google Scholar]
- Schiff, T. , Dotson, M. , Cohen, S. , De Vizio, W. , McCool, J. , & Volpe, A. (1994). Efficacy of a toothpaste containing potassium nitrate, soluble pyrophosphate, PVM/MA copolymer, and sodium fluoride on dentinal hypersensitivity: A twelve‐week clinical study. Journal of Clinical Dentistry, 5(Spec, No), 87–92. [PubMed] [Google Scholar]
- Schiff, T. , He, T. , Sagel, L. , & Baker, R. (2006). Efficacy and safety of a novel stabilized stannous fluoride and sodium hexametaphosphate toothpaste for dentinal hypersensitivity. Journal of Contemporary Dental Practice, 7, 1–10. [PubMed] [Google Scholar]
- Sharma, N. , Roy, S. , Kakar, A. , Greenspan, D. , & Scott, R. (2011). Instant sensitivity relief of a stannous containing sodium fluoride toothpaste. Journal of Dental Research, 90(Sp Iss A), abstract 1476. https://iadr.abstractarchives.com/abstract/2011sandiego-149747/instant-sensitivity-relief-of-a-stannouscontaining-sodium-fluoride-dentifrice [Google Scholar]
- West, N. , Addy, M. , & Hughes, J. (1998). Dentine hypersensitivity: The effects of brushing desensitizing toothpastes, their solid and liquid phases, and detergents on dentine and acrylic: Studies in vitro. Journal of Oral Rehabilitation, 25, 885–895. [DOI] [PubMed] [Google Scholar]
- West, N. X. , Addy, M. , Jackson, R. J. , & Ridge, D. B. (1997). Dentin hypersensitivity and the placebo response. A comparison of the effect of strontium acetate, potassium nitrate and fluoride toothpastes. Journal of Clinical Periodontology, 24, 209–215. [DOI] [PubMed] [Google Scholar]
- West, N. X. , Lussi, A. , Seong, J. , & Hellwig, E. (2013). Dentin hypersensitivity: Pain mechanisms and aetiology of exposed cervical dentin. Clinical Oral Investigations, 17(Suppl 1), S9–S19. 10.1007/s00784-012-0887-x [DOI] [PubMed] [Google Scholar]
- West, N. , Newcombe, R. G. , Hughes, N. , Mason, S. , Maggio, B. , Sufi, F. , & Claydon, N. (2013). A 3‐day randomized clinical study investigating the efficacy of two toothpastes, designed to occlude dentine tubules, for the treatment of DH. Journal of Dentistry, 41, 187–194. 10.1016/j.jdent.2012.11.007 [DOI] [PubMed] [Google Scholar]
- West, N. X. , Seong, J. , & Davies, M. (2014). Dentine Hypersensitivity In Lussi A., & Ganss C. (Eds.), Erosive tooth wear. Monogr Oral Sci (pp. 108–122). Basel, Switzerland: Karger. [Google Scholar]
- West, N. X. , Seong, J. , & Davies, M. (2015). Management of DH: Efficacy of professionally and self‐administered agents. Journal of Clinical Periodontology, 42(Suppl, 16), S256–S302. 10.1111/jcpe.12336 [DOI] [PubMed] [Google Scholar]


