ABSTRACT
Aims
To explore the effect of an online self‐management program in secondary care for men with lower urinary tract symptoms (LUTS).
Methods
We performed a prospective nonrandomized double‐cohort pilot study of consecutive adult men referred with uncomplicated LUTS to three urology outpatient departments. Men in both cohorts received care as usual from a urologist, but men in the intervention cohort also had access to an online self‐management program. Outcomes were assessed after 6 and 12 weeks: LUTS severity was assessed with the International Prostate Symptom Score (IPSS), the Overactive Bladder Questionnaire (OABq), and the Perceived Global Impression of Improvement (PGI‐I). The main outcome of interest was a clear improvement in the PGI‐I scores (“much better” or “very much better”).
Results
Age, symptom severity, and quality of life scores were comparable between the intervention (n = 113) and standard care (n = 54) cohorts. Clear improvement in the PGI‐I scores was reported after 12 weeks in 19.4% and 26.1% of men in the intervention and standard care cohorts, respectively. However, logistic regression analysis indicated that the difference between cohorts was not significant. Multivariable linear regression analysis also indicated no significant differences between cohorts for the IPSS or the OABq score at either assessment point. Notably, the uptake of the intervention was low (53%).
Conclusions
We found no significant benefit from adding an online self‐management program to standard care for men with LUTS, probably due to the low uptake of the intervention that may have resulted from the timing in the care pathway.
Keywords: BPH, conservative treatment, LUTS, self‐management
1. INTRODUCTION
Attempts to improve the self‐management of lower urinary tract symptoms (LUTS) in men are important given the age‐related increase in the prevalence of this often chronic condition,1, 2 and the associated need to reduce both overtreatment (eg, polypharmacy) and costs. To support self‐management among these patients, we have recently developed an online tool for use in secondary care that provides evidence‐based individualized advice.3 This requires that patients input information about LUTS severity, drinking habits, and caffeine intake, as well as information from frequency volume charts. The design of this online tool was based on available research evidence combined with input from general practitioners (GPs) and urologists. To explore the possible effects of this intervention on perceived global improvement and symptom severity among men referred to a Dutch urology outpatient department with LUTS, we conducted a pilot study.
2. PATIENTS AND METHODS
2.1. Study design
In this pilot study, we used a prospective double‐cohort design to compare standard care at a urology outpatient department (standard care cohort) and standard care plus access to our online self‐management program (intervention cohort). As no comparable studies were available we have chosen not to perform a randomized controlled trial. Due to a lack of information on the primary outcome we were unable to perform a power analysis.
A medical ethics review board confirmed that Medical Research Involving Human Subjects Act (WMO) did not apply to this study, which was otherwise conducted in accordance with the ethical standards of the 1964 Declaration of Helsinki and its subsequent revisions. All participants gave informed consent.
2.2. Participants and setting
We recruited consecutive men aged 18 years or older referred with uncomplicated LUTS to the urology outpatient departments of three large teaching nonacademic hospitals in the Netherlands. We have defined uncomplicated LUTS as having LUTS without a history of (recurrent) urinary tract infections, acute or chronic urinary retention, prostate or bladder cancer, neurogenic bladder, or urethral stricture.
The recruitment occurred in two phases: we initially enrolled men into the standard care cohort, before enrolling men into the intervention cohort. Between April 2017 and March 2018, all eligible men received a postal invitation to participate before their first outpatient visit. Information about the study was also made available in a video message.
A member of the research team met with eligible men 20 to 30 minutes before their scheduled outpatient appointment. During this meeting, participants were encouraged to ask questions before being asked to complete an informed consent form and a baseline questionnaire. Men were excluded if they did not comply with the definition of uncomplicated LUTS, had severe comorbidity, or had any inability to understand Dutch.
2.3. Protocol
All men received care as usual from a urologist. In addition, men in the intervention cohort received access to the online self‐management program. The development of the program has described elsewhere,3 but it consisted of nine items (ie, providing general information and education about LUTS including the lack of association with prostate cancer, pelvic floor muscle training, bladder training, urethral milking, double voiding, caffeine management, alcohol management, fluid management, and exercise advice). Based on the individual data input, only relevant advice was shown to participants.
Questionnaires were completed before the first consultation with a urologist (ie, baseline) and after 6 and 12 weeks. At baseline, we collected details of the patient characteristics together with symptom severity according to the International Prostate Symptom Score (IPSS)4, 5 and Overactive Bladder Questionnaire (OABq).6, 7 We also required patients to complete the eHealth Literacy Scale (eHEALS).8 At both follow‐up assessments, we recorded the type of treatment received (patient‐reported only) and repeated the IPSS and OABq questionnaires. At 12 weeks, we also asked participants to complete a Perceived Global Impression of Improvement (PGI‐I) questionnaire.9
2.4. Outcomes
The primary outcome of interest was the PGI‐I at 12 weeks. Because there were no studies available in the literature to provide estimates, we did not perform a sample size calculation. The secondary outcomes changed in symptom severity after 6 and 12 weeks, as assessed by the IPSS and OABq. Differences in the scores from baseline to both follow‐up assessments were calculated for each secondary outcome.
2.5. Statistical analyses
We performed complete case analyses by comparing the baseline characteristics of men who completed follow‐up and who dropped out. We used means and standard deviations for variables with normal distributions, median and interquartile range (IQR) for variables without normal distributions and frequencies and percentages for qualitative variables.
PGI‐I outcomes are presented as frequencies and percentages for both cohorts. In clinical practice, patients and physicians are interested in clear perceived improvement. Therefore, we dichotomized the PGI‐I outcomes into clear improvement (PGI‐I scores “much better” and “very much better”) and no clear improvement (all other PGI‐I scores). This dichotomy was used to explore associations by intervention cohort, age (categorized by tertile), body mass index, baseline symptom severity, and type of treatment received. Concerning treatment, patients were asked about the details of any lifestyle advice given, medication prescribed, physical therapy referrals, or surgery that had been recommended. Univariable logistic regression analyses were performed and variables with a P value of ≤ .25 were input in the multivariable model. Results are presented as odds ratios and 95% confidence intervals. For the final model, we present the percentage of explained variance (Nagelkerke R2).10
Mean changes in symptom severity scores (ie, IPSS and OABq scores) at 6 and 12 weeks are also presented. Linear regression analyses were then performed using the same variables as for the primary outcome. For the final models, we present the percentage of explained variance (adjusted R2).
The uptake of the online intervention was further investigated by identifying those men who actually used the intervention and those who visited the study website. We then compared the baseline characteristics of men who did and did not use the intervention, without performing statistical analysis. Reasons for noncompliance were identified using the questionnaire at week 12.
3. RESULTS
3.1. Participant characteristics
In total, 187 of 349 invited men (54%) agreed to participate, of whom 127 were enrolled in the standard care cohort and 60 were enrolled in the intervention cohort (Figure 1). Twenty participants were later excluded because of suspected bladder or prostate cancer (n = 15), a history of prior surgery for LUTS (n = 1), the presence of urethral stricture (n = 2), or failure to complete the baseline questionnaire (n = 2).
Figure 1.
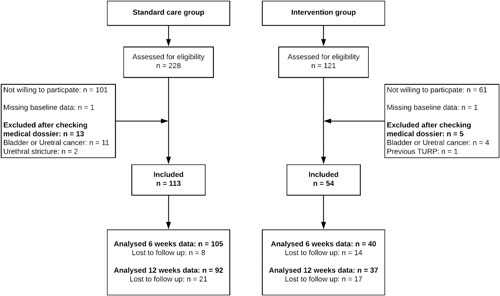
Flow chart of participant recruitment, inclusion, and follow‐up. TURP, transurethral resection of the prostate.
The baseline characteristics of each cohort are presented in Table 1. Age, symptom severity, and quality of life scores were comparable between the intervention and standard care cohorts, but participants in the intervention cohort scored significantly higher on the eHEALS questionnaire (29.0, IQR 8.3) compared with the standard care cohort (24.0, IQR 10.0) (P < .005). At 6 weeks, the dropout rate was significantly higher in the intervention cohort (25.9%) than in the standard care cohort (7.1%) (P = .01); although the pattern reversed at 12 weeks, it was no longer significant (18.6% and 31.5%, respectively; P = .063). Men who dropped out were significantly younger than those who remained in the study (mean 59.2 ± 1.7 years vs 65.2 ± 12.4 years; P = .04 at 6 weeks; 58.1 ± 14.5 years vs 66.2 ± 11.6 years; P < .001 at 12 weeks). However, body mass index, baseline IPSS score, and education levels did not differ between the men who dropped out and those who completed the study.
Table 1.
Baseline characteristics of the study population
| Control | Intervention | ||
|---|---|---|---|
| (N = 113) | (N = 54) | P value | |
| Age | |||
| Mean | SD | 65.2 | 13.5 | 62.7 | 10.9 | .243 |
| ≤60 | 31 (27.4) | 24 (44.4) | |
| 60‐70 | 31 (27.4) | 12 (22.2) | |
| ≥70 | 51 (45.1) | 18 (33.3) | |
| IPSS | |||
| Mean | SD | 19.0 (6.3) | 17.7 (6.1) | .232 |
| Mild (0‐7) | 5 (4.4) | 3 (5.6) | |
| Moderate (8‐19) | 58 (51.3) | 29 (53.7) | |
| Severe (>20) | 50 (44.2) | 22 (40.7) | |
| IPSS QoL | |||
| Median | IQR | 4.0 | 2.0 | 4.0 | 2.0 | .604 |
| OABq | |||
| Median | IQR | 40.0 | 26.7 | 38.3 | 30.0 | .625 |
| Education | |||
| None or elementary school only | 14 (12.4) | 4 (7.4) | .666 |
| Lower education | 32 (28.3) | 13 (24.1) | |
| Secondary education | 27 (23.9) | 14 (24.6) | |
| Higher education | 40 (35.4) | 23 (42.6) | |
| eHEALS | |||
| Median | IQR | 24.0 | 10.0 | 29.0 | 8.3 | .004 |
| Comorbidity | |||
| One or more | 53 (46.9) | 21 (38.9) | .405 |
| Cardiovascular diseases | 31 (27.4) | 12 (22.2) | |
| Diabetes Mellitus | 18 (15.9) | 8 (14.8) | |
| Parkinson disease | 0 (0.0) | 0 (0.0) | |
| COPD | 9 (8.0) | 5 (9.3) | |
| Sleep apnoea | 16 (14.2) | 4 (7.4) | |
| CVA | 4 (3.5) | 2 (3.7) | |
| Treatment by GP | 73 (64.6) | 29 (53.7) | 0.235 |
| BMI | |||
| Median | IQR | 26.2 | 5.4 | 26.0 | 4.4 | 0.640 |
| ≤25 | 43 (38.1) | 16 (29.6) | |
| 25‐30 | 53 (46.9) | 27 (50.0) | |
| ≥30 | 17 (15.0) | 10 (18.5) |
Note: Outcomes are presented as frequencies and percentages unless otherwise stated.
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; eHEALS, eHealth literacy scale; GP, general practitioner; IPSS, International Prostate Symptom Score; IQR, interquartile range; OABq, Overactive Bladder Questionnaire; QoL, quality of life; SD, standard deviation.
3.2. Primary outcome: changes in the PGI‐I
Figure 2 shows the PGI‐I results in both cohorts. Clear improvements were reported by 19.4% of men in the intervention cohort and by 26.1% of men in the standard care cohort. Both the univariable and the multivariable logistic regression analyses yielded no differences between the cohorts on this outcome (Table 2). The Nagelkerke R2 was 0.12 for the final model.
Figure 2.
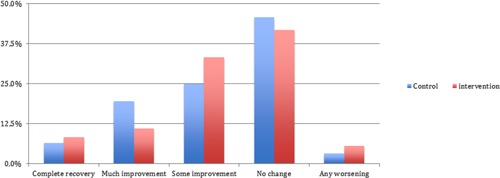
PGI‐I results at 12 weeks in the intervention and standard care cohorts. The above figure is only shown to 50% on the vertical axis. PGI‐I, Perceived Global Impression of Improvement.
Table 2.
Linear regression analysis of the predictors of clear improvement in the PGI‐I at 12 weeks
| Univariable analyses | Multivariable model | |||
|---|---|---|---|---|
| OR | (95% CI) | OR | (95% CI) | |
| Intervention group (ref = control) | 0.71 | (0.27; 1.83) | 0.73 | (0.26‐2.07) |
| Age (reference age is ≤60 y) | ||||
| 60‐70 y | 0.60 | (0.18; 1.97) | NI | |
| ≥70 y | 1.21 | (0.46; 3.23) | NI | |
| Baseline severity LUTS (IPSS) | 0.93 | (0.86; 1.00) | 0.94 | (0.87‐1.01) |
| BMI (reference point is ≤25) | ||||
| 25‐30 | 0.73 | (0.30; 1.78) | NI | |
| ≥30 | 0.66 | (0.18; 2.42) | NI | |
| Lifestyle advice | 1.10 | (0.45; 2.70) | NI | |
| Medication | 1.44 | (0.61; 3.38) | NI | |
| Surgery | 0.26 | (0.08; 0.80) | 0.32 | (0.10‐1.04) |
| Physical therapy | 1.38 | (0.55‐3.42) | NI | |
Note: The PGI‐I outcome scores were dichotomized into clear improvement (“much better” and “very much better”) and no clear improvement (all other PGI‐I scores).
Abbreviations: CI, confidence interval; IPSS, International Prostate Symptom Score; NI, not included in the analysis (univariable P > .25); OR, odds ratio; PGI‐I Perceived Global Impression of Improvement.
3.3. Secondary outcomes: changes in the IPSS and OABq
After 6 and 12 weeks, the respective IPSS changes were −4.0 ± 5.9 and −4.8 ± 6.4 in the control cohort and −2.2 ± 4.5 and −3.2 ± 4.8 in the intervention cohort. The respective changes in the OABq at 6 and 12 weeks were −9.3 ± 14.8 and −9.8 ± 17.3 in the control cohort and −8.3 ± 14.0 and −6.8 ± 14.9 in the intervention cohort. No linear regression analysis showed significant differences between the cohorts for either outcome (Tables S2 and S3). The adjusted R2 values for the final models were 0.14 (IPSS change at 6 weeks), 0.16 (IPSS change at 12 weeks), 0.26 (OABq change at 6 weeks), and 0.26 (OABq change at 12 weeks).
3.4. Analysis of the intervention cohort
Only 43 of the 60 participants enrolled in the intervention cohort completed one or both follow‐up questionnaires, and of these, only 23 (53%) used the intervention. There were no statistically significant differences in baseline characteristics between the users and nonusers (Table S1). The main reasons cited for not using the website were a lack of time to use it (40%), a lack of interest in using it (30%), and not being an internet user (15%).
4. DISCUSSION
In this pilot study, we found no benefit from adding a newly developed online self‐management program to care as usual for men with uncomplicated LUTS in a secondary care setting. Specifically, the changes in the perceived improvement and symptom severity did not differ between cohorts. The lack of improvement in the intervention group may be explained by the low uptake of the online program in the intervention group, with only half of all men actually using the intervention.
There has been limited research in the published literature, so the data reported by Brown et al serves as the main point of reference for our findings.9 Brown et al compared the impact of self‐management with that of care as usual in a hospital setting.9 In that study, the self‐management program consisted of supervised group sessions,11, 12 and the main outcome was “treatment failure,” defined as one or more of the following: symptom‐worsening (three points or more on the IPSS), use of medication to control LUTS, acute urinary tract infection, or surgical intervention. At 3 months, treatment failure had occurred in 10% of the self‐management group and in 42% of the standard care group.9 Notably, our results contrast with those reported by Brown et al, but the comparison of our data is hampered by several important differences.
First, Brown et al excluded men who had received any form of medical treatment (alpha‐blockers, 5‐alpha‐reductase inhibitors, or anticholinergics) in the preceding 3 months.9 In our study, consistent with real‐world practice advocated by the Dutch guideline on Male LUTS,13 many participants (61%) had received treatment from their GP before referral to the urologist. This is an important distinction because the effect of self‐management may be larger in untreated men. The second difference is that standard care in the study by Brown et al12 began with watchful waiting for all participants. By contrast, we did not dictate or intervene with the care provided by the urologist. This resulted in a major difference between studies, with only 19% of participants receiving active treatment in the study by Brown et al compared with 81% in the current study. In the Netherlands, most receive an alpha‐blocker before referral to a urologist, so we believe that a new period of watchful waiting would be unhelpful and would have reduced the ecological validity of our research. Finally, men participating in the study by Brown et al may have felt more obliged to follow the intervention because it was provided in small group sessions. No such impetus was present in our study, and patients were free to engage or not engage with the intervention.
Despite the finding that our online intervention provided no additional benefit to care as usual in the present cohorts, we believe that the efforts of this pilot study still have merit. For example, although we wanted to determine the potential for benefit in secondary care, this placement in the care pathway may be the most relevant explanation for the lack of effectiveness. Indeed, various guidelines have established that self‐management is relevant at the start of a clinical encounter,1, 2, 13 but most men in our research were already on an established care pathway and may have already received self‐management advice and drug treatment from a GP. Therefore, these men will probably have been less sensitive to the effects of our intervention. Recently, Norton et al14 summarized the outcomes of a working group of the National Institute of Diabetes and Digestive and Kidney Disease, focusing on the understanding of self‐management for male LUTS. In that research, the authors mentioned that a self‐management program based on internet and video platforms was being evaluated among more than 250 000 men with LUTS by Kaiser Permanente, Southern California. Until that research is published, our intervention provides details of the first evidence‐based online intervention for the self‐management of LUTS.
Our study has several limitations that limit us to draw firm conclusions. First, we used a nonrandomized design for feasibility reasons, which will have hampered our ability to interpret the outcomes. Nevertheless, given that care, as usual, was similar in both cohorts, we still believe that this study gives important insights into the potential effect of our online intervention. Second, we encountered considerable levels of nonresponse and dropout. Although this may reflect a lack of interest in this population, it was notable that dropout rates were higher in the intervention cohort. If men considered noncompliance with the online intervention as justification for not completing the questionnaires, it might explain their apparent dropout. In future studies, we will explain more clearly that active study participation (eg, completing questionnaires) does not depend on treatment adherence. Third, the different cohort sizes resulted from different enrollment periods. Enrollment for the standard care cohort began with study inception, at which point the intervention was still under construction. Therefore, enrollment into the first cohort was prolonged and enrollment into the second cohort was cut short due to time restraints. The resulting small intervention cohort prevented in‐depth subgroup analyses (eg, based on adherence to the intervention).
Our intervention provides a new, evidence‐based, online tool that offers men access to important and personalized information on their most bothersome symptoms. Given the issues associated with introducing this intervention after patients were referred to secondary care, further research will be needed before they reach this stage. To this end, we have planned a new trial in which we will assess the impact of the online self‐management program on healthcare‐seeking behavior among men with LUTS in primary care, including the impact on referrals to secondary care. Specifically, adult men who have symptoms of LUTS and who either are considering visiting a GP or are being managed by their GP will be targeted.
Supporting information
Supporting information
Supporting information
Supporting information
Supporting information
Blanker MH, Admiraal A, Brandenbarg P, et al. Effectiveness of a newly developed online self‐management program for male patients with uncomplicated lower urinary tract symptoms. Neurourology and Urodynamics. 2019;38:2273‐2279. 10.1002/nau.24131
References
REFERENCES
- 1. National Clinical Guideline Centre (NICE) . Lower urinary tract symptoms in men: Management (clinical guideline CG97). 2015;CG97(01/09).
- 2. Gratzke C, Bachmann A, Descazeaud A, et al. EAU guidelines on the assessment of nonneurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol. 2015;67(6):1099‐1109. [DOI] [PubMed] [Google Scholar]
- 3. Blanker MH, Brandenbarg P, Slijkhuis BGC, Steffens MG, van Balken MR, Jellema P. Development of an online personalized self‐management intervention for men with uncomplicated LUTS. Neurourol Urodyn. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barry MJ, Williford WO, Chang Y, et al. Benign prostatic hyperplasia specific health status measures in clinical research: how much change in the american urological association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients? J Urol. 1995;154(5):1770‐1774. [DOI] [PubMed] [Google Scholar]
- 5. Barry MJ, Fowler FJ Jr, O'leary MP, et al. The American urological association symptom index for benign prostatic hyperplasia. J Urol. 1992;148(5):1549‐1557. [DOI] [PubMed] [Google Scholar]
- 6. Coyne KS, Matza LS, Kopp Z, Abrams P. The validation of the patient perception of bladder condition (PPBC): a single‐item global measure for patients with overactive bladder. Eur Urol. 2006;49(6):1079‐1086. [DOI] [PubMed] [Google Scholar]
- 7. Coyne KS, Matza LS, Thompson CL. The responsiveness of the Overactive Bladder Questionnaire (OAB‐q. Qual Life Res. 2005;14(3):849‐855. [DOI] [PubMed] [Google Scholar]
- 8. van der Vaart R, van Deursen AJ, Drossaert CH, Taal E, van Dijk JA, van de Laar MA. Does the eHealth literacy scale (eHEALS) measure what it intends to measure? Validation of a dutch version of the eHEALS in two adult populations. J Med Internet Res. 2011;13(4):e86 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3222202/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Viktrup L, Hayes RP, Wang P, Shen W. Construct validation of patient global impression of severity (PGI‐S) and improvement (PGI‐I) questionnaires in the treatment of men with lower urinary tract symptoms secondary to benign prostatic hyperplasia. BMC Urol. 2012;12:30. 30‐2490‐12‐30, PMID: 23134716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nagelkerke NJ. A note on a general definition of the coefficient of determination. Biometrika. 1991;78(3):691‐692. [Google Scholar]
- 11. Brown C, van der Meulen J, Mundy AR, O'Flynn E, Emberton M. Defining the components of a self‐management programme for men with uncomplicated lower urinary tract symptoms: a consensus approach. Eur Urol. 2004;46(2):254‐263. [DOI] [PubMed] [Google Scholar]
- 12. Brown CT, Yap T, Cromwell DA, et al. Self management for men with lower urinary tract symptoms: randomised controlled trial. BMJ. 2007;334(7583):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Blanker MH, Klomp MA, van den Donk M, van der Heide WK, Opstelten W, Burgers JS. Summary of the NHG practice guideline “lower urinary tract symptoms in men”. Ned Tijdschr Geneeskd. 2013;157(18):6178. [PubMed] [Google Scholar]
- 14. Norton JM, Bavendam TG, Elwood W, et al. Research needs to understand self‐management of lower urinary tract symptoms: summary of NIDDK workshop. J Urol. 2018;199(6):1408‐1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Supporting information
Supporting information
Supporting information


